Abstract
An oral prodrug of GS 4071, a potent and selective inhibitor of influenza neuraminidases, is currently under clinical development for the treatment and prophylaxis of influenza virus infections in humans. To investigate the potential development of resistance during the clinical use of this compound, variants of the human influenza A/Victoria/3/75 (H3N2) virus with reduced susceptibility to the neuraminidase inhibitor GS 4071 were selected in vitro by passaging the virus in MDCK cells in the presence of inhibitor. After eight passages, variants containing two amino acid substitutions in the hemagglutinin (A28T in HA1 and R124M in HA2) but no changes in the neuraminidase were isolated. These variants exhibited a 10-fold reduction in susceptibility to GS 4071 and zanamivir (GG167) in an in vitro plaque reduction assay. After 12 passages, a second variant containing these hemagglutinin mutations and a Lys substitution for the conserved Arg292 of the neuraminidase was isolated. The mutant neuraminidase enzyme exhibited high-level (30,000-fold) resistance to GS 4071, but only moderate (30-fold) resistance to zanamivir and 4-amino-Neu5Ac2en, the amino analog of zanamivir. The mutant enzyme had weaker affinity for the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid and lower enzymatic activity compared to the wild-type enzyme. The viral variant containing the mutant neuraminidase did not replicate as well as the wild-type virus in culture and was 10,000-fold less infectious than the wild-type virus in a mouse model. These results suggest that although the R292K neuraminidase mutation confers high-level resistance to GS 4071 in vitro, its effect on viral virulence is likely to render this mutation of limited clinical significance.
Influenza virus infections remain a serious seasonal health concern and the potential of severe pandemics due to the emergence of new influenza strains, such as the H5N1 “bird flu” recently identified in Hong Kong (39), provides additional impetus to develop potent and effective antiviral agents (24). At present, only amantadine and, in some countries, rimantadine are approved for the treatment and prophylaxis of influenza A infections. However, the usefulness of these two compounds is limited by their lack of activity against influenza B viruses and their rapid selection of drug-resistant mutants which remain transmissible and pathogenic (10, 25).
The influenza neuraminidase, which is expressed on the virus surface, has long been considered a valid target for antiviral therapy. This enzyme, which cleaves terminal sialic acid residue from glycoconjugates, is essential for virus proliferation and infectivity (3, 17, 19, 27, 28). The observation that the structural and catalytic amino acids which line the enzyme active site are highly conserved among different influenza neuraminidase types and subtypes (reviewed in reference 6) suggests that inhibitors of this enzyme would be active against a broad range of influenza viruses.
Based on information gained from crystallographic studies of influenza neuraminidases complexed with sialic acid or the transition state analog Neu5Ac2en (2, 41, 43), several potent and selective inhibitors of the influenza neuraminidases have been synthesized (15, 16, 43). Two of these, GS 4071 ([3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]1-cyclohexene-1-carboxylic acid), in the form of its oral prodrug GS 4104, and zanamivir (GG167, 4-guanidino-Neu5Ac2en) are currently under clinical development for the prophylaxis and treatment of influenza virus infections. Both compounds have demonstrated efficacy against influenza A and B viruses in vitro (13, 23, 40, 43, 45), in animal models of influenza virus infection (23, 31, 32, 34), and in experimental influenza virus infection in humans (11, 12, 14) when GS 4104 is taken orally and zanamivir is delivered topically to the respiratory tract as an inhalant.
Although the development of resistance to zanamivir in animals or people treated with the drug has not been reported, influenza variants resistant to zanamivir due to mutations within their hemagglutinin or neuraminidase genes have been selected in vitro (1, 7, 8, 20, 38). In general, zanamivir-resistant hemagglutinin mutants have been easier to generate than neuraminidase mutations. These hemagglutinin mutants commonly contain amino acid substitutions in or near the sialic acid binding site and are believed to make the virus replication less dependent on neuraminidase activity (7, 20, 33). However, these mutations likely only affect the in vitro, not the in vivo, susceptibility to zanamivir (29).
The most common neuraminidase mutation which arises in vitro under selective pressure of zanamivir has been that of the conserved Glu119 residue in the neuraminidase active site (1, 7, 38). Mutations of Glu119, which interacts with the guanidino side chain of zanamivir but not with the natural substrate (43), cause a 100-fold reduction in the sensitivity of the enzyme to zanamivir (1, 7, 38). Viruses containing mutations at this position remain infectious (8) and capable of inducing a febrile response in ferrets (5). Recently, a Lys substitution for the conserved Arg at position 292 has also been reported for a variant selected in the presence of zanamivir (8). The neuraminidase containing this mutation exhibited only a 10-fold reduction in sensitivity to zanamivir. A reassortant virus containing the mutant neuraminidase was 500-fold less infectious than wild-type virus in mice (8).
In this report, we describe the first in vitro isolation and characterization of variants of a human influenza virus, A/Victoria/3/75 (H3N2), selected in the presence of GS 4071. In contrast with the experience with amantadine and rimantadine, with which drug-resistant variants can be selected after one or two passages in culture (26), variants with decreased susceptibility to GS 4071 did not readily occur. In the eighth passage, a variant containing two mutations in the stalk region of the hemagglutinin was isolated. This variant exhibited a minor decrease in susceptibility to neuraminidase inhibitors in general. A second variant, containing a conservative substitution of a Lys for an Arg at amino acid 292 of the neuraminidase enzyme active site, was isolated later in the selection process. This mutation caused a marked decrease in the susceptibility of the virus and the sensitivity of the enzyme to GS 4071. However, this mutation also adversely affected neuraminidase enzyme activity, compromised the ability of the virus to replicate in tissue culture, and reduced the infectivity of the virus 10,000-fold in mice.
MATERIALS AND METHODS
Cells, virus, and neuraminidase inhibitors.
Madin-Darby canine kidney (MDCK) cells, from the American Type Culture Collection (ATCC) (Bethesda, Md.), were grown in Eagle’s minimum essential medium containing Earle’s salts, Fungi-Bac (Irvine Scientific, Santa Ana, Calif.), and 10% fetal bovine serum (Irvine Scientific). The human influenza A/Victoria/3/75 (H3N2) virus was obtained from ATCC and was propagated in the allantoic cavity of fertilized specific-pathogen-free hen eggs (SPAFAS, Norwich, Conn.) or in MDCK cells in maintenance medium composed of Eagle’s minimum essential medium containing Earle’s salts, Fungi-Bac, 15 mM HEPES (pH 7.4), and tolylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin (Worthington Biochemical, Freehold, N.J.) at a final concentration of 2 μg/ml. The influenza neuraminidase inhibitor GS 4071, zanamivir, and 4-amino-Neu5Ac2en were synthesized at Gilead Sciences, Inc., in accordance with previously published procedures (16, 42).
Isolation of influenza variants with decreased susceptibility to GS 4071.
MDCK cells in 24-well tissue culture plates were infected with egg-grown virus at a low multiplicity of infection (0.001 to 0.03 PFU per cell) in maintenance medium. In order to minimize the probability of selecting hemagglutinin mutants with weak affinity for cellular receptors, virus was allowed to adsorb for only 15 min at 37°C, after which time the inoculum was removed and the cells were washed with prewarmed phosphate-buffered saline (PBS) (1). After the PBS wash, fresh maintenance medium containing GS 4071 was added, and the cultures were incubated at 35°C until greater than 90% of the cells were lysed. At that time, the virus titer in the culture supernatant cleared of cellular debris was determined by plaque assay or hemagglutination of human erythrocytes. The culture supernatant was then used as inoculum to infect a new cell monolayer, again at a low multiplicity of infection, and the virus was allowed to grow in the presence of a higher concentration of GS 4071. The concentration of GS 4071 in the first round of selection was 0.6 nM, which is the 50% inhibitory concentration (IC50) for egg-grown A/Victoria/3/75 virus in a plaque reduction assay (23). The concentration of GS 4071 in the second round of selection was 2.4 nM, and it was doubled in each subsequent passage. Every few passages, plaque reduction assays were performed to determine whether the harvested virus exhibited decreased susceptibility to GS 4071. The 12-B1 and 12-S3 variants, isolated from the virus pool harvested after 12 passages in the presence of GS 4071, were plaque purified through three rounds in the presence of inhibitor.
Tissue culture assays.
Plaque reduction assays to determine susceptibility to the neuraminidase inhibitors were performed as described previously (9, 16). Briefly, confluent monolayers of MDCK cells in six-well tissue culture plates were inoculated with 30 PFU per well of virus in maintenance medium containing 0.2% bovine serum albumin. After 30 min at 37°C, the inoculum was removed, and fresh maintenance medium containing 0.2% bovine serum albumin, the desired concentration of inhibitor, and 1% agarose was added. After 2 to 3 days at 37°C, the overlay was removed, and the cell monolayer was stained with 0.1% crystal violet in 20% methanol. The IC50 was taken to be the concentration of inhibitor required to reduce the number of plaques to 50% of that in duplicate wells containing no inhibitor. No attempt was made to quantitate plaque size.
Virus yield assays were performed in 24-well tissue culture plates containing confluent monolayers of MDCK cells. Cells were infected as described for the plaque reduction assays, using a low multiplicity of infection (0.001 PFU per cell). After removal of the inoculum, cells were fed with 1 ml of maintenance medium. At the indicated times after infection, samples were harvested and the virus titers determined by plaque assay as described above.
Neuraminidase enzymatic assay.
Neuraminidase activity was assayed by using previously described modifications (23) to the method of Potier et al. (30). Briefly, virus in culture supernatants from fully cytopathic cultures of MDCK cells was cleared of cellular debris by centrifugation at 800 × g for 10 min, solubilized with the addition of Nonidet P-40 to a final concentration of 0.1%, and used without further modification as the source of enzyme. Assays to determine sensitivity (IC50) to neuraminidase inhibitors were performed at 37°C as 100-μl reactions containing 50 μM 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA) as substrate, 33 mM MES (morpholineethanesulfonic acid) (pH 6.5), 4 mM CaCl2, and a concentration of solubilized virus empirically determined to maintain a constant level of enzyme activity throughout the 20-min reaction time. After stopping reactions with the addition of 150 μl of 14 mM NaOH in 83% ethanol, fluorescent product was quantitated in a Perkin-Elmer fluorimeter (model LS50B). Experiments to determine inhibition constants (Ki) were performed as described previously (23) with the exception that Nonidet P-40-treated tissue culture supernatant was used as the source of enzyme. Determinations of IC50 and Ki values were made after a 45-min preincubation of enzyme with inhibitor.
Investigation of the time-dependent change in the inhibitory activity of GS 4071 and other neuraminidase inhibitors was based on analysis of product formation progress plots as described previously (1, 7) by using Nonidet P-40-treated tissue culture supernatants as the source of enzyme. The amount of each culture supernatant used was determined empirically to ensure that the rate of product formation in the uninhibited samples was constant throughout the 90-min reaction period.
Sequencing of neuraminidase and hemagglutinin genes.
Viral RNA was prepared from tissue culture supernatant or allantoic fluid with a QIAamp viral RNA kit (Qiagen). The synthetic oligonucleotide 5′-AGCAAAAGCAGG-3′ was used as primer to generate cDNAs of the eight viral RNA segments by using Ready-To-Go You-Prime First-Strand Beads (Pharmacia). PCR amplification of the neuraminidase gene was accomplished by using the Expand PCR System (Boehringer Mannheim) and the oligonucleotides 5′-GGAGTGAAGATGAATCCAA-3′ and 5′-GTAGAAACAAGGAGTTTTTTC-3′ as coding and noncoding primers, respectively. The hemagglutinin gene was amplified in a similar manner by using the oligonucleotides 5′-GCAGGGGATAATTCTATTAACCATG-3′ and 5′-AGGGTGTTTTTAATTACTAATACAC-3′ as coding and noncoding primers, respectively. PCR products were purified with the Wizard PCR DNA purification system (Promega) and sequenced manually by using the Thermo Sequenase system (Amersham).
Determination of viral infectivity.
Groups of six female specific-pathogen-free BALB/c mice (8 to 10 g; B&K International, Fremont, Calif.) were inoculated intranasally with 100 μl of 10-fold serial dilutions of the wild-type virus, or the plaque-purified 12-B1 or 12-S3 variants in PBS. Three days after infection, three mice from each group were sacrificed, and their lungs were weighed and scored from 0 (normal) to 4 (maximum lung coloration) for the appearance of consolidation. The lungs were then homogenized, and serial dilutions of the lung homogenate were assayed in MDCK cells for infectious virus as described previously (35). Seven days after infection, the remaining three mice from each group were sacrificed, and their lungs were analyzed as described above.
RESULTS
Isolation of variants with decreased susceptibility to GS 4071.
The human influenza A/Victoria/3/75 (H3N2) virus, propagated in embryonated hen eggs, was passaged in MDCK cells in the presence of concentrations of GS 4071 that were increased twofold at each passage. By the third passage, the earliest tested, the susceptibility of the virus pool to GS 4071 and zanamivir in a plaque reduction assay was eightfold lower than that of the wild-type virus. Sequence analysis of the neuraminidase gene of the virus pool indicated no differences from that of the wild-type virus. A similar decrease in susceptibility to the neuraminidase inhibitors was observed for virus passaged for the same number of rounds in the absence of inhibitor, suggesting that this change is due to an adaptation of the egg-grown virus to the tissue culture system.
After eight passages in the presence of GS 4071, the virus exhibited a further decrease in susceptibility to GS 4071. Genotypic analysis of plaque purified variants from this passage did not detect mutations in the neuraminidase gene but revealed two mutations in the hemagglutinin gene resulting in an Ala→Thr substitution at amino acid 28 of HA1 (A28T) and an Arg→Met substitution at amino acid 124 of HA2 (R124M). These two mutations, which are located in the stalk region of the hemagglutinin, not the sialic acid binding site, were not detected in virus passaged in the absence of inhibitor or at earlier passages in the presence of inhibitor. The variants were approximately 10-fold less susceptible to both GS 4071 and zanamivir in a plaque reduction assay (Table 1). These data suggest that the hemagglutinin mutations, though caused by the selective pressure exerted by GS 4071, reduce the susceptibility of the virus to neuraminidase inhibitors in general and not to GS 4071 in particular.
TABLE 1.
Inhibition of wild-type and variant viruses in a plaque reduction assay
| Virus | Encoded amino acida
|
Fold change in IC50b
|
|||
|---|---|---|---|---|---|
| 292 (NA) | 28 (HA1) | 124 (HA2) | GS 4071 | Zanamivir | |
| A/Victoria/3/75 (MDCK adapted) | Arg (wt) | Ala (wt) | Arg (wt) | 1 (0.008 μM) | 1 (0.033 μM) |
| Passage 8c | Arg | Thr | Met | 8.6 | 8.5 |
| 12-B1 | Arg | Thr | Met | 6.9 | 6.4 |
| 12-S3 | Lys | Thr | Met | 3,230 | 60 |
Genotypic results are indicated for amino acid 292 of the neuraminidase (NA), amino acid 28 of subunit 1 of the hemagglutinin (HA1), and amino acid 124 of subunit 2 of the hemagglutinin (HA2).
Data are shown as fold change in IC50 relative to values obtained for the wild-type virus passaged in MDCK cells in the absence of inhibitor. Numbers shown in parentheses indicate IC50 values for wild-type virus passaged in MDCK cells. IC50 values are from a single experiment in which all samples were tested in parallel.
IC50 values are given for a plaque-purified variant isolated from virus harvested after eight passages in the presence of GS 4071.
By the 11th passage, there was a further decrease in susceptibility to GS 4071. Genotypic and phenotypic analysis of eight plaque-purified variants from the 12th passage indicated the presence of two virus populations, with the 12-B1 and 12-S3 variants being representative of each of the two populations. The 12-B1 variant had the A28T and R124M hemagglutinin mutations seen in virus isolated from passage 8 but did not have neuraminidase mutations. The 12-S3 variant had a Lys substitution for the conserved Arg at amino acid 292 (R292K) of neuraminidase in addition to the A28T and R124M mutations in hemagglutinin. Passage of the 12-S3 variant for 10 rounds in the absence of inhibitor had no effect on its genotype or phenotype, indicating that both the hemagglutinin and neuraminidase mutations were stable.
To determine the effect of the hemagglutinin and neuraminidase mutations on the susceptibility of the virus to different neuraminidase inhibitors, the susceptibility of the 12-S3 variant was compared to that of the 12-B1 variant and wild-type virus that had been passaged for 12 rounds in MDCK cells in the absence of inhibitor. The results shown in Table 1 indicate that in a plaque reduction assay the 12-B1 variant exhibited the same modest decrease in susceptibility to GS 4071 and zanamivir as the passage 8 virus containing the same two hemagglutinin mutations. The 12-S3 variant, containing the neuraminidase mutation in addition to the hemagglutinin mutations, exhibited a further reduction in susceptibility to both GS 4071 and zanamivir compared to the 12-B1 variant, with the effect more dramatic for GS 4071 (Table 1).
Growth of the 12-B1 and 12-S3 variants in culture.
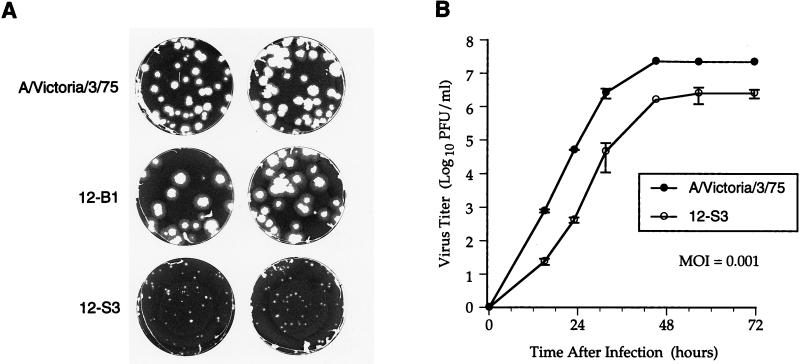
Although the wild-type virus and the 12-B1 variant grew well in the allantoic cavity of fertilized hen eggs, the 12-S3 variant did not grow in eggs. To determine the effect of the hemagglutinin and neuraminidase mutations on the ability of the viruses to replicate in tissue culture, the growth of wild-type or variant viruses in a plaque assay was investigated in the absence of inhibitor. As shown in Fig. 1, the 12-B1 variant containing the two hemagglutinin mutations was able to replicate well in MDCK cells, producing plaques which were similar in size to those formed by the wild-type virus. In contrast, the 12-S3 variant containing both the hemagglutinin and neuraminidase mutations formed much smaller plaques, indicating that this virus was compromised for growth as determined by plaque assay.
FIG. 1.
The 12-S3 variant is compromised for growth in culture. (A) MDCK cells were infected with the wild-type A/Victoria/3/75 virus, the 12-B1 variant, or the 12-S3 variant in a standard plaque assay. After 3 days at 37°C, the cell monolayers were stained to reveal the size of the plaques. (B) MDCK cells were infected with the wild-type virus or the 12-S3 variant at a multiplicity of infection of 0.001 PFU per cell. At the indicated times after infection, the titer of virus in the culture supernatant was determined. The data points are means and standard deviations obtained from duplicate wells in a representative experiment.
Comparison of the growth of the wild-type virus and the 12-S3 variant in a virus yield assay also indicated that the mutant was compromised for growth in cell culture (Fig. 1). Although the two viruses had similar growth rates at mid-log phase, the 12-S3 mutant exhibited an initial lag in its growth and achieved approximately 10-fold-lower peak titers.
Effect of the R292K mutation on neuraminidase activity.
The effect of the R292K neuraminidase mutation on the properties of the neuraminidase enzyme was determined by comparing the wild-type and mutant enzymes in an in vitro assay using the fluorogenic substrate MUNANA. As shown in Table 2, the affinity of the mutant neuraminidase for the substrate was reduced approximately fivefold relative to that of the wild-type enzyme as indicated by the increase in Km value. The R292K mutation also affected the activity of the enzyme, causing a >10-fold decrease in the turnover rate as indicated by measurements of Vmax. These assays were performed with a standardized amount of detergent-solubilized virus based on measurements of total virus protein and hemagglutinating activity. Based on these data, the catalytic efficiency (Vmax/Km ratio) of the mutant neuraminidase was approximately 2% of that of the wild-type enzyme. Similar results (unpublished data) indicating that the R292K mutation causes a decrease in enzyme activity and affinity for substrate were obtained when assays were performed at a pH of 5.0, the pH optimum of the mutant enzyme (8).
TABLE 2.
Characterization of wild-type and R292K mutant neuraminidases
| Enzyme | Substratea
|
Ki (nM)b
|
|||
|---|---|---|---|---|---|
| Km (μM) | Relative Vmax | GS 4071 | Zanamivir | 4-Amino-Neu5Ac2en | |
| Wild type | 84 | 17 | 0.13 (3.25 × 10−6) | 0.33 (8.25 × 10−6) | 100 (2.50 × 10−3) |
| Mutant | 337 | 1 | 3,600 (2.00 × 10−2) | 8.0 (4.44 × 10−5) | 3,000 (1.67 × 10−2) |
Km and relative Vmax values were determined by using MUNANA as substrate and are average values from at least three separate experiments using different virus preparations.
Ki values were determined as described in reference 23 and are average values from two independent experiments. Numbers shown in parentheses indicate the Ki/Km ratio as an indicator of relative inhibitory efficiency.
To determine whether the R292K mutation affects enzyme stability, the neuraminidase activities of purified wild-type and 12-S3 viruses after incubation for up to 72 h at 4, 37, or 42°C were compared. The stability of neuraminidase activity of the variant was comparable to that of the wild-type virus at all temperatures (data not shown). Unlike the zanamivir-resistant neuraminidase containing a Glu→Gly substitution at amino acid 119 (4, 21), these results indicate that the effect of the R292K mutation on enzyme activity is not due to a decrease in enzyme stability.
Drug sensitivity of the R292K mutant neuraminidase.
The sensitivity of the wild-type and mutant neuraminidases to inhibition by GS 4071, zanamivir, and 4-amino-Neu5Ac2en, the amino analog of zanamivir, was determined in an enzymatic assay. As shown in Table 2, the inhibition constant (Ki) for GS 4071 was 30,000-fold higher for the mutant enzyme than for the wild-type enzyme. In contrast, there was only a 30-fold increase in the Ki value for zanamivir and 4-amino-Neu5Ac2en.
The dramatic effect of the R292K mutation on the sensitivity to GS 4071 was surprising due to the conservative nature of the substitution and the fact that amino acid 292 does not interact with the 3-pentyloxy side chain of GS 4071 (16). To attempt to understand the role of the R292K mutation, we compared the sensitivities of the wild-type and mutant enzymes to a series of GS 4071 analogs which have different side chains and activities against the wild-type enzyme. As shown in Table 3, the R292K mutant enzyme was less sensitive to all the compounds tested than the wild-type enzyme. However, the R292K mutation had a much more pronounced effect on sensitivity to GS 4071 analogs containing branched side chains than those with linear or cyclic side chains.
TABLE 3.
Inhibition of wild-type and mutant neuraminidases by analogs of GS 4071
| Compound | R | IC50 (nM)a
|
Fold increase in IC50 | |
|---|---|---|---|---|
| Wild type | Mutant | |||
| GS 4071 |  |
0.2 | 6,500 | 32,500 |
| GS 4047 | 0.7 | 8,500 | 12,145 | |
| GS 4048 | 0.8 | 22,500 | 28,125 | |
| GS 3768 | 60 | 14,000 | 235 | |
| GS 4926 | 290 | 21,000 | 70 | |
| GS 4993 | 750 | 44,500 | 60 | |
| GS 4093 | 21 | 17,500 | 835 | |
| GS 4383 | 0.09 | 6,500 | 72,220 | |
Data shown are average values from two independent experiments using 50 μM MUNANA as substrate.
To further investigate the effect of the R292K mutation on the sensitivity of the mutant neuraminidase to GS 4071, the kinetics of inhibition by this compound of the wild-type and mutant neuraminidases were determined. As shown in Fig. 2, progress plots for the hydrolysis of MUNANA by the wild-type virus exhibit slow-binding inhibition by GS 4071 as indicated by the time-dependent change in the rate of product formation in reactions containing inhibitor. In contrast, similar experiments performed with the mutant neuraminidase indicate that GS 4071 is not a slow-binding inhibitor of this enzyme. For comparison, zanamivir is a slow-binding inhibitor of both the wild-type and R292K mutant neuraminidases (data not shown), indicating that the loss of slow-binding activity is specific to GS 4071.
FIG. 2.
Kinetics of inhibition of wild-type and mutant neuraminidase by GS 4071. Progress plots depicting the hydrolysis of MUNANA by the wild-type (A) and mutant (B) neuraminidase in the presence of varying concentrations of GS 4071. The concentration of GS 4071 present in each of the reactions is indicated.
The R292K neuraminidase mutation reduces virulence.
Gubareva et al. (8) have recently reported that reassortants containing an avian N2 neuraminidase with the R292K mutation are less infectious than the wild-type virus in mice. To investigate the effect of the R292K neuraminidase mutation on the virulence of the human influenza virus used in this study and to determine whether hemagglutinin mutations selected in combination with the R292K neuraminidase mutation in vitro could restore virulence to a variant containing the mutant neuraminidase, we investigated the virulence of the wild-type, 12-B1, and 12-S3 viruses in mice. The infectivity of the wild-type virus and 12-B1 variant were similar, with mean infectious doses of 5 and 8 PFU per mouse, respectively, as measured by the presence of virus in lung homogenates of animals prepared 3 days after infection. The two viruses also were similar in terms of their ability to induce an increase in lung weight and the gross appearance of consolidation at autopsy, indicating that the A28T and R124M hemagglutinin mutations had no effect on virulence. In contrast, even at an inoculum of 40,000 PFU per mouse, infectious virus was recovered from the lung homogenate of only 1 of 3 mice infected with the 12-S3 variant. These results indicate that the R292K neuraminidase mutation, even in the presence of hemagglutinin mutations, severely compromises the virulence of the virus.
DISCUSSION
The specific influenza neuraminidase inhibitors GS 4071, as its oral prodrug GS 4104, and zanamivir, a drug being developed for topical administration, represent a new class of antiviral compounds which are promising agents for the clinical management of influenza virus infection. As with all antiviral agents, it is possible that drug-resistant variants may arise. To gain a preliminary understanding of the development and molecular basis for resistance to these compounds, several groups have selected mutant viruses in vitro with decreased susceptibility to neuraminidase inhibitors. To date, most of the in vitro selections have been carried out in the presence of zanamivir.
In this report, we describe the in vitro selection of influenza variants in the presence of GS 4071, the active form of the oral prodrug GS 4104 which is currently in phase III clinical trials for the prophylaxis and treatment of influenza virus infections. The selection of variants with decreased susceptibility to GS 4071 in these studies follows a pattern similar to what has been observed with zanamivir. The first drug-selected variant generated in the presence of GS 4071 was detected in the eighth round of passage and exhibited an approximately 10-fold decrease in susceptibility to all neuraminidase inhibitors tested. Although virus was passaged under conditions designed to reduce the likelihood of selecting for hemagglutinin binding site mutations (1), this variant contained mutations in the hemagglutinin but not in the neuraminidase gene. Notably, the hemagglutinin mutations (A28T in HA1 and R124M in HA2) detected in this variant are not associated with the receptor binding site, as has often been the case in previous studies (7, 20, 33). Instead, they are located in the stalk region of the hemagglutinin where mutations often affect processing of the molecule, including changes in the pH of membrane fusion and protease sensitivity (18). Several amino acid changes to this region of the hemagglutinin have been reported to occur as egg-grown influenza viruses adapt to tissue culture (18). The effect of these mutations on susceptibility to neuraminidase inhibitors in culture remains to be determined. However, we and others (7) have found that adaptation of egg-grown influenza viruses to MDCK cells does reduce nonspecifically their susceptibility to neuraminidase inhibitors.
A neuraminidase mutation in the A/Victoria/3/75 (H3N2) virus passaged in the presence of GS 4071 was detected at the 11th round of selection. The mutation which appeared was a Lys substitution for the conserved Arg at amino acid 292 of the neuraminidase active site. This same mutation has been selected as a rare event in an avian influenza A/turkey/Minnesota/833/80 (H4N2) virus passaged in the presence of zanamivir (8). This mutation caused only a moderate (30-fold) decrease in sensitivity of the enzyme to zanamivir and its amino analog 4-amino-Neu5Ac2en. The decreased sensitivity of the mutant enzyme to these inhibitors is presumably due to a weaker interaction between Lys292 and the carboxylate of the inhibitors. Surprisingly, even though X-ray crystallographic studies of the influenza N9 neuraminidase complexed with zanamivir, 4-amino-Neu5Ac2en, or GS 4071 indicate that the interaction between Arg292 and the carboxylate of the three inhibitors is very similar (16, 43), the R292K mutation caused a significantly greater decrease in sensitivity to GS 4071 at the enzyme level compared to zanamivir or 4-amino-Neu5Ac2en.
Previous studies have indicated that the potency of the GS 4071 analogs depends on the strength of the hydrophobic interactions between the hydrocarbon groups at the C-3 position of the inhibitor and conserved amino acid residues in the enzyme active site (16, 44). In wild-type enzyme, the formation of the induced hydrophobic pocket with which the 3-pentyloxy side chain of GS 4071 interacts depends on a reorientation of the side chain of the conserved Glu276 residue upon binding of the inhibitor. In the native enzyme, the carboxylate of Glu276 faces into the active site, interacting with hydroxyl groups on the glycerol side chain of the natural substrate and the sialic acid-based inhibitors, such as zanamivir (2, 41, 43). However, upon binding GS 4071, the carboxylate of Glu276 is forced out of the active site, exposing the hydrocarbon portion of the side chain of Glu276 for hydrophobic interactions with the inhibitor (16). The observation that the R292K mutation has a much more pronounced effect on sensitivity to GS 4071 analogs containing branched side chains, which interact with Glu276 in the reoriented position (16a), suggests that the R292K mutation likely affects the ability of Glu276 to reorient.
The proposed role of the R292K mutation in affecting the reorientation of the Glu276 side chain upon binding of GS 4071 in the enzyme active site is also consistent with the observed kinetics of inhibition of the wild-type and mutant enzymes by GS 4071. In separate studies, we have determined that the slow-binding characteristic of GS 4071 is likely dependent upon its ability to induce the reorientation of the side chain of Glu276 (6a). The observation that GS 4071 is not a slow-binding inhibitor of the R292K mutant neuraminidase therefore suggests that in the presence of the R292K mutation the side chain of Glu276 cannot reorient in order to accommodate the inhibitor. The R292K mutation could block the reorientation of the Glu276 side chain if a Lys at residue 292 makes a stronger interaction with the carboxylate of Glu276 in its native configuration than does an Arg at residue 292.
Further evidence that the R292K mutation affects the formation of the induced hydrophobic pocket in the enzyme is provided by the recent report that this mutation has also been identified in variants of the NWS-G70C (H1N9) virus passaged in the presence of a 6-carboxamide analog of zanamivir (22). The 6-carboxamide analogs of zanamivir also derive much of their binding energy from hydrophobic interactions with the same induced hydrophobic pocket occupied by the 3-pentyloxy side chain of GS 4071 (36, 37).
In an earlier study, Gubareva et al. (8) used an in vitro selected variant of the influenza A/turkey/Minnesota/833/80 (H4N2) virus containing the R292K neuraminidase mutation to investigate the effect of this mutation on infectivity in mice. Their results, obtained by using reassortants of the influenza A/NWS/1/33 (H1N1) virus containing the mutant neuraminidase, indicate that the R292K mutation reduces the infectivity of the virus. In the studies described here, we also found that the R292K neuraminidase mutation severely compromises the infectivity of the virus, although the effect was much more dramatic than that reported previously (8). It is unlikely that the difference is due to the presence of hemagglutinin mutations in the virus used herein since the hemagglutinin mutations by themselves had no effect on infectivity. Instead, it is more likely that the R292K mutation had a more pronounced effect on the activity of the neuraminidase of the virus used in this study than the one used previously (8). This is supported by the observation that the growth of the 12-S3 variant described in this study is compromised whereas that of the A/turkey/Minnesota/833/80 (H4N2) variant was not (8). Importantly, in both viruses the R292K neuraminidase mutation reduced infectivity at least 500-fold, suggesting that any influenza viruses containing this mutation would be severely compromised.
In summary, we have isolated a GS 4071 drug-resistant influenza variant by in vitro passage in the presence of drug. After 12 passages in the presence of inhibitor, a substitution of a Lys for the conserved Arg at amino acid 292 of the neuraminidase was selected. This mutation caused a marked decrease in the susceptibility of the enzyme and virus to GS 4071. The mechanism by which this conservative substitution affects the activity of GS 4071 is likely due to an effect on the formation of an induced hydrophobic pocket in the enzyme active site which is critical for binding of the inhibitor. Although this mutation has a dramatic effect on the sensitivity of the neuraminidase to GS 4071, the fact that the mutation also drastically reduces the infectivity of the virus in cell culture and in mice suggests that this mutation may be of limited clinical significance.
ACKNOWLEDGMENTS
This work was supported in part by contracts NO1-AI-35178 and NO1-AI-65291 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Jay Toole and S. Swaminathan for helpful discussions during the course of these studies.
ADDENDUM
While the manuscript was under review, we performed a second, independent selection of tissue culture-adapted influenza A/Victoria/3/75 (H3N2) variants in the presence of GS 4071. Again, we isolated a variant containing the R292K neuraminidase mutation, but this time there were no mutations in the hemagglutinin gene. This demonstrates that mutations within the hemagglutinin gene are not required for neuraminidase mutations to occur, even in natural (nonreassortant) viruses. Notably, the variant containing the R292K neuraminidase mutation with no hemagglutinin mutations was also compromised for growth in culture and in eggs and was not infectious in mice at the highest inoculum tested (25,000 PFU/mouse). The observation that the variant containing the R292K neuraminidase mutation with no hemagglutinin mutations behaved the same as the 12-S3 variant indicates that the R292K neuraminidase mutation is responsible for the compromised behavior of the variants.
REFERENCES
- 1.Blick T J, Tiong T, Sahasrabudhe A, Varghese J N, Colman P M, Hart G J, Bethell R C, McKimm-Breschkin J L. Generation and characterization of an influenza virus neuraminidase variant with decreased sensitivity to the neuraminidase-specific inhibitor 4-guanidino-Neu5Ac2En. Virology. 1995;214:475–484. doi: 10.1006/viro.1995.0058. [DOI] [PubMed] [Google Scholar]
- 2.Burmeister W P, Ruigrok R W, Cusack S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992;11:49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnet F M. Mucins and mucoids in relation to influenza virus action. Aust J Exp Biol Med Sci. 1948;26:381–387. doi: 10.1038/icb.1948.39. [DOI] [PubMed] [Google Scholar]
- 4.Colacino J M, Chirgadze N Y, Garman E, Murti E G, Loncharich R J, Baxter A J, Staschke K A, Laver W G. A single sequence change destabilizes the influenza virus neuraminidase tetramer. Virology. 1997;236:66–75. doi: 10.1006/viro.1997.8709. [DOI] [PubMed] [Google Scholar]
- 5.Colacino, J. M., W. G. Laver, and G. M. Air. 1997. Selection of influenza A and B viruses for resistance to 4-guanidino-Neu5Ac2en in cell culture. J. Infect. Dis. 176(Suppl. 1):S66–S68. [DOI] [PubMed]
- 6.Colman P M. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 1994;3:1687–1696. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Escarpe, P., and D. Mendel. Unpublished results.
- 7.Gubareva L V, Bethell R, Hart G J, Murti K G, Penn C R, Webster R G. Characterization of mutants of influenza A virus selected with the neuraminidase inhibitor 4-guanidino-Neu5Ac2en. J Virol. 1996;70:1818–1827. doi: 10.1128/jvi.70.3.1818-1827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubareva L V, Robinson M J, Bethell R C, Webster R G. Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J Virol. 1997;71:3385–3390. doi: 10.1128/jvi.71.5.3385-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden F, Cote K, Douglas R G., Jr Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother. 1980;17:865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden F G, Couch R B. Clinical and epidemiological importance of influenza A viruses resistant to amantadine and rimantadine. Rev Med Virol. 1992;2:89–96. [Google Scholar]
- 11.Hayden F G, Lobo M, Treanor J J, Miller M, Mills R G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Efficacy and tolerability of oral GS 4104 for early treatment of experimental influenza in humans, abstr. LB-14. [Google Scholar]
- 12.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 13.Hayden F G, Rollins B S, Madren L K. Anti-influenza virus activity of the neuraminidase inhibitor 4-guanidino-Neu5Ac2en in cell culture and in human respiratory epithelium. Antiviral Res. 1994;25:123–131. doi: 10.1016/0166-3542(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 14.Hayden F G, Treanor J J, Betts A F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 15.Jedrzejas M J, Singh S, Brouillette W J, Laver W G, Air G M, Luo M. Structures of aromatic inhibitors of influenza virus neuraminidase. Biochemistry. 1995;34:3144–3151. doi: 10.1021/bi00010a003. [DOI] [PubMed] [Google Scholar]
- 16.Kim C U, Lew W, Williams M A, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen M S, Mendel D B, Tai C Y, Laver W G, Stevens R C. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 16a.Kim C U, Lew W, Williams M A, Wu H, Zhang L, Chen X, Escarpe P A, Mendel D B, Laver W G, Stevens R C. Structure-activity relationship studies of novel carbocyclic in influenza neuraminidase inhibitors. J Med Chem. 1998;41:2451–2460. doi: 10.1021/jm980162u. [DOI] [PubMed] [Google Scholar]
- 17.Klenk H-D, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–280. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y P, Wharton S A, Martin J, Skehel J J, Wiley D C, Steinhauer D A. Adaptation of egg-grown and transfectant viruses for growth in mammalian cells: selection of hemagglutinin mutants with elevated pH of membrane fusion. Virology. 1997;233:402–410. doi: 10.1006/viro.1997.8626. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKimm-Breschkin J L, Blick T J, Sahasrabudhe A, Tiong T, Marshall D, Hart G J, Bethell R C, Penn C R. Generation and characterization of variants of NWS/G70C influenza virus after in vitro passage in 4-amino-Neu5Ac2en and 4-guanidino-Neu5Ac2en. Antimicrob Agents Chemother. 1996;40:40–46. doi: 10.1128/aac.40.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKimm-Breschkin J L, McDonald M, Blick T J, Colman P M. Mutation in the influenza virus neuraminidase gene resulting in decreased sensitivity to the neuraminidase inhibitor 4-guanidino-Neu5Ac2en leads to instability of the enzyme. Virology. 1996;225:240–242. doi: 10.1006/viro.1996.0595. [DOI] [PubMed] [Google Scholar]
- 22.McKimm-Breschkin J L, Sahasrabudhe A, Blick T J, McDonald M, Colman P M, Hart G J, Bethell R C, Varghese J N. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac2en-derived inhibitors. J Virol. 1997;72:2456–2462. doi: 10.1128/jvi.72.3.2456-2462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendel D B, Tai C Y, Escarpe P A, Li W-X, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monto, A. S., I. A. Iacuzio, and J. R. LaMontague. 1997. Pandemic influenza:confronting a reemergent threat. J. Infect. Dis. 176(Suppl. 1):51–53.
- 25.Monto A S, Arden N H. Implications of viral resistance to amantadine in control of influenza A. Clin Infect Dis. 1992;15:362–367. doi: 10.1093/clinids/15.2.362. [DOI] [PubMed] [Google Scholar]
- 26.Oxford J S, Logan I S, Potter C W. Passage of influenza strains in the presence of aminoadamantane. Ann N Y Acad Sci. 1970;173:300–313. [Google Scholar]
- 27.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 28.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature sensitive influenza virus mutants. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 29.Penn C R, Barnett J M, Bethell R C, Fenton R, Gearing K L, Healy N, Jowett A J. Selection of influenza virus with reduced sensitivity in vitro to the neuraminidase inhibitor GG167 (4-guanidino-Neu5Ac2en): changes in haemagglutinin may compensate for loss of neuraminidase activity. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier; 1996. pp. 735–740. [Google Scholar]
- 30.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon S B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 31.Ryan D M, Ticehurst J, Dempsey M H. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother. 1995;39:2583–2584. doi: 10.1128/aac.39.11.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan D M, Ticehurst J, Dempsey M H, Penn C R. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase) Antimicrob Agents Chemother. 1994;38:2270–2275. doi: 10.1128/aac.38.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahasrabudhe A, Blick T, McKimm-Breschkin J. Influenza virus variants resistant to GG167 with mutations in the haemagglutinin. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier; 1996. pp. 748–752. [Google Scholar]
- 34.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M-H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza A virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res, 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 35.Sidwell R W, Huffman J H, Call E W, Alaghamandaan H, Cook P D, Robins R K. Effect of selenazofurin on influenza A and B virus infections of mice. Antiviral Res. 1986;6:343–353. doi: 10.1016/0166-3542(86)90016-1. [DOI] [PubMed] [Google Scholar]
- 36.Smith P W, Sollis S L, Howes P D, Cherry P C, Cobley K N, Taylor H, Whittington A R, Scicinski J, Bethell R C, Taylor N, Skarzynski T, Cleasby A, Singh O, Wonacott A, Varghese J, Colman P. Novel inhibitors of influenza sialidases related to GG167. Structure-activity, crystallographic and molecular dynamic studies with 4H-pyran-2-carboxylic acid 6-carboxamides. Bioorg Med Chem Lett. 1996;6:2931–2936. [Google Scholar]
- 37.Sollis S L, Smith P W, Howes P D, Cherry P C, Bethell R C. Novel inhibitors of influenza sialidase related to GG167 synthesis of 4-amino and guanidino-4H-pyran-2-carboxylic acid-6-propylamides; selective inhibitors of influenza A virus sialidase. Bioorg Med Chem Lett. 1996;6:1805–1808. [Google Scholar]
- 38.Staschke K A, Colacino J M, Baxter A J, Air G M, Bansal A, Hornback W J, Munroe J E, Laver W G. Molecular basis for the resistance of influenza viruses to 4-guanidino-Neu5Ac2En. Virology. 1995;214:642–646. doi: 10.1006/viro.1995.0078. [DOI] [PubMed] [Google Scholar]
- 39.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 40.Thomas G P, Forsyth M, Penn C R, McCauley J W. Inhibition of the growth of influenza viruses in vitro by 4-guanidino-2,4-dideoxy-N-acetylneuraminic acid. Antiviral Res. 1994;24:351–356. doi: 10.1016/0166-3542(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 41.Varghese J N, McKimm-Breschkin J L, Caldwell J B, Kortt A A, Colman P M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992;14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 42.von Itzstein M, Wan Yang M, Jin B. The synthesis of 2,3-didehydro-2,4-dideoxy-4-guanidinyl-N-acetylneuraminic acid: a potent influenza virus sialidase inhibitor. Carbohydr Res. 1994;259:301–305. doi: 10.1016/0008-6215(94)84065-2. [DOI] [PubMed] [Google Scholar]
- 43.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Cyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 44.Williams M A, Lew W, Mendel D B, Tai C Y, Escarpe P A, Laver W G, Stevens R C, Kim C U. Structure-activity relationship of carbocyclic influenza neuraminidase inhibitors. Bioorg Med Chem Lett. 1997;7:1837–1842. [Google Scholar]
- 45.Woods J M, Bethell R C, Coates J A V, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, Penn C R. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]