Abstract
Hypothalamic kisspeptin neurons are master regulators of mammalian reproduction via direct stimulation of gonadotropin-releasing hormone and consequent gonadotropin release. Here, we generated novel Kiss1 (kisspeptin gene)-Cre rats and investigated the developmental changes and sex differences in visualized Kiss1 neurons of Kiss1-Cre-activated tdTomato reporter rats. First, we validated Kiss1-Cre rats by generating Kiss1-expressing cell-specific Kiss1 knockout (Kiss1-KpKO) rats, which were obtained by crossing the current Kiss1-Cre rats with Kiss1-floxed rats. The resulting male Kiss1-KpKO rats lacked Kiss1 expression in the brain and exhibited hypogonadotropic hypogonadism, similar to the hypogonadal phenotype of global Kiss1 KO rats. Histological analysis of Kiss1 neurons in Kiss1-Cre-activated tdTomato reporter rats revealed that tdTomato signals in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) were not affected by estrogen, and that tdTomato signals in the ARC, AVPV, and medial amygdala (MeA) were sexually dimorphic. Notably, neonatal AVPV tdTomato signals were detected only in males, but a larger number of tdTomato-expressing cells were detected in the AVPV and ARC, and a smaller number of cells in the MeA was detected in females than in males at postpuberty. These findings suggest that Kiss1-visualized rats can be used to examine the effect of estrogen feedback mechanisms on Kiss1 expression in the AVPV and ARC. Moreover, the Kiss1-Cre and Kiss1-visualized rats could be valuable tools for further detailed analyses of sexual differentiation in the brain and the physiological role of kisspeptin neurons across the brain in rats.
Keywords: Hypogonadotropic hypogonadism, Kisspeptin, Neuroendocrinology, Reproduction, Sex differences
Kisspeptin is a neuropeptide encoded by the Kiss1 gene that plays an indispensable role in reproductive functions such as puberty onset, gametogenesis, and ovulation, via the direct stimulation of gonadotropin-releasing hormone (GnRH) secretion in the mammalian hypothalamus [1,2,3,4]. Pioneering studies by Seminara et al. and de Roux et al. demonstrated that mutations in GPR54 (kisspeptin receptor gene) caused hypogonadotropic hypogonadism in humans [5, 6]. Topaloglu et al. subsequently showed that a mutation of KISS1 also replicated hypogonadotropic hypogonadism in humans [7]. Kiss1 global knockout (KO) rats [8] and mice [9,10] showed pubertal failure and infertility in both sexes. Kisspeptin administration potently stimulates gonadotropin release in all mammalian species, such as rodents [11,12,13], sheep [14], goats [15], cows [16], and primates [17], including humans [18]. These results suggest that kisspeptin neurons are targets for therapeutic approaches in hypothalamic reproductive disorders, such as premature ovarian failure and anovulation disease. Indeed, it has been reported that at least 25% of women suffer from reproductive disorders due to hypothalamic dysfunction [19,20,21].
The cell bodies of kisspeptin neurons are primarily located in two distinct regions of the rodent hypothalamus: the arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV) [13, 22,23,24,25]. ARC kisspeptin neurons are involved in the generation of pulsatile GnRH/gonadotropin release, which controls gametogenesis and steroidogenesis in male and female rodents [26,27,28,29,30,31,32]. ARC kisspeptin neurons are a major site of gonadal steroid negative feedback that suppresses tonic (pulsatile) luteinizing hormone (LH) release because ARC kisspeptin neurons express estrogen receptor α (ERα) and estrogen downregulates ARC Kiss1 expression in female rats and mice [24, 33]. Estrogen failed to suppress ARC Kiss1 expression in kisspeptin neuron-specific ERα KO mice [34]. Notably, LH pulses and gonadectomy-induced increase in LH and follicle-stimulating hormone (FSH) completely disappeared in Kiss1 global KO male and female rats [8]. On the other hand, kisspeptin neurons in the AVPV in rodents or the preoptic area (POA) in other species are responsible for estrogen positive feedback that generates a GnRH/LH surge to trigger ovulation in female mammals [2,3,4, 35]. Estrogen upregulates Kiss1 expression in AVPV/POA kisspeptin neurons in rodents [13, 24, 33, 36], pigs [37], and Japanese monkeys [38]. Estrogen activates AVPV/POA kisspeptin neurons in rats, goats, and Japanese monkeys [24, 38, 39] and fails to upregulate AVPV Kiss1 expression in kisspeptin neuron-specific ERα KO mice [34]. Indeed, Kiss1 global KO rats fail to show spontaneous or estrogen-induced LH surge [8].
AVPV/POA kisspeptin neurons exhibit sexual dimorphism in rodents and other mammals. A larger number of AVPV/POA kisspeptin neurons are found in adult females than in males in mice [40, 41], rats [36, 42], and Japanese monkeys [38]. The sexual dimorphism of AVPV kisspeptin neurons is responsible for sex differences in their ability to exert a GnRH/LH surge induced by the preovulatory level of estrogen in rodents. Estrogen largely induces AVPV Kiss1/kisspeptin expression and triggers an LH surge in female rats, but not in male rats [42]. Notably, AVPV kisspeptin neurons play a physiological role in male rats because mating behavior increases the number of AVPV kisspeptin neurons in male rats [43]. AVPV Kiss1 expression was examined in adult animals in most studies. On the other hand, some studies reported AVPV Kiss1 expression on postnatal Day 11 in female and male rats [44] and postnatal Day 10 in female and male mice [45]. Notably, kisspeptin-immunoreactive (IR) cells were evident from embryonic Day 17 (E17) to postnatal Day 1 in the AVPV of male mice, but few kisspeptin-IR cells were found in the AVPV of female mice during the perinatal and prepubertal periods [46]. Pre- and postnatal AVPV Kiss1 induction in male rodents may be due to the enhanced effect of estrogen derived from perinatal testosterone in rodents [47].
ARC kisspeptin neurons do not exhibit any apparent sexual dimorphism. ARC kisspeptin expression is evident during the late embryonic period in rodents of both sexes. Kisspeptin-IR cells were found in the ARC of female and male mice at E13 [48], and in female and male rats at E14.5 [49]. The physiological role of the embryonic or perinatal ARC Kiss1/kisspeptin has not been clarified in female rodents, but neonatal ARC Kiss1/kisspeptin expression is responsible for the neonatal testosterone surge in male rodents [50], which masculinizes the male rodent brain [47]. Notably, ARC Kiss1/kisspeptin expression is suppressed during the prepubertal period in female rats, and an increase in ARC Kiss1/kisspeptin expression triggers puberty onset [51, 52].
Kiss1 expression has also been reported in several brain regions of adult male and female rats and mice, including the medial amygdala (MeA) [53]. MeA kisspeptin neurons may modulate behavioral responses to olfactory cues, sexual partner preferences, and anxiety [54, 55]. Weak Kiss1 expression was reported in the ventromedial nucleus of the hypothalamus (VMH) on postnatal Days 7 and 19 in male and female rats [44], and a small number of Kiss1/kisspeptin-IR cells were detected in the VMH of adult rats [56]. Our previous study revealed that Kiss1-tdTomato rats show little tdTomato fluorescence in the VMH of adult female rats [8]. However, the physiological role of Kiss1 cells in the VMH is not yet fully understood.
Kiss1 neurons were reported in the lateral septum (LS) [57] in γ-aminobutyric acid (GABA) B1 receptor KO male and female mice, but few Kiss1 neurons were detected in the LS of either sex of wild-type mice. Kisspeptin-IR cell bodies are also found in the bed nucleus of the stria terminalis (BNST) of male and female rats [56] and Kiss1-expressing cells are found in the BNST of mice of both sexes [57]. Kisspeptin-IR cell bodies were also found in the dorsal part of the dorsomedial nucleus (DMD) of male and female rats [58] and female mice [59], as well as the posterior hypothalamus (PH) of female mice [60]. Kisspeptin-IR fibers were detected in the paraventricular nucleus (PVN), reticular thalamic nucleus (Rt), medial POA (MPOA), retrochiasmatic area (RCh) [58], and medial tuberal nucleus (MTu) of female rats [56] and the MPOA [60] and PVN [41, 59, 60] of male and female mice. Kiss1-Cre reporter signals were detected in the cortex of female mice [61]. However, little is known about the physiological role of Kiss1/kisspeptin neurons in the brain nuclei, except for the AVPV and ARC, because weak Kiss1 expression in most of these nuclei, such as the MeA, VMH, LS, and BNST, makes it difficult to detect Kiss1-expressing cells. Therefore, genetically engineered Kiss1-visualized rats could be a useful model to investigate the physiological roles of potential Kiss1/kisspeptin neurons in these nuclei because Kiss1-visualized rats allow us to identify potential kisspeptin neurons by tdTomato fluorescence, regardless of Kiss1/kisspeptin expression.
The present study generated novel Kiss1-Cre rats and investigate developmental changes and sex differences in visualized Kiss1 neurons in Kiss1-Cre-activated tdTomato reporter rats. First, we validated Kiss1-Cre rats by generating Kiss1-expressing cell-specific Kiss1 knockout (Kiss1-KpKO) rats, which were obtained by crossing Kiss1-Cre rats with Kiss1-floxed rats [26]. The resulting male Kiss1-KpKO rats were subjected to the evaluation of reproductive indicators, such as testicular weights, ARC Kiss1 expression, pulsatile LH release and pituitary Lhb, Fshb and Gnrhr mRNA expression. We examined sex differences in the distribution and number of kisspeptin neurons in the brain during several developmental periods using Kiss1-Cre-activated tdTomato reporter rats. We generated Kiss1-visualized rats by crossing Kiss1-Cre rats with Cre-dependent tdTomato reporter rats [LE-Tg(Gt(ROSA)26SorCAG-tdTomato)24Jfhy] [62]. The resulting Kiss1-visualized female rats underwent histological analysis by double staining for Kiss1 expression and tdTomato immunoreactivity to examine whether tdTomato signals co-localized with Kiss1-expressing cells in the AVPV and ARC. Kiss1-Cre-activated tdTomato fluorescence signals were examined in adult gonadectomized male and female rats treated with or without estrogen to confirm whether tdTomato fluorescence was detectable in an estrogen-independent manner. The tdTomato signals were examined in the hypothalamic and extra-hypothalamic areas of male and female rats during several developmental periods, such as postnatal Days 1 and 7, 3 weeks (prepubertal), 7 weeks (postpubertal), and 10–13 weeks (adulthood), to determine developmental changes and sex differences in tdTomato-positive cells. We further analyzed sex differences in the distribution and number of tdTomato-positive cells in detail in the AVPV, where kisspeptin neurons exhibit sexual dimorphism.
Materials and Methods
A detailed description of the methodologies, including the generation of gene-modified rats, radioimmunoassay, qRT‒PCR and histological analysis, is provided in the Supplementary Material.
Results
Kiss1-KpKO male rats obtained by crossing newly generated Kiss1-Cre rats with Kiss1-floxed rats showed hypogonadotropic hypogonadism
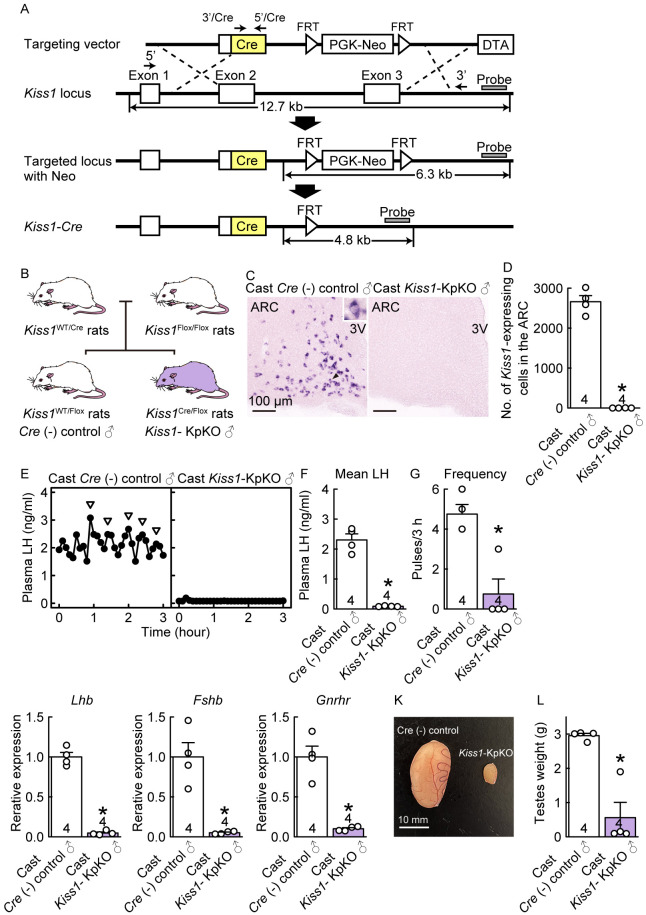
We generated Kiss1-Cre rats in which the Cre recombinase was inserted into the endogenous Kiss1 locus by homologous recombination (Fig. 1A and Supplementary Fig. 1). To confirm that the Cre-loxP system functions in Kiss1-expressing cells in Kiss1-Cre rats, Kiss1-Cre rats were crossed with Kiss1-floxed rats (Fig. 1B) [26]. No Kiss1-expressing cells were detected in the ARC of castrated (Cast) Kiss1-KpKO male rats (n = 4), whereas many Kiss1-expressing cells were found in the ARC of Cast Cre (–)/Kiss1-floxed control male rats (n = 4) during adulthood (8 weeks of age) (Figs. 1C, D). Cast Cre (–)/Kiss1-floxed control adult male rats showed frequent LH pulses, whereas Cast Kiss1-KpKO adult male rats showed severe suppression of pulsatile LH release (Fig. 1E). The mean LH levels and LH pulse frequency in Cast Kiss1-KpKO male rats were significantly lower than those in Cre (–)/Kiss1-floxed control male rats (n = 4) (P < 0.05; Figs. 1F, G). Furthermore, pituitary Lhb, Fshb, and Gnrhr mRNA expression levels were significantly lower in Cast Kiss1-KpKO male rats than in Cast Cre (–)/Kiss1-floxed control male rats (P < 0.05; Figs. 1H, I, J). The testes of Kiss1-KpKO rats were smaller than those of Cre (–)/Kiss1-floxed control rats (Fig. 1K), and the testicular weights of Kiss1-KpKO rats were significantly lower than those of Cre (–)/Kiss1-floxed control rats (n = 4) (P < 0.05; Fig. 1L). These findings suggest that the Cre-loxP system functions in Kiss1-expressing cells in the current Kiss1-Cre rats.
Fig. 1.
Kiss1-expressing neuron-specific Kiss1 knockout (Kiss1-KpKO) male rats obtained by crossing newly generated Kiss1-Cre rats with Kiss1-floxed rats showed hypogonadotropic hypogonadism. (A) Structures of the targeting vector for the generation of Kiss1-Cre rats, the wild-type Kiss1 allele, and the targeted Kiss1 allele with a neomycin resistance (Neo) selection cassette, resulting from replacement at dotted lines. Structure of the targeted Kiss1 allele without Neo resulting from enhanced flippase (FLPe)-flippase recognition target (FRT) recombination in rat embryonic stem (ES) cells. A diphtheria toxin A (DTA) expression cassette in the targeting vector was used for negative selection. (B) Schematic illustration showing the generation of Kiss1-KpKO rats. To generate Kiss1-KpKO rats and Cre (–)/Kiss1-floxed control rats, Kiss1-Cre heterozygous rats were crossed with Kiss1-flox homozygous rats. (C) Kiss1 mRNA-expressing cells in the ARC of a representative Cast Cre (–)/Kiss1-floxed control rat (left) and Kiss1-KpKO rat (right) in adulthood. Scale bars, 100 µm. (D) The numbers of Kiss1 mRNA-expressing cells in the ARC of Cast Cre (–)/Kiss1-floxed control rats and Kiss1-KpKO rats in adulthood. (E) Plasma LH profiles in a representative Cast Cre (–)/Kiss1-floxed control rat (left) and Kiss1-KpKO rat (right) in adulthood. Arrowheads indicate LH pulses identified by the PULSAR computer program. Mean LH concentration (F) and the frequency (G) of LH pulses in Cast Cre (–)/Kiss1-floxed control rat and Kiss1-KpKO rats in adulthood. Pituitary Lhb (H), Fshb (I), and Gnrhr (J) mRNA expression. (K) Photographs of testes obtained from a representative Cre (–)/Kiss1-floxed control rat (left) and Kiss1-KpKO rat (right) in adulthood. Scale bars, 10 mm. (L) Testicular weights in Kiss1-KpKO rats and Cre (–)/Kiss1-floxed control rats in adulthood. Sample size, n = 4. Values are the means ± SEMs. * Significant difference between groups (P < 0.05, Welch’s t test). See also Supplementary Fig. 1.
Colocalization of Kiss1 and Kiss1-Cre-activated tdTomato-IR signals and the lack of the effect of estrogen on tdTomato fluorescence signals in the AVPV and ARC of Kiss1-visualized rats
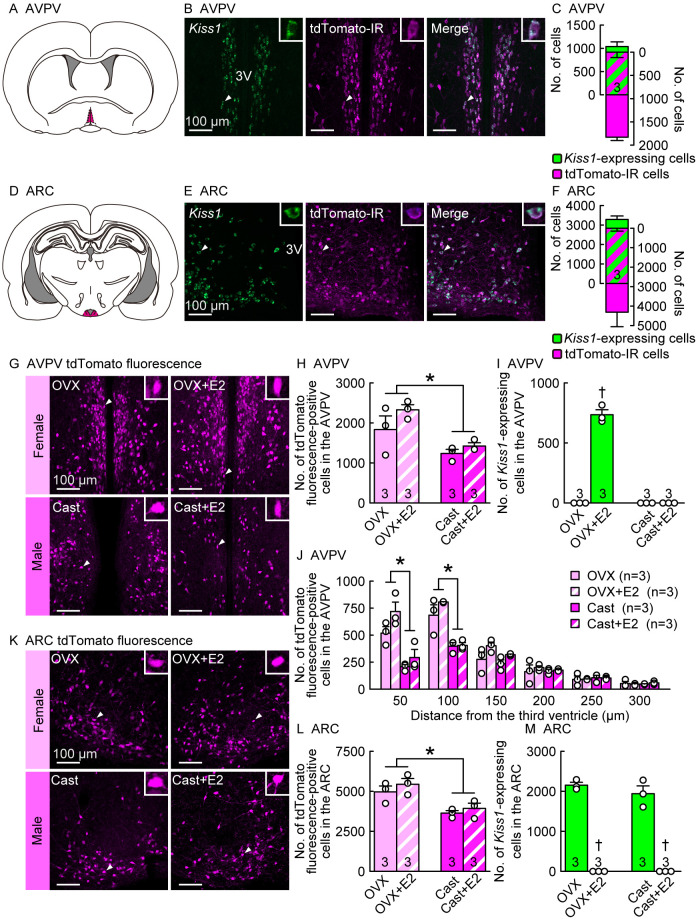
Theoretically, cells that have expressed Kiss1/Cre at least once should persistently express tdTomato fluorescence following Cre recombination, regardless of Kiss1 expression in Kiss1-Cre-activated tdTomato reporter rats. To confirm that tdTomato fluorescence co-localized with Kiss1 in the AVPV and ARC, we used in situ hybridization for Kiss1 expression and immunohistochemistry for tdTomato-IR. Immunohistochemistry for tdTomato was used only for double staining, because tdTomato fluorescence could not be detected after Kiss1in situ hybridization. A number of Kiss1-expressing and tdTomato-IR cells were found in the AVPV of estradiol-17β (E2)-treated ovariectomized (OVX) adult (8–16 weeks of age) female rats (Fig. 2, A and B). Most (82.0 ± 3.0%) Kiss1-expressing cells co-expressed tdTomato-IR signals in the AVPV, and approximately half (49.8 ± 4.3%) of tdTomato-IR cells co-expressed Kiss1 signals in the AVPV of the E2-treated OVX female rats (Fig. 2C). Many Kiss1-expressing and tdTomato-IR cells were found in the ARC of adult OVX female rats (Fig. 2, D and E), and most (86.4 ± 1.3%) Kiss1-expressing cells co-expressed tdTomato-IR signals in the ARC of OVX female rats (Fig. 2F). Approximately two-thirds (68.3 ± 7.1%) of tdTomato-IR cells co-expressed Kiss1 signals in the ARC of OVX female rats (Fig. 2F).
Fig. 2.
Colocalization of Kiss1 and Kiss1-Cre-activated tdTomato-immunoreactive (IR) signals and lack of estrogen effect on tdTomato fluorescence signals in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) of rats. (A) An illustrated brain section showing the AVPV highlighted in magenta. (B) Kiss1 mRNA-expressing (green) and tdTomato-IR (magenta) cells in the AVPV of a representative ovariectomized (OVX) rat treated with a positive feedback level of estradiol-17β (E2) in adulthood (8–16 weeks of age). Insets are magnified images showing a representative Kiss1 and tdTomato-IR double-positive cell (indicated by arrowheads in main images). (C) The number of Kiss1-expressing (green), tdTomato-IR (magenta), or double-positive (striped) cells in the AVPV of OVX+E2 rats in adulthood. (D) An illustration of the brain section showing the ARC highlighted in magenta. (E) Kiss1-expressing (green) and tdTomato-IR (magenta) cells in the ARC of a representative OVX rat in adulthood. (F) The number of Kiss1-expressing (green), tdTomato-IR (magenta), or double-positive (striped) cells in the ARC of OVX rats in adulthood. (G) Kiss1-Cre-activated tdTomato fluorescence in the AVPV of representative OVX and castrated (Cast) rats with or without E2 in adulthood. (H and I) The numbers of (H) tdTomato fluorescence-positive cells and (I) Kiss1-expressing cells in the AVPV of OVX and Cast rats with or without E2 in adulthood. (J) The number of tdTomato fluorescence-positive cells in every 50-µm layer (from the medial to lateral) of the AVPV of OVX and Cast rats with or without E2. (K) Kiss1-Cre-activated tdTomato fluorescence in the ARC of representative OVX and Cast rats with or without E2 in adulthood. (L and M) The number of (L) tdTomato fluorescence-positive cells and (M) Kiss1-expressing cells in the ARC of OVX and Cast rats with or without E2 in adulthood. Sample size, n = 3. Scale bars, 100 µm. 3V, third ventricle. Values are the means ± SEMs. * Significant difference between sexes (P < 0.05, two- or three-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test). † Significant difference by E2 treatment (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test). See also Supplementary Fig. 2.
To confirm that circulating E2 did not affect tdTomato expression in the AVPV and ARC, we compared the number of AVPV and ARC tdTomato fluorescence-positive cells in OVX females and Cast males with and without E2 treatment in adulthood (8–16 weeks of age). Many tdTomato fluorescence-positive cells were found in the AVPV of OVX and Cast rats regardless of E2 treatment (Fig. 2, G and H), whereas Kiss1 expression was found only in the AVPV of E2-treated OVX rats (Fig. 2I). E2 treatment did not significantly affect the number of AVPV tdTomato fluorescence-positive cells in the OVX females or Cast males (Fig. 2H). The number of tdTomato fluorescence-positive cells in the AVPV was significantly higher in females than in males, regardless of E2 treatment (P < 0.05; Fig. 2H). Detailed analyses of the distribution of AVPV tdTomato cells revealed that the number of tdTomato fluorescence-positive cells in the medial part of the AVPV (0–100 µm lateral to the third ventricle [3V]) was significantly higher in females than in males, regardless of E2 treatment (Fig. 2J), whereas no sex difference was found in the number of tdTomato fluorescence-positive cells in the lateral part of the AVPV (100–300 µm lateral to the 3V). Notably, colocalization of Kiss1 and tdTomato-IR signals was primarily found in the medial part of the AVPV of E2-treated OVX females (0–100 µm lateral to the 3V) (Supplementary Fig. 2).
Many tdTomato fluorescence-positive cells were found in the ARC of OVX and Cast rats, regardless of E2 treatment (Fig. 2, K and L), whereas Kiss1-expressing cells were found only in the ARC of OVX and Cast rats without E2 treatment (Fig. 2M). E2 treatment did not significantly affect the number of ARC tdTomato fluorescence-positive cells in OVX females or Cast males. The number of tdTomato fluorescence-positive cells in the ARC was significantly higher in female rats than in male rats, regardless of E2 treatment (P < 0.05, Fig. 2L). Many ARC Kiss1-expressing cells were found in OVX and Cast rats, and E2 treatment significantly reduced these numbers (P < 0.05; Fig. 2M).
Developmental increase and sex difference in Kiss1-Cre-activated tdTomato fluorescence signals in the AVPV and ARC with earlier AVPV tdTomato expression in males and higher AVPV and ARC tdTomato expression in females during the postpubertal period
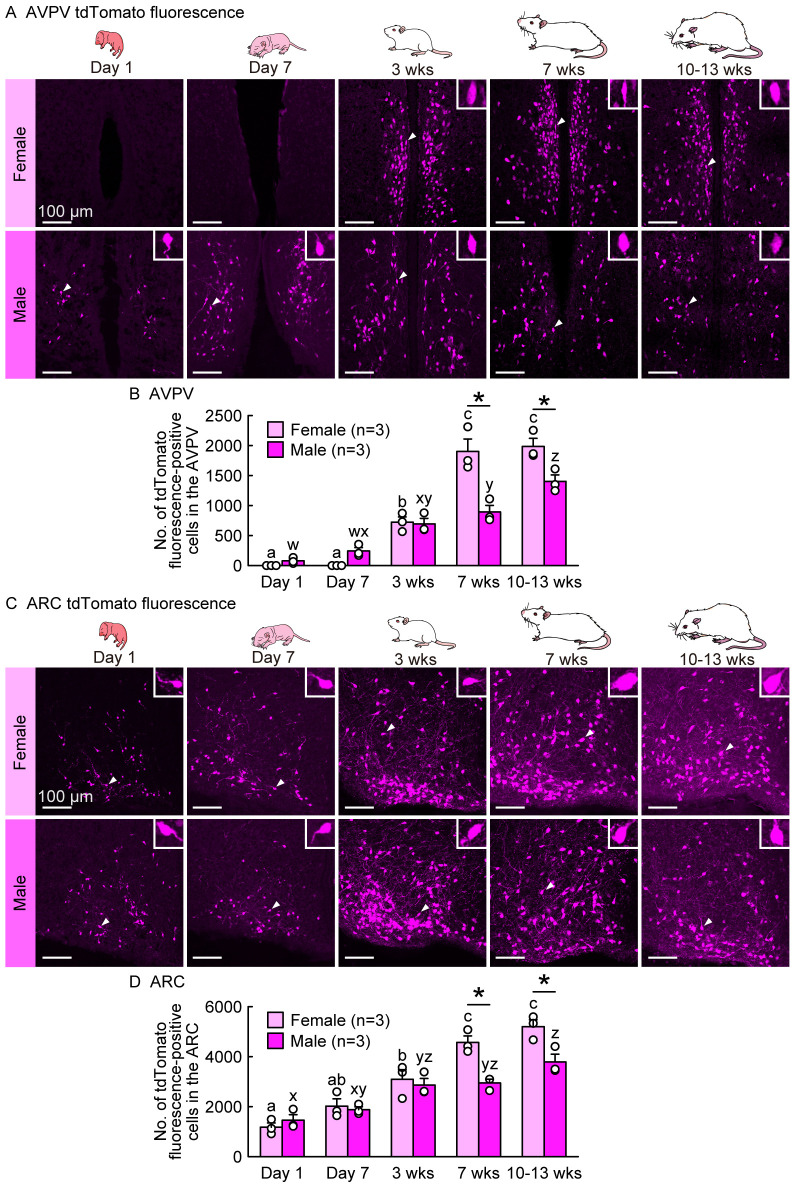
To examine sex differences in the distribution and number of potential kisspeptin neurons during the developmental periods, we compared the number of tdTomato fluorescence-positive cells in the AVPV and ARC between females and males on postnatal Days 1 and 7, and 3 (prepubertal), 7 (postpubertal), and 10–13 weeks (adulthood) of age in gonad-intact rats. Few AVPV tdTomato-positive cells were found on postnatal Days 1 and 7 in female rats, and many AVPV tdTomato fluorescence-positive cells were found at 3, 7, and 10–13 weeks of age when the females were in the diestrus phase (Fig. 3A). Notably, AVPV tdTomato fluorescence-positive cells were found on postnatal Days 1 and 7 and thereafter (3, 7, and 10–13 weeks of age) in male rats. The number of AVPV tdTomato fluorescence-positive cells significantly increased in both sexes during the developmental period and was significantly higher in females than in males at 7 and 10–13 weeks of age (P < 0.05; Fig. 3B). In the ARC, tdTomato fluorescence-positive cells were found in gonad-intact female and male rats on postnatal Day 1 and thereafter (Day 7 and 3, 7, and 10–13 weeks of age) (Fig. 3C). The number of ARC tdTomato fluorescence-positive cells increased significantly in both sexes with increasing age and was significantly higher in females than in males at 7 and 10–13 weeks of age (P < 0.05; Fig. 3D).
Fig. 3.
Developmental increases and sex differences in Kiss1-Cre-activated tdTomato fluorescence signals in the AVPV and ARC with earlier AVPV tdTomato expression in males and higher AVPV and ARC tdTomato expression in females during the postpubertal period. (A) Kiss1-Cre-activated tdTomato fluorescence in the AVPV of representative gonad-intact female and male rats at postnatal Days 1 and 7, and 3 (prepubertal), 7 (postpubertal), and 10–13 weeks (adulthood) of age (in the diestrus phase for 7- and 10–13-week-old females). Insets are magnified images showing a representative Kiss1-Cre-activated tdTomato fluorescence-positive cell (indicated by arrowheads in main images). (B) Developmental changes in the number of tdTomato fluorescence-positive cells in the AVPV of gonad-intact female and male rats. (C) Kiss1-Cre-activated tdTomato fluorescence in the ARC of representative gonad-intact female and male rats. (D) Developmental changes in the number of tdTomato fluorescence-positive cells in the ARC of female and male rats. Sample size, n = 3. Scale bars, 100 µm. Values are the means ± SEMs. Values with different letters (a–c for females and w–z for males) are significantly different between ages (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test). * Significant difference between sexes (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test).
Developmental increase and sex difference in Kiss1-Cre-activated tdTomato fluorescence signals in the MeA of rats with higher MeA tdTomato expression in males than in females in the postpubertal period
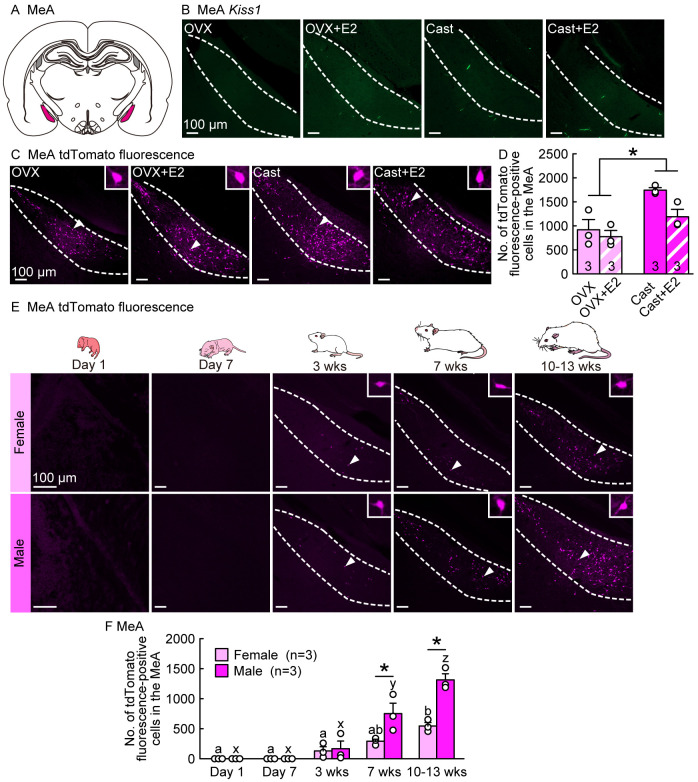
Because we found a large number of tdTomato fluorescence-positive cells in the MeA region of Kiss1-visualized rats, we examined Kiss1 expression and tdTomato fluorescence signals in the MeA of gonadectomized adult male and female rats in the presence or absence of E2. No Kiss1-expressing cells were detected in the MeA of OVX females and Cast males regardless of E2 treatment (Figs. 4A and 4B); however, some Kiss1-Cre-activated tdTomato fluorescence-positive cells were detected in the MeA of both sexes regardless of E2 treatment in adulthood (Fig. 4C). E2 treatment did not significantly affect the number of MeA tdTomato fluorescence-positive cells in OVX females or Cast males (Fig. 4D). Notably, the number of MeA tdTomato fluorescence-positive cells was significantly higher in males than in females (P < 0.05; Fig. 4D).
Fig. 4.
Developmental increases and sex differences in Kiss1-Cre-activated tdTomato fluorescence signals in the medial amygdala (MeA) of rats with higher MeA tdTomato expression in males than in females in the postpubertal period. (A) An illustrated brain section showing the MeA highlighted in magenta. (B) Few Kiss1-expressing cells were detected in the MeA of OVX and Cast rats with or without E2 in adulthood (8–16 weeks of age). (C) Kiss1-Cre-activated tdTomato fluorescence in the MeA of representative OVX and Cast rats with or without E2 in adulthood. Insets are magnified images showing a tdTomato fluorescence-positive cell (indicated by arrowheads in main images). (D) The numbers of tdTomato fluorescence-positive cells in the MeA of OVX and Cast rats with or without E2 in adulthood. (E) Kiss1-Cre-activated tdTomato fluorescence-positive cells in the MeA of representative gonad-intact female and male rats at postnatal Days 1 and 7, and 3 (prepubertal), 7 (postpubertal), and 10–13 weeks (adulthood) of age (in the diestrus phase for 7- and 10–13-week-old females). (F) Developmental changes in the number of tdTomato-positive cells in the MeA of gonad-intact female and male rats. Sample size, n = 3. Scale bars, 100 µm. Values are the means ± SEMs. * Significant difference between sexes (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test). Values with different letters (a–b for females and x–z for males) are significantly different (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test).
We further examined the developmental changes and sex differences in the number of tdTomato fluorescence-positive cells in the MeA of gonad-intact male and female rats. No tdTomato fluorescence-positive cells were found in the MeA of either sex on postnatal Days 1 or 7, whereas tdTomato-positive cells were found in the MeA of both sexes at 3 weeks of age and thereafter (7 and 10–13 weeks of age) (Fig. 4E). The number of MeA tdTomato fluorescence-positive cells significantly increased in both sexes with increasing age and was significantly higher in males than in females at 7 and 10–13 weeks of age (P < 0.05; Fig. 4F).
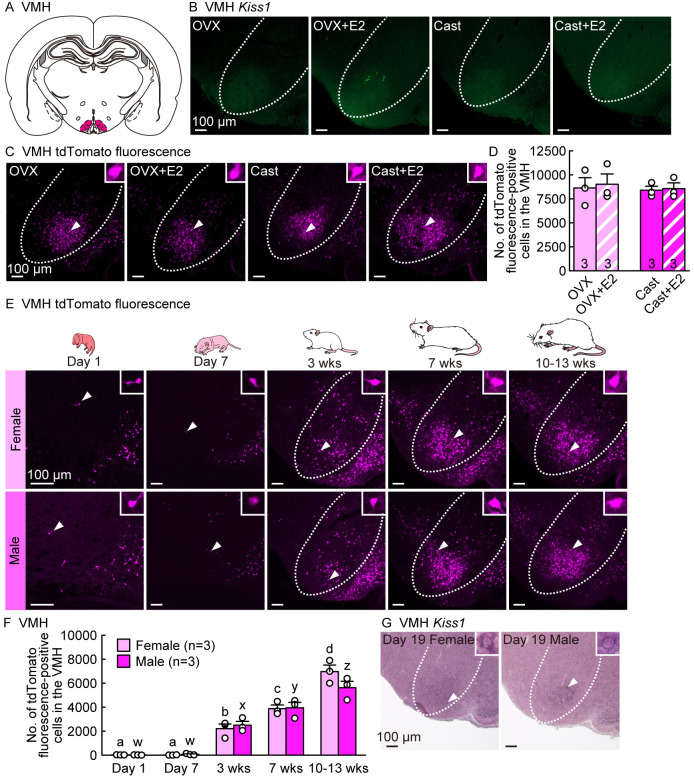
Developmental increase but no sex difference in Kiss1-Cre-activated tdTomato fluorescence signals in the VMH of female and male rats
We also examined Kiss1 expression and tdTomato fluorescence signals in the VMH of male and female rats, because we found a large number of tdTomato fluorescence-positive cells in the VMH of Kiss1-visualized rats. Few Kiss1-expressing cells were detected in the VMH of adult OVX females and Cast males, regardless of E2 treatment (Figs. 5A and 5B), whereas a large number of tdTomato fluorescence-positive cells were found in the VMH of adult rats of both sexes (Fig. 5C). No sex differences or estrogen effects were observed in the number of tdTomato fluorescence-positive cells in the VMH (Fig. 5D).
Fig. 5.
Developmental increases but no sex differences in Kiss1-Cre-activated tdTomato fluorescence signals in the ventromedial nucleus of the hypothalamus (VMH) of female and male rats. (A) An illustrated brain section showing the VMH highlighted in magenta. (B) No Kiss1-expressing cells were detected in the VMH of OVX and Cast rats with or without E2 in adulthood (8–16 weeks of age). (C) Kiss1-Cre-activated tdTomato fluorescence-positive cells in the VMH of representative OVX and Cast rats with or without E2 in adulthood. Insets are magnified images showing tdTomato-positive cells (indicated by arrowheads in the main images). (D) The number of tdTomato-positive cells in the VMH of OVX and Cast rats with or without E2 in adulthood. (E) Kiss1-Cre-activated tdTomato fluorescence-positive cells in the VMH of representative gonad-intact female and male rats at postnatal Days 1 and 7, and 3 (prepubertal), 7 (postpubertal), and 10–13 weeks (adulthood) of age (in the diestrus phase for 7- and 10–13-week-old females). (F) Developmental changes in the number of tdTomato-positive cells in the VMH of gonad-intact female and male rats. (G) Kiss1-expressing cells in the VMH of representative female and male rats on postnatal Day 19. Insets are magnified images showing Kiss1-expressing cells. Sample size, n = 3. Scale bars, 100 µm. Values are the means ± SEMs. Values with different letters (a–d for females and w–z for males) are significantly different (P < 0.05, two-way ANOVA followed by Tukey‒Kramer’s multiple comparisons test).
We also examined developmental changes and sex differences in the number of VMH tdTomato fluorescence-positive cells in gonad-intact male and female rats. Few VMH tdTomato fluorescence-positive cells were found in both sexes at postnatal Days 1 and 7, whereas many VMH tdTomato fluorescence-positive cells were found in both sexes at 3 weeks and thereafter (7 and 10–13 weeks of age) (Fig. 5E). The number of VMH tdTomato fluorescence-positive cells increased significantly in both sexes during the developmental period (P < 0.05; Fig. 5F), and no sex differences were observed in the number of VMH tdTomato fluorescence-positive cells throughout the developmental stages (Fig. 5F). Notably, we found weak Kiss1 mRNA signals at 19 days of age in female and male gonad-intact rats (Fig. 5G), suggesting that transient Kiss1 expression in the VMH during the prepubertal phase (19 days of age) triggers VMH tdTomato expression in both sexes.
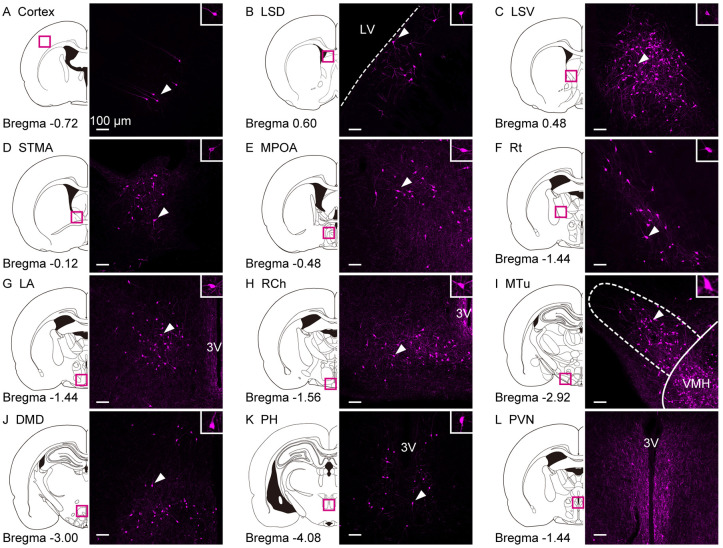
Exploration of Kiss1-Cre-activated tdTomato-IR signals in other forebrain regions of the female rats
We also examined Kiss1-Cre-activated tdTomato and Kiss1 mRNA signals in other brain regions of female rats using double-stained forebrain sections for Kiss1 mRNA and tdTomato-IR. Kiss1-Cre-activated tdTomato-IR cells were found in the cortex (Fig. 6A), LS dorsal part (LSD, Fig. 6B), LS ventral part (LSV, Fig. 6C), BNST medial division anterior part (STMA, Fig. 6D), MPOA (Fig. 6E), Rt (Fig. 6F), lateroanterior hypothalamic nucleus (LA, Fig. 6G), RCh (Fig. 6H), MTu (Fig. 6I), DMD (Fig. 6J), and PH (Fig. 6K) in adult OVX + E2 female rats. However, the Kiss1 mRNA was not detected in these regions. Dense tdTomato-IR fibers were observed in the medial part of the PVN in OVX + E2 female rats (Fig. 6L).
Fig. 6.
Exploration of Kiss1-Cre-activated tdTomato-IR signals in other regions of the female rat forebrain. TdTomato-IR cells in the (A) cortex, (B) LSD, (C) LSV, (D) STMA, (E) MPOA, (F) Rt, (G) LA, (H) RCh, (I) MTu, (J) DMD, and (K) PH in representative OVX+E2 female rats in adulthood (8–16 weeks of age). Note that no Kiss1 mRNA signals were detected in these nuclei by in situ hybridization. (L) The tdTomato-IR fibers in the PVN in a representative OVX+E2 female rat in adulthood. Insets are magnified images showing tdTomato-IR positive cells (indicated by arrowheads in the main images). LSD, lateral septal nucleus dorsal part; LSV, lateral septal nucleus ventral part; STMA, bed nucleus of the stria terminalis medial division anterior part; MPOA, medial preoptic area; Rt, reticular thalamic nucleus; LA, lateroanterior hypothalamic nucleus; RCh, retrochiasmatic area; MTu, medial tuberal nucleus; DMD, dorsal part of dorsomedial hypothalamic nucleus; PH, posterior hypothalamus; PVN, paraventricular hypothalamic nucleus. Scale bars, 100 µm.
Discussion
The present study demonstrated the successful generation of Kiss1-Cre rats exhibiting genetically induced hypogonadotropic hypogonadism by crossing them with Kiss1-floxed rats. Specifically, adult Kiss1-KpKO male rats presented atrophic testes and lacked ARC Kiss1 expression, with severe suppression of LH release even after castration and profound suppression of pituitary Lhb, Fshb, and Gnrhr mRNA expression. These findings suggest that Cre recombinase in the Kiss1-Cre rats successfully knocked out the Kiss1 gene in Kiss1-floxed rats, resulting in a hypogonadotropic hypogonadism phenotype similar to that observed in global Kiss1 KO rats [63]. Using Kiss1-Cre rats, we generated genetically modified rats with Kiss1-Cre-triggered cells visualized by tdTomato fluorescence in various brain regions and found that most of the Kiss1-expressing cells co-expressed tdTomato immunoreactivity in the AVPV of E2-treated OVX rats and the ARC of non-E2-treated OVX rats in adulthood. Notably, E2 treatment did not affect tdTomato fluorescence signals during adulthood. The numbers of tdTomato fluorescence-positive cells in the AVPV and ARC were comparable between OVX and E2-treated OVX rats. In contrast, the number of AVPV Kiss1-expressing cells was decreased by ovariectomy, whereas E2 treatment decreased the number of ARC Kiss1-expressing cells in OVX Kiss1-Cre-activated tdTomato reporter rats. These results suggest that AVPV and ARC kisspeptin neurons can be identified by tdTomato fluorescence, regardless of E2-induced changes in Kiss1 expression. The potential kisspeptin neurons visualized by tdTomato fluorescence provide a useful tool for investigating the mechanism mediating the downregulation of Kiss1/kisspeptin expression because Kiss1-visualized rats allowed us to identify potential kisspeptin neurons by tdTomato fluorescence regardless of Kiss1 and kisspeptin expression. To the best of our knowledge, this is the first study to report the generation of Kiss1-Cre rats, which are useful for investigating the physiological and molecular mechanisms regulating kisspeptin neurons as key players in mammalian reproduction.
The current developmental analysis of Kiss1-Cre-activated tdTomato fluorescence-positive cells revealed that AVPV tdTomato expression during the early postnatal period, such as 1 or 7 days after birth, was found only in male rats, and that these tdTomato fluorescence-positive cells remained until adulthood (10–13 weeks of age). This result suggests that Kiss1 mRNA is transiently expressed in the AVPV of male rats and that kisspeptin neurons in the AVPV survive until the adult stage, when Kiss1 expression is strongly suppressed by the organizing effect of perinatal testosterone in male rats. Perinatal testosterone likely transiently induces AVPV Kiss1 expression and irreversibly inhibits AVPV Kiss1 expression in male rats. In contrast, the earliest AVPV tdTomato fluorescence was observed at 3 weeks of age in females, and the number of tdTomato fluorescence-positive cells gradually increased by adulthood (10–13 weeks of age). The pubertal increase in the number of tdTomato fluorescence-positive cells was largely consistent with the increase in AVPV Kiss1-expressing cells in female rats, as reported in our previous study, which showed an increase in the number of AVPV Kiss1-expressing cells at puberty onset and thereafter in female rats [52]. Notably, compared to male rats, female rats showed a significantly greater number of tdTomato fluorescence-positive cells in the medial part of the AVPV region along the edge of 3V. This finding suggests that Kiss1 neurons in the medial part of the AVPV are responsible for inducing a GnRH/LH surge in response to preovulatory estrogen in female rats. This hypothesis is consistent with our previous study showing that a preovulatory level of E2 induces Kiss1 expression in the medial part of the AVPV, which is associated with an E2-induced LH surge in OVX rats and neonatally castrated male rats [42].
Kiss1-Cre-activated tdTomato fluorescence-positive cells in the ARC were found in the perinatal period (1 day of age) and thereafter in female and male rats, suggesting that ARC Kiss1 expression is initiated in the perinatal period in both sexes. This result is consistent with a previous study showing that kisspeptin immunoreactivity is evident at E14.5 in female and male rats [49]. The number of ARC tdTomato fluorescence-positive cells continuously increased to the adult stage (10–13 weeks of age) in female rats, but was stable after puberty (7, 10–13 weeks of age) in male rats, which suggests that greater numbers of potential ARC kisspeptin neurons exist in female rats than in males in adulthood (10–13 weeks of age). This result is consistent with a previous study, which found a larger number of ARC kisspeptin neurons in gonad-intact female rats than in gonad-intact male rats during adulthood [64]. Notably, Kiss1/kisspeptin expression is largely suppressed by gonad-derived steroids during the prepubertal period in female rats [51]. Therefore, the current visualized potential ARC kisspeptin neurons would be a useful tool to investigate the inhibitory mechanism of the steroid-dependent prepubertal restraint of ARC Kiss1/kisspeptin expression in rodents.
Notably, in the present study, Kiss1 mRNA signals were not detected in a large population of tdTomato fluorescence-positive cells, even in the AVPV of OVX females treated with a positive feedback level of E2, and in the ARC of OVX female rats without E2 treatment. This suggests that many potential kisspeptin neurons lacking Kiss1 expression still exist in the nuclei of adult female rats. Alternatively, tdTomato expression driven by a strong CAG promoter allowed us to easily detect tdTomato signals, even if Kiss1 mRNA expression was undetectable in some kisspeptin neurons. In this context, the coexistence of Kiss1 and tdTomato signals may be detected through an increase in the sensitivity of in situ hybridization for Kiss1. Notably, a small population of Kiss1-expressing cells failed to show tdTomato immunoreactivity in the AVPV and ARC in female rats. Theoretically, cells that have expressed Kiss1/Cre at least once should exhibit persistent tdTomato expression after Kiss1 expression in Kiss1-Cre-activated tdTomato reporter rats. We speculated that the tdTomato-IR signals might be below the threshold for tdTomato immunohistochemistry in some Kiss1-expressing cells. Improvement of the methodology to detect Kiss1 mRNA and tdTomato immunoreactivity may solve the inconsistency between Kiss1-expressing and tdTomato-IR cells in the future.
The current study found a large number of Kiss1-Cre-activated tdTomato fluorescence-positive cells in the MeA of adult male and female rats, while Kiss1 mRNA signals were rarely detected in the nucleus. This suggests that potential MeA Kiss1 neurons can be easily detected using the tdTomato signal as an indicator. The earliest tdTomato fluorescence signals in the MeA were observed at 3 weeks of age, and the number of MeA tdTomato-positive cells increased thereafter in the adult stage (10–13 weeks of age). The number of MeA tdTomato fluorescence-positive cells showed sex differences, with a larger number of tdTomato-positive cells in males than in females. This result is largely consistent with previous studies, which found that the number of MeA Kiss1 neurons was higher in male rats than in female rats [53] and that the number of MeA Kiss1-expressing cells increased throughout development in male mice [65]. MeA kisspeptin neurons receive neuronal projections from the accessory olfactory bulb (AOB), where pheromonal information from the vomeronasal organ is relayed [54]. A previous study showed that selective activation of the posterior dorsal subnucleus of the MeA (MePD) kisspeptin neurons by the DREADD system enhanced sexual partner preference and social interaction and decreased anxiety in male mice [55]. Taken together, the potential kisspeptin neurons visualized by tdTomato fluorescence provide a useful tool for investigating the role of MeA kisspeptin neurons as modulators of behavioral responses to pheromones in male rodents.
The present study found a large number of Kiss1-Cre-activated tdTomato fluorescence-positive cells in the VMH of female and male rats, suggesting that potential kisspeptin neurons are abundantly located in the VMH. Previous studies showed Kiss1 promoter-dependent tdTomato fluorescence in the VMH of adult female rats [8], and Kiss1 expression in the VMH of adult OVX female rats treated with high-dose E2 [56]. The current study detected weak Kiss1 expression on postnatal Day 19 in both sexes of wild-type rats as reported in a previous study [44]. These findings suggest that an increase in sex steroids released from the gonads during the prepubertal period (around postnatal Day 19) triggers Kiss1-Cre-activated tdTomato fluorescence in the VMH because plasma E2 levels increase in female and male rats at 2 weeks of age [66,67,68]. Interestingly, there was no obvious Kiss1-Cre-dependent reporter gene expression in the VMH of Kiss1-Cre mice [40], suggesting species-specific differences in VMH Kiss1 expression. Taken together, Kiss1 was temporally expressed in the VMH during the prepubertal period and Kiss1 expression was turned off in most potential VMH kisspeptin neurons in rats. Further studies are required to clarify the physiological role of kisspeptin neurons in the VMH of both sexes.
The current study demonstrates that a large number of potential kisspeptin cells visualized by Kiss1-Cre-activated tdTomato exist in several other regions of the rat forebrain. Specifically, tdTomato-IR cell bodies were detected in the cortex, LS, BNST, MPOA, RCh, Mtu, DMD, and PH, where Kiss1 and/or Kiss1-Cre reporter signals were previously reported in mice and rats [56, 57, 61]. TdTomato-IR fibers were detected in the medial part of the PVN and kisspeptin-IR fibers were reported in the mouse brain [41, 59, 60]. Therefore, the current Kiss1-Cre-activated tdTomato reporter rats may be useful models for investigating kisspeptin neuronal projections that cannot be examined using Kiss1in situ hybridization. The current study also showed tdTomato-IR cell bodies in the Rt and LA, where Kiss1- and/or kisspeptin-positive cells have not been previously reported. Further studies are needed to clarify the physiological roles of potential kisspeptin neurons originating from the cortex, LS, BNST, MPOA, RCh, Mtu, DMD, and PH, and neurons projecting to the PVN.
In conclusion, the present study demonstrated that the newly generated Kiss1-Cre rats and Kiss1-Cre-activated tdTomato reporter rats could be useful tools for further detailed analyses of potential Kiss1/kisspeptin neurons across the brain. Specifically, AVPV and ARC kisspeptin neurons identified by tdTomato fluorescence, regardless of E2 levels, could be used to investigate the mechanisms mediating the positive and negative effects of E2 on Kiss1/kisspeptin expression in the AVPV and ARC, respectively. Furthermore, Kiss1-Cre rats have an advantage over Kiss1-Cre mice because of their larger body size, which enables frequent blood sampling to detect LH pulses and E2-induced LH surges, and the monitoring of activities of ARC or AVPV kisspeptin neurons using Kiss1-Cre-dependent expression of calcium indicators, such as GCaMP. Moreover, the current Kiss1-Cre rats may also be used to investigate the physiological role of kisspeptin neurons located in brain regions other than the AVPV and ARC, because Kiss1-Cre-activated tdTomato fluorescence-positive cells are more easily detected than Kiss1 mRNA signals. In addition, Kiss1-Cre-dependent infection of tracer genes, such as sensor probe genes, and/or the deletion of certain genes using the Cre/loxP system in potential Kiss1 neurons in Kiss1-Cre rats would provide powerful tools to further improve our understanding of the role of kisspeptin neurons as master regulators of mammalian reproduction.
Conflicts of interests
The authors declare no conflict of interest.
Supplementary
Acknowledgments
We thank the National BioResource Project - Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing the rat strains (LE-Tg(Gt(ROSA)26Sor-CAG-tdTomato)24Jfhy rats NBRP Rat No. 0734). We thank Ms. Kyoko Yogo and Mr. Yuki Enomoto for their technical support. This work was supported in part by the Japan Society for the Promotion of Science KAKENHI grant numbers JP21H05031, JP21K19186 (to H. Tsukamura), JP19H03103 (to N.I.), JP20H03127, JP22K19245 (to Y.U.), and JP22J10728 (to M.N.); Cooperative Study Program (No. 19-256) of National Institute for Physiological Sciences (to H. Tsukamura); “Nagoya University Interdisciplinary Frontier Fellowship” supported by Nagoya University and the Japan Science and Technology Agency, the establishment of university fellowships towards the creation of science technology innovation, grant number JPMJFS2120 (to K.Y.); Graduate Program of Transformative Chem-Bio Research at Nagoya University, supported by the Ministry of Education, Culture, Sports, Science and Technology (Doctoral Program for World-leading Innovative & Smart Education) (to M.N., H. Tsuchida); and Mizutani Scholarship (Graduate School of Bioagricultural Sciences, Nagoya University) (to M.N.).
References
- 1.Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy Neurons Expressing Kisspeptin, Neurokinin B, and Dynorphin A as a GnRH Pulse Generator Controlling Mammalian Reproduction. Front Endocrinol (Lausanne) 2021; 12: 724632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uenoyama Y, Inoue N, Nakamura S, Tsukamura H. Kisspeptin neurons and estrogen–estrogen receptor α signaling: Unraveling the mystery of steroid feedback system regulating mammalian reproduction. Int J Mol Sci 2021; 22: 9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukamura H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen Comp Endocrinol 2022; 315: 113755. [DOI] [PubMed] [Google Scholar]
- 4.Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J Neuroendocrinol 2022; 34: e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 6.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 2012; 366: 629–635. [DOI] [PubMed] [Google Scholar]
- 8.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda K-I, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 9.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 10.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 2007; 104: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077. [DOI] [PubMed] [Google Scholar]
- 12.Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 2004; 145: 4565–4574. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- 14.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 16.Naniwa Y, Nakatsukasa K, Setsuda S, Oishi S, Fujii N, Matsuda F, Uenoyama Y, Tsukamura H, Maeda K, Ohkura S. Effects of full-length kisspeptin administration on follicular development in Japanese Black beef cows. J Reprod Dev 2013; 59: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 2005; 102: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 2005; 90: 6609–6615. [DOI] [PubMed] [Google Scholar]
- 19.Robert L. Barbieri. Female infertility. In: Jerome F. Strauss, Barbieri RL (eds.), Yen and Jaffe’s Reproductive Endocrinology. Elsevier; 2019: 556–581. [Google Scholar]
- 20.Barbieri RL. Clinical applications of GnRH and its analogues. Trends Endocrinol Metab 1992; 3: 30–34. [DOI] [PubMed] [Google Scholar]
- 21.Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA 2003; 290: 1767–1770. [DOI] [PubMed] [Google Scholar]
- 22.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146: 2976–2984. [DOI] [PubMed] [Google Scholar]
- 23.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006; 26: 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 25.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008; 28: 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, Majarune S, Nakamura S, Sanbo M, Hirabayashi M, Kobayashi K, Inoue N, Tsukamura H. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA 2021; 118: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA 2017; 114: E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikegami K, Goto T, Nakamura S, Watanabe Y, Sugimoto A, Majarune S, Horihata K, Nagae M, Tomikawa J, Imamura T, Sanbo M, Hirabayashi M, Inoue N, Maeda K-I, Tsukamura H, Uenoyama Y. Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. J Reprod Dev 2020; 66: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minabe S, Nakamura S, Fukushima E, Sato M, Ikegami K, Goto T, Sanbo M, Hirabayashi M, Tomikawa J, Imamura T, Inoue N, Uenoyama Y, Tsukamura H, Maeda KI, Matsuda F. Inducible Kiss1 knockdown in the hypothalamic arcuate nucleus suppressed pulsatile secretion of luteinizing hormone in male mice. J Reprod Dev 2020; 66: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore AM, Coolen LM, Lehman MN. In vivo imaging of the GnRH pulse generator reveals a temporal order of neuronal activation and synchronization during each pulse. Proceedings of the National Academy of Sciences2022; 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology 2019; 160: 557–567. [DOI] [PubMed] [Google Scholar]
- 32.McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology 2019; 160: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 34.Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology 2015; 156: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci 2022; 16: 953252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 37.Tomikawa J, Homma T, Tajima S, Shibata T, Inamoto Y, Takase K, Inoue N, Ohkura S, Uenoyama Y, Maeda K, Tsukamura H. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod 2010; 82: 313–319. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol 2014; 26: 909–917. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, Wakabayashi Y, Okamura H, Maeda KI, Uenoyama Y, Tsukamura H, Ohkura S. The luteinising hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol 2015; 27: 57–65. [DOI] [PubMed] [Google Scholar]
- 40.Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, Chen C, Colledge WH. Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. J Neuroendocrinol 2016; 28: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marraudino M, Miceli D, Farinetti A, Ponti G, Panzica G, Gotti S. Kisspeptin innervation of the hypothalamic paraventricular nucleus: sexual dimorphism and effect of estrous cycle in female mice. J Anat 2017; 230: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe Y, Ikegami K, Nakamura S, Uenoyama Y, Ozawa H, Maeda K-I, Tsukamura H, Inoue N. Mating-induced increase in Kiss1 mRNA expression in the anteroventral periventricular nucleus prior to an increase in LH and testosterone release in male rats. J Reprod Dev 2020; 66: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. J Comp Neurol 2011; 519: 2954–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 2010; 151: 5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarkson J, Busby ER, Kirilov M, Schütz G, Sherwood NM, Herbison AE. Sexual differentiation of the brain requires perinatal kisspeptin-GnRH neuron signaling. J Neurosci 2014; 34: 15297–15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura S, Watanabe Y, Goto T, Ikegami K, Inoue N, Uenoyama Y, Tsukamura H. Kisspeptin neurons as a key player bridging the endocrine system and sexual behavior in mammals. Front Neuroendocrinol 2022; 64: 100952. [DOI] [PubMed] [Google Scholar]
- 48.Knoll JG, Clay CM, Bouma GJ, Henion TR, Schwarting GA, Millar RP, Tobet SA. Developmental profile and sexually dimorphic expression of kiss1 and kiss1r in the fetal mouse brain. Front Endocrinol (Lausanne) 2013; 4: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desroziers E, Droguerre M, Bentsen AH, Robert V, Mikkelsen JD, Caraty A, Tillet Y, Duittoz A, Franceschini I. Embryonic development of kisspeptin neurones in rat. J Neuroendocrinol 2012; 24: 1284–1295. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Minabe S, Munetomo A, Magata F, Sato M, Nakamura S, Hirabayashi M, Ishihara Y, Yamazaki T, Uenoyama Y, Tsukamura H, Matsuda F. Kiss1-dependent and independent release of luteinizing hormone and testosterone in perinatal male rats. Endocr J 2022; 69: 797–807. [DOI] [PubMed] [Google Scholar]
- 51.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537. [DOI] [PubMed] [Google Scholar]
- 52.Majarune S, Nima P, Sugimoto A, Nagae M, Inoue N, Tsukamura H, Uenoyama Y. Ad libitum feeding triggers puberty onset associated with increases in arcuate Kiss1 and Pdyn expression in growth-retarded rats. J Reprod Dev 2019; 65: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011; 152: 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala kisspeptin neurons: putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology 2017; 104: 223–238. [DOI] [PubMed] [Google Scholar]
- 55.Adekunbi DA, Li XF, Lass G, Shetty K, Adegoke OA, Yeo SH, Colledge WH, Lightman SL, O’Byrne KT. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol 2018; 30: e12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, Inoue K, Adachi AA. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J 2012; 59: 161–171. [DOI] [PubMed] [Google Scholar]
- 57.Di Giorgio NP, Semaan SJ, Kim J, López PV, Bettler B, Libertun C, Lux-Lantos VA, Kauffman AS. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology 2014; 155: 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol 2005; 481: 314–329. [DOI] [PubMed] [Google Scholar]
- 59.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 2009; 21: 673–682. [DOI] [PubMed] [Google Scholar]
- 61.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011; 173: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Igarashi H, Koizumi K, Kaneko R, Ikeda K, Egawa R, Yanagawa Y, Muramatsu S, Onimaru H, Ishizuka T, Yawo H. A novel reporter rat strain that conditionally expresses the bright red fluorescent protein tdTomato. PLoS One 2016; 11: e0155687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 64.Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci 2011; 43: 138–145. [DOI] [PubMed] [Google Scholar]
- 65.Stephens SBZ, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology 2016; 157: 4021–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell MR. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 2018; 159: 2596–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 1975; 97: 898–907. [DOI] [PubMed] [Google Scholar]
- 68.Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod 2012; 87: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.