Abstract
Objective
The effect of sarcopenia on the prognosis of patients undergoing chemotherapy for unresectable pancreatic ductal adenocarcinoma remains largely unexplored. In this retrospective study, we investigated the relationship between sarcopenia and the prognosis of patients receiving first-line nanoparticle albumin-bound paclitaxel plus gemcitabine for unresectable pancreatic ductal adenocarcinoma.
Methods
We enrolled 251 patients with unresectable metastatic or locally advanced pancreatic ductal adenocarcinoma who had received chemotherapy between January 2015 and December 2020 at Kitasato University Hospital. Univariate and multivariate analyses were performed using the stratified Cox proportional hazards model to determine variables significantly associated with the progression-free and overall survival. Propensity score matching was performed to mitigate selection bias effects.
Results
In the propensity score-matched cohort, the progression-free and overall survival were not significantly different between the sarcopenia and non-sarcopenia groups (p=0.335, and 0.679 respectively). The skeletal muscle index decreased by 4.4% and 6.5% in the sarcopenia and non-sarcopenia groups, respectively, during the early treatment phase (p=0.084). There were no significant differences between groups with regard to major adverse events or drug toxicity occurrences. Both the progression-free and overall survival were significantly shorter in the skeletal muscle index loss group than in the non-skeletal muscle index loss group (p=0.026 and 0.045, respectively).
Conclusion
Skeletal muscle index loss during the initial treatment phase may be an early marker for the long-term prognosis of patients receiving nanoparticle albumin-bound paclitaxel plus gemcitabine as first-line treatment for unresectable pancreatic ductal adenocarcinoma.
Keywords: nab-paclitaxel, gemcitabine, pancreatic ductal adenocarcinoma, prognostic factor, sarcopenia
Introduction
Combination chemotherapy with nanoparticle albumin-bound paclitaxel (nab-PTX) plus gemcitabine (GEM) is a common first-line treatment for unresectable locally advanced (UR-LA) or unresectable metastatic (UR-M) pancreatic ductal adenocarcinoma (PDAC) (1). Predicting the efficacy of chemotherapy at the beginning of treatment or during the early post-treatment stages will facilitate the development of optimum treatment strategies; however, the prognostic factors pertaining to nab-PTX+GEM have not been sufficiently validated (2-4).
Sarcopenia is a progressive, systemic disease characterized by decreased skeletal muscle mass and muscle weakness. Recently, the effects of sarcopenia on the prognosis of patients undergoing cancer treatment have attracted the attention of researchers. Based on its etiology, sarcopenia, coined in 1989 (5), can be classified as primary or secondary. Primary sarcopenia is associated with aging, whereas secondary sarcopenia is associated with the presence of systemic diseases, physical inactivity, and poor nutrition (6,7). Secondary sarcopenia is common in patients with pancreatic cancer. Malabsorption, resulting from pancreatic insufficiency or physical factors, such as duodenal stenosis, may lead to malnutrition as cancer progresses. Many patients with pancreatic cancer are underweight at the time of the diagnosis, and patients with weight loss exceeding 10% of the normal adult body weight at the diagnosis have a significantly reduced survival (8). Therefore, it is important to determine effective treatment strategies for such patients by investigating the association between unresectable pancreatic cancer and sarcopenia.
To date, only two studies have investigated the effect of sarcopenia on the prognosis of patients receiving first-line nab-PTX+GEM for unresectable PDAC (9,10). Furthermore, the impact of variation in the skeletal muscle index (SMI) during the early phase of treatment on the long-term prognosis has not been studied.
We therefore investigated the association between clinical factors (such as the presence of sarcopenia at the diagnosis and variation in the SMI in the early phase of treatment) and the long-term prognosis in patients receiving first-line nab-PTX+GEM for unresectable PDAC.
Materials and Methods
Patient eligibility
We enrolled patients with UR-M or UR-LA PDAC who had undergone chemotherapy between January 2015 and December 2020 at Kitasato University Hospital. The inclusion criteria were: 1) age ≥20 years old; 2) a pathological diagnosis of PDAC; 3) UR-LA or UR-M PDAC; and 4) first-line chemotherapy regimen comprising nab-PTX+GEM. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status ≥3 were excluded. Pancreatic cancer was diagnosed histologically using endoscopic ultrasound (EUS)-guided fine-needle aspiration. Contrast-enhanced computed tomography (CE-CT), EUS, and gadolinium-enhanced magnetic resonance imaging were used to assess the pre-treatment tumor stage, local tumor extension, and the presence of distant and lymph node metastases. TNM staging was evaluated using the 8th edition of the Union for International Cancer Control guidelines.
Study design and endpoint
The protocol for this retrospective, single-center study was approved by the Kitasato University Ethics Committee (approval number B22-003). Consent was not deemed necessary, as this was an observational study that used existing data; however, patients were given the opportunity to opt out of the study.
The primary endpoints were the retrospectively assessed the progression-free survival (PFS) and overall survival (OS) in patients with PDAC who underwent first-line nab-PTX+GEM treatment. The endpoints were evaluated after propensity score (PS) matching in patients with and without sarcopenia (sarcopenia and non-sarcopenia groups, respectively). Secondary endpoints included the tumor reduction rate, biological tumor response, prognostic factors, and comparison of the PFS and OS in patients with or without SMI loss (the SMI loss and non-SMI loss groups, respectively) during the early phases of treatment. The tumor response was assessed using CE-CT in accordance with the response evaluation criteria in solid tumors (RECIST) (11). The number of patients who experienced at least one grade ≥3 adverse event (AE) was compared between the groups. Drug toxicity was graded based on the Common Terminology Criteria for Adverse Events version 4.0 (12).
Measuring the skeletal muscle mass
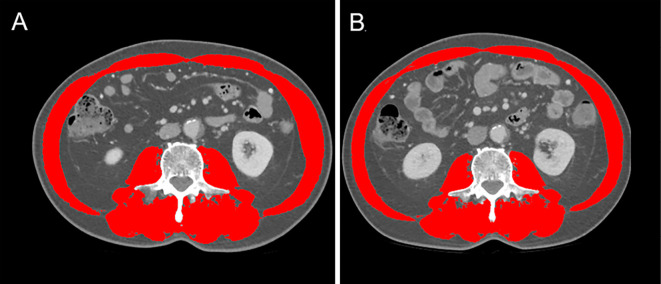
The skeletal muscle area was measured via cross-sectional CT images at the third lumbar vertebra (L3) level using the Slice-O-Matic software program (version 5.0; Tomovision, Montreal, Canada) on available pre-treatment CT scans. The skeletal muscle area was normalized for the square of the height and expressed as the SMI (Fig. 1). An L3 CT scan shows the following skeletal muscles: psoas major, erector spinae, quadratus lumborum, rectus abdominis, transversus abdominis, and internal and external oblique muscles. The cut-off values for low SMI are <42 cm2/m2 for men and <38 cm2/m2 for women (13). These cut-off values were used to assign patients to the sarcopenia or non-sarcopenia groups. Generally, the early treatment phase SMI, obtained using CT after chemotherapy induction, was evaluated after two or three cycles of chemotherapy following treatment initiation.
Figure 1.
The skeletal muscle index calculated using computed tomographic images. Axial computed tomographic images of the third lumbar vertebra region, with the skeletal muscle area highlighted in red. Images of the same patient before (A) and early after (B) chemotherapy induction.
Imaging assessments of body composition were performed and confirmed via a consensus between authors (A.I and K.O).
Biochemical assessments
We retrospectively assessed the results of the following biochemical blood tests performed prior to commencing chemotherapy: total white blood cell count (cells/μL), absolute neutrophil and lymphocyte counts (cells/μL), platelet count (cells/μL), hemoglobin (g/dL), C-reactive protein (CRP, mg/dL), albumin (Alb, g/dL), carcinoembryonic antigen (CEA, ng/mL), and carbohydrate antigen 19-9 (CA19-9, UI/mL) levels. The variables were used to calculate the following prognostic scores: the neutrophil/lymphocyte ratio (NLR), prognostic nutritional index (PNI; calculated as 10× Alb levels+0.005×lymphocyte count), modified Glasgow prognostic score (m-GPS; m-GPS=0 was assigned when CRP levels ≤1.0 and Alb levels ≥3.5, m-GPS=1 was assigned when CRP >1.0 or Alb levels <3.5, and m-GPS=2 was assigned when CRP >1.0 and Alb levels <3.5), and platelet/lymphocyte ratio (PLR). The following parameters were reported to be associated with poor prognoses for patients with pancreatic cancer and other cancers: NLR ≥5, CRP/Alb ratio ≥1, PNI <46, PLR ≥150, and m-GPS ≥1 (14-21).
Treatment
Almost all patients were treated with nab-PTX (125 mg/m2) and GEM (1,000 mg/m2) as first-line chemotherapy on days 1, 8, and 15, every 4 weeks, which corresponded to 1 treatment cycle. In some patients, doses were reduced based on certain factors, such as the age and performance status. The relative dose intensity (RDI) was defined as the ratio of the administered cumulative dose to the planned cumulative dose (both expressed in milligrams). First-line chemotherapy was discontinued for patients who developed progressive disease (PD). In addition, after initiating treatment, patients underwent careful follow-up and were subjected to imaging and tumor marker measurements. Patients with PD were placed on a second-line chemotherapy regimen or underwent adequate supportive care. The start date of follow-up was set as the date of initiation of first-line chemotherapy. The end date of follow-up was set as the final follow-up date in June 2021 or the date of death.
Measurement definitions
The tumor diameter on CE-CT was measured before initiating chemotherapy. The patient response to chemotherapy was classified based on the RECIST guidelines (version 1.1) as follows: complete response, partial response (PR), stable disease (SD), and PD (11). The treatment efficacy of chemotherapy was examined once every two to three cycles during treatment. The non-SMI loss group comprised the cases in which the SMI calculated using the first CE-CT images after the start of treatment was equal to or greater than the SMI calculated from the pre-treatment CT images.
Statistical analyses
Owing to the different clinical characteristics of patients in the sarcopenia and non-sarcopenia groups, PS matching was performed to mitigate the effects of selection bias. PSs were estimated using the following covariates as predictors for sarcopenia and non-sarcopenia in the logistic regression model: age, sex, ECOG performance status, tumor location, tumor size, tumor stage (UR-LA or UR-M), metastatic site (lung, liver, or peritoneum), number of metastatic sites, CRP/Alb ratio, NLR, PLR, m-GPS, PNI, CEA level, CA19-9 level, and body mass index (BMI). The discrimination of the PS model was assessed using the area under the receiver-operating characteristic curve. PS matching was performed using the following algorithm: nearest neighbor and one-to-one pair matching with a ±0.1 caliper and no replacement. The absolute standardized difference (ASD) was used to measure covariate balance. Covariates with an ASD <0.1 were considered to have good balance.
In the PS-matched cohorts, the PFS and OS were estimated using the Kaplan-Meier method. The differences between the Kaplan-Meier curves were evaluated using the stratified log-rank test, and the hazard ratio (HR) was determined using the Cox proportional hazards model. The quartile categories of PS were used as stratification factors. Univariate and multivariate analyses were performed using the stratified Cox proportional hazards model to determine variables significantly associated with the PFS and OS. After calculating the HR and 95% confidence interval (CI), a multivariate analysis using the forced-entry method was performed for significant factors in the univariate analysis to identify factors that were independently associated with the PFS and OS. Categorical variables were statistically analyzed using the Fisher's exact probability and Mann-Whitney U tests. p values <0.05 were considered statistically significant.
Statistical analyses were performed using the SPSS software program, ver. 24.0 (IBM Japan, Tokyo, Japan).
Results
Patient characteristics
During the study period, 251 patients with UR-M or UR-LA PDAC underwent first-line nab-PTX+GEM treatment at Kitasato University Hospital. All 251 patients were included in the analysis. The demographic and baseline characteristics of the included patients are summarized in Table 1.
Table 1.
Patient Characteristics and Comparisons between Patients with and without Sarcopenia before and after Propensity Score Matching.
| Characteristics | Full cohort | Propensity score-matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Sarcopenia | Non-sarcopenia | ASD | Number of patients | Sarcopenia | Non-sarcopenia | ASD | ||
| n=251 | n=140 | n=111 | n=180 | n=90 | n=90 | ||||
| Age, years | <65 ≥65 |
75 176 |
37 103 |
38 73 |
0.170 | 60 120 |
30 60 |
30 60 |
0.000 |
| Sex | Male Female |
141 110 |
66 74 |
75 36 |
0.422 | 119 61 |
61 29 |
58 32 |
0.070 |
| ECOG performance status | 0 1-2 |
135 116 |
72 68 |
63 48 |
0.107 | 97 83 |
48 42 |
49 41 |
0.022 |
| Tumor location | Head body-tail | 110 141 |
57 83 |
53 58 |
0.142 | 79 101 |
39 51 |
40 50 |
0.022 |
| Tumor size, mm | <30 ≥30 |
66 185 |
37 103 |
29 82 |
0.007 | 54 126 |
27 63 |
27 63 |
0.000 |
| Tumor stage | UR-LA UR-M |
95 156 |
51 89 |
44 67 |
0.066 | 64 116 |
31 59 |
33 57 |
0.046 |
| Metastatic site | Lung Liver Peritoneum |
24 104 43 |
15 57 22 |
9 47 21 |
0.089 0.033 0.085 |
12 81 26 |
6 39 12 |
6 42 14 |
0.000 0.067 0.063 |
| Number of metastatic sites | 1-3 >3 |
246 5 |
136 4 |
110 1 |
0.144 | 177 3 |
88 2 |
89 1 |
0.087 |
| CRP/Alb | <0.18 ≥0.18 |
153 98 |
86 54 |
67 44 |
0.022 | 111 69 |
56 34 |
55 35 |
0.023 |
| NLR | <5 ≥5 |
198 53 |
113 27 |
85 26 |
0.101 | 144 36 |
73 17 |
71 19 |
0.056 |
| PLR | <150 ≥150 |
102 149 |
59 81 |
43 68 |
0.069 | 75 105 |
39 51 |
36 54 |
0.068 |
| m-GPS | 0 1-2 |
144 107 |
77 63 |
67 44 |
0.109 | 106 74 |
51 39 |
55 35 |
0.090 |
| PNI | <45 ≥45 |
126 125 |
70 70 |
56 55 |
0.009 | 94 86 |
47 43 |
47 43 |
0.000 |
| CEA, ng/mL | <5 ≥5 |
132 119 |
69 71 |
63 48 |
0.150 | 92 88 |
44 46 |
48 42 |
0.089 |
| CA19-9, UI/mL | <37 ≥37 |
45 206 |
25 115 |
20 91 |
0.004 | 33 147 |
17 73 |
16 74 |
0.029 |
| BMI, kg/m2 | <25 ≥25 |
226 25 |
135 5 |
91 20 |
0.479 | 171 9 |
85 5 |
86 4 |
0.051 |
Alb: albumin, ASD: absolute standardized difference, BMI: body mass index, CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, CRP: C-reactive protein, ECOG: Eastern Cooperative Oncology Group, NLR: neutrophil-to-lymphocyte ratio, m-GPS: modified Glasgow prognostic score, PLR: platelet-to-lymphocyte ratio, PNI: prognostic nutritional index, UR-LA: unresectable locally advanced cancer, UR-M: unresectable metastatic cancer
After PS matching, 90 patients were assigned to each group (Fig. 2). Furthermore, all covariates were well-balanced between the groups, with ASDs <0.1.
Figure 2.
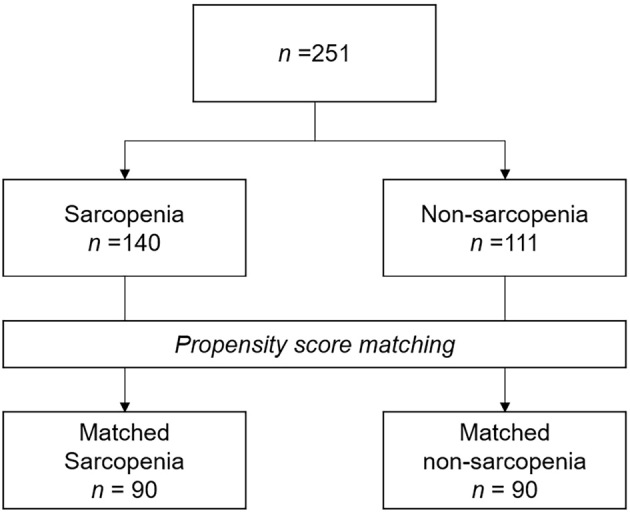
Study flow chart. Propensity score (PS) matching was performed to mitigate selection bias resulting from different patient characteristics. PSs were estimated using the characteristics of patients with and without sarcopenia as predictors in the logistic regression model for sarcopenia or non-sarcopenia; based on the PSs, 90 of 140 and 90 of 111 patients were assigned to the sarcopenia and non-sarcopenia groups, respectively.
The PFS and OS in the sarcopenia and non-sarcopenia groups
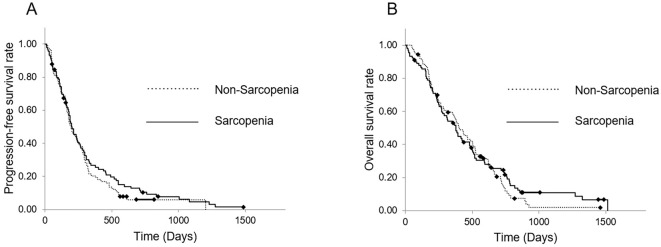
Fig. 3 shows the PFS and OS of patients in the sarcopenia and non-sarcopenia groups. The median PFS of patients in the sarcopenia and non-sarcopenia groups was 200 (95% CI, 160.2-239.8) and 194 (95% CI, 152.7-235.3) days, respectively. The 6-month and 1-year PFS rates of patients in the sarcopenia group were 56.9% and 26.7%, respectively, whereas those of patients in the non-sarcopenia group were 53.1% and 19.2%, respectively. The median OS of patients in the sarcopenia and non-sarcopenia groups was 377 (95% CI, 297.2-456.8) and 404 (95% CI, 318.6-489.4) days, respectively. The 1- and 2-year OS rates of patients in the sarcopenia group were 52.9% and 24.4%, respectively, whereas those of patients in the non-sarcopenia group were 57.1% and 16.2%, respectively. Neither the PFS nor the OS were significantly different between the sarcopenia and non-sarcopenia groups (p=0.335, HR 0.858 and p=0.679, HR 0.908, respectively; Fig. 3).
Figure 3.
Kaplan-Meier curves of the sarcopenia and non-sarcopenia groups in the PS-matched cohort. (A) The PFS from the time of first-line nab-PTX+GEM initiation. The median PFS was 200 and 194 days in the sarcopenia and non-sarcopenia groups, respectively, with no significant differences between groups (p=0.335, HR 0.858). (B) The OS from the time of first-line nab-PTX+GEM initiation. The median OS was 377 and 404 days, with no significant differences between groups (p=0.679, HR 0.908). PS: propensity score, PFS: progression-free survival, PTX: paclitaxel, GEM: gemcitabine, OS: overall survival
Prognostic factors
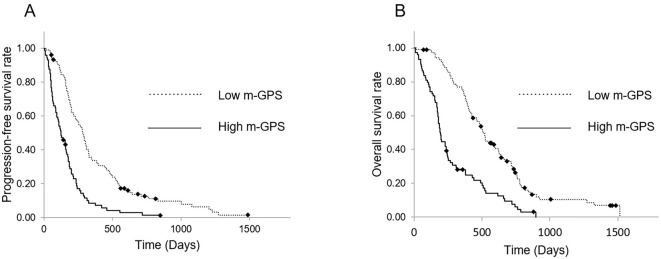
Tables 2, 3 show the results of univariate and multivariate analyses of prognostic factors related to patient outcomes. The multivariate analysis revealed that the stage (UR-M; HR, 2.127; 95% CI, 1.314-3.444) and m-GPS (1 or 2; HR, 2.708; 95% CI, 1.863-3.935) were significantly correlated with a poor PFS (Table 2). In addition, the PS (PS 1-2; HR, 1.414; 95% CI, 1.012-1.976), stage (HR, 1.807; 95% CI, 1.111-2.942), and m-GPS (HR, 2.698; 95% CI, 1.855-3.923) were each independently correlated with a poor OS (Table 3). The median PFS of patients in the high m-GPS and low m-GPS (m-GPS=0) groups was 124 (95% CI, 76.0-172.0) and 284 (95% CI, 242.7-325.3) days, respectively, whereas the median OS was 196 (95% CI, 173.0-219.0) and 510 (95% CI, 466.8-553.2) days, respectively. Both the PFS and OS were significantly shorter in the high m-GPS group than in the low m-GPS group (p<0.001, HR 2.598 and p<0.001, HR 2.743 respectively; Fig. 4).
Table 2.
Univariate and Multivariate Analyses for Progression-free Survival in the Propensity Score-matched Cohort.
| Univariate analysis | Multivariate analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||||||||
| Age, years | ≥65 | 0.887 | 0.636-1.237 | 0.479 | ||||||||||
| Sex | Male | 1.280 | 0.818-2.003 | 0.280 | ||||||||||
| ECOG performance status | 1-2 | 1.359 | 0.989-1.866 | 0.058 | ||||||||||
| Tumor location | Pancreas body-tail | 1.179 | 0.858-1.622 | 0.310 | ||||||||||
| Tumor size, mm | ≥30 | 1.066 | 0.763-1.489 | 0.710 | ||||||||||
| Stage | UR-M | 1.853 | 1.334-2.575 | 0.000 | 2.127 | 1.314-3.444 | 0.002 | |||||||
| Metastatic sites | Lung Liver Peritoneum |
0.567 1.723 0.926 |
0.293-1.098 1.247-2.383 0.585-1.467 |
0.093 0.001 0.744 |
0.930 | 0.581-1.487 | 0.761 | |||||||
| Number of metastatic sites | ≥3 | 2.210 | 0.663-7.368 | 0.197 | ||||||||||
| CRP/Alb | ≥0.18 | 0.980 | 0.713-1.347 | 0.900 | ||||||||||
| NLR | ≥5 | 1.060 | 0.706-1.592 | 0.777 | ||||||||||
| PLR | ≥150 | 1.033 | 0.753-1.416 | 0.842 | ||||||||||
| m-GPS | 1-2 | 2.490 | 1.739-3.565 | 0.000 | 2.708 | 1.863-3.935 | 0.000 | |||||||
| PNI | ≥45 | 0.930 | 0.680-1.273 | 0.652 | ||||||||||
| CEA, ng/mL | ≥5 | 1.199 | 0.877-1.639 | 0.255 | ||||||||||
| CA19-9, UI/mL | ≥37 | 1.393 | 0.926-2.094 | 0.111 | ||||||||||
| BMI, kg/m2 | ≥25 | 0.563 | 0.275-1.155 | 0.117 | ||||||||||
| Sarcopenia | Yes | 0.858 | 0.627-1.173 | 0.336 | ||||||||||
HR: hazard ratio, CI: confidence interval, CRP: C-reactive protein, NLR: neutrophil/lymphocyte ratio, PLR: platelet/lymphocyte ratio, m-GPS: modified Glasgow prognostic score, PNI: prognostic nutritional index, CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, BMI: body mass index, SMI: skeletal muscle index
Table 3.
Univariate and Multivariate Analyses for Overall Survival in the Propensity Score-matched Cohort.
| Univariate analysis | Multivariate analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||||||||
| Age, years | ≥65 | 0.951 | 0.673-1.343 | 0.775 | ||||||||||
| Sex | Male | 1.497 | 0.924-2.426 | 0.102 | ||||||||||
| ECOG performance status | 1-2 | 1.473 | 1.067-2.034 | 0.019 | 1.414 | 1.012-1.976 | 0.042 | |||||||
| Tumor location | Pancreas body-tail | 1.332 | 0.958-1.853 | 0.088 | ||||||||||
| Tumor size, mm | ≥30 | 1.128 | 0.801-1.588 | 0.490 | ||||||||||
| Stage | UR-M | 1.848 | 1.307-2.612 | 0.001 | 1.807 | 1.111-2.942 | 0.017 | |||||||
| Metastatic sites | Lung Liver Peritoneum |
0.813 1.928 0.961 |
0.420-1.574 1.377-2.699 0.608-1.521 |
0.540 0.000 0.866 |
1.103 | 0.688-1.769 | 0.683 | |||||||
| Number of metastatic sites | ≥3 | 3.587 | 1.032-12.467 | 0.044 | 3.355 | 0.931-12.084 | 0.064 | |||||||
| CRP/Alb | ≥0.18 | 1.296 | 0.936-1.795 | 0.119 | ||||||||||
| NLR | ≥5 | 1.299 | 0.860-1.961 | 0.213 | ||||||||||
| PLR | ≥150 | 0.921 | 0.667-1.272 | 0.618 | ||||||||||
| m-GPS | 1-2 | 2.816 | 1.968-4.029 | 0.000 | 2.698 | 1.855-3.923 | 0.000 | |||||||
| PNI | ≥45 | 0.936 | 0.679-1.291 | 0.688 | ||||||||||
| CEA, ng/mL | ≥5 | 1.368 | 0.989-1.891 | 0.058 | ||||||||||
| CA19-9, UI/mL | ≥37 | 1.217 | 0.798-1.858 | 0.362 | ||||||||||
| BMI, kg/m2 | ≥25 | 0.394 | 0.192-0.809 | 0.011 | 0.499 | 0.232-1.074 | 0.075 | |||||||
| Sarcopenia | Yes | 0.935 | 0.680-1.286 | 0.680 | ||||||||||
HR: hazard ratio, CI: confidence interval, CRP: C-reactive protein, NLR: neutrophil/lymphocyte ratio, PLR: platelet/lymphocyte ratio, m-GPS: modified Glasgow prognostic score, PNI: prognostic nutritional index, CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, BMI: body mass index, SMI: skeletal muscle index
Figure 4.
Kaplan-Meier curves of the high m-GPS and low m-GPS groups in the PS-matched cohort. (A) The PFS from the time of first-line nab-PTX+GEM initiation. The median PFS was 124 and 284 days in the high m-GPS and low m-GPS groups, respectively. The PFS was significantly shorter in the high m-GPS group than in the low m-GPS group (p<0.001, HR 2.598). (B) The OS from the time of first-line nab-PTX+GEM initiation. The median OS was 196 and 510 days in the high m-GPS and low m-GPS groups, respectively. The OS was significantly shorter in the high m-GPS group than in the low m-GPS group (p<0.001, HR 2.743). m-GPS: modified Glasgow prognostic score, PS: propensity score, PFS: progression-free survival, OS: overall survival, GEM: gemcitabine, PTX: paclitaxel, HR: hazard ratio
Tumor reduction rates
The tumor reduction rates, evaluated in accordance with the RECIST guidelines version 1.1, revealed PR, SD, PD, and disease control in 37%, 42%, 21%, and 79% of patients in the sarcopenia group, respectively; in the non-sarcopenia group, PR, SD, PD, and disease control were observed in 28%, 47%, 26%, and 74% of patients, respectively. Patients in the sarcopenia and non-sarcopenia groups underwent 7 [interquartile range (IQR), 4-12] and 7 (IQR, 3-10) chemotherapy cycles, respectively, with no significant differences detected between groups (p=0.437). The median RDI of nab-PTX was 60.1% (IQR, 43.6-74.3%) in the sarcopenia group and 61.2% (IQR, 50.2-73.0%) in the non-sarcopenia group (sarcopenia vs. non-sarcopenia group, p=0.739). Similarly, the RDI of GEM was 69.0% (IQR, 56.8-83.9%) in the sarcopenia group and 66.8% (IQR, 54.5-77.5%) in the non-sarcopenia group (sarcopenia vs. non-sarcopenia group, p=0.263).
Variation in the SMI
In patients who were evaluable by CT after 2 or 3 cycles of chemotherapy (166 patients in both groups), the decrease in the SMI was 4.4% (IQR, -0.4-9.7%) in the sarcopenia group and 6.5% (IQR, 1.6%-12.0%) in the non-sarcopenia group during the early phase of treatment across the median evaluation period of 1.8 (IQR, 1.6-2.2) months, with no significant difference detected between groups (p=0.084). The tumor reduction rates, evaluated in accordance with the RECIST guidelines version 1.1, revealed PR, SD, PD, and disease control in 36%, 42%, 22%, and 78% of the patients in the SMI loss group, respectively. In the non-SMI loss group, PR, SD, PD, and disease control were observed in 24%, 72%, 4%, and 96% of the patients, respectively.
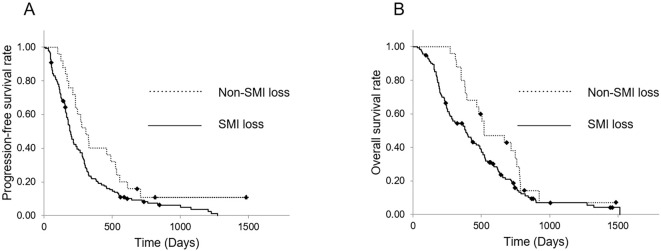
Fig. 5 shows variations in the PFS and OS of patients in the SMI loss and non-SMI loss groups. The median PFS of patients in the SMI loss and non-SMI loss groups was 190 (95% CI, 163.1-217.0) and 308 (95% CI, 212.3-403.7) days, respectively, whereas the median OS was 386 (95% CI, 285.8-486.2) and 520 (95% CI, 271.3-768.7) days, respectively. Both the PFS and OS were significantly shorter in the SMI loss group than in the non-SMI loss group (p=0.026, HR 1.670 and p=0.045, HR 1.603 respectively; Fig. 5).
Figure 5.
Kaplan-Meier curves of the SMI loss and non-SMI loss groups in the PS-matched cohort. (A) The PFS from the time of first-line nab-PTX+GEM initiation. The median PFS was 190 and 308 days in the SMI loss and non-SMI loss groups, respectively. The PFS was significantly shorter in the SMI loss group than in the non-SMI loss group (p=0.026, HR 1.670). (B) The OS from the time of first-line nab-PTX+GEM initiation. The median OS was 386 and 520 days in the SMI loss and non-SMI loss groups, respectively. The OS was significantly shorter in the SMI loss group than in the non-SMI loss group (p=0.045, HR 1.603). SMI: skeletal muscle index, PS: propensity score, PFS: progression-free survival, OS: overall survival, GEM: gemcitabine, PTX: paclitaxel, HR: hazard ratio
Evaluation of AEs
Major grade 3 AEs were observed in 50 patients (56%) in the sarcopenia group, which included hematological toxicities in 45 (50%) and non-hematological ones in 22 (24%). In the non-sarcopenia group, major grade 3 AEs were observed in 66 patients (73%), which included hematological toxicities in 53 (59%) and non-hematological ones in 28 (31%). Major grade 3 AEs were significantly more frequent in the non-sarcopenia group than in the sarcopenia group (p=0.019).
Biliary and duodenal obstruction
The incidence of initial or recurrent biliary obstruction requiring biliary drainage occurring from the date of pre-treatment CT to the observation period was 53.3% in the sarcopenia group and 40.0% in the non-sarcopenia group, with no significant difference noted between the groups (p=0.100). Similarly, the incidence of duodenal obstruction requiring treatment was 20.0% and 18.9%, respectively, with no significant difference noted between the groups (p=1.000).
Second-line chemotherapy
Forty-two percent of patients underwent second-line chemotherapy after nab-PTX+GEM treatment; a significantly greater proportion of these patients were from the sarcopenia group than from the non-sarcopenia group (72% vs. 43%, p<0.001). The regimens used as second-line chemotherapy in the sarcopenia group were S1 (22) in 10 patients, modified FOLFIRINOX (mFFX) in 7 patients, gemcitabine+S1 (GS) (23,24) in 4 patients, liposomal irinotecan+5-fluorouracil+leucovorin (nal-IRI/5-FU/LV) (25) in 2 patients, and S1+oxaliplatin+irinotecan (SOXIRI) (26-28) in 1 patient. In the non-sarcopenia group, S1 was administered to 21 patients, SOXIRI to 10 patients, mFFX to 9 patients, nal-IRI/5-FU/LV to 5 patients, gemcitabine to 4 patients, GS to 1 patient, and S1-based chemoradiotherapy to 1 patient. Second-line chemotherapy was administered to 57% of patients in the SMI loss group and 52% of patients in the non-SMI loss group, with no marked difference in induction rates noted between the groups (p=0.669).
Discussion
In the present study, we retrospectively examined predictive factors for the long-term prognosis of patients who underwent first-line nab-PTX+GEM chemotherapy for unresectable PDAC. While the presence of sarcopenia at the start of treatment was not a prognostic factor for the PFS or OS, we discovered that a reduced UR-M and high m-GPS were independent adverse prognostic factors for the PFS and OS in patients receiving first-line nab-PTX+GEM treatment.
The pathogenesis of sarcopenia involves a combination of factors triggered by tumor cells and the host's immune response to the tumor cells. For example, previous studies on sarcopenia have reported metabolic abnormalities caused by multiple inflammatory cytokines, such as TNF-α, IL-1, and IL-6 (29); increased degradation and a reduction in skeletal muscle protein synthesis induced by proteolysis-inducing factor (PIF) and reactive oxygen species of tumor origin (30,31); lipolysis and inefficient energy expenditure triggered by lipid-mobilizing factor (LMF) and parathyroid hormone-related protein of tumor origin (32); appetite suppression resulting from resistance to ghrelin signaling (33). Nab-PTX+GEM chemotherapy may inhibit the proliferation of tumor cells, induce their apoptosis, and regulate the production of inflammatory cytokines, PIF, and LMF by the tumor. This inhibits the degradation of skeletal muscle proteins, thereby causing inefficient energy expenditure. Thus, variations in the SMI following the initiation of treatment may indirectly reflect the therapeutic effects of nab-PTX+GEM. Our findings also suggest that SMI loss during the early phase of treatment is associated with not only the PFS but also the OS. The variations in the SMI may be useful for predicting the long-term prognosis, similar to the findings in the report by Chiorean et al. (3), which suggested that a reduction in CA19-9 expression at week 8 was an early marker for chemotherapy efficacy. Furthermore, for patients whose SMI decreases during the early phase of treatment, a combination of pharmacotherapy with anamorelin (34) and exercise (35) may enhance patient adherence to chemotherapy and improve the prognosis.
Emori et al. (9) recently reported that the presence of sarcopenia at the start of treatment is an adverse prognostic factor for the PFS and OS in patients receiving first-line nab-PTX+GEM chemotherapy for unresectable PDAC. Furthermore, Asama et al. (10) reported that sarcopenia at the start of treatment was an independent poor prognostic factor for the OS only in elderly patients (>70 years old) who had received first-line nab-PTX+GEM treatment for unresectable PDAC. While the prevalence of sarcopenia was comparable among these studies and our own (53.4% in Emori et al., 50.8% in Asama et al., and 55.7% in this study), contrary to their findings, we found no association between the presence of sarcopenia at the start of treatment and the prognosis, despite performing a similar PS matching analysis as Emori et al. (9) with a larger sample size. Numerous reports have described initial (pre-treatment) sarcopenia as an independent adverse prognostic factor in several malignancies (9,10,36-40). Almost all patients with PDAC located in the pancreatic head require biliary drainage before or during chemotherapy (41,42). Furthermore, locally advanced PDAC of the pancreatic head often involves the duodenum, which leads to loss of appetite and may necessitate gastrojejunostomy or endoscopic duodenal stent placement (42-45). These events can result in hospitalization and aggravation of the general condition or nutritional state. However, no marked difference in the proportion of these events occurring after the date of pre-treatment CT was observed between the sarcopenia and non-sarcopenia groups. Thus, why initial sarcopenia was an adverse prognostic factor in this study is unclear; the target population of this study may have differed from the standard population, despite the PS matching. Our findings suggest that a decrease in skeletal muscle mass following chemotherapy is more reflective of the long-term prognosis than the occurrence of sarcopenia prior to the pancreatic cancer diagnosis (i.e., the presence or absence of pre-treatment sarcopenia). However, it is difficult to draw such a conclusion from currently available evidence. A large-scale, multicenter, prospective study should be conducted to clarify our study findings.
In the present study, a high m-GPS was identified as another independent adverse prognostic factor for the PFS and OS. This result is consistent with the findings of several previous studies showing that a high m-GPS is an adverse prognostic factor in patients with PDAC receiving first-line nab-PTX+GEM chemotherapy (9,46-50).
We found no relationship between the presence or absence of second-line chemotherapy and the prognosis in our present study. The median OS in the second-line chemotherapy group was 481 (95% CI 411.9-550.1) days, whereas the median OS in the group that did not receive second-line chemotherapy was 292 (95% CI, 193.2-390.8) days, with no significant difference between the groups (p=0.461). These results indicate that, although the treatment regimens used for second-line chemotherapy varied and were not consistent in the population of this study, second-line chemotherapy was not effective enough to contribute to OS prolongation.
Several limitations associated with the present study warrant mention. First, considering that it was a single-center, retrospective study, there may have been bias pertaining to patient inclusion. Second, all patients underwent first-line treatment with nab-PTX+GEM, whereas only 42% of patients underwent second-line treatment; hence, there was non-uniformity between the number of patients who underwent first- and second-line treatment. A significantly higher proportion of patients who received second-line treatment belonged to the sarcopenia group than to the non-sarcopenia group. Although the analysis showed no association between the use or non-use of second-line treatment and the prognosis, the OS may have been influenced in cases wherein second-line treatment was used; thus, the OS findings should be interpreted with caution. Third, we only focused on determining SMI loss as a nutritional measurement after chemotherapy treatment. Variations in other nutritional parameters, such as the m-GPS, PNI, NLR, and PLR, warrant a further investigation to identify the factors most strongly associated with the prognosis, which was challenging owing to the retrospective nature of this study. Fourth, there was some variation in the timing of the first imaging evaluation, which was performed after the initiation of treatment with nab-PTX+GEM; because of this, the most appropriate timing for assessing SMI variation could not be determined in this study. Finally, the SMI was used as an indicator of sarcopenia in this study; according to revised diagnostic criteria for sarcopenia, of the many indicators of sarcopenia (6,51-53), muscle strength is a more reflective parameter than loss of skeletal muscle mass (54). Unfortunately, the retrospective nature of this study made assessing the muscle strength impossible. Further studies are thus warranted to determine whether or not the use of other indicators would support our findings.
In conclusion, the presence of sarcopenia at the start of treatment is not a prognostic factor for the PFS and OS; however, the comparison of pre-treatment and early post-treatment CT results of skeletal muscle mass at the L3 level may be useful for establishing the long-term prognosis of patients receiving first-line nab-PTX+GEM chemotherapy for unresectable PDAC.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369: 1691-1703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 20: 143-150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiorean EG, Von Hoff DD, Reni M, et al. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 27: 654-660, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Izumo W, Higuchi R, Furukawa T, et al. Evaluation of early prognostic factors in patients with pancreatic ductal adenocarcinoma receiving gemcitabine together with nab-paclitaxel. Cancer Diagn Progn 5: 399-409, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 127: 990-991, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39: 412-423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48: 16-31, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nemer L, Krishna SG, Shah ZK, et al. Predictors of pancreatic cancer-associated weight loss and nutritional interventions. Pancreas 46: 1152-1157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emori T, Itonaga M, Ashida R, et al. Impact of sarcopenia on prediction of progression-free and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology 22: 277-285, 2022. [DOI] [PubMed] [Google Scholar]
- 10. Asama H, Ueno M, Kobayashi S, et al. Sarcopenia: prognostic value for unresectable pancreatic ductal adenocarcinoma patients treated with gemcitabine plus nab-paclitaxel. Pancreas 51: 148-152, 2022. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247, 2009. [DOI] [PubMed] [Google Scholar]
- 12. NCI common terminology criteria for adverse events (CTCAE) version 4 [Internet]. [cited 2022 Mar]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/
- 13. Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46: 951-963, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 197: 466-472, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 200: 197-203, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer 47: 2633-2641, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109: 416-421, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szkandera J, Stotz M, Absenger G, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 110: 183-188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piciucchi M, Stigliano S, Archibugi L, et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci 18: 730, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikeguchi M, Goto K, Watanabe J, et al. Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg 23: 372-376, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 24: 561-568, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31: 1640-1648, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Ozaka M, Ishii H, Sato T, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. J Cancer Chemother Pharmacol 81: 1017-1023, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Sawada M, Kasuga A, Mie T, et al. Modified FOLFIRINOX as a second-line therapy following gemcitabine plus nab-paclitaxel therapy in metastatic pancreatic cancer. BMC Cancer 20: 449, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387: 545-557, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki M, Ueno H, Shiba S, et al. Phase I study of S-1, irinotecan plus oxaliplatin combination therapy for advanced pancreatic cancer. Ann Oncol 26: IX42, 2015. [Google Scholar]
- 27. Yanagimoto H, Satoi S, Sho M, et al. Phase I study assessing the feasibility of the triple combination chemotherapy of SOXIRI (S-1/oxaliplatin/irinotecan) in patients with unresectable pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol 77: 35-41, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Akahori T, Sho M, Yanagimoto H, et al. Phase II study of the triple combination chemotherapy of SOXIRI (S-1/oxaliplatin/irinotecan) in patients with unresectable pancreatic ductal adenocarcinoma. Oncologist 24: 749-e224, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bian AL, Hu HY, Rong YD, et al. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res 22: 25, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki H, Amitani A, Amitani H, et al. Cancer cachexia - pathophysiology and management. J Gastroenterol 48: 574-594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399-412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513: 100-104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol 29: ii18-ii26, 2018. [DOI] [PubMed] [Google Scholar]
- 34. Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 124: 606-616, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo H, Galvão DA, Newton RU, et al. Exercise medicine in the management of pancreatic cancer: a systematic review. Pancreas 50: 280-292, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan BHL, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15: 6973-6979, 2009. [DOI] [PubMed] [Google Scholar]
- 37. Okumura S, Kaido T, Hamaguchi Y, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery 159: 821-833, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One 10: e0139749, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang G, Meng S, Li R, et al. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget 8: 102474-102485, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boshier PR, Heneghan R, Markar SR, et al. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 31: doy047, 2018. [DOI] [PubMed] [Google Scholar]
- 41. Huguet F, Mukherjee S, Javle M. Locally advanced pancreatic cancer: the role of definitive chemoradiotherapy. Clin Oncol (R Coll Radiol) 26: 560-568, 2014. [DOI] [PubMed] [Google Scholar]
- 42. Kruse EJ. Palliation in pancreatic cancer. Surg Clin North Am 90: 355-364, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Yoshida Y, Fukutomi A, Tanaka M, et al. Gastrojejunostomy versus duodenal stent placement for gastric outlet obstruction in patients with unresectable pancreatic cancer. Pancreatology 17: 983-989, 2017. [DOI] [PubMed] [Google Scholar]
- 44. Troncone E, Fugazza A, Cappello A, et al. Malignant gastric outlet obstruction: which is the best therapeutic option? World J Gastroenterol 26: 1847-1860, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Azemoto N, Ueno M, Yanagimoto H, et al. Endoscopic duodenal stent placement versus gastrojejunostomy for unresectable pancreatic cancer patients with duodenal stenosis before introduction of initial chemotherapy (GASPACHO study): a multicenter retrospective study. Jpn J Clin Oncol 52: 134-142, 2022. [DOI] [PubMed] [Google Scholar]
- 46. Jamieson NB, Mohamed M, Oien KA, et al. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol 19: 3581-3590, 2012. [DOI] [PubMed] [Google Scholar]
- 47. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109: 416-421, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang D, Luo H, Qiu M, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 29: 3092-3100, 2012. [DOI] [PubMed] [Google Scholar]
- 49. Strijker M, van Veldhuisen E, van der Geest LG, et al. Readily available biomarkers predict poor survival in metastatic pancreatic cancer. Biomarkers 26: 325-334, 2021. [DOI] [PubMed] [Google Scholar]
- 50. Fujiwara Y, Haruki K, Shiba H, et al. C-reactive protein-based prognostic measures are superior at predicting survival compared with peripheral blood cell count-based ones in patients after curative resection for pancreatic cancer. Anticancer Res 38: 6491-6499, 2018. [DOI] [PubMed] [Google Scholar]
- 51. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249-256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15: 95-101, 2014. [DOI] [PubMed] [Google Scholar]
- 53. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 69: 576-583, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21: 300-307.E2, 2020. [DOI] [PubMed] [Google Scholar]