Abstract
Primary tracheal adenoid cystic carcinoma (TACC) is a rare malignancy without an established treatment. Central airway obstruction due to TACC often decreases the quality of life and has life-threatening consequences. A 19-year-old man with unresectable TACC and central airway obstruction suffered from progressive cough and dyspnea after exercise. Proton beam therapy (PBT) was selected as the preferred treatment over systemic anti-cancer chemotherapy for TACC. PBT led to complete remission of TACC and the almost complete disappearance of the respiratory symptoms without adverse events. PBT is a useful and safe treatment for unresectable primary TACC.
Keywords: primary adenoid cystic carcinoma, trachea, proton beam therapy, unresectable, young
Introduction
Primary tracheal adenoid cystic carcinoma (TACC) is a rare malignant tumor that accounts for approximately 0.04-0.2% of primary lung tumors (1). TACC is a low-grade malignancy that originates from the salivary glands in the mucosa, representing approximately 10-15% of all salivary neoplasms (2,3). Central airway obstruction due to TACC often decreases the quality of life and can be life-threatening, although the tumor may exhibit slow progression and local infiltration. These tumors are diagnosed late, as they present with non-specific symptoms during the early stage and are difficult to detect on chest X-ray. Thus, TACC is often mis-diagnosed as asthma or other respiratory disorders.
The treatment strategy for TACC is a multi-modal approach that includes surgery, radiotherapy, chemotherapy, or a combination of those therapies. Although surgery is considered the first-line and standard treatment for TACC (4,5), we often experience unresectable cases. However, standard chemotherapy regimens for TACC have not been established. Generally, X-ray radiotherapy is not recommended, as although irradiation can cure TACC, it can also damage radiation-sensitive normal tracheal epithelial cells as a severe adverse event (4,6). Among available radiation therapies, proton beam therapy (PBT) targets the tumor while sparing the surrounding normal tissues, a well-known feature of the Bragg peak.
We herein report successful PBT for unresectable TACC located in the carina and main bronchus in a young Japanese man.
Case Report
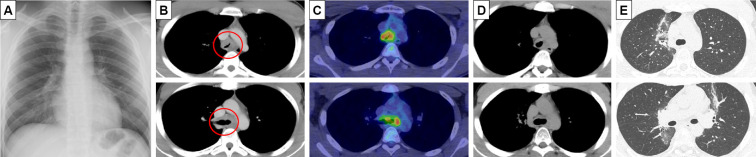
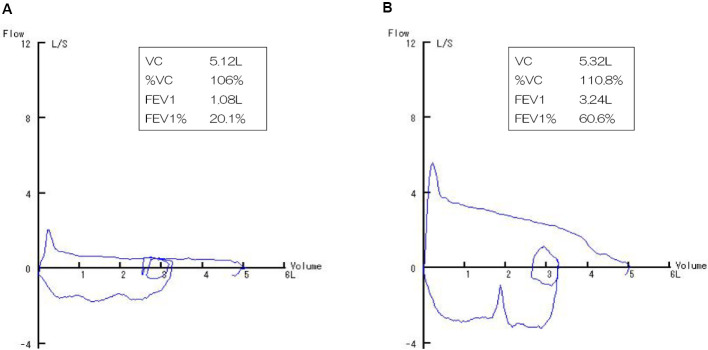
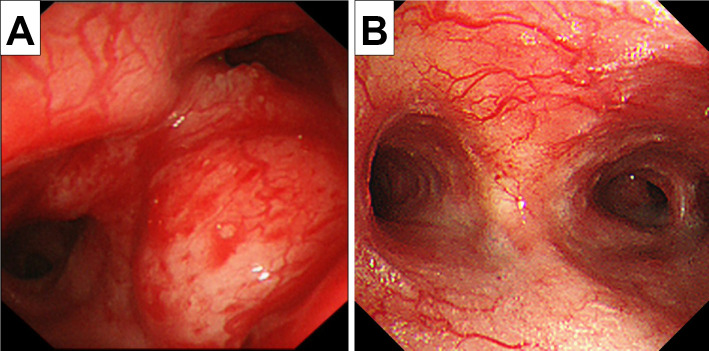
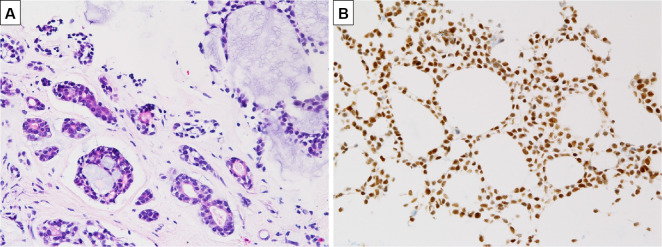
A 19-year-old, non-smoking, male college student suffering from progressive cough and dyspnea after exercise for the past few months underwent an emergency visit to our clinic. His lung sounds were biphasic inspiratory and expiratory rhonchi with prolonged respiratory phases, mainly from the anterior neck to upper-middle chest. Abnormal findings were not found on a chest radiogram (Fig. 1A). 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) showed thickened walls at the carina and bilateral main bronchi (Fig. 1B) with an FDG uptake [maximum standardized uptake value (SUVmax)=5.1] and no abnormal findings in any other organs (Fig. 1C). His flow-volume curves showed plateau peak flows (Fig. 2A). Flexible endobronchial ultrasound bronchoscopy showed a partially protruded submucosa with mucosal vascular ectasia from the carina to bilateral main bronchi (Fig. 3A). A fine-needle aspiration biopsy specimens from the protruded submucosal tissue showed a tubular and cribriform structure with mucus and a chordal arrangement with bilaminar atypical glandular ducts (Fig. 4A). Immunohistochemistry showed a strong expression of MYB in the tumor cell nuclei, with a MYB index >90% (Fig. 4B). Based on these findings, the definitive diagnosis was TACC as stage IIIA with T4N0M0. The tumor was diagnosed as curatively unresectable because of its spread from the carina to bilateral main bronchus.
Figure 1.
A: A chest radiogram demonstrated no abnormal findings. B: Chest computed tomography (CT) showed thickened walls at the carina and bilateral main bronchi. C: 18F-FDG positron emission tomography revealed an FDG uptake (SUVmax=5.1). D: The tumor had disappeared 12 months after PBT. E: Pneumonia (grade 2), as an adverse event, was observed two months after PBT.
Figure 2.
A: The flow-volume curves showed plateau peak flows. B: The flow-volume curves at 12 months after PBT are shown.
Figure 3.
A: Flexible endobronchial ultrasound bronchoscopy showed a partially protruded submucosa with mucosal vascular ectasia from the carina to bilateral main bronchi. B: The therapeutic outcome at 12 months after PBT was evaluated by bronchoscopy.
Figure 4.
A: Submucosal tissue showed a tubular and cribriform structure with mucus and a chordal arrangement with bilaminar atypical glandular ducts. B: Immunohistochemistry showed the strong expression of MYB.
Radiation therapy was considered as curative treatment, and PBT was selected (the target field is shown in Fig. 5). A complete response of the tumor and disappearance of his respiratory symptoms were maintained for 12 months after PBT at 70 Gy (RBE) in 35 fractions. The therapeutic outcome was evaluated by bronchoscopy (Fig. 3B), chest CT (Fig. 1D), and flow-volume curves (Fig. 2B). Although dermatitis (grade 2) during the acute phase (2 weeks after PBT) and pneumonia (grade 2) (Fig. 1E) during the late phase (2 months after PBT) were observed as adverse events of PBT, both toxicities were well resolved by short-term intervention with systemic corticosteroids.
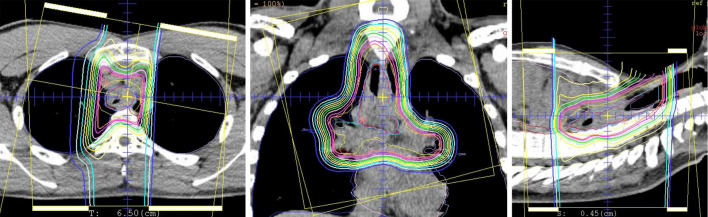
Figure 5.
The target field was treated with PBT at 70 Gy (RBE) in 35 fractions.
Discussion
TACC is a relatively rare low-grade tumor derived from the tracheal and bronchial wall glands. To our knowledge, there are no reports of cases of a teenage onset of TACC. Our case was judged to be unresectable because the tumor extended from the carina to bilateral main bronchi. The best treatment for adenoid cystic carcinoma (ACC) depends on the tumor location, extent, stage, and biological features and patient comorbidities. The combination of surgery and radiotherapy, rather than either modality alone, is recommended as the gold standard for TACC (7-9). Mallick et al. reported a significantly better survival after definitive surgery at three years than with radiotherapy at two years as local therapies for malignant tumors of the trachea. There was no significant difference in the survival after surgery versus radiotherapy for TACC (2). In inoperable cases, radiotherapy alone can achieve sufficient local control of the disease, but the results are not comparable to those of surgery. Bonner Millar et al. reported that ACC patients treated with PBT at 80 Gy (RBE) had no evidence of disease at 11 months of follow-up (10). Wang et al. reported a patient with inoperable TACC who experienced complete remission without symptomatic radiation-induced toxicity (grade ≥2) after hypofractionated radiotherapy at a total dose of 60 Gy in 12 fractions (11). The effectiveness of molecular-targeted drugs and immune checkpoint inhibitors has also been reported, but ACC patients lack gene mutations (12-15). The current systemic chemotherapies and targeted molecular therapies cannot cure advanced ACC.
Some case reports highlight the fact that particle therapies, such as proton beams, neutrons, and carbon ions, show interesting results in treating ACC. PBT is covered by medical insurance for head and neck tumors including ACC, pediatric tumors, prostate cancer, and bone and soft tissue tumors. PBT is a form of charged particle therapy. Protons have very rapid energy loss beyond the first few millimeters of penetration, which results in a highly localized peak radiation dose, known as the Bragg peak. The penetration depth of PBT is directly related to the initial energy of the charged particle, and this can be predetermined during treatment planning to avoid damage to critical structures deep in the target volume. Furthermore, the dose distribution can be tailored to the tumor size. This is of particular value in patients, as it limits irradiation of the spinal cord (16). In 39 patients with unresectable nasal cavity and paranasal sinus tumors (only 5 ACC cases) treated with PBT, Zenda et al. showed promising results with good local control and good progression-free survival and overall survival rates, with no grade 3 toxicities (17). Pommier et al. demonstrated excellent results of passive scatter PBT combined with five-field three-dimensional conformal photon therapy for ACC arising from the nasopharynx and paranasal sites. No patient experienced neck recurrence (18). Some authors have also demonstrated that PBT is effective for disease control (19-21). PBT exhibits a good local disease control rate and a better toxicity profile than neutron therapy (22). Ono et al. reported that high-dose hypofractionated PBT is a safe and feasible treatment for central lung cancer. All patients received PBT at 80 Gy (relative biological effectiveness) in 25 fractions over 5 weeks. However, there were no grade ≥3 toxicities, including no cases of bronchial stricture, obstruction, or fistula (23). PBT can increase the local dose while reducing the lung dose. PBT with intensity modulation should be used to facilitate dose escalation and minimize morbidities. However, there have been no randomized trials to determine the optimal dose and fractionation of radiotherapy for TACC.
PBT in our case was covered by insurance because the patient was under 20 years old and thus classified as a pediatric case. The radiation field was planned as a spread-out Bragg peak for all main bronchial tubes, and the spinal cord dose was reduced to less than 30%. We achieved excellent disease control with a good quality of life at the one-year follow-up examination following PBT. However, the patient had slight asthma-like symptoms, such as cough. Bronchoscopy, pulmonary function assessments, and imaging examinations were performed to determine the effects of treatment. Overall flow-volume curve was improved, but the central airway obstruction remained. The tumors had completely disappeared on bronchoscopy at one year after PBT. In the future, it will be necessary to perform follow-up observations and consider the placement of stents if necessary.
In conclusion, treatment of unresectable TACC is challenging. In our case of unresectable TACC, PBT was used as a curative treatment. This is the first report to document the use of PBT as a treatment strategy for TACC and to evaluate functional outcomes. In the future, it will be necessary to evaluate not only the therapeutic effect but also dysfunction caused by the treatment.
The publication of this case report was approved by the patient and the local ethics board of Kurume University Hospital. We obtained permissions concerning the publication of the case report from the patient (in Japanese).
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hu MM, Hu Y, He JB, Li BL. Primary adenoid cystic carcinoma of the lung: clinicopathological features, treatment and results. Oncol Lett 9: 1475-1481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mallick S, Benson R, Giridhar P, Rajan Singh A, Rath GK. Demography, patterns of care and survival outcomes in patients with malignant tumors of trachea: a systematic review and individual patient data analysis of 733 patients. Lung Cancer 132: 87-93, 2019. [DOI] [PubMed] [Google Scholar]
- 3. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 34: 32-37, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Honings J, Gaissert HA, van der Heijden HF, Verhagen AF, Kaanders JH, Marres HA. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 130: 763-772, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 78: 1889-1896, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Alongi F, Di Muzio N, Motta M, et al. Adenoid cystic carcinoma of trachea treated with adjuvant hypofractionated tomotherapy. Case report and literature review. Tumori 94: 121-125, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Matsuzaki H, Yanagi Y, Hara M, et al. Minor salivary gland tumors in the oral cavity: diagnostic value of dynamic contrast-enhanced MRI. Eur J Radiol 81: 2684-2691, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. ACC of the head and neck an update. Oral Oncol 51: 652-661, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Terhaard CH, Lubsen H, Van der Tweel I, Dutch Head and Neck Oncology Cooperative Group, et al. Salivary glands carcinoma: independent prognostic factors for locoregional control, distant metastases and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2: 681-692, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Bonner Millar LP, Stripp D, Cooper JD, Both S, James P, Rengan R. Definitive radiotherapy for unresected adenoid cystic carcinoma of the trachea. Chest 141: 1323-1326, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Wang D, Bi N, Chen D, Wang L. Complete remission after hypofractionated radiotherapy for a patient with inoperable adenoid cystic carcinoma of bronchus: a case report. Medicine (Baltimore) 97: e13463, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Cai S, Gao S, et al. Tracheobronchial adenoid cystic carcinoma: 50-year experience at the National Cancer Center, China. Ann Thorac Surg 108: 873-882, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Allen AM, Rabin MS, Reilly JJ, Mentzer SJ. Unresectable adenoid cystic carcinoma of the trachea treated with chemoradiation. J Clin Oncol 25: 5521-5523, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Huo Z, Wu H, Li S, Liang Z. Molecular genetic studies on EGFR, KRAS, BRAF, ALK, PIK3CA, PDGFRA, and DDR2 in primary pulmonary adenoid cystic carcinoma. Diagn Pathol 10: 161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tapias LF, Shih A, Mino-Kenudson M, et al. Programmed death ligand 1 and CD8+ immune cell infiltrates in resected primary tracheal malignant neoplasms. Eur J Cardiothorac Surg 55: 691-698, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Exley R, Bernstein JM, Brennan B, Rothera MP. Rhabdomyosarcoma of the trachea: first reported case treated with proton beam therapy. J Laryngol Otol 126: 966-969, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Zenda S, Kohno R, Kawashima M, et al. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 81: 1473-1478, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg 132: 1242-1249, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Linton OR, Moore MG, Brigance JS, Summerlin DJ, McDonald MW. Proton therapy for head and neck adenoid cystic carcinoma: initial clinical outcomes. Head Neck 37: 117-124, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Fukumitsu N, Okumura T, Mizumoto M, et al. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys 83: 704-711, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy - dose-volume histogram analysis. Radiology 213: 489-494, 1992. [DOI] [PubMed] [Google Scholar]
- 22. Castello A, Olivari L, Lopci E. Adenoid cystic carcinoma: focus on heavy ion therapy and molecular imaging. Am J Nucl Med Mol Imaging 8: 1-14, 2018. [PMC free article] [PubMed] [Google Scholar]
- 23. Ono T, Yabuuchi T, Nakamura T, et al. High dose hypofractionated proton beam therapy is a safe and feasible treatment for central lung cancer. Radiol Oncol 51: 324-330, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]