Abstract
Trypanosoma cruzi is the protozoan parasite that causes Chagas’ disease, a frequently fatal illness affecting the heart and gastrointestinal systems. An estimated 16 million to 18 million people in Latin America and 50,000 to 100,000 people in the United States are infected with this pathogen. Treatment options for T. cruzi infections are suboptimal due to the toxicities and limited effectiveness of the available drugs. Azole antimicrobial agents have been discovered to have antitrypanosomal activity by inhibition of ergosterol synthesis. The triazole itraconazole was recently shown to produce a parasitologic cure rate of 53% in chronically infected patients (W. Apt et al., Am. J. Trop. Med. Hyg. 59:133–138, 1998), a result which may lead to more use of this family of drugs for the treatment of T. cruzi infections. In the experiments reported on here, resistance to azoles was induced in vitro by serial passage of mammalian-stage parasites in the presence of fluconazole for 4 months. These parasites were cross resistant to the other azoles, ketoconazole, miconazole, and itraconazole. They remained susceptible to benznidazole and amphotericin B. The azole-resistant phenotype was stable for more than 2 months of in vitro serial passage without fluconazole. In addition, the parasites resisted treatment in mice receiving ketoconazole. The rapid development of azole resistance in T. cruzi in vitro suggests that resistance to azole drugs has the potential to occur in patients and may pose an impediment to the progress being made in the treatment of T. cruzi infection.
An estimated 16 million to 18 million people in Latin America and 50,000 to 100,000 people in the United States are infected with Trypanosoma cruzi (21, 47). This vector-borne protozoan is the etiologic agent of Chagas’ disease, which manifests as potentially fatal cardiomyopathy or dilations in the digestive tract. Treatment options for Chagas’ disease are limited due to the poor efficacies and toxicities of the available drugs (11). Azole antimicrobial agents have been discovered to have antitrypanosomal activity by inhibition of synthesis of ergosterol, which is integral to the parasite cell membrane (14, 41). Azoles, which were developed as antifungal drugs, are widely used clinically to treat mycotic infections. In fungi, azoles inhibit the cytochrome P-450 enzyme lanosterol 14α-demethylase, causing the accumulation of 14α-methylsterols and the decreased production of ergosterol (20, 46). Miconazole and econazole were the first of these inhibitors tested in T. cruzi and showed potent growth inhibition (13). Ketoconazole was shown to inhibit ergosterol synthesis in T. cruzi epimastigotes (44). Other studies showed that ketoconazole, itraconazole, and fluconazole were active in inhibiting intracellular multiplication of parasites and in protecting against lethal infection in mice (14, 17, 24–27, 41, 42). Studies with ketoconazole in patients with Chagas’ disease at doses used to treat deep mycoses failed to induce cure, as demonstrated by parasitologic and serologic tests (7). A clinical trial of itraconazole at 400 mg daily for 4 months led to parasitologic cures in 53% of patients with cardiomyopathic Chagas’ disease, although a comparison to benznidazole was not done (3). Newer azoles that are not yet clinically available have even greater potency against T. cruzi in vitro and in studies with animals (41). Specifically, D0870, a bistriazole derivative, prevented death and induced parasitologic cure in 70 to 90% of mice with both short- and long-term disease (43).
Microbial drug resistance is a tremendous clinical problem that affects all classes of microorganisms. Resistance to azole antimicrobial agents, specifically, has become an impediment to the effective treatment of infections due to Candida and Torulopsis species (20). This problem is particularly serious in the population with AIDS, in whom it is estimated that >33% of candida isolates are resistant to fluconazole (23). Poor clinical outcome and in vitro resistance have been correlated (35). In yeast several different mechanisms of azole resistance have been discovered (20), the most common being efflux pumps which extrude the drug from the intracellular space (36).
Drug resistance is a particularly important problem in pathogenic protozoa. For example, Leishmania spp., which are of the same phylogenetic order as T. cruzi (Kinetoplastida), develop resistance to first-line drugs (pentavalent antimonials) in 5 to 70% of treated individuals from areas of endemicity (39). Models used to study resistance in Leishmania show that gene amplification occurs in association with resistance (5, 18, 32, 33, 39). Trypanosoma brucei, the agent of African sleeping sickness, has become increasingly resistant to clinical drugs (4). Resistance to melarsoprol and to the diamidines is well recognized and is believed to be mediated by altered drug transport (4). Malaria caused by drug-resistant Plasmodium falciparum has become the leading health threat to populations in tropical and subtropical regions (37). Resistance to antifolate drugs is principally due to structural changes in the target enzymes (16). The mechanism(s) for chloroquine resistance in Plasmodium has yet to be delineated (6). Giardia lamblia and Trichomonas vaginalis are other protozoan species known to have clinically significant drug resistance (19, 40).
Drug resistance in T. cruzi has also been reported. A variety of T. cruzi strains were shown to be inherently resistant to the widely used anti-T. cruzi drugs benznidazole and nifurtimox (15). In addition, strains VL-10 and Columbiana were found to be inherently resistant to the azole D0870 (29). Irradiation treatment of T. cruzi resulted in resistance to the purine analogue tubercidin (31). Resistance was determined to be secondary to defects in the transport of tubercidin. Of note is the recent discovery that a multidrug resistance gene of the P-glycoprotein family is present in T. cruzi, although its function or association with drug resistance has not been characterized (12).
The present studies were undertaken to determine if T. cruzi would develop resistance to azole antibiotics in vitro and to study the characteristics of the resistant parasites.
MATERIALS AND METHODS
Test compounds.
Fluconazole (Diflucan) solution (2 mg/ml) in buffered saline for parenteral administration was purchased from Pfizer (New York, N.Y.). Itraconazole, ketoconazole, and miconazole (pure solids) were purchased from Research Diagnostics, Inc. (Flanders, N.J.). Amphotericin B (Amphocin) for injection was purchased from Adria (Columbus, Ohio). Benznidazole (Rochagan) tablets (100 mg) from Roche (Rio de Janeiro, Brazil) were pulverized and dissolved in water. Itraconazole, ketoconazole, and miconazole powders were brought into solution with dimethyl sulfoxide and 1 N HCl and were then diluted in water.
Parasites and culture procedures.
The Tulahuen strain of T. cruzi was provided by S. Reed (Infectious Diseases Research Institute, Seattle, Wash.). Trypomastigotes and amastigotes were grown on monolayers of mouse 3T3 fibroblasts in Dulbecco’s modified Eagle’s medium (BioWhittaker, Walkersville, Md.) supplemented with either 10% heat-inactivated fetal calf serum (HyClone Laboratories, Inc., Logan, Utah) or 10% heat-inactivated Cosmic Calf Serum (HyClone Laboratories, Inc.) plus glutamine, penicillin, and streptomycin as described previously (45). Epimastigotes were grown in liver infusion tryptone broth with 10% heat-inactivated fetal calf serum, penicillin, and streptomycin (LIT medium) as described previously (45). The parasites used in these experiments were clones that had been stably transfected with the Escherichia coli β-galactosidase gene lacZ (8, 9). These genetically altered parasites were used because the expression of β-galactosidase allowed easy quantification in subsequent experiments. This gene was integrated into the genome with linkage to the calmodulin-ubiquitin locus (1). The β-galactosidase-expressing parasites were unaltered in their in vitro growth characteristics and in vivo virulence (8). Trypomastigotes were converted to epimastigotes by inoculating approximately 106 tissue culture-derived trypomastigotes into LIT medium (see above) and incubating the flask at 28°C. Epimastigotes were converted to trypomastigotes by inoculation of a flask containing a nonconfluent monolayer of 3T3 fibroblasts in Dulbecco’s modified Eagle’s medium–10% fetal calf serum with approximately 106 epimastigotes and incubation at 37°C.
Derivation of fluconazole-resistant T. cruzi.
A 75-cm2 tissue culture-treated flask (Corning Costar Corp., Cambridge, Mass.) containing a nonconfluent monolayer of 3T3 fibroblast cells was inoculated with 3 × 107 trypomastigotes. Fluconazole was added to the culture medium at 2 μg/ml, and the medium was replaced every 7 days. After 20 days it was evident that a small number of parasites had burst from the infected cells, and these were transferred to a fresh 3T3 monolayer containing 5 μg of fluconazole per ml. After 15 days, the emerging parasites were passed to a flask containing fluconazole at 10 μg/ml. After 7 days, epimastigotes began to dominate the cultures. Because we have observed that heat-inactivated Cosmic Calf Serum lyses epimastigotes at 37°C (unpublished observations), we altered the medium to contain Cosmic Calf Serum instead of fetal calf serum. The growth of epimastigotes was suppressed, and the trypomastigotes that emerged from the infected cells were serially passaged an additional six times in the presence of 10 μg of fluconazole per ml until there arose a population with growth kinetics similar to that of the parent clone. The fluconazole-resistant population was then cloned by limiting dilution.
Determinations of drug susceptibilities.
Mammalian-stage parasites were tested for their susceptibilities to drugs by previously described methods (8). Briefly, trypomastigotes were inoculated onto monolayers of 3T3 fibroblasts in 96-well plates with dilutions of drug in quadruplicate. After 7 days, the β-galactosidase-containing parasites were quantified by the addition of chlorophenol red β-d-galactopyranoside (CPRG) (final concentration, 100 μM; Boehringer-Mannheim, Indianapolis, Ind.) and 0.1% Nonidet P-40 (Sigma Chemical Co., St. Louis, Mo.). Following a 4-h incubation at 37°C, the colorimetric reaction (yellow to red) was quantified with an enzyme-linked immunosorbent assay reader at an optical density at 570 nm.
Mouse experiments.
Six-week-old female BALB/c mice (B&K Universal, Kent, Wash.) were infected by intraperitoneal injection of T. cruzi trypomastigotes. Ketoconazole was suspended in water containing 0.2% Bacto Agar (Difco Laboratories, Detroit, Mich.) to provide viscosity. Ketoconazole was administered by oral gavage at 30 mg/kg of body weight in 200-μl volumes. Parasitemia was quantified by examining 2 drops of tail blood under a coverslip at a magnification of ×400.
Statistical methods.
Comparisons in the mouse experiment were performed by the unpaired t test with Prism software (version 2.0) from GraphPad Software Inc., San Diego, Calif.
RESULTS
Derivation of a fluconazole-resistant line of T. cruzi in vitro.
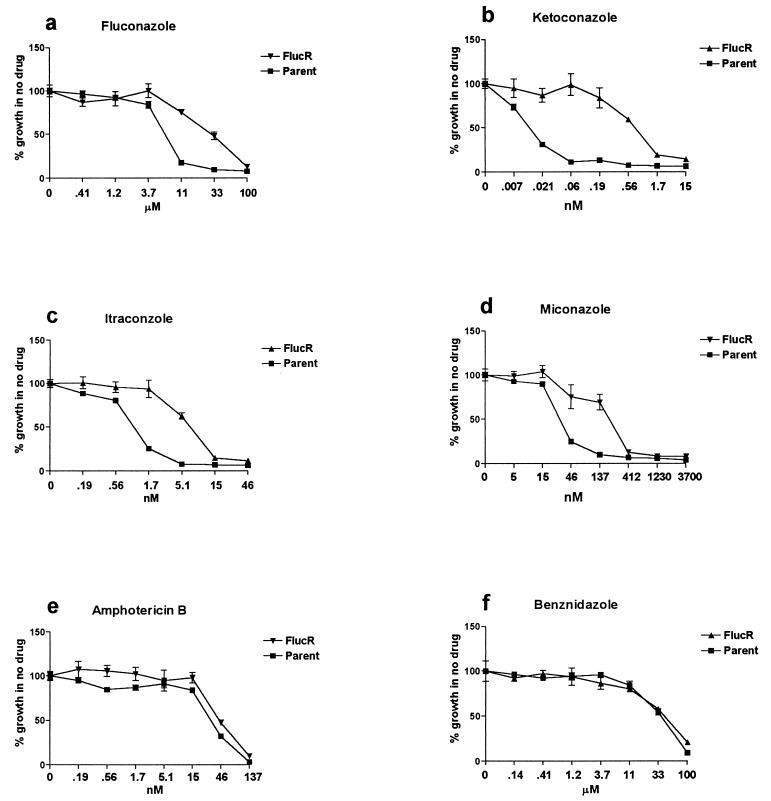
T. cruzi Tulahuen (transfected with the β-galactosidase gene) was grown in the presence of fluconazole at escalating drug concentrations for 4 months. The concentration of fluconazole causing 90% growth inhibition of the parent line was 10 μM (3 μg/ml). This increased to 100 μM (30 μg/ml) in the drug-selected line, referred to as Flucr (Fig. 1a). The concentrations of fluconazole used were nontoxic to the host 3T3 fibroblast cells. The Flucr line was cloned by limiting dilution, and a single clone was used for subsequent experiments.
FIG. 1.
Drug susceptibilities of resistant and parent lines of T. cruzi. Mammalian-stage parasites containing the E. coli β-galactosidase gene were allowed to infect mouse 3T3 fibroblasts in microtiter plates in the presence of the indicated drug dilutions. At day 6, the growth of parasites was quantitated by the addition of the substrate CPRG and reading of the results of the colorimetric reaction at an optical density at 570 nm on an enzyme-linked immunosorbent assay reader. The Flucr line of T. cruzi, derived by long-term in vitro passage in the presence of fluconazole, was resistant to four different azole drugs in comparison to the parent line from which it was derived (a to d). There was no cross-resistance to amphotericin B (e) or benznidazole (f).
Demonstration of cross-resistance to other azole antifungal agents but not to unrelated drugs.
The Flucr T. cruzi clone was compared to the parent line for susceptibility to other azole drugs, specifically, miconazole, ketoconazole, and itraconazole. Each of these drugs was found to be more potent than fluconazole in vitro against T. cruzi, but the resistance of the Flucr parasites to these other azole agents was maintained (Fig. 1b, c, and d). In fact, the 50% inhibitory concentration of ketoconazole was approximately 100-fold greater for the Flucr parasites than for the parent line, indicating even greater relative resistance of the Flucr parasites to this drug than to fluconazole. Susceptibility to compounds unrelated to azoles in structure and mode of antitrypanosomal action was also tested; specifically, amphotericin B and benznidazole were examined (Fig. 1e and f, respectively). There was no difference in the growth inhibition caused by these drugs when the Flucr parasites and the parent line were compared, indicating that cross-resistance to azoles and to these other drugs did not occur. The concentrations of drugs used were not toxic to the host 3T3 fibroblast cells.
Stability of the fluconazole-resistant phenotype out of drug pressure.
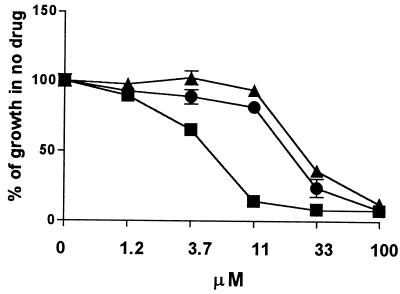
The fluconazole susceptibilities of the Flucr T. cruzi clone carried in tissue culture in the absence of fluconazole for 12 weeks were found to be unchanged in vitro compared to those of Flucr maintained in the presence of fluconazole (data not shown). Similarly, when the Flucr parasites were inoculated into untreated mice and recovered in tissue culture after 2 weeks, the parasites maintained their fluconazole-resistant phenotype (Fig. 2). In addition, Flucr parasites were transformed to the insect-stage epimastigotes by changing the culture conditions (in the absence of fluconazole) and were then converted back to mammalian-stage parasites, and these, too, continued to be fluconazole resistant (data not shown).
FIG. 2.
Stability of the fluconazole-resistant phenotype. A clone of the Flucr parasite line was inoculated into an untreated mouse and was then recovered from blood after 2 weeks. After expansion in vitro (in the absence of fluconazole), the clone (▴) was tested for its susceptibility to fluconazole. It was compared to Flucr parasites which were passaged continuously in vitro in the presence of fluconazole (•) and to the parent line of T. cruzi (■). The experiment has been repeated with three separate mice with similar results.
Azole drug resistance in vivo.
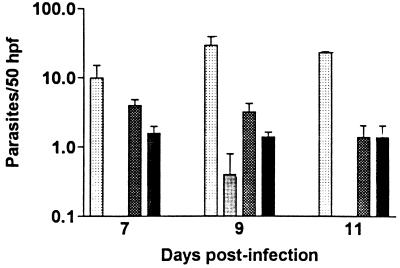
The in vitro-derived azole-resistant T. cruzi (Flucr) clone was tested in mice for its resistance to the azole ketoconazole. This azole was used in the experiment because of the greater potency of ketoconazole observed in vitro against this strain of T. cruzi and because the relative resistance of the Flucr parasites compared to that of the parent line was greatest for ketoconazole. In pilot experiments, it was observed that the Flucr clone was moderately attenuated in its ability to produce parasitemia and death compared to that of the parent line of Tulahuen strain parasites. Thus, the experiment was designed so that two control groups of mice received the parent line of parasites: one in which mice were given the same inoculum (2 × 106) of parasites as the mice infected with the Flucr parasites and one in which mice were given a lower inoculum (1 × 104) of the parent line so that the parasitemia more closely paralleled the parasitemia observed in the mice infected with the Flucr parasites. Mice were divided into groups that received ketoconazole or placebo; hence, there were a total six groups (five mice per group). Mice infected with the parent line (2 × 106) developed a very high level of parasitemia (103 ± 19 trypomastigotes/50 high-power fields on day 9) and died by day 10 postinoculation (data not shown). The comparable mice receiving ketoconazole (30 mg/kg/day by oral gavage) developed low-level parasitemia (peak, 2.4 ± 1.0 trypomastigotes/50 high-power fields on day 11) and survived to 28 days, when the experiment was terminated (data not shown). The data for mice infected with the parent line of T. cruzi (1 × 104) and for the mice infected with the Flucr parasites (2 × 106) are presented in Fig. 3. Mice infected with the parent line and treated with placebo developed high-level parasitemia (peak, 30 ± 10 parasites/50 high-power fields on day 9) and were all dead before day 28, whereas all except one of the mice infected with the parent line and treated with ketoconazole had no detectable parasites on day 9, and all mice in the latter group survived to the end of the experiment (day 28) (differences in the levels of parasitemia between the two groups were significant [P < 0.05] on days 7, 9, and 11). Mice infected with the Flucr T. cruzi (2 × 106) and treated with placebo developed a relatively low level of parasitemia; however, the same level of parasitemia was observed in mice given these parasites and treated with ketoconazole (Fig. 3) (P > 0.10 for days 7, 9, 11). This demonstrates that the Flucr parasites were resistant to ketoconazole treatment in vivo. The parasitemia fell below detectable levels after day 11.
FIG. 3.
Infection of mice with azole-resistant T.
cruzi. Mice were infected with either the parent clone (1 ×
104 trypomastigotes) or the in vitro-derived
fluconazole-resistant clone of T. cruzi (2 ×
106 trypomastigotes). Mice were treated with either placebo
or ketoconazole at 30 mg/kg by oral gavage for the first 7 days of
infection (four groups; n = 5 per group). The levels of
parasitemia were significantly different between the placebo-treated
( ) and ketoconazole-treated
(
) and ketoconazole-treated
( ) mice infected with the parent
line on all 3 days (P < 0.05), whereas the levels of
parasitemia were not significantly different between the
placebo-treated (▩) and ketoconazole-treated (■) mice infected with
the azole-resistant T. cruzi strain on all 3 days
(P > 0.10). hpf, high-power field.
) mice infected with the parent
line on all 3 days (P < 0.05), whereas the levels of
parasitemia were not significantly different between the
placebo-treated (▩) and ketoconazole-treated (■) mice infected with
the azole-resistant T. cruzi strain on all 3 days
(P > 0.10). hpf, high-power field.
DISCUSSION
Azole antifungal drugs have excellent in vitro and in vivo activities against T. cruzi. Anecdotal reports of their efficacies in the treatment of patients with Chagas’ disease and their superior side effect profiles compared to those of the available antitrypanosomal therapeutic agents have led to increased interest in the use of azoles for the treatment of Chagas’ disease in Latin America (14, 30, 38). A recent study showed 53% parasitologic cure rates in patients with chronic Chagas’ disease treated with itraconazole (3), which may lead to more use of this family of drugs to treat T. cruzi infections. As has been seen with fungi, the development of resistance to azole drugs in the clinical setting is likely to occur as selection pressure becomes more widespread. The fact that these drugs require long treatment courses (months) and in some cases are marginally effective against T. cruzi creates a situation in which resistance is prone to develop.
The experiments reported here demonstrated that the resistance of T. cruzi to one azole drug, fluconazole, developed readily in vitro. The mammalian stages, as opposed to the insect stage, of T. cruzi were studied to more closely approximate the in vivo situation in treated humans. A relatively small starting inoculum of parasites (3 × 107) was used to generate a resistant line by serial passage of the parasites in the presence of increasing doses of fluconazole. The doses of fluconazole used (beginning at 2 μg/ml and increasing to 10 μg/ml) were in the range of the peak concentrations that are achieved in human serum following the administration of standard doses (i.e., 4 to 9 μg/ml) (28). Resistance to fluconazole by Candida albicans has been generated in vitro by serial passage in the presence of drug (2, 10). A comparable number of culture passages (four to seven) in the presence of drug was necessary to derive a resistant Candida line (10). Since resistance of Candida to azole drugs frequently develops in patients receiving long-term azole therapy, it is very possible that the resistance of T. cruzi to azoles will also develop in the clinical setting.
The fluconazole-resistant T. cruzi strains were determined to be cross-resistant to three other azole antifungal drugs, specifically, ketoconazole, miconazole, and itraconazole. This suggests a resistance mechanism that is not uniquely selective for the molecular structure of fluconazole. The finding that the azole-resistant parasites were not resistant to benznidazole indicates selectivity in the drug resistance mechanism. Benznidazole, which is chemically distinct from the azole antifungal agents and which is not believed to act on sterol biosynthesis, has been shown to arrest RNA and protein synthesis in T. cruzi (34). In addition, the azole-resistant parasites remained susceptible to amphotericin B. Since amphotericin B works by direct interaction with ergosterol in the cell wall (at least in fungi), the data suggest that the Flucr T. cruzi strains contain ergosterol in their membranes.
The azole-resistant phenotype of T. cruzi was observed to be stable in the absence of drug pressure. The parasite clone propagated in medium without azole drug or passed through untreated mice retained the same level of azole resistance as the clone carried continuously in the presence of fluconazole. From a mechanistic standpoint, this argues against the possibility that amplification of an extrachromosomal element confers resistance because such elements are typically lost in the absence of drug pressure (22).
The resistance to ketoconazole observed in vivo demonstrated that the factors responsible for resistance were expressed by the parasite in the mammalian host. In addition, the experiment with mice demonstrated a level of drug resistance by the parasites sufficient to be clinically relevant. The dose of ketoconazole used to treat the mice (30 mg/kg) was considerably higher than that typically used to treat humans (e.g., the recommended pediatric dosage is 3.3 to 6.6 mg/kg/day). Thus, even considering differences in the pharmacokinetics of ketoconazole between the two species, the data suggest that it would not be easy to achieve in humans drug levels that would overcome the resistance. Because ketoconazole was the most potent of the tested azoles against T. cruzi Tulahuen (Fig. 2), the use of other currently available azole drugs (i.e., fluconazole, itraconazole, or miconazole) would not likely overcome the resistance of the parasites in vivo. It remains to be tested whether the newer azoles undergoing clinical development, such as D0870 (29), have sufficiently greater activity to adequately treat infections caused by resistant T. cruzi strains.
Azole-resistant T. cruzi was moderately attenuated in its ability to produce parasitemia in mice. Whether the factors responsible for resistance are also responsible for the altered virulence is not known. Studies are under way to determine whether the resistance of T. cruzi develops in infected mice undergoing azole treatment to determine (i) whether this phenomenon can, in fact, occur in vivo and (ii) whether the resulting resistant parasites develop the same cross-resistance phenotype (and the same mechanism of resistance) as the in vitro-derived azole-resistant parasites.
In summary, it was shown that mammalian-stage parasites rapidly developed resistance to fluconazole in vitro, and cross-resistance to other azole drugs but not to benznidazole or amphotericin B was observed. The resistant phenotype was stable out of the presence of drug pressure, and the parasites resisted treatment with ketoconazole when they were tested in vivo in mice. These findings suggest that T. cruzi resistance to azoles may occur clinically in humans, particularly because therapies involve long courses to effect a cure. Resistance to azole drugs has the potential to be an impediment to the progress being made in the treatment of T. cruzi infections.
ACKNOWLEDGMENTS
We thank Lynn Barret for technical assistance and Peggie Sue O’Brien for secretarial assistance.
This work was supported, in part, by National Institutes of Health grant AI01258 to F.S.B. and American Heart Association grant-in-aid 95006700 to W.C.V.
REFERENCES
- 1.Ajioka J, Swindle J. The calmodulin-ubiquitin (CUB) genes of Trypanosoma cruziare essential for parasite viability. Mol Biochem Parasitol. 1996;78:217–225. doi: 10.1016/s0166-6851(96)02627-8. [DOI] [PubMed] [Google Scholar]
- 2.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicansfluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sanchez G, Zulantay I, Cortes P, Rodriguez J, Juri D. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59:133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi C J. Resistance to clinical drugs in African trypanosomes. Parasitol Today. 1993;9:190–193. doi: 10.1016/0169-4758(93)90145-6. [DOI] [PubMed] [Google Scholar]
- 5.Beverley S M. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- 6.Borst P, Ouellette M. New mechanisms of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 7.Brener Z. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem Inst Oswaldo Cruz. 1993;88:149–153. doi: 10.1590/s0074-02761993000100023. [DOI] [PubMed] [Google Scholar]
- 8.Buckner F S, Verlinde C L M J, La Flamme A C, Van Voorhis W C. Efficient technique for screening drugs for activity against Trypanosoma cruziusing parasites expressing β-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner F S, Wipke B T, Van Voorhis W C. Trypanosoma cruziinfection does not impair major histocompatibility complex class I presentation of antigen to cytotoxic T lymphocytes. Eur J Immunol. 1997;27:2541–2548. doi: 10.1002/eji.1830271012. [DOI] [PubMed] [Google Scholar]
- 10.Calvet H M, Yeaman M R, Filler S G. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob Agents Chemother. 1997;41:535–539. doi: 10.1128/aac.41.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft S L. The current status of antiparasitic chemotherapy. Parasitology. 1997;114:S3–S15. [PubMed] [Google Scholar]
- 12.Dallagiovanna B, Gamarro F, Castanys S. Molecular characterization of a P-glycoprotein-related tcpgp2 gene in Trypanosoma cruzi. Mol Biochem Parasitol. 1996;75:145–147. doi: 10.1016/0166-6851(95)02519-7. [DOI] [PubMed] [Google Scholar]
- 13.Docampo R. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol Biochem Parasitol. 1981;3:169–180. doi: 10.1016/0166-6851(81)90047-5. [DOI] [PubMed] [Google Scholar]
- 14.Docampo R, Schmunis G A. Sterol biosynthesis inhibitors: potential chemotherapeutics against Chagas disease. Parasitol Today. 1997;13:129–130. doi: 10.1016/s0169-4758(97)01021-1. [DOI] [PubMed] [Google Scholar]
- 15.Filardi L S, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzistrains to drugs used clinically in Chagas’ disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 16.Foote S J, Cowman A F. The mode of action and the mechanisms of resistance to antimalarial drugs. Acta Trop. 1994;56:157–171. doi: 10.1016/0001-706x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 17.Goad L J, Berens R L, Marr J J, Beach D H, Holz G G. The activity of ketoconazole and other azoles against Trypanosoma cruzi: biochemistry and chemotherapeutic action in vitro. Mol Biochem Parasitol. 1989;32:179–190. doi: 10.1016/0166-6851(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 18.Guieros-Filho F, Viola A P B, Gomes F C A, Farina M, Lins U, Bertho A L, Wirth D F, Lopes U G. Leishmania amazonensis: multidrug resistance in vinblastine-resistant promastigotes is associated with rhodamine 123 efflux, DNA amplification, and RNA overexpression of a Leishmania mdr 1gene. Exp Parasitol. 1995;81:480–490. doi: 10.1006/expr.1995.1141. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P J. Metronidazole and drug resistance. Parasitol Today. 1993;9:183–186. doi: 10.1016/0169-4758(93)90143-4. [DOI] [PubMed] [Google Scholar]
- 20.Joseph-Horne T, Hollomon D W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149:173–180. doi: 10.1111/j.1574-6968.1997.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff L V. American trypanosomiasis (Chagas’ disease)—a tropical disease now in the United States. N Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 22.La Flamme A C, Buckner F S, Swindle J, Ajioka J, Van Voorhis W C. Trypanosoma cruzi: expression of interleukin-2 utilizing both supercoiled plasmids and linear DNA. Exp Parasitol. 1996;83:159–163. doi: 10.1006/expr.1996.0061. [DOI] [PubMed] [Google Scholar]
- 23.Law D, Moore C B, Wardle H M, Ganguli L A, Keaney M G, Denning D W. High prevalence of antifungal resistance in Candidaspp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–668. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 24.Lazardi K, Urbina J A, de Souza W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1990;34:2097–2105. doi: 10.1128/aac.34.11.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado R A, Molina J, Payares G, Urbina J A. Experimental chemotherapy with combinations of ergosterol biosynthesis inhibitors in murine models of Chagas’ disease. Antimicrob Agents Chemother. 1993;37:1353–1359. doi: 10.1128/aac.37.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCabe R E, Remington J S, Araujo F G. Ketoconazole promotes parasitological cure of mice infected with Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1987;81:613–615. doi: 10.1016/0035-9203(87)90430-5. [DOI] [PubMed] [Google Scholar]
- 27.McCabe R E, Remmington J S, Araujo F G. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg. 1986;35:280–284. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- 28.Medical Economics Data Production Co. Physicians Desk Reference. Montvale, N.J: Medical Economics Data Production Co; 1997. [Google Scholar]
- 29.Molina J T, Araujo M S S, Pereira M E S, Brener Z, Urbina J A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Activity of the bis-triazole D0870 against drug-resistant Trypanosoma cruzi strains, abstr. B-416; p. 34. [Google Scholar]
- 30.Moreira A A, de Souza H B, Amato-Neto V, Matsubara L, Pinto P L, Tolezano J E, Nunes E V, Okumura M. Evaluation of the therapeutic activity of itraconazole in chronic infections, experimental and human, by Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1992;34:177–180. [PubMed] [Google Scholar]
- 31.Nozaki T, Dvorak J A. Molecular biology studies of tubercidin resistance in Trypanosoma cruzi. Parasitol Res. 1993;79:451–455. doi: 10.1007/BF00931581. [DOI] [PubMed] [Google Scholar]
- 32.Ouellette M, Fase-Fowler F, Borst P. The amplified H circle of methotrexate-resistant Leishmania tarentolaecontains a novel P-glycoprotein gene. EMBO J. 1990;9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouellette M, Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993;9:150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 34.Polak A, Richle R. Mode of action of the 2-nitroimidazole derivative benznidazole. Ann Trop Med Parasitol. 1978;72:45–54. doi: 10.1080/00034983.1978.11719278. [DOI] [PubMed] [Google Scholar]
- 35.Ruhnke M, Eigler A, Engelmann E, Geiseler B, Trautmann M. Correlation between antifungal susceptibility testing of Candidaisolates from patients with HIV infection and clinical results after treatment with fluconazole. Infection. 1994;22:132–136. doi: 10.1007/BF01739024. [DOI] [PubMed] [Google Scholar]
- 36.Sanglard D, Kuchler K, Pagani J L, Nonod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicansisolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schapira A, Beales P F, Halloran M E. Malaria: living with drug resistance. Parasitol Today. 1993;9:168–174. doi: 10.1016/0169-4758(93)90140-b. [DOI] [PubMed] [Google Scholar]
- 38.Solari A, Saavedra H, Sep’ulveda C, Odd’o D, Acuna G, Labarca J, Munoz S, Cuny G, Grengues C, Veas F. Successful treatment of Trypanosoma cruziencephalitis in a patient with hemophilia and AIDS. Clin Infect Dis. 1993;16:255–259. doi: 10.1093/clind/16.2.255. [DOI] [PubMed] [Google Scholar]
- 39.Ullman B. Multidrug resistance and P-glycoproteins in parasitic protozoa. J Bioenerg Biomembr. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- 40.Upcroft J A, Upcroft P. Drug resistance and Giardia. Parasitol Today. 1993;9:187–190. doi: 10.1016/0169-4758(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 41.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114:S91–S99. [PubMed] [Google Scholar]
- 42.Urbina J A, Lazardi K, Aguirre T, Piras M M, Piras R. Antiproliferative synergism of the allylamine SF 86-327 and ketoconazole on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother. 1988;32:1237–1242. doi: 10.1128/aac.32.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbina J A, Payares B, Molina J, Sanoja C, Liendo A, Lazardi K, Piras M M, Piras R, Wincker P, Ryley J F. Cure of short- and long-term experimental Chagas’ disease using D0870. Science. 1996;273:969–971. doi: 10.1126/science.273.5277.969. [DOI] [PubMed] [Google Scholar]
- 44.Urbina J A, Vivas J, Ramos H, Larralde G, Aguilar Z, Avilan L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruziepimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988;30:185–196. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Voorhis W C, Eisen H. FL-160: a surface antigen of Trypanosoma cruzithat mimics mammalian nervous tissue. J Exp Med. 1989;169:641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. WHO Technical Report Series no. 811. Geneva, Switzerland: World Health Organization; 1991. Control of Chagas’ disease; pp. 1–93. [PubMed] [Google Scholar]