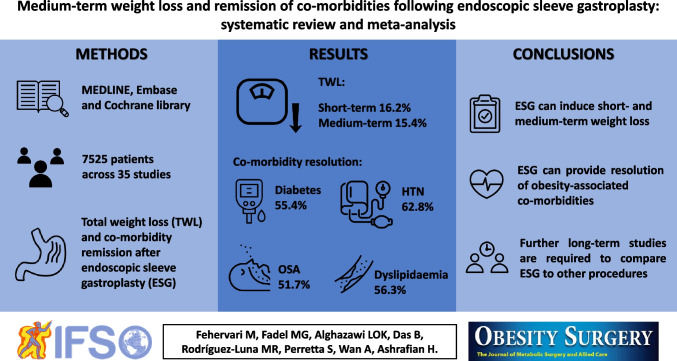
Abstract
This systematic review and meta-analysis aimed to determine the short- and medium-term weight loss outcomes and comorbidity resolution following endoscopic sleeve gastroplasty. Our search identified 35 relevant studies containing data from 7525 patients. Overall, pooled short-term (12 months) total weight loss (TWL) was 16.2% (95% CI 13.1–19.4%) in 23 studies (n = 5659). Pooled medium-term TWL was 15.4% (95% CI 13.7–17.2%) in 10 studies (n = 4040). Diabetes resolution was 55.4% (95% CI 46–64%), hypertension resolution was 62.8% (95% CI 43–82%), dyslipidaemia resolution was 56.3% (95% CI 49–63%), and obstructive sleep apnoea resolution was 51.7% (95% CI 16.2–87.3%) in four studies (n = 480). This pooled analysis demonstrates that ESG can induce durable weight loss and resolution of obesity-associated comorbidities in patients with moderate obesity.
Graphical Abstract
Keywords: Obesity, Endoscopic sleeve gastroplasty, Weight loss, Comorbidities
Introduction
The management of obesity has significantly evolved over the last decade. The first effective surgical treatment of morbid obesity and associated conditions was Roux-en-Y gastric bypass. This was followed by laparoscopic sleeve gastrectomy (LSG), which rapidly became the most performed bariatric operation globally [1–3]. These surgical procedures are associated with significant short- and long-term weight loss as well as remission of obesity-related comorbidities [4, 5].
More recently, less invasive procedures such as endoscopic sleeve gastroplasty (ESG) have been introduced [6]. This procedure aims to create a sleeve-like stomach by intraluminal suturing but keeps the fundus and antrum intact. The reduction of the gastric volume leads to delayed gastric emptying and early satiety. The procedure generates similar gut hormonal changes to conventional bariatric surgery [7, 8]. This fully endoscopic procedure has several appealing advantages compared to laparoscopic operations, including a truly scarless technique, shorter hospital stay and improved perioperative outcomes [9–11]. Endoscopic bariatric procedures could be an alternative solution to bariatric surgery in elderly or surgical unfit patients [12]. Given the increasing utilisation of ESG, it is important to understand the impact on clinically relevant outcomes. Thus, we performed a systematic review and meta-analysis of reported weight loss outcomes and rates of remission of obesity-related comorbidities following ESG over a 5-year period.
Methods
Search Strategy
A systematic literature search was performed using the following electronic databases: Medline, Embase and the Cochrane library between January 1995 and December 2022. The full search strategy has been provided as supplementary material (Supp. 1). The following MeSH terms along with their synonyms were used in all possible combinations: ‘endoscopic sleeve gastroplasty’, ‘endoscopic gastric sleeve’, ‘non-surgical sleeve’, ‘non-surgical gastric sleeve’, ‘morbidity’, ‘quality of life’, ‘diabetes mellitus’, ‘hypertension’, 'dyslipidaemia' and ‘obstructive sleep apnoea’. Studies identified from the search strategy were entered into Covidence (Victoria, Australia) for bibliographic management and duplicates removal. Two authors (MF and MGF) independently identified relevant studies, and any discrepancies were resolved by consensus with the help of a third author (HA).
This systematic literature search and meta-analysis was conducted based on a prospectively developed protocol and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. The review was registered on PROSPERO Centre for Reviews and Dissemination in December 2022 (registration number: CRD42022387320).
Inclusion and Exclusion Criteria
The following criteria were applied for inclusion in the study:
-
i)
Randomised controlled trials (RCT), prospective or retrospective cohort studies, case (control) studies, cross-sectional studies
-
ii)
Patients who had undergone ESG for obesity
-
iii)
Reported outcomes of interest: weight loss and resolution of comorbidities
-
iv)
Original full-text articles in the English language
Animal studies, reviews, abstracts, conference presentations, case reports, editorials and unpublished studies were excluded from the analysis.
Data Extraction and Quality Assessment
A standardised data extraction form was developed on Covidence, and three authors (MF, MGF and LA) independently extracted all relevant data: study design, sample size, patient gender and age, comorbidities, mean body mass index (BMI), mean percentage of excess weight loss (%EWL) and mean percentage of total weight loss (%TWL) at 12 months (short-term) and 2–5 years (medium-term) and resolution of obesity-related comorbidities.
The longest available follow-up data was collected from each study unless there was a significant loss to follow-up reported. The majority of the studies reported outcomes according to Brethauer et al.; however, not all studies described how weight loss, comorbidities and remission outcomes were reported [14]. As the majority of studies were non-randomised, the risk of bias was assessed using the Newcastle-Ottawa Scale [15]. Any discrepancy was resolved by group discussion. The definition of medium-term was based on Mahawar 2018, and all articles were included in this category if they had at least 18 months of follow-up data [16]. The strength of clinical data and subsequent recommendations were graded according to Ho et al. and Bellomo et al. [17, 18].
Statistical Analysis
Data were analysed using Stata Software (Version 15.1. StataCorp LCC, TX). Pooled weighted mean differences, and standardised mean differences were analysed by random effects meta-analysis. All studies with relevant data were included in the analysis. Statistical heterogeneity was calculated using the I2 statistic. This was graded as low (I2 < 30%), moderate (I2 = 30–60%) or high (I2 > 60%) based on the Cochrane Handbook for Systematic Reviews of Intervention.
Results
Study Selection
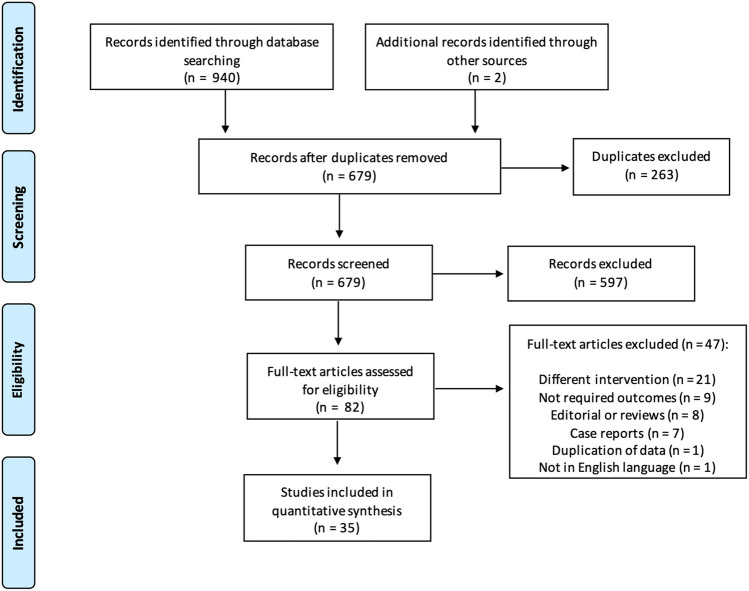
The search identified 940 relevant citations. After removing duplicate results, 679 articles were screened for titles and abstracts, and 82 studies were included in the full-text review. A total of 46 articles were excluded; thus, 32 non-randomised and 3 randomised studies were eligible for inclusion in the meta-analysis [6, 8, 12, 19–50]. The process of study selection is reported in Fig. 1.
Fig. 1.
Flow diagram: process for selection of studies. Adapted from PRISMA 2009 flow diagram [13]
Characteristics of Studies
The included studies contained data from 7525 patients (mean age 42.2 years, 6461 (85.9%) female patients, mean BMI = 37.7 kg/m2). Twenty-eight of the studies were considered high quality, and four studies had high risk of bias according to the Newcastle-Ottawa scale (Supp 2.). The mean follow-up time following ESG was 17.4 months (range 6–60 months). The exact characteristics of the included studies are presented in Table 1.
Table 1.
Summary of published series of endoscopic sleeve gastroplasty. BMI body mass index, ESG endoscopic sleeve gastroplasty, GORD gastro-oesophageal reflux disease, HTN hypertension, LSG laparoscopic sleeve gastrectomy, OSA obstructive sleep apnoea, SD standard deviation
| Study | Year | Sample size (n) | % Male (n) | Mean age ± (SD), years | Mean Weight ± (SD), kg | Mean BMI ± (SD), kg/m2 | Mean HbA1c ± (SD), % | Incidence of diabetes (n) | Incidence of HTN (n) | Incidence of OSA (n) | Incidence of other comorbidities (n) | Mean follow-up period, months | Study design and grade of study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lopez-Nava et al. [36] | 2017 | 248 | 27% (67) | 44.5 ± 10 | 37.8 ± 5.6 | 24 |
Retrospective cohort Level III |
||||||
| Abu Dayyeh et al. [8] | 2017 | 25 | 16% (4) | 47.6 ± 10 | 35.5 ± 2.6 | 20 |
Prospective cohort Level III |
||||||
| Sharaiha et al. [38] | 2017 | 91 | 32% (29) | 43.86 ± 11.26 | 38.6 ± 7 | 6.1 ± 1.1 | 18 | 31 | Abnormal LFTs 65 | 24 |
Prospective cohort Level III |
||
| Saumoy et al. [39] | 2018 | 128 | 33% (42) | 43.62 ± 11.37 | 38.92 ± 6.95 | 12 |
Prospective cohort Level III |
||||||
| Morales et al. [29] | 2018 | 148 | 18% (27) | 41.53 ± 10 | 98.7 ± 17 | 35.11 ± 5.5 | 18 |
Retrospective cohort Level III |
|||||
| Sartoretto et al. [41] | 2018 | 112 | 31% (35) | 45.1 ± 11.7 | 37.9 ± 6.7 | 14 | 26 | 16 | GORD 34 | 6 |
Retrospective cohort Level III |
||
| Kumar et al. [42] | 2018 | 77 | 36% (28) | 41.3 ± 1.1 | 99.4 ± 1.8 | 36.1 ± 0.6 | 12 |
Prospective cohort Level III |
|||||
| Novikov et al. [27] | 2018 | 91 | 23% (21) | 42 ± 12 | 38.6 ± 6.9 | 5.8 ± 0.98 | 20 | 18 | 15 | Dyslipidaemia 13 | 12 |
Retrospective cohort Level III |
|
| Alqahtani et al. [22] | 2019 | 1000 | 10% (103) | 34.4 ± 9.5 | 33.3 ± 4.5 | 17 | 17 | Dyslipidaemia 32 | 18 |
Prospective cohort Level III |
|||
| Fayad et al. [23] | 2019 | 108 | 52% (23) | 48 | 43.07 | ESG 2 | ESG 15 | LSG 1 | GORD 22 | 6 |
Retrospective cohort Level III |
||
| Barrichello et al. [40] | 2019 | 193 | 23% (45) | 42.3 ± 9.6 | 93.4 ± 10.31 | 34.11 ± 2.97 | 12 |
Retrospective cohort Level III |
|||||
| Cheskin et al. [43] | 2019 | 105 | 29% (30) | 47.58 ± 11.98 | 40.5 ± 7.8 |
Retrospective cohort Level III |
|||||||
| Alqahtani et al. [30] | 2019 | 109 | 9% (10) | 17.6 ± 2.2 | 33 ± 4.7 | 2 | 6 | 24 |
Retrospective cohort Level III |
||||
| Fayed et al. [32] | 2019 | 58 | 41% (24) | 48.2 ± 11.8 | 41.5 ± 8.2 | 3 | 17 | 9 | GORD 11 | 12 |
Retrospective cohort Level III |
||
| James et al. [24] | 2020 | 100 | 14% (14) | 45 ± 9 | 106.39 ± 20.24 | 38.41 ± 5.44 | 4 | 29 | Dyslipidaemia 13 | 12 |
Retrospective cohort Level III |
||
| Bhandari et al. [28] | 2020 | 53 | 19% (10) | 40.54 ± 13.79 | 89.12 ± 16.2 | 34.78 ± 5.2 | 10 | 15 | 6 | Hypothyroidism 14 , GORD 4 | 12 |
Retrospective cohort Level III |
|
| Lopez-Nava et al. [19] | 2020 | 11 | 64% (7) | 42.7 ± 5.6 | 111.1 ± 12.3 | 36.9 ± 2.8 | 6 |
Prospective cohort Level III |
|||||
| Espinet-Coll et al. [31] | 2020 | 88 | 31% (27) | 46.1 ± 12.3 | 110.71 ± 17.9 | 39.4 ± 4.69 | 11 | 35 | 8 | Arthropathy 13 | 12 |
Retrospective cohort Level III |
|
| Fiorillo et al. [33] | 2020 | 23 | 30% (7) | 41 | 115.5 ± 29.6 | 39.5 | 2 | 3 | 5 | Arthropathy 7 | 6 |
Retrospective cohort Level III |
|
| Huberty et al. [34] | 2021 | 49 | 6% (3) | 37.6 ± 9.9 | 93.3 ± 8.8 | 34.8 ± 2.7 | 12 |
Randomised Control Trial Level I |
|||||
| Pizzicannella et al. [20] | 2021 | 86 | 29% (25) | 46.6 ± 12.8 | 120.9 ± 25.6 | 43.2 ± 8.6 | 27 | 30 | 48 |
Dyslipidaemia 11 Arthropathy 7 |
12 |
Retrospective cohort Level III |
|
| Li et al. [35] | 2021 | 24 | 75% (18) | 55.6 ± 9.2 | 157.9 ± 49.1 | 49.9 ± 14.4 | 15 | 29 | 17 | GORD 3 | 12 |
Prospective cohort Level III |
|
| Jagtap et al. [25] | 2021 | 26 | 38% (10) | 41.5 ± 9.58 | 99.43 ± 21.89 | 36.55 ± 5.07 | 8.71 ± 1.55 | 13 | 19 | Dyslipidaemia 17 | 12 |
Prospective cohort Level III |
|
| Asokkumar et al. [21] | 2021 | 35 | 43% (15) | 43.6 ± 11.3 | 93.2 ± 16 | 34 ± 4.9 | 8 ± 0.8 | 8 | 17 |
Fatty liver 14 Liver cirrhosis 3 |
6 |
Retrospective cohort Level III |
|
| Lopez-Nava et al. [48] | 2021 | 199 | 29% (58) | 44.6 ± 10 | 110 ± 19.7 | 39.4 ± 5.4 | 24 |
Retrospective cohort Level III |
|||||
| Sharaiha et al. [37] | 2021 | 216 | 32% (70) | 46 ± 13 | 39 ± 6 | 5.8 ± 1 | 67 | 60 |
Prospective cohort Level III |
||||
| Sarkar et al. [50] | 2022 | 90 | 39% (35) | 39.7 ± 11.6 | 38.7 | 46 | 46 | 21 | 27.2 |
Retrospective cohort Level III |
|||
| Alqahtani et al. [26] | 2022 | 6036 | 11% (664) | 34 ± 10 |
ESG 32.5 ± 3.1 LSG 32.9 ± 3.5 |
ESG 112 LSG 350 |
ESG 101 LSG 118 |
Dyslipidaemia ESG 62, LSG 163 |
36 |
Retrospective cohort Level III |
|||
| Polese et al. [44] | 2022 | 27 | 36 ± 9 | 10 |
Prospective cohort Level III |
||||||||
| Abu Dayyeh et al. [45] | 2022 | 77 | 12% (9) | 47.3 ± 9.3 | 98.4 ± 12.3 | 35.5 | 5.8 ± 0.8 | 18 | 38 | 12 |
Randomised control trial Level I |
||
| Bhandari et al. [46] | 2022 | 612 | 19% (188) | 40.7 ± 12.6 | 34.3 ± 5 | 48 |
Prospective cohort Level III |
||||||
| Gkolfakis et al. [47] | 2022 | 48 | 17% (8) | 41.9 ± 9.5 | 34 ± 2.5 |
Randomised control trial Level I |
|||||||
| Matteo et al. [12] | 2022 | 18 | 44% (8) | 41.2 ± 5.9 | 41.2 ± 5.9 | 4 | 12 | 6 | 24 |
Retrospective cohort Level III |
|||
| Manos et al. [49] | 2022 | 191 | 9% (18) | 36.9 | 33.7 | 12 |
Retrospective cohort Level III |
Short- and Medium-Term Weight Loss
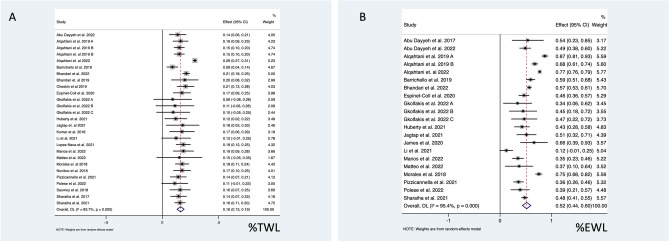
Short-term (1 year) %TWL data was published following 5659 ESGs and 1 year %EWL after 4852 interventions. Pooled analysis of the 23 studies recording %TWL demonstrated 16.2% (95% CI 13.1–19.4%; I2 = 83.7%) reduction of total body weight, and the 18 studies reporting %EWL suggested 51.7% (95% CI 43.5–59.9%; I2 = 95.4%) reduction in excess weight (Fig. 2).
Fig. 2.
Forest plot of A %TWL and B %EWL 12 months after ESG
Medium-term %TWL following ESG was recorded in ten studies investigating outcomes for 4040 patients. Random effect analysis demonstrated 15.4% (95% CI 13.7–17.2%; I2 = 14.5%) reduction of total body weight over the medium-term period. Percentage EWL over the medium-term was recorded in eight studies of 3837 patients and suggested a 51.8% (95% CI 47.9–62.9%; I2 = 87.5%) reduction of excess weight (Fig. 3.). One article described a loss of more than 50% of patients from follow-up year 3 to year 4 [46]. Hence, in our analysis, we included outcome following 3 years of follow-up but did perform the analysis with the 4 years follow-up data that did not show any difference.
Fig. 3.
Forest plot of A %TWL and B %EWL over medium term (2–5 years) following ESG
Remission of Obesity-Related Comorbidities
Remission of diabetes mellitus was reported in 12 studies (5034 patients including 461 diabetics) (Fig. 2). Pooled analysis demonstrated that 55.4% (95% CI 46–64%; I2 = 97.0%) of diabetic individuals went into remission. Four of these studies presented baseline and 12 months post-ESG follow-up HbA1c results [25, 37, 45, 50], and authors of one study provided these results separately [21]. Pooled analysis demonstrated 0.71% (95% CI − 1.06 to − 0.35%; I2 = 97.0%) decrease in the concentration of HbA1c which corresponds to 7.8% (95% CI 4–11%; I2 = 75.0%) improvement (Fig. 4).
Fig. 4.
Forest plot of A diabetes mellitus remission and B reduction of HbA1c reduction 12 months after ESG
Hypertension outcomes were reported in 11 studies (4933 patients with 606 diagnosed with hypertension) (Fig. 3). Remission of hypertension was observed in 383 individuals corresponding to a 62.8% (95% CI 43−82%) success rate. Three studies recorded pre- and postoperative systolic blood pressure. Alongside decreasing antihypertensive medication, pooled analysis suggested a 6.8 mmHg (95% CI 9.5−4.1%; I2 = 99.7%) decrease in resting systolic blood pressure (Fig. 5).
Fig. 5.

Forest plot of remission of hypertension following ESG
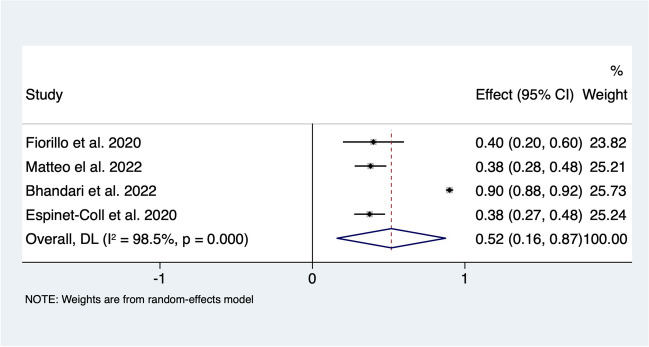
Dyslipidaemia outcomes were reported in eight studies (4835 ESGs with 580 patients diagnosed with dyslipidaemia) (Fig. 4). Pooled data analysis demonstrated remission of dyslipidaemia in 401 patients corresponding to a 56.3% (95% CI 49−63%; I2 = 93.1%) weighted mean reduction in disease prevalence. Change in the serum level of low-density lipoprotein (LDL) was investigated in 199 patients in three studies demonstrating a 3.7 mg/dl (95% CI − 13.6–6.1%; I2 = 56.9) or 1.3% reduction (95% CI − 5–3%). One study reported an increase in the level of LDL after ESG [38]. The same three articles also published outcomes of triglyceride for the same patients before and after ESG. All of these studies demonstrated a reduction of 48.3 mg/dl (95% CI − 3.4 to − 93.1%; I2 = 91.9) in the serum level of triglyceride following ESG which corresponds to a 21.6% (95% CI 6−37%) improvement (Fig. 5).
Pre-ESG obstructive sleep apnoea was recorded in 480 patients from 4 articles investigating 807 ESGs (Fig. 6). Overall, 51.7% (95% CI 16.2–87.3%; I2 = 98.5) of patients went into remission following intervention. No studies directly investigated liver disease, but there were four studies (n = 235 patients) which recorded alanine transaminase (ALT) and aspartate aminotransferase (AST) before and after ESG. A reduction of 18.2 IU (95% CI 8–28%) in ALT level and 14.6 IU (95% CI 1.9–27.2%) in AST level were observed following intervention. This corresponds to a 36.8% (95% CI 19.7–54%) improvement in ALT level and 27.9% improvement in AST level following ESG (Fig. 7.).
Fig. 6.
Forest plot of A resolution of dyslipidaemia, B change in triglyceride, and C low-density lipoprotein following ESG
Fig. 7.
Forest plot of resolution of obstructive sleep apnoea following ESG
In terms of medium-term durability of ESG, data was extracted from all articles publishing results for at least 2 years. Out of the ten studies, five did not present the frequency of revision. In the other five articles, there were 148 out of the 4032 patients that required revision including 96 patients who underwent re-do endoscopic intervention and 52 patients who were converted to LSG. Indication for revision included insufficient weight loss, weight regain and abdominal pain. The overall weighted mean for revision was 3.67% (95% CI 3.61–3.69%).
Discussion
This systematic review and meta-analysis reports weight loss outcomes and obesity-associated comorbidity resolution following ESG up to 5 years. The ultimate aim of all bariatric interventions is to achieve sustainable and clinically relevant weight loss. The pooled analysis of data from 35 studies containing data from 7525 patients confirmed that ESG can lead to sustained weight loss in the medium-term which is associated with significant improvements in obesity-associated comorbidities.
Although surgery is the most effective treatment for morbid obesity [51], a significant proportion of patients may not wish to undergo and/or be unsuitable for conventional surgery (e.g. due to multiple previous abdominal operations). Therefore, a non-surgical weight loss procedure with a favourable risk profile and durable weight loss addresses the needs of this patient group. By reducing the size of the gastric reservoir, ESG delays gastric emptying and induces early satiety which are thought to be the primary mechanisms for the sustained weight loss identified in this meta-analysis [8]. A large multicentre RCT (‘MERIT’) comparing ESG with lifestyle intervention has now shown that ESG can provide 45.1% EWL at 12 months compared with lifestyle modification alone, demonstrating the superiority of ESG over conservative treatment [52]. The accumulated data in this meta-analysis from multiple independent centres performing this procedure confirms that ESG is effective at inducing both obesity-associated comorbidity resolution and clinically significant weight loss.
Although there were no RCTs directly comparing ESG with LSG, resection of the fundus during a LSG removes the major source of ghrelin, a key orexigenic hormone, whereas ESG is a fundus-sparing procedure. ESG might therefore be expected to induce less weight loss than LSG. Whereas LSG can achieve a mean TWL of 23.7% after 5 years [53], our analysis found an average TWL of 15.4% after ESG in the medium-term. Data from comparative non-randomised studies comparing ESG with LSG, including a propensity-score matched analysis of 3018 patient pairs [26], suggested a mean difference in TWL of ~10% between LSG and ESG in the medium-term. It has also been proposed that weight loss and metabolic enhancement may be greater in patients with type 1 diabetes mellitus who are undergoing definitive bariatric surgery due to the immediate BRAVE (bile flow alteration, reduction of gastric size, anatomical gut rearrangement and altered flow of nutrients, vagal manipulation and enteric hormonal modulation) effects of surgery. These features, which include ESG intervention, can result in cascade effects on gut microbiome and local metabolism (intestinal gluconeogenesis and adipokine fluxes) enhancing weight loss [54]. Studies understanding the mechanism of ESG mapped on to the BRAVE effects and its downstream effectors should be considered.
Although the results of this study suggest that weight loss induced by ESG is less than LSG (pending robust clinical comparison trials), ESG has the added advantages of being an endoluminal, safe and organ-preserving procedure. These characteristics are likely to appeal to patients with obesity who would otherwise be reluctant to undergo conventional bariatric surgery. In addition, the average length of stay following ESG is an overnight stay which translates to reduced service costs [20]. Although the equipment can carry a higher cost than some surgical equipment on account of being in its early commercial cycle, the shorter recovery time and few reported adverse events support ESG in becoming a cost-effective solution. In terms of the durability of the procedure, our results demonstrated similar frequency for revision as described following LSG [55].
However, there is considerable heterogeneity in the outcomes that have been reported, which may be due to the heterogeneity of eligibility criteria and patient demographics amongst the mainly nonrandomised observational studies. Another important factor may be the lack of robust quality assurance of the procedures being performed which is becoming an essential component of intervention-based trials [52]. Saumoy et al. showed that efficacy of ESG was obtained after a minimal procedural volume of 38 ESGs, confirming that procedural quality is directly linked to outcome [39]. The MERIT RCT did include standardised training and proctoring of endoscopists, but did not include performance monitoring to ensure procedural quality was maintained throughout the trial. Future RCTs should include methods to ensure procedural quality to reduce variation in clinical outcome and improve the reliability of study findings.
This data supports the role of ESG as an option for patients who choose not to undergo and/or are unsuitable for conventional surgery, but there may be additional indications for ESG. Although the mean BMI of included studies was 37.7 kg/m2, one study evaluated ESG in high-risk patients (BMI > 50 kg/m2, unfit and/or impenetrable abdomen) and found that ESG could induce EWL of 29.1% at 12 months in this patient group [35]. ESG may also be an option as a safe revisional procedure after LSG in the setting of sleeve dilatation [56]. This is particularly important given the chronic nature of obesity and the likelihood of revisional procedures being required after LSG, particularly those performed in younger patients. Thus, the safety and organ-sparing nature of ESG makes it attractive as an initial revisional procedure after LSG. Further studies are warranted to explore the role of ESG for these indications.
The multidisciplinary team must have an active role in patient selection and follow-up to support weight loss following ESG, and the approach should be tailored to each individual patient to optimise outcomes. The decision to proceed with ESG should be guided by preoperative assessment, including medical and weight history, physical examination, laboratory tests, nutritional and psychological or psychiatric counselling. Lopez-Nava et al. [57] demonstrated that a high compliance with the follow-up post-ESG is associated with higher weight loss at one year. Furthermore, ESG is expected to have implications on endoscopy and surgery training programmes. It is a procedure that requires experience and training in endoscopic suturing in order to effectively and safely place full-thickness sutures in the stomach. Specialist bariatric gastroenterologists and surgeons will require training courses in endoscopic suturing, such as in ex vivo porcine specimens or virtual reality, at an early stage as recommended by the American Society for Gastrointestinal Endoscopy [58, 59]. Strengths of this review include the large number of included studies and the length of follow-up of up to 5 years. Limitations include the high heterogeneity of data, unavoidable duplication of data, difficult standardisation of the data limited quality assurance and risk of bias as the majority of studies were non-randomised unblinded cohort studies without sham control arms. This review has not directly analysed the risk of long-term weight recurrence after ESG after initial weight loss, short and long-term complications (e.g. acid reflux), need for revision of ESG (e.g. re-tightening or surgery) or cost-effectiveness of ESG.
In conclusion, through a meta-analysis of outcomes that have been reproduced in multiple independent centres, this review has demonstrated that ESG can generate sustained weight loss in the short- and medium-term and resolution of obesity-associated comorbidities for patients with moderate obesity. Future larger and higher quality studies, including results of ongoing RCTs [60], are needed to evaluate the role of ESG compared to other procedures and for additional indications, such as for patients with super obesity, high-risk patients and as a revisional procedure.
Acknowledgements
The authors would like to thank Mr. Phillip Barlow, NHS Support Librarian, for his invaluable help with the literature search.
Declarations
Ethical Approval
For this type of study, formal consent is not required.
Conflict of Interest
HA is a Chief Scientific Officer of Preemptive Health and Medicine, Flagship Pioneering. All other authors declare no conflict of interest.
Footnotes
Highlights
• Thirty-five articles publishing outcomes of 7525 ESGs with a mean follow-up of 17.6 (6-60) months
• Short-term total weight loss = 16.2%; medium-term total weight loss = 15.4%
• Resolution of comorbidities: diabetes mellitus 55.4%, hypertension 62.8%, dyslipidaemia 56.3%, obstructive sleep apnoea 51.7%
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–3794. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 2.Roth AE, Thornley CJ, Blackstone RP. Outcomes in bariatric and metabolic surgery: an updated 5-year review. Curr Obes Rep. 2020;9(3):380–389. doi: 10.1007/s13679-020-00389-8. [DOI] [PubMed] [Google Scholar]
- 3.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4(4):353–357. doi: 10.1381/096089294765558331. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risi R, Rossini G, Tozzi R, et al. Sex difference in the safety and efficacy of bariatric procedures: a systematic review and meta-analysis. Surg Obes Relat Dis. 2022;18(7):983–996. doi: 10.1016/j.soard.2022.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78(3):530–535. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 7.Vargas EJ, Rizk M, Gomez-Villa J, et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: a comparative prospective study. Gut. 2022;72(6):1073–1080. doi: 10.1136/gutjnl-2022-327816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol. 2017;15(1):37–43 e1. doi: 10.1016/j.cgh.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, et al. Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18(5):1043–53 e4. doi: 10.1016/j.cgh.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Gudur AR, Geng C, Hallowell P, et al. Impact of proceduralist specialty on outcomes following endoscopic sleeve gastroplasty. Obes Surg. 2022;32(11):3714–3721. doi: 10.1007/s11695-022-06282-8. [DOI] [PubMed] [Google Scholar]
- 11.Beran A, Matar R, Jaruvongvanich V, et al. Comparative effectiveness and safety between endoscopic sleeve gastroplasty and laparoscopic sleeve gastrectomy: a meta-analysis of 6775 individuals with obesity. Obes Surg. 2022;32(11):3504–3512. doi: 10.1007/s11695-022-06254-y. [DOI] [PubMed] [Google Scholar]
- 12.Matteo MV, Bove V, Pontecorvi V, et al. Outcomes of endoscopic sleeve gastroplasty in the elder population. Obes Surg. 2022;32(10):3390–3397. doi: 10.1007/s11695-022-06232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA SB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Mahawar KK. Defining short-term, medium-term, long-term, and very long-term follow-up after bariatric surgery. Obes Surg. 2018;28(5):1425–1426. doi: 10.1007/s11695-018-3183-2. [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Bagshaw SM. Evidence-based medicine: classifying the evidence from clinical trials--the need to consider other dimensions. Crit Care. 2006;10(5):232. doi: 10.1186/cc5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho PM, Peterson PN, Masoudi FA. Evaluating the evidence: is there a rigid hierarchy? Circulation. 2008;118(16):1675–1684. doi: 10.1161/CIRCULATIONAHA.107.721357. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Nava G, Asokkumar R, Rull A, et al. Safety and feasibility of a novel endoscopic suturing device (EndoZip TM) for treatment of obesity: first-in-human study. Obes Surg. 2020;30(5):1696–1703. doi: 10.1007/s11695-019-04370-w. [DOI] [PubMed] [Google Scholar]
- 20.Pizzicannella M, Fiorillo C, Barberio M, et al. Endoscopic assessment of morphological and histopathological upper gastrointestinal changes after endoscopic sleeve gastroplasty. Surg Obes Relat Dis. 2021;17(7):1294–1301. doi: 10.1016/j.soard.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Asokkumar R, Lim CH, Tan AS, et al. Safety and early efficacy of endoscopic sleeve gastroplasty (ESG) for obesity in a multi-ethnic Asian population in Singapore. JGH Open. 2021;5(12):1351–1356. doi: 10.1002/jgh3.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alqahtani A, Al-Darwish A, Mahmoud AE, et al. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc. 2019;89(6):1132–1138. doi: 10.1016/j.gie.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Fayad L, Adam A, Schweitzer M, et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc. 2019;89(4):782–788. doi: 10.1016/j.gie.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 24.James TW, Reddy S, Vulpis T, et al. Endoscopic sleeve gastroplasty is feasible, safe, and effective in a non-academic setting: short-term outcomes from a community gastroenterology practice. Obes Surg. 2020;30(4):1404–1409. doi: 10.1007/s11695-019-04331-3. [DOI] [PubMed] [Google Scholar]
- 25.Jagtap N, Kalapala R, Katakwar A, et al. Endoscopic sleeve gastroplasty - minimally invasive treatment for non-alcoholic fatty liver disease and obesity. Indian J Gastroenterol. 2021;40(6):572–579. doi: 10.1007/s12664-021-01202-7. [DOI] [PubMed] [Google Scholar]
- 26.Alqahtani AR, Elahmedi M, Aldarwish A, et al. Endoscopic gastroplasty versus laparoscopic sleeve gastrectomy: a noninferiority propensity score-matched comparative study. Gastrointest Endosc. 2022;96(1):44–50. doi: 10.1016/j.gie.2022.02.050. [DOI] [PubMed] [Google Scholar]
- 27.Novikov AA, Afaneh C, Saumoy M, et al. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic band for weight loss: how do they compare? J Gastrointest Surg. 2018;22(2):267–273. doi: 10.1007/s11605-017-3615-7. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari M, Jain S, Mathur W, et al. Endoscopic sleeve gastroplasty is an effective and safe minimally invasive approach for treatment of obesity: First Indian experience. Dig Endosc. 2020;32(4):541–546. doi: 10.1111/den.13508. [DOI] [PubMed] [Google Scholar]
- 29.Graus Morales J, Crespo Perez L, Marques A, et al. Modified endoscopic gastroplasty for the treatment of obesity. Surg Endosc. 2018;32(9):3936–3942. doi: 10.1007/s00464-018-6133-0. [DOI] [PubMed] [Google Scholar]
- 30.Alqahtani A, Elahmedi M, Alqahtani YA, Al-Darwish A. Endoscopic sleeve gastroplasty in 109 consecutive children and adolescents with obesity: two-year outcomes of a new modality. Am J Gastroenterol. 2019;114(12):1857–1862. doi: 10.14309/ajg.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 31.Espinet-Coll E, Nebreda-Duran J, Galvao-Neto M, et al. Suture pattern does not influence outcomes of endoscopic sleeve gastroplasty in obese patients. Endosc Int Open. 2020;8(10):E1349–E1E58. doi: 10.1055/a-1221-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fayad L, Cheskin LJ, Adam A, et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: efficacy, durability, and safety. Endoscopy. 2019;51(6):532–539. doi: 10.1055/a-0852-3441. [DOI] [PubMed] [Google Scholar]
- 33.Fiorillo C, Quero G, Vix M, et al. 6-Month Gastrointestinal Quality of Life (QoL) Results after endoscopic sleeve gastroplasty and laparoscopic sleeve gastrectomy: a propensity score analysis. Obes Surg. 2020;30(5):1944–1951. doi: 10.1007/s11695-020-04419-1. [DOI] [PubMed] [Google Scholar]
- 34.Huberty V, Boskoski I, Bove V, et al. Endoscopic sutured gastroplasty in addition to lifestyle modification: short-term efficacy in a controlled randomised trial. Gut. 2020;gutjnl-2020-322026. [DOI] [PubMed]
- 35.Li R, Veltzke-Schlieker W, Adler A, et al. Endoscopic sleeve gastroplasty (ESG) for high-risk patients, high body mass index (> 50 kg/m(2)) patients, and contraindication to abdominal surgery. Obes Surg. 2021;31(8):3400–3409. doi: 10.1007/s11695-021-05446-2. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. 2017;27(10):2649–2655. doi: 10.1007/s11695-017-2693-7. [DOI] [PubMed] [Google Scholar]
- 37.Sharaiha RZ, Hajifathalian K, Kumar R, et al. Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Clin Gastroenterol Hepatol. 2021;19(5):1051–7 e2. doi: 10.1016/j.cgh.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 38.Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol. 2017;15(4):504–510. doi: 10.1016/j.cgh.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Saumoy M, Schneider Y, Zhou XK, et al. A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc. 2018;87(2):442–447. doi: 10.1016/j.gie.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Barrichello S, Hourneaux de Moura DT, Hourneaux de Moura EG, et al. Endoscopic sleeve gastroplasty in the management of overweight and obesity: an international multicenter study. Gastrointest Endosc. 2019;90(5):770–780. doi: 10.1016/j.gie.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Sartoretto A, Sui Z, Hill C, et al. Endoscopic sleeve gastroplasty (ESG) is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: a large, international multicenter study. Obes Surg. 2018;28(7):1812–1821. doi: 10.1007/s11695-018-3135-x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159–2164. doi: 10.1007/s00464-017-5869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheskin LJ, Hill C, Adam A, et al. Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: a case-matched study. Gastrointest Endosc. 2020;91(2):342–9 e1. doi: 10.1016/j.gie.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Polese L, Prevedello L, Belluzzi A, et al. Endoscopic sleeve gastroplasty: results from a single surgical bariatric centre. Updat Surg. 2022;74(6):1971–1975. doi: 10.1007/s13304-022-01385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441–451. doi: 10.1016/S0140-6736(22)01280-6. [DOI] [PubMed] [Google Scholar]
- 46.Bhandari M, Kosta S, Reddy M, et al. Four-year outcomes for endoscopic sleeve gastroplasty from a single centre in India. J Minim Access Surg. 2022;19(1):101–106. doi: 10.4103/jmas.jmas_3_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gkolfakis P, Van Ouytsel P, Mourabit Y, et al. Weight loss after endoscopic sleeve gastroplasty is independent of suture pattern: results from a randomized controlled trial. Endosc Int Open. 2022;10(9):E1245–E1E53. doi: 10.1055/a-1880-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Nava G, Asokkumar R, Bautista-Castano I, et al. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic greater curve plication: do they differ at 2 years? Endoscopy. 2021;53(3):235–243. doi: 10.1055/a-1224-7231. [DOI] [PubMed] [Google Scholar]
- 49.Manos T, Costil V, Karsenty L, et al. Safety of endoscopic sleeve gastroplasty with a single-channel endoscope. Obes Surg. 2022;32(9):3074–3078. doi: 10.1007/s11695-022-06210-w. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar A, Tawadros A, Andalib I, et al. Safety and efficacy of endoscopic sleeve gastroplasty for obesity management in new bariatric endoscopy programs: a multicenter international study. Ther Adv Gastrointest Endosc. 2022;15:26317745221093883. doi: 10.1177/26317745221093883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;2014(8):CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster JD, Mackenzie H, Nelson H, et al. Methods of quality assurance in multicenter trials in laparoscopic colorectal surgery: a systematic review. Ann Surg. 2014;260(2):220–229. doi: 10.1097/SLA.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 53.Wolnerhanssen BK, Peterli R, Hurme S, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS) Br J Surg. 2021;108(1):49–57. doi: 10.1093/bjs/znaa011. [DOI] [PubMed] [Google Scholar]
- 54.Ashrafian H, Harling L, Toma T, et al. Type 1 diabetes mellitus and bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2016;26(8):1697–1704. doi: 10.1007/s11695-015-1999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazzati A, Bechet S, Jouma S, et al. Revision surgery after sleeve gastrectomy: a nationwide study with 10 years of follow-up. Surg Obes Relat Dis. 2020;16(10):1497–1504. doi: 10.1016/j.soard.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Maselli DB, Alqahtani AR, Abu Dayyeh BK, et al. Revisional endoscopic sleeve gastroplasty of laparoscopic sleeve gastrectomy: an international, multicenter study. Gastrointest Endosc. 2021;93(1):122–130. doi: 10.1016/j.gie.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Nava G, Asokkumar R, Rull A, et al. Bariatric endoscopy procedure type or follow-up: what predicted success at 1 year in 962 obese patients? Endosc Int Open. 2019;7(12):E1691–E16E8. doi: 10.1055/a-1007-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ASGE. https://www.asge.org/home/education/advanced-education-training/star-certifcate-programs. 2023
- 59.Bazarbashi AN. Training in Bariatric Endoscopy. ACG Case Rep J. 2020;7(3):e00358. doi: 10.14309/crj.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neto MG, Moon RC, de Quadros LG, Grecco E, Filho AC, de Souza TF, et al. Safety and short-term effectiveness of endoscopic sleeve gastroplasty using overstitch: preliminary report from a multicenter study. Surg Endosc. 2020;34(10):4388–4394. doi: 10.1007/s00464-019-07212-z. [DOI] [PubMed] [Google Scholar]