Abstract
Parvalbumin interneurons belong to the major types of GABAergic interneurons. Although the distribution and pathological alterations of parvalbumin interneuron somata have been widely studied, the distribution and vulnerability of the neurites and fibers extending from parvalbumin interneurons have not been detailly interrogated. Through the Cre recombinase-reporter system, we visualized parvalbumin-positive fibers and thoroughly investigated their spatial distribution in the mouse brain. We found that parvalbumin fibers are widely distributed in the brain with specific morphological characteristics in different regions, among which the cortex and thalamus exhibited the most intense parvalbumin signals. In regions such as the striatum and optic tract, even long-range thick parvalbumin projections were detected. Furthermore, in mouse models of temporal lobe epilepsy and Parkinson’s disease, parvalbumin fibers suffered both massive and subtle morphological alterations. Our study provides an overview of parvalbumin fibers in the brain and emphasizes the potential pathological implications of parvalbumin fiber alterations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-023-01083-0.
Keywords: Parvalbumin interneuron, Neuronal fiber, Spatial distribution, Pathological alteration, Temporal lobe epilepsy, Status epilepticus, Parkinson’s disease
Introduction
Fast-spiking parvalbumin (PV) interneurons are one of the major types of regulatory GABAergic interneurons, accounting for nearly 40% of all GABAergic interneurons in the neocortex [1]. PV interneurons are morphologically characterized by long, thick, and multiple dendrites, as well as extensive arborization of axons [2]. This ensures that PV interneurons integrate multiple inputs and generate divergent inhibitory outputs, casting powerful inhibitory control over the local networks [2]. Moreover, dendrites of PV interneurons in the cortex and hippocampus are highly connected by gap junctions to form a syncytial organization, which is fundamental to the generation of gamma oscillation [2–6]. Previous studies have reported that the somata of PV interneurons are widely distributed throughout the brain [7–13]. However, the distribution of PV fibers has scarcely been demonstrated.
In addition to crucial regulatory functions under physiological conditions, PV interneurons are highly vulnerable in neurological diseases [14, 15]. In both patients and animal models of temporal lobe epilepsy (TLE), the most common type of adult epilepsy, the number and functionality of PV interneurons are reduced in the hippocampus [16–18], while in models of Parkinson’s disease (PD), the immunoreactivity and the number of PV interneurons is also significantly decreased in the substantia nigra [19, 20]. However, due to the limitations of routine methods such as immunohistochemistry, previous studies have mainly focused on the changes in the number or immunoreactivity of PV somata, rather than those of PV fiber networks. This may give rise to the neglect of subtle changes in the processes of PV interneurons which may have important pathological implications.
In the current study, we visualized the PV fibers by using transgenic mice with Cre-driven fluorescent tdTomato expression [21], and observed the distribution of PV fibers in this mouse brain. We focused on the morphological features of PV fiber networks in the grey matter and several white matter structures. Furthermore, we observed the pathological changes of PV fibers in the pilocarpine model of TLE and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD. Subtle alterations in PV fibers that are easy to neglect were detected via this Cre-driven fluorescence strategy. In conclusion, our work provides an overview of the distribution of PV fibers in the mouse brain and demonstrates the subtle changes in PV fibers under pathological conditions.
Materials and Methods
Animals
To visualize PV somata and fibers clearly and objectively, we crossed PV-Cre mice with Ai9 mice [B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, JAX stock #007909, The Jackson Laboratory, Bar Harbor, USA] to generate PV-Cre+/−:ROSA26Sortm9(CAG-tdTomato)+/− mice (referred as PV-tdTomato in the following text). The specific and proper labeling of PV interneurons by this strategy was previously verified by Monteiro et al. [22]. All mice used in the study were 8–10 weeks old.
All animals were housed with temperature and humidity monitored and under 12/12-h light/dark cycles. Four to six mice were kept per cage with ad libitum access to food and water. All experimental procedures were approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University.
The Establishment of Models of TLE and PD
TLE was modeled by the induction of status epilepticus (SE) as previously described with slight modifications [23]. Briefly, mice were injected intraperitoneally (i.p.) with 10 mEq/kg lithium chloride (L9650, Sigma-Aldrich, St. Louis, USA) in normal saline. Eighteen hours later, mice were injected i.p. with 100 mg/kg of pilocarpine hydrochloride (14487, Cayman Chemical, Ann Arbor, USA). Methscopolamine bromide (S129958, Aladdin, Shanghai, China) at 1 mg/kg was subcutaneously administered 30 min before pilocarpine to avoid peripheral cholinergic effects and reduce mortality during SE. The severity of convulsive seizures was classified with the Racine scale: Stage 0, no response; Stage 1, mouth and facial movements; Stage 2, head nodding; Stage 3, unilateral forelimb clonus; Stage 4, bilateral forelimb clonus and rearing; Stage 5, generalized tonic-clonic seizure characterized by rearing and falling [24]. Mice that did not develop continuous seizure activity were excluded from further studies. The onset of SE was defined as the time point when mice reached stage 4 without returning to normal behavior. SE was allowed for 60 min and then it was terminated with 30 mg/kg of diazepam (Jin Yao Pharmaceutical Co., Tianjin, China). Mice that did not receive pilocarpine hydrochloride were used as naïve controls.
The MPTP model of Parkinson’s disease was established as previously described [19, 20]. Mice were injected i.p. with MPTP (10 mg/kg, HY-15608, MedChemExpress, Monmouth Junction, USA) four times at intervals of 1 h, and the total dose per mouse was 40 mg/kg. A pole test was applied 7 days after the establishment of the PD model to assess the motor coordination deficit as previously described [25]. Briefly, each mouse was placed head upward near the top of a vertical iron pole with a rough surface (diameter 10 mm, height 60 mm). The time until the mouse descended to the floor was recorded (referred to as TLA) and compared between PD mice and control mice that received saline. The longer the TLA, the more severe the motor coordination deficit. All animals underwent euthanasia 3 days and 7 days after the establishment of SE and MPTP models for immunohistochemistry.
Stereotaxic Surgery and Virus Injection
PV-tdTomato mice were anesthetized with isoflurane (4% for induction and 1.5% for maintenance) and mounted in a stereotaxic apparatus (RWD Life Science Inc., Shenzhen, China). The skull was exposed with a small incision and holes were drilled. To explore the origin of long-range PV projections in the caudate-putamen (CPu), rAAV-EF1α-DIO-EYFP-WPRE-hGH pA (titer: 2.40 × 1012 vector genomes per mL; Cat#: PT-0012, BrainVTA, Wuhan, China) was injected using a microinjection needle with a 10 μL microsyringe (Gaoge Industry and Trade Co., Ltd., Shanghai, China) at a rate of 30 nL/min controlled by a microsyringe pump (Kd Scientific Inc., Holliston, USA) at the following coordinates according to the 2nd edition of The Mouse Brain in Stereotaxic Coordinates by Paxinos and Franklin [26]: bilateral primary somatosensory cortex (in mm: AP 0.38, ML 2.6, DV 1.8; 200 nL), bilateral primary motor cortex (in mm: AP 1.1, ML 1.2, DV 1.5; 200 nL), bilateral reticular thalamic nuclei (in mm: AP 0.82, ML 1.6, DV 3.5; 100 nL), bilateral lateral globus pallidus (in mm: AP −0.46, ML 1.85, DV 4; 120 nL). The microsyringe had a glass pipette of 15–25 μm in diameter at the tip to avoid excessive tissue injury. The needle was left in place for another 8–10 min before withdrawal following injection. Mice were carefully handled and were allowed to recover for three weeks after virus injection, after which mice were perfused for immunohistochemistry.
Immunohistochemistry
Mice were perfused transcardially with 4% paraformaldehyde after normal saline and brains were removed and soaked in 30% sucrose in phosphate-buffered saline (PBS) until sinking. Serial coronal cryo-sections (30 μm) and sagittal cryo-sections (40 μm) were cut. In order to present the distribution of PV-positive fibers and somata in different brain regions, we chose representative coronal sections according to the 2nd edition of The Mouse Brain in Stereotaxic Coordinates by Paxinos and Franklin [26], anteroposterior from bregma (in mm) 1.98, 1.54, 0.38, − 0.34, − 1.94, − 3.16, including the cerebral cortex, hippocampus, amygdala, septal nucleus, striatal structures, thalamus, hypothalamus, and midbrain. Sagittal sections were cut sequentially to observe the distribution of the fibers and somata that expressed EYFP.
Sections were washed three times in PBS and blocked with 10% normal donkey serum (SL050, Solarbio, Beijing, China) along with 0.3% Triton-X100 (Sigma-Aldrich) in PBS for 30 min at room temperature (RT). Sections were then incubated with rabbit anti-NeuN primary antibody (1:500, ABN78, Sigma-Aldrich), rabbit anti-GFAP antibody (1:2000, Z0334, Agilent Technologies, Santa Clara, USA), rabbit anti-PV antibody (1:500, GTX134110, GeneTex, Irvine, USA), rabbit anti-tyrosine hydroxylase (TH) primary antibody (1:1000, AB152, Sigma-Aldrich), and rat anti-myelin basic protein (1:500, ab7349, Abcam, UK) at 4 °C overnight in humidified incubation chambers followed by Alexa Fluor Plus 488 donkey anti-rabbit (1:500, A32790, Invitrogen, Carlsbad, USA) and donkey anti-rat (1:500, A48269, Invitrogen) IgG secondary antibody. Sections were then counterstained with Hoechst 33258 (C0021, Solarbio) and mounted on coverslips.
Confocal Imaging and Section Scanning
Prepared sections were examined under a laser scanning confocal microscope (FV3000, Olympus, Tokyo, Japan), and images of brain regions of interest were captured with appropriate magnification. For visualization of the 3D structure of PV fibers, Z-stacks were established by scanning images 1 μm apart, and the 3D structure was reconstructed with Imaris software (v.9.5.0, Oxford Instruments, Oxford, UK). For the coronary and sagittal overview of the PV signals, sections were scanned using SLIDEVIEW VS200 (Olympus) to generate landscapes of PV signals. Selected coronary landscapes of PV signals were stacked according to the stereotaxic frame of the mouse brain rostrally to caudally to visualize the general distribution of PV-positive signals across the brain. For statistical analysis of PV signal densities in certain brain regions, integrated densities of PV signals in that region from representative sections were divided by the area of this region to obtain the relative signal density, as measured with ImageJ (v.1.52a, National Institutes of Health, Bethesda, USA). To determine the alterations of PV somata in the TLE and PD models, the numbers of PV- and NeuN-positive somata from sections at similar bregma locations were counted with Image J (v.1.52a, National Institutes of Health) and compared between groups. The morphological changes in PV fibers in the TLE and PD models were investigated thoroughly as follows. PV fiber abundance was defined as the percentage of the area of PV fibers to the total area of the 20× confocal field, to describe the overall complexity of the PV fiber network. The areas of PV fibers were obtained by subtracting the area of PV somata from the area of all PV-positive signals, as measured with Imaris software (v.9.5.0, Oxford Instruments plc). TH fiber abundance was calculated using the methods for PV fiber abundance. The diameters of PV fibers were measured with Imaris software (v.9.5.0, Oxford Instruments plc). To obtain the volume of the PV fiber tract in the CPu, PV signals were first 3D-reconstructed based on a series of thirty sequential Z-stack images at intervals of 1 μm. The volumes of PV fiber tracts were obtained by subtracting the volume of PV somata from the volume of all PV-positive signals, as reconstructed and measured with Imaris software (v.9.5.0, Oxford Instruments plc). The PV fiber density in the myelin basic protein (MBP)-positive fiber tract in the CPu was calculated by dividing the number of PV fibers by the area of MBP signals. The number of PV fibers was counted with ImageJ (v.1.52a, National Institutes of Health), and the areas of MBP signals were measured with OlyVIA (v3.2.1, Olympus).
Statistical Analysis
Data are expressed as the mean ± SEM. Student’s t-test was used to compare the difference between the two groups. One-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons and the Kruskal-Wallis test with Dunn’s multiple comparisons were used to compare the differences between the three groups for normal distribution and skewed distribution when appropriate. Repeated measures of two-way ANOVA with Bonferroni’s multiple comparisons were used to compare the difference in seizure behavior between the control and SE groups. Statistical analysis was conducted with SPSS 25.0 software (SPSS Inc., Chicago, IL, USA).
Results
The General Distribution of PV Fibers in the Mouse Brain
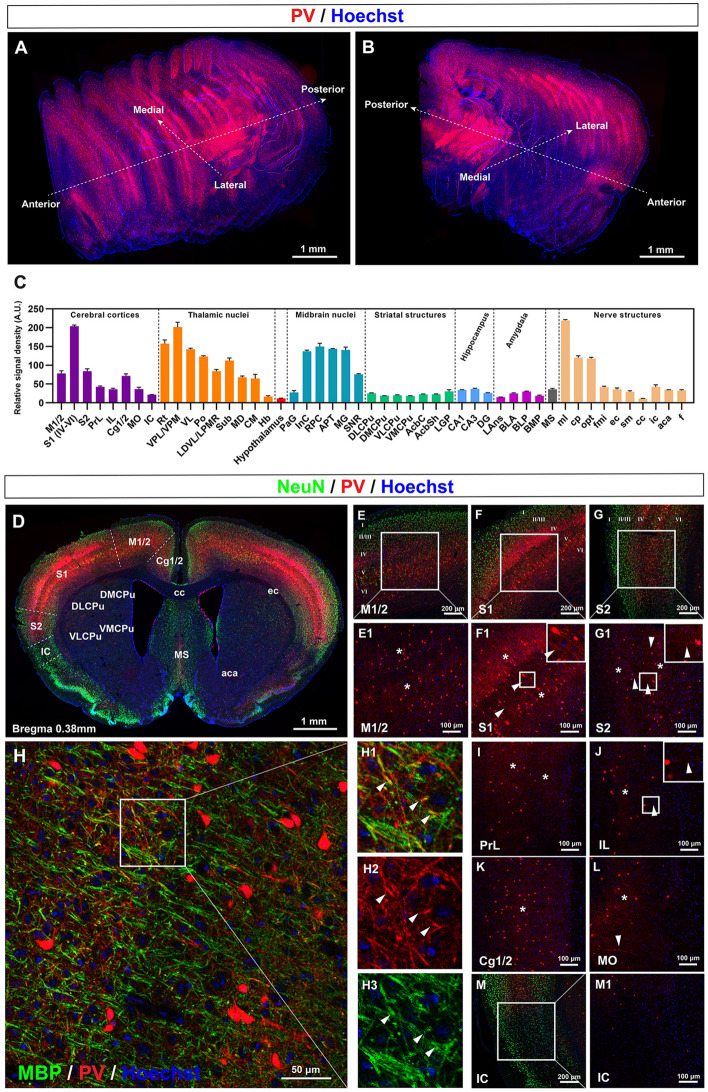
Before we inspected the distribution of PV fibers in specific brain regions, we started by outlining the general distribution of PV fluorescence in the mouse brain. We attained this goal by stacking selected coronal sections and reconstructing them three-dimensionally (Fig. 1A, B) based on standard coordinates from The Mouse Brain Atlas by Paxinos and Franklin [26]. We found that PV fluorescence was widely distributed across the brain at different densities. The somata and fibers mingled, and they were particularly enriched in the cerebral cortex (especially the primary somatosensory cortex) and thalamic nuclei, which was verified by quantification (Fig. 1A–C). PV signals were also enriched in the midbrain, especially in specific nuclei. In comparison, PV signals in the hippocampus and the striatal structures were relatively sparse, and those in the hypothalamus were nearly undetectable. Signals in areas caudal to the midbrain were not observed. Dense PV signals were also detected in certain white matter structures such as the medial lemniscus (ml), cerebral peduncle (cp), and the optic tract (opt) (Fig. 1C). This global view offered us a general idea of PV expression and we then observed the details of different regions from the areas with the highest PV expression levels to those with the lowest levels. The specificity of PV-Cre mice was verified by co-staining tdTomato with PV on brain sections (Fig. S1).
Fig. 1.
General distribution of PV fibers in the brain and in the cerebral cortex. A, B 3D reconstruction of PV signals throughout the mouse brain using selected coronal sections. C Quantification of the PV fluorescence density in different regions of the mouse brain. D Coronal section at bregma 0.38 mm demonstrating the cortex subfields and territories of the caudate-putamen where PV fibers are found. E–G PV fibers and somata in the M1/2, S1, and S2 subfields of the cerebral cortex. E1–G1 Magnification of boxes in E–G. Asterisks in E1–G1 show local PV fiber networks. Arrowheads in F1 and G1 show long PV neurites. H, H1–H3 Myelination of PV fibers in the somatosensory cortex. Arrowheads in H1–H3 indicate myelinated PV fibers. I–M PV fibers and somata in the PrL, IL, Cg1/2, MO, and IC subfields of the cerebral cortex. Asterisks in I–L show the local PV fiber networks. Arrowheads in J and L show long PV neurites. M1 Magnification of box in M. Abbreviations: A.U. arbitrary unit; Cg1/2 cingulate cortex area 1 and area 2; IC insular cortex; IL infralimbic cortex; MO medial orbital cortex; M1/2 primary and secondary motor cortex; PrL prelimbic cortex; S1 primary somatosensory cortex; S2 secondary somatosensory cortex. Scale bars, 1 mm (A, B, D), 200 μm (E–G, M), 100 μm (E1–G1), 50 μm (H), 100 μm (I–L, M1). n = 3 mice.
The Distribution of PV Fibers is Most Abundant in the Cortex and the Thalamus
We started our observations in the cortex and thalamus, the two regions with the most prominent PV signals. As these brain regions are essentially involved in sensory information processing, both the somatosensory cortex and the thalamus exhibited the most prominent PV fluorescence relative to the whole brain, which indicated the essential role of PV somata and fibers in the regulation of sensory information.
The Cerebral Cortex
Compared with the ventral part of the cortex, the dorsal part, i.e., the neocortex, had more intense PV signals, with the somatosensory cortex showing the greatest abundance (Fig. 1D). PV fluorescence signals were widely distributed from layer II to layer VI (Fig. 1E–G). Layer IV and deeper layer V exhibited more intense PV fluorescence than other layers (Fig. 1E–G). To be more specific, in the primary and secondary motor cortex (M1/2), the primary somatosensory cortex (S1), and the secondary somatosensory cortex (S2), densely intertwined PV-positive networks (Fig. 1E1–G1, asterisks) formed two parallel PV-fiber bands through the neocortex (Fig. 1D–G). These parallel bands in layers IV and V likely regulate the relay of information, since layer IV is the primary receptive region of thalamic sensory-motor signaling [27–29], while layer V receives inputs from all cortical layers and projects both intracortically and subcortically [30–33]. What is more, in layer V of S1 and S2, interlaminar PV fibers were distributed vertically to the brain surface, building communications between layers (inserts in Fig. 1F1 and G1, arrowheads). Interestingly, MBP-positive myelin was found to wrap a minority of PV-positive fibers in the somatosensory cortex (Fig. 1H, H1–H3, arrowheads).
In the prefrontal cortex [including the prelimbic cortex (PrL) and infralimbic cortex (IL)], the cingulate cortices (Cg1/2), and the medial orbital cortex (MO), PV-positive somata and fibers also mingled and generated clear fluorescent signals (Fig. 1I–L). In these regions, PV fibers mainly extended locally to form interconnected networks (Fig. 1I–L, asterisks). Occasionally, interlaminar PV fibers pointed vertically to the brain surface (Fig. 1J and L, arrowheads). By contrast, in the insular cortex (IC) PV somata were sparsely distributed and PV fibers were nearly undetectable (Fig. 1M and M1).
The Thalamic Nuclei
We next interrogated the distribution of PV fibers in the nuclei of the thalamus in detail. As shown in Fig. 2A, PV signals gradually weakened from the lateral to medial thalamus. The strongest PV expression was seen in the reticular thalamic nucleus (Rt) where PV interneurons were the major neuronal type and were arranged in a reticular formation (Fig. 2A, B, B1, and B2). PV fibers extended between somata to form fiber connections (Fig. 2B2, asterisks). In regions next to the Rt, PV expression was still strong, although weaker than that in the Rt. These regions included the ventral posterolateral thalamic nucleus (VPL), the ventral posteromedial thalamic nucleus (VPM), the ventrolateral thalamic nucleus (VL), the posterior thalamic nuclear group (Po), the laterodorsal thalamic nucleus, ventrolateral part (LDVL), and the lateral posterior thalamic nucleus, mediorostral part (LPMR), where PV somata were diffusely distributed, and PV fibers intertwined to form a local network (Fig. 2C–F, asterisks). Particularly, short bush-like PV neurites arborizing in a limited territory were noted in the VL (Fig. 2D, arrow and insert). In the thalamic submedius nucleus (Sub, Fig. 2G), PV fluorescence was also relatively strong. However, PV somata were not recognized here, and NeuN-positive PV-negative neurons were embedded in the dense network formed by thin short PV fibers and thick PV clumps (Fig. 2G).
Fig. 2.
PV fibers in the thalamus and hypothalamus. A Coronal section at bregma − 1.58 mm demonstrating the nuclei of the thalamus and hypothalamus. B Distribution of PV fibers and somata in the Rt. B1, B2 Magnification of box in B. Asterisks show a local PV fiber network with embedded PV somata. C–F PV fibers and somata in the VPL, VPM, VL, Po, LDVL, and LPMR of the thalamus. Asterisks demonstrate the locally intertwined PV fiber network. Arrow in D shows a PV-positive soma with a short basket-like arborization. G–J, I1, J1 PV fibers and somata of Sub, MD, CM, and Hb, in the medial part of the thalamus. Asterisks in H and I1 show locally intertwined PV fibers. Arrowheads in I1 indicate long horizontal PV fibers connecting the contralateral CM. K, K1 PV signals are hardly seen in the hypothalamus. Abbreviations: CM central medial thalamic nucleus; Hb habenular nucleus; HT hypothalamus; LDVL laterodorsal thalamic nucleus, ventrolateral part; LHb lateral habenular nucleus; LPMR lateral posterior thalamic nucleus, mediorostral part; MD mediodorsal thalamic nucleus; MDC mediodorsal thalamic nucleus, central part; MDM mediodorsal thalamic nucleus, medial part; MDL mediodorsal thalamic nucleus, lateral part; MHb medial habenular nucleus; Po posterior thalamic nuclear group; Rt reticular thalamic nucleus; VL ventrolateral thalamic nucleus; VPL ventral posterolateral thalamic nucleus; VPM ventral posteromedial thalamic nucleus. Scale bars, 1 mm (A), 100 μm (B–F), 50 μm (B1 and B2), 50 μm (G), 100 μm (H–J, I1–J1), 200 μm (K and K1). n = 3 mice.
In the more medial part of the thalamus, including the mediodorsal thalamic nucleus (MD) and the central medial thalamic nucleus (CM), PV signals further weakened but were still detectable (Fig. 2H, I, and I1). In the MD, PV fibers in the central part were denser than those in the lateral and medial parts (Fig. 2H, asterisks). In the CM, PV fibers constituted a local network positioned symmetrically (Fig. 2I and I1, asterisks). Long horizontal PV fibers (Fig. 2I1, arrowheads in insert) traveled between the contralateral parts, indicating contralateral connections. Last, in the most central part of the thalamus, the habenular nucleus showed nearly no PV fluorescence (Fig. 2J and J1).
PV Fluorescence in Other Grey Matter Areas Varies from Medium to Almost No Expression
Other regions also demonstrated some PV fibers, although PV somata were not as evident and the PV fiber fluorescence signals were not as intense as those in the cortex and the thalamus. We described these regions according to their PV fluorescence intensity from medium to nearly no expression, involving the midbrain nuclei, the striatal structures, the hippocampus, the medial septal nucleus in the basal forebrain, the amygdala, and the hypothalamus.
The Midbrain Nuclei
As shown in Fig. 3A, PV signals in the midbrain nuclei varied among different regions. Interestingly, the strongest PV signals were in the ml (Fig. 3A and B), which is a white matter structure. In the ml, dense PV fibers gathered to form thick bundles (Fig. 3B) while sparse PV somata were mingled within them (inserts in Fig. 3B, arrow). This strong PV fluorescence corresponded to the strong PV signals in the thalamus since the ml transmits proprioceptive information and the sense of discriminative touch to the thalamus [34]. In the grey matter of the midbrain, most nuclei contained diffusely distributed PV somata with PV fiber forming local networks. Typical areas included the anterior pretectal nucleus (Fig. 3C and D, asterisk for fiber networks and arrows for somata), the dorsal and ventral parts of the medial geniculate nucleus (Fig. 3C and E, asterisks for fiber networks and arrows for somata), the interstitial nucleus of Cajal (Fig. 3F), the parvicellular part of the red nucleus (Fig. 3G) above the ml, and the reticular part of the substantia nigra (SNR, Fig. 3H, H1–H3) under the ml, while in the periaqueductal grey, both PV somata and fibers were hardly seen (Fig. 3I).
Fig. 3.
PV fibers in the midbrain nuclei. A Coronal section at bregma − 3.16 mm demonstrating the midbrain nuclei locations where the detailed images were captured. B PV fibers in ml are intertwined to form large fiber bundles. PV somata with faint fluorescent signals are detectable between large PV fiber bundles (arrow in the inset). C–E PV fibers in the APT and MG form local networks (asterisks) with embedded PV somata (arrows). F–H PV fibers and somata in the InC, RPC, and SNR. PV fibers form local networks (asterisk) with faint PV somata embedded within the fiber network (arrows). H1–H3 Magnifications of the inset h from H demonstrating the PV somata. I In the PAG, both PV somata and fibers are hardly detectable. Abbreviations: APT anterior pretectal nucleus; ml medial lemniscus; MG medial geniculate nucleus; MGD medial geniculate nucleus, dorsal part; MGV medial geniculate nucleus, ventral part; InC interstitial nucleus of Cajal; PAG periaqueductal gray; RPC red nucleus, parvicellular part; SNR substantia nigra, reticular part. Scale bars, 1 mm (A), 100 μm (B–I). n = 3 mice.
The Striatal Structure
Striatal structures, including the striatum, the globus pallidus, and the internal capsule, are subcortical structures that are essential in the interaction with the cortex, and play important roles in the regulation of motor, motivational, and cognitive behaviors [35, 36]. We next investigated the distribution of PV fibers in the striatal structures (Figs. 1D and 4A, B). Overall, PV fluorescence signals here were at a medium expression level.
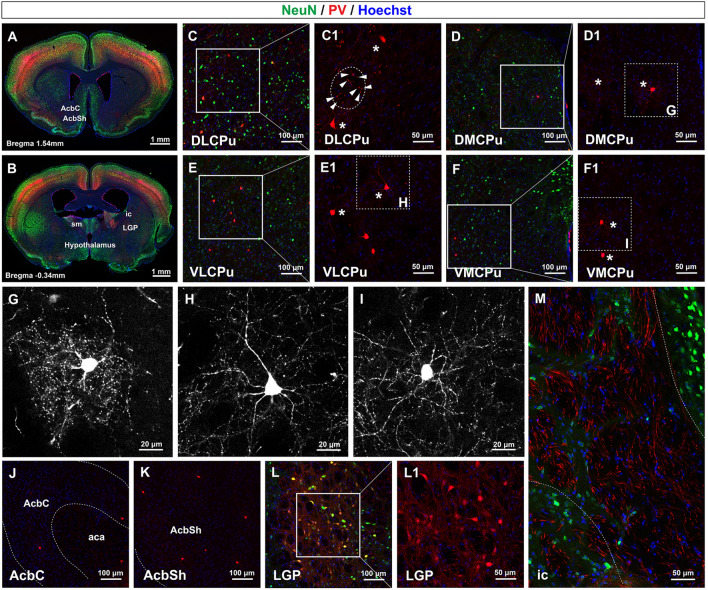
Fig. 4.
PV fibers in the striatal structures. A, B Coronal sections demonstrating the locations where the detailed images were captured. C–F PV fibers and somata in the DLCPu, DMCPu, VLCPu, and VMCPu. C1–F1 Magnification of boxes from C–F. Basket-like PV fibers extend from the soma and form a localized network (asterisks). PV fibers orthogonal to the coronal sections are also detectable (arrowheads in C1). G–I Magnified images of boxes from D1–F1 show complex local fiber fields formed by single basket-like PV interneurons. J, K PV signals in the AcbC and AcbSh. L PV signals in the LGP. L1 Magnification of box in L. M PV fibers in the ic. Abbreviations: ic internal capsule; AcbC accumbens nucleus core; AcbSh accumbens nucleus shell; DLCPu dorsolateral caudate putamen; DMCPu dorsomedial caudate putamen; LGP lateral globus pallidus; VLCPu ventrolateral caudate putamen; VMCPu ventromedial caudate putamen. Scale bars, 1 mm (A and B), 100 μm (C–F), 50 μm (C1–F1), 20 μm (G–I), 100 μm (J–L), 50 μm (L1 and M). n = 3 mice.
First, in the dorsal striatum, i.e., the CPu, PV somata were diffusely distributed in the dorsolateral, dorsomedial, ventrolateral, and ventromedial parts of the CPu (Fig. 4C–F and C1–F1). Interestingly, basket-like PV fiber extended radially from PV somata, forming a much broader field than the somata themselves and targeting adjacent medium spiny neurons (Fig. 4C1–F1, asterisks). The enlarged and high-contrast images (Fig. 4G–I) clearly demonstrated this feature. Besides the somata and the branching fibers, we also noted some brighter and stronger PV grains as shown by arrowheads in the circle of Fig. 4C1. 3D reconstructed images confirmed that these PV-positive grains were actually PV fibers that were orthogonal to the coronal sections, and they were wrapped in MBP-positive myelin sheaths (Fig. S2). In the core and shell parts of the nucleus accumbens in the ventral striatum, PV somata and fibers were sparsely distributed (Fig. 4J and K). Overall, the PV fiber network was a little denser in the dorsal striatum than in the ventral striatum.
Then in the lateral globus pallidus (LGP, Fig. 4L and L1), numerous PV somata were detected. Long and intertwined PV fibers extended from PV somata, and they were mixed with points of high fluorescence density. Lastly, in the internal capsule (ic, Fig. 4M), the typical white matter in the striatum, long slender PV fibers were distributed in parallel to form clusters of bundles, while PV somata were not detected.
The Hippocampus, Medial Septum, and Amygdala
In the hippocampus, PV interneurons are responsible for the generation of gamma oscillations that are crucial for memory encoding and retrieval [37, 38]. We next observed the distribution of PV fibers in different subdomains of the hippocampus (Fig. 5A). In the CA1 and CA3 regions, PV somata were sparsely localized at the edge of the pyramidal cell layer. PV fibers were buried in the pyramidal layer and formed a dense fiber network surrounding pyramidal cells (Fig. 5B, B1, C, C1, asterisks). In the dentate gyrus, PV somata were mainly distributed in the junction between the granular layer and the polymorph layer, while PV fibers formed networks among the granular cells (Fig. 5D and D1, asterisk). Interestingly, some PV neurons here sent long thin projections toward the molecular layer through the granular cells (Fig. 5D1, arrowhead). In the fimbria of the hippocampus, neither PV somata nor fibers could be discerned (Fig. 5E and E1).
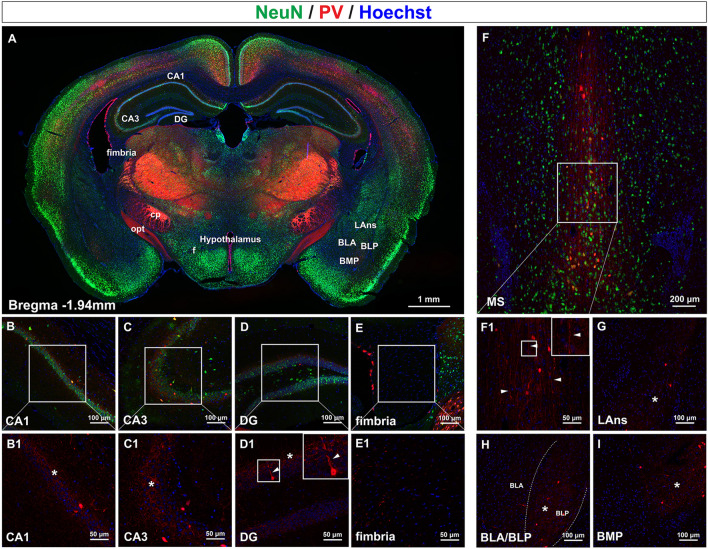
Fig. 5.
PV fibers in the hippocampus, medial septal nucleus, and nuclei of the amygdala. A Coronal section at bregma − 1.94 mm showing the subfields of the hippocampus and nuclei of the amygdala. B–E Distribution of PV fibers in the CA1, CA3, and DG subfields as well as the fimbria of the hippocampus. B1–E1 Magnification of boxes in B–E. PV fibers are interconnected within the pyramidal and granular layers (asterisks), and vertical PV fibers pass through the granular layer (arrowheads). F, F1 Distribution of PV fibers in the medial septal nucleus. PV somata as well as other types of neurons are distributed among the long chain-like fibers (arrowheads in F1). G–I Distribution of PV fibers in the nuclei of the amygdala, including the LAns, BLA, BLP, and BMP. PV fibers arborize locally to form an interconnected network (asterisks). Abbreviations: BLA/BLP basolateral amygdaloid nucleus, anterior/posterior part; BMP basomedial amygdaloid nucleus, posterior part; DG dentate gyrus; LAns lateral amygdaloid nucleus; MS medial septal nucleus. Scale bars, 1 mm (A), 100 μm (B–E), 50 μm (B1–E1), 200 μm (F), 50 μm (F1), 100 μm (G–I). n = 3 mice.
The basal forebrain is located ventral to the basal ganglia and anterior to the hypothalamus [39]. With the medial septal nucleus (MS, Fig. 1C) located at the center, the basal forebrain is characterized by large cholinergic neurons and is involved in feeding, drinking, rewarding, reproductive, and affiliative behaviors [40, 41]. Interestingly, in the MS, we observed that long waterfall-like PV fibers stretched throughout the dorsal-ventral axis (Fig. 5F, F1 arrowheads), with PV somata and other types of neurons seated in between (Fig. 5F).
The basal forebrain extends caudally and laterally to the amygdala [39] (Fig. 5A), where we next observed the distribution of PV fibers. PV somata were diffusely distributed in the lateral amygdaloid nucleus (LAns), basolateral amygdaloid nucleus anterior part and posterior part (BLA/BLP), and basomedial amygdaloid nucleus (BMP). PV fibers ramified radially from PV somata to form a local fiber network (Fig. 5G–I, asterisks). The density of the network formed by PV fibers gradually increased from ventral to dorsal, with the density in the BMP higher than that in the LAns (Fig. 5G, I), and also from medial to lateral, with the density in the BLP higher than that in the BLA (Fig. 5H).
The Hypothalamus
As shown in Fig. 2A, 4B, and 5A, PV fluorescence density in the hypothalamus was scarce; in the enlarged images, we could barely identify any PV fiber or somata (Fig. 2K and K1).
PV Fibers in White Matter Structures
Finally, after we observed the distribution of PV fibers in the main nuclei, we observed PV fibers in white matter structures.
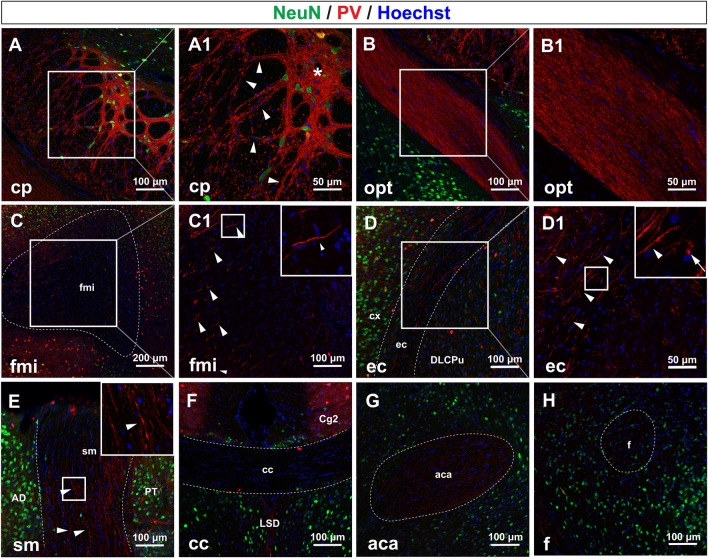
Besides the ml (Fig. 3A) we have mentioned above, another white matter structure with the most prominent PV fluorescence was the cp followed by the opt (Fig. 5A). In the cp, interestingly, PV fibers knitted together to form thick fiber bundles (Fig. 6A and A1, arrowheads) and spreading networks (Fig. 6A1, asterisks). In the opt (Fig. 6B, and B1), we were surprised to find that PV-fibers ran parallel with each other, and they formed a thick fiber bundle which composed part of the opt.
Fig. 6.
PV fibers in some white matter structures. A PV fibers in the cp. A1 Magnification of box in A. In the cp, PV fiber bundles mingle to form dense networks (asterisk) and fiber tracts (arrowheads). B PV fibers in the opt. B1 Magnification of the box in B. In the opt, a fiber bundle composed of PV fibers identifies the opt. C, D PV fibers in the fmi and ec. C1, D2 Magnification of boxes in C and D. Long slender PV fibers are detectable (arrowheads). E PV fibers in the sm. Long, varicose PV fibers run parallel to each other in the sm (arrowheads). F–H PV signals in the cc, aca, and f. In the cc, no PV fibers and somata are seen, while faint and thin PV fibers are seen in the aca and f. Abbreviations: aca anterior commissure, anterior part; cc corpus callosum; cp cerebral peduncle; ec external capsule; f fornix; fmi forceps minor of the corpus callosum; opt optic tract; sm stria medullaris of the thalamus. Scale bars, 100 μm (A, B), 50 μm (A1–B1), 200 μm (C), 100 μm (C1 and D), 50 μm (D1), 100 μm (E–H). n = 3 mice.
Other white matter structures showed sparse PV signals, such as the forceps minor of the corpus callosum (fmi), the external capsule (ec), and the stria medullaris of the thalamus (sm). In the fmi, we found that long, thick, but isolated PV fibers were distributed at the boundary rather than the center of the fmi (Fig. 6C and C1, arrowheads). In the ec (Fig. 6D and D1), many long thick PV fibers ran along the ec (Fig. 6D1, arrowheads), and we found that some PV grains were actually PV fibers orthogonal to the coronal plane (Fig. 6D1, arrow). In the sm, we detected long varicose PV fibers parallel to each other (Fig. 6E, arrowheads).
By contrast, PV fibers were hard to detect in some white matter structures. A PV signal could not be detected in the corpus callosum (Fig. 6F), while in the anterior part of the anterior commissure (Fig. 6G) there were only some thin, sparse PV fibers. In the fornix (Fig. 6H) there were only some PV puncta which were probably the cross-sections of isolated fibers.
The Origins of Long-Range PV Projections in the CPu
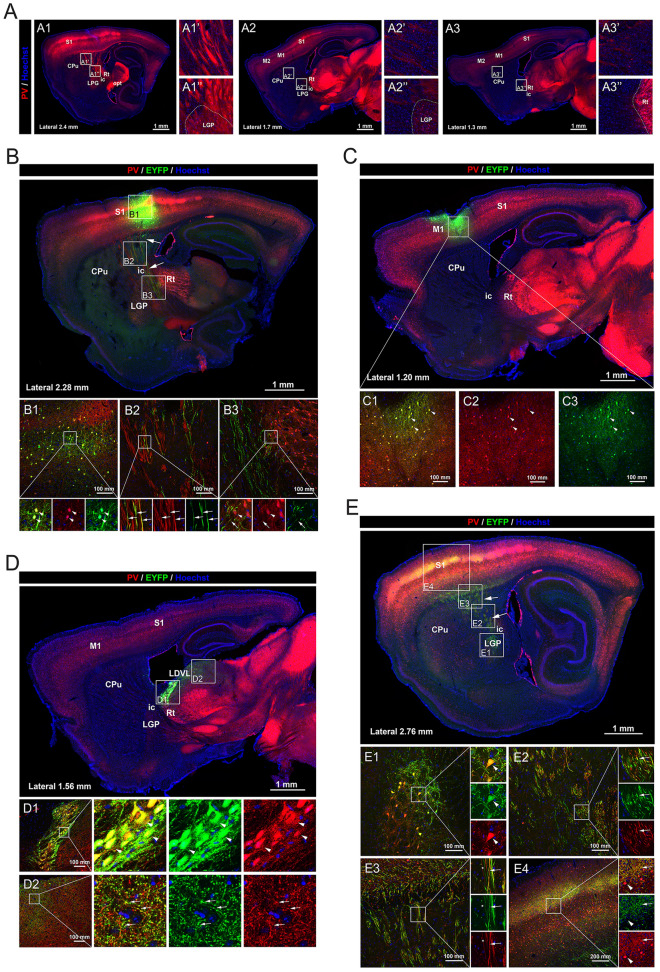
In the previous section, we observed thick myelinated PV fibers that were orthogonal to the coronal sections of the CPu (Fig. S2). This inspired us to hypothesize that these fibers might be long-range projections rather than local neurites. To test this hypothesis and to further investigate the morphology of these PV fibers, sagittal sections of the CPu from the reporter mice were examined. To our astonishment, we found that in sagittal sections, large PV fiber bundles traveled through the CPu, possibly connecting the S1 or M1/2 cortices and subcortical regions including the LGP or Rt (Fig. 7A). This result confirmed the existence of long-range projection of PV fibers in the CPu and suggested that the origins of these projections might be S1 or M1/2 in the cortex, or LGP or Rt in subcortical fields.
Fig. 7.
The origins of the long-range PV projections in the caudate-putamen. A Sagittal sections showing the long-range PV projections in the caudate-putamen and their surrounding structures. B Sagittal section at lateral 2.28 mm showing the distribution and co-localization with PV signals of EYFP-positive signals in PV-tdTomato reporter mice injected with DIO-EYFP virus in S1. EYFP is expressed in the PV somata at the injection site in S1 (arrowheads in B1) and the long-range PV projections in the caudate-putamen (arrows in B2). EYFP projections terminate at the Rt in the thalamus (arrows in B3), surrounding PV somata in the Rt (arrowheads in B3). C Sagittal section at lateral 1.20 mm showing the distribution and co-localization with PV signals of EYFP-positive signals in PV-tdTomato reporter mice injected with DIO-EYFP virus at M1. EYFP signals are limited to the PV neurons at the injection site and surrounding local PV fibers as shown in the magnifications. D Sagittal section at lateral 1.56 mm showing the distribution and co-localization with PV signals of EYFP-positive signals in PV-tdTomato reporter mice injected with DIO-EYFP virus into the Rt. EYFP is expressed in the PV somata at the injection site in the Rt (arrowheads in D1) and PV fibers in the LDVL in the dorsal thalamus (arrows in D2). E Sagittal section at lateral 1.20 mm showing the distribution and co-localization with PV signals of EYFP-positive signals in PV-tdTomato reporter mice injected with DIO-EYFP virus into the LGP. EYFP is expressed in the PV somata at the injection site in the LGP (arrowheads in E1), in a proportion of the long-range PV fibers in the internal capsule (arrows in E2) and the caudate-putamen (arrows in E3), as well as in PV fiber networks in layer IV of S1 (arrows in E4). PV somata at S1 are not co-localized with EYFP (arrowheads in E4). Abbreviations: S1 primary somatosensory cortex; M1/2 primary and secondary motor cortex; CPu caudate putamen; LGP lateral globus pallidus; ic internal capsule; Rt reticular thalamic nucleus; LDVL laterodorsal thalamic nucleus, ventrolateral part. Scale bars, 1 mm (A–E), 100 μm (B1–B3, C1–C3, D1–D2, E1–E3), 200 μm (E4). n = 3 mice.
To verify the possible origins of the long-range PV projection in CPu, adeno-associated virus carrying a DIO-EYFP component was independently injected into S1, M1, Rt, and LGP of the PV-tdTomato reporter mice, and the distribution of EYFP-positive somata and their projecting fibers were observed. In mice with the virus injected at S1 (Fig. 7B), EYFP was finely colocalized with PV somata at the injection site (Fig. 7B1, arrowheads). Importantly, we detected EYFP-positive long-range projections in the CPu colocalizing with part of the PV fibers (Fig. 7B2, arrows), suggesting that these EYFP-positive long-range PV fibers were projected from PV somata in S1. We also found that these EYFP-positive projections terminated around the PV somata in the Rt (Fig. 7B3, arrowheads for PV somata and arrows for PV fiber terminals). By contrast, in mice with the virus injected at M1, EYFP signals were confined to the injection site, colocalizing with local PV somata and PV fiber networks (Fig. 7C). In mice with the virus injected at Rt (Fig. 7D), EYFP was expressed in PV somata within Rt (Fig. 7D1, arrowheads). Interestingly, EYFP was also detected in PV fiber networks in the LDVL of the dorsal thalamus (Fig. 7D2, arrows), suggesting that PV somata in Rt projected towards the dorsal thalamus. In mice with the virus injected at LGP (Fig. 7E), EYFP was expressed in PV somata within the LGP (Fig. 7E1). In the CPu, a large proportion of long-range PV projections expressed EYFP (Fig. 7E2–E3, arrows), suggesting the LGP origin of these long-range PV projections. To our surprise, the EYFP-positive fibers terminated in extensive areas in the S1, mainly colocalizing with PV-positive fibers in layer IV (Fig. 7E4, arrowheads denote PV somata in S1 without EYFP expression and arrows denote the colocalization of EYFP and PV fibers). Taken together, we conclude that the long-range PV projections in the CPu have two components. One originates from LGP and projects upward to an extensive region in S1, and the other originates from S1 and projects downward to the thalamus.
Alterations of Hippocampal PV Fibers in the Li-Pilocarpine Model of SE
PV signals in many regions were characterized by the contrast between the few somata versus the abundant fibers, such as in the hippocampus (Fig. 5B–D, B1–D1), the CPu (Fig. 4C–F and C1–F1), and the substantia nigra (SN, in Fig. 3H). Thus, routine methods for detecting somata, such as immunohistochemistry, may not be sensitive enough to detect subtle alterations in fibers in these regions under pathological conditions. We then took advantage of these PV reporter mice to assess the fiber changes in the Li-pilocarpine model of TLE and the MPTP model of PD, in which the hippocampus and the SN were injured, respectively. The successful establishment of the TLE model was confirmed by the induction of SE behavior and activation of astrocytes in the hippocampus 3 days after model establishment (Fig. S3). The successful establishment of the PD model was confirmed by jeopardized motor coordination in the pole test 7 days after modeling and by the activation of astrocytes in the SN 3 days after modeling (Fig. S4).
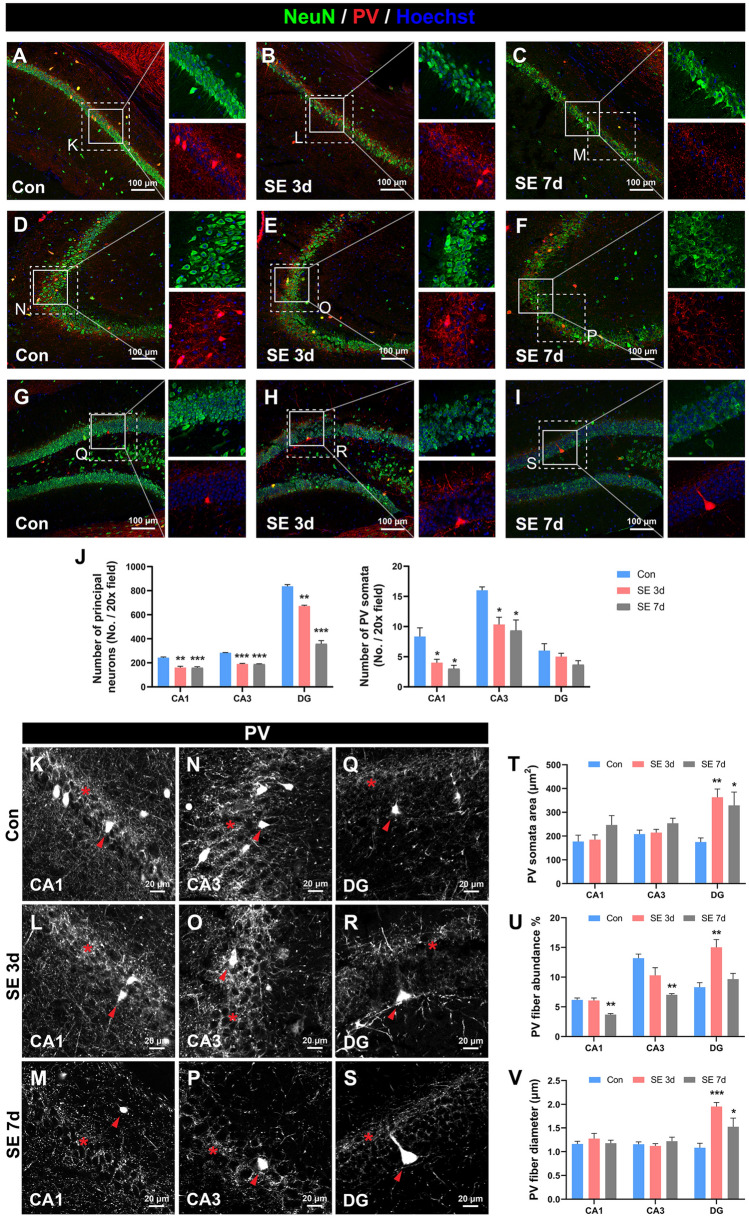
Hippocampal PV interneurons are vulnerable in epilepsy [17, 18, 42] but the alteration of PV fibers has not been reported. We assessed the hippocampal PV fluorescence at 3 days and 7 days after the induction of SE. As expected, SE caused a significant decrease in the number of excitatory principal neurons, including pyramidal neurons in CA1 (Fig. 8A–C, J) and CA3 (Fig. 8D–F, J), and granule cells in the dentate gyrus (DG) (Fig. 8G–I, J) at both 3 days and 7 days after SE. By contrast, the alterations of PV somata are region-dependent. SE induced a significant reduction in the number of PV somata in CA1 (Fig. 8A–C, J) and CA3 (Fig. 8D–E, J), but spared the number of PV somata in the DG (Fig. 8G–I, J). Interestingly, we found that the area of PV somata in the DG significantly increased at 3 days and 7 days after SE (Fig. 8Q–T). As for PV fibers, in CA1 and CA3, the abundance of PV fibers remained unchanged at 3 days after SE but significantly decreased at 7 days after SE, suggesting reduced PV fiber network complexity and jeopardized PV arborization (Fig. 8K–P, U). Interestingly, the abundance of PV fibers in the DG significantly increased at 3 days after SE but returned to baseline at 7 days after SE (Fig. 8Q–S, U). The diameters of PV fibers were also measured. In CA1 and CA3, PV fiber diameters were unchanged after SE (Fig. 8K–P, V). However, PV fiber diameter in the DG significantly increased at both 3 days and 7 days after SE (Fig. 8Q–S, V). Overall, although the abundance of PV fibers was reduced in CA1 and CA3, the case in the DG was different. The increases in PV somata area, PV fiber diameter, and PV fiber abundance together suggested a hypertrophic reaction of PV interneurons in the DG after the hyperexcitability insults of SE.
Fig. 8.
Alteration of PV fibers in the mouse model of SE. A–C Representative images showing the changes in CA1 pyramidal neurons and PV interneurons at different time points after SE [control (Con), 3 days post-SE (SE 3d), and 7 days post-SE (SE 7d)]. D–F Representative images showing the alterations in CA3 pyramidal neurons and PV interneurons at different time points after SE (control, 3- and 7-days post-SE). G–I Representative images showing the changes in DG granule cells and PV interneurons at different time points after SE (control, 3- and 7-days post-SE). J Statistical analyses of the number of NeuN-positive principal neurons (left) and the number of PV somata (right) at different subfields and time points after SE. One-way ANOVA with Dunnett’s multiple comparisons. *P <0.05, **P <0.01, **P <0.001, versus control group. n = 3 mice per group. K–S Enlarged high-contrast images from the dashed-line boxes in A–I demonstrating the morphological characteristics of PV somata and PV fibers, with asterisks denoting PV fiber networks and arrowheads denoting PV somata. T Statistical analyses of the area of individual PV somata. n = 8 somata from 3 mice per group. U Statistical analyses of the PV fiber abundance. n = 3 mice per group. V Statistical analyses of the PV fiber diameter. n = 15 PV fibers from 3 mice per group. One-way ANOVA with Dunnett’s multiple comparisons in T–V. *P <0.05, **P <0.01, ***P <0.001, vs control group. Abbreviations: Con control; SE status epilepticus. Scale bars, 100 μm (A–I); 20 μm (K–S).
Alterations of PV Fibers in the CPu and SN in the MPTP Model of PD
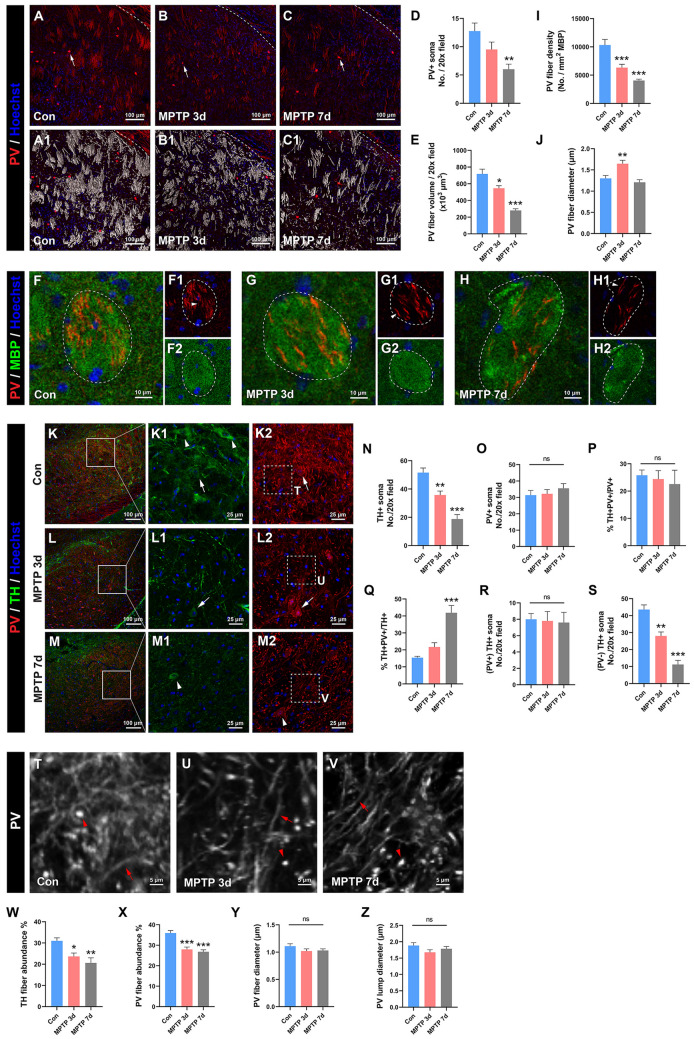
We next investigated the vulnerability of PV somata and fibers in the MPTP model of PD, focusing on their pathological changes in the CPu and SN. In the CPu, the number of PV somata was unchanged at 3 days after modeling but significantly decreased at 7 days after modeling (Fig. 9A–D, arrows). Interestingly, we noted remarkable morphological alterations in the myelinated long-range PV projections in the CPu after PD. Three-dimensional reconstruction analysis revealed that the volume of these PV projections significantly decreased after PD in a time-dependent manner (Fig. 9A1–C1, E). Moreover, the density of PV fibers in each MBP-positive fiber tract also became significantly looser after PD in a time-dependent manner (Fig. 9F–H, I). Further analyses of these PV fibers revealed that their diameter underwent a temporary increase at 3 days after PD and returned to baseline at 7 days after PD (Fig. 9F1, G1, H1, J; arrowheads). We then observed the changes of PV signals in the SN in the MPTP models. As expected, TH cells in the SN significantly decreased after PD in a time-dependent manner (Fig. 9K–M, K1–M1 arrowheads, N). However, the number of PV somata was unaltered after PD (Fig. 9K2–M2, O). Surprisingly, colocalization analyses revealed that TH+ and PV+ somata showed some colocalization in the SN, with 25.81% ± 1.94% of PV+ somata also expressing TH (Fig. 9P) and 15.46% ± 0.77% of TH+ somata also expressing PV (Fig. 9Q). As the PD progressed, the proportion of TH+PV+/PV+ neurons remained unchanged (Fig. 9P) while the proportion of TH+PV+/TH+ neurons increased and reached statistical significance at 7 days after modeling (Fig. 9Q). Further analyses revealed that the number of PV/TH double-positive cells was unchanged after PD (Fig. 9R), but the number of PV-negative TH-positive cells significantly declined in a time-dependent manner after PD (Fig. 9S) which underlay the rising proportion of TH+PV+/TH+ neurons in Fig. 9Q. Taken together, these results suggest that in the SN, TH somata are more vulnerable than PV somata after the attack of PD. Meanwhile, the abundance of TH fibers (Fig. 9K1–M1) and PV fibers (Fig. 9K2–M2) both significantly declined after PD (Fig. 9W–X), suggesting that the complexity of TH and PV fiber networks decreases after PD. We noted that in the SN, PV fibers could be classified into two categories according to their morphological characteristics. One was the PV fibers with long thin morphology (Fig. 9T–V arrows) and the other was the thick bright PV clumps (Fig. 9T–V arrowheads). We measured each of their diameters and found no significant change in either the diameter of the thin PV fibers or that of the PV clumps (Fig. 9Y–Z). Taken together, these results in the CPu and SN demonstrate that, unlike PV somata, PV fibers are rather vulnerable in PD, which provides more information about PD pathology than the observation of PV somata only.
Fig. 9.
Alteration of PV fibers in the mouse MPTP model of PD. A–C Representative images showing the PV signals in the caudate-putamen at different time points after model establishment [control (Con), 3 days post-MPTP (MPTP 3d), and 7 days post-MPTP (MPTP 7d)]. Arrows in A–C denote the PV somata. A1–C1 3D reconstruction of the volume of PV fibers from A–C, respectively. D, E Statistical analyses of the number of PV somata (D) and PV fiber volume in the caudate-putamen (E). n = 4 mice per group. F Statistical analysis showing the PV fiber density per MBP-positive myelinated fiber tract in the caudate-putamen in each group. n = 16 MBP-positive fiber tracts from 4 mice per group. G Statistical analysis showing the PV fiber diameter in the MBP-positive fiber tracts in the caudate-putamen in each group. n = 16 PV fibers from 4 mice per group. H–J Representative images demonstrating the location and morphological characteristics of PV fibers within the MBP-positive fiber tract in the caudate-putamen. Arrowheads denote individual PV fibers. n = 4 mice per group. K–M Representative images showing the co-localization and changes in TH-positive neurons and PV-positive neurons in the substantia nigra at different time points after model establishment (control, 3- and 7-days post-MPTP). Arrowheads in K1–M1 denote TH-positive neurons while arrowheads in K2–M2 denote PV-positive neurons. Arrows in K1, K2, L1, and L2 indicate the co-localization of PV and TH somata. N, O Statistical analyses showing the number of TH+ somata and PV+ somata in the substantia nigra. n = 4 mice. P, Q Statistical analyses showing the proportion of neurons with both PV- and TH-positive in the total PV population (P) and TH population (Q). n = 4 mice. R, S Statistical analyses showing the number of neurons with both PV- and TH-positive (R) and with PV-negative but TH-positive (S) in the substantia nigra. n = 4 mice. T–V Enlarged and high-contrast images from dashed-line boxes in K2–M2 showing the morphological characteristics of PV fibers in the substantia nigra. Arrowheads denote the PV-positive clumps and arrows denote PV fibers. W, X Statistical analyses showing the TH fiber abundance and PV fiber abundance in the substantia nigra. n = 4 mice per group. Y, Z Statistical analyses showing the diameter of PV fiber and PV clumps in the substantia nigra. n = 20 fibers (Y) and 20 clumps (Z) from 4 mice per group. One-way ANOVA with Dunnett’s multiple comparisons in E–G, N–S, and W–Z. *P <0.05, **P <0.01, ***P <0.001, ns, no statistically significant difference, vs the control group. Abbreviations: Con control; MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH tyrosine hydroxylase. Scale bars, 100 μm (A–C, A1–C1, K–M), 10 μm (F−H), 25 μm (K1–M1, K2–M2), 5 μm (T–V).
Discussion
In this study, by using PV-tdTomato reporter mice, we thoroughly examined the distribution of PV fibers in the mouse brain. Above all, the most prominent PV signals were found in the cortex (especially the somatosensory cortex), the thalamus, and the ml, which are all structures involved in the conduction and processing of sensory information [43–46]. The high density of PV fibers in these regions indicated the vital role of the PV system in the regulation of sensory information. Moreover, PV fibers were not only distributed in the grey matter, but were also detected in typical white matter such as the cp, and opt. Particularly, we demonstrated myelinated long-range PV projections in the CPu, connecting S1 and the thalamus downward, and connecting the LGP and S1 upward. In the hippocampus of the mouse model of TLE and the CPu and SNR of the mouse model of PD, PV fibers demonstrated subtle morphological changes. Taken together, the current study provides an overview of the spatial atlas of PV fibers in the mouse brain and the pathological alterations of PV signals in disease models of TLE and PD, providing a reference for future studies of the functions and plasticity of PV interneurons and their arborizations.
Although the morphological features of PV fibers varied among different brain regions, commonly shared features could be abstracted into 7 categories, which we named as follows: (1) Polarized, (2) Basket-like, (3) Beads-on-string, (4) Smooth reticular formation (SRF), (5) Rough reticular formation (RRF), (6) Parallel cable, and (7) Large bundle (Fig. S5). Polarized PV fibers clearly extended from the soma in a definite direction (Fig. S5A). They were mainly detected in S1/2, MO, and IL of the cortex, and the DG of the hippocampus, and they were the dendritic shafts of local PV interneurons. Basket-like PV fibers extended from the soma with a basket-like arborization (Figs S5B and 4G–I). They were solely detected in PV interneurons of the CPu. Basket-like PV arborization represents the extensive inhibitory regulation by PV interneurons of medium spiny neurons, the dominant output neurons of the CPu [47]. Beads-on-string PV fibers were noted in the MS, with long PV fibers going dorsally/ventrally with embedded PV somata (Figs S5C and 5F). We surmised that these PV interneurons were strung successively, playing the role of a relay hub of information. The SRF is the most frequently seen morphological feature with PV fibers, characterized by a network structure formed by thin PV fibers around the somata (Fig. S5D). They were detected in M1/2, PrL, and Cg1/2 of the cortex, Rt, MD, and CM in the thalamus, midbrain brain nuclei except for the SN, subfields of the hippocampus, and subfields of the amygdala. These fine, thin PV fibers represent the widespread and coordinated regulation of local principal neurons, subsequently controlling the signal output. In the RRF PV fibers are network structures formed by thick, irregular, and varicose PV fibers, mixed with PV-positive clumps and somata (Fig. S5E). This form of PV fibers is mostly seen in the thalamus, including nuclei of the VPL/VPM, LDVL/LPMR, Sub, VL, and Po, as well as the LGP and SNR. The exact composition and origin of these rough reticular structures are still enigmatic. Based on their morphology, we suggest that they are a mixture of long-range PV fibers and local thin networks, on the one hand representing the long-range projection of PV interneurons, and on the other hand representing local PV regulation of other neurons. The parallel cable is a thick, isolated, and parallel PV fiber that goes a long distance (Fig. S5F). They were noted within the white matter structures of the fmi, ec, ic, and sm, and they are long-range fibers extending from distant somata. The large bundle is a dense fiber bundle formed by densely-packed PV fibers (Fig. S5G). PV fibers in the opt and cp belong to this category. PV interneurons are the major type of GABAergic interneurons, accounting for ~ 40% of all cortical GABAergic interneurons [1]. The different morphological features of PV fibers in different brain regions reflect the diverse functional implications of PV neurons.
PV is conventionally regarded as the chemical marker of fast-spiking PV interneurons, which provide both feedforward and feedback inhibition of local circuit activity [2]. However, recent advances have revealed that PV-containing neurons can also send long-range projections, challenging the conventional concept. In their study, Zhang et al. [48] found that PV-positive neurons in the subthalamic nucleus send long-range and excitatory projections to the SNR and globus pallidus. Their findings are enlightening in that PV-containing neurons, which are traditionally regarded as inhibitory interneurons, express the excitatory glutamate transporter VGLUT3 and send long-range excitatory projections [48]. Moreover, Wang et al. [49] reported that PV-positive neurons in the ventral zona incerta send long-range projections to the posterior complex of the thalamus, and cannabinoid type 1 receptors expressed at the axon terminals of these projections regulate nocifensive behaviors. In the current study, we also observed long-range PV projections, especially those traveling through the CPu. We found that these long-range PV projections had at least two components, one originating from PV-positive neurons in S1 and downwardly projecting to the Rt in the thalamus, the other originating from PV-positive neurons in the LGP and upwardly projecting to extensively to layer IV in S1. Although currently we could not determine the exact function of these long-range projections, we surmise that they are involved in the sensory information possessing, since S1 is deeply involved in processing sensory information from various parts of the body [50–52], as well as emotion regulation [50] and the integration of motor and sensory signals [53]. Besides these, we also detected long-range PV projections originating from PV-positive neurons in the Rt towards the LDVL in the dorsal thalamus. Taken together, our results provide solid evidence of the existence of long-range PV projections in the mouse brain.
An increasing number of studies have revealed the myelination of PV interneurons [54]. In non-human primates, myelinated PV interneurons have been detected in the somatosensory cortex [54], visual cortex [55, 56], entorhinal cortex [57], hippocampus [58, 59], and striatum [60]. Specifically, in the somatosensory cortex of mice, 25%–50% of myelinated axons are GABAergic, nearly all of which are PV-positive [61]. Myelination not only speeds up action potential (AP) propagation and optimizes the fidelity of APs in PV interneurons, but also provides metabolic and trophic support to facilitate the energy status of PV interneurons for their high-frequency activity and gamma oscillation [54, 62, 63]. Here, we found that some PV fibers in the somatosensory cortex were myelinated. We also noted that in the striatum, PV fibers run along the thick myelinated fiber tract. The myelination of the PV fiber tract further supported the long-range projection and the signal transduction of PV fibers.
Another key feature of the dendrites of PV interneurons is their interconnection via gap junctions [60, 64–67]. Gap junctions allow PV interneurons to detect inputs with a wider spatial range, including inputs that are unconnected to a certain PV interneuron but are connected to adjacent interneurons [12]. Moreover, gap junctions between PV interneurons speed up the transmission of their fast-spiking activity and provide rapid postsynaptic depolarization followed by delayed synaptic inhibition. This mechanism facilitates the gamma oscillation of the interneuron networks [3]. We observed that PV fibers were intertwined throughout the whole mouse brain with regional characteristics. The existence of gap junctions raises the possibility that PV interneurons in a certain brain region comprise a regional-scale syncytial organization, and even the possibility that PV interneurons between different regions connect to form a massive, whole-brain-scale syncytial organization, rendering a comprehensive and coordinated regulation of the central nervous system.
PV interneurons are vulnerable under pathological conditions [14]. Epilepsy, characterized by a lasting predisposition to generate spontaneous seizures and comorbidity of cognitive and psychosocial consequences, is one of the most common brain disorders, affecting >70 million people worldwide [68]. PV interneurons are highly vulnerable to epilepsy. Consistent with previous reports [17, 18, 42, 69], we found that SE induced a significant decrease in the number of PV interneurons in the CA1 and CA3 subfields of the hippocampus but not in the DG. Another interesting finding in the current study is the hypertrophic alteration of PV somata and fibers in the DG subfield after SE, suggesting a reactive response of PV interneurons after a seizure attack. As previously reported, PV interneurons provide potent inhibitory regulation within local networks [70] and have antiepileptic effects in models of epilepsy and hyperexcitability [71–75]. Whether the hypertrophic alteration of PV fibers after SE is a protective adaption or merely pathological damage remains to be elucidated. Single-cell RNA sequencing of PV interneurons after SE might provide more clues.
PD is the second most common neurodegenerative disease [76]. One of the crucial pathological features of PD is the loss of dopaminergic neurons within the SN [77, 78], which is considered to be the cause of motor symptoms [79]. Apart from dopaminergic neurons, PV interneurons have also been reported to be vulnerable in the nigral-striatal pathway in PD. In the MPTP models of PD, the number of nigral PV interneurons has been reported to decrease within one week after PD induction while that of striatal PV interneurons remains unchanged [19, 20]. In the 6-hydroxydopamine hydrobromide (6-OHDA) model of PD, one study reported that the number of PV interneurons in the striatum decreased four weeks after PD induction [80], while another study reported that the number of PV interneurons remained unchanged around two weeks after 6-OHDA injection [81]. In our MPTP model of PD, although the number of SNR TH somata significantly declined after PD induction, the number of PV somata remained unchanged. By contrast, the complexity of both PV fiber and TH fiber networks significantly decreased. These results suggest that TH-positive neuronal somata are more vulnerable than PV-positive neuronal somata in PD, while fibers of both TH and PV neurons are affected by PD lesions. In the current study, the immunohistochemistry of TH was inefficient for visualizing TH fibers, which limited us from analyzing the colocalization of TH and PV fibers. Further studies where TH and PV fibers are simultaneously visualized, such as TH virus-driven visualization in the PV-Cre-tdTomato system, are warranted to objectively investigate the colocalization of TH and PV fibers. In the PD model, we also found a significant decrease in the PV fiber volume and density in the thick PV fiber tracts in the striatum, suggesting impaired signal transduction along these long-range PV projections. Taken together, our results suggest that PV fibers are more vulnerable than somata in the MPTP model of PD. The pathological relevance of PV fiber alteration with motor symptoms in PD remains to be elucidated in future studies.
Several limitations in this study should be noted. Firstly, the current study was conducted based on selected coronal brain sections. This inevitably led to a biased selection of brain regions and made it hard to track long fibers without interruption. Techniques such as fluorescent micro-optical sectioning tomography would definitely facilitate a more comprehensive, detailed, and tridimensional observation. Moreover, the current study mainly focused on the PV fibers in the forebrain and midbrain. Future studies investigating PV fibers in more caudal parts such as the brainstem and cerebellum should be fruitful. Secondly, instead of visualizing the PV fibers of a single neuron, we visualized the local PV fiber networks formed by a group of PV interneurons. This provided a measure of the local PV network, but in some brain regions with dense networks, it was hard to distinguish PV fibers extending from a certain individual soma. Sparse labeling of neurons by a fluorescent vector virus with PV-specific promoters might help with this dilemma. Lastly, as discussed above, some of the fibers of PV interneurons are highly myelinated. In the current study, we did not focus on the myelination of PV fibers throughout the brain, thus we did not measure the percentage of myelinated PV fibers. Further studies demonstrating the myelination of PV fibers in detail throughout the brain would provide more information.
In conclusion, in this study, we thoroughly observed the distribution of PV fibers throughout the mouse brain and the pathological alterations of PV fibers in models of TLE and PD. We not only found locally-intertwined PV fiber networks and long-range PV projections, but also demonstrated the SE- and PD- induced changes in PV fibers. This work provides an overview of PV fibers in the mouse brain, with the cortex (especially the somatosensory cortex) and thalamus having the most intense PV signals. Our work also demonstrated the feasibility of visualizing the pathological changes of PV fibers under disease conditions with the aid of Cre-based fluorescent transgenic mice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study is supported by the Ministry of Science and Technology of China (2021ZD0201005), the National Natural Science Foundation of China (82071529 and 81974204), and the Key R&D Program of Shaanxi Province (2021SF-238).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Changgeng Song, Yan Zhao, and Jiajia Zhang contributed equally to this work.
Change history
8/28/2023
A Correction to this paper has been published: 10.1007/s12264-023-01105-x
Contributor Information
Wen Jiang, Email: jiangwen@fmmu.edu.cn.
Shengxi Wu, Email: shengxi@fmmu.edu.cn.
Fang Gao, Email: fanggao@fmmu.edu.cn.
References
- 1.Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345: 1255263. [DOI] [PubMed]
- 3.Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–681. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsáki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBeau FEN, Traub RD, Monyer H, Whittington MA, Buhl EH. The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res Bull. 2003;62:3–13. doi: 10.1016/j.brainresbull.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsch C, Scotti AL, Nitsch FM. Distribution of parvalbumin-containing interneurons in the hippocampus of the gerbil—a qualitative and quantitative statistical analysis. J Chem Neuroanat. 1995;9:135–147. doi: 10.1016/0891-0618(95)00076-J. [DOI] [PubMed] [Google Scholar]
- 8.Nassar M, Simonnet J, Lofredi R, Cohen I, Savary E, Yanagawa Y, et al. Diversity and overlap of parvalbumin and somatostatin expressing interneurons in mouse presubiculum. Front Neural Circuits. 2015;9:20. doi: 10.3389/fncir.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delevich K, Tucciarone J, Huang ZJ, Li B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci. 2015;35:5743–5753. doi: 10.1523/JNEUROSCI.4565-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drexel M, Preidt AP, Kirchmair E, Sperk G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–329. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan RL, Wilson CJ, Emson PC, Heizmann CW. Parvalbumin-containing GABAergic interneurons in the rat neostriatum. J Comp Neurol. 1990;302:197–205. doi: 10.1002/cne.903020202. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Okamoto M, et al. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience. 2017;359:196–208. doi: 10.1016/j.neuroscience.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Ruden JB, Dugan LL, Konradi C. Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology. 2021;46:279–287. doi: 10.1038/s41386-020-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahar L, Delacroix BM, Nam HW. The role of parvalbumin interneurons in neurotransmitter balance and neurological disease. Front Psychiatry. 2021;12:679960. doi: 10.3389/fpsyt.2021.679960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ábrahám H, Molnár JE, Sóki N, Gyimesi C, Horváth Z, Janszky J, et al. Etiology-related degree of sprouting of parvalbumin-immunoreactive axons in the human dentate gyrus in temporal lobe epilepsy. Neuroscience. 2020;448:55–70. doi: 10.1016/j.neuroscience.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Soukupová M, Binaschi A, Falcicchia C, Zucchini S, Roncon P, Palma E, et al. Impairment of GABA release in the hippocampus at the time of the first spontaneous seizure in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2014;257:39–49. doi: 10.1016/j.expneurol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, et al. Cerebral alterations in a MPTP-mouse model of Parkinson’s disease—an immunocytochemical study. J Neural Transm (Vienna) 2003;110:1129–1144. doi: 10.1007/s00702-003-0021-y. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Muramatsu Y, Kurosaki R, Michimata M, Matsubara M, Imai Y, et al. Protective effects of neuronal nitric oxide synthase inhibitor in mouse brain against MPTP neurotoxicity: An immunohistological study. Eur Neuropsychopharmacol. 2004;14:93–104. doi: 10.1016/S0924-977X(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 21.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro P, Barak B, Zhou Y, McRae R, Rodrigues D, Wickersham IR, et al. Dichotomous parvalbumin interneuron populations in dorsolateral and dorsomedial striatum. J Physiol. 2018;596:3695–3707. doi: 10.1113/JP275936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Huang L, Chen L, Hao D, Chen J. Neuroprotection by tetrahydroxystilbene glucoside in the MPTP mouse model of Parkinson’s disease. Toxicol Lett. 2013;222:155–163. doi: 10.1016/j.toxlet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. pp. 63–110. [Google Scholar]
- 27.DeFelipe J, Jones EG. Parvalbumin immunoreactivity reveals layer IV of monkey cerebral cortex as a mosaic of microzones of thalamic afferent terminations. Brain Res. 1991;562:39–47. doi: 10.1016/0006-8993(91)91184-3. [DOI] [PubMed] [Google Scholar]
- 28.Kharazia VN, Weinberg RJ. Glutamate in thalamic fibers terminating in layer IV of primary sensory cortex. J Neurosci. 1994;14:6021–6032. doi: 10.1523/JNEUROSCI.14-10-06021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ZH, Chang MH, Yang JW, Sun JJ, Lee HC, Shyu BC. Layer IV of the primary somatosensory cortex has the highest complexity under anesthesia and cortical complexity is modulated by specific thalamic inputs. Brain Res. 2006;1082:102–114. doi: 10.1016/j.brainres.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 30.Ghazanfar AA, Nicolelis MAL. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex. 1999;9:348–361. doi: 10.1093/cercor/9.4.348. [DOI] [PubMed] [Google Scholar]
- 31.Killackey HP, Koralek KA, Chiaia NL, Rhodes RW. Laminar and areal differences in the origin of the subcortical projection neurons of the rat somatosensory cortex. J Comp Neurol. 1989;282:428–445. doi: 10.1002/cne.902820309. [DOI] [PubMed] [Google Scholar]
- 32.Koralek KA, Olavarria J, Kellackey HP. Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol. 1990;299:133–150. doi: 10.1002/cne.902990202. [DOI] [PubMed] [Google Scholar]
- 33.Schubert D, Staiger JF, Cho N, Kötter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 2001;21:3580–3592. doi: 10.1523/JNEUROSCI.21-10-03580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro-Orozco D, Bollu PC. Neuroanatomy, Medial Lemniscus (Reils Band, Reils Ribbon) Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 35.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal Ganglia: A critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 36.Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal Ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- 38.Antonoudiou P, Tan YL, Kontou G, Upton AL, Mann EO. Parvalbumin and somatostatin interneurons contribute to the generation of hippocampal gamma oscillations. J Neurosci. 2020;40:7668–7687. doi: 10.1523/JNEUROSCI.0261-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Detari L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–277. doi: 10.1016/S0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 40.Zahm DS. The evolving theory of basal forebrain functional—Anatomical ‘macrosystems’. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Záborszky L, Gombkoto P, Varsanyi P, Gielow MR, Poe G, Role LW, et al. Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J Neurosci. 2018;38:9446–9458. doi: 10.1523/JNEUROSCI.1676-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuruba R, Hattiangady B, Parihar VK, Shuai B, Shetty AK. Differential susceptibility of interneurons expressing neuropeptide Y or parvalbumin in the aged hippocampus to acute seizure activity. PLoS One. 2011;6:e24493. doi: 10.1371/journal.pone.0024493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood KC, Blackwell JM, Geffen MN. Cortical inhibitory interneurons control sensory processing. Curr Opin Neurobiol. 2017;46:200–207. doi: 10.1016/j.conb.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol. 2002;539:567–578. doi: 10.1113/jphysiol.2001.013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Sabri MM, Arabzadeh E. Information processing across behavioral states: Modes of operation and population dynamics in rodent sensory cortex. Neuroscience. 2018;368:214–228. doi: 10.1016/j.neuroscience.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Tepper JM, Koós T, Ibanez-Sandoval O, Tecuapetla F, Faust TW, Assous M. Heterogeneity and diversity of striatal GABAergic interneurons: Update 2018. Front Neuroanat. 2018;12:91. doi: 10.3389/fnana.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Roy DS, Zhu Y, Chen Y, Aida T, Hou Y, et al. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature. 2022;607:321–329. doi: 10.1038/s41586-022-04806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Dong P, He C, Feng XY, Huang Y, Yang WW, et al. Incerta-thalamic circuit controls nocifensive behavior via cannabinoid type 1 receptors. Neuron. 2020;107:538–551.e7. doi: 10.1016/j.neuron.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Kropf E, Syan SK, Minuzzi L, Frey BN. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz J Psychiatry. 2019;41:261–269. doi: 10.1590/1516-4446-2018-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013;503:51–58. doi: 10.1038/nature12654. [DOI] [PubMed] [Google Scholar]
- 53.Petersen CCH. Sensorimotor processing in the rodent barrel cortex. Nat Rev Neurosci. 2019;20:533–546. doi: 10.1038/s41583-019-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stedehouder J, Kushner SA. Myelination of parvalbumin interneurons: A parsimonious locus of pathophysiological convergence in schizophrenia. Mol Psychiatry. 2017;22:4–12. doi: 10.1038/mp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somogyi P, Soltész I. Immunogold demonstration of GABA in synaptic terminals of intracellularly recorded, horseradish peroxidase-filled basket cells and clutch cells in the cat’s visual cortex. Neuroscience. 1986;19:1051–1065. doi: 10.1016/0306-4522(86)90122-3. [DOI] [PubMed] [Google Scholar]
- 57.Wouterlood FG, Härtig W, Brückner G, Witter MP. Parvalbumin-immunoreactive neurons in the entorhinal cortex of the rat: Localization, morphology, connectivity and ultrastructure. J Neurocytol. 1995;24:135–153. doi: 10.1007/BF01181556. [DOI] [PubMed] [Google Scholar]
- 58.Brauer K, Härtig W, Gärtner U, Brückner G, Arendt T. Different myelination of rat septohippocampal fibres as revealed by immunofluorescence double-labelling. Brain Res. 2000;878:188–193. doi: 10.1016/S0006-8993(00)02653-6. [DOI] [PubMed] [Google Scholar]
- 59.Gärtner U, Härtig W, Brauer K, Brückner G, Arendt T. Electron microscopic evidence for different myelination of rat septohippocampal fibres. Neuroreport. 2001;12:17–20. doi: 10.1097/00001756-200101220-00011. [DOI] [PubMed] [Google Scholar]
- 60.Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: A light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-S. [DOI] [PubMed] [Google Scholar]
- 61.Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife. 2016;5:e15784. doi: 10.7554/eLife.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci. 2001;21:2687–2698. doi: 10.1523/JNEUROSCI.21-08-02687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 67.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 68.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 69.Cameron S, Lopez A, Glabman R, Abrams E, Johnson S, Field C, et al. Proportional loss of parvalbumin-immunoreactive synaptic boutons and granule cells from the hippocampus of sea lions with temporal lobe epilepsy. J Comp Neurol. 2019;527:2341–2355. doi: 10.1002/cne.24680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson BR, Gao WJ. PV interneurons: Critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37. doi: 10.3389/fncir.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fabene PF, Andrioli A, Priel MR, Cavalheiro EA, Bentivoglio M. Fos induction and persistence, neurodegeneration, and interneuron activation in the hippocampus of epilepsy-resistant versus epilepsy-prone rats after pilocarpine-induced seizures. Hippocampus. 2004;14:895–907. doi: 10.1002/hipo.20003. [DOI] [PubMed] [Google Scholar]
- 72.Khan AA, Shekh-Ahmad T, Khalil A, Walker MC, Ali AB. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br J Pharmacol. 2018;175:2097–2115. doi: 10.1111/bph.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen W, Luo B, Gao N, Li H, Wang H, Li L, et al. Neddylation stabilizes Nav1.1 to maintain interneuron excitability and prevent seizures in murine epilepsy models. J Clin Invest 2021, 131: e136956. [DOI] [PMC free article] [PubMed]
- 74.Colasante G, Lignani G, Brusco S, Di Berardino C, Carpenter J, Giannelli S, et al. dCas9-based Scn1a gene activation restores inhibitory interneuron excitability and attenuates seizures in dravet syndrome mice. Mol Ther. 2020;28:235–253. doi: 10.1016/j.ymthe.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Călin A, Ilie AS, Akerman CJ. Disrupting epileptiform activity by preventing parvalbumin interneuron depolarization block. J Neurosci. 2021;41:9452–9465. doi: 10.1523/JNEUROSCI.1002-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 77.Jankovic J, Tan EK. Parkinson’s disease: Etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 78.Li T, Le W. Biomarkers for parkinson’s disease: How good are they? Neurosci Bull. 2020;36:183–194. doi: 10.1007/s12264-019-00433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 80.Gomez G, Escande MV, Suarez LM, Rela L, Belforte JE, Moratalla R, et al. Changes in dendritic spine density and inhibitory perisomatic connectivity onto medium spiny neurons in L-dopa-induced dyskinesia. Mol Neurobiol. 2019;56:6261–6275. doi: 10.1007/s12035-019-1515-4. [DOI] [PubMed] [Google Scholar]
- 81.Ma Y, Zhan M, OuYang L, Li Y, Chen S, Wu J, et al. The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav Brain Res. 2014;266:37–45. doi: 10.1016/j.bbr.2014.02.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.