Abstract
Opioid use disorder (OUD) has become a considerable global public health challenge; however, potential medications for the management of OUD that are effective, safe, and nonaddictive are not available. Accumulating preclinical evidence indicates that antagonists of the dopamine D3 receptor (D3R) have effects on addiction in different animal models. We have previously reported that YQA14, a D3R antagonist, exhibits very high affinity and selectivity for D3Rs over D2Rs, and is able to inhibit cocaine- or methamphetamine-induced reinforcement and reinstatement in self-administration tests. In the present study, our results illustrated that YQA14 dose-dependently reduced infusions under the fixed-ratio 2 procedure and lowered the breakpoint under the progressive-ratio procedure in heroin self-administered rats, also attenuated heroin-induced reinstatement of drug-seeking behavior. On the other hand, YQA14 not only reduced morphine-induced expression of conditioned place preference but also facilitated the extinguishing process in mice. Moreover, we elucidated that YQA14 attenuated opioid-induced reward or reinforcement mainly by inhibiting morphine-induced up-regulation of dopaminergic neuron activity in the ventral tegmental area and decreasing dopamine release in the nucleus accumbens with a fiber photometry recording system. These findings suggest that D3R might play a very important role in opioid addiction, and YQA14 may have pharmacotherapeutic potential in attenuating opioid-induced addictive behaviors dependent on the dopamine system.
Keywords: Opioid use disorder, D3 receptors, Dopamine, Self-administration, Conditioned place preference
Introduction
Opioid abuse is a serious problem worldwide and has major social and public health implications [1]. According to the 2020 Annual Report on Drug Control in China, heroin remains the second most common type of illicit drug, accounting for >37.5% of addiction cases [2]. Several agonists or antagonists (naltrexone, methadone, and buprenorphine) targeting opioid receptors have been developed and used to control withdrawal symptoms and prevent relapse into opioid addiction [3, 4]. They have certain limitations, including limited success in relapse prevention, poor compliance, abuse potential, and depression [5, 6]. The dopamine D3 receptor (D3R) is one of the highlighted targets on the list of the 10 highest-priority pharmacological targets for medications against drug addiction [7]. Therefore, the identification of highly selective antagonists for D3Rs is an important pathway to the regulation of opioid addiction.
D3Rs are mainly expressed in the mesolimbic system compared to dopamine D2 receptors with broader distribution and have the highest affinity for endogenous dopamine of all known dopamine receptor subtypes, which makes them a potential target for drug addiction treatment [8–14]. Because of these important characteristics, D3R antagonists for treating psychostimulant addiction (cocaine and methamphetamine) have been extensively studied [15–18]. Recently, growing evidence has shown that D3R antagonists, such as SB277011A, CAB2-015, BAK4-54, and (±) VK4-116, attenuate opioid-induced addictive behaviors in opioid self-administration and reinstatement [19–21]. Moreover, our previous studies and Li’s work demonstrated that the knockout of D3R blocks the development of behavioral sensitization with morphine [22, 23]. Therefore, D3R antagonists may be therapeutically beneficial for preventing and/or treating OUD.
We have reported that YQA14 is a patented, selective D3R antagonist designed and synthesized at our institute, and exhibits a high affinity for D3R, displaying ~5,000,000-fold (high affinity) and 150-fold (low affinity) selectivity over D2R without binding to opioid receptors (δ, μ, and κ) [17, 24]. Regarding pharmacokinetics, YQA14 shows better acceptable pharmacokinetic properties, possesses a longer half-life than SB-277011A (another D3R antagonist) in rodents, and has better metabolic stability in humans. In pharmacological investigations, researchers have extensively studied YQA14 in cocaine- and methamphetamine-induced self-administration and behavioral sensitization in mice and rats [15, 17, 25]. In particular, researchers have reported the inhibitory effect of YQA14 in cocaine-induced conditioned place preference (CPP) and morphine-induced locomotor behavioral sensitization in wild-type mice, but not in D3R-knockout mice [16, 22]. Furthermore, YQA14 is also effective in attenuating the expression and preventing the drug-primed relapse of morphine-induced CPP in rats [26]. These studies demonstrated that YQA14 exerts its anti-addiction effects by specifically binding to D3Rs; this deserves further study as a candidate medication for the treatment of opioid abuse and addiction. However, the role of YQA14 in opioid-induced reinforcement or reinstatement in self-administration and the neurobiological mechanism by which YQA14 blocks opioid addiction is still unknown. With the development of optical fiber recording, scientists can record the dynamic changes in dopaminergic (DAergic) neurons in the brain using the genetically encoded Ca2+ indicator GCaMP6. New optical methods and indicators provide a wide dynamic range, rapid kinetics, single-cell resolution, and exquisite selectivity to detect the activity of DAergic neurons in freely-behaving animals with high spatial resolution [27].
Morphine and heroin bind to the same opioid receptors in the mesolimbic DA system, disinhibiting DA neurons in the ventral tegmental area (VTA) and increasing DA release in its projections to the nucleus accumbens (NAc); this leads to rewarding effects and initiates the process of addiction. D3Rs are expressed in the VTA and NAc. Thus, YQA14 may exert anti-opioid addiction effects by primarily affecting the activation of the DA reward system induced by opioids. Thus, in the present study, we assessed the effects of YQA14 treatment on heroin self-administration in rats and morphine-induced CPP in mice. Despite differences in the pharmacokinetic characteristics of morphine and heroin, there may be similar neurochemical events involved in their reinforcing effects. Finally, we clarified a neurobiological mechanism to account for the attenuation by YQA14 of the morphine-elevated activation of DAergic neurons and DA release in the NAc with a fiber photometry system.
Materials and Methods
Drugs and Chemicals
YQA14 was synthesized and provided by the Beijing Institute of Pharmacology and Toxicology [17] and was dissolved in a vehicle of 25% 2-hydroxypropyl-β-cyclodextrin (Xi’an Deli Biologic & Chemical Industry Co., Ltd, Xi’an, China). Morphine sulfate (Qinghai Pharmaceutical Factory, Xining, China) and free base heroin was obtained from the Beijing Public Security Bureau Forensic Medical Examination Centre (China). Morphine was dissolved in sterile 0.9% saline. Heroin was dissolved in HCl and diluted with saline to the experimental concentration (0.025 mg/kg, 0.05 mg/kg, and 0.25 mg/kg), and then the pH was adjusted to 7.5.
Animals
Male Sprague-Dawley rats weighing 250–300 g were used for the self-administration experiments, and C57BL/6J mice weighing 18–22 g (Beijing Animal Centre, Beijing, China) were used for the CPP and fiber photometry experiments. They were kept in a temperature (22°C)- and humidity (50%)-controlled room on a 12-h light/dark cycle (lights on from 07:00 to 19:00) with food and water available ad libitum except during training sessions. Experiments were all conducted during the animal’s light cycle. All procedures conformed to protocols approved by the guidelines of the Institutional Review Committee for the Use of Animals.
Heroin Self-administration Experiments in Rats
Intravenous Surgery
Rats were prepared for intravenous heroin self-administration by surgical catheterization of the unilateral external jugular vein. Each jugular catheter was constructed of Micro-Renathane (Braintree Scientific, Braintree, USA); catheterization was performed under sodium pentobarbital anesthesia using standard aseptic surgical techniques as described previously [15, 17]. Each catheter ran subcutaneously to the top of the skull, where it connected to a stainless-steel cannula (modified 22-gauge cannula, RWD Life Science, Shenzhen, China) fixed to the skull with four stainless steel jeweler’s screws (RWD Life Science) and dental acrylic cement. To prevent clogging and bacterial infection, each catheter was flushed twice a day with a gentamicin-heparin-saline solution (30 IU/mL heparin, Sinopharm Pharmaceutical Co., Ltd., Beijing, China) to maintain catheter patency, and it was capped after flushing. In addition, we monitored the rats at least four times per day to ensure that they were all in good condition according to the score sheet [28]. The rats were euthanized if the score was ≥7. After the surgical procedure, each animal was housed individually in its home cage and allowed to recover for 5–7 days before the start of procedures. Each rat was placed in a test chamber and allowed to nose-poke for i.v. heroin infusion (0.05 mg/kg per infusion) on a fixed ratio 1 (FR1) reinforcement schedule in 4-h sessions daily for 3 days. During every self-administration period, the number of available infusions was limited to 50 to lessen the likelihood of unintended overdose. With the completion of this program, rats were switched to FR2 reinforcement, in which two active nose pokes resulted in one self-administration i.v. infusion of heroin (0.025 mg/kg per infusion). The dose of heroin was chosen based on previous research showing that 0.025 mg/kg per injection of heroin shows good sensitivity in evaluating changes in drug-seeking behavior [29]. The rats were under FR2 reinforcement training (7–10 days) until the following criteria of stable heroin-maintained responding were met: <10% variability in the inter-response interval and <10% variability in the number of active responses for at least 3 consecutive days [17, 30, 31].
Apparatus
Intravenous heroin self-administration experiments were conducted in standard dual-level experimental chambers (300 mm × 300 mm × 300 mm) (Anilab SuperState Version 4.0) equipped with two nose-poke holes (one active, one inactive) located 45 mm above the floor; a cue-light located above the active nose-poke hole and a house light was configured as previously described [15]. Each chamber was housed individually in a sound-attenuating cubicle with a ventilation fan. After poking the active nose-poke hole, the injection pump was activated, and inactive nose-pokes were recorded to provide an index of general activity but had no consequences.
The Effects of YQA14 on Heroin Self-administration Under FR2 Reinforcement
After FR2 self-administration was established, according to the average infusions of the last three days, the animals were divided into three groups: YQA14 12.5 mg/kg (i.p., n = 11), YQA14 25 mg/kg (i.p., n = 11), or vehicle (25% 2-hydroxypropyl-cyclodextrin, n = 11). Then, the three groups randomly received YQA14 (12.5 or 25 mg/kg, i.p.) or vehicle 20 min prior to the test session.
The Effects of YQA14 on the Motivation of Heroin Under Progressive Ratio Self-administration
After the completion of heroin self-administration under the FR2 procedure, the naïve rats were arranged under progressive ratio (PR) reinforcement, a protocol of reinforcement reported previously [31, 32], to investigate whether YQA14 altered the motivation for the heroin reward. Then, according to the average infusions of the last three days, they were divided into three groups: YQA14 12.5 mg/kg (i.p., n = 7), 25 mg/kg (i.p., n = 7), or vehicle (n = 7). The three groups randomly received YQA14 (12.5 mg/kg or 25 mg/kg, i.p.) or vehicle 20 min prior to the test session. The breakpoint levels were then compared among animals receiving YQA14 (12.5 mg/kg or 25 mg/kg, i.p.) or vehicle.
The Effects of YQA14 on the Reinstatement of Heroin-seeking Behaviors Induced by Heroin
Finally, we assessed the effect of YQA14 on heroin-induced reinstatement after stable heroin self-administration under the FR2 schedule in naïve rats as described above. All the rats experienced daily 4-h extinction sessions for 14 days, during which the heroin pump and the heroin-associated cue light were turned off, and nose pokes (without limitation) were recorded but not responded to. Thereafter, all rats were given 0.25 mg/kg of heroin (i.p.) to arouse relapse to drug-seeking behaviors. During the 4-h reinstatement test, active nose pokes resulted in the heroin-associated cue light, but no heroin was administered, and all nose pokes were recorded. On the reinstatement test day, each group of rats received either vehicle (n = 8 per group) or one dose of YQA14 (12.5 mg/kg or 25 mg/kg i.p., n = 8). At 20 min after vehicle or YQA14 administration, all rats were given a priming injection of heroin (0.25 mg/kg s.c.) immediately before the reinstatement test in the same self-administration chambers. During the reinstatement test, heroin-seeking behavior induced by heroin was assessed under FR2 conditions when the heroin-associated cue light, house light, and pump were turned on. Heroin-induced nose-poke responses were recorded and compared between different dose groups of rats.
Morphine-induced Conditioned Place Preference (CPP) in Mice
Apparatus
CPP training and testing were conducted using a conventional apparatus (CPP-VR01, AniLab Instrument Co., Ltd, Ningbo, China) with two compartments of the same dimension (250 mm × 350 mm × 640 mm) and a central tunnel (150 mm × 350 mm × 640 mm) joining the chambers, with each chamber distinguished by different colors and floor textures as described previously [26]. An LED light and camera were placed on the top of each compartment. All data were recorded via a computerized video tracking system (AniLab v 2.43, AniLab Software & Instrument Co. Ltd., Ningbo, China) and were analyzed in a blinded manner.
CPP Procedure
The methods for CPP in mice were carried out as previously described with minor modifications [25]. We investigated the effects of YQA14 on acquisition, expression, extinction, and reinstatement. First, in the preconditioned phase, the mice were given free access to all compartments of the apparatus for 15 min. The time spent in each compartment was recorded. This habituation was to eliminate biased mice (defined operationally as spending >60% of the 15 min in either box). Then, all the mice were assigned in a counterbalanced manner within each group. Second, morphine conditioning was conducted for 8 days (4 drug sessions and 4 saline sessions). Each animal received morphine (10 mg/kg, s.c.) or saline (10 mL/kg, s.c.) injections alternately every other day. The mice were then immediately confined to the appropriate drug- or saline-paired compartment for 45 min.
The Effects of YQA14 on the Acquisition of Morphine-induced CPP
The naïve animals received vehicle (n = 12 per group) or YQA14 (12.5 or 25 mg/kg, i.p., n = 12 per group) 20 min prior to each morphine (10 mg/kg, s.c., n = 12 per group) or saline (10 mL/kg, s.c., n = 12) injection during the morphine conditioning phase (Fig. 3A). The CPP test was conducted 24 h after the last injection. There was no drug treatment on the test day. The CPP score was assigned a value by subtracting the time spent in the saline-paired compartment from the time spent in the drug-paired compartment.
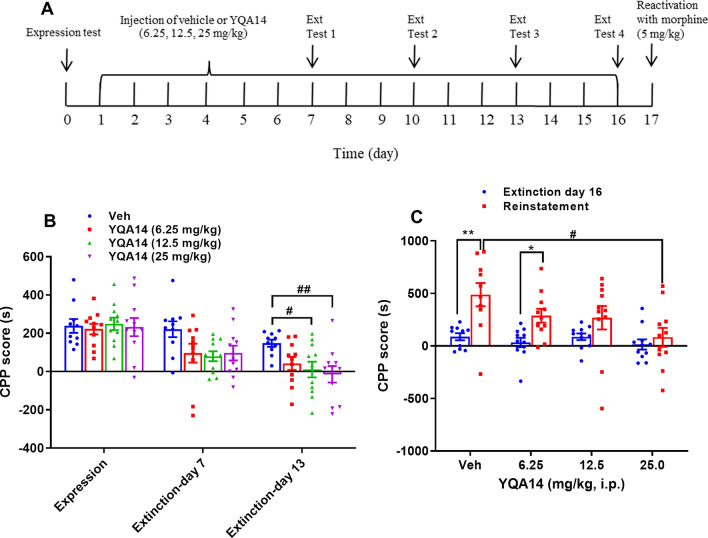
Fig. 3.
Effect of YQA14 (6.25 mg/kg, 12.5 mg/kg, and 25 mg/kg, i.p.) on the acquisition and expression of morphine-induced CPP in mice. Experimental protocol for saline, morphine, and YQA14 injections. A The CPP procedure of Panel B. B YQA14 does not change the acquisition of morphine-induced CPP in mice (mean ± SEM, n = 12, *P <0.05, **P <0.01, compared with the preconditioning test, paired t-tests). C The CPP procedure of Panel D. D YQA14 inhibits the expression of morphine-induced CPP in mice (mean ± SEM, n = 14, **P <0.01, compared with the preconditioning phase, paired t-tests; #P <0.05, compared with the vehicle-treated group, Bonferroni post hoc test)
The Effects of YQA14 on the Expression of Morphine-induced CPP
Either vehicle (n = 14) or YQA14 (6.25, 12.5, and 25.0 mg/kg, i.p., n = 14 per group) was administered 20 min prior to the CPP test to additional groups of naïve mice that did not receive chronic YQA14 treatment during morphine conditioning (Fig. 3C). The mice were placed in the center corridor and were given free access to the other two compartments for 15 min.
The Effects of YQA14 on the Extinction and Reconstruction of Morphine-induced CPP
Naïve mice were trained with morphine. Following the establishment of morphine-induced CPP, mice were separated into 3 groups and then underwent extinction in the home cage from extinction day 1 to extinction day 16. During each session, the mice received vehicle (n = 10) or YQA14 (6.25, 12.5, 25 mg/kg, i.p., n = 11 per group) daily until the CPP behaviors were extinguished. The training outline of CPP is shown in Fig. 4A. The reinstatement test was performed on extinction day 17 after the extinction was achieved according to the CPP score of the post-extinction test on extinction day 16. Each mouse was immediately placed in the central tunnel and permitted to move freely in each chamber for 15 min after the morphine (5 mg/kg, s.c.) injection.
Fig. 4.
Effect of YQA14 (6.25 mg/kg, 12.5 mg/kg, and 25 mg/kg, i.p.) on extinction and reinstatement of morphine-induced CPP in mice. A The CPP procedure for B and C. Ext, extinction. B YQA14 accelerates the extinction of morphine-induced CPP in mice (mean ± SEM, n = 10–11, #P <0.05, ##P <0.01, compared with the vehicle group on the same day, Bonferroni post hoc test). C YQA14 inhibits the reinstatement of morphine-induced CPP in mice (mean ± SEM, n = 10–11, *P <0.05, **P <0.01 compared with the extinction phase, paired t-tests; #P <0.05 compared with the vehicle group, Bonferroni post hoc test)
Recording of the Activation of DAergic Neurons in the VTA and Dopamine Levels in the NAc with Fiber Photometry
The administration of opioids, either systemically or directly into the VTA [33], leads to an increase in DA levels in the NAc. This effect is regulated by the disinhibition of DAergic neurons in the VTA via activation of Gi-coupled µ-opioid receptors located on the cell bodies and terminals of GABAergic neurons that normally provide inhibitory tone [34, 35]. Therefore, we investigated the effects of YQA14 on the morphine-induced activation of DAergic neurons in the VTA and DA levels in the NAc to elucidate the neurobiological mechanism of the anti-opioid effects of YQA14. Fiber photometry, together with the genetically encoded Ca2+ indicator GCaMP6 and DA sensors (G-protein-coupled receptor-based, GRABDA), in the VTA and NAc separately permitted the tracking of the activity dynamics of cell-type-specific neurons and the neurotransmission DA levels with sub-second temporal resolution. These ease-of-use techniques are well suited to freely-moving mice during drug infusion in the present study.
Virus Injection and Fiber Implantation
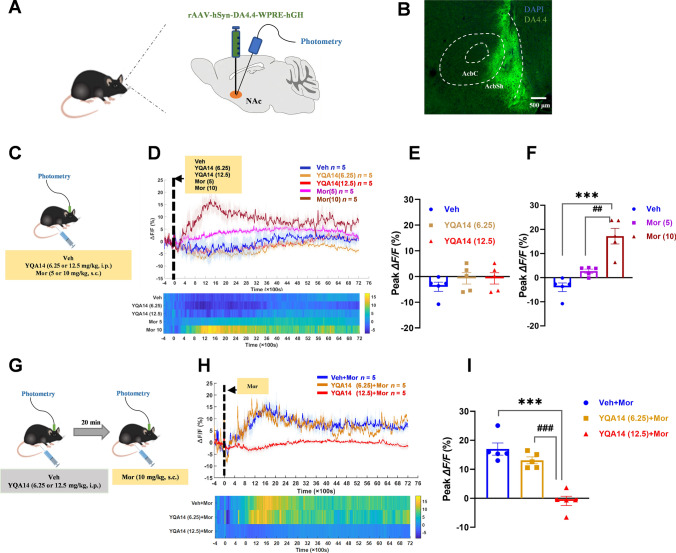
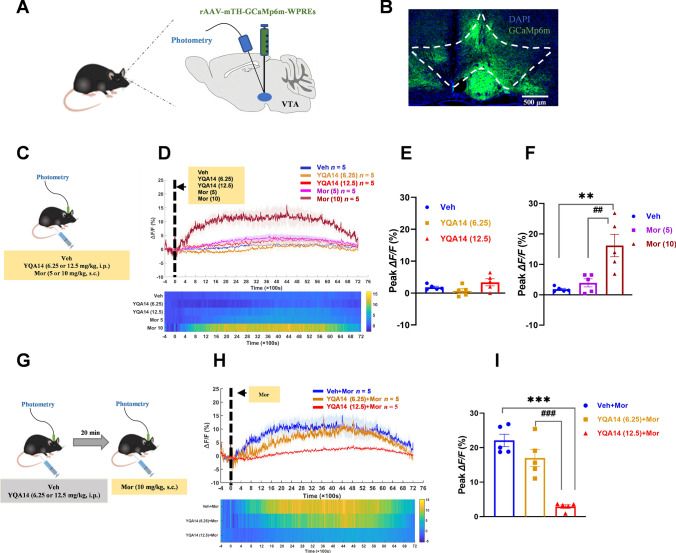
Animals were anesthetized with xylazine (12.5 mg/kg, i.p.) and ketamine (100 mg/kg, i.p.) and then placed in a stereotaxic device (RWD Life Science, Shenzhen, China) for virus injection and optical fiber insertion. To record the activity of DA neurons in the VTA in the fiber photometry experiments, rAAV-mTH-GCaMP6m-WPRE virus was unilaterally microinjected into the VTA (AP, −3.2 mm; ML, −0.5 mm; DV, −4.2 mm; from the dura). On the other hand, to investigate the DA levels in the NAc, rAAV-hSyn-DA4.4 was injected (300 nL per site) into the NAc using the following coordinates relative to bregma: AP, 1.4 mm; ML, −0.8 mm; DV, −4.5 mm. All the above injections were performed with a WPI Nanoliter 2000 injector at a rate of 30 nL/min. The injector remained in place for an additional 10 min. Subsequently, a single optical fiber (200 µm O.D., numerical aperture (NA) = 0.37, Shanghai Fiblaser) was implanted unilaterally above the VTA and NAc, at the above coordinates. Stereotaxic surgery was performed according to the mouse brain stereotaxic atlas of Franklin and Paxinos.
Apparatus
The fiber photometry system was produced by Thinker Tech Nanjing Biotech Co., Ltd (Nanjing, China). To record fluorescence signals, 470-nm excitation light was reflected by a dichroic mirror (MD498; Thorlabs, USA) and focused by a 20 × 0.4 objective. An optical fiber cable (200 mm O.D., NA = 0.37, 2 m long) guided the light between the objective and the implanted optical fiber. The laser power was adjusted to 20 watts at the exit of the implant tip for each animal. The GCaMP6m and GRABDA fluorescence were bandpasses filtered (MF525-39, Thorlabs) and collected by a photomultiplier tube (H10721-210, Hamamatsu). An amplifier was used to convert the photomultiplier tube current output to voltage signals, which were further filtered through a 40-Hz low-pass filter.
Recording
A script provided by Thinker Tech was used to obtain ∆F/F traces. The ∆F/F values were computed as (F − F0)/F0, where F0 is the mean value of the integral of the pre-administration signal lasting for 400 s. Fluorescence signals were sampled at 50 Hz. In the data analysis, we used MATLAB and applied the baseline correction strategy to analyze the recorded data. To reduce the photobleaching effect caused by long-term recording, we used the polynomial fitted correction. ∆F/F values are presented as average plots, with the shaded area indicating the SEM. Mice with off-target fiber-tip locations were excluded from the analysis.
The Effects of YQA14 on Morphine-induced DA Activation in the VTA
Ca2+ signals were recorded using fiber photometry in naïve animals expressing GCaMP6m (n = 40). The effect of different doses of YQA14 or morphine on VTA DAergic activity was first examined. Briefly, the mice (n = 25) were connected to the optical fiber recording and injected with vehicle, YQA14 (6.25 mg/kg and 12.5 mg/kg, i.p.), or morphine (5 mg/kg and 10 mg/kg, s.c.) after 20 min. Then the Ca2+ signals of VTA DA neurons were collected for 2 h.
To investigate the effect of YQA14 on morphine-induced DA activation in the VTA, the rest of the mice (n = 15) were randomly assigned to three groups and given either vehicle or YQA14 (6.25 mg/kg and 12.5 mg/kg, i.p.). Twenty minutes after administration, the mice were connected to the optical fiber recording system, and morphine (10 mg/kg, s.c.) was injected. The Ca2+ signals of VTA DA neurons were then collected for 2 h.
The Effects of YQA14 on the Morphine-induced Dopamine Increase in the NAc
DA signals were recorded with the fiber photometry system in naïve animals expressing GRABDA (n = 40). The effect of different doses of YQA14 or morphine on DA release in the NAc was first examined. Briefly, the mice (n = 25) were connected to the optical fiber recording system and injected with vehicle, YQA14 (6.25 mg/kg and 12.5 mg/kg, i.p.) and morphine (5 mg/kg and 10 mg/kg, s.c.) after 20 min. Then, the DA signals in the NAc were collected for 2 h.
To investigate the effect of YQA14 on the morphine-induced DA increase in the NAc, the rest of the mice (n = 15) were randomly assigned to three groups and given either vehicle or YQA14 (6.25 mg/kg and 12.5 mg/kg, i.p.). Twenty minutes after the administration, the mice were connected to the optical fiber recording, and morphine (10 mg/kg, s.c.) was injected. The DA signals in the NAc were then collected for 2 h.
Data Analyses
All data are presented as the mean ± SEM. One-way analysis of variance (ANOVA) and paired t-tests were used to analyze the data reflecting the effects of YQA14 on heroin self-administration, morphine-induced CPP, and reinstatement of heroin/morphine seeking. The behavioral data were analyzed using one-way ANOVA. Individual group comparisons were made with the Bonferroni post hoc test to determine specific group differences. For all analyses, P-values <0.05 were considered significant. Statistical analyses were applied with GraphPad Prism 8.
Results
YQA14 Inhibits Heroin-induced Reinforcement Under FR2 and Heroin-induced Motivation Under PR in Self-administration in Rats
Pretreatment with YQA14 (12.5 mg/kg or 25 mg/kg, i.p.) significantly reduced the self-administration behavior induced by 0.025 mg/kg per heroin injection (Fig. 1A, C). One-way ANOVA revealed that the infusions (F2, 30 = 3.355, P = 0.0484) and active pokes (F2, 30 = 3.651, P = 0.0381) had a treatment main effect. Individual group comparisons using the post hoc Bonferroni test showed a significant reduction in heroin (0.025 mg/kg per infusion) self-administration (t = 2.406, P = 0.045) and active pokes (t = 2.631, P = 0.0266) after administration of 25 mg/kg YQA14 compared with the vehicle group. Meanwhile, inactive pokes under the same experimental conditions were not changed by YQA14 (Fig. 1D, F2, 30 = 1.284, P = 0.2916).
Fig. 1.
Effect of YQA14 (12.5 mg/kg and 25 mg/kg, i.p.) on intravenous heroin self-administration under FR2 and PR reinforcement schedules in rats. And experimental protocols for heroin and YQA14 injections. A A significant reduction in the FR2 infusion following pretreatment with YQA14 (mean ± SEM, n = 11 per group. *P <0.05, compared with the vehicle (Veh) group, Bonferroni post hoc test). B Systemic administration of YQA14 significantly reduces active pokes under the FR2 schedule in rats (*P <0.05, compared with the vehicle group, n = 11 per group, Bonferroni post hoc test). C YQA14 has no effect on the frequency of inactive pokes (n = 11 per group, one-way ANOVA). D A significant reduction in the percentage of the breakpoint pre-test following pretreatment with YQA14 (mean ± SEM, n = 7 per group, *P <0.05, compared with the vehicle group, Bonferroni post hoc test). E The representative individual responding to heroin after vehicle or 25 mg/kg YQA14 pretreatment.
Then the effects of YQA14 on the motivation for heroin intake behavior were examined under the PR reinforcement model, in which the reinforcer was incrementally imposed. Pretreatment with YQA14 lowered the PR breakpoint (shown as % change) induced by heroin (0.025 mg/kg per injection), as shown in Fig. 1B and E. One-way ANOVA revealed a reduction in the percentage of the pre-test breakpoint (F2, 18 = 4.231, P = 0.0312) and active pokes (F2, 18 = 3.584, P = 0.0489) after YQA14 administration. Post hoc Bonferroni comparisons showed a decrease in the percentage of pre-test breakpoint (t = 2.888, P = 0.0196) and active pokes (t = 2.673, P = 0.0310) in the YQA14 25 mg/kg group compared with the vehicle group. Inactive pokes under the same experimental conditions were not changed by YQA14 (Fig. 1F, F2, 18 = 0.1688, P = 0.8460).
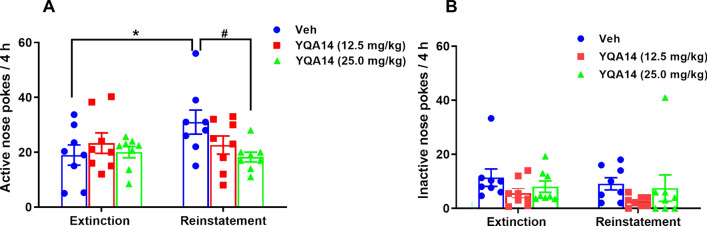
YQA14 Inhibits Reinstatement of the Heroin-seeking Behavior Induced by Heroin in Rats
Based on the above attenuation of YQA14 on morphine-induced reinforcement, we then investigated the effects of YQA14 on the reinstatement of the heroin-seeking behavior in rats. The total number of active nose pokes was recorded during the extinction phase and the reinstatement test in all groups (Fig. 2A). Compared to the extinction session, a single dose of heroin (0.25 mg/kg, i.p.) evoked robust reinstatement of heroin-seeking behavior in rats in the vehicle group after forced abstinence (t = 3.309, P = 0.0130, paired t-test vs extinction phase). One-way ANOVA revealed that YQA14 injection had a significant effect on responses (F2, 21 = 3.834, P = 0.0381). Individual group comparisons indicated that 25 mg/kg YQA14 injection resulted in a statistically significant reduction in active pokes during the reinstatement test (t = 2.725, P = 0.0254 vs vehicle). In contrast, YQA14 pretreatment did not alter inactive pokes under the same experimental conditions (Fig. 2B, two-way ANOVA, dose factor F2, 21 = 2.291, P = 0.1259; time factor F1, 21 = 0.7862, P = 0.3853), suggesting a specific effect on heroin-seeking behavior.
Fig. 2.
Effects of YQA14 (12.5 mg/kg and 25 mg/kg, i.p.) on heroin-triggered drug-seeking behavior in rats. A Systemic administration of YQA14 (25 mg/kg, i.p., 20 min prior to the test) significantly inhibits heroin-triggered drug-seeking behavior in rats (mean ± SEM, n = 8, *P <0.05, compared with the extinction phase, paired t-tests; #P <0.05, compared with the vehicle group, Bonferroni post hoc test). B YQA14 pretreatment does not alter inactive pokes under the same experimental conditions (mean ± SEM, n = 8, one-way ANOVA)
YQA14 Inhibits the Expression but Does Not Change the Acquisition of Morphine-induced CPP in Mice
We next examined the effect of YQA14 on the expression of morphine-induced CPP. During the training session, mice were not treated with YQA14. In the expression test, 20 min prior to the test, injection of YQA14 (6.25 mg/kg, 12.5 mg/kg, and 25 mg/kg, i.p.) significantly attenuated morphine-induced CPP in a dose-dependent manner (F3, 52 = 2.947, P = 0.0413, Fig. 3D). Post hoc analyses revealed that administration of 25 mg/kg YQA14 (t = 2.742, P = 0.0251 vs vehicle) led to a significant reduction in CPP score in mice.
We also determined whether repeated administration of YQA14 with morphine during the CPP training session would alter the acquisition of morphine-induced CPP in mice. The results showed that morphine produced a robust place preference regardless of YQA14 treatment (P <0.01, P <0.05, and P <0.05 for vehicle, 12.5 mg/kg and 25 mg/kg YQA14, respectively, Fig. 3B), indicating that chronic administration of YQA14 did not influence the acquisition of morphine-induced CPP (F2, 33 = 0.53, P = 0.5935 vs vehicle).
YQA14 Accelerates Extinction and Then Attenuates Morphine-induced Restoration of CPP in Mice
We used naïve mice to establish the CPP model induced by morphine (10 mg/kg, s.c.). Before the extinction session, mice were not pretreated with YQA14 during the acquisition and expression sessions. The results showed that morphine (10 mg/kg, s.c.) significantly increased the CPP score compared with the preconditioning phase, which indicated that the CPP model was established. Then, during the extinction phase, the mice were treated daily with vehicle or YQA14 in their home cages. The procedure of this experiment is displayed in Fig. 4A.
Repeated pretreatment with YQA14 resulted in a decrease in reward-seeking behavior during abstinence, i.e., the time spent in the drug-paired compartment on extinction-day 13 (F3, 39 = 3.757, P = 0.0184, vs vehicle, Fig. 4B). Individual group comparisons using the post hoc Bonferroni test showed a significant reduction in morphine-seeking behavior after administration of 12.5 mg/kg (t = 2.655, P = 0.0343) and 25 mg/kg (t = 3.14, P = 0.0096) YQA14 compared with the vehicle group.
We then proceeded to conduct daily extinction for an additional 3 days because the CPP response was not completely extinguished. Next, we tested the influence of chronic YQA14 treatment on the morphine-induced restoration of CPP. On the restoration test day, priming with morphine (5 mg/kg, s.c.) in the vehicle group induced a robust morphine-induced CPP (t = 4.276, P = 0.002) that was significantly attenuated by YQA14 (6.25 mg/kg, 12.5 mg/kg, and 25 mg/kg, i.p.) in a dose-dependent manner (F3, 39 = 2.919, P = 0.0460, Fig. 4C). Individual group comparisons revealed that 25 mg/kg YQA14 resulted in a rapid decrease in the restoration of morphine-seeking after abstinence (t = 2.956, P = 0.0158).
YQA14 Attenuates the Activation of DAergic Neurons in the VTA and the Enhanced Extracellular Dopamine Levels in the NAc by Morphine in Mice
Given the important role of the mesolimbic DA system in the opioid-induced reward effect, we further investigated whether the DAergic neurons in the VTA and extracellular DA levels in the NAc were affected by the actions of YQA14 on basal and post-morphine responses using fiber photometry assays. One-way ANOVA indicated that YQA14 did not affect DAergic neuronal excitability in the VTA (F2, 12 = 2.903, P = 0.0937, Fig. 5E) or the level of DA content in the NAc (F2, 12 = 0.8267, P = 0.4610, Fig. 6E). However, one-way ANOVA revealed that morphine showed dose-dependent changes in DA neuronal activity (F2, 12 = 11.66, P = 0.009, Fig. 5F) and DA levels (F2, 12 = 24.486, P <0.001, Fig. 6F). Individual group comparisons using the post hoc Bonferroni test showed that 10 mg/kg morphine, but not 5 mg/kg morphine, significantly strengthened DAergic neuronal activity (t = 4.472, P = 0.0023 vs vehicle, Fig. 5F) and increased basal extracellular DA levels in the NAc (t = 6.843, P <0.001 vs vehicle, Fig. 6F). One-way ANOVA revealed that YQA14 showed dose-dependent inhibition of morphine-induced excitation of DA neuronal activity in the VTA (F2, 12 = 30.935, P <0.001, Fig. 5I) and elevation of DA levels in the NAc (F2, 12 = 31.316, P <0.001, Fig. 6I). Post hoc analysis showed that pretreatment with 12.5 mg/kg YQA14 but not 6.25 mg/kg YQA14 significantly inhibited morphine-induced activation of DAergic neurons (t = 7.590, P <0.001 vs vehicle+morphine group, Fig. 5I) and attenuated the increase in extracellular DA levels after administration of 10 mg/kg morphine (t = 7.526, P <0.001 vs vehicle+morphine group, Fig. 6I).
Fig. 5.
Effects of YQA14 on morphine-induced changes in DA content in the NAc of mice. A Diagram of virus injection. B Fluorescence microscopy image showing DA4.4 (green) expressed in the NAc. Nuclei are counterstained with DAPI (blue). Scale bar, 500 μm. C Experimental protocol for morphine. D Average ΔF/F (upper) and heatmap (lower) of DA4.4 fluorescence in response to Sal and Mor (5 mg/kg and 10 mg/kg). E YQA14 does not affect the DA content of the NAc (n = 5 per group, one-way ANOVA ). F Morphine (10 mg/kg, s.c.) significantly increases the level of DA in the NAc (n = 5 per group, one-way ANOVA, mean ± SEM, ***P <0.001 compared with vehicle; ##P <0.01 compared with 5 mg/kg morphine, Bonferroni post hoc test). G Experimental protocol for morphine and YQA14 injections. H Average ΔF/F (upper) and heatmap (lower) of DA4.4 fluorescence in response to morphine (10 mg/kg) and YQA14 (6.25 mg/kg and 12.5 mg/kg) against morphine (10 mg/kg). I YQA14 (12.5 mg/kg, i.p.) significantly inhibits the morphine-induced increase in DA in the NAc (n = 5 per group, one-way ANOVA, ***P <0.001 compared with Veh+Mor, ###P < 0.001 compared with YQA14 (6.25)+Mor, Bonferroni post hoc test)
Fig. 6.
Effects of YQA14 on morphine-induced changes in the activity of DA neurons in the VTA of mice. A Diagram of virus injection. B Fluorescence microscopy image showing GCaMP6m (green) expressed in the VTA. Nuclei are counterstained with DAPI (blue). Scale bar, 500 μm. C Experimental protocol for morphine. D Average ΔF/F (upper) and heatmap (lower) of GCaMP6m fluorescence in response to Veh, YQA14 (6.25 mg/kg and 12.5 mg/kg), and Mor (5 mg/kg and 10 mg/kg). E YQA14 does not affect the excitability of DAergic neurons in the VTA. n = 5 per group, one-way ANOVA, mean ± SEM, Bonferroni post hoc test. F Morphine (10 mg/kg, s.c.) significantly increases the excitability of DAergic neurons in the VTA. n = 5 per group, one-way ANOVA, mean ± SEM. **P <0.01, compared with vehicle. ##P <0.01 compared with morphine (5 mg/kg), Bonferroni post hoc test. G Experimental protocol for morphine and YQA14 injections. H Average ΔF/F (upper) and heatmap (lower) of GCaMP6m fluorescence in response to morphine (10 mg/kg) and YQA14 (6.25 mg/kg and 12.5 mg/kg) against morphine (10 mg/kg). I YQA14 (12.5 mg/kg, i.p.) significantly inhibits morphine-induced elevation of DAergic neuronal excitability in VTA brain regions. n = 5 per group, one-way ANOVA, mean ± SEM. ***P <0.001 compared with Veh+Mor. ###P <0.001 compared with YQA14 (6.25)+Mor, Bonferroni post hoc test
Thus, based on the above results of the fiber photometry assays, in summary, morphine-induced reward and reinforcement by up-regulating the activity of DAergic neurons in the VTA and increasing the level of DA in the NAc; and pretreatment with YQA14 inhibited or attenuated the up-regulation and increase in the above DA system (Fig. 7).
Fig. 7.
Blockade of D3Rs attenuates opioid-induced rewarding associated with inhibiting the mesolimbic DA system. A Opioids inhibit GABAergic interneurons in the VTA to disinhibit mesolimbic DAergic neurons and increase somatodendritic and axonal DA release in the NAc. B Pretreatment with YQA14 attenuates the opioid-induced increase in the DA neuron activity in the VTA and DA levels in the NAc. Red arrows, DA release; green arrows, GABA release
Discussion
In the present study, we investigated the effects of YQA14 on opioid-seeking behavior and uncovered the neural mechanisms by applying self-administration and CPP models in rats and mice and using an optical fiber recording system. The results of this study suggested that (1) YQA14 not only significantly attenuated heroin self-administration behaviors under FR2 or PR reinforcement conditions but also inhibited the cue or heroin-primed reinstatement of drug seeking; (2) the chronic administration of YQA14 also facilitated the extinction process and decreased the morphine-induced reconstruction of CPP; and (3) pretreatment with YQA14 decreased the activation of DAergic neurons in the VTA and diminished the elevation of DA levels in the NAc induced by acute morphine exposure. Importantly, to the best of our knowledge, this is the first study to use new fiber photometry assays to study the neural mechanisms underlying the effects of YQA14 on opioid-seeking behavior. These findings indicated that the D3R antagonist YQA14 attenuated opioid-seeking behavior, mediated at least in part by the influence of the mesolimbic DA reward system.
In preclinical animal models of drug-seeking, intravenous drug self-administration is the classical method to emulate the drug-seeking process, which assesses well the reinforcement and motivation for the drug [36]. In the self-administration models, YQA14 decreased the active pokes under the FR2 schedule in a dose-dependent manner and lowered the breakpoint under the PR schedule in heroin self-administration in rats. This is consistent with a recent report that ABS01-113, a newly-developed D3R antagonist, significantly attenuated heroin self-administration and (heroin + cue)-induced reinstatement of drug-seeking behavior [37]. FR reinforcement mainly reflects positive reinforcement and animals’ voluntary drug-taking behaviors, whereas PR reinforcement measures the rewarding efficacy of drugs and the enhancement of motivation for drug-seeking [36, 38–40]. Our results suggested that YQA14 might not only reduce the reinforcement of opioids to attenuate drug-taking behavior but also decrease the animals’ motivation for drug-seeking in heroin self-administration models. In addition, we also used CPP, an animal model based on Pavlovian stimulus learning for drug-seeking investigation, to assess the effects of YQA14 on morphine-induced CPP. The results also showed that YQA14 dose-dependently inhibited the expression of CPP but not CPP acquisition in mice. These results are consistent with our earlier studies in morphine-induced CPP in rats [26]. Therefore, based on the previous and present results, YQA14 displays stable and reliable pharmacological efficiency in anti-opioid addiction in different species.
Opioid addiction is well characterized by a persistent susceptibility to drug relapse. Drug re-exposure has been identified as the most consequential determinant of recurrence in humans and in experimental animals [41–43]. Thus, the long-lasting prevention of predisposition to drug relapse is necessary for addiction therapy since the long-lasting predisposition is the most notable attribution of drug relapse. Using classical reinstatement models, including heroin-induced reinstatement in self-administration and morphine-induced restoration in CPP, we found that YQA14-treated animals displayed a blunted motivation for heroin or morphine in self-administration or CPP, respectively. This finding is also consistent with previous studies that used other D3R antagonists (PF-4363467, VK4-116, BAK4-54, and CAB2-015) to reduce opioid (fentanyl or oxycodone)-induced self-administration in rats [19, 20, 44].
The models of self-administration and CPP both involve the mesolimbic DA reward system. The DA system, which originates from DA neurons in the VTA and projects to the NAc and prefrontal cortex, plays an important role in the reinforcement, motivation, and learning processes. In this system, DA has been identified as an essential component that initiates rewarding effects to promote the motivation to opioid-seeking [45, 46]. Studies have shown that opioid injection activates DA neurons in the medial part of the VTA and increases DA release primarily in the medial shell of the NAc, causing transcriptomic changes in the NAc, suggesting an important role of the VTA and NAc in opioid reward. Many studies have shown that a change in extracellular DA levels in the NAc is a potential mechanism of opioid-induced self-administration or CPP [47, 48]. Benefiting from optical emission technology, recent studies have demonstrated that opioids bind to μ-opioid receptors in the VTA or substantia nigra pars compacta, thus inhibiting GABAergic interneurons, causing the disinhibition of mesolimbic DAergic neurons, and finally leading to an increase in DA release in somatodendritic and axons [49–53], especially in the NAc, where rewards initiate the addiction process [54, 55]. Although recent studies suggest that GABA neurons in the substantia nigra pars reticulata play a critical role in opioid reward and relapse, which partially explains the mechanism of opioid addiction, VTA GABA neurons could still be one of the major targets for opioids [52, 56]. Furthermore, we speculate that the reduction in opioid-seeking behavior after YQA14 is mainly achieved by affecting the DA system. As predicted, we demonstrated that YQA14 not only inhibited the morphine-induced excitation of DA neurons in the VTA but also decreased the morphine-induced increase in extracellular DA in the NAc using a fiber photometry recording system. D3Rs are abundantly expressed in the NAc [11, 12, 57]. Diaz and colleagues showed that D3R mRNA (messenger RNA) is expressed in medium-sized spiny neurons of the ventromedial shell of the NAc in rats using in situ hybridization histochemistry [58]. Therefore, we speculate that YQA14 may bind to D3Rs in GABAergic neurons in the NAc, disinhibiting GABAergic neurons, increasing GABA release in projections to the VTA, and inhibiting the activity of DA neurons. As the major inhibitory inputs (estimated 50%−70% of all afferents) to DA neurons, GABAergic neurons are important for controlling the activity of DA neurons [59]. A feedback neural circuit of the inhibitory effects exerted on neurons originates from the NAc and projects to the VTA. The latest literature identifies new functional and organizational principles of afferent-specific regulation in mesolimbic DA neurons. The results showed that the NAc medial shell could inhibit mesolimbic DA neurons via direct actions on GABAA receptors [60]. Therefore, the architecture and function of inhibitory feedback projecting from the NAc to the VTA may well support our speculation. However, given that D3Rs are also autoreceptors expressed in presynaptic DA neuron terminals, YQA14 may also bind to DA D3 autoreceptors to influence DA neuronal activity. A previous study found that the ligand preferentially binds to postsynaptic D3Rs and mediates downstream signaling when DA levels in the synaptic cleft are significantly elevated; therefore, we speculate that in our study, YQA14 may have preferentially bound to postsynaptic D3Rs and then regulated the activity of GABAergic neurons, thus regulating the activity of DA neurons in the VTA, when the morphine-induced DA level increased rapidly and massively in the VTA; thus, the presynaptic D3Rs, which are expressed in the axon terminals of VTA DA neurons, do not play a major role at this time [61]. However, the neurobiological mechanism still needs to be further studied.
In summary, our study found that YQA14, a highly selective D3R blocker, significantly attenuated heroin-induced drug administration and potentially addictive behavior in rats and mice, and this was mainly achieved by decreasing the morphine-induced excitability of DA neurons in the VTA and increasing DA levels in the NAc. It is expected that YQA14 will be a potential candidate for the clinical treatment of opioid addiction, and is worth further study.
Acknowledgments
We thank Rifang Yang (Beijing Institute of Pharmacology and Toxicology) for providing YQA14. This work was supported by the National Natural Science Foundation of China (81573405 and U1502225), the Natural Science Foundation of Beijing (7212159), the National Key R&D Program of China (2016YFC0800907 and 2017YFC131040), the Medical Innovation Program (16CXZ033), and the Beijing Nova Program (xx2014A014).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Rong-Rong Hu, Meng-Die Yang, and Xiao-Yan Ding contributed equally to this work.
Contributor Information
Jin Li, Email: jinli9802@163.com.
Rui Song, Email: songrui1983@yeah.net.
References
- 1.Zhang XY, Li Q, Dong Y, Yan W, Song K, Lin YQ, et al. Mu-opioid receptors expressed in glutamatergic neurons are essential for morphine withdrawal. Neurosci Bull. 2020;36:1095–1106. doi: 10.1007/s12264-020-00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HY, Kharrazi H, Bodycombe D, Weiner JP, Alexander GC. Healthcare costs and utilization associated with high-risk prescription opioid use: A retrospective cohort study. BMC Med. 2018;16:69. doi: 10.1186/s12916-018-1058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallersnes OM, Jacobsen D, Ekeberg Ø, Brekke M. Mortality, morbidity and follow-up after acute poisoning by substances of abuse: A prospective observational cohort study. Scand J Public Health. 2019;47:452–461. doi: 10.1177/1403494818779955. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Li CY, Xi W, Jin S, Wu ZH, Jiang P, et al. Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron. 2019;101:748–761.e5. doi: 10.1016/j.neuron.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Valentinova K, Tchenio A, Trusel M, Clerke JA, Lalive AL, Tzanoulinou S, et al. Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nat Neurosci. 2019;22:1053–1056. doi: 10.1038/s41593-019-0421-4. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen K, White DA, Acri JB. NIDA’s medication development priorities in response to the Opioid Crisis: Ten most wanted. Neuropsychopharmacology. 2019;44:657–659. doi: 10.1038/s41386-018-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile M, Lin R, Kabbani N, Karpa K, Kilimann M, Simpson I, et al. Paralemmin interacts with D3 dopamine receptors: Implications for membrane localization and cAMP signaling. Arch Biochem Biophys. 2006;446:60–68. doi: 10.1016/j.abb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- 10.Diaz J, Lévesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, et al. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-C. [DOI] [PubMed] [Google Scholar]
- 11.Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N, N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-B. [DOI] [PubMed] [Google Scholar]
- 13.Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- 14.Levant B. The D3 dopamine receptor: Neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 15.Chen Y, Song R, Yang RF, Wu N, Li J. A novel dopamine D3 receptor antagonist YQA14 inhibits methamphetamine self-administration and relapse to drug-seeking behaviour in rats. Eur J Pharmacol. 2014;743:126–132. doi: 10.1016/j.ejphar.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 16.Song R, Zhang HY, Peng XQ, Su RB, Yang RF, Li J, et al. Dopamine D3 receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, et al. YQA14: A novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2012;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrero-Bautista R, Franco-García A, Hidalgo JM, Fernández-Gómez FJ, Ribeiro Do Couto B, Milanés MV, et al. Distinct regulation of dopamine D3 receptor in the basolateral amygdala and dentate gyrus during the reinstatement of cocaine CPP induced by drug priming and social stress. Int J Mol Sci. 2021;22:3100. doi: 10.3390/ijms22063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You ZB, Gao JT, Bi GH, He Y, Boateng C, Cao J, et al. The novel dopamine D3 receptor antagonists/partial agonists CAB2-015 and BAK4-54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology. 2017;126:190–199. doi: 10.1016/j.neuropharm.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, et al. Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology. 2019;44:1415–1424. doi: 10.1038/s41386-018-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boateng CA, Bakare OM, Zhan J, Banala AK, Burzynski C, Pommier E, et al. High affinity dopamine D3 receptor (D3R)-selective antagonists attenuate heroin self-administration in wild-type but not D3R knockout mice. J Med Chem. 2015;58:6195–6213. doi: 10.1021/acs.jmedchem.5b00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv Y, Hu RR, Jing M, Zhao TY, Wu N, Song R, et al. Selective dopamine D3 receptor antagonist YQA14 inhibits morphine-induced behavioral sensitization in wild type, but not in dopamine D3 receptor knockout mice. Acta Pharmacol Sin. 2019;40:583–588. doi: 10.1038/s41401-018-0153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Hou Y, Cao W, Yan CX, Chen T, Li SB. Role of dopamine D3 receptors in basal nociception regulation and in morphine-induced tolerance and withdrawal. Brain Res. 2012;1433:80–84. doi: 10.1016/j.brainres.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Wang X, Li Z, Li J, Zhuang X, Zhang Z. P-Glycoprotein (ABCB1) limits the brain distribution of YQA-14, a novel dopamine D3 receptor antagonist. Chem Pharm Bull (Tokyo) 2015;63:512–518. doi: 10.1248/cpb.c15-00089. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Song R, Chen Y, Yang RF, Wu N, Su RB, et al. A selective D3 receptor antagonist YQA14 attenuates methamphetamine-induced behavioral sensitization and conditioned place preference in mice. Acta Pharmacol Sin. 2016;37:157–165. doi: 10.1038/aps.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu R, Song R, Yang R, Su R, Li J. The dopamine D3 receptor antagonist YQA14 that inhibits the expression and drug-primed reactivation of morphine-induced conditioned place preference in rats. Eur J Pharmacol. 2013;720:212–217. doi: 10.1016/j.ejphar.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunamaker EA, Anderson RJ, Artwohl JE, Lyubimov AV, Fortman JD. Predictive observation-based endpoint criteria for mice receiving total body irradiation. Comp Med. 2013;63:313–322. [PMC free article] [PubMed] [Google Scholar]
- 29.Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- 30.Zhan J, Jordan CJ, Bi GH, He XH, Gardner EL, Wang YL, et al. Genetic deletion of the dopamine D3 receptor increases vulnerability to heroin in mice. Neuropharmacology. 2018;141:11–20. doi: 10.1016/j.neuropharm.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Self-administration of heroin in rats: Effects of low-level lead exposure during gestation and lactation. Psychopharmacology. 2004;174:203–210. doi: 10.1007/s00213-003-1742-1. [DOI] [PubMed] [Google Scholar]
- 32.Belin-Rauscent A, Lacoste J, Hermine O, Moussy A, Everitt BJ, Belin D. Decrease of cocaine, but not heroin, self-administration and relapse by the tyrosine kinase inhibitor masitinib in male Sprague Dawley rats. Psychopharmacology (Berl) 2018;235:1545–1556. doi: 10.1007/s00213-018-4865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steidl S, Wasserman DI, Blaha CD, Yeomans JS. Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons. Neurosci Biobehav Rev. 2017;83:72–82. doi: 10.1016/j.neubiorev.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: Human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Galaj E, Bi GH, Klein B, Hempel B, Shaik AB, Gogarnoiu ES, et al. A highly D3R-selective and efficacious partial agonist (S)-ABS01-113 compared to its D3R-selective antagonist enantiomer (R)-ABS01-113 as potential treatments for opioid use disorder. Neuropsychopharmacology. 2022;47:2309–2318. doi: 10.1038/s41386-022-01379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol Psychiatry. 2016;79:39–46. doi: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts DCS, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuro Psychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 41.Stewart J. Pathways to relapse: The neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 42.Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: Studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- 43.Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- 44.Wager TT, Chappie T, Horton D, Chandrasekaran RY, Samas B, Dunn-Sims ER, et al. Dopamine D3/D2 receptor antagonist PF-4363467 attenuates opioid drug-seeking behavior without concomitant D2 side effects. ACS Chem Neurosci. 2017;8:165–177. doi: 10.1021/acschemneuro.6b00297. [DOI] [PubMed] [Google Scholar]
- 45.Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkow ND, Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 47.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 48.Juarez B, Han MH. Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology. 2016;41:2424–2446. doi: 10.1038/npp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron. 2014;82:1346–1356. doi: 10.1016/j.neuron.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34:14707–14716. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galaj E, Han X, Shen H, Jordan CJ, He Y, Humburg B, et al. Dissecting the role of GABA neurons in the VTA versus SNr in opioid reward. J Neurosci. 2020;40:8853–8869. doi: 10.1523/JNEUROSCI.0988-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, et al. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. eLife. 2018;7:e39945. doi: 10.7554/eLife.39945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–225. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galaj E, Xi ZX. Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits. Pharmacol Biochem Behav. 2021;200:173072. doi: 10.1016/j.pbb.2020.173072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 58.Diaz J, Lévesque D, Griffon N, Lammers CH, Martres MP, Sokoloff P, et al. Opposing roles for dopamine D2 and D3 receptors on neurotensin mRNA expression in nucleus accumbens. Eur J Neurosci. 1994;6:1384–1387. doi: 10.1111/j.1460-9568.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 59.Henny P, Brown MTC, Northrop A, Faunes M, Ungless MA, Magill PJ, et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–619. doi: 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron. 2018;97:434–449.e4. doi: 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]