Abstract
The amygdala is an important hub for regulating emotions and is involved in the pathophysiology of many mental diseases, such as depression and anxiety. Meanwhile, the endocannabinoid system plays a crucial role in regulating emotions and mainly functions through the cannabinoid type-1 receptor (CB1R), which is strongly expressed in the amygdala of non-human primates (NHPs). However, it remains largely unknown how the CB1Rs in the amygdala of NHPs regulate mental diseases. Here, we investigated the role of CB1R by knocking down the cannabinoid receptor 1 (CNR1) gene encoding CB1R in the amygdala of adult marmosets through regional delivery of AAV-SaCas9-gRNA. We found that CB1R knockdown in the amygdala induced anxiety-like behaviors, including disrupted night sleep, agitated psychomotor activity in new environments, and reduced social desire. Moreover, marmosets with CB1R-knockdown had up-regulated plasma cortisol levels. These results indicate that the knockdown of CB1Rs in the amygdala induces anxiety-like behaviors in marmosets, and this may be the mechanism underlying the regulation of anxiety by CB1Rs in the amygdala of NHPs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-023-01081-2.
Keywords: Cannabinoid type-1 receptor, Amygdala, Marmoset, Anxiety, CRISPR/Cas9
Introduction
The amygdala is crucial for regulating emotions [1–5] and plays a pivotal role in the pathophysiology of various mental diseases, such as depression [6, 7], social fear [8], and anxiety [9, 10]. In the central nervous system, the endocannabinoid (eCB) system functions to guard against negative emotions through the cannabinoid type-1 receptor (CB1R) [11]. CB1Rs encoded by the cannabinoid receptor 1(CNR1) gene are highly expressed in the amygdala and modulate synaptic transmission by suppressing the release of neurotransmitters [12, 13]. CB1R dysfunction is associated with some mental disorders [14, 15]. The pharmacological activation/inactivation of CB1Rs can bidirectionally regulate rodent anxiety-like behavior [16–18]. Circuit-specific manipulation of CB1Rs in the amygdala of mice induces depression-like behavior [7]. In addition, mental diseases including depression are accompanied by sleep disorders, and CB1Rs have been found to regulate sleep [19–21]. However, it remains unknown whether decreased CB1Rs in the amygdala induce mental disease in non-human primates (NHPs).
The marmoset (Callithrix jacchus) has garnered broad interest in the studies of social communication, emotion, and cognition as it is a highly social New World monkey with extensive vocal communication and rich emotional behavior [22–27]. Marmosets are also invaluable in biomedical research due to their genetic and physiological similarities to humans, and they are relatively easy to house in captivity [28]. Recent advances in the adeno-associated virus (AAV)-mediated delivery technique of CRISPR/Cas9 in adult macaques [29, 30] highlight the potential application of genetically modified adult marmosets in neuroscience. In the current study, we investigated the function of CB1Rs in the amygdala of marmosets through regional delivery of AAV-SaCas9-gRNA to knock down CB1Rs in the amygdala. We found that marmosets with amygdala CB1R knockdown displayed anxiety-like behaviors. These results revealed the emotion-specific function of the amygdala CB1Rs in NHPs.
Materials and Methods
Animal Ethics
All experiments were conducted under the guidelines of Zhejiang University (ZJU) Committee for the Care and Use of Laboratory Animals. All the experimental protocols were approved by the Animal Advisory Committee at ZJU and followed the National Institutes of Health (NIH) guidelines. Animal details are provided in Table 1. Male and female marmosets (350–450 g, 2–4 years old) were purchased from Johnbio (Jiangsu, China) and kept in pairs in the Non-human Primate Center at ZJU [31]. The monkeys were housed in a colony room with a maintained temperature of 26–28°C and humidity of 45%–55% with a 12-h light/dark cycle. The marmosets were fed daily with 30–40 g of nutritious food.
Table 1.
Detailed information on marmosets used in this study
| Group | ID | Age (years) | Sex | Virus injected |
|---|---|---|---|---|
| Control | Ctrl1 | 5 | Male | gRNA(empty) |
| Control | Ctrl2 | 3 | Male | gRNA(empty) |
| Control | Ctrl3 | 3 | Female | None |
| KD | KD1 | 4 | Male | gRNA(cjCB1R) |
| KD | KD2 | 3 | Male | gRNA(cjCB1R) |
| KD | KD3 | 3 | Female | gRNA(cjCB1R) |
KD, knockdown; Ctrl, control; gRNA, guide RNA; cjCB1R, AAV2/9-hSyn-saCas9-hU6-gRNA; empty, AAV2/9-hSyn-saCas9-pA-hU6-gRNA.
Viruses
All viruses were constructed by and purchased from Taitool Bioscience (Shanghai, China). AAV2/9-hSyn-saCas9-hU6-gRNA (cjCB1R) [1.21 × 1013 viral genomes (vg)/mL] was used to knockdown the CNR1 gene, AAV2/9-hSyn-saCas9-pA-hU6-gRNA (empty) (1.58 × 1013 vg/mL) was used as the control, and AAV2/9-hSyn-EGFP-WPRE-pA (1.42 × 1013 vg/mL) was co-injected with the above viruses (1:9) to display the location of injection.
Magnetic Resonance Imaging (MRI) for the Amygdala Coordinates
MRI data were acquired on a 7T research scanner (Siemens Healthcare, Erlangen, Germany) with a single loop coil (RAPID MR International, Columbus, OH, USA) for signal reception and transmission. Structural images were obtained at an isotropic voxel size of 0.33 mm. Briefly, animals were anesthetized with alfaxalone [intramuscular (i.m.), 0.1 mg/kg, Jurox Inc., North Kansas City, USA] and maintained by isoflurane (0.5%–1.2%, RWD, Shenzhen, China). Two glass pipettes (OD: 1.0 mm, ID: 0.58 mm; Sutter Instruments, Novato, USA) filled with lidocaine gel were inserted into the hollow ear bars as markers of the interaural axis. After the animal was fixed in a home-made MRI-compatible stereotaxic apparatus, the scan procedure was started. The MRI data were analyzed using MRIcron v1.0 (NITRC, Washington, DC, USA) to calculate the coordinates of the amygdala and guide virus injection.
Virus Injections
The surgery and virus injection were performed under aseptic conditions. The animals were initially anesthetized with alfaxalone (i.m., 0.1 mg/kg, Jurox Inc.) and maintained by isoflurane (0.5%–1.2%, RWD). The head was fixed in a stereotaxic frame (RWD) and a craniotomy was made according to the MRI-based coordinates. A cocktail of viruses was infused through a Hamilton syringe (Hamilton, Reno, USA) placed in a syringe pump (KD Scientific, Holliston, USA) at a speed of 80 nL/min for a total of 1 μL at each injection site. After infusion, the syringe was left in place for 5 min. Totally, 10 sites were injected with viruses (5 injections on each hemisphere) across all the amygdala coordinates. After injection, the wound was carefully cleaned and the skin was placed back and sutured. Buprenorphine (i.m., 0.005 mg/kg, Pharmaceutical Research Institute, Tianjin, China) was applied for 3 days to reduce pain, and antibiotic (i.m., ceftriaxone sodium, 22 mg/kg CHUNG-HWA Pharmacology, Suzhou, China) was applied once per day for one week. The animals were given special care for two weeks with additional nutritional supplements and physiological examinations. Measurements were made two months after injection.
Immunohistochemistry
Animals were first euthanized by administering an overdose of sodium pentobarbitone (i.m., 100 mg/kg, Jurox Inc.) and then transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde overnight at 4°C, and then immersed in 30% sucrose in PBS for 72 h. The brains were embedded in the Optimal Cutting Temperature compound (Sakura Finetek, Torrance, USA), 20-μm sections were cut on a cryostat (Leica Microsystems, Wetzlar, Germany), and the sections were then washed in PBS for 5 min.
RNAscope in situ hybridization was applied using an RNAscope Multiplex Fluorescent Reagent Kit v2 (ACDbio, Newark, USA) and RNAscope Probe-cj-CNR1 (Cat No. 565041; ACDbio) according to the manufacturer’s standard protocols.
For immunofluorescence staining, sections first underwent target retrieval pretreatment, and were then blocked with 3% bovine serum albumin in PBST (0.3% Triton X-100 in PBS) for 1 h and incubated with primary antibodies overnight at 4°C. The sections were then incubated with fluorescent secondary antibodies (1:400, Invitrogen, Carlsbad, USA) for 2 h at room temperature. The primary antibody was anti-GFAP (1:800, BioLegend, San Diego, SMI-21R, USA). Anti-GFP was used to show EGFP expression in the amygdala. Sections were mounted after 4′,6-diamidino-2-phenylindole (DAPI) staining (1:5000, Sigma-Aldrich, St. Louis, USA). Confocal images were captured under a 20× objective on an A-1R confocal microscope (Nikon, Tokyo, Japan). Cells were counted with ImageJ v1.52 (NIH, Bethesda, USA).
In Vivo Mutation Efficiency Detection
To determine whether CB1R gene editing occurred in the marmoset brain near the injection sites, fresh fluorescent-positive brain tissue was collected from brain slices and then digested by protease K. Genome DNA was extracted on a HiPure DNA column and the purified DNA was checked by agarose gel analysis. Sanger sequencing (Easy-Do Biotech, Hangzhou, China) was applied after the proximal regions of the small guide RNA (sgRNA) targeting sites were amplified by polymerase chain reaction (PCR). Results were compared with the control to confirm that the CNR1 gene was edited near the injection sites.
Behavioral Assays
We used six behavioral assays, all of which have been used in previous marmoset studies: sleep measurement in the home cages [32–34], agitated psychomotor activity in a new environment [35, 36], vocalizations [37, 38], sucrose preference test [39], three-chamber social test [40], and snake intruder test [41, 42]. Among them, the sucrose preference test and three-chamber social test are adapted from rodents and used for testing anhedonia and social ability. The others are unique to marmosets.
Daily Activity and Sleep Measurement in the Home Cage
Daily activity and sleep patterns were assessed using an ActiWatch Mini (CamNtech, Cambridgeshire, UK) worn on the neck of each animal [31]. Animals were allowed to habituate to the device for 2 days and this was followed by 7 days of data collection. Daytime was defined as 07:00 to 19:00 and nighttime was defined as 19:00 to 07:00 according to the light/dark cycle (lights on at 07:00 and off at 19:00). Daily activity was analyzed in 1-min epochs using Sleep Analysis v7 (CamNtech) offline. First, an immobility threshold was set. Animals were defined as wakeful if they moved more than the immobility threshold. Otherwise, they were considered immobile. Sleep was defined if the animal remained immobile for >6 min (6 epochs). Sleep latency was defined as the time from the light-off to the time that the animal fell asleep. Sleep duration was defined as the time that an animal spent between falling asleep at night and waking up in the morning. Sleep efficiency was defined as the percentage of actual sleep to sleep duration. The sleep fragmentation index was defined as the sum of wakefulness (awake time/sleep duration) and immobility (1-min immobility epochs/total immobility epochs), which was used as an indicator of restlessness. Calculation details are provided in the Sleep Analysis v7 software guidelines.
Agitated Psychomotor Activity in a New Environment
Video recordings were carried out in the marmoset colony. The animals were transferred into a new cage (850 mm × 800 mm × 800 mm) with one wall transparent. The video recording started 5 min after the animal entered the cage and its movement was recorded for 10 min by a video camera (ORDRO HDV-Z80, Shenzhen, China) 1.5 m from the cage via the transparent wall. Trajectories and distances moved were analyzed using a custom MatLab 2017b (MathWorks, Natick Massachusetts, USA) program (provided by Prof. Xinjian Li). Average velocity was defined as distance moved per second. Stationary time was defined as the total time of immobile phases that lasted >1 s.
Three-chamber Social Test
The social ability of marmosets was assessed by the three-chamber social test modified from that used for mice [43]. In brief, the subject animal was first introduced to the chamber in the center and allowed to habituate to the apparatus for 20 min. Animals with left- or right-side preferences were excluded from the following experiments. Next, a stranger marmoset (opposite sex, non-cage-mate) was introduced to the left or right chamber and left for 20 min (stranger introduced session). Finally, the cage-mate of the animal was introduced to the side chamber different from the stranger and left for 20 min (cage mate introduced session). The behavior of the subject animal was recorded by a video camera (ORDRO HDV-Z80). The three-chamber social test was applied three times on different days. The introduced chambers for the stranger and cage mate were switched to avoid the influence of location preference. We analyzed the time that the subject animal spent in each chamber in different sessions.
Recordings of Marmoset Vocalizations
The calls of the marmoset monkeys were recorded in a double-walled soundproof chamber by a microphone (Audio-Technica AT2020, Tokyo, Japan). The subject animal was transferred to the vocal recording room and allowed 10 min to adapt to the environment. A microphone was placed in front of the transfer cage for 20 min of vocal recordings in an alone scenario. Adobe Audition CS6 v5.0 (Adobe, San Jose, USA) was used to record and analyze the calls. The phee calls were identified according to the spectrogram.
Sucrose Preference Test
Sucrose preference tests were applied in the home cages in the morning. Each marmoset was provided with two bottles containing 100 mL of 10% sucrose solution and 100 mL of water. The sucrose preference test lasted for 2 h. The weights of the sucrose and water were measured and the positions of the two bottles were exchanged every 30 min. The sucrose preference index was calculated by the formula below:
Snake Intruder Test
Animals were first habituated to the test apparatus for 10 min. A rubber snake was then introduced in the corner and monkey behavior was recorded by a video camera (ORDRO HDV-Z80). We calculated the time that the animal stayed at the same corner as the snake, which is defined as the snake-paired corner. Snake inspection time was defined as the time spent staring at the snake. Immobile time was defined as the time staying immobile for >1 s.
Blood Sample Collection and Cortisol Test
Blood samples (0.5 mL) were collected intravenously in EDTA-coated tubes at 10:00 on two successive days. The blood samples were immediately centrifuged at 1000× g for 10 min at room temperature. The plasma was collected and stored at − 80°C until use. Using standard protocols, plasma cortisol levels were measured using an ELISA toolkit (Enzo, ADI-901-071, New York, USA).
Quantification and Statistical Analysis
The sample size for statistical comparisons was as in previous research [29, 30]. All data were analyzed with GraphPad Prism v6.01 (GraphPad Software, San Diego, USA). Differences in individuals before and after virus injection were tested using the two-tailed paired t-test and differences between groups were tested using the two-tailed unpaired t-test. Repeated two-way analysis of variance (ANOVA) was used to test differences in the three-chamber test. Data are shown as the mean ± SEM. Differences were considered statistically significant at *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
All data generated or analyzed in the current study are included in the manuscript and supporting files; source data files have been provided.
Results
Validation of CB1R Knockdown by AAV-Mediated CRISPR/Cas9 Virus
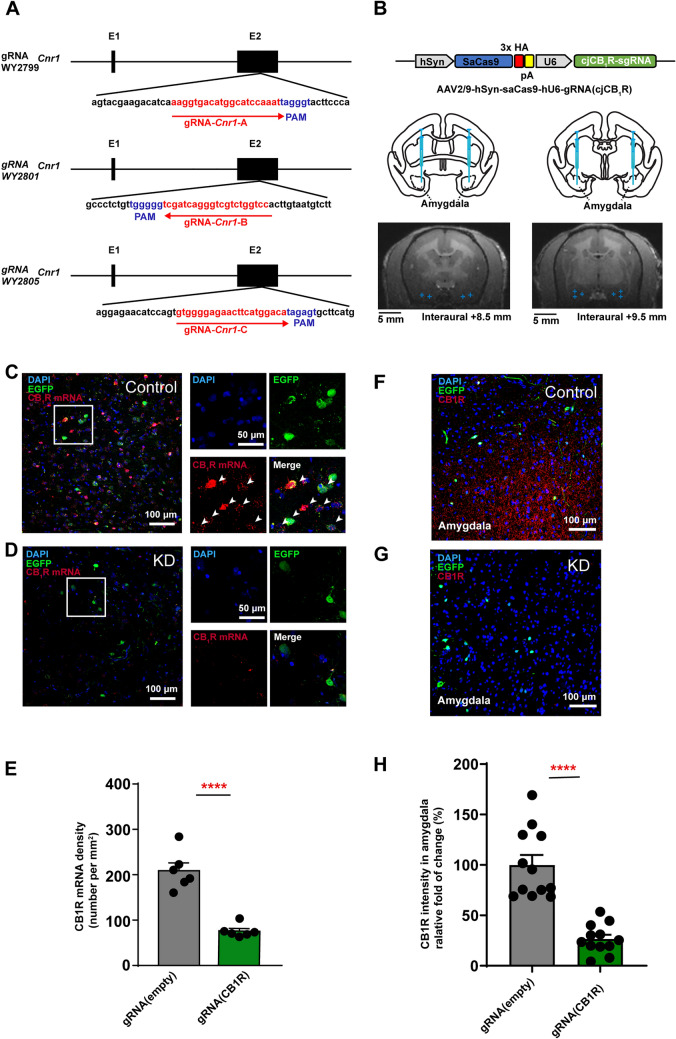
To study the function of CB1Rs in the amygdala of NHPs, we constructed an AAV-mediated CRISPR/Cas9 virus to knock down the CNR1 gene in the amygdala of adult marmosets in vivo, and then examined the animal’s behaviors. The marmoset CNR1 gene has two exons localized to chromosome 4. The CB1R protein is mainly transcribed by exon 2 with ~ 1400 bp. So, we mainly targeted exon 2. To knock down the CNR1 gene, three guide RNAs (gRNAs, Fig. 1A) were synthesized and individually inserted into the AAV2/9-hSyn-SaCas9-U6-gRNA vector, by harboring the human synapsin promoter to drive SaCas9 and the U6 promoter, and then to drive the sgRNA cassette. In contrast, we used AAV2/9-hSyn-saCas9-pA-hU6-gRNA (empty) as the control virus. To indicate the range of infection in the brain, the target virus and control virus were each mixed with AAV2/9-hSyn-EGFP-WPRE-pA, and co-injected into the amygdala. Considering the deep location and individual variation of the amygdala in NHPs, MRI imaging was applied to localize and target the amygdala during virus injection (Fig. 1B). Six marmosets were divided into two groups based on virus difference (Control group and KD group), with two males and one female in each group (Table 1). To validate the efficiency of the virus, one marmoset (Ctrl 1) that had received control virus injections and two marmosets that had received knockdown (KD) virus injections were sacrificed via transcardial perfusion after anesthesia with pentobarbital sodium (80 mg/kg). Then, the brain tissue and slices of the amygdala were collected for Sanger sequencing, in situ hybridization, and fluorescence staining to evaluate knockdown efficiency at the gene, messenger RNA (mRNA), and protein levels. First, the fluorescence-positive brain region in the amygdala of CB1R KD marmosets was collected and Sanger sequencing was applied after the sgRNA-targeting gene was amplified via polymerase chain reaction (PCR). We identified predicted mutated CNR1 gene sequences in these brain regions (Fig. S1A–E). Second, using RNAscope staining, the mRNA level was tested in both the control and KD groups (Fig. 1C, D). We found, in comparison with the control group, fewer CB1R mRNA-positive cells in the amygdala of CB1R-gene-edited marmosets (Fig. 1E). Last, we applied immunofluorescence staining to the brain slices of CB1R KD and control marmosets (Fig. 1F, G), which showed that the CB1R was significantly decreased in the amygdala of the CB1R gene-edited marmosets than that of the control (Fig. 1H). Therefore, our results indicated that the CB1Rs in the amygdala were knocked down at the gene, mRNA, and protein levels.
Fig. 1.
Construction, injection, and validation of AAV-mediated SaCas9 viruses in the amygdala of adult marmosets. A Targeting sites of gRNA for the marmoset CNR1 gene (red). Peptidylglycine alpha-amidating monooxygenase (PAM) sequences are indicated in blue. B Upper, schematic of KD virus; middle, atlas of the marmoset brain with injection sites in the amygdala; lower, examples of MRI images with amygdala injection sites (indicated with blue crosses). C, D Left, RNAscope staining for CB1R mRNA (red, indicated by white arrows). EGFP (green), and DAPI (blue) in the amygdala of control (C) and KD (D) marmosets. Right, magnified view of the rectangle on left. Scale bars, 100 μm (left); 50 μm (right). E Density of CB1R mRNA-expressing cells in the amygdala, n = 6 slices, two-tailed unpaired t-test, t(10) = 7.209, P < 0.0001; data are the mean ± SEM. F, G Immunostaining of amygdala sections for Cas9-CB1R in control (F) and KD (G) marmosets for EGFP (green), CB1R (red), and DAPI (blue). H The relative density of fluorescence intensity of CB1R protein in the amygdala of marmosets. n = 12 slices from one control marmoset (Ctrl 1); n = 12 slices from two CB1R KD monkeys (KD1, KD3) , two-tailed unpaired t-test, t(10) = 6.876, P < 0.0001; data are expressed as the mean ± SEM. ****P < 0.0001.
Disrupted Night Sleep in CB1R KD Marmosets
Sleep disturbance is one of the major clinical symptoms of mood disorders, such as depression [44, 45] and anxiety [16] which has been reported to be associated with the amygdala. In addition, previous clinical studies have shown that eCBs promote sleep through CB1Rs [20, 21, 46]. So, sleep disturbance may be one of the symptoms after CB1R KD in marmosets. To examine this possibility, we measured the sleep of marmosets using an actigraphy device based on previous studies [32, 34, 47]. We found that sleep latency to light-off in control marmosets did not significantly change after virus injections (Fig. 2A, B and D, n = 3), however, it was prolonged in KD marmosets after virus injections (Fig. 2F, G, and I, n = 3). In addition, the duration of night sleep in control marmosets did not change (Fig. 2C, E) but was shortened in KD marmosets with a decreasing trend after virus injection (Fig. 2H, J). Also, we found that the KD group showed prolonged sleep latency (Fig. 2K) and decreased sleep duration in comparison with the control group (Fig. 2L). We examined the daytime sleep and found that daytime nap time was not changed in KD marmosets (Fig. S2). We also examined sleep efficiency [48] and the fragmentation index [32], which contribute to sleep quality. Our results showed that sleep efficiency and fragmentation index did not change in control marmosets after virus injections (Fig. S3A, B). However, KD marmosets exhibited a decreasing trend of sleep efficiency (Fig. S3C, E) and an increasing trend of the fragmentation index (Fig. S3D, F) after virus injections, suggesting decreased sleep quality. These results indicate that the knockdown of CB1Rs in the amygdala disrupts night sleep quantity and quality, which may affect the emotional behaviors in marmosets.
Fig. 2.
Disrupted night sleep in CB1R knockdown marmosets. A, F Representation of sleep patterns at night (19:00 to 07:00) before virus injection (upper) and after virus injection (lower) in a control (A) and a KD (F) marmoset. The sleep phase is indicated by the black box and the awake phase is indicated by the white box. B, G Sleep latency in a control (B) and a KD (G) marmoset. Mann-Whitney U test, U = 21, P = 0.7104 (B); U = 4, P = 0.0064 (G). C, H Night sleep duration in a control (C) and a KD (H) marmoset. Mann-Whitney U test, U = 22, P = 0.8048 (C); U = 7, P = 0.0239 (H). D, I Comparison of 7-day average sleep latency before and after virus injection for individuals in control (D) and KD (I) groups. n = 3, two-tailed paired t-test, t(2) = 2.858, P = 0.1037 (D); t(2) = 2.104, P = 0.1700 (I). E, J Comparison of 7-day average night sleep duration for individuals in control (E) and KD (J) groups. n = 3, two-tailed paired t-test, t(2) = 1.529, P = 0.2659 (E); t(2) = 2.387, P = 0.1396 (J). K Comparison of changes in sleep latency between control and KD groups. n = 3, two-tailed unpaired t-test, t(4) = 3.116, P = 0.0357. L Comparison of changes in night sleep duration between control and KD groups. n = 3, two-tailed unpaired t-test, t(4) = 2.832, P = 0.0472. *P < 0.05; n.s., not significant; data are the mean ± SEM.
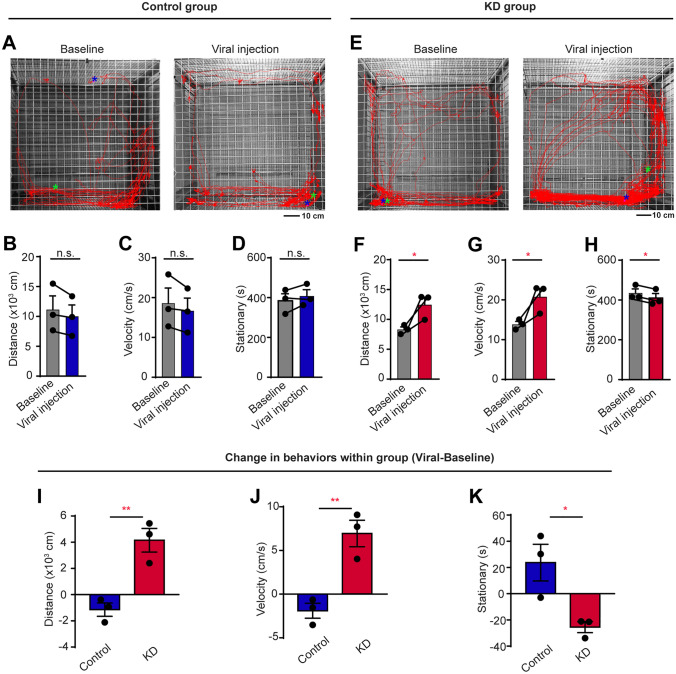
Agitated Psychomotor Activity in a New Environment of CB1R Knockdown Marmosets
The ability to adapt to a new environment is vital for the survival of humans and animals. However, the new environment may induce stress and abnormal behaviors in patients with mental diseases [49, 50]. Meanwhile, humans with anxiety usually display changes in both emotion and locomotion and sometimes they exhibit increased locomotor behaviors. So, we transferred individual marmosets to a new environment to compare their locomotor behaviors before and after the knockdown of CB1Rs (Fig. 3A, E). We found that their distance moved, average velocity, and stationary time did not change after virus injection in the control group (Fig. 3B–D). However, KD marmosets showed increased distance moved and velocity (Fig. 3F, G) as well as decreased stationary time after virus injection (Fig. 3H). Meanwhile, in comparison with the control group, KD marmosets exhibited greater agitation with the increased distance moved (Fig. 3I) and velocity (Fig. 3J), as well as decreased stationary time (Fig. 3K). However, no significant changes were found in the time of locomotion before and after the knockdown of CB1Rs when the locomotion was measured in their home cages by actigraphy (Fig. S4). These results suggest that CB1R knockdown in the amygdala of marmosets specifically induces agitated psychomotor activity in new environments but not in familiar surroundings, which is similar to the symptoms of human patients with anxiety.
Fig. 3.
Agitated psychomotor activity in a new environment by CB1R KD marmosets. A, E Representative movement trajectories in a new environment before virus injection (left) and after virus injection (right) in a control (A) and a KD (E) marmoset. The start and end positions are indicated by green and blue asterisks, respectively. Scale bar, 10 cm. B, F Seven-day average travel distance in a new environment before and after virus injection in control (B) and KD (F) groups. n = 3, two-tailed paired t-test, t(2) = 2.227, P = 0.1558 (B); t(2) = 4.577, P = 0.0446 (F). C, G Seven-day average velocity in a new environment before and after virus injection in control (C) and KD (G) groups. n = 3, two-tailed paired t-test, t(2) = 2.227, P = 0.1558 (C); t(2) = 4.577, P = 0.0446 (G). D, H Seven-day average stationary time in a new environment before and after virus injection in control (D) and KD (H) groups. n = 3, two-tailed paired t-test, t(2) = 1.703, P = 0.2307 (D); t(2) = 6.078, P = 0.0260 (H). I–K Comparison of changes in travel distance (I), average velocity (J), and stationary time (K) in new environment between control and KD groups. n = 3 marmosets per group, two-tailed unpaired t-test, t(4) = 5.080, P = 0.0071 (I); t(4) = 5.080, P = 0.0071 (J); t(4) = 3.379, P = 0.0278 (K). *P < 0.05; **P < 0.01; n.s., not significant; data are the mean ± SEM.
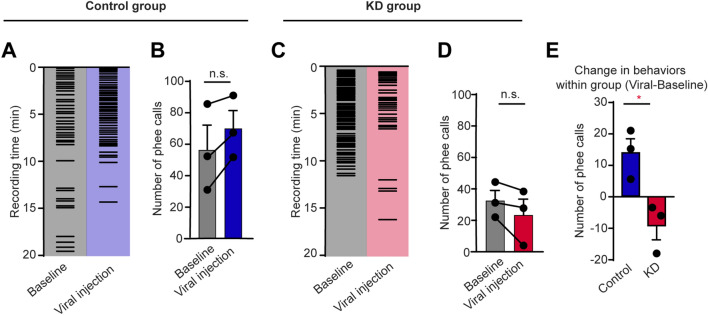
Decreased Social Desire During Vocal Communication in CB1R Knockdown Marmosets
Anxiety usually induces a decreased desire for social communication. The marmoset is a highly social NHP with extensive vocal communication [24]. They produce phee calls to engage in long-distance vocal communication to contact each other [51, 52]. We hypothesized that CB1R knockdown in the amygdala might induce an abnormal desire for social communication in marmosets, reflected by a decrease in vocal communication. We recorded phee calls of marmosets in an alone scenario to test their social desire by vocalizations. Interestingly, while the number of phee calls did not change significantly in the control group before and after virus injections (Fig. 4A, B), the KD marmosets tended to produce fewer phee calls after virus injections, indicating decreased vocal social behavior in CB1R-knockdown marmosets (Fig. 4C, D). Comparison between KD and control groups showed that the knockdown of CB1Rs resulted in a decrease in phee calls (Fig. 4E), suggesting decreased social desire during vocal communication.
Fig. 4.
Decreased social desire for vocal communication in CB1R KD marmosets. A, C Example raster plots of phee calls during vocal recordings before and after virus injection in a control (A) and a KD (C) marmosets. B, D Five-day average number of phee calls before and after virus injection in control (B) and KD (D) groups. n = 3, two-tailed paired t-test, t(2) = 3.058, P = 0.0923 (B); t(2) = 0.1793, P = 2.031 (D). E Comparison of changes in phee calls between control and KD groups. n = 3 marmosets per group, two-tailed unpaired t-test, t(4) = 3.598, P = 0.0228. *P < 0.05; data are the mean ± SEM.
The three-chamber social test is widely used in rodents to test social behaviors [53]. To better understand the role of amygdala CB1Rs in social behavior, we modified the rodent three-chamber protocol and applied it to marmosets. The marmoset first went through the stranger-introduced phase followed by the cage mate-introduced phase (See Methods and Fig. S5A). We found that amygdala CB1R knockdown did not change marmoset social behaviors in the three-chamber paradigm (Fig. S5B–D). A possible explanation is that the rodent-designed social behavior test was not suitable for testing sociability in NHPs since the two species use different sensory modalities to recognize and communicate with conspecifics [24]. Taken together, our results suggest that the knockdown of CB1Rs in the amygdala decreases social desire during vocal communication in marmosets, implying anxiety-like social avoidance.
Unaffected Hedonic and Fear Behaviors After Amygdala CB1R Knockdown
In a previous study, we have shown that the disruption of circuit-specific amygdala CB1Rs induces depression-related anhedonia in mice [7]. To further test this possibility, we assessed the hedonic state via an adapted sucrose preference test [39]. Marmosets consumed sucrose and water freely for >2 h (Fig. S6) and sucrose preference was assessed in the first 30 min and 120 min. Surprisingly, the preference for sucrose or water was not changed after virus injection in both the control and KD groups (Fig. 5A–D), suggesting no depression-like behavior. Previous studies have also indicated that the amygdala is a key region for the fear response in both rodents and humans [2, 54]. To measure the fear response of marmosets after virus injection, we applied the snake intruder test (Fig. 5E) based on previous protocols [42]. Unexpectedly, the knockdown of CB1Rs in the amygdala did not change the fear response (Fig. 5F–H). Those results suggest that the knockdown of CB1Rs in the amygdala does not change the hedonic state and innate fear response in marmosets.
Fig. 5.
Unaltered hedonic state and fear response in CB1R KD marmosets. A Paradigm of the sucrose preference test. B, C Individual sucrose preference in the first 30 min (left) and 120 min (right) before and after virus injection in control (B) and KD (C) groups. n = 3, two-tailed paired t-test, t(2) = 0.2001, P = 0.8599 (B, left); t(2) = 0.1724, P = 0.8790 (B, right); t(2) = 0.1042, P = 0.9265 (C, left); t(2) = 0.2166, P = 0.8486 (C, right). D Comparison of changes in sucrose preference in the first 30 min (left) and 120 min (right) between control and KD groups. n = 3, two-tailed unpaired t-test, t(4) = 0.0057, P = 0.9957 (left); t(4) = 0.2760, P = 0.7962 (left). E Paradigm of the snake intruder test. F, G Individual time spent in the snake corner (left), time spent inspecting the snake (middle), and immobile time (right) before and after virus injection in control (F) and KD (G) groups. n = 3, two-tailed paired t-test, t(2) = 1.000, P = 0.4226 (F, left); t(2) = 1.527, P = 0.2662 (F, middle); t(2) = 1.273, P = 0.3309 (F, right); t(2) = 0.7155, P = 0.5486 (G, left); t(2) = 1.492, P = 0.2742 (G, middle); t(2) = 0.5324, P = 0.6476 (G, right). H Comparison of changes in time spent in the snake corner (left), time spent inspecting the snake (middle), and immobile time (right) between control and KD groups. n = 3, two-tailed unpaired t-test, t(4) = 0.5368, P = 0.6198 (left); t(4) = 0.7391, P = 0.5009 (middle); t(4) = 1.222, P = 0.2889 (left). n.s., not significant; data are the mean ± SEM.
Increased Plasma Cortisol Level in CB1R KD Marmosets
Mental diseases usually accompany abnormal hormone secretion, such as increased cortisol due to anxiety [55]. Our behavioral results have shown that the knockdown of CB1Rs in the marmoset amygdala induces anxiety-like behaviors, which may change the plasma cortisol. Thus, morning blood was collected before and after the knockdown of CB1Rs in the amygdala, and plasma cortisol was measured using ELISA analysis (Fig. 6A). Interestingly, we found that the amygdala CB1R knockdown significantly increased the plasma cortisol level (Fig. 6B–D). Thus, these results further indicate that CB1R knockdown in the amygdala induces anxiety-like behaviors.
Fig. 6.
Increased plasma cortisol level in CB1R KD marmosets. A Paradigm of plasma cortisol level evaluation. B, C Individual plasma cortisol level before and after virus injection in control (B) and KD (C) groups. n = 3, two-tailed paired t-test, t(2) = 1.346, P = 0.3106 (B); t(2) = 2.990, P = 0.0960 (C). D Comparison of changes in plasma cortisol level between control and KD groups. n = 3, two-tailed unpaired t-test, t(4) = 2.933, P = 0.0427. *P < 0.05; data are the mean ± SEM.
Discussion
Using in vivo gene editing, Sanger sequencing, in situ hybridization, fluorescence staining, behavioral testing, and biochemical measurement, we studied the function of CB1Rs in the amygdala of adult marmosets. We found that AAV-mediated delivery of the CRISPR/Cas9 system successfully knocked down CB1Rs in the amygdala of adult marmosets and this induced anxiety-like behaviors. These symptoms included disrupted night sleep, agitated psychomotor activity in a new environment, decreased desire for vocal communication, and increased plasma cortisol levels. This improves our understanding of the eCB system in the amygdala of NHPs and advances the clinical application of eCB for diagnosing and treating of psychiatric disorders, such as anxiety.
Although rodents are the most commonly used animal models in neuroscience research, they differ from humans in multiple aspects, which may hinder us from understanding the brain mechanisms underlying human emotion, cognition, and mental disorders [56–58]. As such, studying the brains of NHPs is critical for translational research due to their similarity to humans [59]. Electrolytic lesions and pharmacological manipulations via cannulas are commonly used methods to explore the general function of the amygdala in NHPs [60–62]. But these methods cause non-negligible side effects and lack molecular marker specificity. Recently, the gene editing techniques widely used in rodents are not well established in NHPs, which greatly hinders us from understanding the brain mechanisms of human mental disorders. Here, for the first time, we established an in vivo gene-editing method via the CRISPR/Cas9 system to study the function of CB1Rs in the amygdala of marmosets. We validated that the CB1Rs in the amygdala were knocked down at the gene (Fig. S1A–E), mRNA (Fig. 1C, D), and protein levels (Fig. 1F, G) by using Sanger sequencing, in situ hybridization, and fluorescence staining. Western blotting is one of the most commonly used techniques to detect specific proteins in biological samples. However, we did not use this method because the marmoset brain was fixed with 4% paraformaldehyde when the animals were sacrificed. Paraformaldehyde treatment affects the depolymerization of proteins so that they cannot be separated by gels. Furthermore, Western blotting antibodies generally only recognize the primary structure of proteins, so they may not recognize formaldehyde-treated proteins either. We demonstrated the feasibility of virus-mediated gene editing in adult marmosets, thus providing a novel strategy to rapidly and cost-effectively develop transgenic marmoset models [28]. This approach can be applied rapidly to develop transgenic marmoset models for neuroscience research and the treatment of human gene-related diseases.
CB1R knockdown in the amygdala of marmosets induced various anxiety-like behaviors, which is consistent with previous reports that the amygdala and CB1Rs are involved in the onset and development of anxiety [2, 11, 63]. Notably, we detected sleep disturbance and agitation in the CB1R knockdown marmosets, a key pathological manifestation of human patients with anxiety disorders [64]. However, these have rarely been found in rodent models. Moreover, we found increased plasma cortisol levels in the KD marmosets, a representative biomarker of anxiety disorders in humans [55]; in contrast, this has rarely been found in rodents, either [65, 66]. Our results suggest that NHP models are crucial for identifying specific molecular biomarkers of human mental diseases for their diagnosis and treatment.
As CB1Rs work through synaptic modulation, and not all neurons in the amygdala express CB1Rs [7], a potential explanation for our findings may be that neurons that regulate the anxiety state may express higher levels of CB1Rs. Thus, the knockdown of CB1Rs may disrupt the anxiogenic-anxiolytic balance, thereby potentiating anxiety transmission by enhancing amygdala-related anxiogenic circuit activity. However, further circuit-based studies are required to test this hypothesis.
Inconsistent with previous rodent studies [7, 67], CB1R knockdown in marmosets did not lead to depression-like or fear behaviors. There are several possible reasons. First, the genetic and behavioral differences between rodents and NHPs may explain the different outcomes to some extent. Second, previous rodent studies knocked out CB1Rs in a circuit-specific, cell-type-specific manner, or in amygdala subregions, which currently is difficult to carry out in NHPs. Different amygdala subregions may functionally compensate for each other, which resulted in anxiety-like behavior after CB1R knockdown in the marmosets. Last, but not least, since CB1Rs are mainly distributed in the presynaptic membrane [11], the intra-amygdala infusion of CB1R agonists and antagonists may affect CB1Rs in the amygdala-projecting terminals rather than the amygdala itself, which may complicate the effects. In a word, the underlying mechanism still awaits further investigation.
There are several limitations in the current study. As mentioned above, we treated the amygdala as a whole, and different types of cells and subregions in the amygdala may compensate in function, leading to less dramatic changes in marmoset behavior. Thus, future studies are needed to target the different amygdala subregions or different types of neurons in the NHP. The use of Cre-expressing marmosets may also be insightful [23]. The small sample size (n = 3) is another limitation of the current study. Due to the coronavirus disease 2019 (COVID-19) epidemic, the animal sources for marmosets were completely cut off, which limited our study to meet the minimal number of animals for comparison between groups, which may explain why some behavioral results showed increasing or decreasing trends but did not reach significance. For example, individual KD animals had a significantly increased sleep latency (Fig. 2G) and decreased sleep duration (Fig. 2H) after the knockdown of CB1 in the amygdala. However, the comparison within the KD group (n = 3) only showed increasing (Fig. 2I) and decreasing trends (Fig. 2J) which did not reach the level of significance. The same is true for vocal behavior (Fig. 4D, E). We believe the number of animals is crucial for behavioral testing. A larger sample size can lead to statistically more reliable results than what we have now.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Zilong Qiu for providing the AAV-SaCas9 vector. We are grateful to Research Assistant Shuangshuang Liu from the Core Facilities of Zhejiang University School of Medicine, as well as Dr. Sanhua Fang and Research Assistant Li Liu from the Core Facilities of Zhejiang University Institute of Neuroscience. This work was supported by the Zhejiang Province Natural Science Foundation of China (LD22H090003), Key-Area Research and Development Program of Guangdong Province (2019B030335001 and 2018B030334001), the National Natural Science Foundation of China (31871070, 82090031, 32071097, 31871056, and 32170991), the Key R&D Program of Zhejiang Province (2020C03009), Fundamental Research Funds for the Central Universities (2021FZZX001-37), and the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-057).
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Lin Zhu, Di Zheng, and Rui Li contributed equally to this work.
Contributor Information
Lixia Gao, Email: lxgao10@zju.edu.cn.
Xiao-Ming Li, Email: lixm@zju.edu.cn.
References
- 1.Morrison SE, Salzman CD. Re-valuing the amygdala. Curr Opin Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gothard KM. Multidimensional processing in the amygdala. Nat Rev Neurosci. 2020;21:565–575. doi: 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunsmoor JE, Paz R. Fear generalization and anxiety: Behavioral and neural mechanisms. Biol Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Fan XC, Ma CN, Song JC, Liao ZH, Huang N, Liu X, et al. Rac1 signaling in amygdala astrocytes regulates fear memory acquisition and retrieval. Neurosci Bull. 2021;37:947–958. doi: 10.1007/s12264-021-00677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen CJ, Zheng D, Li KX, Yang JM, Pan HQ, Yu XD, et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–349. doi: 10.1038/s41591-018-0299-9. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara NC, Trask S, Rosenkranz JA. Maturation of amygdala inputs regulate shifts in social and fear behaviors: A substrate for developmental effects of stress. Neurosci Biobehav Rev. 2021;125:11–25. doi: 10.1016/j.neubiorev.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayakar R, Tone EB, Crosson B, Turner JA, Anderson PL, Phan KL, et al. Amygdala volume and social anxiety symptom severity: Does segmentation technique matter? Psychiatry Res Neuroimaging. 2020;295:111006. doi: 10.1016/j.pscychresns.2019.111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Choi K, Le T, McGuire J, Xing G, Zhang L, Li H, et al. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res. 2012;46:882–889. doi: 10.1016/j.jpsychires.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- 16.Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Häring M, Kaiser N, Monory K, Lutz B. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS One. 2011;6:e26617. doi: 10.1371/journal.pone.0026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Solís A, Vásquez KG, Prospéro-García O. Acute and subchronic administration of anandamide or oleamide increases REM sleep in rats. Pharmacol Biochem Behav. 2010;95:106–112. doi: 10.1016/j.pbb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Rueda-Orozco PE, Soria-Gómez E, Montes-Rodríguez CJ, Pérez-Morales M, Prospéro-García O. Intrahippocampal administration of anandamide increases REM sleep. Neurosci Lett. 2010;473:158–162. doi: 10.1016/j.neulet.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Morales M, De La Herrán-Arita AK, Méndez-Díaz M, Ruiz-Contreras AE, Drucker-Colín R, Prospéro-García O. 2-AG into the lateral hypothalamus increases REM sleep and cFos expression in melanin concentrating hormone neurons in rats. Pharmacol Biochem Behav. 2013;108:1–7. doi: 10.1016/j.pbb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Okano H, Sasaki E, Yamamori T, Iriki A, Shimogori T, Yamaguchi Y, et al. Brain/MINDS: A Japanese national brain project for marmoset neuroscience. Neuron. 2016;92:582–590. doi: 10.1016/j.neuron.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Okano H. Current status of and perspectives on the application of marmosets in neurobiology. Annu Rev Neurosci. 2021;44:27–48. doi: 10.1146/annurev-neuro-030520-101844. [DOI] [PubMed] [Google Scholar]
- 24.Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, Wang X. Marmosets: A neuroscientific model of human social behavior. Neuron. 2016;90:219–233. doi: 10.1016/j.neuron.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samandra R, Haque ZZ, Rosa MGP, Mansouri FA. The marmoset as a model for investigating the neural basis of social cognition in health and disease. Neurosci Biobehav Rev. 2022;138:104692. doi: 10.1016/j.neubiorev.2022.104692. [DOI] [PubMed] [Google Scholar]
- 26.Galvão-Coelho NL, Silva HP, Leão Ade C, de Sousa MB. Common marmosets (Callithrix jacchus) as a potential animal model for studying psychological disorders associated with high and low responsiveness of the hypothalamic-pituitary-adrenal axis. Rev Neurosci. 2008;19:187–201. doi: 10.1515/REVNEURO.2008.19.2-3.187. [DOI] [PubMed] [Google Scholar]
- 27.Kaas JH. Comparative functional anatomy of marmoset brains. Ilar J. 2020;61:260–273. doi: 10.1093/ilar/ilaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx V. Neurobiology: Learning from marmosets. Nat Methods. 2016;13:911–916. doi: 10.1038/nmeth.4036. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Wu S, Ma X, Li X, Cheng T, Chen Z, et al. Co-editing PINK1 and DJ-1 genes via adeno-associated virus-delivered CRISPR/Cas9 system in adult monkey brain elicits classical parkinsonian phenotype. Neurosci Bull. 2021;37:1271–1288. doi: 10.1007/s12264-021-00732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu SH, Li X, Qin DD, Zhang LH, Cheng TL, Chen ZF, et al. Induction of core symptoms of autism spectrum disorder by in vivo CRISPR/Cas9-based gene editing in the brain of adolescent rhesus monkeys. Sci Bull (Beijing) 2021;66:937–946. doi: 10.1016/j.scib.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Cao X, Zhu L, Qi R, Wang X, Sun G, Ying Y, et al. Effect of a high estrogen level in early pregnancy on the development and behavior of marmoset offspring. ACS Omega. 2022;7:36175–36183. doi: 10.1021/acsomega.2c03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Sharma J, Ke Q, Landman R, Yuan J, Chen H, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature. 2019;570:326–331. doi: 10.1038/s41586-019-1278-0. [DOI] [PubMed] [Google Scholar]
- 33.Farsi H, Harti D, Achaâban MR, Piro M, Ouassat M, Challet E, et al. Validation of locomotion scoring as a new and inexpensive technique to record circadian locomotor activity in large mammals. Heliyon. 2018;4:e00980. doi: 10.1016/j.heliyon.2018.e00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross CN, Adams J, Gonzalez O, Dick E, Giavedoni L, Hodara VL, et al. Cross-sectional comparison of health-span phenotypes in young versus geriatric marmosets. Am J Primatol. 2019;81:e22952. doi: 10.1002/ajp.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging. 2006;27:1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Yabumoto T, Yoshida F, Miyauchi H, Baba K, Tsuda H, Ikenaka K, et al. MarmoDetector: A novel 3D automated system for the quantitative assessment of marmoset behavior. J Neurosci Methods. 2019;322:23–33. doi: 10.1016/j.jneumeth.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: Antiphonal calling in common marmosets. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- 38.Miller CT, Beck K, Meade B, Wang X. Antiphonal call timing in marmosets is behaviorally significant: Interactive playback experiments. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:783–789. doi: 10.1007/s00359-009-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander L, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, Cockcroft GJ, et al. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron. 2019;101:307–320.e6. doi: 10.1016/j.neuron.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagami A, Yasue M, Nakagaki K, Nakamura M, Kawai N, Ichinohe N. Reduced childhood social attention in autism model marmosets predicts impaired social skills and inflexible behavior in adulthood. Front Psychiatry. 2022;13:885433. doi: 10.3389/fpsyt.2022.885433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montardy Q, Kwan WC, Mundinano IC, Fox DM, Wang L, Gross CT, et al. Mapping the neural circuitry of predator fear in the nonhuman primate. Brain Struct Funct. 2021;226:195–205. doi: 10.1007/s00429-020-02176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melamed JL, de Jesus FM, Maior RS, Barros M. Scopolamine induces deficits in spontaneous object-location recognition and fear-learning in marmoset monkeys. Front Pharmacol. 2017;8:395. doi: 10.3389/fphar.2017.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu P, Yue Y, Su J, Sun X, Du H, Liu Z, et al. Pattern decorrelation in the mouse medial prefrontal cortex enables social preference and requires MeCP2. Nat Commun. 2022;13:3899. doi: 10.1038/s41467-022-31578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandi-Perumal SR, Monti JM, Burman D, Karthikeyan R, BaHammam AS, Spence DW, et al. Clarifying the role of sleep in depression: A narrative review. Psychiatry Res. 2020;291:113239. doi: 10.1016/j.psychres.2020.113239. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Rawtaer I, Fam J, Jiang MJ, Feng L, Kua EH, et al. Sleep correlates of depression and anxiety in an elderly Asian population. Psychogeriatrics. 2016;16:191–195. doi: 10.1111/psyg.12138. [DOI] [PubMed] [Google Scholar]
- 46.Jumpertz R, Wiesner T, Blüher M, Engeli S, Bátkai S, Wirtz H, et al. Circulating endocannabinoids and N-acyl-ethanolamides in patients with sleep apnea—specific role of oleoylethanolamide. Exp Clin Endocrinol Diabetes. 2010;118:591–595. doi: 10.1055/s-0030-1253344. [DOI] [PubMed] [Google Scholar]
- 47.Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30:1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8. [DOI] [PubMed] [Google Scholar]
- 49.Spasojevic N, Stefanovic B, Jovanovic P, Dronjak S. Anxiety and hyperlocomotion induced by chronic unpredictable mild stress can be moderated with melatonin treatment. Folia Biol (Praha) 2016;62:250–257. doi: 10.14712/fb2016062060250. [DOI] [PubMed] [Google Scholar]
- 50.Penninx BW, Pine DS, Holmes EA, Reif A. Anxiety disorders. Lancet. 2021;397:914–927. doi: 10.1016/S0140-6736(21)00359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller CT, Wren Thomas A. Individual recognition during bouts of antiphonal calling in common marmosets. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:337–346. doi: 10.1007/s00359-012-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller CT, Mandel K, Wang X. The communicative content of the common marmoset phee call during antiphonal calling. Am J Primatol. 2010;72:974–980. doi: 10.1002/ajp.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rein B, Ma K, Yan Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat Protoc. 2020;15:3464–3477. doi: 10.1038/s41596-020-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi Y, Wu X, Zhou J, Cui W, Wang J, Hu Q, et al. Single-nucleus RNA sequencing reveals that decorin expression in the amygdala regulates perineuronal nets expression and fear conditioning response after traumatic brain injury. Adv Sci (Weinh) 2022;9:e2104112. doi: 10.1002/advs.202104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Feng G, Jensen FE, Greely HT, Okano H, Treue S, Roberts AC, et al. Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proc Natl Acad Sci U S A. 2020;117:24022–24031. doi: 10.1073/pnas.2006515117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, et al. Brains, genes, and primates. Neuron. 2015;86:617–631. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camus S, Ko WK, Pioli E, Bezard E. Why bother using non-human primate models of cognitive disorders in translational research? Neurobiol Learn Mem. 2015;124:123–129. doi: 10.1016/j.nlm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Jennings CG, Landman R, Zhou Y, Sharma J, Hyman J, Movshon JA, et al. Opportunities and challenges in modeling human brain disorders in transgenic primates. Nat Neurosci. 2016;19:1123–1130. doi: 10.1038/nn.4362. [DOI] [PubMed] [Google Scholar]
- 60.Braesicke K, Parkinson JA, Reekie Y, Man MS, Hopewell L, Pears A, et al. Autonomic arousal in an appetitive context in primates: A behavioural and neural analysis. Eur J Neurosci. 2005;21:1733–1740. doi: 10.1111/j.1460-9568.2005.03987.x. [DOI] [PubMed] [Google Scholar]
- 61.Wellman LL, Forcelli PA, Aguilar BL, Malkova L. Bidirectional control of social behavior by activity within basolateral and central amygdala of primates. J Neurosci. 2016;36:8746–8756. doi: 10.1523/JNEUROSCI.0333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dal Monte O, Costa VD, Noble PL, Murray EA, Averbeck BB. Amygdala lesions in rhesus macaques decrease attention to threat. Nat Commun. 2015;6:10161. doi: 10.1038/ncomms10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramikie TS, Patel S. Endocannabinoid signaling in the amygdala: Anatomy, synaptic signaling, behavior, and adaptations to stress. Neuroscience. 2012;204:38–52. doi: 10.1016/j.neuroscience.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SC, Kim YK. Anxiety disorders in the DSM-5: Changes, controversies, and future directions. Adv Exp Med Biol. 2020;1191:187–196. doi: 10.1007/978-981-32-9705-0_12. [DOI] [PubMed] [Google Scholar]
- 65.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 67.Roche M, O'Connor E, Diskin C, Finn DP. The effect of CB1 receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.