Abstract
Hepatic arterial infusion chemotherapy (HAIC) using a combination of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) has shown promise for hepatocellular carcinoma (HCC) patients classified under Barcelona Clinic Liver Cancer (BCLC) stage C. In China, the combined therapy of camrelizumab and apatinib is now an approved first-line approach for inoperable HCC. This study (NCT04191889) evaluated the benefit of combining camrelizumab and apatinib with HAIC-FOLFOX for HCC patients in BCLC stage C. Eligible patients were given a maximum of six cycles of HAIC-FOLFOX, along with camrelizumab and apatinib, until either disease progression or intolerable toxicities emerged. The primary outcome measured was the objective response rate (ORR) based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Thirty-five patients were enrolled. Based on RECIST v1.1 criteria, the confirmed ORR stood at 77.1% (95% CI: 59.9% to 89.6%), with a disease control rate of 97.1% (95% CI: 85.1% to 99.9%). The median progression-free survival was 10.38 months (95% CI: 7.79 to 12.45). Patient quality of life had a transient deterioration within four cycles of treatment, and generally recovered thereafter. The most frequent grade ≥3 or above treatment-related adverse events included reduced lymphocyte count (37.1%) and diminished neutrophil count (34.3%). The combination of camrelizumab, apatinib, and HAIC demonstrated encouraging results and manageable safety concerns for HCC at BCLC stage C.
Subject terms: Gastrointestinal cancer, Drug development
Introduction
Worldwide, primary liver cancer ranks as the sixth most frequent cancer diagnosis and stands third in terms of cancer-related mortality, with hepatocellular carcinoma (HCC) accounting for 75–80% of these primary liver cancer cases.1 Over 80% of HCC instances in China originate from Hepatitis B virus (HBV) infections, and the 5-year rates of overall survival (OS) range between a mere 10% to 18%.2–4 HCC that has progressed to the Barcelona Clinic Liver Cancer (BCLC) stage C, which is marked by a performance status of 1–2, significant vascular invasion such as portal vein tumor thrombosis (PVTT), and/or had extrahepatic metastasis, typically excludes surgical interventions.5 Besides, the presence of tumor thrombus and extra-hepatic metastasis in HCC are risk factors for poor prognosis.5 Despite the recent increase in therapeutic options BCLC stage C patients, there remains a need for new treatment strategies.
The union of PD-(L)1 inhibitors with agents targeting VEGF has marked a pivotal shift in HCC management, as evidenced initially in the IMbrave150 study.6 When antiangiogenic drugs are paired with anti-PD-1 treatments, there’s a suppression of immune checkpoint activity and an enhancement in T-cell functionality, leading to a more potent antitumor response compared to anti-PD-1 treatment alone.7,8 A combination of camrelizumab, a PD-1 antagonist, with apatinib, a tyrosine kinase inhibitor (TKI) focusing on VEGFR-2, has shown notable antitumor effects in advanced HCC cases.9 Recently, the international randomized controlled CARES-310 trial demonstrated that camrelizumab plus apatinib conferred a survival advantage over sorafenib for HCC patients with unresectable HCC.10 These findings have led to the approval of camrelizumab plus apatinib in China as an first-line treatment approach for unresectable HCC.
Hepatic arterial infusion chemotherapy (HAIC) using a combination of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) is effective in decreasing the intrahepatic tumor burden, as it allows for the targeted delivery of chemotherapy drugs to the arteries supplying the tumor.11 HAIC-FOLFOX yielded significantly prolonged OS with a better overall safety profile compared to transarterial chemoembolization (TACE) for large HCC.12 The combined use of HAIC-FOLFOX and sorafenib has shown improved survival rates in HCC patients, in contrast to using sorafenib alone.13 HAIC-FOLFOX plus lenvatinib and toripalimab (an anti-PD-1 antibody) were found to be feasible in treating advanced HCC patients with high-risk features in a recent single-arm trial.14
With all above mentioned, it is hypothesized that camrelizumab and apatinib in combination with HAIC may further improve outcomes. Consequently, this investigation was designed to evaluate the therapeutic benefit of camrelizumab and apatinib when used alongside HAIC-FOLFOX in HCC patients at BCLC stage C.
Results
Patients
From April 13, 2020 to May 10, 2022, 35 eligible patients were enrolled (Fig. 1 and Supplementary Fig. S1). Most of the participants (91.4%) were male, with 42.9% being above the age of 50. All the cases originated from HBV infection. A total of 16 patients (45.7%) had a PVTT of Vp 3 or 4, and five patients (14.3%) developed extrahepatic metastasis prior to enrollment (Table 1).
Fig. 1.
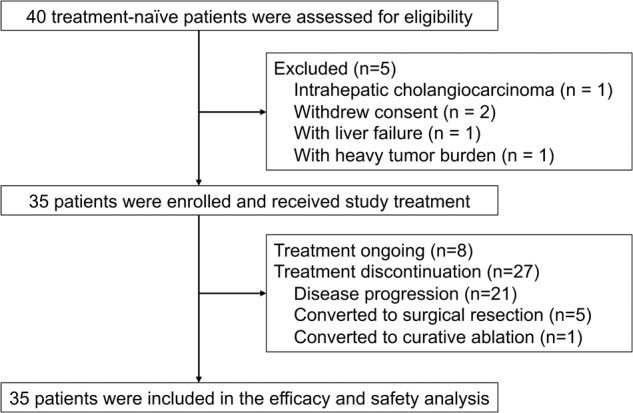
Patient flowchart
Table 1.
Baseline characteristics of patients
| Variables | All patients (n = 35) |
|---|---|
| Age, years, median (range) | 46 (27–67) |
| ≥50 | 15 (42.9%) |
| <50 | 20 (57.1%) |
| Sex, n (%) | |
| Male | 32 (91.4%) |
| Female | 3 (8.6%) |
| BMI, kg/m2, median (range) | 20.6 (17.3–28.8) |
| ≥24 | 10 (28.6%) |
| <24 | 25 (71.4%) |
| Etiology, n (%) | |
| Hepatitis B | 35 (100.00%) |
| ECOG performance status score, n (%) | |
| 0 | 16 (45.7%) |
| 1 | 18 (51.4%) |
| 2 | 1 (2.9%) |
| Child-Pugh score, n (%) | |
| 5 | 32 (91.4%) |
| 6 | 3 (8.6%) |
| ALBI grade, n (%) | |
| Grade 1 | 29 (82.9%) |
| Grade 2 | 6 (17.1%) |
| AFP, ng/mL, n (%) | |
| ≥400 | 19 (54.3%) |
| <400 | 16 (45.7%) |
| PIVKA-II, mAU/mL, median (range) | 8392.4 (42.0–75000.0) |
| Tumor size, cm, median (range) | 10.5 (4.7–18.9) |
| ≥10 | 21 (60.0%) |
| <10 | 14 (40.0%) |
| Venous tumor thrombus, n (%) | 31 (88.6%) |
| PVTT, n (%) | |
| Vp1 | 3 (8.6%) |
| Vp2 | 6 (17.1%) |
| Vp3 | 8 (22.9%) |
| Vp4 | 8 (22.9%) |
| Absent | 10 (28.6%) |
| IVCTT, n (%) | |
| Hepatic vein invasion | 8 (22.9%) |
| IVC invasion | 2 (5.7%) |
| Absent | 25 (71.4%) |
| Extrahepatic metastasis, n (%) | 5 (14.3%) |
BMI body mass index, ECOG Eastern Cooperative Oncology Group, ALBI albumin-bilirubin, AFP alpha-fetoprotein, PIVKA-II prothrombin in vitamin K absence II, PVTT portal vein tumor thrombosis, IVCTT inferior vena cava tumor thrombosis, IVC inferior vena cava
Efficacy
Up to September 30, 2022, the median duration of follow-up stood at 23.10 months (95% confidence interval [CI], 17.44 to 28.76). The median cycles of HAIC were 6 (range, 4 to 6); the median cycles of camrelizumab were 9 (range, 4 to 32), and the median duration for apatinib was 9.1 months (range, 1.8 to 27.9).
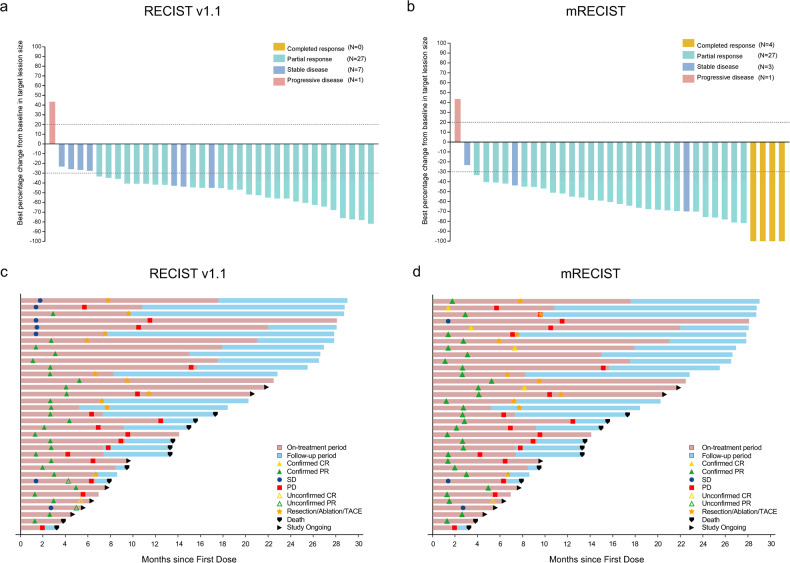
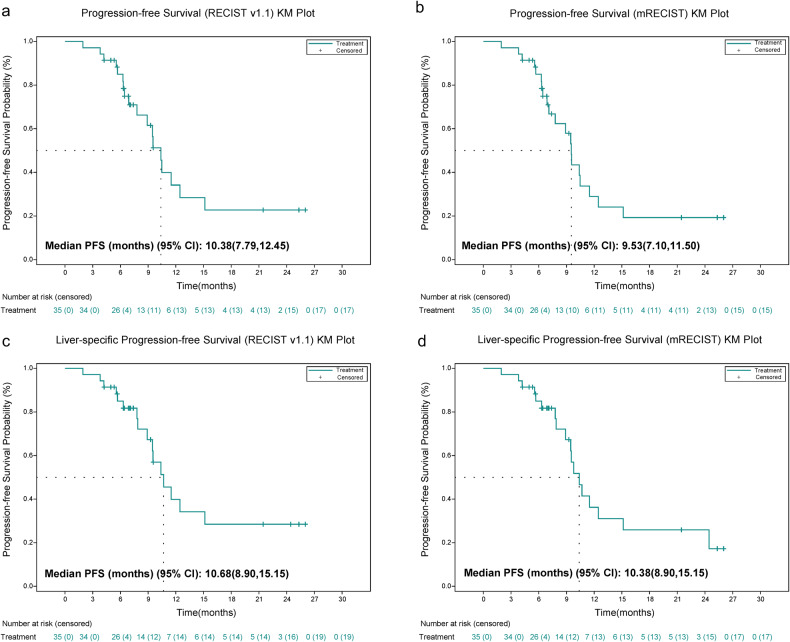
Based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, the confirmed objective response rate (ORR) stood at 77.1% (95% CI: 59.9% to 89.6%), and the disease control rate (DCR) was 97.1% (95% CI: 85.1% to 99.9%) (Fig. 2). The confirmed ORR per modified RECIST (mRECIST) was 88.6% (95%CI, 73.3% to 96.8%). The subgroup analysis of ORR is presented in Supplementary Fig. S2. The ORR was consistent among all subgroups, including those with tumor size ≥10 cm or PVTT of Vp 3 or 4. The median time for progression-free survival (PFS) was established at 10.38 months (95% CI: 7.79 to 12.45), with the 6-, 12-, 18-month PFS rate of 85.0%, 34.2%, and 22.8%, respectively. When focusing on liver-specific PFS, the median duration was 10.68 months (95% CI: 8.90 to 15.15) (Fig. 3). The time to response (TTR) was 2.66 months (95% CI: 2.10 to 2.89), and the median duration of response (DoR) was 7.52 months (95% CI: 4.83 to 12.52). The median OS remains not reached, and the 6-, 12-, 24-month OS rates were 94.3%, 87.4%, and 65.0%, respectively (Table 2 and Supplementary Fig. S3). After the triple combination treatment, six of 35 patients (17.1%) achieved disease downstaging and received curative therapy (five patients underwent R0 resection, and one patient received curative ablation).
Fig. 2.
Treatment response and duration. a Best percentage changes from baseline in target lesions per RECIST v1.1; b best percentage changes from baseline in target lesions per mRECIST; c treatment exposure and response duration per RECIST v1.1; and d treatment exposure and response duration per mRECIST
Fig. 3.
Survival analysis. a Kaplan–Meier curves of progression-free survival (PFS) per RECIST v1.1; b Kaplan–Meier curves of PFS per mRECIST; c Kaplan–Meier curves of liver-specific PFS per RECIST v1.1; d Kaplan–Meier curves of liver-specific PFS per mRECIST
Table 2.
Tumor response
| Variables | All patients (n = 35) | |
|---|---|---|
| RECIST v1.1 (n = 35) | mRECIST (n = 35) | |
| Best objective response, n (%) | ||
| Complete response | 0 | 4 (11.4%) |
| Partial response | 27 (77.1%) | 27 (77.1%) |
| Stable disease | 7 (20.0%) | 3 (8.6%) |
| Progressive disease | 1 (2.9%) | 1 (2.9%) |
| Objective response rate, n (%) | 27 (77.1%) | 31 (88.6%) |
| 95% CI | (59.9%, 89.6%) | (73.3%, 96.8%) |
| Disease control ratea, n (%) | 34 (97.1%) | 34 (97.1%) |
| 95% CI | (85.1%, 99.9%) | (85.1%, 99.9%) |
| TTR, months, median (95% CI) | 2.66 (2.10, 2.89) | 2.63 (1.38, 2.73) |
| DOR, months, median (95% CI) | 7.52 (4.83, 12.52) | 6.70 (5.09, 9.66) |
| PFS, months, median (95% CI) | 10.38 (7.79, 12.45) | 9.53 (7.10, 11.50) |
| 6-month PFS rate | 85.0% | 85.0% |
| 12-month PFS rate | 34.2% | 28.9% |
| 18-month PFS rate | 22.8% | 19.3% |
| Liver-specific PFS, months, median (95% CI) | 10.68 (8.90, 15.15) | 10.38 (8.90, 15.15) |
| 6-month PFS rate | 85.0% | 85.0% |
| 12-month PFS rate | 39.9% | 36.3% |
| 18-month PFS rate | 28.8% | 25.9% |
TTR time to response, DOR duration of response, PFS progression-free survival, CI confidence interval, RECIST Response Evaluation Criteria in Solid Tumors, mRECIST modified Response Evaluation Criteria in Solid Tumors
aFor patients with stable disease, it should persist for a minimum of 6 weeks
Exploratory endpoints
All patients were included in the quality of life (QoL) analysis. The global health status, as well as all functioning and most symptoms had a transient deterioration within four cycles of treatment, and generally recovered thereafter (Supplementary Fig. S4). The time to deterioration (TTD) was not reached (Supplementary Fig. S5). Following treatment, we observed a substantial reduction in the levels of prothrombin in vitamin K absence II and alpha-fetoprotein. During treatment, some patients exhibited a transient deterioration in liver function, as indicated by a temporary rise in the albumin-bilirubin (ALBI) score to level 2. However, following administration of liver-supportive care, the majority of these patients experienced a restoration or even improvement of liver function, with ALBI score returning to level 1 (Supplementary Fig. S6).
Safety
All patients experienced treatment-related adverse events (TRAEs) (Table S1). TRAEs of grade ≥3 or above were evident in 26 patients (74.3%), with the predominant events being decreased lymphocyte count (37.1%) and reduced neutrophil count (34.3%) (Table 3). Reactive cutaneous capillary endothelial proliferation (RCCEP) was observed in 13 patients (37.1%), with the majority presenting at grade 1 (Table S2). Hypertension related to apatinib manifested in 14 (40.0%) patients, primarily at grades 1 and 2 (Table S3). HAIC-induced reductions in neutrophil and lymphocyte counts were observed in 29 (82.9%) and 27 (77.1%) patients, respectively (Table S4).
Table 3.
Treatment-related adverse events of all grades occurring in more than 15% of patients
| Events, n (%) | All patients (n = 35) | ||
|---|---|---|---|
| Any grade | Grade 1–2 | Grade 3 or higher | |
| Aspartate aminotransferase increased | 34 (97.1) | 24 (68.6) | 10 (28.6) |
| Alanine aminotransferase increased | 33 (94.3) | 26 (74.3) | 7 (20.0) |
| Hypoalbuminemia | 31 (88.6) | 31 (88.6) | 0 |
| Neutrophil count decreased | 29 (82.9) | 17 (48.6) | 12 (34.3) |
| Lymphocyte count decreased | 27 (77.1) | 14 (40.0) | 13 (37.1) |
| Anemia | 25 (71.4) | 23 (65.7) | 2 (5.7) |
| Platelet count decreased | 23 (65.7) | 15 (42.9) | 8 (22.9) |
| Blood bilirubin increased | 22 (62.9) | 19 (54.3) | 3 (8.6) |
| Proteinuria | 22 (62.9) | 22 (62.9) | 0 |
| Abdominal pain | 21 (60.0) | 21 (60.0) | 0 |
| White blood cell decreased | 20 (57.1) | 14 (40.0) | 6 (17.1) |
| Weight loss | 17 (48.6) | 17 (48.6) | 0 |
| Anorexia | 17 (48.6) | 17 (48.6) | 0 |
| Hyperglycemia | 16 (45.7) | 16 (45.7) | 0 |
| Hyponatremia | 15 (42.9) | 14 (40.0) | 1 (2.9) |
| Hyperuricemia | 15 (42.9) | 15 (42.9) | 0 |
| Hypokalemia | 14 (40.0) | 12 (34.3) | 2 (5.7) |
| Rash | 14 (40.0) | 12 (34.3) | 2 (5.7) |
| Hypertension | 14 (40.0) | 9 (25.7) | 5 (14.3) |
| Hand-foot syndrome | 13 (37.1) | 10 (28.6) | 3 (8.6) |
| RCCEP | 13 (37.1) | 12 (34.3) | 1 (2.9) |
| Diarrhea | 13 (37.1) | 12 (34.3) | 1 (2.9) |
| Vomiting | 12 (34.3) | 12 (34.3) | 0 |
| Fatigue | 11 (31.4) | 11 (31.4) | 0 |
| Hematuria | 11 (31.4) | 11 (31.4) | 0 |
| Upper respiratory infection | 9 (25.7) | 9 (25.7) | 0 |
| Gingival hemorrhage | 9 (25.7) | 9 (25.7) | 0 |
| Fever | 8 (22.9) | 8 (22.9) | 0 |
| Oral mucositis | 8 (22.9) | 8 (22.9) | 0 |
| Gingivitis | 8 (22.9) | 8 (22.9) | 0 |
| Ascites | 7 (20.0) | 7 (20.0) | 0 |
| Epistaxis | 7 (20.0) | 7 (20.0) | 0 |
| Cough | 6 (17.1) | 6 (17.1) | 0 |
| Headache | 6 (17.1) | 6 (17.1) | 0 |
RCCEP reactive cutaneous capillary endothelial proliferation
AEs prompted dose reductions in two patients, while four patients ceased treatment due to AEs (Table S1). A total of 13 patients experienced serious AE (SAE), with platelet count decreased (14.3%) as the most common (Table S5). Five (14.3%) patients experienced immune-related SAEs, including one case of immune dermatitis (grade 3), one case of RCCEP (grade 3), one case of thrombocytopenia (grade 3), and two cases of immune hepatitis (grade 3, grade 4, respectively); all of them recovered after camrelizumab discontinuation and steroid treatment.
Discussion
The therapeutic landscape of HCC has undergone significant advancements with the integration of antiangiogenic agents and immunotherapy. As demonstrated in the IMbrave150 study, atezolizumab and bevacizumab achieved an ORR of 27.3% and a median PFS spanning 6.8 months for HCC.6 Likewise, the CARES-301 study showed that camrelizumab plus apatinib yielded an ORR of 25.4% and a median PFS of 5.6 months.10 The triple combination treatment in our study showed a numerically higher ORR (77.1% per RECIST v1.1) and prolonged PFS (10.38 months) than that of above studies, which suggested that HCC in BCLC stage C may benefit from the addition of HAIC to camrelizumab and apatinib. A synergistic antitumor effect of HAIC-FOLFOX, apatinib, and camrelizumab may be responsible for survival benefit in our study.7,15,16 In HAIC, chemotherapy agents are infused directly into tumors for about 50 h. The oxaliplatin and fluorouracil in HAIC-FOLFOX induces tumor cell death, which release tumor antigens.17 Apatinib is started on day 8 after HAIC treatment to allow for a recovery period, which is beneficial for the transient liver function damage caused by HAIC, and to reduce the liver toxicity of combined chemotherapy and other drugs. Additionally, low-dose apatinib induces prolonged vascular normalization, which reduces tumor hypoxia and acidosis and enhanced the efficacy of the infiltrating immune cells.18 Anti-PD-1 therapy targets immune checkpoint and activates cytotoxic T lymphocyte function, thereby providing a more favorable antitumor activity.7,8 Therefore, camrelizumab is initiated in the second cycle of treatment.
The prognosis of HCC patients with high-risk features remains suboptimal. Our study also enrolled patients at high-risk, including eight patients with Vp 4 PVTT and 21 with tumors larger than 10 cm. According to the subgroup analysis, the combination of camrelizumab, apatinib and HAIC-FOLFOX yielded high response rate for these patients. Notably, one patient who initially met the inclusion criteria with an ECOG PS score of 1 experienced a deterioration to 2 before treatment. Despite this, the patient showed improvement in ECOG PS score and achieved a PR after treatment. This observation suggests that the regimen may extend benefits to patients with poorer ECOG PS scores, although further research is needed to confirm this. Consistent with our results, Lai et al. also demonstrated that combining HAIC-FOLFOX with lenvatinib and toripalimab is a viable option for HCC patients exhibiting high-risk characteristics.14 Taking these results into account, camrelizumab, apatinib and HAIC was beneficial for patients with unresectable HCC, even for those at high risk.
After the triple combination treatment, six patients (17.1%) achieved disease downstaging and received curative therapy, including five patients who underwent R0 resection, and one patient who received curative ablation. This advantage has also been discussed in several other studies about HAIC. According to a study in patients with large HCC, a notably increased rate of curative surgical resection was evident in the HAIC-FOLFOX group compared to the TACE group (24% vs. 12%).12 Furthermore, disease downstaging was achieved in up to 12% of HCC patients who underwent treatment with either HAIC in combination with sorafenib or HAIC as a standalone therapy.13,19
The QoL of patients with malignancies are of paramount importance, particularly since it is proven to affect the long-term prognosis of the patients.20 In our analysis, compared with the baseline, most patients experienced a transient deterioration in QoL within four cycles of treatment, followed by a gradual improvement. Interestingly, it appeared that the improvement of QoL coincided with the control of disease. The REFLECT study also found that responders were related to a lower risk of deterioration and better scores in QoL than non-responders.21 This emphasized the importance of effective tumor control to improve the QoL of patients.
In our findings, the combination of HAIC with camrelizumab and apatinib was generally well-tolerated. The frequency and severity of AEs encountered in our cohort aligned with the established safety profiles of HAIC and the combination of camrelizumab and apatinib, as indicated in prior research.9,22 The combination of agents did not appear to induce any unusual overlapping toxicity. The occurrence of grade 3 TRAEs was consistent with the results from the RESCUE study.9 What is noteworthy is that we made some modifications in HAIC by reducing the dose oxaliplatin from 130 mg/m2 to 85 mg/m2, and administering an analgesic agent concurrently with the infusion to avoid substantial abdominal pain caused by direct injection of oxaliplatin into the intrahepatic artery.
This trial is not without its limitations. First, the lack of a control arm in our single-arm design makes it difficult to definitively attribute the observed benefits solely to the addition of systemic therapy following HAIC. Second, the study’s sample size was limited, and although we met the predetermined criteria of Simon’s two-stage design, early termination of the study could potentially result in an overestimation of the ORR. To comprehensively evaluate the merits of the triple combination, we have initiated a randomized controlled phase 3 trial (NCT05313282) aimed at assessing its benefit compared to camrelizumab plus apatinib in HCC patients with BCLC Stage C. Third, the study exclusively enrolled patients with HBV infection, representing 85–90% of HCC cases in China.23,24 While this focus aligns with the prevalent etiological factors of HCC in China, it may restrict the extrapolation of our findings to HCC cases stemming from other causes. It’s important to highlight that China accounts for half of both the global incidence and mortality of HCC.1,25 Thus, the study’s focus on HBV-related HCC holds significant implications not just for China, but for global HCC treatment strategies as well. Forth, the average age of the patients was 46, which may appear young but is actually reflective of the typical HCC patient demographic in China. The mean age of HCC diagnosis in China is 52, significantly younger than that in Western countries and Japan.26,27 This variation can largely be ascribed to the chronic HBV infection, which is a primary factor leading to HCC in China. In our cohort, 57.1% of the patients were under the age of 50, and 42.9% were 50 or older, mirroring the age distribution for HBV-related HCC in China.
In summary, the regimen combining camrelizumab, apatinib, and HAIC demonstrated efficacy and safety in treating BCLC stage C HCC patients. Conclusions with more powerful evidence may be obtained from the phase 3 study in the near future.
Methods
Patients
TRIPLET represents a single-arm trial. Essential criteria for patient inclusion encompassed: age between 18 and 70 years; a clinical or pathological HCC diagnosis based on the American Association for the Study of Liver Diseases criteria;28 classification within BCLC stage C;5 no prior anti-tumor treatment exposure; presence of at least one measurable intrahepatic tumor as per RECIST v1.1; an Eastern Cooperative Oncology Group (ECOG) performance status score of either 0 or 1; and a Child-Pugh score of ≤7. Patients with autoimmune disease, uncontrolled hypertension or high risk of bleeding were ruled out. Detailed eligibility criteria can be found in protocol.
Ethics statements
All participants gave their written consent prior to being included in the study. The study protocol was approved by the Institutional Review Boards (B2019-187-01). This research has been registered on ClinicalTrials.gov under the identifier NCT04191889.
Procedures
After enrollment, patient underwent up to six cycles of HAIC, each lasting 21 days, with the FOLFOX regimen. This regimen consisted of a 2-h infusion of oxaliplatin at 85 mg/m2, a 2–3-h administration of leucovorin at 400 mg/m2, and a 46-h delivery of fluorouracil at 2500 mg/m2. In addition, all participants were administered camrelizumab (200 mg intravenously, commencing on day 4 of the second HAIC cycle and repeated every 21 days) and apatinib (250 mg daily, taken orally, beginning on day 8 of the initial HAIC cycle) until disease progression or unacceptable toxicities (Supplementary Fig. S1).
HAIC was performed by inserting a 5-French Yashiro catheter (Terumo Corporation, Tokyo, Japan) through the femoral artery with a 2.7-French microcatheter inside, and then, advancing the tip of the microcatheter to the tumor-feeding artery, guided by concurrent arteriography. Tumors were accessed via the right or left hepatic artery. When a tumor demonstrated additional blood supply from extrahepatic sources, catheter tip was positioned in the main feeding artery. Besides, branch arteries were embolized with blank microspheres. In the event that there is a short access path from intrahepatic arteries leading to chemical agents flowing into the gastroduodenal artery, coils would be used to embolize it. The administration of chemotherapeutic agents for HAIC was completed within 3 days after hepatic catheter placement. The catheter and sheath were removed after the completion of each HAIC.
Combination therapy was discontinued when disease progression, disease downstaging to have an opportunity to perform curative treatment, unacceptable toxicities, or death occurred. In the event of grade ≥3 or serious TRAEs, the related study treatment should be discontinued, and the other two were allowed to continue.
Every 6 weeks until treatment completion, tumor responses were evaluated using dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), following both RECIST v1.1 and mRECIST criteria. In the event that a patient achieves complete response (CR) or partial response (PR), the response must be confirmed no less than 4 weeks of the initial evaluation. AEs during the treatment were recorded or graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) 4.0.
Outcomes
The primary objective was to determine the ORR according to RECIST v1.1, which was defined as the percentage of participants experiencing either a CR or PR. Secondary outcomes encompassed ORR as determined by mRECIST, DCR, TTR, DoR, PFS, liver-specific PFS, OS, along with 6-month and 12-month PFS and OS rates. A comprehensive definition of these secondary endpoints is provided in the study protocol. An exploratory objective of this study was to evaluate the QoL, gauged using the European Organization for Research and Treatment of Cancer QoL questionnaire (EORTC QLQ-C30) [30]. This assessment was conducted at the study’s onset and then every 6 weeks until the treatment concluded. Scores from the EORTC QLQ-C30 were converted to a scale ranging from 0 to 100. On this scale, a higher score indicates improved functioning but increased symptom severity. The TTD in QoL was determined as treatment initiation to the first observed decline of 10 or more points from the baseline score.
Statistical analysis
A Simon’s 2-stage design was adopted in this study, with a one-sided α of 2.5% and to guarantee the power over 80%. The null hypothesis of ORR per RECIST v1.1 was 40%, and the alternative hypothesis was 60.8%. If a response is observed in over 11 out of the initial 26 evaluated patients during the first phase, an additional 21 patients would be recruited for the study. The treatment would be deemed worthy of further investigation if more than 25 patients exhibit a response.
All participants who underwent at least one study treatment were considered for both efficacy and safety analyses. Both ORR and DCR were presented with their two-sided 95% CI: calculated using the Clopper-Pearson approach. The median values for time-to-event variables were determined through the Kaplan–Meier technique, and their respective 95% CI were derived using the Brookmeyer and Crowley method. All statistical evaluations were carried out using SAS® software (version 9.4, SAS Institute Inc, Cary, USA).
Supplementary information
Acknowledgements
This study was supported by Guangzhou Science and Technology Program Civic Technology Research Plan (No. 201903010037) and Guangdong Natural Science Foundation (No. 2020A1515011539). This study was also supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd. We express our gratitude to Zhiguo Hou (Jiangsu Hengrui Pharmaceuticals) for his guidance in study formulation, to Shaofen Huo (Jiangsu Hengrui Pharmaceuticals) for her insights in data interpretation, and to Ni Guan and Xinwei Zhong (Jiangsu Hengrui Pharmaceuticals) for their statistical expertise. Additionally, we appreciate Zhongjiang Chen (Jiangsu Hengrui Pharmaceuticals) for his assistance in medical writing.
Author contributions
T.-Q.Z., Z.-J.G., and M.-X.Z. equally contributed to the data acquisition, analysis, and interpretation. They were responsible for crafting the figures and tables and played a primary role in drafting the manuscript. Z.-L.H. reviewed all patient data. J.-B.L. completed the statistical analysis. Y.-K.G., P.-H.W., and J.-H.H. performed critical revisions of the manuscript. Y.-K.G. and T.-Q.Z. have taken responsibility to address any queries related to the manuscript’s accuracy and integrity. Both ensured that issues, if any, were thoroughly investigated and resolved. Y.-K.G. and P.-H.W. significantly contributed to the study’s conception and design and granted final approval of the manuscript version intended for publication. All authors have read and approved the article.
Data availability
The datasets supporting the conclusions of this manuscript are available in the Sun Yat-sen University Cancer Center Research Data Deposit repository (RDDA2020001690).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Tian-Qi Zhang, Zhi-Jun Geng, Meng-Xuan Zuo.
Contributor Information
Pei-Hong Wu, Email: wuph@sysucc.org.cn.
Yang-Kui Gu, Email: guyk@sysucc.org.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-023-01663-6.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. Worldwide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob. Health. 2018;6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Reig M, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 7.Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med.9, 10.1126/scitranslmed.aak9679 (2017). [DOI] [PMC free article] [PubMed]
- 8.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin. Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 10.Qin, S. et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet10.1016/S0140-6736(23)00961-3 (2023). [DOI] [PubMed]
- 11.Lyu N, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J. Hepatol. 2018;69:60–69. doi: 10.1016/j.jhep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Li QJ, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J. Clin. Oncol. 2022;40:150–160. doi: 10.1200/JCO.21.00608. [DOI] [PubMed] [Google Scholar]
- 13.He M, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai Z, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur. J. Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res. 2015;3:436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol. Res. 2019;7:630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 19.Lyu N, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1) J. Clin. Oncol. 2022;40:468–480. doi: 10.1200/JCO.21.01963. [DOI] [PubMed] [Google Scholar]
- 20.Quinten C, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J. Natl Cancer Inst. 2011;103:1851–1858. doi: 10.1093/jnci/djr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel A, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021;6:649–658. doi: 10.1016/S2468-1253(21)00110-2. [DOI] [PubMed] [Google Scholar]
- 22.Hua X, et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002) Clin. Cancer Res. 2022;28:637–645. doi: 10.1158/1078-0432.CCR-21-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan SL, Wong VW, Qin S, Chan HL. Infection and cancer: the case of hepatitis B. J. Clin. Oncol. 2016;34:83–90. doi: 10.1200/JCO.2015.61.5724. [DOI] [PubMed] [Google Scholar]
- 24.The Chinese Society of Clinical Oncology (CSCO) Expert Committee. CSCO Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma. Beijing, China: People’s Medical Publishing House. 2022.
- 25.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J. Hepatol. 2020;72:250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021;15:1295–1307. doi: 10.1080/17474124.2021.1991792. [DOI] [PubMed] [Google Scholar]
- 27.Park JW, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimbach JK, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this manuscript are available in the Sun Yat-sen University Cancer Center Research Data Deposit repository (RDDA2020001690).