Abstract
Several antibiotics have been reported to lessen the ovarian suppression produced by oral contraceptive agents, as a result of drug interactions. The present investigation was designed to study the likelihood of the occurrence of any such interaction between the fluoroquinolone antibiotic ciprofloxacin (Ciproxin) at a dosage of 500 mg twice a day and the “low-dose” oral contraceptive Marvelon (30 μg of ethinyl estradiol [EE] plus 150 μg of desogestrel). Twenty-four healthy female volunteers were studied in a double-blind, placebo-controlled, randomized crossover trial. There were no significant differences between measurements of the area under the concentration-time curve of EE up to 24 h after oral contraceptive intake during placebo and ciprofloxacin administration on days 11 and 16 of the cycles, indicating the absence of pharmacokinetic interaction. Similarly, no clinically significant differences in the levels of sex hormone binding globulin were found between the placebo and ciprofloxacin cycles, indicating no major variation in EE levels during ciprofloxacin and placebo treatment. Ten subjects in each of the placebo and ciprofloxacin groups had early-follicular-phase levels of 17-β estradiol (<184 ng/liter) at one or more points during their cycles, but none had values above the early-follicular-phase range, indicating no significant ovarian activity. In addition, all subjects had progesterone levels of <2 ng/ml, indicating the absence of ovulation. Only two subjects, who received the placebo, had evidence of sustained follicular growth to a potentially ovulatory follicle (∼18 mm). We conclude that ciprofloxacin does not interfere with the ovarian suppression produced by the low-dose oral contraceptive Marvelon.
Since the introduction of oral contraceptives in 1961, there has been a trend of reducing the estrogen content of the preparations in order to minimize side effects. With the lowering of ethinyl estradiol (EE) levels in the development of the “low-dose” pill in order to lessen the risk of complications, the estrogen content of oral contraceptives may have reached its minimal therapeutic level (2, 14); further lowering of the concentration of EE in the pill may no longer guarantee effective contraception. This can occur during the concomitant use of certain drugs, either as a consequence of interference with the enterohepatic circulation or by induction of hepatic microsomal drug-metabolizing enzymes (2, 3, 5, 14). A retrospective analysis concluded that 41% of unwanted pregnancies among oral-contraceptive users occurred during the concomitant use of antibiotics (excluding tuberculostatics) (13). In one study investigating the possible interaction of ofloxacin (200 mg twice a day for 7 days) with a low-dose oral contraceptive (Microgynon), no indications of ovulation were detected (4).
In view of the potential impact of this type of drug interaction on the reliability of oral contraceptive treatment, we have undertaken a single-center, double-blind, placebo-controlled, randomized crossover trial to evaluate the possible interaction of the fluoroquinolone antibiotic ciprofloxacin (Ciproxin), administered orally, with an oral low-dose contraceptive (Marvelon).
MATERIALS AND METHODS
This phase I study was designed to assess whether ciprofloxacin affects the contraceptive effect of Marvelon (containing 30 μg of EE plus 150 μg of desogestrel; Organon Laboratories Ltd., Cambridge, United Kingdom) by lowering the levels of these exogenous hormones (e.g., EE) in plasma and thus permitting the increase of the circulating levels of the endogenous hormones estradiol and progesterone, thereby blocking the effective suppression of the reproductive cycle. The study also evaluated follicle ripening in ovaries, which is suppressed by Marvelon, by measuring the increase in mean follicular diameter in the subovulatory cycles (∼18 mm) during treatment with ciprofloxacin.
Patients.
The study was a double-blind, placebo-controlled, single-center crossover trial involving 24 healthy female volunteers, aged 19 to 32 years, with body weights between 51 and 82 kg (within 90 to 120% of the normal range). Based upon the sample size calculation 12 volunteers was sufficient to prove that there was no interaction, up to a geometric standard deviation of 1.61 for the ratio of the area under the concentration-time curve (AUC) of EE. In view of the secondary reliability parameter (induction of follicle ripening) an increase to at least 24 volunteers was recommended.
Each individual had regular, normal menstrual cycles before starting contraceptive use or had proven ovulatory cycles (i.e., was postpartum). All subjects had used Marvelon without gynecological problems or side effects for ∼3 months. No other medication was being taken by the study subjects, and no antibiotic had been taken for ∼2 months. Each subject was willing to use an additional contraceptive (e.g., condoms or an intrauterine device). All subjects gave written consent after receiving detailed written information. The study was approved by the ethics committee (Commissie Wetenschappelijk Onderzoek bij Mensen) of the University Hospital of Utrecht, Utrecht, The Netherlands.
The volunteers were divided into two blocks of 12 individuals, who were randomly allocated to one of two treatment order groups, with six individuals per group (Table 1). All subjects received Marvelon, one tablet daily, on days 8 to 28 (a total of 21 days) of each cycle (day 1 being the first day of the stop week). Ciprofloxacin (500 mg) or the placebo was administered twice daily on days 8 to 17 (a total of 10 days) of cycles I and III. The placebo matched the active drug in appearance, taste, and weight. The first morning dose of ciprofloxacin or the placebo was taken with the Marvelon tablet.
TABLE 1.
Random allocation of 24 subjects
| Block (no. of subjects) | Treatment order group (no. of subjects) | Treatment received during cycle
|
||
|---|---|---|---|---|
| I | II | III | ||
| I (12) | 1 (6) | Ciprofloxacin | Washout | Placebo |
| 2 (6) | Placebo | Washout | Ciprofloxacin | |
| II (12) | 1 (6) | Ciprofloxacin | Washout | Placebo |
| 2 (6) | Placebo | Washout | Ciprofloxacin | |
Measurements.
All 24 subjects had a transvaginal ultrasound (ALOKA ultrasonograph with a 5-MHz transducer) performed at days 8, 10, 12, and 14. If on day 14 the diameters of the follicles seemed to be ≥10 mm, additional measurements were performed.
The 12 subjects in block I had levels of the following in their blood measured on cycle days 11 and 16: EE, by radioimmunoassay (with a kit from Hazleton, Washington, D.C.); sex hormone binding globulin (SHBG), by time-resolved immunoassay (DELFIA kit; Wallac); and ciprofloxacin, by high-performance liquid chromatography (8) with a calibration range of 0.010 to 3.00 mg/liter. Samples were obtained 0, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 23.59 h after dosing.
All 24 subjects during both cycles had progesterone levels measured by time-resolved fluoroimmunoassay (DELFIA kit; Wallac) on day 20 and additionally on days 16, 24, and 28 if the follicles had a diameter of ≥10 mm.
Single measurements of 17-β estradiol (E2) in serum, by time-resolved fluoroimmunoassay (DELFIA kit; Wallac), were done for all 24 subjects on days 1, 8, 10, 12, and 14. These measurements were repeated on days 16, 20, 24, and 28 for subjects with a follicle diameter of ≥10 mm on day 14.
Pharmacokinetic parameters were calculated in accordance with the guidelines found in reference 2a. Hemoglobin and hematocrit values were obtained on days 8 and 20 of cycle I and were repeated during cycle III if thought to be necessary by the investigator. Adverse reactions were recorded.
Criteria of evaluation.
The primary criteria assessed in the 12 subjects of block I included the AUC from 0 to 24 h (AUC0–24) of EE on day 11 of cycles I and III. Secondary criteria included the following: AUC0–24 of EE on day 16 (based on similar measurements for the 12 volunteers of block I); progesterone levels on day 20 of the cycle (for all 24 subjects); SHBG levels on cycle days 1, 8, 10, 12, and 14 of both treatment periods (for all 24 subjects); and single measurements of serum E2 levels on days 1, 8, 10, 12, and 14 for all volunteers. If on day 14 the diameters of the follicles were ∼10 mm, measurements of E2 levels were repeated on days 16, 20, 24, and 28. The diameters of the follicles on day 10 of the cycle were determined by vaginal ultrasound (for all 24 subjects). A diameter of ∼18 mm was considered potentially ovulatory, and a diameter of ∼10 mm indicated ovarian activity. In addition, the AUC0–24, the maximum concentration in serum (Cmax), and half-life (t1/2) of EE on days 11 and 16 (based on data from the 12 volunteers of block I) were calculated.
Statistical methods.
The purpose of this trial was to show that levels of EE in plasma during ciprofloxacin treatment are, on average, identical to those seen during placebo treatment. To establish average equivalence between the two treatments in the protocol, the two one-sided tests procedure was performed; log-transformed parameters were used for analysis. Typically, confidence intervals of 80 to 125% for bioequivalence are used. When considering potential therapeutic consequences (AUCEE+ciprofloxacin/AUCEE) of an interaction (dosage reduction or increases), the acceptance range indicating a lack of interaction may be wider and was calculated to be 67 to 150%, based on a log Δ(AUCcombination/AUCmonodrug) of 1.50. This analysis was done for EE at day 11 (confirmatory) and day 16 (exploratory).
RESULTS
At the conclusion of the study, all 24 subjects met the criteria for statistical evaluation. Protocol compliance was good.
Ciprofloxacin pharmacokinetics.
All concentrations and derived pharmacokinetic parameters for the first 12 subjects were noncontributory and therefore were not reported. In addition, the ciprofloxacin concentrations were consistent with good compliance with the treatment.
EE pharmacokinetics.
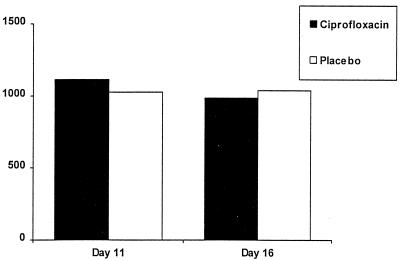
There was no difference in the AUC0–24s of EE on day 11 after dosing with ciprofloxacin or the placebo in the first 12 subjects (Fig. 1; Table 2). The estimated average intraindividual value (ciprofloxacin/placebo) of EE AUCs was 102%. The corresponding 90% confidence interval was 80 to 130%, which falls within the predefined range for equivalence (i.e., 67 to 150%).
FIG. 1.
Mean EE AUC0–24 (nanograms per hour per liter) on cycle days 11 and 16 in 12 patients taking ciprofloxacin or the placebo.
TABLE 2.
Mean AUC0–24 in 12 volunteers receiving ciprofloxacin or the placebo
| Day | Treatment | AUC0–24 (ng · h/liter)
|
M | |||
|---|---|---|---|---|---|---|
| Arithmetic mean | SD | Minimum | Median | |||
| 11 | Ciprofloxacin | 1,115.45 | 737.18 | 525.69 | 753.89 | 28 |
| Placebo | 1,027.24 | 568.28 | 606.30 | 855.28 | 26 | |
| 16 | Ciprofloxacin | 986.04 | 326.10 | 687.12 | 852.98 | 15 |
| Placebo | 1,040.50 | 414.35 | 640.97 | 890.79 | 19 | |
On day 11, the geometric mean values for AUC0–12 were 638.8 and 629.5 ng · h/liter and for Cmax they were 101.0 and 98.9 ng/liter during ciprofloxacin and placebo treatment, respectively (Table 3). The geometric mean t1/2 of EE was shorter after ciprofloxacin treatment (6.5 h) than after placebo treatment (11.2 h); this difference was due to the variability of the assay. The median time to Cmax was 2.0 h after ciprofloxacin treatment and 1.8 h after placebo treatment. The values for AUC0–24 of EE on day 16 show equivalence between the two treatment groups (Fig. 1; Table 2).
TABLE 3.
EE pharmacokinetic parameters for 12 volunteers
| Cycle day and calculation | AUC0–12 (ng · h/liter)
|
Cmax (ng/liter)
|
t1/2 (h)
|
|||
|---|---|---|---|---|---|---|
| Cipro-floxacin | Placebo | Cipro-floxacin | Placebo | Cipro-floxacin | Placebo | |
| 11 | ||||||
| Geometric mean | 638.85 | 629.52 | 100.97 | 98.92 | 0.47 | 11.18 |
| Geometric SD | 1.60 | 1.49 | 1.55 | 1.80 | 0.45 | 1.77 |
| CVa | 41.0 | 64.0 | 8.3 | 62.0 | ||
| 16 | ||||||
| Geometric mean | 656.17 | 677.48 | 108.97 | 101.68 | 11.21 | 10.46 |
| Geometric SD | 1.39 | 1.48 | 1.46 | 1.77 | 1.75 | 1.58 |
| CV | 40.0 | 62.0 | 48.0 | |||
CV, coefficient of variation.
On day 16, the geometric mean values for AUC0–12 were 656.2 and 677.5 ng · h/liter and Cmax values were 109.0 and 101.7 ng/liter after ciprofloxacin and placebo treatments, respectively. The t1/2 values were also similar (11.2 and 10.5 h, respectively) (Table 3). The median values for time to Cmax were 1.5 h after ciprofloxacin treatment and 2.0 h after placebo treatment.
Progesterone pharmacokinetics.
Levels of circulating progesterone could not be evaluated, because many were below the limit of determination (10 nmol/liter = 0.31 ng/ml). All subjects had progesterone levels of <0.31 ng/ml on day 20 of both treatment periods, except for four subjects, three of whom were receiving the placebo. The progesterone levels of those subjects were 0.38 ng/ml (ciprofloxacin) and 0.77, 0.67, and 0.48 ng/ml (placebo). These levels are all below 2 ng/ml, indicating the absence of ovulation.
Serum estradiol pharmacokinetics.
Ten subjects in each of the groups receiving placebo and ciprofloxacin had early-follicular-phase levels of E2 (<184 ng/liter) at one or more points during their cycles, but none had values above the early-follicular-phase range, indicating that there was no significant ovarian activity. Therefore, pharmacokinetic parameters were not assessed. The median of the highest E2 levels was below the level of detection (<13.6 ng/liter) in both the placebo and ciprofloxacin cycles. The highest level detected in the placebo cycles was 232 ng/liter; in the ciprofloxacin cycles it was 32.9 ng/liter.
HBG pharmacokinetics.
The levels of SHBG in the patients receiving ciprofloxacin and the patients receiving placebo were similar (Table 4).
TABLE 4.
Mean values for SHBG
| Treatment | Cycle day | No. of subjectsa | Level of SHBG (μmol/ml)
|
M | ||
|---|---|---|---|---|---|---|
| Mean | SD | Minimum | ||||
| Ciprofloxacin | 1 | 23 | 265.26 | 82.30 | 141.0 | 2 |
| 8 | 22 | 175.68 | 64.55 | 94.0 | 1 | |
| 10 | 23 | 170.26 | 60.70 | 97.0 | 1 | |
| 12 | 23 | 187.96 | 64.51 | 107.0 | 1 | |
| 14 | 22 | 206.95 | 67.85 | 115.0 | 1 | |
| Placebo | 1 | 24 | 274.96 | 89.83 | 145.0 | 2 |
| 8 | 24 | 182.25 | 65.62 | 100.0 | 1 | |
| 10 | 24 | 174.33 | 61.09 | 98.0 | 1 | |
| 12 | 23 | 203.74 | 65.33 | 103.0 | 1 | |
| 14 | 24 | 223.38 | 63.17 | 126.0 | 2 | |
In some cases, sampling errors caused the number of subjects to be fewer than 24.
Ovarian activity.
Four subjects had an indication of ovarian activity, with maximal follicle diameters of ∼10 mm on day 14 during ciprofloxacin coadministration, as did two subjects during coadministration of the placebo. Two of the placebo-treated subjects were potentially ovulatory (maximal follicle diameter of ∼18 mm). The mean follicle diameters on day 10 are presented in Table 5.
TABLE 5.
Maximal follicle diameter on day 10 in 22 volunteersa
| Treatment | Diam (mm)
|
|||
|---|---|---|---|---|
| Mean | SD | Minimum | Median | |
| Ciprofloxacin | 8.55 | 2.39 | 5.0 | 8.0 |
| Placebo | 10.59 | 5.69 | 5.0 | 10.0 |
Two volunteers had ultrasonographic measurements below the level of detection.
Safety results.
The most common adverse event was mild nausea in four individuals receiving ciprofloxacin and three subjects during coadministration of the placebo. Moderate diarrhea was reported by two individuals receiving ciprofloxacin. There were individual reports of symptoms such as tiredness, vomiting, pyrosis, meteorism, anomalies of taste, and anorexia in all treatment groups. There were no deaths or dropouts.
DISCUSSION
Concern was aroused when interaction between rifampin and oral contraceptives during the treatment of tuberculosis was reported in 1971 (7). Subsequently, rifampin-associated pregnancies were reported (6, 10). It has been proposed that rifampin, a potent inducer of hepatic P-450 enzymes, induces the increased degradation of EE by ethinyl estradiol 2-hydroxylase. A later review, summarizing reports to the United Kingdom Committee on Safety of Medicines, implicated penicillin and tetracyclines in 70% of the pregnancies occurring during antibiotic–oral-contraceptive coadministration (1). In other reports, 23 and 18% of oral-contraceptive failures in reliable pill takers were attributed to the coadministration of antibiotics (11, 12).
With concentrations of EE in plasma as an index of contraceptive efficacy, AUC measurements up to 24 h after oral contraceptive intake were not different during coadministration of ciprofloxacin or the placebo, reflecting the absence of a pharmacokinetic interaction during ciprofloxacin coadministration. SHBG levels in the ciprofloxacin and placebo groups were also similar. Since SHBG increases with increasing EE levels, these results concur with the finding of no major variation in EE levels during coadministration of ciprofloxacin. In addition, none of the subjects had estradiol levels above the early-follicular-phase range (184 to 227 ng/liter), indicating that there was no significant ovarian activity. Moreover, all subjects had progesterone levels of <2 ng/ml, reflecting the absence of ovulation. Follicular development into potentially ovulatory follicles was observed only in two subjects, both receiving the coadministered placebo.
In conclusion, ciprofloxacin does not appear to alter ovarian activity when coadministered with the low-dose oral contraceptive Marvelon. No evidence of pharmacokinetic interaction between the antibiotic and the contraceptive agent was detected.
ACKNOWLEDGMENT
We thank H. J. Kloosterboer, Organon Int., Oss, The Netherlands, for his active contribution.
REFERENCES
- 1.Back D J, Grimmer S F M, Orme M L E, Proudlove C, Mann R D, Breckenridge A M. Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics. Br J Clin Pharmacol. 1988;25:527–532. doi: 10.1111/j.1365-2125.1988.tb03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back D J, Orme M L E. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472–484. doi: 10.2165/00003088-199018060-00004. [DOI] [PubMed] [Google Scholar]
- 2a.Bayer AG. Harmonisation of data evaluation in pharmacokinetics—a task force report. 5747 (P). Leverkusen, Germany: Bayer AG; 1989. [Google Scholar]
- 3.British Medical Journal. Drug interactions with oral contraceptive steroids. Br Med J. 1980;6233:93–94. doi: 10.1136/bmj.281.6233.93. . (Editorial.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csemiczky G, Alvendal C, Landgren B M. Risk for evaluation in women taking a low-dose oral contraceptive (Microgynon) when receiving antibacterial treatment with a fluoroquinolone (ofloxacin) Adv Contracept. 1996;12:101–109. doi: 10.1007/BF01849631. [DOI] [PubMed] [Google Scholar]
- 5.D’Arcy P F. Drug interactions with oral contraceptives. Drug Intell Clin Pharm. 1986;20:353–362. doi: 10.1177/106002808602000504. [DOI] [PubMed] [Google Scholar]
- 6.Nocke-Finck, L., H. Breuer, and D. Reimers. 1973. Effects of antibiotics on oestrogen excretion in women taking oral contraceptives. Acta Endocrinol. 177(Suppl.):136.
- 7.Reimers D, Jezek A. Rifampicin und andere Antituberkulotika bei gleichzeitiger oraler Kontrazeption. Prax Pneumol. 1971;25:255–262. [PubMed] [Google Scholar]
- 8.Scholl H, Schmidt K, Weber B. Sensitive and selective determination of picogram amounts of ciprofloxacin and its metabolites in biological samples using high-performance liquid chromatography and photothermal post-column derivatization. J Chromatogr. 1987;416:321–330. doi: 10.1016/0378-4347(87)80515-7. [DOI] [PubMed] [Google Scholar]
- 9.Schuirmann D J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 10.Skolnick J L, Stoler B S, Katz D B, Anderson W H. Rifampicin, oral contraceptives, and pregnancy. JAMA. 1976;236:1382. [PubMed] [Google Scholar]
- 11.Sparrow M J. Pill method failures. N Z Med J. 1987;100:102–105. [PubMed] [Google Scholar]
- 12.Sparrow M J. Pregnancies in reliable pill takers. N Z Med J. 1989;102:575–577. [PubMed] [Google Scholar]
- 13.Szoka, P. R., and R. A. Edgren. 1988. Drug interactions with oral contraceptives: compilation and analysis of an adverse experience report database. Fertil. Steril. 49(Suppl.):31S–39S. [PubMed]
- 14.van der Kuy A, Merkus J M W M. The interaction between OAC and antibiotics. J Drug Ther Res. 1989;14:255–256. [Google Scholar]