Abstract
Background & Aims:
The hepatitis E virus (HEV) is a small positive-sense RNA virus that encodes a cytoplasmic form of the capsid protein (ORF2c), essential for virion structure, and a secreted glycosylated form (ORF2s) that accumulates at high titer in serum and can mask neutralizing epitopes. We explored the contribution of ORF2s to HEV replication, and its role in generating antibodies against ORF2 in a nonhuman primate.
Approach and Results:
We used a recombinant HEV genotype 3 variant that does not express ORF2s due to the introduction of stop codons (ORF2smut). Rhesus macaques (RM) were given intrahepatic injections of infectious wildtype HEV (ORF2swt) RNA or a variant lacking ORF2s expression (ORF2smut). The replication of ORF2smut virus was delayed by approximately 2 weeks compared with ORF2swt and peak titers were nearly 10-fold lower. Reversions of the 3 mutations that blocked ORF2s expression were not detected in the ORF2smut genomes, indicating genetic stability. However, serum antibodies against ORF2 were transiently detected in RMs infected with ORF2smut, whereas they were long lasting in RMs infected with ORF2swt. Moreover, RMs infected with ORF2smut were more susceptible to re-infection, as evidenced by the viral RNA detected in fecal samples and the expansion of HEV-specific CD8+ T cells.
Conclusions:
These findings indicate ORF2s may be dispensable for viral replication in vivo but is required for long-lived antibody-mediated responses that protect against HEV re-exposure.
Keywords: Hepatitis E virus (HEV), soluble ORF2 protein, immune response, antibody, T cell response
Graphical Abstract

Introduction
Hepatitis E Virus (HEV) is the leading cause of acute hepatitis worldwide and is caused by at least 4 different Paslahepevirus balayani genotypes (1,2). Most cases in developed countries are caused by HEV genotypes 3 and 4, which are transmitted zoonotically through consumption of contaminated meat products. HEV genotypes 3 and 4 can establish persistent infections in immunocompromised individuals, such as solid-organ transplant recipients, which can progress rapidly to liver cirrhosis (1).
The mechanisms of viral clearance and prevention of persistent infection are poorly understood. Results of epidemiology, immunology, and vaccinology studies indicate that HEV-specific immune responses are required for protective immunity (4). However, the contribution of HEV-specific antibodies and T-cell responses to viral clearance and prevention of persistent infection are unclear.
The HEV ORF2 encodes the capsid protein (ORF2c) and a secreted protein (ORF2s) (4–6). Conflicting reports have indicated that ORF2s and ORF2c are generated either by translation initiation at the same start codon and subsequent proteolytic cleavage or translation initiation at different start codons, leading to the generation of a signal peptide on the N-terminus of ORF2s and differential cellular protein localization and processing (4–6). Although ORF2c is an essential component of virion structure, the function of ORF2s is unknown. ORF2s is the main antigen found in serum from patients with HEV infection, and it inhibits antibody-mediated neutralization of the virus in vitro (4–6). Moreover, serum ORF2s levels increase during persistent infection vs acute infection (7). The contribution of ORF2s to virus replication and the immune response to HEV infection has not been defined in vivo.
Rhesus macaques (RMs) are susceptible to infection with HEV genotypes 1–4; their infection resembles many aspects of human HEV infection, making RMs a suitable animal model for studies of pathogenesis (1,3). Using a recombinant HEV genotype 3 variant that does not express ORF2s due to the introduction of stop codons (ORF2smut), we investigated the contribution of ORF2s to virus replication and the immune response during acute HEV infection in RMs.
HEV-specific T cells have been previously considered to be important for HEV clearance (3). HEV-specific CD4+ and CD8+ T cells are detectable during acute HEV infection (8–11). Expansion of HEV-specific T cells correlates with virus clearance, and their frequency decreases in peripheral blood following resolution of viral infection (8–13). Depletion of CD8+ T cells prolongs shedding of HEV into the feces by a week (14), indicating that HEV-specific CD8+ T cells contribute to rapid virus clearance but are not essential to prevent persistent infection. Furthermore, we evaluated the frequency of HEV-specific CD8+ T cells in RMs infected with ORF2smut, potentially compensating for the attenuated and transient HEV-specific antibody responses seen in these RMs.
Our data shown here indicate that ORF2s although not essential, may be required for efficient viral replication in vivo and the induction of a long-lasting antibody response, thereby mediating protective immunity. HEV-specific CD8+ T cells can counterbalance the transient responses of HEV-specific antibodies, generated due to a lack of ORF2s expression, to control virus replication during primary infection.
Methods
RMs
We selected 4 female Indian RMs (Macaca mulatta), 12–14 years old, for the study. All were negative for HEV-specific IgG and expressed Mamu-A*0101. RM studies were performed at the Emory National Primate Research Center, Atlanta, an AAALAC accredited institution. All protocols were reviewed and approved by the Institutional animal Care and Use Committee (IACUC), protocol number 201900102.
Cell lines and cell culture
Hepatoma-based Huh7.5 cells (gift from Dr. Charles M. Rice at Rockefeller Institute, NY (15) and human embryonic kidney 293T cells (HEK293T, ATCC CRL-1573) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 100U/mL penicillin, and 100μg/mL streptomycin and maintained at 37°C and 5% CO2.
Generation of an ORF2s mutant HEV (ORF2smut)
Infectious clones of the HEV genotype 3 Kernow-C1/p1 strain (JQ679014) and its cell culture-adapted Kernow-C1/p6 strain (JQ679013) (16,17) were used in this study. Six single nucleotide mutations were introduced into HEV genotype 3 Kernow-C1/p1 genome: A5359U, U5386A, C5387G, U5389A, C5360G, and G5361C. The ORF2s mutant HEV (ORF2smut) gene was inserted into the pUC57-Amp vector using the ValueGENE Synthesis platform (Genewiz).
For in vitro analyses, the introduced mutations were cloned from a HEV Kernow-C1/p1 into an HEV Kernow-C1/p6 vector (16,17). Restriction digest was used to excise an 1141bp fragment flanking the introduced mutations. Because the nucleotides 5072 and 5554 contained in the 1141bp fragment are different in p6 than in p1, these nucleotides were first mutated by site directed mutagenesis to prevent the introduction of any unwanted mutations into p6.
Generating a replication-deficient HEV Kernow-C1/p6 GAA
A replication deficient HEV Kernow-C1/p6 ORF2swt GAA was generated from HEV Kernow-C1/p6 ORF2swt by site directed mutagenesis of a highly conserved GDD motif at nucleotide 4674–4675 in the HEV polymerase ORF1 (18,19).
Replication of ORF2smut in vitro
HEV p6 wildtype (ORF2swt), ORF2smut, and ORF2swt GAA RNA was transcribed in vitro using mMessage mMachine T7 (Mirus Bio) and purified using the RNeasy Kit (Qiagen). RNA concentration was measured spectroscopically, and RNA integrity was analyzed by agarose gel electrophoresis. Cells were transfected with in vitro transcribed ORF2swt, ORF2smut and ORF2swt GAA RNA using the TransIT-mRNA Transfection Kit (Mirus Bio). After 24hrs, cells were seeded for RNA and protein lysate collection. RNA was extracted from cell lysate using RNeasy kit (Qiagen) and from supernatants using QIAmp Viral RNA Mini Kit (Qiagen). HEV RNA in cell lysates were quantified by qRT-PCR using the TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher Scientific). The QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific) was used for amplification and detection. Proteins were detected by immunoblot, using anti-ORF2, anti-ORF3 and anti-beta-actin. Rabbit polyclonal anti-ORF3 antibody was generated through a commercial source (GeneScript) by immunizing animals with a KLH-conjugated synthetic peptide corresponding to amino acids 81–113 of the ORF3 protein of the Kernow-C1/p6 strain. HEV antigen was quantified in supernatant of transfected Huh7.5 cells by ELISA using the anti-ORF2 1E6 antibody.
HEV infection of RMs
HEV p1 wildtype (ORF2swt) and ORF2smut RNA was transcribed in vitro using mMessage mMachine T7 (Mirus Bio). Each RM was given 0.75mg of viral RNA, by ultrasound-guided intrahepatic inoculation, at 3 sites in the right and 1 site in the left lobe of the liver.
Inoculum for the second infection was prepared from fecal suspensions from an RM persistently infected with HEV genotype 3 Kernow-C1 (HQ389543). Fecal suspensions were filtered and diluted to 2×107GE/mL. Fecal suspensions were injected intravenously at week 27 after the first infection.
Quantification of HEV in Feces
Feces was collected by animal care staff into conical tubes containing PBS/1% BSA and 10% fecal suspensions were generated. Insoluble particles were precipitated by centrifugation. Supernatants were 0.45μm filtered and stored at −80°C. RNA was isolated from feces using TriReagent (Sigma-Aldrich). Genomic DNA was removed from fecal-derived RNA using dsDNase (Thermo Fisher Scientific). HEV RNA was quantified as described above.
Cloning and expression of p239-mcherry
HEV p239 (ORF2 aa 368–606) from HEV gt3 Kernow-C1 was cloned upstream of mCherry2 ORF and a protein A-FLAG (PA) tag and downstream of a signal peptide sequence enabling protein secretion. p239-mCherry-PA protein was purified from supernatants of transfected HEK293T cells by IgG affinity column. p239-mCherry-PA was then proteolytically digested to remove the PA tag. Products were resolved by in 10% acrylamide SDS denaturing gel and analyzed by Coomassie stain.
Isolation of Plasma and Blood Mononuclear Cells (PBMCs) from blood
Blood was collected by animal care staff into EDTA-coated tubes. Plasma was separated from the cell fraction by centrifugation and stored at −80°C. PBMCs were isolated from the diluted blood cell fraction by density-gradient centrifugation using Ficoll paque (Cytiva). PBMCs were cryopreserved in 90% FCS, 10% DMSO.
Detection of HEV-specific antibodies
ELISA plates (Thermo Fisher Scientific) were coated overnight with HEV p239-mcherry, genotype 3, 1μg/mL in coating buffer (0.1M Na2CO3, pH 9.5). Plates were washed with PBS-T (PBS, 0.05% tween-20) and blocked with blocking buffer (5% milk and 1% normal goat serum (NGS), PBS-T). Plasma samples were diluted 1:10 in binding buffer (0.5% milk, 0.1% NGS, PBS-T). HEV-specific antibodies were detected using biotinylated anti-human IgG or IgM and streptavidin–horseradish peroxidase. The ELISA was developed by addition of peroxidase substrate (BD). The reaction was stopped with sulfuric acid (Sigma-Aldrich) and antibody concentration was determined by measuring absorbance at 450 nm.
Neutralization assays
HEV neutralization assays were performed as described previously (4). Serum samples collected before intrahepatic inoculation (week 0) and post infection (6169: week 8; Ryz11, RnN11, Rsp11: week 10) were diluted 1:100, 1:300 and 1:900. Dilutions were incubated with gradient purified naked HEV particles (3*107GE of HEV Kernow-C1/p6) before transfer onto HepG2-shMAVS cells (described previously (20). HEV antigen was quantified in the culture medium 5 days later using the WANTAI HEV antigen ELISA kit (Wantai). Based on measured HEV antigen levels, neutralization was calculated as percentage of inhibition of virus infection.
Sequencing of the ORF2 start region
To analyze the sequence of the ORF2 start region, RNA was isolated from the feces at an early and a late timepoint of infection as described above. cDNA was generated using the Maxima reverse transcriptase kit (Thermo Fisher Scientific). The ORF2 start region was amplified using a nested PCR approach, amplifying a 585bp fragment from 4930–5515bp. High-fidelity polymerase Q5 and Taq polymerase (New England Biolabs) were used for amplification and adenylation. Purified PCR products were cloned into TOPO-TA Vectors (Thermo Fisher Scientific) and colonies were screened by PCR and restriction digest. Eleven to 12 plasmids per monkey per timepoint and monkey were sequenced. Sequencing results were analyzed using Clustal Omega and compared to the HEV gt3 Kernow-C1/p1 reference sequence (JQ679014.1).
Detection of HEV-specific CD8+ T cells
HEV-specific CD8+ T cells were detected in cryopreserved PBMCs isolated from peripheral blood by flow cytometry using HEV ORF1 and ORF2 dextramers containing mapped Mamu-A*A0101 epitopes (HEV X917 (epitope sequence: LTPRPIIHAV), Pol1732 (epitope sequence: VSPGLVHNL), ORF22088 (epitope sequence: YTSTARHRL) (Immudex ApS) as described elsewhere (14). Surface levels of CD3, CD8, CD14, CD16, CD19, and PD-1 were analyzed by staining with fluorophore-conjugated antibodies. Dead cells and CD14+/CD16+/CD19+ cells were excluded from the analysis. Levels of cell surface markers were analyzed by flow cytometry, using an LSRII or Fortessa instrument (Becton, Dickinson and Company). Per sample, 1,000,000–2,000,000 events were acquired. Data were analyzed using FlowJo.
Results
ORF2smut HEV Replicates At Comparable levels To ORF2swt HEV in vitro but Does Not Produce Soluble ORF2
To analyze the contribution of ORF2s to viral replication, we constructed ORF2smut HEV, by changing the start codon at position 5359 of the HEV genome from adenine to uracil—this changed the amino acid methionine to leucine. Five more single-nucleotide mutations were introduced, at positions 5386, 5387, 5389, 5360, and 5361; this generated 2 stop codons between the start codons of ORF2s and capsid ORF2 (ORF2c), at positions 5359 and 5404, respectively, and reduced the risk of reversion to the wildtype HEV sequence (Figure 1A). Importantly, the introduced mutations resulted in no amino acid changes in ORF3, translated from a different reading frame (21), and ORF2c, which is translated from a start codon at position 5404 (4).
Figure 1: ORF2smut HEV replicates in vitro but does not produce soluble ORF2.

A) RNA sequence of the ORF2/3 start region of HEV ORF2swt and HEV ORF2smut; start codons of ORF3, ORF2s, and ORF2c are indicated. Also shown is the amino acid sequence of ORF2swt and introduced amino acid changes. B-E) Huh7.5 cells were transfected with HEV p6 ORF2swt, ORF2smut, or ORF2swt GAA. B, C) Analysis of the effect of introduced mutations on HEV replication in vitro. HEV RNA levels in B) lysates and C) supernatants of transfected cells at day 10 after transfection were quantified by qRT-PCR. Standard error mean (SEM) of biological triplicates is indicated by error bars. At least three independent experiments were performed, of which one representative is shown. D, E) Effects of introduced mutations on ORF2 and ORF3 expression and secretion in vitro. D) Immunoblot to detect intra- and extracellular HEV proteins ORF2 and ORF3 at day 10 post transfection. E) Levels of HEV ORF2 in cell supernatants and lysates collected at indicated time points. Two independent experiments were performed, of which one representative is displayed here. GE: genome equivalents. Blue: HEV ORF2swt transfected cells. Red: HEV ORF2smut transfected cells.
HEV Kernow-C1/p1 replicates only at low levels in vitro (16,17), so the introduced mutations were cloned into the cell culture-adapted HEV Kernow-C1/p6 backbone, generating HEV p6 ORF2smut. To analyze the contribution of ORF2s to viral replication in vitro, we transfected Huh7.5 cells with in vitro-transcribed HEV p6 ORF2swt, ORF2smut, or replication-deficient ORF2swt GAA RNA (18,19); supernatants and cell lysates were collected for up to 10 days. Virus RNA isolated from cell lysates, collected at the end of the experiment, were quantified using a qRT-PCR assay with primers and probes specific to HEV ORF2 (described in the Materials and Methods section). Additionally, HEV RNA was quantified in the supernatant at day 4, day 7, and day 10 after transfection. HEV RNA yields were similar in lysates and supernatants of cells transfected with ORF2swt and ORF2smut for the duration of the experiment, suggesting that ORF2smut replicates efficiently in vitro and its replicative fitness is comparable to ORF2swt (Figure 1B, C). Small differences observed were not significant as judged by overlapping 95% confidence intervals.
To test whether the introduced mutations prevented production of ORF2s, we analyzed ORF2 expression and secretion by immunoblotting (Figure 1D). Intracellular levels of ORF2 were lower in cells transfected with ORF2smut vs ORF2swt, whereas ORF2 was undetectable in the supernatant of cells transfected with ORF2smut, indicating that the introduced mutations prevented ORF2s expression and secretion (Figure 1D). As expected, intra- and extracellular levels of ORF3 were not affected by the introduced mutations (Figure 1D).
To confirm these results, HEV ORF2 levels in cell lysates and supernatants were analyzed using a commercial HEV ORF2 ELISA kit. HEV ORF2 increased over time in the supernatant of cells transfected with ORF2swt but remained below the detection limit in cells transfected with ORF2smut. Although the commercial HEV ORF2 ELISA does not discriminate between ORF2c and ORF2s, it appears likely that vast majority ORF2 detected in the supernatant is soluble, since the majority of ORF2 detected in cell culture supernatants is not virion associated (4). ORF2 was detectable in the cell lysates of cells transfected with ORF2swt and cells transfected with ORF2smut (Figure 1E). These results indicate that the introduced mutations prevent ORF2s expression, but do not affect HEV replication in vitro.
Since ORF2 has been reported to inhibit RIG-I signaling in vitro (22), we analyzed the effect of ORF2s on the induction of interferon stimulated genes (ISGs). HepG2 hepatoma cells were inoculated with ORF2swt and ORF2smut p6 HEV (Supplementary Figure 1). CXCL10 induction as well as HEV replication were comparable in both samples, indicating that the deletion of ORF2s does not affect ISG induction in vitro.
ORF2s Expression is Dispensable for HEV Replication in RMs
To analyze the in vivo contribution of ORF2s to virus replication and the immune response, we infected 2 RMs with HEV p1 ORF2swt and 2 RMs with ORF2smut HEV, by intrahepatic inoculation of in vitro-transcribed HEV RNA, similar to the approach used in early HEV studies (23). Due to the COVID-19 pandemic, RMs were prioritized for SARS-CoV2 research and only a total of four RMs were available for use in this study. However, key conclusions are supported by statistical analyses despite the small sample size. A percutaneous injection of HEV RNA avoids the accumulation of mutations associated with extended propagation of HEV in cell culture, which is often required to obtain sufficient viral yield for intravenous inoculation. In vitro-transcribed RNAs from ORF2swt and ORF2smut HEV have comparable integrity, determined by a tape station assay and visualized by gel electrophoresis (Supplementary Figure 2A). Importantly, the RNA samples had similar levels of intensity in the electropherogram, at 7200 nucleotides (the size of the HEV genome)—these findings confirm that the in vitro-transcribed ORF2swt and ORF2smut RNAs are quantitively and qualitative comparable (Supplementary Figure 2B).
Following intrahepatic inoculation of RMs, blood and feces were collected weekly, from weeks 0–12, at week 20 and at week 27 after infection (Figure 2A). We quantified fecal viral loads using qRT-PCR. Virus shedding was detected in fecal samples from RMs infected with ORF2swt, from weeks 2–4 for 6169 and weeks 3–6 for Ryz11, peaking at 266,000 and 85,000GE/g feces for 6169 and RYz11, respectively (Figure 2B). Replication of ORF2smut was delayed when compared to ORF2swt (weeks 5–8 and weeks 5–9 after infection for RNn11 and Rsp11, respectively), and peak virus shedding was substantially reduced in fecal samples from RMs infected with ORF2smut (25,000 and 9000GE/g feces for RNn11 and Rsp11, respectively) (Figure 2B). Due to the low levels of ORF2s during acute infection (7), we were unable to detect ORF2s in ORF2swt or ORF2smut transfected RMs (data not shown). In addition, ALT and AST liver enzyme levels were largely within the normal range in ORF2swt and ORF2smut infected RMs, indicating that the absence of ORF2 did not drastically change the pathogenicity in this experiment (Supplementary Figure 3).
Figure 2: ORF2s expression is dispensable for HEV replication in RMs.

A) Experimental schedule: 2 RMs per group were given intrahepatic injections of 0.75 mg of in vitro-transcribed viral RNA; 6169, Ryz11 were infected with HEV p1 ORF2swt RNA; RnN11, Rsp11 were infected with HEV p1 ORF2smut RNA. Blood and feces were collected at indicated timepoints. B) RNA was isolated from fecal suspension and HEV genomes were quantified using a qRT-PCR assay. All samples were run in duplicates. GE: genome equivalents. Blue: HEV ORF2swt infected RMs. Red: HEV ORF2smut infected RMs.
C) Consensus sequence of analyzed samples and resulting ORF2s and ORF2c amino acid sequences are shown. HEV genotype 3, Kernow-C1/p1 strain is shown as reference sequence. In the sequence area analyzed and displayed, all clones shared the consensus sequence. Gray: Sequence present in all sequences. Blue: Sequence present in reference sequence, but not in all sequences analyzed. Red: Sequence does not match reference sequence (introduced changes of ORF2smut). 6169 and Ryz11: RMs infected with ORF2swt. RnN11, Rsp11: RMs infected with ORF2smut.
To ensure that no reversions to ORF2smut had occurred, we isolated viral RNA from the feces of all RMs at an early and a late timepoint following infection and amplified the ORF2 nucleotide sequences from positions 4930–5515 by RT-PCR, which spans the nucleotide stretch containing the engineered mutations. Amplicons were cloned into TOPO-TA vectors and sequenced. The sequence of the ORF2 start region was identical in all clones from samples collected at early and late timepoints from both RMs infected with ORF2swt (RMs 6169 and Ryz11), and matched the HEV genotype 3 Kernow-C1/p1 reference sequence (JQ679014.1) (Figure 2C). Moreover, all clones from both RMs infected with ORF2smut retained all 6 original mutations (including the mutated start codon and insertion of tandem stop codons). Therefore, ORF2s mutations were stable and did not revert to wildtype sequences during infection with ORF2smut HEV. These results demonstrate that the expression of ORF2s is not required for HEV replication in vivo. However, its absence led to attenuation of virus replication in vivo.
HEV-specific Antibodies Detected Following Infection with ORF2swt and ORF2smut Viruses
To study the effect ORF2s on the humoral immune response, we analyzed HEV-specific antibodies from peripheral blood of infected RMs. We generated a truncated ORF2 bait by fusing amino acids 368–606 of the HEV ORF2 protein, referred to as p239 (24), with a C-terminal mCherry2 tag (Figure 3A). p239 elicits a robust antibody response (24) following immunization, making it an effective immunogen in the Hecolin vaccine (25,26). Importantly, the p239 fragment of the bait generated in this study derives from HEV gt3 Kernow-C1 strain and is produced in mammalian cells, as opposed to p239 from previous studies, which originates from gt1 HEV and is produced in bacteria (24). As shown in figure 3B, p239-mCherry with an expected size of 57kDa was successfully expressed and purified from supernatants of a stably transduced HEK293T cell line.
Figure 3: HEV-specific antibodies are transiently detected, and at lower levels in RMs infected with ORF2smut virus.
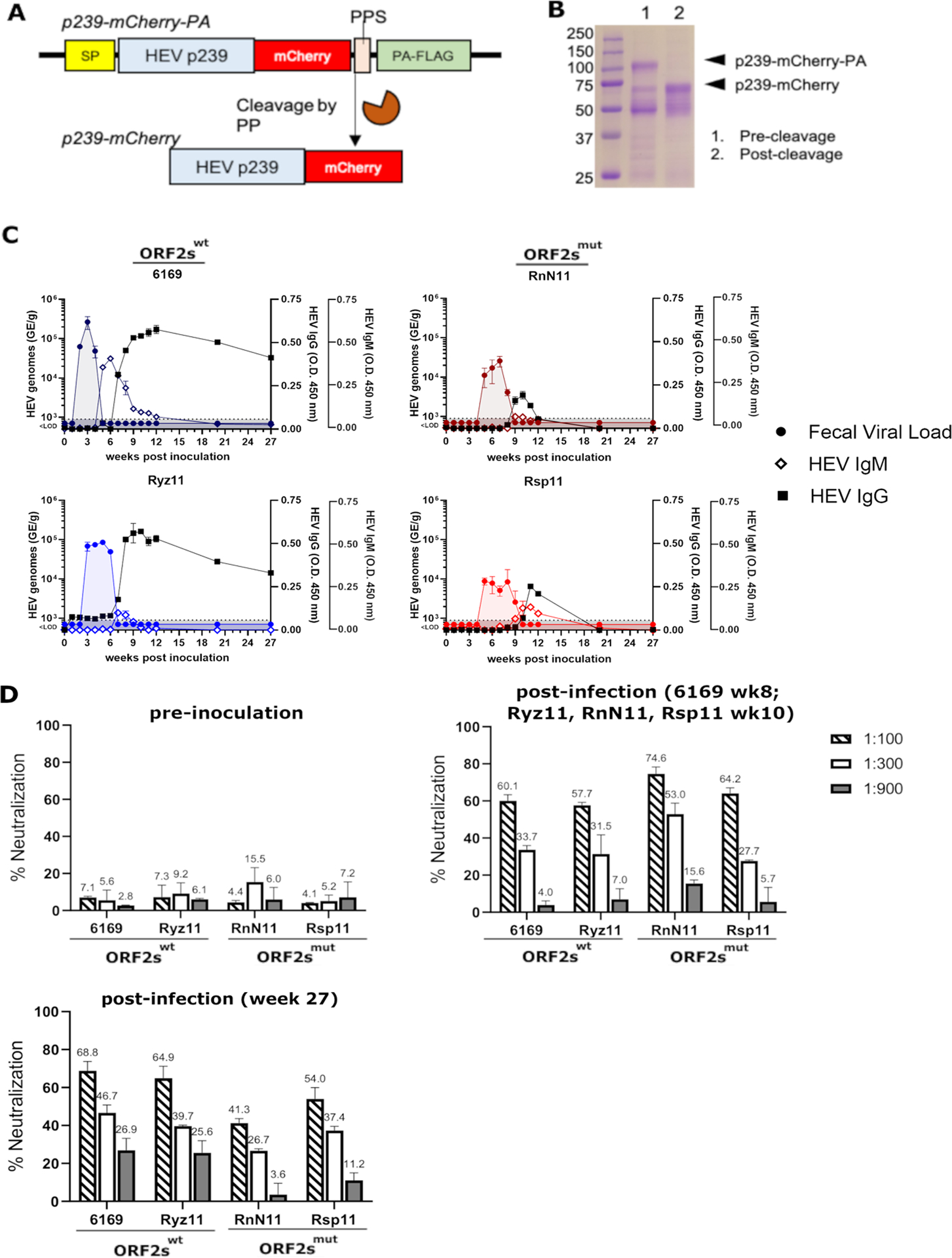
A) The HEV p239-mCherry-PA construct. p239 (ORF2 aa 368–606) from HEV gt3 Kernow strain was cloned in-frame and upstream of the mCherry2 ORF and a protein A-FLAG (PA-FLAG) tag for affinity purification, which was then cleaved by PreScission protease (PP) at the PP cleavage site (PPS). The entire fusion protein is downstream of a signal peptide (SP) sequence that enables protein secretion. (B) p239-mCherry-PA protein was purified, using an IgG affinity column, from supernatants of HEK293T cells transfected with p239-mCherry construct. p239-mCherry-PA was then proteolytically digested in-column to remove PA tag. Lane 1, tagged p239-mCherry-PA prior to proteolysis; Lane 2, cleaved p239-mCherry. C) HEV-specific antibodies (IgM and IgG) were detected in serum using an ELISA. ELISA plates were coated with HEV p239-mcherry. D) Neutralization of HEV p6 with sera of RMs pre-inoculation and post infection was analyzed using an ELISA based neutralization assay. 6169: week 8 post inoculation. Ryz11, RnN11, Rsp11: week 10 post inoculation as well as week 0 and week 27 (all RMs) were analyzed. 6169 and Ryz11, RMs infected with ORF2swt; RnN11 and Rsp11, RMs infected with ORF2smut. Blue: HEV ORF2swt infected RMs. Red: HEV ORF2smut infected RMs.
We compared levels of p239-specific plasma IgM and IgG from infected RMs (samples collected weeks 0–12) using an ELISA coated with p239-mCherry. In samples from RMs infected with ORF2swt, we detected HEV-specific IgM at the end of the period of virus shedding into feces (week 5 for 6169 and week 7 for Ryz11). HEV-specific IgG was first detected either simultaneously with HEV-specific IgM (Ryz11) or 2 weeks after the first detection of IgM (6169) (Figure 3C). The duration and magnitude of HEV-specific IgG was long lasting and sustained close to peak levels through week 20 and week 27. In plasma from RMs infected with ORF2smut, HEV-specific IgM was also detected at the end of the period of virus shedding into feces (week 9 for RnN11 and week 10 for Rsp11). HEV-specific IgG were first detected simultaneously with HEV-specific IgM. However, levels of HEV-specific IgG were about 2-fold lower than those of RMs infected with ORF2swt. Furthermore, levels of HEV-specific IgM and IgG decreased rapidly in these animals, until they were undetectable by week 20 after infection (Figure 3C).
To analyze whether infected RMs developed neutralizing antibodies in the absence of ORF2s, neutralization was analyzed. Neutralizing antibodies were not present at the timepoint of inoculation, but detectable in the serum of ORF2swt (60.1% and 57.7% for 6169 and Ryz11, respectively, at 1:100 dilution) and ORF2smut (74.6% and 64.2% for RnN11 and Rsp11, respectively, at 1:100 dilution) transfected RMs following viral clearance, indicating that neutralizing antibodies are generated in the presence and absence of ORF2s (Figure 3D). Neutralizing antibodies persist in all four RMs until week 27 post inoculation, albeit neutralization is slightly reduced in ORF2smut transfected RMs (41.3% and 54.0% for RnN11 and Rsp11, respectively) compared to ORF2swt transfected RMs (68.8% and 64% for 6169 and Ryz11, respectively).
These results indicate that ORF2s expression is required for generating an optimal ORF2-specific antibody response following HEV infection. Notably, the ORF2smut virus elicited only a very weak and transient antibody response that became undetectable within 3 months after virus clearance.
Frequency of HEV-specific CD8+ T cells in RMs Infected with ORF2smut
Our data indicate that the magnitude and duration of ORF2-specific antibodies were reduced in in the absence of ORF2s, raising the question of whether HEV-specific T cell response is also reduced in these animals despite virus clearance. HEV-specific CD8+ T cells are detectable in peripheral blood of patients with acute HEV infection (genotype 3), and the frequency of these T cells decreases following viral clearance (8,11). We therefore measured the frequency of HEV-specific CD8+ T cells in the peripheral blood of ORF2swt and ORF2mut-infected RMs with HEV ORF1- and ORF2-specific multimers described in our recent study (14) (Figure 4, Supplementary Figure 4). HEV-specific CD8+ T cells were detectable and had an activated phenotype (indicated by the presence of surface PD-1) in PBMCs from RMs infected with ORF2swt or ORF2smut virus (Figure 4A). CD8+ T cells from RMs infected with ORF2swt virus reacted to either ORF1 or ORF2 multimers, whereas CD8+ T cells from RMs infected with ORF2smut virus reacted exclusively with the ORF1-specific multimers (Figure 4). However, due to the limited number of RMs used in this study we cannot conclude whether is due to the absence of ORF2s in ORF2smut transfected RMs or stochastically. Similar to kinetics of p239-specific plasma antibodies, the frequency of HEV-specific CD8+ T cell peaked rapidly after viral clearance in RMs infected with either ORF2swt or ORF2smut virus (Figure 4B). However, peak frequencies of multimer+ CD8+ T cells were higher in both RMs infected with ORF2smut virus (1.64% and 0.5% for RNn11 and Rsp11, respectively) compared to RMs infected with ORF2swt virus (0.21% and 0.30% for 6169 and Ryz11, respectively). This increase might be important for virus clearance ORF2smut infected RMs potentially compensating the attenuated and transient production of HEV-specific antibodies. However, due to the relatively low frequency of HEV-specific CD8+ T cell numbers and in only two animals per group, it needs to be interpreted cautiously.
Figure 4: Frequency of HEV-specific CD8+ T cells in RMs infected with ORF2smut virus.

A, B) HEV-specific CD8+ T cells were detected by flow cytometry using HEV ORF1 and ORF2 dextramers at indicated time points following intrahepatic inoculation. A) Overlayed FACS blots showing HEV multimer+ cells (red) in CD3+ population, displayed as PD-1 against CD-8 expression. The panel shows the percent of multimer+PD1+CD8+ cells. B) Frequency of HEV ORF1 or ORF2 multimer+CD8+ T cells as percentage of CD8+PD1+ population graphed against weeks since intrahepatic inoculation. 6169, Ryz11, RMs infected with ORF2swt virus; RnN11, Rsp11, RMs infected with ORF2smut. Blue: HEV ORF2swt infected RMs. Red: HEV ORF2smut infected RMs.
RMs infected with ORF2smut HEV do not develop sterilizing immunity
HEV-specific antibody has a well-established role in mediating protective immunity in most individuals (3). Because the HEV-specific antibody response was attenuated and short-lived in RMs infected with the ORF2smut HEV (Figure 3C and 5A), we reasoned that the absence of ORF2s might affect the development of protective immunity. Since HEV-specific CD8+ T cells were higher in ORF2smut transfected RMs (Figure 4), it appears unlikely that the reduced HEV-specific antibody levels following primary infection were solely due to reduced viral replication, but at least in part a direct consequence of the absence of ORF2s in ORF2smut transfected RMs. To test whether infection with ORF2smut virus reduces protection against re-infection, we challenged all RMs with wildtype HEV genotype 3 Kernow-C1, via intravenous injection at week 27 after the primary infection (Figure 5B). Blood and feces were collected at days 0, 3, 7, 10, 14, 21, and 28 after this second infection, and HEV RNA and HEV-specific IgM and IgG were quantified. HEV genomes were detectable in the feces of RnN11 at days 7 and 10 after the challenge, peaking at day 7 (≈54000 GE/g feces) (Figure 5C). No shedding of virus into the feces was detected for RMs 6169, Ryz11, or Rsp11. ALT and AST liver enzyme levels were inapparent throughout the study (Supplementary Figure 5).
Figure 5: Contribution of ORF2s to Immune Memory Production of Antibodies and Protective Immunity.

A) Comparison of relative HEV-specific IgG titers 27 weeks after intrahepatic transfection, measured using an HEV IgG ELISA and displayed as absorbance at 450 nm. B) Experimental design. RMs previously infected with ORF2swt or ORF2smut viruses were given intravenous injections of 2*107 GE HEV genotype 3 Kernow-C1. Blood and feces were collected at indicated time points. C) RNA was isolated from fecal suspensions, as described in Figure 4. D) Neutralization of HEV gt3 p6 with sera of RMs at d0 of the second inoculation and d14 post second inoculation was analyzed using an ELISA based neutralization assay. Blue: HEV ORF2swt infected RMs. Red: HEV ORF2smut infected RMs.
HEV-specific IgM increased to comparable levels in plasma samples from RMs 6169, RnN11, and Rsp11, but was sustained until the end of the experiment in only RMs infected with ORF2smut virus—levels decreased rapidly in plasma from RM 6169. However, levels of HEV-specific IgG increased rapidly in plasma from RMs infected with ORF2swt and ORF2smut viruses and were detected at comparable levels 22 days following the second infection (Figure 5C). The increase in levels of HEV-specific IgG following the second infection was significantly higher in RMs infected with ORF2smut HEV (ΔOD450 nm=0.5) than in RMs infected with ORF2swt HEV (ΔOD450 nm=0.1 for 6169 and ΔOD450 nm=0.2 for Ryz11).
To assess whether HEV neutralizing antibodies contributed to protective immunity, we analyzed the neutralizing capacity of serum of all four animals at d0 post second inoculation (week 27 post first inoculation) and d14 post second infection (Figure 5D). HEV neutralizing antibody levels were lower in the serum of ORF2smut transfected RMs at the timepoint of second inoculation, potentially contributing to the observed lack of sterilizing immunity. Interestingly, HEV neutralizing antibody levels correlate with protective immunity, since they were lowest in RnN11 (41.1%), the RM that showed fecal viral shedding upon second inoculation, intermediate in Rsp11 (54.0%) and highest in previously ORF2swt inoculated RMs 6169 and Ryz11 (68.8% and 64.9%), which did not display any signs of active viral replication following second inoculation. Therefore, neutralizing antibody levels potentially contribute to the degree of protective immunity seen upon reinfection. However, it is a challenge to draw strong conclusions here given the observed small differences in neutralizing antibody levels and the low number of available animals.
Although virus was detected in the feces of only 1 RM previously infected with ORF2smut (RNn11), the kinetics of p239-specific antibody recall responses were similar between Rsp11 and RNn11 (Figure 5C), so we proposed that transient reinfection might have also occurred in Rsp11. We investigated expansion of HEV-specific CD8+ T cells in all RMs as a marker of viral replication, given that CD8+ memory T cells expand following an encounter with specific antigens presented by infected cells (27).
Interestingly, expansion of CD8+ T cells specific to ORF1 (a nonstructural protein) was detected in both RMs infected previously with ORF2smut HEV (peak frequency 5.44% in RnN11 and 0.43% in Rsp11, respectively), but not RMs infected with ORF2swt HEV (peak frequency 0.01% in 6169 and 0.04% in Ryz11, respectively) (Figure 6A, B), suggesting that low level of reinfection had occurred in Rsp11 as well. Moreover, the expansion of ORF1-specific CD8+ T cells was about 10-fold higher in RnN11 than in Rsp11 (Figure 6A, B), correlating with the differences observed in virus shedding into feces. It must be noted that following the second inoculation HEV-specific IgG serum levels increased also in ORF2swt transfected RMs (Figure 5C). However, in contrast to HEV-specific CD8+ T cells, the expansion and differentiation of HEV-specific B cells does not indicate active viral replication but could be due to presented ORF2 contained in the inoculum. Taken together, these results indicate that RMs infected with ORF2smut HEV failed to develop sterilizing immunity and became reinfected following intravenous challenge, whereas RMs infected with ORF2swt HEV were fully protected against reinfection.
Figure 6: Reinfection occurs only in RMs infected with ORF2smut HEV.

A, B) HEV-specific CD8+ T cells were detected by flow cytometry using ORF1 and ORF2 HEV multimers at indicated time points following intravenous challenge with HEV genotype 3 Kernow-C1. A) Overlayed FACS blots showing HEV multimer+ cells (red) in CD3+ population, displayed as PD-1 against CD-8 expression. Panel shows percent of multimer+ PD1+CD8+ cells. B) Frequency of ORF1 or ORF2 multimer+CD8+ T cells as percentage of CD8+PD1+ population, graphed against days since intravenous challenge. 6169 and Ryz11, RMs infected with ORF2swt; RnN11 and Rsp11, RMs infected with ORF2smut. Blue: HEV ORF2swt infected RMs. Red: HEV ORF2smut infected RMs.
Discussion
ORF2s is the main viral antigen detectable in the serum during HEV infection (4,6). Conflicting data has been reported regarding the origin of different ORF2 variants. ORF2c and ORF2s were reported to be generated either by proteolytic processing or translation initiation at different start codons (4,6). We reported that mutation of the upstream start codon from methionine to leucine, as well as the introduction of 2 stop codons between the presumptive start codon of ORF2s and ORF2c, eliminated the secretion of ORF2s in vitro but remained replication competent both in vitro and in vivo. These results provide strong evidence that ORF2s and ORF2c are generated independently by translation initiation at different start codons.
The reasons for attenuated replication of the ORF2smut virus in vivo remains unclear. Fecal viral shedding was delayed by approximately two weeks and 10-fold lower level in ORF2smut infected RMs. It is possible that ORF2s facilitate HEV replication by modulating innate immune responses. Supporting this notion, a recently published report shows that the activation of the interferon beta promoter is significantly reduced when ORF2 is overexpressed in RIG-I expressing HEK293 cells, indicating that ORF2 perhaps inhibits innate immune signaling (22). This may provide a potential explanation for the delayed and reduced viral replication that is observed in ORF2smut infected RMs. However, we did not detect any differences in ISG induction in HepG2 cells infected with ORF2swt or ORF2smut HEV, indicating that inhibition of innate immunity by ORF2s may not be the reason for the attenuated replication of ORF2smut in RMs. However, we cannot exclude the possibility that modification of ORF2swt by introducing six nucleotide mutations may also have contributed to the attenuations of fecal shedding. Future experiments to decrease the number of engineered nucleotide mutations may shed some light on viability and/or stability of the ORF2smut virus.
Our study sheds light on the function of ORF2s in modulating adaptive immune responses. Compared to ORF2swt-infected RMs, HEV-specific antibody response was much weaker and detected only transiently in ORF2smut-infected RMs, indicating that ORF2s is critical for eliciting a robust and long-lasting antibody response that is capable of mediating protection against HEV re-infection. In addition, HEV neutralizing antibody levels were reduced in ORF2smut transfected RMs at the timepoint of second inoculation, indicating that ORF2s is important for eliciting a strong and long-lasting neutralizing antibody response. Importantly, the short-lived antibody response generated without ORF2s appears to be the reason why these animals failed to generate sterilizing immunity, since HEV-ORF2 specific antibody and HEV neutralizing levels correlated with protective immunity. At the timepoint of rechallenge, ORF2-specific antibodies were undetectable in ORF2smut infected RMs while neutralizing antibodies were still detectable. One could speculate that a significant portion of ORF2 antibodies in ORF2swt infected RMs were non-neutralizing antibodies induced by ORF2s. However, how non-neutralizing ORF2-specific antibodies elicited by ORF2s contribute to protective immunity requires further investigation.
Unexpectedly, higher levels of HEV-specific CD8+ T cells were detected in RMs infected with ORF2smut. It was recently reported that depletion of CD8+ T cells during HEV infection of RMs did not cause persistence (14), indicating that HEV-specific antibodies can compensate for the lack of HEV-specific CD8+ T cells. Thus enhanced HEV-specific CD8+ T cell responses seen in ORF2smut-infected RMs may potentially compensate for their attenuated antibody responses and promote HEV clearance. However, due to the relatively low frequency of HEV-specific CD8+ T cells and in only two animals per group, it needs to be interpreted cautiously.
Interestingly, ORF2s is detected at significantly higher levels during persistent infection as opposed to during acute infection (7), indicating that ORF2s might have a role in immune evasion and establishing persistent infection. Considering that most HEV infections are acute and self-limiting, and persistent infections occur only in immunocompromised individuals (1), ORF2s may have differential impact on immunity during acute vs persistent HEV infection. Whether ORF2s may inhibit antibody-mediated neutralization in a chronic infection setting requires further investigation. ORF2smut appears to be an important tool for studies of HEV immune evasion and persistence (1,28). Persistent HEV infection is a growing global health problem and further research into immune therapies for persistent HEV infection is needed to address this significant, unmet need.
Supplementary Material
Acknowledgements:
This project was supported by NIH grants, R01AI126890, R01AI136533, U19AI159819, 1R01AI175800 to A.G., and ORIP/OD P51OD011132 (formerly NCRR P51RR000165) to the Emory National Primate Research Center (A.G.), R01AI139511 and R21AI159735 to Z.F. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations:
- HEV
Hepatitis E virus
- RM
rhesus macaque
- ORF2swt
wildtype HEV
- ORF2smut
recombinant HEV lacking ORF2s expression
References
- 1.Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, Dalton HR. Hepatitis E virus infection. Nat Rev Dis Primers 2017;3:17086. [DOI] [PubMed] [Google Scholar]
- 2.Purdy M, Drexler J, Meng X, Norder H, Okamoto H, Van der Poel W, Reuter G, et al. ICTV Virus Taxonomy Profil: Hepeviridae 2022. J Gen Virol 2022;103;9. [DOI] [PubMed] [Google Scholar]
- 3.Walker CM. Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb Perspect Med 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin X, Ying D, Lhomme S, Tang Z, Walker CM, Xia N, Zheng Z, et al. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc Natl Acad Sci U S A 2018;115:4773–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankavay M, Montpellier C, Sayed IM, Saliou JM, Wychowski C, Saas L, Duvet S, et al. New insights into the ORF2 capsid protein, a key player of the hepatitis E virus lifecycle. Sci Rep 2019;9:6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montpellier C, Wychowski C, Sayed IM, Meunier JC, Saliou JM, Ankavay M, Bull A, et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 2018;154:211–223 e218. [DOI] [PubMed] [Google Scholar]
- 7.Behrendt P, Bremer B, Todt D, Brown RJ, Heim A, Manns MP, Steinmann E, et al. Hepatitis E Virus (HEV) ORF2 Antigen Levels Differentiate Between Acute and Chronic HEV Infection. J Infect Dis 2016;214:361–368. [DOI] [PubMed] [Google Scholar]
- 8.Brown A, Halliday JS, Swadling L, Madden RG, Bendall R, Hunter JG, Maggs J, et al. Characterization of the Specificity, Functionality, and Durability of Host T-Cell Responses Against the Full-Length Hepatitis E Virus. Hepatology 2016;64:1934–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ayoubi J, Behrendt P, Bremer B, Suneetha PV, Gisa A, Rinker F, Manns MP, et al. Hepatitis E virus ORF 1 induces proliferative and functional T-cell responses in patients with ongoing and resolved hepatitis E. Liver Int 2018;38:266–277. [DOI] [PubMed] [Google Scholar]
- 10.Gisa A, Suneetha PV, Behrendt P, Pischke S, Bremer B, Falk CS, Manns MP, et al. Cross-genotype-specific T-cell responses in acute hepatitis E virus (HEV) infection. J Viral Hepat 2016;23:305–315. [DOI] [PubMed] [Google Scholar]
- 11.Suneetha PV, Pischke S, Schlaphoff V, Grabowski J, Fytili P, Gronert A, Bremer B, et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology 2012;55:695–708. [DOI] [PubMed] [Google Scholar]
- 12.Husain MM, Aggarwal R, Kumar D, Jameel S, Naik S. Effector T cells immune reactivity among patients with acute hepatitis E. J Viral Hepat 2011;18:e603–608. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy AS, Das R, Rathod SB, Arankalle VA. Cytokine profiles, CTL response and T cell frequencies in the peripheral blood of acute patients and individuals recovered from hepatitis E infection. PLoS One 2012;7:e31822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremer W, Blasczyk H, Yin X, Salinas E, Grakoui A, Feng Z, Walker C. Resolution of hepatitis E virus infection in CD8+ T cell-depleted rhesus macaques. J Hepatol 2021;75:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science 2000;290:1972–1974. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen HT, Torian U, Faulk K, Mather K, Engle RE, Thompson E, Bonkovsky HL, et al. A naturally occurring human/hepatitis E recombinant virus predominates in serum but not in faeces of a chronic hepatitis E patient and has a growth advantage in cell culture. J Gen Virol 2012;93:526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla P, Nguyen HT, Faulk K, Mather K, Torian U, Engle RE, Emerson SU. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J Virol 2012;86:5697–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graff J, Nguyen H, Kasorndorkbua C, Halbur PG, St Claire M, Purcell RH, Emerson SU. In vitro and in vivo mutational analysis of the 3’-terminal regions of hepatitis e virus genomes and replicons. J Virol 2005;79:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao D, Huang YW, Meng XJ. The nucleotides on the stem-loop RNA structure in the junction region of the hepatitis E virus genome are critical for virus replication. J Virol 2010;84:13040–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin X, Li X, Ambardekar C, Hu Z, Lhomme S, Feng Z. Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog 2017;13:e1006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff J, Nguyen H, Yu C, Elkins WR, St Claire M, Purcell RH, Emerson SU. The open reading frame 3 gene of hepatitis E virus contains a cis-reactive element and encodes a protein required for infection of macaques. J Virol 2005;79:6680–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingane S, Joshi N, Surjit M, Ranjith-Kumar CT. Hepatitis E Virus ORF2 Inhibits RIG-I Mediated Interferon Response. Front Microbiol 2020;11:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emerson SU, Zhang M, Meng XJ, Nguyen H, St Claire M, Govindarajan S, Huang YK, et al. Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc Natl Acad Sci U S A 2001;98:15270–15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005;23:2893–2901. [DOI] [PubMed] [Google Scholar]
- 25.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376:895–902. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015;372:914–922. [DOI] [PubMed] [Google Scholar]
- 27.Martin MD, Badovinac VP. Defining Memory CD8 T Cell. Front Immunol 2018;9:2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardinali NR, Guimaraes JR, Melgaco JG, Kevorkian YB, Bottino FO, Vieira YR, da Silva AC, et al. Cynomolgus monkeys are successfully and persistently infected with hepatitis E virus genotype 3 (HEV-3) after long-term immunosuppressive therapy. PLoS One 2017;12:e0174070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


