Abstract
Background
Beta‐blockers are one of the classes of drugs frequently used to treat hypertension. Quantifying the blood pressure (BP) lowering effects of nonselective beta‐blockers provides important information that aids clinical decision making.
Objectives
To quantify the dose‐related effects of nonselective beta‐adrenergic receptor blockers (beta‐blockers) on systolic blood pressure (SBP) and diastolic blood pressure (DBP) as compared with placebo in people with primary hypertension.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and ClinicalTrials.gov for randomized controlled trials up to October 2013.
Selection criteria
Randomized, double‐blind, placebo‐controlled, parallel or cross‐over trials. Studies had to contain a nonselective beta‐blocker monotherapy arm with a fixed dose. Participants enrolled into the studies had to have primary hypertension at baseline. Duration of studies had to be between three and 12 weeks.
Data collection and analysis
Two review authors (GW and AL) independently confirmed the inclusion of studies and extracted the data.
Main results
We included 25 RCTs that evaluated the BP lowering effects of seven nonselective beta‐blockers in 1264 people with hypertension. Among the 25 RCTs, four were parallel studies and 21 were cross‐over studies. Overall, nonselective beta‐blockers lowered systolic BP and diastolic BP compared with placebo. Nonselective beta‐blockers, in the recommended dose range, did not showed a convincing dose‐response relationship by direct comparison. The once (1x) and twice (2x) starting dose subgroups contained the largest sample size. The estimate of BP lowering efficacy for nonselective beta‐blockers by combining the 1x and 2x starting dose subgroup was ‐10 mmHg (95% CI ‐11 to ‐8) for systolic BP and ‐7 mmHg (95% CI ‐8 to ‐6) for diastolic BP (low‐quality evidence). Nonselective beta‐blockers starting at the 1x recommended starting doses lowered heart rate by 12 beats per minute (95% CI 10 to 13) (low‐quality evidence). The dose‐response relationship in heart rate was evident by both direct and indirect comparison. Due to imprecision, there was no clear evidence of an effect of nonselective beta‐blockers on pulse pressure in any dose subgroups except for a small reduction with the 2x starting dose (‐2.2 mmHg, 95% CI ‐3.7 to ‐0.7) (very low quality evidence). The point estimates in the 1x, four times (4x) and eight times (8x) starting dose subgroups were similar to the 2x starting dose subgroup. Therefore, it would appear that if nonselective beta‐blockers do lower pulse pressure, the magnitude is likely to be about 2 mmHg. There were very limited data (two studies) on withdrawals due to adverse effects (risk ratio (RR) 0.84; 95% CI 0.38 to 1.82).
Authors' conclusions
In people with mild‐to‐moderate hypertension, nonselective beta‐blockers lowered peak BP by a mean of ‐10/‐7 mmHg (systolic/diastolic) and reduced heart rate by 12 beats per minute. Propranolol and penbutolol were the two drugs that contributed to most of the data for nonselective beta‐blockers. This estimate is likely exaggerated due to the presence of extreme outliers and other sources of bias. If we removed the extreme outliers from the analysis, the estimate for non‐selective beta‐blockers was lower (‐8/‐5 mmHg (systolic/diastolic)). Nonselective beta‐blockers did not show a convincing graded dose‐response in the recommended dose range for systolic BP and diastolic BP, while higher dose nonselective beta‐blockers provided greater reduction of heart rate. Using higher dose nonselective beta‐blockers might cause more side effects, such as bradycardia, without producing an additional BP lowering effect. The effect of nonselective beta‐blockers on pulse pressure was likely small, at about 2 mmHg.
Plain language summary
Nonselective beta‐blockers for treatment of high blood pressure
Background
Beta‐blockers are a class of drug commonly used to treat high blood pressure. Nonselective beta‐blockers are a subclass of beta‐blockers including propranolol (Inderal), nadolol (Corgard), etc. We asked how much this subclass of drugs lower blood pressure.
Study characteristics
We developed a comprehensive search strategy of all relevant scientific databases to identify all clinical trials to answer this question. Participants had to have a baseline systolic blood pressure (the top number of a blood pressure reading) of at least 140 mmHg or a diastolic blood pressure (the bottom number of a blood pressure reading) of at least 90 mmHg, or both of these. We did not restrict participants by age, gender, baseline risk or any other medical conditions.
Key results
We found 25 clinical trials that compared the blood pressure lowering effect of seven nonselective beta‐blockers with placebo in 1264 people with high blood pressure. On average, nonselective beta‐blockers lowered blood pressure by about 10 mmHg systolic and 7 mmHg diastolic, and reduced heart rate by 12 beats per minute. This estimate is likely greater than the true effect because of biases in the running and reporting of the trials. We did not find convincing evidence that higher doses of nonselective beta‐blockers lowered blood pressure more than lower doses. However, higher doses of nonselective beta‐blockers significantly lowered heart rate compared with lower doses, which could lead to more side effects. Since the blood pressure lowering effect for systolic is similar to the blood pressure lowering effect of diastolic, the effect of this subclass on pulse pressure (the difference between systolic and diastolic) was small at about 2 mmHg.
Quality of the evidence
The quality of the evidence is low due to the presence of extreme outliers and high risk of biases.
Summary of findings
Summary of findings for the main comparison. Nonselective beta‐blockers compared with placebo for primary hypertension.
| Nonselective beta‐blockers compared with placebo for primary hypertension | |||
|
Patient or population: adults with primary hypertension Intervention: nonselective beta‐blockers Comparison: placebo | |||
| Outcomes | Mean estimates of combining 1x and 2x starting dose (95% CI) | Number of participants in the subgroups (# of studies) | Quality of the evidence (GRADE) |
| SBP (mmHg) | ‐9.5 (‐10.9 to ‐8.1)1,2,3,7 | 948 (16) | Low4,5 |
| DBP (mmHg) | ‐6.6 (‐7.4 to ‐5.8)1,2,3,7 | 948 (16) | Low4,5 |
| Heart rate (beats per minute) | ‐11.8 (‐12.9 to ‐10.7)1,2 | 864 (13) | Low4,5 |
| Pulse pressure (mmHg) | ‐2.0 (‐3.2 to ‐0.9)1,2,3 | 948 (16) | Very low4,5,6 |
| Withdrawal due to adverse effect | 0.84 (0.38 to 1.82) | 729 (2) | Low8,9 |
| CI: confident interval; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
| Footnotes | |||
| |||
Background
Description of the condition
Elevated blood pressure (BP) is a highly prevalent condition that is associated with an increased risk of adverse cardiovascular events including stroke, myocardial infarction (MI), congestive heart failure (CHF) and renal failure. Antihypertensive drug treatment has been shown to reduce the incidence of these adverse events in people aged 60 years and over with moderate‐to‐severe elevations of BP greater than 160/100 mmHg (Musini 2009). There are a number of classes of antihypertensive drugs used to treat elevated BP. Beta‐adrenergic receptor blockers (beta‐blockers) are one of those classes of drugs.
Description of the intervention
Beta‐blockers were originally marketed and used to treat angina. During their use in people with angina, it was discovered that they also lowered BP (Prichard 1966; Prichard 1969). Since then, they have received clinical attention because of their proven effectiveness to prevent recurrence in people who have recently had a MI (Teo 1993), and for people with heart failure (Brophy 2001; Ko 2004). Five published systematic reviews are relevant to this proposed review. Wright 2000 assessed the mortality and morbidity associated with different types of beta‐blockers. He found that post MI patients treated with nonselective beta‐blockers had a statistically significant reduction in total mortality compared with placebo, whereas those people treated with beta1‐selective beta‐blockers or partial agonist beta‐blockers did not. One review assessed the effects of beta‐blockers on morbidity and mortality in adults with hypertension (Wiysonge 2007). The review concluded that beta‐blockers were not the best class of drugs to use as first‐line antihypertensive therapy. However, it is possible that this applies primarily to beta1‐selective beta‐blockers, as atenolol was the beta‐blocker used in 75% of the trials.
Wright 2009 examined the mortality and morbidity outcomes of different classes of antihypertensive drugs. He found that beta‐blockers significantly reduced stroke and cardiovascular events but not all‐cause mortality and coronary heart disease (CHD). The clinical outcome results of beta‐blockers were inferior compared with low‐dose thiazide, angiotensin‐converting enzyme (ACE) inhibitors and calcium channel blockers.
Three further systematic reviews have assessed the effects of beta‐blockers on BP. One Cochrane systematic review of beta‐blockers for hypertension during pregnancy showed that oral beta‐blockers decreased the incidence of severe hypertension and the need for additional antihypertensive therapy (Magee 2003). One systematic review of the dose‐response relationship of BP lowering effects of beta‐blockers and other antihypertensive drugs was limited by the fact that it did not differentiate between the different classes of beta‐blockers (Law 2005). Finally, one Cochrane systematic review of the BP lowering efficacy of beta‐blockers as a second‐line treatment did not have enough trials to be able to differentiate between the different classes of beta‐blockers (Chen 2010).
How the intervention might work
Beta‐adrenergic receptors are present in many body systems including the heart, blood vessels, kidneys and nervous system. Presently, the mechanism whereby beta‐blockers lower BP is unknown. However, many hypothetical mechanisms have been proposed. Beta‐blockers could lower BP by decreasing cardiac output, reducing renin production, modulating the sympathetic nervous system or other mechanisms. It is likely that a combination of these mechanisms is responsible for the BP lowering effect.
Beta‐blockers were designed to inhibit beta‐receptors competitively and thus modulate sympathetic nervous system activity. Nonselective beta‐blockers were the first group of beta‐blockers to be developed and marketed. Drugs in this class, such as propranolol, competitively inhibit both beta1 and beta2 receptors with similar affinity and have no intrinsic sympathomimetic (partial agonist) activity or alpha‐receptor blocking property.
Why it is important to do this review
Since it is possible that beta‐blockers with different mechanisms of action have different effects on morbidity and mortality, it is crucial to determine whether they have different BP lowering abilities. No published review has compared the BP lowering effect of beta‐blockers based on their mechanism of action. If beta‐blockers with different beta‐receptor selectivity lower BP differently, this review could provide useful information toward understanding the mechanisms by which they lower BP in humans.
Furthermore, since BP measurement is used on a daily basis by physicians as a surrogate indicator to manage hypertension, it is important to know accurately the mean BP lowering effect of beta‐blockers, both individually and as a subclass. Nonselective beta‐blockers were the first subclass of beta‐blockers to be used clinically, and, thus, were the first subclass to be profiled. The protocol for this review was published previously (Wong 2008). This review will provide the basic BP lowering data to which the other subclasses can be compared. The information found in this review will also be useful to clinicians, researchers designing future drug trials and authors of other systematic reviews.
Objectives
Primary objective
To quantify the dose‐related effects of nonselective beta‐adrenergic receptor blockers (beta‐blockers) on systolic blood pressure (SBP) and diastolic blood pressure (DBP) as compared with placebo in people with primary hypertension.
Secondary objectives
To determine the effects of nonselective beta‐blockers on variability of BP.
To determine the effects of nonselective beta‐blockers on pulse pressure.
To quantify the dose‐related effects of nonselective beta‐blockers on heart rate.
To quantify the effects of nonselective beta‐blockers on withdrawals due to adverse effects (WDAE).
Methods
Criteria for considering studies for this review
Types of studies
We included studies that met the following criteria:
placebo‐controlled;
random allocation to nonselective beta‐blocker group and placebo group;
parallel or cross‐over design;
double blinded;
duration of follow‐up of at least three weeks;
BP measurements at baseline (following washout) and at one or more time points between three and 12 weeks after starting treatment.
Types of participants
Participants had a baseline SBP of at least 140 mmHg or a DBP of at least 90 mmHg, or both, measured in a standard way. Participants must not have had creatinine levels greater than 1.5 times the normal level. We did not restrict participants by age, gender, baseline risk or any other comorbid conditions.
Types of interventions
Monotherapy with any non‐selective beta‐blocker, including amosulalol, arotinolol, befunolol, betaxolol, bevantolol, bucumolol, bufetolol, bufuralol, bunitrolol, bupranolol, butofilolol, carazolol, carteolol, celiprolol, cetamolol, cloranolol, epanolol, indenolol, levobunolol, mepindolol, metipranolol, moprolol, nadolol, nadoxolol, nifenalol, penbutolol, practolol, pronethalol, propranolol, sotalol, sulfinalol, talinolol, tertatolol, tilisolol, timolol, toliprolol and xibenolol.
Data from trials in which titration to a higher dose was based on BP response were not eligible.
Types of outcome measures
Primary outcomes
Change in trough (13 to 26 hours after the dose) or peak (1 to 12 hours after the dose) (or both) SBP and DBP compared with placebo. If BP measurements were available at more than one time within the acceptable window, we used the weighted means of BPs taken in the three to 12 week range.
Secondary outcomes
Change in standard deviation compared with placebo.
Change in pulse pressure compared with placebo.
Change in heart rate compared with placebo.
Number of participants who WDAE compared with placebo.
Search methods for identification of studies
We searched the following databases for randomized controlled trials (RCTs):
The Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 9);
MEDLINE (1946 to 11 October 2013);
EMBASE (1974 to 11 October 2013);
ClinicalTrials.gov (all years to 11 October 2013).
We applied no language restrictions.
We searched the Database of Abstracts of Reviews of Effects (DARE) for related reviews. We used previously published meta‐analyses on dose‐response of beta‐blockers to help identify references to trials.
We used a modified, expanded version of the standard search strategy of the Cochrane Hypertension Group with additional terms related to beta‐blockers in general and all the specific drugs listed above to identify relevant articles. We adapted the MEDLINE strategy (Appendix 1) for searches of CENTRAL (Appendix 2), EMBASE (Appendix 3) and ClinicalTrials.gov (Appendix 4).
The initial search of all the databases was performed to identify citations with potential relevance. The initial screen of these abstracts excluded articles whose titles or abstracts, or both, were clearly irrelevant. We retrieved the full text of remaining articles (and translated them into English where required). We searched the bibliographies of pertinent articles, reviews and texts for additional citations. Two independent review authors assessed the eligibility of the trials using a trial selection form. A third review author resolved any discrepancies.
We identified and collected relevant studies for all beta‐blockers. The review authors separated the trials into four different subclasses of beta‐blockers (nonselective beta‐blockers, dual receptor blockers, partial agonists and beta‐1 selective blockers). We published the results of each subclass separately.
Data collection and analysis
Selection of studies
We imported references and abstracts of search results into Reference Manager 11 software. Selection of studies was based on the criteria listed above.
Data extraction and management
Two review authors independently extracted data using a standard form, and then cross‐checked each entry. A second review author confirmed all numeric calculations and graphic interpolations.
Assessment of risk of bias in included studies
Standard quality measures were not useful to distinguish between trials meeting the strict entry criteria of this review (Jadad 1996). We assessed risk of bias using the 'Risk of bias' tables for each trial as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
The position of the person during BP measurement may affect the BP lowering effect. However, in order to prevent loss of valuable data, if data from only one position were reported, then data from that position were collected. When BP measurement data were available in more than one position, we used sitting BP as the first preference. If both standing and supine were available, we used standing BP.
Dealing with missing data
In case of missing information in the included studies, we contacted investigators (using email, letter, fax, or a combination of these) to obtain the missing information.
In the case of missing standard deviations of the change in BP, we imputed the standard deviations based on the information in the same trial or from other trials using the same drug. We used the following hierarchy (listed from high to low preference) to impute standard deviation values:
standard deviation of change in BP taken in a different position to that of the BP data used;
standard deviation of BP at the end of treatment;
standard deviation of BP at the end of treatment measured in a different position to that of the BP data used;
standard deviation of BP at baseline (except if this measure is used for entry criteria);
mean standard deviation of change in BP from other trials using the same drug.
Assessment of heterogeneity
We used a standard Chi2 test and I2 statistic to test for heterogeneity of treatment effect between the trials. We applied the fixed‐effect model to obtain summary statistics of pooled trials, unless significant between‐study heterogeneity was present, in which case we used the random‐effects model.
Assessment of reporting biases
We assessed reporting biases using the 'Risk of bias' table in Characteristics of included studies table for each study.
Data synthesis
We performed data synthesis and analyses using Review Manager 5 (RevMan 2012). We combined data for changes in BP and heart rate using a generic inverse variance method. We analyzed drop‐outs due to side effects using risk ratio (RR) with 95% confidence interval (CI). When there is a significant difference in risk ration for withdrawal due to adverse effects, we would provide risk difference (RD) and number needed to treat for an additional harmful outcome (NNTH).
Subgroup analysis and investigation of heterogeneity
If possible, subgroup analyses included:
different regimens of the same active chemical entity;
gender, age and race;
comorbid conditions: ischemic heart disease, peripheral vascular disease, diabetes;
baseline severity of hypertension: mild, moderate, severe.
Sensitivity analysis
We tested the robustness of the results using several sensitivity analyses, including:
trials that were industry sponsored versus non‐industry sponsored;
trials with BP data measured in the sitting position versus other measurement positions;
trials with reported standard deviations of BP change versus imputed standard deviations.
Results
Description of studies
Results of the search
All four reviews used the same study inclusion criteria. In order to save time and effort, we developed a comprehensive search strategy so that all four subclasses of beta‐blockers were searched simultaneously (Appendix 1; Appendix 2; Appendix 3; Appendix 4). We then sorted citations according to their respective subclasses afterward. The search was first run in May 2010, and was updated twice in August 2011 and September 2012. In May 2010, the search strategy identified 18,604 citations from MEDLINE, EMBASE and CENTRAL. In August 2011, we identified an additional 2462 citations. In September 2012, we identified 248 new citations. Thus, we identified 21,314 citations in all three searches since May 2010, of which 8353 were confirmed to be duplicates. The review authors then screened 12,961 titles and abstracts and excluded 12,366 citations. We judged 595 citations to potentially meet the inclusion criteria based on title and abstract. These were retrieved for detailed review. After reading the full‐text articles, 480 did not meet our inclusion criteria and were excluded. One citation was a separate publication of the same data, and both are included in Lepantalo 1985. One hundred and fourteen trials met our inclusion criteria but 18 of them were excluded for reasons listed in the Characteristics of excluded studies table. Ninety‐six trials were included in all four reviews. Twenty‐five studies examining BP lowering efficacy of seven nonselective beta‐blockers in people with primary hypertension were included in this review. Figure 1 shows the PRISMA flow diagram. We contacted the primary author of Sica 2004 by email to request information on the time of BP measurement, but did not get a response.
1.
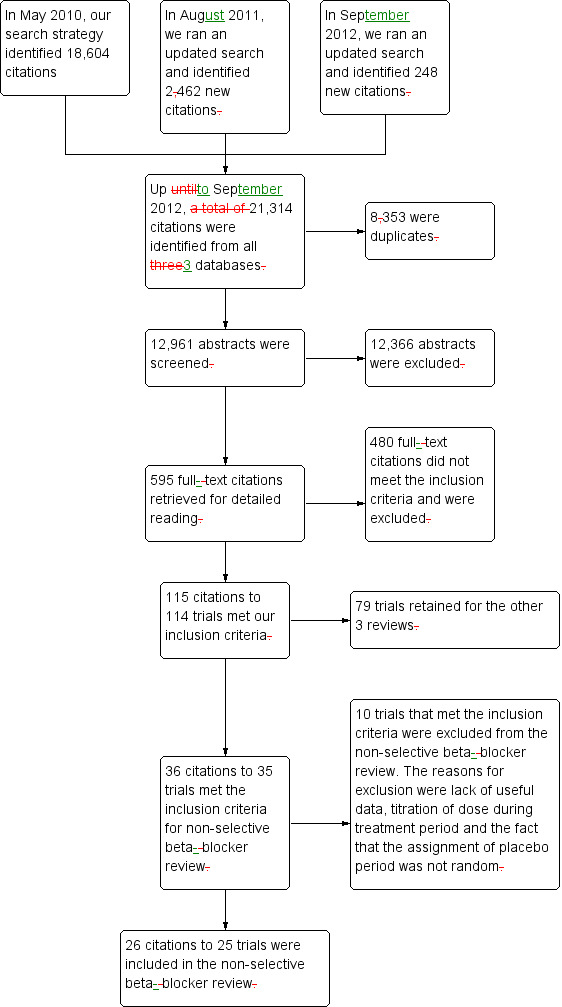
PRISMA diagram.
Included studies
This review comprised 25 RCTs with 1279 randomized participants (four parallel studies and 21 cross‐over studies). The duration of treatment ranged between three and 12 weeks, with the majority of trials lasting for four weeks. All studies were prospective, randomized, double‐blind, placebo‐controlled fixed‐dose trials. Propranolol was the most studied nonselective beta‐blocker with 18 trials in doses ranging from 60 to 640 mg/day. Participants enrolled into these studies were mostly between 52 to 59 year‐old with mild‐to‐moderate hypertension. The mean baseline BP of the included studies was 158/104 mmHg. Included trials are itemized in the Characteristics of included studies table.
Excluded studies
We excluded 10 studies that met the inclusion criteria from the nonselective beta‐blocker review. Reasons for exclusion included lack of useful data and titration of dose according to BP response or side effect (see Characteristics of excluded studies table).
Risk of bias in included studies
Figure 2 summarizes the overall risk of bias of the included studies. The assessment shows an unclear or high risk of bias in over 50% of the items suggesting that the findings of this review are likely an exaggeration of the real effect.
2.
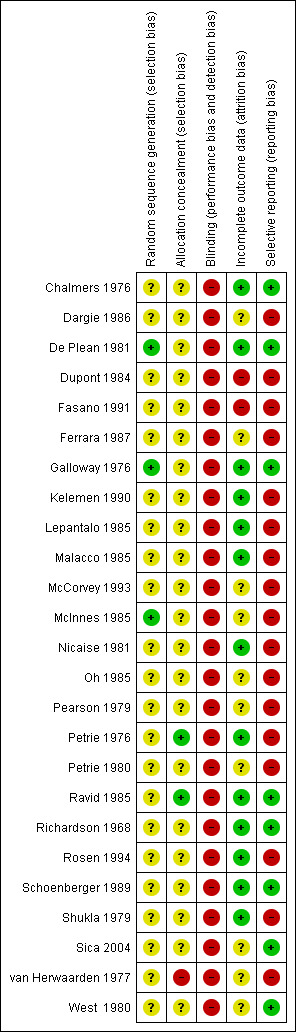
Allocation
All of the studies stated that participants were randomized and allocation of treatment was concealed. There was no obvious difference in baseline parameters between parallel groups suggesting that randomization was achieved in these trials. Therefore, we judged that there was a low risk of selection bias in the parallel trials. However, these baseline data were not provided for the cross‐over studies so we could not be confident that randomization and allocation concealment was achieved in these studies.
Blinding
All the studies used a double‐blind design. However, since beta‐blockers significantly lower heart rate and most of the studies measured BP by mercury sphygmomanometer, there was a high likelihood that the investigators could detect the assignment of intervention by the effect on the heart rate. Therefore, the risk of detection and performance bias was high in this review. This would also lead to performance bias. Because the auscultatory method is affected by the bleed rate of the mercury column, a slower heart rate also has the potential to lead to an overestimation of the decrease in SBP and an underestimation of the decrease in DBP.
Incomplete outcome data
We did not include participants who withdrew in the analysis for all the studies who reported drop‐outs. The dropout rate was low (total dropout rate was 7.5%) and, therefore, we judged that there was a low risk of attrition bias in our estimates.
Selective reporting
Only two studies reported WDAE in this review. WDAE is an important outcome in all RCTs. Not reporting WDAE could lead to high risk of reporting bias.
Other potential sources of bias
Publication bias
Nadolol, indenolol and moprolol studies did not provide any useful SBP and DBP data for our review. Nadolol is indicated for hypertension in both the US and Canada. In order to satisfy regulatory requirement in US and Canada, nadolol must have been tested in RCTs for BP lowering efficacy. The studies published before 1985 showed much greater BP lowering effect compared to studies published after 1985. They used same drug and same design for these trials. However, the patient population was different. In the older studies, the participants had moderate‐to‐severe hypertension according to recent definitions, whereas in the more recent studies, the participants had mild‐to‐moderate hypertension. In addition, the funnel plots showed presence of extreme positive outliers. Excluding these outliers from the analysis considerably lowered the estimate in some subgroups. This suggests that the estimate could be exaggerated due to extreme positive outliers and the high risk of publication bias.
Effects of interventions
See: Table 1
Effects of propranolol on systolic blood pressure, diastolic blood pressure, heart rate and pulse pressure
The recommended daily dose of propranolol according to the Canadian Pharmacists Association product monograph is 80 to 320 mg for extended release pill once daily for treatment of hypertension (eCPS). We included 18 studies examining the BP lowering effect of propranolol at doses ranging from 60 mg/day to 640 mg/day, in durations of four to 12 weeks in 837 people with hypertension. The majority of included studies measured peak BP using a mercury sphygmomanometer. The weighted mean baseline BP of the included studies was 163 mmHg SBP and 107 mmHg DBP. Analysis 1.1; Analysis 1.2; Analysis 1.3 and Analysis 1.4 summarize the results of propranolol.
1.1. Analysis.
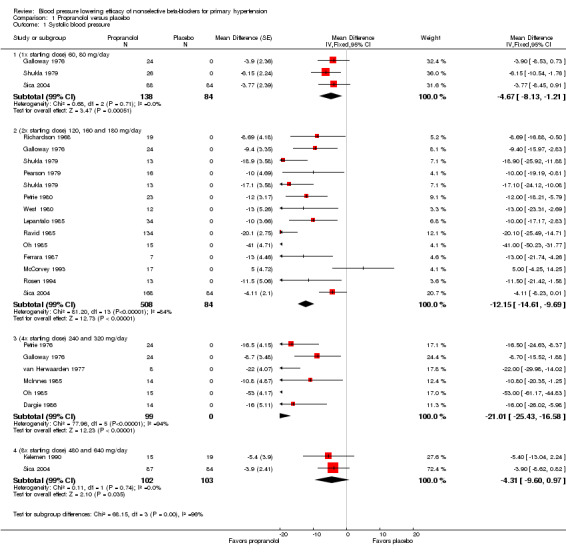
Comparison 1 Propranolol versus placebo, Outcome 1 Systolic blood pressure.
1.2. Analysis.
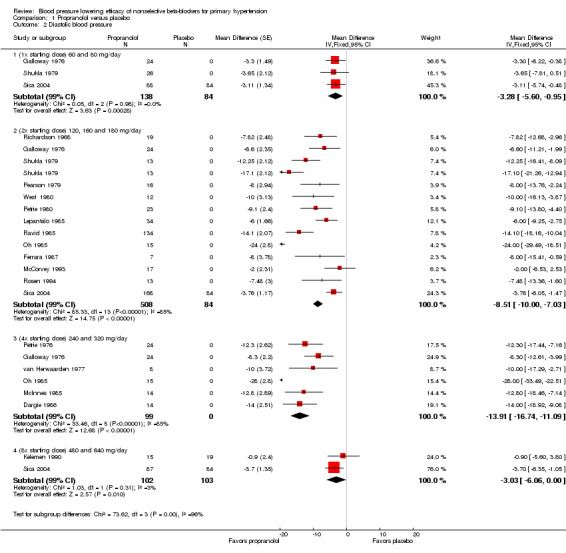
Comparison 1 Propranolol versus placebo, Outcome 2 Diastolic blood pressure.
1.3. Analysis.
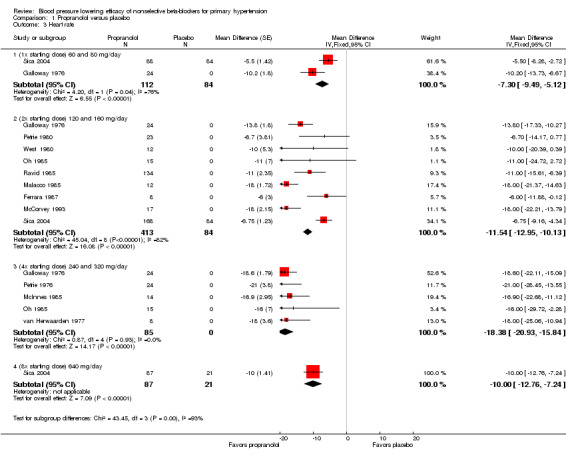
Comparison 1 Propranolol versus placebo, Outcome 3 Heart rate.
1.4. Analysis.
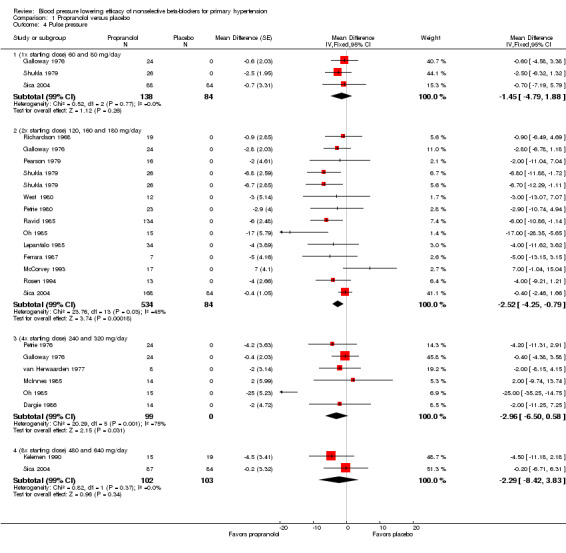
Comparison 1 Propranolol versus placebo, Outcome 4 Pulse pressure.
Propranolol significantly lowered SBP and DBP compared with placebo at doses equivalent to the recommended starting dose, twice (2x) the starting dose and four times (4x) times the starting dose. There was a significant degree of heterogeneity at 2x and 4x the starting dose in both SBP and DBP. The cause of heterogeneity is explored in the Discussion.
Not all studies included in propranolol analyses reported the change in heart rate. All doses of propranolol significantly lowered heart rate compared with placebo. There was a significant degree of heterogeneity at the starting dose and 2x the starting dose.
Only propranolol at 2x the starting dose significantly lowered pulse pressure. The magnitude of the effect on pulse pressure for the other doses was similar but not statistically significant compared with placebo. When all four doses were pooled, the result was statistically significant (‐2 mmHg).
We were able to use the data from Galloway 1976; Shukla 1979; and Sica 2004 to assess the additional BP lowering effect of propranolol directly when doses were doubled. Doubling the dose of propranolol did not cause a greater BP lowering effect for SBP or DBP. However, doubling the dose of propranolol significantly reduced heart rate further. The test for subgroup differences by direct comparison in heart rate was significant (P value < 0.00001). Oh 1985 also compared the effect of propranolol directly when dose was doubled. However, the BP lowering effect, a mean of ‐53 mmHg SBP and ‐28 mmHg DBP, seen in this study disagreed with all the other data. We judged this trial to be an extreme outlier, as of questionable validity and, therefore, we did not use it in the direct comparison analyses.
Funnel plots
Funnel plots were prepared for SBP and DBP at 2x the starting dose to explore heterogeneity (Figure 3; Figure 4). The funnel plots did not show the normal pattern with a number of extreme outliers and with the largest studies off the mean effect size. A possible explanation for this finding is discussed in Summary of main results.
3.
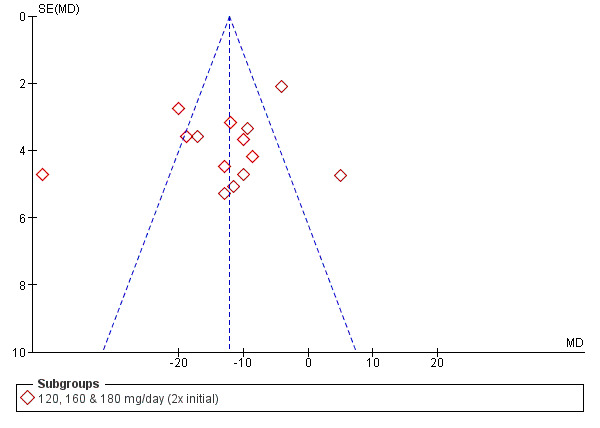
Funnel plot of systolic blood pressure of 2x starting dose propranolol.
4.
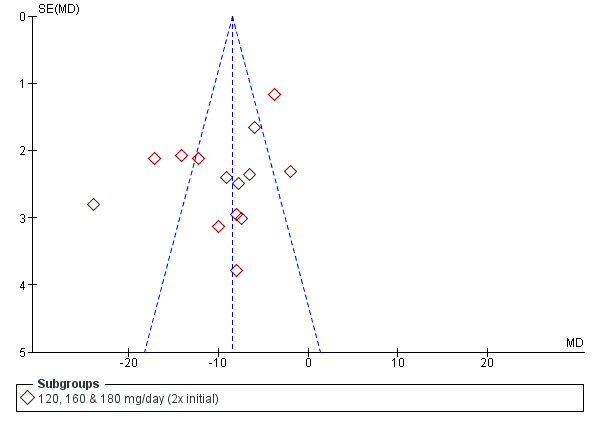
Funnel plot of diastolic blood pressure of 2x starting dose propranolol.
Effects of timolol on systolic blood pressure, diastolic blood pressure, heart rate and pulse pressure
The recommended daily dose of timolol for hypertension is 5 mg twice daily to 20 mg twice daily (eCPS; FDA). One study examining the BP lowering efficacy of 30 mg/day timolol in 20 people with hypertension was included (Chalmers 1976). It was a cross‐over study with an eight‐week treatment period. BP was measured by mercury sphygmomanometer. Timolol 30 mg/day significantly lowered both SBP and DBP compared with placebo. The treatment also significantly lowered heart rate but did not significantly lower pulse pressure. Analysis 2.1; Analysis 2.2; Analysis 2.3 and Analysis 2.4 summarize the results of timolol.
2.1. Analysis.
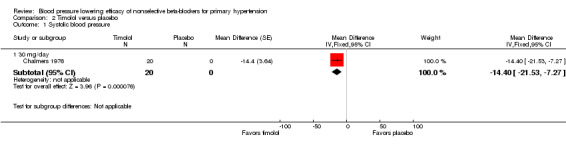
Comparison 2 Timolol versus placebo, Outcome 1 Systolic blood pressure.
2.2. Analysis.
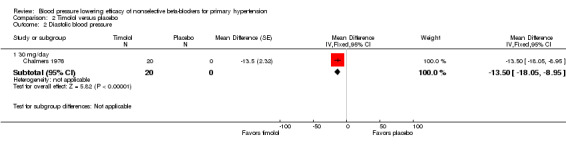
Comparison 2 Timolol versus placebo, Outcome 2 Diastolic blood pressure.
2.3. Analysis.
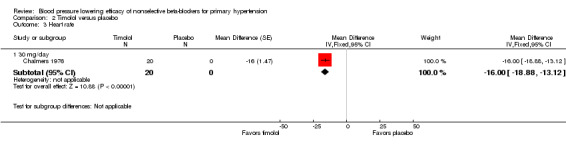
Comparison 2 Timolol versus placebo, Outcome 3 Heart rate.
2.4. Analysis.
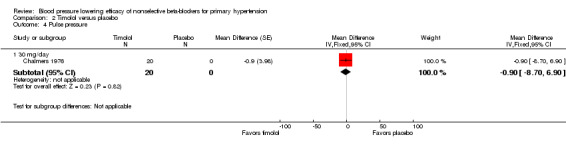
Comparison 2 Timolol versus placebo, Outcome 4 Pulse pressure.
Effects of penbutolol on systolic blood pressure, diastolic blood pressure, heart rate and pulse pressure
The recommended dose of penbutolol for hypertension is 20 mg once daily to 80 mg once daily (FDA). Two studies with 312 participants treated with penbutolol 20 mg/day, 40 mg/day, 80 mg/day or placebo were included. Mean baseline BP was 151.9/100 mmHg. De Plean 1981 was a cross‐over study lasting for four week. Schoenberger 1989 was a parallel study with treatment lasting for six weeks. Both studies measured BP using mercury sphygmomanometer in the standing position. Most of the BP was measured at trough times (13 to 24 hours after last dose). Analysis 3.1; Analysis 3.2; Analysis 3.3 and Analysis 3.4 summarize the results of penbutolol.
3.1. Analysis.
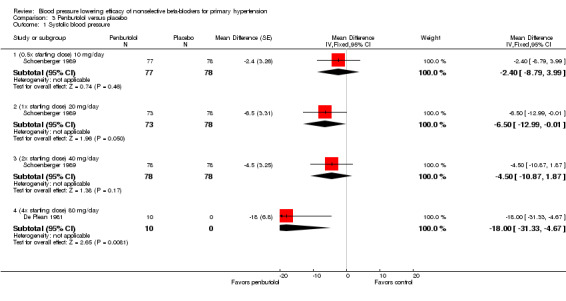
Comparison 3 Penbutolol versus placebo, Outcome 1 Systolic blood pressure.
3.2. Analysis.
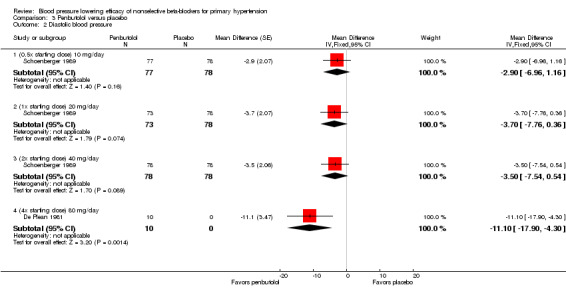
Comparison 3 Penbutolol versus placebo, Outcome 2 Diastolic blood pressure.
3.3. Analysis.
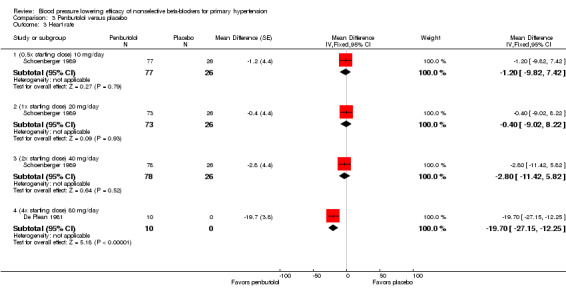
Comparison 3 Penbutolol versus placebo, Outcome 3 Heart rate.
3.4. Analysis.
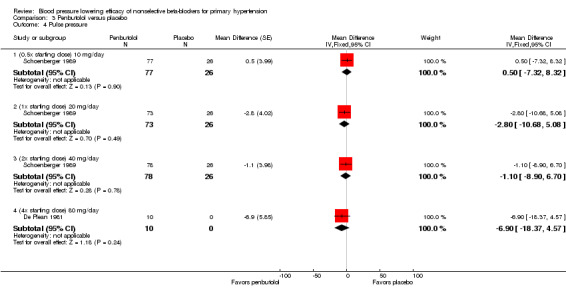
Comparison 3 Penbutolol versus placebo, Outcome 4 Pulse pressure.
Only penbutolol 80 mg/day significantly reduced both SBP and DBP compared with placebo. Direct comparison of dose effect was possible using data from Schoenberger 1989. There was no significant difference in BP lowering effect of SBP (P value = 0.68), DBP (P value = 0.96) or heart rate (P value = 0.93) for 10 to 40 mg/day penbutolol.
Effects of tertatolol on systolic blood pressure, diastolic blood pressure, heart rate and pulse pressure
One study with 20 participants was included for tertatolol. Fasano 1991 was a parallel study examining the BP lowering effect of tertatolol 5 mg/day for four weeks. The authors measured peak BP in the standing position using an automated device. The baseline BP was 154.5/104 mmHg.
Tertatolol 5 mg/day significantly lowered SBP and DBP compared with placebo. It also significantly lowered heart rate but did not lower pulse pressure compared with placebo. Analysis 4.1; Analysis 4.2; Analysis 4.3 and Analysis 4.4 summarize the results of tertatolol.
4.1. Analysis.
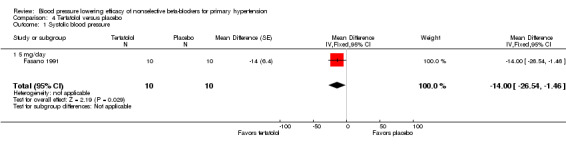
Comparison 4 Tertatolol versus placebo, Outcome 1 Systolic blood pressure.
4.2. Analysis.
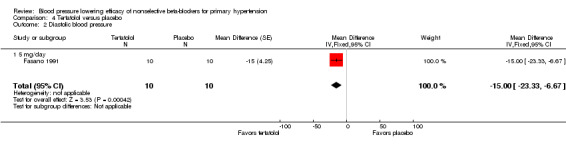
Comparison 4 Tertatolol versus placebo, Outcome 2 Diastolic blood pressure.
4.3. Analysis.
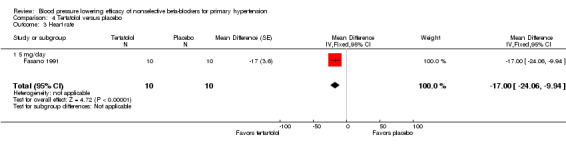
Comparison 4 Tertatolol versus placebo, Outcome 3 Heart rate.
4.4. Analysis.
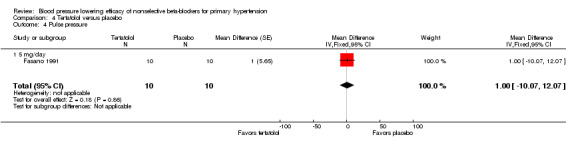
Comparison 4 Tertatolol versus placebo, Outcome 4 Pulse pressure.
Effect on heart rate of other nonselective beta‐blockers
Moprolol, indenolol and nadolol studies only provided data on heart rate. Indenolol and nadolol 80 mg/day significantly lowered heart rate compared with placebo but moprolol had no significant effect (Analysis 5.1; Analysis 6.1; Analysis 7.1).
5.1. Analysis.
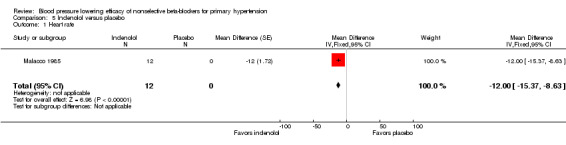
Comparison 5 Indenolol versus placebo, Outcome 1 Heart rate.
6.1. Analysis.
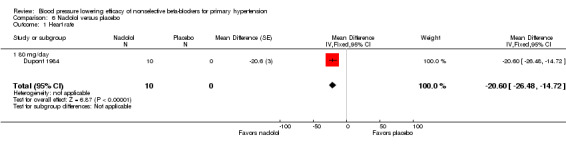
Comparison 6 Nadolol versus placebo, Outcome 1 Heart rate.
7.1. Analysis.
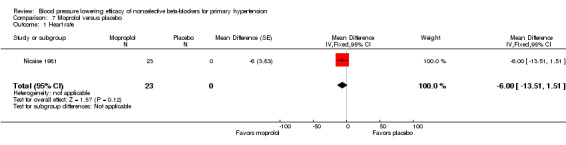
Comparison 7 Moprolol versus placebo, Outcome 1 Heart rate.
Pooled effects of nonselective beta‐blockers
We pooled the BP, heart rate and pulse pressure data of all available nonselective beta‐blockers based on the recommended starting doses (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4). The analyses showed that 0.5 times the starting dose did not significantly lower SBP, DBP, heart rate or pulse pressure, whereas the recommended starting doses and higher doses significantly lowered SBP, DBP and heart rate. Pulse pressure was not significantly reduced at any dose except for the 2x the starting dose subgroup.
8.1. Analysis.
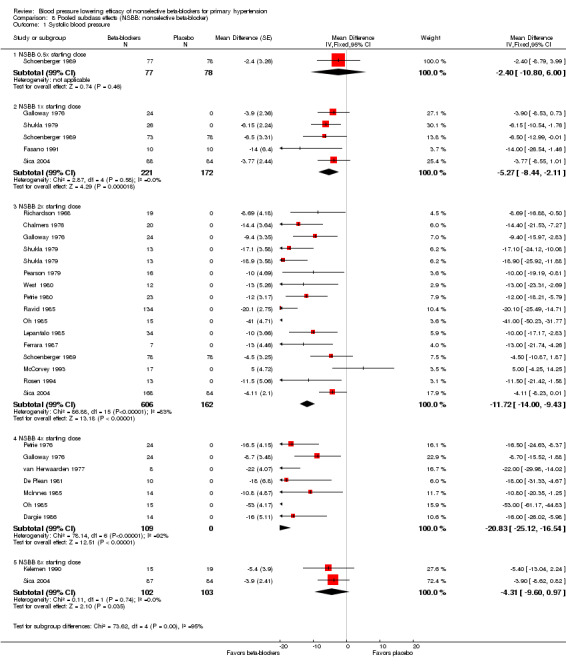
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 1 Systolic blood pressure.
8.2. Analysis.
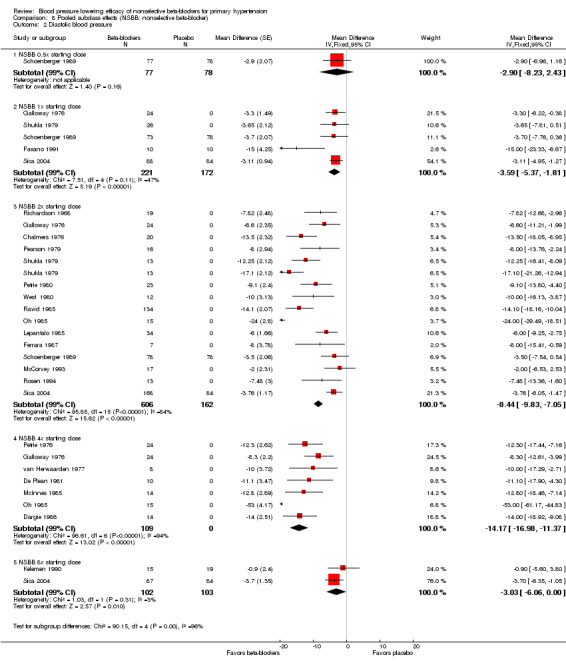
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 2 Diastolic blood pressure.
8.3. Analysis.
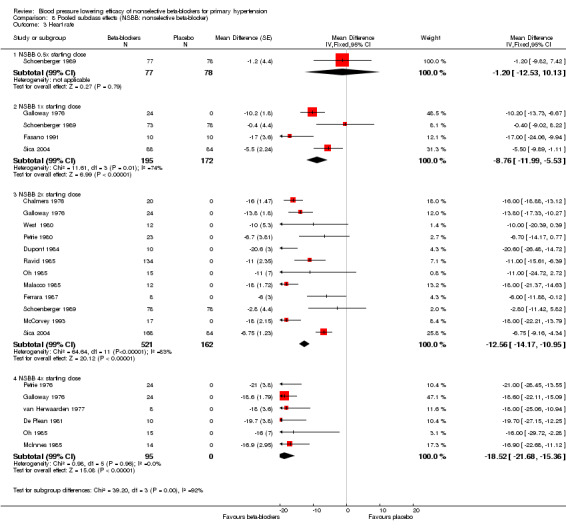
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 3 Heart rate.
8.4. Analysis.
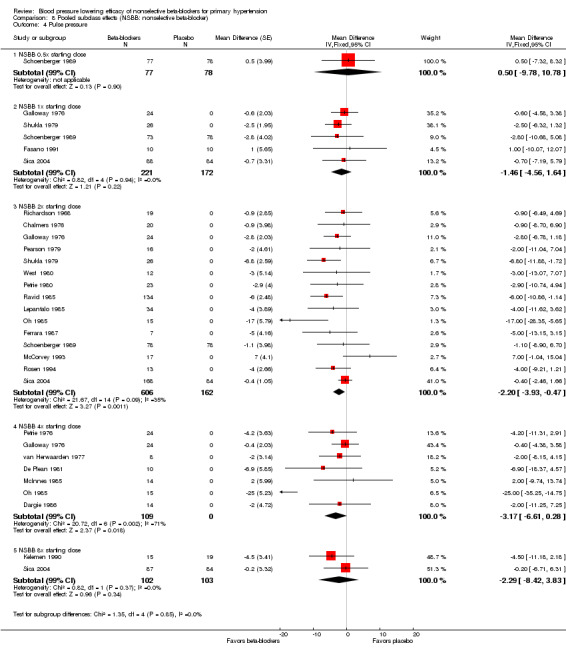
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 4 Pulse pressure.
Test for subgroup differences by direct comparison were not significant in the recommended dose range (once (1x), 2x, 4x and eight times (8x) the starting dose) for SBP, DBP and pulse pressure. Test for subgroup differences by direct comparison was significant in the recommend dose range for heart rate demonstrating a relationship between dose and effect for heart rate.
None of the studies included provided pulse pressure data. We calculated the pulse pressure by subtracting DBP from SBP. The pooled analysis confirmed the findings for effect of propranolol on pulse pressure.
Withdrawal due to adverse effects
Only two studies, one using propranolol and the other using penbutolol, provided data regarding WDAE (Analysis 8.5). WDAE were not significantly different with either propranolol or penbutolol compared with placebo. Due to the lack of information, no conclusion can be made about the effect of nonselective beta‐blockers on WDAE.
8.5. Analysis.
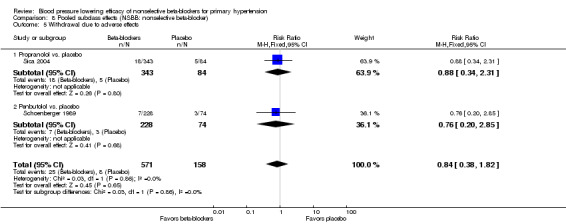
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 5 Withdrawal due to adverse effects.
Blood pressure variability
We assessed the BP variability between treatment and placebo by comparing the end treatment standard deviation with placebo by unpaired t‐test. Thirteen studies provided end‐treatment standard deviation of beta‐blocker and placebo group for this analysis. The overall mean end‐treatment standard deviation (SBP/DBP) for treatment group was 20.9/13.0 mmHg and 19.8/12.5 mmHg for placebo group. The BP variability for both SBP and DBP was not significantly different between treatment and placebo group. The P value for SBP variability was 0.23 and DBP variability was 0.77. The weighted mean variance ratio for SBP/DBP was 1.12/1.05. The majority of the data that contributed to the mean standard deviation came from propranolol.
Subgroup analysis
We were unable to perform subgroup analysis for race or comorbid condition due to lack of data reported separately for these parameters. There was no difference between trials in terms of age and severity and, therefore, we could not perform a subgroup analysis for these two parameters either. There was one study, Ravid 1985, which reported data for men and women separately. This study examined the BP lowering efficacy of propranolol 160 mg/day in 66 men and 68 women. The weighted mean baseline BP was 168/112 mmHg for men and 166/110 mmHg for women. The mean SBP reduction was ‐28 mmHg (95% CI ‐35 to ‐21) for men and ‐13 mmHg (95% CI ‐21 to ‐5) for women; the mean DBP reduction was ‐15 mmHg (95% CI ‐21 to ‐10) for men and ‐13 mmHg (95% CI ‐19 to ‐7) for women. The reduction of SBP but not DBP was significantly greater in men than women. In addition, propranolol significantly lowered pulse pressure in men but not in women.
Discussion
Summary of main results
Effect of nonselective beta‐blockers on systolic blood pressure, diastolic blood pressure, heart rate and pulse pressure
Propranolol
This review provides the most up‐to‐date estimates of the dose‐related BP lowering efficacy of nonselective beta‐blockers. Most of the data contributing to the estimates came from propranolol trials. Propranolol significantly lowered resting peak SBP and DBP compared with placebo. Similarly, all propranolol doses significantly lowered heart rate compared with placebo.
Direct comparison of doses was possible by pooling three studies that examined the effect of doubling the dose of propranolol. This analysis showed that doubling the dose of propranolol did not significantly lower BP further. However, this dose‐response analysis is limited due to the small sample size.
Significant heterogeneity and the relatively large effect size of propranolol 4x starting dose subgroup were caused by an extreme outlier (Oh 1985). The point estimate of the 4x starting dose would change from ‐21/‐15 mmHg to ‐14/‐12 mmHg if we exclude Oh 1985. Although this was still a large effect, the wide 95% CI indicated that this estimate was imprecise.
If there is any dose‐response relationship for propranolol, it is less with the starting dose, peaks at 4x the starting dose and then decreases with higher doses. This would be an unusual effect and there are few if any proven dose responses of this pattern. If true, an explanation might be that, at higher doses, the beta‐2 blocking effect leads to antagonism of the BP lowering effect by blocking vasodilation in the arteries to muscles. More carefully conducted trials are needed to elucidate this dose‐response pattern properly. In contrast, this pattern was not seen with any of the other nonselective beta‐blockers.
Heterogeneity in propranolol 2x starting dose subgroup
The 2x starting dose subgroup of SBP contained the largest sample size in the analysis and manifested a very unusual pattern in the funnel plots (Figure 3; Figure 4). The random‐effects estimates (SBP ‐13 mmHg (95% CI ‐19 to ‐7); DBP ‐10 mmHg (95% CI ‐14 to ‐6) was not significantly different from fixed effect estimates (SBP ‐12 mmHg (95% CI ‐15 to ‐10); DBP ‐9 mmHg (95% ‐10 to ‐7) in the 2x starting dose subgroup. In order to explore the heterogeneity, we arranged the studies according to publication year and then divided them into two groups based on their publication year, one before 1985 and one after 1985. In the older studies, the funnel plot showed that Oh 1985 and Ravid 1985 were two extreme outliers and the main contributors to heterogeneity. In the newer studies, the funnel plot identified McCorvey 1993 as an extreme outlier.
We conducted the same procedure in order to explore the heterogeneity in DBP analysis of 2x starting dose subgroup. In the older studies, the funnel plot identified Oh 1985; Ravid 1985; and Shukla 1979 as extreme outliers in this subgroup. There was no significant heterogeneity among the newer studies.
The estimates for the two subgroups were significantly different from each other (‐16/‐11 mmHg for the older studies and ‐5/‐4 mmHg for the newer studies). The difference in effect size between these two groups is possibly due to the difference in baseline BP. The weighted mean baseline BP was higher in the older studies (170/113 mmHg) compared to the newer studies (154/101 mmHg). This reflects the fact that the definition of hypertension had changed over time. In the older studies, hypertension was defined as greater than 160/100 mmHg and in the more recent studies as greater than 140/90 mmHg. Higher baseline BP would be expected to be associated with producing greater absolute BP reductions.
Another factor that may have added to the difference is a higher risk of publication bias in the older studies and that older studies were influenced by active marketing of the drug. Studies published at the time were more likely to show greater effect. In contrast, in the newer studies, propranolol was being studied as an active comparator to a newer drug with a possible negative bias against propranolol. The older studies could also have been subject to greater loss of blinding leading to detection bias, which would also tend to exaggerate the effect size. It also could be a combination of these factors that led to the difference in effect size and caused significant heterogeneity.
The baseline BP in the newer studies was similar to the baseline in the RCTs of the ACE inhibitor and angiotensin receptor blocker (ARB) reviews by Heran 2008 (ACEI) and Heran 2008 (ARB). Thus, when comparing the BP lowering effect between nonselective beta‐blockers and ACE inhibitors and ARBs, it is more appropriate to use the estimates from the newer studies.
Penbutolol
The larger study in penbutolol analysis, Schoenberger 1989, showed no statistically significant BP lowering effect in doses ranging from 10 mg/day to 40 mg/day. The relatively large effect of the smaller RCT, De Plean 1981, in penbutolol 80 mg/day was out of keeping with the other data. The larger effect in the 80 mg/day subgroup could be because De Plean 1981 measured peak BP while Schoenberger 1989 measured trough BP. Moreover, there are insufficient published evidence supporting the product monograph recommendation of penbutolol 20 mg/day and 40 mg/day in the treatment of hypertension.
Other included non‐selective beta‐blockers
There were few data available for other nonselective beta‐blockers. It was not possible to draw any definitive conclusions based on such small data sets. Some of these drugs, such as nadolol and timolol, have been approved for clinical use in US, Europe and Canada since late 1970. Therefore, we are certain that studies were completed in order to fulfill regulatory requirements in these countries and that these studies remain unpublished at this time. Despite our effort to search for unpublished data, we were unable to find any trial data for these drugs. It is important that these trial results be made available for scientific analysis and are available to contribute to systematic reviews.
Pooled effects of nonselective beta‐blockers
We pooled the data from all available nonselective beta‐blockers based on the recommended starting dose in order to obtain the overall subclass effect. We recognized that this method presented certain limitations as different manufacturers might have used different methods to determine with the recommended starting doses. However, this method provided the most logical way of data standardization for combining different drugs to be analyzed as a whole class.
Propranolol contributed to 65% of the data. The pooled data also presented similar characteristics of heterogeneity as in the propranolol analyses because propranolol was the main contributor of data in the pool. The 1x and 2x starting dose subgroups contained the largest sample size. The estimate of BP lowering efficacy for nonselective beta‐blockers by combining the 1x and 2x starting dose subgroup was ‐10/‐7 mmHg. If we exclude Oh 1985, the estimate would change from ‐10/‐7 mmHg to ‐9/‐6 mmHg. However, other than it being an extreme outlier, due to the lack of information, we found no flaws in the trial that would justify excluding it from the estimate.
Dose‐response analysis was inconclusive. Nonselective beta‐blockers, in the recommended dose range, did not showed a convincing dose‐response relationship by direct comparison. There was little evidence to support using a dose higher than 2x starting dose of nonselective beta‐blockers to lower BP further.
Heart rate
Nonselective beta‐blockers starting at the 1x recommended starting doses significantly lowered heart rate. The dose‐response relationship for heart rate was evident by both direct and indirect comparison.
Pulse pressure
Nonselective beta‐blockers did not significantly reduce pulse pressure in any dose subgroups except for the 2x starting dose. We calculated a minimum sample size of 442 was needed to provide adequate power (alpha = 0.05, beta = 0.8) to detect a difference of 2 mmHg in pulse pressure with a SD of 15. Only the 2x starting dose subgroup contained a sufficient number of participants according to the power analysis. The point estimates in the 1x, 4x and 8x starting dose subgroups were similar to the 2x starting dose subgroup. Therefore, it would appear that if nonselective beta‐blockers do lower pulse pressure, the magnitude is likely about 2 mmHg.
This relative lack of effect on pulse pressure may explain why beta‐blockers appear to be less effective at reducing mortality than other drug classes as shown by Musini 2009; Wiysonge 2007; and Wright 2000; . However, we recognize that most of the data in people with hypertension included in the Musini 2009 and Wiysonge 2007 reviews used atenolol. Furthermore, Wright 2000 found that only nonselective beta‐blockers demonstrated a mortality benefit in people post MI. This suggests that the effect of nonselective beta‐blockers on mortality could be different to the beta‐1 selective, atenolol. More studies evaluating the effect on mortality and morbidity of nonselective beta‐blockers are needed to elucidate this question.
Blood pressure variability
Nonselective beta‐blockers did not significantly change end treatment BP variability compared with placebo. The SBP variance ratio in our analysis was smaller than the one found in Webb 2011 (1.12 versus 1.34). One analysis on the BP variability of beta‐blockers suggested that nonselective beta‐blockers might significantly increase SBP variability (Webb 2011). There were several important differences between our analysis and that of Webb 2011 that could have caused the differences in findings. First, Webb 2011 included people with hypertension, post MI and CHF in their analysis whereas our analysis only included people with primary hypertension. The effect of beta‐blockers on BP variability could be different in primary hypertension compared to people post MI and with CHF.
Second, Webb 2011 also compared beta‐blockers with other antihypertensive treatments whereas we compared beta‐blockers with placebo alone. Beta‐blockers could tend to increase BP variability compared with placebo, while other antihypertensive drugs could tend to lower BP variability compared with placebo. By comparing beta‐blockers with other antihypertensive drugs directly, the larger difference could have led to a statistically significant result. A systematic review of BP variability of all antihypertensive drugs classes compared with placebo in people with hypertension is needed to determine the truth.
None of the studies included in the analysis was design to examine the difference in BP variability. Future RCT designed to evaluate the BP variability of beta‐blockers are needed in order to clarify this issue.
Subgroup analysis
The planned subgroup analyses were not possible due to lack of data. Ravid 1985 provided some data for subgroup analysis in man and women. We summarized the findings in this study in the results section. However, we should not draw any conclusion based on the findings of one single study. In addition, the effect sizes of Ravid 1985 were extreme, raising concern about bias in this study. More large‐scale RCT reporting outcome separately by sex are needed before we can determine whether or not there is a significant difference in effect size between difference sexes.
Overall completeness and applicability of evidence
This review provided a reasonable amount of information regarding BP lowering efficacy of nonselective beta‐blockers. Most of the studies included in this review had the same primary objectives, which were to examine the BP lowering efficacy of respective beta‐blockers in the studies compared with placebo. Therefore, we are confident that the evidence found in this review is the best evidence available to answer our objectives.
Quality of the evidence
Table 1 summarizes the combined effect size of the combined starting and 2x the starting dose of nonselective beta‐blockers. In addition, it provides a judgment of the quality of evidence in this review. This review included 25 RCTs with 1279 people with hypertension. The sample size should provide adequate power to draw robust conclusions. However, as for all systematic reviews, we were limited by the data available to us.
Most of the identified studies did not use an automated machine to measure BP. An automated machine could mitigate the risk of clinicians or participants detecting the intervention assignment. Therefore, the risk of detection bias remains high in our review.
None of the studies reported pulse pressure as an outcome. We calculated pulse pressure by subtracting DBP from SBP. This meant that the data on pulse pressure did not come from direct measurement in the studies. Indirectness of this outcome is the reason the evidence was further downgraded for pulse pressure.
In addition, the risk of publication bias was high in this review. For these and other reasons outlined in the discussion, the estimates of the BP lowering effect shown in the Table 1 are very likely an exaggeration of the true effect. This is reflected in the table by the low to very low judgment of the quality of the evidence.
Potential biases in the review process
The rigid methodology of this review and the comprehensive search strategies minimized potential for bias by the authors in the selection process of included studies. The search strategies were developed and performed by the Trial Search Co‐ordinator of the Cochrane Hypertension Group. The inclusion criteria were designed to minimize any biases that could have been introduced during the selection process.
There were occasions that the review authors had to obtain data from figures instead of numeric tables. This could introduce potential for bias, as the measurements on the figure could be different from person to person. In order to minimize this type of bias, two review authors extracted data independently and then the mean of the two values were used for the analyses.
Agreements and disagreements with other studies or reviews
Two published reviews, Chen 2010 and Law 2005, also examined the BP lowering effect of beta‐blockers. Chen 2010 examined the additional effect of beta‐blockers in combination with other antihypertensive drugs. We specifically examined beta‐blockers as monotherapy; therefore, it is not appropriate to compare our review to that of Chen 2010.
In addition, both Chen 2010 and Law 2005 pooled all the beta‐blockers together by assuming that they had similar effects. Our review is quite unique in the sense that we have not assumed all beta‐blockers behave similarly. We examined them individually and, in the case of this review, found that they were different. We will be able to assess the differences in BP lowering effect of each subclass to one another when all four beta‐blocker reviews in this Cochrane series are published.
Nonselective beta‐blockers lowered peak SBP by a smaller degree (‐10/‐7 mmHg) than the peak measurement of ACE inhibitors (‐11 mmHg) and ARBs (‐12 mmHg) (Heran 2008 (ACEI); Heran 2008 (ARB)). However, they lowered peak DBP by a similar degree compared to ARBs (‐7 mmHg) and greater degree compared to ACE inhibitors (‐6 mmHg). The effect of nonselective beta‐blockers is likely exaggerated due to the presence of extreme outliers and other biases. If we remove the extreme outliers from the analysis, the estimate for nonselective beta‐blockers is lower (‐8/‐5 mmHg). The most striking difference between nonselective beta‐blockers and the other classes is the smaller effect on pulse pressure, which is the result of the relative larger DBP effect size compare to SBP.
Authors' conclusions
Implications for practice.
This review provides the most up‐to‐date clinical evidence on the hemodynamic effects of nonselective beta‐blockers in people with hypertension. Propranolol and penbutolol contributed to most of the data. In people with mild‐to‐moderate hypertension, nonselective beta‐blockers lowered peak blood pressure (BP) by a mean of ‐10/‐7 mmHg and reduced heart rate by 12 beats per minute. This estimate is likely exaggerated due to the presence of extreme outliers and other sources of bias. Mean estimate from studies published before 1985 were much higher compared to recent studies. Baseline BP, time of measurement and publication bias help to explain the differences in effect size.
Nonselective beta‐blockers showed no convincing dose‐response relationship for systolic blood pressure and diastolic blood pressure. The evidence for an increasing magnitude of effect at higher doses was weak. However, it was evident that nonselective beta‐blockers showed a dose‐response relationship on heart rate. This suggests that nonselective beta‐blockers cause more bradycardia at higher doses compared with lower doses but do not provide additional BP lowering effects. There is very low quality evidence that twice the starting dose of nonselective beta‐blockers leads to a small reduction in pulse pressure (2 mmHg). The estimates in other dose subgroups showed similar effect sizes; however, these were imprecise and compatible with no effect or small differences. This suggests that if nonselective beta‐blockers significantly lower pulse pressure, the effect is likely small.
Implications for research.
Many of the drugs in this beta‐blocker class have no data available for their BP lowering efficacy despite the fact that they are marketed for the treatment of hypertension. It should be mandatory that all clinical trial results be published in detail.
Manufacturers should provide the justification for recommended dosages. Data should be made available to show additional effects at higher doses if a dose range is recommended.
The procedure of randomization and blinding must be described in detail in published reports.
Trial should report the method and time that BP was measured. Peak and trough BP should be reported separately. Future randomized controlled trials should use 24‐hour ambulatory BP measurements, which would include both peak and trough measurements.
Reporting of withdrawal due to adverse effects should be mandatory for all trials.
The results of this review should be compared with those of the other three subclasses and the BP lowering effects of different subclasses of beta‐blockers should be examined.
History
Protocol first published: Issue 4, 2008 Review first published: Issue 2, 2014
| Date | Event | Description |
|---|---|---|
| 18 June 2010 | Amended | Added second author; added double blind and crossover studies in inclusion criteria. Updated background, search methods and reference. |
Acknowledgements
The review authors would like to acknowledge the help provided by the Cochrane Hypertension Group and thank Stephen Adams for retrieving the full text of the studies. Alexandra Laugerotte confirmed the eligibility of included studies and independently extracted the data.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to 11 October 2013 with Daily Update ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp adrenergic beta‐antagonists/ 2 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 3 acebutolol.mp. 4 exp alprenolol/ 5 alprenolol.mp. 6 amosulalol.mp. 7 arotinolol.mp. 8 atenolol.mp. 9 befunolol.mp. 10 betaxolol.mp. 11 bevantolol.mp. 12 exp bisoprolol/ 13 bisoprolol.mp. 14 bopindolol.mp. 15 bucindolol.mp. 16 bucumolol.mp. 17 bufetolol.mp. 18 bufuralol.mp. 19 bunitrolol.mp. 20 exp bupranolol/ 21 bupranolol.mp. 22 butofilolol.mp. 23 carazolol.mp. 24 exp carteolol/ 25 carteolol.mp. 26 carvedilol.mp. 27 exp celiprolol/ 28 celiprolol.mp. 29 cetamolol.mp. 30 cloranolol.mp. 31 cyanopindolol.mp. 32 deacetylmetipranolol.mp. 33 dihydroalprenolol.mp. 34 dilevalol.mp. 35 epanolol.mp. 36 esmolol.mp. 37 indenolol.mp. 38 iodocyanopindolol.mp. 39 exp labetalol/ 40 labetalol.mp. 41 landiolol.mp. 42 exp levobunolol/ 43 levobunolol.mp. 44 mepindolol.mp. 45 exp metoprolol/ 46 metoprolol.mp. 47 exp metipranolol/ 48 metipranolol.mp. 49 moprolol.mp. 50 exp nadolol/ 51 nadolol.mp. 52 nadoxolol.mp. 53 nebivolol.mp. 54 nifenalol.mp. 55 nipradilol.mp. 56 oxprenolol.mp. 57 exp penbutolol/ 58 penbutolol.mp. 59 exp pindolol/ 60 pindolol.mp. 61 exp practolol/ 62 practolol.mp. 63 pronethalol.mp. 64 exp propranolol/ 65 propranolol.mp. 66 proxodolol.mp. 67 exp sotalol/ 68 sotalol.mp. 69 sulfinalol.mp. 70 talinolol.mp. 71 tertatolol.mp. 72 tilisolol.mp. 73 exp timolol/ 74 timolol.mp. 75 toliprolol.mp. 76 xibenolol.mp. 77 or/1‐76 78 hypertension/ 79 hypertens$.tw. 80 exp blood pressure/ 81 (blood pressure or bloodpressure).mp. 82 or/78‐81 83 randomized controlled trial.pt. 84 controlled clinical trial.pt. 85 randomized.ab. 86 placebo.ab. 87 clinical trials as topic/ 88 randomly.ab. 89 trial.ti. 90 or/83‐89 91 animals/ not (humans/ and animals/) 92 90 not 91 93 77 and 82 and 92
Appendix 2. CENTRAL search strategy
Database: (Wiley) Cochrane Central Register of Controlled Trials <Issue 9, 2013> Search date: 11 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ID Search #1MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees #2(acebutolol):ti,ab,kw in Trials #3 MeSH descriptor: [Alprenolol] explode all trees #4 alprenolol:ti,ab,kw in Trials #5 amosulalol:ti,ab,kw in Trials #6 arotinolol:ti,ab,kw in Trials #7 atenolol:ti,ab,kw in Trials #8 befunolol:ti,ab,kw in Trials #9 betaxolol:ti,ab,kw in Trials #10 bevantolol:ti,ab,kw in Trials #11 MeSH descriptor: [Bisoprolol] explode all trees #12 bisoprolol:ti,ab,kw in Trials #13 bopindolol:ti,ab,kw in Trials #14 bucindolol:ti,ab,kw in Trials #15 bucumolol:ti,ab,kw in Trials #16 bufetolol:ti,ab,kw in Trials #17 bufuralol:ti,ab,kw in Trials #18 bunitrolol:ti,ab,kw in Trials #19 MeSH descriptor: [Bupranolol] explode all trees #20 bupranolol:ti,ab,kw in Trials #21 butofilolol:ti,ab,kw in Trials #22 carazolol:ti,ab,kw in Trials #23 MeSH descriptor: [Carteolol] explode all trees #24 carteolol:ti,ab,kw in Trials #25 carvedilol:ti,ab,kw in Trials #26 MeSH descriptor: [Celiprolol] explode all trees #27 celiprolol:ti,ab,kw in Trials #28 cetamolol:ti,ab,kw in Trials #29 cloranolol:ti,ab,kw in Trials #30 cyanopindolol:ti,ab,kw in Trials #31 deacetylmetipranolol:ti,ab,kw in Trials #32 dihydroalprenolol:ti,ab,kw in Trials #33 dilevalol:ti,ab,kw in Trials #34 epanolol:ti,ab,kw in Trials #35 esmolol:ti,ab,kw in Trials #36 indenolol:ti,ab,kw in Trials #37 iodocyanopindolol:ti,ab,kw in Trials #38 MeSH descriptor: [Labetalol] explode all trees #39 labetalol:ti,ab,kw in Trials #40 nebivolol:ti,ab,kw in Trials #41 MeSH descriptor: [Levobunolol] explode all trees #42 mepindolol:ti,ab,kw in Trials #43 mepindolol:ti,ab,kw in Trials #44 MeSH descriptor: [Metoprolol] explode all trees #45 metoprolol:ti,ab,kw in Trials #46 MeSH descriptor: [Metipranolol] explode all trees #47 levobunolol:ti,ab,kw in Trials #48 nifenalol:ti,ab,kw in Trials #49 MeSH descriptor: [Nadolol] explode all trees #50 nadolol:ti,ab,kw in Trials #51 nadoxolol:ti,ab,kw in Trials #52 nebivolol:ti,ab,kw in Trials #53 metipranolol:ti,ab,kw in Trials #54 nipradilol:ti,ab,kw in Trials #55 oxprenolol:ti,ab,kw in Trials #56 MeSH descriptor: [Penbutolol] explode all trees #57 penbutolol:ti,ab,kw in Trials #58 MeSH descriptor: [Pindolol] explode all trees #59 pindolol:ti,ab,kw in Trials #60 MeSH descriptor: [Practolol] explode all trees #61 practolol:ti,ab,kw in Trials #62 pronethalol:ti,ab,kw in Trials #63 MeSH descriptor: [Propranolol] explode all trees #64 propranolol:ti,ab,kw in Trials #65 MeSH descriptor: [Sotalol] explode all trees #66 sotalol:ti,ab,kw in Trials #67 sulfinalol:ti,ab,kw in Trials #68 talinolol:ti,ab,kw in Trials #69 tertatolol:ti,ab,kw in Trials #70 tilisolol:ti,ab,kw in Trials #71 MeSH descriptor: [Timolol] explode all trees #72 timolol:ti,ab,kw in Trials #73 toliprolol:ti,ab,kw in Trials #74 xibenolol:ti,ab,kw in Trials #75 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 in Trials #76 MeSH descriptor: [Hypertension] this term only #77 hypertens*:ti,ab in Trials #78 MeSH descriptor: [Blood Pressure] explode all trees #79 blood pressure:ti,ab,kw in Trials #80 #76 or #77 or #78 or #79 in Trials #81 #75 and #80 in Trials
Appendix 3. EMBASE search strategy
Database: EMBASE <1974 to 11 October 2013> ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp beta adrenergic receptor blocking agent/ 2 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 3 acebutolol.mp. 4 exp alprenolol/ 5 alprenolol.mp. 6 amosulalol.mp. 7 arotinolol.mp. 8 atenolol.mp. 9 befunolol.mp. 10 betaxolol.mp. 11 bevantolol.mp. 12 exp bisoprolol/ 13 bisoprolol.mp. 14 bopindolol.mp. 15 bucindolol.mp. 16 bucumolol.mp. 17 bufetolol.mp. 18 bufuralol.mp. 19 bunitrolol.mp. 20 exp bupranolol/ 21 bupranolol.mp. 22 butofilolol.mp. 23 carazolol.mp. 24 exp carteolol/ 25 carteolol.mp. 26 carvedilol.mp. 27 exp celiprolol/ 28 celiprolol.mp. 29 cetamolol.mp. 30 cloranolol.mp. 31 cyanopindolol.mp. 32 deacetylmetipranolol.mp. 33 dihydroalprenolol.mp. 34 dilevalol.mp. 35 epanolol.mp. 36 esmolol.mp. 37 indenolol.mp. 38 iodocyanopindolol.mp. 39 exp labetalol/ 40 labetalol.mp. 41 landiolol.mp. 42 exp levobunolol/ 43 levobunolol.mp. 44 mepindolol.mp. 45 exp metoprolol/ 46 metoprolol.mp. 47 exp metipranolol/ 48 metipranolol.mp. 49 moprolol.mp. 50 exp nadolol/ 51 nadolol.mp. 52 nadoxolol.mp. 53 nebivolol.mp. 54 nifenalol.mp. 55 nipradilol.mp. 56 oxprenolol.mp. 57 exp penbutolol/ 58 penbutolol.mp. 59 exp pindolol/ 60 pindolol.mp. 61 exp practolol/ 62 practolol.mp. 63 pronethalol.mp. 64 exp propranolol/ 65 propranolol.mp. 66 proxodolol.mp. 67 exp sotalol/ 68 sotalol.mp. 69 sulfinalol.mp. 70 talinolol.mp. 71 tertatolol.mp. 72 tilisolol.mp. 73 exp timolol/ 74 timolol.mp. 75 toliprolol.mp. 76 xibenolol.mp. 77 or/1‐76 78 exp hypertension/ 79 hypertens$.tw. 80 (blood pressure or bloodpressure).mp. 81 or/78‐80 82 randomized controlled trial/ 83 crossover procedure/ 84 double‐blind procedure/ 85 (randomized or randomly).ab. 86 (crossover$ or cross‐over$).tw. 87 placebo$.ab. 88 (doubl$ adj blind$).tw. 89 or/82‐88 90 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 91 89 not 90 92 77 and 81 and 91
Appendix 4. ClinicalTrials.gov search strategy
Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search date: 11 October 2013 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Study type: Interventional Studies Conditions: hypertension Interventions: (beta blocker) or (adrenergic beta‐antagonist) Outcome Measures: blood pressure Search terms: randomized
Data and analyses
Comparison 1. Propranolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 18 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (1x starting dose) 60, 80 mg/day | 3 | 222 | Mean Difference (Fixed, 95% CI) | ‐4.67 [‐8.13, ‐1.21] |
| 1.2 (2x starting dose) 120, 160 and 180 mg/day | 13 | 592 | Mean Difference (Fixed, 95% CI) | ‐12.15 [‐14.61, ‐9.69] |
| 1.3 (4x starting dose) 240 and 320 mg/day | 6 | 99 | Mean Difference (Fixed, 95% CI) | ‐21.01 [‐25.43, ‐16.58] |
| 1.4 (8x starting dose) 480 and 640 mg/day | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐4.31 [‐9.60, 0.97] |
| 2 Diastolic blood pressure | 18 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (1x starting dose) 60 and 80 mg/day | 3 | 222 | Mean Difference (Fixed, 95% CI) | ‐3.28 [‐5.60, ‐0.95] |
| 2.2 (2x starting dose) 120, 160 and 180 mg/day | 13 | 592 | Mean Difference (Fixed, 95% CI) | ‐8.51 [‐8.00, ‐7.03] |
| 2.3 (4x starting dose) 240 and 320 mg/day | 6 | 99 | Mean Difference (Fixed, 95% CI) | ‐13.91 [‐16.74, ‐11.09] |
| 2.4 (8x starting dose) 480 and 640 mg/day | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐3.03 [‐6.06, 0.00] |
| 3 Heart rate | 12 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (1x starting dose) 60 and 80 mg/day | 2 | 196 | Mean Difference (Fixed, 95% CI) | ‐7.30 [‐9.49, ‐5.12] |
| 3.2 (2x starting dose) 120 and 160 mg/day | 9 | 497 | Mean Difference (Fixed, 95% CI) | ‐11.54 [‐12.95, ‐10.13] |
| 3.3 (4x starting dose) 240 and 320 mg/day | 5 | 85 | Mean Difference (Fixed, 95% CI) | ‐18.38 [‐20.93, ‐15.84] |
| 3.4 (8x starting dose) 640 mg/day | 1 | 108 | Mean Difference (Fixed, 95% CI) | ‐10.0 [‐12.76, ‐7.24] |
| 4 Pulse pressure | 18 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (1x starting dose) 60 and 80 mg/day | 3 | 222 | Mean Difference (Fixed, 95% CI) | ‐1.45 [‐4.79, 1.88] |
| 4.2 (2x starting dose) 120, 160 and 180 mg/day | 13 | 618 | Mean Difference (Fixed, 95% CI) | ‐2.52 [‐4.25, ‐0.79] |
| 4.3 (4x starting dose) 240 and 320 mg/day | 6 | 99 | Mean Difference (Fixed, 95% CI) | ‐2.96 [‐6.50, 0.58] |
| 4.4 (8x starting dose) 480 and 640 mg/day | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐2.29 [‐8.42, 3.83] |
| 5 Direct comparison 2x doses | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 Systolic blood pressure | 3 | 222 | Mean Difference (Fixed, 95% CI) | ‐3.01 [‐5.91, ‐0.11] |
| 5.2 Diastolic blood pressure | 3 | 222 | Mean Difference (Fixed, 95% CI) | ‐1.53 [‐3.38, 0.32] |
| 5.3 Heart rate | 2 | 196 | Mean Difference (Fixed, 95% CI) | ‐3.37 [‐5.55, ‐1.20] |
1.5. Analysis.
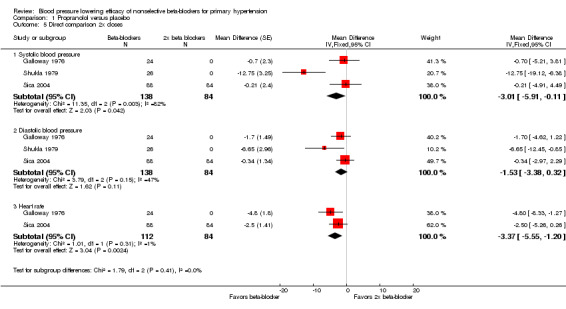
Comparison 1 Propranolol versus placebo, Outcome 5 Direct comparison 2x doses.
Comparison 2. Timolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 30 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐14.4 [‐21.53, ‐7.27] |
| 2 Diastolic blood pressure | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 30 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐13.50 [‐18.05, ‐8.95] |
| 3 Heart rate | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 30 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐16.0 [‐18.88, ‐13.12] |
| 4 Pulse pressure | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 30 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐0.9 [‐8.70, 6.90] |
Comparison 3. Penbutolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 (0.5x starting dose) 10 mg/day | 1 | 155 | Mean Difference (Fixed, 95% CI) | ‐2.4 [‐8.79, 3.99] |
| 1.2 (1x starting dose) 20 mg/day | 1 | 151 | Mean Difference (Fixed, 95% CI) | ‐6.5 [‐12.99, ‐0.01] |
| 1.3 (2x starting dose) 40 mg/day | 1 | 156 | Mean Difference (Fixed, 95% CI) | ‐4.5 [‐10.87, 1.87] |
| 1.4 (4x starting dose) 80 mg/day | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐18.0 [‐31.33, ‐4.67] |
| 2 Diastolic blood pressure | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 (0.5x starting dose) 10 mg/day | 1 | 155 | Mean Difference (Fixed, 95% CI) | ‐2.9 [‐6.96, 1.16] |
| 2.2 (1x starting dose) 20 mg/day | 1 | 151 | Mean Difference (Fixed, 95% CI) | ‐3.7 [‐7.76, 0.36] |
| 2.3 (2x starting dose) 40 mg/day | 1 | 156 | Mean Difference (Fixed, 95% CI) | ‐3.50 [‐7.54, 0.54] |
| 2.4 (4x starting dose) 80 mg/day | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐11.1 [‐17.90, ‐4.30] |
| 3 Heart rate | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 (0.5x starting dose) 10 mg/day | 1 | 103 | Mean Difference (Fixed, 95% CI) | ‐1.2 [‐9.82, 7.42] |
| 3.2 (1x starting dose) 20 mg/day | 1 | 99 | Mean Difference (Fixed, 95% CI) | ‐0.40 [‐9.02, 8.22] |
| 3.3 (2x starting dose) 40 mg/day | 1 | 104 | Mean Difference (Fixed, 95% CI) | ‐2.80 [‐11.42, 5.82] |
| 3.4 (4x starting dose) 80 mg/day | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐19.7 [‐27.15, ‐12.25] |
| 4 Pulse pressure | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 (0.5x starting dose) 10 mg/day | 1 | 103 | Mean Difference (Fixed, 95% CI) | 0.5 [‐7.32, 8.32] |
| 4.2 (1x starting dose) 20 mg/day | 1 | 99 | Mean Difference (Fixed, 95% CI) | ‐2.8 [‐10.68, 5.08] |
| 4.3 (2x starting dose) 40 mg/day | 1 | 104 | Mean Difference (Fixed, 95% CI) | ‐1.1 [‐8.90, 6.70] |
| 4.4 (4x starting dose) 80 mg/day | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐6.9 [‐18.37, 4.57] |
Comparison 4. Tertatolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐14.0 [‐26.54, ‐1.46] |
| 1.1 5 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐14.0 [‐26.54, ‐1.46] |
| 2 Diastolic blood pressure | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐15.0 [‐23.33, ‐6.67] |
| 2.1 5 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐15.0 [‐23.33, ‐6.67] |
| 3 Heart rate | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐17.0 [‐24.06, ‐9.94] |
| 3.1 5 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐17.0 [‐24.06, ‐9.94] |
| 4 Pulse pressure | 1 | 20 | Mean Difference (Fixed, 95% CI) | 1.0 [‐10.07, 12.07] |
| 4.1 5 mg/day | 1 | 20 | Mean Difference (Fixed, 95% CI) | 1.0 [‐10.07, 12.07] |
Comparison 5. Indenolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate | 1 | 12 | Mean Difference (Fixed, 95% CI) | ‐12.0 [‐15.37, ‐8.63] |
Comparison 6. Nadolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐20.6 [‐26.48, ‐14.72] |
| 1.1 80 mg/day | 1 | 10 | Mean Difference (Fixed, 95% CI) | ‐20.6 [‐26.48, ‐14.72] |
Comparison 7. Moprolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate | 1 | 23 | Mean Difference (Fixed, 95% CI) | ‐6.0 [‐13.51, 1.51] |
Comparison 8. Pooled subclass effects (NSBB: nonselective beta‐blocker).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 NSBB 0.5x starting dose | 1 | 155 | Mean Difference (Fixed, 95% CI) | ‐2.4 [‐10.80, 6.00] |
| 1.2 NSBB 1x starting dose | 5 | 393 | Mean Difference (Fixed, 95% CI) | ‐5.27 [‐8.44, ‐2.11] |
| 1.3 NSBB 2x starting dose | 15 | 768 | Mean Difference (Fixed, 95% CI) | ‐11.72 [‐14.00, ‐9.43] |
| 1.4 NSBB 4x starting dose | 7 | 109 | Mean Difference (Fixed, 95% CI) | ‐20.83 [‐25.12, ‐16.54] |
| 1.5 NSBB 8x starting dose | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐4.31 [‐9.60, 0.97] |
| 2 Diastolic blood pressure | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 NSBB 0.5x starting dose | 1 | 155 | Mean Difference (Fixed, 95% CI) | ‐2.9 [‐8.23, 2.43] |
| 2.2 NSBB 1x starting dose | 5 | 393 | Mean Difference (Fixed, 95% CI) | ‐3.59 [‐5.37, ‐1.81] |
| 2.3 NSBB 2x starting dose | 15 | 768 | Mean Difference (Fixed, 95% CI) | ‐8.44 [‐9.83, ‐7.05] |
| 2.4 NSBB 4x starting dose | 7 | 109 | Mean Difference (Fixed, 95% CI) | ‐14.17 [‐16.98, ‐11.37] |
| 2.5 NSBB 8x starting dose | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐3.03 [‐6.06, 0.00] |
| 3 Heart rate | 17 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 NSBB 0.5x starting dose | 1 | 155 | Mean Difference (Fixed, 95% CI) | ‐1.2 [‐12.53, 10.13] |
| 3.2 NSBB 1x starting dose | 4 | 367 | Mean Difference (Fixed, 95% CI) | ‐8.76 [‐11.99, ‐5.53] |
| 3.3 NSBB 2x starting dose | 12 | 683 | Mean Difference (Fixed, 95% CI) | ‐12.56 [‐14.17, ‐10.95] |
| 3.4 NSBB 4x starting dose | 6 | 95 | Mean Difference (Fixed, 95% CI) | ‐18.52 [‐21.68, ‐15.36] |
| 4 Pulse pressure | 22 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 NSBB 0.5x starting dose | 1 | 155 | Mean Difference (Fixed, 95% CI) | 0.5 [‐9.78, 10.78] |
| 4.2 NSBB 1x starting dose | 5 | 393 | Mean Difference (Fixed, 95% CI) | ‐1.46 [‐4.56, 1.64] |
| 4.3 NSBB 2x starting dose | 15 | 768 | Mean Difference (Fixed, 95% CI) | ‐2.20 [‐3.93, ‐0.47] |
| 4.4 NSBB 4x starting dose | 7 | 109 | Mean Difference (Fixed, 95% CI) | ‐3.17 [‐6.61, 0.28] |
| 4.5 NSBB 8x starting dose | 2 | 205 | Mean Difference (Fixed, 95% CI) | ‐2.29 [‐8.42, 3.83] |
| 5 Withdrawal due to adverse effects | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.82] |
| 5.1 Propranolol vs. placebo | 1 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.31] |
| 5.2 Penbutolol vs. placebo | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.20, 2.85] |
| 6 Combined 1x and 2x starting dose estimates | 18 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Systolic blood pressure | 16 | 948 | Mean Difference (Fixed, 95% CI) | ‐9.50 [‐10.91, ‐8.09] |
| 6.2 Diastolic blood pressure | 16 | 948 | Mean Difference (Fixed, 95% CI) | ‐6.60 [‐7.43, ‐5.76] |
| 6.3 Heart rate | 13 | 864 | Mean Difference (Fixed, 95% CI) | ‐11.80 [‐12.90, ‐10.71] |
| 6.4 Pulse pressure | 16 | 948 | Mean Difference (Fixed, 95% CI) | ‐2.02 [‐3.17, ‐0.87] |
8.6. Analysis.
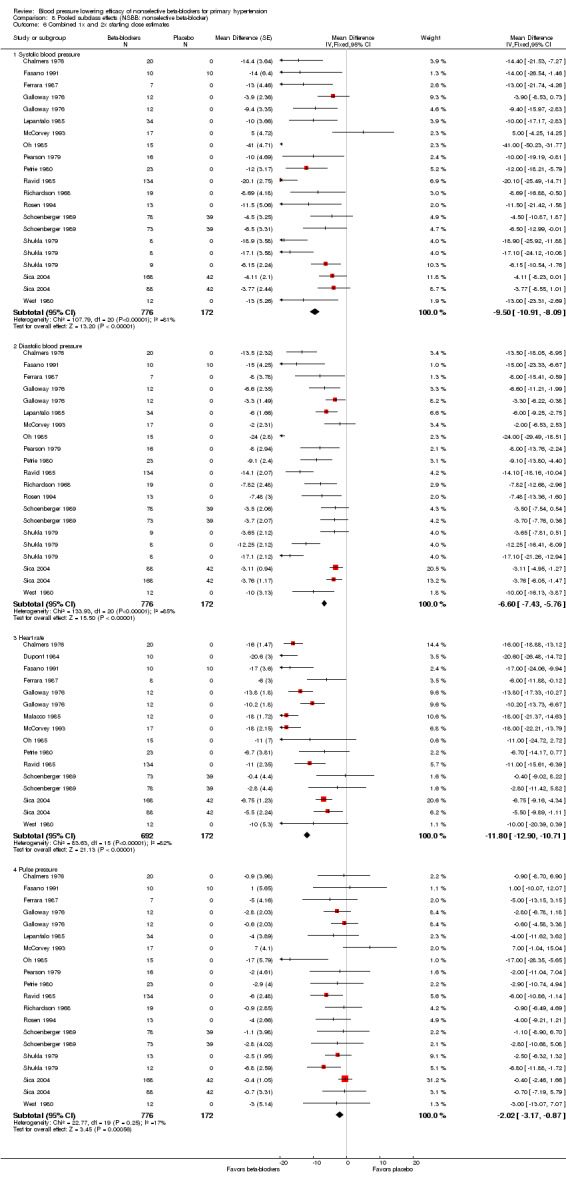
Comparison 8 Pooled subclass effects (NSBB: nonselective beta‐blocker), Outcome 6 Combined 1x and 2x starting dose estimates.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chalmers 1976.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over study. 8‐week run‐in period followed by 4 × 8‐week treatment phases | |
| Participants | 23 participants Age range: 30‐60 years, 20/23 participants completed the study Inclusion criteria: DBP 95‐120 mmHg Exclusion criteria: pre‐existing condition: obstructive airway diseases, diabetes |
|
| Interventions | Timolol 10 mg 3 times daily, hydrochlorothiazide 50 mg once daily, hydrochlorothiazide 50 mg plus timolol 10 mg, placebo | |
| Outcomes | Supine, standing, casual SBP, DBP, mean BP Resting HR Plasma renin activity WDAE |
|
| Notes | No WDAE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo pill was used. Participants took the same number of pills every time. Physicians were also blinded but unblinded by effect on pulse rate |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Reported how many participants dropped out |
| Selective reporting (reporting bias) | Low risk | SBP, DBP, HR were primary outcomes. WDAE were reported |
Dargie 1986.
| Methods | Randomized, double‐blind, cross‐over study. Each period of treatment lasted for 4 weeks | |
| Participants | 14 participants (9 women, 5 men) Mean age (range): 51.6 years (30‐65) Inclusion criteria: DBP 95‐110 mmHg on single‐drug therapy |
|
| Interventions | Verapamil 120 mg 3 times daily, p ropranolol 80 mg 3 times daily, v erapamil 120 mg plus propranolol 80 mg 3 times daily, placebo |
|
| Outcomes | Supine and standing SBP, DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No Information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information other than stated the study was double blind |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No report of follow‐up procedure or number of drop‐outs |
| Selective reporting (reporting bias) | High risk | HR and WDAE not reported |
De Plean 1981.
| Methods | Randomized, double‐blind, cross‐over study. Each treatment period lasted for 4 weeks. BP was measured about 6 hours after drug intake. Mean of 2 or 3 readings is used for BP data | |
| Participants | 16 participants entered the study. 10/16 participants completed the study. 1 person WDAE Age range: 30‐56 years Baseline: mean SBP 163.9 mmHg, mean DBP 117.1 mmHg, mean HR 88.8 beats per minute, mean body weight 83.2 kg |
|
| Interventions | Penbutolol 80 mg once daily, hydrochlorothiazide 100 mg once daily, placebo | |
| Outcomes | Supine and standing SBP, DBP, HR Biochemical data Total withdrawal and WDAE |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of the active treatments was selected according to a predetermined random code" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Both active drugs and placebo were available in capsules of identical size, shape and color", but pulse would unblind BP measurements |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Reported SBP, DBP and HR |
| Selective reporting (reporting bias) | Low risk | SBP and DBP were primary outcomes HR and WDAE were reported |
Dupont 1984.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. Each treatment period lasted 4 weeks | |
| Participants | 10 participants Mean age (range): 54.4 years (45‐62) Inclusion criteria: ambulatory DBP 95‐115 mmHg |
|
| Interventions | Nadolol 80 mg once daily, placebo | |
| Outcomes | Mean ABP, HR, CO, GFR, renal blood flow, renal vascular resistance | |
| Notes | SBP and DBP data were not reported. Only HR data were relevant to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | High risk | SBP and DBP data were not reported |
| Selective reporting (reporting bias) | High risk | SBP and DBP data were not reported |
Fasano 1991.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel study. 4‐week washout followed by 4‐week treatment period | |
| Participants | 20 participants (12 male) Mean age (range:) 39 years (18‐57) Inclusion criteria: primary hypertension Exclusion criteria: secondary hypertension; cardiovascular diseases; chronic diseases; metabolic diseases; history of severe side effects with beta‐blockers; pregnancy, lactation or use of oral contraceptive |
|
| Interventions | Tertatolol 5 mg once daily, placebo | |
| Outcomes | Supine and standing SBP, DBP, HR, cardiac workload | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of placebo pills |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | High risk | 2 participants dropped out without any information on follow‐up |
| Selective reporting (reporting bias) | High risk | SBP, DBP and HR were primary outcomes WDAE was not reported |
Ferrara 1987.
| Methods | Randomized, double‐blind, cross‐over study. Washout period for 2 weeks followed by 3‐week test period for each treatment | |
| Participants | 7 participants (3 women, 4 men) Age range: 26‐62 years Inclusion criteria: primary hypertension |
|
| Interventions | Propranolol 80 twice daily, placebo | |
| Outcomes | Supine SBP, DBP, HR, oral glucose tolerance test, insulin sensitivity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of placebo pills |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | WDAE was not reported |
Galloway 1976.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. Eligible participants were seen in hypertension clinic 2 weeks after discharge from hospital. Each treatment period lasted for 4 weeks | |
| Participants | 24 participants (6 men) Mean age (range): 46 years (19‐65) Mean body weight: 62.6 kg Inclusion criteria: DBP > 90 mmHg but < 105 mmHg |
|
| Interventions | Propranolol 20 mg 3 times daily, propranolol 40 mg 3 times daily, propranolol 80 mg 3 times daily, placebo | |
| Outcomes | Supine, standing, postexercise SBP, DBP, HR | |
| Notes | Drop‐outs and WDAE not reported. However, author stated that no dose‐related adverse event was recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of administration was determine by a random code" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Each pill was identical in appearance |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Data analysis was based on data from all 24 participants. There was no report of drop‐outs |
| Selective reporting (reporting bias) | Low risk | SBP, DBP and HR were primary outcomes. No drop‐outs |
Kelemen 1990.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel study. After screening, participant entered a 4‐week single‐blind placebo washout period. During which all antihypertensive drugs were replaced by placebo. After washout, patients were tested for baseline readings. Eligible participants were then randomized to a double‐blind treatment group for 11 weeks | |
| Participants | Inclusion criteria: DBP > 90 mmHg but < 105 mmHg Exclusion criteria: history of coronary, valvular, renal, cerebral vascular, hepatic or neurological diseases; history of alcohol or drug abuse; any contraindication for propranolol and diltiazem and exercise |
|
| Interventions | Participants first started on propranolol 160 mg, diltiazem 240 mg or placebo in the first week. Then they were force titrated to propranolol 240 mg or diltiazem 360 mg per day or equal amount of placebo pills. After titration, all participants entered a 10‐week exercise training program | |
| Outcomes | Echocardiogram; resting supine and exercise SBP, DBP, HR; plasma lipid and lipoprotein | |
| Notes | Only exercise HR was reported. Resting HR was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo pills were in the same number of active treatments, but pulse rate effect would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | 1 participant withdrew from propranolol group |
| Selective reporting (reporting bias) | High risk | HR was not reported |
Lepantalo 1985.
| Methods | After 4‐week washout period, participants were randomly allocation to 3x 4‐week treatment period. Participants would cross‐over to another treatment at the end of each treatment period without washout | |
| Participants | 34 participants (17 male) Mean age (range): 54.1 years (52‐68) Inclusion criteria: mild primary hypertension Exclusion criteria: diabetes, peripheral arteries disease |
|
| Interventions | Propranolol 80 mg twice daily, metoprolol 100 mg twice daily, placebo | |
| Outcomes | BP, ankle:arm systolic BP ratio, calf blood flow, peripheral resistance | |
| Notes | HR was not reported. There were no drop‐outs. Same as Lepantalo 1983 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | "All pills were of the same appearance" but pulse rate would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | HR was not reported |
Malacco 1985.
| Methods | After 3‐week washout period, participants were randomized to 3x 4‐week treatment periods. Each treatment period was separated by a 7‐day washout period. BP was measured at the end of each period | |
| Participants | 12 participants (6 male) Aged: < 60 years Inclusion criteria: SBP ≥ 150 mmHg, DBP ≥ 100 mmHg Exclusion criteria: congestive heart failure, lung disease, ischemic heart disease, chronic alcoholism, diabetes, renal insufficiency, bradycardia |
|
| Interventions | Propranolol 80 mg twice daily, indenolol 60 mg twice daily, placebo | |
| Outcomes | MBP, HR, CO | |
| Notes | Only HR data were used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo was given at same frequency as active treatments, but pulse rate would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | SBP and DBP were not reported |
McCorvey 1993.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. Eligible participants discontinued all previous antihypertensive treatment and were then randomized to treatment or placebo immediately. All participants were forced titrated to maximum dose 3 days after randomization. The dosage remained the same for the next 4 weeks. All cross‐over procedures were the same as stated above | |
| Participants | 17 participants Inclusion criteria: primary hypertension with documented elevated BP for the previous 12 months Exclusion criteria: history of stroke, MI, angina, hepatic dysfunction, serum creatinine > 1.5 mg/dL, history of bronchospasm, AV block greater than first degree, congestive heart failure, gout, alcohol and drug abuse, participants who were unable to comply with protocol |
|
| Interventions | Propranolol 120 mg once daily, hydrochlorothiazide 25 mg once daily, enalapril 10 mg once daily, placebo for first 3 days. Then dose was double for the reminder of the treatment period | |
| Outcomes | BP, HR, QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | "All drugs were dispensed in similar appearing gelatin capsules, but pulse rate would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | WDAE not reported |
McInnes 1985.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 4 weeks of washout period, participants were randomized to 4 × 4‐week treatment periods. BP was measured at the end of each period | |
| Participants | 14 participants Inclusion criteria: DBP 95‐110 mmHg with single‐drug therapy |
|
| Interventions | Verapamil 120 mg 3 times daily, propranolol 80 mg 3 times daily, verapamil 120 mg plus propranolol 80 mg 3 times daily, placebo | |
| Outcomes | SBP, DBP, HR, PR interval, end systolic dimension, end diastolic dimension, fractional shortening | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of treatment is systematically varied to minimize any effect of sequence" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | WDAE was not reported |
Nicaise 1981.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 1 month of placebo washout, participants were randomized to treatment for 2 months and then crossed‐over to the other treatment for another 2 months | |
| Participants | 30 participants (8 males) Mean age (range): 79 years (69‐93) Inclusion criteria: SBP ≥ 160 mmHg, DBP ≥ 95 mmHg, or both |
|
| Interventions | (‐)moprolol 75 mg twice daily, placebo | |
| Outcomes | MAP, HR, drop‐outs, WDAE | |
| Notes | 7 people dropped out, 5 WDAE (3 in placebo, 2 in moprolol) SD not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Participants who dropped out were not included |
| Selective reporting (reporting bias) | High risk | SBP, DBP and SD were not reported |
Oh 1985.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 2 weeks of washout, participants were randomized to treatment or placebo for 4 weeks | |
| Participants | 15 Chinese participants Mean age (range): 39.3 years (23‐57) Inclusion criteria: BP > 144/94 but < 200/115 mmHg |
|
| Interventions | Propranolol 160 mg once daily, propranolol 320 mg once daily, placebo | |
| Outcomes | Resting, exercise and after exercise SBP, DBP, HR, adverse effects | |
| Notes | There were 4 groups (A, B, C, D); however, only group A data were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double dummy, but decreased pulse rate would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | WDAE not reported |
Pearson 1979.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. Without washout period, participants were randomized to treatment upon entry. Each treatment period lasted for 6 weeks | |
| Participants | 16 participants (8 males) Mean age: 54 years Inclusion criteria: DBP ≥ 100 mmHg with single‐drug therapy Exclusion criteria: angina, MI, heart failure, stroke, COPD, gout, women of child‐bearing potential or using oral contraceptive pills |
|
| Interventions | Propranolol 80 mg twice daily, tienilic acid 250 mg every morning, propranolol 80 mg plus tienilic acid 250 mg, placebo | |
| Outcomes | SBP, DBP, HR, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The tablets in each treatment period were identical in number and appearance", but pulse rate would unblind BP measurement |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | SEM were extracted from figures. WDAE not reported |
Petrie 1976.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 14‐day washout period, participants were randomized to a sequence of 4 week treatments | |
| Participants | 24 participants (13 males) Inclusion criteria: DBP > 105 mmHg but < 125 mmHg Exclusion criteria: recent MI, evidence of heart failure, heart block, gross ischemia, grade III or IV retinopathy, diabetes, gout, impaired liver function, or creatinine clearance < 60mL/minute or if they were on any other drug treatment |
|
| Interventions | Methyldopa 250 mg 3 times daily, practolol 200 mg 3 times daily, propranolol 80 mg 3 times daily, methyldopa 250 mg plus propranolol 80 mg both 3 times daily, methyldopa 250 mg plus practolol 200 mg both 3 times daily, placebo | |
| Outcomes | SBP, DBP, HR, QoL | |
| Notes | SD were imputed using the mean of other studies that provided SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Package were pre‐packed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants took the same number of pills |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | SD were not reported |
Petrie 1980.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 4‐week run‐in period, participants were randomized to a sequence of 4 week treatments. There were no washout periods between treatments | |
| Participants | 23 participants (12 males) Mean age (years): 40.9 years (21‐59) Inclusion criteria: DBP > 90 mmHg |
|
| Interventions | Atenolol 100 mg once daily, sustained‐release oxprenolol 160 mg once daily, long‐acting propranolol 160 mg once daily, placebo | |
| Outcomes | 5‐minute supine and 2‐minute standing SBP, DBP, HR. Measurement were made 20‐22 hours after dose | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Matching placebo was used as the same frequency |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | Safety data not reported |
Ravid 1985.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 2‐week placebo run‐in period, participants were randomly allocated to series of 5 × 4‐week treatments | |
| Participants | 134 participants (66 males) Mean age: men 52 years, women 54 years Inclusion criteria: DBP > 95 mmHg but < 120 mmHg, normal renal function, absent of liver diseases and gross obesity |
|
| Interventions | Slow‐release propranolol 160 mg once daily, slow‐release oxprenolol 160 mg once daily, atenolol 100 mg once daily, metoprolol 200 mg once daily, placebo | |
| Outcomes | SBP, DBP, HR, adverse effects | |
| Notes | Study reported male and female data separately. The data had larger than average SEM, which cause the SD to be large | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | "Treatment was unknown to patients and physicians" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo was taken as the same frequency as active treatment |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Participants who dropped out were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Richardson 1968.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 1‐week run‐in period, participants were randomized to sequence of 5 week treatment periods | |
| Participants | 19 participants (5 males) Inclusion criteria: moderate‐to‐severe hypertension |
|
| Interventions | Propranolol 40 mg 3 times daily, chlorthalidone 50 mg twice daily, propranolol 40 mg 3 times daily plus chlorthalidone 50 mg twice daily, placebo | |
| Outcomes | SBP, DBP, HR, WDAE, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Dummy for both drugs were given |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Only participants who completed the study were included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Rosen 1994.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 1‐month single‐blind run‐in period, participants were randomized to series of 1 month treatments. Treatment periods were separated by 2‐week washout period | |
| Participants | 13 participants (100% male) Inclusion criteria: males with prior history of hypertension and sexual dysfunction were recruited. Participants had 2 consecutive sitting DBP 90‐100 mmHg Exclusion criteria: people with diabetes, congestive heart failure, renal disease, alcoholism, or other medical or psychiatric disorders |
|
| Interventions | α‐methyldopa 500 mg twice daily, propranolol 80 mg twice daily, atenolol 100 mg once daily, dyazide 50/25 mg twice daily, placebo | |
| Outcomes | SBP, DBP, sleep study, sexual function, hormone study | |
| Notes | HR data were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Data from participant who withdrawn were analyzed separately |
| Selective reporting (reporting bias) | High risk | HR were not reported |
Schoenberger 1989.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel study. After 4 weeks of single‐blind run‐in period, participants were randomized to 6‐week treatment period, followed by 2‐week tapering off period | |
| Participants | 302 participants (160 men) Inclusion criteria: adults with mild‐to‐moderate hypertension, defined as supine DBP 95‐115 mmHg Exclusion criteria: pregnancy, child‐bearing potential, significant hepatic or renal disorders, COPD, asthma, thyroid disorder, bradycardia, psychosis, use of drugs that interfere with beta‐blockers 2 weeks before study, metabolic acidosis, unstable diabetes, continued need for concomitant antihypertensive medications or diuretics, heart failure, AV block, conditions that affect compliance, concomitant administration of investigational drugs |
|
| Interventions | Penbutolol 10 mg once daily, 20 mg once daily, 40 mg once daily, placebo | |
| Outcomes | Supine and standing SBP, DBP, HR 24 hours after last dose, WDAE, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebos were used at same frequency as active drugs |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | Participants who dropped out were not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Shukla 1979.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After 2‐week run‐in period, eligible participants were randomized to series of 4 week treatments | |
| Participants | 26 participants Inclusion criteria: DBP 90‐114 mmHg Exclusion criteria: recent MI, heart failure, heart block, grade III/IV retinopathy, impaired hepatic or renal functions, diabetes, COPD |
|
| Interventions | Propranolol 60 mg 3 times daily, propranolol 120 mg 3 times daily, propranolol 180 mg 3 times daily, placebo | |
| Outcomes | SBP, DBP | |
| Notes | HR not reported. No drop‐outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Pills of Identical appearance were used |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | HR not reported |
Sica 2004.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel study. After 2‐3 weeks of single‐blind placebo run‐in, eligible participants were randomized to 1 of the treatments for 8 weeks. Participants who were randomized to propranolol 120 mg or 160 mg would start at 80 mg then force titrated to respective dose in the next 2 weeks. Participants who were randomized to propranolol 640 mg/day started at 160 mg and then up titrated to 640 mg in the next 3 weeks | |
| Participants | Inclusion criteria: DBP 96‐114 mmHg during 2 readings while sitting Exclusion criteria: pregnancy, lactating, renal artery stenosis, gastrointestinal diseases, kidney or liver diseases, cardiac diseases within the last 6 months, asthma, COPD, nonallergic bronchospasms, insulin‐dependent diabetes, concomitant administration of drugs that affect BP |
|
| Interventions | Propranolol 80 mg once daily, propranolol 120 mg once daily, propranolol 160 mg once daily, propranolol 640 mg once daily, placebo | |
| Outcomes | SBP, DBP, HR, plasma concentration, safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo pills were taken at the same frequency |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
van Herwaarden 1977.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. There was no run‐in period. Participants were randomized to 4 consecutive treatments, each lasting 4 weeks. At the end of each treatment period, BP was measured 2 hours after the morning dose | |
| Participants | 8 participants Inclusion criteria: supine DBP after 15 minutes of rest 100‐120 mmHg |
|
| Interventions | Propranolol 80 mg 3 times daily, metoprolol 100 mg 3 times daily, placebo. Each beta‐blocker treatment was alternated with placebo treatment | |
| Outcomes | SBP, DBP, HR, effect of adrenaline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | High risk | Randomization might be compromised as placebo was alternated with active treatments |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo was used at the same frequency |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | High risk | WDAE not reported |
West 1980.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study. After a 6‐week run‐in period, participants received, in random order, 5 × 6‐week test phase. The BP data werethe mean of the last 4 weeks of each treatment period | |
| Participants | 12 participants Inclusion criteria: untreated DBP 95‐120 mmHg |
|
| Interventions | Methyclothiazide 5 mg once daily, labetalol 300 mg twice daily, propranolol 80 mg twice daily, hydralazine 50 mg twice daily, placebo | |
| Outcomes | SBP, DBP, HR, adverse effects, WDAE | |
| Notes | SD were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo was given for every active treatment at respective frequency |
| Incomplete outcome data (attrition bias) Primary and secondary outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
ABP: ambulatory blood pressure; AV: atrioventricular; BP: blood pressure; CO: cardiac output; COPD: chronic obstructive pulmonary disease; DBP: diastolic blood pressure; GFR: glomerular filtration rate; HR: heart rate; MAP: mean arterial pressure; MBP: mean blood pressure; MI: myocardial infarction; QoL: quality of life; SBP: systolic blood pressure; SD: standard deviation; SEM: standard error of the mean; WDAE: withdrawals due to adverse effects.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ades 1988 | Data during treatment were not reported. Available data reported were 4‐7 days after the last dose |
| Ades 1990 | Same as Ades 1988, did not provided data until after 4‐7 days of last dose |
| Agnes 1989 | Did not have drugs versus placebo data |
| Beilin 1972 | Dose adjusted to side effects |
| Cherchi 1985 | Did not have parallel placebo group |
| Costa 1984 | Nonfixed dose |
| Davidson 1976 | Nonfixed dose |
| Kubik 1984 | Not randomized to placebo |
| Smith 1988 | Provided ambulatory blood pressure measurement data only |
| Weigmann 1998 | Provided ambulatory blood pressure measurement data only |
Contributions of authors
James Wright formulated the idea for the review, developed the basis for the protocol, supervised the data analysis and interpretation, and contributed to the final draft of the review.
Gavin Wong took the lead role in searching, identifying and assessing studies; in data extraction and analyses; and in writing the first draft of the review.
Sources of support
Internal sources
University of British Columbia, Department of Anesthesiology, Pharmacology & Therapeutics, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
None known.
New
References
References to studies included in this review
Chalmers 1976 {published data only}
- Chalmers J, Horvath J, Tiller D, Bune A. Effect of timolol and hydrochlorothiazide on blood pressure and plasma renin activity. Double blind factorial trial. Lancet 1976;2(7981):328‐31. [NSC016‐TIMOLOL315] [DOI] [PubMed] [Google Scholar]
Dargie 1986 {published data only}
- Dargie H, Cleland J, Findlay I, Murray G, McInnes G. Combination of verapamil and beta blockers in systematic hypertension. American Journal of Cardiology 1986;57:80D‐2D. [NSC‐Propranolol‐546] [DOI] [PubMed] [Google Scholar]
De Plean 1981 {published data only}
- Plean JF, Vander Elst E, Ypersele de Strihou C. Penbutolol or hydrochlorothiazide once a day in hypertension. A controlled study with home measurements. British Journal of Clinical Pharmacology 1981;12:215‐21. [NSC‐Penbutolol‐380] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dupont 1984 {published data only}
- Dupont AG, Bossuyt AM, Jonckheer MH, Six RO. Effect of nadolol on ambulatory blood pressure, renal haemodynamics and cardiac function in hypertension. Proceedings of BPS. 1984:129P. [NSC‐Nadolol‐527] [DOI] [PMC free article] [PubMed]
Fasano 1991 {published data only}
- Fasano ML, Gaeta I, Simone G, Iannuzzi R, Ferrara LA. Cardiovascular response to adrenergic simulation during treatment with tertatolol. A new non cardioselective beta blocking agent in primary hypertensive patients. Japanese Heart Journal 1991;32(4):435‐44. [NSP005‐TERTATOLOL161] [DOI] [PubMed] [Google Scholar]
Ferrara 1987 {published data only}
- Ferrara LA, Capaldo B, Rivellese A, Genovese S, Iovine C, Mastranzo P, et al. Adrenergic system and carbohydrate metabolism. Effect of beta receptor blockade on insulin secretion and peripheral insulin sensitivity in normoglycemic patients . European Journal of Clinical Pharmacology 1987;33:273‐7. [NSC‐Propranolol‐459] [DOI] [PubMed] [Google Scholar]
Galloway 1976 {published data only}
- Galloway DB, Glover SC, Hendry WG, Logie AW, Petrie JC, Smith MC, et al. Propranolol in hypertension: a dose response study. BMJ 1976;2:140‐2. [NSC015‐PROPRANOLOL‐318] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelemen 1990 {published data only}
- Kelemen MH, Effron MB, Valenti SA, Stewart KJ. Exercise training combined with antihypertensive drug therapy. JAMA 1990;263(20):2766‐71. [NSP‐propranolol‐469] [PubMed] [Google Scholar]
Lepantalo 1985 {published data only}
- Lepantalo M, Totterman KJ. Effect of long term beta adrenergic blockade on calf blood flow in hypertensive patients. Clinical Physiology 1983;3:35‐42. [NSC018‐PROPRANOLOL263] [DOI] [PubMed] [Google Scholar]
- Lepantalo MJA, Totterman KJ. Lower limb hemodynamics during antihypertensive treatment with metoprolol and propranolol. Inter Angio 1985;4:225‐8. [NSC011‐PROPRANOLOL‐238] [PubMed] [Google Scholar]
Malacco 1985 {published data only}
- Malacco E, Mailland F, Bevilacqua M, Bosisio E, Meroni R. Long term hemodynamic effects of indenolol in hypertension at rest and during isometric exercise. Current Therapeutic Research 1985;37(3):537‐44. [NSC‐Propranolol/indenolol‐439] [Google Scholar]
McCorvey 1993 {published data only}
- McCorvey E, Wright JT, Culbert JP, McKenney JM, Proctor JD, Annett MP. Effect of hydrochlorothiazide, enalapril and propranolol on quality of life and cognitive and motor function in hypertensive patients. Clinical Pharmacy 1993;12:300‐5. [NSC014‐PROPRANOLOL‐363] [PubMed] [Google Scholar]
McInnes 1985 {published data only}
- McInnes GT, Findlay IN, Murray G, Cleland JGF, Dargie HJ. Cardiovascular responses to verapamil and propranolol in hypertensive patients. Journal of Hypertension 1985;3(Suppl 3):S219‐21. [NSC‐Propranolol‐236] [PubMed] [Google Scholar]
Nicaise 1981 {published data only}
- Nicaise J, Desmedt J, Kunstler M, Vryens R, Wuestenberghs A. (‐)Moprolol as antihypertensive therapy in the elderly. Current Therapeutic Research 1981;30(1):93‐7. [NSC‐Moprolol‐379] [Google Scholar]
Oh 1985 {published data only}
- Oh VMS, Chia BL, Taylor EA. Effect of long acting propranolol on blood pressure and heart rate in hypertensive Chinese. British Journal Clinical Pharmacology 1985;20:144‐7. [NSC‐Propranolol‐243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearson 1979 {published data only}
- Pearson RM, Bulpitt CJ, Havard CWH. Propranolol and tienilic acid in essential hypertension. Postgraduate Medical Journal 1979;55(Suppl 3):115‐9. [NSC‐Propranolol‐447] [PubMed] [Google Scholar]
Petrie 1976 {published data only}
- Petrie JC, Galloway DB, Jeffers TA, Millar HR, Smith MC, Wood RA, Lewis JA, Simpson WT. Methyldopa and propranolol or practolol in moderate hypertension. BMJ 1976;2:137‐139. [NSC‐Propranolol/practolol‐443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrie 1980 {published data only}
- Petrie JC, Jeffers TA, Robb OJ, Scott AK, Webster J. Atenolol, sustained release oxprenolol and long acting propranolol in hypertension. BMJ 1980;280(6231):1573‐4. [NSC017‐PROPRANOLOL‐278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravid 1985 {published data only}
- Ravid M, Lang R, Jutrin I. The relative antihypertensive potency of propranolol, oxpranolol, atenolol and metoprolol given once daily. Archive Internal Medicine 1985;145:1321‐3. [NSC021‐PROPRANOLOL248] [PubMed] [Google Scholar]
Richardson 1968 {published data only}
- Richardson DW, Freund J, Gear AS, Mauck HP, Preston LW. Effect of propranolol on elevated arterial blood pressure. Circulation 1968;37:534‐42. [NSC020‐PROPRANOLOL354] [DOI] [PubMed] [Google Scholar]
Rosen 1994 {published data only}
- Rosen RC, Kostis JB, Jekelis A, Taska LS. Sexual sequelae of antihypertensive drugs: treatment effects on self report and physiological measures in middle aged male hypertensive. Archives of Sexual Behavior 1994;23(2):135‐51. [NSC‐Propranolol‐115] [DOI] [PubMed] [Google Scholar]
Schoenberger 1989 {published data only}
- Schoenberger JA. Usefulness of penbutolol for systemic hypertension. American Journal of Cardiology 1989;63:1339‐42. [NSP‐PENBUTOLOL‐197] [DOI] [PubMed] [Google Scholar]
Shukla 1979 {published data only}
- Shukla SK, Bhatnagar MK, Agarwal RK, Dwivedi RN, Gupta R. Beta blocker (propranolol) in mild hypertension. A dose response study. Indian Heart Journal 1979;31(6):330‐2. [NSC012‐PROPRANOLOL419] [PubMed] [Google Scholar]
Sica 2004 {published data only}
- Sica DA, Neutel JM, Weber MA, Manwitz N. The antihypertensive efficacy and safety of a chronotherapeutic formulation of propranolol in patients with hypertension. Journal of Clinical Hypertension 2004;6:231‐41. [NSP003‐PROPRANOLOL042] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Herwaarden 1977 {published data only}
- Herwaarden CLA, Fennis JFM, Binkhorst RA, t'Laar A. Haemodynamic effects of adrenaline during treatment of hypertensive patients with propranolol and metoprolol. European Journal of Clinical Pharmacology 1977;12:397‐402. [NSC‐Propranolol‐446] [DOI] [PubMed] [Google Scholar]
West 1980 {published data only}
- West MJ. Comparison of labetalol, hydrallazine, and propranolol in the therapy of moderate hypertension. Medical Journal of Australia 1980;1:224‐5. [NSC‐Propranolol‐451] [PubMed] [Google Scholar]
References to studies excluded from this review
Ades 1988 {published data only}
- Ades PA, Gunther PGS, Meacham CP, Handy MA. Hypertension, exercise and beta adrenergic blockade. Annals of Internal Medicine 1988;109:629‐34. [NSP‐PROPRANOLOL‐209] [DOI] [PubMed] [Google Scholar]
Ades 1990 {published data only}
- Ades PA, Gunther PGS, Meyer WL, Gibson TC, Maddalena J, Orfeo T. Cardiac and skeletal muscle adaptation to training in systemic hypertension and effect of beta blockade (metoprolol or propranolol). American Journal of Cardiology 1990;66:591‐6. [DOI] [PubMed] [Google Scholar]
Agnes 1989 {published data only}
- Agnes E, Vandermecken C, Prost JF, Safar ME. The tertatolol international multicenter study. American Journal of Hypertension 1989;2(11):296S‐302S. [DOI] [PubMed] [Google Scholar]
Beilin 1972 {published data only}
- Beilin LJ, Juel‐Jensen BE. Alpha and beta adrenergic blockade in hypertension. Lancet 1972;1:979‐82. [DOI] [PubMed] [Google Scholar]
Cherchi 1985 {published data only}
- Cherchi A. Controlled study of the antihypertensive efficacy of the combination of nadolol and bendroflumethiazide with respect to nadolol alone and placebo. Clinica Terapeutica 1985;113:301‐6. [PubMed] [Google Scholar]
Costa 1984 {published data only}
- Costa FV, Caldari R, Borghi C, Bassein L, Ambrosioni E. Acebutalol, atenolol and nadolol: comparison of their antihypertensive efficacy at rest and during exercise. Current Therapeutics Research 1984;35(6):961‐73. [Google Scholar]
Davidson 1976 {published data only}
- Davidson C, Thadani U, Singleton W, Taylor SH. Comparison of antihypertensive active of beta blocking drugs during chronic treatment. BMJ 1976;2:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubik 1984 {published data only}
- Kubik MM, Coote JH. Propranolol vs metoprolol vs labetalol: a comparative study in essential hypertension. European Journal of Clinical Pharmacology 1984;26:1‐6. [DOI] [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith SA, Littler WA. The effect of epanolol on intra‐arterial ambulatory blood pressure and baroreceptor heart rate reflex in essential hypertension. Clinical and Experimental Pharmacology and Physiology 1988;15:327‐31. [DOI] [PubMed] [Google Scholar]
Weigmann 1998 {published data only}
- Weigmann I, Terhaag B, Wierich W, Herrmann WM. Antihypertensive effects of different talinolol dosages after four weeks of treatment in comparison with placebo. Drug Research 1998;48(3):240‐4. [PubMed] [Google Scholar]
Additional references
Brophy 2001
- Brophy JM, Joseph L, Rouleau JL. Beta blockers in congestive heart failure. A Bayesian meta‐analysis. Annals of Internal Medicine 2001;134:550‐60. [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen JMH, Heran BS, Perez MI, Wright JM. Blood pressure lowering efficacy of beta‐blockers as second‐line therapy for primary hypertension. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007185.pub2] [DOI] [PubMed] [Google Scholar]
eCPS
- Canadian Pharmacist Association. e‐CPS drug monograph. www.e‐therapeutics.ca/cps.showMonograph.action (assessed 21 February 2014).
FDA
- FDA Online Label Repository. labels.fda.gov/ (accessed 21 February 2014).
Heran 2008 (ACEI)
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008 (ARB)
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Ko 2004
- Ko DT, Hebert PR, Coffey CS, Curtis JP, Foody JM, Sedrakyan A, et al. Adverse effects of beta‐blocker therapy for patients with heart failure. Archives of Internal Medicine 2004;164:1389‐94. [DOI] [PubMed] [Google Scholar]
Law 2005
- Law M, Morris JK, Jordan R, Wald N. Headaches and the treatment of blood pressure results from a meta‐analysis of 94 randomized placebo‐controlled trials with 24 000 participants. Circulation 2005;112:2301‐6. [DOI] [PubMed] [Google Scholar]
Magee 2003
- Magee LA, Duley L. Oral beta‐blockers for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002863] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000028.pub2] [DOI] [PubMed] [Google Scholar]
Prichard 1966
- Prichard BNC, Gillam PMS. Propranolol in hypertension. American Journal of Cardiology 1966;18(3):387‐91. [DOI] [PubMed] [Google Scholar]
Prichard 1969
- Prichard BNC, Gillam PMS. Treatment of hypertension with propranolol. BMJ 1969;1:7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Teo 1993
- Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. JAMA 1993;270:1589‐95. [PubMed] [Google Scholar]
Webb 2011
- Webb AJS, Fischer U, Rothwell PM. Effect of beta‐blocker selectivity on blood pressure variability and stroke. Neurology 2011;77:731‐7. [DOI] [PubMed] [Google Scholar]
Wiysonge 2007
- Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, et al. Beta blockers for hypertension. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong GWK, Laugerotte A, Wright JM. Blood pressure lowering efficacy of non‐selective beta blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007452] [DOI] [Google Scholar]
Wright 2000
- Wright JM. Choosing a first‐line drug in the management of elevated blood pressure: what is the evidence? 2: β‐Blockers. CMAJ 2000;163(2):188‐92. [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]


