Abstract
Beta‐blocker usage is inconsistently associated with increased fall risk in the literature. However, due to age‐related changes and interindividual heterogeneity in pharmacokinetics and dynamics, it is difficult to predict which older adults are more at risk for falls. Therefore, we wanted to explore whether elevated plasma concentrations of selective and nonselective beta‐blockers are associated with an increased risk of falls in older beta‐blocker users. To answer our research question, we analyzed samples of selective (metoprolol, n = 316) and nonselective beta‐blockers (sotalol, timolol, propranolol, and carvedilol, n = 179) users from the B‐PROOF cohort. The associations between the beta‐blocker concentration and time to first fall were assessed using Cox proportional hazard models. Change of concentration over time in relation to fall risk was assessed with logistic regression models. Models were adjusted for potential confounders. Our results showed that above the median concentration of metoprolol was associated with an increased fall risk (HR 1.55 [1.11–2.16], p = .01). No association was found for nonselective beta‐blocker concentrations. Also, changes in concentration over time were not associated with increased fall risk. To conclude, metoprolol plasma concentrations were associated with an increased risk of falls in metoprolol users while no associations were found for nonselective beta‐blockers users. This might be caused by a decreased β1‐selectivity in high plasma concentrations. In the future, beta‐blocker concentrations could potentially help clinicians estimate fall risk in older beta‐blockers users and personalize treatment.
Keywords: accidental falls, adrenergic beta‐antagonists, metoprolol, risk assessment
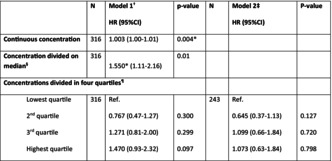
The table shows the associations between metoprolol concentration and fall risk. Continuous and median concentrations are associated with an increased fall risk.
Abbreviations
- ACE
angiotensin converting enzyme
- AT2
angiotensin 2 receptor
- ATC
anatomical therapeutical chemical
- B‐PROOF
B‐vitamins for the prevention of osteoporotic fractures
- BMI
body mass index
- DAG
directed acyclic graph
- eGFR
estimated glomerular filtration rate
- FRIDs
fall‐risk‐increasing drugs
- GDS
geriatric depression scale
- HRs
hazard ratios
- MMSE
mini‐mental state examination
- ORs
odds ratios
- SKF
Stichting Farmaceutische Kengetallen
1. INTRODUCTION
Falls are a major health problem in older adults. One third of adults over 65 years, and half of adults over 80 years fall at least once a year, often resulting in injury, hospital admissions, and reduced quality of life. 1 , 2 The number of older adults is expected to increase in the upcoming years. In 2050, one in four persons living in Western countries is expected to be 65 years or over, and the number of adults over 80 years will be 3 times as high compared with now. 3 Thus, the number of older adults require medical care due to fall incidents will likely increase substantially in the upcoming decades.
Many different fall‐risk factors have been identified. A well‐established and potentially modifiable risk factor for falling is the use of Fall‐Risk‐Increasing Drugs (FRIDs). 4 An important group of FRIDs is cardiovascular drugs such as vasodilators, antiarrhythmics, and antihypertensives. 5 Beta‐blockers are among the most commonly used drugs in older adults. A large cohort study (n = 4961) found that almost half of community‐dwelling older hypertensive individuals used beta‐blockers. 6 Beta‐blockers are prescribed for hypertension, angina pectoris, arrhythmia, and heart failure and have established benefits in cardiovascular outcomes. 7 However, beta‐blockers have also been shown to have frequent negative side effects, especially in older adults. 8 These adverse effects include bradycardia and hypotension, both risk factors for falling. A meta‐analysis by Woolcott et al. showed that beta‐blocker use in older adults was associated with a 14% increase in fall risk. 9 These results were challenged by a more recent systematic review, which found beta‐blockers to be protective of falls. 5 However, a narrative synthesis showed that a subgroup of beta‐blockers, nonselective beta‐blockers in fact increased fall risk. 10 The conflicting results might however also be explained by the fact that the latest review performed meta‐analyses of adjusted data but it also included more recent studies and studies of higher quality. Also, prescription patterns might have changed over the past years due to newly obtained knowledge on cardiovascular drug use in older adults. Given the contradictory outcomes in the available literature, international experts on geriatric pharmacology and FRIDs have not reached a consensus on whether or not beta‐blockers increase fall risk. 11
Previous studies have found no dose–response relation for beta‐blockers in older fallers. 12 Interindividual heterogeneity in pharmacokinetics and dynamics might explain these findings. 13 For example, individuals using metoprolol with poor metabolism phenotype of CYP2D6 have lower blood pressures and pulse rates due to a prolonged effect of metoprolol. 14 , 15 These side effects can contribute to an increased fall risk.
In general, plasma concentrations can be viewed as the result of pharmacokinetic processes in an individual patient. The plasma concentration dictates the pharmacological treatment effect (pharmacodynamics) but also the risk of adverse drug effects. Since the beta‐blocker dosage is a poor predictor of falls ‐because it does not take CYP2D6 polymorphism and other potential factors into account‐ quantification of beta‐blocker plasma concentrations might have the potential to guide clinical decision‐making. However, whether the beta‐blocker plasma concentration is indeed related to fall risk in older adults has not been studied before. Therefore, we explored whether there is an association between beta‐blocker plasma concentration and fall risk in beta‐blocker users. As it has been shown previously that nonselective beta‐blockers are particularly associated with increased fall risk, we studied also concentrations of nonselective beta‐blockers in relation to fall risk.
2. METHODS
2.1. Trial design and participants
For this study, a subgroup of beta‐blocker users from the multicenter B‐PROOF (B‐Vitamins for the PRevention of Osteoporotic Fractures) study was used. A detailed description of the B‐PROOF trial was published previously. 16 In short, this was a randomized, placebo‐controlled, double‐blind trial that studied the effect of vitamins B12, D, and folic acid supplementation on osteoporotic fractures in 2919 community‐dwelling participants aged ≥65 years, having mildly elevated serum homocysteine levels (12–50 μmol/L). Fall risk between the intervention and placebo groups did not differ. 17 Therefore, for the current analyses, the data set was treated as cohort data. Participants were recruited from August 2008 until March 2011, for a follow‐up period of 2–3 years. The study protocol was approved by the Medical Ethical Committee of Wageningen University, The Netherlands. All participants gave their written informed consent prior to study participation.
2.2. Beta‐blocker users
Beta‐blocker usage was based on pharmacy dispensing records obtained from the Dutch Foundation for Pharmaceutical Statistics (SFK), containing data from ~95% of all Dutch community pharmacies and self‐reported usage data. Beta‐blocker use was defined according to the Anatomical Therapeutical Chemical (ATC) code. Metoprolol (C07AB02 or C07BB02) is the most commonly used selective beta‐blocker in the Netherlands in general 18 and in this cohort in particular. Therefore, the analyses were restricted to this subgroup of selective beta‐blockers. In the B‐PROOF data set, we identified 683 metoprolol users and we aimed to include a randomly selected sample of approximately 300 metoprolol users. In terms of nonselective beta‐blockers, the B‐PROOF data set did not contain 300 users of a single nonselective beta‐blocker, so therefore, we included all sotalol (C07AA07), timolol (S01ED01 or S01ED51), propranolol (C07AA05), and carvedilol (C07AG02) users.
Participants having prescriptions up to 30 days prior to blood withdrawal at baseline and/or follow‐up visits were selected. To capture more potential users, participants with a prescription of up to 30 days after the withdrawal date were selected as some participants might not had a refill in the 30 days before. We also included participants that self‐reported using a nonselective beta‐blocker both at baseline and/or follow‐up blood sampling.
2.3. Beta‐blocker concentration
Blood samples were obtained from participants in the morning at baseline and follow‐up 2 years later. Participants were in a fasted state or had had a light breakfast. Venous blood was drawn in an EDTA tube at baseline and at follow‐up visits and stored at −80°C until analysis.
Metoprolol, sotalol, propranolol, carvedilol, and timolol plasma concentrations were analyzed using liquid chromatography with mass spectrometric detection. The method was validated over the following concentration ranges: 100–50 000 ng/mL (metoprolol), 100–5000 ng/mL (sotalol), 10–5000 ng/mL (propranolol), 1–5000 ng/mL (carvedilol), and 0.075–2500 ng/mL (timolol). In these concentration ranges, accuracy ranged from 96% to 110%, intraday imprecision was ≤4.3% and interday imprecision was ≤7.2% for all compounds.
If use of a beta‐blocker was reported (self‐reported and/or pharmacy‐based), and no beta‐blocker concentration was detectable, the respective concentration was set at half of the lower limit of detection: 0.5 ng/mL (metoprolol), 50 ng/mL (sotalol), 5.0 ng/mL (propranolol), 0.5 ng/mL (carvedilol), and 0.0145 ng/mL (timolol).
In addition, we calculated delta concentrations by subtracting the concentration at follow‐up minus the concentration at baseline.
2.4. Primary and secondary outcomes
The primary outcome was time to first fall. Falls were defined as “an unintentional change in position resulting in coming to rest at a lower level or on the ground” as advised by the Prevention of Falls Network Europe. 19 Falls were reported prospectively using fall calendars, which were filled in on a weekly basis by participants and returned to the research team every 3 months. In case of missing or unclear data, participants were contacted via telephone. The study had a follow‐up duration of 2 to 3 years. Participants were followed until their drop‐out date or the date of their last calendar, date of death, or the end of the study, whatever came first. 16 The secondary outcome was the occurrence of fall during follow‐up in relation to the change in plasma concentration over time.
2.5. Covariables
During the B‐PROOF trial, participant characteristics were assessed at baseline using a questionnaire (including age, gender, use of a walking aid, alcohol consumption, smoking, and medical history), and measurements were performed (including height, weight, blood pressure, estimated Glomerular Filtration Rate (eGFR), hand grip strength, cognitive performance, and depressive symptoms). Cognitive performance was assessed using the Mini‐Mental State Examination (MMSE). Depressive symptoms were assessed with the Geriatric Depression Scale (GDS‐15). Physical performance was measured using a walking test, a chair stand test, and a balance test. Participants could score a maximum of 12 points with a maximum of 4 points per item. Cardiovascular disease was defined as having self‐reported arrhythmia, angina pectoris, myocardial infarction, heart failure, valve dysfunction, atrial septum defect, pericarditis, aneurysm, or pulmonary hypertension. Participants were asked if they had a history of hypertension. Polypharmacy was defined as using five or more medications. Other FRIDs included: psychotropic drugs (antidepressants, benzodiazepines or benzodiazepine‐like drugs, antiepileptics, Parkinson drugs, antipsychotics, opioids, and/or anticholinergics); and cardiovascular drugs (cardiac glycosides, class I and III antiarrhythmics, nitrates, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin 2 receptor (AT2) antagonists, and/or diuretics).
2.6. Statistical analyses
Baseline characteristics were calculated for fallers and nonfallers using Chi‐square tests, Mann–Whitney U‐tests, and t‐tests for categorical, continuous nonnormally distributed, and normally distributed data, respectively.
Plasma concentration levels were analyzed continuously and categorically. For the categorical analysis, the plasma concentrations were divided into concentrations above and below the median and in quartiles. The category below the median and the lowest quartile category were set as references.
We combined continuous nonselective beta‐blocker concentrations of different agents using z‐scores. Second, the median of sotalol, timolol, propranolol, and carvedilol were calculated individually after which participants were grouped into two categories (below and above median). Also, concentrations were categorized into quartiles. If more than 25% of the users of an individual beta‐blocker had a nondetectable concentration, these individuals with nondetectable concentrations were grouped into the lowest percentile. The remaining concentrations were divided equally over the remaining three percentiles (25th–50th, 50th–75th, and highest percentile, respectively). If a participant used two or more nonselective beta‐blockers, the highest plasma concentration value was used in the analysis. This concerned in total one participant at baseline and two participants at follow‐up. In all three cases, timolol was the analyzed concentration. If the number of users of a specific drug group exceeded 50, subgroup analyses were performed.
For the delta concentration, all participants with a negative delta or a delta equal to zero were given the value 0, which was set as the reference category. All participants with a positive delta were given the value 1.
Cox regression models were used to calculate hazard ratios (HRs) for time to first fall based on beta‐blocker concentration at baseline. To analyze the association between the delta plasma concentration and fall occurrence during follow‐up logistic regression models were used to calculate odds ratios (ORs). In model 1, beta‐blocker concentration was adjusted for age and gender. Potential confounders were added to model 2 if they changed the effect size by 10% or more. Potential confounders were selected based on a Directed Acyclic Graph (DAG) and included region, alcohol use, smoking, body mass index (BMI), walking aid use, performance, polypharmacy, number of medications, experiencing pain, eGFR, hand grip strength, GDS, MMSE, cardiovascular disease, arrhythmia's, cardiovascular medication use (minus beta‐blockers), and psychotropic medication use. If covariates could not be added, due to the lack of 10 fall events per covariate, 20 only model 1 is presented. If there were not 10 fall events per covariate in the final model, we chose the most clinically relevant covariates.
If we found an association between plasma concentration and fall risk, we explored whether this could potentially be related to blood pressure levels by plotting the mean systolic or diastolic blood pressure against plasma concentrations.
P‐values of ≤.05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows, version 26.0.0.1 (IBM Corp.).
3. RESULTS
3.1. Study population
Baseline samples of 316 metoprolol and 179 nonselective beta‐blocker users (sotalol, timolol, propranolol, and carvedilol) were analyzed. After the exclusion of incomplete cases, we calculated the delta concentrations in 302 metoprolol and 124 nonselective beta‐blocker users.
Baseline characteristics are shown in Tables 1 and 2. Metoprolol users who experienced a fall during follow‐up were significantly older and had a lower hand grip strength and estimated Glomerular Filtration Rate (eGFR) compared with metoprolol users who did not fall during the follow‐up. Also, more fallers had a history of falls and the use of walking aids was more prevalent in this group compared with the nonfallers (Table 1). In the nonselective beta‐blocker users, fallers had a significantly lower body mass index (BMI) and reported more often experiencing a fall in the 12 months prior to study enrollment (Table 2).
TABLE 1.
Patient characteristics of metoprolol users.
| N overall | Nonfallers (n = 162) | Fallers (n = 154) | |
|---|---|---|---|
| Age (years) a | 316 | 73 (69–77) | 74 (70–80)* |
| Gender b | 316 | ||
| Male | 83 (51.2%) | 70 (45.5%) | |
| Female | 79 (48.8%) | 84(54.6%) | |
| BMI (kg/m2) c | 315 | 28.5 (4.3) | 27.8 (4.4) |
| Alcohol use (yes) b | 316 | 162 (87.7%) | 154 (82.5%) |
| Smoking b | 316 | ||
| Never | 52 (32.1%) | 52 (33.8%) | |
| Current | 12 (7.4%) | 6 (3.9%) | |
| Former | 98 (60.5%) | 98 (62.3%) | |
| Fall in the past 12 months prior to study enrollment (yes) b | 248 | 29 (22.7%) | 55 (45.8%)* |
| Using a walking aid (yes) b | 315 | 16 (9.9%) | 27 (17.5%)* |
| Hand grip strength (kg) c | 312 | 31.8 (25.1–40.9) | 28.9 (23.1–37.5)* |
| Physical performance a | 312 | 9 (6–10) | 8 (4–10) |
| Polypharmacy (yes) b | 315 | 83 (51.6%) | 81 (52.6%) |
| Number of medications a | 315 | 5 (3–6) | 5 (3–7) |
| Psychotropic medication use (yes) b | 315 | 17 (10.6%) | 25 (16.2%) |
| MMSE a | 316 | 28 (27–29) | 29 (28–29) |
| GDS‐15 a | 314 | 1 (0–2) | 1 (0–2) |
| History hypertension (yes) b | 248 | 86 (67.2%) | 84 (70.0%) |
| Cardiovascular disease (yes) b | 245 | 72 (57.0%) | 55 (46.6%) |
| Systolic blood pressure (in mmHg) c | 258 | 149 (20.8) | 150 (20.5) |
| Diastolic blood pressure (in mmHg) c | 258 | 80 (12.0) | 80 (10.4) |
| eGFR (mL/min/1.73 m2) c | 314 | 75.5 (23.2) | 68.0 (20.0)* |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; GDS, geriatric depression scale; MMSE, mini‐mental state examination.
Presented as median (range).
Presented as n (%).
Presented as mean (standard deviation [SD]).
p‐value ≤.05 (comparison of nonfallers to fallers).
TABLE 2.
Patient characteristics nonselective beta‐blocker users.
| N overall | Nonfallers (n = 75) | Fallers (n = 104) | |
|---|---|---|---|
| Age (years) a | 179 | 75.0 (71–80) | 75.0 (69–82) |
| Gender b | 179 | ||
| Male | 46 (61.3%) | 51 (49.0%) | |
| Female | 29(38.7%) | 53 (51.0%) | |
| BMI (kg/m2) c | 178 | 28.3 (4.4) | 26.6 (3.9)* |
| Alcohol use (yes) b | 179 | 57 (76.0%) | 88 (84.6%) |
| Smoking b | 179 | ||
| Never | 29 (38.7%) | 39 (37.5%) | |
| Current | 7 (9.3%) | 11 (10.6%) | |
| Former | 39 (52.0%) | 54 (51.9%) | |
| Fall in the past 12 months prior to study enrollment (yes) b | 138 | 11 (21.2%) | 42 (48.8%)* |
| Using a walking aid (yes) b | 177 | 16 (21.6%) | 16 (15.5%) |
| Hand grip strength (kg) c | 176 | 32.4 (10.4) | 30.9 (10.9) |
| Physical performance a | 179 | 8 (4–10) | 7.5 (5–10) |
| Polypharmacy (yes) b | 179 | 47 (62.7%) | 62 (59.6%) |
| Number of medications a | 179 | 5 (3–6) | 5 (3–7) |
| Psychotropic medication use (yes) b | 179 | 11 (14.7%) | 22 (21.1%) |
| MMSE a | 178 | 28 (27–29) | 29 (27–29) |
| GDS‐15 a | 179 | 1 (0–3) | 1 (0–2) |
| Elevated blood pressure (yes) b | 139 | 20 (37.7%) | 37 (43.0%) |
| Cardiovascular disease (yes) b | 138 | 31 (58.5%) | 46 (54.1%) |
| Systolic blood pressure (in mmHg) c | 148 | 142 (15.0) | 144 (23.9) |
| Diastolic blood pressure (in mmHg) c | 148 | 78 (9.2) | 78 (11.9) |
| eGFR (mL/min/1.73 m2) c | 178 | 71.6 (22.9) | 68.6 (19.9) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; GDS, geriatric depression scale; MMSE, mini‐mental state examination.
Presented as median (range),
Presented as n (%),
Presented as mean (standard deviation [SD]).
p‐value ≤.05 (comparison of nonfallers to fallers).
3.2. Association between metoprolol concentration and fall risk
In the metoprolol group (n = 316), 154 users (49%) experienced a fall during the follow‐up. Cox regression analyses showed that within users of metoprolol, a higher plasma concentration was associated with an increased fall risk per ng/mL (HR: 1.003, [1.00–1.01] per ng/mL, p = .004, model 1). Also, when dividing the concentration into two groups, above and below the median concentration, a significant association was found (HR: 1.550, [1.11–2.16], p = .01). When dividing the concentrations into quartiles, no significant associations were found (Table 3).
TABLE 3.
Baseline metoprolol concentration and time to first fall.
| N | Model 1 c HR (95%CI) | p‐value | N | Model 2 d HR (95%CI) | p‐value | |
|---|---|---|---|---|---|---|
| Continuous concentration | 316 | 1.003 (1.00–1.01) | .004* | |||
| Concentration divided on median a | 316 | 1.550* (1.11–2.16) | .01* | |||
| Concentrations divided in four quartiles b | ||||||
| Lowest quartile | 316 | Ref. | 243 | Ref. | ||
| Second quartile | 0.767 (0.47–1.27) | .300 | 0.645 (0.37–1.13) | .127 | ||
| Third quartile | 1.271 (0.81–2.00) | .299 | 1.099 (0.66–1.84) | .720 | ||
| Highest quartile | 1.470 (0.93–2.32) | .097 | 1.073 (0.63–1.84) | .798 | ||
Note: Data presented as hazard ratio with a 95% confidence interval. Number of events model 1: n = 154. Number of events model 2: n = 118.
Abbreviations: N, number of analyzed plasma concentrations; CI, confidence interval.
Median concentration selective beta‐blocker: 16.95 ng/mL.
Concentration selective beta‐blocker 25th percentile: 8.61 ng/mL; 75th percentile: 46.5 ng/mL.
Model 1 was adjusted for age and gender.
Model 2 was adjusted for age, gender, eGFR, and CV disease. No model 2 was constructed for the continuous and binary metoprolol concentration because none of the added variables changed the outcome more than 10%.
p‐value ≤.05.
No correlation was observed between systolic and diastolic blood pressures and the plasma concentration of metoprolol (Figures S1 and S2).
3.3. Association between nonselective beta‐blocker concentration and fall risk
We analyzed baseline samples of 179 nonselective beta‐blocker users of whom 104 (58%) experienced a fall during the follow‐up. Analyzing the nonselective beta‐blocker plasma concentrations continuously, divided on the median or on quartiles, no associations with fall risk were observed (Table 4).
TABLE 4.
Baseline nonselective beta‐blocker concentration and time to first fall.
| N | Model 1 c HR (95%CI) | p‐value | N | Model 2 d HR (95%CI) | p‐value | |
|---|---|---|---|---|---|---|
| Continuous concentration | ||||||
| Nonselective beta‐blocker a | 179 | 0.936 (0.76–1.15) | .523 | |||
| Timolol | 70 | 1.006 (0.69–1.47) | .974 | 54 | 1.075 (0.71–1.62) | .729 |
| Sotalol | 73 | 1.000 (0.99–1.00) | .948 | |||
| Concentration divided on median b | ||||||
| Nonselective beta‐blocker a | 179 | 0.953 (0.65–1.40) | .809 | |||
| Timolol | 70 | 1.131 (0.61–2.08) | .692 | 54 | 1.247 (0.63–2.48) | .530 |
| Sotalol | 73 | 1.042 (0.56–1.95) | .896 | |||
| Concentrations are divided into four quartiles | ||||||
| Nonselective beta‐blocker a | 179 | 138 | ||||
| First quartile | Ref. | Ref. | ||||
| Second quartile | 1.020 (0.60–1.73) | .941 | 1.183 (0.64–2.19) | .593 | ||
| Third quartile | 1.319 (0.80–2.17) | .278 | 1.413 (0.80–2.49) | .233 | ||
| Fourth quartile | 0.725 (0.41–1.29) | .275 | 0.779 (0.40–1.50) | .455 | ||
| Timolol | 70 | |||||
| First quartile | Ref. | |||||
| Second quartile | 0.904 (0.37–2.23) | .827 | ||||
| Third quartile | 1.616 (0.69–3.80) | .271 | ||||
| Fourth quartile | 0.996 (0.38–2.62) | .994 | ||||
| Sotalol | 73 | |||||
| First quartile | Ref. | |||||
| Second quartile | 1.398 (0.45–4.31) | .560 | ||||
| Third quartile | 1.384 (0.45–4.22) | .568 | ||||
| Fourth quartile | 0.793 (0.24–2.58) | .700 | ||||
Note: Data presented as hazard ratio with a 95% confidence interval. Nonselective beta‐blockers: timolol, sotalol, propranolol, carvedilol.
Abbreviations: N, number of analyzed plasma concentrations; CI, confidence interval.
Number of events model: nonselective beta‐blockers = 104; timolol = 43; sotalol = 44. Number of events model 2: timolol = 35; nonselective beta‐blockers = 85. A systemic timolol concentration was measured in 45% of our timolol users.
Median timolol: 0.0145 ng/mL. Median sotalol: 704.00 ng/mL.
Model 1 was adjusted for age and gender.
Model 2 was adjusted for age, gender, and: timolol continue and median: cardiovascular (CV) disease; nonselective beta‐blockers quartiles: CV disease. No model 2 was constructed for nonselective beta‐blockers and sotalol continuous and median concentrations or for timolol and sotalol quartiles because either none of the variables changed the outcome more than 10%, or the number of events was too low to add covariates to the model.
Seventy participants (43 fallers and 27 nonfallers) used timolol eye drops and in 32 participants (45%), we detected a systemic timolol concentration. We included 73 sotalol users in our analyses of whom 44 experienced a fall. No significant association between baseline timolol or sotalol plasma concentration and fall risk was observed (Table 4).
3.4. Plasma concentration changes over time
We calculated the delta concentration for 302 metoprolol users of whom 194 had a positive delta concentration, indicating an increase in the plasma concentration over time. In the nonselective beta‐blocker group (n = 124), 58 participants had a positive delta. Also, we performed a subgroup analysis for timolol (n = 56) and sotalol (n = 45) where, respectively, 19 and 22 positive deltas were found.
In the analyses of both metoprolol and nonselective beta‐blocker users and subgroup analysis of timolol and sotalol users, no association between plasma concentration change over time and fall risk was observed (Table S1).
4. DISCUSSION
Our results showed that the plasma concentration of the selective beta‐blocker metoprolol at baseline was significantly associated with an increased fall risk during follow‐up in users. In contrast, no association with falls was found for plasma concentrations of nonselective beta‐blockers, nor for the individual agents timolol and sotalol. Furthermore, a changing plasma concentration over time (delta concentration) was not associated with falls among metoprolol or nonselective beta‐blocker users or timolol or sotalol users.
To our knowledge, this is the first study to address the role of beta‐blocker plasma concentrations in fall risk. Previous studies have only assessed the characteristics of different beta‐blockers in relation to their role in beta‐blocker‐related fall risk.
Previous analyses of the B‐PROOF cohort showed that selective beta‐blocker use was not associated with an increased fall risk while the use of a nonselective beta‐blocker did increase fall risk. 10 This was attributed to the receptor binding profile and the negative effects caused by beta‐2‐antagonism for example on muscle function. However, when comparing the results between these studies, it should be remembered that we analyzed fall risk among users and Ham et al. investigated risk related to the use of beta‐blockers. Moreover, we analyzed only metoprolol users from the B‐PROOF study whereas Ham et al. included also other selective beta‐blocker users.
Nonselective beta‐blocker users are thus most likely more at risk for falls because they use a nonselective beta‐blocker but based on our results having a higher concentration does not further increase fall risk compared to users with a lower concentration. Our results indicate that within users, metoprolol users with higher plasma concentrations had an increased fall risk compared to individuals with lower concentrations. In general, it is known that the risk of adverse effects of selective beta‐blockers increases when plasma concentration increases. 21 In a previous analysis of the B‐PROOF cohort, no dose–response was found. We also did not observe a correlation between plasma concentration and blood pressure. Thus, other pathways might have played a role, such as heartrate (bradycardia) or orthostatic hypotension. Orthostatic hypotension is consistently associated with an increased risk of falling. 22 The sudden drop in blood pressure when standing up is normally corrected by increasing the heart rate and subsequently, the cardiac output. Selective beta‐blockers inhibit the increase in heart rate resulting in an uncorrected low blood pressure and therefore, potentially increase the risk of falling. A lower heart rate and a higher risk of orthostatic hypotension due to the high concentration of metoprolol might explain why we found an association between fall risk and a high concentration of metoprolol. Also, importantly, at higher plasma concentrations, metoprolol is less cardio‐selective. 23 This could explain why we found the fall risk increased in persons with higher concentrations as it is in line with previous research which demonstrated the importance of selectivity.
Thus, in summary, nonselectivity of beta‐blockers (or loss of selectivity in higher blood concentrations) may be the underlying pathway for fall risk. On the other hand, confounding by indication cannot fully be ruled out regarding the risk difference of the subgroups. For example, nonselective beta‐blockers such as sotalol are predominantly used for the treatment of arrhythmia's.
4.1. Strengths and limitations
The major strength of our study was that data on falls was documented using prospective fall calendars that participants had to fill in weekly, reducing the chance of recall bias.
Our study also had some limitations. Blood was drawn during baseline and follow‐up measurements while participants fell somewhere in the 2‐ to 3‐year follow‐up period. During follow‐up period, dosage changes or gaps in usage might have occurred that could have influenced plasma concentration and subsequently, fall risk. Baseline or follow‐up plasma concentrations give an indication but might not be a representative estimate of the plasma concentration at the time of the fall. Also, the sample size of nonselective beta‐blocker users was relatively small. Therefore, we had to combine data of four nonselective beta‐blockers concentrations and we were only able to perform subgroup analysis of sotalol and timolol. Although we corrected the analysis for multiple cardiovascular diseases, we cannot fully rule out bias by indication in the analysis of nonselective beta‐blocker concentrations. Propranolol can be prescribed for essential tremor and tremor in Parkinson's disease. The latter is a risk factor for falls, 24 , 25 the B‐PROOF data set did not contain information on these diseases and therefore, we could not test this as a possible confounder in our models. In addition, we had no data on heart rate or orthostatic hypotension, so we could not investigate whether the association between metoprolol concentration and falls is related to these factors. We also did not know the time a patient took their medication or the time blood was drawn. Therefore, we cannot estimate if concentrations have reached a steady state or not. However, all participants were instructed to take their medication according to their normal schedule with a light breakfast and all blood withdrawals took place in the morning, making it likely that concentrations reached a steady state though variations are possible. Moreover, we were able to measure systemic concentrations only in 45% of our timolol users. The systemic absorption of timolol is dependent on the technical ability of the patient to administer the eye drops but also on the time between the administration of the eye drops and the blood withdrawal. Also, gellanous timolol eye drops are less absorbed systemically than aqueous eye drops, though most of our participants used aqueous eye drops.
4.2. Clinical implications and future perspectives
Ideally, beta‐blocker monitoring will take place based on clinical parameters such as heart rate and blood pressure. However, in complex cases in which clinical parameters do not provide sufficient knowledge to adjust beta‐blocker treatment, analyzing plasma concentration of metoprolol may potentially help clinicians predict which individual metoprolol user has an increased risk of falling. Based on the plasma concentration, dosage can be altered or the medication can switched to a safer alternative. Plasma concentrations of nonselective beta‐blocker do not seem good markers for fall risk. However, our findings should be confirmed in studies with more frequent blood measurements to obtain representative blood concentrations around the time of the fall. Furthermore, future studies might further look into the pathogenesis of falls due to a high plasma concentration of metoprolol including, for example, the influence on heart rate, rhythm control, orthostatic hypotension. Also, other individual selective and nonselective beta‐blockers should be investigated to understand if these medications increase fall risk when present in high concentrations.
5. CONCLUSION
To conclude, our study has shown that metoprolol concentration is associated with an increased risk of falling in users. In contrast, for nonselective beta‐blocker plasma concentrations, no association was found among users nor was an association found for the changing concentration over time among both metoprolol and nonselective beta‐blocker users. We showed that plasma concentrations of metoprolol could be potentially used as a predictor of fall risk. However, our findings should be confirmed in studies with more frequent plasma concentration measurements surrounding the time of the fall.
FUNDING INFORMATION
This work was supported by the Clementine Brigitta Maria Dalderup fund (project numbers 20713 and 7303, receiver N. van der Velde), which is an Amsterdam University fund (https://www.auf.nl/en/about‐the‐fund/about.html). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The B‐PROOF was funded by the Netherlands Organization for Health Research and Development (ZonMw, Grant 6130.0031), the Hague; an unrestricted grant from NZO (Dutch Dairy Association), Zoetermeer; Orthica, Almere; NCHA (Netherlands Consortium Healthy Ageing), Leiden/Rotterdam; Ministry of Economic Affairs, Agriculture and Innovation (project KB‐15‐004‐003), the Hague; Wageningen University, Wageningen; VUmc, Amsterdam; Erasmus Medical Center, Rotterdam; Unilever, Colworth, UK. The sponsors and patients had no role in the design or implementation of the study, data collection, data management, data analysis, data interpretation, or in the preparation, review, or approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
KMAS is an employee of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for the government and related healthcare authorities and several pharmaceutical companies.
ETHICS STATEMENT AND PATIENT CONSENT
The study protocol was approved by the Medical Ethical Committee of Wageningen University, The Netherlands. All participants gave their written informed consent prior to study participation.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We thank D. van der Laan and M. Pistorius of the hospital pharmacy department of the Amsterdam Academic Medical Center, for analyzing the included blood samples and providing us with the data to investigate our research question. Also, we thank the participants of the B‐PROOF study for their enthusiasm and cooperation. Furthermore, we thank the dedicated team that conducted the study. Especially, R. Dhonukshe‐Rutten, A.W. Enneman, A.C. Ham. and P. Lips, S. Smits, and the research assistants.
Ploegmakers KJ, van Poelgeest EP, Seppala LJ, et al. The role of plasma concentrations and drug characteristics of beta‐blockers in fall risk of older persons. Pharmacol Res Perspect. 2023;11:e01126. doi: 10.1002/prp2.1126
Primary laboratory of origin: AmsterdamUMC, location AMC Pharmacy Laboratory, Meibergdreef 9, 1105AZ Amsterdam.
DATA AVAILABILITY STATEMENT
Because of restrictions based on privacy regulations and the informed consent of the participants, data cannot be made freely available in a public repository. B‐PROOF data can be obtained upon request. Requests should be directed toward the principal investigators of the studies, who have a protocol for approving data requests.
REFERENCES
- 1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701‐1707. [DOI] [PubMed] [Google Scholar]
- 2. Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long‐term reduced quality of life. J Trauma—Inj Infect Crit Care. 2011;71(3):748‐753. [DOI] [PubMed] [Google Scholar]
- 3. United Nations, Department of Economic and Social Affairs, population division . World Population Ageing 2015. 2015.
- 4. Montero‐Odasso M, van der Velde N, Martin FC, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51(9):1‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Vries M, Seppala LJ, Daams JG, et al. Fall‐risk‐increasing drugs: a systematic review and meta‐analysis: I. cardiovascular drugs. J Am Med Dir Assoc. 2018;19(4):371.e1‐371.e9. [DOI] [PubMed] [Google Scholar]
- 6. Tinetti ME, Han L, Lee DSH, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol. 2011;8(1):13‐28. [DOI] [PubMed] [Google Scholar]
- 8. Cai A, Calhoun DA. Antihypertensive medications and falls in the elderly. Am J Hypertens. 2018;31(3):281‐283. [DOI] [PubMed] [Google Scholar]
- 9. Woolcott JC. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons (archives of internal medicine (2009) 169, 21 (1952–1960)). Arch Intern Med. 2010;170(5):477. [DOI] [PubMed] [Google Scholar]
- 10. Ham AC, Swart KMA, Enneman AW, et al. Medication‐related fall incidents in an older, ambulant population: the B‐PROOF study. Drugs and Aging. 2014;31(12):917‐927. [DOI] [PubMed] [Google Scholar]
- 11. Seppala LJ, Petrovic M, Ryg J, et al. STOPPFall (screening tool of older persons prescriptions in older adults with high fall risk): a Delphi study by the EuGMS task and finish group on fall‐risk‐increasing drugs. Age Ageing. 2021;50(4):1189‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ham AC, van Dijk SC, Swart KMA, et al. Beta‐blocker use and fall risk in older individuals: original results from two studies with meta‐analysis. Br J Clin Pharmacol. 2017;83(10):2292‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta‐adrenergic blockers in humans. J Pharm Pharm Sci. 2001;4(2):185‐200. [PubMed] [Google Scholar]
- 14. Nagele P, Liggett SB. Genetic variation, β‐blockers, and perioperative myocardial infarction. Anesthesiology. 2011;115(6):1316‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blake CM, Kharasch ED, Schwab M, Nagele P. A meta‐analysis of CYP2D6 metabolizer phenotype and metoprolol pharmacokinetics. Clin Pharmacol Ther. 2013;94(3):394‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Wijngaarden JP, Dhonukshe‐Rutten RA, van Schoor NM, et al. Rationale and design of the B‐PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatr. 2011;11(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swart KMA, Ham AC, van Wijngaarden JP, et al. A randomized controlled trial to examine the effect of 2‐year vitamin B12 and folic acid supplementation on physical performance, strength, and falling: additional findings from the B‐PROOF study. Calcif Tissue Int. 2016;98(1):18‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ZorginstituutNederland . “Top 500 van geneesmiddelen o.b.v. het aantal gebruikers in 2021.”.
- 19. Lamb SE, Jørstad‐Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618‐1622. [DOI] [PubMed] [Google Scholar]
- 20. Bujang MA, Sa'at N, Sidik TMITAB, Lim CJ. Sample size guidelines for logistic regression from observational studies with large population. Malaysian J. Med. Sci. 2018;25(4):122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rau T, Wuttke H, Michels LM, et al. Impact of the CYP2D6 genotype on the clinical effects of metoprolol: a prospective longitudinal study. Clin Pharmacol Ther. 2009;85(3):269‐272. [DOI] [PubMed] [Google Scholar]
- 22. Mol A, Bui Hoang PTS, Sharmin S, et al. Orthostatic hypotension and falls in older adults: a systematic review and meta‐analysis. J Am Med Dir Assoc. 2019;20(5):589‐597.e5. [DOI] [PubMed] [Google Scholar]
- 23. Dean L. Maraviroc therapy and CCR5 genotype . 2012. [PubMed]
- 24. Bloem BR, Steijns JAG, Smits‐Engelsman BC. An update on falls. Curr Opin Neurol. 2003;16(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 25. Rao AK, Louis ED. Ataxic gait in essential tremor: a disease‐associated feature? Tremor Other Hyperkinet Mov. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Because of restrictions based on privacy regulations and the informed consent of the participants, data cannot be made freely available in a public repository. B‐PROOF data can be obtained upon request. Requests should be directed toward the principal investigators of the studies, who have a protocol for approving data requests.


