Abstract
We report a diagnostically challenging case of a SMARCA4‐deficient undifferentiated tumour to emphasize its potential to mimic other malignant tumours on histology, especially in small biopsies and where rhabdoid morphology is lacking. A 48‐year‐old man, who was known for chronic obstructive pulmonary disease and polysubstance use, presented with dyspnoea and an anterior mediastinal mass that had grown rapidly over a seven‐month period. The rapid growth and location in the anterior mediastinum raised clinical suspicion for lymphoma or a germ cell tumour. Microscopic examination of a transthoracic, ultrasound‐guided, core needle biopsy revealed relatively uniform, malignant epithelioid cells with clear cytoplasm, but lacking any rhabdoid features. Tumour necrosis was prominent. The immunohistochemistry panel was negative for lymphoma markers, but positive for SALL4 (a marker typically associated with germ cell tumours), CD34, EMA, and HepPar1, while expression of SMARCA4 and claudin‐4 was entirely lost. Only focal cytokeratin expression was demonstrated. SMARCB1 (INI1) expression was retained. The diagnosis of SMARCA4‐DUT was made based on these findings. Unfortunately, the tumour was already at an advanced stage at diagnosis (stage IVA) and the patient had a poor performance status. He was treated with palliative radiotherapy with no significant improvement in performance status and passed away 3 months after diagnosis. The case highlights the importance of considering SMARCA4‐DUT in the differential diagnosis of an undifferentiated, rapidly growing thoracic tumour and the potential for misdiagnosis on a small tissue sample, particularly as rhabdoid morphology may be absent.
Keywords: lung, SMARCA4, thoracic, undifferentiated tumour
A 48‐year‐old man with chronic obstructive pulmonary disease and polysubstance use presented with dyspnoea and a fast‐growing mass in the anterior mediastinum, which raised suspicion of lymphoma or a germ cell tumour. Following histopathological examination and with the aid of a large immunohistochemical panel, SMARCA4‐deficient undifferentiated tumour (SMARCA4‐DUT) was diagnosed. This case emphasizes the need to consider SMARCA4‐DUT when evaluating undifferentiated thoracic tumours, as misdiagnosis can occur, especially when rhabdoid morphology is absent.
INTRODUCTION
SMARCA4‐deficient thoracic sarcoma, characterized by the loss of SMARCA4 expression and aggressive clinical behaviour, was first described in 2015 by Le Loarer et al. 1 It is associated with heavy smoking, male sex, and is often associated with emphysema/bullae.
The 5th edition of the World Health Organization Classification of Thoracic Tumours replaced the term SMARCA4‐deficient thoracic sarcoma with SMARCA4‐deficient undifferentiated tumour (SMARCA4‐DUT), an entity that is separate from SMARCA4‐deficient non‐small cell lung cancer. 2
The histological diagnosis of SMARCA4‐DUT requires an undifferentiated thoracic tumour consisting of round to epithelioid cells with or without rhabdoid morphology and complete loss of SMARCA4 by immunohistochemistry. Desirable diagnostic criteria include loss of SMARCA2, expression of CD34, SOX2 and/or SALL4, and absent or focal claudin‐4. 2
Currently, there is no standardized treatment for SMARCA4‐DUT. Early phase studies with the H3K27 histone methyltransferase EZH2 inhibitor in tumours related to the SWI/SNF complex show promise 3 and recent case reports have reported some success with immunotherapy. 4
In general, the prognosis of patients with SMARCA4‐DUT is dismal with a reported median survival of 5 months (range 1–13). 5
The correct diagnosis may be challenging on a small tissue sample. We report a case of thoracic SMARCA4‐DUT diagnosed on transthoracic needle core biopsy, with emphasis on the differential diagnosis and potential for misdiagnosis which may lead to unnecessary treatment.
CASE REPORT
A 48‐year‐old man presented with complaints of worsening dyspnoea and a productive cough for 2 weeks. He was known with a previous medical history of chronic obstructive pulmonary disease and polysubstance use (cigarettes, cannabis, methamphetamine and methaqualone). On clinical examination, he was in respiratory distress with a respiratory rate of 28 per minute, with diminished breath sounds bilaterally, crackles over the right middle zone, wheezes bilaterally and anterior central dullness on percussion. There were no features of cor pulmonale or peripheral lymphadenopathy. An acute exacerbation of chronic obstructive pulmonary disease was diagnosed.
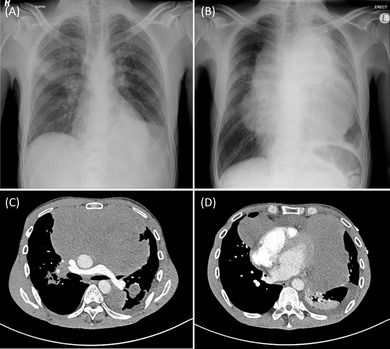
Further workup consisted of a chest radiograph (Figure 1B) which revealed a new, large anterior mediastinal mass when compared to a chest radiograph taken 7 months earlier (Figure 1A). Post‐contrasted computed tomography chest (Figure 1C,D) confirmed a large anterior mediastinal mass with extension to the middle and posterior mediastinum. Calcifications were absent. The main consideration was lymphoma due to its smooth, lobulated contour, lack of calcifications, rapid growth and the presence of small pleural and pericardial effusions. The differential diagnosis based on the clinical and radiological findings also included thymic or germ cell tumours.
FIGURE 1.
(A) A supine AP chest radiograph taken 7 months prior did not show mediastinal widening. (B) An erect PA chest radiograph taken at this presentation demonstrated a very large, multi‐lobulated central mass causing significant mediastinal widening. An axial post‐contrasted CT chest at the subcarinal level (C) and at the level of the heart (D) showed this non‐enhancing mass to be centred in the anterior mediastinum and to displace and encase mediastinal structures including the pulmonary arteries, the aorta and the heart.
Blood tests revealed an elevated white cell count (neutrophilia), anaemia of chronic disease, raised C‐reactive protein, and markedly elevated lactate dehydrogenase with normal alpha fetoprotein and ß‐ human chorionic gonadotropin levels.
The diagnostic approach was specifically aimed at investigating for potentially curable tumours such as lymphoma and germ cell tumour. A transthoracic, ultrasound‐guided core needle biopsy was obtained. Microscopic examination of haematoxylin and eosin stained sections revealed sheets of relatively uniform, epithelioid cells with irregular nuclei with mostly fine chromatin and inconspicuous nucleoli and pale eosinophilic to clear cytoplasm (Figure 2). Tumour necrosis was prominent but rhabdoid features were absent. The histological differential diagnosis included high‐grade lymphoma, germ cell tumour, thymic tumours, NUT carcinoma, undifferentiated carcinoma, mesothelioma, metastatic melanoma and sarcoma.
FIGURE 2.
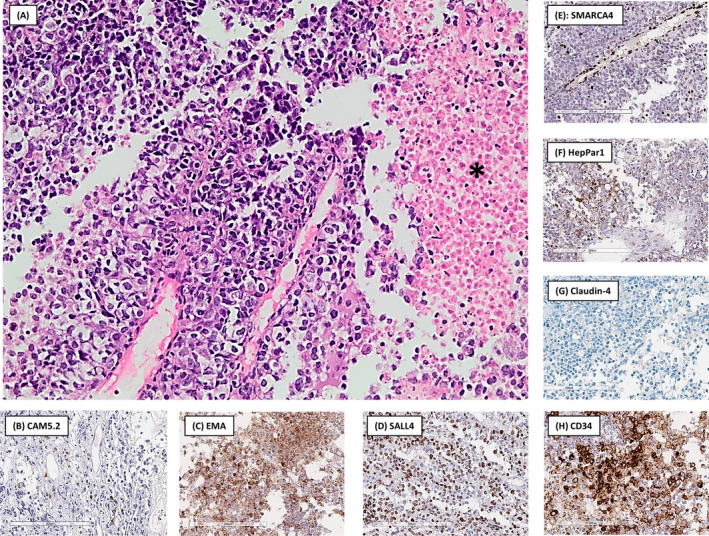
(A) The tumour was composed of sheets of epithelioid cells with clear cytoplasm. Tumour necrosis, a prominent finding in this specimen, is present on the right, indicated by a black asterisk (haematoxylin and eosin stain, original magnification 200×). Immunohistochemistry showed only focal positive staining in isolated cells with CAM5.2 (B), while convincing cytoplasmic expression was found with EMA (C) and nuclear expression with SALL4 (D). SMARCA4 (E) was completely lost in the tumour cells while still being expressed in endothelial cells (internal control). HepPar1 (F) showed patchy granular cytoplasmic staining in ±10% of tumour cells. Claudin‐4 (G) was completely lost while CD34 (H) stained positive in the majority of tumour cells.
The immunohistochemistry workup (Table 1) was initially aimed at mediastinal Hodgkin and non‐Hodgkin lymphoma and germ cell tumour but all lymphoma‐directed markers were negative. SALL4, considered to be a pan‐germ cell marker, was positive but more specific germ cell markers for seminoma (CD117 & PLAP), embryonal carcinoma (CD30) and yolk sac tumour (alpha fetoprotein) were all negative. Other positive markers were EMA, CD34, HepPar1 (focal staining) and CAM5.2 (very focal staining). SMARCA4 expression was entirely lost, while SMARCB1 (INI1) showed retained staining. Claudin‐4 was negative. SMARCA2 and SOX2 were not available.
TABLE 1.
Summary of immunohistochemistry results for this case.
| Antibody | Clone | Source | Result |
|---|---|---|---|
| Broad spectrum cytokeratin | MNF116 | Dako | Negative |
| Low‐molecular weight cytokeratin | CAM5.2 | BD Biosciences | Focal staining in <5% of cells |
| CK7 | OV‐TL 12/30 | Novocastra | Negative |
| CK20 | Ks 20.8 | Dako | Negative |
| CK19 | RCK108 | Dako | Negative |
| EMA | E29 | Dako | Positive |
| SALL4 | 6E3 | Cell Marque | Positive |
| Alpha‐fetoprotein | (polyclonal) | Dako | Negative |
| CD117 | (polyclonal) | Dako | Negative |
| PLAP | 8A9 | Leica | Negative |
| CD45 | 2B11 & PD7/26 | Dako | Negative |
| CD3 | (polyclonal) | Dako | Negative |
| CD5 | 4C7 | Novocastra | Negative |
| CD20 | L26 | Dako | Negative |
| PAX‐5 | 1EW | Novocastra | Negative |
| CD30 | Ber‐H2 | Dako | Negative |
| CD15 | MMA | Ventana | Negative |
| CD34 | QBend‐10 | Dako | Positive in majority of cells |
| SOX‐10 | EP268 | Abcam | Negative |
| TTF‐1 | SPT24 | Bond | Negative |
| Napsin A | IP64 | Novocastra | Negative |
| CD1a | MTB1 | Novocastra | Negative |
| Ki‐67 | MIB‐1 | Dako | ±30% |
| HepPar1 | OCH1E5 | Dako | Cytoplasmic staining in ±10% of cells |
| NUT | (polyclonal) | Abcam | Negative |
| SMARCA4 | EPNCIR111A | Abcam | Loss of staining |
| SMARCB1/INI1 | MRQ‐27 | Cell Marque | Retained expression |
| Claudin‐4 | EP417 | Cell Marque | Negative |
Abbreviations: EMA, epithelial membrane antigen; HepPar1, hepatocyte specific antigen; INI1, integrase interactor 1; NUT1, nuclear protein in testis 1; PAX‐5, paired box 5; PLAP, placental alkaline phosphatase; SALL4, Spalt‐like transcription factor 4; SMARCA4, SWI/SNF related, matrix associated, actin dependent regulator of chromatin subfamily A member 4; SMARCB1, SWI/SNF related, matrix associated, actin dependent regulator of chromatin subfamily B member 1; SOX‐10, sex region Y‐related HMG‐box 10; TTF‐1, thyroid transcription factor 1.
Based on these findings, thoracic SMARCA4‐DUT was diagnosed.
The malignancy was staged as stage IVA (T4N3M1a) due to the presence of malignant pleural effusions. Upon evaluation at the oncology unit, the patient already exhibited signs and symptoms of significant superior vena cava obstruction. His ECOG performance status was 3, making him unsuitable for systemic platinum‐based doublet chemotherapy. Regrettably, our facility lacks access to immunotherapy, and there is limited available data on its potential survival benefit for patients with a poor performance status. Due to cost considerations, our institution does not offer endovascular stent insertions for palliative lung cancer patients with superior vena cava syndrome. The patient underwent a course of palliative radiotherapy to his mediastinum, receiving 20 Gy in 5 fractions, along with high‐dose intravenous dexamethasone. Unfortunately, this treatment did not lead to a significant improvement in his performance status. He passed away three months after diagnosis.
DISCUSSION
This report highlights the difficulties in making a correct diagnosis of SMARCA4‐DUT on small tissue samples, especially when rhabdoid morphology is lacking, and demonstrates the potential for misdiagnosis. It also documents how rapidly these tumours can grow in a relatively short time‐span.
The clinical features of a middle‐aged man with a strong smoking history, chronic obstructive pulmonary disease and a large mediastinal‐centred mass on imaging are typical, although non‐specific, for SMARCA4‐DUT. 2 These tumours tend to arise in a younger age group than lung cancer and may be centred in the anterior mediastinum, which can raise suspicion for a lymphoma, mediastinal germ cell tumour, thymic tumour or NUT carcinoma.
On histopathology, the presence of epithelioid cells with clear cytoplasm, in combination with SALL4 expression, may lead to a misdiagnosis of a germ cell tumour such as seminoma, embryonal carcinoma or yolk sac tumour, especially if too little tissue is available for additional, more specific immunohistochemical markers.
One of the most helpful diagnostic clues for SMARCA4‐DUT on microscopy is the finding of rhabdoid morphology, 6 but this feature may be variable and was completely absent in this case. While SALL4 expression may represent a diagnostic pitfall to the unwary, its expression, together with at least focal cytokeratin, EMA, and/or CD34 staining can also be a particularly useful clue to the diagnosis of SMARCA4‐DUT in small tissue samples. 6
SMARCA4‐DUT also has to be distinguished from SMARCA4‐deficient non‐small cell lung cancer and other types of non‐thoracic malignancies. 7 Absent or only focal claudin‐4 expression helps to exclude SMARCA4‐deficient adenocarcinoma 2 while clinico‐radiological correlation is essential to exclude other non‐thoracic SMARCA4‐deficient tumours metastasizing to the thorax.
HepPar1 expression has not previously been reported in SMARCA4‐DUT but has been reported in the setting of SMARCA4‐deficient adenocarcinoma. 8 Its expression may lead to misdiagnosis as metastatic hepatocellular carcinoma.
The histological differential diagnosis with the expected, typical immunohistochemical profile for each entity is summarized in Table 2.
TABLE 2.
Typical expected immunohistochemical expression profiles for selected entities in the differential diagnosis of mediastinal SMARCA4‐DUT.
| Tumour type | Pan‐CK | EMA | CD45 | SOX‐10 | CD30 | SALL4 | CD34 | SOX2 | Claudin‐4 | Other pertinent expressed markers |
|---|---|---|---|---|---|---|---|---|---|---|
| SMARCA4‐DUT | Neg/Focal | Pos | Neg | Neg | Neg | Pos | Pos | Pos | Neg | SMARCB1/INI1 |
| Non‐Hodgkin B‐cell lymphoma | Neg | Neg | Pos | Neg | Neg/Pos | Neg | Neg | Neg | Neg | PAX‐5, CD20 |
| Classic Hodgkin lymphoma | Neg | Neg | Neg | Neg | Pos | Neg | Neg | Neg | Neg | CD15, PAX‐5 (weak) |
| Seminoma | Neg/Pos | Neg | Neg | Neg | Neg | Pos | Neg | Neg | Neg | OCT3/4, CD117, PLAP |
| Embryonal carcinoma | Pos | Neg | Neg | Neg/ Focal | Pos | Pos | Neg | Pos | Neg | OCT3/4 |
| Yolk sac tumour | Pos | Neg | Neg | Neg | Neg | Pos | Neg | Neg | Neg | Glypican 3, alpha‐ fetoprotein |
| SMARCA4‐ deficient adenocarcinoma | Pos | Pos | Neg | Neg | Neg | Neg | Neg | Neg/ Pos | Pos | CK7, HepPar1 |
| Thymic carcinoma | Pos | Pos | Neg | Neg | Neg | Neg | Neg | Pos | Pos | CK5/6, p63, CD5, CD117 |
| NUT carcinoma | Pos | Pos | Neg | Neg | Neg | Neg | Neg/ Pos | Pos | Neg | p63, p40, NUT1 |
| Mesothelioma | Pos | Pos | Neg | Neg | Neg | Neg | Neg | Neg/ Pos | Neg | CK5/6, Calretinin, D2‐40, WT‐1 |
| Metastatic melanoma | Neg | Neg | Neg | Pos | Neg | Neg | Neg | Pos/ Neg | Neg | S100, Melan A, HMB‐45 |
| Metastatic hepatocellular carcinoma | Pos/Neg* | Neg/Pos | Neg | Pos/Neg | Neg | Neg/Pos | Neg** | Pos/Neg | Neg | Arginase1, Glypican 3, HepPar1 |
Abbreviations: CK, cytokeratin; EMA, epithelial membrane antigen; HepPar1, hepatocyte specific antigen; HMB‐45, human melanoma black 45; INI1, integrase interactor 1; Neg, negative; NUT1, nuclear protein in testis 1; OCT3/4, octamer‐binding transcription factor 3/4; PAX‐5, paired box 5; PLAP, placental alkaline phosphatase; Pos, positive; SALL4, Spalt‐like transcription factor 4; SMARCA4‐DUT, SWI/SNF‐related, matrix associated actin dependent regulator of chromatin subfamily A member 4 deficient undifferentiated tumour; SMARCB1, SWI/SNF related, matrix associated actin dependent regulator of chromatin subfamily B member 1; SOX10, sex region Y‐related HMG‐box 10; SOX2, sex region Y‐related HMG‐box 2; WT‐1, Wilms tumour 1.
Depends on the specific cytokeratin clone, usually positive for MNF116 and CAM5.2, negative for AE1/AE3.
Tumour cells negative but positive in endothelial cells lining sinusoids.
In conclusion, the diagnosis of SMARCA4‐DUT remains challenging on limited tissue with many potential pitfalls but awareness of the entity and close correlation of clinical, imaging and pathological findings, along with an appropriate immunohistochemical panel, can lead to the correct diagnosis of this aggressive tumour type.
AUTHOR CONTRIBUTIONS
Deborah Johanna Maartens made the histopathological diagnosis, wrote the first draft and assisted in formatting the presented material. Sibusiso Ndaba and Muhammad Saadiq Moolla were directly involved in the clinical care of this patient, obtained informed consent and provided the clinical information. Sucari Susanna Catherina Vlok provided the radiological images and reviewed the radiological findings. Firzana Hendricks provided information on the oncological decision making and treatment. Coenraad Frederik Nicolaas Koegelenberg reviewed the clinical findings and assisted with editing. Abraham Christoffel van Wyk reviewed the histopathological findings, provided the histopathological images and reviewed the first draft to produce the second draft. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
We would like to thank Mrs Ursula Paulsen for performing the immunohistochemistry and Mr Muneeb Adonis for digitally scanning the histology slides.
Maartens DJ, Moolla MS, Ndaba S, Vlok SSC, Hendricks F, Koegelenberg CFN, et al. Thoracic SMARCA4‐deficient undifferentiated tumour: Diagnostic challenges and potential for misdiagnosis in small tissue samples. Respirology Case Reports. 2023;11:e01238. 10.1002/rcr2.1238
Associate Editor: Sita Andarini
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Le Loarer F, Watson S, Pierron G, De Montpreville VT, Ballet S, Firmin N, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF‐deficient sarcomas. Nat Genet. 2015;47:1200–1205. 10.1038/ng.3399 [DOI] [PubMed] [Google Scholar]
- 2. Yoshida A, Boland JM, Le Loarer F, Jain D, Rekhtman N. Thoracic SMARCA4‐deficient undifferentiated tumour. WHO classification of Tumours editorial board. Thoracic Tumours [Internet]. Volume 5. 5th ed. Lyon (France): International Agency for Research on Cancer. (WHO Classification of Tumours Series ; 2021. https://tumourclassification.iarc [Google Scholar]
- 3. Orlando KA, Nguyen V, Raab JR, Walhart T, Hill C, Hill C, et al. Remodeling the cancer epigenome: mutations in the SWI/SNF complex offer new therapeutic opportunities. Expert Rev Anticancer Ther. 2019;19:375–391. 10.1080/14737140.2019.1605905.Remodeling [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi L, Lin L, Ding Y, Zeng Y, Chen X. Case report: a rapid response to immunotherapy in a thoracic SMARCA4‐deficient undifferentiated tumor with respiratory failure. Front Oncol. 2022;12:1–7. 10.3389/fonc.2022.1020875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crombé A, Alberti N, Villard N, Pilleul F, Buy X, Le Loarer F, et al. Imaging features of SMARCA4‐deficient thoracic sarcomas: a multi‐centric study of 21 patients. Eur Radiol. 2019;29:4730–4741. 10.1007/s00330-019-06017-x [DOI] [PubMed] [Google Scholar]
- 6. Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, et al. SMARCA4‐deficient thoracic sarcomas. Am J Surg Pathol. 2019;43:455–465. 10.1097/PAS.0000000000001188 [DOI] [PubMed] [Google Scholar]
- 7. Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, et al. SMARCA4‐deficient thoracic sarcomatoid tumors represent primarily smoking‐related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15:231–247. 10.1016/j.jtho.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. SMARCA4‐deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar‐1pos immunophenotype. Virchows Arch. 2017;471:599–609. 10.1007/s00428-017-2148-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


