Version Changes
Revised. Amendments from Version 1
-Added the main results in the Abstract -Expanded the Machine Learning Introduction in the extended data -Added Table 1 related to class of stimuli class -Added a Study limitations paragraph
Abstract
Machine learning approaches have been fruitfully applied to several neurophysiological signal classification problems. Considering the relevance of emotion in human cognition and behaviour, an important application of machine learning has been found in the field of emotion identification based on neurophysiological activity. Nonetheless, there is high variability in results in the literature depending on the neuronal activity measurement, the signal features and the classifier type. The present work aims to provide new methodological insight into machine learning applied to emotion identification based on electrophysiological brain activity. For this reason, we analysed previously recorded EEG activity measured while emotional stimuli, high and low arousal (auditory and visual) were provided to a group of healthy participants. Our target signal to classify was the pre-stimulus onset brain activity. Classification performance of three different classifiers (LDA, SVM and kNN) was compared using both spectral and temporal features. Furthermore, we also contrasted the performance of static and dynamic (time evolving) approaches. The best static feature-classifier combination was the SVM with spectral features (51.8%), followed by LDA with spectral features (51.4%) and kNN with temporal features (51%). The best dynamic feature classifier combination was the SVM with temporal features (63.8%), followed by kNN with temporal features (63.70%) and LDA with temporal features (63.68%). The results show a clear increase in classification accuracy with temporal dynamic features.
Keywords: EEG, brain anticipatory activity, machine learning, emotion
Introduction
In last decades, the vision of the brain has moved from a passive stimuli elaborator to an active reality builder. In other words, the brain is able to extract information from the environment, building up inner models of external reality. These models are used to optimize the behavioural outcome when reacting to upcoming stimuli 1– 4 .
One of the main theoretical models assumes that the brain, in order to regulate body reaction, runs an internal model of the body in the world, as described by embodied simulation framework 5 . A much-investigated hypothesis is that the brain functions as a Bayesian filter for incoming sensory input; that is, it activates a sort of prediction based on previous experiences about what to expect from the interaction with the social and natural environment, including emotion 6 . In light of this, it is possible to consider emotions, not only as a reaction to the external world, but also as partially shaped by our internal representation of the environment, which help us to anticipate possible scenarios and therefore to regulate our behaviour.
The construction model of emotion 7 argues that the human being actively builds-up his/her emotions in relation to the everyday life and social context in which they are placed. We actively generate a familiar range of emotions in our reality, based on their usefulness and relevance in our environment. In this scenario, in a familiar context we are able to anticipate which emotions will be probably elicited, depending on our model. As a consequence, the study of the anticipation of/preparation for forthcoming stimuli may represent a precious window for understanding the individual internal model and emotion construction process, resulting in a better understanding of human behaviour.
A strategy to study preparatory activity could be related to the experimental paradigm in which cues are provided regarding the forthcoming stimuli, allowing the investigation of the brain activity dedicated to the elaboration of incoming stimuli 8, 9 . A cue experiment to predict the emotional valence of the forthcoming stimuli showed that the brain’s anticipatory activation facilitates, for example, successful reappraisal via reduced anticipatory prefrontal cognitive elaboration and better integration of affective information in the paralimbic and subcortical systems 10 . Furthermore, preparation for forthcoming emotional stimuli also has relevant implications for clinical psychological conditions, such as mood disorders or anxiety 11, 12 .
Recently, the study of brain anticipatory activity has been extended to statistically unpredictable stimuli 13– 15 , providing experimental hints of specific anticipatory activity before stimuli are randomly presented. Starting from the abovementioned studies, we focused on the extension of brain anticipatory activity to statistically unpredictable emotional stimuli.
According to the so-called dimensional model, emotion can be defined in terms of three different attributes (or dimensions): valence, arousal and dominance. Valence measures the positiveness (ranging from unpleasant to pleasant), arousal measures the activation level (ranging from boredom to frantic excitement) and dominance measures the controllability (i.e. the sense of control) 16 .
Emotions can be estimated from various physiological signals 17, 18 , such as via skin conductance, electrocardiogram (ECG) and electroencephalogram (EEG). The latter has received a considerable amount of attention in the last decade, introducing several machine learning and signal processing techniques, originally developed in other contexts, such as text mining 19 , data processing 20 and brain computer interfaces 21, 22 . Emotion recognition has been re-drawn as a machine learning problem, where proper EEG related features are used as inputs to specific classifiers.
State of the art
According to the literature, the most common features belong the spectral domain, in the form of spectral powers in delta, theta, alpha and gamma bands 23 , as well as power spectral density (PSD) bins 24 . The remaining belong to the time domain, in the form of event-related de/synchronizations (ERD/ERS) and event-related potentials (ERP) 23 , as well as shape related indices such as the Hjorth parameters and the fractal dimension 24 .
The most commonly used classifier is the support vector machine (SVM) with the radial basis function (RBF) kernel, followed by the k-nearest neighbour (kNN) and the linear discriminant analysis (LDA). When compared with neural network (NN) based classfiers (e.g., CNN, MLP-BP, ANN), SVM, kNN and LDA are adopted, complexively, 79.3% (NN 3.17%) 23 , 84% (NN 15.5%) 24 , and 44.4% (NN 5.6%) 25 of the times. Finally, most of the classifiers are implemented as non-adaptive (i.e. static) 23 , in contrast to the dynamic versions that take into account the temporal variability of the features 26 .
The classification performances are very variable because of the different features and classifiers adopted. The following examples are taken from 23 - in particular, from the subset (17 out of 63) of reviewed papers that focused on arousal classification. Using an SVM (RBF kernel) and spectral features (e.g. short-time Fourier transform), Lin and colleagues obtained 94.4% accuracy (i.e. percentage of corrected classification) 27 , while using similar spectral features (e.g. PSD) and classifier (SVM with no kernel), Koelstra and colleagues obtained an accuracy of 55.7% 28 . Liu and Sourina obtained an accuracy of 76.5% using temporal features (e.g. fractal dimension) with an SVM (no kernel) 29 , while Murugappan and Murugappan obtained a an accuracy of 63% using similar temporal features and an SVM with a polynomial kernel 30 . Finally, Thammasan and collegues obtained an accuracy of 85.3% using spectral features (e.g. PSD), but with a kNN (with k=3) 31 .
The purpose of the present work is to provide new methodological advancements on the machine learning classification of emotions, based on the brain anticipatory activity.
Our main research question can be summarised as: what is the best classifier/features combination for the classification of the emotions in the brain anticipatory activity framework? For this purpose, we compared the performances of the tree most common classifiers (namely LDA, SVM, kNN) trained using two types of EEG features (namely, spectral and temporal). In addition to their popularity, the classifiers were selected as representative of 3 different families, namely parametric (LDA), discriminative (SVM) and non-parametric (kNN) classifiers, as well as 2 additional families, namely linear (LDA) and non-linear (SVM, kNN) classifiers. Each classifier was also trained followed a dynamic approach, to take into account the temporal variability of the features.
The results provide useful insights regarding the best classifier features configuration to better discriminate emotion-related brain anticipatory activity.
Within the extended data of the present article 32 we included a document (titled Machine Learning Introduction) briefly describing the classification problem. We are aware that the treatment is far from being fully exhaustive, but we hope it will serve as a comprehensive and self contained starting point for novice readers.
Methods
Ethical statement
The data of the present study were obtained in the experiment described in 33, which was approved by the Ethical Committee of the Department of General Psychology, University of Padova (No. 2278). Before taking part in the experiment, each subject gave his/her informed consent in writing after having read a description of the experiment. In line with department policies, this re-analysis of an original study approved by the ethics committee did not require new ethical approval.
Stimuli and experimental paradigm
In the present study, we reanalysed the EEG data 32 of the experiment described in 33, applying a static and dynamic classification approach by using the three different classifiers and two different feature types.
A more detailed description of the experimental design is available in the original study. Here we describe only the main characteristics.
Two sensory categories of stimuli (i.e. visual and auditory), were extracted according to their arousal value from two standardized international archives. Visual stimuli consisted of pictures of 28 faces, 14 neutral faces and 14 fearful faces were extracted from the NIMSTIM archive 34 , whereas auditory stimuli consisted of 28 sounds, and 14 low- and 14 high-arousal sounds were chosen from the International Affective Digitized Sounds (IADS) archive 35 . The stimuli were labelled as low or high arousal if the corresponding mean arousal score was lower or higher than 5, respectively.
To all 28 adult healthy participants, two different experimental tasks, which were delivered in separate blocks were presented. Within each task, the stimuli were randomly presented and equally distributed according to either sensory category (faces or sounds) and arousal level (high or low). Full details of these tasks have been described previously in 33.
EEG recording
During the entire experiment, the EEG signal was continuously recorded using a Geodesic high density EEG system (EGI GES-300) through a pre-cabled 128-channel HydroCel Geodesic Sensor Net (HCGSN-128) referenced to the vertex (CZ), with a sampling rate of 500 Hz. The impedance was kept below 60kΩ for each sensor. To reduce the presence of EOG artefacts, subjects were instructed to limit both eye blinks and eye movements, as much as possible.
EEG preprocessing
The continuous EEG signal was off-line band-pass filtered (0.1–45Hz) using a Hamming windowed sinc finite impulse response (FIR) filter (order = 16500) and then downsampled at 250 Hz. The EEG was epoched starting from 200 ms before the cue onset and ending at the stimulus onset. The initial epochs were 1300 ms long from the cue onset, including 300 ms of cue/fixation cross presentation and 1000 ms of interstimulus interval (ISI).
All epochs were visually inspected to remove bad channels and rare artefacts. Artefact reduced data were then subjected to independent component analysis (ICA) 35 . All independent components were visually inspected, and those that related to eye blinks, eye movements, and muscle artefacts, according to their morphology and scalp distribution, were discarded. The remaining components were back-projected to the original electrode space to obtain cleaner EEG epochs.
The remaining ICA-cleaned epochs that still contained excessive noise or drift (±100 μV at any electrode) were rejected and the removed bad channels were reconstructed. Data were then re-referenced to the common average reference (CAR) and the epochs were baseline corrected by subtracting the mean signal amplitude in the pre-stimulus interval. From the original 1300 ms long epochs, final epochs were obtained only from the 1000 ms long ISI.
Spectral features for static classification
From each epoch and each channel k, the PSD was estimated by a Welch’s periodogram using 250 points long Hamming’s windows with 50% overlapping. PSD was first log transformed to compensate the skewness of power values 36 , then the spectral bins corresponding to alpha, beta and theta bands – defined as 6~13 Hz, 13~30 Hz and 4~6 Hz, respectively 37 – were summed together. Finally, alpha, beta and theta total powers were computed as:
As a measure of emotional arousal, we computed the ratio between beta and alpha total powers 38 , while to measure cognitive arousal, we computed the ratio between beta and theta total powers 39 .
For each epoch, the feature (with a dimensionality of 256) was obtained, concatenating the beta-over-alpha and beta-over-theta ratio of all the channels:
Temporal features for static classification
It has been previously shown that arousal level (high or low) can be estimated from the contingent negative variation potentials 33 . The feature extraction procedure, therefore, follows the classical approach for event-related potentials 40 . Each epoch from each channel was first band pass filtered (0.05~10 Hz) using a zero-phase 2 nd order Butterworth filter and decimated to a sample frequency of 20 Hz. EEG signal was thus normalized (i.e. z-scored) according to the temporal mean and the temporal standard deviation:
where is the raw signal from i-th channel at time point t k , and m i and s i are, respectively, the temporal mean and the temporal standard deviation of the i-th channel. For each epoch, the feature (with a dimensionality of 2560) was obtained, concatenating all normalized signal from each channel:
Dynamic approach
Each epoch was partitioned into 125 temporal segments, 500 ms long and shifted by 1/250 s (one sample). Within each time segment, we extracted the spectral and temporal features for dynamic classification, following the same approaches described in Spectral features for static classification and Temporall features for static classification sub-sections, respectively. Temporal features for dynamic classification had a dimensionality of 1280, corresponding to 0.5 × 20 = 10 samples per channel. Spectral features for dynamic classification had the same dimensionality as their static counterparts (256), but the Welch’s periodogram was computed using a 16 points long Hamming’s window (zero-padded to 250 points) with 50% overlapping.
Feature reduction and classification
The extracted features (for both the static and dynamic approaches) were grouped according to the stimulus type (sound or image) and the task (active or passive), in order to classify the group-related arousal level (high or low). A total of four binary classification problems (high arousal vs low arousal) were performed: active image (Ac_Im), active sound (Ac_So), passive image (Ps_Im) and passive sound (Ps_So). For each classification problem, the two classes were approximatively balanced, as shown in the following Table 1. We chose a binary classification since it is associated to lower computational costs than the multiclass alternatives, that are usually obtained by a cascade of binary classifications (see Machine Learning Introduction within the Extended data 32 ).
Table 1. Class distribution.
Distribution of the two classes (High arousal and Low arousal) for each classification problem.
| Classification problem | # High arousal (%) | #Low arousal (%) |
|---|---|---|
| Ac_Im | 1294 (51%) | 1243 (49%) |
| Ac_So | 1223 (49%) | 1270 (51%) |
| Ps_Im | 1215 (48%) | 1305 (52%) |
| Ps_So | 1279 (49%) | 1302 (51%) |
Features for static classification were reduced by means of the biserial correlation coefficient r 2 with the threshold set at 90% of the total feature score. In order to identify the discriminative power of each EEG channel, a series of scalp plots (one for each feature type and each group) of the coefficients were drawn. Since each channel is associated with N > 1 features (as well as N r 2 coefficients), the coefficients (one coefficient for each channel) are calculated as a mean value. In other words, spectral and temporal features had two and 20 scalar features, respectively, for each EEG channel. To compute their scalp plots, we averaged 2 and 20 r 2 coefficients of each channel. To enhance the visualization of the plots, the coefficients were finally normalized to the total score and expressed as a percentage.
Each classification problem was addressed by the mean of three classifiers: LDA with pseudo-inverse covariance matrix; soft-margin SVM with penalty parameter C = 1 and RBF kernel; and kNN with Euclidean distance and k=1. Additionally, a random classifier, giving a uniform pseudo-random class (Pr{HA} = Pr{LA} = 0.5), served as a benchmark 41 . Since we were interested in the overall correct classification without distinguishing between the different classes, the accuracy was selected as evaluation metric 42 . As long as the classes are approximatively balanced, the accuracy can be considered a straightforward and robust metric to assess the classifier performance (see ML Introduction in the Extended data 32 ).
The accuracy of the classifiers was measured, repeating 10 times for a 10 fold cross validation scheme. The feature selection was computed within each cross validation step, to avoid overfitting and reduce biased results 43 .
For each group (Ac_Im, Ac_So, Ps_Im, Ps_So) and each feature type (static spectral, static temporal), the classification produced a 10 × 4 matrix containing the mean accuracies (one for each of the 10-fold cross-validation repetitions) of each classifier.
Features for dynamic classification were reduced and classified similarly to the static ones. For each temporal segment, the associated features were reduced by means of the biserial correlation coefficient (threshold at 90%) and the classifiers (SVM, kNN, LDA and random) were evaluated using a 10-fold cross-validation scheme – repeated 10 times.
For each group, each feature type (dynamic spectral, dynamic temporal), each temporal segment and each classifier, the classification produced 10 sequences of mean accuracies – one for each repetition of the 10-fold cross-validation scheme.
Data analysis
The syntax in MATLAB used for all analyses is available on GitHub along with the instructions on how to use it (see Software availability) 44 . The software can also be used with the open-source program Octave.
Statistical analysis
The results of the static classifications were compared against the benchmark classifier by means of a two-sample t-test (right tail).
The results of dynamic classifications were compared following a segment-by-segment approach. For each group, the accuracy sequences of the dynamic classifiers (SVM, kNN and LDA) were compared with the benchmark accuracy sequence. Each sample , with k = {SVM, kNN, LDA}, was tested against by means of two-sample t-tests (right tail). The corresponding p-value sequences were Bonferroni-Holm corrected for multiple comparisons. Finally, the best accuracy point was detected as the left extreme of the temporal window corresponding to the highest significant accuracy.
Results
Static approach
In Figure 1 and Figure 2, the scalp distributions of r 2 coefficients for each binary static classification problem, grouped for feature (spectral, temporal) and groups (Ps_Im, Ps_So, Ac_Im, Ac_So), are shown.
Figure 1. Spectral features.

Scalp distribution of the r 2 coefficients (normalized to the total score and expressed as percentage) grouped for tasks and stimulus type. ( a) Active task: left Image, right Sound; ( b) Passive task: left Image, right Sound.
Figure 2. Temporal features.

Scalp distribution of the r 2 coefficients (normalized to the total score and expressed as percentage), grouped for tasks and stimulus type. ( a) Active task: left Image, right Sound; ( b) Passive task: left Image, right Sound.
The temporal feature gave the most consistent topographical pattern, showing that the regions that best differentiate between high vs low stimuli (auditory and visual) were located over the central-parietal electrodes, whereas a more diffuse pattern in the scalp topography emerged for the spectral features.
In Figure 3 and Figure 4, box plots of the accuracies of static temporal and spectral classifications, grouped for condition, are shown. Note that SVM accuracies (the 2 nd boxplot from the left) are always shown as lines because the accuracies were constant within each cross-validation step (see also Table 2, Table 3 and Table 4).
Figure 3. Box-plots of the accuracies of the static spectral classifications.
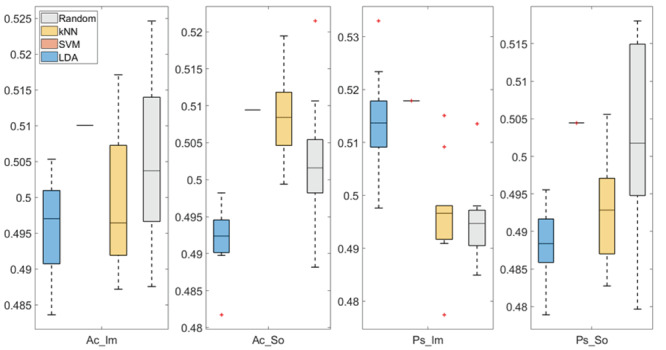
From left: Active Image (Ac_Im), Active Sound (Ac_So), Passive Image (Ps_Im) and Passive Sound (Ps_So).
Figure 4. Box-plots of the accuracies of the static temporal classifications.

From left: Active Image (Ac_Im), Active Sound (Ac_So), Passive Image (Ps_Im) and Passive Sound (Ps_So).
Table 2. Static features.
Ordered accuracies grouped for classifier, feature and group.
| Classifier | Accuracy | Feature | Group |
|---|---|---|---|
| SVM | 51.80% | Spectral | Ps_Im |
| LDA | 51.40% | Spectral | Ps_Im |
| kNN | 51% | Temporal | Ac_So |
| kNN | 50.90% | Spectral | Ac_So |
| SVM | 50.90% | Spectral | Ac_So |
| SVM | 50.90% | Temporal | Ac_So |
| SVM | 50.40% | Temporal | Ps_So |
SVM, support vector machine; LDA, linear discriminant analysis; kNN, k-nearest neighbour.
Table 3. Mean (M) and standard deviations (SD) of the accuracies of the static spectral classifications.
Active Image (Ac_Im), Active Sound (Ac_So), Passive Image (Ps_Im) and Passive Sound (Ps_So).
| Group | LDA | SVM | kNN | Random |
|---|---|---|---|---|
| Ac_Im | M=0.496, SD=0.007 | M=0.510, SD=0.000 | M=0.500, SD=0.010 | M=0.505, SD=0.011 |
| Ac_So | M=0.492, SD=0.004 | M=0.509, SD=0.000 | M=0.509, SD=0.007 | M=0.503, SD=0.009 |
| Ps_Im | M=0.514, SD=0.010 | M=0.518, SD=0.000 | M=0.496, SD=0.010 | M=0.495, SD=0.008 |
| Ps_So | M=0.488, SD=0.005 | M=0.504, SD=0.000 | M=0.493, SD=0.007 | M=0.503, SD=0.013 |
SVM, support vector machine; LDA, linear discriminant analysis; kNN, k-nearest neighbour.
Table 4. Mean (M) and standard deviations (SD) of the accuracies of the static temporal classifications.
Active Image (Ac_Im), Active Sound (Ac_So), Passive Image (Ps_Im) and Passive Sound (Ps_So).
| Group | LDA | SVM | kNN | Random |
|---|---|---|---|---|
| Ac_Im | M=0.492, SD=0.010 | M=0.510, SD=0.000 | M=0.500, SD=0.008 | M=0.498, SD=0.007 |
| Ac_So | M=0.501, SD=0.007 | M=0.509, SD=0.000 | M=0.510, SD=0.006 | M=0.498, SD=0.012 |
| Ps_Im | M=0.500, SD=0.012 | M=0.518, SD=0.000 | M=0.492, SD=0.005 | M=0.499, SD=0.006 |
| Ps_So | M=0.499, SD=0.008 | M=0.504, SD=0.000 | M=0.492, SD=0.006 | M=0.498, SD=0.008 |
SVM, support vector machine; LDA, linear discriminant analysis; kNN, k-nearest neighbour.
Note that all the accuracies refer to the same static classification problem (high arousal vs low arousal), performed using different classifiers (SVM, LDA, kNN) and features (spectral, temporal), on different groups (Ps_Im, Ps_So, Ac_Im, Ac_So).
Using spectral features, in only two groups some classifiers showed an accuracy greater than the benchmark. In the Ac_So group, ACC SVM = 50.9% (t(18)=2.371, p=0.015) and ACC kNN = 50.9% (t(18)=1.828, p=0.042), while for Ps_Im, ACC LDA = 51.4% (t(18)=4.667, p<0.001) and ACC SVM = 51.8% (t(18)=9.513, p<0.001).
Using temporal features, in all the groups some classifiers showed an accuracy greater than the benchmark. In the Ac_So group, ACC SVM = 50.9% (t(18)=2.907, p=0.005) and ACC kNN = 51% (t(18)=2.793, p=0.006) and in the Ps_So group, AAC SVM = 50.4% (t(18)=9.493, p<0.001).
Dynamic approach
In Figure 5– Figure 11, the results of the significant dynamic classifications are shown. In the upper section of the plots, the mean (bold line) and the standard deviation (shaded) of the accuracy sequence are shown. In the lower section of the plot (black dashed line), the Bonferroni-Holm corrected p-values sequence, discretized (as a stair graph) as significant (p<0.05) or non-significant (p>0.05) is shown.
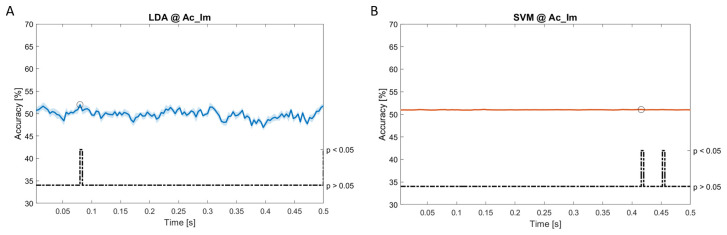
Figure 5. Spectral dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ac_Im group for LDA ( a) and SVM ( b) classifiers.
Figure 11. Temporal dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ps_So group for LDA ( a) and kNN ( b) classifiers.
Note that all the accuracy plots refer to the same dynamic classification problem (high arousal vs low arousal), performed using different classifiers (SVM, LDA, kNN) and features on different groups. Spectral: Ac_Im ( Figure 5), Ac_So ( Figure 6), Ps_Im ( Figure 7) and Ps_So ( Figure 8); temporal: Ac_So ( Figure 9), Ps_Im ( Figure 10) and Ps_So ( Figure 11).
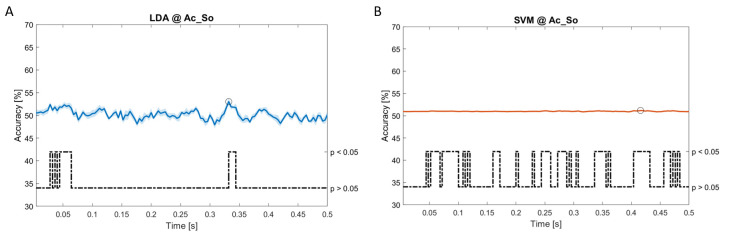
Figure 6. Spectral dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ac_So group for LDA ( a) and SVM ( b) classifiers.
Figure 7. Spectral dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ps_Im group for LDA ( a) and SVM ( b) classifiers.
Figure 8. Spectral dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ac_So group for SVM ( a) and kNN ( b) classifiers.
Figure 9. Temporal dynamic features.

Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ac_So group for LDA classifier.
Figure 10. Temporal dynamic features.
Accuracy (mean value, coloured line; standard deviation, shaded line) and p-values (black dotted line) in Ps_Im group for LDA ( a), SVM ( b) and kNN ( c) classifiers.
Using spectral features, in all the groups some classifiers showed an accuracy greater than the benchmark. In the Ac_Im group, ACC LDA = 51.97% @ t = 0.080 s (t(18)=6.291, p<0.001) and ACC SVM = 51.07% @ t = 0.416 s (t(18)=6.531, p<0.001). In the Ac_So group, ACC LDA = 53.04% @ t = 0.332 s (t(18)=8.583, p<0.001) and ACC SVM = 51.16% @ t = 0.146 s (t(18)=8.612, p<0.001). In the Ps_Im group, ACC LDA = 53.12% @ t = 0.156 s (t(18)=6.372, p=0.000) and ACC SVM = 51.83% @ t = 0.140 s (t(18)=6.668, p<0.001). In the Ps_So group, ACC SVM = 50.62% @ t = 0.024 s (t(18)=5.236, p=0.003) and ACC kNN = 51.41% @ t = 0.476 s (t(18)=4.307, p=0.026).
Using temporal features, in only three groups did some classifiers show an accuracy greater than the benchmark. In the Ac_So group, ACC SVM = 63.80% @ t = 0.100 s (t(18)=6.113, p=0.001). In the Ps_Im group, ACC LDA = 63.68% @ t = 0.024 s (t(18)=12.108, p<0.001) and ACC SVM = 51.43% @ t = 0.084 s (t(18)=4.881, p=0.008). In the Ps_So group, ACC LDA = 64.30% @ t = 0.0276 s (t(18)=11.092, p<0.001) and ACC kNN = 63.70% @ t = 0.480 s (t(18)=16.621, p<0.001).
Table 5 reports the accuracies for dynamic features, ordered in descending order and grouped for classifier, feature group and time.
Table 5. Dynamic features.
Ordered accuracies grouped for classifier, feature and group.
| Classifier | Accuracy | Time [s] | Group | Feature |
|---|---|---|---|---|
| SVM | 63.80% | 0.1 | Ac_So | Temporal |
| kNN | 63.70% | 0.048 | Ps_So | Temporal |
| LDA | 63.68% | 0.024 | Ps_Im | Temporal |
| LDA | 63.30% | 0.0276 | Ps_So | Temporal |
| LDA | 53.12% | 0.156 | Ps_Im | Spectral |
| LDA | 53.04% | 0.3332 | Ac_So | Spectral |
| LDA | 51.97% | 0.08 | Ac_Im | Spectral |
| SVM | 51.83% | 0.14 | Ps_Im | Spectral |
| SVM | 51.43% | 0.084 | Ps_Im | Temporal |
| kNN | 51.41% | 0.476 | Ps_So | Spectral |
| SVM | 51.16% | 0.146 | Ac_So | Spectral |
| SVM | 51.07% | 0.416 | Ac_Im | Spectral |
| SVM | 50.62% | 0.024 | Ps_So | Spectral |
SVM, support vector machine; LDA, linear discriminant analysis; kNN, k-nearest neighbour.
Discussion
The aim of the study was to provide new methodological insights regarding machine learning approaches for the classification of anticipatory emotion-related EEG signals, by testing the performance of different classifiers on different features.
From the ISIs (i.e. the 1000 ms long window preceding each stimulus onset), we extracted two kinds of “static” features, namely spectral and temporal, the most commonly used features in the field of emotion recognition 23, 24 . As spectral features, we used the beta-over-alpha and the beta-over-theta ratio, whereas for the temporal feature we concatenated the decimated EEG values.
Additionally, we extracted the temporal sequences of both static spectral and temporal features, using a 500 ms long window moving along the ISI to build dynamic spectral and temporal features, respectively. This step is crucial for our work since, considering the temporal resolution of the EEG, an efficient classification should take into account the temporal dimension, to provide information about when the difference between two conditions are maximally expressed and therefore classified.
We trained and tested three different classifiers (LDA, SVM, kNN, the most commonly used in the field of emotion recognition 23, 24 ) following both static and dynamic approaches, comparing their accuracies against a random classifier that served as benchmark.
Our goal was to identify the best combination of approach (static vs dynamic), classifier (LDA vs SVM vs kNN) and feature (spectral vs temporal) to classify the arousal level (high vs low) of 56 auditory/visual stimuli. The stimuli, extracted from two standardized datasets (NIMSTIM 45 and IADS 34 ), for visual and auditory stimuli, respectively) were presented in a randomized order, triggered by a TrueRNG™ hardware random number generator.
Considering the number of groups (four), the number of classifiers (three) and the number of feature types (two), each classification (static or dynamic) produced a total of 24 accuracies, whose significances were statistically tested (using a two-sample t-test and the benchmark’s accuracies).
Within the nine significant accuracies obtained using a static approach, the classifier that obtained the highest number of accuracies was the SVM (six significant accuracies), followed by kNN (two significant accuracies) and LDA (one significant accuracy). The most frequent feature was the temporal (five significant accuracies). Finally, the best (static) feature-classifier combination was the SVM with spectral features (51.8%), followed by LDA with spectral features (51.4%) and kNN with temporal features (51%).
Within the 13 significant accuracies obtained using a dynamic approach, the classifier that obtained the highest number of accuracies was the SVM (six significant accuracies), followed by LDA (four significant accuracies) and kNN (three significant accuracies). The most frequent feature was the spectral (eight significant accuracies). Finally, the best (dynamic) feature-classifier combination was the SVM with temporal features (63.8%), followed by kNN with temporal features (63.70%) and LDA with temporal features (63.68%). Spectral features produced only the 5th highest accuracy (53.12% with LDA). The three best accuracies were all within the first 100ms of the ISI, although a non-significant Spearman’s correlation between accuracy and time was observed (r=-0.308, p=0.306).
Table 6, summarises the three best (in terms of accuracy) classifier/features combinations for both the static and dynamic approaches.
Table 6. Best classifier/features combinations for static and dynamic approach.
| Static classification | Dynamic classification | ||||
|---|---|---|---|---|---|
| Classifier | Classifier | Classifier | Classifier | Features | Accuracy |
| SVM | SVM | SVM | SVM | Temporal | 63.87% |
| LDA | LDA | LDA | kNN | Temporal | 63.70% |
| kNN | kNN | kNN | LDA | Temporal | 53.12% |
Our results show that globally the SVM presents the best accuracy, independent from feature type (temporal or spectral). This is in line with previous studies were SVM outperformed other classifiers such as NN and Random Forests 46 . More importantly, the combination of SVM with the dynamic temporal feature produced the best classification performance. This finding is particularly relevant, considering the application of EEG in cognitive science. In fact, due to its high temporal resolution, EEG is often applied to investigate the timing of neural processes in relation to behavioural performance.
Our results therefore suggest that, in order to best classify emotions based on electrophysiological brain activity, the temporal dynamic of the EEG signal should be taken into account with a dynamic classifier. In fact, by including also time evolution of the feature in the machine learning model, it is possible to infer when two different conditions maximally diverge, allowing possible interpretation of the timing of the cognitive processes and the behaviour of the underlying neural substrate.
Finally, the main contribution of our results for the scientific community is that they provide a methodological advancement that is generally valid both for the investigation of emotion based on a machine learning approach with EEG signals and also for the investigation of preparatory brain activity.
Study limitations
Nevertheless, the present study presents some limitations.
Despite being comparable with previous studies, the obtained accuracy is lower than those obtained with more complex classifiers, such as those based on Convolutional Neural Networks (CNNs). For example, feeding temporal features into a CNN classifier, some authors reported accuracies up to 86.5% 47 , while others reported accuracies up 98.9% by training a CNN classifier with spectral features 48 .
Additionally, the evaluation of the proposed classifiers is based on the accuracy, that can be still considered robust because of the class balance within each dataset. However, by considering other metrics (such as the MCC, the F-score, the Cohen’s kappa) or analysing the confusion matrices, the misclassifications could be more deeply analysed and, therefore, the classifiers could be more effectively tuned to an optimal point.
Finally, the discretization of the stimuli into two classes only (low and high) instead of multiple ones (e.g., low, medium, high) lowered the training/test computational costs but could represent a sub-optimal solution in terms of classification accuracies. By adding multiple classes, that is by discretizing the stimuli into finer arousal levels, the accuracy could be boosted, at the cost of lowering the number of available instances per class and increasing the chance of overfitting the models.
Data availability
Underlying data
Figshare: EEG anticipation of random high and low arousal faces and sounds. https://doi.org/10.6084/m9.figshare.6874871.v8 32
This project contains the following underlying data:
-
-
EEG metafile (DOCX)
-
-
EEG data related to the Passive, Active and Predictive conditions (CSV)
-
-
Video clips of the EEG activity before stimulus presentation (MPG)
Extended data
Figshare: EEG anticipation of random high and low arousal faces and sounds. https://doi.org/10.6084/m9.figshare.6874871.v8 32
This project contains the following extended data:
-
-
Detailed description of LDA, SVM and kNN machine learning algorithms (DOCX)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Software availability
Source code available from: https://github.com/mbilucaglia/ML_BAA
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3666045 44
License: GPL-3.0
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved with reservations]
References
- 1. Friston K: A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nobre AC: Orienting attention to instants in time. Neuropsychologia. 2001;39(12):1317–1328. 10.1016/s0028-3932(01)00120-8 [DOI] [PubMed] [Google Scholar]
- 3. Mento G, Vallesi A: Spatiotemporally dissociable neural signatures for generating and updating expectation over time in children: A High Density-ERP study. Dev Cogn Neurosci. 2016;19:98–106. 10.1016/j.dcn.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mento G, Tarantino V, Vallesi A, et al. : Spatiotemporal neurodynamics underlying internally and externally driven temporal prediction: A high spatial resolution ERP study. J Cogn Neurosci. 2015;27(3):425–439. 10.1162/jocn_a_00715 [DOI] [PubMed] [Google Scholar]
- 5. Barsalou LW: Grounded Cognition. Annu Rev Psychol. 2008;59:617–645. 10.1146/annurev.psych.59.103006.093639 [DOI] [PubMed] [Google Scholar]
- 6. Barrett LF: The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 2017;12(11):1833. 10.1093/scan/nsx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruner JS: Acts of meaning. Harvard University Press,1990. Reference Source [Google Scholar]
- 8. Miniussi C, Wilding EL, Coull JT, et al. : Orienting attention in time. Modulation of brain potentials. Brain. 1999;122(Pt 8):1507–1518. 10.1093/brain/122.8.1507 [DOI] [PubMed] [Google Scholar]
- 9. Stefanics G, Hangya B, Hernádi I, et al. : Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci. 2010;30(41):13578–13585. 10.1523/JNEUROSCI.0703-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denny BT, Ochsner KN, Weber J, et al. : Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Soc Cogn Affect Neurosci. 2014;9(4):403–411. 10.1093/scan/nss148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abler B, Erk S, Herwig U, et al. : Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;41(6):511–522. 10.1016/j.jpsychires.2006.07.020 [DOI] [PubMed] [Google Scholar]
- 12. Morinaga K, Akiyoshi J, Matsushita H, et al. : Anticipatory anxiety-induced changes in human lateral prefrontal cortex activity. Biol Psychol. 2007;74(1):34–38. 10.1016/j.biopsycho.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 13. Duma GM, Mento G, Manari T, et al. : Driving with Intuition: A Preregistered Study about the EEG Anticipation of Simulated Random Car Accidents. PLoS One. 2017;12(1):e0170370. 10.1371/journal.pone.0170370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radin DI, Vieten C, Michel L, et al. : Electrocortical activity prior to unpredictable stimuli in meditators and nonmeditators. Explore (NY). 2011;7(5):286–299. 10.1016/j.explore.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 15. Mossbridge JA, Tressoldi P, Utts J, et al. : Predicting the unpredictable: critical analysis and practical implications of predictive anticipatory activity. Front Hum Neurosci. 2014;8:146. 10.3389/fnhum.2014.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunes H, Pantic M: Automatic, Dimensional and Continuous Emotion Recognition. Int J Synth Emot. 2010;1(1):32. 10.4018/jse.2010101605 [DOI] [Google Scholar]
- 17. Shu L, Xie J, Yang M, et al. : A Review of Emotion Recognition Using Physiological Signals. Sensors (Basel). 2018;18(7): pii: E2074. 10.3390/s18072074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cimtay Y, Ekmekcioglu E, Caglar-Ozhan S: Cross-subject multimodal emotion recognition based on hybrid fusion. IEEE Access. 2020;8:168865–168878. 10.1109/ACCESS.2020.3023871 [DOI] [Google Scholar]
- 19. Halim Z, Waqar M, Tahir M: A machine learning-based investigation utilizing the in-text features for the identification of dominant emotion in an email. Knowledge-Based Systems. 2020;208:106443. 10.1016/j.knosys.2020.106443 [DOI] [Google Scholar]
- 20. Cimtay Y, Ilk HG: A novel derivative-based classification method for hyperspectral data processing. Advances in Electrical and Electronic Engineering. 2017;15(4):657–662. 10.15598/aeee.v15i4.2381 [DOI] [Google Scholar]
- 21. Calvo RA, D’Mello S: Affect detection: An interdisciplinary review of models, methods, and their applications. IEEE Trans Affect Comput. 2010;1(1):18–37. 10.1109/T-AFFC.2010.1 [DOI] [Google Scholar]
- 22. Ullah S, Halim Z: Imagined character recognition through EEG signals using deep convolutional neural network. Med Biol Eng Comput. 2021;59(5):1167–1183. 10.1007/s11517-021-02368-0 [DOI] [PubMed] [Google Scholar]
- 23. Alarcao SM, Fonseca MJ: Emotions Recognition Using EEG Signals: A Survey. IEEE Trans Affect Comput. 2017;3045:1–20. 10.1109/TAFFC.2017.2714671 [DOI] [Google Scholar]
- 24. Al-Nafjan A, Hosny M, Al-Ohali Y, et al. : Review and Classification of Emotion Recognition Based on EEG Brain-Computer Interface System Research: A Systematic Review. Appl Sci. 2017;7(12):1239. 10.3390/app7121239 [DOI] [Google Scholar]
- 25. Suhaimi NS, Mountstephens J, Teo J: EEG-based emotion recognition: A state-of-the-art review of current trends and opportunities. Comput Intell Neurosci. 2020;2020:8875426. 10.1155/2020/8875426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lotte F, Congedo M, Lécuyer A, et al. : A review of classification algorithms for EEG-based brain-computer interfaces. J Neural Eng. 2007;4(2):R1–R13. 10.1088/1741-2560/4/2/R01 [DOI] [PubMed] [Google Scholar]
- 27. Lin YP, Wang CH, Wu TL, et al. : EEG-based emotion recognition in music listening: A comparison of schemes for multiclass support vector machine.In: Proceedings of the ICASSP, IEEE International Conference on Acoustics, Speech and Signal Processing - Proceedings.2009. 10.1109/ICASSP.2009.4959627 [DOI] [Google Scholar]
- 28. Koelstra S, Yazdani A, Soleymani M, et al. : Single trial classification of EEG and peripheral physiological signals for recognition of emotions induced by music videos.In: Proceedings of the Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics).2010. 10.1007/978-3-642-15314-3_9 [DOI] [Google Scholar]
- 29. Liu Y, Sourina O: EEG-based valence level recognition for real-time applications.In: Proceedings of the Proceedings of the 2012 International Conference on Cyberworlds, Cyberworlds.2012;2012. 10.1109/CW.2012.15 [DOI] [Google Scholar]
- 30. Murugappan M, Murugappan S: Human emotion recognition through short time Electroencephalogram (EEG) signals using Fast Fourier Transform (FFT).In: Proceedings of the Proceedings - 2013 IEEE 9th International Colloquium on Signal Processing and its Applications, CSPA.2013;2013. 10.1109/CSPA.2013.6530058 [DOI] [Google Scholar]
- 31. Thammasan N, Fukui KI, Numao M: Application of deep belief networks in EEG-based dynamic music-emotion recognition.In: Proceedings of the Proceedings of the International Joint Conference on Neural Networks.2016. 10.1109/IJCNN.2016.7727292 [DOI] [Google Scholar]
- 32. Tressoldi P, Duma GM, Mento G: EEG anticipation of random high and low arousal faces and sounds. figshare. 2018; Dataset. 10.6084/m9.figshare.6874871.v8 [DOI] [Google Scholar]
- 33. Duma GM, Mento G, Semenzato L, et al. : EEG anticipation of random high and low arousal faces and sounds [version 2; peer review: 1 approved, 1 not approved]. F1000Research. 2019;8(1):1508. 10.12688/f1000research.20277.2 [DOI] [Google Scholar]
- 34. Stevenson RA, James TW: Affective auditory stimuli: characterization of the International Affective Digitized Sounds (IADS) by discrete emotional categories. Behav Res Methods. 2008;40(1):315–21. 10.3758/brm.40.1.315 [DOI] [PubMed] [Google Scholar]
- 35. Stone JV: Independent component analysis: an introduction. Trends Cogn Sci. 2002;6(2):59–64. 10.1016/s1364-6613(00)01813-1 [DOI] [PubMed] [Google Scholar]
- 36. Allen JJ, Coan JA, Nazarian M: Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol Psychol. 2004;67(1–2):183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 37. Babiloni C, Stella G, Buffo P, et al. : Cortical sources of resting state EEG rhythms are abnormal in dyslexic children. Clin Neurophysiol. 2012;123(12):2384–2391. 10.1016/j.clinph.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 38. Mert A, Akan A: Emotion recognition from EEG signals by using multivariate empirical mode decomposition. Pattern Anal Appl. 2018;21:81–89. 10.1007/s10044-016-0567-6 [DOI] [Google Scholar]
- 39. Clarke AR, Barry RJ, Karamacoska D, et al. : The EEG Theta/Beta Ratio: A marker of Arousal or Cognitive Processing Capacity? Appl Psychophysiol Biofeedback. 2019;44(2):123–129. 10.1007/s10484-018-09428-6 [DOI] [PubMed] [Google Scholar]
- 40. Blankertz B, Lemm S, Treder M, et al. : Single-trial analysis and classification of ERP components - A tutorial. Neuroimage. 2011;56(2):814–825. 10.1016/j.neuroimage.2010.06.048 [DOI] [PubMed] [Google Scholar]
- 41. Bilucaglia M, Pederzoli L, Giroldini W, et al. : EEG correlation at a distance: A re-analysis of two studies using a machine learning approach [version 2; peer review: 2 approved]. F1000Res. 2019;8:43. 10.12688/f1000research.17613.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sokolova M, Japkowicz N, Szpakowicz S: Beyond accuracy, F-score and ROC: a family of discriminant measures for performance evaluation. In Australasian joint conference on artificial intelligence. 2006;1015–1021. 10.1007/11941439_114 [DOI] [Google Scholar]
- 43. Müller K, Krauledat M, Dornhege G, et al. : Machine learning techniques for brain-computer interfaces. Biomed Tech (Biomed Tech). 2004.49:11–22. 10.13109/9783666351419.11 [DOI] [Google Scholar]
- 44. Marco B: BAA - Matlab Code (Version 1). Zenodo. 2020. 10.5281/zenodo.3666045 [DOI] [Google Scholar]
- 45. Tottenham N, Tanaka JW, Leon AC, et al. : The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halim Z, Rehan M: On identification of driving-induced stress using electroencephalogram signals: A framework based on wearable safety-critical scheme and machine learning. Information Fusion. 2020;53:66–79. 10.1016/j.inffus.2019.06.006 [DOI] [Google Scholar]
- 47. Cimtay Y, Ekmekcioglu E: Investigating the use of pretrained convolutional neural network on cross-subject and cross-dataset EEG emotion recognition. Sensors (Basel). 2020;20(7):2034. 10.3390/s20072034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maheshwari D, Ghosh SK, Tripathy RK, et al. : Automated accurate emotion recognition system using rhythm-specific deep convolutional neural network technique with multi-channel EEG signals. Comput Biol Med. 2021;134:104428. 10.1016/j.compbiomed.2021.104428 [DOI] [PubMed] [Google Scholar]