Abstract
Since the first approval for immune checkpoint inhibitors (ICIs) for the treatment of cutaneous melanoma more than a decade ago, immunotherapy has completely transformed the treatment landscape of this chemotherapy-resistant disease. Combination regimens including ICIs directed against programmed cell death protein 1 (PD-1) with anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) agents or, more recently, anti-lymphocyte-activation gene 3 (LAG-3) agents, have gained regulatory approvals for the treatment of metastatic cutaneous melanoma, with long-term follow-up data suggesting the possibility of cure for some patients with advanced disease. In the resectable setting, adjuvant ICIs prolong recurrence-free survival, and neoadjuvant strategies are an active area of investigation. Other immunotherapy strategies, such as oncolytic virotherapy for injectable cutaneous melanoma and bispecific T-cell engager therapy for HLA-A*02:01 genotype-positive uveal melanoma, are also available to patients. Despite the remarkable efficacy of these regimens for many patients with cutaneous melanoma, traditional immunotherapy biomarkers (ie, programmed death-ligand 1 expression, tumor mutational burden, T-cell infiltrate and/or microsatellite stability) have failed to reliably predict response. Furthermore, ICIs are associated with unique toxicity profiles, particularly for the highly active combination of anti-PD-1 plus anti-CTLA-4 agents. The Society for Immunotherapy of Cancer (SITC) convened a panel of experts to develop this clinical practice guideline on immunotherapy for the treatment of melanoma, including rare subtypes of the disease (eg, uveal, mucosal), with the goal of improving patient care by providing guidance to the oncology community. Drawing from published data and clinical experience, the Expert Panel developed evidence- and consensus-based recommendations for healthcare professionals using immunotherapy to treat melanoma, with topics including therapy selection in the advanced and perioperative settings, intratumoral immunotherapy, when to use immunotherapy for patients with BRAFV600-mutated disease, management of patients with brain metastases, evaluation of treatment response, special patient populations, patient education, quality of life, and survivorship, among others.
Keywords: guidelines as topic, melanoma
Introduction
Immunotherapy has dramatically transformed the management and prognosis of cutaneous melanoma. Prior to the first United States Food and Drug Administration (US FDA) approval for high-dose interleukin-2 (HD IL-2) to treat metastatic melanoma in 1998, standard of care systemic treatment with chemotherapy offered only a short-term survival benefit to few patients with advanced disease,1 2 and many patients with resectable disease relapsed following surgery.3 Although HD IL-2 offered a small minority of patients long-term survival,4 the toxicity and logistics associated with its administration limited availability to a few specialized centers. The advent of immune checkpoint inhibitors (ICIs), which can be administered in the outpatient setting, led to clinically significant improvements in overall survival (OS) for patients with advanced cutaneous melanoma.5
In the contemporary era, approved frontline ICI regimens for melanoma include agents targeting programmed cell death protein 1 (PD-1) or its ligand (PD-L1) (ie, nivolumab, pembrolizumab, and atezolizumab) as a backbone. Combination regimens targeting additional checkpoints such as cytotoxic lymphocyte antigen-4 (CTLA-4, ie, ipilimumab) and lymphocyte activation gene-3 (LAG-3, ie, relatlimab) have demonstrated superior progression-free survival (PFS) outcomes compared with ICI monotherapy.6 7 Although the potential impact of subsequent therapies cannot be ignored, long-term OS and PFS curves for metastatic cutaneous melanoma treated with immunotherapy often plateau at 3–4 years, raising the question of optimal duration of treatment and the very real possibility of cure.8 The application of immunotherapy to the high-risk resectable disease setting has further improved recurrence-free survival (RFS) for patients with cutaneous melanoma. Thanks to these advances, courageous patients who have now survived advanced melanoma following treatment with immunotherapy can help the medical community tailor survivorship programs to optimize life post treatment by addressing affective disorders such as anxiety and depression, financial turmoil, and the ongoing management of chronic immune-related adverse events (irAEs).
Despite tremendous progress, the incidence of melanoma is predicted to rise over the next 20 years to 510,000 new cases and 96,000 deaths globally by the year 2040, representing a more than 50% increase.9 It is incumbent upon the entire medical community to deliver effective therapies safely to the patients most likely to benefit from treatment. Because immunotherapies act on the immune system as opposed to the tumor itself, patient selection, administration, response monitoring, and quality of life (QOL) support considerations are radically different compared to traditional modalities such as chemotherapy and targeted therapies. Patient populations warranting special consideration include those with rare non-cutaneous melanomas for whom response rates to ICIs are historically lower than for cutaneous disease, and patients with melanoma and altered immune systems who have been historically excluded from trials of immunotherapy. To assist the oncology community in navigating these and other challenging clinical questions, the Society for Immunotherapy of Cancer (SITC) convened a multidisciplinary panel of experts to develop an updated and expanded clinical practice guideline (CPG). This guideline represents an update to SITC’s 2018 CPG focusing on immunotherapy for the treatment of cutaneous melanoma, including new data on treatment sequencing, early-stage disease, rare melanoma subtypes, and other topics.
SITC’s CPGs are developed to assist providers in clinical decision-making and do not mandate a particular course of treatment or medical care. The CPGs are not intended to supplant sound judgment by the treating physician with respect to particular patients or special clinical situations and cannot always account for individual variations among patients. SITC considers adherence to the guidance to be voluntary, with the ultimate determination for the selected course of action to be made by the physician in light of each patient’s individual circumstances.
Guideline development methods
This CPG was developed by the SITC Melanoma Immunotherapy Guideline Expert Panel, under the governance of the SITC Cancer Immunotherapy Guidelines Oversight Committee. The Institute of Medicine’s (IOM) Standards for Developing Trustworthy Clinical Practice Guidelines were used as a model for guideline development.
Expert Panel composition
The guideline development group was multidisciplinary and balanced. Members were selected based on their expertise and experience in the field, including medical oncology, nursing, and patient advocacy, as well as other specialties as needed to support recommendation development.
Conflict of interest management
Disclosures of all financial relationships that might result in actual, potential, or perceived conflicts of interest were individually reported prior to the onset of manuscript development as well as at all key decision points during manuscript development. Those with significant financial connections that may compromise the ability to fairly weigh evidence (either actual or perceived) were not eligible to participate in guideline development. Any non-disqualifying conflicts of interests among members of the SITC Melanoma Immunotherapy Guideline Expert Panel were managed as outlined in SITC’s disclosure and conflict of interest resolution policies.
The financial support for the development of this guideline was provided solely by SITC. No commercial funding was received.
Recommendation development
Panel recommendations are based on literature evidence, where possible, and clinical experience, where appropriate. Literature searches in relevant databases were performed and publications were screened for inclusion in the evidence base for the guideline recommendations. Recommendations were developed based both on literature review and expert opinion presented during open communication and scientific debate. Subsequently, recommendations were refined through a modified Delphi process as described by the RAND/University of California, Los Angeles (UCLA) Appropriateness Method, Expert Panel consensus discussions, and review and editing of manuscript drafts.
Evidence rating
The level of evidence (LE) for a given consensus recommendation is expressed in parentheses following the recommendation (eg, LE:1). Evidence supporting panel recommendations was graded according to the Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence Working Group ‘The Oxford Levels of Evidence 2’. A summary of the OCEBM grading scale may be found in box 1.
Box 1. Summary of ‘The Oxford levels of evidence 2’ (adapted from the Oxford Centre for Evidence-Based Medicine levels of evidence working group).
Level 1
Systematic review or meta-analysis.
Level 2
Randomized trial or observational study with dramatic effect.
Level 3
Non-randomized, controlled cohort, or follow-up study.
Level 4
Case series, case–control, or historically controlled study.
Level 5
Mechanism-based reasoning.
External review
A draft of this CPG was made publicly available to provide an opportunity for stakeholders potentially affected by guidelines to review and comment on the content. All comments were evaluated by the Expert Panel and considered for inclusion into the final manuscript.
Diagnostic tests and biomarkers
Staging and initial workup
If an adequate biopsy of a suspicious skin lesion confirms a diagnosis of melanoma, then further histopathologic assessment of the lesion, including deep and peripheral margin status, ulceration, invasion of surrounding neurovascular structures, microsatellitosis, mitotic rate, and Breslow thickness, is necessary. Once a patient’s disease has been fully staged with or without lymph node (LN) assessment and imaging, assessment for several stage-specific biochemical markers should be considered, including BRAFV600 mutation testing for stage III and IV (and stage II in select cases) disease and lactate dehydrogenase (LDH) for metastatic disease.
Immunotherapy-specific biomarkers
Cutaneous melanoma has been a paradigm of success for immunotherapy treatment, with stage IV disease demonstrating an encouraging response to ICIs regardless of PD-L1 expression, tumor mutational burden (TMB), or microsatellite or mismatch repair status. As such, these assays are not routinely obtained for patients with melanoma, and their role in the management of melanoma has yet to be defined. Gene expression profiling (GEP) may be useful for predicting recurrence risk and has been prospectively validated for uveal melanoma.10 However, its use has not been validated in large studies nor has it been directly compared with risk prediction tools that use current standard pathologic and clinical data for cutaneous melanoma.11 Circulating tumor DNA (ctDNA) is another potentially predictive and prognostic biomarker being evaluated in studies of resectable cutaneous melanoma but is not currently validated for use outside of a clinical trial.
PD-L1 expression
The predictive and prognostic value of PD-L1 expression for melanoma has been conflicting. The primary outcome of objective response rate (ORR) with pembrolizumab treatment was higher for patients with higher PD-L1-staining tumors (by the 22C3 antibody) in KEYNOTE-001 (p<0.001), however, responses were noted in patients with PD-L1 negative (22C3 staining <10%) tumors as well. Survival outcomes (a secondary endpoint) were also improved for patients with higher PD-L1-expressing tumors (HR for PFS 0.76; 95% CI 0.71 to 0.82; p<0.001; HR for OS 0.76; 95% CI 0.69 to 0.83; p<0.001).12 On the other hand, the survival advantage with ipilimumab plus nivolumab or nivolumab monotherapy over ipilimumab monotherapy in CheckMate 067 was not predicted by PD-L1 expression alone.13 Furthermore, RFS did not differ significantly between the ipilimumab plus nivolumab and nivolumab arms, regardless of PD-L1 expression (HR 0.91; 95% CI 0.73 to 1.14 for PD-L1 <1%; HR 0.92; 97.295% CI 0.77 to 1.09 for the intention-to-treat [ITT] population) in the adjuvant setting in CheckMate 915.14
Meta-analyses also report conflicting results on the predictive and prognostic value of PD-L1 expression in melanoma. For example, one meta-analysis of patients with melanoma found that positive PD-L1 expression was significantly associated with OS (HR 0.57; 95% CI 0.46 to 0.70) in the metastatic melanoma subgroup only.15 Another meta-analysis of 1,062 patients from 13 studies demonstrated no association between PD-L1 expression and either PFS (HR 0.82; 95% CI 0.43 to 1.54; p=0.535) or OS (HR 0.93; 95% CI 0.57 to 1.52; p=0.781), however, PD-L1 expression did correlate with an absence of LN metastases (OR 0.46; 95% CI 0.22 to 0.95; p=0.036).16 At the time of guideline publication, PD-L1 expression should not be used to determine eligibility for ICIs, nor to predict response to ICIs in melanoma.
Tumor mutational burden
TMB is high in most cutaneous melanomas given the direct role of ultraviolet exposure in melanoma carcinogenesis, with a median TMB of >10 mutations per megabase (mut/Mb).17 TMB correlates highly with neoantigen load (r=0.90) but is not associated with PD-L1 expression (r=0.049; p=0.6473) in melanoma.18 High TMB may be associated with response to ICIs independent of sex, age, BRAF mutation status, and treatment line.19 High numbers of non-synonymous single nucleotide variants do not predict response of melanoma to ICI monotherapy, however, having a tumor with a mutational load in the top tertile (compared with the bottom tertile) has been significantly associated with survival.20 A significant association between higher TMB and PFS was also noted in an exploratory, retrospective analysis of CheckMate 066 and 067.21 As of February 2022, the FoundationOne CDx next generation sequencing (NGS) assay is an US FDA-approved measurement of TMB, however, other Clinical Laboratory Improvement Amendments (CLIA)-certified assays are available. While high TMB has demonstrated an association with response to ICIs, TMB should not be used to guide clinical decision-making with ICIs in melanoma. For a discussion of desmoplastic melanoma, which is associated with a high mutational load, please refer to the ICI monotherapy section.
Microsatellite and mismatch repair status
Although microsatellite instability-high (MSI-H) tumors commonly have a high TMB, the converse is not true.22 While most melanomas do have a high TMB, the frequency of MSI-H is much lower. In a small study, only 11% of 56 primary cutaneous melanomas and 21% of 42 metastatic melanomas were MSI-H.23 Mismatch repair protein deficiency (dMMR) has been reported in around 13% of cutaneous melanomas, with pathologically-relevant MMR deficiency rates of <1% for uveal melanoma.24 25 Melanoma was not represented in any of the five KEYNOTE studies leading to the US FDA’s tissue-agnostic approval for pembrolizumab monotherapy for all pretreated MSI-H/dMMR solid tumors, nor was it represented in the GARNET study leading to US FDA approval for dostarlimab monotherapy for all pretreated dMMR solid tumors. Although small, single-institution studies have demonstrated some association between MSI-H/dMMR and response to ICIs26 or survival,27 there are no large-scale, prospective studies evaluating the predictive or prognostic value of microsatellite/mismatch repair status for patients with melanoma. Use of this biomarker to select for ICI therapy or predict response to immunotherapy in melanoma is not recommended.
Emerging immunotherapy biomarkers
Predictive biomarkers for ICIs
Despite the tremendous improvements in melanoma-specific survival (MSS) in the past decade, more than one-third of patients with advanced disease do not respond to checkpoint blockade.28 No validated predictive biomarkers to identify the patients who will benefit from ICIs were available for routine clinical use at the time of guideline publication. However, several biomarkers have demonstrated promising initial signals of utility, including GEP, ctDNA, granzyme-B positron emission tomography (PET) imaging, and analysis of the gut microbiome, underscoring the need for clinical trials so that these biomarkers can be prospectively assessed.
Variants in key genes involved in cell cycle regulation or DNA damage response may also perturb the tumor immune microenvironment toward infiltration of cytotoxic cells and inflammatory signaling. For example, mutations in CDKN2A and BRCA2—loci that are typically included in NGS panels—have been linked with response to ICIs in small studies.20 29 Transcriptional profiling may also provide insight into the immunologic status of a tumor. Multigene expression signatures are being developed based on RNA sequencing of tissue from responding versus non-responding melanomas,21 30 31 which may be useful to help predict response to ICIs if validated in large, prospective trials.
Multiple non-invasive strategies for tumor assessment and ICI response prediction were being evaluated at the time of guideline publication. Circulating or ‘cell-free’ mutant BRAF or NRAS DNA levels obtained from a blood draw may be useful as a surrogate marker for disease burden for risk-stratification of melanoma in the unresectable/metastatic32 and adjuvant settings.33 Dynamic changes in the levels of ctDNA may also predict response to ICIs34 and differentiate pseudoprogression from true progression of disease.35 At the time of guideline publication, however, ctDNA measurement for response assessment was strictly investigational. Imaging-based biomarkers are another emerging non-invasive strategy to assess response to ICI therapy in real time. A PET granzyme B-specific imaging probe developed in murine models36 has been used to radiographically distinguish tumor microenvironments more or less conducive to ICI response37 and to visualize organs affected by irAEs.38 The ongoing phase I NCT04169321 trial is prospectively evaluating granzyme B PET imaging to predict response to pembrolizumab in patients with advanced melanoma. Finally, the composition of the gut microbiome offers a rich and varied catalog of potential biomarkers. Differential enrichment for specific bacterial taxa in the gut have been linked to ICI response, resistance, and the development of irAEs.39 40
Biomarkers to predict irAEs associated with ICIs
Severe (≥grade 3) irAEs occur in more than half of patients receiving ipilimumab plus nivolumab.28 Severe toxicity occurs (although at lower rates) with relatlimab plus nivolumab and single-agent anti-PD-1 therapy as well.41 42 No validated biomarkers exist to predict the development of irAEs. As immunotherapy is increasingly used in the adjuvant setting for stage II and stage III disease, it will be critical to identify biomarkers for toxicity to potentially inform risk-benefit discussions for therapy selection and for effective management of therapy-limiting toxicities. Baseline somatic and germline GEP,43 44 circulating inflammatory markers,45 T-cell clonality,46 body mass index,47 and distinct gut microbiome profiles39 48 49 have all predicted the development of ICI-mediated irAEs in exploratory studies of patients with melanoma. Granzyme B-based PET imaging may also play a role in measuring or predicting irAEs in the future.
Panel recommendations
For all patients with stage III and stage IV melanoma, BRAF mutation status should be obtained (LE:2).
Patients with stage IIB/C melanoma have a high risk of recurrence, therefore BRAF mutation testing can be considered on a case-by-case basis so that treatment options are known at the time of recurrence.
For all patients with unresectable/metastatic melanoma, NGS is recommended if feasible.
Although PD-L1 tumor proportion score (TPS) and TMB are associated with ICI response in melanoma, they should not be used for clinical decision-making at the time of manuscript publication.
MSI and MMR status should not be routinely obtained as a standalone test for patients with melanoma.
ctDNA is an exciting new tool to track antitumor response to ICIs and is being explored in research settings, however, this biomarker is not routinely used to guide clinical decision-making for patients with melanoma at the time of manuscript publication.
There are many biomarkers under investigation in melanoma (eg, interferon [IFN]γ gene expression signatures, granzyme B PET imaging, gut microbiome profiling) to predict response to ICIs, but none of those are clinically validated and were not routinely used to guide clinical decision-making at the time of manuscript publication.
Studies to identify biomarkers to predict risk of developing irAEs and to inform treatment of irAEs are ongoing, but none of these biomarkers were routinely used to guide clinical decision-making at the time of manuscript publication.
Stage II cutaneous melanoma
Recurrence assessment for stage II disease
While most melanoma cases are diagnosed as stage I, an estimated 10–20% may present at stage II.50 In accordance with the 8th edition American Joint Committee on Cancer (AJCCv8) Tumor, Node, Metastasis (TNM) system, stage II melanoma includes tumors that are at least 1 mm in depth with ulceration or 2 mm or greater in depth and are without nodal or clinically apparent metastatic disease.51 Stage II melanomas are further subcategorized by their depth (>1–2 mm for T2, >2–4 mm for T3, and >4 mm for T4 disease) and the presence of ulceration (no ulceration for Ta disease versus ulceration present for Tb disease). While the AJCCv8 5-year MSS rates for stage IIA (T2b to T3a) disease are 94%, this rate falls to 87% for stage IIB (T3b to T4a) and 82% for stage IIC (T4b) disease.51 In comparison, the AJCCv8 5-year MSS rate for stage IIIA disease is 93%—better than the stage IIB and IIC subcategories. Although the AJCCv8 staging system is widely used, it has important limitations. Some analyses have revealed a higher recurrence rate for stage II disease than what might be extrapolated from AJCCv8 MSS rates.52 53 Furthermore, 10-year MSS rates reported for over 17,000 patients in the Central Malignant Melanoma Registry (CMMR) were worse for all stage I and II subcategories compared with the AJCCv8 cohort (80.7–83.1% versus 88% for stage IIA, 72.0–79.9% versus 82% for stage IIB, 57.6–64.7% versus 75% for stage IIC).54 Regardless of thickness, stage I and II melanomas can recur following surgery.
Management of stage I/IIA disease
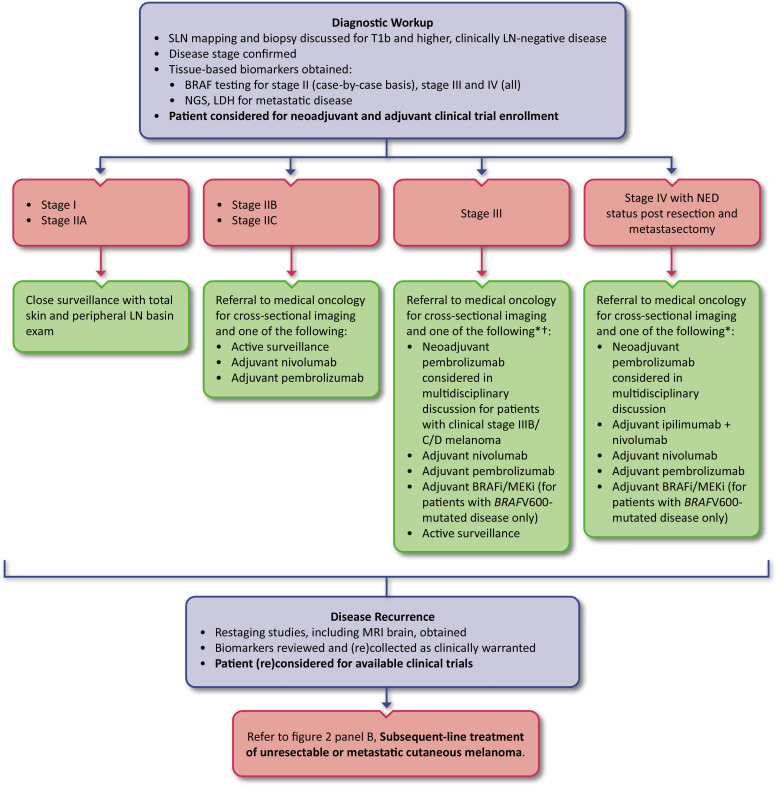
The standard of care for stage I or IIA resected melanoma remains close surveillance (figure 1). Because the majority (83%) of melanomas are diagnosed as stage I or IIA, most melanoma-related deaths also occur in this group by virtue of case volume.50 Given the 5-year AJCCv8 MSS rates of 96–99% for stage I disease and 93–94% for stage IIA disease, adjuvant systemic treatment is not recommended.51 Research to identify biomarkers to select for patients with early-stage disease who may benefit from adjuvant therapy is ongoing (see the Ongoing trials section, below).
Figure 1.
Adjuvant treatment of cutaneous melanoma. Algorithm for resected stage I through resected stage IV (with NED) cutaneous melanoma. *There are no head-to-head prospective data directly comparing initial adjuvant anti-PD-1 ICI therapy to targeted therapy for patients with BRAFV600-mutated melanoma, and there are no prospective data to support the use of adjuvant targeted therapy for resected stage IV BRAFV600-mutated melanoma. The toxicity profile of each of these approaches is different, and (potentially long-term) adverse events associated with each therapy should be weighed against absolute benefit. †Adjuvant systemic treatment should be considered for stage IIIA and strongly considered for stage IIIB and higher melanoma. ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; LN, lymph node; NED, no evidence of disease; NGS, next generation sequencing; PD-1, programmed cell death protein 1; SLN, sentinel lymph node
Management of stage IIB/IIC disease
KEYNOTE-716 was the first study to evaluate the efficacy of adjuvant immunotherapy selecting exclusively for sentinel lymph node (SLN) biopsy-negative, resected stage IIB and IIC melanoma.55 This study randomized 976 patients (64% stage IIB, 34.8% stage IIC) 1:1 to receive adjuvant pembrolizumab versus adjuvant placebo and met the RFS endpoint showing benefit for the pembrolizumab arm. After a median follow-up of 14.4 months, pembrolizumab significantly prolonged RFS compared to placebo (HR 0.65; 95% CI 0.46 to 0.92; p=0.00658; median not reached for both arms). At the second interim analysis (median follow-up of 20.9 months for both arms), 15% of patients who received pembrolizumab and 24% of patients who received placebo had a first recurrence or died (HR 0.61; 95% CI 0.45 to 0.82), and the median RFS was still not reached in either group.56 It is important to note, however, that MSS was not reported in KEYNOTE-716, and the optimal strategy for achieving cure—adjuvant immune checkpoint blockade versus immune checkpoint blockade at the time of relapse—has yet to be determined. While the rate of grade ≥3 treatment-related adverse events (TRAEs) was higher for the pembrolizumab arm (16.1% vs 4.3% for placebo), there were no treatment-related deaths associated with adjuvant checkpoint inhibition. In December of 2021, the US FDA approved adjuvant pembrolizumab for the treatment of completely resected stage IIB and IIC melanoma. At the time of the third interim analysis (median follow-up 27.4 months), pembrolizumab had significantly improved distant metastasis-free survival (DMFS) versus placebo (HR 0.64; 95% CI 0.47 to 0.88; p=0.0029; median DMFS not reached in either arm), with the risk of recurrence remaining lower in the pembrolizumab arm as well (HR 0.64; 95% CI 0.50 to 0.84).57
The phase III CheckMate 76K trial (NCT04099251) evaluating adjuvant nivolumab for resected, SLN-negative, stage IIB and IIC melanoma, met its primary RFS endpoint. Although CheckMate 76K was not yet published at the time of guideline publication, at the first interim analysis presented at the 2022 Society for Melanoma Research Congress, adjuvant nivolumab reduced the risk of recurrence or death by 58% compared with placebo (HR 0.42, 95% CI 0.30 to 0.59, p<0.0001). The 12-month RFS rates for nivolumab and placebo, respectively, by stage were 93% versus 84% in stage IIB and 84% versus 72% in stage IIC. Although pembrolizumab and nivolumab are similar, these two drugs had not been compared directly for the adjuvant treatment of stage IIB and IIC melanoma, and at time of publication only pembrolizumab was US FDA approved for this indication.
See figure 1 for management options for stage IIB and IIC cutaneous melanoma in the adjuvant setting and table 1 for a complete list of trials supporting the US FDA approvals for adjuvant ICIs. Prior to KEYNOTE-716, adjuvant IFNα-2b had historically been considered for some patients with stage II melanoma, but is no longer a treatment option.52 58
Table 1.
Landmark trials leading to US FDA approvals of adjuvant ICIs for cutaneous melanoma.
| Trial with US FDA approval date | Key inclusion criteria | Study arms | Key outcomes | Treatment-related adverse events |
| EORTC 18071 (NCT00636168)62 October, 2015 |
Completely resected stage III melanoma with >1 mm of regional LN tumor involvement. | Adjuvant ipilimumab* for up to 3 years (n=475). Adjuvant placebo for up to 3 years (n=476). |
Median RFS: 26.1 vs 17.1 months (HR 0.75; 95% CI 0.64 to 0.90; p=0.0013). 1-year RFS: 63.5% vs 56.1%. |
Grade 3–4: 42% vs 2.5%. Grade 5: 1% vs 0%. |
| CheckMate 238 (NCT02388906)74
December, 2017 |
Stage IIIB, IIIC, or IV melanoma with complete regional lymphadenectomy or resection (including resection of distant metastases). | Adjuvant nivolumab† for up to 1 year (n=453). Adjuvant ipilimumab* for up to 1 year (n=453). |
Median RFS: NR for either arm (HR 0.65; 97.56% CI 0.51 to 0.83; p<0.001). 12-month RFS: 70.5% vs 60.8%. |
Grade ≥3: 14.4% vs 45.9%. Grade 5: 0% vs 0.4%. |
| EORTC 1325/KEYNOTE-054 (NCT02362594)63 February, 2019 |
Resected (including complete regional lymphadenectomy) stage IIIA (N1a with ≥1 micrometastasis measuring >1 mm), IIIB, or IIIC melanoma with no ITMs. | Adjuvant pembrolizumab‡ for up to 1 year (n=514). Adjuvant placebo for up to 1 year (n=505). |
Median RFS: NR for either arm (HR 0.57; 98.4% CI 0.43 to 0.74; p<0.001). 12-month RFS: 75.4% vs 61.0%. |
Grade ≥3: 14.7% vs 3.4%. Grade 5: 0.2% vs 0%. |
| KEYNOTE-716 (NCT03553836)55 December, 2021 |
Completely resected stage IIB or IIC melanoma. | Adjuvant pembrolizumab‡ for up to 1 year (n=487). Adjuvant placebo for up to 1 year (n=489). |
Median RFS: NR for either arm (0.65; 95% CI 0.46 to 0.92; p=0.00658). 12-month RFS: 90.5% vs 83.1%. |
Grade ≥3: 16.1% vs 4.3%. Grade 5: 0% for both arms. |
Information presented in this table is based on data available at the time of each corresponding US FDA approval. Experimental arm data are listed first.
*Ipilimumab was dosed at 10 mg/kg every 3 weeks for four doses and then every 12 weeks.
†Nivolumab was dosed at 3 mg/kg every 2 weeks.
‡Pembrolizumab was dosed at 200 mg every 3 weeks.
CI, confidence interval; EORTC, European Organisation for Research and Treatment of Cancer; HR, hazard ratio; ICI, immune checkpoint inhibitor; ITMs, in-transit metastases; LN, lymph node; NR, not reached; RFS, recurrence-free survival; US FDA, United States Food and Drug Administration.
Ongoing trials
Several studies of adjuvant treatment for stage II melanoma were ongoing at the time of publication. The phase III NivoMela trial (NCT04309409) is using a GEP selection assay to identify patients with stage II melanoma (including stage IIA disease) who have a high risk of relapse and then assigns these ‘high-risk’ patients to randomization to receive adjuvant PD-1 inhibition versus observation. Although not an immunotherapy study, the phase III COLUMBUS-AD trial (NCT05270044) is evaluating encorafenib plus binimetinib for BRAFV600-mutated stage IIB/C melanoma. In the neoadjuvant setting, the phase II, NCT03757689 study is exploring the impact of a single dose of pembrolizumab administered 3 weeks prior to resection on the rate of SLN positivity for patients with stage IIB and IIC melanoma. Clinical trial enrollment both prior to and following SLN assessment is imperative to inform pathological and clinical endpoints.
Panel recommendations
For patients with T1b and higher, clinically LN-negative melanoma, SLN mapping and biopsy should be discussed and offered, when feasible (LE:2).
For patients with resected stage IIB and IIC melanoma, a referral to medical oncology and surveillance with cross-sectional imaging are recommended (LE:3).
For patients with resected stage IIB and IIC melanoma, adjuvant pembrolizumab (LE:2) or nivolumab (LE:2), surveillance alone, or clinical trial enrollment are all options. A discussion about the potential risks and benefits associated with adjuvant PD-1 inhibition is recommended as part of a shared decision-making process.
For patients with resected stage I and stage IIA melanoma, close surveillance with total skin examination and physical examination of peripheral LN basins should be continued. These patients may also be considered for clinical trials. Routine imaging for these patients in the absence of symptoms is not recommended.
Stage III and resected stage IV cutaneous melanoma
Recurrence assessment for stage III disease
Patients with stage III melanoma have LN involvement and/or in-transit metastases (ITMs) without distant metastatic disease. The prognosis of stage III disease varies widely and while adjuvant therapy can incur significant morbidity and cost, undertreatment carries the risk for progression to stage IV disease and death. The subset of patients with AJCCv8 stage IIIA melanoma (T1a/b-T2a, N1/2a, M0) with a non-ulcerated primary (T1a or T2a) and a SLN containing <1 mm of tumor have been identified as having a lower risk for distant metastases, and a thorough risk-benefit discussion of adjuvant (ICI or targeted) systemic therapy is required for these patients.51 59 None of the landmark trials leading to US FDA approvals for adjuvant ICI therapy for stage III and stage IV melanoma included stage IIIA disease with <1 mm of LN involvement. Furthermore, these trials defined disease stage by AJCCv7 definitions, therefore T3 or T4 disease may have been designated as stage IIIA. All patients with SLN-positive disease, including those with stage IIIA melanoma who forgo adjuvant systemic treatment, require high intensity surveillance with regular physical examinations and serial CT or PET scans with or without nodal basin ultrasound.60 It should be noted that stage IIIA disease in the AJCCv7 included T1 to T4a disease,61 therefore studies designed to include stage IIIA disease prior to the switch to the 8th edition staging system in January, 2018 (European Organisation for Research and Treatment of Cancer [EORTC] 18071 and EORTC 1325/KEYNOTE-054) may have overestimated disease risk in this stage group. Furthermore, all patients with resected stage IIIA melanoma enrolled in these trials of adjuvant ICI therapy were required to undergo complete regional lymphadenectomy prior to receiving treatment.62 63
While SLN tumor involvement of <1 mm remained a good differentiator of survival for stage IIIA melanoma from the 7th to the 8th edition of AJCC, survival for stage IIIA patients remained heterogeneous overall.59 A recent prospective study identified patients with stage IIIA melanoma and a SLN metastatic tumor deposit of ≥0.3 mm as a relatively high-risk subgroup, with a 5-year disease-specific survival rate of 80.3% (vs 94.1% for patients with SLN deposits <0.3 mm; HR 1.26; 1.11 to 1.44; p<0.0001).64 Additionally, MSS rates for stage IIIA disease demonstrated by CMMR and EORTC were lower compared with those estimated by the AJCCv8 (5-year MSS: 80% vs 93%; 10-year MSS: 71% vs 88%, respectively).65 Lower MSS rates were demonstrated by CMMR versus AJCCv8 for some higher substages as well: 5-year MSS: 75% versus 83% and 10-year MSS: 61% versus 77% for stage IIIB disease; 5-year MSS: 56% versus 69%; 10-year MSS: 45% versus 60% for stage IIIC disease; and 5-year MSS: 30% versus 32% and 10-year MSS: 30% versus 24% for stage IIID disease.
Upon resection of stage III melanoma, it is incumbent upon the oncologist to provide education regarding the recurrence risk associated with specific disease substages (eg, IIIA, IIIB) as well as the potential risks (eg, irAEs) and RFS benefits of adjuvant systemic treatment. The decision for adjuvant treatment versus active surveillance should be made in collaboration with well-informed patients and caregivers. Although patient care decisions should always be made on a case-by-case basis, the 5-year and 10-year substage-specific MSS rates discussed in the preceding paragraph may help to inform these decisions. Cross-sectional and regional LN ultrasound surveillance should be obtained as directed by the National Comprehensive Cancer Network (NCCN) guidelines, which are supported by data from the MSLT-II and DeCOG-SLT studies.60 66 67 A retrospective analysis of 1,918 American and Australian patients with stage III melanoma demonstrated a 15.8% cumulative incidence of central nervous system (CNS) metastases at 5 years,68 making a compelling argument for regular brain imaging, ideally with MRI, following resection of stage III disease. This Expert Panel recommends regular surveillance with brain imaging for resected stage IV melanoma as well.
Available agents and indications
Once the decision has been made to proceed with adjuvant systemic treatment for stage III or resected stage IV melanoma, there are several immunotherapeutic options to consider. In 1995, the US FDA approved the use of high-dose IFNα-2b for the adjuvant treatment of melanoma based on results of ECOG 1684, a randomized controlled study of resected stages IIB, IIC, and III disease that demonstrated improved RFS (1.72 years with IFNα vs 0.98 years with observation; p=0.0023) and OS (3.82 years with IFNα vs 2.78 years with observation; p=0.0237).69 Adjuvant administration of the better-tolerated pegylated formulation of IFNα was approved by the US FDA in 2011 based on the EORTC 18991 study of resected stage III melanoma, which again demonstrated a significant RFS benefit (4-year RFS rate with pegylated IFNα 45.6% vs observation 38.9%; p=0.01) but no OS benefit (4-year OS rate 56.8% with pegylated IFNα vs 55.7% with observation; p=0.78).70 71 However, at the time of guideline publication, ICIs had supplanted IFN therapy in the treatment of melanoma due to their superior survival outcomes, tolerability, favorable toxicity profiles, and ease of administration.
ICIs
In October 2015, adjuvant ipilimumab gained US FDA approval for completely resected stage III cutaneous melanoma with >1 mm of regional LN tumor involvement based on the EORTC 18071 trial of patients with resected stage III disease randomized to receive adjuvant ipilimumab 10 mg/kg versus placebo.62 This was the first trial to demonstrate a survival benefit with adjuvant ICI therapy, with an RFS benefit (primary endpoint) demonstrated after 2.74 years of follow-up (26.1 months vs 17.1 months; p=0.0013) and an OS advantage demonstrated after 6.9 years of follow-up (OS not reached vs 7.8 years; p=0.0021).72 However, 52% of patients in the ipilimumab group discontinued treatment due to adverse events, including 5 (1%) treatment-related deaths. In the phase III E1609 study of patients with stage IIIB, IIIC, M1a, or M1b disease randomized to receive 3 mg/kg ipilimumab versus 10 mg/kg ipilimumab versus high dose IFNα, grade ≥3 adverse events favored ipilimumab dosed at 3 mg/kg and improved OS was observed with ipilimumab 3 mg/kg vs high dose IFN-α.73
In December 2017, the US FDA approved nivolumab for the adjuvant treatment of patients with resected cutaneous melanoma and LN involvement based on results of the double-blind, phase III CheckMate 238 trial.74 In this study, 906 patients with stage IIIB, IIIC, and resected stage IV melanoma were randomized 1:1 to receive either nivolumab 3 mg/kg every 2 weeks for up to 1 year or ipilimumab 10 mg/kg every 3 weeks for four doses, followed by every 12 weeks for up to 1 year. At a minimum follow-up of 18 months, the 12-month RFS rate (primary endpoint) was 70.5% for nivolumab versus 60.8% for ipilimumab (HR 0.65; 97.56% CI 0.51 to 0.83; p<0.001). Only 14.4% of patients receiving nivolumab experienced a grade ≥3 TRAE versus 45.9% of patients receiving ipilimumab (including two treatment-related deaths); 9.7% of the patients receiving nivolumab discontinued treatment due to an adverse event versus 42.6% in the ipilimumab group. While RFS benefit persisted on subsequent analysis (4-year RFS rate 51.7% for nivolumab vs 41.2% for ipilimumab; HR 0.71; 95% CI 0.60 to 0.86; p=0.0003), the study was underpowered to determine a difference in the secondary OS endpoint and there were minimal numerical differences between the two groups (4-year OS rate 77.9% for nivolumab vs 76.6% for ipilimumab; HR 0.87; 95% CI 0.66 to 1.14; p=0.31).75 However, there were a higher number of patients in the ipilimumab arm versus the nivolumab arm who received subsequent immunotherapy in the ITT population (34% vs 23%, respectively). The superior RFS of nivolumab versus ipilimumab persisted across all stage, PD-L1 (<5% versus ≥5%), and BRAF subgroups.76
In February 2019, the US FDA approved pembrolizumab for the adjuvant treatment of resected melanoma with LN involvement based on results of the double-blind EORTC1325/KEYNOTE-054 trial, which randomized 1,019 patients with stage IIIA (>1 mm LN metastasis), IIIB, or IIIC (without ITMs) disease 1:1 to receive either adjuvant pembrolizumab or placebo for 1 year.63 With a median follow-up of 15 months, the primary endpoint was met with a 1-year RFS rate in all randomized patients of 75.4% for pembrolizumab versus 61.0% for placebo (HR 0.57; 98.4% CI 0.43 to 0.74; p<0.001). The 3.5-year DMFS (a secondary endpoint) was also significantly higher for pembrolizumab versus placebo on subsequent analysis in both the ITT population (65.3% vs 49.4%; HR 0.60; 95% CI 0.49 to 0.73; p<0.0001) and in the subgroup of patients with PD-L1-positive tumors (66.7% vs 51.6%; HR 0.61; 95% CI 0.49 to 0.76; p<0.0001).77 Grade ≥3 TRAEs occurred in 14.7% of patients in the pembrolizumab group (including one death) and in 3.4% in the placebo group, while irAEs of any grade occurred in 37.3% versus 9.0% of patients receiving pembrolizumab versus placebo, respectively.63 The phase III intergroup S1404 study also demonstrated a significant RFS benefit with adjuvant pembrolizumab versus a standard of care control arm (pooled ipilimumab or high-dose IFNα) with a HR of 0.740 (99.618% CI 0.571 to 0.958) but no significant OS benefit, regardless of PD-L1 status.78
With superior RFS outcomes established for adjuvant anti-PD-1 ICIs for the treatment of advanced resected melanoma, CheckMate 915 evaluated the addition of CTLA-4 blockade for additional benefit. In this phase III trial, 1,844 patients with completely resected stage IIIB, IIIC, IIID, or IV melanoma were randomized to receive nivolumab 240 mg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks versus nivolumab 480 mg every 4 weeks for up to 1 year.79 Of note, patients with resected CNS lesions with or without adjuvant radiation therapy were permitted to enroll in this study. With a minimum follow-up of 24 months, there was no significant difference in RFS rate for the overall ITT population (24-month RFS rate: 64.6% for ipilimumab plus nivolumab vs 63.2% for nivolumab; HR 0.92; 97.295% CI 0.77 to 1.09; p=0.269) or in the PD-L1 <1% ITT population (24-month RFS rate: 53.6% for ipilimumab plus nivolumab vs 52.4% for nivolumab; HR 0.91; 95% CI 0.73 to 1.14). The difference in DMFS rates in patients with stage III disease (an exploratory endpoint) was similarly non-significant between the two treatment groups. In the ipilimumab plus nivolumab arm, 33% of patients experienced a grade 3 or 4 TRAE, 32% discontinued therapy due to a TRAE, and four patients experienced a fatal TRAE. Nivolumab monotherapy, as expected, was much better tolerated, with only 13% of patients experiencing a grade 3 or 4 TRAE and only 10% of patients discontinuing therapy due to a TRAE. There were no treatment-related deaths reported in the nivolumab arm.
The phase II, double-blind IMMUNED study evaluated the optimal adjuvant treatment regimen for patients with stage IV melanoma who had no evidence of disease (NED) after surgery or radiotherapy.80 In this study, 167 patients (including 22 with a history of brain metastases) were randomized to receive adjuvant nivolumab (1 mg/kg every 3 weeks) plus ipilimumab (3 mg/kg every 3 weeks), nivolumab (3 mg/kg every 2 weeks), or placebo. With a median follow-up of 28.4 months (interquartile range [IQR] 17.7–36.8), the 1-year and 2-year RFS rates, respectively, were 75% and 70% in the nivolumab plus ipilimumab arm, 52% and 42% in the nivolumab arm, and 32% and 14% in the placebo arm, with a significant benefit for the addition of ipilimumab to nivolumab on exploratory analysis (HR 0.40; 97.5% CI 0.20 to 0.79). With 49.2 months (IQR 34.9–58.1) of follow-up, the median OS had not been reached for any of the three study arms of IMMUNED, but the HR for OS was significantly improved for ipilimumab plus nivolumab versus placebo (HR 0.41; 95% CI 0.17 to 0.99; p=0.040).81 And while there was no OS benefit demonstrated for nivolumab versus placebo (HR 0.75; 0.36 to 1.56; p=0.44), most study participants in the placebo arm received subsequent anti-PD-1-based therapy, emphasizing once again that it is not known whether OS is improved with upfront adjuvant therapy or treatment at the time of recurrence. Grade 3 and 4 TRAEs occurred in 71% of patients receiving ipilimumab plus nivolumab versus 27% of those receiving nivolumab, and three deaths due to adverse events were determined to be unrelated to study drug.
CheckMate 238, EORTC1325/KEYNOTE-054, CheckMate 915, and IMMUNED did not enroll patients with uveal melanoma. No significant difference in disease recurrence or death for nivolumab versus ipilimumab was observed in the 29 patients with mucosal melanoma enrolled in CheckMate 238 (HR 1.57; 95% CI 0.57 to 4.33).74 The adjuvant treatment of rare melanoma subtypes is discussed further in the Patients with non-cutaneous melanoma section.
Patients with stage III melanoma and regional metastatic disease (ie, ITMs or satellite lesions) are at increased risk for distant disease recurrence82 83 and a multidisciplinary discussion should inform the treatment strategy (eg, regional or intratumoral therapy vs resection followed by systemic therapy) for this population. Available data have demonstrated a clinical benefit with anti-PD-1 therapy for patients with ITMs. Although both EORTC 18071 (adjuvant ipilimumab) and EORTC1325/KEYNOTE-054 (adjuvant pembrolizumab) excluded patients with ITMs,62 63 Southwest Oncology Group (SWOG) 1404 (adjuvant pembrolizumab versus high dose interferon [HDI] or ipilimumab)78 and CheckMate 238 (adjuvant nivolumab vs ipilimumab)74 did include patients with ITMs. A post-hoc 4-year analysis of the 164 patients with ITMs in each treatment arm of CheckMate 238 demonstrated a significant improvement in RFS with nivolumab versus ipilimumab in patients with synchronous nodal involvement and a trend toward favoring nivolumab for patients without nodal involvement.84
Selection of targeted therapy versus immunotherapy
The combination of dabrafenib and trametinib for the adjuvant treatment of resected melanoma with LN involvement and a BRAFV600E or V600K mutation is also US FDA-approved, based on COMBI-AD.85 Dabrafenib plus trametinib-associated serious adverse events were observed at a rate of 36% in COMBI-AD, most commonly fever, fatigue, and nausea, leading to permanent discontinuation in 26%, dose reduction in 38%, and dose interruption in 66% of patients. At the 5-year data analysis cut-off, the median RFS (primary endpoint) was not reached in the dabrafenib plus trametinib group versus 16.6 months for the placebo arm (HR 0.51; 95% CI 0.42 to 0.61) and there were not enough events to analyze OS.86 Importantly, while trials of adjuvant nivolumab and pembrolizumab included (and demonstrated benefit for) resected BRAFV600-mutated melanoma, there have been no head-to-head studies of adjuvant BRAFi/MEKi versus ICIs for resected, BRAFV600-mutated melanoma. Selection of adjuvant therapy in this population should therefore involve shared decision-making between the patient and the provider incorporating a thorough discussion of the unique side effect profile of each drug regimen, patient comorbidities (eg, underlying autoimmune disease or receipt of organ transplant),87 the underlying biology of the cancer (eg, stage III versus stage IV disease), and the feasibility of drug delivery (eg, continuous oral versus intermittent intravenous). This risk-benefit discussion is particularly important for patients with relatively low-risk stage III disease for whom the risk of irAEs (including long-term sequelae) may be higher than the risk of distant disease recurrence.51 88
Emerging data for adjuvant therapy
Additional ongoing trials of adjuvant therapy for advanced melanoma are evaluating a variety of strategies, including the addition of an adjuvant tumor lysate, particle-loaded, dendritic cell vaccine.89 In the phase IIb KEYNOTE-942/mRNA-4157-P201 trial (NCT03897881) of adjuvant pembrolizumab administered with or without the personalized mRNA-4157 vaccine following complete resection of stage III or stage IV melanoma, the primary endpoint of RFS was met (risk of recurrence or death reduced by 44% with the addition of vaccine [HR=0.56; 95% CI, 0.31 to 1.08; one-sided p=0.0266]).90 Serious TRAEs occurred in 14.4% versus 10%, respectively, of patients who received the vaccine plus pembrolizumab versus pembrolizumab alone.
Emerging data for neoadjuvant therapy
Although not US FDA-approved at the time of guideline publication, neoadjuvant immunotherapy is an active area of investigation for high-risk (clinically evident) stage III/IV resectable melanoma. Benefits of neoadjuvant immunotherapy may include abundant antigen availability in the in situ tumor at the time of treatment, increasing the rates of recurrence-free and distant metastases-free (and ultimately melanoma-specific) survival by early introduction of systemic therapy, identifying patients with a favorable treatment response who may be spared extensive surgery and/or adjuvant treatment, sparing patients with biologically aggressive disease who progress rapidly during neoadjuvant treatment from a futile surgery, and facilitating the identification of biomarkers of response (including pathologic response) that may inform adjuvant therapy selection.91 Combination relatlimab and nivolumab is approved for the treatment of unresectable or metastatic melanoma, and trials of this combination in the perioperative setting are ongoing. In a study of 30 patients with clinical stage IIIB–IV(M1a) resectable melanoma, neoadjuvant nivolumab plus relatlimab continued into the adjuvant setting resulted in a 57% pCR rate (the study’s primary endpoint) and a 70% overall pathologic response rate, with a 2-year RFS rate of 92% for patients with any pathologic response (vs 55% for patients no pathologic response; p = 0.005).92 93 The phase II OpACIN-neo trial of patients with macroscopic stage III melanoma evaluated rates of high-grade toxicity and pathologic response for patients receiving one of three neoadjuvant immunotherapy regimens: two cycles of ipilimumab 3 mg/kg plus nivolumab 1 mg/kg, two cycles of ipilimumab 1 mg/kg plus nivolumab 3 mg/kg, or two cycles of ipilimumab 3 mg/kg followed by two cycles of nivolumab 3 mg/kg.94 Compared with the standard dosing of ipilimumab 3 mg/kg plus nivolumab 1 mg/kg, the ‘flipped dose’ neoadjuvant regimen of ipilimumab 1 mg/kg plus nivolumab 3 mg/kg resulted in a lower rate of grade ≥3 irAEs (20% vs 40%) while maintaining a similar radiographic (60% vs 60%) and pathologic response rate (77% [57% pCR] vs 80% [43% pCR]). Patients with stage III melanoma in the PRADO extension of OpACIN-neo then underwent therapeutic LN dissection (TLND) or no TLND, adjuvant systemic treatment or no adjuvant systemic treatment, with or without radiotherapy, based on the index LN pathologic response to at least one dose of neoadjuvant nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (the optimal regimen identified in OpACIN-neo).95 Patients who had an index LN major pathologic response (MPR, ≤10% viable tumor) to the combination (n=60) proceeded without TLND, achieving a 2-year RFS rate of 93.3% and a DMFS rate of 100%.96 These and other phase II studies (including the study of neoadjuvant continued into adjuvant relatlimab plus nivolumab) have demonstrated high pCR rates following neoadjuvant immunotherapy combinations and a correlation between pathologic response and RFS.92 94 97 Interestingly, a pooled analysis of patients with stage IIIB and IIIC melanoma demonstrated improved 2-year RFS rates for patients who received neoadjuvant immunotherapy versus neoadjuvant targeted therapy.98
The randomized phase II SWOG S1801 study measured event-free survival (EFS), with the following protocol-defined events: documented progression that renders the patient unable to receive planned protocol surgery, failure to begin adjuvant therapy within 84 days of surgery, relapse after surgery, or death due to any cause.99 A total of 313 patients with resectable stage IIIB through IV melanoma were randomized 1:1 to receive three doses of neoadjuvant pembrolizumab continued into the adjuvant setting versus upfront surgery and adjuvant pembrolizumab.100 In a landmark analysis after a median follow-up of 14.7 months, EFS was significantly improved for the neoadjuvant-adjuvant versus adjuvant only arm (p=0.004). Similar rates of resection and similar rates of pembrolizumab-related vs surgery-related adverse events were observed in both arms. Several other phase II and phase III trials evaluating the efficacy and safety of neoadjuvant immunotherapy for resectable melanoma were ongoing at the time of guideline publication (eg, NCT04207086 [Neo PeLe], NCT04949113 [NADINA]).
Patients with high-risk stage III/IV melanoma should be enrolled in clinical trials of neoadjuvant immunotherapy whenever possible. Consideration for neoadjuvant therapy requires a multidisciplinary assessment at the time of patient presentation, with surgical oncology engagement to determine the feasibility and utility of resection. Neoadjuvant ICIs are associated with a risk of toxicity or disease progression that may preclude or delay resection as was seen in some patients in OpACIN-neo.101 For example, although therapeutic lymphadenectomy following neoadjuvant therapy may be predicted to be more technically challenging, surgery following neoadjuvant treatment was ultimately more often perceived as easier compared with the surgeon’s baseline impression in a substudy of NeoACTIVATE (NCT03554083).102 It is also critical to standardize pathologic assessment of disease response using a predefined International Neoadjuvant Melanoma Consortium (INMC)-endorsed research pathology methodology designed to assess LN tumor burden following neoadjuvant treatment103 as well as uniform surgical oncologic assessment of the difficulty and morbidity of surgery91 102 post-neoadjuvant therapy. Without standardized pathological and surgical measures, it will be difficult to determine the true benefit of neoadjuvant therapy for high risk, resectable melanoma.
Panel recommendations
For patients with resected stage IIIA melanoma, adjuvant systemic therapy with either an anti-PD-1 ICI (LE:2) or BRAF-targeted therapy (for patients with BRAFV600-mutated disease) (LE:2) should be considered. For patients with resected stage IIIB and above melanoma without contraindications, adjuvant systemic therapy with either an anti-PD-1 ICI (LE:2) or BRAF-targeted therapy (LE:2) should be strongly considered. A discussion about the potential risks and benefits associated with adjuvant therapy versus active surveillance is recommended as part of a shared decision-making process.
For patients with resected stage III BRAFV600-mutated melanoma, while both treatments have shown a similar RFS benefit, there are no head-to-head prospective data directly comparing initial adjuvant anti-PD-1 ICI therapy to targeted therapy. The toxicity profile of each of these approaches is different, therefore consideration of potential long-term/permanent adverse events associated with each of these approaches should be weighed against the absolute benefit.
For patients with resected stage IV BRAFV600-mutated melanoma, ICIs have shown an RFS benefit in the adjuvant setting and both ICIs and targeted therapy have shown an OS benefit in the metastatic setting. Although adjuvant targeted therapy for patients with completely resected stage IV BRAFV600-mutated disease may also be considered (LE:5), data for this approach are lacking. The toxicity profile of adjuvant ICI versus adjuvant targeted therapy is different, therefore consideration of potential long-term/permanent adverse events associated with each of these approaches should be weighed against the absolute benefit.
For patients with resected stage III/IV melanoma, ipilimumab 10 mg/kg or high-dose IFN therapy should no longer be used as adjuvant treatment.
For patients with resectable stage IIIB to IV (without brain metastases) melanoma, while there were no approved neoadjuvant therapies at the time of manuscript publication, neoadjuvant pembrolizumab continued into the adjuvant setting demonstrated improved EFS compared with adjuvant therapy alone in a randomized, phase II trial (LE:2). Neoadjuvant approaches may be considered after multidisciplinary discussion for patients with high-risk stage III and resectable stage IV melanoma. Consideration for clinical trial enrollment is still preferred for eligible patients with high-risk stage III disease.
For patients with resectable clinically or radiographically detectable stage III disease, standard of care treatment includes TLND (LE:2). There were no positive clinical trial data to support de-escalating the extent of operation, regardless of receipt of neoadjuvant or adjuvant systemic therapy, at the time of manuscript publication.
Stage IV cutaneous melanoma
Prior to the advent of ICIs for the treatment of melanoma in 2011, the 5-year survival rate for patients with metastatic disease was <10%, with a median survival from time of stage IV diagnosis of only 6 to 7.5 months.104 Immunotherapy and targeted therapy have dramatically improved long-term survival outcomes for patients with stage IV disease, with a median OS of up to 72.1 months with ipilimumab plus nivolumab reported in CheckMate 067.6
Initial assessment
In 2018, AJCCv8 further subdivided stage IV melanoma according to metastatic sites and serum LDH level in order to more accurately inform prognosis. The presence of brain metastases (AJCCv8 M1d) confers a particularly poor prognosis and unique treatment challenges. Therefore, in addition to whole body imaging (with either CT chest/abdomen/pelvis or PET) and serum LDH, brain imaging should also be included in the initial staging work up of metastatic melanoma.
Available agents and indications for treatment-naïve disease
Historical use of HD IL-2
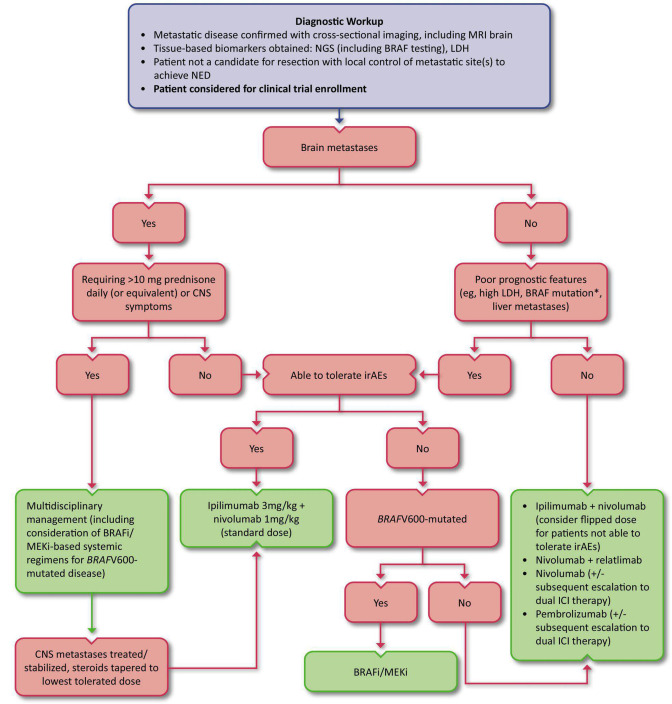
HD IL-2 was the first treatment to provide a life-saving, durable response for a small subset of patients with metastatic melanoma, however, the ORRs were low at only 16%.4 While responses were durable, with 44% of responders surviving beyond 5 years, treatment with HD IL-2 was also associated with a host of life-threatening TRAEs, including hypotension, cardiac arrhythmias, oliguria, volume overload, and bacterial sepsis. The incidence of treatment-related mortality with HD IL-2 is 2%. At the time of guideline publication, as ICIs were widely available and have demonstrated efficacy with tolerable safety, HD IL-2 should not be used in the frontline setting, and numerous alternative options should be considered first. See figure 2 for an algorithm for the first line treatment of unresectable and metastatic melanoma.
Figure 2.
First-line treatment algorithm for unresectable or metastatic cutaneous melanoma. *While the presence of a BRAF mutation is not necessarily a poor prognostic indicator of OS in the context of contemporary BRAF/MEK inhibition, the presence of this mutation does predict a lower response rate to single-agent anti-PD-1. BRAFi, BRAF inhibitor; CNS, central nervous system; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; MEKi, MEK inhibitor; NED, no evidence of disease; NGS, next generation sequencing
ICI monotherapy
Although no longer recommended as frontline monotherapy, the CTLA-4 inhibitor ipilimumab was the first ICI approved by the US FDA in 2011 (see table 2 for a summary of registrational trial data for ICIs in melanoma) based on a phase III study of ipilimumab with or without a glycoprotein peptide vaccine.5 In this study of subsequent-line therapy for patients with advanced melanoma, the median OS for ipilimumab alone was 10.1 months and significantly improved compared with the vaccine arm (HR compared with vaccine alone 0.66; p=0.003). Another phase III study evaluating first-line therapy for patients with advanced melanoma demonstrated a significantly longer 3-year OS rate with ipilimumab plus dacarbazine (20.8%) versus dacarbazine alone (12.2%; HR 0.72; p<0.001).105 Subsequent studies have demonstrated superior efficacy and improved safety with pembrolizumab and nivolumab compared with ipilimumab, and PD-1 inhibitors with or without anti-CTLA-4 therapy have replaced ipilimumab monotherapy in the frontline standard of care setting.
Table 2.
Landmark trials leading to US FDA approvals of ICIs for the treatment of unresectable/advanced/metastatic cutaneous melanoma.
| Trial and US FDA approval date | Key inclusion criteria | Study arms* | Key outcomes | Treatment-related adverse events |
| (NCT00094653)5
March, 2011 Note: first-line ipilimumab monotherapy is no longer considered standard of care in this setting |
HLA-A*0201–positive with unresectable stage III or IV melanoma progressed on a prior regimen for metastatic disease containing one or more of the following: dacarbazine, temozolomide, fotemustine, carboplatin, or IL-2 | Ipilimumab (n=137)† gp100 vaccine (n=136) |
ORR: 10.9% vs 1.5% (p=0.001); CR 1.5% vs 0%; DCR 28.5% vs 11.0% PFS: median 2.86 vs 2.76 months (HR 0.64; p<0.001) OS: median 10.1 vs 6.4 months (HR 0.66; p=0.003); 12-month 45.6% vs 25.3% |
Grade 3–4 irAE: 14.5% vs 3.0% Grade 5: 3.1% vs 1.5% |
| KEYNOTE-002 (NCT01704287)106 September, 2014 |
Progressive disease within 24 weeks after ≥2 ipilimumab doses and, if BRAFV600 mutated, previous treatment with a BRAF or MEK inhibitor or both | Pembrolizumab 2 mg/kg (n=180) Pembrolizumab 10 mg/kg (n=181) ICC with paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or temozolomide (n=179) |
ORR: 21% vs 25% vs 4% Median PFS: 2.9 (HR vs ICC 0.57; 95% CI 0.45 to 0.73, p<0.0001) vs 2.9 (HR vs ICC 0.50; 95% CI 0.39 to 0.64, p<0.0001) vs 2.7 months Median DOR: 3.7 vs 5.4 vs 2.6 months |
Grade 3–4: 11% vs 14% vs 26% Grade 5: 0% for all arms |
| CheckMate 069 (NCT01927419)115 October, 2015‡ |
Advanced melanoma with no prior systemic therapy for unresectable or metastatic disease | Ipilimumab plus nivolumab (n=72 patients with BRAF wild-type disease)§¶ Ipilimumab (n=37 patients with BRAF wild-type disease) § |
ORR: 59.7% vs 10.8% (p<0.0001); CR 16.7% vs 0%; DCR 73.6% vs 43.2% PFS: median 8.9 vs 4.7 months (HR 0.40, 95% CI 0.22 to 0.71; p=0.0012) |
Grade 3–4 (for BRAF WT and MT): 51.1% vs 19.6% |
| CheckMate 066 (NCT01721772)108 November, 2015 |
Unresectable, previously untreated stage III or IV BRAF wild-type melanoma | Nivolumab (n=210) Dacarbazine (n=208) |
ORR: 40.0% vs 13.9% (p<0.001); CR 7.6% vs 1.0%; DCR 56.7% vs 36.1% PFS: median 5.1 vs 2.2 months (HR 0.43; 95% CI 0.34 to 0.56; p<0.001) OS: median NR vs 10.8 months (HR 0.42; 99.79% CI 0.25 to 0.73, p<0.001); 1-year 72.9% vs 42.1% |
Grade 3–4: 11.7% vs 17.6% Grade 5: 0% for both arms |
| KEYNOTE-006 (NCT01866319)137 December, 2015 |
Unresectable stage III or IV melanoma and no more than one previous systemic therapy for advanced disease | Pembrolizumab** (n=277) Ipilimumab (n=278) |
ORR: 32.9% vs 11.9% (p<0.001); CR 6.1% vs 1.4% PFS: median 4.1 mo vs 2.8 months (HR 0.58; 95% CI 0.47 to 0.72; p<0.001) OS: median NR in any arm (HR 0.69; 95% CI 0.52 to 0.90; p=0.0036); 12-month 68.4% vs 58.2% |
Grade ≥3: 10.1% vs 19.9% Grade 5: 0% vs 0.4% |
| CheckMate 067 (NCT01844505)116 January, 2016 |
Stage III (unresectable) or stage IV melanoma and no prior systemic treatment for advanced disease | Ipilimumab plus nivolumab (n=313) ¶ Ipilimumab (n=311) Nivolumab (n=313) |
ORR: 57.6% (ipi/nivo) vs 19.0% (ipi) vs 43.7% (nivo); CR 11.5% (ipi/nivo) vs 2.2% (ipi) vs 8.9% (nivo) PFS: median 11.5 (ipi/nivo; HR vs ipi 0.42 [99.5% CI 0.31 to 0.57; p<0.001]) vs 6.9 (nivo; HR vs ipi 0.57 [99.5% CI 0.43 to 0.76; p<0.001]) vs 2.9 months (ipi) |
Grade 3–4: 55% (ipi/nivo) vs 27.3% (ipi) vs 16.3% (nivo) Grade 5: 0% (ipi/nivo) vs 0.3% (ipi) vs 0.3% (nivo) |
| IMspire150 (NCT02908672)122 July, 2020 |
Unresectable stage IIIC–IV, BRAFV600-mutated melanoma | Atezolizumab plus vemurafenib plus cobimetinib (n=256) Vemurafenib plus cobimetinib (n=258) |
ORR: 66.3% vs 65%; CR 15.7% vs 17.1% Median DOR: 21.0 vs 12.6 months PFS: median 15.1 vs 10.6 months (HR 0.78; 95% CI 0.63 to 0.97, log-rank p=0.025) OS: HR 0.85; 95% CI 0.64 to 1.11; log-rank p=0.23 |
Grade 3–4: 79% vs 73% Grade 5: 0.8% vs 0.4% |
| RELATIVITY-047 (NCT03470922)42 March, 2022 |
Previously untreated advanced melanoma | Nivolumab plus relatlimab (n=355) Nivolumab (n=359) |
PFS: 10.1 vs 4.6 months (HR 0.75, 95% CI 0.6 to 0.9; p=0.0055); 12-month 47.7% vs 36% OS: median NR vs 34.1 months (HR 0.80; 95% CI 0.64 to 1.01) |
Grade 3–4: 18.9% vs 9.7% Grade 5: 0.8% vs 0.6% |
Information presented in this table is based on investigator reviewed data available at the time of each corresponding US FDA approval. Experimental arm data are listed first and in the order in which they appear in the study arms column.
*Therapy-matched placebos are not reported here. With the exception of KEYNOTE-002, patients enrolled in the above trials had not received prior treatment with ICIs.
†gp100 is an HLA-A*0201-restricted melanoma-associated peptide vaccine. gp100 and ipilimumab (dosed at 3 mg/kg) were administered every 3 weeks for up to four (induction) treatments, with re-induction available to eligible patients. Data from a third trial arm, ipilimumab plus gp100, are not reported here as there was no OS difference between the two ipilimumab-containing arms (HR 1.04; p=0.76).
‡Note: this US FDA approval was for BRAF wild-type disease only and was subsequently approved for BRAF-unselected advanced disease in 2016 based on the results of CheckMate 067.
§Patients with BRAFV600-mutated disease were included in this study but are not reported here due to small sample size.
¶Ipilimumab was dosed at 3 mg/kg combined with nivolumab 1 mg/kg every 3 weeks for four doses, followed by nivolumab 3 mg/kg every 2 weeks.
**Pembrolizumab was administered at a dose of 10 mg/kg every 3 weeks. Data from a third trial arm, pembrolizumab 10 mg/kg administered every 2 weeks, are not reported here.
CI, confidence interval; CR, complete response; DCR, disease control rate (complete and partial responses plus stable disease); DOR, duration of response; HR, hazard ratio; ICC, investigator’s choice chemotherapy; ICI, immune checkpoint inhibitor; IL, interleukin; ipi, ipilimumab; irAE, immune-related adverse event; MT, mutated; nivo, nivolumab; NR, not reached; ORR, objective response rate (complete plus partial response); OS, overall survival; PFS, progression-free survival; US FDA, United States Food and Drug Administration; WT, wildtype.
In 2015, the US FDA approved pembrolizumab monotherapy for the treatment of advanced melanoma based on KEYNOTE-006.106 In this phase III open-label study, 834 patients with advanced melanoma were randomized 1:1:1 to receive pembrolizumab 10 mg/kg every 2 weeks, pembrolizumab 10 mg/kg every 3 weeks, or four doses of ipilimumab (3 mg/kg) every 3 weeks. The 6-month PFS rates were similar for the two pembrolizumab groups (47.3% and 46.4% for every 2 and 3 weeks, respectively) and significantly higher compared with the ipilimumab group (26.5%) with a HR for disease progression of 0.58 (p<0.001) for both pembrolizumab groups versus ipilimumab. Estimated 12-month OS rates were 74.1% for pembrolizumab every 2 weeks (HR vs ipilimumab 0.63; 95% CI 0.47 to 0.83; p<0.0005), 68.4% for pembrolizumab every 3 weeks (HR vs ipilimumab 0.69; 95% CI 0.52 to 0.90; p=0.0036), and 58.2% for ipilimumab. The independent data and safety monitoring committee recommended stopping the study early to allow for patients in the ipilimumab group to be offered treatment with pembrolizumab. Furthermore, grade ≥3 TRAEs (a secondary endpoint) were lower in the pembrolizumab groups (13.3% for every 2 weeks and 10.1% for every 3 weeks) than in the ipilimumab group (19.9%). Patients remained on treatment with pembrolizumab for a median of 6.0 months (IQR 2.8–20.3) and ipilimumab for a median of 2.1 months (IQR 1.4–2.1) with 19% of patients in the pembrolizumab groups completing 2 years of treatment.107 At a median follow-up of 57.7 months (IQR 56.7–59.2), the median OS was 32.7 months for the combined pembrolizumab groups and 15.9 months for the ipilimumab group (HR 0.73; 95% CI 0.61 to 0.88; p=0.00049); median PFS also favored the pembrolizumab groups (8.4 months vs 3.4 months; HR 0.57; 95% CI 0.48 to 0.67; p<0.0001).
In 2015, nivolumab monotherapy gained US FDA approval for the first-line treatment of BRAF wild-type, advanced melanoma based on CheckMate 066.108 In this phase III, double-blind study, 418 treatment-naive patients with BRAF wild-type, metastatic melanoma were randomized to receive either nivolumab or dacarbazine. One-year OS rates (72.9% vs 42.1%; HR for death 0.42; 99.79% CI 0.25 to 0.73; p<0.001) and median PFS (5.1 months vs 2.2 months; HR for death or progression of disease 0.43; 95% CI 0.34 to 0.56; p<0.001) both significantly favored nivolumab versus dacarbazine, respectively, and held across multiple prespecified subgroups (including PD-L1 expression). The ORR was 40.0% for nivolumab versus 13.9% for dacarbazine (OR 4.06; p=0.001), with a 7.6% complete response (CR) rate for nivolumab versus 1.0% for dacarbazine. Although both drugs were associated with a high incidence of TRAEs of any grade (74.3% for nivolumab and 75.6% for dacarbazine), only 11.7% of patients experienced a grade 3 or 4 adverse event with nivolumab versus 17.6% of patients in the dacarbazine arm. With a median follow-up of 32.0 months for nivolumab and 17.6 months for dacarbazine, the 5-year OS rates were 39% and 17%, respectively.109 Among 75 nivolumab-treated patients alive and evaluable at the 5-year analysis, 83% had not received subsequent therapy; 23% were still on study treatment, and 60% were treatment-free.
Of note, desmoplastic melanoma, which is characterized by a dense fibrous collagen matrix, is associated with a high mutational load secondary to ultraviolet exposure and is particularly susceptible to anti-PD-(L)1 monotherapy. In one study of 60 patients with advanced desmoplastic melanoma who received an anti-PD-1 ICI, an objective response rate of 70% (95% CI 57% to 81%) and a complete response rate of 32% was observed, as well as a higher percentage of PD-L1-positive cells in the tumor parenchyma of desmoplastic versus non-desmoplastic melanomas.110 Furthermore, a prospective study of 27 patients with resectable desmoplastic melanoma who received neoadjuvant pembrolizumab and underwent a wide resection had a pathologic CR (pCR) rate of 56% (95% CI 35% to 75%) and none of the patients became inoperable.111
ICI combinations
Compared with ICI monotherapy, combination immune checkpoint blockade increases ORR and duration of response (DOR)6 112 either by maximizing the chance of response to either drug independently113 or through true synergy.114 In 2015 the US FDA granted accelerated approval to the combination of nivolumab plus ipilimumab for treatment-naïve, advanced BRAF wild-type melanoma, and in 2016 this indication was expanded to include BRAFV600-mutated melanoma. The accelerated approval for BRAF wild-type disease was based on CheckMate 069, a double-blind phase II study in which treatment-naïve patients with advanced melanoma were randomized 2:1 to receive ipilimumab 3 mg/kg plus nivolumab 1 mg/kg (combination group) versus ipilimumab 3 mg/kg plus placebo (ipilimumab group).115 The ORR for BRAF wild-type tumors was 60% (including 16.7% CRs) in the combination group versus 11% (and no CRs) in the ipilimumab group (p<0.0001), with a median PFS of 8.9 months for the combination versus 4.7 months for ipilimumab (HR 0.40; 95% CI 0.22 to 0.71; p=0.0012). In the double-blind phase III CheckMate 067 study, 945 treatment-naïve patients with advanced melanoma were randomized 1:1:1 to receive nivolumab plus ipilimumab versus either nivolumab or ipilimumab alone.116 The median PFS (a primary endpoint) was significantly longer for nivolumab compared with ipilimumab (6.9 vs 2.9 months) with a HR of 0.57 (99.5% CI 0.43 to 0.76; p<0.001); median PFS was also significantly longer for the combination arm compared with ipilimumab monotherapy (11.5 months vs 2.9 months; HR 0.42; 99.5% CI 0.31 to 0.57; p<0.001). Of note, CheckMate 067 was not designed for formal statistical comparison between the nivolumab and ipilimumab plus nivolumab arms. A sufficient number of patients with BRAFV600-mutated disease (n=298) were included in CheckMate 067 for survival analyses, which demonstrated that median PFS was similar for patients with BRAFV600-mutated and BRAF wild-type disease (11.7 months and 11.2 months, respectively). Furthermore, the survival benefit with combination immunotherapy has been remarkably durable. With a minimum follow-up of 6.5 years, the median OS for patients in the ipilimumab plus nivolumab arm was 72.1 months versus 36.9 months for patients in the nivolumab arm and 19.9 months for patients in the ipilimumab arm.6 Consistent with other studies, the incidence of grade 3–4 TRAEs was highest in the ipilimumab plus nivolumab arm (55.0%), and 36.4% of patients receiving this combination discontinued the study drug due to a TRAE. Grade 3 or 4 TRAEs occurred at a rate of 16.3% in the nivolumab group (with 7.7% treatment discontinuation due to a TRAE) and 27.3% in the ipilimumab group (with 14.8% treatment discontinuation due to a TRAE).
Alternate dosing regimens have also been evaluated with the goal of reducing the incidence of TRAEs. In CheckMate 511, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (ie, ‘flipped dose’) demonstrated a significant decrease in grade ≥3 TRAEs (33.9% vs 48.3%; OR 0.55; 95% CI 0.36 to 0.84) compared with the standard dosing of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.117 While this trial was not designed for formal comparisons of efficacy endpoints across arms, median OS was not reached in either group and median PFS was 10.2 (IQR 6.2–21.9) and 10.0 (IQR 6.3–40.9) months for flipped and standard dosing, respectively. The ORRs for standard dosing and flipped dosing were 53% and 47%, respectively. These data must be considered in context (eg, patients with active Stage M1D and uveal melanoma were excluded and mucosal melanomas were not represented) and are less robust (median follow-up 44.4 months, n=180, and median OS not reached [NR] for flipped dose) compared with the 6.5-year minimum follow-up available from CheckMate 067 (n=314 and median OS 72.1 months for standard dosing).6
Furthermore, when considering frontline combination ICI regimens, there are no head-to-head data comparing nivolumab plus ipilimumab at either flipped or standard dosing to nivolumab plus relatlimab (for more details on nivolumab plus relatlimab, see discussion of the RELATIVITY-047 trial, in subsequent paragraphs). The Expert Panel strongly recommended the standard dosing of nivolumab plus ipilimumab for patients with melanoma brain metastases (MBMs) eligible for systemic ICI therapy and for patients with high-risk features (eg, high LDH, mucosal or acral subtypes, and liver metastases). The majority of the Expert Panel considered standard dosing to be the default regimen for patients without brain metastases as well, noting that this is the dosing supported by the US FDA. However, considering the favorable toxicity profile of flipped dose nivolumab plus ipilimumab in context of the unknown OS data with this regimen, many among the Expert Panel had adapted their practice at the time of guideline publication to use standard dosing for patients with higher risk disease and reserve flipped dosing or nivolumab plus relatlimab for patients with lower risk disease or who may not tolerate high-grade irAEs associated with higher doses of ipilimumab (eg, colitis).
Sequencing induction therapy with nivolumab followed by a planned switch to ipilimumab (or vice versa) has been evaluated as an alternative to combined PD-1/CTLA-4 blockade.118 In the open-label phase II CheckMate 064 trial, 140 patients were randomized 1:1 to receive induction with six doses of nivolumab followed by four doses of ipilimumab versus four doses of ipilimumab followed by six doses of nivolumab. Both groups received nivolumab maintenance thereafter until progression of disease or dose-limiting toxicity. No treatment-related deaths occurred in either group and the treatment-related grade ≥3 adverse event rate occurring during the induction period (ie, until week 25, a primary endpoint) was similar in the nivolumab followed by ipilimumab group (50%; 95% CI 37.6 to 62.4) versus the ipilimumab followed by nivolumab group (43%; 95% CI 31.1 to 55.3). The 25-week response rate (secondary endpoint), however, was higher for nivolumab followed by ipilimumab (41%; 95% CI 29.4 to 53.8) than for ipilimumab followed by nivolumab (20%; 95% CI 11.4 to 31.3). At week 25, progression was reported in only 38% of patients in the nivolumab followed by ipilimumab group versus 60% of patients in the ipilimumab followed by nivolumab group. After a median follow-up of 19.8 months (IQR 12.8–25.7) and 14.7 months (IQR 5.6–23.9), respectively, the median OS (a prespecified exploratory endpoint) was not reached in the nivolumab followed by ipilimumab group versus 16.9 months in the ipilimumab followed by nivolumab group (HR 0.48; 95% CI 0.29 to 0.80). The 12-month OS rate was similarly higher in the nivolumab followed by ipilimumab group (76%; 95% CI 64% to 85% versus 54%; 95% CI 42% to 65%). Final analyses from CheckMate 064 are pending. Studies evaluating the combination of pembrolizumab with ipilimumab, including KEYNOTE-029, may provide other PD-1/CTLA-4 inhibitor combinations with acceptable efficacy and safety profiles.119
In March 2022, the US FDA approved a fixed-dose combination of relatlimab (an anti-LAG-3 checkpoint inhibitor) and nivolumab for the treatment of unresectable or metastatic melanoma based on RELATIVITY-047.42 In this phase II/III, double-blind study, 714 patients with treatment-naïve advanced melanoma were stratified by LAG-3 and PD-L1 expression, BRAF mutation status, and AJCC M stage and randomized to receive either nivolumab plus relatlimab or nivolumab monotherapy. At a median follow-up of 19.3 months, median PFS (primary study endpoint) was reported at 10.2 months versus 4.6 months for relatlimab plus nivolumab and nivolumab, respectively (HR 0.78; 95% CI 0.64 to 0.94).120 In prespecified exploratory subgroup analyses, the difference in PFS between treatment arms was not significant for tumors expressing PD-L1≥1%, however, the PFS for PD-L1-negative tumors significantly favored the nivolumab plus relatlimab combination over nivolumab monotherapy (HR 0.68; 95% CI 0.53 to 0.86). While the incidence of grade 3 and 4 TRAEs was higher in the relatlimab plus nivolumab group (21.1% vs 11.1% for nivolumab monotherapy), this rate is much lower than rates of severe TRAEs reported with ipilimumab plus nivolumab. Studies of other LAG-3/PD-1 checkpoint inhibitor combinations are ongoing.121
Triplet regimens combining BRAFi/MEKi and PD-(L)1 inhibitors have been studied as first-line options for patients with advanced BRAFV600-mutated melanoma. Although one such regimen has gained US FDA approval, triplet therapy is not commonly used due to inconsistent PFS data between studies122–124 and a lack of OS data,122 as well as increased toxicity.123 124 In the phase III IMspire150 trial122 leading to US FDA approval (July 2020) of atezolizumab plus cobimetinib and vemurafenib, the investigator-assessed median PFS (primary endpoint) was significantly improved at 15.1 months for the atezolizumab plus BRAFi/MEKi group versus 10.6 months for the BRAFi/MEKi control group (HR 0.78; 95% CI 0.63 to 0.97; p=0.0249). However, PFS assessed by the independent review committee (secondary endpoint) failed to reach statistical significance (16.1 vs 12.3 months; HR 0.85; 95% CI 0.67 to 1.07; log-rank p=0.16). Rates of severe TRAEs were similar at 33.5% for the atezolizumab group versus 28.8% for the control group. The phase II KEYNOTE-022 study of 120 patients with BRAFV600-mutated advanced melanoma similarly demonstrated improved efficacy with the addition of pembrolizumab to dabrafenib plus trametinib (median PFS was 16.9 months with triplet therapy vs 10.7 months with doublet therapy; HR 0.53; 95% CI 0.34 to 0.83).123 However, the rate of grade ≥3 TRAEs was substantially higher for the triplet regimen (58% for triplet therapy vs 25% for doublet therapy), and this regimen is not US FDA approved. COMBI-I, a phase III study evaluating dabrafenib and trametinib with or without the anti-PD-1 spartalizumab for BRAFV600-mutated advanced treatment-naïve melanoma124 did not meet its primary endpoint of PFS (16.2 months for the spartalizumab group vs 12.0 months for dabrafenib and trametinib alone; HR 0.82; 95% CI 0.655 to 1.027; p=0.042). Grade ≥3 TRAEs occurred in 55% of the patients receiving spartalizumab versus 33% of patients in the control arm. Study investigators noted that ‘adverse event management was challenging and resulted in frequent dose adaptations’. The addition of a MEKi to PD-1 inhibition in patients with advanced BRAF wild-type disease in the phase III IMspire170 trial did not improve PFS and doubled the rate of grade ≥3 TRAEs.125 The Expert Panel agreed that the remarkable benefit of ipilimumab plus nivolumab in this subgroup as well as the toxicity of a triplet regimen outweighed any potential PFS benefit for triplet therapy, and sequential ICI therapy followed by targeted therapy is strongly preferred for patients with advanced BRAFV600-mutated melanoma (for more discussion of treatment sequencing, see the Sequencing of targeted therapy and ICIs for BRAFV600-mutated disease section). However, there were some specific circumstances in which the use of triplet therapy for patients with advanced, BRAFV600-mutated disease might be considered. These scenarios included treatment-naïve patients with rapidly progressing, bulky, or highly symptomatic disease who have a low chance of survival to second-line therapy or pretreated patients whose disease is progressing on BRAFi/MEKi and may worsen with removal of targeted therapy. Triplet therapy may also be considered in some cases for treatment-naïve patients with symptomatic brain metastases who may be requiring steroids based on data from the phase II TRICOTEL study.126
Sequencing of targeted therapy and ICIs for BRAFV600-mutated disease
ICIs (including combination ipilimumab plus nivolumab) and BRAF/MEK inhibition are both recognized as preferred first-line strategies for the treatment of BRAFV600-mutated melanoma and many patients will eventually receive both therapies. Before 2022, however, sparse data were available to guide optimal sequencing of these two different treatment strategies. The phase III DREAMseq/ECOG-ACRIN 6134 study (NCT02224781) demonstrated superior PFS outcomes when immunotherapy is administered in the first line and targeted therapy is given upon progression of disease for advanced, untreated, BRAFV600-mutated melanoma. With a median follow-up of 27.7 months, the 2-year OS rates (the study’s primary endpoint) were 72% for the ipilimumab plus nivolumab first group versus 52% for the dabrafenib plus trametinib first group (log-rank p=0.0095), prompting the safety monitoring committee to recommend halting study accrual given the dramatic survival improvement demonstrated for the ICI first arm.127 Notably, ORRs for the immunotherapy component were much lower when administered after targeted therapy (see the Progression following BRAF-targeted treatment section, below). Grade ≥3 TRAEs were slightly higher for the ipilimumab plus nivolumab first group at 60% with two treatment-related deaths (vs 52% with one treatment-related death for the dabrafenib plus trametinib first group). The phase II SECOMBIT study evaluated sequencing of ipilimumab plus nivolumab and binimetinib plus encorafenib, and is notable for the inclusion of a ‘sandwich’ arm where an 8-week induction course of binimetinib plus encorafenib was given followed by a planned switch to ipilimumab plus nivolumab until progression of disease (POD) followed by binimetinib plus encorafenib (Arm C), a treatment strategy that may be well-suited to rapidly progressive disease.128 With a median follow-up of 32.2 months, the primary OS endpoint was met for all arms, with 2-year OS rates of 65% for targeted therapy followed by dual ICI on POD, 73% for dual ICI followed by targeted therapy on POD, and 69% for the sandwich arm (Arm C).129 Similar to DREAMseq, ORRs to ipilimumab plus nivolumab in SECOMBIT were lower in the arm where patients received targeted therapy until POD compared with the first-line immunotherapy and sandwich arms.
While the above data suggest that ICI therapy should be administered prior to targeted therapy for patients with advanced BRAFV600-mutated melanoma, there are certain instances in which frontline BRAF/MEK inhibition might be considered. One such instance is for patients who are unable to safely taper their immunosuppression (eg, patients with symptomatic brain metastases who are unable to reduce their corticosteroid dose to ≤10 mg prednisone per day or equivalent). The sequencing of therapy for patients with BRAFV600-mutated melanoma must be considered on a case-by-case basis, taking into account patient comorbidities, disease characteristics, and drug toxicity profiles.
Panel recommendations
Regardless of BRAFV600 mutation status, either single-agent anti-PD-1 therapy (LE:2) or front-line combination therapy with either ipilimumab plus nivolumab (LE:2) or nivolumab plus relatlimab (LE:2) is recommended, depending on the clinical scenario.
For first-line therapy of stage IV melanoma, ipilimumab plus nivolumab is preferred over other anti-PD-1-based regimens in patients with poor prognostic features such as liver metastases, brain metastases, BRAF mutation, or high LDH.
For patients with melanoma with poor prognostic features in whom combination therapy is desired but who may not tolerate TRAEs (ie, elderly patients or patients with poor Eastern Cooperative Oncology Group performance status [ECOG PS]), treatment with nivolumab plus relatlimab is a preferred combination regimen.
For patients with low volume melanoma or histology that has demonstrated exceptional responses to anti-PD-1 monotherapy (desmoplastic melanoma), or for patients who are less likely to tolerate high-grade irAEs (eg, patients with a poor ECOG PS or concurrent autoimmune comorbidities), single agent anti-PD-1 therapy may be considered in the frontline.
For patients with BRAFV600-mutated melanoma, despite the approval for vemurafenib, cobimetinib, and atezolizumab, the role of triplet therapy (as opposed to sequential combination ICI therapy followed by targeted therapy) is not clear but may be considered in selected patients (LE:2).
Patients with CNS metastases
About 28.2% of patients with de novo metastatic melanoma have CNS metastases,130 with higher rates of CNS involvement further in disease progression.131 Patients with treated, small, or minimally symptomatic MBMs may be considered for upfront systemic treatment to address both intracranial and extracranial disease. An intracranial clinical benefit rate of 57% (26% CR, 30% partial response [PR], 2% stable disease [SD]) and an extracranial clinical benefit rate of 56% was demonstrated among the 94 patients with asymptomatic MBMs receiving ipilimumab plus nivolumab in CheckMate 204, with CNS grade 3–4 TRAEs observed in only 7% of participants.132 Targeted therapy must also be considered for patients with MBMs who have BRAFV600-mutated disease, as the phase II COMBI-MB trial demonstrated an investigator-assessed intracranial response rate of 58% in the cohort of patients with asymptomatic, V600E-mutated melanoma and no prior local brain therapy and 59% in the cohort of patients with symptomatic disease with or without prior local brain therapy.133 Of note, when dual ICI therapy and targeted therapy are both feasible and safe options for patients with MBMs, this panel prefers dual ICI therapy due to the improved PFS rates reported in CheckMate 204. A meta-analysis of 15 trials including 1,132 patients with MBMs (both symptomatic and asymptomatic) demonstrated a statistically significant improvement in both PFS and OS with combination anti-PD-1 plus anti-CTLA-4 ICIs compared with ICI monotherapy or targeted therapy.134 Of note, patients receiving high-dose corticosteroids were not included in this analysis. In contrast, cohort 2 of the phase II Tricotel study evaluated the safety and efficacy of a triplet regimen (atezolizumab plus cobimetinib plus vemurafenib) for patients with BRAFV600-mutated melanoma metastatic to the brain, including patients with symptomatic MBMs.126 A total of 65 enrolled patients (17% of whom were receiving corticosteroids and 40% of whom had symptomatic CNS metastases) achieved an intracranial ORR of 42% per independent review committee (IRC; primary study endpoint) and 49% by investigator assessment, with an IRC assessed intracranial ORR of 35% in patients with symptomatic CNS metastases at baseline and 46% in asymptomatic patients. The ongoing randomized phase II SWOG S2000 study is evaluating triplet therapy (binimetinib plus encorafenib plus nivolumab) versus ipilimumab plus nivolumab for patients with BRAFV600-mutated melanoma and MBMs (NCT04511013). Notably, patients enrolled in this study may be using up to 8 mg of dexamethasone per day and may have leptomeningeal disease (LMD).
Limited data are available to inform treatment decisions for LMD, which confers a particularly poor prognosis with a median survival of 1.8 months from the time of diagnosis of CNS involvement.135 Ongoing studies are evaluating the efficacy and safety of systemic ipilimumab plus nivolumab (NCT02939300), pembrolizumab (NCT03091478), and avelumab with radiotherapy (NCT03719768) for the treatment of LMD. A median OS of 9.1 months was reported in one early study of intrathecal IL-2 used to treat 42 patients with LMD.136 Treatment with intrathecal immune checkpoint blockade is now being evaluated in the NCT03025256 study of intrathecal plus intravenous nivolumab for patients with melanoma LMD.
Panel recommendations
For patients with MBMs, initial evaluation should include patient factors such as neurological symptoms, performance status, and corticosteroid use. The optimal combination and sequencing of treatments such as surgery, stereotactic radiosurgery, and systemic treatment, is currently not established and warrants expeditious multidisciplinary discussion and collaborative management. For patients with MBMs for whom systemic therapy is considered appropriate following expedited multidisciplinary evaluation, the sequencing of targeted therapy (for BRAFV600-mutated disease) versus ICIs should be considered on a case-by-case basis.
For patients with asymptomatic MBMs for whom steroids have been tapered to the lowest tolerated dose and for whom potential toxicities are tolerable, ipilimumab plus nivolumab is recommended in the frontline (LE:1). There are no data supporting the use of nivolumab plus relatlimab in patients with MBMs. Multidisciplinary management is required for management of all patients with MBMs.
For patients with MBMs, ipilimumab should be dosed at 3 mg/kg in combination with nivolumab 1 mg/kg (ie, standard dosing).
Available agents and indications for previously-treated disease
Upon progression of disease, patients with melanoma should undergo restaging—including cross-sectional body imaging and an MRI of the brain—and progression should be confirmed with subsequent imaging (see the Evaluation and management of response to immunotherapy section). NGS results should be reviewed to help inform selection of subsequent-line treatment, which may entail clinical trial enrollment. See figure 3 for a testing and treatment algorithm for previously-treated, unresectable/metastatic cutaneous melanoma.
Figure 3.
Subsequent line treatment algorithm for unresectable or metastatic cutaneous melanoma. *There are no data to guide treatment for progression on first-line nivolumab plus relatlimab. Referral for clinical trial is preferred in this scenario. †The decision to continue ICI therapy beyond initial radiographic progression should be based on melanoma-associated symptoms, disease kinetics, and the presence or absence of irAEs. ‡Progression of disease on BRAF/MEK inhibition that may worsen with removal of targeted therapy is one specific circumstance in which this Expert Panel would consider the use of triplet therapy. §Primary resistance is defined as: best response of PD or SD for <6 months following at least 6 weeks of drug exposure. Secondary resistance is defined as: CR, PR, or SD for >6 months following at least 6 weeks of drug exposure.139 ¶ Ipilimumab monotherapy and ipilimumab plus pembrolizumab are other regimens that have demonstrated some efficacy for patients with advanced melanoma that has progressed on anti-PD1 therapy. BRAF/MEK inhibition could be considered as well in patients with BRAFV600-mutated disease in need of rapid response. CNS, central nervous system; CR, complete response; CTLA-4, cytotoxic T lymphocyte antigen-4; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; LDH, lactate dehydrogenase; NED, no evidence of disease; NGS, next generation sequencing; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein 1; PR, partial response; SD, stable disease.
Progression following prior anti-CTLA-4 monotherapy
Ipilimumab monotherapy is no longer recommended for the first-line treatment of metastatic melanoma. However, responses to anti-PD-1 ICIs have been demonstrated in tumors that progress after anti-CTLA-4 therapy. In the phase II KEYNOTE-002 study, patients with advanced, ipilimumab-refractory melanoma were randomized to receive pembrolizumab (2 mg/kg or 10 mg/kg) or investigator’s choice of chemotherapy.137 PFS in the ITT population, the study’s primary endpoint, was significantly improved for both the 2 mg/kg (HR 0.57; 95% CI 0.45 to 0.73; p<0.0001) and the 10 mg/kg (HR 0.50; 95% CI 0.39 to 0.64; p<0.0001) pembrolizumab groups compared chemotherapy at second interim analysis. At the time of final analysis, with a median follow-up of 28 months, however, the median OS was not significantly different between the three arms.138
Progression following prior anti-PD-1 ICI treatment
ICIs are now the standard of care frontline treatment for metastatic melanoma. Additionally, an ever-increasing number of patients with resectable disease receive ICIs in the adjuvant setting and on clinical trial in the neoadjuvant setting, yet sparse data are available to inform selection of therapy for anti-PD-1-resistant disease. Definitions of resistance to anti-PD-1 therapy have been developed by SITC to inform future trial design and drug development in this challenging setting.139–141 These definitions differentiate between primary and secondary resistance based on drug exposure and maximal benefit obtained, with confirmed progressive disease (CPD, see the Evaluation and management of response to immunotherapy section) or SD lasting less than 6 months after at least 6 weeks on treatment defining primary resistance and progression after clinical benefit (ie, CR, PR, or SD lasting longer than 6 months) defining secondary resistance.
Rechallenge with ICI
While subsequent response rates are generally lower, rechallenge with a second course of anti-PD-1 therapy may produce responses for some patients, particularly for those whose tumors do not have primary resistance. Furthermore, patients who had an initial complete or partial response to anti-PD-1 therapy have demonstrated higher rates of response to anti-PD-1 rechallenge compared with patients whose best response to initial therapy was SD. In the EORTC 1325/KEYNOTE-054 study, 20 patients whose disease recurred (without brain metastases) ≥6 months following completion of 1-year of adjuvant pembrolizumab received a second course of pembrolizumab.142 With a median follow-up time of 19 months, the median PFS for these rechallenge patients was 4.1 months. Among the nine patients with evaluable stage IV recurrent tumors, there was one CR, three SDs, and five PDs by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. In KEYNOTE-006, 13 patients who were initially randomized to receive pembrolizumab received a second course of the drug.107 In an exploratory analysis there were three CRs (two of which were surgical CRs prior to initiation of second course pembrolizumab), four PRs, three SDs, and one PD, with assessment pending for two patients.
Standard of care treatment for patients whose disease has progressed during or following adjuvant anti-PD-1 therapy is less established, and clinical trial participation is preferred in this instance. If clinical trial enrollment is not feasible, then available treatment options for these patients include ipilimumab plus nivolumab, ipilimumab plus pembrolizumab, nivolumab plus relatlimab, combination BRAF plus MEK inhibition for patients with BRAFV600-mutated disease, or single agent anti-PD-1 rechallenge (for patients who experience late recurrence). A large, multi-institutional study that followed about 850 consecutive patients with resected stage III/IV melanoma who received adjuvant anti-PD-1 therapy reported that 17% of patients experienced a recurrence of their disease, and cutaneous melanoma recurrences occurred during receipt of adjuvant therapy 76% of the time.143 For the patients whose disease recurred during receipt of adjuvant therapy and then went on to receive subsequent systemic therapy, none (0 out of 6) responded to single agent anti-PD-1, 24% (8 out of 33) responded to ipilimumab (alone or in combination with an anti-PD-1 ICI), and 78% (18 out of 23) responded to BRAFi/MEKi. For those patients whose disease recurred following discontinuation of adjuvant anti-PD-1 therapy, 40% (2 out of 5) responded to single agent anti-PD-1, 40% (2 out of 5) responded to ipilimumab-based therapy, and 90% (9 out of 10) responded to BRAF/MEK inhibitors.
Rechallenge with an anti-PD-1 agent in combination with anti-CTLA-4 or anti-LAG-3 may offer benefit to some patients by overcoming immune suppression due to alternate checkpoints. A large, multicohort, retrospective study including 355 patients whose unresectable stage III or IV melanoma had progressed following anti-PD-1 therapy received in the adjuvant or metastatic setting demonstrated a significantly higher ORR with ipilimumab plus anti-PD-1 compared with ipilimumab monotherapy (31% vs 13%; p<0.0001).144 SD was achieved in an additional 9% of patients in the ipilimumab plus anti-PD-1 group and an additional 14% of patients in the ipilimumab group. Both median PFS (3.0 vs 2.6 months; HR 0.69; 95% CI 0.55 to 0.87; p=0.0019) and median OS (20.4 vs 8.8 months; HR 0.50; 95% CI 0.38 to 0.66; p<0.0001) were significantly improved for the ipilimumab plus anti-PD-1 group. The incidence of grade ≥3 TRAEs was similar for both groups (31% and 33% for combination and monotherapy, respectively). The phase II SWOG S1616 study randomized 92 patients with advanced melanoma with primary resistance to anti-PD-1 therapy 3:1 to receive ipilimumab plus nivolumab (followed by nivolumab maintenance) versus ipilimumab monotherapy.145 At a median follow-up of 25.3 months, the PFS HR was 0.63 (90% CI 0.41 to 0.97) favoring the combination. Milestone 6-month PFS rate estimates were 34% versus 13% and the ORRs were 28% versus 9% for combination treatment versus ipilimumab monotherapy, respectively. Low-dose (1 mg/kg) ipilimumab plus pembrolizumab demonstrated a response rate of 29% by immune-related RECIST (irRECIST) with a median DOR of 16.6 months in responding patients in a prospective phase II, single-arm, open-label trial that included 70 patients with advanced melanoma whose disease had progressed on prior anti-PD-1 monotherapy or combination therapy.146 Median PFS was 5.0 months and median OS was 24.7 months. Of note, the ORR for the subgroup of patients who had received adjuvant anti-PD-1 therapy (n=13) was 15%. While FDA-approved in the frontline metastatic setting, relatlimab plus nivolumab has also demonstrated activity in the anti-PD-1-resistant setting. In 351 patients with disease that progressed on one prior line of anti-PD-1-based therapy, the ORR for this combination was 12% with a median PFS of 2.1 months.147
HD IL-2
HD IL-2 is no longer a standard of care first-line treatment option, although its use in ICI-refractory disease has been studied retrospectively. One query of the PROCLAIM database identified 40 patients with metastatic melanoma who had received HD IL-2 following anti-PD-(L)1 therapy and found a best overall response of CR in 10%, PR in 13%, and SD in 37%.148 A prospective phase II trial (NCT04562129) is evaluating the efficacy and safety of administering combined HD IL-2 plus low-dose ipilimumab followed by sequential nivolumab in unresectable stage III or stage IV melanoma that progressed on prior anti-PD1 therapy.
Second-line therapy for patients with BRAFV600-mutated melanoma
With 265 out of 300 patients enrolled, the DREAMseq study of patients with advanced, BRAFV600-mutated melanoma demonstrated a lower ORR for nivolumab plus ipilimumab when sequenced after progression of disease on dabrafenib plus trametinib (30% vs 46% when given in the frontline).127 The ORR for dabrafenib plus trametinib was not substantially different whether given after nivolumab plus ipilimumab or in the frontline (48% vs 43%, respectively). Importantly, frontline ICI therapy followed by BRAF/MEK inhibition (vs the reverse sequence) improved 2-year OS for patients with BRAFV600-mutated disease (see the Sequencing of targeted therapy and ICIs for BRAF V600-mutated disease section, above). The single arm phase II TRIDeNT study is evaluating the role of combination dabrafenib/trametinib plus nivolumab triplet therapy in patients with advanced, BRAFV600-mutated melanoma following disease progression on immunotherapy as well as in the frontline setting.149 Among 16 evaluable patients with anti-PD-1-refractory disease, the ORR was 88% (including two CRs) and median PFS was 8.2 months. Median OS was not reached at a median follow-up of 18.4 months. No statistically significant differences in OS were seen across patients with and without brain metastases or prior anti-PD-1 exposure.
Emerging data on immunotherapy for previously-treated stage IV disease
ICI-based combinations
The phase II, single-arm, open-label LEAP-004 study evaluated the efficacy and safety of the multikinase inhibitor lenvatinib plus pembrolizumab in 103 patients with unresectable stage III to IV melanoma and confirmed PD on anti-PD-(L)1 therapy.150 At a median follow-up of 15.3 months, the ORR was 21.4% (including three CRs). The ORRs across subgroups were 33.3% for patients who had received prior anti-PD-1 plus anti-CTLA-4 therapy (n=30), 18.2% for patients who received anti-PD-(L)1 therapy in the adjuvant setting only (n=11), 22.6% for patients with primary anti-PD-(L)1 resistance (n=62), and 22.7% for patients with secondary anti-PD-(L)1 resistance (n=22). The disease control rate (DCR) was 66.0%, the median DOR was 8.2 months, and an estimated 37.2% of patients had an ongoing DOR at ≥9 months. Of note, there was one treatment-related death in the study and the rate of grade 3–4 TRAEs was 45.6%, with 56.3% of patients requiring a lenvatinib dose reduction. Blockade of alternate checkpoints is another strategy to overcome resistance to anti-PD-1 therapy. The anti-LAG-3 relatlimab in combination with nivolumab is US FDA-approved, and other anti-LAG-3 agents are being developed as well.121.
Adoptive cell therapies
Novel immunotherapeutic strategies are under investigation to address the ongoing unmet need for effective treatment of patients with ICI-resistant disease. The tumor-infiltrating lymphocyte (TIL) therapy, lifileucel, has demonstrated promising results for these pretreated patients. In a multicenter, phase II study, 153 patients whose melanoma had progressed after a median of three lines of therapy (81.7% received both anti-PD-1 and anti-CTLA-4 therapy) received a non-myeloablative lymphodepletion regimen, a single lifileucel infusion, and up to six doses of HD IL-2.151 These patients had an ORR of 31.4% (8 CRs and 40 PRs) and at a median follow-up of 27.6 months, 41.7% of the responses had been maintained for ≥18 months. A phase III randomized study compared non-myeloablative, lymphodepleting chemotherapy followed by HD IL-2 followed by TIL therapy versus ipilimumab in 168 patients with melanoma largely (86%) refractory to anti-PD-1 therapy.152 After a median follow-up of 33 months, PFS (primary endpoint) was significantly longer for the TIL arm (7.2 vs 3.1 months; HR 0.50; p<0.001).153
Panel recommendations
Patients with metastatic melanoma should be fully restaged (including a restaging brain MRI) at the time of disease progression.
For all patients with advanced melanoma whose disease has progressed on any anti-PD-1-based ICI therapy without an anti-CTLA-4 agent, there is no clear standard of care subsequent line therapy and thus treatment with ipilimumab plus nivolumab (LE:2), BRAF-targeted agents (if appropriate) if not already done (LE:2), or enrollment in clinical trials evaluating strategies including adoptive cell therapies, novel combinations, and other strategies, should be strongly encouraged in shared decision-making with the patient.
-
For all patients with advanced melanoma whose disease has progressed on anti-PD-1 therapy and clinical trial enrollment is not feasible, dual ICI therapy or BRAF-targeted therapy (if appropriate) should be considered, with choice of therapy taking anticipated toxicities and phenotype of resistance (primary versus secondary) into account.
For patients whose best response is PD or <6 months of SD following at least 6 weeks of therapy with a single anti-PD-1 agent (ie, primary anti-PD-1 ICI resistance), combination ipilimumab plus nivolumab is preferred (LE:2) and ipilimumab monotherapy (LE:2) or ipilimumab plus pembrolizumab (LE:3) can be considered.
For patients who initially benefited from anti-PD-1-based monotherapy for at least 6 months, discontinued therapy, and then ultimately progressed, re-induction with single-agent anti-PD-1 can be considered on progression of disease (LE:3).
Intratumoral immunotherapy in melanoma
Melanoma is a disease characterized by locoregional spread to regional LNs and intralymphatic spread (in-transit and satellite). Satellites around a primary melanoma or ITMs between the primary melanoma site and the regional LN basin may both represent intralymphatic metastases that portend a relatively poor prognosis.82 154 155 Patients with in-transit melanoma are at high risk for further locoregional and distant recurrence, and data suggest that there is no substantial survival difference between melanomas with ITMs and satellites.154 It is also important to note that many patients with melanoma relapse with in-transit/satellite and/or subcutaneous metastases. The optimal management of patients with in-transit/satellite and/or subcutaneous metastases is challenging and depends on the size, number, and clinical behavior of the locoregional disease and the presence of other metastases. Taking these factors into account, intratumoral (IT) therapy should be considered in the multidisciplinary management of locoregionally-advanced melanoma, and other options include isolated limb infusion, isolated limb perfusion, and stereotactic body radiation therapy as well as systemic therapy.
Historical context on local immunotherapy approaches
IT therapy has been used for many years to treat cancer. All IT agents evaluated to date have increased inflammatory cells in the tumor microenvironment (TME) and perhaps enhanced antigen presentation and inflammatory cytokines. Experience with BCG, an attenuated form of Mycobacterium bovis, has demonstrated some efficacy in the treatment of melanoma, but today it is primarily used in the treatment of early bladder cancer. IL-2 has been successfully injected into melanoma lesions with a pCR rate of 32% in a small series. There have also been promising data on the use of the topical immunotherapeutic contact sensitizer diphencyprone (DPCP) and injected 10% Rose Bengal (PV-10) to elicit antitumor T-cell responses via release of danger-associated molecular patterns.156 157 This guideline will focus on the newer agents available for IT therapy, particularly the US FDA-approved agent talimogene laherparepvec (T-VEC).
T-VEC in melanoma
T-VEC is the first and only US FDA-approved oncolytic virus at the time of guideline publication, indicated for the IT treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma. T-VEC is derived from a genetically modified oncolytic type 1 herpes simplex virus (HSV-1), selected for in vitro oncolytic activity. The oncolytic HSV-1 was further modified by deletion of viral infected cell protein (ICP) 34.5 and 47 genes, and insertion of two copies of the human cytokine granulocyte macrophage-colony stimulating factor (GM-CSF) gene, which collectively improves tumor-selective replication, major histocompatibility complex class I-associated antigen presentation, and promotion of dendritic cell recruitment and activation.158 159
Following dose-finding studies, T-VEC was approved by the US FDA for local treatment of melanoma that recurred after initial surgery in 2015 based on the results of OPTiM—a randomized, open-label, phase III study in unresectable stage IIIB through IVM1c melanoma with superficially accessible metastases.160 Four hundred and thirty-six patients were randomized 2:1 to receive either IT T-VEC once every 2 weeks or subcutaneous GM-CSF once every 14 days in 28-day cycles, respectively. Patients were required to have at least one cutaneous, subcutaneous, or nodal lesion ≥10 mm in diameter, with large and new lesions prioritized for injection. Most enrolled patients were HSV-1 seropositive at baseline (59% in the T-VEC arm and 55% in the GM-CSF arm).161 The primary endpoint was durable response rate (DRR), defined as an objective (complete or partial) response per modified WHO criteria that persisted ≥6 months. At the time of primary analysis, the DRR was significantly higher for the T-VEC arm compared with the GM-CSF control arm (16.3%; 95% CI 12.1 to 20.5 vs 2.1%; 95% CI 0 to 4.5; OR 8.9; p<0.001) as was the ORR (26.4%; 95% CI 21.4 to 31.5 vs 5.7%; 95% CI 1.9 to 9.5; p<0.001). At final analysis in 2019 with a median follow-up time of 49 months, the median OS for the T-VEC and GM-CSF arms were 23.3 months (95% CI 19.5 to 29.6) and 18.9 months (95% CI 16.0 to 23.7), respectively (unstratified HR 0.79; 95% CI 0.62 to 1.00; p=0.0494 [descriptive]).161
T-VEC is well-tolerated, with TRAEs occurring most frequently during the first three cycles. The most common TRAEs reported were fatigue, chills, and fever, with only 11.3% of patients in the T-VEC arm experiencing a grade ≥3 TRAE (most commonly cellulitis). There were no treatment-related deaths in the T-VEC arm of OPTiM.
Preclinical rationale supports combination approaches involving IT immunotherapy and systemic ICIs with potential mechanisms of synergy including enhanced neoantigen presentation and modulation of immunosuppressive cells such as regulatory T-cells and myeloid-derived suppressor cells in the TME. A phase Ib study that enrolled 21 patients with advanced melanoma found that the combination of pembrolizumab and T-VEC was well tolerated and associated with an ORR of 62% (including 33% CR).162 Despite promising early clinical data, the largest phase III trial evaluating T-VEC with pembrolizumab to date, MASTERKEY 265/KEYNOTE-034, failed to meet its primary endpoints.163 Although at a median follow-up of 31.0 months median OS was not reached for the T-VEC plus pembrolizumab arm versus 49.2 months for the placebo plus pembrolizumab arm, the difference did not achieve statistical significance (HR 0.96; 95% CI 0.76 to 1.24; p=0.74). Another open-label, randomized phase II study of 198 patients with unresectable stage III/IV melanoma comparing T-VEC combined with ipilimumab versus ipilimumab alone also failed to meet its OS and PFS endpoints.164
Indications for T-VEC
While T-VEC is well tolerated and effective for some patients, careful clinical consideration is required for patient selection, drug delivery, and response monitoring with this agent. For example, although T-VEC exerts both local and systemic effects, the response rate is much lower and time to response much longer for non-injected lesions in cases of visceral metastatic disease.165 Isolated case reports have described successful administration of T-VEC in carefully selected patients with a history of solid organ transplant with close monitoring and multidisciplinary consultation.166–168 However, because T-VEC is a live virus that may cause life-threatening disseminated herpes infection, it is not routinely recommended for immunosuppressed or pregnant patients.169 Additionally, OPTiM excluded patients with a serum LDH >1.5 times the upper limit of normal, >3 visceral metastases (except lung or nodal organ metastases), visceral metastases >3 cm, unstable liver metastases, bone metastases, and active cerebral metastases, therefore careful consideration must be given when administering T-VEC as there were no US FDA approvals for use of this agent in combination with systemic treatment at the time of guideline publication.
Practical considerations for T-VEC administration
Individual institutions determine the biosafety level and precautions needed to handle and administer live viruses. In general, contact precautions are needed and consideration must be made for protection of patients, caregivers, and clinical staff. Furthermore, the US FDA recommends that pregnant or immunosuppressed providers not administer or handle T-VEC due to the potential for disseminated herpes infection. Early studies demonstrated that the virus persists on the dressing placed over the injected lesions, so care must be taken when removing bandages and gauze. In general, it is recommended that a patient keep dressings on for 1 week. Some institutions label the bandage so that contact precautions are evident if the patient becomes hospitalized.
Assessing response to T-VEC
Measuring response to intralesional immunotherapy requires not only traditional cross-sectional imaging but also quantitative and qualitative assessment of both injected and non-injected cutaneous and subcutaneous lesions. For evaluable lesions measured at two or more different time points, OPTiM defined response by a ≥50% decrease (from baseline) in the product of the lesion’s two largest perpendicular diameters. Ongoing hyperpigmentation may occur despite disease response and biopsy of accessible lesions with ongoing pigmentation may be required to determine response to treatment. Atypical changes in lesion morphology may precede shrinkage, therefore monitoring for flattening, softening, or eschar formation has been recommended.169 Serial photographs of injected lesions can be helpful to document evolving qualitative changes. When assessing response with cross-sectional imaging, the Response Criteria for Intratumoral Immunotherapy in Solid Tumors (itRECIST) may be used to measure overall, non-injected, and injected response rates,170 although these criteria are complex and less practical for real-world, off-trial use.
Time to response with T-VEC
Progression prior to response (PPR, defined in OPTiM as the appearance of a new lesion or >25% increase in total baseline tumor area) occurred in nearly half (48%) of patients who eventually achieved a durable response to T-VEC.171 Small patient numbers and a lack of documentation of potentially confounding data precluded a formal analysis of characteristics that may be independently associated with the development of PPR. An informal analysis did identify enrichment for baseline HSV seronegativity in patients who experienced PPR even though high initial doses of T-VEC were administered in an attempt to seroconvert HSV-seronegative patients.160 171 Visible and palpable lesions may take months to respond to T-VEC (median time to response in OPTiM was 4.1 months, with a range of 1.2–16.7 months). Furthermore, median time to onset of durable response occurred later for those patients who experienced PPR (5.8 months [range 1.3–10.6] versus 3.1 months [1.2–9.5]; p=0.004). The immediate risks of rapid systemic disease progression must therefore be carefully weighed against the potential for delayed benefits of T-VEC.
Emerging intratumoral immunotherapy approaches
Although not approved as a neoadjuvant treatment at the time of guideline publication, T-VEC has also demonstrated efficacy when given in this setting. With a median follow-up of 63 months, patients with resectable stage IIIB–IVM1a had improved 5-year Kaplan-Meier estimates of RFS (22.3% vs 15.2%; HR 0.76; 80% CI 0.60 to 0.97) when six doses of neoadjuvant T-VEC were given prior to surgery and investigator’s choice of adjuvant therapy.172 Additional intralesional approaches being advanced through clinical trials include the use of vusolimogene oderparepvec (RP1) as monotherapy or in combination with anti-PD-1,173 174 intratumoral electroporation of tavokinogene telseplasmid, a DNA construct carrying IL-12,175 and intratumorally administered toll-like receptor (TLR) agonists administered as monotherapy and in combination with ICIs.176 177
Panel recommendations
T-VEC monotherapy is well tolerated, easily administered, and should be considered as part of the treatment plan for patients with predominantly injectable disease at any point in the treatment course for melanoma as part of a multidisciplinary approach.
Intratumoral therapies may be considered throughout the treatment course, although with T-VEC, responses in non-injected visceral lesions are rare (LE:2).
Some members of this Expert Panel have used T-VEC in immunosuppressed patients after careful, case-by-case consideration.
Evaluation and management of response to immunotherapy
Imaging
Routine cross-sectional imaging is indicated for all patients with stage III or IV melanoma, and CT scans (with intravenous contrast for areas outside of the thorax) remain the preferred imaging modality to assess response to immunotherapy, with additional imaging of the neck and limbs as clinically indicated. Brain MRI should also be obtained given the high risk of CNS metastasis. Cross-sectional imaging to evaluate symptoms concerning for recurrence or metastatic disease should be considered for any patient with a history of melanoma, although screening studies for asymptomatic patients with stage I or IIA disease are not currently recommended. A small, retrospective study demonstrated that surveillance imaging for asymptomatic patients with resected stage IIB to IIIC melanoma may identify recurrent disease earlier and improve outcomes with subsequent ICI therapy.178 However, these potential benefits must be weighed against the potential risks of false-positive or incidental imaging findings that may lead to invasive confirmatory tests.
Interpreting physiologic information from fluorodeoxyglucose (FDG)-PET/CT obtained during melanoma treatment with ICIs can be challenging. Limited specificity and a lack of anatomic detail have been cited as reasons to prefer CT or MRI over FDG-PET/CT for assessment of response of melanoma to ICIs.179 However, in select situations, FDG-PET/CT imaging may be clinically useful for patients whose disease is more readily apparent with this modality. Metabolic response may also be useful to help determine when ICIs can be electively discontinued,180 181 an approach that is being prospectively evaluated in the PET-Stop trial (NCT04462406) (see the Duration of immunotherapy section for further discussion). Regardless of the modality selected, imaging results obtained during immunotherapy treatment must be considered in the appropriate clinical context. For example, the appearance of enlarging or even new lesions on a CT scan may represent pseudoprogression in a patient whose disease-related symptoms have improved (see the Pseudoprogression section for further discussion of atypical patterns of response).
Although not yet validated in larger trials, other retrospective data suggest that a complete metabolic response (CMR) on FDG-PET/CT at 1 year may be more predictive of an ongoing treatment response compared with disease control as measured by CT scan.182 Long-term survival data from Checkmate 067 suggest that patients who have achieved either a CR or PR to ICI therapy at 12 months have improved long-term survival compared with those patients whose best response is either SD or PD on CT scan.183 In pooled data from neoadjuvant melanoma trials, radiologic responses correlated fairly well, but not perfectly, with pathologic responses to neoadjuvant immunotherapy (Κ=0.306).98 For patients receiving neoadjuvant immunotherapy, all patients with a CR and 83% of patients with a PR by CT had pathologic complete or near-complete responses (see the Pathologic response criteria section for detailed definitions). Pathologic complete or near-complete responses were also seen in 38% of patients receiving immunotherapy with SD by CT. Survival outcomes were excellent for the patients with pathologic response, regardless of radiographic response, suggesting that in some cases, disease evident as a PR or even SD on CT scan may reflect non-viable, treated melanoma.
Evaluation of response to ICIs
ICI treatment may lead to atypical radiographic responses. Apparent increases in tumor size on imaging may represent lymphocytic infiltration and inflammation as opposed to disease progression. For example, one analysis of patients receiving pembrolizumab monotherapy in KEYNOTE-001 estimated that the benefit of ICI therapy was underestimated by the standard RECIST v1.1 criteria by about 15% when compared with immune-related response criteria (irRC).12 The irRC uses bidimensional measurement of target lesions and was developed to address novel patterns of response to ipilimumab in advanced melanoma.184 185 By irRC, progressive disease was defined as a 25% increase from the nadir and new lesions did not define progression. The higher rate of benefit demonstrated by irRC may account for the apparent discordance between rates of long-term survival and response measured with RECIST v1.1 among patients treated with ICIs.
Alternate radiographic response criteria
The irRECIST criteria were developed based on the irRC but using just one dimension to potentially increase reproducibility.185 The immune RECIST (iRECIST) criteria, developed by the RECIST working group for use in trials of immunotherapy for patients with cancer, designates different responses with the prefix ‘i’ to signify ‘immune’. Immune unconfirmed PD (UPD), or ‘iUPD’, is defined by a ≥20% increase in tumor burden or by the appearance of new target or non-target lesions. Progression is then confirmed (iCPD) at next follow-up by a further increase of ≥5 mm of target tumor burden or a new target lesion or any increase in non-target lesions. The immune-modified RECIST (imRECIST) criteria were developed based on the responses seen in non-small cell lung cancer, metastatic urothelial carcinoma, renal cell carcinoma, and melanoma with atezolizumab treatment.186 187 These criteria allowed for best overall response to occur following PD and changed the definition of PD with respect to new lesions and non-target lesions. Compared with RECISTv1.1, imRECIST found a DCR that was 8–13% greater and a median PFS that was 0.5–1.5 months longer.
A modified version of RECIST v1.1 has been used in several studies to evaluate response to ICIs for patients with MBMs.132 133 188 189 This modification compartmentalizes the intracranial and extracranial spaces, allowing for measurement of up to five target intracranial lesions 5 mm to 30 mm in diameter on MRI in addition to the separate measurement of extracranial lesions.190 Response criteria for MBMs have also been proposed by the Response Assessment for Neuro-Oncology Brain Metastases group, which uses a diameter cut-off of 10 mm to define measurable intracranial disease, allowing for a 5 mm diameter cut-off when an MRI slice thickness of 1.5 mm or less is used.191
Pathologic response criteria
Pathologic response in the resection specimen is a widely-used endpoint in clinical trials evaluating the efficacy of neoadjuvant ICI therapy,92 192–194 and may inform selection of adjuvant therapy and need for additional surgery.95 The INMC defines pCR as a ‘complete absence of residual viable tumor’, with pathologic partial response defined as ‘≤50% of the tumor bed occupied by viable tumor cells’ and pathologic non-response (pNR) defined as ‘>50% of the tumor bed occupied by viable tumor cells’.103 A near pCR/MPR may also be defined in some trials as ‘>0% but ≤10% viable tumor cells’. In a pooled analysis of neoadjuvant melanoma trials, 61% and 26% of patients achieved a pCR or near pCR following neoadjuvant immunotherapy (combination or single-agent), respectively.98 An estimated 96% of patients who achieved pCR were recurrence-free at 2 years versus 64% for those who did not (p<0.001). OS rates at 2 years were also significantly improved in patients who achieved any INMC pathologic response to neoadjuvant immunotherapy versus those with pNR (99% vs 72%, respectively; p<0.001). Available data indicate that pathologic response to neoadjuvant therapy in resectable melanoma is a reliable surrogate endpoint for survival and standardization of this endpoint across clinical trials will be critical to estimate the true benefit of neoadjuvant therapy.195 Pathologic response has been shown to correlate with metabolic response on FDG-PET/CT in the neoadjuvant setting,196 however, validation of pathologic biomarkers for use in combination with imaging is needed.
Pathologic response as measured in an on-treatment biopsy specimen may inform therapy for patients with metastatic melanoma as well, although these biopsies may be prone to sampling error and this biomarker requires further, large-scale prospective validation. In one study that included patients with metastatic melanoma receiving anti-PD-1 monotherapy, increasing early on-treatment immune-related pathologic response score (defined by histologic features including TIL density, plasma cells, neovascularization, and proliferative fibrosis) in a biopsy obtained after 22–36 days on treatment was associated with objective response as measured by RECIST v1.1 (p=0.009).197 Furthermore, while tumor necrosis was not associated with response, MPR (≤10% viable tumor cells) in on-treatment biopsy specimens was associated with improved OS (HR 0.13; 95% CI 0.054 to 0.31; p=0.015). Features of immune response on biopsy were seen in 50% of patients with radiographic SD, which may differentiate those who are more likely to achieve ongoing benefit from ICI therapy. Another study identified unique T-cell subsets and expression of checkpoint proteins including PD-(L)1 and LAG-3 in early on-treatment biopsies as predictive of response to anti-PD-1 following progression on anti-CTLA-4.198 Novel approaches fusing high-dimensional radiomics with pathological assessment of biopsy specimens are also being developed along with ultrasound-based strategies to obtain non-invasive prognostic and predictive biomarkers.
Pseudoprogression
During treatment with ICIs, false-positive ‘pseudoprogression’ may appear as new or increased FDG-avid areas on FDG-PET/CT or new or enlarging areas of disease on CT. Typically pseudoprogression has been reported during the first 12 weeks of ICI therapy, although late pseudoprogression may occur as well. Pseudoprogression may be more common with CTLA-4 inhibitors compared to PD-1 inhibitors and has been estimated to occur in about 6.4% of patients with melanoma receiving ICIs.199 While progressive disease, including pseudoprogression, is strictly defined by a ≥20% increase in the sum of the longest dimension with an absolute increase of 5 mm from nadir or the appearance of new lesions, atypical response (defined as a transient increase of 10–19% above the nadir) may also be observed with ICI therapy. When atypical response is included in the definition of ‘pseudoprogression’, rates of this phenomenon are much higher. For example, one retrospective study of patients receiving nivolumab with or without another anticancer agent (including ipilimumab) reported a RECIST v1.1 rate of pseudoprogression (including atypical response) of 31% among 45 patients with melanoma who experienced a clinical benefit from therapy.200 These atypical responses may represent antitumor immune activity—one retrospective study of 96 patients with melanoma treated with pembrolizumab found that an increase of <20% in (iRECIST) tumor burden on CT was associated with improved OS.201
It is critical to distinguish true progression of disease on immunotherapy versus pseudoprogression. If progression on imaging is true or confirmed, then the resistance phenotype (ie, primary vs secondary) may inform subsequent lines of treatment (see the Progression following prior anti-PD-1 treatment section).139 However, distinguishing true progression from pseudoprogression on imaging is challenging. Biopsy of new or enlarging lesions could potentially demonstrate infiltrating immune cells, but this procedure is invasive, may not be feasible or safe, and may be subject to sampling error.202 Measurement of circulating or cell-free tumor DNA is an active area of investigation to differentiate between pseudoprogression and confirmed progression,35 however, this technique has not been validated with large prospective studies.
Treatment beyond progression
The iRECIST guidelines propose that clinically stable patients with progression of disease on imaging may undergo repeat imaging in 4–8 weeks to distinguish true- versus pseudo-progression.203 Clinically relevant increases in disease-related symptoms or a decline in performance status are likely to be associated with true progression, however. Confirmatory imaging may be delayed beyond 8 weeks in certain circumstances, for example, when no salvage therapy is available or when the tumor being treated has a well described association with pseudoprogression (eg, melanoma). Severe laboratory abnormalities and irAEs should both be absent if ICIs are to be continued beyond progression on imaging.204
Management of oligoprogressive disease
Definitive management of isolated sites of metastatic disease progression can improve survival and should be considered for all patients receiving immunotherapy who develop an isolated site of progression. Multiple studies prior to the widespread use of ICIs have demonstrated survival benefit with metastasectomy,205–207 including second metastasectomy.208 In IMMUNED, the median RFS was NR (HR vs placebo 0.23; 97.5% CI 0.12 to 0.45; p<0.0001), 12.4 months (HR vs placebo 0.56; 97.5% CI 0.33 to 0.94; p=0.011), and 6.4 months for patients who received adjuvant ipilimumab plus nivolumab, nivolumab, and placebo, respectively, among 162 patients with treatment-naïve stage IV melanoma and NED status post definitive surgery or radiotherapy.80 The pattern of disease progression and successful completion of definitive treatment have important implications for outcomes. Definitive treatment for progression of established tumors compared with lesions in a new location was associated with significantly improved 3-year PFS (70% vs 6%; p=0.001) and 5-year disease-specific survival (93% vs 31%; p=0.046) in one retrospective study that included 52 patients receiving ICIs for metastatic melanoma.209 Significant improvement in median OS was seen in 237 patients with stage IV or unresectable stage III melanoma receiving ICIs following metastasectomy for those who achieved a complete resection (NR vs 10.8 months; p<0.0001) and for those who underwent resection of a single (vs multiple) metastases (NR vs 7.8 months; p<0.0001).210
Treatment of oligometastatic disease with radiation may be used to address brain lesions and extracranial metastases that are not amenable to surgical resection. Radiation may enhance the efficacy of systemic immunotherapy through the abscopal effect, a phenomenon by which radiation is thought to produce an out-of-field response potentially mediated by immune system activation.211 Although some retrospective studies have demonstrated efficacy and acceptable safety with radiation combined with ICI therapy,212 213 a concurrent radio-immunotherapy approach has yet to be validated in large-scale, prospective trials.
Duration of immunotherapy
Although increasing numbers of patients with advanced melanoma survive long-term with ICI therapy, the optimal duration of treatment has yet to be established. In a small part-prospective, part-retrospective study of 185 patients with advanced melanoma who were observed following elective discontinuation of pembrolizumab or nivolumab in the absence of disease progression or a toxicity limiting treatment, 78% of patients remained progression-free after stopping therapy. Progression occurred in only 14% of patients who had achieved a CR compared to 32% and 50% of patients whose best response was a PR and SD, respectively.214 A retrospective single-institution study where elective discontinuation of treatment was allowed after a median treatment duration of 12 months based on a CR on CT scan, a CMR on FDG-PET/CT, or NED on biopsy of a non-CR/CMR tumor site found a 3-year EFS rate of 95%.180 The ongoing phase II PET-Stop/EA6192 trial (NCT04462406) is prospectively evaluating the utility of FDG-PET/CT and biopsy to determine optimal cessation of ICIs for unresectable stage IIIB to IV melanoma. In the KEYNOTE-001 trial, 655 patients with ipilimumab-naïve or ipilimumab-pretreated advanced/metastatic melanoma received different doses of pembrolizumab, and the 24-month disease-free survival (DFS) rate for the 105 patients who achieved a CR was 90.9%.215 The 24-month DFS rate for the 67 patients who discontinued pembrolizumab after CR for observation was almost identical at 89.9%. In the absence of prospective data, there are proposed algorithms to determine optimal ICI duration, with consideration for cessation of treatment for patients who either achieve a CR for at least 6 months or a PR or SD for at least 6 months with no active disease on FDG-PET/CT or pathologic assessment.181 However, with a lack of large-scale, prospective data to inform the optimal duration of ICI treatment, elective ICI discontinuation mandates a thorough risk-benefit discussion. The duration of ICI therapy should be decided on in consultation with patients who have been educated early about the possibility of and reasons for ICI cessation. Data regarding the chance of response with ICI rechallenge on subsequent relapse (discussed in the Rechallenge with ICI section) and the surveillance plan to monitor for relapse should be proactively discussed with patients and their caregivers.
The decision to rechallenge a patient with ICI therapy following the development of an irAE should be determined by the risks of rechallenge (eg, severity of the initial irAE, requirement for prolonged immunosuppression) versus the potential benefit of ICI therapy (eg, response of disease to initial course of ICI therapy). A more detailed discussion of ICI rechallenge following an irAE can be found in the SITC CPG on ICI-related adverse events.87
Panel recommendations
For patients with melanoma receiving ICIs, radiographic measurement of response is useful but not universally predictive of clinical benefit and the information obtained must be placed in the larger clinical context (LE:3).
The decision to continue ICI therapy beyond initial radiographic progression should be based on melanoma-associated symptoms, disease kinetics, and the presence or absence of irAEs.
For patients with melanoma with clinical symptoms of ongoing disease, irAEs, or who are treated beyond progression, repeated radiographic assessment after a short interval (4–8 weeks) may guide decision-making.
For clinically stable patients with melanoma receiving ICIs who develop an isolated site of progression, local therapy may be considered while systemic ICI therapy is continued.
While the optimal duration of ICI treatment for patients with unresectable or metastatic melanoma has yet to be determined by prospective data, retrospective data support discontinuation of ICI therapy after 1 year using confirmed CR, CMR on FDG-PET/CT, or pCR (LE:3). Prolonged PR or SD may also serve as potential thresholds for treatment discontinuation after 1 year, however, PFS is lower for these patients (LE:3). There are no data to support treatment with ICIs beyond 2 years.
Special patient populations
A number of patient populations have historically been excluded from clinical trials of ICIs and therefore efficacy and safety of immunotherapy for these patients is largely derived from small retrospective analyses or case reports. These populations include patients with pre-existing autoimmune disease, patients requiring immunosuppression (eg, for solid organ transplant), patients with poor performance status, patients living with HIV (PLWH), pregnant patients, and patients with rare non-cutaneous subtypes of melanoma. Due to the paucity of data available to guide the use of ICIs in these populations, shared decision-making between the patient and oncology team is imperative and should include a discussion of the patient’s goals of treatment, potential risks and benefits of treatment, and enrollment in clinical trials.
Panel recommendations
For the special patient populations discussed in this guideline, it is critical to consider clinical trials in all stages of treatment (eg, neoadjuvant, adjuvant, metastatic).
For patients with melanoma, performance status and comorbidities should take precedence over numerical age when determining eligibility for therapy with ICIs (LE:3).
Patients with altered immune systems at baseline
Patients with pre-existing autoimmune disease
ICIs may be considered for patients with melanoma and pre-existing autoimmune disease on a case-by-case basis depending on the organs affected, the severity of the autoimmune condition, cancer prognosis, and need for immunosuppression. SITC guidelines recommend initiating ICI therapy in patients with pre-existing life-threatening autoimmune disease only after a careful risk-benefit discussion with consideration of risk of autoimmune flare versus potential survival benefits of ICIs as well as alternative therapies.87 For patients with non-life-threatening autoimmune disease, counseling on the possibility of autoimmune flare-ups and close monitoring with potential involvement of an appropriate specialist during ICI treatment are recommended.
Patients who are immunosuppressed and/or solid organ transplant recipients
Although solid organ transplant recipients are at increased risk of developing a malignancy, they have historically been excluded from clinical trials of ICIs due to the potential for graft rejection.216 Although clinical benefit of immune checkpoint blockade has been noted in some transplant recipients, an overall allograft rejection rate of 41% following treatment with ICIs was reported in one retrospective analysis of 64 patients.217 Rejection rates were reported at 44% for transplanted kidneys, 39% for transplanted livers, and 20% for cardiac allografts. Prospective data from a phase I study of 17 kidney transplant recipients receiving nivolumab for advanced solid tumors suggest that maintaining baseline immunosuppression during anti-PD-1 therapy may be safe and tolerable with reduced risk of allograft rejection, however, additional studies are needed to establish if ICI efficacy is compromised using this approach.218
Patients who are elderly or have an ECOG PS ≥2
ICIs are well-tolerated in the elderly,219 220 including combination ipilimumab plus nivolumab.219 The safety and efficacy of ICIs in patients with a poor ECOG PS is less clear. In one retrospective cohort study of 519 patients with advanced urothelial cancer, an improved ECOG PS (0–1 versus ≥2) was associated with an improvement in median OS for those receiving ICIs in the first line (15.2 vs 7.2 months; HR 0.62; p=0.01) but not subsequent lines of treatment (9.8 vs 8.2 months; HR 0.78; p=0.27).221 Importantly, ICI initiation within 30 days of death was associated with increased odds of dying in a hospital in this population (OR 2.89; p=0.04).
Patients living with HIV infection
PLWH are at increased risk for developing melanoma even in the post-highly active antiretroviral therapy (HAART) era.222 Clinical benefit and acceptable safety with anti-PD-1 monotherapy have been demonstrated in a cohort study that enrolled patients with advanced non-melanoma cancers and well-controlled HIV (CD4 count ≥100 cells/µL, antiretroviral therapy for ≥4 weeks, HIV viral load <200 copies/mL).41 For patients with poorly-controlled HIV, consultation with an infectious diseases specialist and initiation of HAART is recommended prior to initiation of ICIs.223 A systematic review including 73 PLWH with a variety of advanced solid tumors found an ORR of 27% for ICIs (mostly anti-PD-1 monotherapy) in the melanoma subgroup.224 Across tumor types, for the 34 patients with known paired pretreatment and post-treatment viral titers available, HIV remained suppressed in 26 of the 28 (93%) individuals with undetectable HIV loads at baseline. Although using ICIs to treat melanoma in PLWH requires close co-management with an infectious disease specialist, these patients should be given equal consideration to the general population for treatment with ICIs in the standard of care and clinical trial settings.225–227 While ICIs have been proposed for treatment of HIV given the role of the PD-1 axis in maintaining the latent viral reservoir as well as antiviral T-cell exhaustion, checkpoint blockade as a dual treatment for melanoma and HIV residual after HAART remains theoretical.228–230
Patients who are pregnant
Although case reports have documented pregnancies being carried to term in women with advanced melanoma receiving ICIs,231–234 initiation of ICIs during pregnancy is generally discouraged, with adherence to pregnancy prevention strongly recommended during ICI treatment87 and for 5 months following cessation. Anti-PD-1 agents are pregnancy category D drugs, while ipilimumab is pregnancy category C,234 with evidence for placental transfer of exogenously-administered maternal IgG antibodies.235 One retrospective analysis of seven pregnant patients (and nine neonates) receiving ICIs for advanced melanoma reported an 88.9% rate of premature birth and a 71.4% rate of pregnancy complications, including intrauterine growth retardation, hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, placental insufficiency, and low fetal heart rate.234 The use of T-VEC is also contraindicated during pregnancy.169
Panel recommendations
Patients with melanoma who have altered immune systems at baseline should not be automatically excluded from receiving ICI therapy. Given that immunotherapy is potentially curative for melanoma, these patients should be referred to an experienced cancer center for consideration of treatment. Shared decision-making between patient, provider, and collaborative care team to initiate ICI therapy is essential when discussing risks versus benefits of ICIs for these patients.
For solid organ transplant recipients with melanoma, the shared decision to initiate ICI therapy should be informed by a careful risk-benefit discussion in consultation with the transplantation team, weighing the chance of long-term melanoma specific survival against the substantial risk of allograft loss resulting in the need for life-supporting interventions (eg, dialysis, insulin, etc) or death.
For patients with pre-existing autoimmune disease with melanoma, the shared decision to initiate ICI therapy should be informed by a careful risk-benefit discussion in coordination of care with relevant specialty providers weighing the chance of long-term melanoma specific survival against the risk of flare of autoimmune disease.
PLWH and melanoma should not be routinely excluded from receipt of ICI therapy either on or off clinical trials (LE:1). Data have demonstrated that it is safe to use ICIs in PLWH who are compliant on HAART unless there is a specific contraindication to ICI therapy (eg, low CD4 count or uncontrolled viremia).
For pregnant patients with melanoma, there are no clinical trial data to inform the efficacy or safety of ICIs. Initiation or continuation of ICI treatment in pregnant patients warrants a careful risk-benefit conversation with the patient and the individuals they choose to be involved with their decision-making (eg, family, intimate partner, and friends) along with multidisciplinary evaluation including high-risk obstetrics (LE:4).
For patients with melanoma receiving non-steroid therapeutic immune suppression, reduction or modification of immune suppression should be discussed, when appropriate, prior to initiation of ICI therapy (LE:5).
For patients with melanoma who are receiving high-dose corticosteroids, the dose of corticosteroids should be reduced to ≤10 mg prednisone (or equivalent) per day, if possible, prior to initiation of ICI therapy (LE:3). This does not apply to corticosteroids for solid organ allograft preservation. For patients who are unable to taper to ≤10 mg prednisone per day, individual consideration is required with possible subspecialty co-management.
Patients with non-cutaneous melanoma
Uveal melanoma
Around 5% of all melanomas are uveal, a disease that is molecularly and clinically distinct from cutaneous melanoma and has a lower response rate to ICIs.236 Uveal melanoma has a propensity for metastatic spread—particularly to the liver—which may occur years to decades following initial diagnosis.10 Although primary uveal melanomas may be treated with eye-directed radiotherapy or surgery, 52% of locally treated tumors will eventually metastasize and the prognosis is very poor for distantly recurrent disease.237 Systemic treatment options for metastatic uveal melanoma are limited and clinical trial enrollment for these patients is always preferred when possible. GEP, a test performed by the treating ophthalmologist, can be helpful to determine prognosis and eligibility for clinical trial enrollment.10
The response rate of metastatic uveal melanoma to ICI monotherapy is very low at around 3.6% for anti-PD-(L)1 therapy238 and 2.6% for ipilimumab239 in small retrospective and single-arm studies. Response rates for combination ipilimumab plus nivolumab have been relatively higher at 11.5%240 and 18%241 in separate phase II studies.
In January 2022, the US FDA approved tebentafusp-tebn (IMCgp100), a gp100 peptide-HLA-directed CD3 bispecific T-cell engager, for the treatment of unresectable or metastatic uveal melanoma in patients who are HLA-A*02:01 genotype positive. Tebentafusp-tebn was the first systemic therapy to demonstrate an OS benefit for patients with metastatic uveal melanoma. In the phase III, open-label IMCgp100-202 (NCT03070392) trial, 378 HLA-A*02:01-positive patients with treatment-naïve, unresectable or metastatic uveal melanoma were randomized 2:1 to receive either tebentafusp-tebn or investigator’s choice of systemic treatment (single-agent pembrolizumab, ipilimumab, or dacarbazine).242 Median OS in the tebentafusp-tebn group was 21.7 months versus 16.0 months for investigator’s choice of treatment (HR 0.51; 95% CI 0.37 to 0.71; p<0.001). The 1-year OS and 6-month PFS rates, respectively, were 73% and 31% for the tebentafusp-tebn group versus 59% and 19% in the control group (HR for OS 0.51; 95% CI 0.37 to 0.71; p<0.001; HR for PFS 0.73; 95% CI 0.58 to 0.94; p=0.01). Tebentafusp-tebn was well-tolerated—although most patients in the tebentafusp-tebn group experienced a rash (83%) or fever (76%), only 2% of these patients discontinued treatment due to irAEs and there were no treatment-related deaths.
Uveal melanoma most commonly metastasizes to the liver, and disease surveillance should always include cross-sectional liver imaging (eg, ultrasound, CT, or MRI) at a frequency determined by recurrence risk as determined by a GEP.10 243 When metastatic uveal melanoma is confined to the liver, it may be treated with various types of liver-directed therapy offering a PFS benefit over systemic treatments.244 These treatments may include regional isolated perfusion, embolization, ablation, or radiation, and should be guided by a multidisciplinary discussion. The phase III FOCUS trial of patients with hepatic-dominant ocular melanoma reported an ORR (primary study endpoint) of 35.2% versus 12.5% for patients receiving percutaneous hepatic perfusion versus investigator’s choice of transarterial chemoembolization (TACE), pembrolizumab, ipilimumab, or dacarbazine.245 The study also demonstrated an improvement in median OS, which was 20.53 months (95% CI 16.59 to 24.35) for the patients who received percutaneous hepatic perfusion versus 14.06 months (95% CI 9.99 to 19.78) for the patients who received best alternative care.
Mucosal melanoma
Only around 1% of melanomas are mucosal in origin, with these rare tumors affecting primarily the head and neck, anal/rectal, and female genital tract sites.236 The 5-year survival rate for mucosal melanoma is 25%, with worse survival outcomes compared with other melanoma subtypes.246
For resectable mucosal melanoma, guidelines recommend adjuvant ICIs and clinical trial enrollment for all patients.247 A phase II study of patients with resected stage II and stage III mucosal melanoma randomized 189 patients to receive adjuvant observation, high-dose IFNα-2b, or temozolomide plus cisplatin.248 At a median follow-up time of 26.8 months, the median RFS and estimated median OS were 5.4 months and 21.2 months (observation), 9.4 months and 40.4 months (high-dose IFNα-2b), and 20.8 months and 48.7 months (for temozolomide plus cisplatin), respectively. Data demonstrating benefit for adjuvant immunotherapy for mucosal melanoma are conflicting, however. A multicenter, retrospective analysis of 118 patients with mucosal melanoma who underwent curative-intent surgery with or without radiation and with or without adjuvant radiation and/or adjuvant systemic therapy (immunotherapy [n=70], chemotherapy [n=5], and targeted therapy [n=1]) demonstrated no benefit with systemic adjuvant therapy.249 One single-institution, retrospective analysis identified 36 patients with resectable mucosal melanoma who had received neoadjuvant anti-PD-1 therapy with or without an anti-CTLA-4 agent.250 The ORR was 47% with a pathologic response rate of 35%. One quarter of the patients did not undergo surgery due to a CR (n=3, 8%) or disease progression (n=6, 17%). An ongoing trial is evaluating lenvatinib plus pembrolizumab in the neoadjuvant/adjuvant setting for this rare cancer (NCT04622566).
In the metastatic setting, response rates to pembrolizumab monotherapy were 22% and 15% for ipilimumab-naïve and ipilimumab-pretreated disease, respectively, in 84 patients with mucosal melanoma identified in a post-hoc analysis of KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006.251 Similar response rates were reported in a separate pooled analysis of 86 patients with mucosal melanoma (including some patients pretreated with ipilimumab), including a 23.3% ORR for patients receiving nivolumab monotherapy and an ORR of 37.1% associated with combination ipilimumab plus nivolumab.252 Ongoing studies of ICIs in combination with agents that inhibit the vascular endothelial growth factor (VEGF) pathway have demonstrated high response rates for patients with mucosal melanoma.
Acral lentiginous melanoma
Although rare, acral lentiginous melanoma (ALM) is the most common subtype of melanoma among non-Caucasian patients, most commonly presents on the non-hair-bearing regions (soles, palms, or subungual), and has a worse prognosis than non-acral cutaneous melanoma.253 254 In the phase II CheckMate 172 trial of nivolumab for patients whose advanced melanoma had progressed on ipilimumab, a similar median OS and 18-month survival was observed for patients with non-acral (25.3 months and 57.5%, respectively) and acral (25.8 months and 59.0%, respectively) melanoma.255 Another multi-institutional, retrospective cohort analysis that identified 25 patients with advanced ALM (80% of whom had received prior ipilimumab) reported an ORR for anti-PD-1 therapy (either pembrolizumab or nivolumab) of 32% and a median PFS of 4.1 months (median follow-up time 20.0 months).256 ALM is considered immunologically cold compared with cutaneous melanoma,257 and combination approaches with imiquimod, dacarbazine, or IFN therapy have been proposed to improve the efficacy of ICIs for ALM,258 although large scale prospective data for these strategies are lacking.
Panel recommendations
Patients with non-cutaneous melanoma should be advised that although these are rare subsets of melanoma, clinical trial strategies may be available that lead to long-term, high-quality survival.
For patients with rare melanoma subtypes, referral to an experienced provider at an academic medical center is recommended.
For all patients with advanced rare melanoma subtypes, immunotherapy is recommended in the frontline setting with consideration for potential contraindications and toxicities.
For all patients with rare melanoma subtypes, molecular mutation testing is recommended. The discovery of an actionable mutation offers the opportunity for targeted therapy or enrollment in molecularly-directed clinical trials.
For patients with localized uveal melanoma, eye-directed therapy at a subspecialty center should be considered for primary treatment.
In the surveillance of patients with uveal melanoma, liver monitoring is recommended, which may be tailored to the patient’s risk of recurrence (LE:2).
For patients with uveal melanoma oligometastatic to the liver only, liver-directed therapy should be considered (LE:1) and can be considered for use in conjunction with immunotherapy on multidisciplinary discussion (LE:5).
For adult patients with untreated HLA-A*02:01-positive unresectable or metastatic uveal melanoma, treatment with tebentafusp-tebn (LE:2) or clinical trial enrollment is recommended.
For patients with mucosal melanoma, a risk benefit discussion about definitive surgical resection at a specialty care center as a frontline consideration should be held, with the role of immunotherapy best determined by multidisciplinary evaluation.
Patient education and QOL support
Patient and caregiver education are imperative for effective and safe cancer care, particularly for patients receiving ICIs. Providers should counsel patients on the unique mechanism of action and side effect profile of ICIs both prior to initiation and throughout the course of treatment using a communication style that is best suited to each individual patient.259 260 Oncology nurses and oncology advanced practice providers play a pivotal role in patient education, including management of treatment side effects, and the nurses’ level of knowledge is influential on shared decision-making.261 If the patient/caregiver appears to be struggling to digest information, it may be helpful to proactively address commonly asked questions about immunotherapy or key differences between immunotherapy and chemotherapy. Referral to support groups, counseling, social work, pain management, financial counseling, survivorship, and other resources is important to help maintain patient QOL throughout and after immunotherapy treatment.
Recognition of irAEs
Although most irAEs occur within the first 16 weeks of initiating ICIs, they may also occur years after starting treatment.262 ICIs may continue to influence the immune system following discontinuation and patients should be instructed to alert all medical providers about their present or past receipt of ICIs.260
All patients receiving ICIs and their caregivers should receive frequent counseling about the signs and symptoms of irAEs, with encouragement to ‘call early and call often.’ Patients may be hesitant to report irAE symptoms for fear of treatment discontinuation, therefore it is imperative to educate patients about the importance of early irAE recognition.263 The oncology team should also provide reassurance that most irAEs can be effectively managed and many patients are candidates for subsequent ICI rechallenge. Furthermore, patients and caregivers may benefit from knowing that neither the development of irAEs nor a requirement for steroids to treat irAEs negatively impacts OS.262 Standardized toxicity questionnaires may further facilitate irAE-related discussions between providers and patients/caregivers, and other modalities (such as online reporting) may offer a convenient method for reporting non-emergent symptoms.
Patients receiving ICIs should be encouraged to carry a wallet card with their treatment information and contact information for their primary oncologist.259 263 One ICI wallet card is available for download from the Oncology Nursing Society at: www.ons.org/toolkits/immunotherapy-patient-wallet-card-1. It is also important for patients to know that their oncologist should be alerted in the event of a suspected irAE, regardless of which healthcare provider initially diagnosed the toxicity. Patients may also benefit from a medical bracelet alerting providers to irAEs that may lead to an emergency medical situation (eg, patients with immune-related adrenal insufficiency requiring stress dose steroids during acute illness).260
QOL outcomes with ICIs
Data from registrational trials as well as ‘real world’ studies demonstrate that ICIs, particularly anti-PD-1 monotherapy, are well-tolerated with either a neutral or a positive impact on health-related quality of life (HRQOL). The prespecified exploratory endpoints of EORTC QOL Questionnaire Core 30 (QLQ-C30) global health status (GHS)/QoL and EuroQol 5 dimension 5 level (EQ-5D-5L) visual analogue scale (VAS) scores were stable from baseline to week 48 in both the adjuvant pembrolizumab and adjuvant placebo arms of KEYNOTE-716, with no clinically meaningful decline observed.264 HRQOL outcome data from KEYNOTE-002265 and KEYNOTE-006266 demonstrate superiority of pembrolizumab to chemotherapy and ipilimumab, respectively, in terms of QOL measures. Similarly, no significant deterioration in HRQOL was observed for patients receiving nivolumab with or without ipilimumab in CheckMate 067.267 One prospective observational ‘real world’ study demonstrated more favorable HRQOL outcomes for pembrolizumab compared with ipilimumab plus nivolumab in patients with treatment-naïve metastatic melanoma at 12, 18, and 24 weeks,268 however, the durability of these outcomes was not reported. Increasing numbers of patients are experiencing long-term survival after receiving ICIs. A single institution survey of 90 patients who had completed therapy with an ICI and were alive more than 1 year following treatment initiation269 found that most survivors reported excellent overall QOL, which did not differ based on time since treatment completion or receipt of combination therapy. However, 40% of survivors reported some or moderate anxiety/depression, 31% reported some or moderate pain/discomfort, 28% reported fatigue, 17% reported joint aches, 12% reported muscle aches, and 12% reported difficulty in sleeping.
Financial toxicity
Oncology providers should facilitate open and frequent communication about the cost of care with patients and caregivers early on, with prompt referral for financial assistance when needed. It is important for providers to realize that the cost of immunotherapy treatment may go beyond the price of the anticancer agents and that expenses related to travel for appointments, missed work, and unplanned hospital stays may cause patients unanticipated financial distress. In a Cancer Support Community online questionnaire completed by 57 melanoma survivors, 69% of respondents reported moderate to very serious concern regarding health insurance/money worries with 57% depleting their savings due to medical costs.270 Furthermore, despite 42% of respondents desiring financial assistance, only 28% reported speaking to their care team about the cost of care. In this survey, financial impact was significantly associated with increased distress for patients with an annual income of less than $60,000 USD (p<0.05). The prohibitive cost of healthcare also adversely impacts compliance, with 13% of survivors reporting that they had to skip some doses of medications due to medical expenses.
Fertility considerations
A significant number of patients in the USA under the age of 50 years are diagnosed with melanoma.271 As increasing numbers of patients with melanoma are experiencing long-term survival following ICI therapy, fertility has become an important survivorship issue for these patients. ICIs may impair fertility directly or as a result of immune-related endocrinopathies, including hypothyroidism and primary or secondary hypogonadism.272 273 Hypophysitis has been reported at higher rates with ipilimumab (up to 11%) compared with anti-PD-(L)1 therapy (<1–3%),272 and patients should be counseled on the risk of infertility associated with their specific ICI treatment plan. Furthermore, any patient desiring fertility following ICI treatment should be considered for referral to an oncofertility specialist prior to initiation of therapy to assist with family planning.274 Patients should be advised that insurance coverage for fertility preservation may vary between insurance providers and demographics (eg, patient sex assigned at birth).
Panel recommendations
When providing education to patients with melanoma receiving ICIs, and their caregivers, it is important to present information in a clear, concise, and easily understandable format. The treating oncology team should proactively identify barriers to care (eg, language barrier, lack of access to reliable internet/phone, after-hours communication) and attempt to address these barriers as early as possible.
The oncology team should establish realistic expectations with patients and their caregivers in terms of toxicity management (eg, need for holding doses, initiation of steroids) and goals of treatment.
For patients with melanoma receiving ICIs and their caregivers, a clear, ongoing line of communication with the oncology team is necessary as irAEs may present at any time during or after treatment. It is important to emphasize to patients that irAEs may present with symptoms that could be mistaken as cancer-related (eg, fatigue due to autoimmune adrenal insufficiency), therefore ongoing communication between the oncology team and the patient and their caregivers is imperative for early identification and management. The oncology team should identify and confirm the best method for communication with each patient and their caregivers. Patients and caregivers should be encouraged to contact the oncology team promptly to report new or worsening symptoms suggestive of an irAE. Counseling should be provided on what symptoms would warrant reporting during evening, weekend, and holiday hours.
The oncology team should perform ongoing assessments of the patient’s understanding of the importance of reporting symptoms of irAEs. A thorough review of systems probing for irAEs should be obtained at each follow-up visit.
For patients with melanoma receiving ICIs, QOL support requires a patient-centered approach. The patient and their caregivers should be offered multidimensional resources, including psychosocial support, dietary counseling, pain management, patient and survivor and caregiver support groups, comprehensive skin examinations, physical and/or occupational therapy, and financial counseling. Patients may require these services at any point during their care, including after ICI discontinuation and during survivorship years.
For all patients with melanoma benefiting from immunotherapy, a survivorship plan should be considered early on. Recognizing and managing affective disorders associated with prior cancer treatment and diagnosis, transitioning to a new care team, anxiety associated with surveillance imaging, and defining life ‘post-cancer’ are all key survivorship considerations.
For patients with melanoma with reproductive potential, pregnancy prevention should be addressed prior to initiation of immunotherapy. For patients with melanoma being considered for immunotherapy who wish to preserve fertility, referral to an oncofertility specialist should be strongly considered.
The oncology team should provide support and education for the optimal management of long-term, fixed immune-related toxicities (eg, hypoadrenalism, hypothyroidism, diabetes). This includes management and education about the long-term effects of steroid use with regular assessment of bone health.
Conclusion
Immunotherapy has been improving survival for patients with advanced melanoma for decades, and ICIs are now approved to combat earlier stages of the disease in the adjuvant setting. Patients with advanced melanoma who respond to immunotherapy have a chance for long-term survival, with ICI combinations targeting LAG-3 or CTLA-4 in addition to PD-1 driving up response rates. However, patients whose melanoma is not responsive to immune checkpoint blockade, including those with non-cutaneous disease subtypes, are much less likely to survive their cancer, particularly in the advanced stages of disease. Ongoing studies are evaluating novel agents and combinations—including intratumoral approaches and cell therapies—in order to make immunotherapy effective for all patients with melanoma, whether in the neoadjuvant, adjuvant, or advanced disease setting. Biomarkers are also needed to identify those patients who will respond to ICIs and who are more likely to tolerate the side effects of dual checkpoint blockade or emerging strategies. Immunotherapy is often lifesaving for patients with melanoma, and strategies to expand access to ICIs for patients with altered immune systems such as solid organ transplant recipients, pregnant patients, or PLWH are urgently needed. Numerous studies aimed at addressing these and other obstacles are ongoing, and it is incumbent on the entire oncology community to prioritize clinical trial enrollment in shared decision-making with their patients. This guideline will be updated as highly anticipated new data on immunotherapy for the treatment of melanoma become available.
Acknowledgments
The authors wish to thank SITC staff including Claire Griffiths, MD, MPH, and Sam Million-Weaver, PhD, for medical writing support and Angela Kilbert and Nichole Larson for project management and editorial assistance. Additionally, the authors thank SITC for supporting the manuscript development.
Footnotes
Twitter: @CharlotteAriyan, @diwakardavar, @bizarMD, @jasonlukemd, @HTawbi_MD
Contributors: All authors served on the SITC Melanoma Immunotherapy Guideline Expert Panel, drafted content, and provided critical review during the manuscript development. ACP and RJS provided leadership as Chairs of the Expert Panel and provided guidance on the manuscript structure and content and thus are first and last authors; all other authors are listed alphabetically by last name. JKT was the patient advocate representative.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CA—Other: Stock Pfizer, Advisory Board: Iovance and Merck, Memorial Sloan Kettering Cancer Center. EIB—Consulting Fees: Nektar, Instilbio, Novartis, Xilio, Sanofi, Merck, and Iovance. DD—IP Rights: US Patent 63/124,231, Compositions and Methods for Treating Cancer, December 11, 2020, US Patent 63/208,719, Compositions and Methods For Determining Responsiveness to Immune Checkpoint Inhibitors (ICI), Increasing Effectiveness of ICI and Treating Cancer, June 9, 2021; Consulting Fees: Checkmate Pharmaceuticals, Finch, Shionogi, Vedanta Biosciences; Fees for Non CE Services: Medical Learning Group (MLG), Clinical Care Options (CCO); Contracted Research: Arcus, Checkmate Pharmaceuticals, CellSight Technologies, Immunocore, Merck, Tesaro/GSK. GTG—Consulting Fees: Bristol Myers Squibb, Regeneron, Genentech, Novartis, Merck, Sapience Therapeutics, Exicure, Eisai, Iovance Biotherapeutics, Lyell Immunopharma, and HUYABIO International; Contracted Research: Exelixis (institutional support), Lucerno Dynamics. OH—Researcher: Arcus, Aduro, Akeso, Amgen, Bioatla, BMS, Cytomx, Exelixis, Roche Genentech, GSK, Immunocore, Idera, Incyte, Iovance, Merck, Moderna, Merck Serono, Nextcure, Novartis, Pfizer, Regeneron, Seattle Gen, Torque, Zelluna; Consultant Advisor Speaker: Alkermes, Amgen, Bactonix, Beigene, Bioatla, BMS, Esai, Roche Genentech, Georiamune, GigaGen, Grit Bio, GSK, Idera, Immunocore, Incyte, Instilbio, IO Biotech, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Gen, Tempus, Vial Health Tech, Zelluna; Independent Contractor: Alkermes, Amgen, Bactonix, Beigene, Bioatla, BMS, Esai, Roche Genentech, Georiamune, GigaGen, Grit Bio, GSK, Idera, Immunocore, Incyte, Instilbio, IO Biotech, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Gen, Tempus, Vial Health Tech, Zelluna; Publically Traded Stocks: Bactonix. TJH—Contracted Research: Genentech, SkylineDX. BI—Consulting Fees: Johnson & Johnson, Volastra Therapeutics, Merck, AstraZeneca, Eisai and Janssen Pharmaceuticals; research funding to Columbia University from Alkermes, Arcus Biosciences, Checkmate Pharmaceuticals, Compugen, Immunocore, and Synthekine. DBJ—IP Rights: MHC-II for use as immunotherapy biomarker, Abatacept as treatment for irAEs; Consulting Fees: BMS, Catalyst, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoIngineering, Novartis, Oncosec, Pfizer, Targovax; Contracted Research: BMS, Incyte. RPK—Consulting Fees: Regeneron. JJL—Researcher: AbbVie, Astellas, AstraZeneca, Bristol Myers Squibb, Corvus, Day One, EMD Serono, Fstar, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Next Cure, Numab, Palleon, Pfizer, Replimmune, Rubius, Servier, Scholar Rock, Synlogic, Takeda, Trishula, Tizona, Xencor; Consultant Advisor Speaker: AbbVie, Agenus, Alnylam, Atomwise, Bayer, Bristol Myers Squibb, Castle, Checkmate, Codiak, Crown, Cugene, Curadev, Day One, Eisai, EMD Serono, Endeavor, Flame, G1 Therapeutics, Genentech, Gilead, Glenmark, HotSpot, Kadmon, KSQ, Janssen, Ikena, Inzen, Immatics, Immunocore, Incyte, Instil, IO Biotech, Macrogenics, Merck, Mersana, Nektar, Novartis, Partner, Pfizer, Pioneering Medicines, PsiOxus, Regeneron, Replimmune, Ribon, Roivant, Servier, STINGthera, Synlogic, Synthekine 7 Hills, Affivant, Bright Peak, Exo, Fstar, Inzen, RefleXion, Xilio (stock) Actym, Alphamab Oncology, Arch Oncology, Duke Street Bio, Kanaph, Mavu, NeoTx, Onc.AI, OncoNano, physIQ, Pyxis, Saros, STipe, Tempest, AbbVie, Agenus, Amgen, Immutep, Evaxion. TCM—Consulting Fees: Merck, BMS, GigaGen, OncoSec; Pfizer – Scientific Advisory Board. MJM—Consulting Fees: AstraZeneca, Nektar Therapeutics, Istari Oncology, Immunai, Xilio Therapeutics; Fees for Non CE Services: Bristol Myers Squibb (served as a speaker). ACP—Consulting Fees: Regeneron, BMS, Merck; Fees for Non CE Services: BMS; Contracted Research: Merck, BMS, Iovance, Regeneron, Takeda, Ideaya, Replimune: All payments to institution. KMR—Consulting Fees: BMS, Merck, Eisai, Immunocore. AKSS—Consulting Fees: Novartis, Pfizer, Regeneron, Iovance. Contracted Research: Ascentage, Ideaya, Regeneron, Bristol Myers Squibb, Immunocore, Merck, Nektar, Olatec, Replimune, Seagen. KS—Contracted Research: Trials at Norris Cotton Cancer Center, Syndax, Provectus, OmniSeq, BrightPath Biotherapeutics, Ludwig Institute of Cancer Research, Hlsinn, AbbVie, BMS, OncoSec, Ultimovacs, Altellas, AstraZeneca, Xencor, Exicure, Numab, Checkmate Pharmaceuticals, Natera. RJS—Reseracher: Merck (research funding); Consultant Advisor Speaker: BMS, Merck, Novartis, Pfizer; Other: Faculty for SCION Workshop. JT—Consulting Fees: GSK, Genentech, BMS, Akoya Biosciences, Merck, AstraZeneca, Compugen, Lunaphore; Contracted Research: BMS, Akoya Biosciences; Other: Akoya Biosciences; Other Details: Patent pending for multiplexing imaging strategy; Ownership Interest Less Than 5 Per Cent: Akoya Biosciences. HAT—Consulting Fees: Genentech, BMS, Novartis, Merck, Eisai, Karyopharm, Boxer Capital, Iovance, Jazz Pharmaceuticals, Medicenna; Contracted Research: Genentech, BMS, Novartis, Merck, GSK, EMD Serono, Eisai, Dragonfly Therapeutics, RAPT Therapeutics. JKT—Nothing to Disclose. CV—Nothing to Disclose. SAW—Consulting Fees: Lyell; Contracted Research: research funds to institution from BMS and Apexigen. MKW—Consulting Fees: Merck, Pfizer, Bristol Myers Squibb, Regeneron, EMD-Serono, ExiCure, Castle Biosciences, Adagene. SITC Staff—CG, AK, NL, SM-W—Nothing to Disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Flaherty KT, Lee SJ, Schuchter LM, et al. Final results of E2603: A double-blind, randomized phase III trial comparing carboplatin (C)/Paclitaxel (P) with or without sorafenib (S) in metastatic Melanoma. JCO 2010;28:8511. 10.1200/jco.2010.28.15_suppl.8511 [DOI] [Google Scholar]
- 2. Hill GJ, Krementz ET, Hill HZ. Dimethyl Triazeno Imidazole Carboxamide and combination therapy for Melanoma. IV. late results after complete response to chemotherapy (central oncology group protocols 7130, 7131, and 7131A). Cancer 1984;53:1299–305. : [DOI] [PubMed] [Google Scholar]
- 3. Pasquali S, Haydu LE, Scolyer RA, et al. The importance of adequate primary tumor Excision margins and sentinel node biopsy in achieving optimal Locoregional control for patients with thick primary Melanomas. Ann Surg 2013;258:152–7. 10.1097/SLA.0b013e31828421e1 [DOI] [PubMed] [Google Scholar]
- 4. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic Melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–16. 10.1200/JCO.1999.17.7.2105 [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with Ipilimumab in patients with metastatic Melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Checkmate 067: 6.5-year outcomes in patients (Pts) with advanced Melanoma. JCO 2021;39:9506. 10.1200/JCO.2021.39.15_suppl.9506 [DOI] [Google Scholar]
- 7. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in untreated advanced Melanoma. N Engl J Med 2022;386:24–34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michielin O, Atkins MB, Koon HB, et al. Evolving impact of long-term survival results on metastatic Melanoma treatment. J Immunother Cancer 2020;8:e000948. 10.1136/jitc-2020-000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous Melanoma in 2020 and projections to 2040. JAMA Dermatol 2022;158:495–503. 10.1001/jamadermatol.2022.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onken MD, Worley LA, Char DH, et al. Collaborative ocular oncology group report number 1: prospective validation of a multi-gene Prognostic assay in Uveal Melanoma. Ophthalmology 2012;119:1596–603. 10.1016/j.ophtha.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grossman D, Okwundu N, Bartlett EK, et al. Prognostic gene expression profiling in cutaneous Melanoma: identifying the knowledge gaps and assessing the clinical benefit. JAMA Dermatol 2020;156:1004–11. 10.1001/jamadermatol.2020.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody Pembrolizumab in Melanoma. J Clin Oncol 2016;34:4102–9. 10.1200/JCO.2016.67.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined Nivolumab and Ipilimumab in advanced Melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 14. Long GV, Schadendorf D, Vecchio MD, et al. Abstract Ct004: adjuvant therapy with Nivolumab (NIVO) combined with Ipilimumab (IPI) vs NIVO alone in patients (Pts) with Resected stage IIIB-D/IV Melanoma (Checkmate 915). Cancer Res 2021;81. 10.1158/1538-7445.AM2021-CT004 [DOI] [Google Scholar]
- 15. Xu J, Wang F, Yan Y, et al. Prognostic and Clinicopathological value of PD-L1 in Melanoma: A meta-analysis. Am J Med Sci 2020;359:339–46. 10.1016/j.amjms.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Dong M, Shui Y, et al. A pooled analysis of the Prognostic value of PD-L1 in Melanoma: evidence from 1062 patients. Cancer Cell Int 2020;20:96. 10.1186/s12935-020-01187-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor Genomic biomarkers for PD-1 Checkpoint blockade–based Immunotherapy. Science 2018;362:eaar3593. 10.1126/science.aar3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dousset L, Poizeau F, Robert C, et al. Positive association between location of Melanoma, ultraviolet signature, tumor mutational burden, and response to anti-PD-1 therapy. JCO Precis Oncol 2021;5:PO.21.00084. 10.1200/PO.21.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic features of response to anti-PD-1 therapy in metastatic Melanoma. Cell 2016;165:35–44. 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodi FS, Wolchok JD, Schadendorf D, et al. TMB and inflammatory gene expression associated with clinical outcomes following Immunotherapy in advanced Melanoma. Cancer Immunol Res 2021;9:1202–13. 10.1158/2326-6066.CIR-20-0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmieri G, Rozzo CM, Colombino M, et al. Are molecular alterations linked to genetic instability worth to be included as biomarkers for directing or excluding Melanoma patients to Immunotherapy Front Oncol 2021;11:666624. 10.3389/fonc.2021.666624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmieri G, Ascierto PA, Cossu A, et al. Assessment of genetic instability in Melanocytic skin lesions through Microsatellite analysis of benign Naevi, Dysplastic Naevi, and primary Melanomas and their metastases. Melanoma Res 2003;13:167–70. 10.1097/00008390-200304000-00009 [DOI] [PubMed] [Google Scholar]
- 24. Toomey CB, Phou S, Fraser K, et al. Frequency of mismatch repair gene mutations in Uveal Melanoma. Invest Ophthalmol Vis Sci 2019;60:761.30793208 [Google Scholar]
- 25. Toomey CB, Protopsaltis NJ, Phou S, et al. PREVALENCE of mismatch repair gene mutations in uveal melanoma. Retina 2020;40:2216–20. 10.1097/IAE.0000000000002732 [DOI] [PubMed] [Google Scholar]
- 26. Ponti G, Pellacani G, Tomasi A, et al. Immunohistochemical mismatch repair proteins expression as a tool to predict the Melanoma Immunotherapy response. Mol Clin Oncol 2020;12:3–8. 10.3892/mco.2019.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvino E, Passarelli F, Cannavò E, et al. High expression of the mismatch repair protein Msh6 is associated with poor patient survival in Melanoma. Am J Clin Pathol 2014;142:121–32. 10.1309/AJCPCX2D9YULBBLG [DOI] [PubMed] [Google Scholar]
- 28. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and Ipilimumab versus Ipilimumab in untreated Melanoma. N Engl J Med 2015;372:2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helgadottir H, Ghiorzo P, van Doorn R, et al. Efficacy of novel Immunotherapy regimens in patients with metastatic Melanoma with Germline Cdkn2A mutations. J Med Genet 2020;57:316–21. 10.1136/jmedgenet-2018-105610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-Γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danaher P, Warren S, Lu R, et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from the cancer genome Atlas (TCGA). J Immunother Cancer 2018;6:63. 10.1186/s40425-018-0367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santiago-Walker A, Gagnon R, Mazumdar J, et al. Correlation of BRAF Mutation status in circulating-free DNA and tumor and association with clinical outcome across four Brafi and Meki clinical trials. Clin Cancer Res 2016;22:567–74. 10.1158/1078-0432.CCR-15-0321 [DOI] [PubMed] [Google Scholar]
- 33. Lee RJ, Gremel G, Marshall A, et al. Circulating tumor DNA predicts survival in patients with Resected high-risk stage II/III Melanoma. Ann Oncol 2018;29:490–6. 10.1093/annonc/mdx717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JH, Long GV, Boyd S, et al. Circulating tumour DNA predicts response to anti-Pd1 antibodies in metastatic Melanoma. Ann Oncol 2017;28:1130–6. 10.1093/annonc/mdx026 [DOI] [PubMed] [Google Scholar]
- 35. Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and Pseudoprogression in patients with metastatic Melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol 2018;4:717–21. 10.1001/jamaoncol.2017.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of Immunotherapy response. Cancer Res 2017;77:2318–27. 10.1158/0008-5472.CAN-16-3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. LaSalle T, Austin EE, Rigney G, et al. Granzyme B PET imaging of immune-mediated tumor killing as a tool for understanding Immunotherapy response. J Immunother Cancer 2020;8:e000291. 10.1136/jitc-2019-000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferreira CA, Heidari P, Ataeinia B, et al. Non-invasive detection of Immunotherapy-induced adverse events. Clin Cancer Res 2021;27:5353–64. 10.1158/1078-0432.CCR-20-4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrews MC, Duong CPM, Gopalakrishnan V, et al. Gut Microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med 2021;27:1432–41. 10.1038/s41591-021-01406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut Microbiome modulates response to anti-PD-1 Immunotherapy in Melanoma patients. Science 2018;359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the safety of Pembrolizumab in patients with HIV and advanced cancer—A phase 1 study. JAMA Oncol 2019;5:1332–9. 10.1001/jamaoncol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lipson EJ, Tawbi HA-H, Schadendorf D, et al. Relatlimab (RELA) plus Nivolumab (NIVO) versus NIVO in first-line advanced Melanoma: primary phase III results from RELATIVITY-047 (Ca224-047). JCO 2021;39:9503. 10.1200/JCO.2021.39.15_suppl.9503 [DOI] [Google Scholar]
- 43. Shahabi V, Berman D, Chasalow SD, et al. Gene expression profiling of whole blood in Ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013;11:75. 10.1186/1479-5876-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weidhaas J, Marco N, Scheffler AW, et al. Germline biomarkers predict toxicity to anti-Pd1/Pdl1 Checkpoint therapy. J Immunother Cancer 2022;10:e003625. 10.1136/jitc-2021-003625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim SY, Lee JH, Gide TN, et al. Circulating Cytokines predict immune-related toxicity in Melanoma patients receiving anti-PD-1–based Immunotherapy. Clin Cancer Res 2019;25:1557–63. 10.1158/1078-0432.CCR-18-2795 [DOI] [PubMed] [Google Scholar]
- 46. Lozano AX, Chaudhuri AA, Nene A, et al. T cell characteristics associated with toxicity to immune Checkpoint blockade in patients with Melanoma. Nat Med 2022;28:353–62. 10.1038/s41591-021-01623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang D, Shah N, Cook M, et al. n.d. Association between body mass index and immune-related adverse events (irAEs) among advanced-stage cancer patients receiving immune Checkpoint inhibitors: A pan-cancer analysis. Cancers;13:6109. 10.3390/cancers13236109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dubin K, Callahan MK, Ren B, et al. Intestinal Microbiome analyses identify Melanoma patients at risk for Checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391. 10.1038/ncomms10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCulloch JA, Davar D, Rodrigues RR, et al. Intestinal Microbiota signatures of clinical response and immune-related adverse events in Melanoma patients treated with anti-PD-1. Nat Med 2022;28:545–56. 10.1038/s41591-022-01698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II Melanoma. Cancer 2020;126:1166–74. 10.1002/cncr.32585 Available: https://onlinelibrary.wiley.com/toc/10970142/126/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–92. 10.3322/caac.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agarwala SS, Lee SJ, Yip W, et al. Phase III randomized study of 4 weeks of high-dose interferon-Α-2B in stage T2Bno, T3A-bNO, T4A-bNO, and T1-4N1A-2A (microscopic) Melanoma: A trial of the Eastern cooperative oncology group-American college of Radiology imaging network cancer research group (E1697). J Clin Oncol 2017;35:885–92. 10.1200/JCO.2016.70.2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee AY, Droppelmann N, Panageas KS, et al. Patterns and timing of initial relapse in pathologic stage II Melanoma patients. Ann Surg Oncol 2017;24:939–46. 10.1245/s10434-016-5642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Garbe C, Keim U, Amaral T, et al. Prognosis of patients with primary Melanoma stage I and II according to American joint committee on cancer version 8 validated in two independent cohorts: implications for adjuvant treatment. J Clin Oncol 2022;40:3741–9. 10.1200/JCO.22.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo after complete resection of high-risk stage II Melanoma: efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. Annals of Oncology 2021;32:S1314–5. 10.1016/j.annonc.2021.08.2116 [DOI] [Google Scholar]
- 56. Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely Resected stage IIB or IIC Melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 2022;399:1718–29.:S0140-6736(22)00562-1. 10.1016/S0140-6736(22)00562-1 [DOI] [PubMed] [Google Scholar]
- 57. Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in Resected stage IIB or IIC Melanoma (KEYNOTE-716): distant metastasis-free survival results of a Multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol 2022;23:1378–88. 10.1016/S1470-2045(22)00559-9 [DOI] [PubMed] [Google Scholar]
- 58. Eggermont AMM, Robert C, Ribas A. The new era of adjuvant therapies for Melanoma. Nat Rev Clin Oncol 2018;15:535–6. 10.1038/s41571-018-0048-5 [DOI] [PubMed] [Google Scholar]
- 59. Madu MF, Franke V, Van de Wiel BA, et al. External validation of the American joint committee on cancer 8th edition Melanoma staging system: who needs adjuvant treatment Melanoma Res 2020;30:185–92. 10.1097/CMR.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 60. Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in Melanoma. N Engl J Med 2017;376:2211–22. 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC Melanoma staging and classification. JCO 2009;27:6199–206. 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant Ipilimumab versus placebo after complete resection of high-risk stage III Melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522–30. 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- 63. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus placebo in Resected stage III Melanoma. N Engl J Med 2018;378:1789–801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 64. Moncrieff MD, Lo SN, Scolyer RA, et al. Clinical outcomes and risk stratification of early-stage Melanoma Micrometastases from an international multicenter study: implications for the management of American joint committee on cancer IIIA disease. JCO 2022;40:3940–51. 10.1200/JCO.21.02488 [DOI] [PubMed] [Google Scholar]
- 65. Garbe C, Keim U, Suciu S, et al. Prognosis of patients with stage III Melanoma according to American joint committee on cancer version 8: A reassessment on the basis of 3 independent stage III Melanoma cohorts. JCO 2020;38:2543–51. 10.1200/JCO.19.03034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. NCCN . Melanoma: cutaneous 2023. 2023. Available: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 67. Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive Melanoma (Decog-SLT): a Multicentre, randomised, phase 3 trial. Lancet Oncol 2016;17:757–67. 10.1016/S1470-2045(16)00141-8 [DOI] [PubMed] [Google Scholar]
- 68. Haydu LE, Lo SN, McQuade JL, et al. Cumulative incidence and predictors of CNS metastasis for patients with American joint committee on cancer 8th edition stage III Melanoma. J Clin Oncol 2020;38:1429–41. 10.1200/JCO.19.01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon Alfa-2B adjuvant therapy of high-risk Resected cutaneous Melanoma: the Eastern cooperative oncology group trial EST 1684. J Clin Oncol 1996;14:7–17. 10.1200/JCO.1996.14.1.7 [DOI] [PubMed] [Google Scholar]
- 70. Okuyama S, Gonzalez R, Lewis KD. Pegylated interferon Alpha-2B as adjuvant treatment of stage III malignant Melanoma: an evidence-based review. Core Evid 2010;5:39–48. 10.2147/ce.s8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon Alfa-2B versus observation alone in Resected stage III Melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008;372:117–26. 10.1016/S0140-6736(08)61033-8 [DOI] [PubMed] [Google Scholar]
- 72. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Ipilimumab versus placebo after complete resection of stage III Melanoma: long-term follow-up results the EORTC 18071 double-blind phase 3 randomized trial. JCO 2019;37:2512. 10.1200/JCO.2019.37.15_suppl.2512 [DOI] [PubMed] [Google Scholar]
- 73. Tarhini AA, Lee SJ, Hodi FS, et al. Phase III study of adjuvant Ipilimumab (3 or 10 mg/kg) versus high-dose interferon Alfa-2B for Resected high-risk Melanoma: North American Intergroup E1609. JCO 2020;38:567–75. 10.1200/JCO.19.01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected stage III or IV Melanoma. N Engl J Med 2017;377:1824–35. 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 75. Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected stage IIIB–C and stage IV Melanoma (Checkmate 238): 4-year results from a Multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:1465–77. 10.1016/S1470-2045(20)30494-0 [DOI] [PubMed] [Google Scholar]
- 76. Weber JS, Mandalà M, Del Vecchio M, et al. Adjuvant therapy with Nivolumab (NIVO) versus Ipilimumab (IPI) after complete resection of stage III/IV Melanoma: updated results from a phase III trial (Checkmate 238). JCO 2018;36:9502. 10.1200/JCO.2018.36.15_suppl.9502 [DOI] [Google Scholar]
- 77. Eggermont AMM, Blank CU, Mandalà M, et al. Adjuvant Pembrolizumab versus placebo in Resected stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:643–54. 10.1016/S1470-2045(21)00065-6 [DOI] [PubMed] [Google Scholar]
- 78. Grossmann KF, Othus M, Patel SP, et al. Final analysis of overall survival (OS) and relapse-free-survival (RFS) in the intergroup S1404 phase III randomized trial comparing either high-dose interferon (HDI) or Ipilimumab to Pembrolizumab in patients with high-risk Resected Melanoma. JCO 2021;39:9501. 10.1200/JCO.2021.39.15_suppl.9501 [DOI] [Google Scholar]
- 79. Weber JS, Schadendorf D, Del Vecchio M, et al. Adjuvant therapy of Nivolumab combined with Ipilimumab versus Nivolumab alone in patients with Resected stage IIIB-D or stage IV Melanoma (Checkmate 915). J Clin Oncol 2023;41:517–27. 10.1200/JCO.22.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant Nivolumab plus Ipilimumab or Nivolumab monotherapy versus placebo in patients with Resected stage IV Melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020;395:1558–68. 10.1016/S0140-6736(20)30417-7 [DOI] [PubMed] [Google Scholar]
- 81. Livingstone E, Zimmer L, Hassel JC, et al. Adjuvant Nivolumab plus Ipilimumab or Nivolumab alone versus placebo in patients with Resected stage IV Melanoma with no evidence of disease (IMMUNED): final results of a randomised, double-blind, phase 2 trial. Lancet 2022;400:1117–29. 10.1016/S0140-6736(22)01654-3 [DOI] [PubMed] [Google Scholar]
- 82. Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit Melanoma after sentinel Lymphadenectomy. Ann Surg Oncol 2005;12:587–96. 10.1245/ASO.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 83. Wong JH, Cagle LA, Kopald KH, et al. Natural history and selective management of in transit Melanoma. J Surg Oncol 1990;44:146–50. 10.1002/jso.2930440305 [DOI] [PubMed] [Google Scholar]
- 84. Larkin J, Gogas H, Del Vecchio M, et al. Analysis of patients (Pts) with in-transit metastases treated with Nivolumab (NIVO) or Ipilimumab (IPI) in Checkmate 238. JCO 2021;39:9569. 10.1200/JCO.2021.39.15_suppl.9569 [DOI] [Google Scholar]
- 85. Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-Mutated Melanoma. N Engl J Med 2017;377:1813–23. 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 86. Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant Dabrafenib plus Trametinib in stage III Melanoma. N Engl J Med 2020;383:1139–48. 10.1056/NEJMoa2005493 [DOI] [PubMed] [Google Scholar]
- 87. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of cancer (SITC) clinical practice guideline on immune Checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Patrinely JR, Johnson R, Lawless AR, et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk Resected Melanoma. JAMA Oncol 2021;7:744–8. 10.1001/jamaoncol.2021.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Adams L, Chick R, Clifton G, et al. 300 Final analysis of a prospective, randomized, double-blind, placebo-controlled phase iib trial of tumor lysate, particle-loaded, dendritic cell vaccine in stage III/IV melanoma: 36-month analysis. 35th Anniversary Annual Meeting (SITC 2020); November 2020: 10.1136/jitc-2020-SITC2020.0300 [DOI] [Google Scholar]
- 90. Moderna and Merck announce mRNA-4157/V940, an investigational personalized mRNA cancer vaccine, in combination with KEYTRUDA (Pembrolizumab), met primary efficacy Endpoint in phase 2B KEYNOTE-942 trial [press release]. December 13, 2022. 2022.
- 91. van Akkooi ACJ, Hieken TJ, Burton EM, et al. Neoadjuvant systemic therapy (NAST) in patients with Melanoma: surgical considerations by the International Neoadjuvant Melanoma consortium (INMC). Ann Surg Oncol 2022;29:5241–2. 10.1245/s10434-022-11622-0 [DOI] [PubMed] [Google Scholar]
- 92. Amaria RN, Postow MA, Tetzlaff MT, et al. Neoadjuvant and adjuvant Nivolumab (Nivo) with anti-Lag3 antibody Relatlimab (Rela) for patients (Pts) with Resectable clinical stage III Melanoma. JCO 2021;39:9502. 10.1200/JCO.2021.39.15_suppl.9502 [DOI] [Google Scholar]
- 93. Amaria RN, Postow M, Burton EM, et al. Neoadjuvant Relatlimab and Nivolumab in Resectable Melanoma. Nature 2022;611:155–60. 10.1038/s41586-022-05368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Blank CU, Rozeman EA, Menzies AM, et al. Opacin-Neo: a multicenter phase II study to identify the optimal Neo-adjuvant combination scheme of Ipilimumab (IPI) and Nivolumab (NIVO). Ann Oncol 2018;29:viii734. 10.1093/annonc/mdy424.052 [DOI] [Google Scholar]
- 95. Blank CU, Reijers ILM, Pennington T, et al. First safety and efficacy results of PRADO: A phase II study of personalized response-driven surgery and adjuvant therapy after Neoadjuvant Ipilimumab (IPI) and Nivolumab (NIVO) in Resectable stage III Melanoma. JCO 2020;38:10002. 10.1200/JCO.2020.38.15_suppl.10002 [DOI] [Google Scholar]
- 96. Blank CU, Reijers ILM, Saw RPM, et al. Survival data of PRADO: a phase 2 study of personalized response-driven surgery and adjuvant therapy after Neoadjuvant Ipilimumab (IPI) and Nivolumab (NIVO) in Resectable stage III Melanoma. JCO 2022;40:9501. 10.1200/JCO.2022.40.16_suppl.9501 [DOI] [Google Scholar]
- 97. Rozeman EA, Hoefsmit EP, Reijers ILM, et al. Survival and biomarker analyses from the Opacin-Neo and Opacin Neoadjuvant Immunotherapy trials in stage III Melanoma. Nat Med 2021;27:256–63. 10.1038/s41591-020-01211-7 [DOI] [PubMed] [Google Scholar]
- 98. Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with Neoadjuvant therapy in Melanoma: a pooled analysis from the International Neoadjuvant Melanoma consortium (INMC). Nat Med 2021;27:301–9. 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
- 99. Patel S, Othus M, Prieto V, et al. LBA6 neoadjvuant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Ann Oncol 2022;33:S1408. 10.1016/j.annonc.2022.08.039 [DOI] [Google Scholar]
- 100. Patel SP, Othus M, Chen Y, et al. Neoadjuvant–adjuvant or adjuvant-only Pembrolizumab in advanced Melanoma. N Engl J Med 2023;388:813–23. 10.1056/NEJMoa2211437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of Neoadjuvant Ipilimumab plus Nivolumab in macroscopic stage III Melanoma (Opacin-Neo): a Multicentre, phase 2, randomised, controlled trial. Lancet Oncol 2019;20:948–60. 10.1016/S1470-2045(19)30151-2 [DOI] [PubMed] [Google Scholar]
- 102. Hieken TJ, Price DL, Piltin MA, et al. Surgeon assessment of the technical impact of Neoadjuvant systemic therapy on operable stage III Melanoma. Ann Surg Oncol 2022;29:780–6. 10.1245/s10434-021-11112-9 [DOI] [PubMed] [Google Scholar]
- 103. Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after Neoadjuvant therapy for metastatic Melanoma. Ann Oncol 2018;29:1861–8. 10.1093/annonc/mdy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Keung EZ, Gershenwald JE. The eighth edition American joint committee on cancer (AJCC) Melanoma staging system: implications for Melanoma treatment and care. Expert Rev Anticancer Ther 2018;18:775–84. 10.1080/14737140.2018.1489246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus Dacarbazine for previously untreated metastatic Melanoma. N Engl J Med 2011;364:2517–26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 106. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced Melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 107. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus Ipilimumab in advanced Melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, Multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–51. 10.1016/S1470-2045(19)30388-2 [DOI] [PubMed] [Google Scholar]
- 108. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated Melanoma without BRAF Mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 109. Robert C, Long GV, Brady B, et al. Five-year outcomes with Nivolumab in patients with wild-type BRAF advanced Melanoma. J Clin Oncol 2020;38:3937–46. 10.1200/JCO.20.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Eroglu Z, Zaretsky JM, Hu-Lieskovan S, et al. High response rate to PD-1 blockade in Desmoplastic Melanomas. Nature 2018;553:347–50. 10.1038/nature25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kendra KL, Moon J, Eroglu Z, et al. Neoadjuvant PD-1 blockade in patients with Resectable Desmoplastic Melanoma (SWOG 1512). JCO 2022;40:9502. 10.1200/JCO.2022.40.16_suppl.9502 [DOI] [Google Scholar]
- 112. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced Melanoma. N Engl J Med 2017:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug Additivity or synergy. Cell 2017;171:1678–91. 10.1016/j.cell.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wei SC, Anang N-AAS, Sharma R, et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci USA 2019;116:22699–709. 10.1073/pnas.1821218116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hodi FS, Postow MA, Chesney J, et al. Abstract 2860: improved clinical response in patients with advanced Melanoma treated with Nivolumab combined with Ipilimumab compared to Ipilimumab alone. Cancer Res 2015;75:2860. 10.1158/1538-7445.AM2015-2860 [DOI] [Google Scholar]
- 116. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or monotherapy in untreated Melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lebbe C, Meyer N, Mortier L, et al. Two dosing regimens of Nivolumab (NIVO) plus Ipilimumab (IPI) for advanced (Adv) Melanoma: three-year results of Checkmate 511. JCO 2021;39:9516. 10.1200/JCO.2021.39.15_suppl.9516 [DOI] [Google Scholar]
- 118. Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of Nivolumab and Ipilimumab with a planned switch in patients with advanced Melanoma (Checkmate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55. 10.1016/S1470-2045(16)30126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Carlino MS, Menzies AM, Atkinson V, et al. Long-term follow-up of standard-dose Pembrolizumab plus reduced-dose Ipilimumab in patients with advanced Melanoma: KEYNOTE-029 part 1B. Clin Cancer Res 2020;26:5086–91. 10.1158/1078-0432.CCR-20-0177 [DOI] [PubMed] [Google Scholar]
- 120. Long GV, Stephen Hodi F, Lipson EJ, et al. Overall survival and response with Nivolumab and Relatlimab in advanced Melanoma. NEJM Evidence 2023;2. 10.1056/EVIDoa2200239 [DOI] [PubMed] [Google Scholar]
- 121. Hamid O, Wang D, Kim TM, et al. Clinical activity of Fianlimab (Regn3767), a human anti-LAG-3 Monoclonal antibody, combined with Cemiplimab (anti-PD-1) in patients (Pts) with advanced Melanoma. JCO 2021;39:9515. 10.1200/JCO.2021.39.15_suppl.9515 [DOI] [Google Scholar]
- 122. Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, Vemurafenib, and Cobimetinib as first-line treatment for Unresectable advanced BRAFV600 Mutation-positive Melanoma (Imspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;395:1835–44. 10.1016/S0140-6736(20)30934-X [DOI] [PubMed] [Google Scholar]
- 123. Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of Pembrolizumab, Dabrafenib, and Trametinib in. J Immunother Cancer 2020;8:e001806. 10.1136/jitc-2020-001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nathan P, Dummer R, Long GV, et al. Lba43 Spartalizumab plus Dabrafenib and Trametinib (Sparta-Dabtram) in patients (Pts) with previously untreated BRAF V600–mutant Unresectable or metastatic Melanoma: results from the randomized part 3 of the phase III COMBI-I trial. Ann Oncol 2020;31:S1172. 10.1016/j.annonc.2020.08.2273 [DOI] [Google Scholar]
- 125. Gogas H, Dréno B, Larkin J, et al. Cobimetinib plus Atezolizumab in Brafv600 wild-type Melanoma: primary results from the randomized phase III Imspire170 study. Ann Oncol 2021;32:384–94. 10.1016/j.annonc.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 126. Dummer R, Queirolo P, Gerard Duhard P, et al. Atezolizumab, Vemurafenib, and Cobimetinib in patients with Melanoma with CNS metastases (TRICOTEL): a Multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2023:00334–0.:S1470-2045(23)00334-0. 10.1016/S1470-2045(23)00334-0 [DOI] [PubMed] [Google Scholar]
- 127. Atkins MB, Lee SJ, Chmielowski B, et al. Dreamseq (Doublet, randomized evaluation in advanced Melanoma sequencing): a phase III trial—Ecog-Acrin Ea6134. JCO 2021;39:356154. 10.1200/JCO.2021.39.36_suppl.356154 [DOI] [Google Scholar]
- 128. Ascierto PA, Mandala M, Ferrucci PF, et al. Lba45 first report of efficacy and safety from the phase II study SECOMBIT (sequential combo Immuno and targeted therapy study). Annals of Oncology 2020;31:S1173–4. 10.1016/j.annonc.2020.08.2275 [DOI] [Google Scholar]
- 129. Ascierto PA, Mandalà M, Ferrucci PF, et al. Sequencing of Ipilimumab plus Nivolumab and Encorafenib plus Binimetinib for untreated BRAF-Mutated metastatic Melanoma (SECOMBIT): A randomized, three-arm, open-label phase II trial. J Clin Oncol 2023;41:212–21. 10.1200/JCO.21.02961 [DOI] [PubMed] [Google Scholar]
- 130. Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 2017;19:1511–21. 10.1093/neuonc/nox077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Glitza IC, Heimberger AB, Sulman EP, et al. Chapter 19 - Prognostic factors for survival in Melanoma patients with brain metastases. In: Hayat MA, ed. Brain Metastases from Primary Tumors, Volume 3. San Diego: Academic Press, 2016: 267–97. [Google Scholar]
- 132. Tawbi HA, Chung C, Margolin K. Nivolumab and Ipilimumab in Melanoma metastatic to the brain. N Engl J Med 2018;379:2178. 10.1056/NEJMc1812500 [DOI] [PubMed] [Google Scholar]
- 133. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus Trametinib in patients with BRAFV600-Mutant Melanoma brain metastases (COMBI-MB): a Multicentre, Multicohort, open-label, phase 2 trial. Lancet Oncol 2017;18:863–73. 10.1016/S1470-2045(17)30429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rulli E, Legramandi L, Salvati L, et al. The impact of targeted therapies and Immunotherapy in Melanoma brain metastases: A systematic review and meta-analysis. Cancer 2019;125:3776–89. 10.1002/cncr.32375 [DOI] [PubMed] [Google Scholar]
- 135. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in Melanoma patients with brain metastases. Cancer 2011;117:1687–96. 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- 136. Glitza IC, Rohlfs M, Bassett R, et al. Imct-07Therapeutic outcomes of intrathecal Interleukin-2 in metastatic Melanoma patients with Leptomeningeal disease (Lmd). Neuro Oncol 2015;17:v108. 10.1093/neuonc/nov218.07 [DOI] [Google Scholar]
- 137. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for Ipilimumab-refractory Melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing Pembrolizumab versus investigator-choice chemotherapy for Ipilimumab-refractory advanced Melanoma. Eur J Cancer 2017;86:37–45. 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 139. Kluger HM, Tawbi HA, Ascierto ML, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy resistance Taskforce. J Immunother Cancer 2020;8:e000398. 10.1136/jitc-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rizvi N, Ademuyiwa FO, Cao ZA, et al. Society for Immunotherapy of cancer (SITC) consensus definitions for resistance to combinations of immune Checkpoint inhibitors with chemotherapy. J Immunother Cancer 2023;11:e005920. 10.1136/jitc-2022-005920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Atkins MB, Ascierto PA, Feltquate D, et al. Society for Immunotherapy of cancer (SITC) consensus definitions for resistance to combinations of immune Checkpoint inhibitors with targeted therapies. J Immunother Cancer 2023;11:e005923. 10.1136/jitc-2022-005923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Eggermont AM, Meshcheryakov A, Atkinson V, et al. Crossover and rechallenge with Pembrolizumab in recurrent patients from the EORTC 1325-MG/Keynote-054 phase III trial, Pembrolizumab versus placebo after complete resection of high-risk stage III Melanoma. Eur J Cancer 2021;158:156–68. 10.1016/j.ejca.2021.09.023 [DOI] [PubMed] [Google Scholar]
- 143. Owen CN, Shoushtari AN, Chauhan D, et al. Management of early Melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann Oncol 2020;31:1075–82. 10.1016/j.annonc.2020.04.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pires da Silva I, Ahmed T, Reijers ILM, et al. Ipilimumab alone or Ipilimumab plus anti-PD-1 therapy in patients with metastatic Melanoma resistant to anti-PD-(L)1 monotherapy: a Multicentre, retrospective, cohort study. Lancet Oncol 2021;22:836–47. 10.1016/S1470-2045(21)00097-8 [DOI] [PubMed] [Google Scholar]
- 145. Vanderwalde AM, Moon J, Kendra K, et al. Abstract Ct013: S1616: Ipilimumab plus Nivolumab versus Ipilimumab alone in patients with metastatic or Unresectable Melanoma that did not respond to anti-Pd-1 therapy. Cancer Res 2022;82:CT013. 10.1158/1538-7445.AM2022-CT013 [DOI] [Google Scholar]
- 146. Olson DJ, Eroglu Z, Brockstein B, et al. Pembrolizumab plus Ipilimumab following anti-PD-1/L1 failure in Melanoma. J Clin Oncol 2021;39:2647–55. 10.1200/JCO.21.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ascierto PA, Lipson EJ, Dummer R, et al. Nivolumab and Relatlimab in patients with advanced Melanoma that had progressed on anti–programmed Death-1/programmed death ligand 1 therapy: results from the phase I/IIa RELATIVITY-020 trial. J Clin Oncol 2023;41:2724–35. 10.1200/JCO.22.02072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Buchbinder EI, Dutcher JP, Daniels GA, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic Melanoma and renal cell carcinoma following Pd1 or Pdl1 inhibition. J Immunother Cancer 2019;7:49. 10.1186/s40425-019-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Burton EM, Amaria RN, Glitza IC, et al. Phase II study of triplet combination Nivolumab (N) with Dabrafenib (D) and Trametinib (T) (Trident) in patients (Pts) with PD-1 Naïve or refractory BRAF-Mutated metastatic Melanoma (MM) with or without active brain metastases. JCO 2021;39:9520. 10.1200/JCO.2021.39.15_suppl.9520 [DOI] [Google Scholar]
- 150. Arance AM, de la Cruz-Merino L, Petrella TM, et al. Lenvatinib (Len) plus Pembrolizumab (Pembro) for patients (Pts) with advanced Melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: updated findings of LEAP-004. JCO 2021;39:9504. 10.1200/JCO.2021.39.15_suppl.9504 [DOI] [Google Scholar]
- 151. Chesney J, Lewis KD, Kluger H, et al. Efficacy and safety of Lifileucel, a one-time Autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced Melanoma after progression on immune Checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J Immunother Cancer 2022;10:e005755. 10.1136/jitc-2022-005755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Haanen JBAG, Rohaan M, Borch TH, et al. Lba3 treatment with tumor-infiltrating lymphocytes (TIL) versus Ipilimumab for advanced Melanoma: results from a multicenter, randomized phase III trial. Ann Oncol 2022;33:S1406. 10.1016/j.annonc.2022.08.036 [DOI] [Google Scholar]
- 153. Rohaan MW, Borch TH, van den Berg JH, et al. Tumor-infiltrating lymphocyte therapy or Ipilimumab in advanced Melanoma. N Engl J Med 2022;387:2113–25. 10.1056/NEJMoa2210233 [DOI] [PubMed] [Google Scholar]
- 154. Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American joint committee on cancer staging system for cutaneous Melanoma and proposal of a new staging system. J Clin Oncol 1997;15:1039–51. 10.1200/JCO.1997.15.3.1039 [DOI] [PubMed] [Google Scholar]
- 155. Pawlik TM, Ross MI, Thompson JF, et al. The risk of in-transit Melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol 2005;23:4588–90. 10.1200/JCO.2005.12.245 [DOI] [PubMed] [Google Scholar]
- 156. Lôbo M de M, Calsavara VF, Ricci BV, et al. Response rates of cutaneous Melanoma metastases to Diphencyprone: A meta-analysis. Journal of the American Academy of Dermatology 2020;83:1812–3. 10.1016/j.jaad.2020.04.023 [DOI] [PubMed] [Google Scholar]
- 157. Ross MI. Intralesional therapy with PV-10 (rose Bengal) for in-transit Melanoma. J Surg Oncol 2014;109:314–9. 10.1002/jso.23554 [DOI] [PubMed] [Google Scholar]
- 158. Liu BL, Robinson M, Han Z-Q, et al. Icp34.5 deleted herpes Simplex virus with enhanced Oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003;10:292–303. 10.1038/sj.gt.3301885 [DOI] [PubMed] [Google Scholar]
- 159. Kaufman HL, Shalhout SZ, Iodice G. Talimogene Laherparepvec: moving from first-in-class to best-in-class. Front Mol Biosci 2022;9:834841. 10.3389/fmolb.2022.834841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced Melanoma. J Clin Oncol 2015;33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 161. Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of Optim: a randomized phase III trial of Talimogene Laherparepvec versus granulocyte-macrophage colony-stimulating factor in Unresectable stage III–IV Melanoma. J Immunother Cancer 2019;7:145. 10.1186/s40425-019-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ribas A, Dummer R, Puzanov I, et al. Oncolytic Virotherapy promotes Intratumoral T cell infiltration and improves anti-PD-1 Immunotherapy. Cell 2017;170:1109–19. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ribas A, Chesney J, Long GV, et al. 1037O MASTERKEY-265: A phase III, randomized, placebo (Pbo)-Controlled study of Talimogene Laherparepvec (T) plus Pembrolizumab (P) for Unresectable stage IIIB–Ivm1C Melanoma (MEL). Ann Oncol 2021;32:S868–9. 10.1016/j.annonc.2021.08.1422 [DOI] [Google Scholar]
- 164. Puzanov I, Chesney J, Collichio F, et al. 433 Talimogene Laherparepvec (T-VEC) in combination with Ipilimumab (IPI) versus IPI alone for advanced Melanoma: 4-year interim analysis of a randomized, open-label, phase 2 trial. 35th anniversary annual meeting (SITC 2020). 35th Anniversary Annual Meeting (SITC 2020); November 2020. 10.1136/jitc-2020-SITC2020.0433 [DOI] [Google Scholar]
- 165. Kaufman HL, Amatruda T, Reid T, et al. Systemic versus local responses in Melanoma patients treated with Talimogene Laherparepvec from a multi-institutional phase II study. J Immunother Cancer 2016;4:12. 10.1186/s40425-016-0116-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Schvartsman G, Perez K, Flynn JE, et al. Safe and effective administration of T-VEC in a patient with heart transplantation and recurrent locally advanced Melanoma. J Immunother Cancer 2017;5:45. 10.1186/s40425-017-0250-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hirotsu KE, Hua V, Tran AT, et al. Complete remission from Intralesional Talimogene Laherparepvec for regionally advanced Merkel cell carcinoma in an immunocompromised solid organ transplant patient. JAAD Case Rep 2021;13:144–6. 10.1016/j.jdcr.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ressler J, Silmbrod R, Stepan A, et al. Talimogene Laherparepvec (T-VEC) in advanced Melanoma: complete response in a heart and kidney transplant patient. A case report. Br J Dermatol 2019;181:186–9. 10.1111/bjd.17783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rehman H, Silk AW, Kane MP, et al. Into the clinic: talimogene Laherparepvec (T-VEC), a first-in-class Intratumoral Oncolytic viral therapy. J Immunother Cancer 2016;4:53. 10.1186/s40425-016-0158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Goldmacher GV, Khilnani AD, Andtbacka RHI, et al. Response criteria for Intratumoral Immunotherapy in solid tumors: itRECIST. J Clin Oncol 2020;38:2667–76. 10.1200/JCO.19.02985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Andtbacka RHI, Ross M, Puzanov I, et al. Patterns of clinical response with Talimogene Laherparepvec (T-VEC) in patients with Melanoma treated in the Optim phase III clinical trial. Ann Surg Oncol 2016;23:4169–77. 10.1245/s10434-016-5286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dummer R, Gyorki D, Hyngstrom J, et al. LBA39 final 5-year results of the phase II, multicenter, randomized, open-label trial of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs immediate surgery in patients (pts) with resectable stage IIIB-ivm1a melanoma (MEL). Annals of Oncology 2022;33:S1406. 10.1016/j.annonc.2022.08.037 [DOI] [Google Scholar]
- 173. Emamekhoo H, Patel S, Rodriguez E, et al. IGNYTE: A phase 1/2 multi-cohort clinical trial of Rp1 ± Nivolumab in patients with non-small cell lung cancer and other solid tumors. Int J Radiat Oncol*Biol*Phys 2022;112:e12–3. 10.1016/j.ijrobp.2021.10.184 [DOI] [Google Scholar]
- 174. Luke J, Migden M, Chai-Ho W, et al. 550 ARTACUS: an open-label, multicenter, phase 1B/2 study of Rp1 in solid organ transplant recipients with advanced cutaneous malignancies. J Immunother Cancer 2021;9:A580. 10.1136/jitc-2021-SITC2021.550 [DOI] [Google Scholar]
- 175. Algazi AP, Twitty CG, Tsai KK, et al. Phase II trial of IL-12 Plasmid Transfection and PD-1 blockade in Immunologically quiescent Melanoma. Clin Cancer Res 2020;26:2827–37. 10.1158/1078-0432.CCR-19-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ribas A, Medina T, Kummar S, et al. SD-101 in combination with Pembrolizumab in advanced Melanoma: results of a phase IB, multicenter study. Cancer Discov 2018;8:1250–7. 10.1158/2159-8290.CD-18-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ribas A, Medina T, Kirkwood JM, et al. Overcoming PD-1 blockade resistance with Cpg-A toll-like receptor 9 agonist Vidutolimod in patients with metastatic Melanoma. Cancer Discov 2021;11:2998–3007. 10.1158/2159-8290.CD-21-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ibrahim AM, Le May M, Bossé D, et al. Correction to: imaging intensity and survival outcomes in high-risk Resected Melanoma treated by systemic therapy at recurrence. Ann Surg Oncol 2020;27:976–7. 10.1245/s10434-020-08618-z [DOI] [PubMed] [Google Scholar]
- 179. Sullivan RJ, Atkins MB, Kirkwood JM, et al. An update on the society for Immunotherapy of cancer consensus statement on tumor Immunotherapy for the treatment of cutaneous Melanoma: version 2.0. J Immunother Cancer 2018;6:44. 10.1186/s40425-018-0362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gibney GT, Zaemes J, Shand S, et al. PET/CT scan and biopsy-driven approach for safe anti-PD-1 therapy discontinuation in patients with advanced Melanoma. J Immunother Cancer 2021;9:e002955. 10.1136/jitc-2021-002955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Robert C, Marabelle A, Herrscher H, et al. Immunotherapy discontinuation - how, and when? data from Melanoma as a paradigm. Nat Rev Clin Oncol 2020;17:707–15. 10.1038/s41571-020-0399-6 [DOI] [PubMed] [Google Scholar]
- 182. Tan AC, Emmett L, Lo S, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic Melanoma. Ann Oncol 2018;29:2115–20. 10.1093/annonc/mdy330 [DOI] [PubMed] [Google Scholar]
- 183. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with Nivolumab plus Ipilimumab or Nivolumab alone versus Ipilimumab in patients with advanced Melanoma. J Clin Oncol 2022;40:127–37. 10.1200/JCO.21.02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 185. Somarouthu B, Lee SI, Urban T, et al. Immune-related tumour response assessment criteria: a comprehensive review. Br J Radiol 2018;91:20170457. 10.1259/bjr.20170457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer Immunotherapy. J Clin Oncol 2018;36:850–8. 10.1200/JCO.2017.75.1644 [DOI] [PubMed] [Google Scholar]
- 187. Kataoka Y, Hirano K. Which criteria should we use to evaluate the efficacy of immune-Checkpoint inhibitors Ann Transl Med 2018;6:222. 10.21037/atm.2018.04.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kirkwood JM, Long GV, Trefzer U, et al. BREAK-MB: A phase II study assessing overall intracranial response rate (OIRR) to Dabrafenib (Gsk2118436) in patients (Pts) with BRAF V600E/K Mutation-positive Melanoma with brain metastases (Mets). JCO 2012;30:8501. 10.1200/jco.2012.30.15_suppl.8501 [DOI] [Google Scholar]
- 189. Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active Melanoma brain metastases treated with combination Nivolumab plus Ipilimumab (Checkmate 204): final results of an open-label, Multicentre, phase 2 study. Lancet Oncol 2021;22:1692–704. 10.1016/S1470-2045(21)00545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Tawbi HA, Boutros C, Kok D, et al. New era in the management of Melanoma brain metastases. Am Soc Clin Oncol Educ Book 2018;38:741–50. 10.1200/EDBK_200819 [DOI] [PubMed] [Google Scholar]
- 191. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 2015;16:e270–8. 10.1016/S1470-2045(15)70057-4 [DOI] [PubMed] [Google Scholar]
- 192. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant Ipilimumab plus Nivolumab in macroscopic stage III Melanoma. Nat Med 2018;24:1655–61. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 193. Amaria RN, Reddy SM, Tawbi HA, et al. Publisher correction: Neoadjuvant immune Checkpoint blockade in high-risk Resectable Melanoma. Nat Med 2018;24:1942. 10.1038/s41591-018-0252-y [DOI] [PubMed] [Google Scholar]
- 194. Huang AC, Orlowski RJ, Xu X, et al. A single dose of Neoadjuvant PD-1 blockade predicts clinical outcomes in Resectable Melanoma. Nat Med 2019;25:454–61. 10.1038/s41591-019-0357-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stein JE, Lipson EJ, Cottrell TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res 2020;26:545–51. 10.1158/1078-0432.CCR-19-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. De Barros E. Silva MJ, Liz CD, Teixeira MR, et al. FDG-PET/CT response and outcome of Neoadjuvant Immunotherapy for clinical stage III Melanoma. JCO 2021;39:e21569. 10.1200/JCO.2021.39.15_suppl.e21569 [DOI] [Google Scholar]
- 197. Stein JE, Soni A, Danilova L, et al. Major pathologic response on biopsy (Mprbx) in patients with advanced Melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol 2019;30:589–96. 10.1093/annonc/mdz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Chen P-L, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune Checkpoint blockade. Cancer Discov 2016;6:827–37. 10.1158/2159-8290.CD-15-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Park HJ, Kim KW, Pyo J, et al. Incidence of Pseudoprogression during immune Checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 2020;297:87–96. 10.1148/radiol.2020200443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Thomas R, Somarouthu B, Alessandrino F, et al. Atypical response patterns in patients treated with Nivolumab. AJR Am J Roentgenol 2019;212:1177–81. 10.2214/AJR.18.20938 [DOI] [PubMed] [Google Scholar]
- 201. Nishino M, Giobbie-Hurder A, Manos MP, et al. Immune-related tumor response Dynamics in Melanoma patients treated with Pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 2017;23:4671–9. 10.1158/1078-0432.CCR-17-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor Pseudoprogression after Immunotherapy. Cancer Biol Med 2019;16:655–70. 10.20892/j.issn.2095-3941.2019.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing Immunotherapeutics. The Lancet Oncology 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Tsimberidou AM, Levit LA, Schilsky RL, et al. Trial reporting in Immuno-oncology (TRIO): an American society of clinical oncology-society for Immunotherapy of cancer statement. J Immunother Cancer 2018;6:108. 10.1186/s40425-018-0426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV Melanoma: results of Southwest oncology group clinical trial S9430. Cancer 2011;117:4740–06. 10.1002/cncr.26111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic Melanoma: analysis of data from the first multicenter selective Lymphadenectomy trial (MSLT-I). Ann Surg Oncol 2012;19:2547–55. 10.1245/s10434-012-2398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Faries MB, Mozzillo N, Kashani-Sabet M, et al. Long-term survival after complete surgical resection and adjuvant Immunotherapy for distant Melanoma metastases. Ann Surg Oncol 2017;24:3991–4000. 10.1245/s10434-017-6072-3 [DOI] [PubMed] [Google Scholar]
- 208. Ollila DW, Hsueh EC, Stern SL, et al. Metastasectomy for recurrent stage IV Melanoma. J Surg Oncol 1999;71:209–13. [DOI] [PubMed] [Google Scholar]
- 209. Klemen ND, Wang M, Feingold PL, et al. Patterns of failure after Immunotherapy with Checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic Melanoma. J Immunother Cancer 2019;7:196. 10.1186/s40425-019-0672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bello DM, Panageas KS, Hollmann T, et al. Survival outcomes after Metastasectomy in Melanoma patients categorized by response to Checkpoint blockade. Ann Surg Oncol 2020;27:1180–8. 10.1245/s10434-019-08099-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. D’Andrea MA, Reddy GK. Systemic antitumor effects and Abscopal responses in Melanoma patients receiving radiation therapy. Oncology 2020;98:202–15. 10.1159/000505487 [DOI] [PubMed] [Google Scholar]
- 212. Anderson ES, Postow MA, Wolchok JD, et al. Melanoma brain metastases treated with stereotactic Radiosurgery and concurrent Pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76. 10.1186/s40425-017-0282-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nardin C, Mateus C, Texier M, et al. Tolerance and outcomes of stereotactic Radiosurgery combined with anti-programmed cell Death-1 (Pembrolizumab) for Melanoma brain metastases. Melanoma Res 2018;28:111–9. 10.1097/CMR.0000000000000413 [DOI] [PubMed] [Google Scholar]
- 214. Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced Melanoma. Ann Oncol 2019;30:1154–61. 10.1093/annonc/mdz110 [DOI] [PubMed] [Google Scholar]
- 215. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of Pembrolizumab in patients with metastatic Melanoma. JCO 2018;36:1668–74. 10.1200/JCO.2017.75.6270 [DOI] [PubMed] [Google Scholar]
- 216. Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of Checkpoint inhibitors in transplant recipients: A case series and systematic review of literature. Oncologist 2020;25:505–14. 10.1634/theoncologist.2019-0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Carroll RP, Boyer M, Gebski V, et al. Immune Checkpoint inhibitors in kidney transplant recipients: a Multicentre, single-arm, phase 1 study. Lancet Oncol 2022;23:1078–86. 10.1016/S1470-2045(22)00368-0 [DOI] [PubMed] [Google Scholar]
- 219. Samani A, Zhang S, Spiers L, et al. Impact of age on the toxicity of immune Checkpoint inhibition. J Immunother Cancer 2020;8:e000871. 10.1136/jitc-2020-000871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Archibald WJ, Victor AI, Strawderman MS, et al. Immune Checkpoint inhibitors in older adults with Melanoma or cutaneous malignancies: the Wilmot cancer Institute experience. J Geriatr Oncol 2020;11:496–502. 10.1016/j.jgo.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 221. Khaki AR, Li A, Diamantopoulos LN, et al. Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune Checkpoint inhibitors. Cancer 2020;126:1208–16. 10.1002/cncr.32645 Available: https://onlinelibrary.wiley.com/toc/10970142/126/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Olsen CM, Knight LL, Green AC. Risk of Melanoma in people with HIV/AIDS in the Pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PLoS One 2014;9:e95096. 10.1371/journal.pone.0095096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sahin IH, Kane SR, Brutcher E, et al. Safety and efficacy of immune Checkpoint inhibitors in patients with cancer living with HIV: A perspective on recent progress and future needs. JCO Oncol Pract 2020;16:319–25. 10.1200/JOP.19.00754 [DOI] [PubMed] [Google Scholar]
- 224. Cook MR, Kim C. Safety and efficacy of immune Checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: A systematic review. JAMA Oncol 2019;5:1049–54. 10.1001/jamaoncol.2018.6737 [DOI] [PubMed] [Google Scholar]
- 225. Shan Q, Lu H. Immune Checkpoint inhibitors in special populations. Technol Cancer Res Treat 2021;20:15330338211036526. 10.1177/15330338211036526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American society of clinical oncology-friends of cancer research HIV working group. J Clin Oncol 2017;35:3774–80. 10.1200/JCO.2017.73.7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. G REaS . NCCN guidelines: cancer in people with HIV (version 1.2022 — February 3, 2022). National Comprehensive Cancer Network 2022. [Google Scholar]
- 228. Guihot A, Marcelin A-G, Massiani M-A, et al. Drastic decrease of the HIV reservoir in a patient treated with Nivolumab for lung cancer. Ann Oncol 2018;29:517–8. 10.1093/annonc/mdx696 [DOI] [PubMed] [Google Scholar]
- 229. Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in Cd4+ T cells from ART-suppressed individuals. Nat Commun 2019;10:814. 10.1038/s41467-019-08798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Guaitoli G, Baldessari C, Maur M, et al. Treating cancer with Immunotherapy in HIV-positive patients: A challenging reality. Crit Rev Oncol Hematol 2020;145:S1040-8428(19)30222-7. 10.1016/j.critrevonc.2019.102836 [DOI] [PubMed] [Google Scholar]
- 231. Burotto M, Gormaz JG, Samtani S, et al. Viable pregnancy in a patient with metastatic Melanoma treated with double Checkpoint Immunotherapy. Seminars in Oncology 2018;45:164–9. 10.1053/j.seminoncol.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 232. Xu W, Moor RJ, Walpole ET, et al. Pregnancy with successful foetal and maternal outcome in a Melanoma patient treated with Nivolumab in the first trimester: case report and review of the literature. Melanoma Res 2019;29:333–7. 10.1097/CMR.0000000000000586 [DOI] [PubMed] [Google Scholar]
- 233. Bucheit AD, Hardy JT, Szender JB, et al. Conception and viable twin pregnancy in a patient with metastatic Melanoma while treated with CTLA-4 and PD-1 Checkpoint inhibition. Melanoma Res 2020;30:423–5. 10.1097/CMR.0000000000000657 [DOI] [PubMed] [Google Scholar]
- 234. Andrikopoulou A, Korakiti AM, Apostolidou K, et al. Immune Checkpoint inhibitor administration during pregnancy: a case series. ESMO Open 2021;6:100262. 10.1016/j.esmoop.2021.100262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Palmeira P, Quinello C, Silveira-Lessa AL, et al. Igg Placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646. 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chang AE, Karnell LH, Menck HR. The National cancer data base report on cutaneous and Noncutaneous Melanoma: a summary of 84,836 cases from the past decade. The American college of Surgeons Commission on cancer and the American Cancer society. Cancer 1998;83:1664–78. [DOI] [PubMed] [Google Scholar]
- 237. Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant Uveal Melanoma. Invest Ophthalmol Vis Sci 2003;44:4651–9. 10.1167/iovs.03-0538 [DOI] [PubMed] [Google Scholar]
- 238. Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic Uveal Melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–53. 10.1002/cncr.30258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of Ipilimumab for metastatic Uveal Melanoma: a retrospective review of the Dana-Farber cancer Institute, Massachusetts general hospital, memorial Sloan-Kettering cancer center, and University hospital of Lausanne experience. Cancer 2013;119:3687–95. 10.1002/cncr.28282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Piulats JM, Espinosa E, de la Cruz Merino L, et al. Nivolumab plus Ipilimumab for treatment-Naïve metastatic Uveal Melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma group (GEM-1402). J Clin Oncol 2021;39:586–98. 10.1200/JCO.20.00550 [DOI] [PubMed] [Google Scholar]
- 241. Pelster MS, Gruschkus SK, Bassett R, et al. Nivolumab and Ipilimumab in metastatic Uveal Melanoma: results from a single-arm phase II study. J Clin Oncol 2021;39:599–607. 10.1200/JCO.20.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with Tebentafusp in metastatic Uveal Melanoma. N Engl J Med 2021;385:1196–206. 10.1056/NEJMoa2103485 [DOI] [PubMed] [Google Scholar]
- 243. Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a Prognostic 31-gene expression profile in subpopulations of Melanoma patients with favorable outcomes by standard criteria. Journal of the American Academy of Dermatology 2019;80:149–157. 10.1016/j.jaad.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 244. Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic Uveal Melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular Melanoma study. Ann Oncol 2019;30:1370–80. 10.1093/annonc/mdz176 [DOI] [PubMed] [Google Scholar]
- 245. Zager JS, Orloff MM, Ferrucci PF, et al. FOCUS phase 3 trial results: percutaneous hepatic perfusion (PHP) with Melphalan for patients with ocular Melanoma liver metastases (PHP-OCM-301/301A). JCO 2022;40:9510. 10.1200/JCO.2022.40.16_suppl.9510 [DOI] [Google Scholar]
- 246. Kuk D, Shoushtari AN, Barker CA, et al. Prognosis of Mucosal, Uveal, Acral, Nonacral cutaneous, and unknown primary Melanoma from the time of first metastasis. Oncologist 2016;21:848–54. 10.1634/theoncologist.2015-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Nenclares P, Ap Dafydd D, Bagwan I, et al. Head and neck Mucosal Melanoma: the United Kingdom national guidelines. Eur J Cancer 2020;138:11–8. 10.1016/j.ejca.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 248. Lian B, Si L, Cui C, et al. Phase II randomized trial comparing high-dose IFN-Α2B with Temozolomide plus cisplatin as systemic adjuvant therapy for Resected Mucosal Melanoma. Clin Cancer Res 2013;19:4488–98. 10.1158/1078-0432.CCR-13-0739 [DOI] [PubMed] [Google Scholar]
- 249. Choi Jacob SB. Zhang Hui clinical practices and outcomes of patients with primary Mucosal Melanoma in the adjuvant setting. 2022.
- 250. Ho J, Mattei J, Tetzlaff M, et al. Neoadjuvant Checkpoint inhibitor Immunotherapy for Resectable Mucosal Melanoma. Front Oncol 2022;12:1001150. 10.3389/fonc.2022.1001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Hamid O, Robert C, Ribas A, et al. Antitumour activity of Pembrolizumab in advanced Mucosal Melanoma: a post-hoc analysis of KEYNOTE-001. Br J Cancer 2018;119:670–4. 10.1038/s41416-018-0207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of Nivolumab alone or in combination with Ipilimumab in patients with Mucosal Melanoma: A pooled analysis. J Clin Oncol 2017;35:226–35. 10.1200/JCO.2016.67.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Bello DM, Chou JF, Panageas KS, et al. Prognosis of Acral Melanoma: a series of 281 patients. Ann Surg Oncol 2013;20:3618–25. 10.1245/s10434-013-3089-0 [DOI] [PubMed] [Google Scholar]
- 254. Huang K, Fan J, Misra S. Acral Lentiginous Melanoma: incidence and survival in the United States, 2006-2015, an analysis of the SEER Registry. J Surg Res 2020;251:329–39. 10.1016/j.jss.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 255. Nathan P, Ascierto PA, Haanen J, et al. Safety and efficacy of Nivolumab in patients with rare Melanoma subtypes who progressed on or after Ipilimumab treatment: a single-arm, open-label, phase II study (Checkmate 172). Eur J Cancer 2019;119:168–78. 10.1016/j.ejca.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 256. Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti-PD-1 agents in Acral and Mucosal Melanoma. Cancer 2016;122:3354–62. 10.1002/cncr.30259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Furney SJ, Turajlic S, Stamp G, et al. The mutational burden of Acral Melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res 2014;27:835–8. 10.1111/pcmr.12279 Available: http://doi.wiley.com/10.1111/pcmr.2014.27.issue-5 [DOI] [PubMed] [Google Scholar]
- 258. Nakamura Y, Fujisawa Y. Diagnosis and management of Acral Lentiginous Melanoma. Curr Treat Options Oncol 2018;19:42. 10.1007/s11864-018-0560-y [DOI] [PubMed] [Google Scholar]
- 259. Wood LS, Moldawer NP, Lewis C. Immune Checkpoint inhibitor therapy: key principles when educating patients. Clin J Oncol Nurs 2019;23:271–80. 10.1188/19.CJON.271-280 [DOI] [PubMed] [Google Scholar]
- 260. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune Checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. JCO 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Tariman J, Mehmeti E, Spawn N, et al. Oncology nursing and shared decision making for cancer treatment. CJON 2016;20:560–3. 10.1188/16.CJON.560-563 [DOI] [PubMed] [Google Scholar]
- 262. Robert C, Hwu W-J, Hamid O, et al. Long-term safety of Pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced Melanoma. Eur J Cancer 2021;144:182–91. 10.1016/j.ejca.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Fecher LA, Agarwala SS, Hodi FS, et al. Ipilimumab and its toxicities: A Multidisciplinary approach. Oncologist 2013;18:733–43. 10.1634/theoncologist.2012-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Khattak MA, Luke JJ, Long GV, et al. Adjuvant Pembrolizumab versus placebo in Resected high-risk stage II Melanoma: health-related quality of life from the randomized phase 3 KEYNOTE-716 study. European Journal of Cancer 2022;176:207–17. 10.1016/j.ejca.2022.08.004 [DOI] [PubMed] [Google Scholar]
- 265. Schadendorf D, Dummer R, Hauschild A, et al. Health-related quality of life in the randomised KEYNOTE-002 study of Pembrolizumab versus chemotherapy in patients with Ipilimumab-refractory Melanoma. Eur J Cancer 2016;67:46–54. 10.1016/j.ejca.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 266. Petrella TM, Robert C, Richtig E, et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of Pembrolizumab versus Ipilimumab in patients with advanced Melanoma. Eur J Cancer 2017;86:115–24. 10.1016/j.ejca.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 267. Schadendorf D, Larkin J, Wolchok J, et al. Health-related quality of life results from the phase III Checkmate 067 study. Eur J Cancer 2017;82:80–91. 10.1016/j.ejca.2017.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Joseph RW, Liu FX, Shillington AC, et al. Health-related quality of life (Hrqol) in patients with advanced Melanoma receiving Immunotherapies across real-world clinical practice settings. JCO 2019;37:e21004. 10.1200/JCO.2019.37.15_suppl.e21004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Mamoor M, Postow MA, Lavery JA, et al. Quality of life in long-term survivors of advanced Melanoma treated with Checkpoint inhibitors. J Immunother Cancer 2020;8:e000260. 10.1136/jitc-2019-000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Buzaglo JS, Miller MF, Zaleta AK, et al. Financial toxicity and cancer-related distress among Melanoma survivors. JCO 2017;35:9588. 10.1200/JCO.2017.35.15_suppl.9588 [DOI] [Google Scholar]
- 271. Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of Melanoma in the United States. JAMA Dermatol 2020;156:57–64. 10.1001/jamadermatol.2019.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Duma N, Lambertini M. It is time to talk about fertility and Immunotherapy. Oncologist 2020;25:277–8. 10.1634/theoncologist.2019-0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Garutti M, Lambertini M, Puglisi F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open 2021;6:100276. 10.1016/j.esmoop.2021.100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Woodruff TK. Oncofertility: a grand collaboration between reproductive medicine and oncology. REPRODUCTION 2015;150:S1–10. 10.1530/REP-15-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]