Abstract
Objectives
This study aims to assess the relative of social support and psychological distress in disease activity among patients with Crohn’s disease (CD) in China, and explore whether sex moderates the relationship between disease activity and social support and psychological distress in CD.
Design
Our study has a cross-sectional design.
Setting
This was a single-centre study, which was conducted in Wuhan, China.
Participants
A total of 184 patients with CD at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology were enrolled in this study; of these,162 patients were included in the final analysis.
Primary and secondary outcome measures
The main study outcome was the CD patients’ clinical and questionnaire data. The association of disease activity, social support and psychological distress with patients with CD was also evaluated based on the collected data.
Results
A total of 162 patients with CD were enrolled. Compared with patients with CD in remission (CD-R), the patients with CD in activity (CD-A) had higher C reactive protein (CRP) (p=0.001), anaemia (p<0.001) and relapse rates in the last year (p<0.001). Independent samples t-tests indicated that the CD-A group reported lower Social Support Rating Scale scores and higher Symptom Checklist-90 scores than the CD-R group. Moreover, men with CD had lower somatisation (p=0.030) and anxiety (p=0.050) scores than women. In binary logistic regression models, the subjective support (beta=0.903, p=0.013), the clinical factors of CRP (beta=1.038, p=0.001) and psychological distress factors of anxiety (beta=1.443, p=0.008) and other (beta=1.235, p=0.042) were disease activity predictors.
Conclusion
The findings highlight the importance of the psychological distress and social support factors that may play a role in CD patients’ health. Interventions to address these issues should be part of management in CD.
Keywords: Coeliac disease, Inflammatory bowel disease, MENTAL HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our study improved the understanding of the differences in psychological distress and social support among patients with Crohn’s disease (CD) at different active stages in low/middle-income countries, especially in central China.
Provide evidence for subsequent studies attempting to establish a relationship between social support and psychological well-being and disease activity.
In this study, gender differences were considered in the analysis.
However, this cross-sectional study could not address the causality between disease activity and psychological change factors of patients with CD.
Introduction
Crohn’s disease (CD) is a chronic, non-specific intestinal inflammatory disease characterised by recurrent abdominal pain and diarrhoea that peaks in young adulthood.1 In addition to gastrointestinal manifestations, patients with CD experience other systemic manifestations and complications. As of 2017, inflammatory bowel disease (IBD) affected 6.8 million people worldwide. The USA reported the highest incidence of IBD, followed by the UK.2 Epidemiological studies have shown that the incidence of IBD in China is 3.44 cases per one million people, which is the highest in Asia, and the incidence of IBD in mainland China is higher in the south and lower in the north.3 In recent years, the incidence of CD has increased rapidly in China.4 5
Due to bowel damage and a long medical history, patients have a high prevalence of psychological impairment, such as anxiety and depression, compared with the general population.6 7 Although medical treatments are effective in controlling gastrointestinal inflammation, the relapsing behaviour of CD can cause psychological disorders. Moreover, patients with CD typically require lifelong medication, which seriously affects their quality of life and increases psychological distress. The CD is associated with high medical costs, high rates of psychological disorders and illness burdens associated with reduced productivity and activity. Studies have shown that approximately 20% of patients with IBD may have symptoms of anxiety, and approximately 15% have symptoms of depression.8 9 Large population studies showed that the prevalence of psychological distress and injury in patients with IBD was significantly higher than that in non-IBD adults.10 11 Regarding the relationship between the psychological state of patients with IBD and gender differences, studies have pointed out that female patients with IBD are more prone to anxiety, depression and other psychological problems than male patients.9 For example, the prevalence of comorbidity anxiety and depression in female patients with IBD was 33.8% and 21.2%, respectively, compared with 22.8% and 16.2% in male patients with IBD.12
The uncertainty of treatment results and psychological disorders may lead to disease recurrence, aggravate the course of the disease, and directly lead to the decline of patients’ quality of life and the increase of treatment costs.13 14 Patients with IBD and anxiety or depression have a higher risk of hospitalisation, emergency room visits, readmissions and use of outpatient services than patients without these symptoms.15 Thus, healthcare services for CD are more demanding and costly for patients with symptoms of anxiety and depression. Xu et al reported that poor sleep quality, anxiety and depression were related to inflammatory activity.16 In addition, disease activity was found to be associated with depression and anxiety, and psychological distress may increase the likelihood of disease relapse.17 However, most previous studies have focused on anxiety or depression, rarely focusing on other dimensions. Social support might be another dimension that plays a role in disease severity. Interestingly, there are few studies on the association between social support and disease activity in patients with IBD.18 19
This study aimed to improve the understanding of the differences in psychological distress and social support among patients with IBD at different active stages in China to provide evidence for subsequent research attempts to establish an association between social support and psychological well-being and disease activity. In addition, we considered whether sex moderates the relationship between disease activity and social support and psychological distress in CD. Previous studies of patients with IBD have found that older patients have higher symptoms of anxiety and depression. Therefore, in this study, age was used as a control variable. Thus, we proposed the following hypotheses: there were significant differences in psychological disorders and social support between patients with IBD in activity (CD-A) and CD patients in remission (CD-R), and there may be gender differences as well.
Methods
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Participants
This study was a cross-sectional, single-centre study. Participants were recruited between March 2020 and March 2022 at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology in China. The inclusion criteria were patients with a diagnosis of CD,20 adult patients (aged 18 years or more), patients with sufficient ability in spoken and written Chinese to complete all the questionnaires, patients without a diagnosis of concomitant mental disorders or dementia and patients not taking psychotropic medication for CD. The exclusion criteria were as follows: (1) patients who could not complete the questionnaires; (2) patients who had tumours or other medical comorbidities and (3) patients who were pregnant. The flow diagram of the enrolled patients and healthy controls is shown in figure 1.
Figure 1.
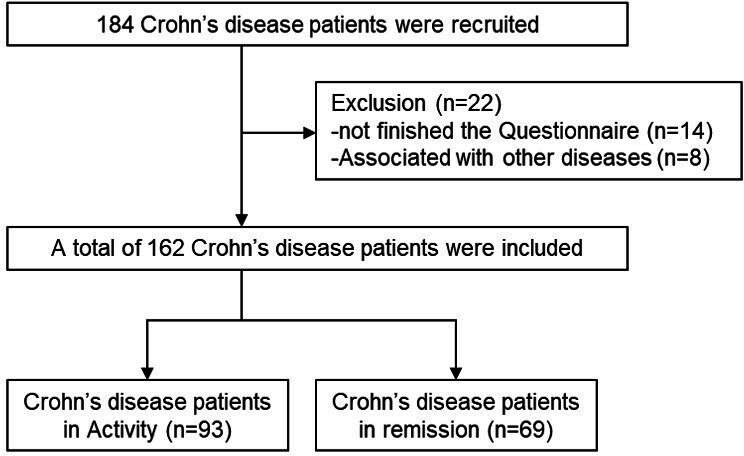
Flow diagram of the enrolled patients.
Data collection
Clinical and demographic data were collected, including age, sex, body mass index, employment status, living status, educational status, marital status and disease duration. The severity of CD was assessed using Crohn’s Disease Activity Index (CDAI) scores.21 A CDAI score of less than 150 was defined as disease in remission. A CDAI score of 150 or more was defined as disease in activity.
Social support was assessed using the Social Support Rating Scale (SSRS).22 23 The participants’ social support was evaluated by the Chinese version of the SSRS, which was previously demonstrated to have reliability and validity. It can measure the characteristics of social support and its relationship with participants’ mental health levels, mental illness, and various physical diseases. The scale has 10 items, including items regarding objective support (3 items), subjective support (4 items) and the utilisation of social support (3 items). The total score ranges from 11 to 59 and is acquired by adding the scores of each item. Lower scores on indices of the SSRS indicate less social support.
The psychological state was assessed using the Symptom Checklist-90 (SCL-90).24 25 The scale has a total of 90 items regarding a wide range of psychiatric symptoms, including feelings, emotions, thinking, consciousness, behaviours, habits, interpersonal relationships, diet and sleep. Ten factors are used to reflect 10 aspects of psychological symptoms, including psychoticism, paranoid ideation, phobic anxiety, hostility, anxiety, depression, interpersonal sensitivity, obsessive–compulsive behaviours and somatisation. The statistical standard of the SCL-90 mainly consists of two items: the total score and the various factor scores. The total score is the sum of the scores of the 90 items, which reflects the severity of the disease. The factor score is the average score of all factors, which ranges from ‘0’ (‘no problem’) to ‘4’ (‘very serious’). Each factor reflects a certain aspect of the participant’s symptoms, so the symptom distribution characteristics of the participants can be understood through the factor score. According to the results of the Chinese norm, if the total score exceeds 160 points, the positive items exceed 43 points or any factor score exceeds 2 points, the participants are considered to have a positive screening, and further examination is needed. This version has excellent internal consistency for all items.
Statistical analysis
All statistical analyses were performed in SPSS V.26.0, GraphPad Prism V.8.0 and Origin 2021 software. The independent sample t-test was used to determine the relationship between disease status and sex differences and scale factors. Correlation analysis was applied to evaluate the relationship among the clinical, psychological and social support factors. Hierarchical multiple regression analysis was used to examine the unique contribution of participant characteristics, psychological distress scores and SSRS factor scores on the composite factors of disease status. A p<0.05 was considered statistically significant.
Results
Sample characteristics
A total of 162patients with CD with complete survey responses were analysed (CD-A n=93, CD-R n=69). Participants in the CD-A group reported a disease course of 13 months, and those in the CD-R group reported a disease course of 11 months. The independent sample t-test and χ2 tests indicated no statistically significant difference in age, employment status, living status, marital status or years of education between the two groups. Compared with the CD-R group, the CD-A group had higher C reactive protein (CRP) (p=0.001) and ESR values (p<0.001). In addition, these patients tended to have higher anaemia rates and relapse rates in the last year (p<0.001), which is shown in table 1. Independent samples t-tests indicated differences between the two groups, with the CD-A group reporting lower SSRS scores and higher SCL-90 scores than the CD-R group, which is shown in table 2. Moreover, women showed higher levels of anaemia rate (p=0.021), relapse rates in the last year (p=0.020) and somatisation (p=0.030) and anxiety (p=0.050) than men, as shown in table 3 and online supplemental table 1.
Table 1.
Baseline characteristics of the study population
| Characteristics | CD-A (n=93) | CD-R (n=69) | P value |
| Age | 31±12 | 35±15 | 0.096 |
| Sex (female) | 36 (38.7%) | 31 (44.9%) | 0.427 |
| Body mass index | 19.5±3.9 | 20.8±4.3 | 0.049 |
| Employment status | – | – | 0.961 |
| No | 27 (29.0%) | 21 (30.5%) | |
| Retired | 6 (6.5%) | 3 (4.3%) | |
| Yes | 60 (64.5%) | 45 (65.2%) | |
| Living status | – | – | 0.248 |
| Alone | 3 (3.2%) | 6 (8.7%) | |
| With others | 90 (96.8%) | 63 (91.3%) | |
| Education | – | – | 0.705 |
| Up to 6 years | 5 (5.4%) | 4 (5.8%) | |
| Up to 9 years | 28 (30.1%) | 18 (26.1%) | |
| Up to 12 years | 44 (47.3%) | 34 (49.3%) | |
| College | 16 (17.2%) | 13 (18.8%) | |
| Marital status | – | – | 0.579 |
| Married/cohabitating | 53 (57.0%) | 34 (49.3%) | |
| Widowed/divorced | 7 (7.5%) | 5 (7.2%) | |
| Single | 33 (35.5%) | 30 (43.5%) | |
| Montreal location | – | – | 0.808 |
| Ileal (L1) | 35 (37.6%) | 28 (40.7%) | |
| Colonic (L2) | 15 (16.2%) | 5 (7.2%) | |
| Ileocolon (L3) | 39 (41.9%) | 33 (47.8%) | |
| Upper gastrointestinal tract (L4) | 1 (1.1%) | 0 (0%) | |
| L4+L1/L2/L3 | 3 (3.2%) | 3 (4.3%) | |
| Montreal behaviour | – | – | 0.401 |
| Inflammatory | 52 (55.9%) | 45 (65.2%) | |
| Structuring | 24 (25.8%) | 16 (23.2%) | |
| Penetrating | 17 (18.3%) | 8 (11.6%) | |
| Perianal disease | 35 (37.6%) | 28 (40.6%) | 0.704 |
| Current therapy | – | – | 0.169 |
| No treatment | 0 (0%) | 2 (2.9%) | |
| Corticosteroids | 8 (8.6%) | 7 (10.1%) | |
| 5-aminosalicylates | 21 (22.6%) | 17 (24.6%) | |
| Immunomodulators | 27 (29.0%) | 22 (31.9%) | |
| Antitumour necrosis factor | 16 (17.2%) | 9 (13.0%) | |
| Combined therapy | 21 (22.6%) | 12 (17.5%) | |
| Anaemia | – | – | <0.001 |
| No | 36 (38.7%) | 55 (79.7%) | |
| Mild | 49 (52.7%) | 10 (14.5%) | |
| Moderate | 6 (6.5%) | 3 (4.3%) | |
| Severe | 2 (2.1%) | 1 (1.5%) | |
| Relapses in the last year | – | – | <0.001 |
| 0 | 13 (14.0%) | 35 (50.7%) | |
| 1–2 | 57 (61.3%) | 24 (34.8%) | |
| 3 | 8 (8.6%) | 8 (11.6%) | |
| ≥4 | 15 (16.1%) | 2 (2.9%) | |
| Disease duration (months) | 13±25 | 11±19 | 0.594 |
| C reactive protein | 24.3±34.4 | 8.8±18.8 | 0.001 |
Note: Data are presented as the number (%) or mean±SD.
CD-A, Crohn’s disease patients in activity; CD-R, CD patients in remission.
Table 2.
Questionnaire survey in for patients with Crohn’s disease
| Variable | CD-A (n=93) | CD-R (n=69) | P value |
| SCL-90 | |||
| Somatisation | 18.8±5.9 | 16.3±4.7 | 0.004 |
| Obsessive–compulsive | 19.0±5.4 | 15.4±4.4 | <0.0001 |
| Interpersonal sensitivity | 15.4±5.8 | 12.1±4.0 | <0.0001 |
| Depression | 24.7±9.1 | 18.3±5.2 | <0.0001 |
| Anxiety | 16.2±5.3 | 12.4±3.2 | <0.0001 |
| Hostility | 10.3±3.8 | 8.8±3.6 | 0.012 |
| Phobic anxiety | 9.7±3.3 | 8.2±2.0 | 0.001 |
| Paranoid | 8.9±3.2 | 7.3±2.3 | <0.0001 |
| Psychoticism | 14.9±4.3 | 12.3±3.6 | <0.0001 |
| Other | 12.6±3.9 | 9.9±2.5 | <0.0001 |
| SSRS | |||
| Objective support | 8.8±2.8 | 9.3±2.4 | 0.239 |
| Subjective support | 15.1±5.6 | 18.0±6.2 | 0.003 |
| Availability | 6.9±1.7 | 7.4±1.7 | 0.080 |
Note: Data are presented as the mean±SD.
CD-A, Crohn’s disease patients in activity; CD-R, Crohn’s disease patients in activity; SCL-90, Symptom Checklist-90; SSRS, Social Support Rating Scale.
Table 3.
Differences in questionnaire survey results between men (n=95) and women (n=67)
| Variable | Men | Women | P value |
| SCL-90 | |||
| Somatisation | 17.2±5.0 | 19.5±6.4 | 0.030 |
| Obsessive–compulsive | 17.2±5.0 | 18.1±5.9 | 0.345 |
| Interpersonal sensitivity | 13.9±5.1 | 14.1±6.2 | 0.811 |
| Depression | 21.2±7.4 | 24.0±10.0 | 0.089 |
| Anxiety | 14.2±4.4 | 15.8±5.6 | 0.050 |
| Hostility | 9.6±3.6 | 9.5±4.3 | 0.932 |
| Phobic anxiety | 8.8±2.6 | 9.7±3.6 | 0.124 |
| Paranoid | 8.1±2.8 | 8.5±3.4 | 0.515 |
| Psychoticism | 13.7±4.1 | 13.9±4.3 | 0.734 |
| Other | 11.4±3.4 | 11.7±4.3 | 0.614 |
| SSRS | |||
| Objective support | 8.9±2.7 | 9.1±2.6 | 0.641 |
| Subjective support | 16.4±5.7 | 16.1±6.6 | 0.757 |
| Availability | 7.0±1.7 | 7.3±1.7 | 0.471 |
Note: Data are presented as the mean±SD.
SCL-90, Symptom Checklist-90; SSRS, Social Support Rating Scale.
bmjopen-2023-076219supp001.pdf (32.4KB, pdf)
Preliminary analyses
Figure 2 shows the correlation between social support and the psychological distress scale. The results showed that objective support was negatively correlated with psychological distress (obsessive–compulsive, interpersonal sensitivity, anxiety, hostility, phobic anxiety, paranoid, psychoticism and other factors) (p<0.05). Subjective support was negatively correlated with psychological distress (somatisation, obsessive–compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid, psychoticism and other) (p<0.05). Availability was negatively correlated with psychological distress (somatisation, obsessive–compulsive, interpersonal sensitivity, depression, anxiety, hostility, paranoid, psychoticism and other factors) (p<0.05).
Figure 2.
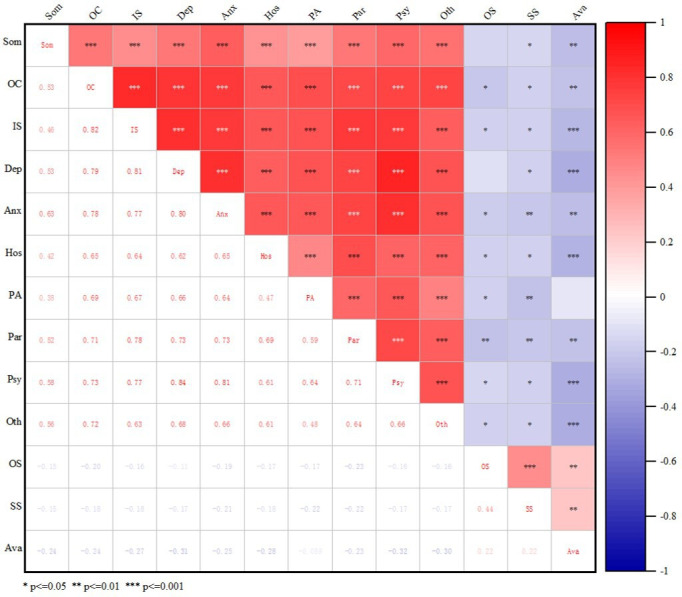
Analysis of the correlation of clinical data with social support and psychological factors. Anx, anxiety; Ava, availability; Dep, depression; Hos, hostility; IS, interpersonal sensitivity; OC, obsessive–compulsive; OS, objective support; Oth,other; PA, phobic anxiety; Par, paranoid; Psy, psychoticism; Som, somatisation; SS, subjective support.
Binary logistic regression models
Univariate analysis suggested that CRP levels (p=0.001), somatisation (p=0.007), obsessive compulsiveness (p<0.001), interpersonal sensitivity (p<0.001), depression (p<0.001), anxiety (p=0.039), hostility (p=0.015), phobic anxiety (p=0.002), paranoid (p=0.001), psychoticism (p<0.001), other factors (p<0.001) and subjective support (p=0.003) were statistically significant and were included in the subsequent binary logistic regression analysis. Binary logistic regression analyses showed that the social support factors of subjective support (beta=0.903, p=0.013), the clinical factors of CRP levels (beta=1.038, p=0.001), the psychological distress factors of anxiety (beta=1.443, p=0.008) and other factors (beta=1.235, p=0.042) were predictors of disease activity, as shown in table 4.
Table 4.
Results of the analysis of binary logistic regression analysis on disease activity
| The factors | Hosmer and Lemeshow test | SE | Wald | Beta | 95% CI | P value |
| Univariate | – | – | – | – | ||
| Age | 0.466 | 0.012 | 2.727 | 0.980 | 0.957 to 1.004 | 0.099 |
| Body mass index | 0.155 | 0.040 | 3.760 | 0.925 | 0.855 to 1.001 | 0.053 |
| Disease duration | 0.494 | 0.007 | 0.284 | 1.004 | 0.990 to 1.018 | 0.594 |
| C reactive protein | 0.000 | 0.011 | 11.378 | 1.039 | 1.016 to 1.062 | 0.001 |
| Objective support | 0.434 | 0.061 | 1.389 | 0.931 | 0.827 to 1.049 | 0.239 |
| Subjective support | 0.339 | 0.028 | 8.551 | 0.921 | 0.872 to 0.973 | 0.003 |
| Availability | 0.504 | 0.095 | 3.017 | 0.849 | 0.705 to 1.021 | 0.082 |
| Somatisation | 0.090 | 0.034 | 7.296 | 1.096 | 1.025 to 1.171 | 0.007 |
| Obsessive compulsive | 0.163 | 0.037 | 16.349 | 1.161 | 1.080 to 1.248 | <0.001 |
| Interpersonal sensitivity | 0.130 | 0.038 | 13.650 | 1.153 | 1.069 to 1.243 | <0.001 |
| Depression | 0.785 | 0.032 | 19.579 | 1.154 | 1.083 to 1.230 | <0.001 |
| Anxiety | 0.039 | 0.058 | 20.748 | 1.301 | 1.162 to 1.456 | 0.039 |
| Hostility | 0.056 | 0.049 | 5.876 | 1.126 | 1.023 to 1.240 | 0.015 |
| Phobic anxiety | 0.601 | 0.079 | 9.578 | 1.275 | 1.093 to 1.488 | 0.002 |
| Paranoid | 0.212 | 0.081 | 10.490 | 1.300 | 1.109 to 1.523 | 0.001 |
| Psychoticism | 0.029 | 0.055 | 13.645 | 1.224 | 1.099 to 1.362 | <0.001 |
| Other | 0.382 | 0.061 | 18.473 | 1.301 | 1.154 to 1.467 | <0.001 |
| Multivariate | – | – | – | – | – | |
| Subjective support | 0.519 | 0.041 | 6.216 | 0.903 | 0.834 to 0.979 | 0.013 |
| C reactive protein | 0.012 | 10.486 | 1.038 | 1.105 to 1.062 | 0.001 | |
| Anxiety | 0.138 | 7.009 | 1.443 | 1.100 to 1.893 | 0.008 | |
| Other | 0.103 | 4.155 | 1.235 | 1.008 to 1.512 | 0.042 |
Note: SE, Durbin-Watson:1.980.
Discussion
In this study, we described clinical, social support and psychological distress differences, and we also assessed the relationships between disease activity and dimensions of psychological distress and social support symptoms in a cohort of patients with CD. As we previously hypothesised, our present results showed that patients with CD-A had higher SCL-90 and lower SSRS scores than patients with CD-R, and social support factors were related to psychological distress factors, both of which had an impact on disease activity. We also found that women showed higher levels of somatisation and anxiety than men, but this was not observed for social support. Finally, we found that CRP, subjective support, anxiety and other factors were relevant determinants of disease activity in patients with CD.
Psychological factors such as anxiety and depression have been studied about CD,26 but the roles of other factors such as social support have been poorly investigated. The high correlations of social support factors with psychological distress symptoms in patients with CD are consistent with a previous study about other illnesses27 and indicate that the three factors of social support are likely a concept that reflects another dimension of psychological states. Social support is defined as behavioural or emotional support provided by family members, other people or other groups.
Social support is a positive health resource that contributes to the well-being of people with chronic diseases. The ability of an actor to derive benefits from his or her membership in a social network or other social structure. This positive support helps individuals overcome difficulties and challenges in life, especially stress related to coping with chronic illness. This is consistent with a biobehavioural model in which patients’ responses to illness and health are influenced by family and peer relationships. Social support can be divided into three categories, namely: objective support, subjective support and availability.3 However, in populations of patients with chronic disease, there are individual differences in the use of social support. Some people can receive support at any time but refuse the help of others. In addition, interpersonal support is a process of individual interaction. Past research has shown that social support has different effects on different diseases.28
IBD is considered a biopsychosocial disease characterised by psychological distress and psychological or psychiatric disorders, which is associated with stress, social interactions and attachment insecurity.29 30 Chronic diseases are thought to affect a patient’s mental capacity and determine the patient’s transition to attachment insecurity. Recently, several studies have begun investigating attachment dimensionality in people with IBD. According to attachment theory and research and social interaction are regulated by individual’s attachment system, which begins to develop in infancy. Individuals with secure attachment styles may form positive relationships, experience a sense of self-confidence and have realistic perceptions of others. Conversely, people with anxious attachment types may have a sense of insecurity in relationships. Sound social support may provide individuals with positive emotional experiences and secure attachment styles.31 32 Social support for family members and friends includes the ability to communicate stress problems, discuss fears and worries, make decisions together, plan social activities together, and get along together in difficult situations. This positive support helps individuals overcome difficulties and challenges in life, especially the stress associated with coping with chronic diseases. On the contrary, patients with insecure attachment may be less able to form positive relationships with doctors and less able to receive help and support from close people, which can lead to worsening disease management.
Consistent with previous research, the depressive, anxiety and somatisation factors of patients with CD are different for different disease severities.33 34 The three factors of social support are negatively correlated with psychological distress (somatisation, anxiety, anxiety and other factors). Somatisation mainly reflects the subjective body discomfort of patients, including discomfort due to cardiovascular, gastrointestinal, respiratory and other systems, as well as headaches, backaches and muscle soreness. Interestingly, the relationship between disease severity and most psychological distress and social support factors in addition to anxiety and subjective support factors was no longer significant after including social support factors in the model.
In binary logistic regression models, we also found that subjective support factors, anxiety, other psychological distress factors and CRP remained significant predictors of disease severity, which may be related to the fluctuating course of progression and remission that characterises this disease. Namely, patients with CD attach particular importance to their subjective feelings and may be inclined to interpret any physical or subjective discomfort they experience as a sign of psychological distress, leading them to report lower levels of subjective support. However, these lower levels of subjective support result in worsening disease activity. This biopsychosocial explanation is consistent with what has been found in people with CD.35 36
In our study, the psychological dimension data were obtained from the SCL-90. The results indicate that the psychological state of CD-A is affected in some dimensions, compared with CD-R. This is consistent with the results of Goodhand et al.37 Neither CD-A nor CD-R met the criteria for anxiety and depression in our study. In recent years, an increasing number of doctors have realised that psychological disorders are common in patients with IBD and may affect the disease condition and quality of life.22 38 However, the aetiology of psychological disorders appears to be multifactorial; for example, environmental factors may include stressful life events, disease activity, disease course, medications, income or marital status.39 40 Regarding clinical factors, the inflammatory performance of CD may play a role in disease activity and quality of life. CD-A had higher anaemia rates, CRP values, erythrocyte sedimentation rate (ESR) values and relapse rates. It is possible to improve the psychological state and quality of life of patients with CD through early identification and intervention.
In recent years, some scholars have also studied the gender difference of IBD. At present, many studies have found that the differences in the psychological performance of patients with IBD are related to gender, and females are predictors of IBD combined anxiety and depression.41 42 However, the study of Nahon et al pointed out that the incidence of anxiety and depression in female patients with IBD was not significantly increased, and gender was not correlated with the occurrence of anxiety and depression.43 In this study, we also found that women with CD tend to report greater depressive symptoms than men, which was consistent with previous research.34 44 At the same time, epidemiological studies have confirmed that there are significant gender differences in the incidence of IBD, and this difference shows significant regional differences. In the USA,45 Canada,46 47 Israel,28 Spain48 and Denmark,49 the incidence of women is higher than that of men. In Asian countries such as South Korea,50 India51 and China,52 the incidence is higher in men than in women. The results indicate that female patients with IBD are more prone to anxiety and depression, which was mainly reflected in three aspects. First, women have a higher rate of anaemia symptoms and disease recurrence than men, which may be more prone to psychological problems due to illness and reduced quality of life. Second, women are less likely than men to use immunosuppressives and biological agents. Although there were no statistically significant differences in these clinical characteristics between genders of patients with CD, which may be due to the insufficient sample size included in this study. Third, women’s psychological activities are more delicate, more concerned about their symptoms, and pregnant with the next generation of problems. Therefore, in daily clinical diagnosis and treatment, more attention should be paid to whether women have mental and psychological abnormalities and their severity, and effective health education and psychological support should be provided according to the specific circumstances.
To our knowledge, this is the first study to compare the relationship between social support and disease activity across men and women with CD. The strengths of this study include the diversity of the sample in terms of social support and psychological distress scores, which allow us to assess somatisation in patients with different levels of disease activity.
Several limitations should be considered in this study. First, this was a single-centre study in which all participants were of Han nationality and from Hubei Province, China. Second, the cross-sectional nature of the data precludes us from concluding the causality of the relationships among social support, psychological distress and disease activity. In the future, longitudinal research should be conducted to establish a more robust connection between various clinical, psychological, and social support factors and disease activity in patients with CD.
Conclusions
In conclusion, this study indicates the importance of considering a broader range of psychological distress and social support factors that may play a role in the health of patients with CD. Further exploration of these factors in longitudinal and intervention studies may help to develop effective CD management models.
Supplementary Material
Footnotes
Contributors: MH and LT substantial contributions to the conception, design, acquisition, analysis and interpretation of data for the work. They also draft the work or revise it critically for important intellectual content. LW, YZ, XL, XY and CH substantial contributions to the acquisition and interpretation of data for the work. PL, QL and PH substantial contributions to revising it critically for important intellectual content. LY and LZ substantial contributions to the conception, design and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LZ is the guarantor.
Funding: This research was supported by Natural Science Foundation of Hubei Province(2021CFB450) and Wuhan Knowledge Innovation Special Basic Research Project (2022020801010462).
Disclaimer: The funding sources had no role in the study conduct, data collection, analyses, data interpretation, and the decision to submit the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the institutional ethics committee of Tongji Medical College of Huazhong University of Science and Technology, and consent was acquired from all participants (protocol number ICH S016). All participants were informed of the purpose and methods of this study and provided written informed consent.
References
- 1.Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. The Lancet 2017;389:1741–55. 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 2.Alatab S, Sepanlou SG, Ikuta K, et al. Global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. The Lancet Gastroenterology & Hepatology 2020;5:17–30. 10.1016/S2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai W, Zeng Y, Liang E, et al. The actuality of resilience, social support and quality of life among patients with inflammatory bowel disease in China. Nurs Open 2022;9:2190–8. 10.1002/nop2.946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Li Y, Wu W, et al. The incidence of inflammatory bowel disease in northern China: a prospective population-based study. PLoS ONE 2014;9:e101296. 10.1371/journal.pone.0101296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 6.van den Brink G, Stapersma L, Vlug LE, et al. Clinical disease activity is associated with anxiety and depressive symptoms in adolescents and young adults with inflammatory bowel disease. Aliment Pharmacol Ther 2018;48:358–69. 10.1111/apt.14832 [DOI] [PubMed] [Google Scholar]
- 7.Xiong Q, Tang F, Li Y, et al. Association of inflammatory bowel disease with suicidal Ideation, suicide attempts, and suicide: a systematic review and meta-analysis. Journal of Psychosomatic Research 2022;160:110983. 10.1016/j.jpsychores.2022.110983 [DOI] [PubMed] [Google Scholar]
- 8.Neuendorf R, Harding A, Stello N, et al. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. Journal of Psychosomatic Research 2016;87:70–80. 10.1016/j.jpsychores.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Barberio B, Zamani M, Black CJ, et al. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021;6:359–70. 10.1016/S2468-1253(21)00014-5 [DOI] [PubMed] [Google Scholar]
- 10.Blackwell J, Saxena S, Petersen I, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case-control study. Gut 2021;70:1642–8. 10.1136/gutjnl-2020-322308 [DOI] [PubMed] [Google Scholar]
- 11.Frolkis AD, Vallerand IA, Shaheen A-A, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2019;68:1606–12. 10.1136/gutjnl-2018-317182 [DOI] [PubMed] [Google Scholar]
- 12.Bernstein CN, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:360–8. 10.1093/ibd/izy235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kok KB, Byrne P, Ibarra AR, et al. Understanding and managing psychological disorders in patients with inflammatory bowel disease: a practical guide. Frontline Gastroenterol 2023;14:78–86. 10.1136/flgastro-2022-102094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordi SBU, Lang BM, Auschra B, et al. Depressive symptoms predict clinical recurrence of inflammatory bowel disease. Inflamm Bowel Dis 2022;28:560–71. 10.1093/ibd/izab136 [DOI] [PubMed] [Google Scholar]
- 15.Dubinsky MC, Dotan I, Rubin DT, et al. Burden of comorbid anxiety and depression in patients with inflammatory bowel disease: a systematic literature review. Expert Review of Gastroenterology & Hepatology 2021;15:985–97. 10.1080/17474124.2021.1911644 [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Chen X, Ma K, et al. Correlation between sleep, life, mood, and diet and severity of inflammatory bowel disease in China: a retrospective study. Med Sci Monit 2021;27:e930511. 10.12659/MSM.930511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med 2004;66:79–84. 10.1097/01.psy.0000106907.24881.f2 [DOI] [PubMed] [Google Scholar]
- 18.Katz L, Tripp DA, Ropeleski M, et al. Mechanisms of quality of life and social support in inflammatory bowel disease. J Clin Psychol Med Settings 2016;23:88–98. 10.1007/s10880-015-9431-x [DOI] [PubMed] [Google Scholar]
- 19.Yılmaz T, Bekaroğlu ET. Does interpersonal sensitivity and paranoid Ideation predict Nomophobia: an analysis with a young adult sample. 2021: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–164K. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 21.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the crohn’s disease activity index (CDAI). Gastroenterology 1979;77:843–6. 10.1016/0016-5085(79)90384-6 [DOI] [PubMed] [Google Scholar]
- 22.Fu H, Kaminga AC, Peng Y, et al. Associations between disease activity, social support and health-related quality of life in patients with inflammatory bowel diseases: the mediating role of psychological symptoms. BMC Gastroenterol 2020;20:11. 10.1186/s12876-020-1166-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JW, Lyu S, et al. Effects of psychological stress and social support on quality of life of patients with ulcerative colitis. Chinese Journal of Digestion 2018;38:613–7. [Google Scholar]
- 24.Schmitz N, Hartkamp N, Kiuse J, et al. The symptom check-List-90-R (SCL-90-R): a German validation study. Qual Life Res 2000;9:185–93. 10.1023/A:1008931926181 [DOI] [PubMed] [Google Scholar]
- 25.Strack MH. The symptom Checklist-90-R (SCL-90-R) for presenting statistically and clinically significant psychotherapy outcome. Psychother Psychosom Med Psychol 1998;48:257–64. [PubMed] [Google Scholar]
- 26.Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol 2016;14:829–35. 10.1016/j.cgh.2015.12.045 [DOI] [PubMed] [Google Scholar]
- 27.Shang X, Li L, Niu C, et al. Relationship between social support and parenting sense of competence in puerperal women: multiple mediators of resilience and postpartum depression. Front Psychiatry 2022;13:986797. 10.3389/fpsyt.2022.986797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slonim-Nevo V, Sarid O, Friger M, et al. Effect of social support on psychological distress and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis 2018;24:1389–400. 10.1093/ibd/izy041 [DOI] [PubMed] [Google Scholar]
- 29.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013;144:36–49. 10.1053/j.gastro.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 30.Agostini A, Scaioli E, Belluzzi A, et al. Attachment and mentalizing abilities in patients with inflammatory bowel disease. Gastroenterol Res Pract 2019;2019:7847123. 10.1155/2019/7847123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guiney JT, Fonagy P, et al. Interpersonal stress regulation and the development of anxiety disorders: an attachment-based developmental framework. Front Behav Neurosci 2011;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colonnello V, Agostini A. Disease course, stress, attachment, and mentalization in patients with inflammatory bowel disease. Med Hypotheses 2020;140:109665. 10.1016/j.mehy.2020.109665 [DOI] [PubMed] [Google Scholar]
- 33.Sitnikova K, Dijkstra-Kersten SMA, Mokkink LB, et al. Systematic review of measurement properties of questionnaires measuring somatization in primary care patients. J Psychosom Res 2017;103:42–62. 10.1016/j.jpsychores.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 34.Regev S, Odes S, Slonim-Nevo V, et al. Differential relationships of somatization, depression, and anxiety to severity of crohn’s disease. J Health Psychol 2021;26:2390–401. 10.1177/1359105320909879 [DOI] [PubMed] [Google Scholar]
- 35.Goldring AB, Taylor SE, Kemeny ME, et al. Impact of health beliefs, quality of life, and the physician-patient relationship on the treatment intentions of inflammatory bowel disease patients. Health Psychol 2002;21:219–28. 10.1037//0278-6133.21.3.219 [DOI] [PubMed] [Google Scholar]
- 36.Veilleux S, Noiseux I, Lachapelle N, et al. Patients' perception of their involvement in shared treatment decision making: key factors in the treatment of inflammatory bowel disease. Patient Educ Couns 2018;101:331–9. 10.1016/j.pec.2017.07.028 [DOI] [PubMed] [Google Scholar]
- 37.Goodhand JR, Wahed M, Mawdsley JE, et al. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflammatory Bowel Diseases 2012;18:2301–9. 10.1002/ibd.22916 [DOI] [PubMed] [Google Scholar]
- 38.Mules TC, Swaminathan A, Hirschfeld E, et al. The impact of disease activity on psychological symptoms and quality of life in patients with inflammatory bowel disease-results from the stress, anxiety and depression with disease activity (SADD) study. Aliment Pharmacol Ther 2022;55:201–11. 10.1111/apt.16616 [DOI] [PubMed] [Google Scholar]
- 39.Peppas S, Pansieri C, Piovani D, et al. The brain-gut axis: psychological functioning and inflammatory bowel diseases. J Clin Med 2021;10:377. 10.3390/jcm10030377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng L, Zhou N, Li Z, et al. Co-occurrence of gut microbiota dysbiosis and bile acid metabolism alteration is associated with psychological disorders in crohn’s disease. FASEB J 2022;36:e22100. 10.1096/fj.202101088RRR [DOI] [PubMed] [Google Scholar]
- 41.Panara AJ, Yarur AJ, Rieders B, et al. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment Pharmacol Ther 2014;39:802–10. 10.1111/apt.12669 [DOI] [PubMed] [Google Scholar]
- 42.Maconi G, Gridavilla D, Viganò C, et al. Perianal disease is associated with psychiatric co-morbidity in crohn’s disease in remission. Int J Colorectal Dis 2014;29:1285–90. 10.1007/s00384-014-1935-6 [DOI] [PubMed] [Google Scholar]
- 43.Nahon S, Lahmek P, Durance C, et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflammatory Bowel Diseases 2012;18:2086–91. 10.1002/ibd.22888 [DOI] [PubMed] [Google Scholar]
- 44.Freitas TH. Associations of sense of coherence with psychological distress and quality of life in inflammatory bowel disease. WJG 2015;21:6713. 10.3748/wjg.v21.i21.6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. 10.1016/j.cgh.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 46.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20:1761–9. 10.1097/MIB.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 47.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol 2006;101:1559–68. 10.1111/j.1572-0241.2006.00603.x [DOI] [PubMed] [Google Scholar]
- 48.Barreiro-de Acosta M, Molero A, Artime E, et al. Epidemiological, clinical, patient-reported and economic burden of inflammatory bowel disease (ulcerative colitis and crohn’s disease) in Spain: a systematic review. Adv Ther 2023;40:1975–2014. 10.1007/s12325-023-02473-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980-2013: a nationwide cohort study. Aliment Pharmacol Ther 2017;45:961–72. 10.1111/apt.13971 [DOI] [PubMed] [Google Scholar]
- 50.Kim S-H, Park Y, Kim SP, et al. Shift to a younger age and regional differences in inflammatory bowel disease in Korea: using healthcare administrative data. Dig Dis Sci 2022;67:5079–89. 10.1007/s10620-021-07328-0 [DOI] [PubMed] [Google Scholar]
- 51.Sood A, Kaur K, Mahajan R, et al. Colitis and crohn’s foundation (India): a first nationwide inflammatory bowel disease registry. Intest Res 2021;19:206–16. 10.5217/ir.2019.09169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Song P, Li J, et al. The disease burden and clinical characteristics of inflammatory bowel disease in the chinese population: a systematic review and meta-analysis. Int J Environ Res Public Health 2017;14:238. 10.3390/ijerph14030238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076219supp001.pdf (32.4KB, pdf)
Data Availability Statement
No data are available.


