Key Points
Question
Does biweekly cabazitaxel, 16 mg/m2 (biweekly CBZ16) plus prophylactic granulocyte colony–stimulating factor (G-CSF) at each cycle induce less grade 3 or higher neutropenia and/or neutropenic complications than the standard regimen (triweekly cabazitaxel, 25 mg/m2 [triweekly CBZ25]) plus G-CSF in patients 65 years or older with metastatic castration-resistant prostate cancer (mCRPC)?
Findings
In this phase 3 randomized clinical trial with 196 heavily pretreated patients 65 years or older with mCRPC, biweekly CBZ16 plus G-CSF significantly reduced by 12-fold the incidence of grade 3 or higher neutropenia and/or neutropenic complications compared with triweekly CBZ25.
Meaning
These findings suggest that biweekly CBZ16 plus G-CSF should be offered to patients 65 years or older with mCRPC for whom standard cabazitaxel regimens are unsuitable.
Abstract
Importance
Many patients 65 years or older with metastatic castration-resistant prostate cancer (mCRPC) are denied taxane chemotherapy because this treatment is considered unsuitable.
Objective
To determine whether biweekly cabazitaxel (CBZ), 16 mg/m2 (biweekly CBZ16), plus prophylactic granulocyte colony–stimulating factor (G-CSF) at each cycle reduces the risk of grade 3 or higher neutropenia and/or neutropenic complications (eg, febrile neutropenia, neutropenic infection, or sepsis) compared with triweekly CBZ, 25 mg/m2 (triweekly CBZ25), plus G-CSF (standard regimen).
Design, Setting, and Participants
A total of 196 patients 65 years or older with progressive mCRPC were enrolled in this prospective phase 3 randomized clinical trial conducted in France (18 centers) and Germany (7 centers) between May 5, 2017, and January 7, 2021. All patients had received docetaxel and at least 1 novel androgen receptor–targeted agent.
Interventions
Patients were randomly assigned 1:1 to receive biweekly CBZ16 plus G-CSF and daily prednisolone (experimental group) or triweekly CBZ25 plus G-CSF and daily prednisolone (control group).
Main Outcome and Measures
The primary end point was the occurrence of grade 3 or higher neutropenia measured at nadir and/or neutropenic complications.
Results
Among 196 patients (97 in the triweekly CBZ25 group and 99 in the biweekly CBZ16 group), the median (IQR) age was 74.6 (70.4-79.3) years, and 181 (92.3%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. The median (IQR) follow-up duration was 31.3 (22.5-37.5) months. Relative dose intensities were comparable between groups (median [IQR], 92.7% [83.7%-98.9%] in the triweekly CBZ25 group vs 92.8% [87.0%-98.9%] in the biweekly CBZ16 group). The rate of grade 3 or higher neutropenia and/or neutropenic complications was significantly higher with triweekly CBZ25 vs biweekly CBZ16 (60 of 96 [62.5%] vs 5 of 98 [5.1%]; odds ratio, 0.03; 95% CI, 0.01-0.08; P < .001). Grade 3 or higher adverse events were more common with triweekly CBZ25 (70 of 96 [72.9%]) vs biweekly CBZ16 (55 of 98 [56.1%]). One patient (triweekly CBZ25 group) died of a neutropenic complication.
Conclusions and Relevance
In this randomized clinical trial, compared with the standard regimen, biweekly CBZ16 plus G-CSF significantly reduced by 12-fold the occurrence of grade 3 or higher neutropenia and/or neutropenic complications, with comparable clinical outcomes. The findings suggest that biweekly CBZ16 regimen should be offered to patients 65 years or older with mCRPC for whom the standard regimen is unsuitable.
Trial Registration
ClinicalTrials.gov Identifier: NCT02961257
This phase 3 randomized clinical trial assesses whether biweekly cabazitaxel, 16 mg/m2, reduces the risk of grade 3 or higher neutropenia and/or neutropenic complications compared with triweekly cabazitaxel, 25 mg/m2, in patients 65 years or older with metastatic castration-resistant prostate cancer.
Introduction
The management of metastatic castration-resistant prostate cancer (mCRPC) has substantially evolved since 2010, with 5 classes of therapies showing improved overall survival (OS): taxanes (docetaxel and cabazitaxel [CBZ]), androgen receptor (AR)–targeted agents (abiraterone and enzalutamide), immunotherapy agents (sipuleucel-T), radiolabeled small molecules (radium-223 and lutetium-177-PSMA-617), and inhibitors of poly-ADP ribose polymerase (olaparib and rucaparib) for patients with BRCA-mutated tumors.1,2 Patients progressing with abiraterone or enzalutamide respond poorly to the alternative AR-targeted agents. However, despite level 1 evidence for cross-resistance,3 many patients with mCRPC, especially those 65 years or older, receive AR-targeted therapies in sequence before receiving chemotherapy, possibly because of concerns about the toxic effects of chemotherapy.4,5,6
In patients 75 years and older, standard regimens of taxanes (docetaxel and CBZ) are commonly associated with more grade 3 or higher adverse events (AEs) and treatment discontinuations due to AEs than in younger patients,7,8 despite providing a survival advantage in older patients3,9,10 and substantial reduction of pain, which is important at such an advanced stage of the disease.7,8,11 Patients receiving CBZ experience less alopecia, peripheral neuropathy, peripheral edema, and nail disorders than those receiving docetaxel, but CBZ is associated with a high rate of grade 3 or higher neutropenia.9 Although the risk of neutropenic complications can be partly mitigated by a lower CBZ dose, like in the PROSELICA (Cabazitaxel at 20 mg/m2 Compared to 25 mg/m2 With Prednisone for the Treatment of Metastatic Castration Resistant Prostate Cancer) trial,12,13 and/or prophylactic G-CSF from cycle 1, like in the CARD (Cabazitaxel Versus the Switch to Alternate AR-Targeted Agent [Enzalutamide or Abiraterone] in Metastatic Castration-Resistant Prostate Cancer Patients Previously Treated With Docetaxel and Who Rapidly Failed a Prior AR-Target Agent) trial,3 this risk remains a challenge in patients 65 years or older with mCRPC associated with comorbidities and/or age-related decline in organ function, polypharmacy, and risk of potentially serious drug-drug interactions.14
To further reduce the incidence of severe neutropenia and its complications, an alternative strategy is to change the schedule of administration. Interestingly, in the PROSTY (Docetaxel and Prednisone in Treating Patients With Hormone-Refractory Metastatic Prostate Cancer) prospective phase 3 trial,15,16 administration of docetaxel, 50 mg/m2, every 2 weeks had a similar efficacy and an improved tolerability when compared with the standard regimen (75 mg/m2 every 3 weeks). This biweekly schedule is now recommended for older patients with mCRPC who cannot tolerate the standard docetaxel regimen.1,17,18
In this context, pilot studies were conducted both at our institution19 and elsewhere20; findings suggested that CBZ administered at a dose of 16 mg/m2 every 2 weeks retained activity and was associated with a reduced incidence of severe neutropenia. Thus, we investigated whether such a biweekly CBZ regimen plus prophylactic G-CSF at each cycle would be better tolerated (ie, reduce the risk of grade ≥3 neutropenia and/or neutropenic complications) and as effective as the standard triweekly regimen in patients 65 years or older with mCRPC.
Methods
Study Design
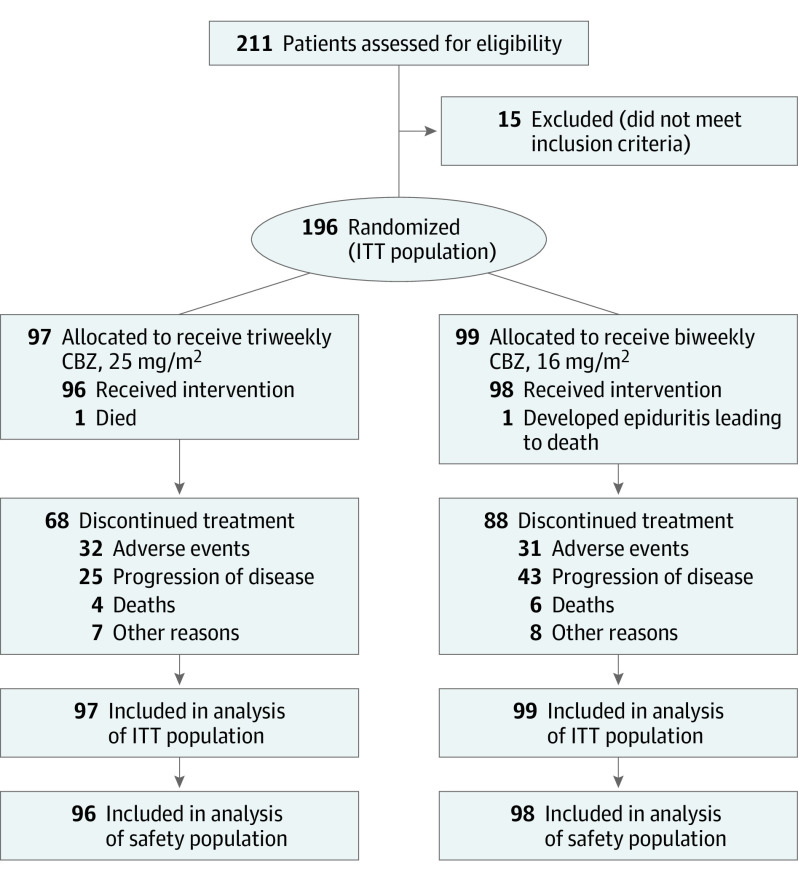
The CABASTY (Trial Evaluating the Safety of 2 Schedules of Cabazitaxel in Elderly Men With mCRPC Previously Treated With a Docetaxel) study was a multicenter prospective open-label phase 3 randomized clinical trial in patients 65 years or older with mCRPC in France (18 centers) and Germany (7 centers). A total of 196 patients were enrolled between May 5, 2017, and January 7, 2021 (Figure 1). The study was conducted in accordance with International Council for Harmonization guidelines,21 the Declaration of Helsinki,22 and all applicable local laws and regulations. The trial protocol (Supplement 1) was approved by the independent ethics committee or institutional review board at each study site, and all participants provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Figure 1. Study Flow Diagram.
CBZ indicates cabazitaxel; and ITT, intention to treat.
Randomization was stratified by age (<70 years vs ≥70 years) and score on the Geriatric 8 (G8) screening tool23 (<14 vs ≥14). Patients were randomly assigned (1:1 ratio) to either receive (1) CBZ, 16 mg/m2, on day 1 and day 15 of a 4-week cycle plus G-CSF from day 3 to day 7 (biweekly CBZ16; experimental group) or (2) CBZ, 25 mg/m2, on day 1 of each week in a 3-week cycle plus G-CSF from day 3 to day 7 (triweekly CBZ25; control group). All patients also received 10 mg of oral prednisolone daily.
Each dose of CBZ was administered intravenously over 1 hour for a maximum of 10 cycles unless there was disease progression or unacceptable toxic effects or the patient requested stopping. The CBZ dose could be reduced in case of unacceptable toxic effects. Only 1 dose reduction was allowed per patient (from 25 mg/m2 to 20 mg/m2 in the triweekly CBZ25 group; from 16 mg/m2 to 13 mg/m2 in the biweekly CBZ16 group). The CBZ treatment could be delayed by no more than 2 weeks to allow recovery from acute toxic effects. The G-CSF prophylaxis could be reduced to 3 days after cycle 1 if neutrophil count was higher than 1000 cells per mm3.
Blood biochemistry was assessed immediately before each treatment cycle, and blood cell counts were measured immediately before and on a weekly basis during each cycle. Serum prostate-specific antigen (PSA) level was measured every 3 weeks in the triweekly CBZ25 group and every 2 weeks in the biweekly CBZ16 group. Tumor imaging was performed every 12 weeks with computed tomography, radiography, and isotope bone scanning. Response to treatment was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1).24 Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0, and monitored every 3 weeks in the triweekly CBZ25 group during treatment, every 2 weeks in the biweekly CBZ16 group during treatment, and at the end of study visits (planned 30 days after the last study treatment administration).
Patients and Procedures
Eligible patients were 65 years or older with histologically confirmed prostate cancer; castrate levels of serum testosterone (<0.5 ng/mL) per European Association of Urology definition1; disease metastatic progression per Prostate Cancer Working Group 2 criteria25; a previous docetaxel-containing regimen; health status allowing chemotherapy (G8 score >14 or ≤14 with geriatric assessment and intervention allowing chemotherapy)14; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2 (ECOG PS 2 related to prostate cancer disease); and adequate hematological, liver, and kidney functions. Exclusion criteria were impaired liver and/or kidney function; blood neutrophil count lower than 1.5 × 109/L; platelet count lower than 100 × 109/L; or hemoglobin concentration lower than 10 g/L. Complete eligibility criteria are provided in the trial protocol in Supplement 1.
Outcomes
The primary end point was the incidence of grade 3 or higher neutropenia (according to Common Toxicity Criteria for Adverse Events criteria) and/or neutropenic complications (eg, febrile neutropenia, neutropenic infection, or sepsis). Secondary end points included (1) dose reductions and dose delays of CBZ; (2) OS, defined as time from randomization to death from any cause; (3) radiographic progression-free survival (PFS), defined as time from randomization to radiological tumor progression (according to RECIST 1.1 criteria); (4) progression of bone lesions (according to Prostate Cancer Working Group 2 criteria) or death from any cause; (5) PSA response defined by a decrease in PSA by 50% or more from baseline, confirmed by a second value at least 4 weeks later, with no clinical or radiographic evidence of disease progression during this period; (6) tumor response in measurable lesions (according to RECIST 1.1 criteria); (7) time to first symptomatic skeletal-related event (SRE), defined as pathological bone fracture, spinal cord compression, surgery to bone, or radiation to bone; and (8) incidence of SREs and quality of life (not reported in this article). The complete list of end points is provided in the trial protocol in Supplement 1. No blinded central review was performed on standard imaging (bone scans, computed tomographic scans, and magnetic resonance imaging of pelvis, abdomen, and chest). After discontinuation of the study, a follow-up of survival status was performed every 3 months for at least 2 years.
Statistical Analysis
To calculate the sample size, we assumed that the incidence of grade 3 or higher neutropenia and/or complications would be 32% with triweekly CBZ25 plus prophylactic G-CSF based on previous trials conducted in our institution.19,26 For an estimate of this incidence with biweekly CBZ16, we based our assumption on 2 pilot studies with a rate of 15% in a Finnish cohort27 and 16.5% in a French cohort.19 To detect a 20% difference in grade 3 or higher neutropenia and/or complications between both groups with 80% power, using a 2-sided Fisher exact test at α = .05 significance level, 70 evaluable patients were required. Assuming 10% nonevaluable patients, 77 patients had to be included in each group.
All efficacy analyses used data obtained at the cutoff date of January 31, 2022. If a radiographic event or death did not occur during the study, then radiographic PFS data were censored at last tumor assessment or at the cutoff date. There was no previous interim analysis. For time-to-event efficacy criteria, comparison between groups was performed using a log-rank test and hazard ratios (HRs) with corresponding 95% CIs and P values derived from adjusted (for age and G8 score) Cox models. Survival curves were generated using Kaplan-Meier estimates. Efficacy analysis was conducted on the intention-to-treat population (all randomized patients), and safety analysis was performed on the safety population (all patients who received at least 1 dose of CBZ). The significance threshold was 1-sided P = .05. Data were analyzed using R statistical software, version 4.3.0 (R Foundation for Statistical Computing).
Results
Among 196 patients (median [IQR] age, 74.6 [70.4-79.3] years), 97 were randomized to the triweekly CBZ25 group and 99 to the biweekly CBZ16 group (Figure 1). Patients’ characteristics at baseline were well balanced between groups (Table 1). A total of 181 patients (92.3%) had an ECOG PS of 0 or 1. All patients had received previous docetaxel. Although previous treatment by AR-targeted agents was not an inclusion requirement, 91 of 189 patients (48.1%) had received at least 3 lines of hormone therapy. Prophylactic G-CSF was given to all patients from cycle 1, regardless of treatment group. Treatment was prematurely discontinued in 68 of 96 patients (70.8%) in the triweekly CBZ25 group, mainly due to AEs, and 88 of 98 patients (89.8%) in the biweekly CBZ16 group, mainly due to disease progression.
Table 1. Baseline Characteristics of Intention-to-Treat Population.
| Characteristica | Patients, No./total No. (%)b | ||
|---|---|---|---|
| Overall (N = 196) | Triweekly CBZ25 group (n = 97) | Biweekly CBZ16 group (n = 99) | |
| Age, y | |||
| Median (IQR) [range] | 74.6 (70.4-79.3) [65.0-95.8] | 74.5 (71.2-79.5) [65.0-92.1] | 74.6 (70.3-78.7) [65.2-95.8] |
| Group | |||
| <75 | 100/196 (51.0) | 49/97 (50.5) | 51/99 (51.5) |
| 75-80 | 54/196 (27.6) | 26/97 (26.8) | 28/99 (28.3) |
| >80 | 42/196 (21.4) | 22/97 (22.7) | 20/99 (20.2) |
| ECOG performance status | |||
| 0-1 | 181/196 (92.3) | 88/97 (90.7) | 93/99 (93.9) |
| 2 | 15/196 (7.7) | 9/97 (9.3) | 6/99 (6.1) |
| Gleason score | |||
| <7 | 96/185 (51.9) | 50/94 (53.2) | 46/91 (50.5) |
| 8-10 | 89/185 (48.1) | 44/94 (46.8) | 45/91 (49.5) |
| PSA baseline measurements, ng/mLc | |||
| Mean (SD) | 194.3 (301.6) | 141.2 (208.6) | 248.5 (366.8) |
| Median (IQR) | 68.4 (23.5-217.0) | 60.5 (23.6-169.8) | 86.1 (21.6-273.7) |
| Range | 2.7-2073.0 | 2.7-1280.0 | 2.9-2073.0 |
| Lactate dehydrogenase higher than ULNd | 100/183 (54.6) | 50/87 (57.5) | 50/96 (52.1) |
| Alkaline phosphatase higher than ULNd | 82/188 (43.6) | 46/92 (50.0) | 36/96 (37.5) |
| Hemoglobin lower than LLNd | 116/191 (60.7) | 58/93 (62.4) | 58/98 (59.2) |
| Neutrophils higher than median (4.10 × 103 cells/mm3)d | 96/191 (50.3) | 41/93 (44.1) | 55/98 (56.1) |
| NLR >3d | 105/191 (55.0) | 47/93 (50.5) | 58/98 (59.2) |
| Metastatic disease at initial diagnostic assessmentd | |||
| Yes | 94/190 (49.5) | 50/95 (52.6) | 44/95 (46.3) |
| No | 96/190 (50.5) | 45/95 (47.4) | 51/95 (53.7) |
| Metastases | |||
| Bone | 155/187 (82.9) | 78/91 (85.7) | 77/96 (80.2) |
| Lymph | 79/187 (42.2) | 39/91 (42.9) | 40/96 (41.7) |
| Lung | 20/187 (10.7) | 12/91 (13.2) | 8/96 (8.3) |
| Liver | 18/187 (9.6) | 7/91 (7.7) | 11/96 (11.5) |
| G8 score group | |||
| <14 | 39/196 (19.9) | 20/97 (20.6) | 19/99 (19.2) |
| ≥14 | 157/196 (80.1) | 77/97 (79.4) | 80/99 (80.8) |
| SIOG group | |||
| Fit | 137/196 (69.9) | 70/97 (72.2) | 67/99 (67.7) |
| Vulnerable | 35/196 (17.9) | 16/97 (16.5) | 19/99 (19.2) |
| Frail | 24/196 (12.2) | 11/97 (11.3) | 13/99 (13.1) |
| Comorbidities | |||
| Hypertension | 69/196 (35.2) | 28/97 (28.9) | 41/99 (41.4) |
| Hypercholesterolemia | 20/196 (10.2) | 9/97 (9.3) | 11/99 (11.1) |
| Diabetes | 25/196 (12.8) | 10/97 (10.3) | 15/99 (15.2) |
| Pain visual analog score classd | |||
| 0-3 | 157/193 (81.3) | 75/94 (79.8) | 82/99 (82.8) |
| 4-10 | 36/193 (18.7) | 19/94 (20.2) | 17/99 (17.2) |
| Previous AR-targeted agentsd | |||
| 1 Line | 47/189 (24.9) | 26/92 (28.3) | 21/97 (21.6) |
| 2 Lines | 51/189 (27.0) | 27/92 (29.3) | 24/97 (24.7) |
| ≥3 Lines | 91/189 (48.1) | 39/92 (42.4) | 52/97 (53.6) |
| Previous chemotherapyd | |||
| 1 Line | 166/195 (85.1) | 83/97 (85.6) | 83/98 (84.7) |
| 2 Lines | 18/195 (9.2) | 9/97 (9.3) | 9/98 (9.2) |
| ≥3 Lines | 11/195 (5.6) | 5/97 (5.2) | 6/98 (6.1) |
| Docetaxel | |||
| Yes | 196/196 (100) | 97/97 (100) | 99/99 (100) |
| Cumulative dose, median (IQR)e | 450.0 (300.0-593.0) | 450.0 (330.0-525.0) | 450.0 (300.0-600.0) |
| Previous AR-targeted agentsd | |||
| Abiraterone | 122/196 (62.2) | 59/97 (60.8) | 63/99 (63.6) |
| Enzalutamide | 111/196 (56.6) | 49/97 (50.5) | 62/99 (62.6) |
| Abiraterone and enzalutamide | 66/196 (33.7) | 29/97 (29.9) | 37/99 (37.4) |
| Apalutamide | 4/196 (2.0) | 4/97 (4.1) | 0 |
| Darolutamide | 5/196 (2.6) | 1/97 (1.0) | 4/99 (4.0) |
Abbreviations: AR, androgen receptor; CBZ16, cabazitaxel, 16 mg/m2; CBZ25, cabazitaxel, 25 mg/m2; ECOG, Eastern Cooperative Oncology Group; G8, Geriatric 8 screening tool; LLN, lower limit of normal; NLR, neutrophil to lymphocyte ratio; PSA, prostate-specific antigen; SIOG, International Society of Geriatric Oncology; ULN, upper limit of normal.
Quantitative variables were analyzed using a t test (normal distribution) or Wilcoxon Mann-Whitney test (nonnormal distribution). Qualitative variables were analyzed using a χ2 test or Fisher exact test.
Denominators differ due to missing data.
Missing data for 12 patients (4 in the biweekly CBZ16 group and 8 in the triweekly CBZ25 group).
Percentages based on total number of patients with baseline level of the parameter.
Missing data for 1 patient in the triweekly CBZ25 group.
The median (IQR) number of treatment cycles received was 6.0 (4.0-10.0) in the triweekly CBZ25 group and 5.0 (4.0-7.0) in the biweekly CBZ16 group (eTable 1 in Supplement 2). The median (IQR) relative dose intensity was 92.7% (83.7%-98.9%) in the triweekly CBZ25 group and 92.8% (87.0%-98.9%) in the biweekly CBZ16 group. The median cumulative dose of CBZ was almost the same in both groups (overall: 142.7 mg/m2 [IQR, 96.4-209.5 mg/m2]). More patients had at least 1 cycle with dose reduction in the triweekly CBZ25 group vs the biweekly CBZ16 group (45 of 96 [46.9%] vs 10 of 98 [10.2%]) (eTable 1 in Supplement 2). The median (IQR) duration of follow-up in the overall population was 31.3 (22.5-37.5) months.
Primary End Point
In the safety population, grade 3 or higher neutropenia measured at nadir and/or neutropenic complications were observed in 60 of 96 patients (62.5%) treated with triweekly CBZ25 vs 5 patients (5.1%) treated with biweekly CBZ16 (odds ratio [OR], 0.03; 95% CI, 0.01-0.08; P < .001) (Table 2). Neutropenic complications occurred in 13 patients (13.5%) in the triweekly CBZ25 group vs 3 (3.1%) in the biweekly CBZ16 group; complications mainly consisted of febrile neutropenia (10 of 13 patients in the triweekly CBZ25 group vs 0 in the triweekly CB16 group; P = .001). One patient in the triweekly CBZ25 group died of febrile neutropenia. Grade 3 or higher neutropenia was more common with increasing age (median [IQR] age with vs without neutropenia: 77.2 [72.8-81.5] years vs 73.5 [69.7-78.2] years; P < .001) (eTable 2 in Supplement 2) and occurred mainly at cycle 1 (42 of 65 patients [64.6%]), while the nadir was usually reached at 8 days for the triweekly CBZ25 group and 12 days for the biweekly CBZ16 group (eTable 3 in Supplement 2). Regression analysis showed that the odds of grade 3 or higher neutropenia and/or neutropenic complications were higher in the triweekly CBZ25 group (OR, 29.99; 95% CI, 11.56-77.77; P < .001) (eFigure 1 in Supplement 2).
Table 2. Incidence of Grade 3 or Higher Neutropenia and/or Neutropenic Complications in Safety Population.
| Resulta | Patients, No./total No. (%) | P value | ||
|---|---|---|---|---|
| Overall (N = 194) | Triweekly CBZ25 group (n = 96) | Biweekly CBZ16 group (n = 98) | ||
| Primary end point | 65/194 (33.5) | 60/96 (62.5) | 5/98 (5.1) | <.001 |
| At least 1 neutropenia grade ≥3 | 62/194 (32.0) | 60/96 (62.5) | 2/98 (2.0) | <.001 |
| At least 1 neutropenia complication | 16/194 (8.2) | 13/96 (13.5) | 3/98 (3.1) | .009 |
| Complications | ||||
| Febrile neutropenia | 10/16 (62.5) | 10/13 (76.9) | 0 | NA |
| Sepsis | 4/16 (25.0) | 2/13 (15.4) | 2/3 (66.7) | NA |
| Septic shock | 2/16 (12.5) | 1/13 (7.7) | 1/3 (33.3) | NA |
Abbreviations: CBZ16, cabazitaxel, 16 mg/m2; CBZ25, cabazitaxel, 25 mg/m2; NA, not available.
Quantitative variables were analyzed using a t test (normal distribution) or Wilcoxon Mann-Whitney test (nonnormal distribution). Qualitative variables were analyzed using a χ2 test or Fisher exact test.
Secondary End Points
The number of patients with 1 or more dose delays (≥14 days) and/or reductions was 47 of 96 (49.0%) in the triweekly CBZ25 group and 14 of 98 (14.3%) in the biweekly CBZ16 group. At the cutoff date, 150 deaths had occurred, 76 of 97 (78.4%) in the triweekly CBZ25 group and 74 of 99 (74.7%) in the biweekly CBZ16 group. The median OS was the same in both groups (14.1 months [95% CI, 11.4-17.4 months] in the triweekly CBZ25 group and 14.1 months [95% CI, 11.8-20.0 months] in the biweekly CBZ16 group; HR for death, 0.87; 95% CI, 0.63-1.20; P = .39) (Table 3; Figure 2). Subgroup analyses of OS did not identify any significant difference between groups (eFigure 2 in Supplement 2).
Table 3. Efficacy End Points in Intention-To-Treat Population.
| Outcomea | Patients, No./total No. (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Overall (N = 196) | Triweekly CBZ25 group (n = 97) | Biweekly CBZ16 group (n = 99) | |||
| Overall survival | |||||
| Median (95% CI), mo | 14.1 (12.3-16.5) | 14.1 (11.4-17.4) | 14.1 (11.8-20.0) | NA | NA |
| Deaths | 150/196 (76.5) | 76/97 (78.4) | 74/99 (74.7) | 0.87 (0.63-1.20) | .39 |
| Radiographic PFS | |||||
| Median (95% CI), mo | 8.57 (7.23-11.40) | 10.25 (7.13-13.90) | 7.82 (6.14-11.70) | NA | NA |
| Progression | 161/196 (82.1) | 80/97 (82.5) | 81/99 (81.8) | 0.98 (0.71-1.34) | .89 |
| PSA response ≥50% | |||||
| Time to response, median (95% CI), mo | 5.71 (4.07-NR) | 4.96 (3.41-NA) | 7.39 (3.94-NA) | NA | NA |
| Not evaluable | 11/184 (6.0) | 3/93 (3.2) | 8/91 (8.8) | NA | NA |
| Response | 75/184 (40.8) | 39/93 (41.9) | 36/91 (39.6) | 0.82 (0.52-1.30) | .40 |
| Skeletal-related events | |||||
| Time to event, median (95% CI), mo | NR | NR | NR | NA | NA |
| Events | 19/196 (9.7) | 10/97 (10.3) | 9/99 (9.1) | 0.75 (0.30-1.88) | .50 |
| Objective tumor response | |||||
| Partial response | 29/196 (14.8) | 15/97 (15.5) | 14/99 (14.1) | NA | .18 |
| Stable disease | 99/196 (50.5) | 51/97 (52.6) | 48/99 (48.5) | ||
| Progressive disease | 48/196 (24.5) | 18/97 (18.6) | 30/99 (30.3) | ||
| Not evaluable | 20/196 (10.2) | 13/97 (13.4) | 7/99 (7.1) | NA | NA |
| Positive objective tumor response | |||||
| Response | 29/176 (16.5) | 15/84 (17.9) | 14/92 (15.2) | NA | .64 |
| Not available | 20/176 (11.4) | 13/84 (15.5) | 7/92 (7.6) | NA | NA |
Abbreviations: CBZ16, cabazitaxel, 16 mg/m2; CBZ25, cabazitaxel, 25 mg/m2; HR, hazard ratio; NA, not applicable; NR, not reached; PFS, progression-free survival; PSA, prostate-specific antigen.
Quantitative variables were analyzed using a t test (normal distribution) or Wilcoxon Mann-Whitney test (nonnormal distribution). Qualitative variables were analyzed using a χ2 test or Fisher exact test.
Figure 2. Kaplan-Meier Curves of Overall Survival and Radiological Progression-Free Survival in the Control and Experimental Groups.
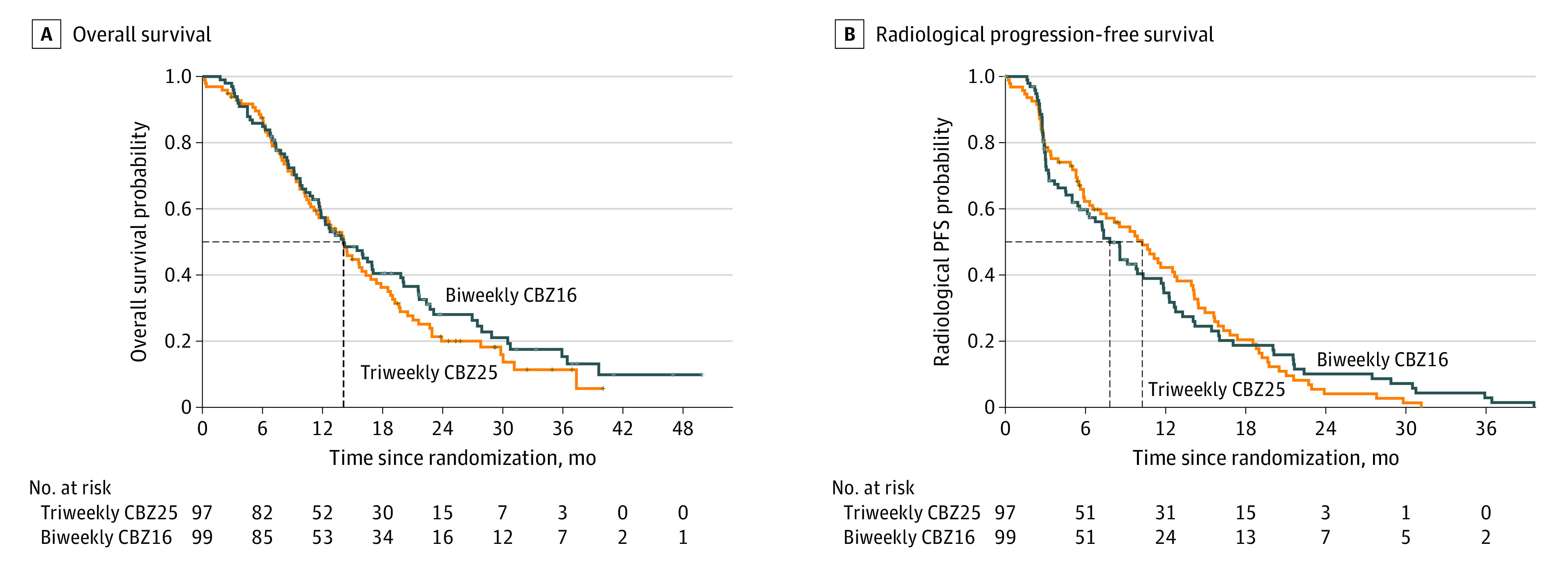
The control group (n = 97) received the standard regimen of triweekly cabazitaxel, 25 mg/m2 (triweekly CBZ25). The experimental group (n = 99) received biweekly cabazitaxel, 16 mg/m2 (biweekly CBZ16). For overall survival, the median (IQR) time since randomization was 14.13 (7.95-22.70) months with triweekly CBZ25 vs 14.06 (8.44-27.47) months with biweekly CBZ16 (hazard ratio, 0.87; 95% CI, 0.63-1.20; log-rank P = .39). For radiological progression-free survival (PFS), the median (IQR) time since randomization was 10.25 (3.94-15.93) months with triweekly CBZ25 vs 7.82 (2.99-14.19) months with biweekly CBZ16 (hazard ratio, 0.98; 95% CI, 0.71-1.34; log-rank P = .89). The dotted lines indicate median time.
Radiographic progression was observed in 161 patients, 80 of 97 (82.5%) in the triweekly CBZ25 group and 81 of 99 (81.8%) in the biweekly CBZ16 group. The median radiographic PFS was 10.25 months (95% CI, 7.13-13.90 months) in the triweekly CBZ25 group vs 7.82 months (95% CI, 6.14-11.70 months) in the biweekly CBZ16 group (HR for progression, 0.98; 95% CI, 0.71-1.34; P = .89) (Table 3; Figure 2).
The PSA response was evaluable in 93 of 97 patients (95.9%) in the triweekly CBZ25 group and 91 of 99 (91.9%) in the biweekly CBZ16 group (eFigure 2 in Supplement 2). A confirmed PSA decline from baseline of at least 50% was observed in 39 of 93 patients (41.9%) in the triweekly CBZ25 group vs 36 of 91 (39.6%) in the biweekly CBZ16 group (P = .40) (Table 3). Time to PSA response was not statistically different between groups (eFigure 3 in Supplement 2).
Objective tumor response was observed in 15 of 84 patients (17.9%) in the triweekly CBZ25 group and 14 of 92 (15.2%) in the biweekly CBZ16 group (P = .64) (Table 3). Skeletal-related events occurred in 10 of 97 patients (10.3%) in the triweekly CBZ25 group and 9 of 99 (9.1%) in the biweekly CBZ16 group (P = .50) (Table 3).
Safety
Overall, 194 patients were exposed to at least 1 cycle of CBZ, 96 in the triweekly CBZ25 group and 98 in the biweekly CBZ16 group (eTable 1 in Supplement 2). Adverse events (of any grade) occurred in almost all patients (193 of 194 [99.5%]) (eTable 4 in Supplement 2). Grade 3 or higher AEs were more frequent in the triweekly CBZ25 group (70 [72.9%]) than in the biweekly CBZ16 group (55 [56.1%]). The incidence of serious AEs of any grade was comparable in both groups (42 [43.8%] with triweekly CBZ25 vs 45 [45.9%] with biweekly CBZ16). The percentage of patients with AEs leading to permanent discontinuation was almost the same in both groups (32 [33.3%] in the triweekly CBZ25 group vs 31 [31.6%] in the biweekly CBZ16 group). Five patients (5.2%) in the triweekly CBZ25 group had AEs leading to death within 30 days of CBZ administration vs 10 patients (10.2%) in the biweekly CBZ16 group (eTable 4 in Supplement 2). Main grade 5 AEs were progression of preexisting disease (n = 5), cardiorespiratory insufficiency (n = 4), and deterioration of general physical health (n = 3). The most common grade 3 or higher AEs were fatigue (13 [13.5%] with triweekly CBZ25 vs 8 [8.2%] with biweekly CBZ16), infection (7 [7.3%] with triweekly CBZ25 vs 11 [11.2%] with biweekly CBZ16), and diarrhea (4 [4.2%] with triweekly CBZ25 vs 6 [6.1%] with biweekly CBZ16) (eTable 4 in Supplement 2).
Discussion
The CABASTY phase 3 randomized clinical trial was designed to test the hypothesis that CBZ, 16 mg/m2, plus G-CSF administered every 2 weeks was associated with a lower incidence of grade 3 or higher neutropenia and/or neutropenic complications compared with the standard regimen (CBZ, 25 mg/m2, plus G-CSF administered every 3 weeks) in patients 65 years or older with mCRPC who had not responded to previous docetaxel and AR-targeted agents. The primary end point was met; the biweekly regimen was associated with an incidence of grade 3 or higher neutropenia measured at nadir that was 12 times lower than the standard triweekly regimen (5.1% vs 62.5%; P < .001). In addition, no patient developed febrile neutropenia with the biweekly regimen compared with 10.4% of patients with the standard regimen. More importantly, in this older and heavily pretreated population (previous docetaxel received by all patients and 3 different lines of AR-targeted agents received by 48.1% of them), median OS reached 14.1 months in both groups. There was no significant difference between groups in radiographic PFS, PSA response, objective tumor response, and time to SREs.
To rigorously compare the incidence of grade 3 or higher neutropenia and/or neutropenic complications between the 2 CBZ schedules, blood neutrophil count was measured every week after infusion to capture the neutrophil nadir, and we checked that the median cumulative dose of CBZ was comparable in both groups. Prophylactic G-CSF was given from cycle 1 to all patients regardless of treatment group because (1) patients enrolled were older (median age, 74.6 years) and heavily pretreated, thus at high risk of neutropenic complications28,29,30; (2) in a large compassionate-use program, neutropenic complications with triweekly CBZ25 mainly occur at cycle 1, especially31 when the absolute neutrophil count before chemotherapy infusion is lower than 4000 cells per mm3; and (3) the benefit of adding prophylactic G-CSF from cycle 1 to triweekly CBZ25 was unambiguously confirmed in the CARD trial with a significantly prolonged OS vs abiraterone or enzalutamide and a low rate of febrile neutropenia (3%).3
The high incidence of grade 3 or higher neutropenia measured at nadir in the CABASTY control group (62.5%; triweekly CBZ25 plus G-CSF) was lower than that of other phase 3 trials, including the TROPIC (XRP6258 Plus Prednisone Compared to Mitoxantrone Plus Prednisone in Hormone Refractory Metastatic Prostate Cancer) trial (82.0%),9 the FIRSTANA (Cabazitaxel Versus Docetaxel Both With Prednisone in Patients With Metastatic Castration Resistant Prostate Cancer) trial (70.6%),13 and the PROSELICA trial (73.3%),12 which did not allow prophylactic G-CSF from cycle 1. The incidence was slightly higher than in the CARD trial (44.7%), in which prophylactic G-CSF was mandatory at every cycle3; however, patients enrolled in CARD were younger (median, 71.0 years vs 74.6 years in CABASTY) and were treated earlier (after failure of 2 lines of life-extending therapies) than in CABASTY (almost one-half of the patients received CBZ in the fifth-line setting8). These 2 reasons may also explain the difference in incidence between the present trial and previous trials that have been conducted in our institution.19,26
In the current trial, the sample size was calculated based on the primary objective (ie, incidence of grade ≥3 neutropenia and/or neutropenic complications) and may not be sufficient to identify a difference in secondary outcomes. However, OS observed in CABASTY (median, 14.1 months in both treatment groups) compared well with OS observed with the standard CBZ regimen in trials conducted either in second- or third-line therapy for mCRPC.3,9,12,32 This finding further supports evidence that CBZ retains its activity in resistant tumors33 and further differentiates CBZ from docetaxel, which seems to lose some activity in patients previously exposed to AR-targeted agents.10,34,35 In addition, the fact that a CBZ biweekly regimen showed an OS that was similar to a triweekly regimen suggests that the activity of CBZ may be related to the cumulative dose administered (similar in both groups in CABASTY) rather than the peak concentration, which is a direct cause of neutropenia. This finding is important because severe neutropenia has been associated with prolonged OS in many tumor types36,37 but at the cost of an increased risk of neutropenic complications.
Limitations
This study has some limitations. Because of the different CBZ schedules, the treatment could not be blinded to patients. This was an open-label trial with no central review of the standard imaging. However, a previous study38 has suggested little variance between a local and a central imaging review in this population. The triweekly dose of 20 mg/m2 CBZ was not chosen as a comparator, but the rate of grade 3 or higher neutropenia and/or neutropenic complications was much higher in the PROSELICA study (41.8%)12 compared with the adapted triweekly schedule of CBZ of 16 mg/m2.
Conclusions
The results of the CABASTY phase 3 randomized clinical trial demonstrated that a biweekly schedule of CBZ, 16 mg/m2, plus prophylactic G-CSF at every cycle, significantly reduced the risk of severe neutropenia and/or neutropenic complications compared with the standard regimen (triweekly CBZ, 25 mg/m2, plus G-CSF). Because the risk of grade 3 or higher neutropenia increased with age and is already high in patients younger than 75 years, these data may be practice changing, especially in patients 65 years or older with mCRPC who are often denied chemotherapy in daily clinical routines.
Trial Protocol and Statistical Analysis Plan
eTable 1. Cabazitaxel Exposure (Safety Patient Population)
eTable 2. Baseline Characteristics of Patients Who Developed or Not Grade ≥3 Neutropenia During Treatment, Regardless of Treatment Arm
eTable 3. First Occurrence of Grade ≥3 Neutropenia per Treatment Cycle (Safety Population)
eFigure 1. Forest Plot of Logistic Regression on Incidence of Grade ≥3 Neutropenia and/or Neutropenic Complications (Safety Population)
eFigure 2. Forest Plot of Treatment Effect on Overall Survival (Intent-to-Treat Population)
eFigure 3. Waterfall Plot of the PSA Response
eTable 4. Adverse Events (AEs) (Safety Population)
Data Sharing Statement
References
- 1.Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part II–2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263-282. doi: 10.1016/j.eururo.2020.09.046 [DOI] [PubMed] [Google Scholar]
- 2.Sartor O, de Bono J, Chi KN, et al. ; VISION Investigators . Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103. doi: 10.1056/NEJMoa2107322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit R, de Bono J, Sternberg CN, et al. ; CARD Investigators . Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. doi: 10.1056/NEJMoa1911206 [DOI] [PubMed] [Google Scholar]
- 4.Caffo O, Maines F, Rizzo M, Kinspergher S, Veccia A. Metastatic castration-resistant prostate cancer in very elderly patients: challenges and solutions. Clin Interv Aging. 2016;12:19-28. doi: 10.2147/CIA.S98143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh WK, Miao R, Vekeman F, et al. Real-world characteristics and outcomes of patients with metastatic castration-resistant prostate cancer receiving chemotherapy versus androgen receptor–targeted therapy after failure of first-line androgen receptor–targeted therapy in the community setting. Clin Genitourin Cancer. 2017;16(1):P50-P57. [DOI] [PubMed] [Google Scholar]
- 6.Oh WK, Cheng WY, Miao R, et al. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor–targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol Oncol. 2018;36(11):500.e1-500.e9. doi: 10.1016/j.urolonc.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Horgan AM, Seruga B, Pond GR, et al. Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J Geriatr Oncol. 2014;5(2):119-126. doi: 10.1016/j.jgo.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg CN, Castellano D, de Bono J, et al. Efficacy and safety of cabazitaxel versus abiraterone or enzalutamide in older patients with metastatic castration-resistant prostate cancer in the CARD study. Eur Urol. 2021;80(4):497-506. doi: 10.1016/j.eururo.2021.06.021 [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, et al. ; TROPIC Investigators . Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 10.van Soest RJ, Nieuweboer AJM, de Morrée ES, et al. ; Dutch Uro-Oncology Studygroup (DUOS) . The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer. 2015;51(17):2562-2569. doi: 10.1016/j.ejca.2015.07.037 [DOI] [PubMed] [Google Scholar]
- 11.Droz JP, Efstathiou E, Yildirim A, et al. First-line treatment in senior adults with metastatic castration-resistant prostate cancer: a prospective international registry. Urol Oncol. 2016;34(5):234.e21-234.e29. doi: 10.1016/j.urolonc.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 12.Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer–PROSELICA. J Clin Oncol. 2017;35(28):3198-3206. doi: 10.1200/JCO.2016.72.1076 [DOI] [PubMed] [Google Scholar]
- 13.Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial–FIRSTANA. J Clin Oncol. 2017;35(28):3189-3197. doi: 10.1200/JCO.2016.72.1068 [DOI] [PubMed] [Google Scholar]
- 14.Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116-136. doi: 10.1016/j.ejca.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 15.Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, et al. ; PROSTY Study Group . 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14(2):117-124. doi: 10.1016/S1470-2045(12)70537-5 [DOI] [PubMed] [Google Scholar]
- 16.Lehtonen M, Sormunen J, Luukkaala T, et al. 2-Weekly versus 3-weekly docetaxel for metastatic castration-resistant prostate cancer: complete quality of life results from the randomised, phase-III PROSTY trial. Acta Oncol. 2022;61(8):963-971. doi: 10.1080/0284186X.2022.2098680 [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(1):71-90. doi: 10.6004/jnccn.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottet N, Cornford P, van den Bergh RCN; Prostate Cancer Guidelines Panel. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. European Association of Urology; 2023. Accessed March 16, 2023. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023_2023-06-13-141145_owmj.pdf
- 19.Clément-Zhao A, Auvray M, Aboudagga H, et al. Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer. BJU Int. 2018;121(2):203-208. doi: 10.1111/bju.13855 [DOI] [PubMed] [Google Scholar]
- 20.Kellokumpu-Lehtinen PL, Marttila T, Jekunen A, et al. Biweekly cabazitaxel is a safe treatment option for metastatic castration-resistant prostate cancer (mCRPC) patients after docetaxel—a final analysis of the Prosty II trial. Anticancer Res. 2020;40(12):6915-6921. doi: 10.21873/anticanres.14715 [DOI] [PubMed] [Google Scholar]
- 21.International Council for Harmonisation. ICH guidelines. International Council for Harmonisation. Accessed May 18, 2023. https://www.ich.org/page/ich-guidelines
- 22.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Bellera CA, Rainfray M, Mathoulin-Pélissier, et al. Screening Older Cancer Patients: First Evaluation of the G-8 Geriatric Screening Tool. Ann Oncol. 2012;23(8):2166-2172. doi: 10.1093/annonc/mdr587 [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. doi: 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oudard S. TROPIC: phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol. 2011;7(4):497-506. doi: 10.2217/fon.11.23 [DOI] [PubMed] [Google Scholar]
- 27.Kellokumpu-Lehtinen PLI, Marttila T, Hervonen P, et al. Biweekly cabazitaxel as post-docetaxel treatment for metastatic castration resistant prostate cancer (mCRPC): findings from an early safety analysis of the PROSTY II trial. J Clin Oncol. 2015;33(7)(suppl):276. doi: 10.1200/jco.2015.33.7_suppl.276 [DOI] [Google Scholar]
- 28.Dranitsaris G, Rayson D, Vincent M, et al. Identifying patients at high risk for neutropenic complications during chemotherapy for metastatic breast cancer with doxorubicin or pegylated liposomal doxorubicin: the development of a prediction model. Am J Clin Oncol. 2008;31(4):369-374. doi: 10.1097/COC.0b013e318165c01d [DOI] [PubMed] [Google Scholar]
- 29.Aapro MS, Bohlius J, Cameron DA, et al. ; European Organisation for Research and Treatment of Cancer . 2010 Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8-32. doi: 10.1016/j.ejca.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 30.Droz JP, Aapro M, Balducci L, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014;15(9):e404-e414. doi: 10.1016/S1470-2045(14)70018-X [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich A, Bracarda S, Mason M, et al. ; European investigators . Safety of cabazitaxel in senior adults with metastatic castration-resistant prostate cancer: results of the European Compassionate-Use Programme. Eur J Cancer. 2014;50(6):1090-1099. doi: 10.1016/j.ejca.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 32.Beer TM, Hotte SJ, Saad F, et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol. 2017;18(11):1532-1542. doi: 10.1016/S1470-2045(17)30605-8 [DOI] [PubMed] [Google Scholar]
- 33.van Soest RJ, de Morrée ES, Kweldam CF, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67(6):981-985. doi: 10.1016/j.eururo.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 34.Al Nakouzi N, Le Moulec S, Albigès L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2015;68(2):228-235. doi: 10.1016/j.eururo.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 35.Delanoy N, Hardy-Bessard AC, Efstathiou E, et al. Clinical progression is associated with poor prognosis whatever the treatment line in metastatic castration resistant prostate cancer: the CATS international database. Eur J Cancer. 2020;125:153-163. doi: 10.1016/j.ejca.2019.10.030 [DOI] [PubMed] [Google Scholar]
- 36.Meisel A, von Felten S, Vogt DR, et al. Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration-resistant prostate cancer (mCRPC): a post-hoc analysis of the TROPIC phase III trial. Eur J Cancer. 2016;56:93-100. doi: 10.1016/j.ejca.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 37.Meisel A, de Wit R, Oudard S, et al. Neutropenia, neutrophilia, and neutrophil-lymphocyte ratio as prognostic markers in patients with metastatic castration-resistant prostate cancer. Ther Adv Med Oncol. 2022;14:17588359221100022. doi: 10.1177/17588359221100022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol. 2015;33(12):1356-1363. doi: 10.1200/JCO.2014.55.3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Cabazitaxel Exposure (Safety Patient Population)
eTable 2. Baseline Characteristics of Patients Who Developed or Not Grade ≥3 Neutropenia During Treatment, Regardless of Treatment Arm
eTable 3. First Occurrence of Grade ≥3 Neutropenia per Treatment Cycle (Safety Population)
eFigure 1. Forest Plot of Logistic Regression on Incidence of Grade ≥3 Neutropenia and/or Neutropenic Complications (Safety Population)
eFigure 2. Forest Plot of Treatment Effect on Overall Survival (Intent-to-Treat Population)
eFigure 3. Waterfall Plot of the PSA Response
eTable 4. Adverse Events (AEs) (Safety Population)
Data Sharing Statement