This nonrandomized controlled trial evaluates the activity and toxicity profile of an antiangiogenic metronomic combinatorial regimen in pediatric patients with relapsed or refractory medulloblastoma.
Key Points
Question
What is the outcome of an antiangiogenic metronomic combinatorial regimen in pediatric patients with recurrent or refractory medulloblastoma?
Findings
In this single-arm, phase 2 nonrandomized controlled trial of 40 patients with previously irradiated recurrent or refractory medulloblastoma, a response rate of 45% was achieved. Six patients achieved a complete response, 9 patients achieved a partial response, and 5 patients had stable disease.
Meaning
The use of an antiangiogenic metronomic combinatorial regimen warrants further investigation.
Abstract
Importance
Medulloblastoma recurrence in patients who have previously received irradiation has a dismal prognosis and lacks a standard salvage regimen.
Objective
To evaluate the response rate of pediatric patients with medulloblastoma recurrence using an antiangiogenic metronomic combinatorial approach (Medulloblastoma European Multitarget Metronomic Anti-Angiogenic Trial [MEMMAT]).
Design, Setting, and Participants
This phase 2, investigator-initiated, multicenter nonrandomized controlled trial assessed 40 patients with relapsed or refractory medulloblastoma without a ventriculoperitoneal shunt who were younger than 20 years at original diagnosis. Patients were enrolled between April 1, 2014, and March 31, 2021.
Interventions
Treatment consisted of daily oral thalidomide, fenofibrate, celecoxib, and alternating 21-day cycles of low-dose (metronomic) oral etoposide and cyclophosphamide, supplemented by intravenous bevacizumab and intraventricular therapy consisting of alternating etoposide and cytarabine.
Main Outcomes and Measures
The primary end point was response after 6 months of antiangiogenic metronomic therapy. Secondary end points included progression-free survival (PFS), overall survival (OS), and quality of life. Adverse events were monitored to assess safety.
Results
Of the 40 patients (median [range] age at treatment start, 10 [4-17] years; 25 [62.5%] male) prospectively enrolled, 23 (57.5%) achieved disease control after 6 months of treatment, with a response detected in 18 patients (45.0%). Median OS was 25.5 months (range, 10.9-40.0 months), and median PFS was 8.5 months (range, 1.7-15.4 months). Mean (SD) PFS at both 3 and 5 years was 24.6% (7.9%), while mean (SD) OS at 3 and 5 years was 43.6% (8.5%) and 22.6% (8.8%), respectively. No significant differences in PFS or OS were evident based on molecular subgroup analysis or the number of prior recurrences. In patients demonstrating a response, mean (SD) overall 5-year PFS was 49.7% (14.3%), and for patients who remained progression free for the first 12 months of treatment, mean (SD) 5-year PFS was 66.7% (16.1%). Treatment was generally well tolerated. Grade 3 to 4 treatment-related adverse events included myelosuppression, infections, seizures, and headaches. One heavily pretreated patient with a third recurrence died of secondary acute myeloid leukemia.
Conclusions and Relevance
This feasible and well-tolerated MEMMAT combination regimen demonstrated promising activity in patients with previously irradiated recurrent medulloblastoma. Given these results, this predominantly oral, well-tolerated, and outpatient treatment warrants further evaluation.
Trial Registration
ClinicalTrials.gov Identifier: NCT01356290
Introduction
Medulloblastoma, an aggressive embryonal tumor arising in the cerebellum or, less frequently, in the dorsal brain stem, is one of the most common malignant central nervous system tumors in children.1 Four clinically relevant molecular subgroups—WNT (wingless-related integration site), SHH (sonic hedgehog signaling molecule), group 3, and group 4—are known2 and have been differentiated into additional subtypes.3,4,5 Unfortunately, identification of these subgroups has not yet translated into more effective targeted therapies. Surgery, craniospinal irradiation, and multiagent chemotherapy remain the standard up-front treatment approach for most patients. Although this treatment is highly effective for most patients, approximately 30% of patients will experience relapse of their disease, often metastatic at the time of relapse, and most will eventually die of their disease.6,7,8,9,10,11,12 A widely used treatment strategy for recurrent medulloblastoma is the combination of temozolomide and irinotecan,13 which was recently augmented with bevacizumab.14 This treatment regimen was well tolerated and had an acceptable response rate but failed to lead to sustained responses in patients who had previously received irradiation.
The advantage of metronomic antiangiogenic therapies targeting the tumor microenvironment is that even drugs to which tumors have previously been exposed at high concentrations and have developed resistance can be used. For example, etoposide and cyclophosphamide given at low, long-term dosing to target normal cells that support tumor proliferation, such as endothelium, can produce substantial inhibition of tumor growth, even in tumors made highly resistant to these drugs.15,16,17 Similar effects can be observed using other inhibitors of endothelial cell function, including cyclooxygenase 2 inhibitors, peroxisome proliferator–activated receptor α agonists, and thalidomide.18,19,20
A retrospective observational study was recently published of an alternative strategy to treat recurrent medulloblastoma, using a modified 5-drug oral metronomic antiangiogenic therapy.21 On the basis of this preliminary experience,22 we evaluated the activity and toxicity profile of this combinatorial approach in a phase 2 trial (Medulloblastoma European Multitarget Metronomic Anti-Angiogenic Trial [MEMMAT]) in pediatric patients with previously irradiated recurrent or refractory medulloblastoma.
Methods
Study Design and Patients
MEMMAT was a prospective, international, multicenter, single-arm, phase 2 study that included sites in Europe and the US. This academic trial depended on local institutions to generate funding to perform the study. The trial was conducted in accordance with the Declaration of Helsinki23 and in compliance with the International Conference on Harmonisation Good Clinical Practice and local regulatory ethics committee guidelines. Written informed consent was required from parent(s) or legal guardian(s) and/or the patients before study enrollment. The Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) reporting guidelines were followed. The trial protocol is available in Supplement 1.
Patients were enrolled from April 1, 2014, to March 31, 2021. Eligible patients were younger than 20 years at original diagnosis, with a histologically confirmed medulloblastoma diagnosis and a documented relapse on magnetic resonance imaging (MRI), biopsy, or cerebrospinal fluid (CSF) cytologic testing. There was no limit to the number of previous relapses or any restrictions to the types of previous therapies administered. Acceptable organ function and bone marrow recovery and a Karnofsky Performance Scale Index score or Lansky Play-Performance Scale score of 50% or higher were required (lower Karnofsky and Lansky scores indicate functional impairment). MEMMAT did not collect data on race or ethnicity. At the time of enrollment, patients were permitted to undergo additional surgical resection. Patients dependent on ventriculoperitoneal shunts were excluded because of the anticipated difficulties in providing adequate intraventricular therapy. The medulloblastoma molecular group was determined by DNA methylation profiling according to previously described methods.24 Forty-one patients met the eligibility criteria and were enrolled, and 1 dropped out, leaving an evaluable population of 40 patients.
Study Treatment
Treatment consisted of daily oral thalidomide, fenofibrate, celecoxib, and alternating 21-day cycles of oral etoposide and cyclophosphamide, supplemented by intravenous bevacizumab and intraventricular therapy via an Ommaya reservoir consisting of alternating etoposide and liposomal or aqueous cytarabine. For an overview of the dosing schedule and dose modifications, see eTables 1 and 2 in Supplement 2. Concomitant pneumocystis prophylaxis was recommended. Treatment was given for 12 months and was continued for an optional second year without oral etoposide or cyclophosphamide and with extended intervals of intraventricular therapy, depending on response and tolerability.
Response Assessments
Magnetic resonance imaging with and without gadolinium enhancement was performed within 2 weeks before the start of protocol therapy. In case of surgery, postoperative MRI was required within 72 hours after surgery. Six months after the start of antiangiogenic treatment, MRI was mandatory to assess response. A CSF cytologic test was performed once at each cycle of intraventricular therapy. Continuing response or stable disease was confirmed every 3 months until disease progression or study discontinuation. The MRIs were collated and centrally reviewed by an experienced panel of pediatric neuroradiologists and pediatric neurooncologists (A.P. and M.S.).
Response Criteria
Complete response (CR) was defined as the total disappearance of all radiologic evidence of tumor, determined by 2 observations not less than 4 weeks apart, and no evidence of malignant cells in the CSF. No evidence of disease (NED) was defined as no recurrence or appearance of new lesions and no malignant cells in the CSF in a patient with complete resection and no measurable disease after surgery, with NED continuing for at least 6 months. Partial response (PR) was defined as regression of at least 50% of all tumor size (the sum of the products of all measured lesions), determined by 2 observations not less than 4 weeks apart. No simultaneous progression of any lesion or the appearance of new lesions may have occurred. Nonmeasurable lesions (eg, diffuse leptomeningeal spread) must have remained stable or regressed for this category. Patients whose CSF results were negative for disease were required to continue to test negative. Stable disease was regression of less than 50% of tumor size or progression less than 25% of at least 4 weeks’ duration, without appearance of new lesions. Progressive disease was worsening of disease, evidenced by enlargement of any existing lesion(s) of 25% or more or appearance of new lesions or the manifestation of new CSF-positive disease.
Health-related quality of life (QOL) data were collected using the KINDL questionnaire in the respective national language at study enrollment and after 6 months. The questionnaire consists of 24 Likert scale items, with 4 items each representing 1 of the following 6 scales: physical well-being, emotional well-being, self-esteem, family, friends, and everyday functioning (school or nursery school or kindergarten). Age-specific self-report versions for children and adolescents and a proxy version for parents were applied. The scale scores and the total score were compared with reference scores of a norm sample of the same age groups.25
Patients were followed up for survival after completion or discontinuation of study treatment. Safety was evaluated and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Adverse events were recorded at study visits and for at least 28 days after the last dose of study treatment.
Statistical Analysis
Data cutoff was on March 7, 2022. Trial enrollment for this phase 2 cohort was planned for 40 patients (1 − β = 0.90; estimated dropout rate, 5%). The clinically sufficient response rate was specified with π1 = 35%; the clinically insufficient response rate was specified with π0 = 15%. In the primary efficacy analysis, tumor responses of all patients were determined by central review and used as the primary assessment in the overall response analysis. The primary end point of response was estimated using the minimax 3-stage design for phase 2 oncology clinical trials with 2 interim analyses (eTable 3 in Supplement 2).26 Binomial proportion of best overall response of a confirmed NED, CR, or PR was reported using a 2-sided multiple testing procedure with α = .05. Both overall survival (OS) and progression-free survival (PFS) were described using the Kaplan-Meier method, and median OS and PFS were calculated using reversed Kaplan-Meier estimator; median times and 95% CIs were calculated. Statistical analyses were conducted using SPSS software, version 27 (SPSS Inc).
Results
Study Patients
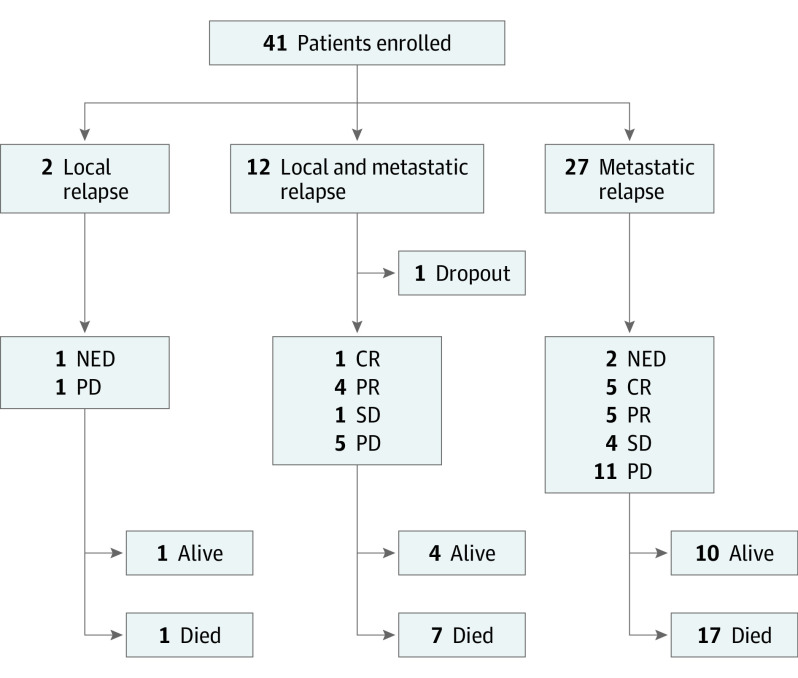
Baseline demographic and disease characteristics of the 40 analyzed patients (median [range] age at treatment start, 10 [4-17] years; 25 [62.5%] male and 15 [37.5%] female) are summarized in Table 1. All patients had received irradiation as part of their previous treatment. Median (range) time to relapse was 20 (1-57) months. Eight patients experienced a second relapse and 2 patients a third relapse. Seven of these patients received temozolomide, irinotecan, bevacizumab, or a combination of those drugs to treat previous relapses. The pattern of relapse was local (2 [5.0%]), metastatic (27 [67.5%]), or combined local and metastatic (11 [27.5%]) (Figure 1). No association between molecular group at diagnosis and individual relapse pattern was found. Twelve patients underwent surgical resection before enrollment, with 4 gross total resections and 8 partial resections. Four patients received focal reirradiation after completion of the 6-month response MRI. MYC(N) amplification was identified in 4 of the 40 patients: 3 (MYC [OMIM 190080]) in group 3 and 1 (MYCN [OMIM 164840]) in SHH, similar to previous studies.9,10
Table 1. Baseline Demographic and Disease Characteristicsa.
| Characteristic | Finding (N = 40) |
|---|---|
| Sex | |
| Female | 15 (37.5) |
| Male | 25 (62.5) |
| Molecular subgroup at original diagnosis | |
| WNT | 1 (2.5) |
| SHH | 4 (10.0) |
| Group 3 | 12 (30.0) |
| Group 4 | 23 (57.5) |
| MYC/MYCN amplification | |
| No | 36 (90.0) |
| MYC | 3 (7.5) |
| MYCN | 1 (2.5) |
| No. of recurrences at study entry | |
| 1 | 29 (72.5) |
| 2 | 9 (22.5) |
| 3 | 2 (5.0) |
| Age at MEMMAT start, median (range), y | 10 (4-17) |
| Pattern of current relapse | |
| Local | 2 (5.0) |
| Metastatic | 28 (70.0) |
| Combined local and metastatic | 10 (25.0) |
Abbreviation: MEMMAT, Medulloblastoma European Multitarget Metronomic Anti-Angiogenic Trial; SHH, sonic hedgehog signaling molecule; WNT, wingless-related integration site.
Data are presented as number (percentage) of patients unless otherwise indicated. Data on race and ethnicity were not collected by MEMMAT.
Figure 1. Study Flow Diagram.
CR indicates complete response; NED, no evidence of disease; PD, progressive disease; PR, partial response; SD, stable disease.
Efficacy
Among the 40 patients, 23 (57.5%) achieved disease control (NED, CR, PR, and stable disease) after 6 months of treatment, whereas 17 patients (42.5%) discontinued treatment because of disease progression within the first 6 months. A total of 16 patients (40.0%) received the MEMMAT treatment for at least 1 year. Three patients had a complete resection of their relapse and remained with NED thereafter (7.5%). Best response was CR in 6 patients (15.0%), PR in 9 patients (22.5%), and stable disease in 5 patients (12.5%). Objective response rate, defined as ongoing NED after complete resection, CR, or PR, was 45%.
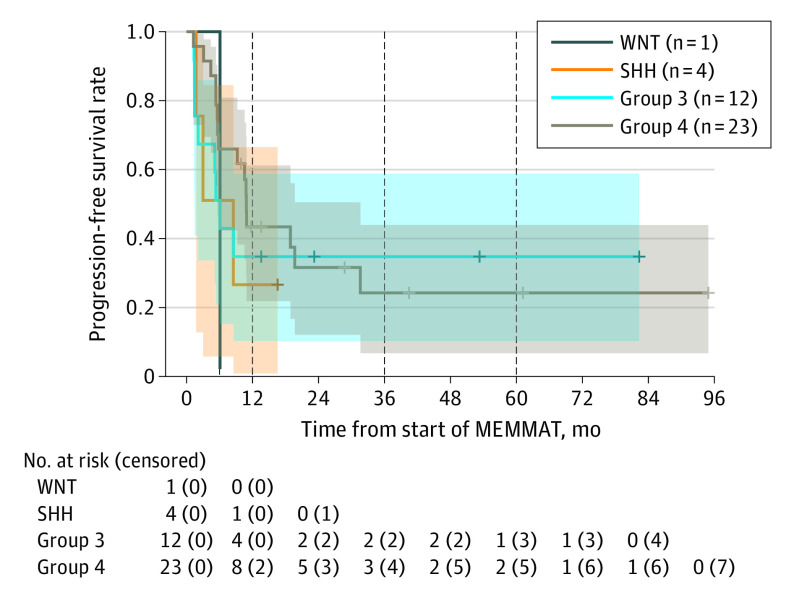
Median follow-up time was 40.5 months (range, 1.3-94.9 months), and mean (SD) PFS at both 3 and 5 years was both 24.6% (7.9%). Mean (SD) OS was 43.6% (8.5%) at 3 years and 22.6% (8.8%) at 5 years. Median PFS and OS were 8.5 months (range, 1.7-15.4 months) and 25.5 months (range, 10.9-40.0 months), respectively (Figure 2). Two patients died of events without progression of the investigated disease. No significant differences in PFS or OS were evident regarding the molecular groups (Figure 3; eTable 4 in Supplement 2), the number of relapses, or the intraventricular treatment with liposomal cytarabine or aqueous cytarabine (eFigures 1-3 in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Survival Outcomes of the 40 Study Participants.
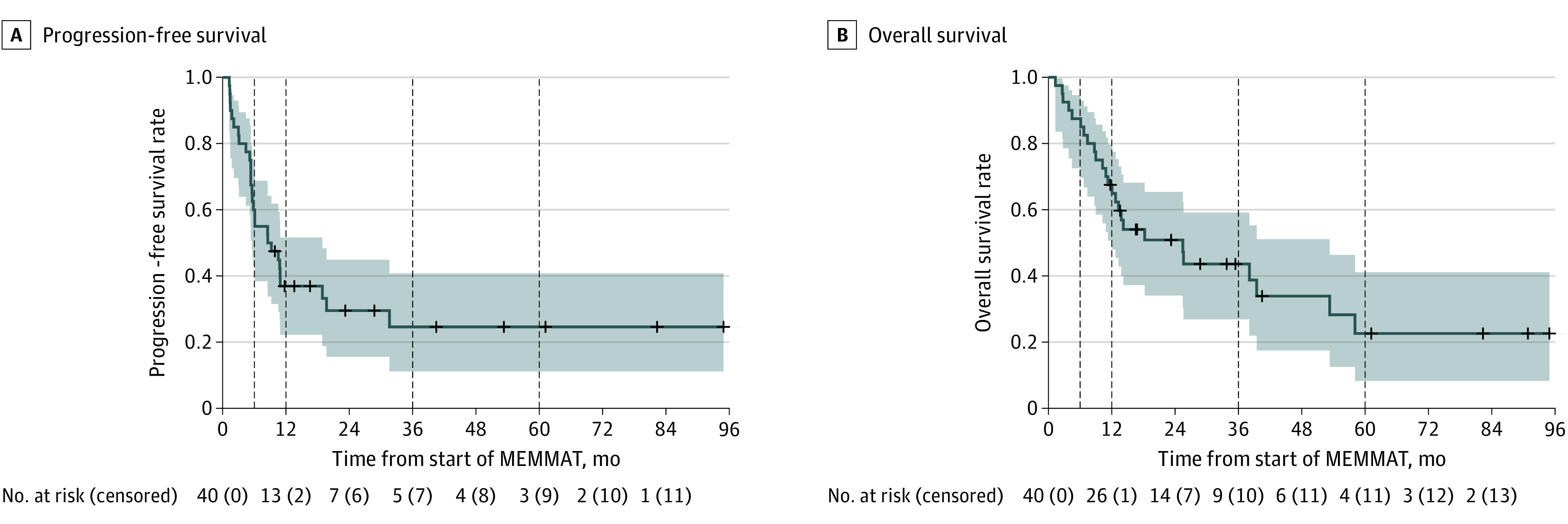
The shaded areas represent 95% CIs; vertical dashed lines indicate the 6-month, 12-month, 36-month, and 60-month time points; and crosses represent censored participants. MEMMAT indicates Medulloblastoma European Multitarget Metronomic Anti-Angiogenic Trial.
Figure 3. Progression-Free Survival After 12 Months for Medulloblastoma Subgroup Distribution.
The shaded areas represent 95% CIs; vertical dashed lines indicate the 12-month, 36-month, and 60-month time points; and crosses represent censored participants. MEMMAT indicates Medulloblastoma European Multitarget Metronomic Anti-Angiogenic Trial; SHH, sonic hedgehog signaling molecule; and WNT, wingless-related integration site.
In an exploratory analysis of patients who had disease control at 6 months, median PFS was 31.6 months (range, 11.0-52.2 months), and 5-year mean (SD) PFS was 42.8 (12.4) months. In the 18 patients (45.0%) who demonstrated any response (NED, CR, or PR), median PFS was 31.6 months (range, 11.0-52.2 months) and mean (SD) 5-year PFS was 49.7 (14.3) months. In the 13 patients (32.5%) who had no progression after 12 months of treatment, mean (SD) 5-year PFS rate was 66.7% (16.1%). Fifteen patients remained alive for a mean of 39 months (range, 12-95 months) after initiation of MEMMAT treatment, 12 of whom were progression free (eFigures 4-6 in Supplement 2).
Safety and QOL
The most frequently reported adverse events of any grade were hematologic disorders (Table 2). One heavily pretreated patient who presented with a third relapse died of secondary acute myeloid leukemia 10 months after starting MEMMAT treatment, having received etoposide, temozolomide, and irinotecan as part of his prior therapy. A complete list of all treatment-related adverse effects is shown in eTable 5 in Supplement 2.
Table 2. Treatment-Related Adverse Events in the Study Patients.
| Adverse event | No. (%) of patients (N = 40) | ||
|---|---|---|---|
| Grade 3 | Grade 4 | Total | |
| Hematologic | |||
| Anemia | 5 (12.5) | 0 | 5 (12.5) |
| Leukopenia | 2 (5.0%) | 8 (20.0) | 10 (25.0) |
| Lymphopenia | 0 | 3 (7.5) | 3 (7.5) |
| Neutropenia | 3 (7.5) | 14 (35) | 17 (42.5) |
| Platelet count decreased | 0 | 4 (10.0) | 4 (10.0) |
| Neurologic | |||
| Fatigue | 1 (2.5) | 0 | 1 (2.5) |
| Headache | 2 (5.0) | 0 | 2 (5.0) |
| Hearing impairment | 0 | 1 (2.5) | 1 (2.5) |
| Seizure | 2 (5.0) | 0 | 2 (5.0) |
| Infection | |||
| Cerebellitis | 1 (2.5) | 0 | 1 (2.5) |
| Chemical arachnoiditis | 0 | 1 (2.5) | 1 (2.5) |
| Febrile neutropenia | 2 (5.0) | 0 | 2 (5.0) |
| Infection, not otherwise specified | 3 (7.5) | 1 (2.5) | 4 (10.0) |
| Meningitis | 2 (5.0) | 0 | 2 (5.0) |
| Mucositis | 1 (2.5) | 0 | 1 (2.5) |
| Sepsis | 0 | 1 (2.5) | 1 (2.5) |
| Urinary tract infection | 1 (2.5) | 0 | 1 (2.5) |
| Laboratory | |||
| Elevated liver enzymes | 2 (5.0) | 0 | 2 (5.0) |
| Hyponatremia | 3 (7.5) | 0 | 3 (7.5) |
| Various | |||
| Hypertension | 2 (5.0) | 0 | 2 (5.0) |
| Proteinuria | 2 (5.0) | 0 | 2 (5.0) |
| Wound dehiscence | 1 (2.5) | 0 | 1 (2.5) |
The QOL results measured by the KINDL questionnaire indicated a medium or low QOL before initiation of protocol therapy. Quality of life did not further decrease considerably once therapy was initiated and slightly improved, although results were heterogeneous (eTable 6 in Supplement 2).
Discussion
Effective treatment for recurrent medulloblastoma remains a significant unmet need, with no approved therapies and only rare long-term survivors. The metronomic antiangiogenic drug combination with intraventricular therapy used in MEMMAT significantly prolonged survival and was able to produce durable responses in patients without a ventriculoperitoneal shunt who had previously received irradiation. The treatment is predominantly oral, well tolerated, outpatient, and easily adapted in these heavily pretreated patients.
In the past, numerous attempts have been made to treat relapsed medulloblastoma. In some of these studies, significant responses have been observed, but these responses lasted only a short period in patients who had previously received irradiation.13,14,27 With a median OS of 25.5 months, our study also compared favorably to the OS of 19 months reported by the Children’s Oncology Group study with the addition of bevacizumab to temozolomide and irinotecan.14 Whether this superior result is attributable to the difference in systemic therapy or the addition of intraventricular therapy needs to be determined. Although half of the infants in one study who had not received irradiation as part of their primary treatment could be saved at relapse,10,28 subsequent irradiation or myeloablative chemotherapy was not successful in patients previously treated with irradiation, with only a limited number of patients alive at the time of reporting of those often highly selected cohorts.29,30,31,32 Most important, the MEMMAT combination enhanced durability of response and facilitated sustained PFS in one-quarter of patients with recurrent medulloblastoma who have undergone heavy pretreatment and previous irradiation.
In accordance with a previous study,10 we observed that our exclusively prior-irradiated cohort also experienced early relapses. A post hoc analysis, however, showed very promising 5-year PFS in patients demonstrating a response (49.7%) and for patients who remained progression free the first 12 months of treatment (66.7%), indicating patients who might benefit from this intervention.
MEMMAT differs from conventional treatment regimens because it applies a metronomic antiangiogenic combinatorial approach to target the tumor microenvironment. Metronomic chemotherapy is defined as the long-term administration of chemotherapy at low, minimally toxic doses, without prolonged drug-free breaks designed to treat vascular cells needed to maintain tumor cell proliferation, migration, and metastatic spread. Beyond its antiangiogenic effect, metronomic chemotherapy appears to also affect other cells within the tumor microenvironment that may play important roles by inducing immunogenic pathways that can activate both innate and adaptive immune responses. Metronomic cyclophosphamide, for example, was shown to deplete regulatory T cells in various tumor types and is a common treatment for nephrotic syndrome and lupus nephritis.33,34,35
Given the redundancy of mechanisms involved in new blood vessel formation by cancer, growth interference via multiple pathways is required for induction and maintenance of tumor response. Therefore, MEMMAT combined metronomic chemotherapy with the repurposing of nonchemotherapeutic drugs, as was previously done in the COMBAT (combined oral metronomic biodifferentiating antiangiogenic treatment) regimen.36,37 The rationale for combining the 5 oral drugs as well as bevacizumab and intraventricular therapy in MEMMAT has previously been described in detail.21,22,38,39
Metronomic chemotherapy has been reported to be better tolerated and to induce less severe adverse effects than conventional chemotherapy. This was also confirmed by MEMMAT, in which this therapy was generally well tolerated in a heavily pretreated cohort in an outpatient setting. The MEMMAT combination was feasible because the patients were adherent even with the frequent necessary visits to the outpatient clinic for intraventricular therapy, and only 1 of all patients dropped out of the study. Most toxic effects were hematologic and consistent with low-dose chemotherapy. Frequent clinical and laboratory checks were used to closely monitor the treatment, and dose modification was added to reduce neutropenic episodes and infections while minimizing prolonged drug-free breaks.
Quality of life during metronomic therapy has rarely been reported but is even more important for patients with an overall poor prognosis.40,41 The QOL questionnaires indicated a medium or low QOL at baseline, likely reflecting the heavy burden of prior therapy and associated sequelae from their original diagnosis and/or prior relapses. Furthermore, the significance of another relapse might have further influenced the low baseline QOL at the start of treatment. However, it appears that QOL did not worsen during MEMMAT treatment and slightly improved. Clearly, additional studies in this area of patient outcome are needed to clarify areas that might help improve QOL.
All but 2 patients in our study had disease recurrence at distant central nervous system sites with or without disease in the original tumor bed, a pattern that is consistent with previous observations.9,11 Prior studies have shown that surgery in isolated medulloblastoma relapses was associated with improved survival,8,9,42 albeit with limited long-term success in patients who had previously received irradiation.7,8,41 To avoid denying patients with complete resection access to the study, MEMMAT allowed patients with recurrent medulloblastoma to undergo resection before enrollment, and maintaining NED after a gross total resection was considered a benefit of the treatment combination. However, only 4 patients in our cohort achieved a gross total resection, 2 with local and 2 with metastatic relapse. One of these patients experienced rapid disease progression (3 months) and died of her disease, whereas the other 3 patients are alive, progression free, and without evidence of disease at 41, 29, and 14 months, respectively, after enrollment in MEMMAT.
Although it was not an original objective of the trial, with the recent subclassification of medulloblastoma,5 we were interested to see whether outcome differed by group. No association between outcome and group assignment was identified; however, this study was not powered to pick up small differences (Figure 2). This result is consistent with the data from our retrospective series21 and the recent Children’s Oncology Group trial for relapsed medulloblastoma,14 although others have reported longer median postrelapse survival for patients with group 4 tumors.11
Limitations
There are some important limitations of our study. The patient population reported in this trial, although all having relapsed medulloblastoma after standard up-front surgery, radiation therapy, and chemotherapy, is heterogeneous with regard to their molecular subgrouping, and the study was underpowered to detect true differences between groups. In contrast to a previous publication,21 we have excluded patients with a ventriculoperitoneal shunt because of the inherent risk of premature diversion of possibly important intraventricularly administered drugs. The ability to identify which drugs were responsible for problematic toxic effects in this group was thus limited, which impacts our ability to make optimal substitutions going forward. Randomized clinical trials are needed to fully evaluate the efficacy of this metronomic regimen, although this will be difficult because there is no clear standard of care for comparison. Perhaps more importantly, all the drugs in this regimen are commercially available, as are those of other frequently used regimens in relapsed medulloblastoma; thus, there are no industry partners incentivized to support such studies at the current time, and the availability of the drugs might depend on the market situation. Relying on comparisons to other single-arm trials, while less than ideal, remains the best current option for identifying new active treatment regimens. Because the aim of initial treatment is to cure and prevent relapse, figuring out how and for which patients to incorporate this approach into frontline therapy remains a long-term goal.43,44
Conclusions
This nonrandomized controlled trial found that the MEMMAT regimen is a feasible and promising strategy for patients who have previously received irradiation. Given this meaningful clinical benefit in a difficult-to-treat population, further evaluation of the combination is warranted. In the future, an improved understanding of the molecular signature and specific pathways of medulloblastoma groups and subgroups may allow us to add targeted therapies to a MEMMAT backbone.
Trial Protocol
eFigure 1. OS After 12 Months for Medulloblastoma Subgroup Distribution
eFigure 2. Kaplan-Meier Estimates of Survival Outcomes Regarding the Number of Relapses
eFigure 3. Kaplan-Meier Estimates of Survival Outcomes Regarding Intraventricular Liposomal Cytarabine (n = 17) and Aqueous Cytarabine (n = 24)
eFigure 4. Kaplan-Meier Estimates for PFS of Patients That Exhibited Disease Control (NED+CR+PR+SD)
eFigure 5. Kaplan-Meier Estimates for PFS of Patients That Exhibited Response (NED+CR+PR)
eFigure 6. Kaplan-Meier Estimates for PFS of Patients That Showed No Progression After 12 Months of MEMMAT Treatment
eTable 1. MEMMAT Regimen: Dosing Schedule
eTable 2. Dose Modifications of the MEMMAT Regimen
eTable 3. Results and Description of the Interim Analyses
eTable 4. Responders and Non-responders by Subgroup
eTable 5. All Treatment-Related AEs in the Study Population (N = 40)
eTable 6. Quality of Life at the Beginning and After 6 Months of the MEMMAT Protocol
Data Sharing Statement
References
- 1.Ostrom QT, Price M, Ryan K, et al. CBTRUS statistical report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol. 2022;24(suppl 3):iii1-iii38. doi: 10.1093/neuonc/noac161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465-472. doi: 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwalbe EC, Lindsey JC, Nakjang S, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 2017;18(7):958-971. doi: 10.1016/S1470-2045(17)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma T, Schwalbe EC, Williamson D, et al. Second-generation molecular subgrouping of medulloblastoma: an international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019;138(2):309-326. doi: 10.1007/s00401-019-02020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200-1207. doi: 10.1016/S1470-2045(13)70449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizer B, Donachie PHJ, Robinson K, et al. Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: results of a Children’s Cancer and Leukaemia Group study. Eur J Cancer. 2011;47(9):1389-1397. doi: 10.1016/j.ejca.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Sabel M, Fleischhack G, Tippelt S, et al. ; SIOP-E Brain Tumour Group . Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol. 2016;129(3):515-524. doi: 10.1007/s11060-016-2202-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RM, Richardson S, Schwalbe EC, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc Health. 2020;4(12):865-874. doi: 10.1016/S2352-4642(20)30246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Smith KS, Deng M, et al. Clinical outcomes and patient-matched molecular composition of relapsed medulloblastoma. J Clin Oncol. 2021;39(7):807-821. doi: 10.1200/JCO.20.01359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huybrechts S, Le Teuff G, Tauziède-Espariat A, et al. Prognostic clinical and biologic features for overall survival after relapse in childhood medulloblastoma. Cancers (Basel). 2020;13(1):53. doi: 10.3390/cancers13010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill RM, Plasschaert SLA, Timmermann B, et al. Relapsed medulloblastoma in pre-irradiated patients: current practice for diagnostics and treatment. Cancers (Basel). 2021;14(1):126. doi: 10.3390/cancers14010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill J, Geoerger B, Gesner L, et al. ; European Consortium Innovative Therapies for Children with Cancer (ITCC) and the European Society for Paediatric Oncology (SIOPE) brain tumor group . Phase II study of irinotecan in combination with temozolomide (TEMIRI) in children with recurrent or refractory medulloblastoma: a joint ITCC and SIOPE brain tumor study. Neuro Oncol. 2013;15(9):1236-1243. doi: 10.1093/neuonc/not097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy AS, Krailo M, Chi S, et al. Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: report of a COG randomized Phase II screening trial. Pediatr Blood Cancer. 2021;68(8):e29031. doi: 10.1002/pbc.29031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878-1886. [PubMed] [Google Scholar]
- 16.Panigrahy D, Kaipainen A, Butterfield CE, et al. Inhibition of tumor angiogenesis by oral etoposide. Exp Ther Med. 2010;1(5):739-746. doi: 10.3892/etm.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashley DM, Meier L, Kerby T, et al. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14(6):1922-1927. doi: 10.1200/JCO.1996.14.6.1922 [DOI] [PubMed] [Google Scholar]
- 18.Panigrahy D, Kaipainen A, Huang S, et al. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci U S A. 2008;105(3):985-990. doi: 10.1073/pnas.0711281105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Panigrahy D, Hwang SH, et al. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci U S A. 2014;111(30):11127-11132. doi: 10.1073/pnas.1410432111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082-4085. doi: 10.1073/pnas.91.9.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slavc I, Mayr L, Stepien N, et al. Improved long-term survival of patients with recurrent medulloblastoma treated with a “MEMMAT-like” metronomic antiangiogenic approach. Cancers (Basel). 2022;14(20):5128. doi: 10.3390/cancers14205128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyrl A, Chocholous M, Kieran MW, et al. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012;59(3):511-517. doi: 10.1002/pbc.24006 [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 24.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469-474. doi: 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. 1998;7(5):399-407. doi: 10.1023/A:1008853819715 [DOI] [PubMed] [Google Scholar]
- 26.Chen K, Shan M. Optimal and minimax three-stage designs for phase II oncology clinical trials. Contemp Clin Trials. 2008;29(1):32-41. doi: 10.1016/j.cct.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Le Teuff G, Castaneda-Heredia A, Dufour C, et al. ; European consortium Innovative Therapies for Children with Cancer (ITCC) . Phase II study of temozolomide and topotecan (TOTEM) in children with relapsed or refractory extracranial and central nervous system tumors including medulloblastoma with post hoc Bayesian analysis: a European ITCC study. Pediatr Blood Cancer. 2020;67(1):e28032. doi: 10.1002/pbc.28032 [DOI] [PubMed] [Google Scholar]
- 28.Erker C, Mynarek M, Bailey S, et al. Outcomes of infants and young children with relapsed medulloblastoma after initial craniospinal irradiation-sparing approaches: an international cohort study. J Clin Oncol. 2023;41(10):1921-1932. doi: 10.1200/JCO.21.02968 [DOI] [PubMed] [Google Scholar]
- 29.Ridola V, Grill J, Doz F, et al. High-dose chemotherapy with autologous stem cell rescue followed by posterior fossa irradiation for local medulloblastoma recurrence or progression after conventional chemotherapy. Cancer. 2007;110(1):156-163. doi: 10.1002/cncr.22761 [DOI] [PubMed] [Google Scholar]
- 30.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer. 2010;54(4):649-651. doi: 10.1002/pbc.22378 [DOI] [PubMed] [Google Scholar]
- 31.Dunkel IJ, Boyett JM, Yates A, et al. ; Children’s Cancer Group . High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. J Clin Oncol. 1998;16(1):222-228. doi: 10.1200/JCO.1998.16.1.222 [DOI] [PubMed] [Google Scholar]
- 32.Tsang DS, Sarhan N, Ramaswamy V, et al. Re-irradiation for children with recurrent medulloblastoma in Toronto, Canada: a 20-year experience. J Neurooncol. 2019;145(1):107-114. doi: 10.1007/s11060-019-03272-2 [DOI] [PubMed] [Google Scholar]
- 33.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641-648. doi: 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7. doi: 10.1038/s41572-019-0141-9 [DOI] [PubMed] [Google Scholar]
- 35.Zotta F, Vivarelli M, Emma F. Update on the treatment of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022;37(2):303-314. doi: 10.1007/s00467-021-04983-3 [DOI] [PubMed] [Google Scholar]
- 36.Sterba J, Valik D, Mudry P, et al. Combined biodifferentiating and antiangiogenic oral metronomic therapy is feasible and effective in relapsed solid tumors in children: single-center pilot study. Onkologie. 2006;29(7):308-313. [DOI] [PubMed] [Google Scholar]
- 37.Zapletalova D, André N, Deak L, et al. Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: a multicenter experience. Oncology. 2012;82(5):249-260. doi: 10.1159/000336483 [DOI] [PubMed] [Google Scholar]
- 38.Robison NJ, Campigotto F, Chi SN, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61(4):636-642. doi: 10.1002/pbc.24794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieran MW, Turner CD, Rubin JB, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27(11):573-581. doi: 10.1097/01.mph.0000183863.10792.d4 [DOI] [PubMed] [Google Scholar]
- 40.El Kababri M, Benmiloud S, Cherkaoui S, et al. Metro-SMHOP 01: metronomics combination with cyclophosphamide-etoposide and valproic acid for refractory and relapsing pediatric malignancies. Pediatr Blood Cancer. 2020;67(9):e28508. doi: 10.1002/pbc.28508 [DOI] [PubMed] [Google Scholar]
- 41.Porkholm M, Toiviainen-Salo S, Seuri R, et al. Metronomic therapy can increase quality of life during paediatric palliative cancer care, but careful patient selection is essential. Acta Paediatr. 2016;105(8):946-951. doi: 10.1111/apa.13338 [DOI] [PubMed] [Google Scholar]
- 42.Gaab C, Adolph JE, Tippelt S, et al. Local and systemic therapy of recurrent medulloblastomas in children and adolescents: results of the P-HIT-REZ 2005 study. Cancers (Basel). 2022;14(3):471. doi: 10.3390/cancers14030471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19(6):768-784. doi: 10.1016/S1470-2045(18)30204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.André N, Orbach D, Pasquier E. Metronomic maintenance for high-risk pediatric malignancies: one size will not fit all. Trends Cancer. 2020;6(10):819-828. doi: 10.1016/j.trecan.2020.05.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. OS After 12 Months for Medulloblastoma Subgroup Distribution
eFigure 2. Kaplan-Meier Estimates of Survival Outcomes Regarding the Number of Relapses
eFigure 3. Kaplan-Meier Estimates of Survival Outcomes Regarding Intraventricular Liposomal Cytarabine (n = 17) and Aqueous Cytarabine (n = 24)
eFigure 4. Kaplan-Meier Estimates for PFS of Patients That Exhibited Disease Control (NED+CR+PR+SD)
eFigure 5. Kaplan-Meier Estimates for PFS of Patients That Exhibited Response (NED+CR+PR)
eFigure 6. Kaplan-Meier Estimates for PFS of Patients That Showed No Progression After 12 Months of MEMMAT Treatment
eTable 1. MEMMAT Regimen: Dosing Schedule
eTable 2. Dose Modifications of the MEMMAT Regimen
eTable 3. Results and Description of the Interim Analyses
eTable 4. Responders and Non-responders by Subgroup
eTable 5. All Treatment-Related AEs in the Study Population (N = 40)
eTable 6. Quality of Life at the Beginning and After 6 Months of the MEMMAT Protocol
Data Sharing Statement