Introduction
Recent advances in systemic therapy for hepatocellular carcinoma (HCC) have been remarkable. Systemic therapies were initially developed for advanced HCC. Today, sorafenib [1], lenvatinib (LEN) [2], atezolizumab plus bevacizumab [3], durvalumab plus tremelimumab [4], and durvalumab alone [4] are approved as first-line agents. Regorafenib [5], ramucirumab [6], and cabozantinib [7] have been approved as second-line agents globally. According to the 21st [8], 22nd [9], 23rd [10], and 24th [11] reports of the HCC registry and follow-up survey data by the Japan Liver Cancer Association, the 5-year survival rates improved to 43% for cases registered in 2001–2005, 50% for cases registered in 2006–2009, and 58% for cases registered in 2010–2013) [12], and the median overall survival (OS) improved to 50, 60, and 80 months, respectively [12]. These data suggest that patients enrolled between 2010 and 2013 have benefited from recently developed effective drugs, leading to long-term survival.
The goal of treatment in intermediate-stage HCC is achieving “cancer-free with drug-free” status. Therefore, for transarterial chemoembolization (TACE)-unsuitable patients [13, 14], upfront systemic therapy with LEN or atezolizumab plus bevacizumab may be initiated. After achieving normalization of abnormal vessels or tumor shrinkage, TACE may be effective. A proof-of-concept study of intermediate-stage HCC with a high tumor burden showed that LEN-TACE is more effective than TACE alone, as suggested by a comparative analysis after adjusting baseline characteristics in both treatment arms using propensity score matching [15]. LEN-TACE sequential therapy was tested in a single-arm phase II trial, TACTICS-L [16], which demonstrated a favorable progression-free survival (PFS) and OS. The REPLACEMENT trial [17], a multicenter prospective single-arm phase II study, confirmed the efficacy of atezolizumab plus bevacizumab in a population that exceeded the up-to-seven criteria. Atezolizumab plus bevacizumab followed by curative (ABC) conversion therapy was assessed in a multicenter proof-of-concept study and validated in a population that exceeded the up-to-seven criteria [18–21]. This multicenter proof-of-concept study showed that clinical complete response (CR) and drug-free status could be achieved in patients at the intermediate stage [21]. The IMbrave050 study [22], a global Phase III trial that was recently presented at the American Association for Cancer Research (AACR) meeting, showed that atezolizumab plus bevacizumab as adjuvant therapy after resection or ablation was effective in preventing recurrence mainly in early-stage HCC [23].
Thus, the synergistic effects of sequential/combination systemic therapy and locoregional therapy provide clinical benefits including OS prolongation in early-stage, intermediate-stage, and advanced-stage HCCs. In this Editorial, this issue will be discussed.
Early-Stage HCC
The results of the Phase III IMbrave050 study presented at the AACR meeting in April 2023 are the first positive adjuvant therapy results reported; all previous adjuvant therapy trials yielded negative results. In that sense, this is a landmark study [22], and the results are encouraging. In this study, patients at a high risk of recurrence after resection or ablation of HCC were randomized to receive atezolizumab plus bevacizumab for 1 year or active surveillance (control). The primary endpoint of recurrence-free survival (RFS) was significantly better in the atezolizumab plus bevacizumab group than in the control group [23].
In the IMbrave050 trial [22], the baseline characteristics of the atezolizumab plus bevacizumab group included a median age of approximately 60 years, 83% were male, 83% were Asian, and 71% were from the Asia Pacific region excluding Japan. Performance status was 0 in 77% and 1 in 23% of patients; hepatitis B was detected in 63%, hepatitis C in 10%, nonviral etiology in 14%, and BCLC stage A in 86% of patients. In this population, 88% of patients underwent resection and 12% underwent ablation. The median tumor diameter in the resection group was 5.3 cm; 91% of the patients had one tumor, and 7% had two tumors. The median diameter of ablation was 2.5 cm, and all cases underwent three or fewer ablations (71% were single tumors). The hazard ratio (HR) of IRF-assessed RFS for the primary endpoint was 0.72 [95% confidence interval (CI), 0.56–0.93, p = 0.012], which was extremely favorable in this population. These results are groundbreaking, and the study is the first in the world to demonstrate the efficacy of adjuvant therapy in HCC. The HR for INV-assessed RFS was 0.70 (95% CI, 0.54–0.91, p = 0.007), similar to that for IRF-assessed RFS. Subgroup analysis also showed mostly favorable results for atezolizumab plus bevacizumab. Of particular note were the HRs for the resection group (n = 585) and the ablation group (n = 83), which were almost all favorable for atezolizumab plus bevacizumab despite the relatively low risk of recurrence for the ablation group (RFS HR, 0.61; 95% CI, 0.26–1.41) compared with the resection group (HR, 0.75; 95% CI, 0.58–0.98). These results indicate that atezolizumab plus bevacizumab is associated with a greater reduction in recurrence with respect to RFS in both high- and relatively low-risk patients, which was also shown in the NIVOLVE trial [23–26]. One key factor potentially associated with the positive results is the ability of bevacizumab to alter the intratumoral immune microenvironment related to a high risk of recurrence in terms of presence of Tregs, WNT/β-catenin pathway activation, and fewer intratumoral CD8+ T-cell infiltration, as demonstrated in the NIVOLVE study [23–26] (Fig. 1).
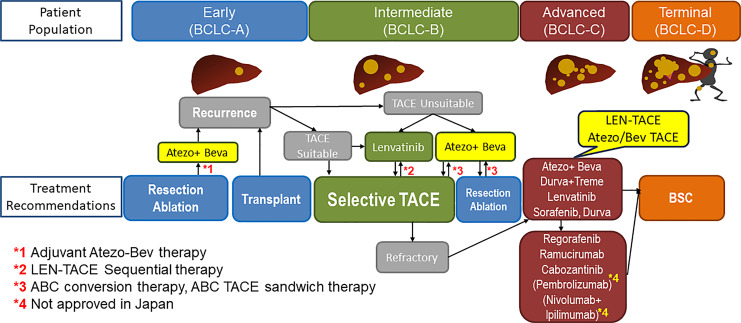
Fig. 1.
Patients in all stages of HCC benefit from systemic therapy combined with locoregional therapy, as shown in several trials in each stage of HCC. HCC, hepatocellular carcinoma.
Intermediate-Stage HCC
LEN-TACE Sequential Therapy: Multicenter Proof-of-Concept Study
The efficacy of LEN in combination with TACE was first demonstrated in a multicenter proof-of-concept study [15] (Fig. 1). The OS of patients treated with LEN plus TACE was 37.9 months, which was better than the 21.3 months for patients treated with TACE alone (HR = 0.48; 95% CI, 0.16–0.79; p < 0.01). PFS was 16.0 months in the LEN arm, which was significantly better than 3.0 months for TACE (HR = 0.19). The objective response rate (ORR) was also significantly better for LEN (73.3%) than that for TACE (33.3%) (p < 0.01). Upfront LEN followed by TACE was also associated with better preservation of liver function than TACE alone, suggesting that LEN preceded by TACE is an efficient treatment option for up-to-seven criteria OUT patients who are not suitable for TACE [15]. Subsequently, the APPLE consensus statement defined the concept of TACE unsuitability, which includes conditions that are not amenable to TACE such as a high tumor burden [13]. According to the APPLE consensus statement, TACE-unsuitable conditions include the following: (A) conditions in which TACE cannot be expected to be effective, (B) conditions that are prone to TACE refractoriness, and (C) conditions in which TACE is likely to reduce liver function. The Japan Society of Hepatology published a consensus statement [14] following the APPLE consensus definition of TACE-unsuitable conditions. This led to the recommendation that systemic therapy, especially drugs with a high response rate such as LEN, be administered first rather than TACE alone in conditions that are not suitable for TACE [13, 14]. LEN may increase the efficacy of subsequent TACE by normalizing the tumor vasculature; drug delivery is thus improved through the normalization of microvascular density, reduction of interstitial pressure, and reduction of vascular permeability [27, 28]. A recent report showed that compared with sorafenib and control, LEN decreases vascular density, increases the percentage of mature vessels, and increases the number of functional vessels after 4 days in rats [29]. It also improves hypoxic conditions and decreases interstitial pressure, thus increasing the efficacy of subsequent TACE [29, 30].
The TACTICS trial [31–33] and the TACTICS-L trial [16], which were both prospective trials, showed that LEN plus TACE is more effective for maintaining high-quality CR than TACE alone (Fig. 2). Therefore, LEN-TACE sequential therapy is commonly used in patients with TACE unsuitable intermediate-stage HCC. In TACE-unsuitable HCC, although LEN is the main player, TACE plays a key role because LEN alone does not provide high-quality CR. In TACE-suitable up-to-seven criteria IN patients and those with simple nodular types, TACE is the main player, but LEN is the key player. This is because high-quality durable CR cannot be achieved with TACE alone, whereas it is possible with upfront administration of LEN, even for a short period of time [30].
Fig. 2.
A high-quality durable complete response (CR) is obtained with LEN-TACE sequential therapy compared with TACE alone. TACE, transarterial chemoembolization therapy.
LEN-TACE Sequential Therapy: TACTICS-L Trial
The TACTICS-L trial was a phase II prospective multicenter single arm study conducted at 21 Japanese centers (Fig. 1). The trial design was almost identical to that of the TACTICS trial except the drug type (sorafenib vs. LEN) and treatment arms (single arm vs. two randomized arms), and LEN was administered 2–3 weeks prior to TACE followed by additional TACE [16]. Despite few differences in background factors between the TACTICS and TACTICS-L trials, TACTICS-L had an extremely high CR rate of 67.7%, which was maintained at 57.2% at 12 months. Furthermore, 50% of patients in the up-to-seven criteria IN and OUT groups achieved CR, even in the up-to-seven criteria OUT group [16]. CR rates of 54%, 63%, and 100% were achieved in patients with the pathological gross types of simple nodular with extranodular growth, confluent multinodular type, and infiltrative type, respectively, which are generally considered resistant to TACE alone. The CR rates of patients with these pathological gross tumor types were comparable with that of the simple nodule type, in which TACE is considered effective, which was 70%. The PFS was 28 months and OS was not reached. After adjusting the baseline characteristics using the inverse probability weighting method, the efficacy of TACE alone (from the control arm of the prospective TACTICS trial) was compared with that of LEN-TACE (from the prospective TACTICS-L trial). The results showed that the PFS HR of LEN-TACE was better in up-to-seven criteria OUT (HR = 0.49) patients than in up-to-seven criteria IN (HR = 0.70) patients (Fig. 3). The OS HR of LEN-TACE was also better in the up-to-seven criteria OUT group (HR = 0.41) than that in the up-to-seven criteria IN group (HR = 0.89), indicating that the up-to-seven criteria OUT group benefited more from LEN-TACE (Fig. 4). These data support that TACE alone has a limited effect in up-to-seven criteria OUT HCC and confirm that up-to-seven criteria OUT HCC is a TACE-unsuitable patient population, in which LEN-TACE is an extremely effective treatment strategy. LEN-TACE is also effective to a certain extent in the up-to-seven criteria IN patient population [16] (Fig. 5, 6).
Fig. 3.
LEN-TACE sequential therapy (from the TACTICS-L trial) achieves better PFS HR than TACE alone (from the TACTICS trial) in HCC patients with a tumor burden exceeding the up-to-seven criteria after adjusting the baseline characteristics in the two trials using the inverse probability weighting (IPW) method. LEN, lenvatinib; TACE, transarterial chemoembolization; PFS, progression-free survival.
Fig. 4.
LEN-TACE sequential therapy (from the TACTICS-L trial) achieves better OS HR than TACE alone (from the TACTICS trial) in HCC patients with a tumor burden exceeding the up-to-seven criteria based on a comparative analysis after adjusting the baseline characteristics in the two trials using the IPW method. LEN, lenvatinib; TACE, transarterial chemoembolization; OS, overall survival.
Fig. 5.
Novel treatment strategy for intermediate-stage HCC. Upfront systemic therapy followed by locoregional therapy achieves high-quality CR.
Fig. 6.
Treatment strategy for intermediate-stage HCC. LEN-TACE and ABC conversion can achieve cancer-free with drug-free status. SNEG, simple nodular with extranodular growth type; CMN, confluent multinodular type; LEN, lenvatinib; TACE, transarterial chemoembolization; ABC conversion, atezolizumab plus bevacizumab followed by curative conversion therapy.
REPLACEMENT Trial
In the IMbrave150 trial, atezolizumab plus bevacizumab showed favorable results in advanced-stage HCC as well as in intermediate-stage HCC. OS was 17.5 months in advanced-stage HCC and 25.8 months in intermediate-stage HCC, whereas PFS was 6.5 months and 12.6 months in advanced- and intermediate-stage HCC, respectively. ORR was also favorable at 27% in advanced-stage HCC versus 44% in intermediate-stage HCC per RECIST v1.1 [34]. Waterfall plots showed that intermediate-stage HCC tumors shrank in 82% of cases, and the depth of response averaged −42.6% in RECIST ver. 1.1. Waterfall plots per mRECIST showed a decrease in tumor blood flow in 87% of cases, and the depth of response averaged −57.2% [35].
Based on these results, the REPLACEMENT trial was conducted prospectively in an up-to-seven criteria OUT intermediate-stage population at 37 sites in Japan [17] (Fig. 1). This was a phase II multicenter, prospective, single arm study of atezolizumab plus bevacizumab conducted in Japan that included 74 patients with up-to-seven criteria OUT intermediate-stage HCC. The ORR was as high as 46%, and 16% of patients achieved CR according to mRECIST. The rate of progressive disease was low at 7%, which was consistent with the results of the IMbrave150 trial showing an intermediate-stage mRECIST CR rate of 17%, PR rate of 33%, ORR of 50%, and progressive disease rate of 4% [17, 35]. The median PFS was 9.1 months and the median OS was not reached. Propensity score matching analysis showed that atezolizumab plus bevacizumab was associated with a better PFS than TACE alone (7.4 months vs. 5.3 months) (HR = 0.59, p = 0.042). The results of inverse probability weighting with adjusted baseline characteristics confirmed that PFS was better in patients receiving atezolizumab plus bevacizumab than in those treated with TACE alone (8.5 months vs. 4.3 months) (HR = 0.54, p = 0.038), supporting that atezolizumab plus bevacizumab has a better outcome than TACE alone.
ABC Conversion: A Multicenter Proof-of-Concept Study
In the multicenter proof-of-concept study (Fig. 1), 38 (35%) of 110 patients with TACE-unsuitable intermediate-stage HCC who underwent curative conversion (resection, ablation, and super-selective TACE) after atezolizumab plus bevacizumab achieved clinical CR (CR on imaging and all tumor markers negative for AFP, PIVKA-II, and AFP-L3), and 25 (23%) achieved pathological CR, successfully achieving drug-free status [21, 36]. In addition, the patients who underwent curative conversion had very good PFS and OS [21] (Fig. 5, 6). The phase III IMPACT trial was initiated in Japan in July 2023 (Registration No.: jRCTs051230037) to compare atezolizumab plus bevacizumab plus on-demand TACE with atezolizumab plus bevacizumab alone in the intermediate-stage HCC population.
Advanced-Stage HCC
The phase III LAUNCH trial comparing LEN-TACE versus LEN alone in advanced-stage HCC [37] showed that LEN-TACE significantly prolonged OS (HR = 0.45, p < 0.001) and PFS (HR = 0.43, p < 0.001) compared with LEN alone in advanced-stage HCC patients with extrahepatic spread and/or vascular invasion. This study demonstrated that locoregional therapy, which can reliably control intrahepatic lesions, in combination with systemic therapy prolongs OS even in advanced-stage HCC, as intrahepatic lesions are usually a prognostic factor.
Hepatic arterial infusion chemotherapy (HAIC) is also beneficial in advanced-stage HCC, and the phase II LEOPARD trial (Fig. 1) combining LEN plus CDDP reported a high response rate of 64.7%, including a CR rate of 29.4% according to mRECIST [38]. In the phase III SILIUS trial, which evaluated the effect of adding the HAIC with low-dose 5-fluorouracil plus cisplatin to sorafenib in advanced-stage HCC, HAIC combination treatment did not result in OS prolongation [39]. However, a phase III trial conducted in China comparing FORFOX HAIC plus sorafenib with sorafenib alone showed that HAIC plus sorafenib significantly prolonged OS (HR = 0.35, p < 0.01) and PFS (HR = 0.33, p < 0.01) [40].
Regarding immune checkpoint inhibitor (ICI) treatment, Duffy et al. [41] showed that in patients with advanced-stage HCC who undergo RFA or TACE in only one of multiple HCCs, some nodules that do not receive RFA/TACE show a partial response to ICIs (abscopal effect). In addition, although untreated nodules showed little infiltration of CD8+ or CD3+ T-cells before RFA/TACE, these nodules showed abundant CD3+ T-cell and CD8+ T-cell infiltration after RFA/TACE; CD3+ T-cell and CD8+ T-cell infiltration was higher in responsive than in nonresponsive nodules. This suggests that RFA/TACE caused tumor antigen release followed by activation of cancer immunity cycles, resulting in infiltration of CD8+ T-cells into the tumor and inhibition of immunosuppressor cells, which may have improved the response to ICIs [41]. Pathological CR was also reported in HCC patients treated with atezolizumab plus bevacizumab followed by TACE, and evidence of pathological CR was found in the resected specimens [21]. This suggests that locoregional therapy has an immunostimulatory effect and potentiates the effects of concurrent ICI therapy [42]. Sequential/combination treatment with locoregional therapy and immunotherapy is expected to improve the prognosis of advanced-stage HCC in the future. The phase III IMPACT study (Registration No.: jRCTs051230037) was designed to assess the prognostic value of adding on demand TACE to atezolizumab plus bevacizumab in intermediate- and advanced-stage HCC.
Conclusion
Recent data suggest that systemic therapy, especially immunotherapy, combined with locoregional therapy (Fig. 7) is beneficial and contributes to prolonging OS not only in advanced-stage HCC but also in intermediate- and early-stage HCCs. Early- and intermediate-stage HCCs have traditionally been treated with locoregional therapy alone. However, the addition of systemic therapy to locoregional therapy is expected to prolong PFS and OS.
Fig. 7.
Potential Combination Immunotherapy combined with Locoregional Therapy.
Systemic therapy has become an effective treatment for all stages of HCC (Fig. 8). In that sense, the key statement for the future treatment of all stages of HCC is “combination/sequential therapy of locoregional and systemic therapies.”
Fig. 8.
New treatment paradigm. Patients in all stages of HCC benefit from systemic therapy combined with locoregional therapy.
Conflict of Interest Statement
Lecture: Eli Lilly, Bayer, Eisai, Chugai, Takeda, AstraZeneca; Grants: Taiho, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, GE Healthcare; Advisory Consulting: Chugai, Roche, AstraZeneca, Eisai. Masatoshi Kudo is the Editor-in-Chief of Liver Cancer.
Funding Sources
There is no funding for this editorial.
Author Contributions
Masatoshi Kudo conceived, wrote, and approved the final manuscript.
Funding Statement
There is no funding for this editorial.
References
- 1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 7. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 21st nationwide follow-up survey of primary liver cancer in Japan (2010-2011). Hepatol Res. 2021;51(4):355–405. 10.1111/hepr.13612. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012-2013). Hepatol Res. 2022;52(1):5–66. 10.1111/hepr.13675. [DOI] [PubMed] [Google Scholar]
- 10. Iijima H, Kudo M, Kubo S, Kurosaki M, Sakamoto M, Shiina S, et al. Report of the 23rd Nationwide Follow-Up Survey of Primary Liver Cancer in Japan (2014-2015). Hepatol Res. 2023(Epub ahead of print). doi: 101111/hepr13953. 10.1111/hepr13953. [DOI] [PubMed] [Google Scholar]
- 11. Liver Cancer Study Group of Japan . Report of the 24th Nationwide follow-up survey of primary liver cancer in Japan (2016-2017) Osaka; 2022. (in Japanese). [Google Scholar]
- 12. Kudo M. Surveillance, diagnosis, and treatment outcome of hepatocellular carcinoma in Japan: 2023 update. Liver cancer. 2023;12(2):95–102. 10.1159/000530079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver cancer. 2020;9(3):245–60. 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver cancer. 2021;10(3):181–223. 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh A liver function: a proof-of-concept study. Cancers. 2019;11(8):1084. 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kudo M, Ueshima K, Saeki I, Ishikawa T, Inaba Y, Morimoto N, et al. A phase 2, prospective, multicenter, single-arm trial of transarterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable intermediate-stage hepatocellular carcinoma: TACTICS-L trial. Liver Cancer. 2023. (Epub ahead of print). https://doiorg/101159/000531377. [DOI] [PMC free article] [PubMed]
- 17. Kudo M, Ueshima K, Tsuchiya K, Kato N, Yamashita T, Shimose S, et al. REPLACEMENT Study: primary analysis of a phase II study of atezolizumab plus bevacizumab for TACE-unsuitable patients with tumor burden beyond up-to-seven criteria in intermediate-stage hepatocellular carcinoma. ASCO Annual Meeting. 2023. Abstr #4125. [Google Scholar]
- 18. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver cancer. 2022;11(5):399–406. 10.1159/000526163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudo M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int J Clin Oncol. 2022;27(7):1110–9. 10.1007/s10147-022-02166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudo M. A Novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion. Liver cancer. 2021;10(6):539–44. 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer. 2023. (Epub ahead of print). https://doiorg/101159/000529574. [DOI] [PMC free article] [PubMed]
- 22. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023. (in press). [DOI] [PubMed] [Google Scholar]
- 23. Kudo M. Adjuvant atezolizumabbevacizumab after resection or ablation for hepatocellular carcinoma. Liver Cancer. 2023. https://doiorg/101159/000531225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kudo M, Ueshima K, Nakahira S, Nishida N, Ida H, Minami Y, et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): a phase 2 prospective multicenter single-arm trial and exploratory biomarker anlysis 2021. ASCO Annual Meeting. 2021. Abstr #4070. [Google Scholar]
- 25. Kudo M, Ueshima K, Nakahira S, Nishida N, Ida H, Minami Y, et al. Final results of adjuvant nivolumab for Hepatocellular Carcinoma (HCC) after Surgical Resection (SR) or Radiofrequency Ablation (RFA) (NIVOLVE): a phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. ASCO-GI. 2022. [Google Scholar]
- 26. Kudo M. Adjuvant immunotherapy after curative treatment for hepatocellular carcinoma. Liver cancer. 2021;10(5):399–403. 10.1159/000518584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 28. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 29. Une N, Takano-Kasuya M, Kitamura N, Ohta M, Inose T, Kato C, et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;38(6):60. 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 30. Kudo M. A New treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver cancer. 2019;8(5):299–311. 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of Transarterial Chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–501. 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Final results of TACTICS: a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver cancer. 2022;11(4):354–67. 10.1159/000522547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kudo M. Implications of the TACTICS trial: establishing the New concept of combination/sequential systemic therapy and transarterial chemoembolization to achieve synergistic effects. Liver cancer. 2022;11(6):487–96. 10.1159/000527404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 35. Kudo M, Finn RS, Galle PR, Zhu AX, Ducreux M, Cheng A-L, et al. IMbrave150: efficacy and safety of atezolizumab plus bevacizumab versus sorafenib in patients with barcelona clinic liver cancer stage B unresectable hepatocellular carcinoma: an exploratory analysis of the phase III study. Liver Cancer. 2023. Epub ahead of print. https://doiorg/101159/000528272. [DOI] [PMC free article] [PubMed]
- 36. Kudo M. Drug-off criteria in patients with hepatocellular carcinoma who achieved clinical complete response after combination treatment with immunotherapy and locoregional therapy. Liver Cancer. 2023. Epub ahead of print. 10.1159/000532023. [DOI] [PMC free article] [PubMed]
- 37. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–27. 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 38. Ikeda M, Yamashita T, Ogasawara S, Kudo M, Inaba Y, Morimoto M, et al. Multicenter phase II trial of lenvatinib plus hepatic intra-arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma: LEOPARD. Liver Cancer. 2023. Epub ahead of print. doi: 101159/000531820. 10.1159/000531820. [DOI] [PMC free article] [PubMed]
- 39. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–32. 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 40. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–60. 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–51. 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh P, Toom S, Avula A, Kumar V, Rahma OE. The immune modulation effect of locoregional therapies and its potential synergy with immunotherapy in hepatocellular carcinoma. J Hepatocell Carcinoma. 2020;7:11–7. 10.2147/JHC.S187121. [DOI] [PMC free article] [PubMed] [Google Scholar]