Abstract
Introduction
Atezolizumab plus bevacizumab therapy is extremely effective in the treatment of intermediate-stage hepatocellular carcinoma (HCC), with a response rate of 44%, as reported in the IMbrave150 trial. When tumor shrinkage is obtained, achieving complete response (CR) is possible in many cases using curative conversion with resection, ablation, or superselective transarterial chemoembolization (TACE) with curative intent. This concept, i.e., curative conversion by combining systemic therapy and locoregional therapy, has not been reported before. This multicenter proof-of-concept study was conducted to show the value of curative conversion in immunotherapy-treated intermediate-stage HCC meeting TACE-unsuitable criteria.
Methods
This study included 110 consecutive Child-Pugh A patients who received atezolizumab plus bevacizumab as first-line treatment for unresectable and TACE-unsuitable intermediate-stage HCC at seven centers in Japan. CR rate, drug-free rate, time to CR, change in liver function, efficacy in positron emission tomography (PET)-positive HCC, progression-free survival (PFS), and overall survival (OS) were assessed in patients who achieved CR using resection, ablation, superselective TACE with curative intent following atezolizumab plus bevacizumab or atezolizumab plus bevacizumab alone.
Results
Clinical or pathological CR was achieved in 38 patients (35%) (median observation period: 21.2 months). The modalities of curative conversion in 35 patients were as follows: resection, 7; ablation, 13; and superselective TACE, 15. Three patients achieved clinical CR with atezolizumab plus bevacizumab therapy alone. Among the 38 CR patients, 25 achieved drug-free status. PFS was not reached, and 3 patients experienced recurrence after reaching CR. Regarding OS, there were no deaths in any of the CR patients. The albumin-bilirubin score did not deteriorate after locoregional therapy or resection. Of seven PET-positive patients who achieved CR with atezolizumab plus bevacizumab followed by curative conversion, five achieved drug-free status.
Conclusion
The achievement of CR rate by curative conversion in patients treated with atezolizumab plus bevacizumab as the preceding therapy for unresectable and TACE-unsuitable intermediate-stage HCC was 35%. Overall, 23% of patients achieved drug-free status and no recurrence was observed from this patient subgroup with CR and drug-free status. Thus, achieving CR and/or drug-free status should be a therapeutic goal for patients with intermediate-stage HCC without vascular invasion or extrahepatic spread.
Keywords: Hepatocellular carcinoma, Atezolizumab plus bevacizumab, Transarterial chemoembolization, Curative conversion, Cancer-free, Treatment-free, Resection, Ablation
Introduction
The approval of sorafenib in 2007 was the result of the successful Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol (SHARP) trial [1] and Asia-Pacific trial [2]; since then, systemic therapy for hepatocellular carcinoma (HCC) includes lenvatinib [3] and atezolizumab plus bevacizumab (Atezo/Bev) [4] in addition to sorafenib as the first-line treatment, and regorafenib [5], ramucirumab [6], and cabozantinib [7] as the second-line treatment. This accounts for a total of six regimens with seven globally approved drugs. Among these regimens, Atezo/Bev, a combination immunotherapy, has shown overwhelming superiority regarding overall survival (OS) and progression-free survival (PFS) over sorafenib, as revealed in the interim analysis of the global Phase III IMbrave150 trial, and is widely used worldwide as the first-line treatment regimen [8]. An updated analysis of IMbrave150 also showed favorable outcomes that surpassed those of sorafenib, with an OS of 19.2 months (hazard ratio [HR], 0.66) and a PFS of 6.2 months (HR, 0.64) [9]. In intermediate-stage and advanced-stage HCC, OS was 25.8 and 17.5 months and PFS was 17.6 and 6.5 months, respectively, which are favorable results. The objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 was 44% in intermediate-stage and 27% in advanced-stage HCC, indicating that the ORR (tumor shrinkage effect) was favorable for intermediate-stage HCC [9]. One in 2 patients achieved partial response (PR) or higher. In addition, in patients receiving Atezo/Bev 28% per RECIST 1.1 and 49% per mRECIST achieved >50% best decrease in sum of longest diameter from baseline[10]. Thus, when tumor shrinkage is obtained, curative conversion, including resection or radiofrequency ablation (RFA)/microwave ablation (MWA), is possible because intermediate-stage HCCs are locally advanced cancers without vascular invasion or extrahepatic spread [11]. In addition, curative conversion is sometimes possible by superselective transarterial chemoembolization (TACE) with curative intent [12, 13] including induction treatment with lenvatinib followed by TACE, i.e., LEN-TACE sequential therapy [14, 15].
Intermediate-stage HCCs are extremely heterogeneous tumors [16, 17]. The Asia-Pacific Primary Liver Cancer Expert (APPLE) consensus [18] and Japan Society of Hepatology (JSH) consensus [19] statements proposed that intermediate-stage HCC could be divided into two groups: TACE-suitable and TACE-unsuitable. According to these consensus statements, the TACE-unsuitable group is defined as following 3 subgroups: (1) TACE-resistant tumors (confluent multinodular type, simple nodular with extra growth type, diffuse or infiltrative type, massive type, or poorly differentiated HCC), (2) patient population prone to failure using TACE (such as tumor conditions exceeding the up-to-seven criteria); and (3) conditions in which liver function is likely to be deteriorated to Child-Pugh B due to TACE (tumors exceeding up-to-seven criteria, especially bilobar multifocal disease, or modified albumin-bilirubin (ALBI) grade 2b). These two consensus statements recommend upfront systemic therapy followed by TACE for TACE-unsuitable patient populations [18, 19]. Recently, following the proposals of the APPLE and JSH consensus statements, global guidelines, including the American Association for the Study of Liver Disease (AASLD) [20], European Society for Medical Oncology (ESMO) [21], and Barcelona Clinic Liver Cancer (BCLC) [22] guidelines, have also recommended prioritizing upfront systemic therapy in TACE-unsuitable intermediate-stage HCC. The first-line treatment for systemic therapy is Atezo/Bev.
For TACE-unsuitable HCC, LEN-TACE sequential therapy is extremely effective [14, 15, 23–27] and is currently the standard of care in Asian countries. Furthermore, in the TACTICS-L trial presented at ASCO-GI 2022, LEN-TACE sequential therapy resulted in a complete response (CR) rate of >50%, even in cases exceeding the up-to-seven criteria [28], making it a promising treatment strategy. Duration of response was very long, lasting >1 year in >50% of cases. In fact, in the proof-of-concept study LEN-TACE achieved cancer-free and drug-free status in 16.7% [15].
The results of LEN-TACE sequential therapy demonstrated that the combination or sequential use of systemic therapy and locoregional therapy can improve the curability rate in HCC. Inpatients receiving Atezo/Bev therapy, resection, and ablation in addition to superselective TACE with curative intent should be possible because of their tumor shrinkage effects [9, 10]. Therefore, CR could be achieved by performing curative conversion during or after Atezo/Bev therapy (Atezo/Bev followed by curative conversion; ABC conversion) [29, 30]. The aim of this multicenter proof-of-concept study was to demonstrate the value of ABC conversion therapy for improving achievement rate of CR with or without drug-free status using locoregional therapy, including resection, ablation, and superselective TACE with curative intent [12–15], during or after Atezo/Bev therapy.
Materials and Methods
This observational multicenter cohort study included unresectable and TACE-unsuitable intermediate-stage HCC patients with Child-Pugh A liver function who received Atezo/Bev as first-line treatment at seven institutes in Japan. To eliminate selection bias, the subjects were consecutive patients for whom Atezo/Bev therapy was introduced as first-line treatment for unresectable and TACE-unsuitable intermediate-stage HCC at each institute. There were 110 Child-Pugh A, unresectable, and TACE-unsuitable consecutive HCC patients who received Atezo/Bev therapy as first-line treatment between May 2018 and April 2022 at these seven institutes (including three patients who were enrolled in the Phase III IMbrave150 trial and underwent curative conversion after the trial). The definition of “TACE-unsuitable” was based on the APPLE [18] and JSH consensus [19] statements.
The criteria defining “Clinical CR” were as follows: (1) CR on computed tomography or magnetic resonance imaging according to modified RECIST, and (2) normalization of the levels of three tumor markers (alpha-fetoprotein [AFP], protein induced by vitamin K absence or antagonists-II [PIVKA-II], and lens culinaris agglutinin-reactive AFP fraction [AFP-L3]) below the standard value for ≥6 weeks when any of three tumor markers is elevated. The “drug-off” criteria were as follows: patients who could be curatively resected or patients who received curative locoregional therapy and fulfilled the following three criteria: (1) patients who achieved CR according to modified RECIST by superselective TACE with curative intent [12, 13] or RFA/MWA, (2) maintaining normalized three tumor markers ≥24 weeks and (3) complete disappearance of intratumoral arterial flow on contrast-enhanced ultrasound (CEUS), which is the most sensitive technique to detect intratumoral arterial blood flow, much more sensitive than dynamic computed tomography or magnetic resonance imaging [31–45].
The curative conversion modalities used were resection, ablation, superselective TACE with curative intent, or a combination of these therapies. “Superselective TACE” was defined as performing TACE from subsegmental feeding artery or more peripheral artery since “selective TACE” is defined as TACE from segmental artery [46]. Patients who underwent curative resection did not receive subsequent Atezo/Bev therapy; however, those who underwent ablation or superselective TACE with curative intent received an additional Atezo/Bev therapy course of at least 4–6 cycles even if tumor marker levels were normalized. The purpose of this was to activate tumor-specific immune responses mediated by the subsequent release of tumor antigens (ABC-TACE sandwich therapy) [47–55]. Atezo/Bev therapy is still ongoing in patients who had not achieved any of the three above-mentioned “drug-off” criteria at the time of submission of this manuscript.
The rates of achievement of clinical CR and drug-free status of the 110 patients were calculated. The efficacy of ABC conversion, time to CR, PFS, OS, change in ALBI score, time to CR in positron emission tomography (PET)-positive HCCs, and changes in AFP levels after CR on imaging were also assessed. PFS and OS in patients who did not receive ABC conversion therapy were also analyzed.
Time to CR, RFS, and OS was estimated by the Kaplan-Meier method. Primary and secondary endpoints of this study are clinical CR and drug-free rates, respectively. This multicenter study was approved by the Ethics Committee of Kindai University Hospital.
Results
Patient Characteristics
Baseline characteristics in all 110 patients are summarized in Table 1. In addition, baseline characteristics in patients who achieved clinical CR (n = 38) and did not achieve clinical CR (n = 72) are shown. There were no significant differences in baseline characteristics between patients who achieved CR and those who did not achieve CR except tumor number (Table 1). Inpatients who did not achieve clinical CR had more numbers of tumor >7.
Table 1.
Patient baseline characteristics
| Factors | Received curative conversion and achieved clinical CR (n = 38) | Did not receive curative conversion or did not achieve clinical CR (n = 72) | p value |
|---|---|---|---|
| Age | |||
| Years old, median (IQR) | 77.0 (71.8, 81.0) | 75.6 (69.3, 81.0) | 0.379 |
| Sex | |||
| Male/female | 32/6 | 50/22 | 0.125 |
| ECOG PS | |||
| 0/1 | 27/11 | 60/12 | 0.105 |
| BMI | |||
| kg/m2, median (IQR) | 25.2 (22.5, 27.8) | 23.7 (22.3, 26.5) | 0.160 |
| Etiology | |||
| HBV/HCV/AL/NAFL/other | 3/15/9/9/2 | 13/26/13/16/4 | 0.172 |
| BCLC stage | |||
| A/B up-to-7 IN/B OUT | 4/9/25 | 2/18/52 | 0.230 |
| Tumor size | |||
| cm, median (IQR) | 4.0 (2.6, 5.1) | 3.8 (2.4, 6.0) | 0.841 |
| Tumor number | |||
| 1/2-3/4-6/7- | 8/16/10/4* | 8/19/18/27* | 0.018 |
| ALBI score | |||
| Median (IQR) | −2.76 (−2.93, −2.38) | −2.65 (−2.87, −2.23) | 0.206 |
| NLR | |||
| Median (IQR) | 2.15 (1.36, 3.25) | 2.74 (1.81, 3.73) | 0.145 |
| PLT | |||
| Median (IQR) | 14.1 (12.8, 18.3) | 14.5 (10.9, 18.0) | 0.624 |
| PT-INR | |||
| Median (IQR) | 1.07 (1.00, 1.15) | 1.1 (0.99, 1.13) | 0.624 |
| ALB | |||
| g/dL, median (IQR) | 4.1 (3.7, 4.3) | 4.0 (3.6, 4.2) | 0.377 |
| T-bil | |||
| mg/dL, median (IQR) | 0.7 (0.5, 0.9) | 0.8 (0.6, 1.1) | 0.354 |
| CRP | |||
| mg/dL, median (IQR) | 0.19 (0.11, 0.42) | 0.19 (0.09, 0.39) | 0.941 |
| ALT | |||
| U/L, median (IQR) | 31.5 (18.8, 49.3) | 32.5 (25, 42.5) | 0.624 |
| AFP | |||
| ng/mL, median (IQR) | 11.0 (3.5, 209) | 75 (7.3, 764) | 0.057 |
| DCP | |||
| mAU/mL, median (IQR) | 382 (40.5, 2026) | 319 (74.9, 2197) | 0.901 |
ALBI; albumin‐bilirubin, NLR; neutrophil to lymphocyte ratio, ALT; alanine aminotransferase, AFP; alpha-fetoprotein, DCP; des-gamma‐carboxy prothrombin.
Achieving CR and Drug-Free Rates
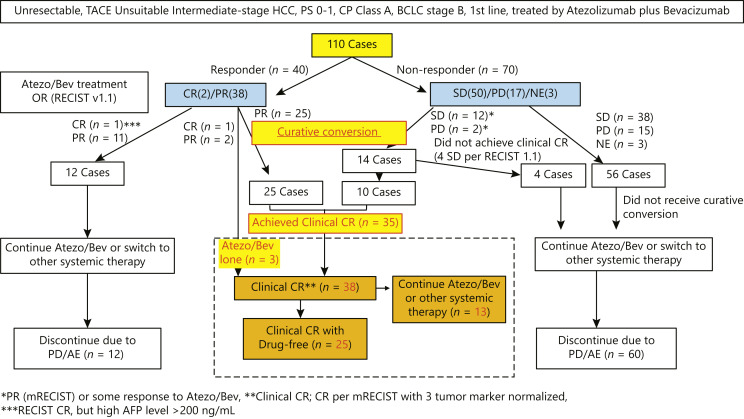
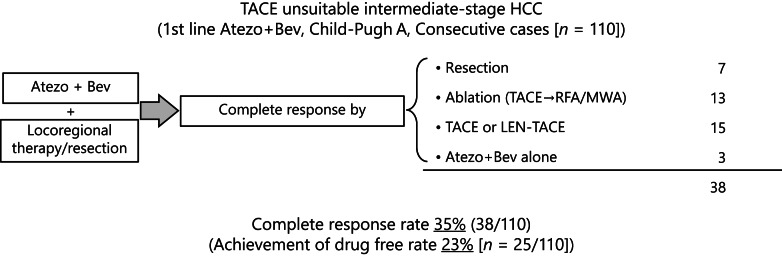
Of 110 patients, 2 patients achieved CR, 38 achieved PR, 50 achieved stable disease (SD), 17 achieved progressive disease (PD) per RECIST v1.1 by Atezo/Bev therapy. Response was not evaluable in 3 patients. ORR by Atezo/Bev was 36.4% (40/110), and disease control rate was 81.8% (Fig. 1.). Curative conversion was performed in 25 patients with PR, 12 patients with SD, and 2 patients with PD. Three patients (1 CR and 2 PR patients) achieved clinical CR with Atezo/Bev alone. As a result, clinical CR was obtained with or without curative conversion in 38 of the 110 Child-Pugh A, TACE-unsuitable intermediate-stage HCC patients in whom Atezo/Bev therapy was introduced as first-line treatment. Four patients with SD did not achieve clinical CR even after curative conversion therapy and excluded from the “clinical CR” group (Fig. 1.). As a result, 8 SD patients and 2 PD patients achieved clinical CR. Thus, successful curative conversion rate was 89.7% (35/39) (Fig. 1). One CR patient and 11 PR patients did not receive curative conversion therapy due to the physicians’ discretion since curative conversion is not a standard of care and continued Atezo/Bev treatment until PD or severe adverse events, i.e., those patients never received locoregional therapy (Fig. 1). The OR per RECIST ver. 1.1 of Atezo/Bev therapy in patients who achieved clinical CR before curative conversion was as follows: CR, 1 case; PR, 27; SD, 8; and PD, 2 (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000529574; Fig. 1). Achieving clinical CR rate by curative conversion among 110 patients was 35% (95% confidence interval [CI], 26–44%) (online suppl. Table 1; Fig. 1–3). The median observation period was 21.2 months (range: 18.8–23.6 months). The time to CR was 7.1 months [95% CI, 5.4–8.8] (online suppl. Fig. 1). At present, 25 (23% [95% CI, 15–32]) of the 110 patients have achieved drug-free status without recurrence because these 25 cases met the “drug-off” criteria.
Fig. 1.
ABC conversion: Patient flow. Atezolizumab followed by curative conversion was performed in 39 patients. Of them, 35 patients achieved clinical complete response (CR) defined by CR per mRECIST and normalized 3 tumor markers ≥6 weeks. Clinical CR with drug-free status was achieved 25 of 38 patients.
Fig. 2.
Achievement rate of complete response in intermediate-stage HCC. Among 110 Child-Pugh A transarterial chemoembolization (TACE)-unsuitable intermediate-stage HCC patients who received Atezo/Bev therapy as first-line treatment, 38 (35%) achieved complete response by curative conversion. Among the 38 patients, 25 (25/110, 23%) are currently drug-free status. ABC, Atezo/Bev therapy followed by curative conversion; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; MWA, microwave ablation.
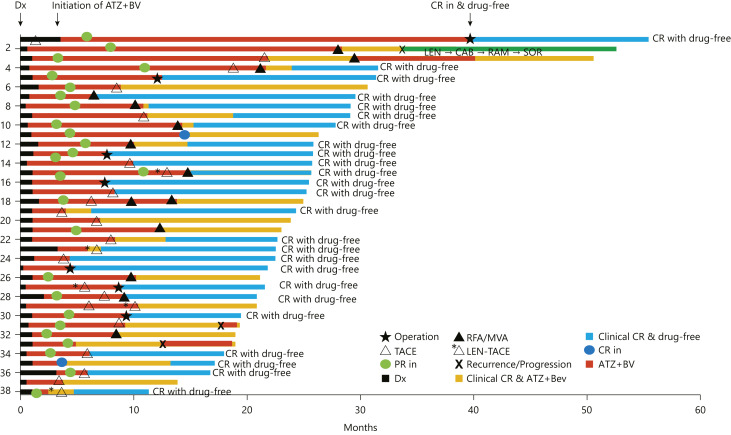
Fig. 3.
Swimmer plot of 38 patients who achieved complete response with or without drug-free status. Complete response was achieved with resection, superselective TACE with curative intent, RFA, and MWA, and 25 cases reached drug-free status (blue color). RFA, radiofrequency ablation; MWA, microwave ablation; LEN-TACE, lenvatinib-transarterial chemoembolization; Dx, diagnosis; ATZ+BV, atezolizumab plus bevacizumab.
There are 13 patients who achieved clinical CR after ablation or superselective TACE with curative intent +/− ablation who are receiving ongoing Atezo/Bev therapy. AFP level normalization was observed in all cases; however, AFP levels increased again in 2 patients who developed recurrence after achieving clinical CR (online suppl. Fig. 2). Additional one case showed recurrence during the longer follow-up period without AFP elevation. The modalities of curative conversion were as follows: resection, 7 cases; ablation (including 5 patients who underwent RFA or MWA after TACE or LEN-TACE), 13; and superselective TACE with curative intent (including 4 patients who underwent LEN-TACE sequential therapy), 15. Three patients became clinical CR with Atezo/Bev therapy alone [modified RECIST CR plus normal tumor marker levels, i.e., clinical CR]. These three cases are still receiving Atezo/Bev therapy with considering some intervention by locoregional therapy or resection (online suppl. Table 1; Fig. 2, 3). Overall, 13 patients did not meet the “drug-off” criteria so far and therefore continued Atezo/Bev treatment. The median cycles/duration of additional Atezo/Bev therapy after curative conversion was 6 cycles (18 weeks). In 25 patients who achieved drug-free status, 7 received resection, 8 received ablation, 10 received superselective TACE or LEN-TACE with curative intent.
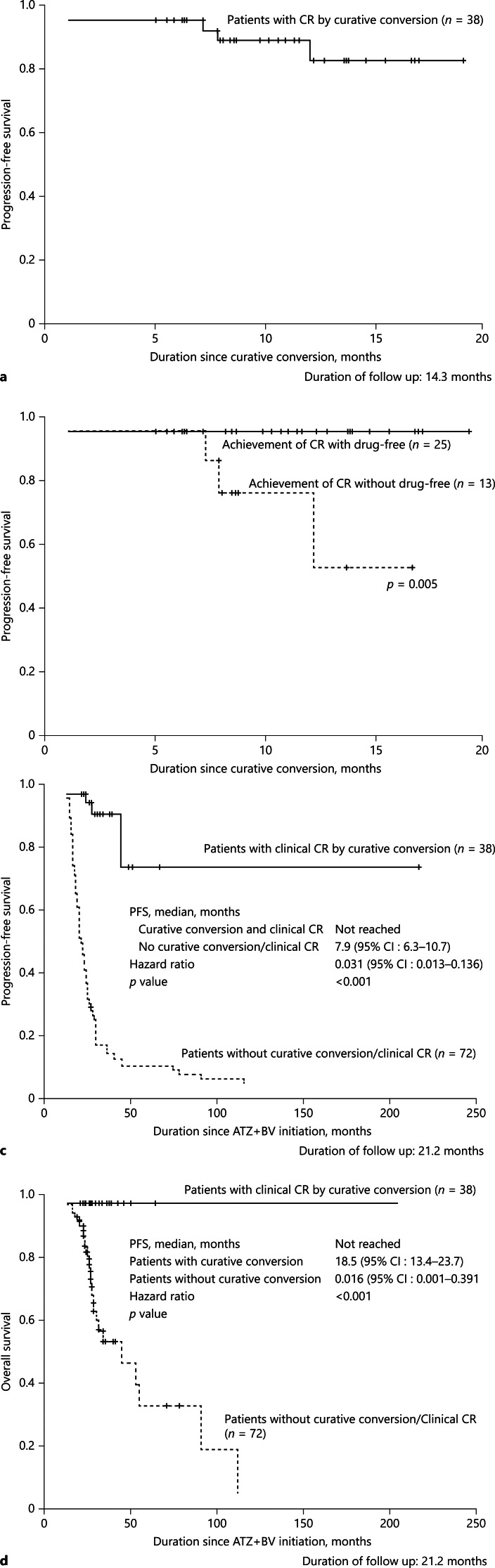
PFS (CR Maintenance Rate)
Recurrence was observed in 3 patients who achieved clinical CR (Fig. 3, 4a). Although the clinical CR criteria were temporarily met in these 3 patients, recurrence occurred at sites other than the treated site by TACE in 2 patients who received TACE alone. New lesion occurred in 1 patient who achieved Atezo/Bev alone. The median PFS in patients with clinical CR was not met (Fig. 4a,b). There was no recurrence in patients who achieved CR with drug-free status (n = 25), whereas recurrence was detected in patients who could not achieve drug-free status because “drug-off” criteria were not met (Fig. 4b). PFS in patients who achieved clinical CR was not met, whereas those who did not receive curative conversion or did not achieve clinical CR were 7.9 months (95% CI, 6.3–10.7) (Fig. 4c) since Atezo/Bev initiation. There was no significant difference in baseline patient characteristics between those 2 groups except body mass index (Table 2). Recurrence was found in clinical CR patients treated with Atezo/Bev alone (n = 1) and superselective TACE alone (n = 2). No recurrence was observed in 25 patients who received resection, ablation, and LEN-TACE sequential therapy during the median follow-up period of 21.2 months (Fig. 4c).
Fig. 4.
PFS (CR maintenance rate). a Median PFS of the 38 cases that achieved clinical CR was not reached. b There was no recurrence from the patients with CR and drug-free status. There were 3 recurrences, who received TACE alone (n = 2) or Atezo/Bev alone (n = 1). c Median PFS since atezolizumab plus bevacizumab initiation. Median PFS in patients who achieved clinical CR by curative conversion was much better than those who did not receive curative conversion or did not achieve CR (HR 0.031, p < 0.001). d Median OS since atezolizumab plus bevacizumab initiation. Median OS in patients who did not receive curative conversion or did not achieve clinical CR was 18.5 months (95% CI, 13.4–23.7). There was no death who achieved clinical CR by curative conversion. CR, complete response; ABC, atezolizumab plus bevacizumab followed by curative conversion; OS, overall survival; PFS, progression-free survival.
Table 2.
Patient baseline characteristics
| Factors | Achieved drug-free status (n = 25) | Not achieved drug-free status (n = 13) | p value |
|---|---|---|---|
| Age | |||
| Years old, median (IQR) | 75.0 (71.0, 79.0) | 78.0 (74.5, 81.0) | 0.247 |
| Sex | |||
| Male/female | 21/4 | 10/3 | 0.084 |
| PS | |||
| 0/1 | 20/5 | 7/6 | 0.092 |
| BMI | |||
| kg/m2, median (IQR) | 23.6 (21.4, 26.9) | 27.4 (25.2, 30.0) | 0.040 |
| Etiology | |||
| HBV/HCV/AL/NAFL/other | 1/10/5/7/2 | 1/6/4/2/0 | 0.645 |
| BCLC stage | |||
| A/B up-to-7 IN/B OUT | 4/20/1 | 2/10/1 | 0.890 |
| Tumor size | |||
| cm, median (IQR) | 4.3 (3.3, 5.2) | 3.8 (1.8, 5.7) | 1.000 |
| Tumor number | |||
| 1/2-3/4-6/7- | 6/12/4/3 | 2/4/6/1 | 0.260 |
| ALBI score | |||
| Median (IQR) | −2.79 (−2.99, −2.56) | −2.45 (−2.85, −2.22) | 0.171 |
| NLR | |||
| Median (IQR) | 1.87 (1.26, 3.18) | 2.20 (1.89, 3.94) | 0.463 |
| PLT | |||
| Median (IQR) | 14.0 (12.8, 21.1) | 14.6 (12.6, 15.2) | 1.000 |
| PT-INR | |||
| Median (IQR) | 1.04 (0.99, 1.16) | 1.09 (1.06, 1.12) | 0.143 |
| ALB | |||
| g/dL, median (IQR) | 4.1 (3.7, 4.3) | 3.8 (3.7, 4.2) | 0.875 |
| T-bil | |||
| mg/dL, median (IQR) | 0.7 (0.5, 1.0) | 0.6 (0.5, 0.8) | 0.730 |
| CRP | |||
| mg/dL, median (IQR) | 0.20 (0.11, 0.36) | 0.17 (0.08, 0.80) | 1.000 |
| ALT | |||
| U/L, median (IQR) | 31.0 (19.0, 50.0) | 32.0 (18.0, 40.0) | 1.000 |
| AFP | |||
| ng/mL, median (IQR) | 18.0 (2.7, 207) | 11.0 (5.15, 208.8) | 0.904 |
| DCP | |||
| mAU/mL, median (IQR) | 728 (43.3, 3933) | 259 (34.4, 1326) | 0.570 |
NLR; neutrophil to lymphocyte ratio, PLT; platelet, PT; prothrombin time, AFP; alpha‐fetoprotein, DCP; des-gamma-carboxy prothrombin.
Overall Survival
There were no deaths in patients who achieved clinical CR during the median follow-up period of 21.2 months (Fig. 4d). However, median OS was 18.5 months (95% CI, 13.4–23.7) in 72 patients who did not receive curative conversion therapy or did not achieve clinical CR (Fig. 4d).
Change in ALBI Score
There was no clear decline in the median ALBI scores during treatment in the 38 patients who achieved clinical CR. Normally, there was no deterioration in liver function due to locoregional therapy, including superselective TACE with curative intent, RFA, or resection, in patients undergoing curative conversion (online suppl. Fig. 3). Pathological CR was achieved in three of the seven resected patients.
Pathological Findings of Resected Specimens
Of the 7 patients who underwent curative resection following Atezo/Bev therapy, two underwent TACE (one was PET-positive and underwent LEN-TACE) before resection. The two patients who underwent TACE before resection received at least six cycles of Atezo/Bev therapy before resection. Microscopic pathological examination did not detect viable cancer cells, and pathological CR was confirmed in both cases (Fig. 5). In these 2 patients, three tumor markers (AFP, AFP-L3 fraction, and PIVKA-II) became negative, and avascular state was confirmed using CEUS before resection. The other patient achieved pathological CR with six cycles of Atezo/Bev therapy alone.
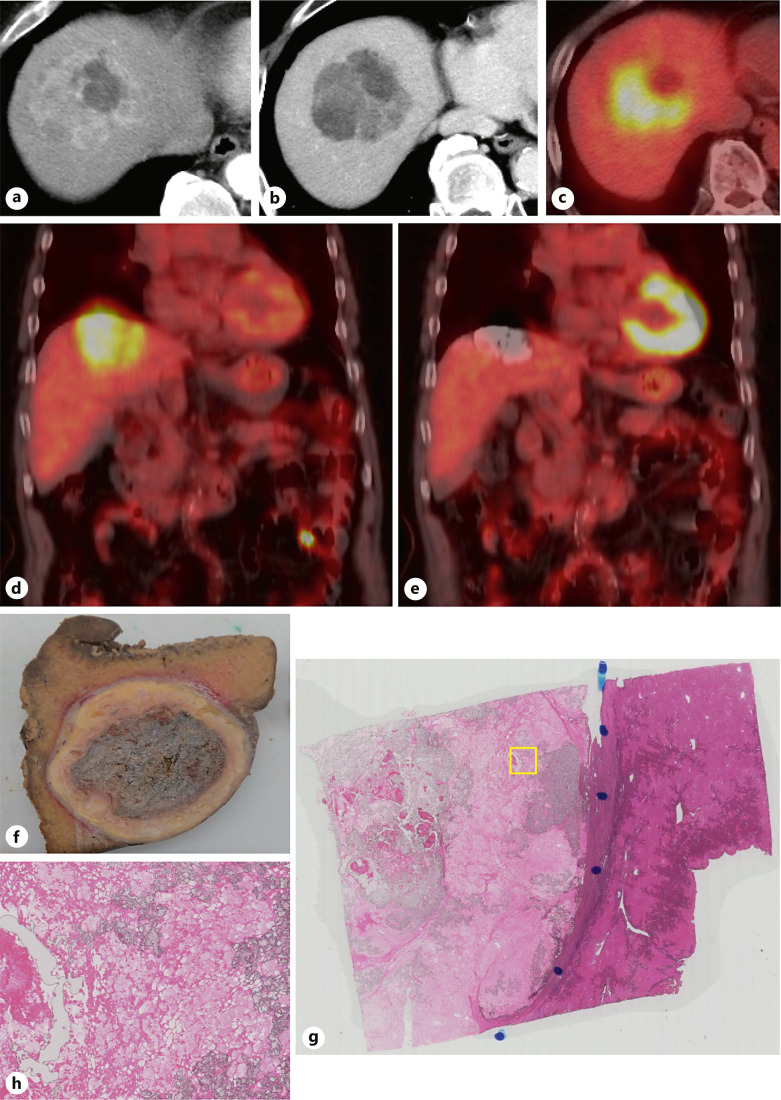
Fig. 5.
Case of a patient with PET-positive HCC who underwent ABC conversion. a A solitary HCC measuring 12 cm in size was observed in segment 8. Heterogeneous staining is shown in the arterial phase of dynamic CT. A necrotic area was observed in the tumor. b Heterogeneous morphology, suggesting confluent multinodular gross pathological type, is also observed in the equilibrium phase of dynamic CT. A necrotic portion was also observed in the tumor. c FDG-PET showed an extremely strong accumulation of FDG except in the necrotic area. d Coronal image of FDG-PET before Atezo/Bev therapy. e Coronal image of FDG-PET-CT 6 months after six cycles of Atezo/Bev therapy followed by LEN-TACE sequential therapy. Disappearance of FDG accumulation and tumor shrinkage is observed. f Laparoscopic subsegmentectomy was performed. A microscopic image of the resected specimen shows complete pathological necrosis. g The tumor was necrotic in a low-magnification view of the resected specimen. h No viable tumor was found in the high-magnification image of the resected specimen. FDG-PET, fluorodeoxyglucose-positron emission tomography; LEN-TACE, lenvatinib-transarterial chemoembolization; HCC, hepatocellular carcinoma; CT, computed tomography.
The seven patients exhibited a clear tumor shrinkage effect, and as mentioned above three of the seven patients achieved pathological CR according to the resected specimens. In the other 4 patients who did not receive TACE before resection, viable tumor cells were detected in the resected specimens. All seven patients achieved drug-free status following resection, and recurrence has not been observed to date even in a patient with PET-positive HCC.
CR and Drug-Free Rates in PET-Positive HCCs
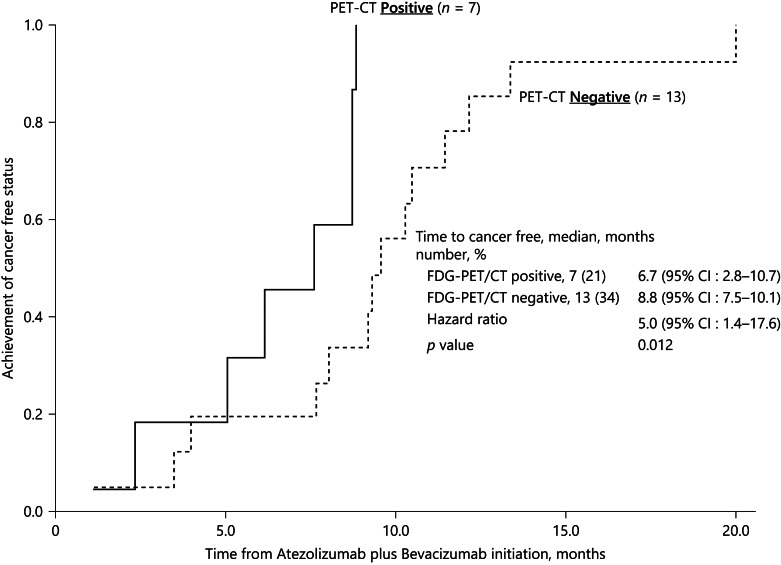
Of 20 patients who underwent PET before Atezo/Bev therapy, 7 showed significant fluorodeoxyglucose accumulation, and 13 showed no abnormal fluorodeoxyglucose accumulation and were PET-negative. The seven PET-positive HCC patients achieved clinical CR by curative conversion and remain in nonrecurrence status. The curative conversion modalities used in the 7 patients were as follows: RFA in two, LEN-TACE plus RFA in one, LEN-TACE followed by resection in one, LEN-TACE in two, and superselective TACE with curative intent in one. Five of seven patients are undergoing follow-up observations in drug-free status, and recurrence has not been observed to date (online suppl. Table 1). The other 2 patients who are still receiving Atezo/Bev therapy remain in clinical CR. The time to clinical CR was as follows: PET-positive HCC, 6.7 months (95% CI, 2.8–10.7), and PET-negative HCC, 8.8 months (95% CI, 7.5–10.1). The time to CR was significantly shorter in PET-positive HCC than in PET-negative HCC patients (HR, 5.0 [95% CI, 1.4–17.6], p = 0.012) (Fig. 6).
Fig. 6.
Time to complete response by FDG-PET positivity. PET-CT was performed in 20 cases. Seven cases were PET-positive HCCs, and 13 were PET-negative HCCs. The time to complete response evaluated by CT/MRI and 3 tumor markers in FDG-PET-positive HCCs was 6.7 months, which was faster than the 8.8 months in PET-negative HCCs (HR = 5.0; 95% CI, 1.4–17.6, p = 0.012). FDG-PET, fluorodeoxyglucose-positron emission tomography.
Reasons for Curative Conversion and Timing
Curative conversion was applied in patients who exhibited tumor shrinkage (RECIST PR) in 25: however, in patients who had SD after at least six treatment cycles (except one case; 3 cycles) (n = 12), in patients who showed slow PD (n = 2), in patients who underwent drug interruption or termination due to adverse event occurrence (n = 1) (4 cases when considering those that overlapped with other reasons), and in patients with PET-positive HCC (n = 7), locoregional therapy was intentionally implemented between Atezo/Bev therapies in order to achieve deep response, resulting in CR. As a result, 35 of 39 patients achieved clinical CR (CR per mRECIST and normalized 3 tumor markers ≥6 weeks).
Adverse Events
No new adverse events were observed during Atezo/Bev therapy and in curative conversion therapy, including resection and ablation or superselective TACE with curative intent, in any of the 38 cases.
Discussion
This study examined the efficacy of curative conversion for achieving high clinical CR- and drug-free rates in intermediate-stage HCC patients who met the criteria for TACE unsuitability. Locoregional therapies, including resection, ablation, and superselective TACE with curative intent, were used during or after Atezo/Bev therapy. The results suggested that this was an excellent treatment strategy for intermediate-stage HCC when considering all patients were TACE-unsuitable and relatively poor prognostic. In these patients, sustained clinical CR or drug-free status usually could not be achieved by locoregional therapy alone in TACE-unsuitable patients [18, 19, 56–58] or Atezo/Bev alone. Actually, in this study 12 responders to Atezo/Bev did not receive curative conversion because of the physicians' discretion since these physicians believed systemic therapy should be continued as long as it is effective. Subsequently, these patients terminated Atezo/Bev due to PD or adverse events and switched to other systemic therapy. PFS and OS were not good in those patients as compared with patients who received curative conversion therapy (Fig. 4c, d).
TACE has been the standard of care for intermediate-stage HCC; however, the evidence for TACE is derived from a meta-analysis of six randomized controlled trials that compared TACE with no treatment in an era when no effective systemic therapy exists [59]. Many effective drugs are currently available, and there is no evidence to determine which is better for OS between locoregional therapies and upfront systemic therapy followed by curative locoregional therapy [11, 29, 30].
The APPLE [18] and JSH consensus [19] statements proposed the concept of TACE unsuitability. They recommend selective TACE after the administration of systemic agents for TACE-resistant tumors, such as confluent multinodular, poorly differentiated, or diffuse type HCCs. These include HCCs with a high tumor burden, such as those exceeding the up-to-seven criteria or those prone to TACE refractoriness [11, 18, 19, 29]. This concept was also included in the updated AASLD, ESMO, and BCLC guidelines [20–22].
In the TACTICS trial, PFS was prolonged by upfront sorafenib followed by its combination with TACE [60]; the clinical benefit was more evident in tumors beyond the up-to-seven criteria than in tumors within the up-to-seven criteria. In addition, time to vascular invasion and time to extrahepatic spread were significantly prolonged in response to the combination with drugs [61]. Furthermore, the proof-of-concept study showed that TACE preceded by lenvatinib therapy (LEN-TACE sequential therapy) extended OS compared with TACE alone [15]. In addition, 16.7% patients who received LEN-TACE achieved cancer-free and drug-free status [15]. This is because upfront lenvatinib normalizes abnormal vessel, microvessel density, vascular permeability, and drug delivery [62–64] resulting in improving TACE efficacy. Many validation studies were subsequently conducted [14, 23–26]; currently, LEN-TACE sequential therapy is the standard of care for TACE-unsuitable HCC in Asian countries, especially in Japan and China [27]. The LAUNCH trial demonstrated that LEN-TACE sequential therapy prolonged PFS and OS more effectively than LEN alone in advanced-stage HCC patients [27]. The results of the TACTICS-L trial, a prospective single-arm multicenter phase II trial, were presented at ASCO-GI 2022 and confirmed the effectiveness of LEN-TACE sequential therapy in intermediate-stage HCC [28].
Atezo/Bev is currently the first choice among first-line HCC treatment agents, given its ability to prolong OS compared with sorafenib in the Phase III IMbrave150 trial [7, 8, 65, 66]. An updated analysis showed that Atezo/Bev therapy prolonged OS and PFS to a greater extent in intermediate-stage HCC than in advanced-stage HCC (25.8 vs. 17.5 months and 12.6 vs. 6.5 months, respectively) [9]. In RECIST ver. 1.1, the ORR was extremely high at 44% for intermediate-stage HCC compared with 27% for advanced-stage HCC, suggesting that unresectable tumors may become resectable, and that unablatable tumors may become ablatable due to the tumor shrinkage effect [10]. Furthermore, the antivascular endothelial growth factor action of bevacizumab may improve drug delivery and increase the efficacy of TACE by normalizing tumor blood vessels, as well as stromal pressure and vascular permeability similar to lenvatinib [62–64]. This may be the reason why initially TACE-unsuitable patients with SD/slow PD to Atezo/Bev responded to superselective TACE after Atezo/Bev or lenvatinib treatment before superselective TACE.
In the present study, of 110 consecutive Child-Pugh A HCC patients who were administered Atezo/Bev as first-line treatment for unresectable and TACE-unsuitable intermediate-stage HCC in seven institutes, 39 underwent curative conversion; 89.7% of them (35/39) achieved clinical CR even in SD or PD patients with Atezo/Bev, and 25 achieved drug-free status. Three patients exhibited recurrence after achieving clinical CR; however, there was no recurrence in patients who achieved CR with drug-free status during the median observation period of 21.2 months. All of 3 cases with recurrence were treated with Atezo/Bev alone (n = 1) or Atezo/Bev followed by superselective TACE (n = 2). CR with drug-free status may be a very good predictor of pathological CR. In that sense, in order to achieve pathological CR, superselective LEN-TACE may be a preferable treatment strategy rather than superselective TACE alone [14, 15], which was shown in resected specimen (Fig. 5). These results suggest that curative conversion during or after Atezo/Bev therapy is an extremely effective treatment strategy for achieving pathological CR.
The therapeutic goal in patients with advanced-stage HCC is prolonging OS, whereas that in patients with intermediate-stage HCC without extrahepatic spread or vascular invasion is achieving CR and drug-free status. Furthermore, curative and powerful therapies, i.e., locoregional therapies including ablation or superselective TACE with curative intent, are available only for the treatment of HCC and not for other solid tumors. Achieving pathological CR with only systemic therapy is extremely difficult [11]. Similarly, it is difficult to achieve pathological CR with locoregional therapy alone in TACE-unsuitable patient population [18, 19, 56–58]. The fact that pathological CR can be obtained by the synergistic effect of combining superselective TACE with curative intent and effective systemic therapy (Fig. 4) [15] suggests that curative conversion during or after Atezo/Bev should be actively and intentionally considered.
Intermediate-stage HCC is potentially a curable disease. Therefore, it is important to abandon the common sense of systemic therapy, which is that when starting systemic therapy, it should be continued as long as it is effective [67]. In other words, considering the high achievement rate of clinical CR and drug-free status, therapeutic strategy to systemic therapy may need to be changed in patients with intermediate-stage HCC. The transition to curative therapy should be considered while the drug is still effective [30]. In fact, patients who achieved objective response to Atezo/Bev per RECIST 1.1, but did not receive curative locoregional therapy, showed poor PFS and OS as compared with those who achieved clinical CR by curative conversion therapy (Fig. 4). Resection was actively performed in this study; pathological evaluation of resected specimens after ABC-TACE sandwich or ABC-LEN-TACE sandwich therapy applied to two cases showed pathological CR (Fig. 5) in all of two cases. In the future, if ABC-TACE sandwich or ABC-LEN-TACE sandwich therapy provides image CR according to mRECIST, negativity for the three tumor markers (AFP, PIVKA-II, and AFP-L3) ≥ 24 weeks, and an avascular state with CEUS, it may be possible to make a clinical diagnosis of pathological CR. In such cases, resection may be avoided in patients with comorbidities or in elderly patients.
Most PET-positive HCCs are poorly differentiated and biologically aggressive [68–73], and recurrence is common even after resection, RFA, selective TACE, or transplantation [68, 74–76]. In addition, they have epithelial-mesenchymal transition type characteristics [77], including keratin 19-positive stem cell-type HCCs [78], and they are frequently associated with vascular invasion [79–84]. Therefore, PET-positive HCCs are generally tumors with a poor prognosis, and the implementation of protocols to improve their prognosis has long been an unmet need. In the present study, among seven PET-positive HCC patients, seven underwent Atezo/Bev therapy followed by RFA (n = 2), superselective TACE with curative intent (n = 1), LEN-TACE (n = 2), LEN-TACE followed by RFA (n = 1), and LEN-TACE followed by resection (n = 1) (Fig. 5) and achieved clinical or pathological CR. Five patients have reached drug-free status with no recurrences observed. The remaining 2 patients have a high possibility of reaching drug-free status and are currently continuing Atezo/Bev therapy. Considering these findings, Atezo/Bev-LEN-TACE sandwich therapy, ABC-TACE sandwich therapy, and combinations of Atezo/Bev with ablation or resection are expected to significantly improve the prognosis of PET-positive HCCs.
Patients who achieved curative conversion after Atezo/Bev therapy showed either of the following five conditions: (1) tumor shrinkage, (2) state of SD or slow PD after 4–6 cycles, even if tumor shrinkage was not obtained, (3) dose interruption or termination was necessary due to an adverse event, (4) TACE-resistant (confluent multinodular type, poorly differentiated HCC, or infiltrative HCC) tumor conditions and (5) PET-positive HCC. In cases in which tumor shrinkage is not achieved even after 4–6 cycles of Atezo/Bev therapy (considering that tumors that responded after four cycles were present in >80% in the Atezo/Bev GO30140 Phase 1b Arm A swimmer plot [85]), it is important to consider curative conversion for SD or slow PD cases if this was the best response after the 4–6 cycles. Achieving clinical CR and drug-free status eliminates the concerns about immune-related adverse events or proteinuria in the future. Even if there is a recurrence, it could be detected at very early stage by intensive follow-up, and curative treatment may be applicable. In patients with SD or slow PD who achieve CR on imaging by superselective TACE with curative intent or RFA, the tumor antigens released by TACE or RFA activate tumor antigen-specific immune response as a result of continuing Atezo/Bev for at least six cycles [47–55, 86–88]. The remaining viable cancer cells are expected to be killed off, resulting in a higher possibility of pathological CR (Fig. 5). In countries where combination immunotherapy regimen other than Atezo/Bev is approved, similar approach may be possible by those combination immunotherapy.
One limitation of this study was the small sample size; however, the number of patients who begin receiving Atezo/Bev as first-line therapy in the intermediate stage is small. Second limitation may be that this was not a comparative study because we focused on a new treatment strategy, Atezo/Bev followed by curative conversion therapy (ABC conversion therapy). However, this could be considered a strength of this study. The results suggest that intentional curative conversion in selected patients who respond well to Atezo/Bev therapy can lead to clinical CR and/or drug-free status (i.e., pathological CR), which is rarely possible by Atezo/Bev alone as shown in 12 responders to Atezo/Bev, who did not receive curative conversion therapy, or locoregional therapy alone (Fig. 1). In that sense, this proposal of ABC conversion therapy may be the strength of this study. Third limitation of this study is a relatively short follow-up period (21.2 months) which led to the many censoring cases. Further long-term follow-up is needed. Finally, this is still an exploratory study and this concept, curative conversion, and whether or not oncological cure is actually achieved by ABC conversion therapy should be further confirmed by longer duration of follow-up and multicenter prospective study. Actually, based on the results of this proof-of-concept study, phase III clinical trial to prove the value of curative conversion after or during Atezo/Bev treatment in intermediate-stage HCC is scheduled to be initiated in the first half of 2023.
In summary, among unresectable and TACE-unsuitable intermediate-stage HCC cases, 35% achieved clinical CR, and 23% achieved drug-free status by curative conversion therapy. A drug-free status can be achieved when significant tumor shrinkage is obtained with Atezo/Bev therapy; at this stage, resection, ablation, and superselective TACE with curative intent are possible by actively performing curative conversion to achieve CR. In some cases, clinical CR and drug-free status could be achieved when the tumor shrinkage effect was not obtained even after 4–6 cycles of Atezo/Bev therapy or when PET-positive HCC was present. Clinical CR was achieved in five PET-positive patients by conducting selective TACE or LEN-TACE sequential therapy followed by Atezo/Bev, which does not decrease liver function during the whole treatment course.
In conclusion, because intermediate-stage HCC is a potentially curable disease (Fig. 7) except extremely high tumor burden such as bilobular multifocal disease >10 or beyond up-to-11 criteria [89], the treatment goal is always to achieve clinical/pathological CR and drug-free status. To that end, the timing of curative conversion during the Atezo/Bev combination therapy for intermediate-stage HCC needs to be determined with cautious intention.
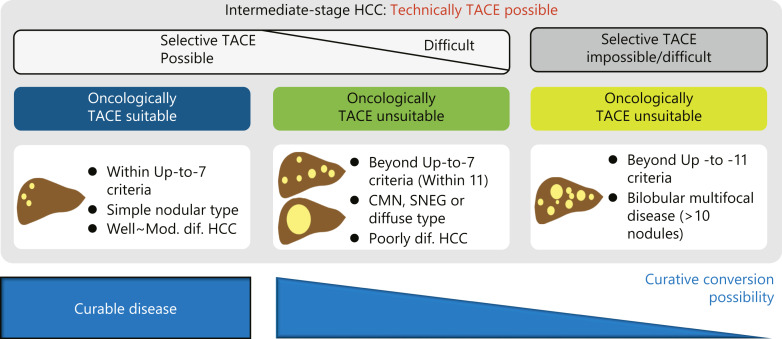
Fig. 7.
Heterogeneity and possibility of curative conversion in intermediate-stage HCC. TACE is technically possible in intermediate-stage HCC. However, since selective TACE is not possible in some population of intermediate-stage HCC, TACE is not suitable oncologically. Curative conversion can achieve complete response in such cases. Curative conversion might be difficult in very high tumor burden, especially in bilobar multifocal disease (>10 nodules) or exceeding up-to-11 criteria. CMN, confluent multinodular type; SNEG, simple nodular with extra growth type.
Statement of Ethics
This multicenter study protocol was reviewed and approved by the Ethics Committee of Kindai University Hospital, approval number R03-218. Written informed consent was not required by the Ethics Committee of Kindai University Hospital since this is an observational study.
Conflict of Interest Statement
Masatoshi Kudo received lecture fee from Eli Lilly, Bayer, Eisai, Chugai, Takeda, and MSD; and grants from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, and GE Healthcare. Masatoshi Kudo is the editor-in-chief of Liver Cancer. Tomoko Aoki, Kazuomi Ueshima, Masahiro Morita, Hirokazu Chishina, Masahiro Takita, Satoru Hagiwara, Yasunori Minami, Hiroshi Ida, Naoshi Nishida (Smoking Research Foundation [Research Grant] Chikara Ogawa), Tetsu Tomonari, Noriaki Nakamura, Hidekatsu Kuroda, Atsushi Takebe, Yoshifumi Takeyama, Masaaki Hidaka, and Susumu Eguchi had no conflict of interest. Kaoru Tsuchiya received lecture fee from Eli Lilly, Bayer, Eisai, Chugai, and Takeda. Stephan L Chan is the advisor for Astra-Zeneca, MSD, Eisai, and Ipsen, and received research funding from Bayer, Eisai, Ipsen, Sirtex, and MSD. Masayuki Kurosaki received lecture fee from Gilead, AbbVie, Eli Lilly, Bayer, Eisai, Chugai, Janssen, and Otsuka. Namiki Izumi received lecturer fee from Chugai, Eisai, Takeda, Lily, and Bayer.
Funding Sources
There is no funding for this study.
Author Contributions
Masatoshi Kudo, Tomoko Aoki, Kazuomi Ueshima, and Kaoru Tsuchiya contributed to the study design, data collection, writing of the manuscript, and final approval of the submitted manuscript. Masahiro Morita, Hirokazu Chishina, Masahiro Takita, Satoru Hagiwara, Yasunori Minami, Hiroshi Ida, Naoshi Nishida, Chikara Ogawa, Tetsu Tomonari, Noriaki Nakamura, Hidekatsu Kuroda, Atsushi Takebe, Yoshifumi Takeyama, Masaaki Hidaka, Susumu Eguchi, Masayuki Kurosaki, and Namiki Izumi contributed to data collection, critical review of the manuscript, and final approval of the submitted manuscript. Stephan L Chan contributed to the critical review at the final approval of the submitted manuscript.
Funding Statement
There is no funding for this study.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 7. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudo M. A new era in systemic therapy for hepatocellular carcinoma: atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2020;9(2):119–37. 10.1159/000505189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 10. Kudo M, Finn RS, Galle PR, Zhu AX, Ducreux M, Cheng AL, et al. IMbrave150: efficacy and safety of atezolizumab plus bevacizumab vs sorafenib in patients with Barcelona clinic liver cancer stage B unresectable hepatocellular carcinoma—an exploratory analysis of the phase III study. Liver Cancer; 2022. 10.1159/000528272 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kudo M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int J Clin Oncol. 2022;27(7):1110–9. 10.1007/s10147-022-02166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyayama S, Mitsui T, Zen Y, Sudo Y, Yamashiro M, Okuda M, et al. Histopathological findings after ultraselective transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Res. 2009;39(4):374–81. 10.1111/j.1872-034X.2008.00465.x. [DOI] [PubMed] [Google Scholar]
- 13. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18(3):365–76. 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299–311. 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh A liver function: a proof-of-concept study. Cancers. 2019;11(8):1084. 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified bolondi’s subclassification (kinki criteria). Dig Dis. 2015;33(6):751–8. 10.1159/000439290. [DOI] [PubMed] [Google Scholar]
- 17. Kudo M. Heterogeneity and subclassification of Barcelona clinic liver cancer stage B. Liver Cancer. 2016;5(2):91–6. 10.1159/000367768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer Expert consensus statements. Liver Cancer. 2020;9(3):245–60. 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158–91. 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 21. Vogel A, Martinelli E; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org, ESMO Guidelines Committee . Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32(6):801–5. 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 22. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020;9(6):756–70. 10.1159/000510299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, et al. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99(8):507–17. 10.1159/000515865. [DOI] [PubMed] [Google Scholar]
- 25. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–75. 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuroda H, Oikawa T, Ninomiya M, Fujita M, Abe K, Okumoto K, et al. Objective response by mRECIST to initial lenvatinib therapy is an independent factor contributing to deep response in hepatocellular carcinoma treated with lenvatinib-transcatheter arterial chemoembolization sequential therapy. Liver Cancer. 2022;11(4):383–96. 10.1159/000522424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023 Jan 1;41(1):117–127. 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 28. Ueshima K, Ishikawa T, Saeki I, Morimoto N, Aikata H, Tanabe N, et al. Transcatheter arterial chemoembolization therapy in combination strategy with lenvatnib in patients with unresectable hepatocellular carcinoma (TACTICS-L) in Japan: final analysis. Gastrointestinal Cancers Symposium. 2022 Jan 20–22. [Google Scholar]
- 29. Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10(6):539–44. 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022;11(5):399–406. 10.1159/000526163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using sonazoid. J Med UltraSound. 2020;28(2):59–82. 10.4103/JMU.JMU_124_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver - update 2020 - WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Stuttgart, Germany: Ultraschall in der Medizin; 2020. Vol. 41. p. 562–85. [DOI] [PubMed] [Google Scholar]
- 33. Shiozawa K, Watanabe M, Takayama R, Takahashi M, Wakui N, Iida K, et al. Evaluation of local recurrence after treatment for hepatocellular carcinoma by contrast-enhanced ultrasonography using Sonazoid: comparison with dynamic computed tomography. J Clin Ultrasound. 2010;38(4):182–9. 10.1002/jcu.20685. [DOI] [PubMed] [Google Scholar]
- 34. Sugimoto K, Saguchi T, Saito K, Imai Y, Moriyasu F. Hemodynamic changes during balloon-occluded transarterial chemoembolization (B-TACE) of hepatocellular carcinoma observed by contrast-enhanced ultrasound. J Med Ultrason. 2014;41(2):209–15. 10.1007/s10396-013-0487-7. [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33(4):605–15. 10.1111/liv.12098. [DOI] [PubMed] [Google Scholar]
- 36. Minami Y, Kudo M. Imaging modalities for assessment of treatment response to nonsurgical hepatocellular carcinoma therapy: contrast-enhanced US, CT, and MRI. Liver cancer. 2015;4(2):106–14. 10.1159/000367733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, et al. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of sonazoid-enhanced harmonic sonography. Oncology. 2008;75(Suppl 1):99–105. 10.1159/000173430. [DOI] [PubMed] [Google Scholar]
- 38. Zhong-Zhen S, Kai L, Rong-Qin Z, Er-Jiao X, Ting Z, Ao-Hua Z, et al. A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall Med. 2012;33:E250–5. 10.1055/s-0032-1325466. [DOI] [PubMed] [Google Scholar]
- 39. Ye J, Huang G, Zhang X, Xu M, Zhou X, Lin M, et al. Three-dimensional contrast-enhanced ultrasound fusion imaging predicts local tumor progression by evaluating ablative margin of radiofrequency ablation for hepatocellular carcinoma: a preliminary report. Int J Hyperthermia. 2019;36(1):55–64. 10.1080/02656736.2018.1530460. [DOI] [PubMed] [Google Scholar]
- 40. Minami Y, Kudo M, Chung H, Kawasaki T, Yagyu Y, Shimono T, et al. Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: prospective randomized controlled trial. AJR Am J Roentgenol. 2007;188(2):489–94. 10.2214/AJR.05.1286. [DOI] [PubMed] [Google Scholar]
- 41. Choi D, Lim HK, Lee WJ, Kim SH, Kim YH, Kim SH, et al. Early assessment of the therapeutic response to radio frequency ablation for hepatocellular carcinoma: utility of gray scale harmonic ultrasonography with a microbubble contrast agent. J Ultrasound Med. 2003;22(11):1163–72. 10.7863/jum.2003.22.11.1163. [DOI] [PubMed] [Google Scholar]
- 42. Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, et al. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;19(6):855–65. 10.3748/wjg.v19.i6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kisaka Y, Hirooka M, Kumagi T, Uehara T, Hiasa Y, Kumano S, et al. Usefulness of contrast-enhanced ultrasonography with abdominal virtual ultrasonography in assessing therapeutic response in hepatocellular carcinoma treated with radiofrequency ablation. Liver Int. 2006;26(10):1241–7. 10.1111/j.1478-3231.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 44. Qu P, Yu X, Liang P, Cheng Z, Han Z, Liu F, et al. Contrast-enhanced ultrasound in the characterization of hepatocellular carcinomas treated by ablation: comparison with contrast-enhanced magnetic resonance imaging. Ultrasound Med Biol. 2013;39(9):1571–9. 10.1016/j.ultrasmedbio.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 45. Frieser M, Kiesel J, Lindner A, Bernatik T, Haensler JM, Janka R, et al. Efficacy of contrast-enhanced US versus CT or MRI for the therapeutic control of percutaneous radiofrequency ablation in the case of hepatic malignancies. Ultraschall Med. 2011;32:148–53. 10.1055/s-0029-1245934. [DOI] [PubMed] [Google Scholar]
- 46. Ikeda M, Arai Y, Inaba Y, Tanaka T, Sugawara S, Kodama Y, et al. Conventional or drug-eluting beads? Randomized controlled study of chemoembolization for hepatocellular carcinoma: jivrosg-1302. Liver cancer. 2022;11(5):440–50. 10.1159/000525500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. den Brok MHMGM, Sutmuller RPM, van der Voort R, Bennink EJ, Figdor CG, Ruers TJM, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64(11):4024–9. 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 48. Iida N, Nakamoto Y, Baba T, Nakagawa H, Mizukoshi E, Naito M, et al. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC Chemokine ligand 3/macrophage inflammatory protein-1alpha. Cancer Res. 2010;70(16):6556–65. 10.1158/0008-5472.CAN-10-0096. [DOI] [PubMed] [Google Scholar]
- 49. Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–57. 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 50. Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006;118(5):1194–204. 10.1002/ijc.21468. [DOI] [PubMed] [Google Scholar]
- 51. Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66(2):1139–46. 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 52. Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914–22. 10.4049/jimmunol.178.3.1914.3 [DOI] [PubMed] [Google Scholar]
- 53. Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53(4):1206–16. 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 54. Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–42. 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 55. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–51. 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuroda C, Sakurai M, Monden M, Marukawa T, Hosoki T, Tokunaga K, et al. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer. 1991;67(1):81–6. . [DOI] [PubMed] [Google Scholar]
- 57. Hashimoto T, Nakamura H, Hori S, Tomoda K, Nakanishi K, Murakami T, et al. Hepatocellular carcinoma: efficacy of transcatheter oily chemoembolization in relation to macroscopic and microscopic patterns of tumor growth among 100 patients with partial hepatectomy. Cardiovasc Intervent Radiol. 1995;18(2):82–6. 10.1007/BF02807227. [DOI] [PubMed] [Google Scholar]
- 58. Yamashita Y, Matsukawa T, Arakawa A, Hatanaka Y, Urata J, Takahashi M. US-guided liver biopsy: predicting the effect of interventional treatment of hepatocellular carcinoma. Radiology. 1995;196(3):799–804. 10.1148/radiology.196.3.7644646. [DOI] [PubMed] [Google Scholar]
- 59. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 60. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–501. 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Final results of TACTICS: a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver cancer. 2022;11(4):354–67. 10.1159/000522547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 63. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 64. Une N, Takano-Kasuya M, Kitamura N, Ohta M, Inose T, Kato C, et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;38(6):60. 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 65. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–72. 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 66. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 67. Kudo M. Sequential therapy for hepatocellular carcinoma after failure of atezolizumab plus bevacizumab combination therapy. Liver cancer. 2021;10(2):85–93. 10.1159/000514312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13(2 Pt 1):427–33. 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 69. Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, et al. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33(3):333–9. [PubMed] [Google Scholar]
- 70. Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36(10):1811–7. [PubMed] [Google Scholar]
- 71. Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996;23(12):1641–74. 10.1007/BF01249629. [DOI] [PubMed] [Google Scholar]
- 72. Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int. 2006;26(7):781–8. 10.1111/j.1478-3231.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 73. Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol. 2011;197(2):W256–259. 10.2214/AJR.10.6331. [DOI] [PubMed] [Google Scholar]
- 74. Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012;19(1):156–62. 10.1245/s10434-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 75. Morio K, Kawaoka T, Aikata H, Namba M, Uchikawa S, Kodama K, et al. Preoperative PET-CT is useful for predicting recurrent extrahepatic metastasis of hepatocellular carcinoma after resection. Eur J Radiol. 2020;124:108828. 10.1016/j.ejrad.2020.108828. [DOI] [PubMed] [Google Scholar]
- 76. Yaprak O, Acar S, Ertugrul G, Dayangac M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J Gastrointest Oncol. 2018;10:336–43. 10.4251/wjgo.v10.i10.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee M, Jeon JY, Neugent ML, Kim JW, Yun M. 18F-Fluorodeoxyglucose uptake on positron emission tomography/computed tomography is associated with metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Clin Exp Metastasis. 2017;34(3–4):251–60. 10.1007/s10585-017-9847-9. [DOI] [PubMed] [Google Scholar]
- 78. Kawai T, Yasuchika K, Seo S, Higashi T, Ishii T, Miyauchi Y, et al. Identification of keratin 19-positive cancer stem cells associating human hepatocellular carcinoma using (18)F-fluorodeoxyglucose positron emission tomography. Clin Cancer Res. 2017;23(6):1450–60. 10.1158/1078-0432.CCR-16-0871. [DOI] [PubMed] [Google Scholar]
- 79. Lin CY, Liao CW, Chu LY, Yen KY, Jeng LB, Hsu CN, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med. 2017;42(4):e183–e187. 10.1097/RLU.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 80. Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: a prospective observational study. HPB (Oxford). 2019;21(6):739–47. 10.1016/j.hpb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 81. Hyun SH, Eo JS, Song BI, Lee JW, Na SJ, Hong IK, et al. Preoperative prediction of microvascular invasion of hepatocellular carcinoma using (18)F-FDG PET/CT: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 2018;45(5):720–6. 10.1007/s00259-017-3880-4. [DOI] [PubMed] [Google Scholar]
- 82. Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40(4):843–51. 10.1007/s00261-014-0256-0. [DOI] [PubMed] [Google Scholar]
- 83. Ünal E, İdilman İS, Akata D, Özmen MN, Karçaaltıncaba M. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol. 2016;22(2):125–32. 10.5152/dir.2015.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9(3):592–600. 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 85. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–20. 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 86. Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45(4):451–8. 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 87. Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40(1):63–70. 10.3892/ijo.2011.1202. [DOI] [PubMed] [Google Scholar]
- 88. Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12(23):3716–21. 10.3748/wjg.v12.i23.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hung YW, Lee IC, Chi CT, Lee RC, Liu CA, Chiu NC, et al. Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: the seven-eleven criteria. Liver Cancer. 2021;10(6):629–40. 10.1159/000517393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplementary material. Further inquiries can be directed to the corresponding author.