Abstract
Mycophenolate mofetil (MMF) has been approved as an immunosuppressive agent in kidney transplant recipients and may thus be used concomitantly with antiherpetic agents, which are used for the treatment of intercurrent herpesvirus infections. We have recently demonstrated that MMF and its parent compound mycophenolic acid (MPA), which is a potent inhibitor of IMP dehydrogenase, potentiate the antiherpesvirus activity of acyclovir, ganciclovir, and penciclovir. We have now evaluated the antiviral efficacy of the combination of MPA and the novel antiherpesvirus agent H2G [(R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine]. When combined with H2G, MPA (at concentrations ranging from 0.25 to 10 μg/ml, which are readily attainable in human plasma) markedly potentiated the antiviral efficacy of H2G against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), as reflected by a 10- to 150-fold decrease in the 50% effective concentration. Moreover, the activity of H2G against a thymidine kinase-deficient strain of HSV-1 (TK− HSV-1) was increased more than 2,500-fold when combined with MPA. MPA by itself had little or no effect on the replication of these viruses. Similar observations were made for varicella-zoster virus. Also, ribavirin (another inhibitor of IMP dehydrogenase) caused a marked enhancement of the activity of H2G against HSV-1 (10-fold), HSV-2 (10-fold), and TK− HSV-1 (>185-fold). Exogenously added guanosine reversed the potentiating effects of MPA on the antiviral activity of H2G, indicating that this potentiating effect resulted from a depletion of the endogenous dGTP pools, thus favoring the inhibitory action of the H2G triphosphate on the viral DNA polymerase.
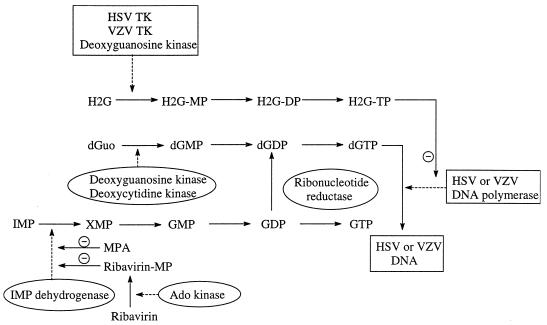
Mycophenolate mofetil (MMF), the morpholinoethyl ester of mycophenolic acid (MPA) is an immunosuppressant that is currently used in kidney transplant recipients. After oral administration, MMF is hydrolyzed to MPA, the active immunosuppressive agent, which is a potent inhibitor of IMP dehydrogenase. IMP dehydrogenase is responsible for the conversion of IMP through XMP to GMP. Since IMP represents an important intermediate in the generation of nucleotides containing guanine as the base, MPA will cause depletion of the intracellular guanine nucleotide pools (Fig. 1). The immunosuppressive effect of MPA has been ascribed to this depletion of the dGTP pools (13, 19, 21, 23, 25).
FIG. 1.
Proposed mechanism by which MPA and ribavirin potentiate the antiherpesvirus activity of H2G. For further information on (i) the phosphorylation of H2G, see reference 14; (ii) the inhibition of viral DNA polymerases by H2G-TP, see references 2 and 14; (iii) the phosphorylation of ribavirin, see reference 26; and (iv) the inhibition of IMP dehydrogenase by MPA and ribavirin monophosphate, see references 19, 23, and 26. MP, monophosphate; DP, diphosphate; TP, triphosphate; Ado, adenosine.
H2G ([(R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine] is an acyclic purine nucleoside analog with activity against herpesviruses, including herpes simplex virus types 1 (HSV-1) and 2 (HSV-2), Epstein-Barr virus, human herpesviruses 6 (HHV-6) and 8 (HHV-8), and particularly varicella-zoster virus (VZV) (1–4, 16, 17, 24). H2G is converted to its monophosphate form by the HSV-1-, HSV-2-, or VZV-encoded thymidine kinase and is then further phosphorylated to its triphosphate form (H2G-TP), which selectively inhibits the viral DNA polymerase. H2G-TP inhibits HSV-1, HSV-2, and VZV DNA polymerases competitively with dGTP (at Kis of 2.8, 2.2, and 0.3 μM, respectively), but it does not serve as an alternative substrate in the polymerization reaction (16). The compound has recently entered phase I/II clinical trials for the treatment of herpesvirus infections, in particular with VZV infections.
We recently showed that the antiherpesvirus activity of acyclovir (ACV), penciclovir (PCV), and ganciclovir (GCV), which, like H2G, are acyclic guanosine nucleoside analogs, is markedly stimulated by MPA and its oral prodrug MMF (18). MMF and MPA are themselves virtually inactive against herpesviruses (with the exception of human cytomegalovirus [CMV], which is slightly inhibited by MPA). ACV, PCV, and GCV, like H2G, are specifically phosphorylated by virus-encoded kinases (HSV-1-, HSV-2-, and VZV-encoded thymidine kinase for all four compounds and, in addition, CMV-encoded UL97 kinase for GCV) to their monophosphate forms and, further on, by cellular kinases to the triphosphate form (11). The triphosphates are specific and strong inhibitors of the viral DNA polymerases. We have demonstrated that a depletion of the dGTP pools brought about by MPA is responsible for the potentiating effect of MPA on the antiherpesvirus activity of ACV, PCV, and GCV (18).
Similarly, ribavirin has been shown to potentiate the anti-HIV activity of 2′3′-dideoxyinosine and other purine nucleoside analogs with anti-HIV activity (7, 8, 13, 14). The increased IMP pools in the ribavirin-treated cells were found to result in an increased phosphorylation of 2′,3′-dideoxyinosine by 5′-nucleotidase, an enzyme that uses IMP as the phosphate donor (9). We have demonstrated that this mechanism does not hold for ACV and GCV (18), although these molecules have been shown to act as a substrate for 5′-nucleotidase (15).
In the present study, we assessed whether MPA also potentiates the antiherpesvirus activity of H2G. Since this compound is currently under clinical study for the therapy of herpesvirus infections, in the future it may well be used concomitantly with the immunosuppressant MMF in those transplant recipients that develop opportunistic herpesvirus infections as a result of the immunosuppressive action of MMF.
Human embryonic lung (HEL) cells and Vero cells were propagated in minimal essential medium (MEM) supplemented with 10% fetal calf serum, l-glutamine, and bicarbonate. Human CMV (strain Davis) and VZV (strains OKA, 07-1, and YS-R) were obtained from the American Type Culture Collection. The origin of HSV-1 (strain KOS) and HSV-2 (strain G) and thymidine kinase-deficient (TK−) HSV-1 (B2006) have been described before (12). Biochemical evidence that the B2006, 07-1, and YS-R strains are TK deficient was obtained from phosphorylation studies with radiolabelled ACV. Levels of the triphosphate form of ACV (ACV-TP) in Vero cells infected with the HSV-1 TK− strain (B2006) were 2.7% of the levels in cells infected with the wild-type virus. The levels of ACV-TP in HEL cells infected with the YS-R and 07-1 strains were ≤3.6% (detection limit) of the levels in cells infected with the wild-type virus.
H2G was kindly provided by A. Molla (Abbott Laboratories, Abbott Park, Ill.), MPA and guanosine were from Sigma (St. Louis, Mo.), and ribavirin was from ICN (Costa Mesa, Calif.). [8-3H]ACV (specific activity, 15 Ci/mmol) was from Moravek Biochemicals (Brea, Calif.). Confluent cultures of Vero cells grown in microtiter trays were inoculated with 100 times the 50% cell culture infective dose of the different HSV strains, whereas confluent cultures of HEL cells were inoculated with 100 PFU of CMV or 20 PFU of VZV. Compounds, either alone or in combination, were added after a 2-h virus adsorption period. Virus-induced cytopathic effect (CPE) was recorded microscopically at 2 to 3 days postinfection (p.i.) for the HSV strains and at 7 days p.i. for CMV. VZV-induced plaque formation was evaluated at 5 days p.i. The 50% effective concentrations (EC50s) were derived from graphical plots.
Cytostatic effects were monitored in Vero cells (in MEM containing 20% fetal calf serum) that were seeded at a density of 4,000 cells/well in 96-well plates and were allowed to attach to the plastic for 24 h. Thereafter, serial dilutions of the test compounds (in MEM containing 2% fetal calf serum), either alone or in combination, were added. The cells were further incubated for 3 days at 37°C, at which time they were trypsinized and counted with a Coulter Counter.
To determine the effect of MPA on intracellular GTP pools, 1-day-old confluent cultures of Vero cells were incubated with different concentrations of MPA for 48 h. After two washes in phosphate-buffered saline, the cells were collected by trypsinization and nucleotides were extracted in 70% ice-cold methanol. Quantification of nucleotide pools was done by high-performance liquid chromatography analysis using a Partisil-sphere radial-compression column (Pharmacia, St. Albans, Hertsfordshire, United Kingdom) as described previously (5). To quantify the levels of ACV-TP generated in cells that had been infected with the different HSV or VZV strains, radiolabeled ACV was added for 24 h at the time that the CPE was ≈60%. Samples were analyzed by high-performance liquid chromatography, and radioactivity in the different fractions was determined by means of liquid scintillation counting.
H2G potently inhibited the replication of HSV-1 and HSV-2 but had, as expected, little or no effect on the replication of the TK− HSV-1 strain (Table 1). At the concentrations used (10, 2.5, 1, and 0.25 μg/ml), MPA had virtually no inhibitory effect on the CPE progression in the HSV-1-, HSV-2-, and TK− HSV-1-infected cultures. However, when H2G was combined with MPA, a pronounced enhancement of the antiviral activity of H2G was noted. In fact, the EC50 of H2G for inhibition of HSV-1 and HSV-2 replication decreased 100- to 150-fold. Of special interest is our observation that H2G, when combined with MPA, efficiently inhibited the replication of the TK− strain of HSV-1. In this case, the increase in antiviral activity was over 2,500-fold. Thus, the EC50 of H2G for inhibition of replication of the TK− HSV-1 strain dropped from values that are not attainable in plasma (>100 μg/ml) to values that could be easily reached (0.04 to 0.8 μg/ml). This pronounced potentiating effect can be explained only by the formation of small amounts of phosphorylated products of H2G in cells infected with the TK− virus. This is most likely accomplished by the residual activity of the defective viral TK, or, alternatively, by cellular TK, or by both. Similar to our findings with HSV, MPA also had a pronounced potentiating effect on the anti-VZV activity of H2G (Table 1). H2G was about 1,000-fold less effective against the TK− strains of VZV (07-1 and YS-R) than against the wild-type strains (OKA and YS). However, when combined with MPA, again at concentrations attainable in plasma, these TK− strains became up to 60-fold more sensitive to H2G.
TABLE 1.
Antiherpesvirus activity of the combination H2G plus MPA
| Virus (strain) | EC50 (μg/ml)a of H2G
|
||||
|---|---|---|---|---|---|
| Alone | In combination with MPA at indicated concn
|
||||
| 10 μg/ml | 2.5 μg/ml | 1.0 μg/ml | 0.25 μg/ml | ||
| HSV-1 (KOS) | 5.3 ± 3.4 | 0.06 ± 0.05 | 0.16 ± 0.18 | 0.24 ± 0.17 | 0.35 ± 0.33 |
| HSV-2 (G) | 11.3 ± 2.9 | 0.07 ± 0.02 | 0.24 ± 0.22 | 0.3 ± 0.17 | 1.6 ± 0.8 |
| TK− HSV-1 (B2006) | >100 | 0.04 ± 0.03 | 0.08 ± 0.01 | 0.25 ± 0.21 | 0.8 ± 0.17 |
| VZV (OKA) | 0.0043 ± 0.004 | 0.0053 ± 0.0049 | 0.0007 ± 0.0006 | 0.0008 ± 0.0006 | 0.0006 ± 0.0003 |
| VZV (YS) | 0.01 ± 0.007 | 0.0068 ± 0.008 | 0.0014 ± 0.0012 | 0.0008 ± 0.0005 | 0.0007 ± 0.0004 |
| TK− VZV (07-1) | 4.6 ± 3.7 | 0.08 ± 0.07 | 0.31 ± 0.26 | 0.33 ± 0.26 | 1.0 ± 1.3 |
| TK− VZV (YS-R) | 11.7 ± 11.7 | 0.35 ± 0.3 | 0.19 ± 0.13 | 0.29 ± 0.28 | 2.8 ± 3.5 |
Concentration required to reduce virus-induced CPE by 50%. The EC50 for inhibition of HSV- or VZV-induced CPE by MPA was >10 μg/ml. Data are mean values ± standard deviations for three to eight separate determinations.
The potentiating effects of MPA on the antiviral activity of H2G against HSV-1, HSV-2, and TK− HSV-1 were efficiently reversed upon addition of guanosine to the culture medium (Table 2). Under the conditions used, MPA also markedly depleted the GTP pools in these cells. Levels of GTP (percent) were 22 ± 4, 37 ± 0.7, 32 ± 9, and 45 ± 6 (mean ± standard deviation) in confluent Vero cell cultures that had been incubated for 48 h with, respectively, 10, 2.5, 1, and 0.25 μg of MMF per ml in comparison to the levels of GTP in untreated control cultures. Our findings thus indicate that the observed potentiating effect of MPA on the antiviral activity of H2G, as shown previously for ACV, PCV, and GCV, must be explained by a depletion of the guanine nucleotide pools, resulting in a decreased competition of dGTP with H2G-TP in the viral DNA polymerization reaction.
TABLE 2.
Effect of exogenously added guanosine on the potentiating effect of MPA on the anti-HSV-1 activity of H2G
| Culture | EC50 (μg/ml)a of H2G
|
||||
|---|---|---|---|---|---|
| Alone | In combination with MPA at indicated concn
|
||||
| 10 μg/ml | 2.5 μg/ml | 1.0 μg/ml | 0.25 μg/ml | ||
| Without guanosine | 8.3 ± 0.14 | 0.06 ± 0.05 | 0.26 ± 0.33 | 0.28 ± 0.3 | 0.45 ± 0.5 |
| With guanosine (25 μg/ml) | 4.9 ± 2.6 | 5.3 ± 2.6 | 6.3 ± 3.6 | 6.5 ± 4.8 | |
The EC50 for inhibition of HSV-1-induced CPE by guanosine was >100 μg/ml and >25 μg/ml for MPA. Data are mean values ± standard deviations for three separate experiments.
In contrast to ACV, GCV, and PCV (18), H2G did not gain a synergistic increase in its already weak anti-CMV activity when combined with MMF (data not shown). This may indicate that (i) virtually no phosphorylated metabolites of H2G are formed in the CMV-infected cells and/or that (ii) H2G-TP is a (very) poor inhibitor of the CMV-encoded DNA polymerase. MMF had by itself some inhibitory effect on CMV replication (EC50, ≈2.5 μg/ml). The anti-CMV activity of azathioprine, another immunosuppressive agent, has been described before (22).
To confirm the observations made for MPA, we also studied the antiherpesvirus activity of the combination of H2G and ribavirin. Ribavirin is a broad-spectrum antiviral agent that has virtually no activity against herpesviruses (20). In its monophosphate form, ribavirin, like MPA, acts as a potent inhibitor of IMP dehydrogenase and thus also causes depletion of the intracellular guanine nucleotide pools (Fig. 1). Similarly to MPA, ribavirin also markedly potentiates the activity of H2G against HSV-1 (10-fold), HSV-2 (10-fold), and TK− HSV-1 (185-fold), although ribavirin did so less efficiently than MPA (Table 3). Ribavirin alone had no activity against the viruses studied. The reason for ribavirin not being as efficient as MPA in potentiating the antiviral activity of H2G can be explained by the fact that ribavirin monophosphate is a less potent inhibitor of IMP dehydrogenase than is MPA (6).
TABLE 3.
Antiherpesvirus activity of the combination H2G plus ribavirin
| Virus (strain) | EC50 (μg/ml)a of H2G
|
||||
|---|---|---|---|---|---|
| Alone | In combination with ribavirin at indicated concn
|
||||
| 100 μg/ml | 50 μg/ml | 25 μg/ml | 10 μg/ml | ||
| HSV-1 (KOS) | 3.3 ± 3.5 | 0.3 ± 0.3 | 0.55 ± 0.60 | 1.5 ± 1.6 | 1.9 ± 2.1 |
| HSV-2 (G) | 13 ± 8.4 | 1.2 ± 0.8 | 1.3 ± 0.6 | 2.6 ± 1.0 | 5.4 ± 2.5 |
| TK− HSV-1 (B2006) | >100 | 0.54 ± 0.39 | 0.75 ± 0.1 | 7.1 ± 8.5 | 13 ± 13 |
Concentration required to reduce virus-induced CPE in Vero cells by 50%. The EC50 for ribavirin alone was >100 μg/ml. Data are mean values ± standard deviations for three to five separate experiments.
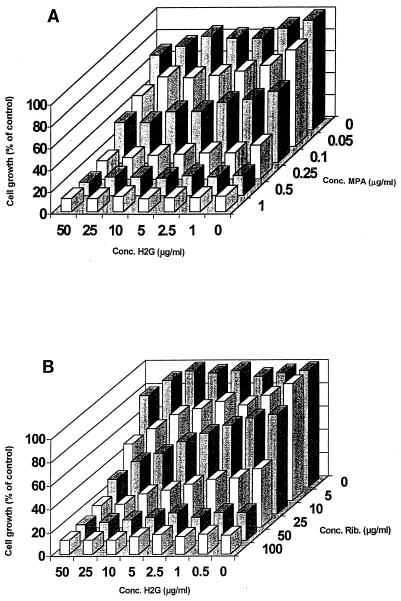
Finally, we wanted to determine whether the combination of H2G and MPA or H2G and ribavirin increased the cytostatic action of H2G. As can be derived from Fig. 2, H2G alone had only a limited cytostatic effect (i.e., a 30 to 40% reduction of Vero cell growth at 50 μg/ml). Addition of MPA or ribavirin at concentrations around their 50% cytostatic concentration for Vero cell growth resulted in an additive, rather than a synergistic, inhibition of cell growth. Similarly, we also observed no potentiation of the cytostatic action of GCV when it was combined with MPA (18). Although we did not observe any stimulation in the cytostatic action of H2G when combined with MPA, we suggest that patients receiving MMF be carefully monitored for a potential increase in the side effects of H2G.
FIG. 2.
Effect of MPA (A) and ribavirin (B) on the cytostatic action of H2G on Vero cells. Data are mean values for at least two separate experiments.
In conclusion, MPA strongly enhances the anti-HSV and anti-VZV activities of the novel purine nucleoside analog H2G. The concentrations required for MPA to potentiate the antiviral activity of H2G do not have to exceed 1 μg/ml, i.e., a concentration that is even below the MPA concentration attained in plasma upon oral dosing of 0.5 to 3 g of MMF (10). The mechanism of action resides in a depletion by MPA of the intracellular dGTP pools, thus favoring the inhibitory effect of H2G-TP on the viral DNA polymerase. Since clinical trials of the efficacy of H2G against herpesvirus infections are currently underway, it will be of interest to investigate whether the antiviral efficacy of H2G is enhanced in patients that are undergoing immunosuppressive therapy with MMF. The use of MMF in transplant recipients may thus be considered a double-edged sword. On the one hand, it may precipitate the reactivation of opportunistic herpesviruses. On the other hand, once the patient receives H2G for this infection, the synergistic action between the two compounds may compensate for the increased risk of developing herpesvirus infections.
Acknowledgments
We thank Miette Stuyck for excellent technical assistance and Christiane Callebaut for dedicated editorial help.
This work was supported by grants from the Belgian Fonds voor Geneeskundig Wetenschappelijk Onderzoek (FGWO), the “Fonds voor Wetenschappelijk Onderzoek (FWO)—Vlaanderen”, and the “Geconcerteerde Onderzoeksacties” (GOA) Vlaamse Gemeenschap. J. Neyts is a postdoctoral research assistant from the “Fonds voor Wetenschappelijk Onderzoek (FWO)—Vlaanderen.”
REFERENCES
- 1.Abele G, Cox S, Bergman S, Lindborg B, Vissgarden A, Karlström A, Harmenberg J, Wahren B. Antiviral activity against VZV and HSV type 1 and type 2 of the (+) and (−) enantiomers of (R,S)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine, in comparison to other closely related acyclic nucleosides. Antivir Chem Chemother. 1991;2:163–169. [Google Scholar]
- 2.Abele G, Eriksson B, Harmenberg J, Wahren B. Inhibition of varicella-zoster virus-induced DNA polymerase by a new guanosine analog, 9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine triphosphate. Antimicrob Agents Chemother. 1988;32:1137–1142. doi: 10.1128/aac.32.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abele G, Karlström A, Harmenberg J, Shigeta S, Larsson A, Lindborg B, Wahren B. Inhibiting effect of (R,S)-9-[4-hydroxymethyl)butyl]guanine on varicella-zoster virus replication in cell culture. Antimicrob Agents Chemother. 1987;31:76–80. doi: 10.1128/aac.31.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Åkesson-Johansson A, Harmenberg J, Wahren B, Linde A. Inhibition of human herpesvirus 6 replication by 9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine (2HM-HBG) and other antiviral compounds. Antimicrob Agents Chemother. 1990;34:2417–2419. doi: 10.1128/aac.34.12.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J, De Clercq E. 9-β-d-Arabinofuranosyladenine 5′-monophosphate (araAMP) is converted directly to its antivirally active 5′-triphosphate form by 5-phosphoribosyl-1-pyrophosphate (PRPP) synthetase. Biochem Biophys Res Commun. 1990;173:781–787. doi: 10.1016/s0006-291x(05)80855-1. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini J, De Clercq E. Assay method for monitoring the inhibitory effects of antimetabolites on the activity of inosinate dehydrogenase in intact human CEM lymphocyte cells. Biochem J. 1992;287:785–790. doi: 10.1042/bj2870785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzarini J, Naesens L, Robins M J, De Clercq E. Potentiating effect of ribavirin on the in vitro and in vivo anti-retrovirus activities of 2′,3′-dideoxyinosine and 2′,3′-dideoxy-2,6-diaminopurine riboside. J Acquired Immune Defic Syndr. 1990;3:1140–1147. [PubMed] [Google Scholar]
- 8.Balzarini J, Lee C-K, Schols D, De Clercq E. 1-β-d-Ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) and 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide (EICAR) markedly potentiate the inhibitory effect of 2′,3′-dideoxyinosine on human immunodeficiency virus in peripheral blood lymphocytes. Biochem Biophys Res Commun. 1991;178:563–569. doi: 10.1016/0006-291x(91)90145-w. [DOI] [PubMed] [Google Scholar]
- 9.Balzarini J, Lee C-K, Herdewijn P, De Clercq E. Mechanism of the potentiating effect of ribavirin on the activity of 2′,3′-dideoxyinosine (ddIno) against human immunodeficiency virus (HIV) J Biol Chem. 1991;266:21509–21514. [PubMed] [Google Scholar]
- 10.Bullingham R, Monroe S, Nicholls A, Hale M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol. 1996;36:315–324. doi: 10.1002/j.1552-4604.1996.tb04207.x. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev Med Virol. 1995;5:149–164. [Google Scholar]
- 12.De Clercq E, Descamps J, Verhelst G, Walker R T, Jones A S, Torrence P F, Shugar D. Comparative efficacy of different antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980;141:563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- 13.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51:278–298. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hartman N R, Ahluwalia G S, Cooney D A, Mitsuya H, Kageyama S, Fridland A, Broder S, Johns D G D A. Inhibitors of IMP dehydrogenase stimulate the phosphorylation of the anti-human immunodeficiency virus nucleosides 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine. Mol Pharmacol. 1991;40:118–124. [PubMed] [Google Scholar]
- 15.Keller P M, McKee S A, Fyfe J A. Cytoplasmic 5′-nucleotidase catalyzes acyclovir phosphorylation. J Biol Chem. 1985;260:8664–8667. [PubMed] [Google Scholar]
- 16.Lowe D M, Alderton W K, Ellis M R, Parmar V, Miller W H, Roberts G B, Fyfe J A, Gaillard R, Ertl P, Snowden W, Littler E. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyts J, De Clercq E. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob Agents Chemother. 1997;41:2754–2756. doi: 10.1128/aac.41.12.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neyts J, Andrei G, De Clercq E. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpes virus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob Agents Chemother. 1998;42:216–222. doi: 10.1128/aac.42.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmesgern E, Fox T, Fleming M A, Thomson J A. Conformational changes and stabilization of inosine 5′-monophosphate dehydrogenase associated with ligand binding and inhibition by mycophenolic acid. J Biol Chem. 1996;271:19421–19427. doi: 10.1074/jbc.271.32.19421. [DOI] [PubMed] [Google Scholar]
- 20.Patterson J L, Fernandez-Larsson R. Molecular mechanism of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 21.Ransom J T. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Shiraki K, Ishibashi M, Okuno T, Namazue J, Yamanishi K, Sonoda T, Takahashi M. Immunosuppressive dose of azathioprine inhibits replication of human cytomegalovirus in vitro. Arch Virol. 1991;117:165–171. doi: 10.1007/BF01310762. [DOI] [PubMed] [Google Scholar]
- 23.Sintchak M D, Fleming M A, Futer O, Raybuck S A, Chambers S P, Caron P R, Murcko M A, Wilson K P. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell. 1996;85:921–930. doi: 10.1016/s0092-8674(00)81275-1. [DOI] [PubMed] [Google Scholar]
- 24.Soike K, Bohm R, Huang J-L, Öberg B. Efficacy of (−)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine in African green monkeys infected with simian varicella virus. Antimicrob Agents Chemother. 1993;37:1370–1372. doi: 10.1128/aac.37.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suthanthiran M, Morris R E, Strom T B. Immunosuppressants: cellular and molecular mechanism of action. Am J Kidney Dis. 1996;28:159–172. doi: 10.1016/s0272-6386(96)90297-8. [DOI] [PubMed] [Google Scholar]
- 26.Willis R C, Carson D A, Seegmiller J E. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci USA. 1978;75:3042–3044. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]