Abstract
Enantioconvergent catalysis has expanded asymmetric synthesis to new methodologies able to convert racemic compounds into a single enantiomer. This review covers recent advances in transition-metal-catalyzed transformations, such as radical-based cross-coupling of racemic alkyl electrophiles with nucleophiles or racemic alkylmetals with electrophiles and reductive cross-coupling of two electrophiles mainly under Ni/bis(oxazoline) catalysis. C–H functionalization of racemic electrophiles or nucleophiles can be performed in an enantioconvergent manner. Hydroalkylation of alkenes, allenes, and acetylenes is an alternative to cross-coupling reactions. Hydrogen autotransfer has been applied to amination of racemic alcohols and C–C bond forming reactions (Guerbet reaction). Other metal-catalyzed reactions involve addition of racemic allylic systems to carbonyl compounds, propargylation of alcohols and phenols, amination of racemic 3-bromooxindoles, allenylation of carbonyl compounds with racemic allenolates or propargyl bromides, and hydroxylation of racemic 1,3-dicarbonyl compounds.
1. Introduction
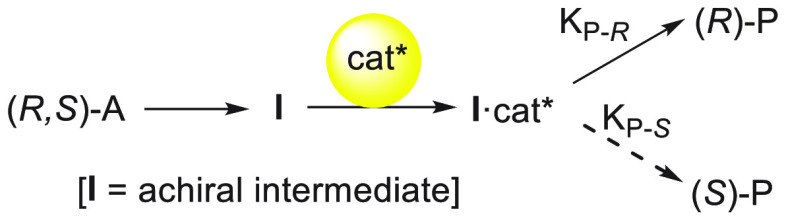
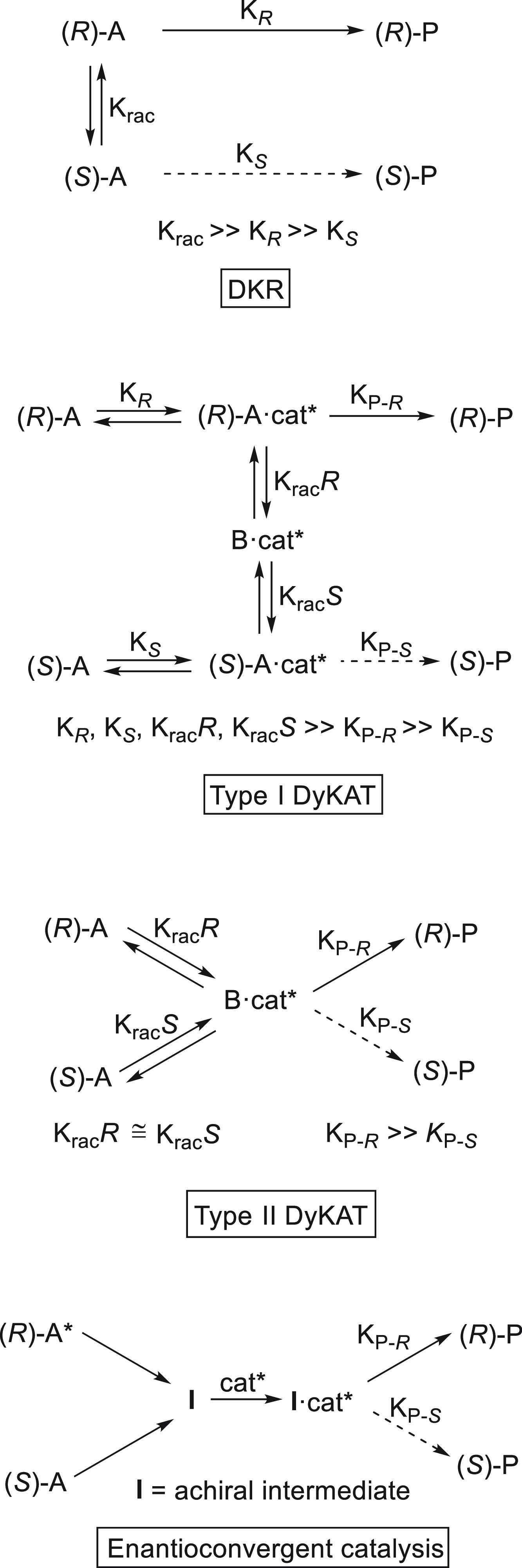
The preparation of enantiopure compounds is a major subject in chemistry. Enantiocatalytic methods are key methodologies nowadays to afford the synthesis of a single enantiomer. Desymmetrization reactions have been extensively applied to achiral or meso-compounds using metal-catalyzed, organocatalyzed, and enzymatic processes.1−19 Conversion of both enantiomers of a racemate into a single enantiomer has been carried out by dynamic kinetic resolutions (DKRs), dynamic kinetic asymmetric transformations (DyKATs), and enantioconvergent processes.20−28 In the case of DKR, the two enantiomers undergo a reversible racemization prior to the selective reaction of one enantiomer with the chiral catalyst, whereas in the DyKATs, the equilibration of both enantiomers is due to the chiral catalyst. In type I DyKATs, both enantiomers are bounded to the catalyst, and these intermediates undergo equilibration, whereas in type II DyKATs, the racemate loses the stereocenter by interaction with the chiral catalyst to form a prochiral intermediate B·cat*. In the case of enantioconvergent reactions, the equilibration is not necessary, and this deracemization methodology does not indicate to control kinetic factors, which are critical in DKRs and DyKATs processes. For enantioconvergent transformations, two enantiomers of the substrate are converted by a stereoablative reaction20 into a prochiral intermediate I that reacts with the chiral catalyst to give one enantiomeric product, for instance, (R)-P (Figure 1).
Figure 1.
Conversion of racemates into a single enantiomer.
In this Review, we will focus on recent developments of enantioconvergent catalysis for asymmetric transformations. This strategy has become a booming methodology in the last 10 years in asymmetric catalysis mainly under transition metal C–C bond-forming reactions and also under organocatalysis for the preparation of enantioenriched compounds.
2. Enantioconvergent Cross-Couplings
In this Section, C–C bond-forming reactions of alkyl and aryl electrophiles with organometals will be considered. Bond-forming reactions by reaction of alkyl electrophiles with other nucleophiles have been performed in enantioconvergent processes. Reductive cross-electrophile couplings of Csp2–Csp3, Csp3–Csp3, and Csp2–Csp2 under Ni catalysis, as well as photoredox radical coupling, will be included. Cu catalysis under a radical mechanism gives enantioconvergent amination processes.
2.1. Racemic Alkyl Electrophiles with Organometals
Carbon–carbon bond formation under transition-metal-catalyzed cross-coupling reactions of acyl and vinyl electrophiles with organometals is an important methodology in organic synthesis.29,30 The development of cross-coupling reactions with alkyl electrophiles, especially secondary systems, was a challenging task because of β-hydride elimination processes31 and has been crucial for asymmetric transformations.32,33 Enantioconvergent cross-coupling reactions of alkyl electrophiles under Ni catalysis form radicals after the oxidative addition, which react with chiral Ni complexes to form the enantioenriched products.21−25,34−36 In this Section, enantioconvergent cross-coupling reactions of secondary alkyl electrophiles with organometals, such as Grignard reagents, organozinc, organoboron, organosilicon, organoindium, organozirconium, organoaluminum, and organotitanium organometallics, will be considered.
2.1.1. Grignard Reagents
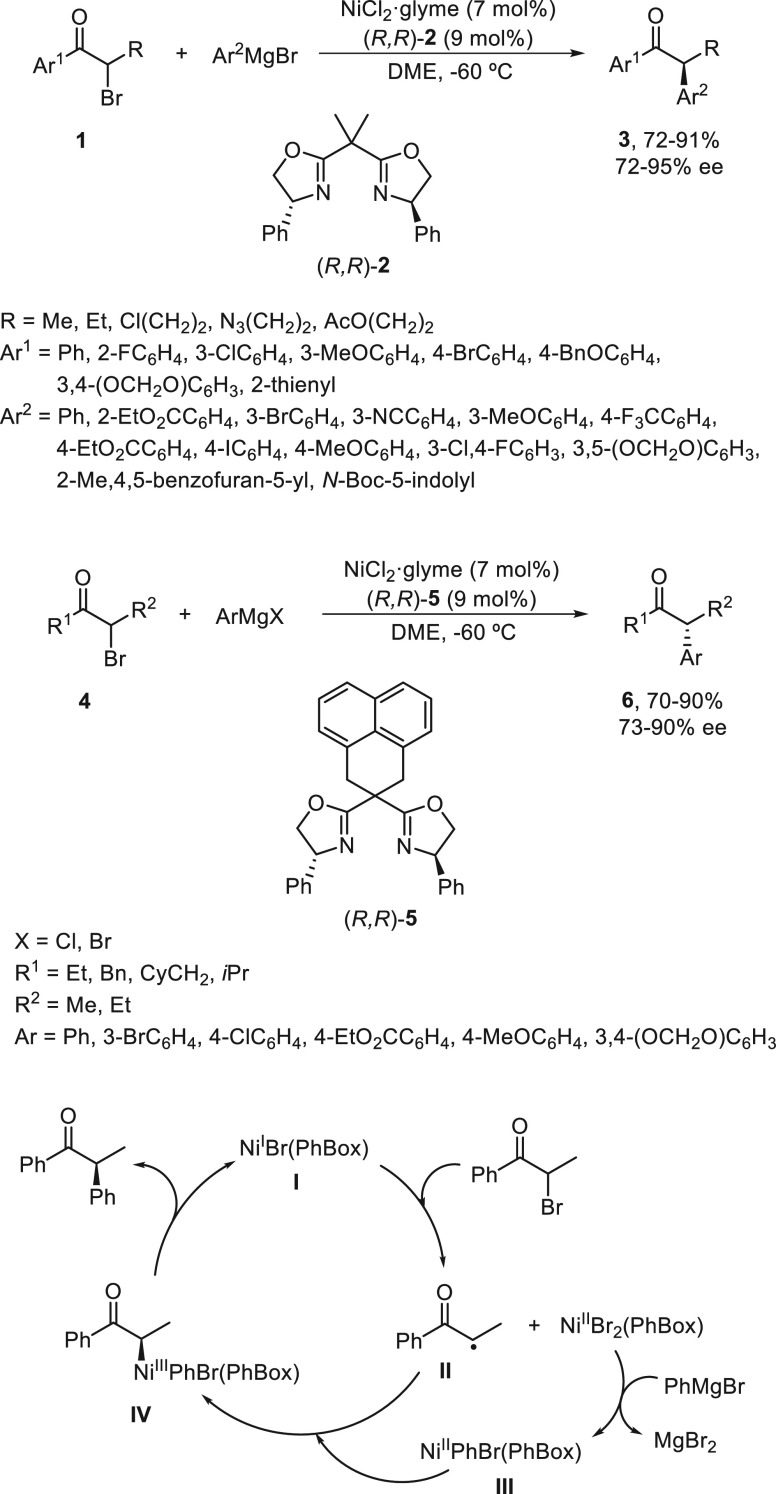
Enantioconvergent Kumada reactions were described for the first time by Lou and Fu.37 Racemic α-bromoketones 1 were coupled with arylmagnesium reagents using chiral nickel/bis(oxazoline) catalyst 2 in dimethoxyethane (DME) at −60 °C (Scheme 1). In the case of alkyl aryl ketones, 7 mol % of NiCl2·glyme and 9 mol % of (R,R)-PhBox (2) were used as catalyst with different arylmagnesium bromides at −60 °C to give the corresponding enantioenriched ketones 3 in good yields (72–91%) and up to 95% ee. When the same reaction conditions were applied to dialkyl ketones 4, ligand 5 gave the best results by working at −40 °C to provide compounds 6 in 70–90% yield and up to 90% ee. Recently, Yin and Fu38 performed mechanistic investigation for the reaction of α-bromo propiophenone with PhMgBr and NiBr2/PhBox as catalyst, thereby establishing that the C–C bond formation process works via a radical chain process. In the proposed catalytic cycle, a nickel radical I abstracts a halogen atom from the α-bromo ketone to generate the radical II and NiBr2(PhBox), which reacts with phenylmagnesium bromide to form intermediate III. Radical II, by reaction with III, gives an organonickel(III) intermediate IV through an out-of-cage pathway. Final reductive elimination of IV affords the coupling product and regenerates the chain-carrying radical I. According to DFT calculations, the coupling between intermediates II and III is the stereochemistry-determining step.
Scheme 1. Enantioconvergent Ni-Catalyzed Kumada Reactions of α-Bromoketones 1 and 4 with Arylmagnesium Halides.
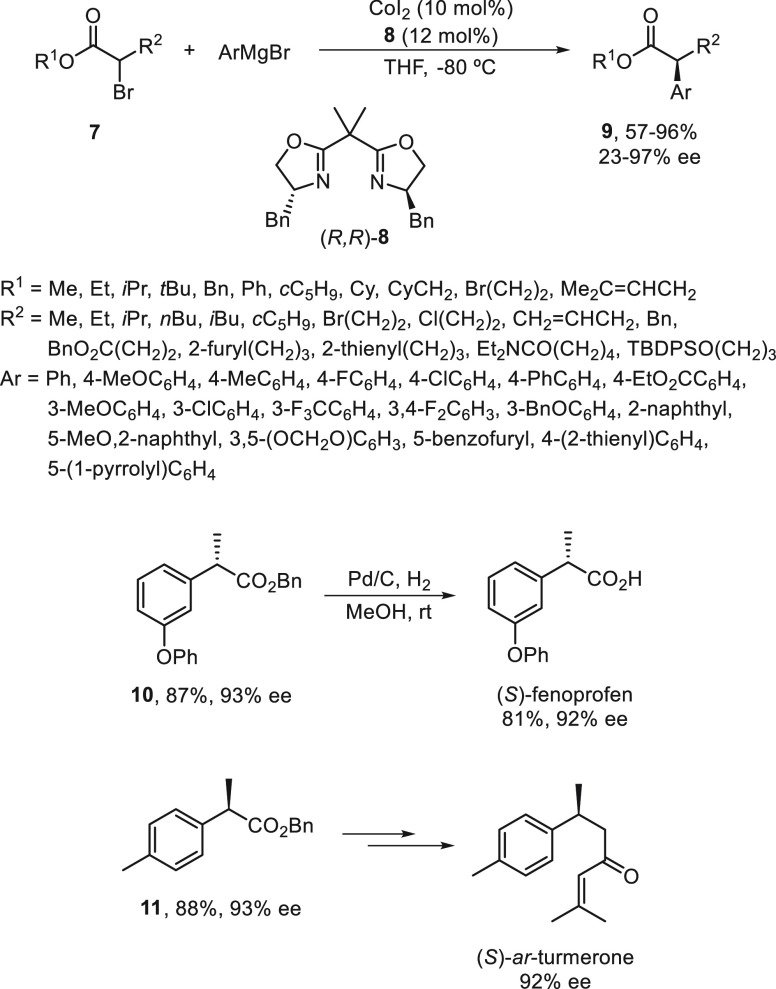
Zhong, Bian, and co-workers39 described the cobalt-catalyzed enantioconvergent Kumada reaction of α-bromo esters 7 using bis(oxazoline) 8 as chiral ligands (Scheme 2). A variety of chiral α-aryl alkanoic esters 9 were prepared using CoI2 (10 mol %) and ligand (R,R)-8 (12 mol %) in THF at −80 °C with good yields (up to 96%) and enantioselectivities (up to 97% ee). This methodology was applied to the synthesis of the nonsteroidal anti-inflammatory drug (NSAID) (S)-fenoprofen by Kumada reaction to give ester 10 followed by debenzylation with hydrogen over Pd/C in 92% ee and 70% overall yield. For the total synthesis of (S)-ar-turmerone ester, (R)-11, which was obtained in 88% yield and 93% ee in gram scale, was further transformed in six steps into this sesquiterpene in 92% ee.
Scheme 2. Enantioconvergent Co-Catalyzed Kumada Reactions of α-Bromo Esters 7 with Arylmagnesium Bromide.
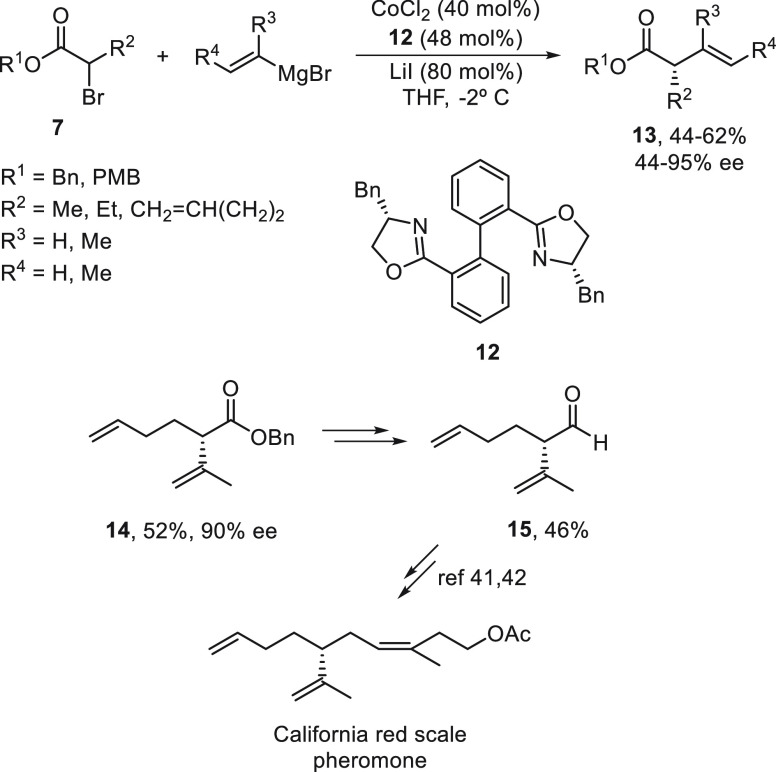
Recently, Zhong’s group40 achieved the Kumada reaction of α-bromo esters 7 with alkenyl Grignard reagents using a cobalt-bis(oxazoline) 12 catalysis to afford highly enantioenriched α-alkyl-β,γ-unsaturated esters 13 (Scheme 3). This enantioconvergent cross-coupling was applied to the formal synthesis of the California red scale pheromone isolated from female Aonidiella aurantia (Maskell). Ester 14 was obtained in 52% yield and 90% ee, and after reduction to the alcohol, oxidation to the aldehyde, and Wittig reaction, (R)-15 was further transformed41,42 into this pheromone. From the radical clock experiments, it could be assumed that this cross-coupling took place via a radical intermediate.
Scheme 3. Enantioconvergent Co-Catalyzed Kumada Reaction of α-Bromo Esters 7 with Alkenyl Grignard Reagents.
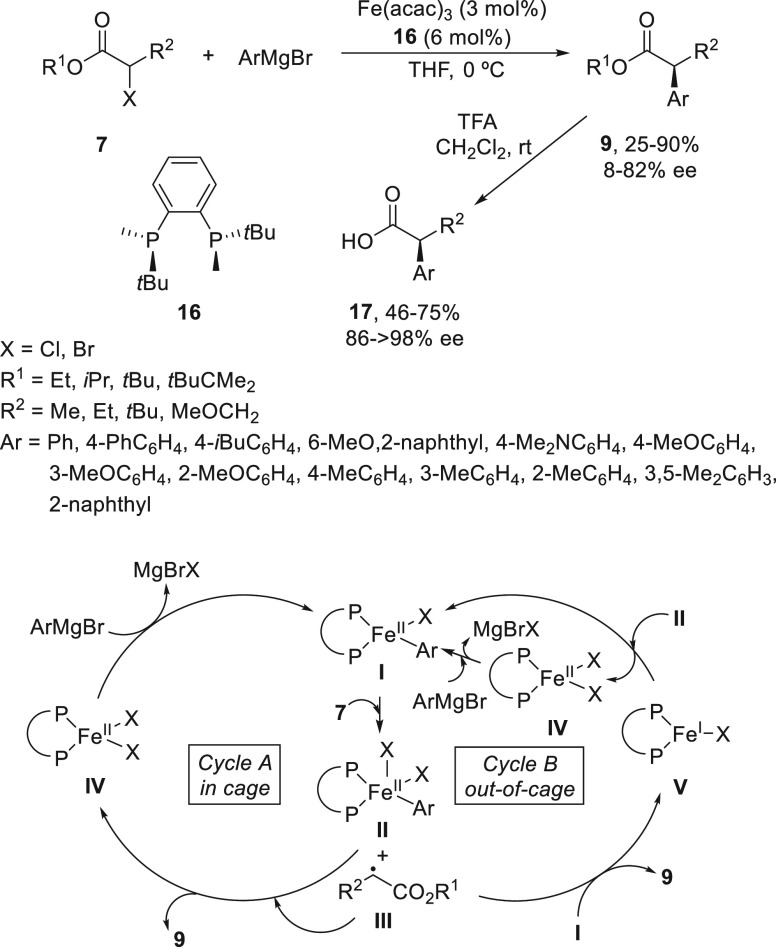
Iron-catalyzed enantioconvergent Kumada reaction of α-chloro and α-bromo alkanoates 7 has been described by Nakamura and co-workers.43 This cross-coupling is catalyzed by Fe(acac)3 and the chiral phosphine (R,R)-BenzP (16) working in THF at 0 °C to give esters 9 in up to 92% yield and 82% ee (Scheme 4). Compounds 9 were readily transformed into the corresponding α-aryl alkanoic acids 17 with up to >98% ee by simple deprotection/recrystallization. Radical probe experiments suggested a catalytic cycle in which the divalent iron species I was generated by partial reduction of Fe(acac)3 by the ligand. This species I abstracts the halogen from the substrate to give the iron species II and radical III. The arylation of intermediate III takes place by the aryl group of the iron species II in the solvent cage to provide the product 9 and the iron complex IV, which undergoes transmetalation with the Grignard reagent to regenerate species I (cycle A). A most favorable alternative process is depicted in cycle B on the basis of a bimetallic mechanism.44 In this out-of-cage mechanism, the radical III escapes from the solvent cage to react with the iron species I to form the coupling product 9 by forming the iron species V. Comproportionation of complexes II and V forms iron(II) species I and IV, or halogen abstraction of V from the α-halo ester 7 forms IV and radical III, which may participate in a chain reaction process.
Scheme 4. Enantioconvergent Fe-Catalyzed Kumada Reactions of α-Chloro and α-Bromo Esters 7 with Arylmagnesium Bromides.
Cross-couplings of α-bromo ketones can be enantioconvergently arylated under Ni/bis(oxazoline) catalysis. This arylation was also performed with α-halogenated esters using arylmagnesium bromides under Co/bis(oxazoline) and by Fe/diphosphine catalysis. Radical processes have been postulated in all these cases.
2.1.2. Organozinc Reagents
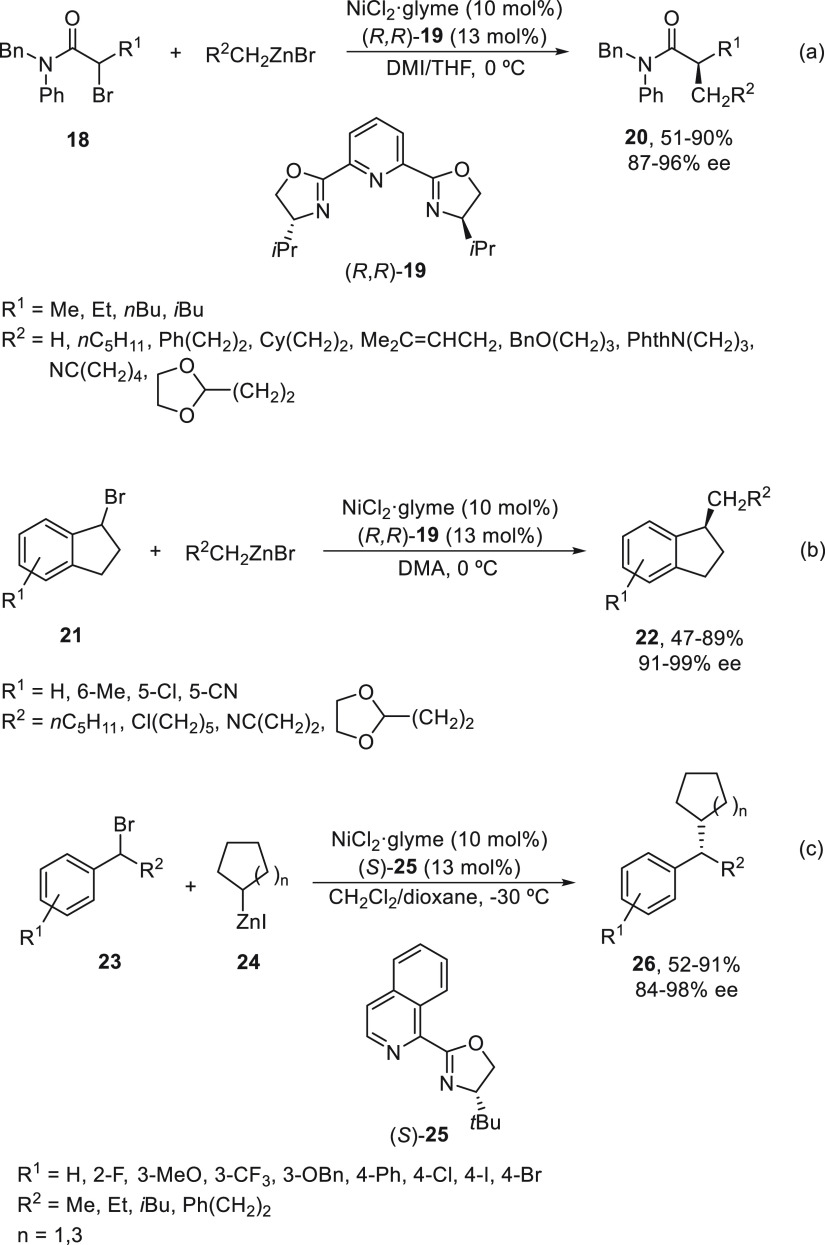
The first advantage in metal-catalyzed enantioconvergent cross-coupling of racemic alkyl electrophiles was performed with organozinc reagents by Fu and co-workers. Using NiCl2·glyme/(R,R)-iPrPyBox (19), α-bromo amides 18 underwent Negishi alkylation with alkylzinc bromides in 1,3-dimethyl-2-imidazolidinone (DMI)/THF at 0 °C to provide products 20 in up to 90% yield (Scheme 5a).45 Secondary benzylic halides, such as bromoindanes 21, reacted with primary alkylzinc bromides to give the corresponding alkylated derivatives 22 using N,N-dimethylacetamide (DMA) as solvent at 0 °C and the same catalyst in up to 89% yield and 99% ee (Scheme 5b).46 Further studies about this sp3–sp3 cross coupling but using secondary alkylzinc iodides 24 showed that an isoquinoline-oxazoline ligand (S)-25 gave the best results for secondary alkyl bromides 23 to afford products 26 in up to 91% yield and 98% ee (Scheme 5c).47
Scheme 5. Enantioconvergent Ni-Catalyzed Negishi Reactions of α-Bromo Amides 18 and Benzylic Halides 21 and 23 with Alkylzinc Reagents.
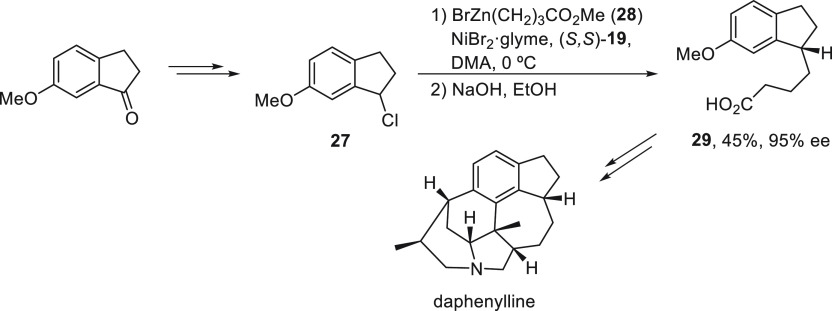
Yokoshima, Fukuyama, and co-workers48 employed this enantioconvergent Negishi reaction in the total synthesis of the alkaloid (−)-daphenylline isolated from the fruit Daphniphyllum longeracemosum.49 The chloroindane 27 reacted with the organozinc reagent 28 using NiBr2·glyme and (S,S)-iPrPyBox (19) as catalyst in DMA at 0 °C to furnish the corresponding acid 29 in 94% ee after hydrolysis and >98% ee after recrystallization of the salt formed with (R)-1-phenylethylamine (Scheme 6).
Scheme 6. Enantioconvergent Ni-Catalyzed Negishi Reaction of Chloroindane 27 with Alkylzinc 28.
Mechanistic studies by DFT calculations were performed by Lin and co-workers50 for the Negishi reaction of bromoindoles 21(46) to corroborate the Ni(I)–Ni(III) mechanism containing sequential oxidative addition–reductive elimination, which is more favorable than the Ni(0)–Ni(II) mechanism. In contrast to the calculations of Fu and co-workers38 for the Kumada reaction, in this case, it was suggested that the reductive elimination is the stereochemistry-determining step and not the coupling of the organonickel(II) complex with the organic radical (see Scheme 1).
Fu and co-workers51 obtained poor results when benzylic halides were allowed to react with arylzinc reagents. Instead, racemic benzylic mesylates were efficiently arylated using NiBr2·diglyme (9 mol %) and the bis(oxazoline) (S,S)-31 (Scheme 7). Starting from benzylic alcohols 30 after mesylation, the subsequent Negishi reaction was carried out in a one-pot process to provide 1,1-diarylalkanes 32 up to 98% yield and 95% ee. This method was applied to a gram-scale synthesis of (S)-sertraline tetralone 34 from alcohol 33, a precursor of sertraline hydrochloride (Zoloft, an antidepressant drug).
Scheme 7. Enantioconvergent Ni-Catalyzed Negishi Reactions of Benzylic Mesylates with Arylzinc Reagents.
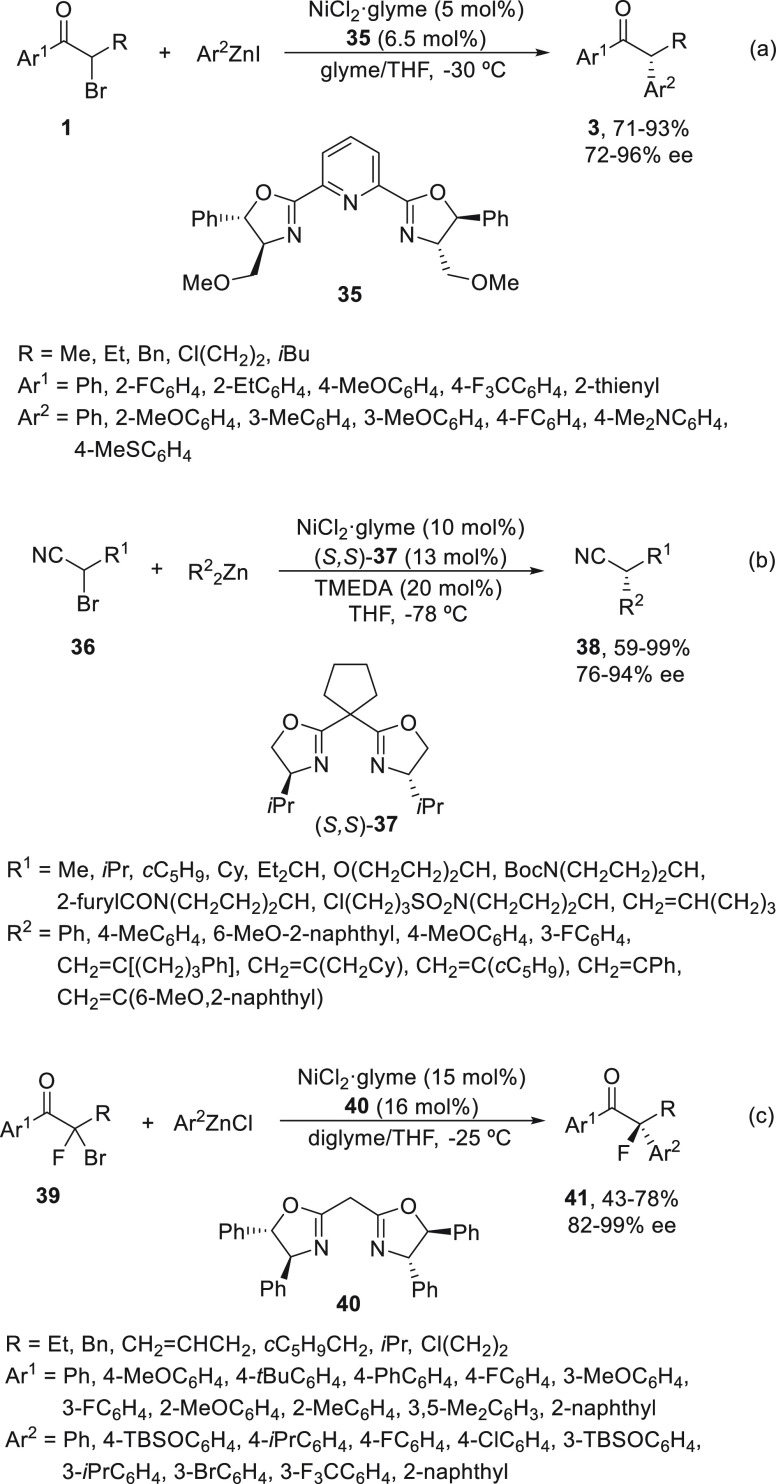
Racemic α-bromo ketones 1 have been arylated to ketones 3 with arylzinc iodides using NiCl2·glyme (5 mol %) and the PyBox ligand 35 with very good yields and enantioselectivities by Fu and co-workers51 (Scheme 8a). If the aryl group of the ketone was bulky, moderate enantioselectivity was observed. The reaction took place under mild reaction conditions with glyme/THF at −30 °C. α-Bromo nitriles 36 underwent enantioconvergent Negishi arylations and alkenylations52 using the PyBox ligand (S,S)-37 in the presence of tetramethylenediamine (TMEDA) (20 mol %) in THF at −78 °C to provide nitriles 38 (Scheme 8b). In this case, substrates prone to undergo cyclization gave acyclic products, which suggests that instead of radical intermediates, this cross-coupling proceeds by conventional oxidative addition. Another case of activated electrophiles is the arylation of α-bromo-α-fluoro ketones 39 using a bis(oxazoline) 40 at −25 °C (Scheme 8c).53 The corresponding tertiary alkyl fluorides 41 were obtained in good yields and high enantioselectivity (up to 99%), which can be further transformed into a variety of organofluorine target molecules.
Scheme 8. Enantioconvergent Ni-Catalyzed Negishi Reactions of α-Bromo Ketones 1, α-Bromo Nitriles 36, and α-Bromo-α-fluoro Ketones 39 with Aryl and Alkenylzinc Reagents.
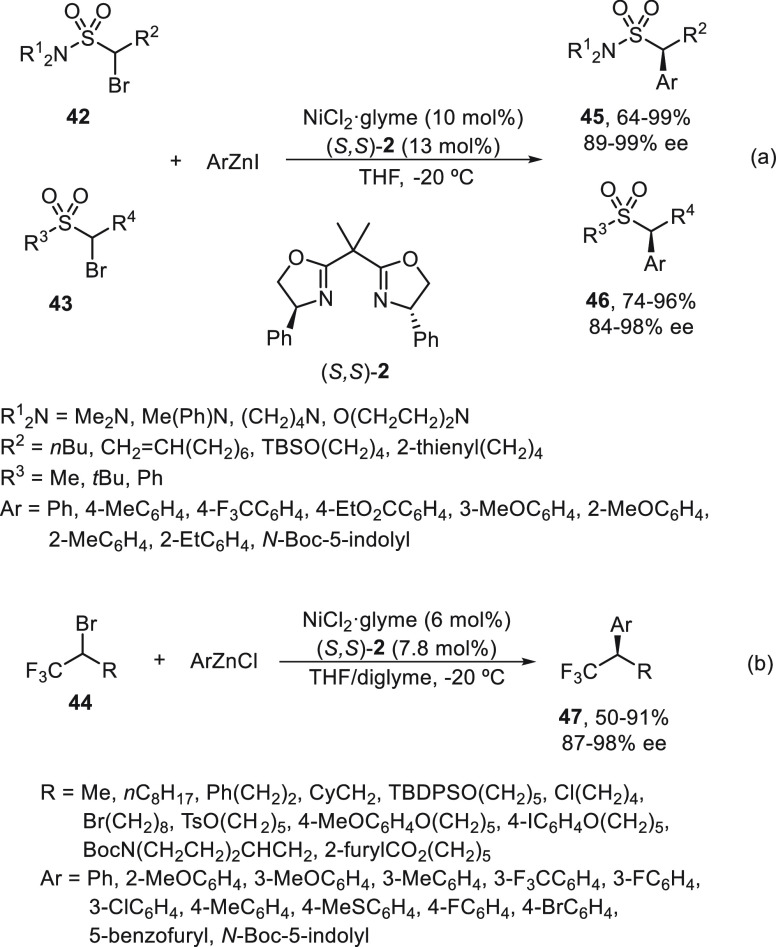
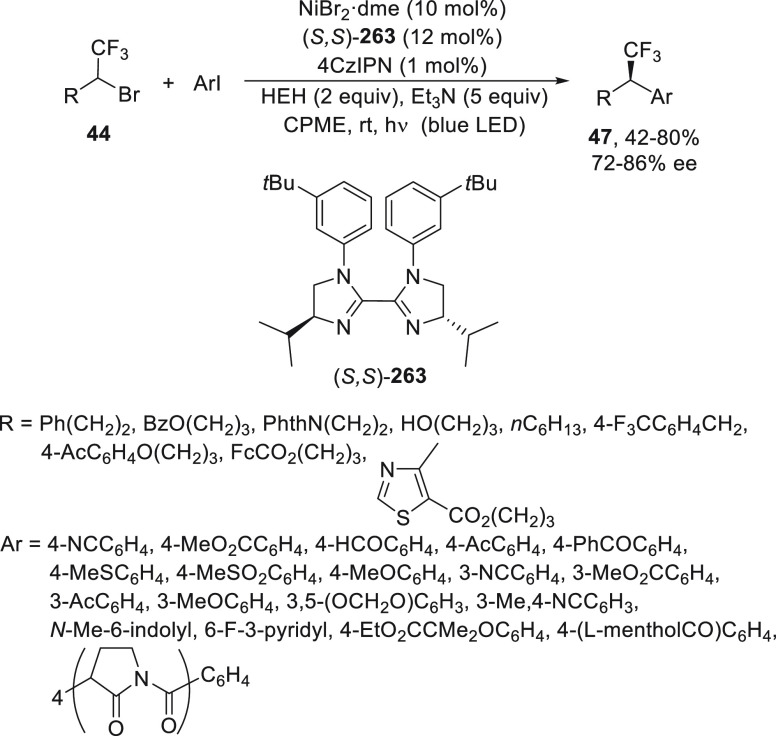
Fu and co-workers54,55 studied the enantioconvergent Negishi reaction of unactivated alkyl electrophiles, such as α-bromo sulfonamides 42(54) and sulfones 43,54 as well as CF3-substituted alkyl bromides 44(55) (Scheme 9). In all cases, NiCl2·glyme/(S,S)-PhBox (2) was used as catalyst at −20 °C with very good enantioselectivity. Sulfonamides 45 and sulfones 46 were obtained by arylation of the starting bromo derivatives 42 and 43, respectively (Scheme 9a).55 Experimental mechanistic studies provided evidence for a radical intermediate that has a sufficient lifetime to escape from the solvent cage and to cyclize onto a pendant olefin. Trifluoromethyl-substituted secondary alkyl bromides 44 were transformed into compounds 47 by reaction with arylzinc chlorides in very good yields and enantioselectivities (Scheme 9b).55 It is noteworthy that the Ni catalyst was able to differentiate between a CF3 and an alkyl substituent in the asymmetric cross-coupling.
Scheme 9. Enantioconvergent Ni-Catalyzed Negishi Reactions of α-Bromoalkyl Sulfonamides 42 and Sulfones 43, as Well as CF3–Substituted Alkyl Bromides 44 with Arylzinc Reagents.
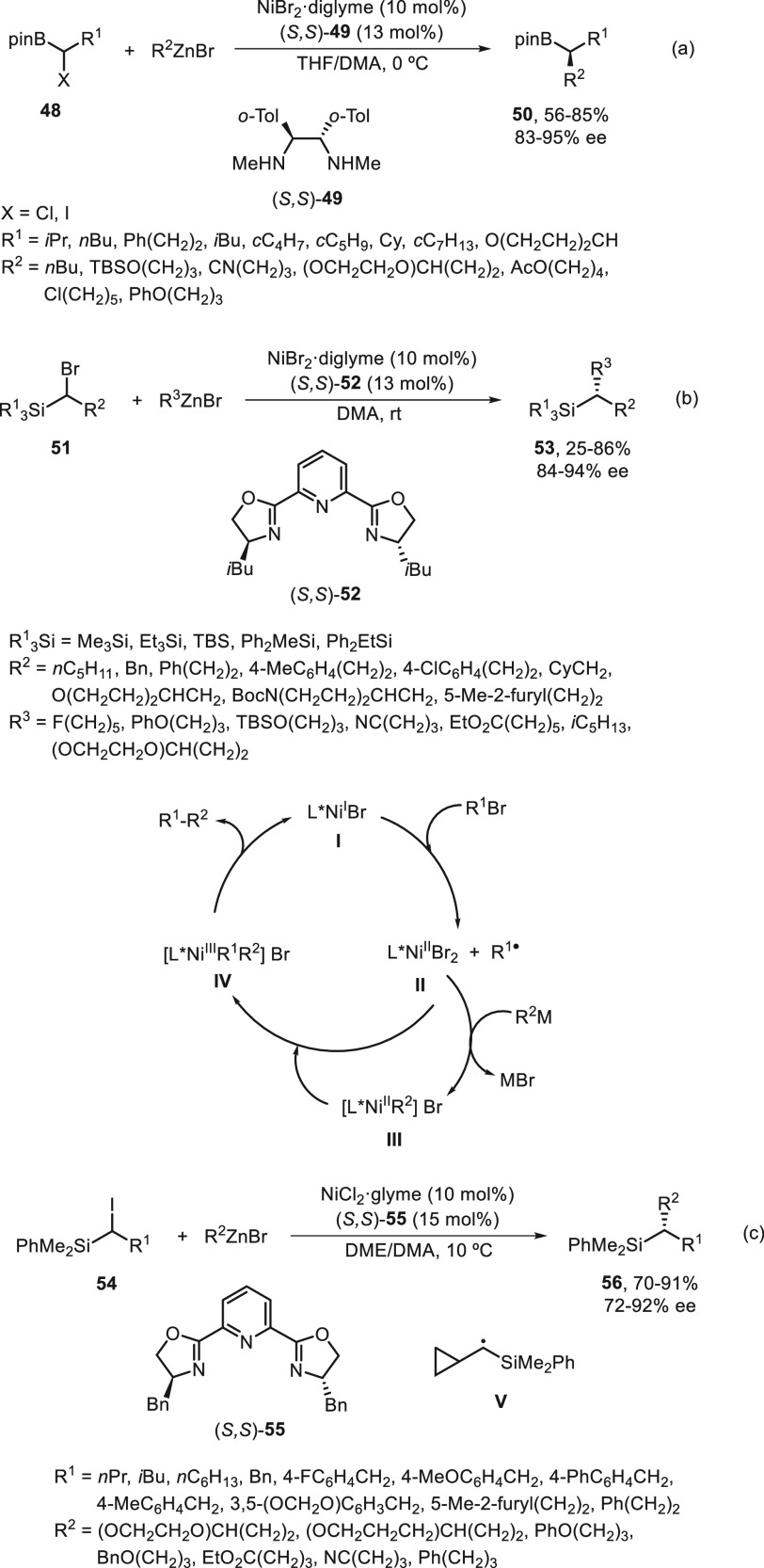
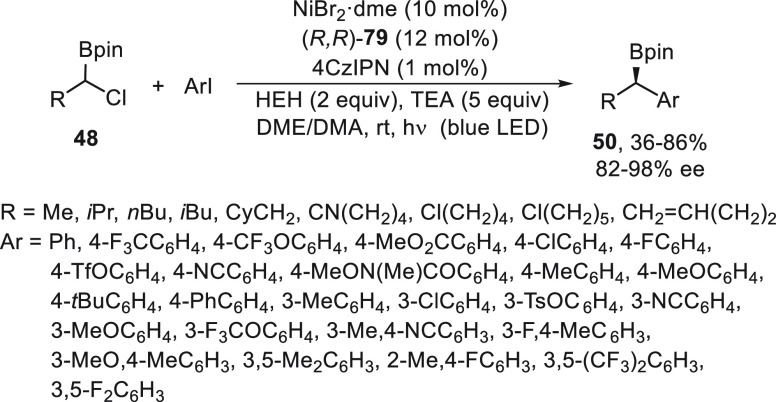
Enantioconvergent substitution reactions of α-haloboronates56 and α-halosilanes57 with alkylzinc reagents catalyzed by nickel have been carried out by Fu and co-workers. Enantioenriched alkylboronate esters are powerful building blocks in synthesis because of the facile transformation of C–B bonds into C–heteroatom bonds in a stereospecific manner. Using racemic α-haloboronates 48, enantioconvergent alkylation with alkylzinc reagents took place under NiBr2·diglyme/(S,S)-diamine 49 catalysis to furnish enantioenriched alkylboronates 50 (Scheme 10a).56 This alkyl–alkyl cross-coupling was carried out under mild reaction conditions THF/DMA at 0 °C and was compatible with different functional groups working with good yields and enantioselectivities. Several transformations to other families of enantioenriched compounds by C–C, C–N, C–halogen, and C–O bond formation were performed with little or no erosion in enantiomeric excess. Because of the synthetic interest of enantioenriched silanes, especially in medicinal chemistry,58−60 Matier, Fu, and Schwarzwalder57 studied the enantioconvergent cross-coupling of α-bromosilanes 51 with alkylzinc reagents in the presence of NiBr2·diglyme/bis(oxazoline) 52 (Scheme 10b). This alkylation proceeded at room temperature in DMA to give enantioenriched organosilanes 53 in moderate to good yields and up to 94% ee. Experiments with an enantioenriched α-bromosilane indicate that racemization occurred under the standard conditions, thereby confirming that the C–Br cleavage is reversible in discarding a DKR. In the proposed mechanism based on ESI-MS analysis and EPR spectrum, the Ni complex III is the predominant resting state during the catalytic cycle in which intermediates I–IV are involved. A consecutive publication of Oestreich and co-workers61 about the enantioconvergent alkylation of racemic α-iodosilanes 54 with alkylzinc bromides used similar reaction conditions (Scheme 10c). In this case, NiCl2/(S,S)-BnPyBox (55) was used as catalyst in a 5:2 mixture of DME/DMA at 10 °C to form the enantioenriched silanes 56 in high yield and slightly lower enantioselectivity. Control experiments with R1 = cyclopropyl revealed a radical clock mechanism supporting the intermediacy of a silicon-stabilized radical V.
Scheme 10. Enantioconvergent Ni-Catalyzed Negishi Reactions of α-Haloboronates 48 and α-Halosilanes 51 and 54 with Alkylzinc Bromides.
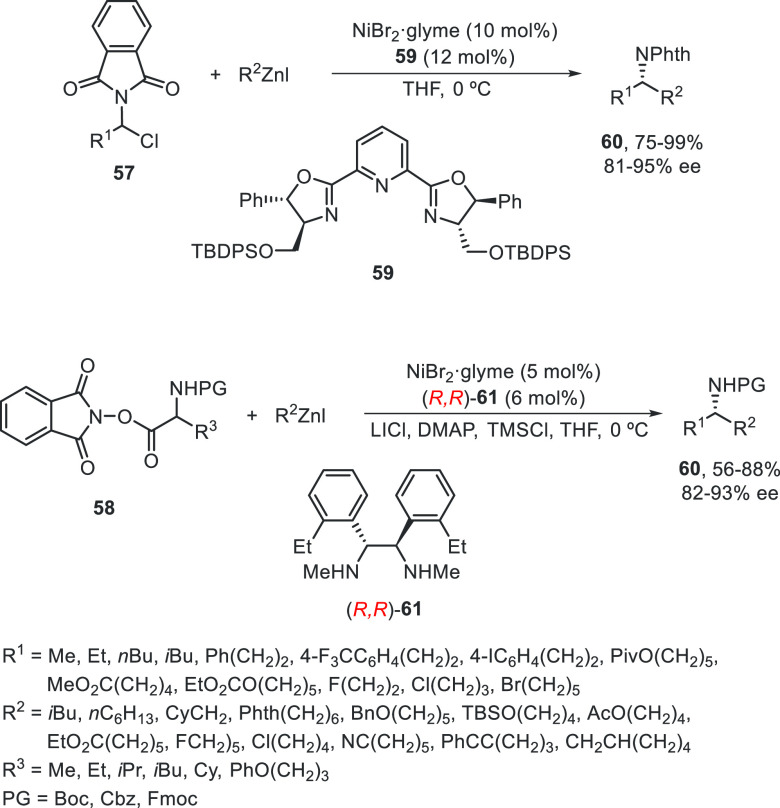
Enantioconvergent synthesis of amines has been achieved by Fu and co-workers using α-phthalimido alkyl chlorides 57 or N-hydroxyphthalimide (NHP) esters 58 of protected α-amino acids as electrophiles.62 Primary amines protected as phthalimides 57 reacted with alkylzinc iodides using NiBr2/bis(oxazoline) 59 as catalyst to provide protected dialkyl carbinamines 60 in good yields and enantioselectivities (Scheme 11). In the case of redox-active NHP esters 58, a decarboxylative coupling with alkylzinc iodides takes place in the presence of NiBr2/diamine 61 as catalyst to afford N-protected dialkyl carbinamines 60 in good yields and enantioselectivities.
Scheme 11. Enantioconvergent Ni-Catalyzed Negishi Reaction of α-Phthalimido Alkyl Chlorides 57 or N-Hydroxyphthalimide Esters 58 of Protected α-Amino Acids with Alkylzinc Iodides.
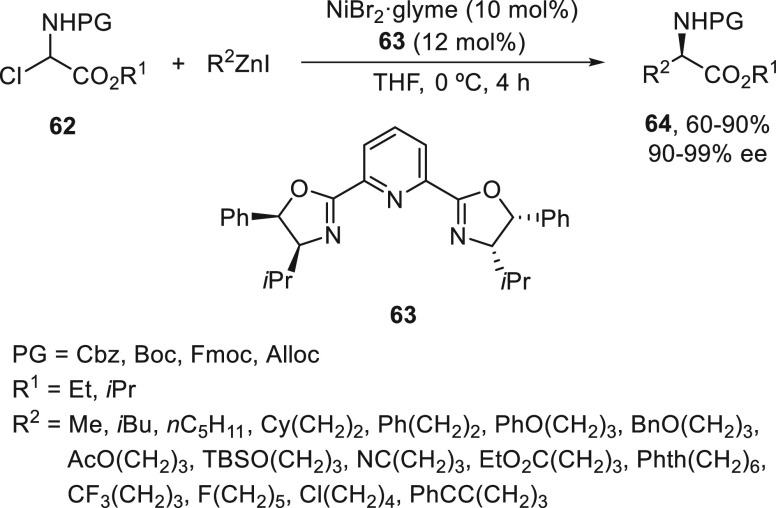
Recently, Fu and co-workers63 applied the Ni-catalyzed enantioconvergent Negishi reaction to the synthesis of α-amino acids. Starting from N-protected α-chloro glycinates 62,64 the alkylation with alkylzinc iodides (1:1.1 molar ratio) using NiBr2·glyme/bis(oxazoline) 63 as catalyst in THF at 0 °C furnished protected α-amino acids 64 in good yields (60–90%) and excellent enantioselectivities (up to 99%) (Scheme 12). These couplings were achieved under mild reaction conditions and are tolerant of air, moisture, and a wide variety of functional groups, and have been applied to the synthesis of intermediates en route to bioactive compounds in gram scale.
Scheme 12. Enantioconvergent Ni-Catalyzed Negishi Reactions of α-Chloro Glycinates 62 with Alkylzinc Iodides.
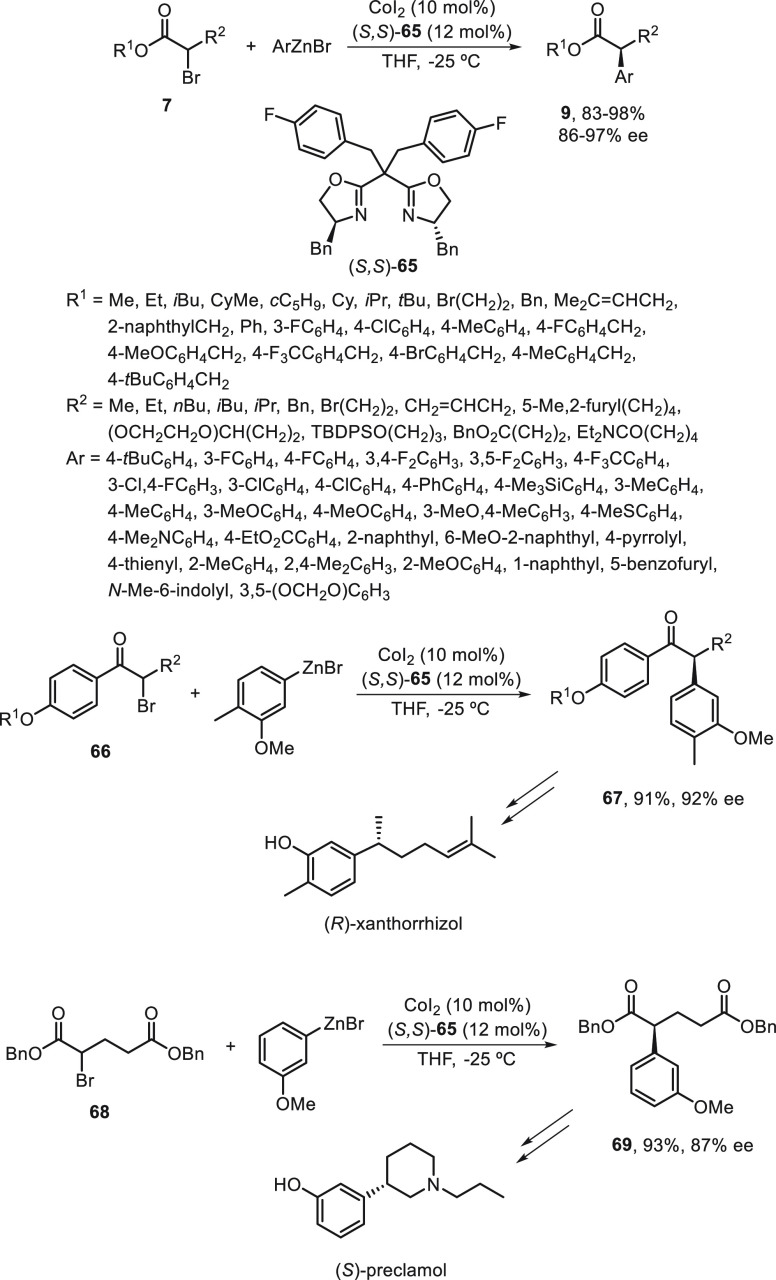
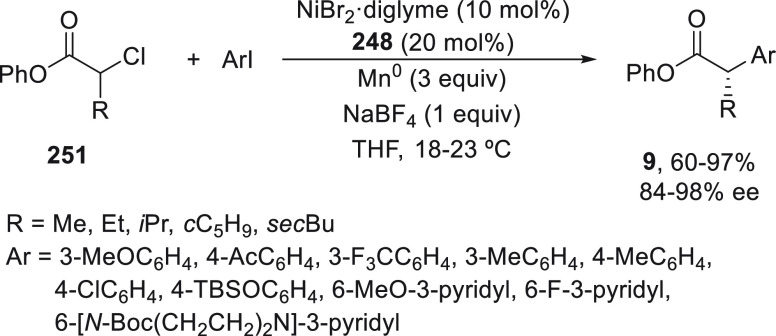
Cobalt-catalyzed enantioconvergent cross-couplings of racemic α-bromo esters 7 with arylzinc reagents, previously described with arylmagnesium reagents (Scheme 2),38 have been performed by Bian and co-workers.65,66 Differently substituted α-bromo esters 7 reacted with arylzinc bromides using CoI2/bis(oxazoline) (S,S)-65 as chiral catalyst in THF at 25 °C to provide compounds 9 in very good yields and ee (Scheme 13). They used radical probes 7 bearing a cyclopropyl and 4-pent-en-1-yl substituents to demonstrate the radical pathway of this Co-catalyzed Negishi reaction. This process was applied in gram scale to the synthesis of the sesquiterpene (R)-xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. rhizome, which has anti-inflammatory, antioxidant and antiestrogenic properties. In this case, the reaction of ester 66 with 4-methyl-3-methoxyphenylzinc bromide gave ester 67 with 91% yield and 92% ee, which is the key precursor of (R)-xanthorrizol. The same group has performed the enantioselective synthesis of (S)-predamol, a central dopamine receptor agonist via a Co-catalyzed enantioconvergent Negishi reaction (Scheme 13).66 The α-bromo ester 68 was allowed to react with 3-methoxyphenylzinc bromide under the previously described reaction conditions to provide 69, a key precursor of (S)-preclamol.
Scheme 13. Enantioconvergent Co-Catalyzed Negishi Reactions of α-Bromo Esters 7 with Arylzinc Bromides.
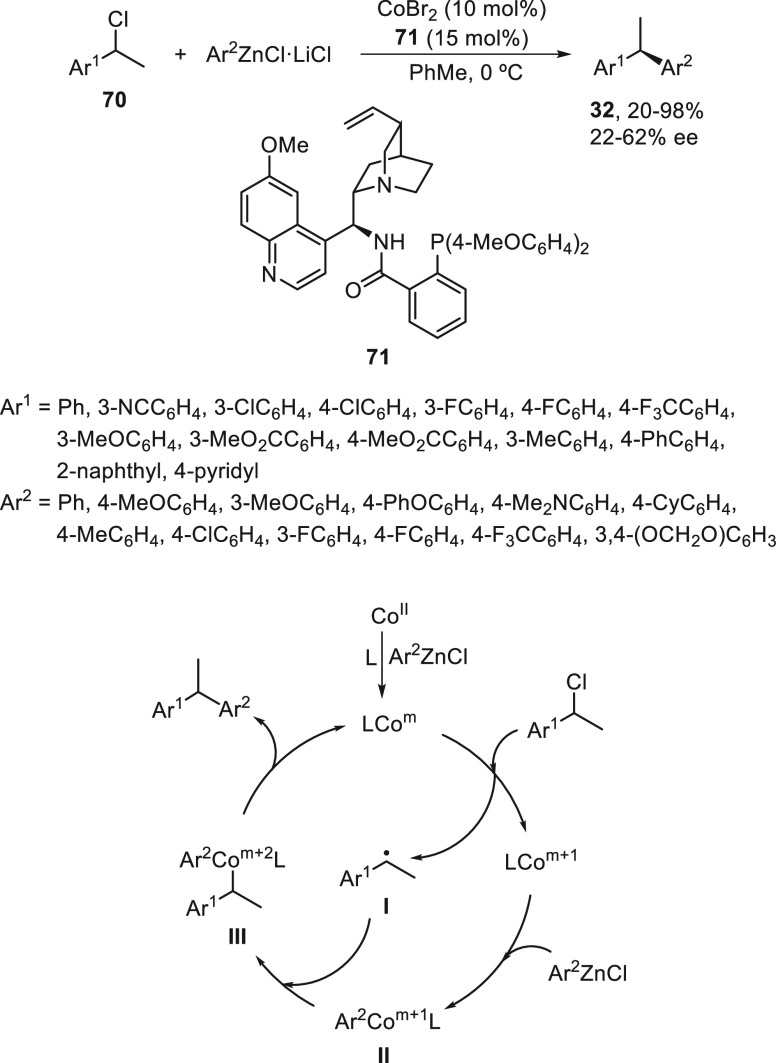
A Negishi C(sp3)–C(sp2) cross-coupling of racemic benzyl chlorides 70 with arylzinc reagents has been carried out under Co catalysis by Gu, Liu, and co-workers.67 In this case, a chiral monodentate anionic ligand 71 and CoBr2 were used as catalysts in toluene at 0 °C to give 1,1-diarylethanes 32 in up to 98% yield and moderate ee (Scheme 14). According to experimental studies, a radical mechanism has been proposed. The CoII is reduced by the arylzinc reagent to the active catalytic species LCoIII, which undergoes an electron-transfer reaction with the benzyl chloride to generate a benzylic radical I and the LCom+1 species. Subsequent transmetalation between the LCom+1 species and the arylzinc reagent provides complex II, which recombines with the benzyl radical I to deliver complex III. Final reductive elimination of III furnished the coupling product and regenerated the catalyst.
Scheme 14. Enantioconvergent Co-Catalyzed Negishi Reactions of Benzyl Chlorides 70 with Arylzinc Reagents.
As a summary of this Section 2.1.2, nickel-catalyzed enantioconvergent cross-coupling reactions allow the C–C bond formation between activated secondary alkyl electrophiles and alkyl or arylzinc reagents to form enantioenriched compounds mainly using mono- and bis(oxazolines) as chiral ligands. In some cases, this Negishi reaction can be also performed under cobalt catalysis. The most favorable mechanism involves the formation of an alkyl radical, which reacts with the chiral organonickel or cobalt intermediates through an out-of-cage pathway to afford the coupling product by final reductive elimination.
2.1.3. Organoboron Reagents
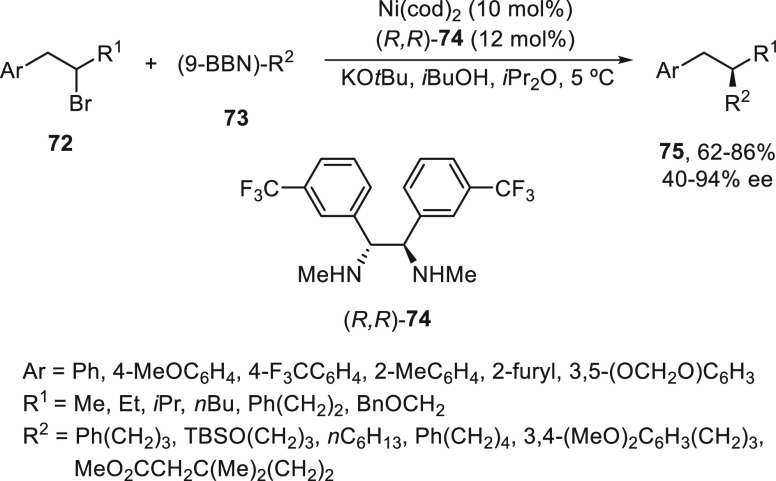
Nickel-catalyzed Suzuki cross-couplings of alkyl halides and alkylboronic acids were first described by Zhou and Fu.68 On the basis of the cross-coupling of unactivated alkyl halides with alkylboranes using trans-N,N′-dimethyl-1,2-cyclohexanediamine,69 Saito and Fu70 performed an enantioconvergent version of this alkyl–alkyl Suzuki reaction using a chiral diamine as ligand. Homobenzylic bromides 72 reacted with alkyl-(9-BBN) 73 using Ni(cod)2/(R,R)-74 as catalyst to give products 75 in good yields (up to 86%) and ee (up to 94%) (Scheme 15). This method was applied to the arylation of racemic α-chloro- and α-bromo amides in which a modest kinetic resolution of the α-chloro amide was observed.71
Scheme 15. Enantioconvergent Ni-Catalyzed Suzuki Reactions of Homobenzylic Bromides 72 with Alkylboranes 73.
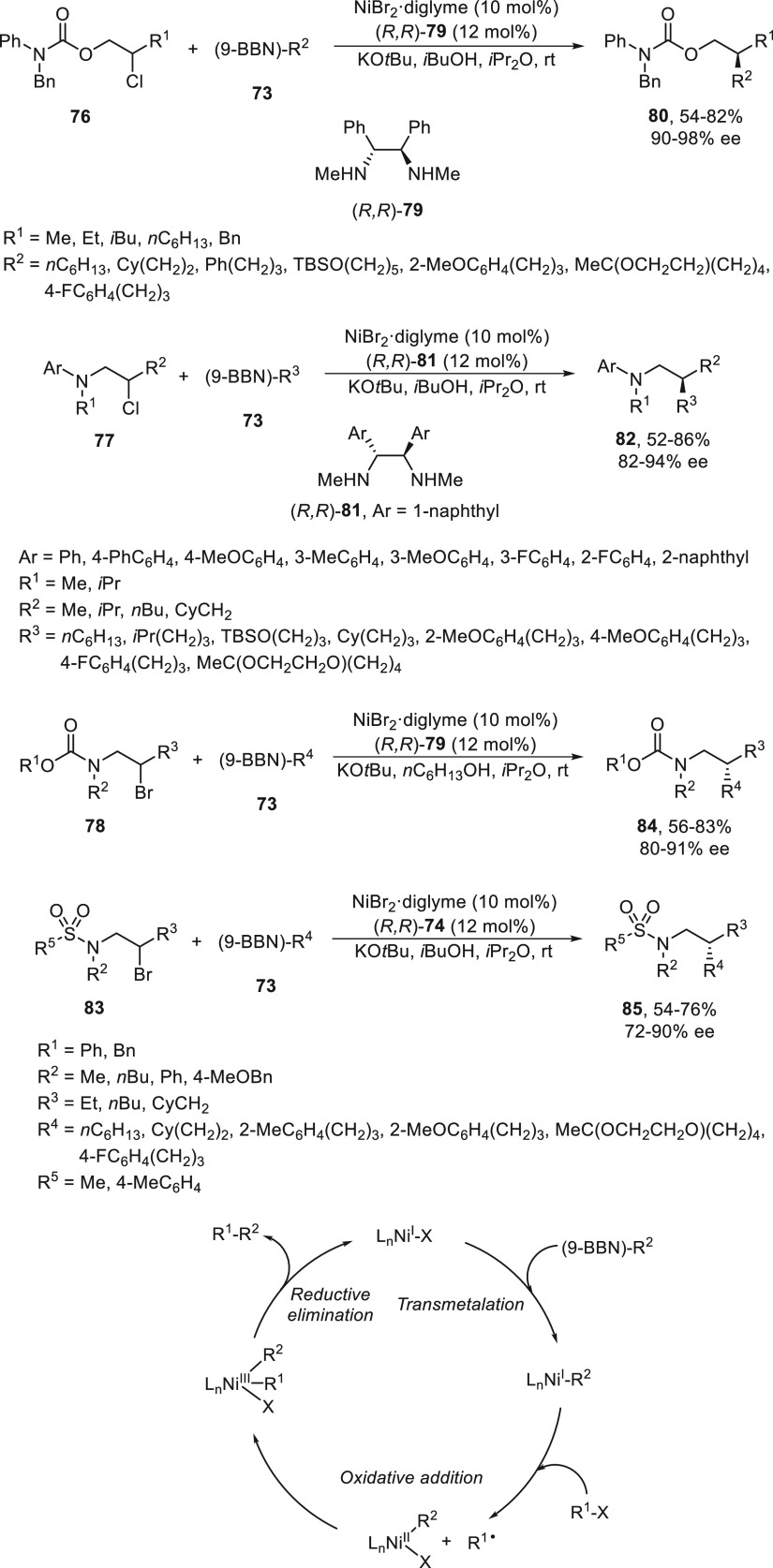
Subsequent studies on enantioconvergent alkyl–alkyl Suzuki reactions by Fu and co-workers were performed with acylated halohydrins 76,72 β-halo alkylanilines 77,73 and N-protected β-bromo alkylamines 78(74) (Scheme 16). In all these cases, the presence of a directing group, which likely interacts with the chiral catalyst, is essential for the high enantioselectivity observed with these unactivated secondary alkyl halides. Acylated halohydrins 76 and alkylboranes 73 reacted in the presence of NiBr2/diamine (R,R)-79 as catalyst, which provided products 80 in up to 82% yield and high enantioselectivity (90–98% ee).72 β-Chloro alkylanilines 77 reacted with boranes 73 using NiBr2/diamine 81 as catalyst to give enantioenriched β-alkylanilines 82 up to 86% yield and up to 94% ee.73 In the case of N-protected β-bromo alkylamines 78 or 83 bearing a carbonate or a sulfonamide group, respectively, the alkylation with boranes 73 was performed with NiBr2 and diamine 79 or 74 as chiral ligands to furnish products 84 or 85, respectively. Experimental evidence showed that the alkyl group of the organoborane is transferred to the reaction product with retention of the configuration consistent with transmetalation with retention. Therefore, the structural integrity for the Ni–R2 bond is maintained during the catalytic cycle depicted in Scheme 16.
Scheme 16. Enantioconvergent Ni-Catalyzed Suzuki Reactions of β-Halogenated Compounds: Reaction of Acylated Halohydrins 76, β-Chloro Alkylanilines 77, and N-Protected β-Bromoalkylamines 78 and 83 with Alkylboranes 73.
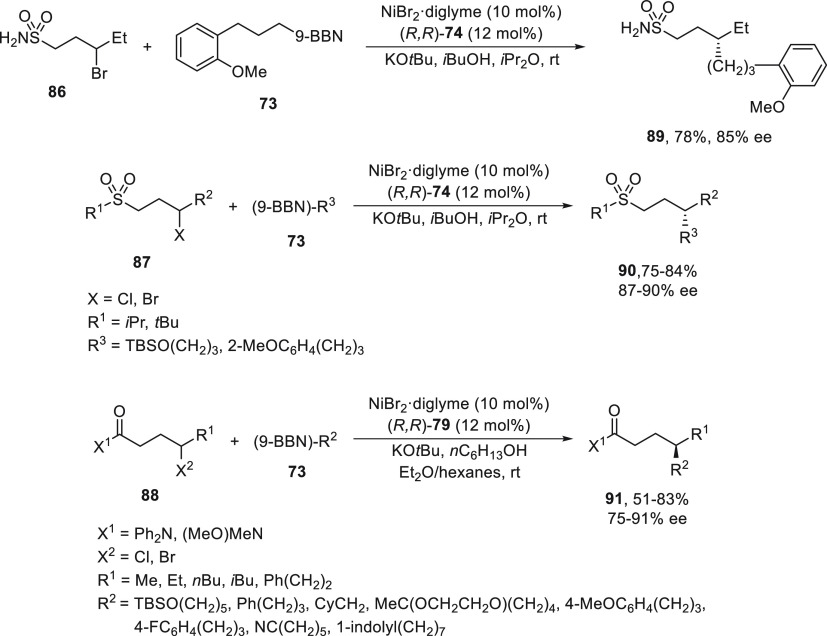
The sulfone group directed the Ni-catalyzed Suzuki reaction at the γ-position of sulfonamide 86 and sulfones 87 under the reaction conditions indicated for sulfonamides 83 (Scheme 16).74 This enantioconvergent γ-alkylation took place with γ-halo carboxamides 88 using NiBr2/diamine (R,R)-79 as catalyst.75 In the case of sulfonamide 86, product 89 was obtained in 78% yield and 85% ee, and sulfones 87 provided compounds 90 in 75–84% yields and 87–90% ee (Scheme 17).74 Carboxamides 88 bearing a bromo or chloro substituents at the γ-position afforded, by reaction with boranes 73, the corresponding cross-coupling products 91 in good yields and enantioselectivities (Scheme 17).75
Scheme 17. Enantioconvergent Ni-Catalyzed Suzuki Reactions of γ-Halogenated Sulfonamide 86, Sulfones 87, and Carboxamides 88 with Alkylboranes 73.
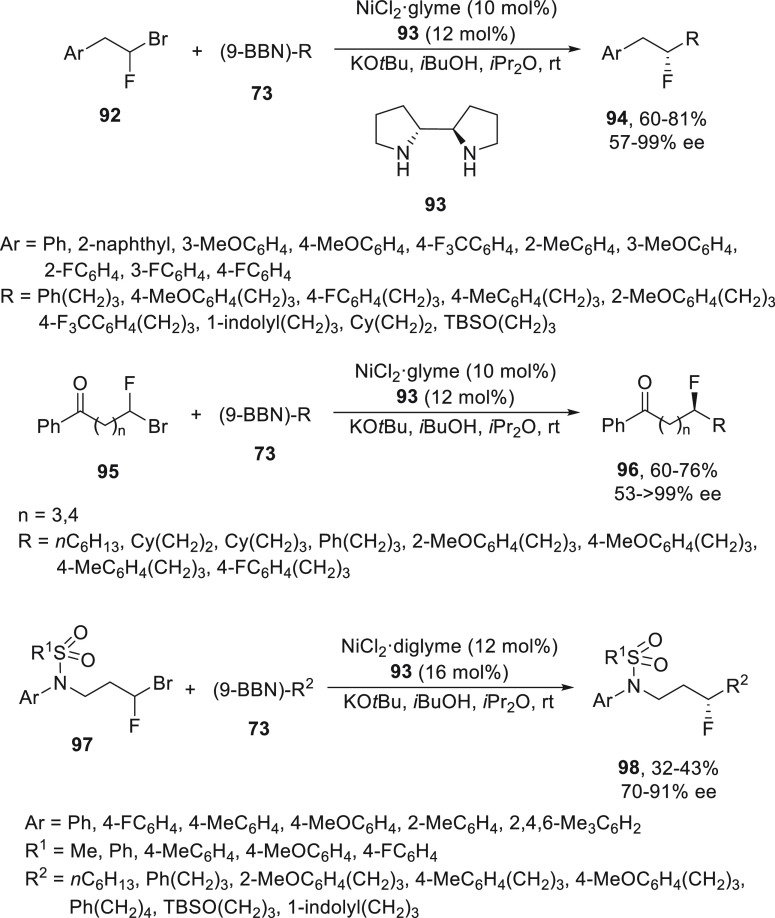
Gandelman and co-workers76,77 described the enantioconvergent synthesis of secondary alkyl fluorides by Suzuki cross-coupling of 1-fluoro-1-haloalkanes with alkylboranes 73. 1-Bromo-1-fluoro-2-arylethanes 92 were alkylated using NiCl2/diamine 93 as catalyst to give products 94 up to 81% yield and up to 99% ee (Scheme 18). Under these reaction conditions, fluorobromoalkanes bearing different directing groups, such as ketones 95, provided chiral δ- and ε-fluoroalkanes 96 after alkylation. 1-Bromo-1-fluoroalkanes bearing a sulfonamide-directing group 97 gave γ-fluorosulfonamides 98 after enantioconvergent cross-coupling with alkylboranes 73 with modest yield and up to 91% ee.
Scheme 18. Enantioconvergent Ni-Catalyzed Suzuki Reactions of 1-Halo-1-fluoroalkanes 92, 95, and 97 with Alkylboranes 73.
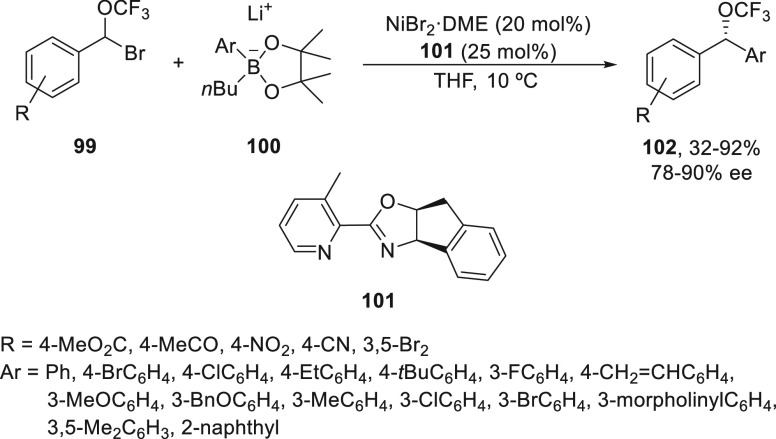
Starting from racemic α-bromobenzyl trifluoromethyl ethers 99, Shen and co-workers78 performed the enantioconvergent cross-coupling with aryl pinacol boronates 100 to form α-trifluoromethoxy-substituted diaryl methanes 102 (Scheme 19). The reaction took place under mild reaction conditions using NiBr2/oxazoline 101 as catalyst to afford products 102 with good yields and up to 90% ee. However, other organometals, such as phenylmagnesium bromide or diphenylzinc, gave compounds 102 with lower yields due to the side reactions of these Lewis acidic organometals with products 102. Several functional groups are tolerated under these reaction conditions, and the reaction can be easily scaled up to grams.
Scheme 19. Enantioconvergent Ni-Catalyzed Suzuki Reactions of α-Bromobenzyl Trifluoromethyl Ethers 99 with Aryl Pinacol Boronates 100.
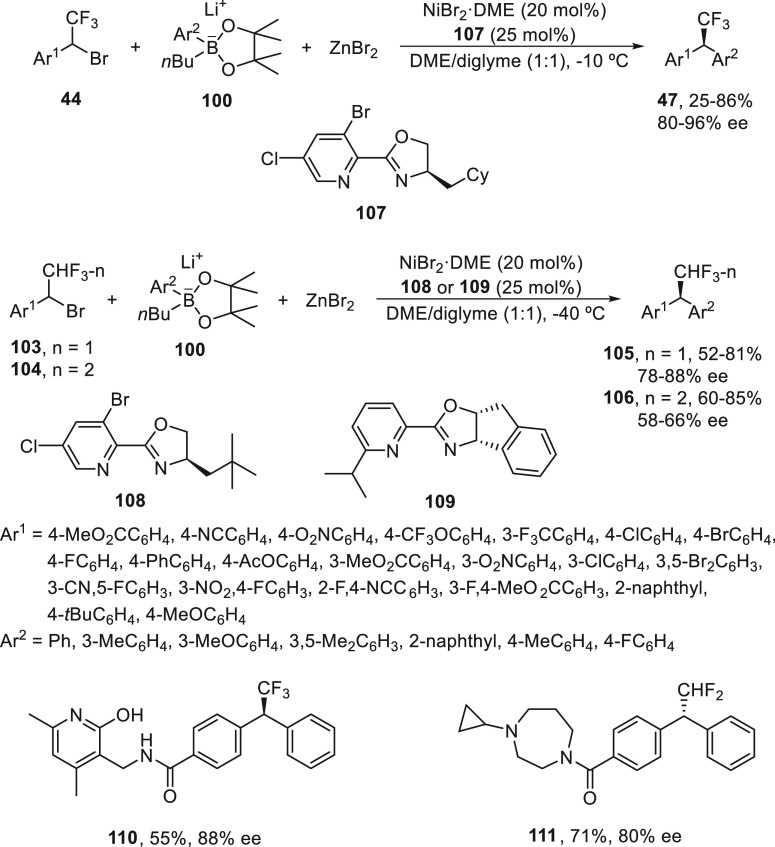
The same group79 has developed an enantioconvergent acylation of secondary α-bromobenzyl trifluoro-/difluoro-/monofluoromethanes 44/103/104 with dilithium aryl zincates, [Ar2ZnBr]Li, generated in situ from lithium organoborates 100 and ZnBr2 (Scheme 20). The use of lithium aryl zincates facilitates the transmetalation step of this nickel-catalyzed cross-coupling reaction, thereby allowing the synthesis of enantioenriched benzhydryl fluoroalkene derivatives 47, 105, and 106 using NiBr2·DME and a pyridine-oxazoline ligand 107 for trifluoromethyl substrates 44, 108 for difluoromethyl compounds 103, and ligand 109 for fluoromethyl reagents. This procedure was applied to the synthesis of 110, a trifluoromethylated mimic of an inhibitor for the histone lysine methyltransferase enhancer of zeste homologue 2 (EZH2)80 in 55% overall yield and with 88% ee. A difluoromethylated compound 111, which is a mimic of histamine H3 receptor,81 was prepared in 71% overall yield and with 80% ee.
Scheme 20. Enantioconvergent Ni-Catalyzed Arylation of Fluorinated Benzylic Bromides 44, 103, and 104 with Lithium Organoboronates 100 and ZnBr2.
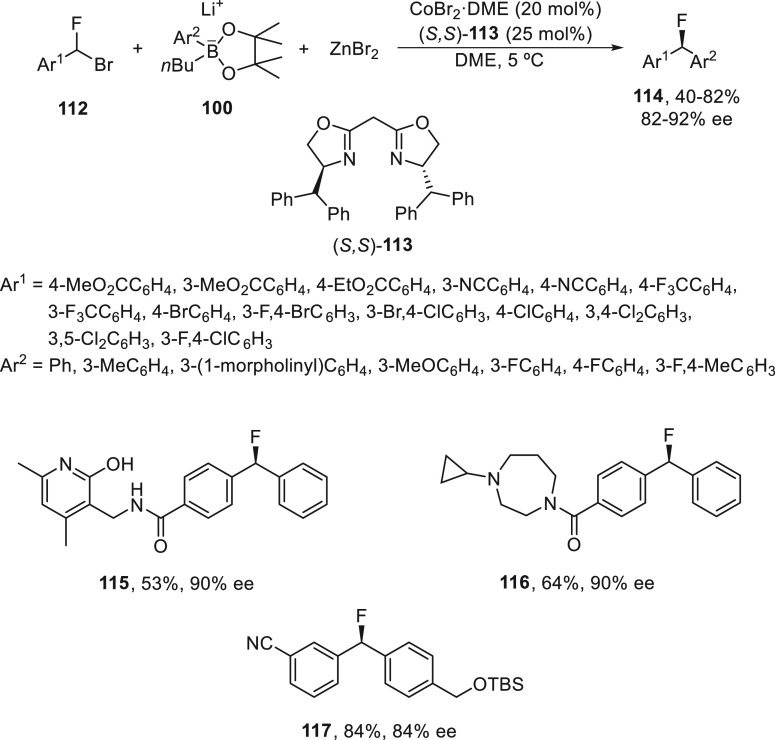
For the cross-coupling of fluorinated secondary benzyl bromides 112, Shen and co-workers82 also employed in situ generated lithium aryl zincates but under cobalt catalysis. In this case, CoBr2·DME and bis(oxazoline) (S,S)-113 were used as chiral catalysts under mild reaction conditions to provide fluorinated diarylmethane derivatives 114 in up to 92% ee (Scheme 21). This methodology was applied to the synthesis of compounds 115 and 116, which are fluorinated mimics of EZH2,80 respectively. Compound 117, a key intermediate of a fluorine-substituted analogue of Lilly’s mGlu 2 receptor potentiators, which is a compound for the treatment of migraine headaches,83 was prepared in 84% yield with 84% ee.
Scheme 21. Enantioconvergent Co-Catalyzed Arylation of Fluorinated Secondary Benzyl Bromides 112 with Lithium Aryl n-Butyl Pinacol Boronates 100 and ZnBr2.
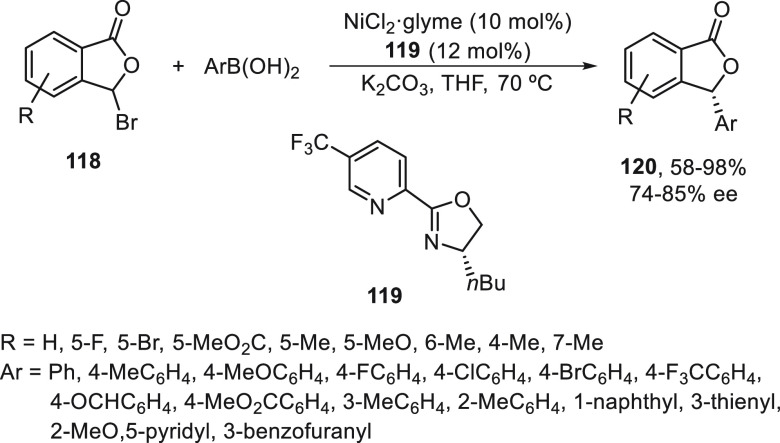
Enantioconvergent Suzuki reactions of 3-bromophthalides 118 with arylboronic acids was recently reported by Zhang, Feng, and co-workers.84 Cross-coupling with arylboronic acids took place using NiCl2/oxazoline 119 as catalyst and K2CO3 as base in THF at 70 °C to give chiral 3-arylphthalides 120 with good yields and up to 85% ee (Scheme 22).
Scheme 22. Enantioconvergent Ni-Catalyzed Suzuki Reaction of 2-Bromophthalides 118 with Arylboronic Acids.
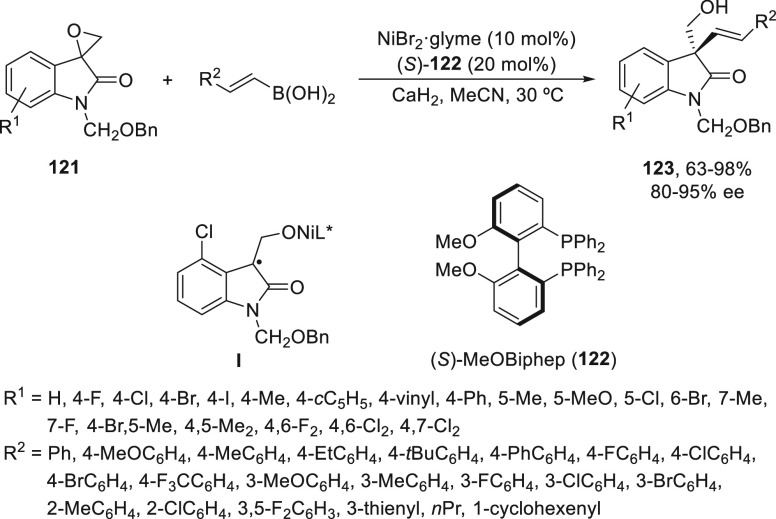
In general, the alkyl electrophiles of these Suzuki reactions are secondary, which give rise to chiral tertiary carbon stereocenters. Recently, Zhang and co-workers85 reported a Ni-catalyzed enantioconvergent coupling of epoxides 121 with alkenylboronic acids. These racemic spiroepoxyindoles 121 afforded chiral oxindoles 123 bearing quaternary carbon stereocenters using NiBr2/(S)-MeOBiphen (122) as catalyst (Scheme 23). In this case, CaH2 was used as base, and MeCN was used as solvent at 30 °C to give products 123 with good yields and enantioselectivities. It has been proposed that the formation of a stabilized tertiary radical intermediate I by a single-electron transfer mechanism is formed during the oxidative addition step.
Scheme 23. Enantioconvergent Ni-Catalyzed Suzuki Reactions of Spiroepoxyindoles 121 with Alkenylboronic Acids.
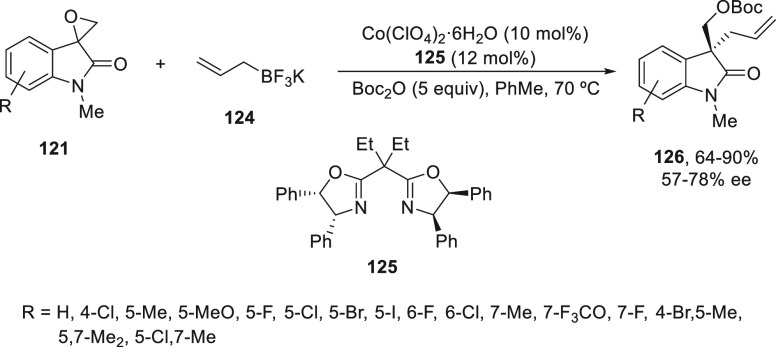
The same group performed this ring-opening reaction of spiroepoxyindoles 121 with allylboron reagents under Co(II) catalysis.86 Potassium allyltrifluoroborate (124) reacted with epoxides 121 using Co(ClO4)2/bis(oxazoline) 125 in the presence of di-tert-butyl dicarbonate (Boc2O) in order to avoid the coordination of the alcohol from the ring-opening product with the chiral catalyst. Chiral oxindoles 126 were obtained with yields of 64–90% and up to 78% ee (Scheme 24).
Scheme 24. Enantioconvergent Co-Catalyzed Suzuki Reactions of Spiroepoxyoxindoles 121 with Potassium Allyltrifluoroborate 124.
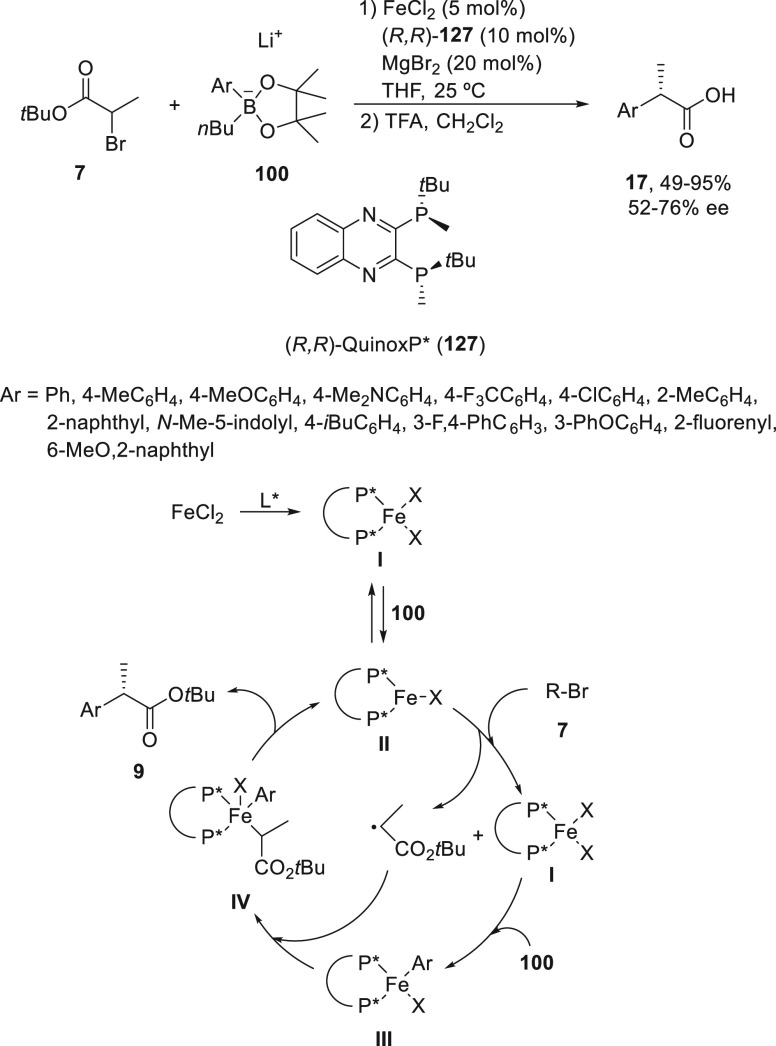
Nakamura and co-workers87 have described the enantioconvergent iron-catalyzed Suzuki reaction of tert-butyl α-bromopropionates 7, which was previously described with Grignard reagents43 (Scheme 4). In this case, lithium aryl pinacol boronates 100 were used as coupling partners, and FeCl2/(R,R)-QuinoxP* (127) was used as catalyst to provide, after TFA hydrolysis of the corresponding esters 9, enantioenriched α-arylpropionic acids 17 with good yields and moderate enantioselectivities (Scheme 25). As mentioned before, α-arylpropionic acids are well-known nonsteroidal anti-inflammatory drugs (NSAIDs). A plausible mechanism based on experimental and theoretical studies is depicted in Scheme 25. Transmetalation of complex I (LFeCl2) with the boron reagent and subsequent reductive elimination gave the active species II. This intermediate abstracts the bromine atom from compound 7 to generate the corresponding alkyl radical, which recombines with complex III generated by transmetalation of I with the boron reagent 100 to produce intermediate IV. Final reductive elimination of complex IV affords the expected product.
Scheme 25. Enantioconvergent Fe-Catalyzed Suzuki Reaction of t-Butyl α-Bromopropionate 7 with Lithium Aryl Pinacol Boronates 100.
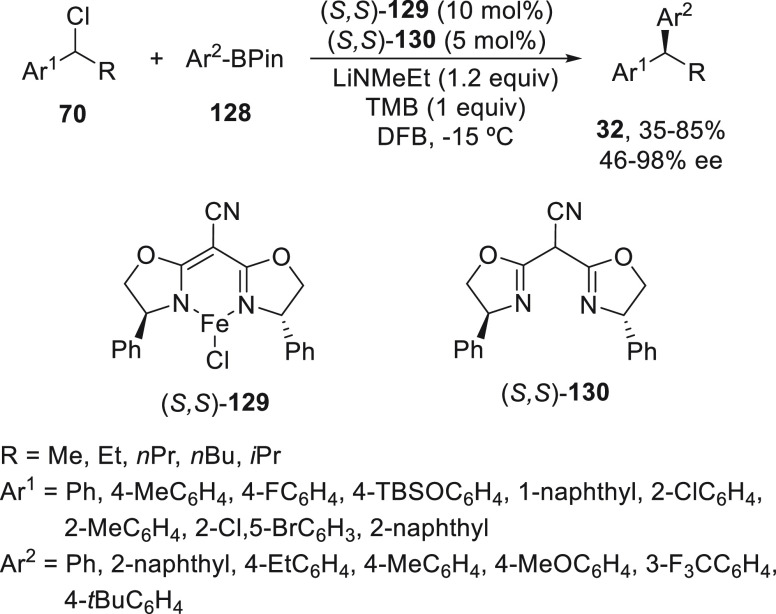
Enantioenriched 1,1-diarylalkanes 32 have been prepared by enantioconvergent Suzuki reaction of benzylic chlorides 70 with arylboronic pinacol esters 128 using cyano[bis(oxazoline)]iron(II) chloride complex 129 and the ligand 130 as catalyst (Scheme 26).88 This cross-coupling took place under mild reaction conditions in the presence of 1,3,5-trimethoxybenzene (TMB) as a stoichiometric additive, LiNMeEt as base, and 1,2-difluorobenzene (DFB) as solvent at −15 °C. Products 32 were obtained with moderate to good yields and enantioselectivities.
Scheme 26. Enantioconvergent Iron-Catalyzed Suzuki Reactions of Benzylic Chlorides 70 with Arylboronic Pinacol Esters 128.
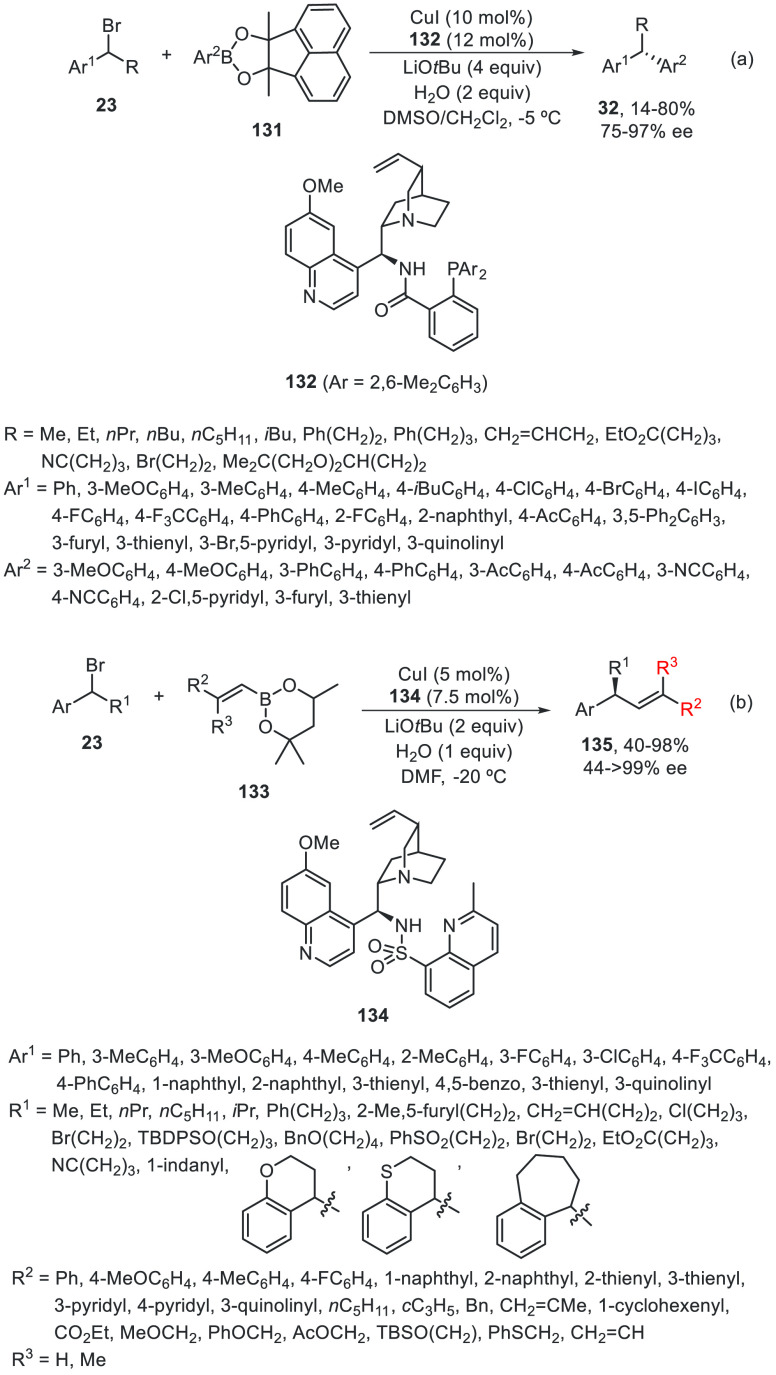
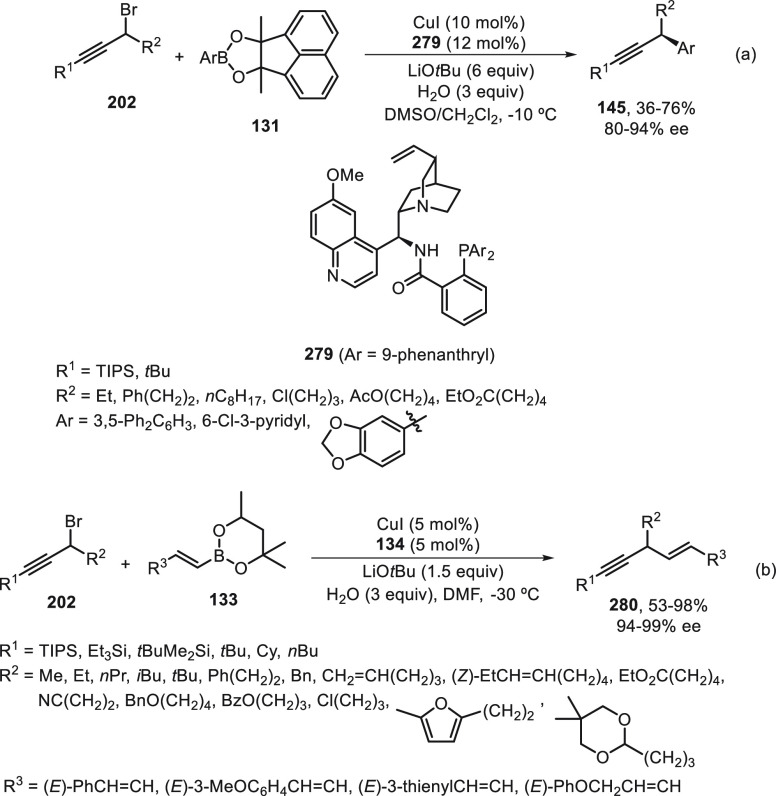
Li, Liu, and co-workers89,90 have developed the Cu-catalyzed enantioconvergent radical Suzuki C(sp3)–C(sp2) cross-coupling. Benzylic bromides 23 have been allowed to react with cyclic B(mac)-derived boronate esters 131 using CuI/N,N,P-ligand 132 as the catalyst to provide enantioenriched 1,1-diarylalkanes 32 in up to 80% yield and 97% ee (Scheme 27a).89 When alkenyl methylpentanediol(mp)-derived boronate esters 133 were used as organometals for the Suzuki cross-coupling with benzylic bromides, CuI/N,N,N-ligand 134 was the best catalyst and gave products 135 in up to 98% yield and up to >99% ee (Scheme 27b).90 In this hemilabile N,N,N-ligand 134, the presence of a methyl group at the ortho position of the sulfonamide quinoline moiety increases the enantioselectivity by steric hindrance probably by elongation of the Cu–N bond. These cross-couplings were also performed with propargyl bromides (see Section 4). Mechanistic studies revealed a radical process depicted in Scheme 27. The CuI complex undergoes a transmetalation process with the boronate ester to give intermediate I, which undergoes a single electron reduction with the benzylic bromide to deliver a radical II and the CuII complex III. Final reaction of these intermediates II and III forms the coupling product and regenerates the catalyst. In the case of alkenylboronates cross-coupling, DFT calculations supported tentatively the favorable transition state (TS) to explain the absolute configuration of the products.
Scheme 27. Enantioconvergent Cu-Catalyzed Suzuki Reactions of Benzylic Bromides 23 with Aryl and Alkenyl Boronates 131 and 133, Respectively.
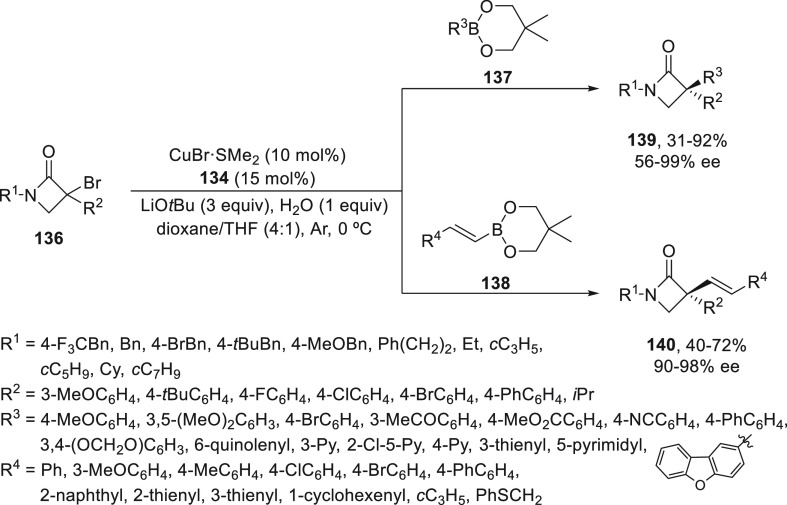
Recently, the same group91 performed a copper-catalyzed enantioconvergent radical C(sp3)–C(sp2) cross-coupling of α-bromo-β-lactams 136 with aryl and alkenyl organoboronate esters 137 and 138 (Scheme 28). In this case, the hemilabile N,N,N-ligand 134 gave products 139 and 140 with a quaternary stereocenter in up to 99% ee. The best results were obtained with neopentyl glycol (neop)-derived aryl-derived aryl and alkenyl boronate esters in the LiOtBu (3 equiv)/H2O (1 equiv) system at 0 °C under an argon atmosphere and using a 4/1 mixture of 1,4-dioxane/THF. Experimental radical trap experiments corroborate the formation of an alkyl radical, which is formed because of the enhanced reducing capability of the Cu(I) catalyst bearing this type of electron-donating ligand.
Scheme 28. Enantioconvergent Cu-Catalyzed Suzuki Reaction of α-Bromo-β-lactams 136 with Aryl and Alkenyl Boronates 137 and 138, Respectively.
Alkyl organoboranes reacted with unactivated secondary alkyl bromides and chlorides bearing a directing group at the α- to ε-position under Ni/chiral diamines catalysis. Boronates and boronic acids have been used for C(sp3)–C(sp2) bond-forming reactions with oxazolines or diphosphines as chiral catalysts. Cobalt, iron, and copper complexes catalyzed the cross-coupling of epoxides and activated bromides with boronates. In all cases, enantioconvergent transformations take place under mild reaction conditions by intermediacy of a radical derived from the electrophile.
2.1.4. Organosilicon Reagents
An enantioconvergent nickel-catalyzed Hiyama cross-coupling reaction can be performed with activated 2,6-di-tert-butyl-4-methylphenyl (BHT) α-bromo esters 7 and organosilanes. Fu and co-workers92 reported this C(sp3)–C(sp2) bond formation using NiCl2/diamine (S,S)-79 as catalyst and aryl or alkenyltrimethoxysilanes to give esters 9 with good yields and up to 99% ee (Scheme 29). This Hiyama reaction took place at room temperature in dioxane promoted by tetrabutylammonium triphenyldifluorosilicate (TBAT) as fluoride activator.
Scheme 29. Enantioconvergent Ni-Catalyzed Hiyama Reactions of α-Bromo Esters 7 with Aryl or Alkenylsiloxanes.
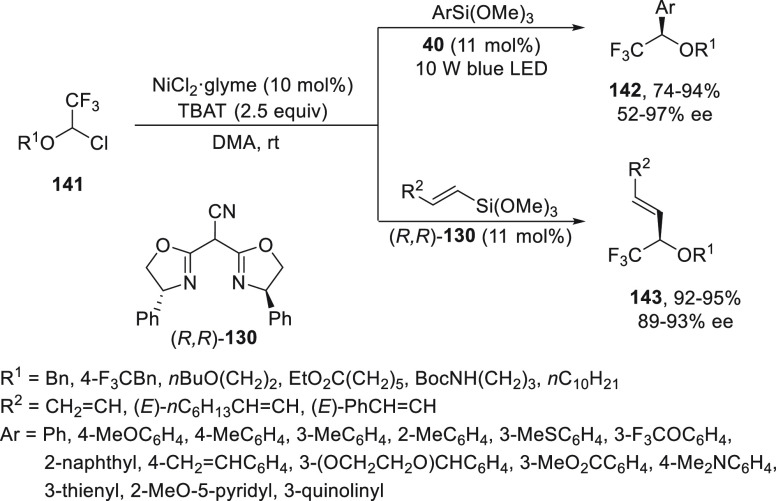
Varenikov and Gandelman93 applied the enantioconvergent Hiyama reaction for the synthesis of enantioenriched α-trifluoromethyl ethers 142 and 143, precursors of α-trifluoromethyl alcohols. Cross-couplings of different α-chloro-α-trifluoromethyl methyl ethers 141 with aryl siloxanes were performed under NiCl2/bis(oxazoline) 40 catalysis and irradiated in darkness with a white or blue LED lamp, which significantly accelerated the process, to provide compounds 142 with very good yields and up to 98% ee (Scheme 30). When alkenyl siloxanes were used as nucleophiles, bis(oxazoline) (R,R)-130 gave compounds 143 in up to 93% ee. The authors presume that the photoinduction facilitates the oxidative addition of electrophile 141 to an excited Ni(I) catalytic species, which likely takes place by radical mechanism, as has been proposed by Fu and co-workers.94 In the case of alkyl ethers, the arylation needed longer reaction times. Presumably, the oxidative addition is the rate-limiting step, and the lower the LUMO of the electrophile, the faster the single-electron transfer (SET) from the catalyst occurs.
Scheme 30. Enantioconvergent Ni-Catalyzed Hiyama Reactions of α-Chloro-α-trifluoromethyl Methyl Ethers 141 with Aryl or Alkenyl Siloxanes.
Enantioconvergent Hiyama reactions can be performed under Ni catalysis with diamines or bis(oxazolines) as chiral ligands and TBAT as fluoride activator. Activated alkyl bromides and chlorides underwent cross-coupling reaction with aryl or alkenyl siloxanes.
2.1.5. Other Organometals
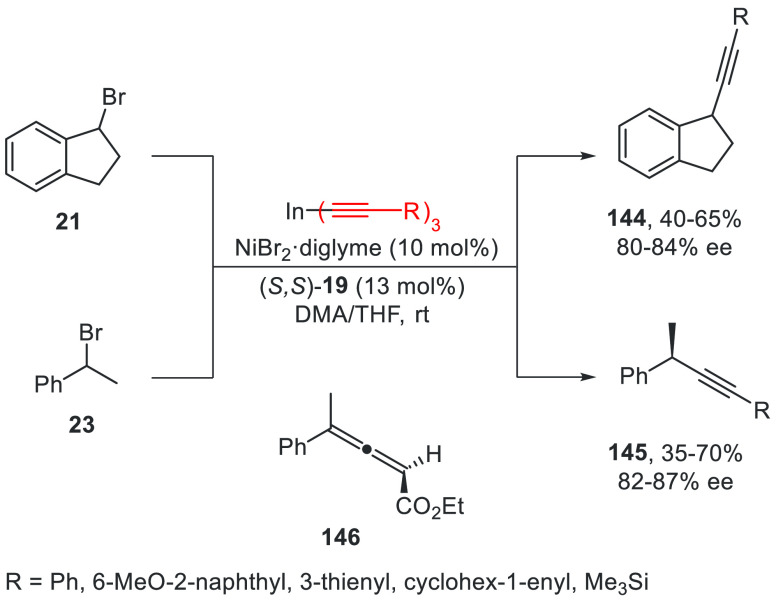
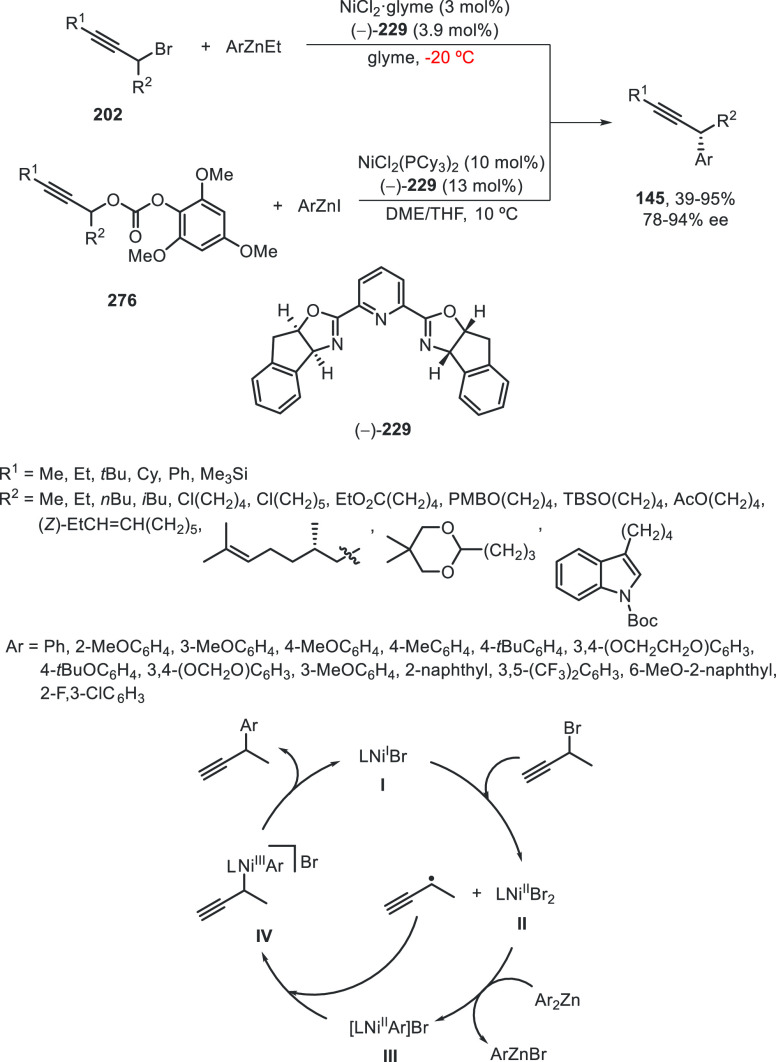
In 2008, Sarandeses and co-workers95 reported the enantioconvergent Ni-catalyzed cross-coupling reaction of trialkynylindium reagents with secondary benzylic bromides 21 and 23 using (S,S)-iPr-Pybox (19) as a chiral ligand (Scheme 31). This alkynylation reaction took place in moderate to good yields and up to 87% ee working at room temperature during 140 h and in a 1:1 mixture of DMA/THF. In the case of products 144, the absolute configuration was not determined. Compounds 145 were obtained without isomerization of the triple bond except in the case of ethyl propiolate, which afforded the allene 146 in 30% yield and 77% ee.
Scheme 31. Enantioconvergent Ni-Catalyzed Cross-Coupling Reactions of Secondary Benzyl Bromides 21 and 23 with Alkynylindium Reagents.
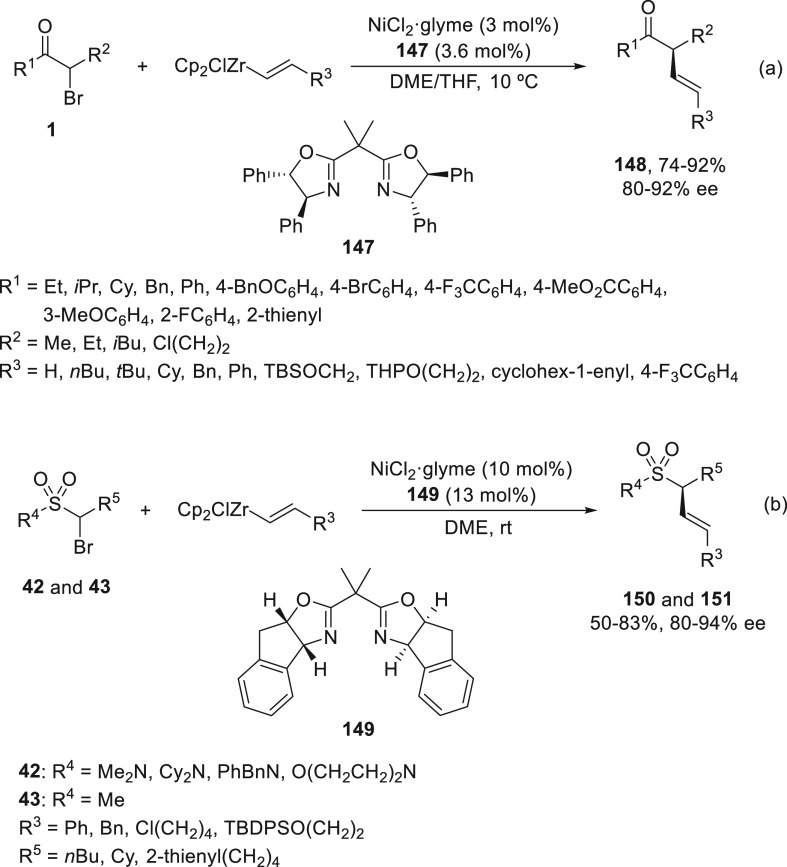
Enantioconvergent and stereospecific Ni-catalyzed alkenylations with organozirconium reagents were described by the Fu group.54,96,97 Initial results were carried out with activated secondary alkyl bromides 1 using (−)-bis(oxazoline) 147 as a chiral ligand under smooth reaction conditions. α-Substituted β,γ-unsaturated ketones 148 were obtained in very good yields and ee with low catalyst loading to keep the (E)-configuration of the starting alkenylzirconium reagent (Scheme 32a). This alkenylation was applied to the cross-coupling of α-bromo sulfonamides 42 and sulfone 43 (R4 = Me; R5 = Cy) with alkenylzirconium reagents using, in this case, ligand 149 to furnish allylic sulfonamides 150 and sulfone 151, respectively (Scheme 32b). In both cases, bis(oxazoline) 149 was the suitable chiral to give the corresponding products in good yields and enantioselectivities.
Scheme 32. Enantioconvergent Ni-Catalyzed Cross-Coupling Reactions of α-Bromo Ketones 1 and α-Bromo Sulfonamides 42 and Sulfones 43 with Alkenylzirconium Reagents.
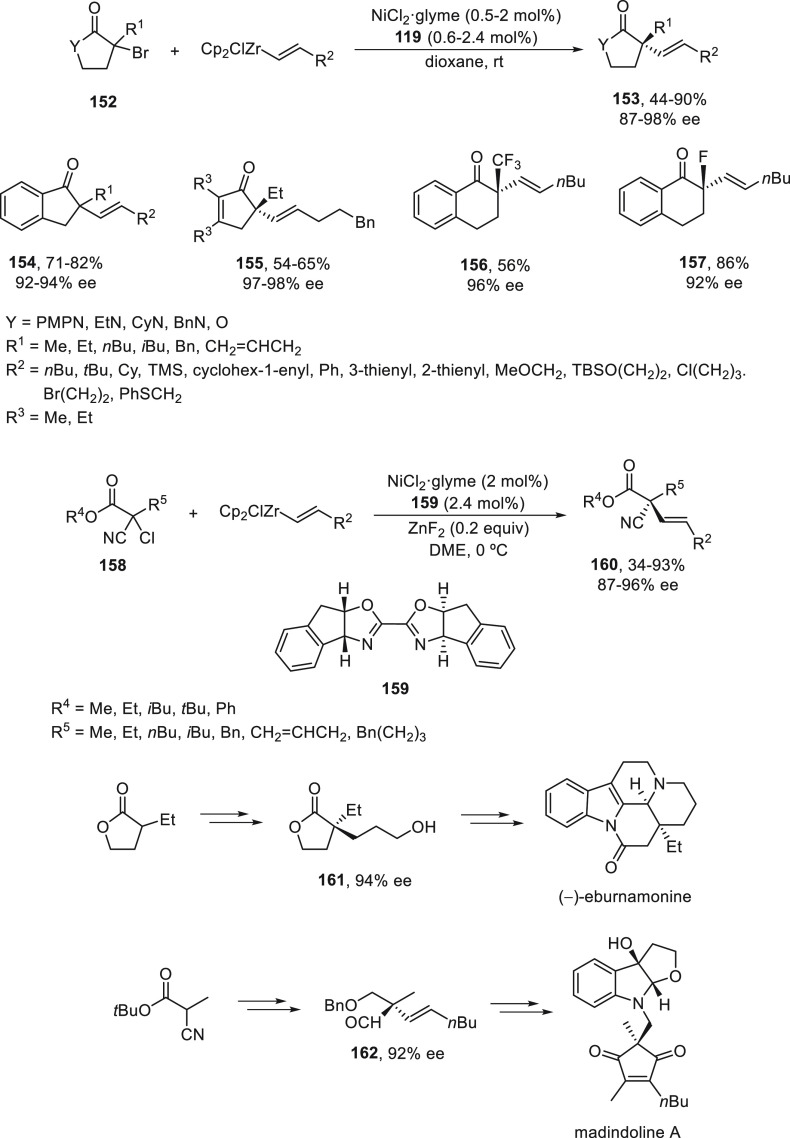
Recently, alkenylzirconium reagents have been used as appropriate nucleophiles for the enantioconvergent challenging alkenylation of activated tertiary alkyl halides.97 Cyclic and acyclic tertiary α-halo carbonyl compounds 152 and 158 reacted with alkenylzirconium reagents to afford enantioenriched α,α-disubstituted products 153–157 and 159, respectively (Scheme 33). For cyclic systems 152, NiCl2/oxazoline 119 was used as catalyst to provide α-alkenylated products 153–157 in good yields and enantioselectivities. In the case of α-chloro-α-cyano esters 158, NiCl2/bis(oxazoline) 159 was the preferred catalyst, and ZnF2 (0.2 equiv) was used as additive to afford product 160, also with good yields and enantioselectivities. As in the case of secondary halides, mechanistic experimental studies suggested the formation of radical intermediates. This method was applied to the formal total synthesis of bioactive natural products, such as (−)-eburnamonine and madindoline A, through the corresponding key intermediates 161 and 162, respectively. Lactone 161 was prepared from racemic 3-ethyloxolan-2-one in four steps with 94% ee, which was previously transformed into the alkaloid (−)-eburnamonine.98 Madindoline A, an inhibitor of interleukin 6, has been previously prepared from aldehyde 162,99 which was prepared in five steps in 92% ee starting from tert-butyl α-cyano propionate.
Scheme 33. Enantioconvergent Ni-Catalyzed Cross-Coupling Reactions of Tertiary Alkyl Halides 152 and 158 with Alkenylzirconium Reagents.
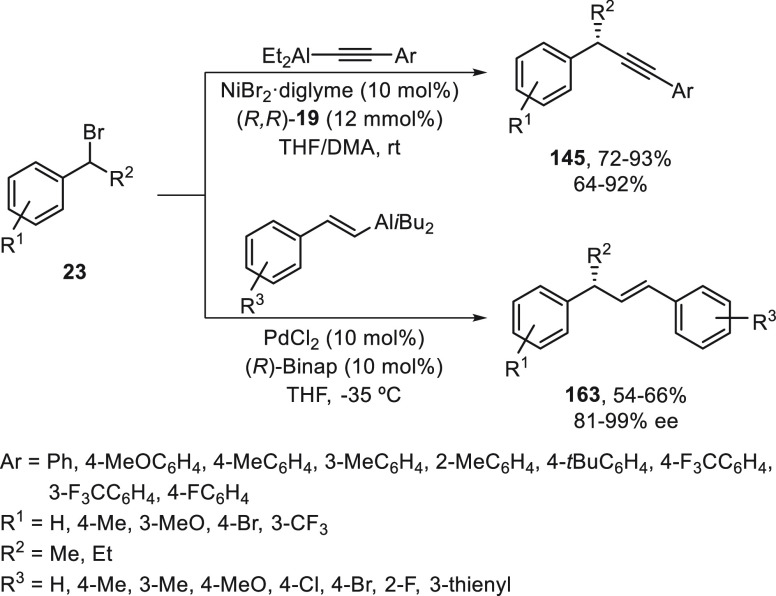
Alkenyl and alkynylaluminum reagents have been employed as nucleophiles in enantioconvergent cross-coupling reactions with secondary benzylic bromides 23 by Zhou and co-workers.100 The alkynylation reaction took place using NiBr2/(R,R)-iPr-Pybox (19) as catalyst in a 1:1 mixture of THF/DMA at room temperature to give products 145 with good yields and enantioselectivities (Scheme 34). However, the alkenylation reaction was carried out under Pd catalysis using (R)-Binap as chiral ligand in THF at −35 °C to provide enantioenriched aryl alkenes 163 with moderate yields and up to 99% ee.
Scheme 34. Enantioconvergent Ni- and Pd-Catalyzed Cross-Coupling of Secondary Benzylic Bromides 23 with Alkynyl and Alkenylaluminum Reagents, Respectively.
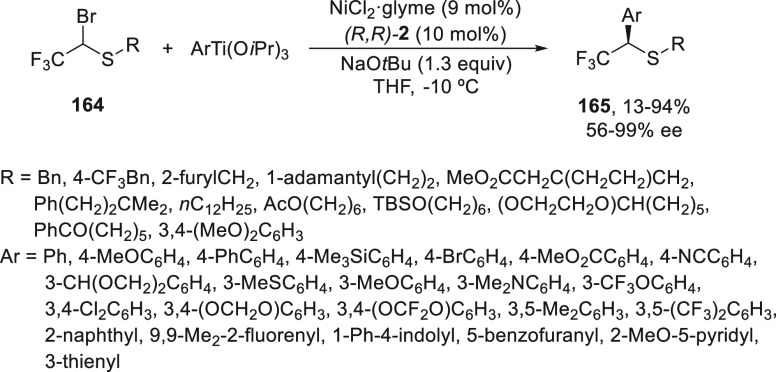
Organotitanium reagents possess a lower nucleophilicity than organomagnesium ones, which allows a greater functional group tolerance, and have higher transmetalation rates than organozinc compounds. Varenikov and Gandelman101 employed for the first time these organometals in asymmetric cross-coupling reactions devoted to the synthesis of enantioenriched α-trifluoromethyl thioethers 165. Initial studies about the enantioconvergent Ni-catalyzed cross-coupling reaction of α-trifluoromethyl-α-bromomethyl thioethers 164 with aryltrimethoxysilanes gave poor results. However, aryltianium(IV) compounds, prepared by mixing Ti(OiPr)4 with arylmagnesium bromides in the presence of NaOtBu, formed titanate complexes able to react with thioethers 164 using NiCl2/PhBox [(R,R)-2] as catalyst in THF at −10 °C to provide a wide range of products 165 in moderate to good yields and high enantioselectivities (Scheme 35).
Scheme 35. Enantioconvergent Ni-Catalyzed Cross-Coupling Reactions of α-Trifluoromethyl-α-bromomethyl Thioethers 164 with Aryltitanium(IV) Reagents.
In summary of this Section 2.1 about enantioconvergent cross-coupling reactions of alkyl electrophiles with organometals, organozinc or organoboron reagents have been largely employed mainly under Ni catalysis and oxazolines or 1,2-diamines as chiral ligands, respectively. For alkenylation reactions, alkenylzirconium reagents using Ni/bis(oxazoline) as catalyst gave the best results, even with tertiary alkyl bromides. In the case of alkynylation reactions of benzylic bromides, alkynylindium and aluminum reagents under Ni catalysis have been successfully used.
2.2. Racemic Alkyl Electrophiles with Other Non-Metallic Nucleophiles
In this Section, enantioconvergent alkylation with alkyl halides of heteroatom-based nucleophiles, such as amines, other carbon nucleophiles, and borylations, mainly under Cu catalysis, will be considered.
2.2.1. Nitrogen Nucleophiles
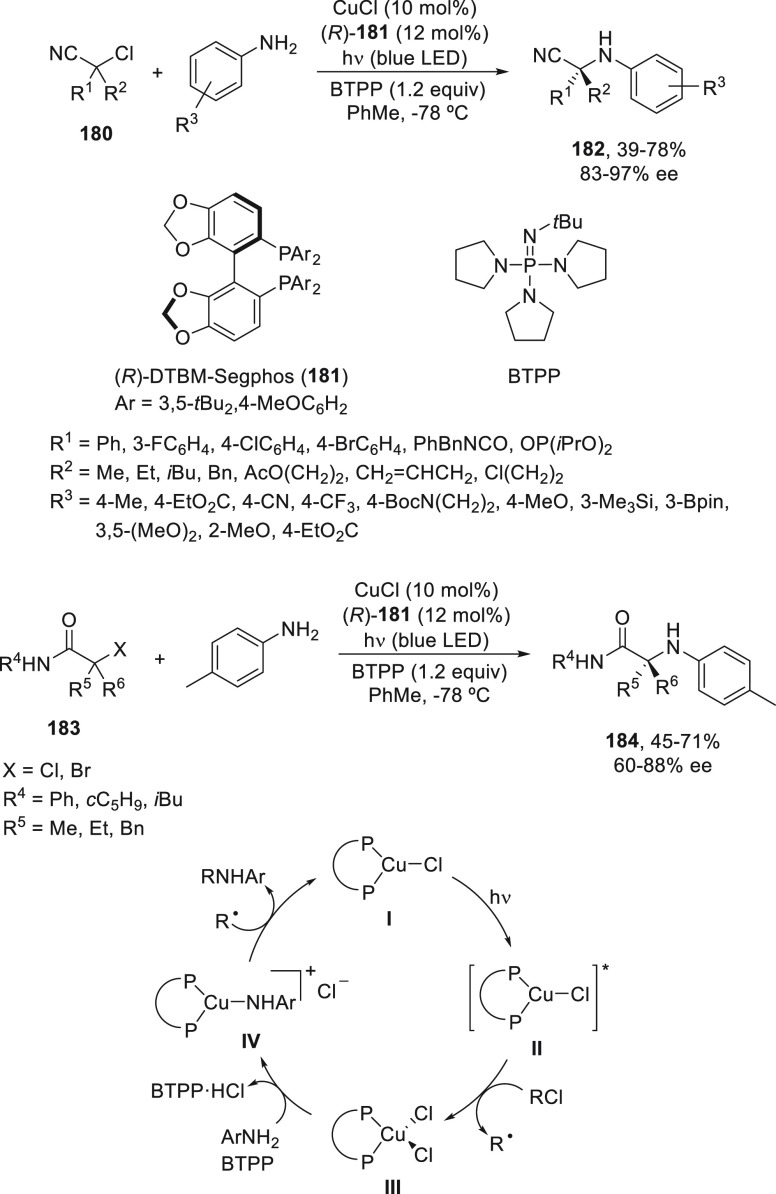
Direct substitution reactions of alkyl electrophiles by nitrogen nucleophiles via SN1 or SN2 processes suffer from many limitations with regard to scope and/or stereoselectivity. Catalytic processes were developed for aryl electrophiles, such as the copper-catalyzed Ullmann reaction and the palladium-catalyzed Buchwald-Hartwig reaction, that achieved a broad scope for C–N formation. The coupling of alkyl halides with amines was achieved by Peters, Fu, and co-workers102 under the combined action of light and copper catalysis. Then, the same group34,103,104 developed challenging enantioconvergent N-alkylations by racemic secondary and tertiary alkyl halides in the presence of light and a chiral copper catalyst. Initial studies were performed with tertiary α-chloro amides 166 and carbazoles and indoles as nitrogen nucleophiles using CuCl/monodentate phosphine 167, LiOtBu as base, and visible light irradiation (blue LED) in toluene at −40 °C (Scheme 36).103 The resulting N-alkylated carbazoles and indoles 168 and 169 were obtained in good yields and enantioselectivities. Experimental and theoretical studies support the proposed catalytic cycle for this enantioconvergent N-alkylation.104 Two key intermediates, the copper(II) metaloradical IV and the tertiary α-amide organic radical R•, have been characterized by EPR and DFT calculations. These two radicals are combined to furnish the C–N coupling in 77% yield and 55% ee. DFT calculations reckon that the organic radical is resistant to radical–radical homocoupling and, therefore, accessible as a free radical in solution. In this detailed pathway, the previously characterized complex I serves as a photoreductant via excitation to II, which reacts with the electrophile to give the radical R• and intermediate III. After ligand substitution by a second carbazolide ligand and loss of one ligand (L), the characterized intermediate IV is formed. Coordination of the radical R• with IV gives intermediate V, which forms the product regenerating the complex I upon binding L.
Scheme 36. Enantioconvergent Photoreduced Cu-Catalyzed N-Alkylation of Carbazoles and Indoles with α-Chloro Amides 166.
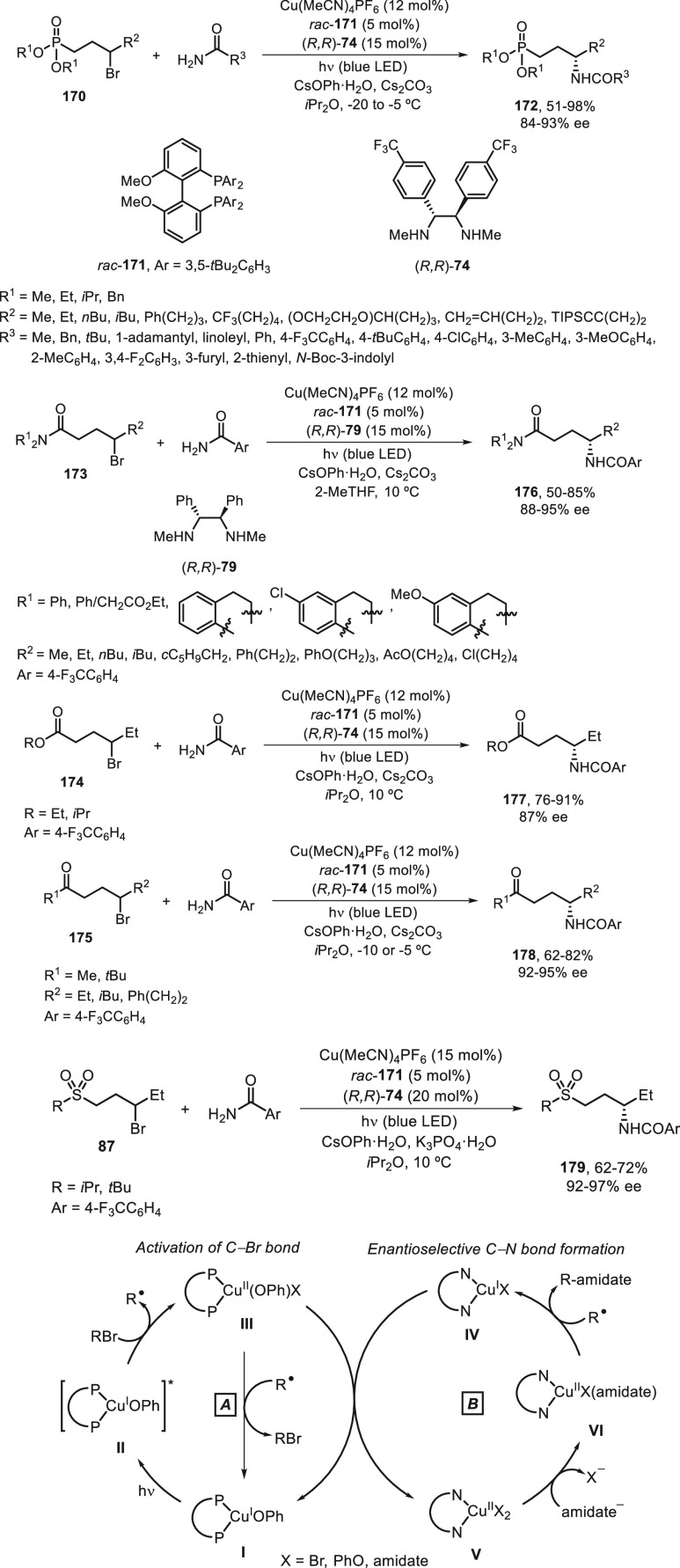
Photoinduced copper-catalyzed amidation of unactivated secondary alkyl halides was initially performed by Peters, Fu, and co-workers with carboxamides105 and carbamates.94 The same group performed an enantioconvergent amidation of racemic secondary alkyl bromides.106 They used a photoinduced copper-catalyzed asymmetric amidation via ligand cooperativity on the basis of three different ligands: a racemic bisphosphine 171, cesium phenoxide, and a chiral diamine (R,R)-79 or (R,R)-74. These ligands assemble in situ to form two distinct catalysts that act cooperatively: a copper/bisphosphine/phenoxide complex I, which serves as photocatalyst, and a chiral copper diamine complex that catalyzes enantioselective C–N bond formation. Alkyl bromides bearing a phosphonoyl group 170 gave γ-aminophosphonic acid derivatives 172 by reaction with carboxamides upon irradiation of the copper-based catalytic system with blue LED lamps in iPr2O at −20 to −5 °C (Scheme 37). Other alkyl bromides 173–175 and 87 bearing Lewis basic functional groups, including γ-bromo amide 173, ester 174, ketone 175 and sulfone 87, provided good enantioselectivity in the nucleophilic substitution reactions and led to products 176–179. The proposed catalytic cycles A and B are based on experimental studies. In cycle A, the photoredox catalyst I gives upon irradiation the excited state of 171·CuIOPh II with a sufficient lifetime to react with the electrophile by an inner-sphere electron-transfer pathway (halogen-atom transfer) to afford the radical R• and intermediate III. Cycle A intersects with cycle B by reaction of intermediate III with the chiral copper complex IV to generate I and V by ligand exchange. Then, a nucleophilic substitution of complex V with the amidate anion leads to complex VI, which reacts with the organic radical R• in an out-of-cage process via coordination of the directing group to CuII followed by C–N bond formation to furnish the product and complex IV.
Scheme 37. Enantioconvergent Photoinduced Cu-Catalyzed N-Alkylation of Carboxamides with γ-Bromo Phosphonates 170, Amides 173, Esters 174, Ketones 175, and Sulfones 87.
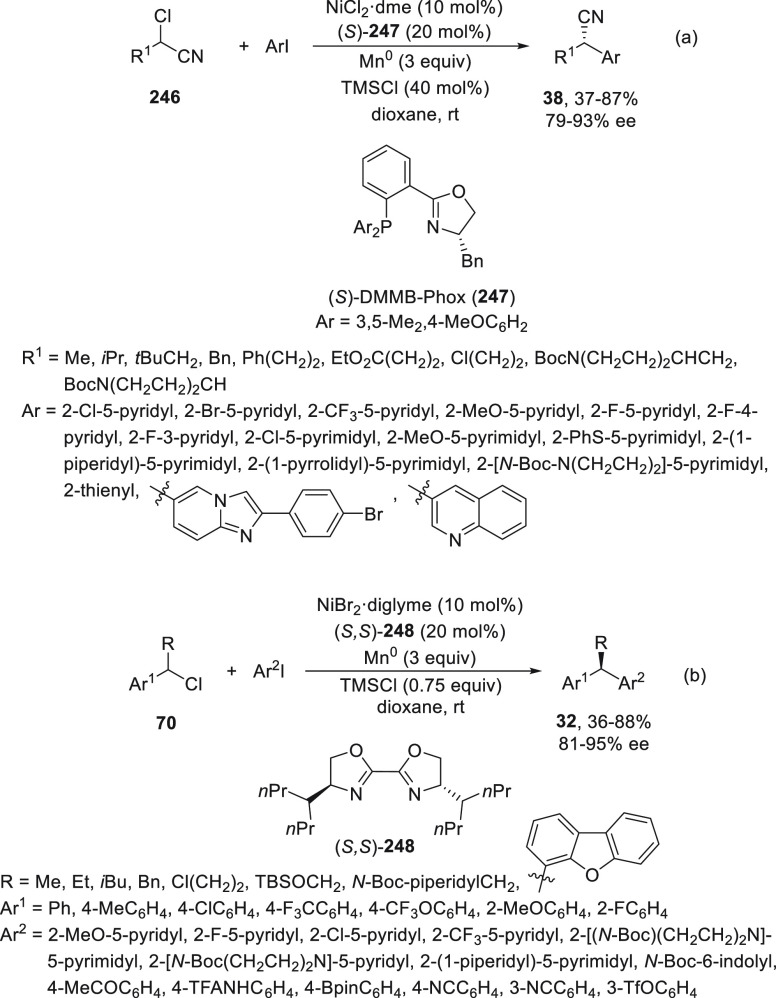
Recently, the same group107 reported the photoinduced copper-catalyzed enantioconvergent alkylation of anilines by activated tertiary α-chloro nitriles 180 (Scheme 38). This substitution reaction took place using CuCl/(R)-DTBM-Segphos (181) as chiral photocatalyst and tert-butylimino-tri(pyrrolidino)phosphane (BTPP) as base in toluene at −78 °C to provide enantioenriched α-amino nitriles 182 with moderate to poor yields and up to 97% ee. Tertiary α-chloro or α-bromo amides 183 gave the corresponding α-amino amides 184 under the same reaction conditions with 45–71% yield and up to 88% ee. Experimental and theoretical studies led to identification of copper-based intermediates, such as the photoreductant L*CuCl (I) and [L*Cu(NHAr)]Cl (IV) as key intermediates. In the proposed catalytic cycle, catalyst I gave intermediate II upon radiation, which abstracts a chlorine atom from the alkyl chloride to form the radical R• and intermediate III. By reaction with the anilido anion intermediate, IV is formed, which reacts with the radical to give the product and regenerates the catalytic complex I.
Scheme 38. Enantioconvergent Photoinduced Cu-Catalyzed N-Alkylation of Anilines with α-Chloro Nitriles 180 and α-Halo Carboxamides 183.
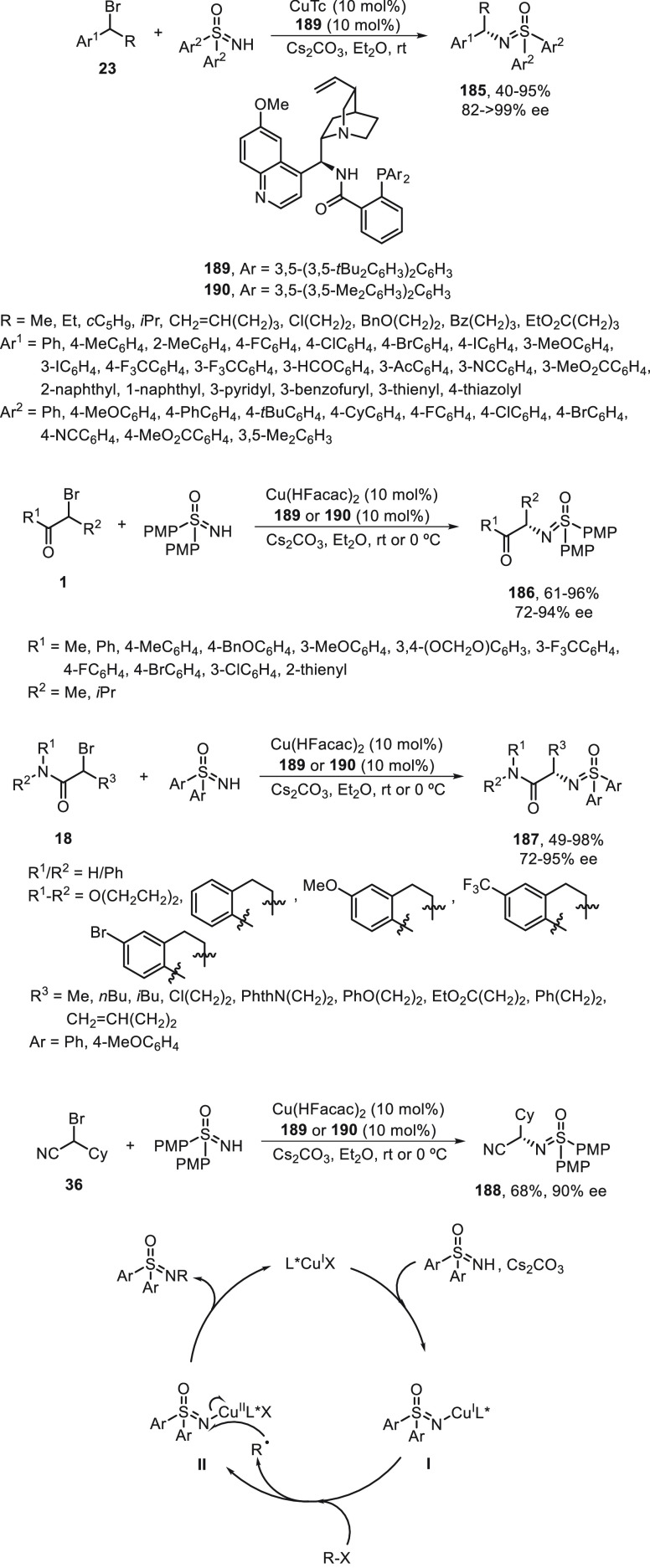
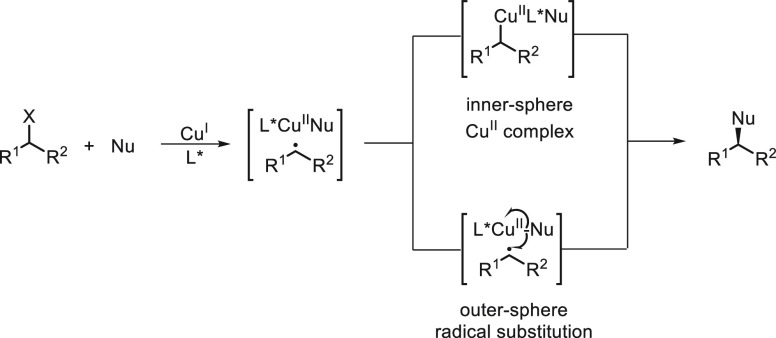
Liu and co-workers108 employed sulfoximines as ammonia surrogates to access α-chiral primary amines. This enantioconvergent Cu-catalyzed radical C–N coupling took place in the absence of light with secondary alkyl halides, such as benzylic bromides 23, α-bromo ketones 1, α-bromo amides 18, and α-bromo nitrile 36, to provide the corresponding N-alkyl sulfoximines 185–188 (Scheme 39). This procedure was carried out under mild thermal conditions using a Cu(I) salt and the bulky N,N,P-ligands 189 or 190 with Cs2CO3 as base in Et2O at room temperature or 0 °C. The authors proposed the formation of complex I able to reduce the alkyl bromide via a single-electron transfer process to generate the alkyl radical R• by an outer-sphere radical substitution to give the product generating the Cu(I) catalyst. Products 185–188 (more than 60 examples) were isolated up to 99% yield and up to >99% ee and were transformed into enantioenriched primary amines by reduction with Mg or with sodium naphthalenide followed by acidic hydrolysis without remarkable losses of enantiopurity. This methodology was applied to the synthesis of commercial drugs, including cinacalcet, dapuxetine, and rivastigmine.
Scheme 39. Enantioconvergent Cu-Catalyzed N-Alkylation of Secondary Benzylic Bromides 23, α-Bromo Ketones 1, α-Bromo Amides 18, and α-Bromo Nitrile 36 with Sulfoximines.
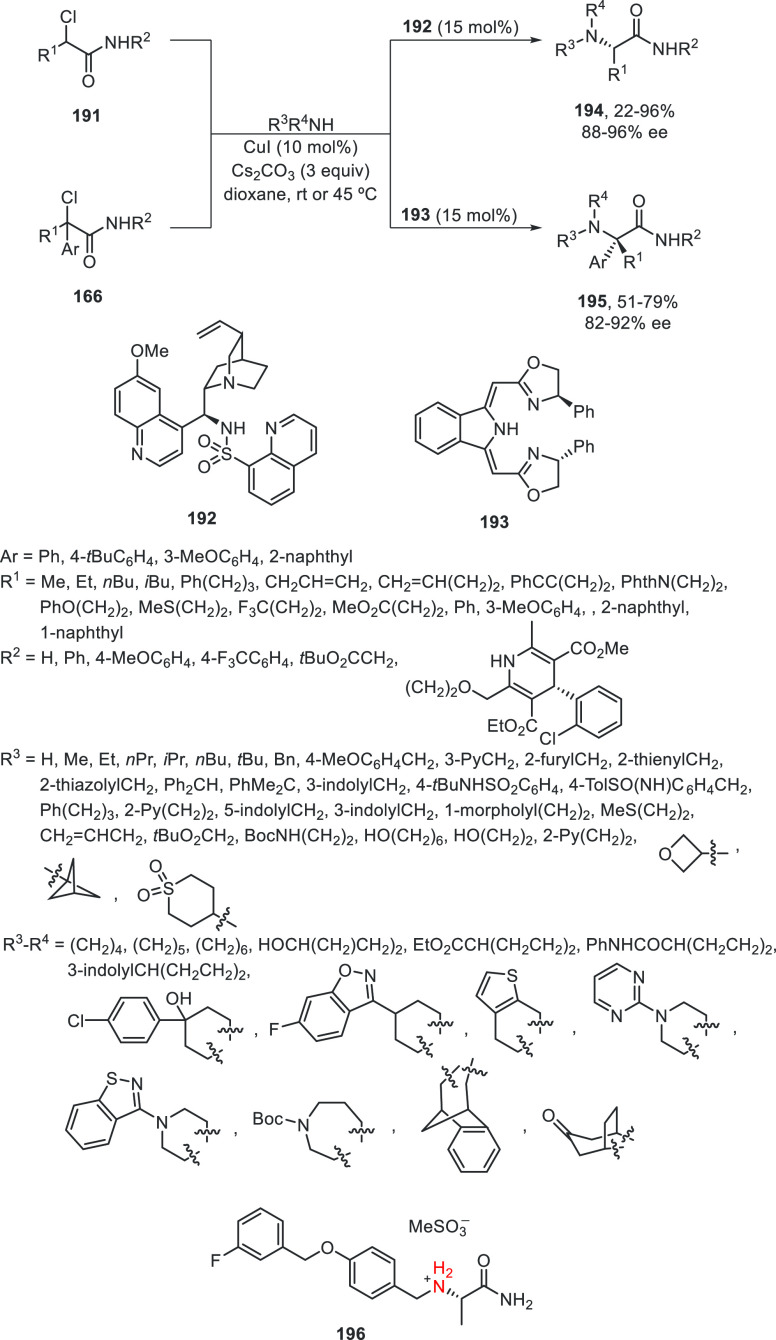
Very recently, Liu and co-workers109 were able to develop the enantioconvergent Cu-catalyzed N-alkylation of aliphatic amines using chiral tridentate anionic ligands 192 and 193. α-Chloro amides 191 and 166 reacted with a wide variety of primary and secondary aliphatic amines, even ammonia (more than 125 examples), to afford α-amino amides 194 and 195, respectively, with 22–99% yields and 88–97% ee (Scheme 40). This procedure was applied to the synthesis of 12 drugs or bioactive molecules, including the anti-Parkinson drug XADAGO 196 with 62% yield and 94% ee. On the basis of experimental studies, the authors proposed the formation of a cuprate intermediate I, which undergoes intramolecular oxidative addition to give II and III in equilibrium. Subsequent outer-sphere amine attack to III delivers IV, which upon ligand exchange with the α-chloro amide forms the N-alkylated product.
Scheme 40. Enantioconvergent Cu-Catalyzed N-Alkylation of α-Chloro Amides 191 and 166 with Aliphatic Amines and Ammonia.
Enantioconvergent cross-coupling amination and amidation of alkyl electrophiles has been mainly performed under photoinduced copper-catalyzed conditions and using phosphines as chiral ligands. In the absence of light, sulfoximines and aliphatic amines are able to perform the N-alkylation of activated alkyl bromides under Cu catalysis.
2.2.2. Oxygen Nucleophiles
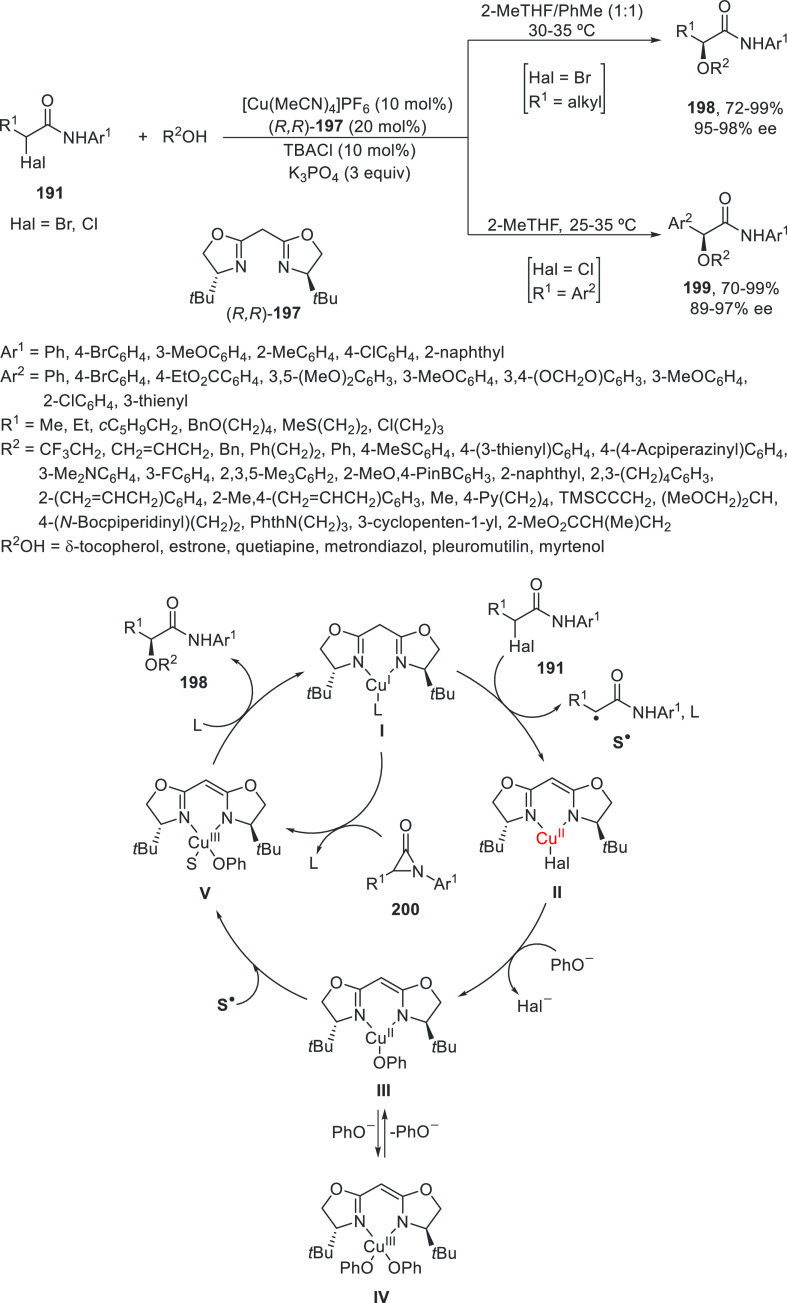
Cross-coupling reactions between α-bromo amides 191 and alcohols under Cu catalysis were reported by Kürti and co-workers in 2018.110 However, just recently, Chen and Fu111 achieved the enantioconvergent process using Cu(II) and a chiral bis(oxazoline) 197 (Scheme 41). The cross-coupling of α-bromo and α-chloro amides 191 took place with aliphatic alcohols and phenols to efficiently give α-alkoxy amides 198 and 199 in very good yields and with high enantioselectivity. These reaction conditions were also applied to the enantioconvergent alkylation of nitrogen nucleophiles, such as aliphatic primary amines and anilines. Experimental studies support that the process proceeds through a free radical pathway. Thus, Cu(I) complex I reacts with the α-halo amide to give the Cu(II) complex II and the alkyl radical S•. This complex II undergoes ligand substitution with the oxygen nucleophile to provide complexes III and IV. Then, the alkyl radical S• reacts at copper to form organocopper(III) complex V via an out-of-cage pathway. This complex V can also be formed by oxidative addition of the aziridinone 200 to complex I along with complexation of phenol. Reductive elimination determines the stereochemistry and affords the product and complex I.
Scheme 41. Enantioconvergent Cu-Catalyzed Cross-Coupling of α-Halo Amides 191 with Oxygen Nucleophiles.
2.2.3. Phosphorus Nucleophiles
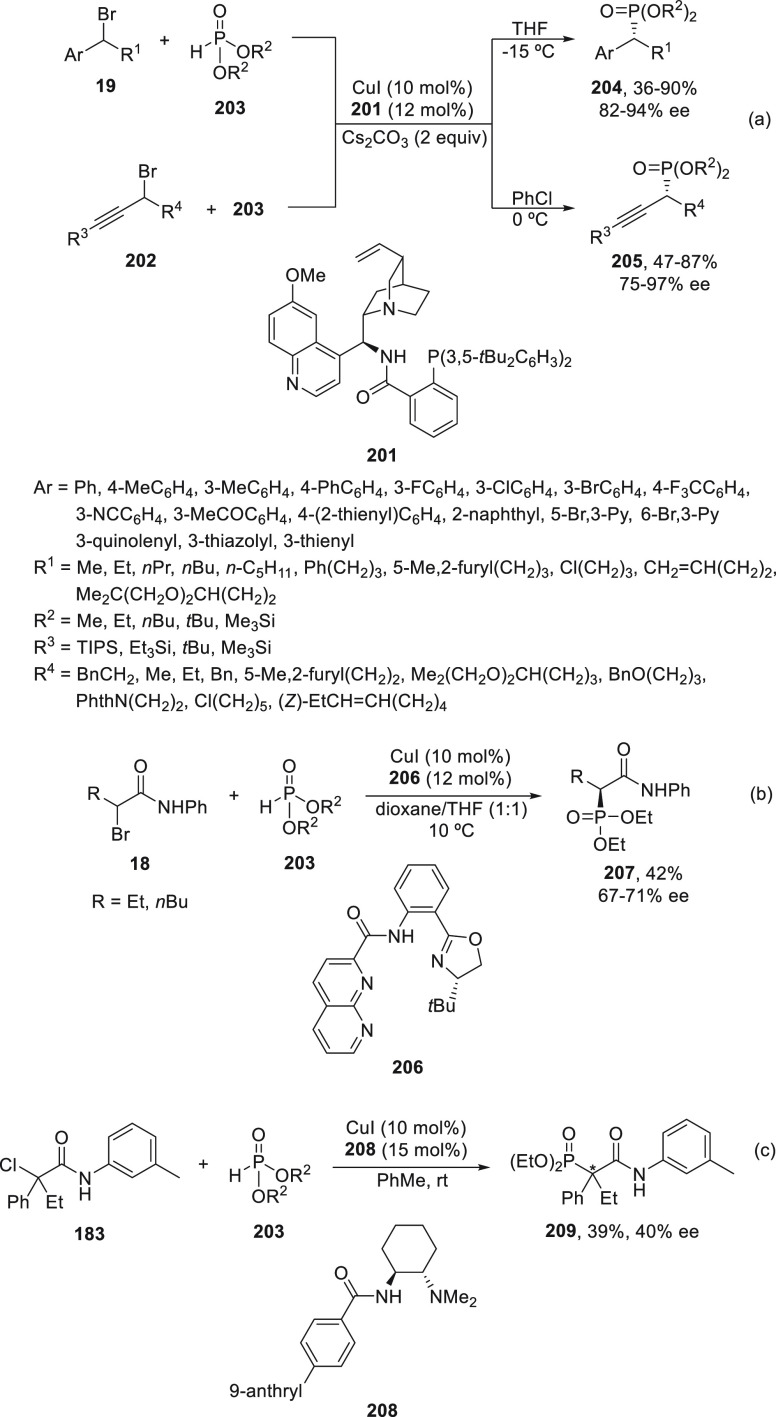
The Michaelis–Becker (M-B) reaction of H-phosphonates with alkyl halides is a direct method for the synthesis of C-phosphonates.112 Only recently, Liu and co-workers113 reported the enantioconvergent copper-catalyzed M-B-type C(sp3)–P cross-coupling reaction. By using the multidentate chiral anionic ligand 201, benzylic bromides 23 and propargylic bromides 202 reacted with H-phosphonates 203 to furnish products 204 and 205, respectively, with remarkable chemo- and enantioselectivity (Scheme 42a). In the case of α-halo carboxamides 18 and 183, ligands 206 and 208 were used, respectively, to provide products 207 and 209 with moderate results (Schemes 42b,c). Concerning the reaction mechanism, a radical trap experiment with TEMPO supports a stereoselective radical pathway over a stereospecific SN2-type process.
Scheme 42. Enantioconvergent Cu-Catalyzed Michaelis–Becker Reaction of Alkyl Halides with H-Phosphonates.
2.2.4. Other Carbon Nucleophiles
Enantioconvergent cross-coupling of racemic alkyl electrophiles with carbon nucleophiles, such as cyanides, acetylides, and nitronates, by generation of achiral radicals via an inner-sphere single-electron transfer (SET) process with a chiral transition-metal catalyst will be considered. In addition, under photoredox catalysis an outer-sphere SET strategy produces alkyl radicals that by enantioselective radical coupling forms C(sp3)–C bonds. In Scheme 43, the Cu-catalyzed enantioconvergent radical C(sp3)–C cross-coupling reactions of alkyl electrophiles with nucleophiles are depicted.114
Scheme 43. Enantioconvergent Strategy for Cu-Catalyzed Radical C(sp3)–C Cross-Coupling Reactions.
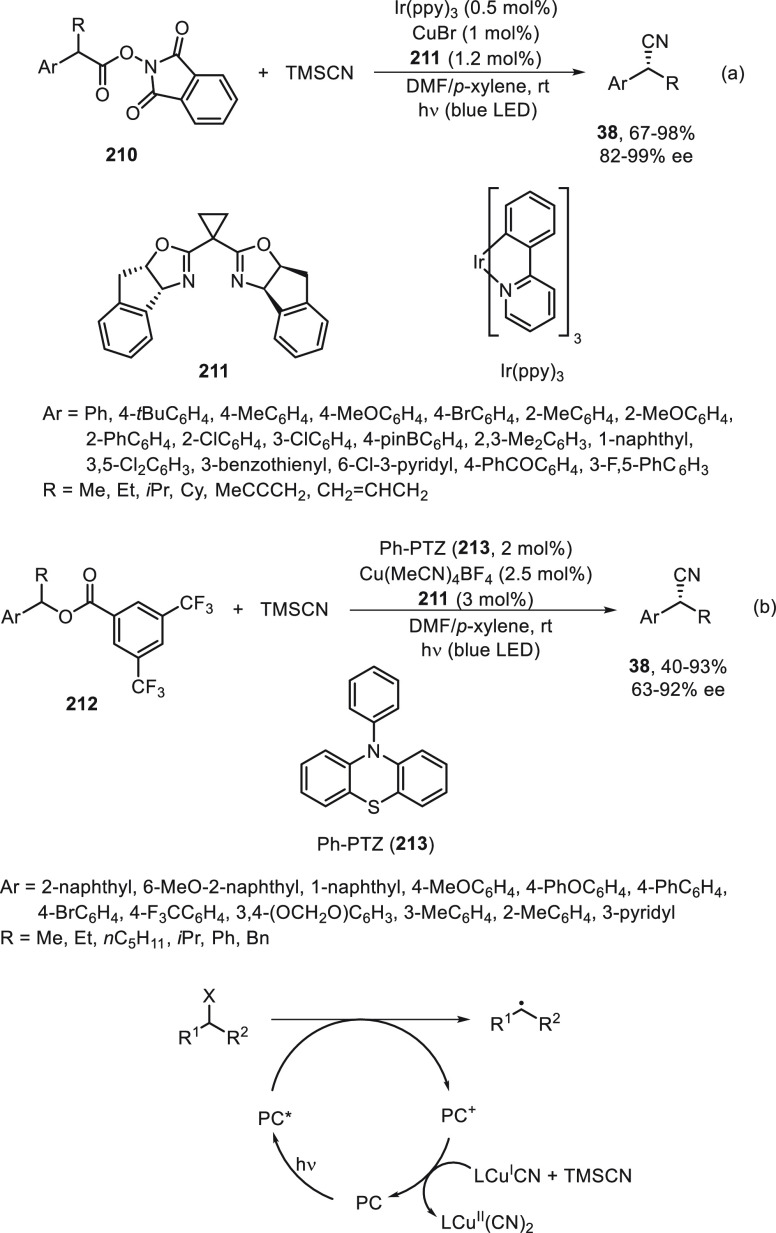
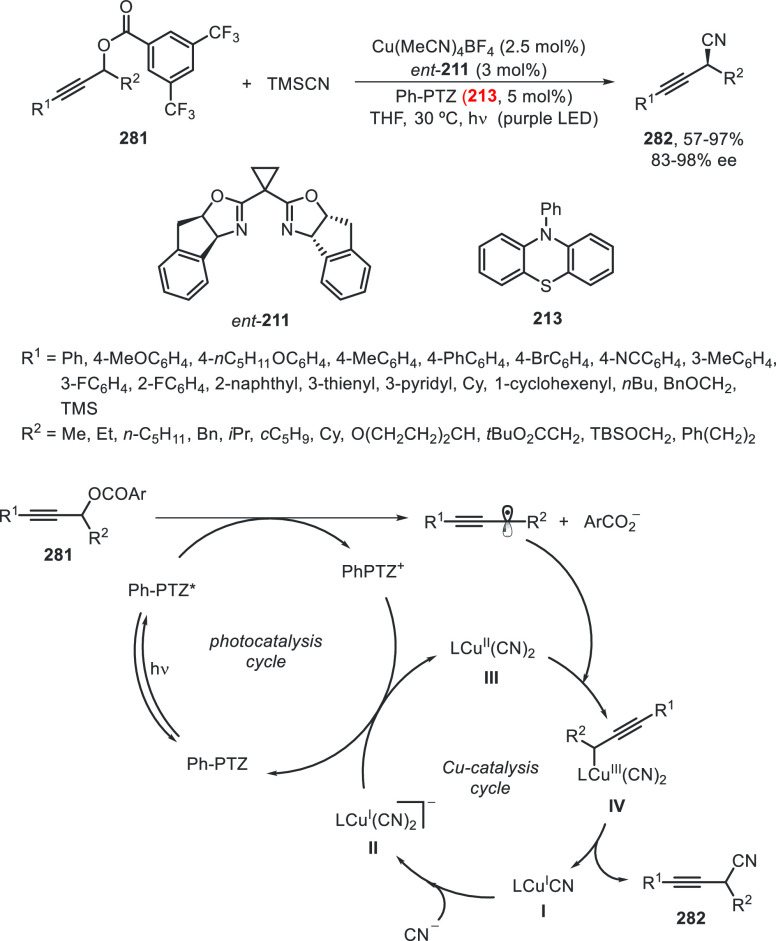
Concerning cyanation reactions, for the synthesis of enantioenriched benzylic nitriles, two types of enantioconvergent photocatalyzed cross-couplings have been reported, either starting from carboxylic acid derivatives 210(115) or benzylic alcohol esters 212.116 Liu and co-workers115 performed decarboxylation of N-hydroxyphthalimide (NHP) esters 210 in the presence of trimethylsilyl cyanide (TMSCN) to give the corresponding benzylic nitriles 38 in up to 98% yield and 99% ee (Scheme 44a). This process was carried out using Ir(ppy)3 as photocatalyst under blue LED irradiation and CuBr/bis(oxazoline) 211 as chiral metal catalyst. Conversely, Xiao and co-workers116 started from 3,5-bis(trifluoromethyl)benzoyl esters 212, which by reaction with TMSCN afforded the corresponding nitriles 38 in up to 93% yield and 92% ee (Scheme 44b). In this case, an organic photocatalyst Ph-PTZ (213) and Cu(MeCN)4BF4/bis(oxazoline) 211 as chiral metal catalyst were used. In a simplified catalytic cycle to explain the initiation of these reactions, starting compounds 210 and 212 gave benzylic radicals by photocatalytic decarboxylation and deoxygenation, respectively, by transfer of one electron of the excited photocatalyst (PC*). This PC* can oxidize L*CuICN to form L*CuIICN, which reacts with TMSCN to form L*CuII(CN)2. Subsequent combination of the benzylic radical with the active species L*CuII(CN)2 by an outer-sphere radical substitution would provide the coupling product 38.
Scheme 44. Enantioconvergent Photocatalyzed Cu-Catalyzed Cyanation of Esters 210 and 212 with TMSCN.
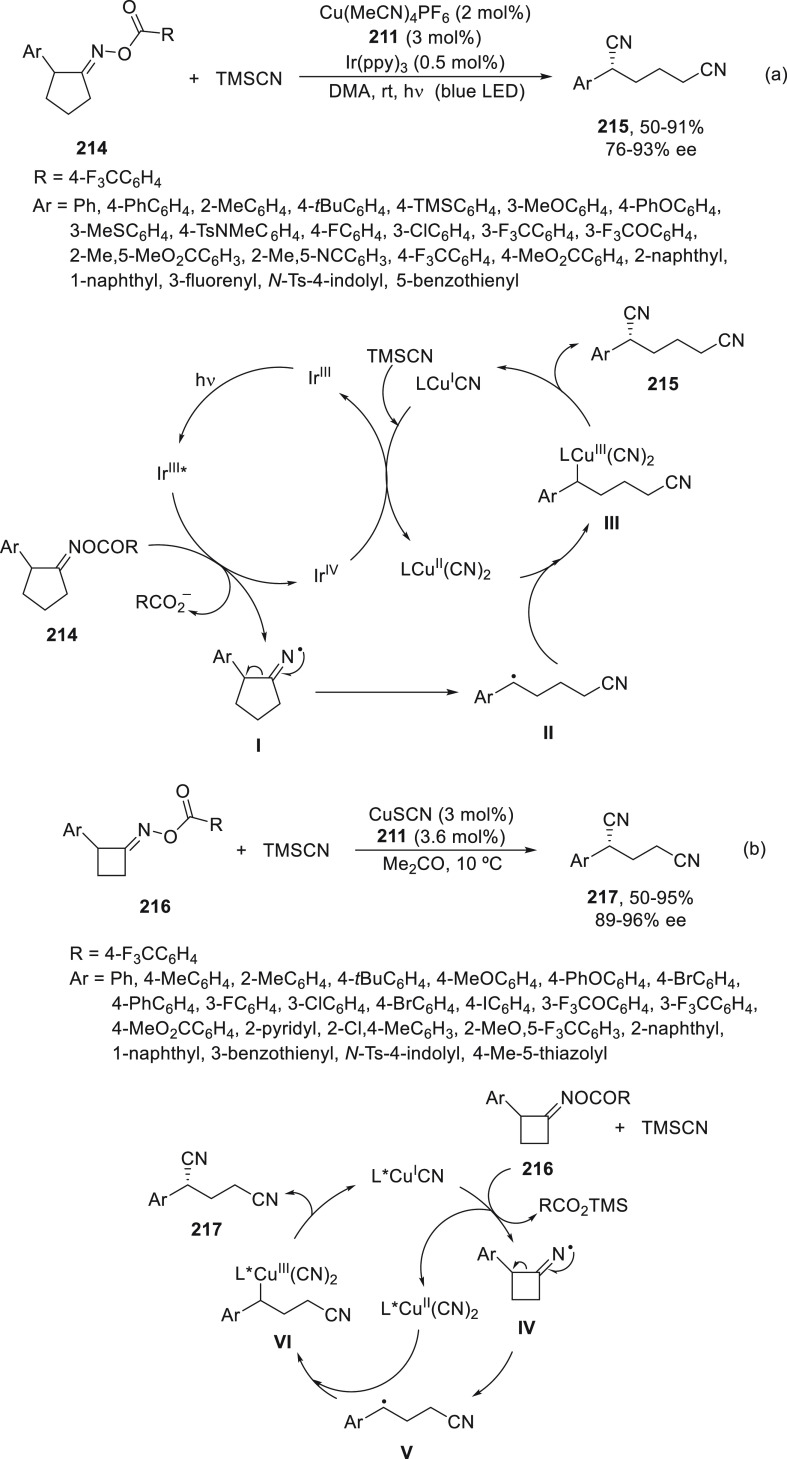
Wang and co-workers117 employed the dual photoredox/copper catalysis for the enantioconvergent ring-opening cyanation of cyclopentanone oxime esters 214 with TMSCN to access enantioenriched 1,6-dinitriles 215 in high yields and enantioselectivities (Scheme 45a). This process is based on the iminyl radical-mediated ring-opening of cyclic oxime derivatives by cleavage of the C–C single bond via β-scission reported by Zard and co-workers.118 Alternatively, cyclobutanone oxime esters 216 were transformed into enantioenriched 1,5-dinitriles 217 only under copper catalysis, presumably because of the higher strain release of four-membered ring than cyclopentanones (Scheme 45b).119 In both cases, bis(oxazoline) 211 was used as chiral ligand. In the proposed mechanism based on experimental studies, in the catalytic cycle for cyclopentanone oxime esters 214, a SET process between the oxime and the excited state of photocatalyst Ir(III)* provided iminyl radical I and the Ir(IV) species, which oxidized L*CuICN to L*CuII(CN)2 by reaction with TMSCN. Intermediate I generates by C–C bond cleavage the benzylic radical II, which is trapped by L*CuII(CN)2 to deliver intermediate III. Final reductive elimination of III gives the desired product 215 and regenerates the catalyst. For the cyclobutanone oxime esters 216, an initial SET process with L*CuICN in the presence of TMSCN affords L*CuII(CN)2 and iminyl radical IV, which evolves to radical V by C–C bond cleavage. Radical V reacts with L*CuII(CN)2 to give species VI followed by reductive elimination to provide dinitrile 217 and the catalyst L*CuICN.
Scheme 45. Enantioconvergent Cu-Catalyzed Cyanation of Cyclopentanone and Cyclobutanone Oxime Esters 214 and 216 with TMSCN to Give Dinitriles 215 and 217.
Xiao, Chen, and co-workers120 independently reported the cyanation of cyclopentanone oxime esters 214 under dual photoredox and Cu catalysis using the reaction conditions depicted in Scheme 44b. In this case, they used a 2 × 3 W purple LED and DMA as solvent at 30 °C to provide dinitriles 215 with 75–99% yield and 81–94% ee.
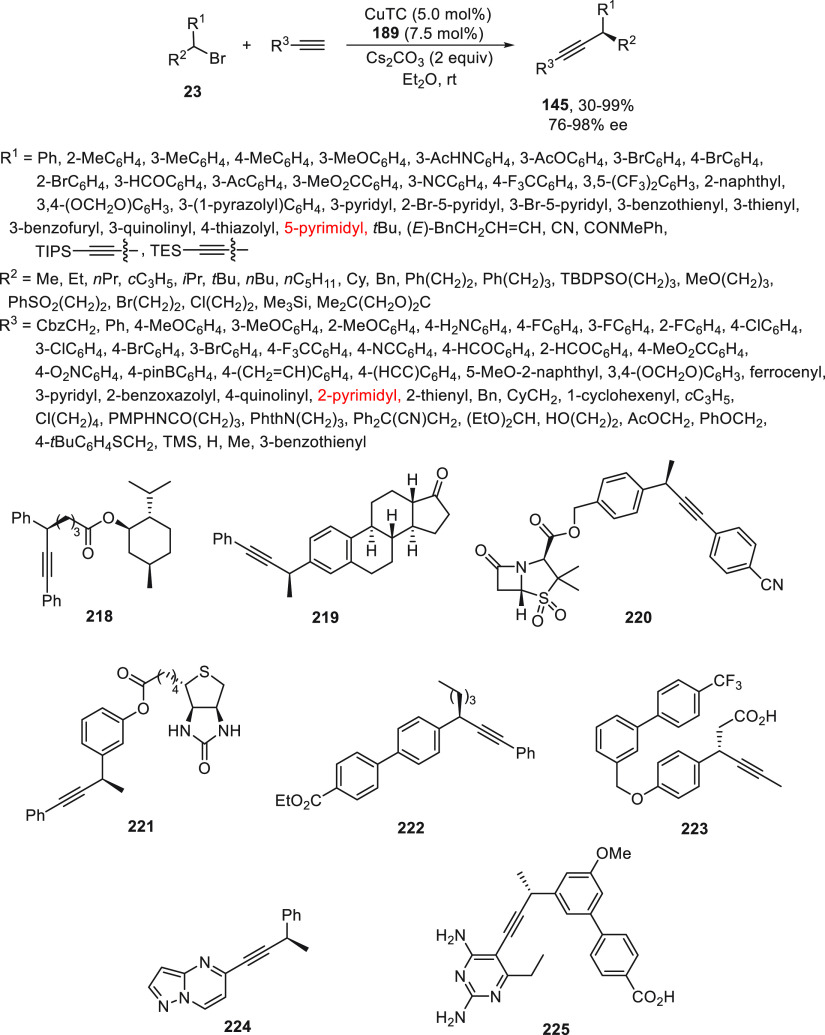
Enantioconvergent C(sp3)–C(sp) cross couplings of secondary alkyl halides with alkynes have been developed by Liu and co-workers121 under CuTC (TC = thiophene-2-carboxylate) catalysis. This radical process needed the strong donating multidentate ligand developed by Dixon et al.122 (189) to enhance the reducing capability of the Cu catalyst, as well as to suppress the Glaser homocoupling.114,121 Benzylic bromides 23 reacted with terminal aromatic and aliphatic acetylenes, including acetylene, itself, using Cs2CO3 as base in ethyl ether at room temperature to provide products 145 (>120 examples) with good yields and enantioselectivities (Scheme 46). Synthetic applications of these transformations employed the core of several bioactive molecules, such as l-menthol, estrone, sulbactam, biotin, and a mesogenic compound 218–222. In addition, they prepared chiral alkyne drug leads, such as 223 (AMG 837), a G-protein coupled receptor GPR40 agonist, and 224, a patented mGluR modulator. They also prepared a dihydrolate reductase (DHFR) inhibitor UCP1172 225 for drug-resistant bacteria treatment and other bioactive molecules. The reaction possibly proceeds by formation of the alkynylcopper(I) complex I, which undergoes a SET process with the racemic alkyl bromide to afford the radical species, and the CuII intermediate II. Subsequent coupling of both species delivers the coupling product and releases the catalyst.
Scheme 46. Enantioconvergent Cu-Catalyzed Reaction of Secondary Benzylic Bromides 23 with Acetylenes.
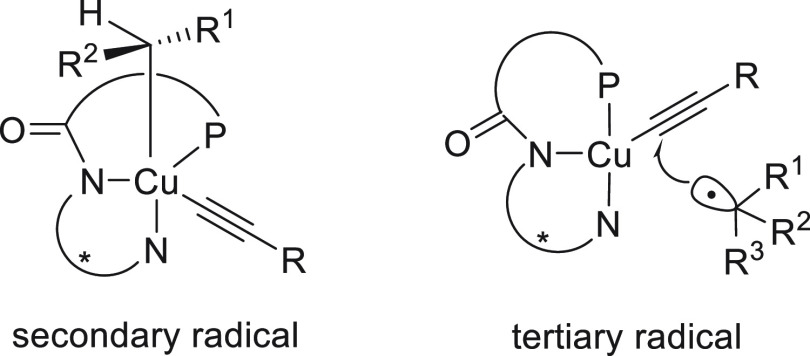
For the Cu-catalyzed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes, Liu and co-workers123 have developed tailor-made N,N,N-ligands on the basis of mechanistic studies. DFT calculations revealed that the coupling of the tertiary alkyl radical and the alkynyl group proceeded via an outer-sphere radical substitution-type C–C bond-formation pathway (Figure 2). However, the secondary alkyl radical is involved in the reductive elimination from an inner-sphere Cu(III) intermediate formed upon radical trapping121 (Figure 2). The enantiodetermining transition states in the outer-sphere C–C bond-formation mechanism are less organized, and therefore, an appropriate ligand should favor the accommodation of the sterically bulky tertiary radical.
Figure 2.
TSs for the Cu-catalyzed cross-coupling of secondary and tertiary radicals with acetylenes.
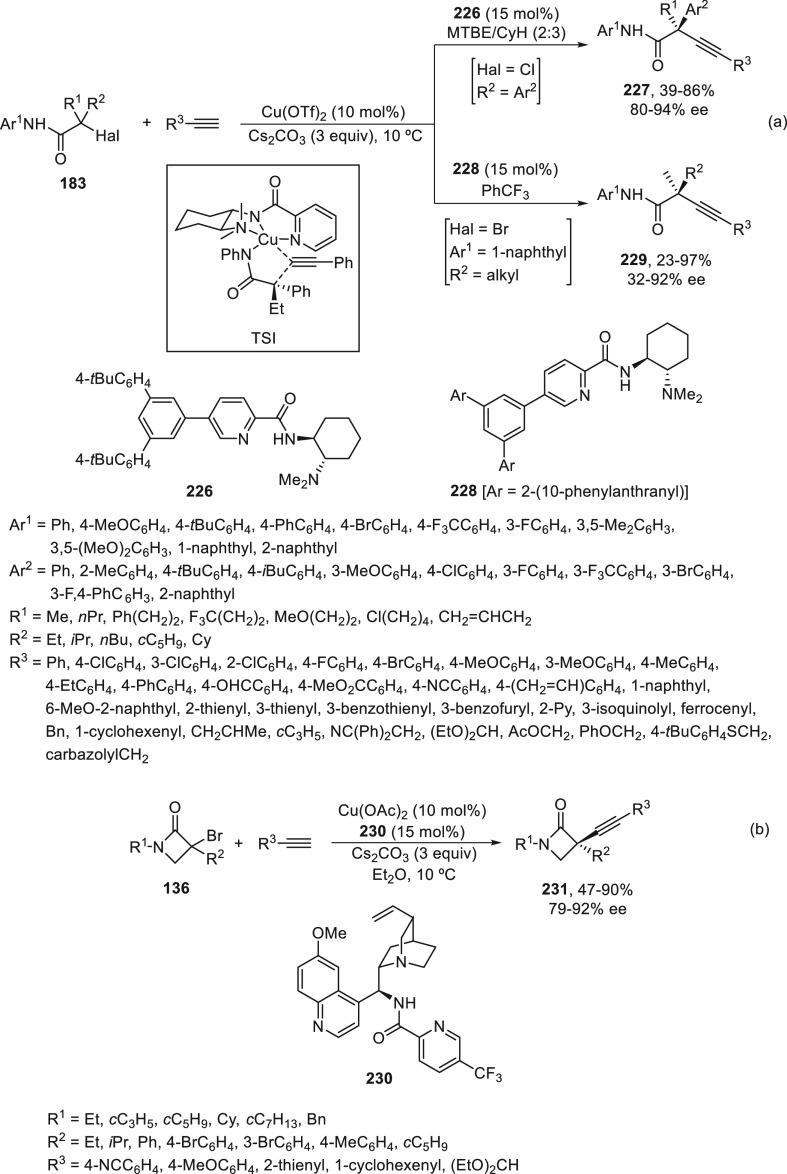
In the case of the cross-coupling of α-chloro amides 183 bearing an aryl group at the α-position, ligand 226 was the most efficient and afforded products 227 in 39–86% yields with 80–94% ee (Scheme 47a).123 α-Bromo amides 183 with two alkyl groups at the α-position were cross-coupled with terminal acetylenes in the presence of ligand 228 to provide products 229 in 23–67% yields with 32–92% ee (Scheme 47a). Cross-coupling of α-bromo-β-lactams 136 with terminal acetylenes was achieved using the chiral ligand 230 to furnish products 231 in good yields (47–90%) with 79–92% ee (Scheme 47b). DFT calculations explained the efficient enantiodiscrimination on the basis of the enantiodetermining outer-sphere radical group transfer pathway (see, TS).
Scheme 47. Enantioconvergent Cu-Catalyzed Sonogashira–Hagihara Reaction of Tertiary α-Halo Amides 183 and 136 with Acetylenes.
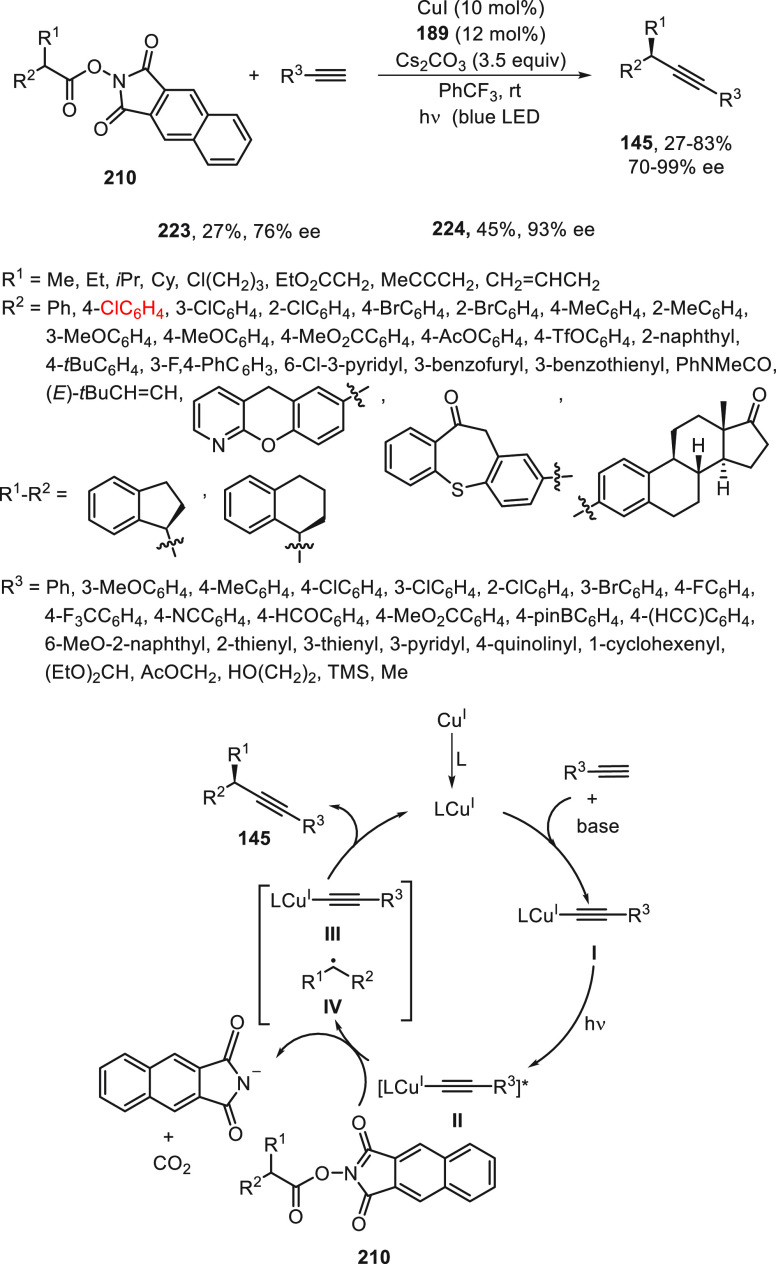
The same group124 performed the enantioconvergent radical decarboxylative C(sp3)–C(sp) cross-coupling by reaction of NHP-esters 210 with terminal alkynes. This photoinduced copper-catalyzed alkynylation of esters 210 as radical precursors used the anionic chiral multidentate N,N,P-ligand 189 for the enantiocontrol over prochiral radical intermediates to avoid their homodimerization. Because of the use of stable and easily available NHP-type esters 210, a broader substrate scope compared with their alkyl halide counterparts 23 was observed, which gave products 145 in moderate to good yields and excellent enantioselectivities (Scheme 48). In addition, a tandem one-pot procedure was developed starting from carboxylic acids, which were esterified and then, without purification, submitted to the standard asymmetric radical decarboxylative alkynylation. On the basis of experimental studies, a possible catalytic cycle was proposed. Thus, intermediate I was excited to give complex II, which transfers one electron to NHP-type ester 210 to deliver the CuII-complex III. The formed anionic radical of ester 210 undergoes a radical decarboxylation to generate the radical intermediate IV. Finally, C(sp3)–C(sp) bond formation with III provides the final product 145 and regenerates the catalyst L*CuI complex.
Scheme 48. Enantioconvergent Photocatalytic and Copper-Catalyzed Decarboxylative Alkynylation of Esters 210.
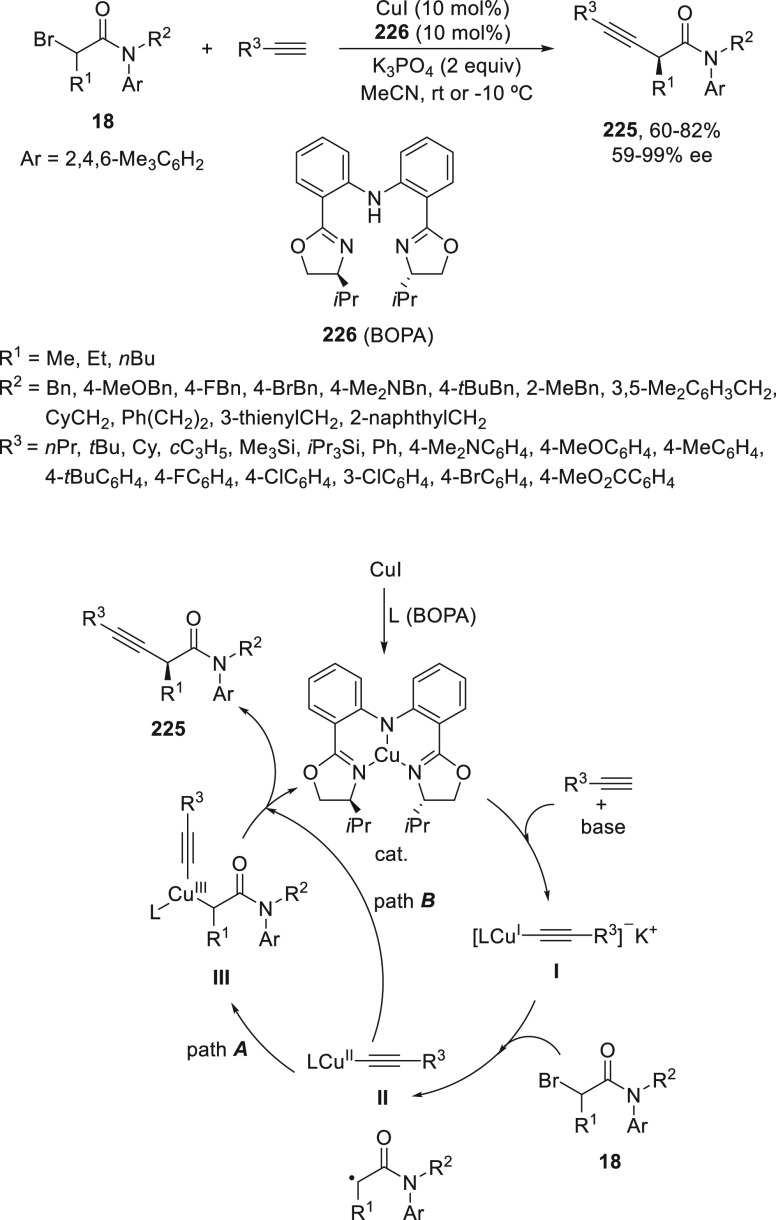
Zhang and co-workers125 reported an enantioconvergent Cu-catalyzed alkynylation reaction of α-bromo amides 18 with terminal alkynes to provide β,γ-alkynyl amides 225 (Scheme 49). They used as anionic chiral ligand114 a bis(oxazoline) diphenylanaline 226 (BOPA) containing a central anionic nitrogen σ-donor and two lone pair donors from the oxazoline units reported by Nakada et al.,126 whereas simple chiral bis(oxazoline) ligands are not effective in this cross-coupling reaction. Racemic α-bromo amides bearing a 2,4,6-trimethyphenyl group at the nitrogen are critical for good stereocontrol to give products 225 with good yields and high enantioselectivities. The authors proposed two possible pathways involving either an inner-sphere CuIII complex III, which undergoes reductive elimination to generate the product (path A), or the radical undergoes direct out-of-cage bond formation with the CuII species II to furnish the product (path B).
Scheme 49. Enantioconvergent Cu-Catalyzed Sonogashira–Hagihara Reaction of α-Bromo Amides 18 with Acetylenes.
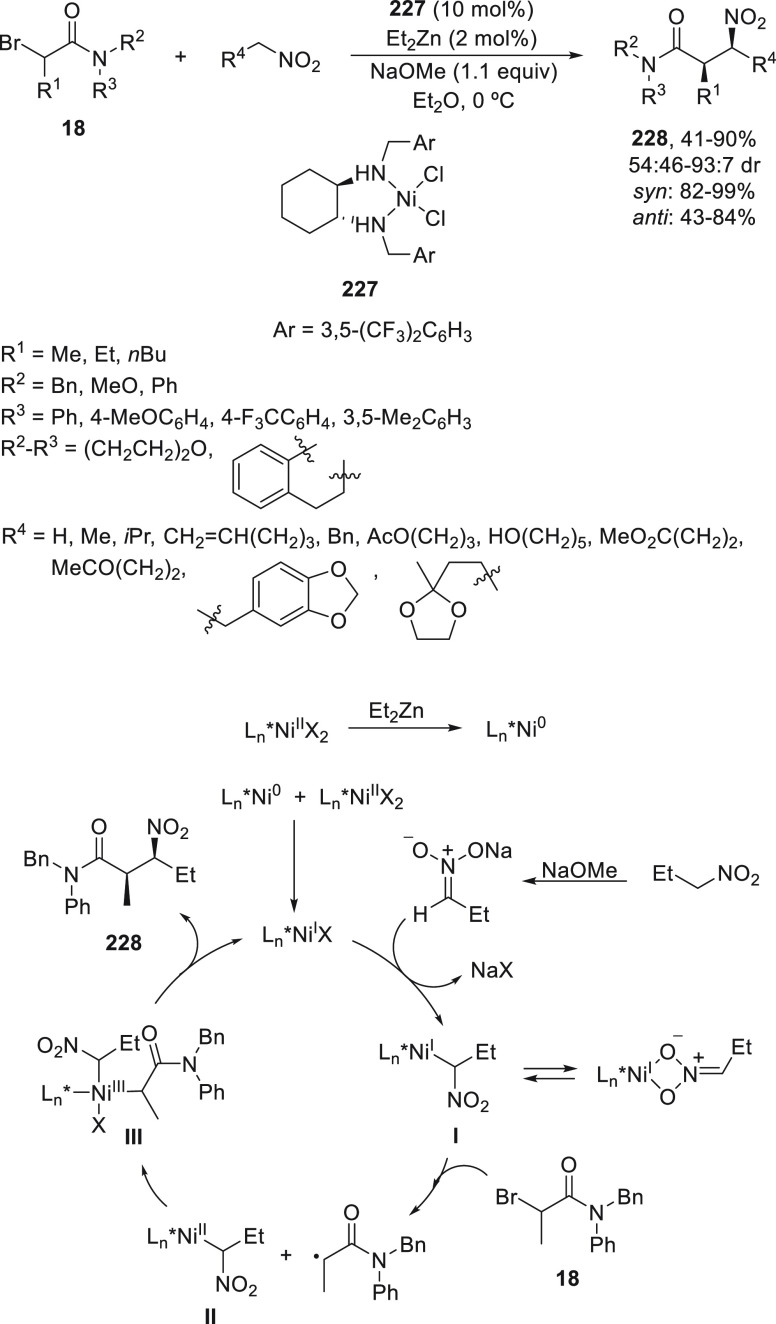
Enantioconvergent alkylation of nitroalkanes with racemic α-bromo amides 18 has been carried out under asymmetric Ni catalysis by Watson and co-workers.127 They employed the Ni-precatalyst 227 (10 mol %), Et2Zn (2 mol %) as in situ reductant, and NaOMe (1.1 equiv) as base in ethyl ether at 0 °C to provide β-nitro amides 228 mainly as syn diastereomers (Scheme 50). In the proposed mechanism, initial reduction of NiII to Ni0 followed by comproportionation with the excess of NiII complex 227 results in a NiI catalyst. Simultaneous deprotonation of the nitroalkane by NaOMe gives a nitronate anion, which undergoes anion exchange with the NiI complex to result in intermediate I. This Ni nitronate reacts with the α-bromo amide via a stepwise oxidative addition to form the NiII intermediate II and subsequently with the radical to form the NiIII species III. Reductive elimination of III provides the product and regenerates the catalyst.
Scheme 50. Enantioconvergent Ni-Catalyzed Reaction of α-Bromo Amides 18 with Nitroalkanes.
For the enantioconvergent decarboxylative cyanation of esters, a cooperative photoredox and copper/bis(oxazoline) catalysis produces enantioenriched benzylic nitriles. Iminyl-radical-triggered C–C bond cleavage of cyclohexanone oxime esters under Cu/bis(oxazoline) catalysis gave chiral 1,6- and 1,5-dinitriles. In the case of alkynylation reactions of benzylic bromides or NHP esters under Cu catalysis, the presence of a chiral multidentate N,N,P-ligand was crucial. Alkynylation reaction under Cu catalysis of α-bromo amides also needs a multidentate anionic N,N,N-ligand. Alkylation of nitroalkanes with α-bromo amides has been performed with NiCl2 and Et2Zn as reductant using a chiral 1,2-diamine ligand. In all these processes, radical intermediates are involved.
2.2.5. Boron Reagents
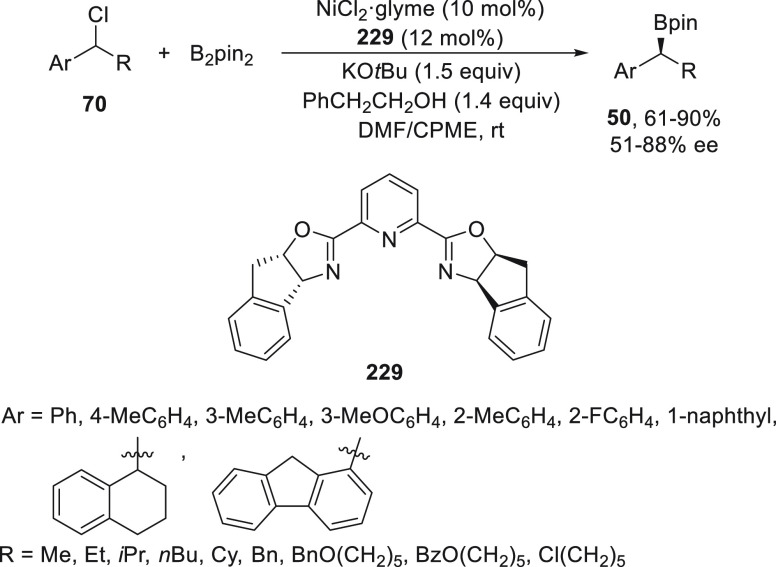
Concerning borylation of alkyl halides under metal catalysis, Miyaura borylation provided C–B bond formation to give alkylboranes. Dunik and Fu128 reported the Ni-catalyzed cross-coupling reaction of primary, secondary, and tertiary alkyl halides with diboron reagents. The resulting boronic esters can be aminated, hydroxymethylated, and arylated. Enantioenriched alkylboron compounds were converted with high retention of the configuration.129 Enantioconvergent Ni-catalyzed borylation of racemic secondary benzylic chlorides 70 was described by Fu and co-workers.130 The corresponding benzylic boronic esters 50 were obtained with good yields and enantioselectivities using NiCl2/bis(oxazoline) 229 as catalyst and B2pin2 as borylating reagent (Scheme 51). The authors demonstrated that enantioenriched benzylic chlorides do not undergo racemization under these reaction conditions. In the proposed mechanism, a radical pathway128 analogous to that described for the Kumada and Negishi reactions (see Section 2.1) was suggested.
Scheme 51. Enantioconvergent Ni-Catalyzed Miyaura Borylation of Benzylic Chlorides 70.
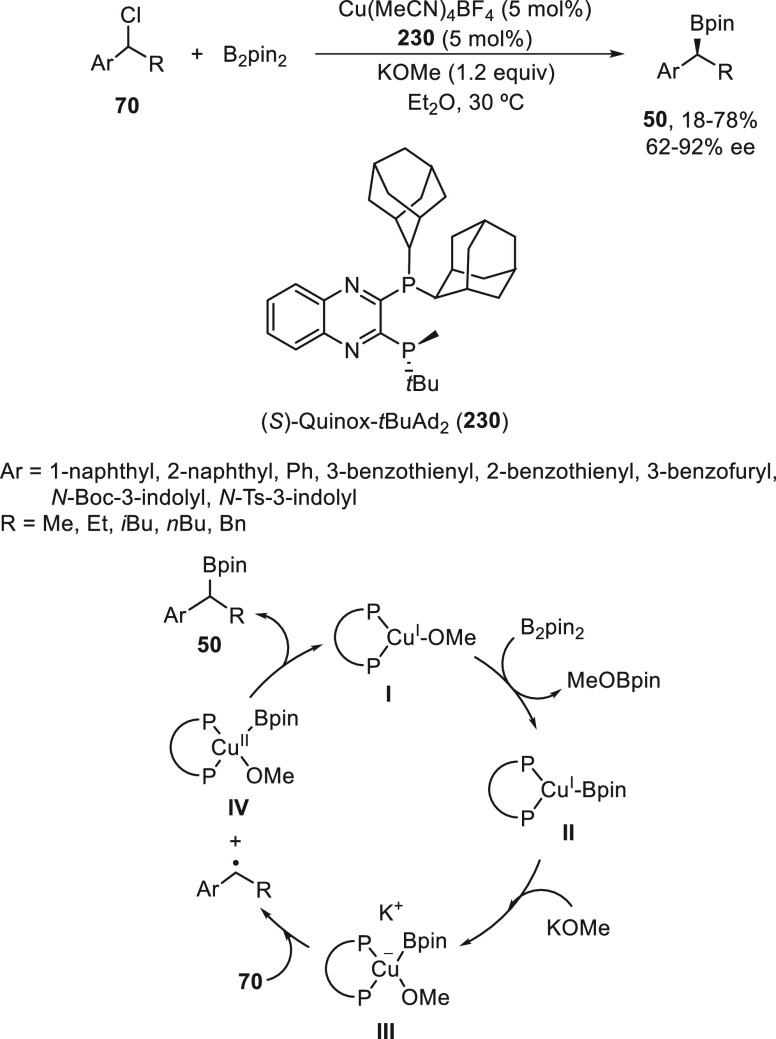
The copper(I)-catalyzed enantioconvergent borylation of racemic benzylic chlorides 70 with B2pin2 has been reported by Ito and co-workers.131 Boronic esters 50 resulted in moderate to good yields and enantioselectivities using Cu(MeCN)4BF4/bisphosphine (S)-quinox-tBuAd2230 as chiral catalyst (Scheme 52). Mechanistic studies on copper(I)-catalyzed borylation reactions have led to a plausible catalytic cycle that involves a radical intermediate.131,132 Catalyst I reacts with B2pin2 to give the borylcopper(I) species II, which reacts with KOMe to provide cuprate III. SET from III to benzylic chloride generates the benzylic radical and the Cu(II) species IV. Subsequent enantioselective borylation of the radical by intermediate IV furnishes the product 50 and regenerates the catalyst I.
Scheme 52. Enantioconvergent Cu-Catalyzed Miyaura Borylation of Benzylic Chlorides 70.
2.3. Racemic Alkylmetals with Electrophiles
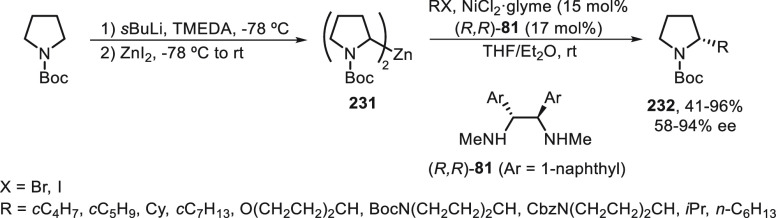
Enantioconvergent cross-coupling reactions for C(sp3)–C bond formation can be also performed through an umpoled strategy using racemic secondary alkyl metals. The first reverse polarity process was described by Kumada and co-workers133 using a racemic benzylic Grignard reagent (PhCHMeMgCl) and vinyl bromide as reaction partners and Ni complexes of chiral (aminoalkylferrocenyl)phosphines to generate enantioenriched 3-methylallylbenzene. Fu and co-workers134 performed the enantioconvergent Negishi reaction of α-zincated N-Boc-pyrrolidine 231 with alkyl halides under NiCl2/diamine 81 catalysis (Scheme 53). The resulting enantioenriched α-alkyl-N-Boc-pyrrolidines 232 were obtained with good yields when alkyl iodides were employed as electrophiles (50–96%), with lower yields with alkyl bromides (41–80%), and with good enantioselectivities, in general.
Scheme 53. Enantioconvergent Ni-Catalyzed Negishi Reactions of Racemic α-Zincated N-Boc-pyrrolidine 231 with Alkyl Halides.
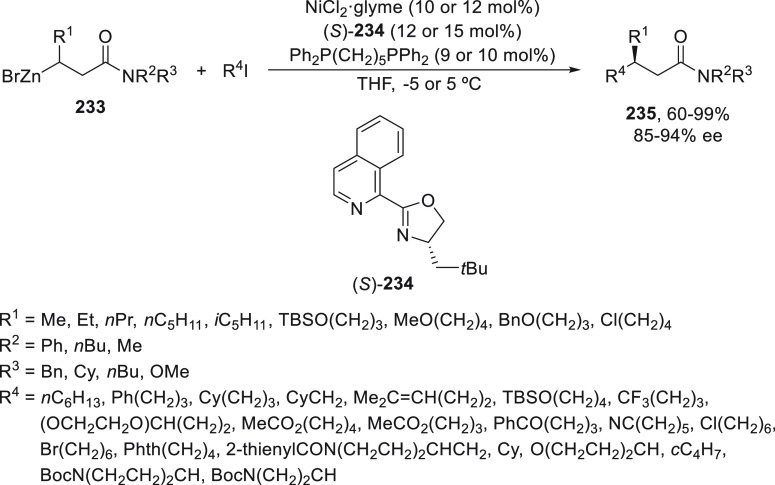
The same group recently reported135 this type of cross-coupling reaction using β-zincated amides 233 and a broad range of alkyl iodides (Scheme 54). The chiral catalyst NiCl2/isoquinoline-oxazoline 234 gave products 235 with good yield and ee. The reaction with primary alkyl groups was performed with 10 mol % of NiCl2 and 12 mol % of ligand at −5 °C, whereas secondary alkyl iodides needed a higher loading, 12 mol % NiCl2, and 15 mol % ligand at 5 °C.
Scheme 54. Enantioconvergent Ni-Catalyzed Negishi Reaction of Racemic β-Zincated Amides 233 with Alkyl Iodides.
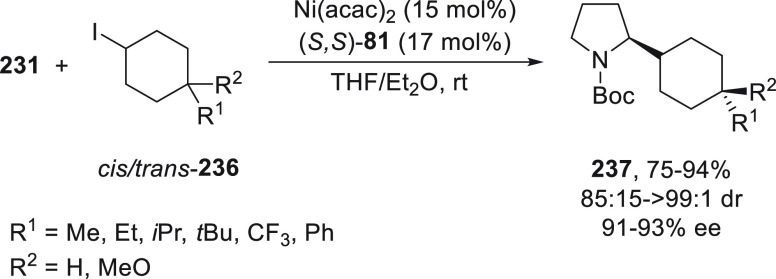
Doubly enantioconvergent cross-coupling of racemic alkyl nucleophiles and electrophiles was also described by Fu and co-workers.136 Vicinal stereocenters are generated with very good stereoselectivity when α-zincated N-Boc-pyrrolidine 231 was allowed to react with 4-substituted cyclohexyl iodides 236 under Ni catalysis (Scheme 55). This Negishi reaction proceeds with good yields to give products 237 with good ee and diastereoselectivity, and it also proceeds with 4,4-disubstituted and 3,5-disubstituted cyclic iodides. With respect to the new C–C bond, the chiral catalyst controls the stereochemistry of the stereocenter in the nucleophile, and the substrate controls the stereochemistry of the stereocenter generated in the electrophile.
Scheme 55. Doubly Enantioconvergent Ni-Catalyzed Negishi Reaction of α-Zincated N-Boc-pyrrolidine 231 with Racemic Alkyl Electrophiles 236.
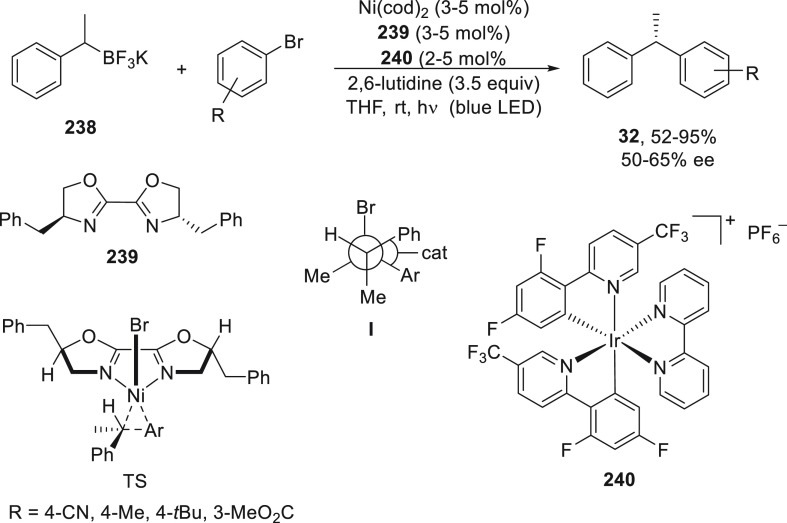
The first enantioconvergent Suzuki reaction of racemic alkylboron reagents with aryl halides was described by Molander and co-workers.137−139 Because of the slow rate of transmetalation of inactivated alkylboron, a photocatalyst 240 and nickel/bis(oxazoline) 239 as dual catalysts enable the cross-coupling of potassium alkyltrifluoroborate 238 with aryl bromides to afford enantioenriched diaryl ethanes 32 in moderate enantioselectivity (Scheme 56). On the basis of DFT calculations, it was proposed to be a DKR of a Ni(III) intermediate wherein the stereodetermining step is the reductive elimination.139 In the lower energy diastereomeric TS, the gauche-like interactions (I) along the forming C–C bond are avoided by rotation of the α-methylbenzyl group.
Scheme 56. Enantioconvergent Photocatalytic and Ni-Catalyzed Suzuki Reactions of Potassium Alkyltrifluoroborate 238 with Aryl Bromides.
In comparison with enantioconvergent cross-coupling reactions of racemic alkyl halides with nucleophiles, only secondary racemic alkylzinc reagents react with electrophiles in an enantioconvergent manner under Ni catalysis. However, other racemic organometals, such as organoboron reagents, equilibrate under the reaction conditions. Recently, another strategy has involved Ni-catalyzed double enantioconvergent cross-coupling of racemic secondary alkylzincs with racemic secondary alkyl electrophiles to generate two stereocenters.
2.4. Reductive Cross-Couplings
An alternative strategy for enantioconvergent C–C bond formation between one electrophile and one organometallic partner is the cross-electrophile coupling reaction.140,141 This reductive cross-coupling (RCC) reaction has been carried out mainly under Ni catalysis between C(sp3) and C(sp2) electrophiles in the presence of a terminal reductant and alternatively under photoredox catalysis. Enantioconvergent related processes, such as decarboxylative cross-coupling reactions and acyl cross-coupling reactions, will be also considered.
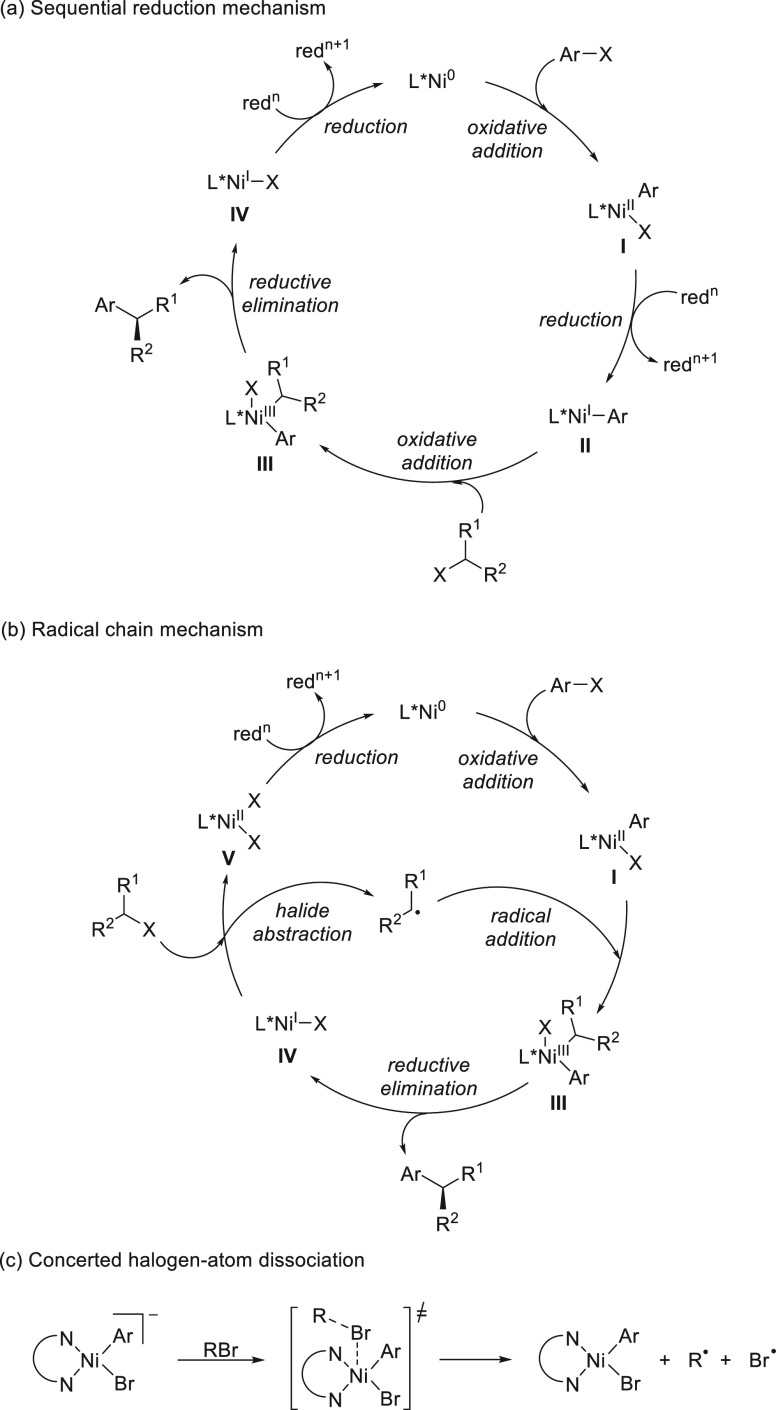
Initial studies on a possible mechanism for conventional RCC reactions are based on a sequential reduction mechanism (Figure 3a) and on a radical chain mechanism (Figure 3b).142,143 In the sequential reduction mechanism, the C(sp2) electrophile undergoes oxidative addition to Ni(0) preferentially to give the Ni(II) complex I, which is then reduced by a metal reductant to Ni(I) intermediate II. This complex II reacts with the C(sp3) electrophile to give the Ni(III) intermediate III, which after reductive elimination provides the enantioenriched product and the Ni(I) complex IV that, after reduction, regenerates the Ni(0) catalyst. In the radical mechanism, intermediate I is formed similarly, which reacts with the alkyl radical to give the Ni(III) complex III, precursor of the final product. Subsequent reductive elimination of III also generates intermediate IV, which undergoes halide abstraction from the alkyl halide to generate the radical and intermediate V. Final reduction of V regenerates the Ni(0) catalyst.
Figure 3.
Proposed mechanisms for conventional reductive cross-coupling reactions.
Diao and co-workers144 recently reported electroanalytical and theoretical studies to elucidate the Ni-mediated radical formation in cross-electrophile coupling reactions. Cyclic voltammetry studies on (bpy)Ni(Mes)Br revealed that instead of outer-sphere electron transfer or two-electron oxidative addition pathways, by using (bpy)Ni catalyst proposed for the halogen-atom abstraction pathway, the inner-sphere electron transfer concerted with halogen-atom dissociation (Figure 3c).
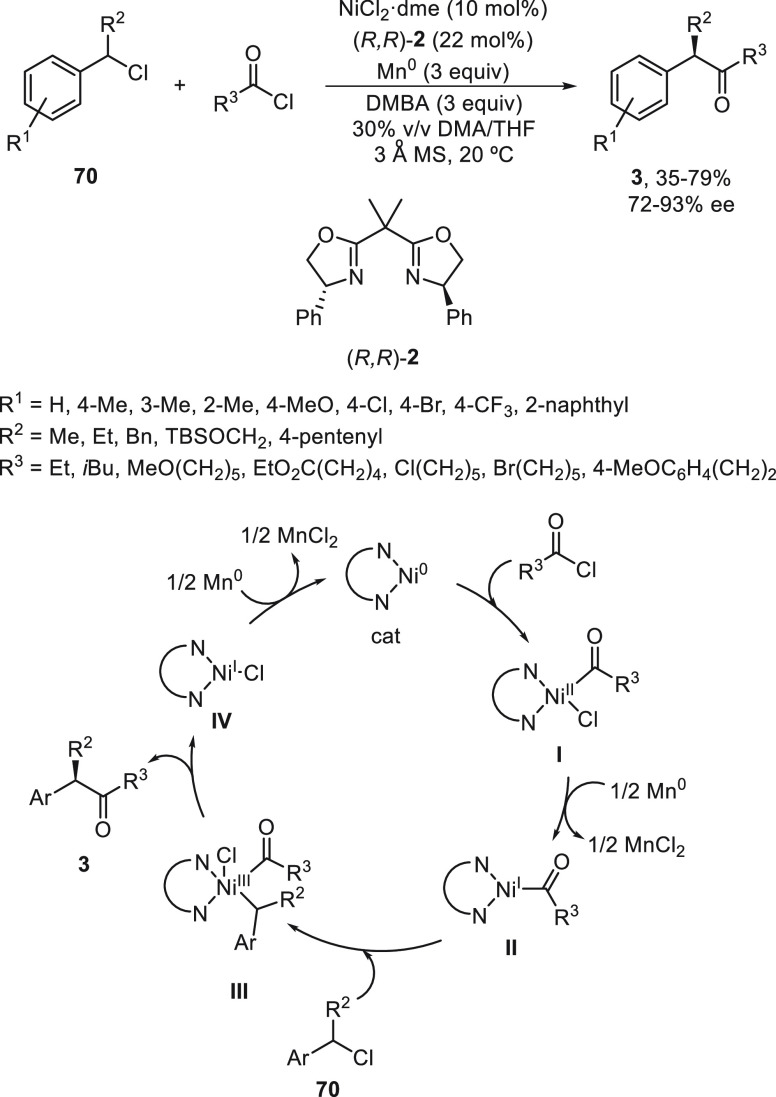
Reisman and co-workers145 reported in 2013 the enantioconvergent acyl cross-coupling of benzylic chlorides 70 with acyl chlorides using NiCl2/bis(oxazoline) Ph-Box (R,R)-2 as catalysts and Mn(0) as the terminal reductant (Scheme 57). The corresponding enantioenriched α-substituted ketones 3 were obtained with moderate to good yields and enantioselectivities in a mixture of THF/DMA and in the presence of dimethylbenzoic acid (DMBA) as additive in order to suppress homocoupling of the benzylic chloride. In the proposed mechanism, intermediate I results from the oxidative addition of the acid chloride, which could be reduced by Mn(0) to give the Ni(I)-acyl species II. Subsequent oxidative addition of a benzyl chloride 70 by a radical process generates the Ni(III) complex III, which undergoes reductive elimination to give intermediate IV and the ketone.
Scheme 57. Enantioconvergent Ni-Catalyzed Reductive Acyl Cross-Coupling of Benzylic Chlorides 70.
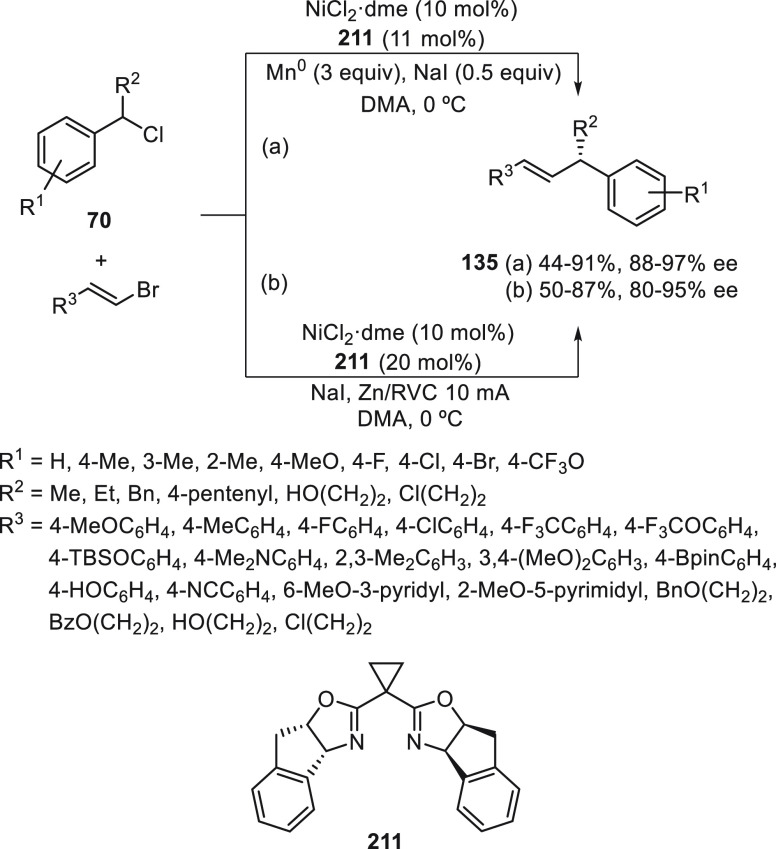
The same group reported the enantioconvergent RCC of benzylic chlorides 70 with alkenyl bromides under NiCl2/bis(oxazoline) 211 and Mn(0) as terminal reductant (Scheme 58a).146 In this case, NaI was an important additive improving the yield of products 135 and decreasing the formation of the dibenzyl homodimer. NaI has been suggested to accelerate the electron-transfer between Mn(0) and Ni or by in situ formation of iodide electrophiles.147 Alkenes 135 were obtained with good yields and enantioselectivities. This process can be driven electrochemically to avoid the use of metal powder as reducing agent.148,149 The corresponding products 135 resulted in up to 87% yield and up to 95% ee (Scheme 58b). Reticulated vitreous carbon (RVC) was used as the cathode, and Zn was used as the sacrificial anode in an undivided cell.148
Scheme 58. Enantioconvergent Ni-Catalyzed Reductive Cross-Coupling of Benzylic Chlorides 70 with Alkenyl Bromides.
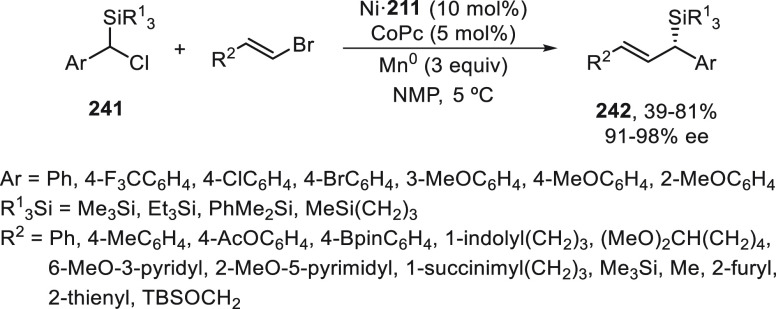
By Ni-catalyzed RCC, Reisman and co-workers150 performed the synthesis of enantioenriched allylic silanes 242 from chloro(arylmethyl)silanes 241 and alkenyl bromides (Scheme 59). In this case, a cobalt phthalocyanine (CoPc) was required for efficient coupling of these bulky benzylic silanes, presumably to favor radical formation.151 This RCC took place in the presence of NiCl2/bis(oxazoline) 211 and Mn(0) as terminal reductant in NMP at 5 °C to provide allylic silanes 242 in moderate to good yields and high enantioselectivities. Stereospecific transformations of these products were applied to the synthesis of (+)-tashiromine.
Scheme 59. Enantioconvergent Ni-Catalyzed RCC of Chloro(arylmethyl)silanes 241 with Alkenyl Bromides.
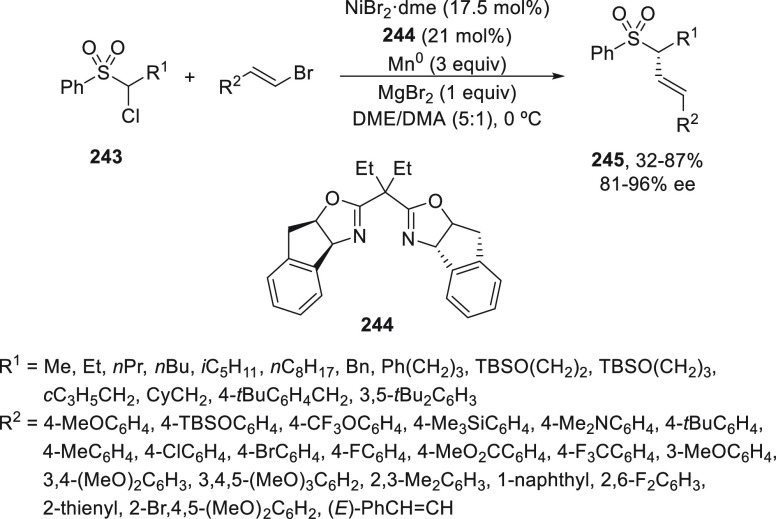
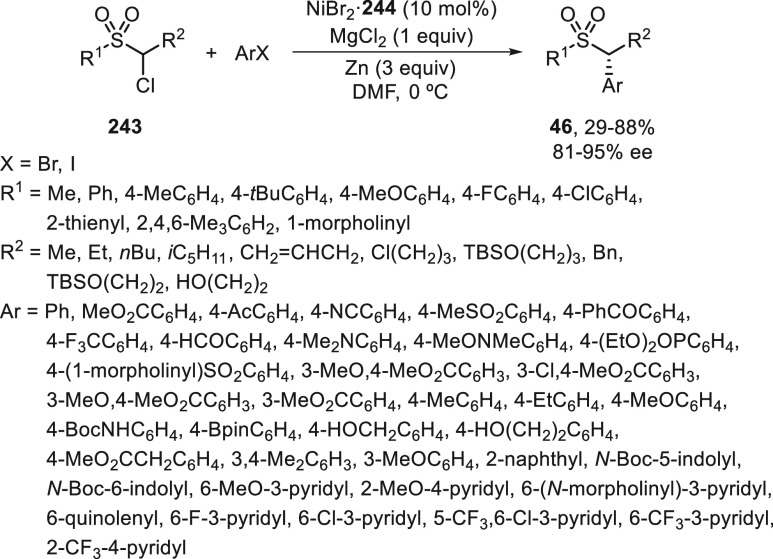
Recently, Sun, Wu, and co-workers152 reported the enantioconvergent reductive alkenylation of α-chloro sulfones 243 under NiBr2/bis(oxazoline) 244 catalysis, Mn as reductant, and MgBr2 as additive (Scheme 60). The resulting enantioenriched allylic sulfones 245 were isolated in up to 87% yield and up to 96% ee and involved radical intermediates.
Scheme 60. Enantioconvergent Ni-Catalyzed RCC of α-Chloro Sulfones 243 with Alkenyl Bromides.
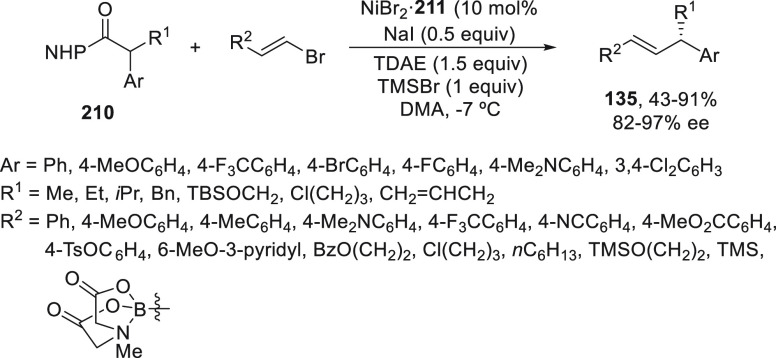
Enantioconvergent reductive alkenylation of N-hydroxyphthalimide (NHP) esters 210 with alkenyl bromides have been carried out by Reisman and co-workers (Scheme 61).153 These esters 210 underwent decarboxylation to generate the corresponding benzylic radicals, which by cross-coupling with alkenyl bromides and using the complex NiBr2/bis(oxazoline) 211 as catalyst furnished enantioenriched alkenes 135 in up to 91% yield and up to 97% ee. The reaction uses tetrakis(N,N-dimethylamino)ethylene (TDAE) as a terminal organic reductant instead of a large excess of metal(0), TMSBr, and NaI as additives. This procedure is an alternative to the use of benzylic chlorides,146 which could be difficult to prepare or unstable, but still gives similar enantioselectivities. According to experimental data, the corresponding mechanism proceeds through a cage-escaped radical.
Scheme 61. Enantioconvergent Ni-Catalyzed Reductive Decarboxylative Cross-Coupling of N-Hydroxyphthalimide Esters 210 with Alkenyl Bromides.
Preliminary enantioconvergent RCC of α-chloroethylbenzene with 4-acetylbromobenzene was described by Weix and co-workers151 using NiBr2/bis(oxazoline) as chiral catalyst and CoPc as cocatalyst to afford the corresponding diarylethane in 41% yield and 43% ee. In 2015, Kadunce and Reisman154 reported the Ni-catalyzed RCC of α-chloro nitriles 246 and aryl iodides (Scheme 62a). This enantioconvergent RCC was performed using NiCl2/phosphinoxazoline (S)-247, Mn(0) as reductant, and TMSCl as additive to provide nitriles 38 in good yields and enantioselectivities. To access 1,1-diarylalkanes 32 from benzylic bromides 70, a different ligand (S)-248 was used by Reisman and co-workers (Scheme 62b).155 Products 32 were obtained under similar reaction conditions as nitriles 38 with moderate to good yields and in up to 95% ee.
Scheme 62. Enantioconvergent Ni-Catalyzed Reductive Cross-Coupling of α-Chloro Nitriles 246 and Benzylic Chlorides 70 with Aryl Iodides.
Doyle, Sigman, and co-workers156 reported the enantioconvergent RCC of racemic styryl aziridine 249 with aryl iodides (Scheme 63). This enantioconvergent C(sp3)–C(sp2) cross-coupling was carried out under NiCl2/bis(oxazoline) 248 catalysis using Mn(0) as a terminal reductant and in the presence of NaI and TMSCl as additives to provide 2,2-diarylethylamines 250 in good yields and enantioselectivities (up to 94%).
Scheme 63. Enantioconvergent Ni-Catalyzed Reductive Cross-Coupling of Styryl Aziridine 249 with Aryl Iodides.
Asymmetric α-sulfonyl arylation of α-chloro sulfones 243 was performed by Lei, Gong, and co-workers.157 They used similar reaction conditions to those previously described for the alkenylation of α-chloro sulfones151 (see Scheme 60). Enantioconvergent Ni-catalyzed RCC of compounds 243 with aryl bromides and iodides yielded enantioenriched α-arylated sulfones 46 in modest to good yields and good enantioselectivities (Scheme 64). In this case, Zn(0) and, in some cases, Mn(0) were used as terminal reductants in DMF as solvent.
Scheme 64. Enantioconvergent Ni-Catalyzed Reductive Cross-Coupling of α-Chloro Sulfones 243 with Aryl Halides.
For the enantioconvergent RCC of α-chloro esters 251 with aryl iodides (1.5 equiv), Reisman and co-workers158 employed NiBr2/bis(oxazoline) 248 as catalyst, Mn(0) as terminal reductant, and NaBF4 as additive to provide esters 9 in good yields and enantioselectivities (Scheme 65). Under these reaction conditions, even β-branched esters (e.g., R = iPr, secBu) gave the corresponding α-aryl esters with good yield and high ee. Experimental studies exclude the participation of a manganese enolate generated in situ from the ester. This procedure was applied to the synthesis of naproxen by reaction of phenyl α-chloro propionate with 6-methoxy-2-naphthyl iodide to give the corresponding ester with 93% yield and 84% ee. DFT calculations using multivariate linear regression model quantitatively relate the cooperative influence of the α-chloro ester and ligand steric profiles on enantioselectivity.
Scheme 65. Enantioconvergent Ni-Catalyzed Reductive Cross-Coupling of α-Chloro Esters 251 with Aryl Iodides.
Dual-nickel/photoredox catalysis159−161 is an alternative strategy for challenging cross-electrophile coupling reactions to promote C(sp3)–C(sp2) bond formation. MacMillan, Doyle, and co-workers162 used carboxylic acid as radical precursors for decarboxylative photocatalyzed/Ni-catalyzed cross-coupling to form C(sp3)–C(sp2) bonds. Enantioconvergent photoredox decarboxylative arylation was carried out by MacMillan, Fu, and co-workers163 starting from racemic α-amino acids and aryl bromides (Scheme 66). Using low loadings of the Ir photocatalyst 240 (2 mol %) and NiCl2/bis(oxazoline) 252 (2–2.2 mol %) as catalyst, the corresponding N-Boc-benzylamines 253 were obtained in up to 84% yield and up to 93% ee. The stereochemical outcome was determined by the configuration of the ligand. This protocol was applied to the synthesis of pharmacophores present in bioactive compounds.
Scheme 66. Enantioconvergent Photoredox and Ni-Catalyzed Decarboxylative Cross-Coupling of α-Amino Acids and Aryl Bromides.
In a similar decarboxylative arylation under photoredox/Ni dual catalysis, Davison and co-workers164 reported an enantioconvergent synthesis of N-benzyl heterocycles 256 from α-heterocyclic carboxylic acids 254 with aryl bromide (Scheme 67). They used an organic photocatalyst 4CzIPN and NiBr2/pyridine-oxazoline (S)-255 as dual catalysts for the C(sp3)–C(sp2) cross-coupling with aryl and hetaryl bromides. The presence of a directing group at the C2 position of the nitrogenated heterocycle increased stereoselectivity in the final product, which was obtained in modest to good yields and up to 88% ee.
Scheme 67. Enantioconvergent Photoredox and Ni-Catalyzed Decarboxylative Cross-Coupling of α-Heterocyclic Carboxylic Acids 254.
Racemic α-chloro imidazol-2-yl ketones 257 reacted with N-aryl glycines under photoredox/rhodium catalysis to form C(sp3)–C(sp3) bonds.165 This enantioconvergent decarboxylative cross-coupling was performed with a chiral rhodium complex 258, which serves as chiral Lewis acid and as photoredox active species upon substrate binding under blue LED irradiation. The resulting products 259 were isolated in up to 80% yield and up to 98% ee. On the basis of extensive studies on Ir and Rh catalysts by Meggers’s group,166,167 the proposed mechanism is depicted on Scheme 68. By coordination of the ketone 257 with the Rh complex, chelate I is formed, which is the photoactive species. Upon irradiation to photoexcited state, intermediate II facilitates a SET from N-aryl glycinates to provide an amine radical cation and an Rh ketyl III. The glycinate radical releases CO2 to furnish an α-aminoalkyl radical, which is coupled with a radical intermediate IV formed by release of chloride from intermediate III, thereby providing the coupling intermediate V. Final release of product 259 and coordination with substrate 257 generates the complex I. The steric model of the Rh-bound radical intermediate IV explains the attack of the α-aminoalkyl radical at the Re face.
Scheme 68. Enantioconvergent Rh-Photocatalyzed Decarboxylative Cross-Coupling of N-Aryl Glycines and α-Chloro Imidazol-2-yl Ketones 257.
Melchiorre and co-workers168 used 1,4-dihydropyridines (1,4-DHPs) as photoreductants in the enantioconvergent photoredox acyl cross-coupling of 4-alkyl-1,4-DHPs 260 and 261 with anhydrides (Scheme 69). This process does not require a photocatalyst and was carried out using NiCl2/bis(oxazoline) (R,R)-2 as catalyst with a single high-power (HP) LED (λmax = 405 nm) to provide ketones 262 and 6 in up to 83% yield and up to 95% ee. Direct excitation of DHPs at 405 nm gave the excited-state intermediate A*, which participates in two sequential SETs with the Ni catalyst to give the Ni(0) intermediate I and the radical cation A+• [E(A+•/A* = 1.6 V and E(NiII/Ni0) = −1.2 V]. This unstable A+• undergoes homolytic cleavage to generate a radical B. Oxidative addition into the acyl anhydride would provide the acylNi(II) complex II, which by radical trapping of B affords the acyl–Ni(III) complex III. Subsequent reductive elimination furnishes the ketone and the Ni(I) complex IV, which by a SET process from A* regenerates the Ni(0) species I.
Scheme 69. Enantioconvergent Photoredox/Ni-Catalyzed Acyl–Akyl Cross-Coupling of 4-Alkyl-1,4-dihydropyridines 260 and 261 with Anhydrides.
An enantioconvergent Ni/photoredox-catalyzed reductive cross-coupling of racemic α-chloro esters 251 with aryl iodides was described by Mao, Walsh, and co-workers.169 They employed the organic dye 4CzIPN (see Scheme 67) as photocatalyst and a Hantzsch ester (HEH) as organic reductant instead of metals, such as Mn and Zn (Scheme 70). As in the case of the enantioconvergent arylation of α-chloro esters 251 (Scheme 65), bis(oxazoline) (S,S)-248 was used as ligand to furnish the corresponding α-aryl esters 9 with good yields and ee. A dual-catalytic mechanism has been proposed to explain this process. The aryl iodide undergoes oxidative addition of the Ni(0) species I to give complex II. Next, the α-chloro ester is reduced by SET to the α-carbonyl radical by the reduced photocatalyst 4CzIPN–. This radical is trapped by intermediate II to generate the Ni(III) species III, which undergoes rapid reductive elimination to form the α-aryl ester. In the left catalytic cycle, 4CzIPN gives by blue light excitation the long-lived photoexcited-state 4CzIPN*, which can be reduced by HEH to give 4CzIPN–. The LnNi(0) species I could be regenerated by a SET from 4CzIPN–.
Scheme 70. Enantioconvergent Photoredox/Ni-Catalyzed Reductive Cross-Coupling of α-Chloro Esters 251 with Aryl Iodides.
Asymmetric borylation reactions of benzylic chlorides with B2pin2 have been carried out under Ni and Cu catalysis by the Fu130 (Scheme 51) and Ito131 (Scheme 52) groups, respectively. Recently, Xu and co-workers170 reported the photoredox/Ni-catalyzed reductive cross-coupling of aryl iodides and α-chloroboranes 48 to furnish benzylic boronic esters 50 with excellent enantioselectivities (Scheme 71). In this case, NiBr2/diamine (R,R)-79 as chiral catalyst, 4CzIPN as photocatalyst, and HEH as reductant were used. A similar mechanism as depicted in Scheme 70 has been proposed.
Scheme 71. Enantioconvergent Photoredox Ni-Catalyzed Reductive Cross-Coupling of α-Chloroboranes 48 with Aryl Iodides.
The same group applied this Ni/photoredox methodology to the synthesis of enantioenriched trifluoromethylated alkanes 47 (Scheme 72).171 Reductive cross-coupling of aryl iodides with racemic α-CF3-substituted alkyl bromides 44 was carried out in the presence of the chiral bis(imidazoline) (S,S)-263172 to provide products 47 in good yields and enantioselectivities under mild conditions. Aryl bromides can be also used to give the products with lower yields. Aryl iodides derived from drugs, such as clofibrate and aniracetam, were transformed into CF3-containing derivatives.
Scheme 72. Enantioconvergent Photoredox/Ni-Catalyzed Reductive Cross-Coupling of α-CF3-Substituted Alkyl Bromides 44 with Aryl Iodides.
Doyle and co-workers173,174 reported a Ni/photoredox-catalyzed enantioconvergent RCC of styrene oxides with aryl iodides. Initial studies173 were carried out with different epoxides using NiBr2/Cp2TiCl2 and 4CzIPN as catalysts. In the enantioconvergent version,174 NiBr2/bis(imidazoline) (S,S)-263 and 4CzIPN were used as catalysts, and MgBr2 was used as Lewis acid (Scheme 73). Enantioenriched 2,2-diarylethanols 264 resulted with high enantioselectivity in correlation with electronic properties of the assayed ligands. Experimental and theoretical mechanistic studies supported that the reductive elimination step is enantiodetermining and that TSS is 1.7 kcal/mol less in energy compared with TSR. One example with 4-ethoxycarbonylphenyl bromide gave the corresponding alcohol 264 with 60% yield and 88% ee. In addition, N-tosyl styrene aziridine 249 reacted with 4-acetylphenyl iodide to furnish the β,β-diaryl N-tosylethanamine 250 with 48% yield and 83% ee.
Scheme 73. Enantioconvergent Photoredox/Ni-Catalyzed Reductive Cross-Coupling of Styrene Oxides with Aryl Iodides.
Recently, XU, Li and co-workers175 applied the previously described photoredox/Ni-catalyzed conditions170 to the synthesis of enantioenriched α-aryl phosphonates 204 by arylation of α-bromophosphonates 265. A wide range of substrates were transformed into products 204 in good to excellent yields and enantioselectivities using NiBr2/bis(imidazoline) (S,S)-263 with HEH as an organic reductant (Scheme 74). From DFT calculations it was found that the oxidative addition of the alkyl radical to the LNi(II) species, and not the reductive elimination, was the enantiodetermining step.
Scheme 74. Enantioconvergent Photoredox Ni-Catalyzed Reductive Cross-Coupling of α-Bromophosphonates 265 with Aryl Iodides.
Conventional Ni-catalyzed enantioconvergent reductive cross-coupling reaction using mainly bis(oxazolines) as chiral ligands allowed the C(sp3)–C(sp2) bond formation of two electrophiles (alkyl with aryl or vinyl halides) in the presence of a terminal reductant. This methodology avoids the use of organometallic reagents and has been used for the formation of stabilized intermediate alkyl radicals, such as benzylic and α-substituted ones, bearing a silyl, sulfonyl or a cyano group. The combination of visible-light photoredox and Ni as dual catalysts is a novel strategy for these reductive cross-coupling reactions, which avoids the use of an excess of metal as terminal reductant. In this case, stabilized radicals are generated with α-amino, α-alkoxycarbonyl, α-boronates, α-trifluoromethyl, and α-phosphoryl groups. The use of bis(oxazolines) as chiral ligand allowed a high control of the enantioselectivity in the arylation of racemic alkyl electrophiles. Organic photocatalysis and reductants have been successfully used for these photoredox Ni-catalyzed transformations.
3. Enantioconvergent Allylic Cross-Couplings
Allylic systems undergo asymmetric allylic substitution reactions mainly under palladium catalysis through DyKAT processes.20−22,24,176−178 Allylic electrophiles are challenging substrates because they face several kinds of selectivity issues, such as chemo-, regio-, Z/E-stereo-, diastereo-, and enantioselectivity. An enantioconvergent cross-coupling reaction of racemic secondary allylic chlorides 266 with alkylzincs was described by Son and Fu in 2008.179 This Negishi reaction was carried out under NiCl2/bis(oxazoline) (S,S)-267 catalysis at −10 °C to provide products 268 in good yields and selectivities (Scheme 75). The enantioselectivity depends strongly on the substituent at the α-position of the chloride. Good ee values are obtained when symmetrical allylic chlorides have sterically low-demand substituents. Unsymmetrical allylic chlorides with R3 = Me and R1 = nBu gave regioselectivity favoring the reaction proximal to the methyl substituent in a ratio 1.9:1. This method was applied to the synthesis of a precursor of the macrocycle fluvirucinine A1.180 Schmidt and Kirschning181 used the same allylic chloride 266 (R1 = CO2Et, R3 = Me) for the synthesis of carolacton, which reduces the number of viable cells in biofilms at nanomolar concentration.
Scheme 75. Enantioconvergent Ni-Catalyzed Negishi Reaction of Allylic Chlorides 266 with Alkylzinc Reagents.
Enantioconvergent Negishi cross-coupling of regioisomeric mixtures of silylated allylic halides 269 and 270 (E/Z > 98:2) to provide enantioenriched vinylsilanes 272 has been described by Oestreich and co-workers (Scheme 76).182 The reaction was performed with alkylzinc bromides NiBr2 or NiI2/Pybox (S,S)-271 as catalysts in DMA at room temperature to give products 272 with good yields and enantioselectivities. The controlling element for the regioconvergence was the silyl group. Following the protocol of Tsubouchi and co-workers183 the cross-coupling of the obtained vinylsilanes 272 with a BnMe2Si substituent with alkyl electrophiles was carried out with alkyl halides to give products 268 with retention of the configuration in the stereocenter and in the C–C double bond. This two-step process is an alternative to the previously described Negishi reaction by Fu and co-workers179 for the synthesis of enantioenriched 1,3-dialkyl-substituted acyclic allylic systems 268.
Scheme 76. Enantioconvergent Ni-Catalyzed Negishi Reaction of Silylated Allylic Halides 269 and 270 with Alkylzinc Bromides.
Doyle and co-workers184 have reported the Ni-catalyzed enantioconvergent Suzuki cross-coupling of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinolines 273 with arylboroxines. This arylation of quinolinium intermediates was carried out in the presence of α,α,α′,α′-tetraaryl-2,2-disubstituted 1,3-dioxolane-4,5-dimethanol (TADDOL)-derived phosphonate 274 as chiral ligand to provide 2-substituted dihydroquinoline derivatives 275 in moderate to high yields and enantioselectivities (Scheme 77). According to previously developed mechanistic studies,185 initial formation of a quinolinium intermediate I is facilitated by Lewis acid assistance from the arylboroxine via an SN1-like mechanism, followed by an unusual ionic oxidative addition of the Ni(0) complex to afford the Ni(II) complex II. Final reaction of II with ArB(OR)3– gives rise to products 275.
Scheme 77. Enantioconvergent Ni-Catalyzed Suzuki Cross-Coupling of 2-Ethoxy-1,2-dihydroquinolines 273 with Arylboroxines.
4. Enantioconvergent Propargylic Alkylations
Smith and Fu reported in 2008 the first enantioconvergent Negishi cross-coupling of secondary propargylic bromides 202 with arylzinc reagents ArZnEt under NiCl2/bis(oxazoline) (−)-229 catalysis.186 The reaction took place with 5 mol % of NiCl2 in glyme at −20 °C to provide alkynes 145 with up to 93% yield and 94% ee (Scheme 78). The same group187 extended this Negishi reaction of propargylic carbonates 276 with ArZnI using 10 mol % of NiCl2(PCy3)2 and 13 mol % of Pybox (−)-229 as chiral ligand in a 1:1 mixture of DME/THF at 10 °C. The resulting alkynes 145 were obtained in up to 95% yield and 93% ee (Scheme 78). This method was also applied to the cross-coupling of racemic TMS-protected propargylic bromides and chlorides.187 Mechanistic studies on the enantioconvergent cross-coupling of propargyl bromides with arylzinc reagents revealed the formation of propargyl radical through an inner-sphere electron transfer reaction with a Ni(I) complex I.188 The resulting LNiBr2 complex II reacts with diarylzinc to provide complex III, which reacts with the propargyl radical to give intermediate IV. Final reductive elimination furnishes the product and regenerates complex I (Scheme 78). In the case of propargylic carbonates, for which a direct SN2 reaction is not viable, the authors suggested that the Ni(I) complex I adds to the carbonyl group to generate a (nickel)ketyl, which then fragments to form the propargyl radical.
Scheme 78. Enantioconvergent Ni-Catalyzed Negishi Reactions of Propargylic Bromides 202 and Carbonates 276 with Arylzinc Reagents.
When propargylic bromides 277 have a silyl moiety at the α-position, an enantioconvergent Ni-catalyzed Negishi reaction results in enantioenriched allenylsilanes 278 (Scheme 79).189 In this case, NiBr2/Pybox (S,S)-19 was used as catalyst for the cross-coupling with primary alkylzinc reagents to regioselectively form allenylsilanes 278 with good yields and moderate enantioselectivities. The high regioselectivity is because of the bulky silyl group directing the cross-coupling at the γ-position of the propargylic system.
Scheme 79. Enantioconvergent Ni-Catalyzed Negishi Reaction of α-Silylated Propargylic Bromides 277 with Primary Alkylzinc Reagents.
As it was mentioned in Section 2.1.3, Li, Liu, and co-workers89,90 reported the enantioconvergent Cu-catalyzed Suzuki reactions of benzylic bromides 19 with aryl and alkenyl boronates 131(89) and 133(90) (Scheme 27). These authors also reported the arylation of propargylic bromides 202 with aryl or heteroaryl B(mac)-derived boronate esters 131 using CuI/N,N,P-ligand 279 (Ar = 9-phenantryl) as catalyst (Scheme 80a).89 Enantioenriched alkynes 145 were obtained in up to 76% yield and 94% ee. Less reactive propargyl chloride of type 202 [R1 = TIPS, R2 = Ph(CH2)2] reacted with 3,5-diphenylB(mac) to give the corresponding alkyne with 42% yield and 95% ee. Alkenyl methylpentanediol (mp)-derived boronate esters 133 were allowed to react with propargylic bromides under the same reaction conditions as benzylic bromides but with ligand 134 (Scheme 24b) to provide enynes 280 in up to 98% yield and 99% ee (Scheme 80b).90
Scheme 80. Enantioconvergent Cu-Catalyzed Suzuki Reactions of Propargylic Bromides 202 with Aryl and Alkenyl Boronates 131 and 133, Respectively.
Lu, Lan, Xiao, and co-workers190 have developed an enantioconvergent propargylic radical cyanation via a synergetic photoredox/copper catalysis strategy. They employed an organophotocatalyst Ph-PTZ (213) to generate propargyl radicals and oxidize Cu(I) species to Cu(II) ones. Propargyl esters 281 were allowed to react with TMSCN using Cu(MeCN)4BF4/bis(oxazoline) ent-211 as chiral catalyst in THF at 30 °C under the irradiation of 2 × 3 W purple LED to give enantioenriched propargylic cyanides 282 in up to 97% yield and 98% ee (Scheme 81). Mechanistic studies based on experiments and DFT calculations suggested that the propargylic ester accepts a single electron from the excited state of Ph-PTZ* to generate propargyl radical and a carboxylate anion. This radical can be captured by LCuII(CN)2 (III) to form the Cu(III) IV. Reductive elimination of intermediate IV would deliver the propargylic cyanide and regenerate the chiral Cu(I) catalyst I. A cyanide anion can be released from TMSCN by the Cu(I) catalyst to form LCuI(CN)2– (II) species.
Scheme 81. Enantioconvergent Photoredox Cu-Catalyzed Cyanation of Propargylic Esters 281.
5. Enantioconvergent C–H Functionalization
Regioselective C–H functionalization processes are important strategies in the direct enantioselective reaction of organic molecules, mainly under transition metal catalysis and especially by means of C–H activation.191−198 This functionalization represents an atom- and step-economic procedure for the generation of structural complexity. In this Section, enantioconvergent C(sp2)–H, C(sp3)–H, and C–H allylic functionalizations will be considered.
5.1. C(sp2)–H Functionalization
Very recently, enantioconvergent cross-coupling of racemic alkyl bromides with azole C(sp2)–H bonds has been disclosed.197 This copper-catalyzed heteroarylation of benzylic bromides 23 has been carried out with azoles, such as 1,3,4-oxadiazoles 283, oxazoles 284, and benzo[d]oxazoles 285 (Scheme 82). CuBH4(PPh3)2/Cinchona alkaloid-derived N,N,P-ligand 286 was the appropriate catalyst in the presence of LiOtBu as base and H2O at 10 °C in DMA/DCM. The resulting enantioenriched azoles 287–289 were obtained in moderate to good yields and enantioselectivities and used for drug discovery. From experimental essays, a tentative mechanism has been proposed in which the LCu(I) complex I reacts with the azole to give intermediate II. This intermediate provides the alkyl radical and the Cu(II) complex III. Finally, after a C(sp3)–C(sp2) coupling via a Cu(III) intermediate and its reductive elimination affords the product.
Scheme 82. Enantioconvergent Cu-Catalyzed Cross-Coupling of Benzylic Bromides 23 with Azoles 283–285.
Independently, Li, Chen, Zhang, and co-workers198 reported a similar enantioconvergent alkylation of azoles under blue-light-promoted reaction conditions. Oxazoles 284 and benzoxazoles 285 reacted with secondary benzylic bromides 23 using CuI/bis(oxazoline) (S,S)-290 as a photo- and chiral catalyst and tBuOLi as base in DCE at −10 °C under blue LED irradiation (Scheme 83). The resulting C–H functionalized oxazoles 288 and benzoxazoles 289 were obtained with moderate to good yields and enantioselectivities. In the proposed mechanism, complex I is formed in situ and undergoes transmetalation with Li-azole to generate intermediate II. After photoexcitation, species III is formed, which delivers by electron transfer LiBr and complex IV. Subsequent transformation of complex IV into intermediate V via enantioselective radical trapping followed by reductive elimination from the Cu(III) center leads to product. Alternatively, intermediate IV can give the product through a direct SET process.
Scheme 83. Enantioconvergent Photo- and Cu-Catalyzed Cross-Coupling of Benzylic Bromides 23 with Oxazoles 284 and 285.
Enantioconvergent [3 + 2] annulation between 1,3-dienes and N-acyl ketimines, generated in situ from 3-aryl-3-hydroxyisoindolin-1-ones 291, proceeded via C(sp2)–H activation under an Ir/chiral diene 292 complex as catalyst (Scheme 84).199 This annulation gave spiroaminoindane derivatives 293 in high yields with high regio- and enantioselectivities. The catalytic cycle is postulated by C–H activation at the ortho position of ketimine II by oxidative addition of the C–H bond to Ir and deprotonation by 1,4-diazabicyclo[2.2.2]octane (DABCO) to provide arylindium(I) species III. The diene, e.g., isoprene, approaches the Ir center from the Re face of the imine to form intermediate IV. Oxidative cyclization in intermediate IV gives the π-allyliridium(III) complex V, and reductive elimination provides VI, which is followed by subsequent protonolysis to form the product and regenerate the cationic iridium catalyst I. This type of asymmetric [3 + 2] annulation has been further performed by the same group with alkynes to give products 294(200) and with 1,3-enynes to provide compounds 295.201
Scheme 84. Enantioconvergent Ir-Catalyzed Annulation of 3-Aryl-3-hydroxyisoindolin-1-ones 291 with 1,3-Dienes.
You and co-workers202 have described a Rh-catalyzed C(sp2)–H functionalization reaction of 4-aryl-5-pyrazolones 296 followed by [3 + 2] annulation reactions with alkynes to furnish highly enantioenriched 4-spiro-5-pyrazolones 298 in up to 99% yield and 98% ee (Scheme 85). These processes were catalyzed by a Rh complex 297 bearing a chiral 1,1′-spirobiindane scaffold SCpRh(C2H4)2. In the proposed catalytic cycle, once the pyrazolone 296 (R1 = Me, R2 = Cy, Ar = Ph) tautomerizes into 296′, the Rh catalyst I deprotonates the hydroxy group of 296′ to form intermediate II. This intermediate II undergoes C–H activation to give rhodacycle III, which forms the eight-membered rhodacycle IV by alkyne coordination and migratory insertion. Final reductive elimination provides product 298 and releases the Rh(I) species, which is oxidized by Cu(OAc)2 to the active Rh(III) catalyst I.
Scheme 85. Enantioconvergent Rh-Catalyzed Annulation of 4-Aryl-5-pyrazolones 296 with Alkynes.
5.2. C(sp3)–H Functionalization
In this Section, enantioconvergent functionalization of racemic compounds at the C(sp3)–H located at the α-, β-, and γ-positions of the functional group will be considered.
5.2.1. α-Functionalization
Racemic α-substituted carbonyl compounds and related systems are transformed into enantioenriched ketones with a quaternary carbon at the α-position, generally by enantioselective metal-catalyzed arylation reactions. This chemistry has been recently covered by Zhou, Yu, and co-workers.203 For the α-arylation of α-carbonyl enolates, aryl bromides, chlorides, and triflates have been used under Pd and chiral phosphines catalysis described by Buchwald and co-workers.204 In the pioneering work of Ma and co-workers,205 the arylation of 2-methylacetoacetates was performed with 2-iodotrifluoroacetanilides under CuI/trans-4-hydroxy-l-proline catalysis. The α-arylation of ketones with chloroarenes was carried out by Ge and Hartwig206 under Ni(cod)2/chiral diphosphines catalysis. Martin and co-workers207 described for the first time the asymmetric α-arylation of cyclic ketones with aryl pivaloyl esters under Ni(cod)2/tol-Binap catalysis. Recently, Li and Wang208 reported the same transformation using cyclic ketones 299 and aryl pyrimidyl ethers 300 under Ni(cod)2/Josiphos 301 catalysis (Scheme 86). The corresponding α-arylated ketones 302 were obtained with good yields and enantioselectivities in the presence of N-Boc-l-phenylalanine and Zn(OTf)2 in p-xylene at 130 °C. According to experimental studies, a plausible mechanism was proposed starting with formation of the chiral Ni complex I, which undergoes ligand exchange with the enolate anion of 299 and the aryl pyrimidyl ethers 300 to provide the anionic Ni(0) intermediate II. This intermediate II evolves to intermediate III by oxidative addition, which after ligand exchange with the chiral amino acid and Zn(OTf)2 provides intermediate IV. Subsequent reductive elimination of the Ni(II) complex IV gives the arylated ketone 302 and complex V to regenerate the catalyst by ligand exchange with 1,5-cyclooctadiene (cod) or with the enolate anion to form intermediates I and II, respectively.
Scheme 86. Enantioconvergent Ni-Catalyzed α-Arylation of Cyclic Ketones 299 with Aryl Pyrimidyl Ethers 300.
Enantioconvergent α-arylation of amides to give enantioenriched oxindoles was first described by Lee and Hartwig209 using Pd(dba)2 and a chiral N-heterocyclic carbene (NHC) as catalyst. Later, Buchwald and co-workers210 developed enantioconvergent α-arylation of 3-alkyl oxindoles with aryl bromides under Pd/chiral biaryl monophosphine ligand catalysis. α-Substituted γ-butyrolactones were enantioconvergently α-arylated with aryl chlorides and bromides in 2002 by Spielvogel and Buchwald211 using Ni(cod)2/Binap as catalyst in the presence of ZnBr2. Stolz, Morgan, and co-workers212 described the enantioconvergent α-arylation of α-alkyl γ-lactams with aryl iodides and bromides using Pd(0)/chiral diphosphines as catalyst. The first Pd-catalyzed enantioconvergent α-arylation of alkylnitriles with aryl bromides to provide enantioenriched α-aryl-α-alkyl nitriles was reported in 2016 by Zhou and co-workers213 using PdCl2/chiral phosphoramidite as catalyst.
Feng and co-workers214 reported the asymmetric C(sp3)–C(sp3) cross-coupling of racemic 3-substituted N-Boc oxindoles 303 with racemic 3-bromo oxindoles 304 for the enantioconvergent synthesis of 3,3′-bisoxindoles 305 (Scheme 87). This reaction took place with high yields and good diastereo- and enantioselectivities under mild reaction conditions catalyzed by chiral Ni(BF4)2/N,N′-dioxide 306 complex as a chiral Lewis acid. The corresponding products 305 were transformed into diverse hexahydropyrroloindole alkaloids, such as (+)-chimonanthidine, (+)-calycanthidine, and related compounds, with potential antiparasitic and anticancer properties.
Scheme 87. Enantioconvergent Ni-Catalyzed Reaction of 3-Bromooxindoles 303 with 3-Substituted N-Boc Oxindoles 304.
Recently, Cai and Shi215 reported an enantioconvergent formal α-arylation of racemic secondary benzylic alcohols 30 to enantioenriched tertiary alcohols 308 by means of a Ni/NHC 307 (ANIPE) catalyst (Scheme 88). This transformation takes place via a dehydrogenation of the secondary alcohol by phenyl triflate followed by addition of arylboronic esters 137 to the intermediate ketones. In the proposed dehydrogenative cycle, phenyl triflate undergoes oxidative addition to Ni(0) to give complex I, which reacts with the secondary alcohol to provide intermediate II. Subsequent β-hydride elimination of II gives benzene and the ketone regenerating Ni(0). In the carbonyl addition cycle, the ketone experiments a Ni-catalyzed enantioselective coupling by oxidative cyclization to form intermediate III followed by transmetalation with the arylboronic ester to provide intermediate IV. Final reductive elimination of complex IV forms the chiral tertiary alcohol and regenerates the Ni(0) catalyst.
Scheme 88. Enantioconvergent Ni-Catalyzed α-Arylation of Secondary Benzylic Alcohols 30 with Arylboronates 137.
5.2.2. β-Functionalization
Recently, metal-catalyzed processes have been used for enantioselective transformations by functionalization of prochiral β-C(sp3)–H bonds.216 Liu and co-workers217 have reported a radical enantioconvergent Cu(I)/chiral phosphoric acid (CPA) 313 dual catalytic protocol for the amination of racemic ketones bearing tertiary C(sp3)–H bonds 309 at the β-position (Scheme 89). By reaction of ketones 309 with arylsulfonylhydrazides 310, the corresponding hydrazones 311 were formed, which were treated with CuCN/CPA 313, perester 312 as oxidant, and ammonium carbonate as additive to provide enantioenriched dihydropyrazoles 314 with moderate to good yields and enantioselectivities. Mechanistic investigations suggest that initially Cu(I) reacts with 312-activated peroxide via a SET process to afford a tert-butoxy radical and the chiral Cu(II) phosphate complex I. Intermolecular hydrogen abstraction of the NH bond in the hydrazone by the tBuO radical provides radical II. A subsequent intramolecular 1,5-hydrogen atom abstraction step forms the tertiary radical III, which associates with complex I to form intermediate IV and promote the enantioselective C–N bond formation.
Scheme 89. Enantioconvergent Cu/CPA-Catalyzed β-Amination of Hydrazones 311 Derived from Ketones 309.
5.2.3. γ-Functionalization
Enantioconvergent amination of racemic tertiary C(sp3)–H bonds has been achieved by Zhang and co-workers218 through an intramolecular radical process via Co(II)-based metalloradical catalysis. This enantioconvergent 1,6-C(sp3)–H amination of sulfamoyl azides 315 was carried out using a Co(II) complex of porphyrin 2,6-DiMeO-QuingPhyrin 316 in benzene at 50 °C to form six-membered cyclic sulfamides 317 in up to 95% yield and 86% ee (Scheme 90). In the proposed mechanism, the efficient H atom abstraction of the tertiary C–H bond occurs by formation of α-Co(III)-aminyl radical I, which gives the carbon-centered radical II. Subsequent radical substitution of intermediate II provides the cyclic product 317. These products have been applied to the stereoselective construction of bicyclic N-heterocycles as sulfamide-fused piperazinone, imidazolone, and tetrahydroquinazoline.
Scheme 90. Enantioconvergent Co-Catalyzed γ-Amination of Sulfamoyl Azides 315.
5.3. Allylic Functionalizations
Asymmetric allylic C–H functionalization under Pd catalysis with unfunctionalized alkenes using an allylic hydrogen atom as the leaving group needs stoichiometric amounts of an oxidant.219 The cleavage of the allylic C–H bond gives the corresponding π-allylpalladium intermediate, which by nucleophilic attack accelerated by a phosphorus-based ligand releases Pd(0) and the product. By means of a stoichiometric amount of an oxidant, the Pd(II) catalyst is regenerated (Scheme 91).
Scheme 91. Pd-Catalyzed Allylic C–H Functionalization.
For the stereoselectivity control, two main strategies have been employed: (a) the use of a chiral ligand compatible with the oxidant and (b) a chiral counterion able to form hydrogen-bonding interactions with the nucleophile and also with a chiral ligand (Figure 4).
Figure 4.
Asymmetric strategies in the Pd-catalyzed allylic C–H functionalization.
These processes can be enantioconvergent when the nucleophile attacking the cationic π-allylpalladium intermediate is an enolate derived from a racemic compound. The first example was described by Trost and co-workers220,221 using 1,3-diketones 318 as nucleophiles, allylbenzenes, phosphoramidite 319 as chiral ligand, 2,6-dimethylbenzoquinone (2,6-DMBQ) as oxidant, and Et3N as base (Scheme 92), The resulting allylated 1,3-diketones 320 were obtained in up to 91% yield and 85% ee, which is lower than the traditional asymmetric allylic alkylation (AAA) with allylic acetates.
Scheme 92. Enantioconvergent Pd-Catalyzed Allylic Alkylation of 1,3-Diketones 318 with Allylbenzenes.
Gong and co-workers219,222 reported the allylation of cyclic β-keto esters 321 with 1,4-dienes in the presence of phosphoramidite 322 to provide enantioenriched α,α-disubstituted β-keto esters 323 with up to 96% ee (Scheme 93). The authors proposed a linear outer-sphere TS to explain the regioselective formation of the linear dienyl products 323. This procedure was applied to the formal synthesis of tanikolide, a brine-shrimp toxin and antifungal marine natural product isolated from the lipid extract of the cyanobacterium.223
Scheme 93. Enantioconvergent Pd-Catalyzed Allylic Alkylation of 1,4-Dienes with Cyclic β-Keto Esters 321.
However, the use of azlactones 324 as nucleophiles resulted in the corresponding branched products 326 (Scheme 94).219,224 Under Pd(dba)2 and phosphoramidite 325 catalysis with the use of 2,5-DMBQ as external oxidant, the allylic C–H alkylation of 1,4-dienes gave α,α-disubstituted α-amino acid surrogates 326 with high yields and excellent levels of Z diastereoselectivities and enantioselectivities. Experimental and computational studies suggest that through the TS the stereo and regioselectivity are governed by the geometry and coordination pattern of the nucleophile. This methodology has been applied to the synthesis of a key intermediate for the synthesis of lepadiformine marine alkaloids described by Rychnovsky and co-workers.225
Scheme 94. Enantioconvergent Pd-Catalyzed Allylic Alkylation of Azlactones 324 with 1,4-Dienes.
Enantioconvergent α-allylation of racemic enolizable aldehydes 327 with terminal alkenes was reported by Gong and co-workers219,226 by using the chiral counteranion strategy. This asymmetric cooperative catalysis was carried out under Pd(PPh3)4, cumylamine, and a CPA (R)-TRIP (328) catalysis with 2,5-BMBQ as oxidant and 3 Å molecular sieve (MS) as additive in methyl tert-butyl ether (MTBE) at 60 °C (Scheme 95). The resulting allylated aldehydes 329 were obtained with good yields and enantioselectivities. In the proposed mechanism, a π-allylpalladium phosphate complex I reacts with enamine II via TS, as demonstrated by Mukherjee and List,227 to give imine III, which generates the aldehyde 329 by hydrolysis. This process was also performed with 1,4-dienes under similar reaction conditions to provide the linear (E,E)-dienyl aldehydes in up to 82% yield, up to 94% ee, and >20:1 E/Z stereoselectivity.219,228
Scheme 95. Enantioconvergent Pd- and CPA-Catalyzed Allylic Alkylation of Aldehydes 327 with Terminal Alkenes.
Pyrazol-5-ones 296 are considered soft nucleophiles and have been allylated with allyl arenes by the cooperative catalysis of a chiral palladium complex and a chiral Brønsted acid by Gong and co-workers.219,229 This allylic C–H alkylation was carried out with Pd(dba)2/phosphoramidite 330 and CPA 331 as catalysts and 2,5-DMBQ as oxidant in toluene at 35 °C to furnish compounds 332 with good yields and enantioselectivities (Scheme 96a). In the proposed catalytic cycle, the π-allylpalladium complex reacts with the tautomer of pyrazole-5-one to form the corresponding TS-I, in which the CPA is bonded to the OH group and also to the Pd. When unactivated terminal alkenes were used, another CPA 333 and the bulkier oxidant 2,5-di-tert-butylbenzoquinone (2,5-DTBQ) were used to provide the corresponding allylated pyrazole-5-ones 334 with good yields and enantioselectivities (Scheme 96b).218,230 For this alkylation with inert allylic C–H bonds, TS-II has been proposed in which a concerted proton and a two-electron transfer process facilitates the allylic C–H cleavage.219,224
Scheme 96. Enantioconvergent Pd- and CPA-Catalyzed Allylic Alkylation of Pyrazol-5-ones 296 with Terminal Alkenes.
For the enantioselective allylic C–H alkylation of 1,4-pentadienes with pyrazole-5-ones 296, Pd(dba)2/phosphoramidite 325 and achiral 2-fluorobenzoic acid (OFBA) as cocatalyst gave the corresponding linear products 335 with high yields and enantioselectivities. With regard to diastereoselectivity, the E/Z ratio was 9:1 to >20:1 (Scheme 97a).219,229 However, substituted 1,4-pentadienes, phosphoramidite 336, and OFBA gave the best results by affording C5-branched and E-dienyl products 337 with up to 93% yield and up to 93% ee (Scheme 97b).219,229 According to DFT calculations, the TS explains the attack of pyrazole-5-one preferentially at the vinyl position via an inner-sphere mechanism in which the nucleophile prefers nitrogen coordination with Pd to afford the C5-branched regioselectively.
Scheme 97. Enantioconvergent Pd- and Achiral Phosphoric Acid-Catalyzed Allylic Alkylation of Pyrazol-5-ones 296 with 1,4-Dienes.
The C5-branched regioselectivity was also observed in the case of 2,5-diarylthiazol-4(5H)-ones 338 using as chiral phosphoramidite 339 and the achiral phosphoric acid OFBA (Scheme 98a).219,231 Under this cooperative catalysis, a broad range of α,α-disubstituted 5H-thiazol-4-ones 340 were isolated in up to 92% yield and up to 91% ee. However, when 5-alkylthiazol-4(5H)-ones 341 were treated with 1,4-dienes using phosphoramidite 342 as chiral ligand and OFBA as Brønsted acid, linear products 343 were mainly formed in up to 96% yield and up to 93% ee (Scheme 98b). This nucleophile-dependent regioselectivity was explained by the difference in the acidity and also by the steric hindrance of the 5-substituted thiazolones. In the case of 5-alkylthiazolones, which have lower acidity and bulkiness than 5-arylthiazolones, the attack on the vinyl π-allylPd intermediate occurs through an outer-sphere mechanism (TS-I) where hydrogen bonding interactions between the counteranion and thiazolone help to form the linear products. For the formation of the C5-branched product, an inner-sphere mechanism (TS-II) was proposed.
Scheme 98. Enantioconvergent Pd- and Achiral Phosphoric Acid-Catalyzed Allylic Alkylation of 5H-Thiazol-4-ones 338 and 341 with 1,4-Dienes.
Copper-catalyzed enantioconvergent cross-coupling of azoles with benzylic bromides through C(sp2)–H functionalization takes place under alkyl radical formation. For enantioconvergent annulation reactions of 3-hydroxyindolinones with dienes and pyrazolones with alkynes, Ir and Rh catalysts are used, respectively. With respect to the C(sp3)–H functionalization of the α-position of carbonyl compounds, Pd, Cu, and Ni catalysts allow their enantioconvergent arylation. In the case of benzylic alcohols under Ni catalysis, the enantioconvergent α-arylation can be carried out with acylboronates. For the enantioconvergent intramolecular β- and γ-amination of hydrazones and sulfamoyl azides, Cu and Co catalysts have been employed, respectively. The allylic C–H enantioconvergent functionalization of terminal alkenes and 1,4-dienes is performed under Pd catalysis and stoichiometric amounts of an oxidant, generally benzoquinones using chiral phosphoric acids. 1,3-Diketones, azlactones, aldehydes via enamines, pyrazolones, and thiazolones have been used as nucleophiles.
6. Enantioconvergent Hydrofunctionalization of Unsaturated Hydrocarbons
In this Section, an alternative to metal-catalyzed enantioconvergent alkyl–alkyl cross-coupling will be considered on the basis of reductive hydroalkylation of racemic alkyl halides with unsaturated hydrocarbons, such as olefins, allenes, and alkynes.
Fu and co-workers232 recently reported the enantioconvergent hydroalkylation of olefins with α-bromo amides. Racemic secondary amides 18 reacted with terminal olefins in combination with triethoxysilane under NiBr2/bis(oxazoline) (R,R)-344 catalysis to provide the alkylated products 20 with good yields and enantioselectivities (Scheme 99a). In a representative example using (Z)-2-hexene as internal alkene, n-alkylation occurred by chain walking. In the case of tertiary bromides derived from β-lactams 136, the corresponding products 345 bearing a quaternary stereocenter were generated with high yields and enantioselectivities under similar mild reaction conditions (Scheme 99b). The starting possible pathway is the Ni-catalyzed hydrosilylation of the olefin,233 and the resulting alkylsilane can serve as nucleophile in a Hiyama-type cross-coupling. However, this possibility was discarded because under these reaction conditions an alkylsilane was not cross-coupled with the alkyl bromide. In the proposed mechanism, the Ni(II) complex I reacts with trimethoxysilane to give a Ni–hydride complex II. After alkene complexation followed by migratory insertion, intermediate III results, which enters in the reaction cycle to undergo cross-coupling with the alkyl bromide. Other families of electrophiles, such as α-bromo esters, underwent very efficient cross-coupling with 1-hexene.
Scheme 99. Enantioconvergent Ni-Catalyzed Hydroalkylation of Olefins with α-Bromo Amides 18 and 136.
Independently, Zhu and co-workers234 reported a similar enantioconvergent hydroalkylation of internal alkenes with racemic α-bromo amides 18 under NiH/oxazoline 346 (Scheme 100). This reaction took place by alkene isomerization followed by the hydroalkylation process to provide products 20 in up to 92% yield and up to 99% ee. By using mixtures of octenes, only a single isomer was obtained with 91% yield and 97% ee. Several terminal alkenes have been used to give products 20 with excellent ee. In the proposed mechanism, after NiH addition to provide complex I, a chain-walking strategy gives the terminal alkylnickel complex II, which is followed by enantioconvergent oxidative addition of the secondary alkyl bromide.
Scheme 100. Enantioconvergent Ni-Catalyzed Hydroalkylation of Alkenes with α-Bromo Amides 18.
When racemic α-heteroatom phosphorus or sulfur alkyl bromides 347 are used as electrophiles, and NiBr2/bis(oxazoline) (R,R)-344 is used as catalyst, the hydroalkylation of terminal olefins takes place with good chemo-, regio-, and enantioselectivity to provide product 348 (Scheme 101).235 This alkyl–alkyl bond formation process was carried out under mild reaction conditions, a broad substrate scope, and good functional group compatibility. A radical-type enantioconvergent reaction mechanism has been proposed according to Fu’s proposal.232
Scheme 101. Enantioconvergent Ni-Catalyzed Hydroalkylation of Olefins with α-Heteroatom Phosphorus and Sulfur Alkyl Bromides 347.
Enantioconvergent hydroalkylation of terminal alkenes with α-acyloxyalkyl bromides 349 was applied to the synthesis of enantioenriched O-arylated alcohols 350 by Yang and Fu236 (Scheme 102a). In this case, NiBr2/bis(oxazolidine) (R,R)-344 was used as catalyst at room temperature to provide products 350 in up to 85% yield and up to 98% ee (Scheme 102b). This process can be also performed by generating in situ bromides 349 from aldehydes and acyl bromides and has been applied to the synthesis of key precursors of paleic acid, an antimicrobial; (R)-4-dodecanolide; and (S)-heptadecan-7-yl propionate, a component of a sex pheromone for the lichen moth.
Scheme 102. Enantioconvergent Ni-Catalyzed Hydroalkylation of Alkenes with α-Bromo O-Acyl Alcohols 349.
Malcolmson and co-workers237 reported the enantioselective Pd-catalyzed hydroalkylation of 1,3-dienes with racemic-activated C-nucleophiles, mainly β-diketones and malononitriles. In one particular example, this process is an enantioconvergent transformation, e.g., in the case of t-butyl α-cyanopropionate 351. The reaction of this racemic nucleophile with (E)-1-phenyl-1,3-butadiene was carried out using chiral Pd-Phox complex 352 as catalyst and Et3N as base in CH2Cl2 at 22 °C to furnish product 353 in moderate diastereoselectivity (Scheme 103). This reaction occurs by nucleophilic attack of the corresponding enolate to the 1,3-disubstituted π-allyl intermediate I.
Scheme 103. Enantioconvergent Pd-Catalyzed Hydroalkylation of (E)-1-Phenyl-1,3-butadiene with t-Butyl α-Cyanopropionate 351.
Trost and co-workers238 reported in 2003 the asymmetric addition of carbon nucleophiles to 1-benzyloxyallenes. Racemic azlactones 324 reacted enantioconvergently with 1-benzyloxyallenes under Pd/ligand 354 as catalyst by using hippuric acid and KOtBu as buffered conditions to form products 355 with good yields and diastereo- and enantioselectivities (Scheme 104). In the proposed catalytic cycle, the catalyst reacts with NuH to give the hydride complex I, which coordinates the allene to give intermediate II. Subsequent formation of the cationic π-allylpalladium complex III followed by nucleophilic attack provides the product.
Scheme 104. Enantioconvergent Pd-Catalyzed Hydroalkylation of 1-Benzyloxyallenes with Azlactones 324.
The same group239 reported the enantioconvergent hydroalkylation of 1-alkoxyallenes with 3-substituted oxindoles 304 under Pd/354 catalysis. In the presence of benzoic acid as cocatalyst, the corresponding enantioenriched oxindoles 356 with two vicinal stereocenters were obtained in excellent chemo-, regio-, diastereo-, and enantioselectivities with high chemical yields (Scheme 105). The authors proposed TS-I as the most favorable mechanism to explain the formation of (R,R)-356 as the major stereoisomer toward TS-II. The 3-indolyl-substituted oxindole 304 was transformed into 357, which was further transformed into the pyrrolidinoindoline core of the gliocladin natural products.
Scheme 105. Enantioconvergent Pd-Catalyzed Hydroalkylation of 1-Alkoxyallenes with 3-Substituted Oxindoles 304.
Jiang and co-workers240 reported an enantioselective regiodivergent241−244 hydroalkylation of 2-alkoxyallenes with pyrazolones 296 using either palladium or Brønsted acid catalysis. Under Trost’s reaction conditions and Pd/354 as catalyst without activator, the branched products 358 were exclusively formed with high regio-, diastereo-, and enantioselectivities and also high yields (Scheme 106). However, using 328 as CPA (S)-TRIP, linear products 359 were formed with high yields and regio- and stereoselectivities. In the first case, it was postulated that the acidity of the H at the C4 in the pyrazolone generates the Pd(II) hydride intermediate I, which evolves to the π-allylpalladium intermediate II to give the branched product 338. The CPA catalyst might enable the nucleophilic addition to form an alkyloxyallyl phosphate III, and after nucleophilic substitution through a hydrogen-bonding interaction in IV, it facilitates the resulted linear allylated pyrazolones 359.
Scheme 106. Enantioconvergent and Regiodivergent Pd- and CPA-Catalyzed Hydroalkylation of 1-Alkoxyallenes with Pyrazolones 296.
Two Spanish groups245,246 reported the enantioconvergent hydroalkylation of aldehydes with allenamides under gold and enamine synergistic catalysis. González and co-workers245 employed IPrAuNTf2361/prolinols 362 or 363 as catalysts in the presence of 2-fluorobenzoic acid in MeCN at room temperature for the hydroalkylation of allenamide 360 to give, after reduction with NaBH4, products 364 with moderate yields and enantioselectivities (Scheme 107a). Conversely, Mascareñas, López, and co-workers246 employed allenamides 365 and 366 and IPrAuNTf2361/prolinol 367 as catalysts in the presence of benzoic acid in toluene at 60 °C followed by reduction with NaBH4 to provide products 368 and 369 with moderate yields and enantioselectivities (Scheme 107b).
Scheme 107. Enantioconvergent Au(I)- and Enamine-Catalyzed Hydroalkylation of Allenamides 360, 365, and 366 with Aldehydes.
Enantioconvergent hydroalkylations of terminal allenes with β-keto carbonyl compounds and aldehydes have also been performed under dual Pd and amine catalysis by Luo and co-workers.247 These reactions were carried out with Pd and the phosphine DpePhos 370 as ligand for the hydrometalation step of the allene and with the chiral primary amine 371 as organocatalyst for the formation of intermediate enamines (Scheme 108). In both cases, the corresponding linear allylic systems 372 and 373 were obtained in up to 96% yield and 96% ee for compounds 372 and up to 82% yield and 91% ee for products 373. In Scheme 108 are depicted the two catalytic cycles for β-keto carbonyl compounds by intermediacy of Pd complexes I and II and by enamine III and imine IV. DFT calculations showed that the bulky tertiary amino group in the enamine III blocks the Re face for the attack of the π-allylpalladium II. The steric effect may explain the exclusive linear selectivity in the allene addition step.
Scheme 108. Enantioconvergent Pd- and Amine-Catalyzed Hydroalkylation of Allenes with β-Keto Carbonyl Compounds and Aldehydes.
Fu and co-workers232 reported the enantioconvergent hydroalkylation of alkynes with racemic secondary bromides under Ni catalysis in combination with triethoxysilane, as previously mentioned for alkenes in Scheme 99. In this case, the corresponding α-vinyl-substituted amides 374 were obtained by reaction of α-bromo amides 18 with 3-hexyne in >15:1 E/Z ratio (Scheme 109). Terminal 1-hexyne gave mainly compound 375 in 5:1 regioisomeric ratio, 65% yield, and 96% ee.
Scheme 109. Enantioconvergent Ni-Catalyzed Hydroalkylation of Alkynes with α-Bromo Amides 18.
For the enantioconvergent hydroalkylation of alkynes with aldehydes, Cruz and Dong248 employed a synergistic catalyst using Rh and Jacobsen’s amine. In this case, a chiral Rh-hydride generates the π-allyl species, and the amine generates the enamine of the racemic aldehyde. Chiral Rh complex (R)-DTBM-Binap 376 was used as ligand, and diamine 377 was used as chiral organocatalyst in the presence of di-n-butylphosphoric acid for the generation of the Rh–H catalyst. They also found a stereodivergent249 process by using enantiomeric amines (R,R)-377. Thus, (S,S)-377 provided the anti-products 378, whereas (R,R)-377 afforded products syn-378 in up to >20:1 regioselectivity (Scheme 110). According to Breit et al.,250 Rh-hydride catalysis can promote isomerization of alkynes to generate allenes, which undergo Rh–H insertion to provide electrophilic π-allylRh species I. In a third catalytic cycle, the formation of the enamine II takes place, and the attack to complex I gives the iminium III precursor of products 378.
Scheme 110. Enantioconvergent and Stereodivergent Rh- and Amine-Catalyzed Hydroalkylation of Alkynes with Aldehydes.
In summary, for the enantioconvergent functionalization of alkenes with racemic alkyl bromides, a Ni-catalyzed enantioselective reaction in the presence of (EtO)3SiH promotes the formation of Ni-hydride for addition to the carbon–carbon double bond for further cross-coupling with the radical formed from the electrophilic alkyl bromide. This process is a suitable alternative to the conventional cross-coupling of alkyl halides with organometallics or nucleophiles and allows the formation of C(sp3)–C(sp3) bonds. However, for the cross-coupling of racemic nucleophiles and allenes, palladium-catalyzed processes are mainly used. In this case, the formation of a Pd-hydride intermediate triggers the reaction with subsequent hydropalladation of the allene to form an electrophilic π-allylpalladium intermediate, which is attacked by the nucleophile. Alternatively, enantioconvergent hydroalkylation of alkynes has been achieved either under the Ni-catalyzed strategy, as in the case of alkenes, or under Rh-hydride isomerization to allenes and the formation of the π-allylrhodium catalyst followed by attack of the racemic nucleophile.
7. Enantioconvergent Hydrogen Autotransfer
Borrowing hydrogen chemistry or hydrogen autotransfer251−254 is a division of hydrogenation reactions in which a catalyst, usually a transition metal [M], first oxidizes an alcohol to form a carbonyl compound and [MH2]. After condensation of the carbonyl compound with the nucleophile (usually an amine or enolate), the borrowed hydrogen is transferred to the intermediate (Scheme 111). Enantioconvergent hydrogen autotransfer converts racemic secondary alcohols into enantioenriched amines through C–N bond-forming reactions or ketones with a stereocenter at the β-position through C–C bond-forming processes. The enantiodetermining step is the hydrogen transfer to the intermediate R1R2C=Nu under transition metal catalysis. This reaction, mainly catalyzed by Ru and Ir complexes, has been used in combination with chiral phosphines and normally releases water as a byproduct, therefore making it an atom-efficient process.
Scheme 111. Hydrogen Autotransfer Catalysis.
7.1. C–N Bond-Forming Reactions
Enantioconvergent amination of racemic alcohols 30 by hydrogen autotransfer was described first by Zhao and co-workers.255 Enantioenriched amines 380 were prepared by cooperative catalysis using the Ir complex (S,S)-379 and CPA (R)-TRIP 328, which promoted the condensation of ketones with anilines in the presence of a 4 Å MS under tert-amyl alcohol reflux (Scheme 112). In the proposed catalytic cycle, complex I is formed from the Ir complex and CPA and reacts with the alcohol to give intermediate II. After formation of the ketone and the iridium hydride III, this complex attacks the iminium species to produce the amine and the catalyst I.
Scheme 112. Enantioconvergent Ir- and CPA-Catalyzed Amination of Alcohols 30 by Hydrogen Autotransfer.
Beller and co-workers256 described the enantioconvergent amidation of diols with urea under Ru3(CO)13/(R)-MeO-Biphep (122) catalysis for the synthesis of oxazolidin-2-ones 381 (Scheme 113). In this process, the sequential formation of C–O and C–N bonds takes place chemo- and regioselectively with good yields and enantioselectivities for terminal diols. The internal 2,3-butanediol gave a 1:1 mixture of cis/trans diastereomers 381′ in 56% yield and 90/92% ee, respectively. In the proposed catalytic cycle, initially the diol reacts with urea to give carbamate I, which would give ketone II by Ru-catalyzed dehydrogenation. Intramolecular condensation provides oxazoline III, which gives rise to the oxazolidin-2-one after reduction.
Scheme 113. Enantioconvergent Ru-Catalyzed Amidation of 1,2-Diols by Hydrogen Autotransfer.
A Ni-catalyzed N-alkylation of hydrazides and anilines has been described by Tang, Zhou, and co-workers.257 The enantioconvergent process was carried out using Ni(OTf)2/(S)-Binapine (382) as catalyst for the amination of benzylic alcohols 30 with hydrazides to furnish enantioenriched products 383 in up to 96% ee (Scheme 114). The reaction was carried out in the presence of acetic acid and a 3 Å MS in tert-amyl alcohol at 110 °C. By further reduction of products 383 with SmI2 or Raney Ni, the N–N single bond can be cleaved to give benzyl amines.
Scheme 114. Enantioconvergent Ni-Catalyzed Amination of Alcohols 30 by Hydrogen Autotransfer.
Zhao and co-workers258 applied the cooperative catalysis by an achiral iridacycle and a CPA to the enantioconvergent intramolecular amination of ortho-substituted anilines 384 to provide tetrahydroquinolines 385 (Scheme 115). This hydrogen autotransfer occurred under iridacycle 386 and CPA 387 catalysis and a 4 Å MS as additive in dimethyl carbonate at 80 °C to give products 385 with high yields and up to 92% ee. A similar catalytic cycle, as depicted in Scheme 112, was proposed. In this case, a concerted transition state (TS) can be formed, which evolves to ketone I, which is a precursor of the tetrahydroquinoline derivative, by intermediacy of the iminium species II.
Scheme 115. Enantioconvergent Ir- and CPA-Catalyzed Intramolecular Amination of Amino Alcohols 384 by Hydrogen Autotransfer.
For the enantioconvergent synthesis of 1,2,3,4-tetrahydroquinoxalines 389, Zhao’s group259 described the reaction of aromatic ortho-diamines 388 with epoxides (Scheme 116). This transformation was carried out under Ir/(R)-Binap catalysis with Zn(OTf)2 as Lewis acid in toluene at 90 or 110 °C. In this method, the Lewis acid catalyzes the epoxide ring opening to give the amino alcohol I, which undergoes Ir-catalyzed hydrogen autotransfer to ketone II. Intramolecular condensation promoted by Zn(OTf)2 gives imine III, and the enantiodetermining reduction of III forms the product.
Scheme 116. Enantioconvergent Iridium- and Zn(OTf)2-Catalyzed Epoxide Opening/Intramolecular Amination with ortho-Diamines 388 by Hydrogen Autotransfer.
Monoamination of 1,2-diols 380 with secondary amines to give β-amino alcohols 390 was described in 2013 by Oe and co-workers.260 The enantioconvergent hydrogen autotransfer took place under [RuCl2(p-cymene)]2/(S,R)-Josiphos 391 catalysis in toluene at 100 °C to furnish products 390 in up to 99% yield and 77% ee (Scheme 117). According to mechanistic studies, it was proposed that the diol is converted into oxo aldehyde I by Ru-catalyzed double dehydrogenation. This oxo aldehyde I reacts with the amine to afford the iminium intermediate II, which is reduced successively to amino ketone III and amino alcohol IV. Absolute configuration of products 390 was not determined.
Scheme 117. Enantioconvergent Ru-Catalyzed Monoamination of 1,2-Diols by Hydrogen Autotransfer.
Diamination of 1,2-diols with anilines to provide 1,2-diamines 392 was recently performed by Zhao’s group.261 For this purpose [Ir(cod)Cl]2/bisphosphine 393 and CDA 394 were used as catalysts to provide diamines 392 in good yields and enantioselectivities (Scheme 118). In the proposed mechanism for this enantioconvergent diamination, the intermediacy of β-amino imine I would give, after reduction, the corresponding diamine. A DyKAT mechanism has been also proposed.
Scheme 118. Enantioconvergent Ir- and CPA-Catalyzed Diamination of 1,2-Diols by Hydrogen Autotransfer.
Indolization/enantioconvergent substitution of alcohols via hydrogen autotransfer of alcohol-containing alkynyl anilines 395 provided enantioenriched 2,3-fused tricyclic indoles 396 (Scheme 119). This tandem transformation described by Zhao’s group262 was enabled by cooperative catalysis of Ir/(S)-Segphos (397) with a 4 Å MS as additive in toluene at 80 °C. A plausible mechanism has been proposed on the basis of experimental studies. First, starting product 395 cyclizes to indole intermediate I catalyzed by cationic iridium phosphate. This indole enters in the borrowing hydrogen catalytic cycle to form intermediate II by Ir-catalyzed dehydrogenation. Subsequent acid-catalyzed intramolecular Friedel–Crafts reaction gives intermediate III and, after dehydration, the α,β-unsaturated imine IV. Final reduction of IV yields the tricyclic indole product 396.
Scheme 119. Enantioconvergent Ir- and CPA-Catalyzed Indolization/Substitution of Alkynyl Anilines 395.
Enantioconvergent C–N bond formation by alkylation of amines with racemic alcohols can be efficiently performed under asymmetric hydrogen autotransfer by a combination of iridium and phosphoric acid catalysis successfully developed by Zhao and co-workers.
7.2. C–C Bond-Forming Reactions
Direct alkylation of enolates with unactivated alcohols using hydrogen autotransfer conditions allows the formation of C–C bonds.251−254 Donohoe and co-workers263 recently reported an enantioconvergent alkylation of pentamethylphenyl (Ph*) ketones 399 with racemic 1,5-diols 400 to give, through a (5 + 1) annulation process, enantioenriched cyclohexanes 401 with good yields and diastereo- and enantioselectivities (Scheme 120a). This annulation was performed under Ir(cod)(acac)/(R)-DTBM-Segphos (181) catalysis in the presence of KOtBu and in tBuOH as solvent at 110 °C. The Ir catalyst oxidized the primary alcohol of 400 to aldehyde I followed by aldol condensation with the ketone 399 to give intermediate II after loss of water. The starting ketones 399 with the Ph* group play a key role by orthogonal orientation to the carbonyl group in avoiding competing reduction and homodimerization processes. Moreover, aryl Ph* derivatives can be transformed via an ipso substitution process into other functional groups.264 The same research group265 further described the intermolecular version of this alkylation of ketones 399 with racemic secondary alcohols to give enantioenriched β-alkylated ketones 402 with moderate enantioselectivities under the previously mentioned reaction conditions (Scheme 120b). The cleavage of the acyl Ph* group provided carboxylic acid esters, thioesters, amides, and alcohols.
Scheme 120. Enantioconvergent Ir-Catalyzed Alkylation of Ketones 399 with Alcohols by Hydrogen Autotransfer.
The Guerbet reaction, which was described more than one century ago by Marcel Guerbet,265,266 is the coupling of two primary alcohols to give a new alcohol. However, only recently have two Chinese groups reported independently the asymmetric Guerbet reaction. Zhao and co-workers267 reported the asymmetric Guerbet reaction of racemic secondary alcohols with primary alcohols at room temperature using a chiral Ru complex 403 as catalyst (Scheme 121). The reaction must be carried out in the presence of 3-pentanone as hydrogen acceptor at the beginning of the reaction and KOtBu as base in tert-amyl alcohol. Alcohols 404 were obtained with moderate yields because kinetic resolution of the starting secondary alcohols occurred simultaneously.
Scheme 121. Enantioconvergent Ru-Catalyzed Guerbet Reaction of Secondary Alcohols with Primary Alcohols.
Conversely, Wang and co-workers268 used commercially available classic Noyori Ru(II)-diamine-diphosphine 405 or 406 as catalysts and KOtBu as base in toluene at 60 °C (Scheme 122). The resulting alcohols 404 were isolated with good yields and enantioselectivities and can be performed at the gram scale. Mechanistic studies support the catalytic cycle depicted in Scheme 122. The two starting alcohols are dehydrogenated to give Ru-hydride intermediates and the two carbonyl compounds. They gave, after aldol condensation, the α,β-unsaturated ketone I, which is then reduced to produce the allylic alcohol II. After a base-catalyzed isomerization, intermediate II affords a ketone III, which is finally reduced by a Ru-hydride to form the enantioenriched alcohol resembling the Noyori asymmetric hydrogenation.
Scheme 122. Enantioconvergent Ru-Catalyzed Guerbet Reaction of Secondary Alcohols with Primary Ones.
In the case of C–C bond-forming reactions, intramolecular aldol condensation has been carried out under Ir/DTBM-Segphos catalysis of pentamethylphenyl ketones with diols to provide cyclohexane derivatives and with secondary alcohols to the corresponding β-alkylated ketones. Enantioconvergent Guerbet reactions have been efficiently performed using chiral Noyori-type Ru catalyst for secondary alcohols with primary alcohols to provide enantioenriched γ-alkylated secondary alcohols.
8. Other Metal-Catalyzed Enantioconvergent Reactions
In this Section, enantioconvergent transition-metal-catalyzed allylations of carbonyl compounds, aminations of 3-bromooxindoles, and other processes, such as addition of 3-bromooxindoles to silyl ketene imines, allenylation of carbonyl groups, and oxidation of β-keto esters, are considered.
8.1. Allylation of Carbonyl Compounds
Enantioconvergent allylation of carbonyl compounds starting from racemic allylic derivatives under transition metal catalysis affords diastereomeric addition products. Krische and co-workers269 described the crotylation of primary alcohols with 3-acetoxy-1-butene via transfer hydrogenative conditions. They employed an ortho-cyclometalated iridium catalyst I generated in situ from [Ir(cod)Cl]2, 4-cyano-3-nitrobenzoic acid, and (S)-Segphos (397) (Scheme 123). This carbonyl crotylation took place with total regioselectivity to provide products 407 with good anti-diastereo- and high enantioselectivity (Scheme 123a). Under the same reaction conditions, but using isopropanol as terminal reductant, the same alcohols 407 were obtained by crotylation of aldehydes (Scheme 123b). In a simplified catalytic mechanism, the cyclometalated iridium hydride I is deprotonated by Cs2CO3 to give the anionic iridacycle II. Oxidative addition of α-methyl allyl acetate forms the π-crotyliridium complex III. Addition of the (E)-α-crotyliridium complex IV to the aldehyde through a chairlike transition state delivers the anti-homoallyl iridium alkoxide V. Exchange of the homoallylic alcohol 407 by isopropanol or the primary alcohol gives intermediate VI, which undergoes β-hydride elimination to regenerate complex I. The same research group270 described an enhancement of the anti-diastereo- and enantioselectivity by using an isolable iridium complex 408 at lower temperature (60 °C) with K3PO4 as base and 5 equiv of H2O to give products 407 with up to 91% yield, >20:1 dr, and 99% ee.
Scheme 123. Enantioconvergent Ir-Catalyzed Diastereoselective Crotylation of Primary Alcohols or Aldehydes.
When they used271 paraformaldehyde as an electrophile under similar reaction conditions, the enantioconvergent reductive coupling with branched allylic acetates 409 provided regioselectively primary homoallylic alcohols 410 in up to 96% ee (Scheme 124). This hydroxymethylation reaction was performed using the Segphos-derived iridacycle (S)-408 in the presence of N-methylmorpholine oxide (NMO) as additive under microwave heating to avoid the formation of catalytic-inactive iridium carbonyl complexes. A similar mechanism as described in Scheme 123 was proposed with the same π-facial discrimination.
Scheme 124. Enantioconvergent Ir-Catalyzed Reductive Coupling of Formaldehyde with Allylic Acetates 409.
Krische’s group272 described the iridium-catalyzed enantioconvergent allylation of carbonyl compounds or their alcohol precursors with α-cyclopropyl allyl acetate 411 (Scheme 125). Under the reaction conditions for iridacycle (R)-408-catalyzed transfer hydrogenation, the corresponding homoallylic alcohols 412 were obtained with total regioselectivity and high enantioselectivity. This (α-cyclopropyl)allylation can be performed with primary alcohols (a) or aldehydes (b) to give the corresponding products 412 with similar results, although with higher diastereoselectivities than in the case of aldehydes.
Scheme 125. Enantioconvergent Ir-Catalyzed Diastereoselective (α-Cyclopropyl)allylation of Primary Alcohols or Aldehydes.
Enantioconvergent redox-triggered C–C coupling of alcohols and vinyl epoxides has been developed by Kirsche’s group.273 Racemic isoprene oxide reacts with primary alcohols under (R)-408 catalysis to furnish products 413 bearing a quaternary stereocenter with high levels of anti-diastereo- and enantioselectivity (Scheme 126a). 1,3-Butadiene epoxide and myrcene oxide reacted with 4-bromobenzyl alcohol to give product 413 with 63% yield, 5:1 dr, and 94% ee in the first case and with 94% yield, 40:1 dr, and 87% ee in the second example. When aldehydes were allowed to react with isoprene oxide in the presence of isopropanol as terminal reductant, prenylated products 413 were isolated with similar yields and diastereoselectivities (Scheme 126b). This tert-(hydroxy)prenylation probably takes place through the (E)-π-allyliridium intermediate I, which minimizes dipole–dipole interactions. Moreover, the reaction would be faster than by intermediacy of the (Z)-σ-allyliridium intermediate II because of the internal coordination of the hydroxy group to iridium. Compounds 413 were further transformed into enantioenriched 2,3,3-trisubstituted oxetanes and applied to the synthesis of an analogue of the antipsychotic agent haloperidol.274
Scheme 126. Enantioconvergent Ir-Catalyzed Diastereoselective tert-(Hydroxy)prenylation of Primary Alcohols or Aldehydes.
Another application of this Ir-catalyzed C–C bond-forming transfer hydrogenation is the coupling of vinyl aziridines with alcohols and aldehydes.275N-(p-Nitrophenylsulfonyl) (Ns) vinyl aziridine 414 reacted with primary alcohols to give γ-amino alcohols 416 using iridacycle (R)-415 as catalyst (Scheme 127a). Alternatively, aldehydes reacted in the presence of isopropanol as terminal reductant to provide branched products of (α-aminomethyl)allylation 416, also with excellent levels of anti-diastereo- and enantioselectivity (Scheme 127b). Chiral 1,3-diols reacted with N-tosylvinyl aziridine under the same reaction conditions to give products 417 with good yields and stereoselectivities (Scheme 127c). These products 417 were converted into 2,4,5-trisubstituted piperidines 418 by intramolecular Mitsunobu reaction.
Scheme 127. Enantioconvergent Ir-Catalyzed Diastereoselective Coupling of Vinyl Aziridine 414 with Alcohols, Aldehydes, or 1,3-Diols.
The asymmetric reductive allylation of aldehydes with allylic carbonates was performed under Ni(cod)2/bis(oxazoline) (S,S)-419 as catalysts using Zn as the terminal reductant.276 In this study, some examples on enantioconvergent processes were included. Racemic carbonates 420 reacted with benzaldehyde under Ni(ClO4)2·6H2O/(S,S)-419 catalysis at 25 °C in DMF to provide mainly anti-407 in moderate ee, even at −25 °C (Scheme 128).
Scheme 128. Enantioconvergent Ni-Catalyzed Diastereoselective Reductive Allylation of Benzaldehyde with Allylic Carbonates 420.
Recently, Chong, Meng, and co-workers277 reported a general enantioconvergent Co-catalyzed reductive allylation of aldehydes with allylic carbonates 421 bearing one or two substituents at the α-position. By means of CoI2/phosphine-oxazoline 422 catalysis, La(OTf)3 as Lewis acid, and Mn powder as the terminal reductant in MeCN at room temperature, the corresponding homoallylic alcohols 407 were obtained with good yields and diastereo- and enantioselectivities (Scheme 129a). In the case of branched allylic carbonates 423, homoallylic alcohols 424 bearing a quaternary stereocenter resulted with good levels of stereoselectivity (Scheme 129b). Racemic allylic alcohols can directly participate in this process to give alcohols 407 with moderate yields and very good diastereo- and enantioselectivities (Scheme 129c). On the basis of experimental observations, the proposed catalytic cycle is depicted in Scheme 129. The Co(I) complex II, generated from the Co(II) complex I through a single-electron reduction by Mn, undergoes oxidative addition to afford the π-allylCo(III) complex III or a single-electron oxidative addition to provide an equilibrium of complexes III and I and the allyl radical IV. This radical IV is captured by complex I, which is followed by a single electron-reduction that delivers the σ-complex V in equilibrium with the π-allylCo complex VI and the more reactive complex VII. Addition of VII to the aldehyde provides the intermediate VIII, which evolves by a single-electron reduction with Mn to give the homoallylic alcoholate and complex II.
Scheme 129. Enantioconvergent Co-Catalyzed Diastereoselective Reductive Allylation of Aldehydes with Allylic Alcohol Derivatives 421 and 423.
Diastereo- and regioselective enantioconvergent allylation of carbonyl compounds are efficiently carried out under Ir catalysis using transfer hydrogenation conditions to provide homoallylic alcohols with high yields and stereoselectivities. Apart from allylic esters, vinyl epoxides and aziridines can also be employed as allylating reagents. Alternatively, Ni and Co complexes work as catalysts under radical conditions.
8.2. Propargylation of Alcohols and Phenols
Transition metal catalysis is a powerful methodology for carbon–carbon bond formation between alcohols and alkyl electrophiles (see Section 2.2.2). Enantioconvergent substitution processes were initially described with propargyl derivatives. Nishibayashi and co-workers278 described the copper-catalyzed enantioconvergent etherification of propargylic carbonates 425 with alcohols and phenols using Py-bis(oxazoline) (S,S)-426 as chiral ligand (Scheme 130). The resulting propargylic ethers 427 were obtained in up to 91% yield and with 78–99% ee. The isolated dicopper complex A explains the observed nonlinear effect. This complex reacts with propargyl carbonate 425 to give intermediate dicopper-acetylide I, which after elimination of a carbonate moiety can form a dicopper-allenylidene complex II. Alcohol attack to complex II at the Si face affords complex III, which evolves to liberate the propargyl ether by ligand exchange with another molecule of propargyl carbonate.
Scheme 130. Enantioconvergent Cu-Catalyzed Etherification of Propargylic Carbonates 425 with Alcohols and Phenols.
Niu and co-workers279 employed Nishibayashi new conditions280 for benzyl alcohols in order to avoid the use of alcohols as solvents. Thus, polyols were propargylated with propargylic carbonates 425 under Cu(I)/borinic acid 428 dual catalysis and bis(oxazoline) PyBox (S,S)-426 as chiral ligand (Scheme 131). Organoborinic acid 428 forms, according to Taylor’s studies,281 a boron “ate” complex A, thereby increasing the nucleophilicity of the diol. The resulting hydroxy ethers 429 were isolated in very good yields and with high enantioselectivity. A similar mechanism was proposed for this propargylation (see Scheme 130). This procedure was also applied to the desymmetrization of meso 1,2-diols.
Scheme 131. Enantioconvergent Cu/Borinic Acid-Catalyzed Etherification of Propargylic Carbonates 425 with Diols.
The same group282 recently reported the enantioconvergent O-propargylation of secondary aliphatic alcohols. In this case, PyBox (R,R)-430 was used as chiral ligand, and Ph2SiF2 was used as a mild Lewis acid additive to form the nucleophilic silicate A. Propargylic ethers 431 were isolated in good yields and with high enantio- and diastereoselectivity (Scheme 132). This method has been applied to derivatize bioactive (natural) products, such as androsterone, etynodiol, vitamin D3, and galantamine trifluoroacetate salt.
Scheme 132. Enantioconvergent Cu-Catalyzed Etherification of Propargylic Carbonates 425 with Secondary Aliphatic Alcohols.
An enantioconvergent nickel/chiral sodium carboxylate dual catalysis has been reported by Guo and co-workers283 for the O-propargylation reaction with hydroxylamines (Scheme 133). Propargylic carbonates 432 reacted with N-hydroxyphthalimides 433 using Ni(cod)2 as catalyst, diphosphine (S)-Cl–MeO-Bipep 434 as chiral ligand, and sodium dicarboxylate (R)-435 as counteranion to provide products 436 in 42–98% yields with 90–98% ee. This strategy was applied, among others, to the total synthesis of (S)-dihydroyashabushiketol isolated initially from young shoots of Alnus firma(284) exhibiting 5α-reductase inhibition.285 From experimental studies, it was proposed that the Ni complex activates the propargylic carbonate to give an allenylnickel species I. After subsequent anion exchange, the nickel complex II is formed, which reacts with the N-hydroxyphthalimide to furnish the product and regenerate the catalyst.
Scheme 133. Enantioconvergent Ni/Sodium Carboxylate 435-Catalyzed Propargylation of N-Hydroxyphthalimides 433 with Propargylic Carbonates 432.
Alcohols and phenols can be propargylated with propargylic carbonates using Cu(I)/bis(oxazoline) catalysts to the corresponding enantioenriched propargylic ethers. In the case of N-hydroxyphthalimides, the propargylation has been carried out under Ni/diphosphine catalysis with a sodium dicarboxylate as chiral counteranion.
8.3. Amination of 3-Bromooxindoles
The 3-substituted 3-aminooxidole unit is present in a large number of alkaloid natural products, drugs, and agrochemical compounds.286−288 Wang and co-workers289 reported an enantioconvergent amination of racemic 3-bromooxindoles 262 with indolines 437 under Ni(OAc)2/bis(oxazoline) 211 catalysis to afford 3-aminooxindoles 438 with good yields and enantioselectivities (Scheme 134). This method was applied to the formal synthesis of the cytotoxic alkaloid (+)-psychotrimine, which exhibits potent antitumor activity against colon and lung cancers290 and antibacterial activity against Gram-positive bacteria.291
Scheme 134. Enantioconvergent Ni-Catalyzed Amination of 3-Bromooxindoles 262 with Indolines 437.
The same group292 reported the enantioconvergent amination of 3-bromooxindoles 262 with anilines under Ni(dppp)Cl2/bis(oxazoline) 211 catalysis to give 3-anilinooxindoles 439 with good yields and moderate enantioselectivities (Scheme 135a). In the proposed mechanism, the racemic 3-bromooxindole undergoes a base-mediated dehydrohalogenative process to provide ortho-azaxylylene I. Coordination of intermediate I with the chiral Ni(II) catalyst gives intermediate II, which suffers the aniline attack to form intermediate III. Final proton transfer and regeneration of the catalyst affords the product. Higher yields and enantioselectivities were obtained by Feng and co-workers293 using Ni(BF4)2/N,N′-dioxide 440 as catalyst (Scheme 135b). Working with DIPEA as base in EtOAc at 0 °C, compounds 439 were isolated in up to 99% yield and up to 96% ee.
Scheme 135. Enantioconvergent Ni-Catalyzed Amination of 3-Bromooxindoles 262 with Anilines.
The enantioconvergent amination of 3-bromooxindoles can be performed in the presence of a base and under Ni(II)/bis(oxazoline) catalysis by intermediacy of an ortho-azaxylylene formed by a dehydrohalogenative process.
8.4. Other Metal-Catalyzed Reactions
Related with the previous Section 8.3 on reactivity of racemic 3-bromooxindoles 262 is the enantioconvergent Ni-catalyzed reaction of these compounds with silyl ketene imines 441 also reported by Feng and co-workers294 (Scheme 136). This reaction was carried out under Ni(BF4)2/N,N′-dioxide 442 catalysis at −20 °C to give products 443 by conjugate addition of silyl ketene imines 441 to the in situ generated indol-2-ones. The resulted enantioenriched β-alkyl nitriles 443 bearing vicinal all-carbon quaternary stereocenters were obtained in up to 90% yield with 23:1 dr and 98% ee. With the N-methyl-protected 3-bromooxindole, no desired product was obtained, which supports the participation of the indol-2-one. In the proposed stereochemical model A, the indol-2-one could be activated by the Ni complex with the Si face shielded by the 2,4,6-triisopropylphenyl group of the ligand. The resulting nitriles 443 have been transformed to enantioenriched pyrroloindoline frameworks and spirocyclohexane oxindole derivatives containing vicinal quaternary stereocenters.
Scheme 136. Enantioconvergent Ni-Catalyzed Reaction of 3-Bromooxindoles 262 with Silyl Ketene Imines 441.
The same group reported295 an enantioconvergent alleno-aldol reaction catalyzed by AuCl3/N,N′-dioxide 445. Racemic allenates 444 reacted with isatins regio-, diastereo-, and enantioselectively under mild reaction conditions to furnish carbinol allenolates 446 (Scheme 137). Complete γ-selectivity was observed to be controlled by the chiral catalyst. In the proposed catalytic cycle, the chiral gold complex [Au] coordinates the allenolate to form the π-adduct I. After γ-deprotonation and enolization to intermediate II, nucleophilic attack to isatin gives adduct III. Final protonation of III followed by elimination of the catalyst provides the product.
Scheme 137. Enantioconvergent Au-Catalyzed Aldol Reaction of Allenolates 444 with Isatins.
Recently, Wang and co-workers296 described the Cr-catalyzed allenylation of aldehydes with racemic propargyl bromides 447 (Scheme 138). In this case, CrCl2/bis(oxazoline) 448 was used as catalyst to furnish α-allenols 449 with adjacent axial and central chiralities. High regio-, diastereo-, and enantioselective control for products 449 were obtained with a broad substrate scope. Experimental studies suggested a radical reaction mechanism initiated by the single-electron reduction of the propargyl bromide by the Cr(II) complex to give the radical I. This radical is captured by the Cr(II) complex to form the propargylic and the allenylic Cr(III) intermediates II and II′, respectively. Subsequent reaction with the aldehyde provides intermediate III, which undergoes dissociation with Cp2ZrCl2 to give intermediate IV, a precursor of the α-allenol by hydrolysis. The Cr(III) complex is reduced to Cr(II) by manganese powder. Probably because of the steric repulsion between the TIPS group and the catalyst in the TS, the asymmetric allenylation, instead of the propargylation, is favored by the Re face attack.
Scheme 138. Enantioconvergent Cr-Catalyzed Allenylation of Aldehydes with Propargylic Bromides 447.
Enantioconvergent aerobic oxidation of β-keto esters and amides 450 to α-hydroxy-β-dicarbonyl compounds 452 has been achieved by Xiao and co-workers297 under photocatalyzed conditions (Scheme 139). In this case, a chiral bis(oxazoline) ligand 451 was grafted with a photosensitive thioxanthone unit, and this ligand with Ni(acac)2 resulted as a powerful catalyst for the α-hydroxylation of compounds 450 using oxygen or air as a green oxidant under visible light. The thioxanthone motif acted as a triple-state sensitizer for the generation of singlet oxygen, and the Ni(II) cation acted as a Lewis acid and coordinated the indanone-derived esters and amides in the enolate form. A possible stereoinduction model A has been proposed in which the coordination enables the Re face attack of either the oxidant 1O2 or the peroxide I.
Scheme 139. Enantioconvergent Photocatalyzed and Ni-Catalyzed Aerobic α-Hydroxylation of β-Keto Esters and Amides.
Under visible light, Meng and co-workers298 performed the enantioconvergent α-hydroxylation of β-keto esters 450 under Cu(OTf)2/salan 453 catalysis. Cyclic substrates 450 were converted into products 454 with yields up to 95% and up to 96% ee by including methyl esters in the presence of air and tetraphenylporphyrin (TPP) as photosensitizer with a white compact fluorescent lamp (CFL) to produce singlet oxygen (Scheme 140). This protocol was applied to the synthesis of a key intermediate of the sodium channel blocker (S)-indoxacarb from compound 455. According to experimental studies, it was suggested that reactive singlet oxygen may participate in this oxidation process, which attacks the enolate from the Si face in intermediate A.
Scheme 140. Enantioconvergent Photocatalyzed and Cu-Catalyzed α-Hydroxylation of β-Keto Esters 450.
Enantioconvergent addition of 3-bromooxindoles to silylketene imines takes place under Ni(II)/N,N′-dioxide catalysis through in situ generated indol-2-ones to provide β-alkyl nitriles. However, aldol reaction of allenoates occurs through an enantioconvergent gold-catalyzed reaction or under Cr-catalyzed addition of propargyl bromides to aldehydes. Enantioconvergent α-hydroxylation of β-keto esters and amides takes place by photo- and Ni- or Cu-catalyzed aerobic oxidations.
Conclusions
Although in each Section a short conclusion sentence has been included, we enclose here a general conclusion. In Section 2, enantioconvergent cross-coupling of activated racemic alkyl electrophiles with aryl organometals can be performed under Ni catalysis by employing chiral bis(oxazoline) as ligands when Grignard and organozinc reagents are the nucleophiles. For organoboron reagents, chiral 1,2-diamines were the best ligands for the enantioconvergent cross-coupling of alkyl bromides and dichlorides with alkyl organoboranes. Bis(oxazolines) or diphosphines were used for aryl boronates and boronic acids under Ni, Co, Fe, and Cu catalysis. The formation of alkyl radicals as intermediates, which reacted with the chiral organonickel or Co, Fe, and Cu intermediates through an out-of-cage pathway, is the key step in these enantioselective transformations. Other organometals, such as organosilicon, organoindium, organozirconium, organoaluminum, and organotitanium reagents have been efficiently used for C(sp3)–C(sp2) bond-forming reactions under Ni(II)/bis(oxazoline) catalysis. Enantioconvergent amination and amidation reactions of racemic-activated alkyl bromides and chlorides can be carried out under photoinduced copper-catalyzed conditions using diphosphines as chiral ligands. Chiral tridentate anionic ligands promote the amination of α-chloro amides with aliphatic amines or ammonia under Cu(I) catalysis. Alcohols and phenols can be used as nucleophiles for the etherification of α-halo amides under Cu(I)/bis(oxazoline) catalysis. Benzylic and propargylic bromides undergo the Michaelis–Becker reaction with H-phosphonates under Cu/N,N-P ligand catalysis. Cyanation reactions require Cu(I)/bis(oxazoline) as catalyst. For the enantioconvergent alkynylation of alkyl bromides with acetylenes, Cu(I)/monophosphine or bis(oxazoline) is used as catalyst. However, tertiary α-chloro amides need chiral tridentate N,N,N-ligands for the alkynylation reaction and for secondary α-bromo amides to react under Cu/bis(oxazoline) catalysis. For the borylation of racemic benzylic bromides, Ni(II)/bis(oxazoline) or Cu(I)/diphosphine is employed as chiral ligand. Racemic alkylzinc reagents are cross-coupled with alkyl halides under Ni(II)/diamine or oxazoline catalysis. Enantioconvergent reductive cross-coupling of two electrophiles can be performed in the presence of a terminal reductant or under photoredox catalysis by intermediacy of stabilized alkyl radicals, which react with acyl chlorides, vinyl bromides, or aryl iodides, thereby avoiding the use of stoichiometric amounts of organometallic reagents. Photoredox decarboxylative enantioconvergent cross-coupling of α-amino acids with aryl bromides occurs mainly under Ni(II)/bis(oxazoline) catalysis. In the case of racemic-activated alkyl chlorides with aryl iodides, photoredox Ni(II)/bis(oxazoline) catalysis is employed. In Sections 3 and 4, racemic allylic and propargylic electrophiles are shown to undergo cross-coupling with organometals with complete regioselectivity and good enantioselectivity using Ni(II)/bis(oxazoline) and Cu(I)/phosphine catalysis. Enantioconvergent C(sp2)–H functionalizations with racemic electrophiles have been achieved under Cu(I)/phosphine or bis(oxazoline) catalysis by formation of radical intermediates. In the case of C(sp3)–H functionalizations, racemic compounds react at the α-, β-, or γ-position under Ni, Cu, or Co catalysis. Allylic alkylation was possible under Pd/phosphoramidite catalysis with racemic nucleophiles, such as 1,3-dicarbonyls, azlactones, aldehydes, pyrazolones, or thiazolones. Hydroalkylation of alkenes with racemic-activated alkyl electrophiles under Ni(II)/bis(oxazoline) catalysis in the presence of triethoxysilane promotes the formation of a Ni-hydride. In the case of racemic nucleophiles, the enantioconvergent hydroalkylation of dienes and allenes has been performed under Pd(II)/diphosphine catalysis. Racemic electrophiles have been employed in the enantioconvergent hydroalkylation of internal acetylenes under Ni or Rh catalysis. Enantioconvergent hydrogen autotransfer of racemic alcohols with nitrogen-containing nucleophiles was carried out mainly under Ir or Ru catalysis. In the case of alkylation of enolates, racemic alcohols react with primary alcohols either under Ir or Ru catalysis. Concerning other metal-catalyzed enantioconvergent reactions, racemic allylic systems have been added to carbonyl compounds or alcohols via transfer hydrogenative conditions, mainly under Ir(I)/diphosphine catalysis, with high regio-, diastereo-, and enantioselectivities. Alcohols and phenols can be propargylated with propargylic carbonates under Cu–bis(oxazoline) catalysis in the presence of borinic acids or diphenyldifluorosilane as alcohol activators. N-Hydroxyphthalimides are propargylated under Ni/diphosphine catalysis with a chiral carboxylate as counteranion. Amination of racemic 3-bromooxindoles were performed under Ni catalysis, other reactions involving allenylation of carbonyl compounds were performed under Au or Cr catalysis with racemic allenolates or propargylic bromides, and hydroxylation of racemic 1,3-dicarbonyl compounds under Ni or Cu catalysis were carried out. This review has attempted to clarify the terminology of enantioconvergent processes and to overview their recent developments and applications to the synthesis of enantioenriched molecules. It is worthy to highlight the impressive development of radical-based enantioconvergent reactions, which have evolved from simple bis(oxazolines) as chiral ligands under nickel catalysis to tridentate anionic ligands under copper catalysis able to facilitate the weak oxidative properties of alkyl halides. A broad array of enantioconvergent reactions have been described so far, but more studies should be carried out to explain the stereochemical results.
Acknowledgments
We gratefully acknowledge financial support from the Spanish Ministerio de Ciencia, Innovación y Universidades (projects CTQ2016-81893REDT and RED2018-102387-T); the Spanish Ministerio de Economía, Industria y Competitividad, Agencia Estatal de Investigación (AEI), and Fondo Europeo de Desarrollo Regional (FEDER, EU) (projects CTQ2016-76782-P, CTQ2016-80375-P, CTQ2017-82935-P, and PID2019-107268GB-I00); Generalitat Valenciana (CIDEGENT/2020/058); and the University of Alicante.
Glossary
Abbreviations
- AAA
asymmetric allylic alkylation
- Ac
acetyl
- acac
acetoacetyl
- Alloc
allyloxycarbonyl
- AMG 837
1-propyn-1-yl-4-{[4′-(trifluoromethyl)-1,1′-biphenyl]-3-yl-methoxy}benzenepropanoic acid
- Ar
aryl
- BARF
tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
- BBN
9-borabicyclo[3.3.1]nonane
- BenzP
12-(t-butylmethylphosphorous)benzene
- BHT
2,6-di-tert-butyl-4-methylphenyl
- Binap
2,2′-bis(diphenylphosphino)-1,1′-binaphthyl
- Bn
benzyl
- BnPyBox
2,6-bis[(4S)-benzyl-2-oxazolin-2-yl]pyridine
- Boc
tert-butyloxycarbonyl
- BOPA
bis(oxazoline)-phenylaniline
- Bpin
bis(pinacolate)diboron
- bpy
2,2′-bipyridine
- BTPP
tert-butylimino-tri(pyrrolidino)phosphane
- Bz
benzyl
- carb
carbazolide
- cat
catalyst
- Cbz
benzyloxycarbonyl
- cC5H9
cyclopentyl
- CFL
compact fluorescent lamp
- cod
1,5-cyclooctadiene
- Cp
ferrocenyl
- CPA
chiral phosphoric acid
- CPME
cyclopentyl methyl ether
- Cy
cyclohexyl
- Cz
carbazolyl
- 4CzIPN
2,4,5,6-tetracarbazolyl-1,3-denzene dinitriles
- DABCO
1,4-diazabicylo[2.2.2]octane
- dba
dibenzylideneacetone
- DCM
dichloromethane
- DFB
1,2-difluorobenzene
- DFT
density functional theory
- DHFR
dihydrolate reductase
- DHP
dihydroxypyridine
- DIAD
diisopropyl azodicarboxylate
- diglyme
bis(2-methoxyethyl) ether
- DIPEA
N,N-diisopropylethylamine
- DKR
dynamic kinetic resolution
- DMA
N,N-dimethylacetamide
- DMAP
4-dimethylaminopyridine
- DMBA
dimethylbenzoic acid
- DMBQ
dimethylbenzoquinone
- DME
dimethoxyethane
- DMF
dimethylformamide
- DMI
1,3-dimethyl-2-imidazolidinone
- DMM-Box
5-benzyl-2-[2-bis(3,5-dimethyl-4-methoxyphenyl)phosphino]oxazoline
- DMPU
N,N′-dimethylpropylurea
- DMSO
dimethyl sulfoxide
- DpePhos
bis[(2-phenylphosphino)phenyl] ether
- dppp
1,3-bis(diphenylphosphino)propane
- dr
diastereomeric ratio
- DTBM-Binap
2,2′-bis(3,5-di-tert-buty-4-methoxy)phenyl-1,1′-binaphthyl
- DTBM-Segphos
5,5′-bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-dibenzodioxole
- DyKAT
dynamic kinetic asymmetric transformations
- ee
enantiomeric excess
- EPR
electron paramagnetic resonance
- equiv
equivalent(s)
- ESI-MS
electrospray ionization-mass spectrometry
- EZH2
enhancer of zeste homologue 2
- Fmoc
fluorenylmethyloxycarbonyl
- glyme
1,2-dimethoxyethane
- GPR 40
fatty acid receptor agonist
- HEH
Hantzsch ester
- Het
heterocycle
- HFacac
hexafluoroacetoacetate
- HP
high-power
- HQ
hydroquinone
- IPrAuNTf2
[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylene] [bis(trifluoromethanesulfonyl)imide] gold
- iPrPyBox
2,6-bis[(4-S)(−)-isopropyl-2-oxazolin-2-yl]pyridine
- L
ligand
- LED
light-emitting device
- LUMO
lowest unoccupied molecular orbital
- M
metal
- M-H
Michaelis–Becker reaction
- MeOBiphen
2,2′-bis(diphenylphosphino)-6,6′-dimethoxy-1,1′-biphenyl
- mGluR
metabotyropic glutamate receptor
- mol
mole(s)
- Ms
methylsulfonyl (mesyl)
- MS
molecular sieve
- MTBE
methyl tert-butyl ether
- NHP
N-hydroxyphthalimide
- NMP
N-methylpyrrolidone
- NSAID
nonsteroidal anti-inflammatory drug
- Nu
nucleophile
- OFBA
2-fluorobenzoic acid
- PC
photocatalyst
- Pc
phthaloccyanine
- PG
protecting group
- PhBox
2 × 2-bis(4-phenyl-2-oxazolin-2-yl)propane
- Phe
phenylalanine
- Phen
1,10-phenanthroline
- Ph-PTZ
N-phenylphenothiazine
- Phth
phthalimido
- Piv
pivaloyl
- PMB
p-methoxybenzyl
- PMP
p-methoxyphenyl
- Por
porphirine
- ppy
2-phenylpyridine
- QuinoxP*
2,3-bis(tert-butylmethylphosphino)quinoxaline
- rac
racemic
- RCC
reductive cross-coupling
- red
reductant
- rt
room temperature
- RVC
reticulated vitreous carbon
- Segphos
5,5′-bis(diphenylphosphino)4,4′-bi-1,3-benzodioxole
- SET
single-electron transfer
- TADDOL
α α,α′,α′-tetraaryl-2,2-disubstituted 1,3-dioxolane-4,5-dimethanol
- TBAF
tetrabutylammonium fluoride
- TBAT
tetrabutylammonium triphenyldifluorosilicate
- TBDPS
tert-butyldiphenylsilyl
- TBS
tert-butyldimethylsilyl
- TC
thiophene-2-carboxylate
- TDAE
tetrakis(N,N-dimethylamino)ethylene
- TEA
triethylamine
- TES
triethylsilyl
- Tf
trifluoromethylsulfonyl (triflyl)
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- THP
tetrahydropyranyl
- TIPS
triisopropylsilyl
- TMB
1,3,5-trimethoxybenzene
- TMEDA
tetramethylenediamine
- TMS
trimethylsilyl
- Tol
tolyl
- TPP
tetraphenylprophyrine
- TRIP
3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogenophosphate
- Ts
4-methylbenzenesulfonyl (tosyl)
- TS
transition state
- UPC1172
4-{3-[(2S)-4-2,4-diamino-6-ethylpyrimidin-5-yl]-5-methoxy}benzoic acid
Biographies
Miguel Yus was born in Zaragoza (Spain) in 1947 and received his B.Sc. (1969), M.Sc. (1971), and Ph.D. (1973) degrees from the University of Zaragoza. After spending two years as a postdoctoral fellow at the Max Planck Institut für Kohlenforschung in Mülheim a. d. Ruhr, he returned to Spain to the University of Oviedo where he became an associate professor in 1977 and was promoted to full professor in 1987 at the same university. In 1988, he moved to a chair in organic chemistry at the University of Alicante. Professor Yus has been a visiting professor at different institutions and universities, including ETH-Zentrum, Oxford, Harvard, Uppsala, Marseille, Tucson, Okayama, Paris, Strasbourg, Bolonia, Sassari, Tokyo, and Kyoto. He is a coauthor of more than 650 papers (and six patents) and has supervised 63 doctoral students (theses already presented). He has delivered about 250 lectures, most of them abroad. His bibliometric data include more than 31 000 citations and an h-index of 82. He has received several international awards and has also been named an active academician by the European Academy of Sciences and Arts and an academic member of the Athens Institute for Education and Research. Professor Yus has been on the advisory board of more than 30 international journals. Professor Yus founded a new chemical company, MEDALCHEMY S.L., to commercialize fine chemicals.
Carmen Nájera was born in Nájera (La Rioja) in 1951 and graduated from the University of Zaragoza in 1973. She obtained her doctorate degree in chemistry from the University of Oviedo in 1979. She had postdoctoral stays at the ETH (Zurich), the Dyson Perrins Laboratory (Oxford), Harvard University, and Uppsala University. She became an associate professor in 1985 at the University of Oviedo and a full professor in 1993 at the University of Alicante. She is a coauthor of more than 400 papers (h-index = 73), 6 patents, and 30 book chapters and has supervised more than 50 Ph.D. students. She is also on the editorial board of several international journals. She has been awarded the 2006 Organic Chemistry Prize by the Spanish Royal Society of Chemistry, the 2006 Rosalind Franklin International Lectureship by the English Royal Society, the SCF 2010 French-Spanish Prize by the Société Chimique de France, the IUPAC 2015 Distinguished Women in Chemistry or Chemical Engineering Award, the 2018 Serratosa lectureship, and the Lilly 2019 lectureship. In 2021, she received the VI Julio Pélaez Awad to Pioneers Women in Physics, Chemistry and Mathematics and the Scientist Award from Valencia Government in 2022. In 2012, she was named a full member of the Royal Spanish Academy of Sciences and was appointed as an active member of the European Academy of Sciences and Arts. Professor Nájera has been on the advisory board of several international journals, and in 2016–2017, she was named a ChemPubSoc Europe Fellow.
Francisco Foubelo was born in 1961. He studied chemistry at the University of Oviedo where he received B.S. (1984), M.S. (1986), and Ph.D. (1989) degrees. After a postdoctoral stay (1989–1991) as a Fulbright fellow at Princeton University, he moved to the University of Alicante where he became an associate professor in 1995 and a full professor in 2002. Dr. Foubelo has coauthored more than 150 papers, and his current research interests are focused on the development of new synthetic methodologies involving chiral sulfinimines and on metal-promoted functionalization of alkenes and alkynes.
José Miguel Sansano was born in Rojales (Alicante) and studied chemistry at the University of Alicante, where he obtained his B.Sc. and Ph.D. degrees in 1988 and 1994, respectively. His thesis was supervised by Prof. C. Nájera and focused on sulfone chemistry. After a two-year postdoctoral stay at the University of Leeds (U.K.) with Prof. R. Grigg, he joined the University of Alicante in 1996, where he was appointed as an associate professor in 2001. In 2010, he was promoted as a full professor in the same university. He was invited as a visiting professor to Chuo University in 2014 and the UFRJ (Brazil). He is a coauthor of more than 180 articles and has supervised 18 Ph.D. students.
The authors declare no competing financial interest.
References
- Willis M. C. Enantioselective Desymmetrization. J. Chem. Soc., Perkin Trans. 1 1999, 1765–1784. 10.1039/a906269b. [DOI] [Google Scholar]
- Garcia-Urdiales E.; Alfonso I.; Gotor V. Enantioselective Enzymatic Desymmetrizations in Organic Synthesis. Chem. Rev. 2011, 111, PR110–PR180. 10.1021/cr100330u. [DOI] [PubMed] [Google Scholar]
- Díaz de Villegas M. D.; Gálvez J. A.; Badorrey R.; López Ram de Víu M. P. Organocatalyzed Enantioselective Desymmetrization of Diols in the Preparation of Chiral Building Blocks. Chem.—Eur. J. 2012, 18, 13920–13935. 10.1002/chem.201202264. [DOI] [PubMed] [Google Scholar]
- Fernández-Pérez H.; Etayo P.; Lao J. R.; Núñez-Rico J. L.; Vidal-Ferran A. Catalytic Enantioselective Reductive Desymmetrization of Achiral and meso-Compounds. Chem. Commun. 2013, 49, 10666–10675. 10.1039/c3cc45466e. [DOI] [PubMed] [Google Scholar]
- Wang M.; Feng M.; Tang B.; Jiang X. Recent Advances of Desymmetrization Protocol Applied in Natural Product Total Synthesis. Tetrahedron Lett. 2014, 55, 7147–7155. 10.1016/j.tetlet.2014.10.152. [DOI] [Google Scholar]
- Manna M. S.; Mukherjee S. Catalytic Asymmetric Desymmetrization Approaches to Enantioenriched Cyclopentanes. Org. Biomol. Chem. 2015, 13, 18–24. 10.1039/C4OB01649A. [DOI] [PubMed] [Google Scholar]
- Petersen K. S. Nonenzymatic Enantioselective Synthesis of All-Carbon Quaternary Centers through Desymmetrization. Tetrahedron Lett. 2015, 56, 6523–6535. 10.1016/j.tetlet.2015.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.-P.; Cao Z.-Y.; Wang Y.-H.; Zhou F.; Zhou J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116, 7330–7396. 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
- Borissov A.; Davies T. Q.; Ellis S. R.; Fleming T. A.; Richardson M. S. W.; Dixon D. J. Organocatalytic Enantioselective Desymmetrization. Chem. Soc. Rev. 2016, 45, 5474–5540. 10.1039/C5CS00015G. [DOI] [PubMed] [Google Scholar]
- Merad J.; Candy M.; Pons J.-M.; Bressy C. Catalytic Enantioselective Desymmetrization of Meso Compounds in Total Synthesis of Natural Products; Towards an Economy and Chiral Reagents. Synthesis 2017, 49, 1938–1954. 10.1055/s-0036-1589493. [DOI] [Google Scholar]
- Suzuki T. Recent Topics in the Desymmetrization of meso-Diols. Tetrahedron Lett. 2017, 58, 4731–4739. 10.1016/j.tetlet.2017.10.048. [DOI] [Google Scholar]
- Nagib D. A. Catalytic Desymmetrization by C-H Functionalization as a Solution to the Chiral Methyl Problem. Angew. Chem., Int. Ed. 2017, 56, 7354–7356. 10.1002/anie.201703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani R. Recent Progress in Catalytic Enantioselective Desymmetrization of Prochiral Organosilanes for the Synthesis of Silicon-Stereogenic Compounds. Synlett 2018, 29, 388–396. 10.1055/s-0036-1591839. [DOI] [Google Scholar]
- Wang Z.; Pan D.; Li T.; Jin Z. N-Heterocyclic Carbene (NHC)-Organocatalyzed Kinetic Resolutions, Dynamic Kinetic Resolutions, and Desymmetrizations. Chem.-Asian J. 2018, 13, 2149–2163. 10.1002/asia.201800493. [DOI] [PubMed] [Google Scholar]
- Metrano A. J.; Miller S. J. Peptide-Based Catalysts Reach the Outer Sphere through Remote Desymmetrization and Atroposelectivity. Acc. Chem. Res. 2019, 52, 199–215. 10.1021/acs.accounts.8b00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Risi C.; Bortolini O.; Di Carmine G.; Ragno D.; Massi A. Kinetic Resolution, Dynamic Kinetic Resolution and Asymmetric Desymmetrization by N-Heterocyclic Carbene Catalysis. Synthesis 2019, 51, 1871–1891. 10.1055/s-0037-1612305. [DOI] [Google Scholar]
- Ding Y.-X.; Zhu Z.-H.; Yu C.-B.; Zhou Y.-G. Recent Advances in Reductive Desymmetrization of Diketones. Asian J. Org. Chem. 2020, 9, 1942–1952. 10.1002/ajoc.202000536. [DOI] [Google Scholar]
- Patti A.; Sanfilippo C. Breaking Molecular Symmetry through Biocatalytic Reactions to Gain Access to Valuable Chiral Synthons. Symmetry 2020, 12, 1454–1479. 10.3390/sym12091454. [DOI] [Google Scholar]
- Nájera C.; Foubelo F.; Sansano J. M.; Yus M. Enantioselective Desymmetrization Reactions in Asymmetric Catalysis. Tetrahedron 2022, 106–107, 132629. 10.1016/j.tet.2022.132629. [DOI] [Google Scholar]
- Turner N. J.Deracemization and Enantioconvergent Processes. In Asymmetric Organic Synthesis with Enzymes; Gotor V.; Alfonso I.; García-Urdiales E., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp 115–131. [Google Scholar]
- Bhat V.; Welin E. R.; Guo X.; Stoltz B. M. Advances in Stereoconvergent Catalysis from 2005 to 2015: Transition-Metal-Mediated Stereoablative Reactions, Dynamic Kinetic Resolutions and Dynamic Kinetic Asymmetric Transformations. Chem. Rev. 2017, 117, 4528–4561. 10.1021/acs.chemrev.6b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney A. H.; Kadunce N. T.; Reisman S. E. Enantioselective and Enantiospecific Transtition-Metal Catalyzed Cross-Coupling Reaction of Organometallic Reagents to Construct C-C Bonds. Chem. Rev. 2015, 115, 9587–9652. 10.1021/acs.chemrev.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J. T.; Moore J. T.; Stoltz B. M. Enantioconvergent Catalysis. Beilstein J. Org. Chem. 2016, 12, 2038–2045. 10.3762/bjoc.12.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencel-Delord J.; Colobert F. Stereoselective Metal-Catalyzed C-C Bond Reactions by Stereoconvergence, Dynamic Kinetic Asymmetric Transformation or Dynamic Kinetic Resolution. Synthesis 2016, 48, 2981–2996. 10.1055/s-0035-1562512. [DOI] [Google Scholar]
- Choi J.; Fu G. C. Transition Metal-Catalyzed Alkyl-Alkyl Bond Formation: Another Dimension in Cross-Coupling Chemistry. Science 2017, 356, eaaf7230 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Tan C.-H. Stereospecific and Stereoconvergent Nucleophilic Substitution Reactions at Tertiary Carbon Centers. Chem. 2021, 7, 1451–1486. 10.1016/j.chempr.2020.11.022. [DOI] [Google Scholar]
- Kikuchi J.; Terada M. Enantioconvergent Substitution Reactions of Racemic Electrophiles by Organocatalysis. Chem.—Eur. J. 2021, 27, 10215–10224. 10.1002/chem.202100439. [DOI] [PubMed] [Google Scholar]
- Dong X.-Y.; Li Z.-L.; Gu Q.-S.; Liu X.-Y. Development for Copper-Catalyzed Enantioconvergent Radical Cross-Coupling of Racemic Alkyl Halides. J. Am. Chem. Soc. 2022, 144, 17319–17329. 10.1021/jacs.2c06718. [DOI] [PubMed] [Google Scholar]
- Metal-Catalyzed Cross-Coupling Reactions; de Meijere A.; Diederich F., Eds.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Magano J.; Dunetz J. R. Large-Scale Applications of Transition Metal-Catalyzed Coupling for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- Jana R.; Pathak T. P.; Sigman M. S. Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-Organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorius F. Asymmetric Cross-Coupling of Non-Activated Secondary Alkyl Halides. Angew. Chem., Int. Ed. 2008, 47, 8347–8349. 10.1002/anie.200803509. [DOI] [PubMed] [Google Scholar]
- Taylor B. L. H.; Jarvo E. R. Construction of Enantioenriched Tertiary Stereogenic Centers by Nickel- and Palladium-Catalyzed Cross-Coupling Reactions of Alkyl Electrophiles. Synlett 2011, 2011, 2761–2765. 10.1055/s-0031-1289871. [DOI] [Google Scholar]
- Fu G. C. Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci. 2017, 3, 692–700. 10.1021/acscentsci.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga A.; Chiba S. Engaging Radicals in Transition Metal-Catalyzed Cross-Coupling with Alkyl Electrophiles: Recent Advances. ACS Catal. 2017, 7, 4697–4706. 10.1021/acscatal.7b01405. [DOI] [Google Scholar]
- Iwasaki T.; Kambe N. Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles. Top. Curr. Chem. 2016, 374, 66. 10.1007/s41061-016-0067-6. [DOI] [PubMed] [Google Scholar]
- Lou S.; Fu G. C. Nickel/Bis(oxazoline)-Catalyzed Asymmetric Kumada Reactions of Alkyl Electrophiles: Cross-Coupling of Racemic α-Bromoketones. J. Am. Chem. Soc. 2010, 132, 1264–1266. 10.1021/ja909689t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.; Fu G. C. Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by Nickel/Bis(oxazoline) Complex. J. Am. Chem. Soc. 2019, 141, 15433–15440. 10.1021/jacs.9b08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Liu F.; Wang N.; Wu L.; Zheng B.; Liu S.; Zhong J.; Bian Q.; Walsh P. J. Cobalt-Bisoxazoline-Catalyzed Asymmetric Kumada Cross-Coupling of Racemic α-Bromo Esters with Alkyl Grignard Reagents. J. Am. Chem. Soc. 2014, 136, 17662–17668. 10.1021/ja5109084. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wang L.; Yuan G.; Liu S.; Sun X.; Yuan C.; Yang Y.; Bian Q.; Wang M.; Zhong J. Cobalt-Bisoxazoline-Catalyzed Enantioselective Cross-Coupling of α-Bromo Esters with Alkenyl Grignard Reagents. Org. Lett. 2020, 22, 4532–4536. 10.1021/acs.orglett.0c01557. [DOI] [PubMed] [Google Scholar]
- Roelofs W.; Gieselmann M.; Carde A.; Tashiro H.; Moreno D. S.; Henrick C. A.; Anderson R. J. Identification of the California Red Scale Sex Pheromone. J. Chem. Ecol. 1978, 4, 211–224. 10.1007/BF00988056. [DOI] [Google Scholar]
- Hutchinson J.-H.; Money T. A Formal Enantiospecific Synthesis of California Red Scale Pheromone. Can. J. Chem. 1985, 63, 3182–3185. 10.1139/v85-526. [DOI] [Google Scholar]
- Jin M.; Adak L.; Nakamura M. Iron-Catalyzed Enantioselective Cross-Coupling Reactions of α-Chloroesters with Alkyl Grignard Reagents. J. Am. Chem. Soc. 2015, 137, 7128–7134. 10.1021/jacs.5b02277. [DOI] [PubMed] [Google Scholar]
- Bauer G.; Wodrich M. D.; Scopelliti R.; Hu X. Iron Pincer Complexes as Catalysts and Intermediates in Alkyl-Aryl Kumada Coupling Reactions. Organometallics 2015, 34, 289–298. 10.1021/om501122p. [DOI] [Google Scholar]
- Fischer C.; Fu G. C. Asymmetric Nickel-Catalyzed Negishi Cross-Coupling of Secondary α-Bromo Amides with Organozinc Reagents. J. Am. Chem. Soc. 2005, 127, 4594–4595. 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- Arp F. O.; Fu G. C. Catalytic Enantioselective Negishi Reactions of Racemic Secondary Benzylic Halides. J. Am. Chem. Soc. 2005, 127, 10482–10483. 10.1021/ja053751f. [DOI] [PubMed] [Google Scholar]
- Binder J. T.; Cordier C. J.; Fu G. C. Catalytic Enantioselective Cross-Coupling of Secondary Alkyl Electrophiles with Secondary Alkylmetal Nucleophiles: Negishi Reactions of Racemic Benzylic Bromides with Achiral Alkylzinc Reagents. J. Am. Chem. Soc. 2012, 134, 17003–17006. 10.1021/ja308460z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R.; Adachi Y.; Yokoshima S.; Fukuyama T. Total Synthesis of (−)-Daphenylline. Angew. Chem., Int. Ed. 2016, 55, 6067–6070. 10.1002/anie.201601958. [DOI] [PubMed] [Google Scholar]
- Shang Q.; Di Y.-T.; Li C.-S.; Fang X.; Tan C.-J.; Zhang Z.; Zhang Y.; He H.-P.; Li S.-L.; Hao X.-J. Daphenylline, a New Alkaloid with an Unusual Skeleton, from. Daphniphyllum longeracemosum. Org. Lett. 2009, 11, 2357–2359. 10.1021/ol9007958. [DOI] [PubMed] [Google Scholar]
- Lin X.; Sun J.; Xi Y.; Lin D. How Racemic Secondary Alkyl Electrophiles Proceed to Enantioselective Products in Negishi Cross-Coupling Reactions. Organometallics 2011, 30, 3284–3292. 10.1021/om1012049. [DOI] [Google Scholar]
- Lundin P. M.; Esquivias J.; Fu G. C. Catalytic Asymmetric Cross-Coupling of Racemic α-Bromoketones with Arylzinc Reagents. Angew. Chem., Int. Ed. 2009, 48, 154–156. 10.1002/anie.200804888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.; Fu G. C. Catalytic Asymmetric Synthesis of Secondary Nitriles via Stereoconvergent Negishi Arylations and Alkenylations of Racemic α-Bromonitriles. J. Am. Chem. Soc. 2012, 134, 9102–9105. 10.1021/ja303442q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Fu G. C. Catalytic Asymmetric Synthesis of Tertiary Alkyl Fluorides: Negishi Cross-Coupling of Racemic α,α-Dihaloketones. J. Am. Chem. Soc. 2014, 136, 5520–5524. 10.1021/ja501815p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.; Martín-Gago P.; Fu G. C. Stereoconvergent Arylations and Alkenylations of Unactivated Alkyl Electrophiles: Catalytic Enantioselective Synthesis of Secondary Sulfonamides and Sulfones. J. Am. Chem. Soc. 2014, 136, 12161–12165. 10.1021/ja506885s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Fu G. C. Stereoconvergent Negishi Arylations of Racemic Secondary Alkyl Electrophiles: Differentiating Between a CF3 and an Alkyl Group. J. Am. Chem. Soc. 2015, 137, 9523–9526. 10.1021/jacs.5b04725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.; Choi J.; Liu A- T.; Slusarczyk M.; Fu G. C. A General Modular Method for the Catalytic Asymmetric Synthesis of Alkylboronate Esters. Science 2016, 354, 1265–1269. 10.1126/science.aai8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzwalder G. M.; Matier C. D.; Fu G. C. Enantioconvergent Cross-Coupling of Alkyl Electrophiles: The Catalytic Asymmetric Synthesis of Organosilanes,. Angew. Chem., Int. Ed. 2019, 58, 3571–3574. 10.1002/anie.201814208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R.; Reddy D. S. Quest for Novel Chemical Entities through Incorporation of Silicon in Drug Scaffolds. J. Med. Chem. 2018, 61, 3779–3798. 10.1021/acs.jmedchem.7b00718. [DOI] [PubMed] [Google Scholar]
- Fujii S.; Hashimoto Y. Progress in the Medicinal Chemistry of Silicon: C/Si Exchange and Beyond. Future Med. Chem. 2017, 9, 485–505. 10.4155/fmc-2016-0193. [DOI] [PubMed] [Google Scholar]
- Tacke R.; Dörrich S. Drug Design Based on the C-Si Switch Strategy. Top. Med. Chem. 2014, 17, 29–59. 10.1007/7355_2014_55. [DOI] [Google Scholar]
- Yi H.; Mao W.; Oestreich M. Enantioselective Construction of α-Chiral Silanes by Ni-Catalyzed C(sp3)-C(sp3) Cross-Coupling. Angew. Chem., Int. Ed. 2019, 58, 3575–3578. 10.1002/anie.201814340. [DOI] [PubMed] [Google Scholar]
- Yang Z.-P.; Freas D. J.; Fu G. C. The Asymmetric Synthesis of Amines via Nickel-Catalyzed Enantioconvergent Substitution Reactions. J. Am. Chem. Soc. 2021, 143, 2930–2937. 10.1021/jacs.0c13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.-P.; Freas D. J.; Fu G. C. Asymmetric Synthesis of Protected Unnatural α-Amino Acids via Enantioconvergent Nickel-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2021, 143, 8614–8618. 10.1021/jacs.1c03903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta S. S.; Roche S. P. Synthesis and Reactivity of α-Haloglycine Esters: Hyperconjugation in Action. Eur. J. Org. Chem. 2019, 2019, 6597–6605. 10.1002/ejoc.201901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Zhong J.; Zhou Y.; Gao Z.; Walsh P. J.; Wang X.; Ma S.; Hou S.; Liu S.; Wang M.; et al. Cobalt-Catalyzed Enantioselective Negishi Cross-Coupling of Racemic α-Bromo Esters with Arylzincs. Chem.—Eur. J. 2018, 24, 2059–2064. 10.1002/chem.201705463. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Liu C.; Wang L.; Han L.; Hou S.; Bian Q.; Zhong J. A Concise Enantioselective Synthesis of (S)-Preclamol via Asymmetric Catalytic Negishi Cross-Coupling Reaction. Synlett 2019, 30, 860–862. 10.1055/s-0037-1611759. [DOI] [Google Scholar]
- Li Z.; Cheng X.-Y.; Yang N. Y.; Chen J.-J.; Tang W.-Y.; Bian J. Q.; Cheng Y. F.; Li Z.-L.; Gu Q.-S.; Liu X.-Y. A Cobalt-Catalyzed Enantioconvergent Radical Negishi C(sp3)-C(sp2) Cross-Coupling with Chiral Multidentate N,N,P-Ligand. Organometallics 2021, 40, 2215–2219. 10.1021/acs.organomet.1c00190. [DOI] [Google Scholar]
- Zhou J.; Fu G. C. Suzuki Cross-Couplings of Unactivated Secondary Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2004, 126, 1340–1341. 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- Saito B.; Fu G. C. Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2007, 129, 9602–9603. 10.1021/ja074008l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito B.; Fu G. C. Enantioselective Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Homobenzylic Halides. J. Am. Chem. Soc. 2008, 130, 6694–6695. 10.1021/ja8013677. [DOI] [PubMed] [Google Scholar]
- Lundin P. M.; Fu G. C. Asymmetric Suzuki Cross-Couplings of Activated Secondary Alkyl Electrophiles: Arylations of Racemic α-Chloroamides. J. Am. Chem. Soc. 2010, 132, 11027–11029. 10.1021/ja105148g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owston N. A.; Fu G. C. Asymmetric Alkyl-Alkyl Cross-Couplings of Unactivated Secondary Alkyl Electrophiles: Stereoconvergent Suzuki Reactions of Racemic Acylated Halohydrins. J. Am. Chem. Soc. 2010, 132, 11908–11909. 10.1021/ja105924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Wilsily A.; Fu G. C. Stereoconvergent Amine-Directed Alkyl-Alkyl Suzuki Reactions of Unactivated Secondary Alkyl Chlorides. J. Am. Chem. Soc. 2011, 133, 8154–8157. 10.1021/ja203560q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsily A.; Tramutola F.; Owston N. A.; Fu G. C. New Directing Groups for Metal-Catalyzed Asymmetric Carbon-Carbon Bond-Forming Processes: Stereoconvergent Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Electrophiles. J. Am. Chem. Soc. 2012, 134, 5794–5797. 10.1021/ja301612y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zultanski S. L.; Fu G. C. Catalytic Asymmetric γ-Alkylation of Carbonyl Compounds via Stereoconvergent Suzuki Cross-Couplings. J. Am. Chem. Soc. 2011, 133, 15362–15364. 10.1021/ja2079515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; Sakthivel S.; Kulbitski K.; Nisnevich G.; Gandelman M. Efficient Synthesis of Secondary Alkyl Fluorides via Suzuki Cross-Coupling Reaction of 1-Halo-1-fluoralkanes. J. Am. Chem. Soc. 2014, 136, 9548–9551. 10.1021/ja504089y. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Gandelman M. Enantioselective Suzuki Cross-Couplings of Unactivated 1-Fluoro-1-haloalkanes: Synthesis of Chiral β-, γ-, δ- and ε-Fluoroalkanes. J. Am. Chem. Soc. 2015, 137, 2542–2547. 10.1021/jacs.5b00473. [DOI] [PubMed] [Google Scholar]
- Huang W.; Wan X.; Shen Q. Enantioselective Construction of Trifluoromethoxylated Stereogenic Centers by a Nickel-Catalyzed Asymmetric Suzuki-Miyaura Coupling of Secondary Benzyl Bromides. Angew. Chem., Int. Ed. 2017, 56, 11986–11989. 10.1002/anie.201706868. [DOI] [PubMed] [Google Scholar]
- Huang W.; Hu M.; Wan X.; Shen Q. Facilitating the Transmetalation Step with Arylzincates in Nickel-Catalyzed Enantioselective Arylation of Secondary Benzylic Halides. Nat. Commun. 2019, 10, 2963. 10.1038/s41467-019-10851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehling V. S.; Vaswani R. G.; Nasveschuk C. G.; Duplessis M.; Iyer P.; Balasubramanian S.; Zhao F.; Good A. C.; Campbell R.; Lee C.; et al. Discovery, Design and Synthesis of Indole-Based EZH2 Inhibitors. Bioorg. Med. Chem. 2015, 25, 3644–3649. 10.1016/j.bmcl.2015.06.056. [DOI] [PubMed] [Google Scholar]
- Allison B. D.; Carruthers N. I.; Levatic M. A.; Santillan J. A.; Shan C. R.. Substituted Benzamide Modulators of the Histamine H3 Receptor. WO2008002816A1, 2008.
- Huang W.; Wan X.; Shen Q. Asymmetric Cross-Coupling Reaction of Fluorinated Secondary Benzyl Bromides with Lithium Aryl Boronates/ZnBr2. Org. Lett. 2020, 22, 4327–4332. 10.1021/acs.orglett.0c01363. [DOI] [PubMed] [Google Scholar]
- Magnus N. A.; Anzeveno P. B.; Coffey D. S.; Hay D. A.; Laurila M. E.; Schkeryantz J. M.; Shaw B. W.; Staszak M. A. Optimized Catalytic Enantioselective Aryl Transfer Process Gives Access to mGlu2 Receptor. Org. Process Res. Dev. 2007, 11, 560–567. 10.1021/op0602268. [DOI] [Google Scholar]
- Xu S.-Y.; Zhang R.; Zhang S.-S.; Feng C.-G. Enantioselective Synthesis of 3-Aryl-Phthalides through a Nickel-Catalyzed Stereoconvergent Cross-Coupling Reaction. Org. Biomol. Chem. 2021, 19, 4492–4496. 10.1039/D1OB00487E. [DOI] [PubMed] [Google Scholar]
- Wu L.; Yang G.; Zhang W. Ni-Catalyzed Enantioconvergent Coupling of Epoxides with Alkenylboronic Acids: Construction of Oxindoles Bearing Quaternary Carbons. CCS Chem. 2020, 2 (2), 623–631. 10.31635/ccschem.019.201900064. [DOI] [Google Scholar]
- Wu L.; Shao Q.; Kong L.; Chen J.; Wei Q.; Zhang W. A Co(II)-Catalyzed Asymmetric Ring Opening Reaction of Spiro-Epoxyoxindoles with Allylboron. Org. Chem. Front. 2020, 7, 862–867. 10.1039/D0QO00072H. [DOI] [Google Scholar]
- Iwamoto T.; Okuzono C.; Adak L.; Jin M.; Nakamura M. Iron-Catalyzed Enantioselective Suzuki-Miyaura Coupling of Racemic Alkyl Bromides. Chem. Commun. 2019, 55, 1128–1131. 10.1039/C8CC09523J. [DOI] [PubMed] [Google Scholar]
- Tyrol C. C.; Yone N. S.; Gallin C. F.; Byers J. A. Iron-Catalyzed Enantioconvergent Suzuki-Miyaura Cross-Coupling to Afford Enantioenriched 1,1-Diarylalkanes. Chem. Commun. 2020, 56, 14661–14664. 10.1039/D0CC05003B. [DOI] [PubMed] [Google Scholar]
- Jiang S.-P.; Dong X.-Y.; Gu Q.-S.; Ye L.; Li Z.-L.; Liu X.-Y. Copper-Catalyzed Enantioconvergent Radical Suzuki-Miyaura C(sp3)-C(sp2) Cross-Coupling. J. Am. Chem. Soc. 2020, 142, 19652–19659. 10.1021/jacs.0c09125. [DOI] [PubMed] [Google Scholar]
- Wang P.-F.; Yu J.; Guo K.-X.; Jiang S.-P.; Chen J.-J.; Gu Q.-S.; Liu J.-R.; Hong X.; Li Z.-L.; Liu X.-Y. Design of Hemilabile N,N,N-Ligands in Copper-Catalyzed Enantioconvergent Radical Cross-Coupling of Benzyl/Propargyl Halides with Alkenylboronate Esters. J. Am. Chem. Soc. 2022, 144, 6442–6452. 10.1021/jacs.2c00957. [DOI] [PubMed] [Google Scholar]
- Wang F.-L.; Liu L.; Yang C.-J.; Luan C.; Yang J.; Chen J.-J.; Gu Q.-S.; Li Z.-L.; Liu X.-Y. Synthesis of α-Quaternary β-Lactams via Copper-Catalyzed Enantioconvergent Radical C(sp3)-C(sp2) Cross-Coupling with Organoboronate Esters. Angew. Chem., Int. Ed. 2023, 62, e202214709 10.1002/anie.202214709. [DOI] [PubMed] [Google Scholar]
- Dai X.; Strotman N. A.; Fu G. C. Catalytic Asymmetric Hiyama Cross-Couplings of Racemic α-Bromo Esters. J. Am. Chem. Soc. 2008, 130, 3302–3303. 10.1021/ja8009428. [DOI] [PubMed] [Google Scholar]
- Varenikov A.; Gandelman M. Synthesis of Chiral α-Trifluoromethyl Alcohols and Ethers via Enantioselective Hiyama Cross-Couplings of Bisfunctionalized Electrophiles. Nat. Commun. 2018, 9, 3566. 10.1038/s41467-018-05946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. M.; Peters J. C.; Fu G. C. Design of a Photoredox Catalyst that Enables the Direct Synthesis of Carbamate-Protected Primary Amines via Photoinduced, Copper-Catalyzed N-Alkylation Reactions of Unactivated Secondary Halides. J. Am. Chem. Soc. 2017, 139, 18101–18106. 10.1021/jacs.7b10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeiro J.; Pérez-Sestelo J.; Sarandeses L. A. Enantioselective Nickel-Catalyzed Cross-Coupling Reactions of Trialkylindium Reagents with Racemic Secondary Benzyl Bromides. Chem.—Eur. J. 2008, 14, 741–746. 10.1002/chem.200701035. [DOI] [PubMed] [Google Scholar]
- Lou S.; Fu G. C. Enantioselective Alkenylation via Nickel-Catalyzed Cross-Coupling with Organozirconium Reagents. J. Am. Chem. Soc. 2010, 132, 5010–5011. 10.1021/ja1017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Yang Z.-P.; Fu G. C. Quaternary Stereocenters via Catalytic Enantioconvergent Nucleophilic Substitution Reactions of Tertiary Alkyl Halides. Nat. Commun. 2021, 13, 236–242. 10.1038/s41557-020-00609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node M.; Nagasawa H.; Fuji K. Chiral Total Synthesis of Indole Alkaloids of the Aspidosperma and Hunteria Types. J. Org. Chem. 1990, 55, 517–521. 10.1021/jo00289a025. [DOI] [Google Scholar]
- Hosokawa S.; Kobayashi S. Total Synthesis of Madindoline A. Synth. Org. Chem. Jpn. 2001, 59, 1103–1108. 10.5059/yukigoseikyokaishi.59.1103. [DOI] [Google Scholar]
- Fang H.; Yang Z.; Zhang L.; Wang W.; Li Y.; Xu X.; Zhou S. Transmetal-Catalyzed Enantioselective Cross-Coupling Reaction of Racemic Secondary Benzylic Bromides with Organoaluminum Reagents. Org. Lett. 2016, 18, 6022–6025. 10.1021/acs.orglett.6b02933. [DOI] [PubMed] [Google Scholar]
- Varenikov A.; Gandelman M. Organotitanium Nucleophiles in Asymmetric Cross-Coupling Reaction: Stereoconvergent Synthesis of Chiral α-CF3 Thioethers. J. Am. Chem. Soc. 2019, 141, 10994–10999. 10.1021/jacs.9b05671. [DOI] [PubMed] [Google Scholar]
- Bissember A. C.; Lundgren R. J.; Creutz S. E.; Peters J. C.; Fu G. C. Transition-Metal-Catalyzed Alkylations of Amines with Alkyl Halides: Photoinduced Copper-Catalyzed Couplings of Carbazoles. Angew. Chem., Int. Ed. 2013, 52, 5129–5133. 10.1002/anie.201301202. [DOI] [PubMed] [Google Scholar]
- Kainz Q. M.; Matier C. D.; Bartoszewicz A.; Zultanski S. L.; Peters J. C.; Fu G. C. Asymmetric Copper-Catalyzed C-N Cross-Couplings Induced by Visible Light. Science 2016, 351, 681–684. 10.1126/science.aad8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Ahn J. M.; Oyala P. H.; Citek C.; Yin H.; Fu G. C.; Peters J. C. Investigation of the C-N Bond-Forming Step in a Photoinduced Copper-Catalyzed Enantioconvergent N-Alkylation; Characterization and Application of a Stabilized Organic Radical as a Mechanistic Probe. J. Am. Chem. Soc. 2022, 144, 4114–4123. 10.1021/jacs.1c13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do H.-Q.; Bachman S.; Bissember A. C.; Peters J. C.; Fu G. C. Photoinduced, Copper-Catalyzed Alkylation of Amides with Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2014, 136, 2162–2167. 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- Chen C.; Peters J. C.; Fu G. C. Photoinduced Copper-Catalyzed Asymmetric Amidation via Ligand Cooperativity. Nature 2021, 596, 250–256. 10.1038/s41586-021-03730-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.; Suematsu H.; Oyala P. H.; Peters J. C.; Fu G. C. Photoinduced, Copper-Catalyzed Enantioconvergent Alkylations of Anilines by Racemic Tertiary Electrophiles: Synthesis and Mechanism. J. Am. Chem. Soc. 2022, 144, 4550–4558. 10.1021/jacs.1c12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-F.; Dong X.-Y.; Cheng J.-T.; Yang N.-Y.; Wang L.-L.; Wang F.-L.; Luan C.; Liu J.; Li Z.-L.; Shuai Q.-S.; et al. Enantioconvergent Cu-Catalyzed Radical C-N Coupling of Racemic Secondary Alkyl Halides to Access α-Chiral Primary Amines. J. Am. Chem. Soc. 2021, 143, 15413–15419. 10.1021/jacs.1c07726. [DOI] [PubMed] [Google Scholar]
- Chen J. J.; Fang J.-H.; Du X.-Y.; Zhang J.-Y.; Bian J.-Q.; Wang F.-L.; Luan C.; Liu W.-L.; Liu J.-R.; Dong X.-Y.; et al. Enantioconvergent Cu-Catalyzed N-Alkylation of Aliphatic Amines. Nature 2023, 618, 294–300. 10.1038/s41586-023-05950-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Behnke N. E.; Kürti L. Copper-Catalyzed Synthesis of Hindered Ethers from α-Bromo Carbonyl Compounds. Org. Lett. 2018, 20, 5452–5456. 10.1021/acs.orglett.8b02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Fu G. C. Copper-Catalyzed Enantioconvergent Alkylation of Oxygen Nucleophiles. Nature 2023, 618, 301–307. 10.1038/s41586-023-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis A.; Becker T. The Structure of Phosphorous acid. Chem. Ber. 1897, 30, 1003–1009. [Google Scholar]
- Wang L. L.; Zhou H.; Cao Y.-X.; Zhang C.; Ren Y.-Q.; Li Z.-L.; Gu Q.-S.; Liu X.-Y. A General Copper-Catalyzed Enantioconvergent Radical Michaelis-Becker-Type C(sp3)-P Cross-Coupling. Nat. Synth. 2023, 2, 430–438. 10.1038/s44160-023-00252-3. [DOI] [Google Scholar]
- Zhou H.; Li Z.-L; Gu Q.-S.; Liu X.-Y. Ligand-Enabled Copper(I)-Catalyzed Asymmetric Radical C(sp3)-C Cross-Coupling Reactions. ACS Catal. 2021, 11, 7978–7986. 10.1021/acscatal.1c01970. [DOI] [Google Scholar]
- Wang D.; Zhu N.; Chen P.; Lin Z.; Liu G. Enantioselective Decarboxylative Cyanation Employing Cooperative Photoredox Catalysis and Copper Catalysis. J. Am. Chem. Soc. 2017, 139, 15632–15635. 10.1021/jacs.7b09802. [DOI] [PubMed] [Google Scholar]
- Chen H.-W.; Lu F.-D.; Cheng Y.; Jia Y.; Lu L.-Q.; Xiao W.-J. Asymmetric Deoxygenative Cyanation of Benzyl Alcohols Enabled by Synergistic Photoredox and Copper Catalysis. Chin. J. Chem. 2020, 38, 1671–1675. 10.1002/cjoc.202000309. [DOI] [Google Scholar]
- Wang T.; Wang Y.-N.; Wang R.; Zhang B.-C.; Yang C.; Li Y.-L.; Wang X.-S. Enantioselective Cyanation via Radical-Mediated C-C Single Bond Cleavage for Synthesis of Chiral Dinitriles,. Nat. Commun. 2019, 10, 5373. 10.1038/s41467-019-13369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J.; Fouquet E.; Zard S.-Z. Ring Opening Induced by Iminyl Radicals Derived from Cyclobutanones: New Aspects of Tin Hydride Cleavage of 5-Phenylsulfenylimines. J. Am. Chem. Soc. 1991, 113, 1055–1057. 10.1021/ja00003a057. [DOI] [Google Scholar]
- Xiao T.; Huang H.; Anand D.; Zhou L. Iminyl-Radical-Triggered C-C Bond Cleavage of Cycloketone Oxime Derivatives: Generation of Distal Cyano-Substituted Alkyl Radicals and Their Functionalization. Synthesis 2020, 52, 1585–1601. 10.1055/s-0039-1690844. [DOI] [Google Scholar]
- Chen J.; Wang P.-Z.; Lu B.; Liang D.; Yu X.-Y.; Xiao W.-J.; Chen J.-R. Enantioselective Radical Ring-Opening Cyanation of Oxime Esters by Dual Photoredox and Copper Catalysis. Org. Lett. 2019, 21, 9763–9768. 10.1021/acs.orglett.9b03970. [DOI] [PubMed] [Google Scholar]
- Dong X.-Y.; Zhang Y.-F.; Ma C.-L.; Gu Q.-S.; Wang F.-L.; Li Z.-L.; Jiang S.-P.; Liu X.-Y. A General Asymmetric Copper-Catalyzed Sonogashira C(sp3)-C(sp) Coupling. Nat. Chem. 2019, 11, 1158–1166. 10.1038/s41557-019-0346-2. [DOI] [PubMed] [Google Scholar]
- Sladojevich F.; Trabocchi A.; Guarna A.; Dixon D. J. A New Family of Cinchona-Derived Amino Phosphine Precatalysts: Application to the High Enantio- and Diastereoselective Silver-Catalyzed Isocyanoacetate Aldol Reaction. J. Am. Chem. Soc. 2011, 133, 1710–1713. 10.1021/ja110534g. [DOI] [PubMed] [Google Scholar]
- Wang F.-L.; Yang C.-J.; Liu J.-R.; Yang N.-Y.; Dong X.-Y.; Jiang R.-Q.; Chang X.-Y.; Li Z.-L.; Xu G.-X.; Yuang D.-L.; et al. Mechanism-Based Ligand Design for Copper-Catalyzed Enantioconvergent C(sp3)-C(sp) Cross-Coupling of Tertiary Electrophiles with Alkynes. Nat. Commun. 2022, 14, 949–957. 10.1038/s41557-022-00954-9. [DOI] [PubMed] [Google Scholar]
- Xia H.-D.; Li Z.-L.; Gu Q.-S.; Dong X.-Y.; Fang J.-H.; Du X.-Y.; Wang L.-L.; Liu X.-Y. Photoinduced Copper-Catalyzed Asymmetric Decarboxylative Alkynylation with Terminal Alkynes. Angew. Chem., Int. Ed. 2020, 59, 16926–16931. 10.1002/anie.202006317. [DOI] [PubMed] [Google Scholar]
- Mo X.; Chen B.; Zhang G. Copper-Catalyzed Enantioselective Sonogashira Type Coupling of Alkynes with α-Bromoamides. Angew. Chem., Int. Ed. 2020, 59, 13998–14002. 10.1002/anie.202000860. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Suzuki T.; Nakada M. Asymmetric Catalysis of Nozaki-Hiyama Allylation and Methallylation with a New Tridentate Bis(oxazolinyl)carbazole Ligand. J. Am. Chem. Soc. 2003, 125, 1140–1141. 10.1021/ja021243p. [DOI] [PubMed] [Google Scholar]
- Devannah V.; Sharma R.; Watson D. A. Nickel-Catalyzed Asymmetric C-Alkylation of Nitroalkanes: Synthesis of Enantioenriched β-Nitroamides. J. Am. Chem. Soc. 2019, 141, 8436–8440. 10.1021/jacs.9b04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudnik A. S.; Fu G. C. Nickel-Catalyzed Coupling Reactions of Alkyl Electrophiles, Including Unactivated Tertiary Halides. J. Am. Chem. Soc. 2012, 134, 10693–10697. 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonori D.; Aggarwal V. K. Stereospecific Couplings of Secondary and Tertiary Boronic Esters. Angew. Chem., Int. Ed. 2015, 54, 1082–1096. 10.1002/anie.201407701. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Bachman S.; Dudnik A. S.; Fu G. C. Nickel-Catalyzed Enantioconvergent Borylation of Racemic Secondary Benzylic Electrophiles. Angew. Chem., Int. Ed. 2018, 57, 14529–14532. 10.1002/anie.201806015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H.; Endo K.; Ozawa Y.; Watanabe Y.; Kubota K.; Imamoto T.; Ito H. Copper(I)-Catalyzed Enantioconvergent Borylation of Racemic Benzyl Chlorides Enabled by Quadrant-by-Quadrant Structure Modification of Chiral Bisphosphine Ligands. Angew. Chem., Int. Ed. 2019, 58, 11112–11117. 10.1002/anie.201906011. [DOI] [PubMed] [Google Scholar]
- Ito H.; Kubota K. Copper(I)-Catalyzed Boryl Substitution of Unsaturated Alkyl Halides. Org. Lett. 2012, 14, 890–893. 10.1021/ol203413w. [DOI] [PubMed] [Google Scholar]
- Hayashi T.; Tajika M.; Tamao K.; Kumada M. High Enantioselectivity in Asymmetric Grignard Cross-Coupling Catalyzed by Nickel Complexes of Chiral (Aminoalkylferrocenyl)phosphines. J. Am. Chem. Soc. 1976, 98, 3718–3719. 10.1021/ja00428a061. [DOI] [Google Scholar]
- Cordier C. J.; Lundgren R. J.; Fu G. C. Enantioconvergent Cross-Couplings of Racemic Alkylmetal Reagents with Unactivated Secondary Alkyl Electrophiles: Catalytic Asymmetric Negishi α-Alkylations of N-Bocpyrrolidine. J. Am. Chem. Soc. 2013, 135, 10946–10949. 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H.; Gorsline B. J.; Fu G. C. Catalyst-Controlled Doubly Enantioconvergent Coupling of Racemic Alkyl Nucleophiles and Electrophiles. Science 2020, 367, 559–564. 10.1126/science.aaz3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X.; Shibata Y.; Makida Y.; Fu G. C. Control of Vicinal Stereocenters through Nickel-Catalyzed Alkyl-Alkyl Cross-Coupling. Angew. Chem., Int. Ed. 2017, 56, 5821–5824. 10.1002/anie.201702402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellis J. C.; Primer D. N.; Molander G. A. Single-Electron Transmetalation in Organoboron Cross-Coupling by Photoredox/Nickel Dual Catalysis. Science 2014, 345, 433–436. 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primer D. N.; Karakaya I.; Tellis J. C.; Molander G. A. Single-Electron Transmetalation: An Enabling Technology for Secondary Alkylboron Cross-Coupling. J. Am. Chem. Soc. 2015, 137, 2195–2198. 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O.; Tellis J. C.; Primer D. N.; Molander G. A.; Kozlowski M. C. Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2015, 137, 4896–4899. 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E. L.; Jarvo E. R. Stereospecific and Stereoconvergent Cross-Coupling between Alkyl Electrophiles. Nat. Rev. Chem. 2017, 1, 0065. 10.1038/s41570-017-0065. [DOI] [Google Scholar]
- Poremba K. E.; Dibrell S. E.; Reisman S. E. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10, 8237–8246. 10.1021/acscatal.0c01842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson D. A.; Jones B. A.; Weix D. J. Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides,. J. Am. Chem. Soc. 2012, 134, 6146–6159. 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q.; Jiang F.; Gong H. DFT Study of the Single Electron Transfer Mechanism in Ni-Catalyzed Reductive Cross-Coupling of Aryl Bromides and Alkyl Bromides,. J. Organomet. Chem. 2014, 770, 130–135. 10.1016/j.jorganchem.2014.08.015. [DOI] [Google Scholar]
- Lin Q.; Fu Y.; Liu P.; Diao T. Monovalent Nickel-Mediated Radical Formation: A Concerted Halogen-Atom Dissociation Pathway Determined by Electroanalytical Studies. J. Am. Chem. Soc. 2021, 143, 14196–14206. 10.1021/jacs.1c05255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney A. H.; Kadunce N. T.; Reisman S. E. Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. J. Am. Chem. Soc. 2013, 135, 7442–7445. 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]
- Cherney A. H.; Reisman S. E. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Vinyl and Benzyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 14365–14368. 10.1021/ja508067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsell M. R.; Everson D. A.; Weix D. J. Nickel-Catalyzed Sodium Iodide- Promoted Reductive Dimerization of Alkyl Halides, Alkyl Pseudohalides, and Allylic Acetates. Chem. Commun. 2010, 46, 5743–5745. 10.1039/c0cc01716g. [DOI] [PubMed] [Google Scholar]
- Delano T. J.; Reisman S. E. Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis. ACS Catal. 2019, 9, 6751–6754. 10.1021/acscatal.9b01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Xu S.; Zhang J.; Kong W. Nickel-Catalyzed Enantioselective Electroreductive Cross-Couplings. Org. Chem. Front. 2020, 7, 3262–3265. 10.1039/D0QO00901F. [DOI] [Google Scholar]
- Hofstra J. L.; Cherney A. H.; Ordner C. M.; Reisman S. E. Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 139–142. 10.1021/jacs.7b11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman L. K. G.; Anka-Lufford L. L.; Naodovic M.; Weix D. J. Cobalt Co-Catalysis for Cross-Electrophile Coupling: Diarylmethanes from Benzyl Mesylates and Aryl Halides,. Chem. Sci. 2015, 6, 1115–1119. 10.1039/C4SC03106G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J.; Sun D.; Song Y.; Tong W.; Wu F. Ni-Catalyzed Asymmetric Reductive Alkenylation of α-Chlorosulfones with Vinyl Bromides. Org. Lett. 2022, 24, 1807–1811. 10.1021/acs.orglett.2c00217. [DOI] [PubMed] [Google Scholar]
- Suzuki N.; Hofstra J. L.; Poremba K. E.; Reisman S. E. Nickel-Catalyzed Enantioselective Cross-Coupling of N-Hydroxyphthalamide Esters with Vinyl Bromides. Org. Lett. 2017, 19, 2150–2153. 10.1021/acs.orglett.7b00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadunce N. T.; Reisman S. E. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Heteroaryl Iodides and α-Chloronitriles. J. Am. Chem. Soc. 2015, 137, 10480–10483. 10.1021/jacs.5b06466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba K. E.; Kadunce N. T.; Suzuki N.; Cherney A. H.; Reisman S. E. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling to Access 1,1-Diarylalkanes. J. Am. Chem. Soc. 2017, 139, 5684–5687. 10.1021/jacs.7b01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B. P.; Orlandi M.; Huang C.-Y.; Sigman M. S.; Doyle A. G. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of Styrenyl Aziridines. J. Am. Chem. Soc. 2017, 139, 5688–5691. 10.1021/jacs.7b03448. [DOI] [PubMed] [Google Scholar]
- Sun D.; Ma G.; Zhao X.; Lei C.; Gong H. Nickel-Catalyzed Asymmetric Reductive Arylation of α-Chlorosulfones with Aryl Halides. Chem. Sci. 2021, 12, 5253–5258. 10.1039/D1SC00283J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano T. J.; Dibrell S. E.; Lacker C. R.; Pancoast A. R.; Poremba K. E.; Cleary L.; Sigman M. S.; Reisman S. E. Nickel-Catalyzed Asymmetric Reductive Cross-Coupling of α-Chloroesters with (Hetero)aryl Iodides. Chem. Sci. 2021, 12, 7758–7762. 10.1039/D1SC00822F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. A.; Phelan J. P.; Badir S. O.; Molander G. A. Recent Advances in Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem., Int. Ed. 2019, 58, 6152–6163. 10.1002/anie.201809431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-H.; Chen H.; Zhu C.; Yu S. A Review of Enantioselective Dual Transition Metal/Photoredox Catalysis. Sci. China Chem. 2020, 63, 637–647. 10.1007/s11426-019-9701-5. [DOI] [Google Scholar]
- Lipp A.; Badir S. O.; Molander G. A. Stereoinduction in Metallaphotoredox Catalysis. Angew. Chem., Int. Ed. 2021, 60, 1714–1726. 10.1002/anie.202007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Ahneman D.; Chu L.; Terrett J.; Doyle A. G.; MacMillan D. W. C. Merging Photoredox with Nickel-Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; Cong H.; Li W.; Choi J.; Fu G. C.; MacMillan D. W. C. Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis. J. Am. Chem. Soc. 2016, 138, 1832–1835. 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzetta C.; Bonifazi D.; Davidson R. W. M. Enantioselective Synthesis of N-Benzylic Heterocycles: A Nickel and Photoredox Dual Catalysis Approach. Org. Lett. 2019, 21, 8957–8961. 10.1021/acs.orglett.9b03338. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Nie X.; Harms K.; Riedel R.; Zhang L.; Meggers E. Enantioconvergent Photoredox Radical-Radical Coupling Catalyzed by a Chiral-at-Rhodium Complex. Sci. China Chem. 2019, 62, 1512–1518. 10.1007/s11426-019-9584-x. [DOI] [Google Scholar]
- Zhang L.; Meggers E. Steering Asymmetric Lewis Acid Catalysis Exclusively with Octahedral Metal-Centered Chirality. Acc. Chem. Res. 2017, 50, 320–330. 10.1021/acs.accounts.6b00586. [DOI] [PubMed] [Google Scholar]
- Huang X.; Meggers E. Asymmetric Photocatalysis with Bis-cyclometalated Rhodium Complexes. Acc. Chem. Res. 2019, 52, 833–847. 10.1021/acs.accounts.9b00028. [DOI] [PubMed] [Google Scholar]
- Gandolfo E.; Tang X.; Roy S. R.; Melchiorre P. Photochemical Asymmetric Nickel-Catalyzed Acyl Cross-Coupling. Angew. Chem., Int. Ed. 2019, 58, 16854–16858. 10.1002/anie.201910168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H.; Zhang Q.; Walsh P. J.; Mao J. Nickel/Photoredox-Catalyzed Asymmetric Reductive Cross-Coupling of Racemic α-Chloro Esters with Aryl Iodides. Angew. Chem., Int. Ed. 2020, 59, 5172–5177. 10.1002/anie.201914175. [DOI] [PubMed] [Google Scholar]
- Zheng P.; Zhou P.; Wang D.; Xu W.; Wang H.; Xu T. Dual Ni/Photoredox-Catalyzed Asymmetric Cross-Coupling to Access Chiral Benzylic Boronic Esters. Nat. Commun. 2021, 12, 1646. 10.1038/s41467-021-21947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P.; Li X.; Wang D.; XU T. Dual Nickel- and Photoredox-Catalyzed Reductive Cross-Coupling to Access Chiral Trifluoromethylated Alkanes. Org. Lett. 2021, 23, 4683–4687. 10.1021/acs.orglett.1c01420. [DOI] [PubMed] [Google Scholar]
- Boland N. A.; Casey M.; Hynes S. J.; Matthews J. W.; Müller-Bunz H.; Wilkes P. Preparation of Enantiopure Bisimidazoline Ligands and Their Use in Asymmetric Catalysis. Org. Biomol. Chem. 2004, 2, 1995–2002. 10.1039/B407743C. [DOI] [PubMed] [Google Scholar]
- Parasram M.; Shields B. J.; Ahmad O.; Knauber T.; Doyle A. G. Regioselective Cross-Electrophile Coupling of Epoxides and (Hetero)aryl Iodides via Ni/Ti/Photoredox Catalysis. ACS Catal. 2020, 10, 5821–5827. 10.1021/acscatal.0c01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. H.; Borden M. A.; Steiman T. J.; Wang L. S.; Parasram M.; Doyle A. G. Ni/Photoredox-Catalyzed Enantioselective Cross-Electrophile Coupling of Styrene Oxide with Aryl Iodides. J. Am. Chem. Soc. 2021, 143, 15873–15881. 10.1021/jacs.1c08105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zheng P.; Wu X.; Li Y.; XU T. Modular and Facile Access to Chiral α-Aryl Phosphates via Dual Nickel- and Photoredox-Catalyzed Reductive Cross-Coupling. J. Am. Chem. Soc. 2022, 144, 3989–3997. 10.1021/jacs.1c12424. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Van Vranken D. J. Asymmetric Transition-Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Crawley M. L. Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis. Chem. Rev. 2003, 103, 2921–2944. 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Gu Q.; You S. Recent Advances in Ni-Catalyzed Allylic Substitution Reactions. Chin. J. Org. Chem. 2019, 39, 15–27. 10.6023/cjoc201809037. [DOI] [Google Scholar]
- Son S.; Fu G. C. Nickel-Catalyzed Asymmetric Negishi Cross-Couplings of Secondary Allylic Chlorides with Alkylzincs. J. Am. Chem. Soc. 2008, 130, 2756–2757. 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]
- Suh Y.-G.; Kim S.-A.; Jung J.-K.; Shin D.-Y.; Min K.-H.; Koo B.-A.; Kim H.-S. Asymmetric Total Synthesis of Fluvirucinine A1. Angew. Chem., Int. Ed. 1999, 38, 3545–3547. . [DOI] [PubMed] [Google Scholar]
- Schmidt T.; Kirschning A. Total Synthesis of Carolacton, a Highly Potent Biofilm Inhibitor. Angew. Chem., Int. Ed. 2012, 51, 1063–1066. 10.1002/anie.201106762. [DOI] [PubMed] [Google Scholar]
- Kranidiotis-Hisatomi N.; Yi H.; Oestreich M. Enantio- and Regioconvergent Nickel-Catalyzed C(sp3)-C(sp3) Cross-Coupling of Allylic Electrophiles Steered by a Silyl Group. Angew. Chem., Int. Ed. 2021, 60, 13652. 10.1002/anie.202102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T.; Matsumura R.; Wasa H.; Tsubouchi A. Copper(I)-Promoted Alkylation of Alkenylbenzyldimethylsilanes. Asian J. Org. Chem. 2014, 3, 838–841. 10.1002/ajoc.201402060. [DOI] [Google Scholar]
- Shields J. D.; Ahneman D. T.; Graham T. J. A.; Doyle A. G. Enantioselective, Nickel-Catalyzed Suzuki Cross-Coupling of Quinolinium Ions. Org. Lett. 2014, 16, 142–145. 10.1021/ol4031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester K. T.; Wu K.; Doyle A. G. Mechanistic Investigation of the Nickel-Catalyzed Suzuki Reaction of N,O-Acetals: Evidence for Boronic Acid-Assisted Oxidative Addition and an Iminium Activation Pathway. J. Am. Chem. Soc. 2012, 134, 16967–16970. 10.1021/ja3079362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. W.; Fu G. C. Nickel-Catalyzed Asymmetric Cross-Coupling of Racemic Propargylic Halides with Arylzinc Reagents. J. Am. Chem. Soc. 2008, 130, 12645–12647. 10.1021/ja805165y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelke A. J.; Sun J.; Fu G. C. Nickel-Catalyzed Enantioselective Cross-Coupling of Racemic Secondary Electrophiles That Bear an Oxygen Leaving Group. J. Am. Chem. Soc. 2012, 134, 2966–2969. 10.1021/ja300031w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schley N. D.; Fu G. C. Nickel-Catalyzed Negishi Arylations of Propargyl Bromides: A Mechanistic Investigation. J. Am. Chem. Soc. 2014, 136, 16588–16593. 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Yi H.; Oestreich M. Enantioconvergent and Regioselective Synthesis of Allenylsilanes by Nickel-Catalyzed C(sp2)-C(sp3) Cross-Coupling Starting from Racemic α-Silylated Propargyl Bromides. Organometallics 2021, 40, 2194–2197. 10.1021/acs.organomet.1c00112. [DOI] [Google Scholar]
- Lu F.-D.; Liu D.; Zhu L.; Lu L.-Q.; Yang Q.; Zhou Q.-Q.; Wei Y.; Lan Y.; Xiao W.-J. Asymmetric Propargylic Radical Cyanation Enabled by Dual Organophotoredox and Copper Catalysis. J. Am. Chem. Soc. 2019, 141, 6167–6172. 10.1021/jacs.9b02338. [DOI] [PubMed] [Google Scholar]
- Newton C. G.; Wang S.-G.; Oliveira C. C.; Cramer N. Catalytic Enantioselective Transformations Involving C-H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- Loup J.; Dhawa U.; Pesciaioli F.; Wencel-Delord J.; Ackermann L. Enantioselective C-H Activation with Earth-Abundant 3d Transition Metals. Angew. Chem., Int. Ed. 2019, 58, 12803–12818. 10.1002/anie.201904214. [DOI] [PubMed] [Google Scholar]
- Woźniak L.; Tan J.-F.; Nguyen Q.-H.; Madron du Vigné A.; Smal V.; Cao Y.-X.; Cramer N. Catalytic Enantioselective Functionalizations of C-H Bonds by Chiral Iridium Complexes. Chem. Rev. 2020, 120, 10516–10543. 10.1021/acs.chemrev.0c00559. [DOI] [PubMed] [Google Scholar]
- Lapuh M. I.; Mazeh S.; Besset T. Chiral Transient Directing Groups in Transition-Metal-Catalyzed Enantioselective C-H Bond Functionalization. ACS Catal. 2020, 10, 12898–12919. 10.1021/acscatal.0c03317. [DOI] [Google Scholar]
- Achar T. K.; Maiti S.; Jana S.; Maiti D. Transition Metal Catalyzed Enantioselective C(sp2)-H Bond Functionalization. ACS Catal. 2020, 10, 13748–13793. 10.1021/acscatal.0c03743. [DOI] [Google Scholar]
- Liao G.; Zhang T.; Lin Z.-K.; Shi B.-F. Transition-Metal-Catalyzed Enantioselective C-H Functionalization via Chiral Transient Directing Group Strategies. Angew. Chem., Int. Ed. 2020, 59, 19773–19786. 10.1002/anie.202008437. [DOI] [PubMed] [Google Scholar]
- Su X.-L.; Ye L.; Chen J.-J.; Liu X.-D.; Jiang S.-P.; Wang F.-L.; Liu L.; Yang C.-J.; Chang X.-Y.; Li Z.-L.; et al. Copper-Catalyzed Enantioconvergent Cross-Coupling of Racemic Alkyl Bromides with Azole C(sp2)-H Bonds. Angew. Chem., Int. Ed. 2021, 60, 380–384. 10.1002/anie.202009527. [DOI] [PubMed] [Google Scholar]
- Li C.; Chen B.; Ma X.; Mo X.; Zhang G. Photo-Promoted Copper-Catalyzed Enantioselective Alkylation of Azoles. Angew. Chem., Int. Ed. 2021, 60, 2130–2134. 10.1002/anie.202009323. [DOI] [PubMed] [Google Scholar]
- Nishimura T.; Nagamoto M.; Ebe Y.; Hayashi T. Enantioselective [3 + 2] Annulation via C-H Activation between Cyclic N-Acyl Ketimines and 1,3-Dienes Catalyzed by Iridium/Chiral Diene Complexes. Chem. Sci. 2013, 4, 4499–4503. 10.1039/c3sc52379a. [DOI] [Google Scholar]
- Nagamoto M.; Yamauchi D.; Nishimura T. Iridium-Catalyzed Asymmetric [3 + 2] Annulation of Aromatic Ketimines with Alkynes via C-H Activation: Unexpected Inversion of the Enantioselectivity Induced by Protic Acids. Chem. Commun. 2016, 52, 5876–5879. 10.1039/C6CC01398H. [DOI] [PubMed] [Google Scholar]
- Nagamoto M.; Sakamoto K.; Nishimura T. Iridium/Chiral Diene-Catalyzed Enantioselective (3 + 2) Annulation of Aromatic Ketimines with 1,3-Dienes via C-H Activation. Adv. Synth. Catal. 2018, 360, 791–795. 10.1002/adsc.201701378. [DOI] [Google Scholar]
- Zheng J.; Wang S.-B.; Zheng C.; You S.-L. Asymmetric Synthesis of Spiropyrazolones by Rhodium-Catalyzed C(sp2)-H Functionalization/Annulation Reactions. Angew. Chem., Int. Ed. 2017, 56, 4540–4544. 10.1002/anie.201700021. [DOI] [PubMed] [Google Scholar]
- Hao Y.-J.; Hu X.-Y.; Zhou Y.; Zhou J.; Yu J.-S. Catalytic Enantioselective α-Arylation of Carbonyl Enolates and Related Compounds. ACS Catal. 2020, 10, 955–993. 10.1021/acscatal.9b04480. [DOI] [Google Scholar]
- Åhman J.; Wolfe J. P.; Troutman M. W.; Palucki M.; Buchwald S. L. Asymmetric Arylation of Ketone Enolates. J. Am. Chem. Soc. 1998, 120, 1918–1919. 10.1021/ja973794z. [DOI] [Google Scholar]
- Xie X.; Chen Y.; Ma D. Enantioselective α-Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-proline at Low Reaction Temperatures. J. Am. Chem. Soc. 2006, 128, 16050–16051. 10.1021/ja066991j. [DOI] [PubMed] [Google Scholar]
- Ge S.; Hartwig J. F. Nickel-Catalyzed Asymmetric α-Arylation and Heteroarylation of Ketones with Chloroarenes: Effect of Halide on Selectivity, Oxidation State, and Room Temperature Reactions. J. Am. Chem. Soc. 2011, 133, 16330–16333. 10.1021/ja2082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornella J.; Jackson E. P.; Martin R. Nickel-Catalyzed Enantioselective C-C Bond Formation through Csp2-O Cleavage in Aryl Esters. Angew. Chem., Int. Ed. 2015, 54, 4075–4078. 10.1002/anie.201412051. [DOI] [PubMed] [Google Scholar]
- Li M.; Wang J. Nickel-Catalyzed Enantioselective C(sp3)-H Arylation of Ketones with Aryl Ethers via Selective CAr-O Cleavage Construct All-Carbon Quaternary Stereocenters. CCC Chem. 2022, 4, 2921–2929. 10.31635/ccschem.022.202101611. [DOI] [Google Scholar]
- Lee S.; Hartwig J. F. Improved Catalysts for the Palladium-Catalyzed Synthesis of Oxindoles by Amide α-Arylation. Rate Acceleration, Use of Aryl Chloride Substrates, and a New Carbene Ligand for Asymmetric Transformations. J. Org. Chem. 2001, 66, 3402–3415. 10.1021/jo005761z. [DOI] [PubMed] [Google Scholar]
- Taylor A. M.; Altman R. A.; Buchwald S. L. Palladium-Catalyzed Enantioselective α-Arylation and α-Vinylation of Oxindoles Facilitated by an Axially Chiral P-Stereogenic Ligand. J. Am. Chem. Soc. 2009, 131, 9900–9901. 10.1021/ja903880q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielvogel D. J.; Buchwald S. L. Nickel-BINAP Catalyzed Enantioselective α-Arylation of α-Substituted γ-Butyrolactones. J. Am. Chem. Soc. 2002, 124, 3500–3501. 10.1021/ja017545t. [DOI] [PubMed] [Google Scholar]
- Jette C. I.; Geibel I.; Bachman S.; Hayashi M.; Sakurai S.; Shimizu H.; Morgan J. B.; Stoltz B. M. Palladium-Catalyzed Construction of Quaternary Stereocenters by Enantioselective Arylation of γ-Lactams with Aryl Chlorides and Bromides. Angew. Chem., Int. Ed. 2019, 58, 4297–4301. 10.1002/anie.201814475. [DOI] [PubMed] [Google Scholar]
- Jiao Z.; Chee K. W.; Zhou J. Palladium-Catalyzed Asymmetric α-Arylation of Akylnitriles. J. Am. Chem. Soc. 2016, 138, 16240–16243. 10.1021/jacs.6b11610. [DOI] [PubMed] [Google Scholar]
- Xu J.; Zhong Z.; Jiang M.; Zhou Y.; Liu X.; Feng X. Asymmetric Catalytic Concise Synthesis of Hetero-3,3′-Bisoxindoles for the Construction of Bispyrroloindoline Alkaloids. CCS Chem. 2021, 3 (7), 1894–1902. 10.31635/ccschem.020.202000443. [DOI] [Google Scholar]
- Cai Y.; Shi S.-L. Enantioconvergent Arylation of Racemic Secondary Alcohols to Chiral Tertiary Alcohols Enabled by Nickel/N-Heterocyclic Carbene Catalysts. J. Am. Chem. Soc. 2021, 143, 11963–11968. 10.1021/jacs.1c06614. [DOI] [PubMed] [Google Scholar]
- Saint-Denis T. G.; Zhu R.-Y.; Chen G.; Wu Q.-F.; Yu J.-Q. Enantioselective C(sp3)-H Bond Activation by Chiral Transition Metal Catalysts. Science 2018, 359, eaao4798 10.1126/science.aao4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-J.; Zhang C.; Gu Q.-S.; Fang J.-H.; Su X.-L.; Ye L.; Sun Y.; Tian Y.; Li Z.-L.; Liu X.-Y. Cu-Catalyzed Intramolecular Radical Enantioconvergent Tertiary β-C(sp3)-H Amination of Racemic Ketones. Nat. Catal. 2020, 3, 539–546. 10.1038/s41929-020-0460-y. [DOI] [Google Scholar]
- Lang K.; Li C.; Kim I.; Zhang X. P. Enantioconvergent Amination of Racemic Tertiary C-H Bonds. J. Am. Chem. Soc. 2020, 142, 20902–20911. 10.1021/jacs.0c11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.-S.; Gong L.-Z. Palladium-Catalyzed Asymmetric C-H Functionalization: Mechanism, Stereo- and Regioselectivities, and Synthetic Applications. Acc. Chem. Res. 2020, 53, 2841–2854. 10.1021/acs.accounts.0c00477. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Thaisrivongs D. A.; Donckele E. J. Palladium-Catalyzed Enantioselective Allylic Alkylations through C-H Activation. Angew. Chem., Int. Ed. 2013, 52, 1523–1526. 10.1002/anie.201207870. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Donckele E. J.; Thaisrivongs D. A.; Osipov M.; Masters J. T. A New Class of Non-C2-Symmetric Ligands for Oxidative and Redox-Neutral Palladium-Catalyzed Asymmetric Allylic Alkylations of 1,3-Diketones. J. Am. Chem. Soc. 2015, 137, 2776–2784. 10.1021/jacs.5b00786. [DOI] [PubMed] [Google Scholar]
- Fan L.-F.; Wang T.-C.; Wang P.-S.; Gong L.-Z. Palladium-Catalyzed Asymmetric Allylic C-H Alkylation of 1,4-Dienes with Cyclic β-Keto Esters. Organometallics 2019, 38, 4014–4021. 10.1021/acs.organomet.9b00363. [DOI] [Google Scholar]
- Singh I. P.; Milligan K. E.; Gerwick W. H. Tanikolide, a Toxic and Antifungal Lactone from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1999, 62, 1333–1335. 10.1021/np990162c. [DOI] [PubMed] [Google Scholar]
- Lin H.-C.; Xie P.-P.; Dai Z.-Y.; Zhang S.-Q.; Wang P.-S.; Chen Y.-G.; Wang T.-C.; Hong X.; Gong L.-Z. Nucleophile-Dependent Z/E and Regioselectivity in the Palladium-Catalyzed Asymmetric Allylic C-H Activation of 1,4-Dienes. J. Am. Chem. Soc. 2019, 141, 5824–5834. 10.1021/jacs.8b13582. [DOI] [PubMed] [Google Scholar]
- Perry M. A.; Morin M. D.; Slafer B. W.; Rychnovsky S. D. Total Synthesis of Lepadiformine Alkaloids using N-Boc α-Amino Nitriles as Trianion Synthons. J. Org. Chem. 2012, 77, 3390–3400. 10.1021/jo300161x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.-S.; Lin H.-C.; Zhai Y.-J.; Han Z.-Y.; Gong L.-Z. Chiral Counterion Strategy for the Asymmetric Oxidative C(sp3)-H/C(sp3)-H Coupling: Enantioselective α-Allylation of Aldehydes with Terminal Alkenes. Angew. Chem., Int. Ed. 2014, 53, 12218–12221. 10.1002/anie.201408199. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; List B. Chiral Counterion in Asymmetric Transition-Metal Catalysis: Highly Enantioselective Pd/Brønsted Acid-Catalyzed Direct α-Allylation of Aldehydes. J. Am. Chem. Soc. 2007, 129, 11336–11337. 10.1021/ja074678r. [DOI] [PubMed] [Google Scholar]
- Zhou X.-L.; Su Y.-L.; Wang P.-S.; Gong L.-Z. Asymmetric Allylic C-H Alkylation of 1,4-Dienes with Aldehydes. Acta Chim. Sinica 2018, 76, 857–861. 10.6023/A18060235. [DOI] [Google Scholar]
- Lin H.-C.; Wang P.-S.; Tao Z.-L.; Chen Y.-G.; Han Z.-Y.; Gong L.-Z. Highly Enantioselective Allylic C-H Alkylation of Terminal Olefins with Pyrazol-5-ones Enabled by Cooperative Catalysis of Palladium Complex and Brønsted Acid. J. Am. Chem. Soc. 2016, 138, 14354–14361. 10.1021/jacs.6b08236. [DOI] [PubMed] [Google Scholar]
- Fan L.-F.; Wang P.-S.; Gong L.-Z. Monodentate Phosphorus Ligand-Enabled General Palladium-Catalyzed Allylic C-H Alkylation of Terminal Alkenes. Org. Lett. 2019, 21, 6720–6725. 10.1021/acs.orglett.9b02325. [DOI] [PubMed] [Google Scholar]
- Wang T.-C.; Han Z.-Y.; Wang P. S.; Lin H.-C.; Luo S.-W.; Gong L.-Z. Enantioselective Synthesis of 5-Alkylated Thiazolidinones via Palladium-Catalyzed Asymmetric Allylic C-H Alkylations of 1,4-Pentadienes with 5H-Thiazol-4-ones. Org. Lett. 2018, 20, 4740–4744. 10.1021/acs.orglett.8b01697. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Yin H.; Fu G. C. Catalytic Enantioconvergent Coupling of Secondary and Tertiary Electrophiles with Olefins. Nature 2018, 563, 379–383. 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.; Huang Z. Advances in Base-Metal-Catalyzed Alkene Hydrosilylation. ACS Catal. 2017, 7, 1227–1243. 10.1021/acscatal.6b02990. [DOI] [Google Scholar]
- Zhou F.; Zhang Y.; Xu X.; Zhu S. NiH-Catalyzed Remote Asymmetric Hydroalkylation of Alkenes with Racemic α-Bromo Amides. Angew. Chem., Int. Ed. 2019, 58, 1754–1758. 10.1002/anie.201813222. [DOI] [PubMed] [Google Scholar]
- He S.-J.; Wang J.-W.; Li Y.; Xu Z.-Y.; Wang X.-X.; Lu X.; Fu Y. Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α-Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc. 2020, 142, 214–221. 10.1021/jacs.9b09415. [DOI] [PubMed] [Google Scholar]
- Yang Z.-P.; Fu G. C. Convergent Catalytic Asymmetric Synthesis of Esters of Chiral Dialkyl Carbinols. J. Am. Chem. Soc. 2020, 142, 5870–5875. 10.1021/jacs.0c01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson N. J.; Wilbur K. C. E.; Malcolmson S. J. Enantioselective Intermolecular Pd-Catalyzed Hydroalkylation of Acyclic 1,3-Dienes with Activated Pronucleophiles. J. Am. Chem. Soc. 2018, 140, 2761–2764. 10.1021/jacs.7b13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M.; Jäkel C.; Plietker B. Palladium-Catalyzed Asymmetric Addition of Pronucleophiles to Allenes. J. Am. Chem. Soc. 2003, 125, 4438–4439. 10.1021/ja029190z. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Xie J.; Sieber J. D. The Palladium-Catalyzed Asymmetric Addition of Oxindoles and Allenes: An Atom Economical Versatile Method for the Construction of Chiral Indole Alkaloids. J. Am. Chem. Soc. 2011, 133, 20611–20622. 10.1021/ja209244m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Wei Z.; Zhang J.; Yang H.; Xia C.; Jiang G. From Palladium to Brønsted Acid Catalysis: Highly Enantioselective Regiodivergent Addition of Alkoxyallenes to Pyrazolones. Angew. Chem., Int. Ed. 2017, 56, 1077–1083. 10.1002/anie.201610473. [DOI] [PubMed] [Google Scholar]
- Nájera C.; Beletskaya I. P.; Yus M. Metal-Catalyzed Regiodivergent Organic Reactions. Chem. Soc. Rev. 2019, 48, 4515–4618. 10.1039/C8CS00872H. [DOI] [PubMed] [Google Scholar]
- Beletskaya I. P.; Nájera C.; Yus M. Catalysis and Regioselectivity in Hydrofunctionalization Reactions of Unsaturated Carbon Bonds. Part I. Russ. Chem. Rev. 2020, 89, 250–275. 10.1070/RCR4916. [DOI] [Google Scholar]
- Beletskaya I. P.; Nájera C.; Yus M. Catalysis and Regioselectivity in Hydrofunctionalization Reactions of Unsaturated Carbon Bonds. Part II. Hydroamination. Russ. Chem. Rev. 2020, 89, 1074–1114. 10.1070/RCR4953. [DOI] [Google Scholar]
- Beletskaya I. P.; Nájera C.; Yus M. Catalysis and Regioselectivity in Hydrofunctionalization Reactions of Unsaturated Carbon Bonds. Part III. Russ. Chem. Rev. 2021, 90, 70–93. 10.1070/RCR4983. [DOI] [Google Scholar]
- Ballesteros A.; Morán-Poladura P.; González J. M. Gold(I) Operational in Synergistic for the Intermolecular α-Addition Reaction of Aldehydes across Allenamides. Chem. Commun. 2016, 52, 2905–2908. 10.1039/C5CC09529H. [DOI] [PubMed] [Google Scholar]
- Fernández-Casado J.; Nelson R.; Mascareñas J. L.; López F. Synergistic Gold and Enamine Catalysis: Intermolecular α-Alkylation of Aldehydes with Allenamides. Chem. Commun. 2016, 52, 2909–2912. 10.1039/C5CC09533F. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Wang Y.; Zhang L.; Cai M.; Luo S. Enantioselective Terminal Addition to Allenes of Dual Primary Amine/Palladium Catalysis. J. Am. Chem. Soc. 2017, 139, 3631–3634. 10.1021/jacs.7b00437. [DOI] [PubMed] [Google Scholar]
- Cruz F. A.; Dong V. M. Stereodivergent Coupling of Aldehydes and Alkynes via Synergistic Catalysis Using Rh and Jacobsen’s Amine. J. Am. Chem. Soc. 2017, 139, 1029–1031. 10.1021/jacs.6b10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletskaya I. P.; Nájera C.; Yus M. Stereodivergent Catalysis. Chem. Rev. 2018, 118, 5080–5200. 10.1021/acs.chemrev.7b00561. [DOI] [PubMed] [Google Scholar]
- Koschker P.; Breit B. Branching Out: Rhodium-Catalyzed Allylation with Alkynes and Allenes. Acc. Chem. Res. 2016, 49, 1524–1536. 10.1021/acs.accounts.6b00252. [DOI] [PubMed] [Google Scholar]
- Reed-Berendt B. G.; Latham D. E.; Dambatta M. B.; Morrill L. C. Borrowing Hydrogen for Organic Synthesis. ACS Cent. Sci. 2021, 7, 570–585. 10.1021/acscentsci.1c00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T.; Hoff O.; Armstrong R. J.; Donohoe T. J. Control of Absolute Stereochemistry in Transition-Metal-Catalyzed Hydrogen Borrowing Reactions. Chem.—Eur. J. 2020, 26, 12912–12925. 10.1002/chem.202001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrgang T.; Kempe R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119, 2524–2549. 10.1021/acs.chemrev.8b00306. [DOI] [PubMed] [Google Scholar]
- Corma A.; Navas J.; Sabater M. J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. 10.1021/acs.chemrev.7b00340. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lim C.-S.; Sim D. S. B.; Pan H.-J.; Zhao Y. Catalytic Enantioselective Amination of Alcohols by the Use of Borrowing Hydrogenation Methodology: Cooperative Catalysis by Iridium and Chiral Phosphoric Acid. Angew. Chem., Int. Ed. 2014, 53, 1399–1403. 10.1002/anie.201307789. [DOI] [PubMed] [Google Scholar]
- Peña-Lopez M.; Neumann H.; Beller M. (Enantio)selective Hydrogen Autotransfer: Ruthenium-Catalyzed Synthesis of Oxazolidin-2-ones from Urea and Diols. Angew. Chem., Int. Ed. 2016, 55, 7826–7830. 10.1002/anie.201600698. [DOI] [PubMed] [Google Scholar]
- Yang P.; Zhang C.; Ma Y.; Zhang C.; Li A.; Tang B.; Zhou J. S. Nickel-Catalyzed N-Alkylation of Acylhydrazines and Arylamines Using Alcohols and Enantioselective Examples. Angew. Chem., Int. Ed. 2017, 56, 14702–14706. 10.1002/anie.201708949. [DOI] [PubMed] [Google Scholar]
- Lim C. S.; Quach T. T.; Zhao Y. Enantioselective Synthesis of Tetrahydroquinolines by Borrowing Hydrogen Methodology: Cooperative Catalysis by an Achiral Iridacycle and a Chiral Phosphoric Acid. Angew. Chem., Int. Ed. 2017, 56, 7176–7180. 10.1002/anie.201703704. [DOI] [PubMed] [Google Scholar]
- Xu G.; Yang G.; Wang Y.; Shao P. L.; Yau J. N. N.; Liu B.; Zhao Y.; Sun Y.; Xie X.; Wang S.; et al. Stereoconvergent, Redox-Neutral Access to Tetrahydroquinoxalines through Relay Epoxide Opening/Amination of Alcohols. Angew. Chem., Int. Ed. 2019, 58, 14082–14088. 10.1002/anie.201906199. [DOI] [PubMed] [Google Scholar]
- Putra A. E.; Oe Y.; Ohta T. Ruthenium-Catalyzed Enantioselective Synthesis of β-Amino Alcohols from 1,2-Diols by “Borrowing Hydrogen. Eur. J. Org. Chem. 2013, 2013, 6146–6151. 10.1002/ejoc.201300692. [DOI] [Google Scholar]
- Pan H.-J.; Lin Y.; Gao T.; Lau K. K.; Feng W.; Yang B.; Zhao Y. Catalytic Diastereo- and Enantioconvergent Synthesis of Vicinal Diamines from Diols through Borrowing Hydrogen. Angew. Chem., Int. Ed. 2021, 60, 18599–18604. 10.1002/anie.202101517. [DOI] [PubMed] [Google Scholar]
- Yang G.; Pan J.; Ke Y.-M.; Liu Y.; Zhao Y. Tandem Catalytic Indolization/Enantioconvergent Substitution of Alcohols by Borrowing Hydrogen to Access Tricyclic Indoles. Angew. Chem., Int. Ed. 2021, 60, 20689–20694. 10.1002/anie.202106514. [DOI] [PubMed] [Google Scholar]
- Armstrong R. J.; Akhtar W. M.; Young T. A.; Duarte F.; Donohoe T. J. Catalytic Asymmetric Synthesis of Cyclohexanes by Hydrogen Borrowing Annulations. Angew. Chem., Int. Ed. 2019, 58, 12558–12562. 10.1002/anie.201907514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar W. M.; Armstrong R. J.; Frost J. R.; Stevenson N. G.; Donohoe T. J. Stereoselective Synthesis of Cyclohexanes via an Iridium Catalyzed (5 + 1) Annulation Strategy. J. Am. Chem. Soc. 2018, 140, 11916–11920. 10.1021/jacs.8b07776. [DOI] [PubMed] [Google Scholar]
- Guerbet M. Action de l’Alcool Amylique de Fermentation Sur Son Derive Sode. C. R. Acad. Sci. Paris 1899, 128, 1002–1004. [Google Scholar]
- Guerbet M. C. R. Condensation de l’Alcool Isopropylique Avec Son Derive Sode; Formation Du Methylisobutylcarbinol et Du Dimethyl-2,4-Heptanol-6. C. R. Acad. Sci. Paris 1909, 149, 129–132. [Google Scholar]
- Ng T. W.; Liao G.; Lau K. K.; Pan H.-J.; Zhao Y. Room Temperature Guerbet Reaction with Unprecedent Catalytic Efficiency and Enantioselectivity. Angew. Chem., Int. Ed. 2020, 59, 11384–11389. 10.1002/anie.202004758. [DOI] [PubMed] [Google Scholar]
- Wang K.; Zhang L.; Tang W.; Sun H.; Xue D.; Lei M.; Xiao J.; Wang C. Asymmetric Guerbet Reaction to Access Chiral Alcohols. Angew. Chem., Int. Ed. 2020, 59, 11408–11415. 10.1002/anie.202003104. [DOI] [PubMed] [Google Scholar]
- Kim I. S.; Han S. B.; Krische M. J. anti-Diastereo- and Enantioselective Carbonyl Crotylation from the Alcohol or Aldehyde Oxidation Level Employing a Cyclometallated Iridium Catalyst: α-Methyl Allyl Acetate as a Surrogate to Preformed Crotylmetal Reagents. J. Am. Chem. Soc. 2009, 131, 2514–2520. 10.1021/ja808857w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Townsend I. A.; Krische M. J. Enhanced anti-Diastereo- and Enantioselectivity in Alcohol-Mediated Carbonyl Crotylation Using an Isolable Single Component Iridium Catalyst. J. Org. Chem. 2011, 76, 2350–2354. 10.1021/jo200068q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza V. J.; Krische M. J. Hydroxymethylation beyond Carbonylation: Enantioselective Iridium-Catalyzed Reductive Coupling of Formaldehyde with Allylic Acetates via Enantiotopic π-Facial Discrimination. J. Am. Chem. Soc. 2016, 138, 3655–3658. 10.1021/jacs.6b01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R.; Hong S.; Krische M. J. Diastereo- and Enantioselective Iridium Catalyzed Carbonyl (α-Cyclopropyl)allylation via Transfer Hydrogenation. Chem.—Eur. J. 2015, 21, 12903–12907. 10.1002/chem.201502499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.; Garza V. J.; Krische M. J. Redox-Triggered C-C Coupling of Alcohols and Vinyl Epoxides: Diastereo- and Enantioselective Formation of All-Carbon Quaternary Centers via tert-(Hydroxy)-Prenylation. J. Am. Chem. Soc. 2014, 136, 8911–8914. 10.1021/ja504625m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-A.; Lee W.; Krische M. J. Enantioselective Synthesis of Oxetanes Bearing All-Carbon Quaternary Centers via Iridium-Catalyzed C-C Bond-Forming Transfer Hydrogenation. Chem.—Eur. J. 2017, 23, 2557–2559. 10.1002/chem.201606046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Franke J.; Ngo C. Q.; Krische M. J. Diastereo- and Enantioselective Iridium Catalyzed Coupling of Vinyl Aziridines with Alcohols: Site-Selective Modification of Unprotected Diols and Synthesis of Substituted Piperidines. J. Am. Chem. Soc. 2015, 137, 7915–7920. 10.1021/jacs.5b04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z.; Wan X.; Zang Z.; Qian Q.; Deng W.; Gong H. Ni-Catalyzed Asymmetric Reductive Allylation of Aldehydes with Allylic Carbonates. Chem. Commun. 2014, 50, 3827–3829. 10.1039/C3CC49859J. [DOI] [PubMed] [Google Scholar]
- Wang L.; Wang L.; Li M.; Chong Q.; Meng F. Cobalt-Catalyzed Diastereo- and Enantioselective Reductive Allyl Additions to Aldehydes with Allylic Alcohol Derivatives via Allyl Radical Intermediates. J. Am. Chem. Soc. 2021, 143, 12755–12765. 10.1021/jacs.1c05690. [DOI] [PubMed] [Google Scholar]
- Nakajima K.; Shibata M.; Nishibayashi Y. Copper-Catalyzed Enantioselective Propargylic Etherification of Propargyl Esters with Alcohols. J. Am. Chem. Soc. 2015, 137, 2472–2475. 10.1021/jacs.5b00004. [DOI] [PubMed] [Google Scholar]
- Li R.-F.; Tang H.; Yang K. R.; Wan L.-Q.; Zhang X.; Liu J.; Fu Z.; Niu D. Enantioselective Propargylation of Polyols and Desymmetrization of meso 1,2-Diols by Copper/Borinic Acid Dual Catalysis. Angew. Chem., Int. Ed. 2017, 56, 7213–7217. 10.1002/anie.201703029. [DOI] [PubMed] [Google Scholar]
- Tsuchida K.; Yuki M.; Nakajima K.; Nishibayashi Y. Copper- and Borinic Acid-Catalyzed Propargylic Etherification of Propargylic Carbonates with Benzyl Alcohols. Chem. Lett. 2018, 47, 671–673. 10.1246/cl.180123. [DOI] [Google Scholar]
- Taylor M. S. Catalysis Based on Reversible Covalent Interactions of Organoboron Compounds. Acc. Chem. Soc. 2015, 48, 295–305. 10.1021/ar500371z. [DOI] [PubMed] [Google Scholar]
- Li R.-Z.; Liu D.-Q.; Niu D. Asymmetric O-Propargylation of Secondary Aliphatic Alcohols. Nat. Catal. 2020, 3, 672–680. 10.1038/s41929-020-0462-9. [DOI] [Google Scholar]
- Xu X.; Peng L.; Chang X.; Guo C. Ni/Chiral Sodium Carboxylate Dual Catalyzed Asymmetric O-Propargylation. J. Am. Chem. Soc. 2021, 143, 21048–21055. 10.1021/jacs.1c11044. [DOI] [PubMed] [Google Scholar]
- Asakawa Y. Chemical Constituents of alnus firma (BETULACEAE) I. Phenyl Propane Derivatives Isolated from Alnus Firma. Bull. Chem. Soc. Jpn. 1970, 43, 2223–2229. 10.1246/bcsj.43.2223. [DOI] [Google Scholar]
- Kim Y. U.; Son H. K.; Song H. K.; Ahn M. J.; Lee S. S.; Lee S. K. Inhibitors of 5α-Reductase Activity by Diarylheptanoids from Alpinia Officinarum. Planta Med. 2003, 69, 72–74. 10.1055/s-2003-37028. [DOI] [PubMed] [Google Scholar]
- Brandâo P.; Burke A. J. Recent Advances in the Asymmetric Catalytic Synthesis of Chiral 3-Hydroxy and 3-Aminooxindoles and Derivatives: Medicinally Relevant Compounds. Tetrahedron 2018, 74, 4927–4957. 10.1016/j.tet.2018.06.015. [DOI] [Google Scholar]
- Kaur J.; Chimni S. S. Catalytic Synthesis of 3-Aminooxindoles via Addition to Isatin Imine: An Update. Org. Biomol. Chem. 2018, 16, 3328–3347. 10.1039/C7OB03002A. [DOI] [PubMed] [Google Scholar]
- Kaur J.; Kaur B. P.; Chimni S. S. Recent Advances in the Catalytic Synthesis of 3-Amonooxindoles: An Update. Org. Biomol. Chem. 2020, 18, 4692–4708. 10.1039/D0OB00777C. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Kang H.; Hong L.; Dong W.; Li G.; Zheng X.; Wang R. Construction of the N1-C3 Linkage Stereogenic Center by Catalytic Asymmetric Amination Reaction of 3-Bromooxindoles with Indolines. Org. Lett. 2014, 16, 2394–2397. 10.1021/ol5007423. [DOI] [PubMed] [Google Scholar]
- Takahashi N.; Ito T.; Matsuda Y.; Kogure N.; Kitajima M.; Takayama H. Determination of Absolute Configuration of Trimeric Indole Alkaloid, Psychotrimine, by First Asymmetric Total Synthesis. Chem. Commun. 2010, 46, 2501–2503. 10.1039/b923918a. [DOI] [PubMed] [Google Scholar]
- Schallenberger M. A.; Newhouse T.; Baran P. S.; Romesberg F. E. The Psychotrimine Natural Products have Antibacterial Activity against Gram-Positive Bacteria and Act via Membrane Disruption. J. Antibiot. 2010, 63, 685–687. 10.1038/ja.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.; Li N.-K.; Zhang Y.-F.; Pan F.-F.; Wang X.-W. Construction of 3-amino-2-oxindoles by Direct Amination of Aniline or α-Amino-Acid Derivatives to 3-Bromooxindoles. Tetrahedron 2016, 72, 1406–1414. 10.1016/j.tet.2016.01.039. [DOI] [Google Scholar]
- Yang W.; Dong P.; Xu J.; Yang J.; Liu X.; Feng X. Enantioselective Synthesis of 3-Substituted 3-Amino-2-oxindoles via Amination with Anilines. Chem.—Eur. J. 2021, 27, 9272–9275. 10.1002/chem.202100829. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Lin L.; Dai L.; Tang Q.; Liu X.; Feng X. Nickel-Catalyzed Conjugate Addition of Silyl Ketene Imines to In Situ Generated Indol-2-ones: Highly Enantioselective Construction of Vicinal All-Carbon Quaternary Stereocenters. Angew. Chem., Int. Ed. 2017, 56, 13107–13111. 10.1002/anie.201705943. [DOI] [PubMed] [Google Scholar]
- Wang G.; Liu X.; Chen Y.; Yang J.; Li J.; Lin L.; Feng X. Diastereoselective and Enantioselective Alleno-Aldol Reaction of Allenoates with Isatins to Synthesis of Carbinol Allenoates Catalyzed by Gold. ACS Catal. 2016, 6, 2482–2486. 10.1021/acscatal.6b00294. [DOI] [Google Scholar]
- Zhang F.-H.; Guo X.; Zeng X.; Wang Z. Catalytic Enantioconvergent Allenylation of Aldehydes with Propargyl Halides. Angew. Chem., Int. Ed. 2022, 61, e202117114 10.1002/anie.202117114. [DOI] [PubMed] [Google Scholar]
- Ding W.; Lu L.-Q.; Zhou Q.-Q.; Wei Y.; Chen J.-R.; Xiao W.-J. Bifunctional Photocatalysis for Enantioselective Aerobic Oxidation of β-Ketoesters. J. Am. Chem. Soc. 2017, 139, 63–66. 10.1021/jacs.6b11418. [DOI] [PubMed] [Google Scholar]
- Yang F.; Zhao J.; Tang X.; Wu Y.; Yu Z.; Meng Q. Visible Light-Induced Salan-Copper(II)-Catalyzed Enantioselective Aerobic α-Hydroxylation of β-Keto Esters. Adv. Synth. Catal. 2019, 361, 1673–1677. 10.1002/adsc.201801263. [DOI] [Google Scholar]