Abstract
The ampD and ampE genes of Pseudomonas aeruginosa PAO1 were cloned and characterized. These genes are transcribed in the same orientation and form an operon. The deduced polypeptide of P. aeruginosa ampD exhibited more than 60% similarity to the AmpD proteins of enterobacteria and Haemophilus influenzae. The ampD product transcomplemented Escherichia coli ampD mutants to wild-type β-lactamase expression.
Most Pseudomonas aeruginosa strains, like nearly all members of the family Enterobacteriaceae, express an inducible chromosomally encoded AmpC β-lactamase (cephalosporinase) (27), which is placed in class C of Ambler’s classification (1) and which is in Bush’s group 1 (3). In enterobacteria, the regulation of AmpC β-lactamase expression is intimately linked to cell wall recycling and involves three genes; ampR, which encodes a transcriptional regulator of the LysR family; ampG, which encodes a transmembrane permease; and ampD, which encodes a cytosolic N-acetyl-anhydromuramyl-l-alanine amidase hydrolyzing 1,6-anhydromuropeptides (9, 12, 15, 25). In the absence of a β-lactam inducer, AmpR is repressed by the murein precursor UDP-MurNAc-pentapeptide (uridine- pyrophosphoryl-N-acetylmuramyl-l-alanyl-d-glutamyl-meso-diaminopimelic acid-d-alanyl-d-alanine) (13). Since β-lactams interfere with murein synthesis, their actions lead to an increased periplasmic accumulation of degradation products such as 1,6-anhydromuropeptides, which are the signal molecules for β-lactamase induction (5, 11). ampG transports these products from periplasm to cytoplasm, where they are cleaved by AmpD, which acts as a negative regulator of AmpC β-lactamase expression (5, 11, 15, 25). In ampD mutants, the constitutive overproduction of AmpC β-lactamase is accompanied by an ac- cumulation of aM-tripeptide (monosaccharide-tripeptide, 1,6-anhydro-N-acetylmuramyl-l-alanyl-d-glutamyl-meso-di-aminopimelic acid) and aM-pentapeptide (monosaccharide-pentapeptide, 1,6-anhydro-N-acetylmuramyl-l-alanyl-d-glu-tamyl-meso-diaminopimelic acid-d-alanyl-d-alanine) in the cytoplasm (5, 11). Jacobs et al. (13) suggested that the aM-tripeptide could be the AmpR-activating ligand, since this product can relieve in vitro the repressed state of AmpR, resulting in the activation of β-lactamase expression. However, potential interactions of the aM-pentapeptide with AmpR have not been investigated. Another gene, ampE, which encodes a transmembrane protein, forms an operon with ampD, but this gene is not involved in β-lactamase expression (10, 19, 24).
In P. aeruginosa, the inducible expression of AmpC β-lactamase is also under the control of the transcriptional regulator AmpR (21). To further elucidate the induction process, the ampD and ampE genes of this organism were cloned and characterized, and complementation analysis was performed with Escherichia coli ampD mutants with the cloned P. aeruginosa ampD and ampE genes. A part of this work was presented before (16).
The strains and plasmids used in this study are described in Table 1. E. coli DH5α was used as the host for construction and propagation of recombinant plasmids. Bacterial cells were grown in tryptic soy broth or tryptic soy agar (Difco Laboratories, Detroit, Mich.). When required, 50 μg of kanamycin/ml, 30 μg of chloramphenicol/ml, and various concentrations of cefotaxime, ampicillin, and cefoxitin were added (Sigma-Aldrich Canada, Oakville, Ontario, Canada).
TABLE 1.
Characteristics of the bacterial strains and plasmids used in this study
| E. coli strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | deoR supE44 Δ(lacZYA-argFV169) (φ80 dlacZΔM15) F−λ−hsdR17 (rk−mk+) recA1 endA1 gyrA96 thi-1 relA1 | 26 |
| JRG582 | Δ(ampDE)2 | 18 |
| STC172 | ampD11E+ | 10 |
| Plasmids | ||
| pEC1C | Cmr; ampC ampR from E. cloacae | 23 |
| pNH5 | Kmr; ampD from E. coli | 10 |
| pBGS19+ | Kmr f1 Ori lacPOZ | 28 |
| pHUL4DE-PH | Kmr; ampDE region of P. aeruginosa PAO1 cloned into pBK-CMV | This study |
| pHUL4DE-1 | Kmr; ampDE + ORF-1 from P. aeruginosa PAO1 cloned into pBGS19+ | This study |
| pHUL4DE-2 | Kmr; ampDE from P. aeruginosa PAO1 cloned into pBGS19+ | This study |
| pHUL4D | Kmr; ampD from P. aeruginosa PAO1 cloned into pBGS19+ | This study |
Cm, chloramphenicol; Km, kanamycin.
Recombinant DNA techniques were performed essentially by standard procedures (26). To clone the ampD and ampE genes of P. aeruginosa PAO1, two degenerated oligonucleotide primers adapted to the P. aeruginosa codon usage and derived from two conserved regions of the E. coli, Citrobacter freundii, and Enterobacter cloacae AmpD amino acid sequences (10, 14, 19) were synthesized on a 394 DNA/RNA synthesizer (PE Applied Biosystems, Foster City, Calif.). The sequences of the primers were as follows: AmpD1, 5′-CGCTGCCSCCSGGCGARTTCG-3′; and AmpD2, 5′-CGGGGCCSGGGTCGGTCTTGC-3′. A 400-bp DNA fragment was amplified by PCR with the Taq DNA polymerase (Promega, Madison, Wis.) and a P. aeruginosa PAO1 lysate, the latter of which was prepared by the freezing-and-boiling method (30). This DNA fragment was used as a probe to screen a λ Zap Express genomic library of P. aeruginosa PAO1. Phage screening and in vivo plasmid excision were performed according to the instructions of the manufacturer (Stratagene, La Jolla, Calif.). A single phage containing a 6.7-kb genomic insert was selected, and plasmid pBK-CMV was excised out of the phage and named pHUL4DE-PH. The ampD gene was located on a 2.6-kb EcoRI fragment of pHULDE-PH, which was cloned into the EcoRI site of pBGS19+ vector to generate plasmid pHUL4DE-1. Both strands of this DNA fragment were sequenced with the Deaza sequencing kit (Pharmacia Biotech, Baie d’Urfé, Québec, Canada) on a Pharmacia LKB Macrophor or the ABI Prism dye terminator cycle sequencing kit with AmpliTaq DNA polymerase, FS (PE Applied Biosystems), on a 373 DNA sequencer (PE Applied Biosystems). Sequence analysis, alignment, the homology study, G + C content calculation, and molecular mass prediction were done with the Genetics Computer Group software package version 9.0 (Madison, Wis.). The PSORT software was used to predict protein localization sites (22).
Sequence analysis of this DNA fragment revealed three complete open reading frames (ORFs) (Fig. 1). The first 567-bp ORF is located 657 nucleotides downstream of the EcoRI site and encodes a 188-amino-acid polypeptide (AmpD) with a predicted molecular mass of 21 kDa. A potential Shine-Dalgarno (SD) sequence (AGGAG) (8) and the consensus −10 (TAGAGT) and −35 (TCGTCA) regions of bacterial promoters (20) were identified 5, 23, and 45 nucleotides upstream of the ampD ATG start codon, respectively. Following ampD, a second ORF (AmpE) of 837 nucleotides, which consists of 278 amino acids with a predicted molecular mass of 31 kDa, was found. The ampE ATG start codon overlaps the ampD TGA termination codon and is preceded by a potential SD sequence (AGGAG) located 5 nucleotides upstream. This strongly suggests that these two genes form an operon, as described for E. coli (10, 19). The G + C contents of ampD and ampE were calculated to be 64 and 68%, respectively, which are typical for P. aeruginosa (32). Finally, 92 nucleotides downstream of the ampE TGA stop codon, a 282-bp ORF (ORF-1), which comprises 93 amino acids with a predicted molecular mass of 10 kDa (Fig. 1), was identified. This ORF is transcribed in the same orientation as ampDE and is preceded by a potential SD sequence (AGGTG) as well as the −10 (CGATAT) and −35 (TTAACC) promoter-like sequences at 11, 25, and 52 nucleotides upstream of its ATG start codon, respectively. The product of ORF-1, like AmpD, possesses the features of a cytoplasmic protein (22). Three other potential ORFs (ORF-a, ORF-b, and ORF-c) from different reading frames, which are incomplete at the 5′ end, were also identified on each side of ampDE (Fig. 1). However, these ORFs, like ORF-1, showed no significant homology to any sequence in the GenBank database.
FIG. 1.
Nucleotide sequence of the P. aeruginosa ampD and ampE genes with ORF-1 and predicted amino acids. The amino acids are presented according to the one-letter code. The putative SD sequences and the potential −10 and −35 regions of promoters are underlined. The stop codons are shown by asterisks. The five transmembrane domains of AmpE are boxed and named I, II, III, IV, and V. The stop codons for the potential ORF-a, ORF-b, and ORF-c are boxed. The oligonucleotide primers used for PCR amplification are shown by dashed arrows.
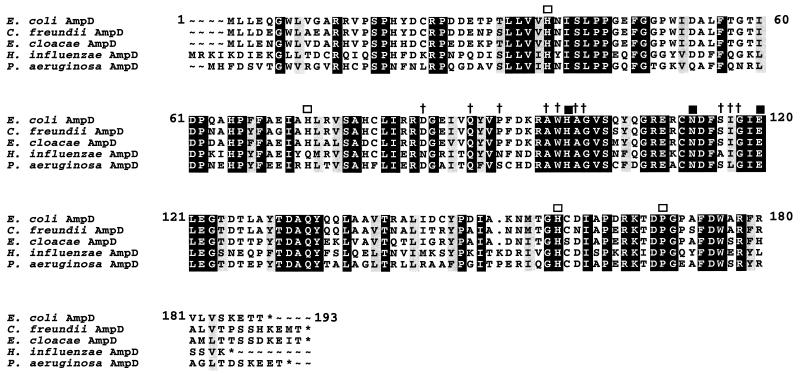
The predicted P. aeruginosa AmpD protein exhibited 65, 63, 62, and 62% similarity to the E. coli, C. freundii, E. cloacae, and putative Haemophilus influenzae AmpD proteins, respectively (7, 10, 14, 19). Amino acid sequence alignment of these AmpD proteins revealed many conserved motifs (Fig. 2). The conserved core region as well as the four strictly identical residues outside of this region, which relate the AmpD proteins of enterobacteria to the cell wall hydrolases of Bacillus spp. (12), were found in the P. aeruginosa AmpD protein. The deduced P. aeruginosa AmpE protein possesses the features of a cytoplasmic membrane protein (22) with five transmembrane-spanning domains (Fig. 1). It showed a low degree of similarity (33%) to its homolog in E. coli (10, 19) and seems to be less conserved than AmpD. Indeed, this difference with regard to AmpE is underlined by the fact that despite the identification of a putative ampD sequence in the genome sequence database of H. influenzae, no ampE sequence could be identified (7).
FIG. 2.
Alignment of the amino acid sequence of P. aeruginosa PAO1 AmpD with those of the E. coli, C. freundii, E. cloacae, and putative H. influenzae AmpD proteins. The similar and identical amino acids are lightly and darkly shaded, respectively. The crosses and the open squares indicate the amino acids conserved in the core and outside region of the Bacillus cell wall hydrolases, respectively. The solid squares show the amino acids strictly conserved in various cell wall hydrolases (12).
To determine the role of the P. aeruginosa ampD and ampE gene products, complementation analyses were performed with E. coli strains JRG582 [Δ(ampDE)2] and STC172 (ampD11E+) (10, 18) with the cloned P. aeruginosa ampD and ampE genes. The MICs of β-lactam antibiotics for transformants were determined by the broth microdilution method as described previously (31). Induction assays and β-lactamase activity measurements were performed as described previously (31). Two plasmids were constructed to do a complementation study. Plasmid pHUL4DE-2, which contains the ampDE operon and the 105- and 209-nucleotide regions located upstream and downstream of this operon, respectively, was constructed as follows: a 1,714-bp DNA fragment was amplified by PCR with the Pfu DNA polymerase (Stratagene) and the oligonucleotide primers AmpD-14 (5′-GGGAATTCCTTTCCTCGAAGCATGTCG-3′) and AmpD-15 (5′-GGGATAGAGTACGGTCTTC-3′) (Fig. 1). This DNA fragment was cloned into the SmaI site of pBGS19+ vector. Plasmid pHUL4D, which contains the complete ampD gene and the first 317 nucleotides encoding AmpE, was constructed by cloning a 984-bp DNA amplification product into the SmaI site of pBGS19+ vector. This fragment was amplified as described above with the oligonucleotide primers AmpD-14 and AmpE-4 (5′-CGCCGCCAGGCGTCGCG-3′) (Fig. 1). The sequences of all cloned PCR DNA fragments were confirmed by complete resequencing.
Since E. coli strains do not contain ampR (24), the E. coli strains STC172 and JRG582 were first transformed with plasmid pEC1C carrying ampC and ampR of E. cloacae (23). The data in Table 2 show that STC172/pEC1C exhibits a high basal β-lactamase activity and is hyperinducible, while JRG582/pEC1C has a fully derepressed phenotype, as shown previously (10). Both of these constructs were highly resistant to ampicillin and cefotaxime. The ampD genes of both E. coli and P. aeruginosa, as expressed from pNH5 and pHUL4D, respectively, transcomplemented the E. coli ampD and ampDE mutants to low-level β-lactam resistance and wild-type β-lactamase expression (low basal level and inducibility) (Table 2). This shows that the cloned P. aeruginosa ampD gene expresses a functional AmpD protein in E. coli cells. These data, as well as the high homology observed among the AmpD proteins, strongly suggest that P. aeruginosa AmpD acts as an N-acetyl-anhydromuramyl-l-alanine amidase, which leads to a decreased amount of anhydromuropeptide, the signal molecule for β-lactamase expression (5, 9, 11, 12). The induced/noninduced ratio of β-lactamase activity was more than 7.5 times lower in cells producing the E. coli AmpD than that in cells containing P. aeruginosa AmpD, and this could be explained by the presence of a very strong promoter behind E. coli ampD.
TABLE 2.
MICs of β-lactam antibiotics and specific activities of E. cloacae AmpC in E. coli STC172 and JRG582 containing the E. coli ampD and P. aeruginosa ampD and ampE genes
| Strain | Relevant genotype | MIC (μg/ml)
|
β-Lactamase activitya
|
Fold increase in activity vs noninduced | ||
|---|---|---|---|---|---|---|
| Ampicillin | Cefotaxime | Noninduced | Inducedb | |||
| STC172/pEC1C/pBGS19+ | ampC+ ampR+ ampD11 ampE+ | 1,024 | 32 | 3,260 ± 190 | 23,490 ± 1,240 | 7.2 |
| STC172/pEC1C/pNH5 | ampC+ ampR+ ampD+ ampE+ | 8 | 0.5 | 59 ± 3 | 86 ± 5 | 1.5 |
| STC172/pEC1C/pHUL4D | ampC+ ampR+ ampD+ ampE+ | 128 | 2 | 116 ± 5 | 2,420 ± 130 | 21 |
| STC172/pEC1C/pHUL4DE-2 | ampC+ ampR+ ampD+ ampE+ | 128 | 1 | 113 ± 5 | 2,930 ± 199 | 26 |
| STC172/pEC1C/pHUL4DE-1 | ampC+ ampR+ ampD+ ampE+ ORF-1 | 64 | 4 | 175 ± 7 | 1,260 ± 60 | 7.2 |
| JRG582/pEC1C/pBGS19+ | ampC+ ampR+Δ(ampDE)2 | 1,024 | 64 | 103,280 ± 6,230 | 109,400 ± 7,110 | 1.1 |
| JRG582/pEC1C/pNH5 | ampC+ ampR+ ampD+ | 8 | <0.5 | 216 ± 14 | 481 ± 29 | 2.2 |
| JRG582/pEC1C/pHUL4D | ampC+ ampR+ ampD+ | 128 | 8 | 226 ± 16 | 8,670 ± 510 | 38 |
| JRG582/pEC1C/pHUL4DE-2 | ampC+ ampR+ ampD+ ampE+ | 128 | 8 | 414 ± 39 | 7,050 ± 400 | 17 |
| JRG582/pEC1C/pHUL4DE-1 | ampC+ ampR+ ampD+ ampE+ ORF-1 | 256 | 2 | 241 ± 19 | 3,970 ± 300 | 17 |
All of the induction experiments were performed in duplicate, and the results presented are averages of two determinations. β-Lactamase activity is expressed as nanomoles of nitrocefin hydrolyzed per minute per milligram of protein.
Induction was carried out with 4 μg of cefoxitin per ml.
The MICs, as well as the basal and induced levels, of β-lactamase were almost the same for cells containing AmpD as those for cells containing AmpD and AmpE. This strongly suggests that similar to the E. coli protein, P. aeruginosa AmpE has no effect on β-lactamase expression (24). This protein, like its homolog in E. coli (10, 19), has the features of an integral inner membrane protein, but its role in the bacterial cell is still unknown.
Expression of ORF-1 together with ampDE from plasmid pHULDE-1 reduced by more than 1.7-fold the induced level of β-lactamase in both E. coli ampD and ampDE mutants, compared to that in strains producing AmpD and AmpE. This suggests that the product of this ORF, which has the features of a cytoplasmic protein, may affect β-lactamase expression in the presence of a β-lactam inducer, perhaps by interacting directly with AmpD or by acting as a regulator of ampD expression, or perhaps by a new unknown mechanism. Further experiments are needed to explore the role of this ORF.
The high degree of homology among the various AmpD proteins shows that AmpD of P. aeruginosa is in its evolution very close to its homologs in enterobacteria, and they probably share a common mechanism of regulation of AmpC β-lactamase expression and murein metabolism. In our approach to characterizing the mechanism of regulation of AmpC β-lactamase production in P. aeruginosa, a putative ampG gene was also cloned and characterized (17). This further strengthens the close relationships between the P. aeruginosa and enterobacterial AmpC β-lactamase induction mechanism and cell wall recycling.
In enterobacteria, three out of four phenotypes of altered β-lactamase expression have been associated with mutations in ampD (2, 6, 10, 14, 19, 29). In clinical and laboratory isolates of P. aeruginosa, three phenotypes of altered β-lactamase expression have also been described (4, 27), and a study is in progress to characterize the ampD gene in some of these isolates.
Nucleotide sequence accession number.
The nucleotide sequence data for the ampD and ampE genes appear in the GenBank database under accession no. AF082575.
Acknowledgments
We thank S. T. Cole, Institut Pasteur, Paris, France, for E. coli JRG582 and STC172 and plasmids pEC1C and pNH5; G. Vézina, Université Laval, for the λ ZAP Express genomic library of P. aeruginosa PAO1; L. Gagnon, Université Laval, for assistance with computer analysis; and M. Goldner, Université Laval, for critically reading the manuscript.
This work was supported by the Canadian Cystic Fibrosis Foundation and, in part, by Canada’s Networks of Centres of Excellence (CBDN).
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc London Ser B. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Bennett P M, Chopra I. Molecular basis of β-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell J I A, Ciofu O, Høiby N. Pseudomonas aeruginosa isolates from patients with cystic fibrosis have different β-lactamase expression phenotypes but are homogeneous in the ampC-ampR genetic region. Antimicrob Agents Chemother. 1997;41:1380–1384. doi: 10.1128/aac.41.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz H, Pfeifle D, Wiedemann B. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob Agents Chemother. 1997;41:2113–2120. doi: 10.1128/aac.41.10.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrhardt A F, Sanders C C, Romero J R, Leser J S. Sequencing and analysis of four new Enterobacter ampD alleles. Antimicrob Agents Chemother. 1996;40:1953–1956. doi: 10.1128/aac.40.8.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 8.Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- 9.Höltje J-V, Kopp U, Ursinus A, Wiedemann B. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-l-alanine amidase. FEMS Microbiol Lett. 1994;122:159–164. doi: 10.1111/j.1574-6968.1994.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 10.Honoré N, Nicolas M H, Cole S T. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol Microbiol. 1989;3:1121–1130. doi: 10.1111/j.1365-2958.1989.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs C, Huang L J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs C, Joris B, Jamin M, Klarsov K, Van Beeumen J, Mengin-Lecreulx D, van Heijenoort J, Park J T, Normark S, Frère J-M. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs C, Frère J-M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 14.Kopp U, Wiedemann B, Lindquist S, Normark S. Sequences of wild-type and mutant ampD genes of Citrobacter freundii and Enterobacter cloacae. Antimicrob Agents Chemother. 1993;37:224–228. doi: 10.1128/aac.37.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korfmann G, Sanders C C. ampG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob Agents Chemother. 1989;33:1946–1951. doi: 10.1128/aac.33.11.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langaee T Y, Huletsky A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Characterization of the ampD gene encoding a regulator of cephalosporinase expression in Pseudomonas aeruginosa PAO1, abstr. A-37; p. 139. [Google Scholar]
- 17.Langaee T Y, Huletsky A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Identification of the ampG gene encoding a signal transducer for induction of the chromosomal AmpC β-lactamase in Pseudomonas aeruginosa PAO1, abstr. A-3; p. 1. [Google Scholar]
- 18.Langley D, Guest J R. Biochemical genetics of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and biochemical properties of deletion mutants. J Gen Microbiol. 1977;99:263–276. doi: 10.1099/00221287-99-2-263. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist S, Galleni M, Lindberg F, Normark S. Signalling proteins in enterobacterial AmpC β-lactamase regulation. Mol Microbiol. 1989;3:1091–1102. doi: 10.1111/j.1365-2958.1989.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 20.Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodge J, Busby S, Piddock L. Investigation of the Pseudomonas aeruginosa ampR gene and its role at the chromosomal ampC promoter. FEMS Microbiol Lett. 1993;111:315–320. doi: 10.1111/j.1574-6968.1993.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakai K, Kanehisha M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 23.Nicolas M H, Honore N, Jarlier V, Philippon A, Cole S T. Molecular genetic analysis of cephalosporinase production and its role in β-lactam resistance in clinical isolates of Enterobacter cloacae. Antimicrob Agents Chemother. 1987;31:295–299. doi: 10.1128/aac.31.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Normark S, Bartowsky E, Erickson J, Jacobs C, Lindberg F, Lindquist S, Weston-Hafer K, Wikström M. Mechanisms of chromosomal β-lactamase induction in Gram-negative bacteria. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 485–503. [Google Scholar]
- 25.Normark S. β-Lactamase induction in Gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb Drug Resist. 1995;1:111–114. doi: 10.1089/mdr.1995.1.111. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.21–1.101. [Google Scholar]
- 27.Sanders C C, Sanders W E., Jr β-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis. 1992;15:825–839. doi: 10.1093/clind/15.5.824. [DOI] [PubMed] [Google Scholar]
- 28.Spratt B G, Hedge P J, Teheesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton P, Shannon K, Phillips I. DNA sequence differences of ampD mutants of Citrobacter freundii. Antimicrob Agents Chemother. 1995;39:2494–2498. doi: 10.1128/aac.39.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starnbach M N, Falkow S, Tompkins L S. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J Clin Microbiol. 1989;27:1257–1261. doi: 10.1128/jcm.27.6.1257-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trépanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob Agents Chemother. 1997;41:2399–2405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West S E H, Iglewski B H. Codon usage in Pseudomonas aeruginosa. Nucleic Acids Res. 1988;6:9323–9335. doi: 10.1093/nar/16.19.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]