Abstract

Acetate derived from electrocatalytic CO2 reduction represents a potential low-carbon synthesis approach. However, the CO2-to-acetate activity and selectivity are largely inhibited by the low surface coverage of in situ generated *CO, as well as the inefficient ethenone intermediate formation due to the side reaction between CO2 and alkaline electrolytes. Tuning catalyst microenvironments by chemical modification of the catalyst surface is a potential strategy to enhance CO2 capture and increase local *CO concentrations, while it also increases the selectivity of side reduction products, such as methane or ethylene. To solve this challenge, herein, we developed a hydrophilic amine-tailed, dendrimer network with enhanced *CO intermediate coverage on Cu catalytic sites while at the same time retaining the in situ generated OH– as a high local pH environment that favors the ethenone intermediate toward acetate. The optimized amine-network coordinated Cu catalyst (G3-NH2/Cu) exhibits one of the highest CO2-to-acetate Faradaic efficiencies of 47.0% with a partial current density of 202 mA cm–2 at −0.97 V versus the reversible hydrogen electrode.
Short abstract
A highly dendritic network with amine terminals was functionalized on Cu nanoparticles as an efficient electrocatalytic CO2 reduction catalyst, which was beneficial for enhancing the *CO intermediate coverage and retaining the in situ generated OH− on the Cu catalytic sites, thus promoting efficient electrocatalytic CO2-to-acetate conversion.
Introduction
Acetate is an important chemical widely used in manufacturing, medicine, and food. The commercial acetate production is mainly based on the thermal carbonylation of methanol and carbon monoxide (CO), which results in a substantial carbon footprint.1,2 The recent development of CO electroreduction has featured a potential alternative means of producing acetate, with a reported acetate Faradaic efficiency (FE) of >70% and partial current density (|jacetate|) over 425 mA cm–2.3 On the other hand, although the direct carbon dioxide reduction reaction (CO2RR) using renewable electricity has the potential of both reducing greenhouse emission and generating value-added chemicals, the selective CO2-to-acetate (or acetic acid) conversion has received only limited progress.
Previous studies in CO electroreduction have suggested that the critical factors of producing acetate include a high coverage of *CO adsorbates on the catalyst surface as well as the formation of ethenone intermediate (*H2CCO) under high alkalinity.3 Nonetheless, in CO2RR, the *CO intermediate is in situ generated from a CO2 source, and thus its coverage is generally lower than the direct use of CO reactant.4 In addition, the side reaction between CO2 and the high alkaline electrolyte also results in the fast depletion of CO2 molecules and generation of (bi)carbonate that can gradually deactivate the catalyst sites,5 further inhibiting the retention of high surface *CO coverage. Even when optimizing the Cu-based electrocatalysts to achieve highest multicarbon (C2+) product selectivities of >80%, such as confinement Cu structures6,7 or metal-doped Cu oxides8,9 to enhance the binding with *CO intermediates, the main C2+ products are ethylene7,8 and ethanol.6,9 To date, the highest partial current density of producing acetate from CO2RR is less than 50 mA cm–2.10
Chemical modification of the catalyst surface has recently been investigated to enhance CO2 capture and increase surface *CO coverage.11−13 Nonetheless, as *CO is a shared critical intermediate for most of the CO2RR products, the scaling relation between different reaction pathways poses a critical limit on the selectivity tuning. For instance, coating of hydrophilic molecules such as polyamides or amino acids on the Cu surface for CO2 capture also increases the *H coverage on the catalytic sites, which not only promotes the competitive hydrogen evolution reaction14 but also can facilitate the formation pathway of the *CHO intermediate toward CH4.15 On the other hand, coating of Cu with hydrophobic molecules for increasing local alkalinity can inevitably increase the (bi)carbonate formation on the surface, thus restricting *CO coverage and resulting in preferable C2H4 selectivity.16−18 Thus, it places a challenging dilemma of both increasing *CO coverage and enhancing the acetate pathway.
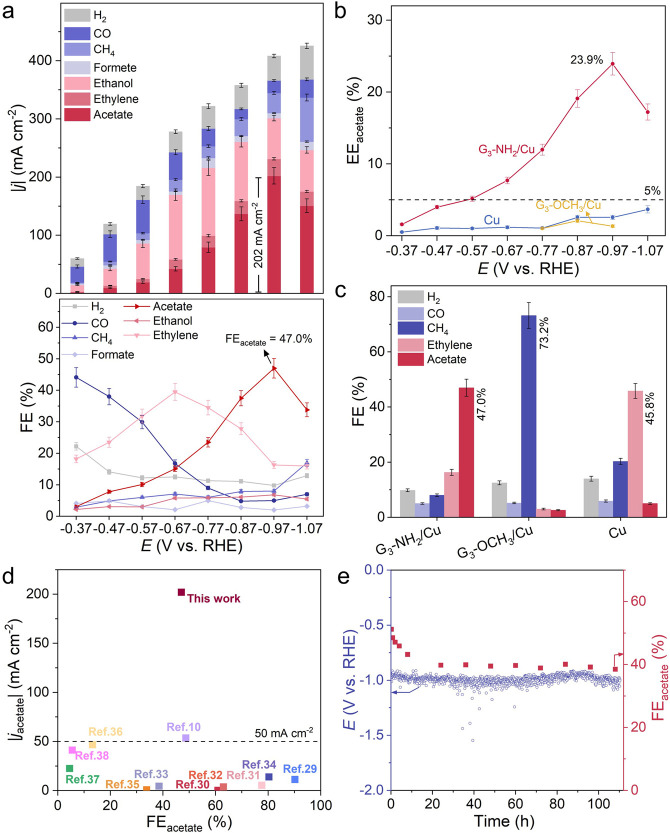
To address this dilemma, we propose that a hydrophilic network can be beneficial for the high coverage of *CO on the catalytic sites while retaining the in situ generated OH– to sustain a high local pH environment that favors the *H2CCO intermediate toward acetate. Herein, we developed a Cu nanoparticle catalyst functionalized with a highly dendritic polymer with amine (-NH2) tails, designated as G3-NH2/Cu, as an efficient CO2-to-acetate electrocatalyst. Compared to pristine (bare) Cu (Figure 1a), the abundant NH2-containing tail chain of the dendritic polymer exhibited a highly intertwined network to capture CO2 toward higher *CO coverage on Cu, and in the meantime allowed strong coordination with the Cu surface to reserve the in situ generated OH–, thus maintaining a stable high local pH (Figure 1b). The dendritic polymer-coated Cu electrocatalyst enabled an outstanding CO2-to-acetate performance, including one of the highest acetate partial current densities (|jacetate|) of 202 ± 14 mA cm–2 with a corresponding Faradaic efficiency (FEacetate) of 47.0 ± 3.1% at −0.97 V versus the reversible hydrogen electrode (vs RHE), suggesting an attractive strategy of surface molecular engineering to tune the scaling relation of different CO2RR products.
Figure 1.
Schematic of the CO2RR process on (a) Cu and (b) -NH2-terminal, dendrimer-functionalized Cu. The abundant -NH2-terminals of the dendritic polymer provide a highly intertwined network to capture CO2 for high *CO coverage on Cu and allow strong coordination with the Cu surface to retain the in situ generated OH–, thus favoring the CO2-to-acetate selectivity.
Results and Discussion
Synthesis and Characterizations of Catalysts
The dendrimer was synthesized via a typical divergent method (Methods in Supporting Information).19 The degree of crossing-linking dendritic branches and functional terminals was controlled by the number of repeated synthesis cycles (Figure S1a), including full generations of -NH2-terminated dendrimers (i.e., G1-NH2, G2-NH2, G3-NH2, G4-NH2) and half generation of the -OCH3-terminated dendrimer (i.e., G3-OCH3, Figure S1b). Fourier transform infrared (FT-IR) spectra (Figure S2) of both G3-NH2 and G3-OCH3 samples showed typical vibrations of N–C=O bonds (1650 and 1557 cm–1) and amide-linked -CH2- (2834, 2838, and 2927 cm–1), assigned to the dendrimer branches.20−22 The G3-NH2 sample showed a broad vibration of -NH2 (∼3285 cm–1),22 while the G3-OCH3 showed vibrations of -COOCH3 (2958, 1735, and 1443 cm–1) and C–C=O bond (1362, 1358, 1328, 1048, and 846 cm–1).22,23
Those synthesized dendrimers were then functionalized onto the Cu nanoparticles via an electrochemical deposition approach (Methods in Supporting Information, Figure 2a), and they were designated as G3-NH2/Cu and G3-OCH3/Cu, respectively. X-ray diffraction of all the samples showed the dominant Cu(111) diffraction at 2θ of 43.3° (JCPDS No. 04-0836, Figure S3). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images displayed the crystalline nanoparticles with diameters of 10–20 nm and lattice spacing of ∼0.21 nm, wrapped in amorphous films of ∼15 nm thickness (Figure 2b and Figure S4). The energy dispersive X-ray spectroscopy (EDS) elemental mapping images showed that Cu, C, O, and N were evenly distributed in both samples (Figures S5 and S6). The obtained EDS atomic N/Cu ratios in G3-NH2/Cu and G3-OCH3/Cu were around 0.70 and 0.32, respectively (Table S1), similar to the X-ray photoelectron spectroscopy (XPS) data (Table S2). The metal contents of these samples were verified by inductively coupled plasma analysis (Table S3). The contact angles of G3-NH2/Cu, G3-OCH3/Cu, and Cu were measured as ∼87°, ∼98°, and ∼145°, respectively (Figure S7).
Figure 2.
(a) Schematic of co-electrodeposition of -NH2-terminal dendrimers and Cu. (b) TEM (inset) and HRTEM images of G3-NH2/Cu. (c) Normalized Cu K-edge XANES spectra of the G3-NH2/Cu, G3-OCH3/Cu, Cu foil, and Cu2O samples. (d) N 1s XPS spectra of the G3-NH2/Cu and G3-OCH3/Cu.
The X-ray absorption spectroscopy of the Cu K-edge was then performed to investigate the chemical state and coordination structure. The X-ray absorption near-edge fine structure (XANES) spectroscopy of Cu K-edge in G3-NH2/Cu and G3-OCH3/Cu showed different shapes of the rising edge and the post edge (Figure 2c), suggesting their different chemical states and local coordination environments.24 The first edge absorption (the first inflection point, black dash in Figure 2c) of G3-NH2/Cu (∼8978.0 eV) was lower than the Cu foil (∼8979.0 eV),25,26 suggesting the electron transfer from the coordination atoms to Cu in G3-NH2/Cu. The N 1s XPS spectra were recorded to investigate the chemical states of N-containing groups (Figure 2d). The G3-OCH3/Cu sample retained a C–N bond (∼399.5 eV), while the N 1s XPS spectrum of G3-NH2/Cu was deconvoluted into two sub-peaks centered at 399.5 and 398.5 eV, ascribed to C–N and Cu–N interactions, respectively,27,28 suggesting the electron transfer from N to Cu in G3-NH2/Cu.
Electrochemical CO2RR Measurements
The electrocatalytic CO2RR performances of G3-NH2/Cu, G3-OCH3/Cu, and pristine Cu were evaluated in flow cells (Methods in the Supporting Information). The G3-NH2/Cu catalyst not only presented the largest total current density among those three samples (Figure S8) but also showed favorable selectivity toward acetate (Figure S9 and Table S4). The peak acetate partial current density (|jacetate|) reached 202 ± 14 mA cm–2 at −0.97 V vs RHE, with corresponding FEacetate of 47.0 ± 3.1% and a half-cell energy efficiency (EE) of 23.9 ± 1.6% (Figure 3a, b). The FEs of ethylene and CH4 were measured as 16.3% and 8.0%, respectively. In comparison, the G3-OCH3/Cu and Cu catalysts showed limited selectivity toward acetate (Figure 3b). For G3-OCH3/Cu, CH4 was the main product with a FECH4 of 73.2 ± 4.7% at −0.97 V vs RHE (Figure 3c and Figure S10), while the FE values of acetate and ethylene were ∼3%. For the Cu catalyst, ethylene was the main product with FEC2H4 of 45.8 ± 2.7% at −0.97 V vs RHE (Figure 3c and Figure S11), and the FE values for acetate and CH4 were 5.0 ± 0.3% and 20.3 ± 1.1%, respectively (Figure 3c and Table S5). Our results featured one of the highest |jacetate| and excellent FEacetate value, substantially exceeding the previously reported results for the CO2-to-acetate production (Figure 3d and Table S6).10,29−38 In addition, by tuning the number of amine tail chains by dendrimer generation (Gi-NH2, i = 1, 2, 3, 4) for optimization of amine network (Table S7), the highest acetate selectivity in C2 products (i.e., FEacetate/(FEacetate + FEethylene + FEethanol)) was obtained from G3-NH2/Cu (Figure S12 and Table S8). It was observed that while the CO2 was captured and converted to the *CO intermediate on G3-NH2, no significant CO2RR performance was observed on G3-OCH3 (Figure S13). Furthermore, after >100 h of a continuous electrochemical CO2RR test at a constant current density of −400 mA cm–2, the potential of G3-NH2/Cu catalyst was stable between −0.9 to −1.1 V vs RHE, and the FEacetate was retained at ∼38.5%, corresponding to ∼82% retention of its initial value (Figure 3e). The morphology (Figure S14) of G3-NH2/Cu was preserved as before electrolysis, suggesting its electrocatalytic stability.
Figure 3.
(a) Partial current densities (the upper panel) and corresponding FE values (the lower panel) of CO2RR products using the G3-NH2/Cu catalyst at various applied constant potentials (without ohmic correction). (b) EE values for acetate at various applied constant potentials with the G3-NH2/Cu, G3-OCH3, and Cu catalysts. (c) FE values at −0.97 V vs RHE on each catalyst. (d) Summary of |jacetate| vs FEacetate of this work with other Cu-based CO2RR catalysts. (e) The stability obtained at a constant negative current density of −400 mA cm–2 (without ohmic correction). Error bars in (a–c) correspond to a mean ± standard deviation of >3 measurements.
Mechanistic Investigation
As the electrochemical active surface areas (ECSAs) of the three electrodes evaluated by double layer capacitance (Cdl)39 were similar (Figure S15), we hypothesized that the distinctive acetate selectivity of the G3-NH2/Cu catalyst was attributed to the high *CO coverage and high local pH. To verify this hypothesis, density functional theory (DFT) calculations were conducted to investigate the CO2-to-acetate pathways in those catalysts. As Cu(111) is the main exposed facet40,41 as well as the dominant facet in the G3-NH2/Cu, G3-OCH3/Cu, and bare Cu, we chose the Cu(111) planes for calculations (Figure S3, Figure S16, Methods in Supporting Information). The adsorption energy of *CO (ΔE*CO) was first compared on these models, as it affects the *CO coverage on Cu(111) surface.7 Compared to pure Cu(111) (−84.9 kJ mol –1), the ΔE*CO values on G3-NH2/Cu(111) and G3-OCH3/Cu(111) were calculated as −108.3 and −109.6 kJ mol–1, respectively (Figure 4a), indicating their enhanced *CO binding capabilities with surface modifications. Then, the *CO-COH coupling pathway and the competitive *CHO pathway were studied.4,42 The *CHO pathway was more efficient than the *CO-COH coupling in G3-OCH3/Cu(111) (Figure S17a), which led to CH4 formation. In contrast, both Cu(111) (Figure S17b) and G3-NH2/Cu(111) (Figure 4b) showed more efficient *CO-COH coupling than the*CHO route, indicating preferable C2 selectivity. The mechanism was then employed on Cu(111), where acetate is formed through the *H2CCO intermediate4,43 and competes with other C2 products.40,44
Figure 4.
(a) Adsorption energies of *CO (ΔE*CO) on Cu(111), G3-OCH3/Cu(111), and G3-NH2/Cu(111) surfaces. (b) Free energy diagrams of *CO reaction pathways on the G3-NH2/Cu(111) surface.
In addition, the Gibbs free energy change (ΔG) value of the *H2CCO formation step was 0.73 eV lower than *CCOH formation step on G3-NH2/Cu(111) (Figure 4b), indicating the acetate route is more favorable than the ethylene or ethanol route. As the adsorption energies of the most favorable configuration of key *CCO and *H2CCO intermediates on G3-NH2/Cu(111) were lower than those on Cu(111) (Figure S18 and Table S9), the *H2CCO species stabilized on G3-NH2/Cu(111) can promote the CO2-to-acetate conversion, while the main product of bare Cu(111) was C2H4, consistent with our experimental results. Thus, the functionalization of G3-NH2 on Cu(111) not only led to a strong *CO binding capability for increasing the surface *CO coverage but also enhanced the adsorption of key *H2CCO intermediates toward acetate production.
The in situ electrochemical surface-enhanced Raman spectroscopy (SERS) was further performed at potentials ranging between −0.37 and −1.07 V vs RHE to confirm the contribution of *CO coverage and OH– confinement on G3-NH2/Cu during CO2RR (Methods in Supporting Information). For G3-NH2/Cu (Figure 5a,b), the Raman peaks of C–N stretching (∼1200 cm–1), C–C stretching (∼1320 cm–1), CO2– stretching (∼1330 and 1545 cm–1), N–H stretching (∼1595 cm–1), and N–H deformation (∼1609 cm–1) showed gradually increasing signals than G3-OCH3/C at more negative potentials,11 confirming the continued CO2 capture by the G3-NH2 network during CO2 electrolysis.45,46 This result was also verified with pure G3-NH2 and G3-OCH3 as electrodes for CO2RR at different applied potentials ranging from −0.37 to −1.07 V vs RHE (Figure S13).
Figure 5.
(a, b) Two-dimensional SERS spectra of (a) G3-NH2/Cu and (b) G3-OCH3/Cu. (c, d) SERS spectra of *CO vibration energy regions on (c) G3-NH2/Cu and (d) G3-OCH3/Cu. (e, f) SERS spectra of *OH and *CO32– vibration energy regions on (e) G3-NH2/Cu and (f) Cu.
In addition, the G3-NH2/Cu exhibited clearer Raman peaks located at 270–350 and 1800–2100 cm–1 (Figure 5c), attributed to atop-adsorbed *CO (*COatop, ∼334 and 2070 cm–1) and bridge-adsorbed *CO (*CObridge, ∼275 and 1850 cm–1).47 As shown in Figure 5c, the*COatop peaks located at 2000–2100 cm–1 showed a red-shift from −0.57 to −0.77 V vs RHE, indicating the *COatop vibration was affected by the vibrational Stark effect.48 In addition, the gradual blue shift of *COatop peaks and the appearance of *CObridge peaks at a more negative potential of −0.77 to −1.07 V vs RHE, suggesting the increased higher *CO coverage.4,49 Furthermore, a weak peak at ∼1970 cm–1 was also detected on G3-NH2/Cu, which was identified as the C=C=O stretching of an *CCO intermediate toward acetate.47 In comparison, for G3-OCH3/Cu (Figure 5d) and Cu (Figure S19), the *CObridge peaks were not observed and the *COatop peaks had much weaker intensities, suggesting their low surface *CO coverage.
Moreover, the Raman peaks observed on both G3-NH2/Cu and Cu showed O–H bending peaks (∼525 cm–1) with the increase of applied negative voltages, assigned to the in situ generated *OH species.50 Those peaks were assigned to the O–H bending modes of the surface *OH species, which are hydrogen-bonded with surrounding water molecules, as suggested by previous Raman studies.51 These peaks were red-shifted from 533 to 517 cm–1 with applied more negative potentials from −0.67 to −1.07 V vs RHE on G3-NH2/Cu (Figure 5e), implying that they were affected by the vibrational Stark effect with a Stark tuning rate of 41 ± 1.5 cm–1/V (Figure S20).47,51,52
While on the Cu surface, another C–O stretching (∼1067 cm–1) was observed for applied negative potentials as small as −0.47 V vs RHE (Figure 5f), corresponding to the CO32– species.16,52,53 However, this peak only became observable on G3-NH2/Cu for potentials more negative than −0.97 V vs RHE along with another peak at ∼1035 cm–1 corresponding to *COOH (Figure 5e).50 The carbonate accumulation on the Cu surface lowers the local pH5 and induces a higher chemical potential (Table S10), which hinders the H2CCO-to-acetate pathway.54 Taken together, the G3-NH2/Cu catalyst was allowed to confine the in situ generated OH– on the Cu surface due to the repulsion between OH– and G3-NH2/G3-NHCOO– during the CO2 electroreduction. Subsequently, the confined OH– reacted with H2CCO to form acetate, instead of reacting with CO2 to form CO32–. As a result, the G3-NH2/Cu enabled a high surface *CO coverage and high local pH with in situ generated OH– to be achieved, which facilitates the production of a *H2CCO intermediate toward acetate, thus boosting the CO2-to-acetate electrosynthesis with a peak FEacetate of 47.0%, a 9.4 times improvement than that of Cu.
Conclusion
In summary, we have demonstrated a -NH2-tailed, dendrimer-functionalized Cu surface aiming to enhance the CO2 capture and increase the local *CO concentration. The -NH2-rich network allowed an increase of the *CO intermediate coverage on the Cu catalytic sites, while at the same time retained the in situ generated OH– and a high local pH environment, favoring the formation of a *H2CCO intermediate toward acetate. The catalyst exhibited a high CO2-to-acetate performance, with an FEacetate of 47.0% and corresponding partial current density of 202 mA cm–2. Our work suggests an attractive strategy of surface molecular engineering to tune the selectivity of acetate from CO2RR.
Acknowledgments
We thank the following funding agencies for supporting this work: the National Key Research and Development Program of China (2018YFA0209402), the National Natural Science Foundation of China (22025502, 21975051), the Natural Science Foundation of Shanghai (23ZR1407000), the Science and Technology Commission of Shanghai Municipality (19XD1420400), and the Shanghai Municipal Education Commission (2019-01-07-00-07-E00045). We thank the BL05U, BL06B, BL11B and BL14W in Shanghai Synchrotron Radiation Facility for XAFS measurement.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.3c00826.
Additional experimental and calculation details; figures of dendrimer structures, FTIR, XRD patterns, TEM and HRTEM images, EDS elemental mapping images, optical images, linear sweep voltammetry curves, representative data on gas products and liquid products distributions, partial current densities and corresponding FE values, FE graphs, CO2RR products distribution and total current densities, CV curves, slab models, free energy diagram, Raman spectra, vibrational frequency data; tables elemental ratios, element contents, FE values, electrochemical performances of CO2 reduction to acetate, absorption energies, impaction of pH condition on the reaction tendency of *H2CCO to acetate formation (PDF)
Author Contributions
G.Z. and L.Z. proposed, designed, and supervised the project. G.Z., L.Z., and L.Y. wrote the manuscript. L.Y., X.L., C.P., S.K., F.H., and Y.T. performed experiments, theoretical calculations, and analyzed the data. All the authors discussed, commented on, and revised the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gunjan D.; Haresh M.. Production pathways of acetic acid and its versatile applications in the food industry. In Biotechnological Applications of Biomass; Thalita Peixoto B., Thiago Olitta B., Luiz Carlos B., Eds.; IntechOpen, 2020; pp 1–10. [Google Scholar]
- Dimian A. C.; Kiss A. A. Novel energy efficient process for acetic acid production by methanol carbonylation. Chem. Eng. Res. Des. 2020, 159, 1–12. 10.1016/j.cherd.2020.04.013. [DOI] [Google Scholar]
- Ji Y.; Chen Z.; Wei R.; Yang C.; Wang Y.; Xu J.; Zhang H.; Guan A.; Chen J.; Sham T.-K.; Luo J.; Yang Y.; Xu X.; Zheng G. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu-Pd sites. Nat. Catal. 2022, 5, 251–258. 10.1038/s41929-022-00757-8. [DOI] [Google Scholar]
- Wei P.; Gao D.; Liu T.; Li H.; Sang J.; Wang C.; Cai R.; Wang G.; Bao X. Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis. Nat. Nanotechnol. 2023, 18, 299–306. 10.1038/s41565-022-01286-y. [DOI] [PubMed] [Google Scholar]
- Lu X.; Zhu C.; Wu Z.; Xuan J.; Francisco J. S.; Wang H. In situ observation of the pH gradient near the gas diffusion electrode of CO2 reduction in alkaline electrolyte. J. Am. Chem. Soc. 2020, 142, 15438–15444. 10.1021/jacs.0c06779. [DOI] [PubMed] [Google Scholar]
- Arán-Ais R. M.; Scholten F.; Kunze S.; Rizo R.; Cuenya B. R. The role of in situ generated morphological motifs and Cu(I) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 2020, 5, 317–325. 10.1038/s41560-020-0594-9. [DOI] [Google Scholar]
- Gu Z.; Shen H.; Chen Z.; Yang Y.; Yang C.; Ji Y.; Wang Y.; Zhu C.; Liu J.; Li J.; Sham T.-K.; Xu X.; Zheng G. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 2021, 5, 429–440. 10.1016/j.joule.2020.12.011. [DOI] [Google Scholar]
- Feng J.; Wu L.; Liu S.; Xu L.; Song X.; Zhang L.; Zhu Q.; Kang X.; Sun X.; Han B. Improving CO2-to-C2+ product electroreduction efficiency via atomic lanthanide dopant-induced tensile-strained CuOx catalysts. J. Am. Chem. Soc. 2023, 145, 9857–9866. 10.1021/jacs.3c02428. [DOI] [PubMed] [Google Scholar]
- Wang P.; Yang H.; Tang C.; Wu Y.; Zheng Y.; Cheng T.; Davey K.; Huang X.; Qiao S. Z. Boosting electrocatalytic CO2-to-ethanol production via asymmetric C-C coupling. Nat. Commun. 2022, 13, 3754. 10.1038/s41467-022-31427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang D.; Li Q.; Dai G.; Zeng M.; Huang Y.; Wei Y. Interface engineering of Mo8/Cu heterostructures toward highly selective electrochemical reduction of carbon dioxide into acetate. Appl. Catal., B 2021, 281, 119426. 10.1016/j.apcatb.2020.119426. [DOI] [Google Scholar]
- Lee G.; Li Y. C.; Kim J.-Y.; Peng T.; Nam D.-H.; Sedighian Rasouli A.; Li F.; Luo M.; Ip A. H.; Joo Y.-C.; Sargent E. H. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 2021, 6, 46–53. 10.1038/s41560-020-00735-z. [DOI] [Google Scholar]
- Zhong H.; Sa R.; Lv H.; Yang S.; Yuan D.; Wang X.; Wang R. Covalent organic framework hosting metalloporphyrin-based carbon dots for visible-light-driven selective CO2 reduction. Adv. Funct. Mater. 2020, 30, 2002654. 10.1002/adfm.202002654. [DOI] [Google Scholar]
- Niu Q.; Dong S.; Tian J.; Huang G.; Bi J.; Wu L. Rational design of novel COF/MOF S-scheme heterojunction photocatalyst for boosting CO2 reduction at gas-solid interface. ACS Appl. Mater. Interfaces 2022, 14, 24299–24308. 10.1021/acsami.2c02439. [DOI] [PubMed] [Google Scholar]
- Ahn S.; Klyukin K.; Wakeham R. J.; Rudd J. A.; Lewis A. R.; Alexander S.; Carla F.; Alexandrov V.; Andreoli E. Poly-amide modified copper foam electrodes for enhanced electrochemical reduction of carbon dioxide. ACS Catal. 2018, 8, 4132–4142. 10.1021/acscatal.7b04347. [DOI] [Google Scholar]
- Xie M. S.; Xia B. Y.; Li Y.; Yan Y.; Yang Y.; Sun Q.; Chan S. H.; Fisher A.; Wang X. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 2016, 9, 1687–1695. 10.1039/C5EE03694A. [DOI] [Google Scholar]
- Chen X.; Chen J.; Alghoraibi N. M.; Henckel D. A.; Zhang R.; Nwabara U. O.; Madsen K. E.; Kenis P. J. A.; Zimmerman S. C.; Gewirth A. A. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 2021, 4, 20–27. 10.1038/s41929-020-00547-0. [DOI] [Google Scholar]
- Li J.; Li X.; Gunathunge C. M.; Waegele M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 9220–9229. 10.1073/pnas.1900761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang Z.; McCallum C.; Xu Y.; Li F.; Wang Y.; Gabardo C. M.; Dinh C.-T.; Zhuang T.-T.; Wang L.; Howe J. Y.; Ren Y.; Sargent E. H.; Sinton D. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2019, 2, 1124–1131. 10.1038/s41929-019-0380-x. [DOI] [Google Scholar]
- Tomalia D. A.; Baker H.; Dewald J.; Hall M.; Kallos G.; Martin S.; Roeck J.; Ryder J.; Smith P. A new class of polymers: starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. 10.1295/polymj.17.117. [DOI] [Google Scholar]
- Maadani M.; Jafari S. H.; Saeb M. R.; Ramezanzadeh B.; Najafi F.; Puglia D. Studying the corrosion protection behavior of an epoxy composite coating reinforced with functionalized graphene oxide by second and fourth generations of poly(amidoamine) dendrimers (GO-PAMAM-2, 4). Prog. Color. Color. Coat. 2020, 13, 261–273. [Google Scholar]
- Beltran V.; Salvado N.; Buti S.; Pradell T. Ageing of resin from Pinus species assessed by infrared spectroscopy. Anal. Bioanal. Chem. 2016, 408, 4073–4082. 10.1007/s00216-016-9496-x. [DOI] [PubMed] [Google Scholar]
- Riaz T.; Zeeshan R.; Zarif F.; Ilyas K.; Muhammad N.; Safi S. Z.; Rahim A.; Rizvi S. A. A.; Rehman I. U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. 10.1080/05704928.2018.1426595. [DOI] [Google Scholar]
- Sivaraman B.; Radhika N.; Das A.; Gopakumar G.; Majumdar L.; Chakrabarti S. K.; Subramanian K. P.; Raja Sekhar B. N.; Hada M. Infrared spectra and chemical abundance of methyl propionate in icy astrochemical conditions. Mon. Not. R. Astron. Soc. 2015, 448, 1372–1377. 10.1093/mnras/stu2602. [DOI] [Google Scholar]
- Kau L.-S.; Spira-Solomon D. J.; Penner-Hahn J. E.; Hodgson K. O.; Solomon E. I. X-ray absorption edge determination of the oxidation state and coordination number of copper: application to the type 3 site in rhus vernicifera lacease and its reaction with oxygen. J. Am. Chem. Soc. 1987, 109, 6433–6442. 10.1021/ja00255a032. [DOI] [Google Scholar]
- Peng C.; Luo G.; Zhang J.; Chen M.; Wang Z.; Sham T. K.; Zhang L.; Li Y.; Zheng G. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580. 10.1038/s41467-021-21901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu S.; Suzuki K.; Gopinath C. S.; Yoshida H.; Hattori T. XPS, XANES and EXAFS investigations of CuO/ZnO/Al2O3/ZrO2 mixed oxide catalysts. Phys. Chem. Chem. Phys. 2002, 4, 1990–1999. 10.1039/b109766k. [DOI] [Google Scholar]
- Xie Y.; Chen Y. Experimental and computational investigation of Cu-N coordination bond strengthened polyaniline for stable energy storage. J. Mater. Sci. 2021, 56, 10135–10153. 10.1007/s10853-021-05920-3. [DOI] [Google Scholar]
- Xu Y.; Li F.; Xu A.; Edwards J. P.; Hung S. F.; Gabardo C. M.; O’Brien C. P.; Liu S.; Wang X.; Li Y.; Wicks J.; Miao R. K.; Liu Y.; Li J.; Huang J. E.; Abed J.; Wang Y.; Sargent E. H.; Sinton D. Low coordination number copper catalysts for electrochemical CO2 methanation in a membrane electrode assembly. Nat. Commun. 2021, 12, 2932. 10.1038/s41467-021-23065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. F.; Huang J. R.; Yu C.; Zhao Z. H.; Zhu H. L.; Ke Z.; Liao P. Q.; Chen X. M. A stable and conductive covalent organic framework with isolated active sites for highly selective electroreduction of carbon dioxide to acetate. Angew. Chem., Int. Ed. 2022, 61, e202206470 10.1002/anie.202206470. [DOI] [PubMed] [Google Scholar]
- Genovese C.; Schuster M. E.; Gibson E. K.; Gianolio D.; Posligua V.; Grau-Crespo R.; Cibin G.; Wells P. P.; Garai D.; Solokha V.; Krick C. S.; Valasco-Velez J. J.; Ampelli C.; Perathoner S.; Held G.; Centi G.; Arrigo R. Operando spectroscopy study of the carbon dioxide electro-reduction by iron species on nitrogen-doped carbon. Nat. Commun. 2018, 9, 935. 10.1038/s41467-018-03138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Chen S.; Quan X.; Yu H. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. 10.1021/jacs.5b02975. [DOI] [PubMed] [Google Scholar]
- De R.; Gonglach S.; Paul S.; Haas M.; Sreejith S. S.; Gerschel P.; Apfel U. P.; Vuong T. H.; Rabeah J.; Roy S.; Schöfberger W. Electrocatalytic reduction of CO2 to acetic acid by a molecular manganese corrole complex. Angew. Chem., Int. Ed. 2020, 59, 10527–10534. 10.1002/anie.202000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.; Sun X.; Yang D.; Ma J.; Kang X.; Zheng L.; Zhang J.; Wu Z.; Han B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851. 10.1038/s41467-019-11599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Zhu Q.; Kang X.; Liu H.; Qian Q.; Ma J.; Zhang Z.; Yang G.; Han B. Design of a Cu(i)/C-doped boron nitride electrocatalyst for efficient conversion of CO2 into acetic acid. Green Chem. 2017, 19, 2086–2091. 10.1039/C7GC00503B. [DOI] [Google Scholar]
- Su X.; Sun Y.; Jin L.; Zhang L.; Yang Y.; Kerns P.; Liu B.; Li S.; He J. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products. Appl. Catal., B 2020, 269, 118800. 10.1016/j.apcatb.2020.118800. [DOI] [Google Scholar]
- Zhang Z.-Y.; Tian H.; Bian L.; Liu S.-Z.; Liu Y.; Wang Z.-L. Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products. J. Energy Chem. 2023, 83, 90–97. 10.1016/j.jechem.2023.04.034. [DOI] [Google Scholar]
- Fang M.; Wang M.; Wang Z.; Zhang Z.; Zhou H.; Dai L.; Zhu Y.; Jiang L. Hydrophobic, ultrastable Cuδ+ for robust CO2 electroreduction to C2 products at ampere-current levels. J. Am. Chem. Soc. 2023, 145, 11323–11332. 10.1021/jacs.3c02399. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Ou P.; Wang X.; Xu Z.; Li Y. C.; Wang Z.; Huang J. E.; Wicks J.; McCallum C.; Wang N.; Wang Y.; Chen T.; Lo B. T. W.; Sinton D.; Yu J. C.; Wang Y.; Sargent E. H. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 2022, 5, 564–570. 10.1038/s41929-022-00788-1. [DOI] [Google Scholar]
- Liu M.; He Q.; Huang S.; Zou W.; Cong J.; Xiao X.; Li P.; Cai J.; Hou L. NiCo-layered double hydroxide-derived B-doped CoP/Ni2P hollow nanoprisms as high-efficiency electrocatalysts for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2021, 13, 9932–9941. 10.1021/acsami.0c20294. [DOI] [PubMed] [Google Scholar]
- Wu Z.-Z.; Zhang X.-L.; Niu Z.-Z.; Gao F.-Y.; Yang P.-P.; Chi L.-P.; Shi L.; Wei W.-S.; Liu R.; Chen Z.; Hu S.; Zheng X.; Gao M.-R. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 2022, 144, 259–269. 10.1021/jacs.1c09508. [DOI] [PubMed] [Google Scholar]
- Luc W.; Fu X.; Shi J.; Lv J.-J.; Jouny M.; Ko B. H.; Xu Y.; Tu Q.; Hu X.; Wu J.; Yue Q.; Liu Y.; Jiao F.; Kang Y. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2019, 2, 423–430. 10.1038/s41929-019-0269-8. [DOI] [Google Scholar]
- Zhang H.; Chang X.; Chen J. G.; Goddard W. A. 3rd; Xu B.; Cheng M. J.; Lu Q. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane. Nat. Commun. 2019, 10, 3340. 10.1038/s41467-019-11292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouny M.; Lv J.-J.; Cheng T.; Ko B. H.; Zhu J.-J.; Goddard W. A.; Jiao F. Formation of carbon-nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 2019, 11, 846–851. 10.1038/s41557-019-0312-z. [DOI] [PubMed] [Google Scholar]
- Li X.; Liu Q.; Wang J.; Meng D.; Shu Y.; Lv X.; Zhao B.; Yang H.; Cheng T.; Gao Q.; Li L.; Wu H. B. Enhanced electroreduction of CO2 to C2+ products on heterostructured Cu/oxide electrodes. Chem. 2022, 8, 2148–2162. 10.1016/j.chempr.2022.04.004. [DOI] [Google Scholar]
- Ma P. C.; Wang S. Q.; Kim J. K.; Tang B. Z. In-situ amino functionalization of carbon nanotubes using ball milling. J. Nanosci. Nanotechnol. 2009, 9, 749–753. 10.1166/jnn.2009.C017. [DOI] [PubMed] [Google Scholar]
- Bergamonti L.; Graiff C.; Tegoni M.; Predieri G.; Bellot-Gurlet L.; Lottici P. P. Raman and NMR kinetics study of the formation of amidoamines containing N-hydroxyethyl groups and investigations on their Cu(II) complexes in water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 515–524. 10.1016/j.saa.2016.07.041. [DOI] [PubMed] [Google Scholar]
- Shao F.; Wong J. K.; Low Q. H.; Iannuzzi M.; Li J.; Lan J. In situ spectroelectrochemical probing of CO redox landscape on copper single-crystal surfaces. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2118166119 10.1073/pnas.2118166119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya D.; Videla P. E.; Palasz J. M.; Tangen I.; Meng J.; Kubiak C. P.; Batista V. S.; Lian T. Sub-nanometer mapping of the interfacial electric field profile using a vibrational Stark shift ruler. J. Am. Chem. Soc. 2022, 144, 14330–14338. 10.1021/jacs.2c05563. [DOI] [PubMed] [Google Scholar]
- Yang P.-P.; Zhang X.-L.; Liu P.; Kelly D. J.; Niu Z.-Z.; Kong Y.; Shi L.; Zheng Y.-R.; Fan M.-H.; Wang H.-J.; Gao M.-R. Highly enhanced chloride adsorption mediates efficient neutral CO2 electroreduction over a dual-phase copper catalyst. J. Am. Chem. Soc. 2023, 145, 8714–8725. 10.1021/jacs.3c02130. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Zhang X.-G.; Bodappa N.; Yang W.-M.; Liang Q.; Radjenovica P. M.; Wang Y.-H.; Zhang Y.-J.; Dong J.-C.; Tian Z.-Q.; Li J.-F. Elucidating electrochemical CO2 reduction reaction processes on Cu(hkl) single-crystal surfaces by in situ Raman spectroscopy. Energy Environ. Sci. 2022, 15, 3968–3977. 10.1039/D2EE01334G. [DOI] [Google Scholar]
- Phelps D. H.; Dalby F. W. Optical observations of the stark effect on OH*. Can. J. Phys. 1965, 43, 144–154. 10.1139/p65-013. [DOI] [Google Scholar]
- Chernyshova I. V.; Somasundaran P.; Ponnurangam S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 9261–9270. 10.1073/pnas.1802256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Wei P.; Gao D.; Wang G. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts. Curr. Opin. Green Sustain. 2022, 34, 100589. 10.1016/j.cogsc.2022.100589. [DOI] [Google Scholar]
- Heenen H. H.; Shin H.; Kastlunger G.; Overa S.; Gauthier J. A.; Jiao F.; Chan K. The mechanism for acetate formation in electrochemical CO2 reduction on Cu: selectivity with potential, pH, and nanostructuring. Energy Environ. Sci. 2022, 15, 3978–3990. 10.1039/D2EE01485H. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.