Abstract
The antibacterial activities of human regimens of cefepime, ceftazidime, and imipenem alone or in combination with amikacin against an isogenic pair of Enterobacter cloacae strains (wild type and its corresponding derepressed cephalosporinase mutant) were compared by using our nonlethal model of pneumonia with 180 immunocompetent rats. Compared with untreated animals, all β-lactam-treated rats, except those inoculated with the mutant isolate and receiving ceftazidime, had significantly lower bacterial counts in their lungs 60 h after the onset of therapy. Although the combination of a β-lactam and amikacin was more bactericidal than each corresponding antimicrobial agent alone, true synergy was noted only with cefepime and imipenem against the constitutive derepressed strain.
Stably derepressed mutant strains of Enterobacter spp. resist most available β-lactams, including ceftazidime, except for the carbapenems (15). Cefepime, a newly extended-spectrum cephalosporin, retains in vitro efficacy against these mutants, but few in vivo data have confirmed these results. Comparing the bactericidal activities of cefepime and amikacin against an isogenic pair of Enterobacter cloacae strains (wild type and its ceftazidime-resistant mutant) in induced experimental pneumonia with rats, we previously reported that each antibiotic alone failed to decrease bacterial counts in the lungs, while combined therapy significantly reduced the number of viable microorganisms (12). The short duration of therapy in these experiments (24 h) as well as the absence of groups treated with a carbapenem or an expanded-spectrum cephalosporin does not permit the definitive proposal of cefepime as the first choice for therapy against stably derepressed cephalosporinase-producing enterobacterial strains. Therefore, the purpose of the present study was to compare the bactericidal activities of 60-h human regimens of cefepime, ceftazidime, and imipenem-cilastatin alone or in combination with amikacin with the same animal model.
Tested organisms.
An isogenic pair of E. cloacae strains (wild type [474S] and its ceftazidime-resistant mutant [474R]) identical to those inoculated into rats in our previous experiments (12) was used for these studies. Each strain was stored at −70°C in Mueller-Hinton broth (bioMérieux, Marçy-l’Etoile, France) supplemented with 10% glycerol. Fresh inocula were prepared for each experiment from cultures grown for 24 h in 10 ml of Trypticase soy broth (bioMérieux) and then rinsed twice and suspended in normal saline prior to use.
Antimicrobial agents.
Cefepime and amikacin were from Bristol-Myers Squibb (Paris, France), ceftazidime was from Glaxo Wellcome (Evreux, France), and imipenem-cilastatin was from Merck Sharp and Dohme-Chibret (Paris, France). Antibiotic powders were freshly diluted with saline before each experiment according to the manufacturer’s instructions.
β-Lactamase assay.
β-Lactamase activity was assayed by UV spectrometry as previously reported (17), with cephalothin as the substrate, and expressed as units of specific activity. One unit of specific activity was defined as the amount of enzyme that hydrolyzed 1 nmol of cephalothin per min/mg of protein.
In vitro studies.
MICs were determined by an agar dilution technique with Mueller-Hinton agar (bioMérieux) and an inoculum of 4 log10 CFU per spot.
Pharmacokinetics.
Preliminary drug-dosing studies were run in uninfected rats as described elsewhere (11) to determine if the subcutaneous dose of 1 mg of uranyl nitrate (Merck, Darmstadt, Germany) per kg of body weight that was previously recommended (11, 12) was optimal to impair their renal function so as to simulate the pharmacokinetics of cefepime, ceftazidime, imipenem, and amikacin in healthy humans. Briefly, 4 days after the uranyl nitrate injection, each rat received a single 1-ml intraperitoneal injection of each antimicrobial agent studied. Multiple blood samples were collected via a femoral catheter during the 8 h following antibiotic administration and immediately centrifuged to separate the plasma. Plasma samples were stored at −70°C and assayed within 7 days. Individual antibiotic pharmacokinetic parameters were determined with the Siphar software package (Simed, Créteil, France).
Antibiotic assay.
Imipenem, ceftazidime, and cefepime concentrations in plasma were determined by using modified versions of high-pressure liquid chromatography assays described elsewhere (1, 6, 7). The amikacin concentration was determined by an immunoenzyme assay (Emit; Syva, Dardilly, France). The lower detection limits of the assays were 0.5, 5, 1, and 1 μg/ml for imipenem, ceftazidime, cefepime, and amikacin, respectively.
Pneumonia model.
The animal model used was previously developed in our laboratory (11, 12). Briefly, male Wistar rats weighting 280 to 300 g were rendered renally insufficient by the subcutaneous administration of 1 mg of uranyl nitrate per kg and intraperitoneally anesthetized 93 h later with phenobarbital (60 mg/kg). Each rat trachea was exposed by a vertical midline incision. A 0.5-ml portion of a bacterial suspension containing 8.9 ± 0.1 log10 CFU (mean ± standard deviation) of E. cloacae was injected intratracheally. Following inoculation, animals were gently shaken for 15 s to help distribute the inoculum in the lungs. Previous studies had shown that, 3 h after bacterial inoculation, all animals had developed bilateral pneumonia with bacterial densities (>7.5 log10 CFU/g of tissue) in both lungs and an intense inflammatory reaction.
Treatment regimens.
Each strain used to induce pneumonia was studied separately. Among the 180 animals utilized in this study, 64 of the 80 wild-type and 90 of the 100 ceftazidime-resistant mutant strain recipients were still alive 3 h after bacterial inoculation; at this time, 8 rats from each study group were killed to document the existence of pneumonia. The remaining rats were randomly assigned to one control group (i.e., no antibiotic) and seven treatment groups. Treatment groups received intraperitoneal injections of either imipenem-cilastatin (30 mg/kg/8 h), ceftazidime (60 mg/kg/8 h), cefepime (60 mg/kg/12 h), or amikacin (25 mg/kg once a day) or a combination of each β-lactam with amikacin given at the same dosages. These dosages were retained so that plasma concentrations could be obtained close to those observed in adult humans receiving 1 g of imipenem three times a day, 2 g of ceftazidime three times a day, 2 g of cefepime twice a day, or 25 mg of amikacin per kg once a day. Therapy was started 3 h after bacterial inoculation and continued for 2.5 days.
Evaluation of antibiotic treatment.
Animals were sacrificed 60 h after the onset of therapy. Blood was obtained by aortic puncture, put in a tube containing EDTA, and centrifuged. The plasma was stored in two or three aliquots at −70°C for determination of antibiotic concentrations and creatinine levels within 7 days after sampling. The plasma containing imipenem was immediately mixed after sampling 1:1 with a stabilizing buffer containing equal volumes of 1 M morphilino-ethane sulfonate and ethylene glycol before freezing. Creatinine levels in plasma were determined to document the fact that renal impairment was well established. The lungs were aseptically removed, gently blotted with sterile absorbent paper to remove blood, weighed, placed in 25 ml of ice-cold saline, and homogenized (Ultraturax, Staufen, Germany). The homogenate was quantitatively cultured after serial dilution on Drigalski agar (bioMérieux) with a Spiral Système plater (Interscience, Saint-Nom-La-Bretèche, France). After overnight incubation at 37°C, viable bacteria were counted and expressed as log10 CFU/g of lung. When no bacterial growth was noted, the value of the detection limit for the specific animal was entered into the statistical analysis.
Determination of emergence of antibiotic resistance during therapy.
Emergence of resistance during β-lactam therapy was examined at the end of each experiment by plating 2 × 200 μl of the lung homogenates onto agar containing imipenem (1 μg/ml), cefepime (1 μg/ml), or ceftazidime (8 μg/ml) for the wild-type strain group and imipenem (2 μg/ml) or cefepime (4 μg/ml) for the ceftazidime-resistant mutant strain group. After incubation in air for 48 h at 37°C, emergence of a resistant strain(s) was defined as growth of at least one colony of E. cloacae.
Statistical analysis.
Results are expressed as medians and their ranges. Bacterial counts in the lungs of the control and treatment groups were compared by one-way nonparametric analysis of variance (Kruskal-Wallis test); when the value of this test was statistically significant, each treatment group was compared to the control group and to each of the other treatment groups by using the Mann-Whitney U test without modifications. For all tests, a P value of <0.05 was considered significant.
The susceptibilities of both organisms to the antimicrobial agents studied are presented in Table 1. The isolates remained susceptible to the four antibiotics tested, except for the stably derepressed cephalosporinase-producing mutant, which was highly resistant to ceftazidime. The cephalosporinase level in the mutant strain was 16,900 U of specific activity, i.e., 110-fold higher than the level determined in the wild-type strain. Such results clearly indicated that overproduction of cephalosporinase was the molecular mechanism explaining resistance to ceftazidime.
TABLE 1.
In vitro susceptibility of the isogenic pair of E. cloacae strains (wild type and stably-derepressed cephalosporinase-producing mutant) to the antibiotics studied
| Antimicrobial agent | MIC (μg/ml) of antibiotic for indicated strain
|
|
|---|---|---|
| Wild type | Mutant | |
| Amikacin | 2 | 2 |
| Ceftazidime | 1 | 128 |
| Cefepime | 0.125 | 1 |
| Imipenem | 0.25 | 0.50 |
The pharmacokinetic parameters of the antibiotics tested in renally insufficient rats simulated those reported in healthy humans given high doses of the same antibiotics (Table 2). Creatinine levels in plasma measured at sacrifice were not statistically different between the study groups, indicating that renal impairment was identical regardless of the treatment received (Table 3). Antibiotic concentrations in plasma observed 60 h after the beginning of therapy did not differ significantly when the antibiotics were administered to animals infected with the two strains. Results for each antibiotic for each strain were then pooled to simplify presentation, and the data are shown in Table 3. Antibiotic levels measured in the plasma of rats were broadly similar to those usually reported in adult humans given high doses of the same antibiotics.
TABLE 2.
Pharmacokinetics for antibiotics given intraperitoneally to noninfected rats with uranyl nitrate-induced renal impairment
| Antimicrobial agent | No. of animals | Dose (mg/kg) | Median (range) antibiotic level in plasma (μg/ml)
|
Half-life (h) | Area under curve (μg × h/ml) | |
|---|---|---|---|---|---|---|
| Peak (30 min after dosing) | Trough (8 h after dosing) | |||||
| Amikacin | 6 | 25 | 80 (60–115) | 15 (10–30) | 2.2 (1.9–2.6) | 288 (224–408) |
| Ceftazidime | 4 | 60 | 197 (139–214) | 15 (5–24) | 1.9 (1.4–2.9) | 346 (266–382) |
| Cefepime | 6 | 60 | 160 (125–220) | 12 (6–28) | 2.0 (1.4–2.8) | 422 (188–615) |
| Imipenem | 5 | 30 | 83 (66–104) | <0.5 (<0.5–1) | 1.0 (0.7–1.3) | 112 (95–145) |
TABLE 3.
Creatinine and antibiotic levels in plasma observed at sacrifice
| Antimicrobial agent(s) | No. of animals | Creatinine level in plasma (μmol/liter) | Median (range) of antibiotic plasma concn (μg/ml)
|
|
|---|---|---|---|---|
| β-Lactama | Amikacinb | |||
| None | 18 | 217 (83–469) | — | — |
| Amikacin | 14 | 169 (72–353) | — | 1 (<1–10) |
| Ceftazidime | 11 | 157 (80–346) | 26 (<5–57) | — |
| Ceftazidime + amikacin | 12 | 215 (89–425) | 31 (13–57) | 1 (<1–13) |
| Cefepime | 18 | 162 (72–288) | 24 (12–48) | — |
| Cefepime + amikacin | 19 | 234 (42–559) | 27 (10–39) | 4 (<1–24) |
| Imipenem | 18 | 194 (60–446) | 1 (<1–15) | — |
| Imipenem + amikacin | 19 | 219 (102–436) | 1 (<1–12) | 3 (<1–17) |
Time from the last β-lactam administration to sacrifice was 4 to 6 h.
Time from the last amikacin administration to sacrifice was 13 to 14 h.
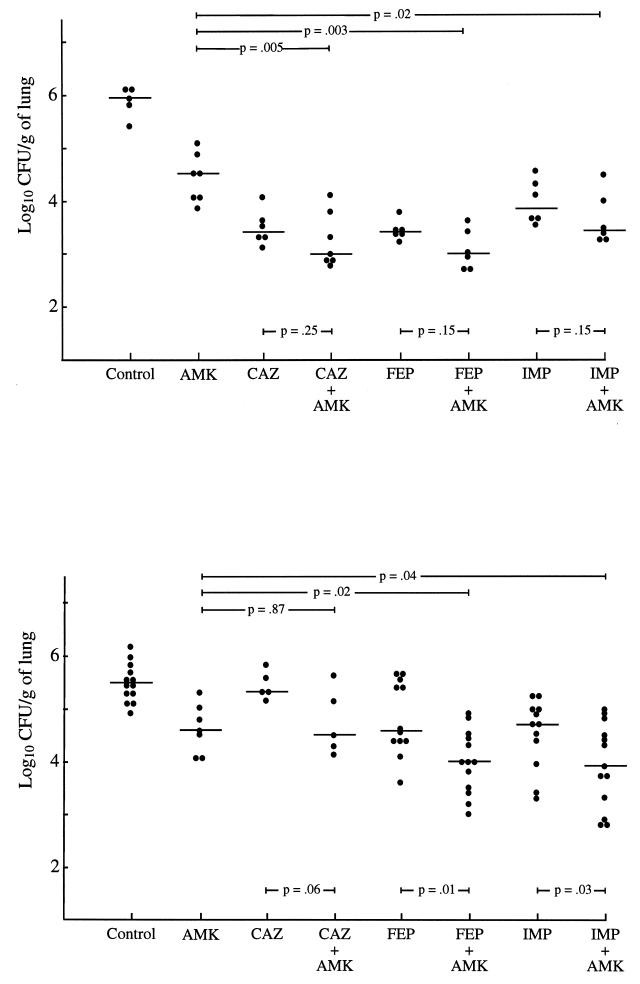
The eight animals from each study group killed at the beginning of therapy presented with bilateral pneumonia, with median E. cloacae counts of log10 8.3 (range, 7.8 to 8.5) and log10 8.1 (range, 8.0 to 8.5) CFU/g of lung for the wild-type strain and its ceftazidime-resistant mutant, respectively. Nine animals died during the antibiotic treatment period (seven rats inoculated with the wild-type strain and two with the mutant strain). At sacrifice, all untreated animals showed a spontaneous decrease in the number of bacteria 60 h after therapy was begun in the treated animals (Fig. 1). Compared with untreated animals, all β-lactam-treated rats, except those inoculated with the mutant isolate and receiving ceftazidime, had significantly (P ≤ 0.02) lower bacterial counts in their lungs 60 h after the onset of therapy. Significantly decreased bacterial titers were also observed in the lungs of animals inoculated with the mutant strain and receiving antibiotic therapy combined with amikacin and imipenem or cefepime compared to those in the lungs of animals receiving each corresponding antimicrobial agent alone (Fig. 1). No E. cloacae isolate resistant to either of the β-lactam agents tested was detected in any of the antibiotic-treated animals.
FIG. 1.
Lung CFU/gram of the isogenic pair of E. cloacae (top panel, wild type; bottom panel, stably-derepressed cephalosporinase-producing mutant) in rats treated with either amikacin (AMK), ceftazidime (CAZ), cefepime (FEP), or imipenem (IMP) or with each β-lactam in combination with amikacin. Each mark represents a single animal. The horizontal bar indicates the median for each group.
Imipenem has been reported to be stable in the presence of chromosomally mediated cephalosporinase and is considered the therapy of choice for infections due to enterobacterial strains which constitutively overproduce β-lactamase (5, 10, 14, 15). Although they were initially regarded as stable, most broad-spectrum cephalosporins such as ceftazidime are hydrolyzed to some extent by high levels of enzyme, rendering them useless against derepressed strains (5, 8, 9). Cefepime retains in vitro activity toward these strains, possibly because of a combination of factors, including faster penetration of the outer membrane of gram-negative bacteria, poor affinity for most β-lactamases, and increased resistance to hydrolysis (2, 8, 9, 16). Using an in vitro infection model to compare the bactericidal activities of human regimens of various β-lactams against a ceftazidime-resistant Enterobacter strain, Palmer and colleagues reported a reduction in bacterial titer over the first 6 h that was similar for all regimens, but significant regrowth occurred with ceftazidime, cefotaxime, and ceftriaxone, whereas no regrowth was observed with cefepime during the 48 h of therapy (13). In this study, performed in immunocompetent animals, the antimicrobial activities of human regimens of cefepime and imipenem were broadly similar against the wild-type E. cloacae strain as well as its corresponding stably derepressed mutant, and their bactericidal effects were reduced against the latter. These encouraging results were recently supported by a clinical study in which 15 of 17 infections due to Enterobacter spp. with low susceptibility or resistance to ceftazidime but with susceptibility to cefepime were successfully treated with cefepime. In particular, cefepime was successfully used to manage chronic infections that had responded poorly to repeated therapy with imipenem, aminoglycosides, or ciprofloxacin (19). However, more clinical experience is required before cefepime can be systematically used in this setting.
Antibiotic combinations including a β-lactam and an aminoglycoside have frequently produced an increased bactericidal effect in vivo in experimental models of aerobic gram-negative bacillary infections that has generally paralleled an increased rate of killing observed in vitro (3). It has been suggested that such combinations are necessary in order to prevent the emergence of resistance during therapy; however, recent clinical experience with older cephalosporins, such as cefotaxime, indicates that the addition of an aminoglycoside does not always prevent resistance (4). Selection of resistance was not observed with any antimicrobial regimen in our model. It is possible that our inoculum was too low to detect resistant subpopulations, as these mutants are reported to occur in 1 of 105 to 108 wild-type strains possessing inducible cephalosporinase (18). In addition, the duration of our experiments (60 h) may not have been sufficient for the selection of such resistant clones.
Acknowledgments
This work was supported in part by a grant-in-aid from Bristol-Myers Squibb, Paris, France.
REFERENCES
- 1.Barbhaiya R H, Forgue S T, Shyu W C, Papp E A, Pittman K A. High pressure liquid chromatographic analysis of BMY 28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31:55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellido F, Péchère J C, Hancock R E W. Reevaluation of the factors involved in the efficacy of new β-lactams against Enterobacter cloacae. Antimicrob Agents Chemother. 1991;35:73–78. doi: 10.1128/aac.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calandra, T., and M. P. Glauser. 1986. Immunocompromised animal models for the study of antibiotic combinations. Am. J. Med. 80(Suppl. 5C):45– 52. [PubMed]
- 4.Chow J W, Fine M J, Shlaes D M, Quinn J P, Hooper D C, Johnson M P, Ramphal R, Wagener M M, Miyashiro D K, Yu V L. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 5.de Champs C, Guelon D, Joyon D, Sirot D, Chanal M, Sirot J. Treatment of a meningitis due to an Enterobacter aerogenes producing a derepressed cephalosporinase and a Klebsiella pneumoniae producing an extended-spectrum beta-lactamase. Infection. 1991;19:181–183. doi: 10.1007/BF01643247. [DOI] [PubMed] [Google Scholar]
- 6.Gravallese D A, Musson D G, Pauliukonis L T, Bayne W F. Determination of imipenem (N-formimidoyl thienamycin) in human plasma and urine by high-performance liquid chromatography: comparison with microbiological methodology and stability. J Chromatogr. 1984;310:71–84. doi: 10.1016/0378-4347(84)80069-9. [DOI] [PubMed] [Google Scholar]
- 7.Jehl F, Gaillon C, Monteil H. High-performance liquid chromatography of antibiotics. J Chromatogr. 1990;531:509–548. doi: 10.1016/s0378-4347(00)82293-8. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., and P. C. Flushs. Activity of cefepime (BMY-28142) and cefpirome (HR 810) against gram-negative bacilli resistant to cefotaxime or ceftazidime. J. Antimicrob. Chemother. 23:163–165. [DOI] [PubMed]
- 9.Knapp C C, Washington J A. Activity of cefepime, ceftazidime, and ceftizoxime against mutants of Enterobacteriae and Pseudomonas aeruginosa derepressed for Class I β-lactamase. J Antimicrob Chemother. 1989;24:1011–1012. doi: 10.1093/jac/24.6.1011. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Beltran, J., C. Calderon, M. P. Sierra, M. Alvarez, and R. Canton. 1997. In vitro activity of carbapenems against Enterobacteriaceae and Pseudomonas aeruginosa hyperproducers of group 1 chromosomal beta-lactamases. Enferm. Infecc. Microbiol. Clin. 15(Suppl. 1):20–26. [PubMed]
- 11.Mimoz O, Jacolot A, Padoin C, Caillon J, Louchahi K, Tod M, Samii K, Petitjean O. Cefepime and amikacin synergy against a cefotaxime-susceptible strain of Enterobacter cloacae in vitro and in vivo. J Antimicrob Chemother. 1997;39:363–369. doi: 10.1093/jac/39.3.363. [DOI] [PubMed] [Google Scholar]
- 12.Mimoz O, Jacolot A, Padoin C, Tod M, Samii K, Petitjean O. Cefepime and amikacin synergy in vitro and in vivo against a ceftazidime-resistant strain of Enterobacter cloacae. J Antimicrob Chemother. 1998;41:367–372. doi: 10.1093/jac/41.3.367. [DOI] [PubMed] [Google Scholar]
- 13.Palmer S M, Kang S L, Cappeletty D M, Rybak M J. Bactericidal killing of cefepime, ceftazidime, cefotaxine and ceftriaxone against Staphylococcus aureus and β-lactamase-producing strains of Enterobacter aerogenes and Klebsiella pneumoniae in an in vitro infection model. Antimicrob Agents Chemother. 1995;39:1764–1771. doi: 10.1128/aac.39.8.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pechère, J. C. 1991. Why are carbapenems active against Enterobacter cloacae resistant to third generation cephalosporins? Scand. J. Infect. Dis. 78(Suppl.):17–21. [PubMed]
- 15.Pfaller M A, Jones R N, Marshall S A, Coffman S L, Hollis R J, Edmond M B, Wenzel R P. Inducible Amp C β-lactamase producing Gram-negative bacilli from blood-stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE) Diagn Microbiol Infect Dis. 1997;28:211–219. doi: 10.1016/s0732-8893(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 16.Phelps D J, Carlton D D, Farrell C A, Kessler R E. Affinity of cephalosporins for β-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986;29:845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philippon L N, Naas T, Bouthors A T, Barakett V, Nordmann P. Oxa-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders C C, Sanders W E., Jr Type I β-lactamases of Gram-negative bacteria: interactions with β-lactam antibiotics. J Infect Dis. 1986;154:792–806. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- 19.Sanders W E, Tenney J H, Kessler R E. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis. 1996;23:454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]