Abstract
Cancer is a widespread and incurable disease caused by genetic mutations, leading to uncontrolled cell proliferation and metastasis. Connexins (Cx) are transmembrane proteins that facilitate intercellular communication via hemichannels and gap junction channels. Among them, Cx46 is found mostly in the eye lens. However, in pathological conditions, Cx46 has been observed in various types of cancers, such as glioblastoma, melanoma, and breast cancer. It has been demonstrated that elevated Cx46 levels in breast cancer contribute to cellular resistance to hypoxia, and it is an enhancer of cancer aggressiveness supporting a pro-tumoral role. Accordingly, Cx46 is associated with an increase in cancer stem cell phenotype. These cells display radio- and chemoresistance, high proliferative abilities, self-renewal, and differentiation capacities. This review aims to consolidate the knowledge of the relationship between Cx46, its role in forming hemichannels and gap junctions, and its connection with cancer and cancer stem cells.
Keywords: Connexin46, GJA3, cancer stem cells, breast cancer, gap junction channels
1. Introduction
Cancer is a devastating non-communicable disease that claims millions of lives worldwide each year [1]. Despite significant clinical and scientific efforts, a cure for cancer remains elusive. One key factor contributing to this challenge is the development of resistance by some cancer cells to conventional treatments such as chemotherapy, radiotherapy, and immunotherapy [2,3,4,5]. Normally, cells in our body divide to repair and maintain healthy tissues, but in cancer, this process becomes disrupted, leading to uncontrolled cell division and the formation of solid tumors in most cases. As these tumors grow, certain cancer cells detach and spread to distant parts of the body through the bloodstream or lymphatic system, a complex process known as metastasis [6]. Although there are many types of cancer, they all share common features such as cell dedifferentiation, significant changes in cell metabolism, loss of contact with neighboring cells, and the presence of cancer stem cells (CSCs) [7,8]. Although CSCs make up a small fraction of cells within a tumor, they are critical to cancer progression, recurrence, metastasis, and resistance to treatment [9,10]. Similar to other types of stem cells, CSCs possess the ability to self-renew and differentiate, which are key characteristics known as stemness in cancer cells [11]. Additionally, CSCs exhibit a high level of drug resistance, making them particularly challenging to eliminate [12]. Hence, a proposed model for chemoresistance and cancer relapse posits that, while conventional chemotherapy induces the death of cancer cells, cancer stem cells (CSCs) manage to survive. Over time, these CSCs repopulate the tumor with both more CSCs and new cancer cells. Consequently, this leads to the development of resistance to chemotherapy within the entire tumor [13]. On the other hand, Connexins (Cxs) are transmembrane proteins involved in the progression of cancer. Some of these proteins inhibit cancer cell aggressiveness, while others exacerbate it. Despite extensive research, the precise role of these proteins in cancer biology remains incompletely understood. However, in the past decade, certain studies have begun to shed light on the potential role of a specific type of Cx, namely Cx46, as an enhancer of CSC characteristics. In this review, our primary objective is to explore the relationship between the presence of Cx46 and the aggressiveness of cancer, with a particular focus on how it contributes to the gain of function in CSCs. We also propose potential molecular mechanisms that may underlie this phenomenon. Additionally, we address some of the controversies that have arisen between basic research findings and clinical observations.
2. Connexins General Characteristics
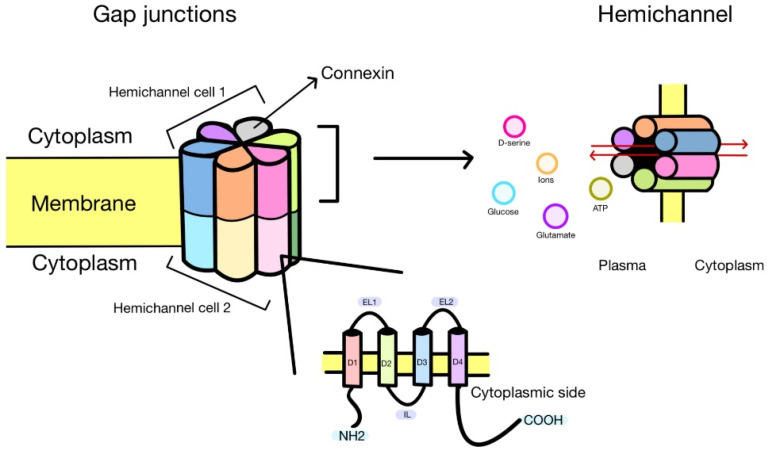
Connexins (Cxs) are a family of proteins that share a common plasma membrane structure, characterized by four transmembrane domains, two extracellular loops, one intracellular loop, and both the C- and N-termini located on the cytoplasmic side [14] (Figure 1). In humans, 21 isoforms of Cxs have been identified [15], and these isoforms are named based on their predicted molecular weight (e.g., Cx46 is predicted to have a molecular weight of ~46 kDa). While Cx isoforms display significant homology, their C-terminus is the most variable region in terms of length and amino acid sequence. Accordingly, the C-terminus of each Cx type contains diverse regulatory sites, such as consensus phosphorylation sites [16,17], pH [18], protein–protein interaction sites [19,20], and cleavage sites [21,22], among others. With the exception of Cx23 [23], almost all Cxs have six conserved extracellular cysteines, which are thought to form intramolecular disulfide bridges, crucial for hemichannel docking and the formation of gap junction channels (GJCs) [24]. However, the role of these extracellular cysteines in hemichannel function seems to be more complex, thus Cx43 hemichannels formed by a protein without extracellular cysteines remain functional [25]; however, substitution of a single cysteine for alanine in Cx46 results in hemichannels with highly reduced permeability to DAPI [26]. Moreover, these extracellular cysteines have been proposed as redox sensors in the Cx46 hemichannel [27]. In mammals, almost all cell types express at least one type of Cx, although the level of expression varies among different Cx types. Among these, Cx43 is the most widely expressed [28,29,30,31]. On the other hand, Cx46 is predominantly limited to the eye lens [32,33]. Interestingly, despite the differences in regulation and expression observed among Cxs, all of them exert their physiological and pathological functions via three distinct mechanisms: hemichannel, GJCs, and engagement in protein–protein interactions. In the next sections, we will discuss each of these mechanisms of action with some degree of detail.
Figure 1.
When two hemichannels from different cells come into contact, they form a gap junction channel, facilitating the flow of molecules and ions between these cells. Each hemichannel is composed of six connexins, each consisting of four transmembrane domains and three loops. One loop is intracellular (IL), while the other two are extracellular (EL1–EL2). D1–D4 denote transmembrane segments of a Cxs. Additionally, both the NH2 and COOH terminals face the cytoplasm. Furthermore, each hemichannel allows the bidirectional exchange (red arrows) of ions and molecules between the intracellular and extracellular environments.
Hemichannels play a crucial role in cell-to-cell communication and are composed of six Cx monomers. It depends on the type of Cx, whether it forms in the endoplasmic reticulum, Golgi apparatus, or post-Golgi vesicles, and is subsequently transported to the plasma membrane [34,35]. The presence of undocked hemichannels on the plasma membrane has been demonstrated using various techniques such as Cryo-EM, biochemical assays, electrophysiological measurements, freeze-fracture, and functional assays including dye uptake and ATP release [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. These hemichannels act as channels that are permeable not only to ions but also to molecules such as ATP [47], glutamate [48], glucose [41], and D-serine [50], among others (Figure 1). This property is based on the fact that hemichannels possess relatively large por456+es. For instance, the pore of Cx26 has an approximate diameter of 14 Å [51]. However, their selectivity appears to be complex and depends on molecular characteristics such as size, charge, and shape [52]. Currently, the understanding of the physiological roles of hemichannels is expanding each year. Thus, they have been implicated in synaptic regulation [53], memory consolidation [54], and the release of neuroactive molecules [42,55]. Furthermore, they have been found to be involved in osmotic regulation [56], light processing in the retina [57], CO2 sensing [58], and PGE2 release [59]. Many of these functions are enabled by the release of signaling molecules, as previously discussed. However, it is important to note that the controlled opening of hemichannels is crucial for these physiological processes, as uncontrolled opening can lead to cell damage due to excessive entry of Na+ and Ca2+ [60,61,62] or even cell lysis [63]. Despite the significance of controlled hemichannel opening, the precise mechanisms that regulate them under such conditions remain not yet fully understood. One possible regulatory mechanism involves transient elevations of intracellular Ca2+ concentration [64,65]. Further research is needed to fully understand the hemichannel control mechanisms in the physiological contexts.
GJCs form when two hemichannels dock at the junctional membrane between adjacent cells, where each hemichannel is contributed by different cells. As hemichannels, GJCs enable the passive exchange of ions and small molecules, facilitating communication between the cytoplasm of neighboring cells [14]. Several molecules have demonstrated some degree of permeability via GJCs, including second messengers (i.e., cAMP and IP3), metabolites (i.e., glucose), and even small peptides [66,67,68] (Figure 1). However, the solute selectivity of GJCs is determined by specific Cx isoforms [69,70]. For instance, Cx32 GJCs have been shown to be approximately 100 times less permeable to ATP than Cx43 GJCs, but they exhibit more effective transferring of adenosine compared to Cx43 GJCs [71]. As hemichannels, the variation in GJC permeability between Cx isoforms is likely attributed to the size, shape, and charge of the ions or molecules passing through these channels [72]. Under physiological conditions, GJCs play a crucial role in coordinating metabolic and signaling responses among groups of cells. For instance, it is widely accepted that Cx43 GJCs coordinate the flow of action potentials between cardiomyocytes, ensuring proper heart rhythm and function [73]. However, when changes in the permeability of Cx43 GJCs may occur due to a health problem such as a heart attack, the conduction of action potentials across the GJCs is hindered, leading to slowed conduction and the potential emergence of arrhythmias [74,75]. It is important to note that Cx43-mediated arrhythmias are a complex phenomenon that extends beyond alterations in ion conduction via GJCs. It has been also demonstrated that Cx43 can form hemichannels that can open to the plasma membrane, allowing ion flux into the extracellular space and affecting cardiomyocyte excitability [76]. Consequently, Cx43 hemichannels have been proposed as potential targets for the arrhythmia treatment [74,77]. Another example is Cx46 and Cx50 GJC in the eye lens, where they play a crucial role in the metabolic maintenance of lens cells [78]. The lens, a transparent structure without blood supply, relies on an intercellular circuit facilitated by Cx46 and Cx50 GJCs to ensure the exchange of nutrients, oxygen, and metabolic waste [79,80,81]. Specifically, Cx46 GJCs are instrumental in enabling the transport of reduced glutathione (GSH) from cortical fiber cells to nuclear cells via diffusion [82]. Consequently, any mutations or oxidative stress affecting Cx46 and Cx50 can lead to alterations in GJCs’ properties and may contribute to cataract formation [83,84]. These two cases exemplify the role of GJCs in physiological conditions, which is the exchange of metabolites and signaling molecules for the correct cell functioning and ultimately the tissue, as well as the organ. Alterations in this communication are usually associated with diseases or, in extreme cases, cell death.
In addition to the canonical functions of Cxs in forming hemichannels and GJCs, it is crucial to acknowledge that Cxs can also exert cellular effects in a channel-independent manner. Cxs have the ability to physically interact with several proteins. Based on the available data, nowadays it is possible to suggest two types of protein–protein interaction mechanisms involving Cxs. The first mechanism involves interactions that encompass the entire Cx protein, allowing it to mainly interact with proteins located near or within the plasma membrane. The second mechanism involves protein–protein interactions between the Cx-free C-terminal and various cytoplasmic proteins. This type of interaction presents an exciting topic of research and opens up new possibilities for understanding additional cellular functions mediated by Cxs.
As illustrative examples of the first proposed mechanism, Cx43 has been found to interact with β-catenin at the plasma membrane of cardiomyocytes [85]. This interaction involves the Cx43 C-terminal and is inhibited by a Src-mediated Cx43 phosphorylation at Y265 and Y313 [86]. Interestingly, this interaction plays an important role in sequestering β-catenin at the plasma membrane, resulting in a reduction in Wnt/β-catenin signaling pathway strength [85]. Another significant instance involves the interaction between Cx43 and ZO-1, which is mediated by the Cx43 C-terminal and the second PDZ domain of ZO-1 [87,88]. Notably, this protein–protein interaction appears to be regulated during the cell cycle in a rat kidney cell line (NRK) [88] and plays a pivotal role in controlling Cx43 GJC formation [89]. Furthermore, building upon this knowledge, a mimetic peptide called aCT-1 has been developed, which mimics the specific segment responsible for the interaction between Cx43 and ZO-1. This peptide has shown promising applications in skin wound healing [31,90], where its potential to modulate cellular interactions and signaling pathways holds great therapeutic value. On the other hand, Cxs have been detected in the mitochondria of various cell types, such as cardiomyocytes [91], mice vascular endothelial cells [92], and rat retinal endothelial cells [93]. Notably, mass spectrometry analyses of mice cardiomyocyte mitochondria have uncovered intriguing interactions between Cx43 and apoptosis-inducing factor (AIF) as well as the β-subunit of the electron transfer protein (ETFB) [94]. These findings strongly suggest that Cx43-mediated protein–protein interactions are not confined to specific cell membranes but can be observed in different cellular contexts. The presence of Cxs within mitochondria adds another layer of complexity to their functional roles beyond their well-established involvement in cell-to-cell communication. Understanding the significance of these protein interactions in the mitochondrial compartment could offer novel insights into mitochondrial function, cellular signaling, and cell fate regulation.
Regarding the second proposed mechanism, compelling evidence has demonstrated that the Cx C-terminal region can be transcribed independently from the rest of the Cxs [95,96], facilitated by an mRNA internal ribosome entry site (IRES) [97]. This unique C-terminal peptide demonstrates the ability to establish its own protein interactions [98,99]. In this context, the free C-terminal region of Cx43 has been shown to increase the migratory capacity of glioma cells via its interaction with the actin cytoskeleton [100]. Conversely, expression of the Cx43 C-terminal in U2OS (an osteosarcoma cell line) and HeLa cells were found to decrease their rate of cell division [101,102], potentially via its interaction with S-phase kinase-associated protein 2 (Skp2) [101]. Similarly, a peptide derived from the Cx43 C-terminal (TAT-Cx43 266-283) has been demonstrated to reduce the cancer stem cell (CSC) phenotype by inhibiting c-Src in patient-derived glioma models [103,104] and in vivo mouse models [104]. Moreover, a peptide encompassing the Cx43 C-terminal (285–363) interacts with Akt within its pleckstrin homology (PH) domain, leading to the inhibition of Akt’s function [105]. Additionally, the Cx C-terminal also plays a role in regulating the expression of various proteins [106], including N-cadherin [107] and p53 [108], as well as certain miRNAs [108]. The suggested mechanism by which the Cx C-terminal region exerts its transcriptional regulation is via its localization within the cell nucleus [109,110], where it can modulate the activity of nuclear proteins [111,112]. In the case of Cx43 C-terminal, it is proposed to act directly as a transcription factor [107].
These findings highlight the multifaceted nature of the Cx C-terminal region, extending beyond its traditional role in Cx-mediated communication. Understanding the diverse protein interactions and transcriptional regulatory functions of the Cx C-terminal region provides valuable insights into the broader cellular mechanisms orchestrated by Cxs. Further investigations in this area hold significant potential for uncovering novel therapeutic targets and strategies for various diseases and conditions associated with Connexin dysfunction. For more details on Cx-based protein–protein interaction, see previous reviews [20,98,112].
3. Cx46 in Cancer
In the 1950s, the idea that Connexins (Cxs) might influence the cell division rate was introduced [113]. Early observations indicated that the downregulation of Cx32 and Cx43, along with the loss of GJC-mediated communication, correlated with neoplastic activity in the liver and brain [114,115,116]. However, as research has progressed, it has become evident that the role of Cxs in cancer is far more complex, with some Cxs exhibiting pro-tumorigenic effects while others demonstrate anti-tumorigenic properties [117,118]. Despite numerous studies that have associated the expression of specific Cxs with changes in cancer cell characteristics, such as cell division rate, cell migration, and the CSC phenotype [118,119], only a limited number of these investigations have successfully identified the molecular mechanisms underlying the involvement of Cxs in cancer. Understanding the precise molecular mechanisms through which Cxs impact tumorigenesis may offer crucial insights into the development of targeted therapeutic approaches and potential biomarkers for various types of cancer.
Similar to many other Cxs, Cx46 has the ability to form both functional GJCs and hemichannels. In the case of Cx46-mediated GJCs, they exhibit a conductance ranging from 148 to 192 pS when studied in N2A cells, and they display sub-conductances in the range of 10 to 60 pS [120,121]. In contrast, when considering hemichannels formed by Cx46, recordings indicate a conductance of approximately 250–300 pS [122,123,124] with a sub-conductance of about 40 pS [122]. Regarding their ionic permeabilities, Cx46 hemichannels show a preference for cations over anions [124]. In terms of the permeability to larger molecules, Cx46 expressed in Xenopus oocytes was shown to allow the passage of carboxyfluorescein, Lucifer Yellow, and Ethidium [125], whereas Cx46 expressed in HeLa cells has demonstrated permeability to DAPI [126]. Additionally, Cx46-based GJCs appear to facilitate the flux of the antioxidant molecule GSH (glutathione) between lens cells, indicating a role in the transport of this vital cellular component [82]. In summary, it is evident that Cx46-based channels share several characteristics with channels formed by other connexins, highlighting the versatility and commonalities in Cx channel behavior across various contexts.
For many years, the attention on Cx46 was only focused on its role in physiological and pathological phenomena in the eye lens [33,79,84]. However, since the 2000s, the study of its possible role in cancer progression began. These studies involved animal models of lung and bone cancer, which revealed a significant decrease in both Cx46 mRNA and protein levels [127,128]. These findings strongly suggested that Cx46 may have a potential anti-cancer role. However, in 2010, a groundbreaking article was published, associating Cx46 with human cancer [129]. This study demonstrated a significant increase in Cx46 levels, as measured by Western blot and immunohistochemistry in samples of human infiltrating breast carcinoma [129]. Furthermore, in a mouse xenograft model, the injection of siRNA against Cx46 inhibited the growth of the MCF-7 human breast cancer cell line [129] and the Y79 human retinoblastoma cell line [130]. Interestingly, Cx46 is expressed in the lens, a hypoxic tissue characterized by its limited blood irrigation. This shared hypoxic condition between the lens and certain solid tumors, such as breast cancer, led to the initial hypothesis that Cx46 might function as a protective factor against hypoxia. To test this hypothesis, Banerjee et al. overexpressed Cx43GFP and Cx46GFP in N2A cells (a mouse neuroblastoma cell line that does not express any type of Cx) and subjected these cells to 1% oxygen (hypoxia). After 24 h in this condition, approximately 90% of the N2A wild-type and N2A cells transfected with Cx43GFP died, while the percentage was only 50% in cells transfected with Cx46GFP [129].
As previously mentioned, Cx46 has been implicated in promoting tumor growth in xenograft models [129,130]. Interestingly, only CSCs have the unique ability to initiate tumor growth in xenograft models [131,132], which suggests a potential link between Cx46 and the CSC phenotype. Supporting this idea, studies have exclusively identified Cx46 expression in CSCs of human glioblastoma, where it plays a crucial role in self-renewal [133]. Given the limited understanding of the underlying mechanism, our laboratory has been investigating whether Cx46 enhances the CSC phenotype in other types of cancer cells. Our research findings indicate that MCF-7 cells expressing Cx46GFP display elevated levels of Sox2 and Oct4 mRNA, along with the formation of larger tumorspheres and clonogenic colonies when compared to Cx46-negative MCF-7 cells [134]. These observations strongly suggest that Cx46 acts as a pro-tumorigenic factor, although the precise mechanism remains elusive. However, Acuña et al. recently shed some light on this matter by demonstrating that MCF-7 cells expressing Cx46 release more exosomes containing Cx46 than Cx46-negative MCF-7 cells [135]. Of greater significance, the Cx46 present on the exosomal membrane enhances the transfer of critical “information” between the exosome and the recipient cell. These findings suggest that Cx46-mediated exosomal communication may play a role in promoting the CSC phenotype and tumorigenic behavior. Additional research is needed to fully understand the molecular mechanisms by which Cx46 affects CSC function and contributes to tumor progression.
4. Unraveling the Mechanisms of Action of Cx46 in Cancer Cells
4.1. The Possible Role for Cx46-GJCs
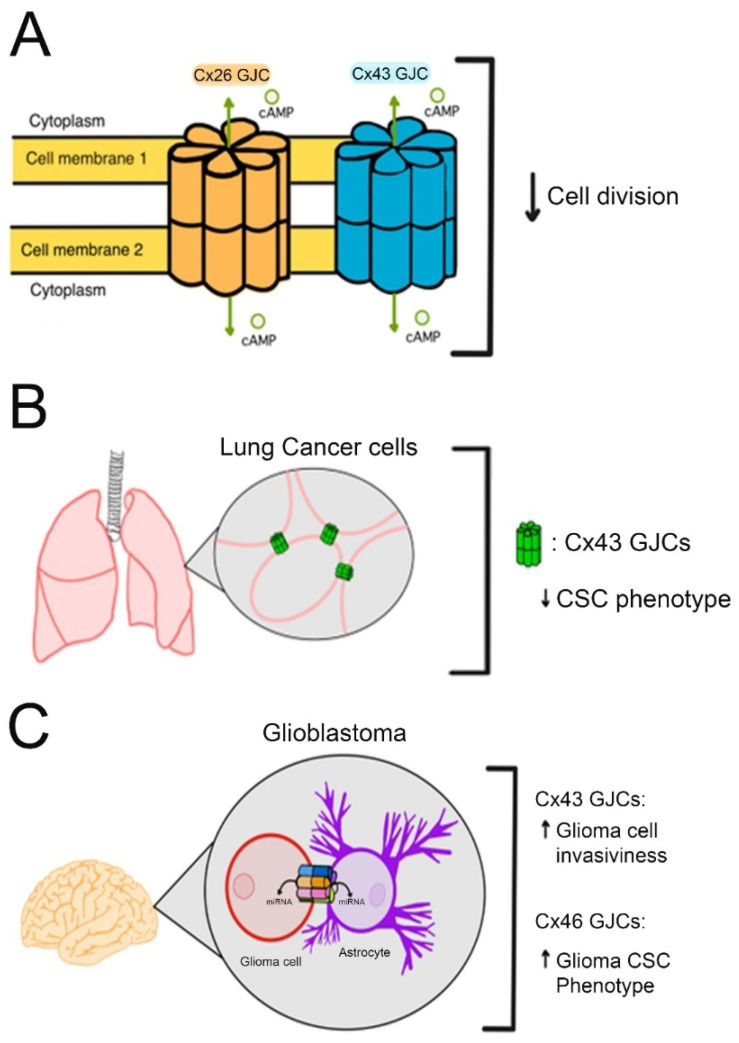
In general, it is well accepted that Cxs do not form functional GJCs in cancer cells [136] and that their re-expression and formation of GJCs can reduce cancer cell proliferation and tumor formation in vivo [137,138,139]. The most plausible mechanism for this anti-tumoral effect is allowing the diffusion of second messengers through them [140,141,142]. For example, the cAMP flow through Cx26- and Cx43-GJCs is associated with a decrease in cell division [143,144]. Similarly, Cx43 expression and GJC formation in lung cancer cells inhibit the CSC phenotype [145]. However, GJCs are not always associated with anti-tumoral effects. Thus, Cx43-GJCs expressed in glioma cells increase their invasiveness via the exchange of miRNAs between glioma cells and astrocytes [146]. In the case of Cx46, its expression was strongly associated with CSC self-renewal, and propagation in human glioblastoma [133]. Interestingly, a molecule that inhibits Cx46-GJCs (clofazimine) reduced the CSC phenotype [147], suggesting an important role of Cx46-GJCs in the maintenance of CSCs (Figure 2).
Figure 2.
Role of Cx-GJCs in regulating cancer cell aggressiveness. (A) illustrates the relationship between the flow of cyclic adenosine monophosphate (cAMP) via connexin 26 (Cx26) and connexin 43 (Cx43) gap junction channels (GJCs) and its impact on cell division. (B). shows that the formation of Cx43 GJCs in lung cancer cells has an inhibitory effect on the Cancer Stem Cell (CSC) phenotype. (C) However, in glioblastoma, Cx43 GJCs are involved in the increase in glioma cells’ invasiveness capacity by facilitating the exchange of microRNAs (miRNAs) between glioma cells and astrocytes. Additionally, Cx46 GJC seems to enhance CSC phenotype, however, the mechanism remains unknown.
4.2. The Possible Role for Cx46 Hemichannels
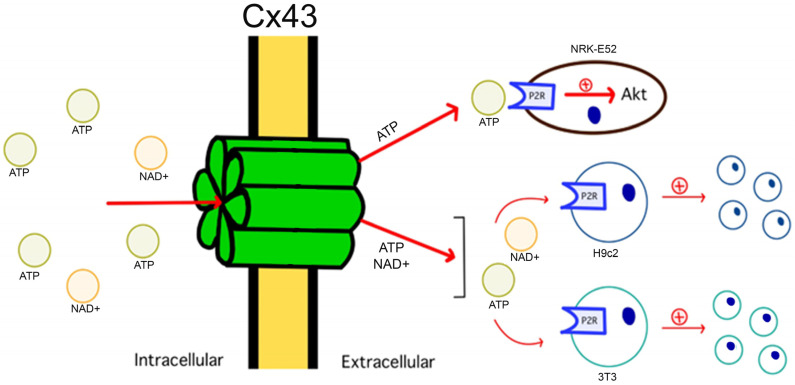
The role of hemichannels in cancer has been poorly investigated. As previously mentioned, Cx hemichannels enable the exchange of signaling molecules between the cytoplasm and the extracellular environment [38,148,149]. For example, Cx43 hemichannels regulate H9c2 and 3T3 cell proliferation via ATP [150] and NAD+ [151] release. Additionally, it has been proposed that Cx43 hemichannels can activate Akt in NRK-E52 cells via the release of ATP [152]. Similarly, only functional Cx37 hemichannels have been found to suppress cell proliferation in rat insulinoma [153]. Regarding Cx46 hemichannels, their permeability to biological molecules like ATP has been suggested [154] but has not yet been proven. Because Cx46 hemichannels are permeable to synthetic molecules with molecular weight and size comparable to those of the biological molecules listed above [126,155,156], there is no reason to expect that Cx46 hemichannels are not permeable to them. Therefore, Cx46 hemichannels could increase the CSC phenotype via the exchange of biological molecules between the cytoplasm and the extracellular milieu (Figure 3).
Figure 3.
Role of Cx-Hemichannels in cell division regulation via the release of signaling molecule Release. Cx43 hemichannels are regulators of cell division by allowing the controlled release of signaling molecules, such as ATP and NAD+. These molecules, upon reaching the extracellular space, activate specific receptors and initiate signaling cascades, such as those controlled by Akt, which in turn modulates cell proliferation and impacts tissue homeostasis and development.
4.3. The Possible Role of Cx46 Protein–Protein Interactions
As previously mentioned, Cxs can have intracellular effects that are independent of their role as channels, mainly via interactions with other proteins [20,112,157,158,159,160,161,162,163,164,165,166,167], and their C-terminus mediates the vast majority of these Cx–protein interactions [19,157,158,162]. Regarding CSCs, the Cx26 C-terminal interacts with Nanog, promoting CSC renewal in triple-negative breast cancer cells [164]. Likewise, the accumulation of Cx32 in the cytoplasm has been shown to enhance CSC renewal in HuH7 hepatoma cells [163], likely via protein–protein interactions. On the other hand, Cx26, which has been associated with a pro-tumorigenic role [118] increases PI3K/Akt activity in NSCLC cells in a channel-independent way [164]. Conversely, Cx43, which, in general, is considered anti-tumorigenic [145,167] via the interaction of its C-terminal with Akt [106], induces its inhibition [167]. Despite that, Cx46 can interact with several proteins [168,169,170,171,172], but the potential interaction between Cx46 and cancer-relevant proteins such as PI3K/Akt has not been investigated.
5. Cx46 in Human Cancer Samples
Unfortunately, only a few studies have investigated the expression of Cx46 in human breast cancer and its association with patient survival. Remarkably, utilizing fluorescent microscopy revealed that Cx46 expression in human breast cancer samples is significantly associated with improved overall survival (OS) for patients [173]. Nevertheless, other studies have found no significant correlation between Cx46 mRNA levels and patient OS [174]. Such contradictions between cell culture studies, where the presence of Cx46 is linked to a more aggressive phenotype of breast cancer cells and the results obtained from human samples warrant further investigation. A possible explanation for these conflicting results is that there might not be a direct correlation between Cx46 mRNA and protein levels. This possibility is supported by the fact that at least in myeloid leukemia cells, there is no correlation between the mRNA and protein levels for Cx26, Cx32, Cx37, Cx43, and Cx45 [175]. Additionally, in HUVEC cells exposed to laminar flow, there is an increase in Cx40 mRNA levels with no significant changes in protein levels [176]. Additionally, from our own laboratory experience, we recognize the crucial importance of selecting an appropriate and validated antibody to ensure reliable and representative results when Western blot analyses and immunofluorescence studies are performed. Therefore, in-depth and comprehensive experiments are indispensable to gain a clear understanding of the significance of Cx46 in breast cancer. By conducting further research, including larger clinical studies and meticulous examination of Cx46 expression at both the mRNA and protein levels, we can hope to unravel the complex role of Cx46 in breast cancer more accurately.
6. Discussion
In the last years, the role of Cx46 in cancer has risen, mostly because results in animal models and human cell lines point out that this protein could be pro-tumorigenic and, moreover, it can be a key factor for the enhancement of EMT and CSC in cancer cells. However, studies correlating human samples of patients with breast cancer suggest that the presence of Cx46 is linked to better overall survival. Therefore, further studies are needed to determine whether Cx46 protein levels are associated with a favorable or unfavorable patient prognosis. Studies correlating the levels of Cx46 mRNA with protein levels could shed some light and obtain better antibodies to help to determine the real Cx46 protein levels in human samples.
Author Contributions
Conceptualization, M.A.R.; investigation, M.A.R.; writing—original draft preparation, M.A.R.; writing—review and editing, M.A.R., I.M.L.-F., M.G.S.-G. and M.S.N.; visualization, M.A.R., I.M.L.-F., M.G.S.-G. and M.S.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We will send all the figures and results as requested.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Francescangeli F., De Angelis M.L., Rossi R., Cuccu A., Giuliani A., De Maria R., Zeuner A. Dormancy, stemness, and therapy resistance: Interconnected players in cancer evolution. Cancer Metastasis Rev. 2023;42:197–215. doi: 10.1007/s10555-023-10092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willers H., Azzoli C.G., Santivasi W.L., Xia F. Basic Mechanisms of Therapeutic Resistance to Radiation and Chemotherapy in Lung Cancer. Cancer J. 2013;19:200. doi: 10.1097/PPO.0b013e318292e4e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J., Li L., Guo B., Liu D., Shi J., Wu C., Chen J., Zhang X., Wu J. Mechanisms of resistance to chemotherapy and radiotherapy in hepatocellular carcinoma. Transl. Cancer Res. 2018;7:765–781. doi: 10.21037/tcr.2018.05.20. [DOI] [Google Scholar]
- 5.Said S.S., Ibrahim W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics. 2023;15:1143. doi: 10.3390/pharmaceutics15041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch D.R. Do we need to redefine a cancer metastasis and staging definitions? Breast Dis. 2006;26:3–12. doi: 10.3233/BD-2007-26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birchmeier C., Birchmeier W., Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 1996;156:217–226. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
- 8.Aponte P.M., Caicedo A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017;2017:5619472. doi: 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafi M., Farhood B., Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2018;234:8381–8395. doi: 10.1002/jcp.27740. [DOI] [PubMed] [Google Scholar]
- 10.Ayob A.Z., Ramasamy T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav A.K., Desai N.S. Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects. Stem Cell Rev. Rep. 2019;15:331–355. doi: 10.1007/s12015-019-09887-2. [DOI] [PubMed] [Google Scholar]
- 12.Schöning J.P., Monteiro M., Gu W. Drug resistance and cancer stem cells: The shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin. Exp. Pharmacol. Physiol. 2017;44:153–161. doi: 10.1111/1440-1681.12693. [DOI] [PubMed] [Google Scholar]
- 13.Shrestha S., Banstola A., Jeong J.H., Seo J.H., Yook S. Targeting Cancer Stem Cells: Therapeutic and diagnostic strategies by the virtue of nanoparticles. J. Control. Release. 2022;348:518–536. doi: 10.1016/j.jconrel.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Sáez J.C., Berthoud V.M., Brañes M.C., Martínez A.D., Beyer E.C. Plasma Membrane Channels Formed by Connexins: Their Regulation and Functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 15.Söhl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Lampe P.D., Lau A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solan J.L., Lampe P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 2018;1860:83–90. doi: 10.1016/j.bbamem.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trexler E.B., Bukauskas F.F., Bennett M.V.L., Bargiello T.A., Verselis V.K. Rapid and direct effects of pH on connexins revealed by the connexin 46 hemichannel preparation. J. Gen. Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervé J.C., Bourmeyster N., Sarrouilhe D. Diversity in protein-protein interactions of connexins: Emerging roles. Biochim. Biophys. Acta Biomembr. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Sorgen P., Trease A., Spagnol G., Delmar M., Nielsen M. Protein–Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018;19:1428. doi: 10.3390/ijms19051428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J.S., Fitzgerald S., Dong Y., Knight C., Donaldson P., Kistler J. Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur. J. Cell Biol. 1997;73:141–149. [PubMed] [Google Scholar]
- 22.Zhang X., Qi Y. Role of intramolecular interaction in connexin50: Mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch. Biochem. Biophys. 2005;440:111–117. doi: 10.1016/j.abb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Iovine M.K., Gumpert A.M., Falk M.M., Mendelson T.C. Cx23, a connexin with only four extracellular-loop cysteines, forms functional gap junction channels and hemichannels. FEBS Lett. 2008;582:165–170. doi: 10.1016/j.febslet.2007.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl G., Levine E., Rabadan-Diehl C., Werner R. Cell/cell channel formation involves disulfide exchange. Eur. J. Biochem. 1991;197:141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- 25.Bao X., Chen Y., Reuss L., Altenberg G.A. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J. Biol. Chem. 2004;279:9689–9692. doi: 10.1074/jbc.M311438200. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Olivares A., Durán-Jara E., Verdugo D.A., Fiori M.C., Altenberg G.A., Stehberg J., Alfaro I., Calderón J.F., Retamal M.A. Extracellular Cysteines Are Critical to Form Functional Cx46 Hemichannels. Int. J. Mol. Sci. 2022;23:7252. doi: 10.3390/ijms23137252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Retamal M.A., García I.E., Pinto B.I., Pupo A., Báez D., Stehberg J., Del Rio R., González C. Extracellular cysteine in connexins: Role as redox sensors. Front. Physiol. 2016;7:1. doi: 10.3389/fphys.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orellana J.A., Froger N., Ezan P., Jiang J.X., Bennett M.V.L., Naus C.C., Giaume C., Sáez J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boengler K., Schulz R. Advances in Experimental Medicine and Biology. Volume 982. Springer; New York, NY, USA: 2017. Connexin 43 and mitochondria in cardiovascular health and disease; pp. 227–246. [DOI] [PubMed] [Google Scholar]
- 30.Straub A.C., Billaud M., Johnstone S.R., Best A.K., Yemen S., Dwyer S.T., Looft-Wilson R., Lysiak J.J., Gaston B., Palmer L., et al. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery J., Ghatnekar G.S., Grek C.L., Moyer K.E., Gourdie R.G. Connexin 43-based therapeutics for dermal wound healing. Int. J. Mol. Sci. 2018;19:1778. doi: 10.3390/ijms19061778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul D.L., Ebihara L., Takemoto L.J., Swenson K.I., Goodenough D.A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthoud V.M., Ngezahayo A. Focus on lens connexins. BMC Cell Biol. 2017;18:6. doi: 10.1186/s12860-016-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zong Y.-J., Liu X.-Z., Tu L., Sun Y. Cytomembrane Trafficking Pathways of Connexin 26, 30, and 43. Int. J. Mol. Sci. 2023;24:10349. doi: 10.3390/ijms241210349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin P.E.M., Evans W.H. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 2004;62:378–387. doi: 10.1016/j.cardiores.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.J., Jeong H., Hyun J., Ryu B., Park K., Lim H.H., Yoo J., Woo J.S. Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020;6:eaba4996. doi: 10.1126/sciadv.aba4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan A.K., Jagielnicki M., Bennett B.C., Purdy M.D., Yeager M. Cryo-EM structure of an open conformation of a gap junction hemichannel in lipid bilayer nanodiscs. Structure. 2021;29:1040–1047.e3. doi: 10.1016/j.str.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plotkin L.I., Manolagas S.C., Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 39.Retamal M.A., Cortés C.J., Reuss L., Bennett M.V.L., Sáez J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Retamal M.A., Yin S., Altenberg G.A., Reuss L. Voltage-dependent facilitation of Cx46 hemichannels. Am. J. Physiol.-Cell Physiol. 2010;298:C132–C139. doi: 10.1152/ajpcell.00258.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras J.E., Saez J.C., Bukauskas F.F., Bennett M.V.L. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zampighi G.A. Distribution of connexin50 channels and hemichannels in lens fibers: A structural approach. Cell Commun. Adhes. 2003;10:265–270. doi: 10.1080/cac.10.4-6.265.270. [DOI] [PubMed] [Google Scholar]
- 44.Sáez J.C., Schalper K.A., Retamal M.A., Orellana J.A., Shoji K.F., Bennett M.V.L. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen B.S., Zonta F., Farkas T., Litman T., Nielsen M.S., MacAulay N. Structural determinants underlying permeant discrimination of the Cx43 hemichannel. J. Biol. Chem. 2019;294:16789–16803. doi: 10.1074/jbc.RA119.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puhar A., Sansonetti P. Dye-uptake Experiment through Connexin Hemichannels. Bio-Protocol. 2014;4:e1221. doi: 10.21769/BioProtoc.1221. [DOI] [Google Scholar]
- 47.Stout C.E., Costantin J.L., Naus C.C.G., Charles A.C. Intercellular Calcium Signaling in Astrocytes via ATP Release through Connexin Hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 48.Ye Z.-C., Wyeth M.S., Baltan-Tekkok S., Ransom B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Retamal M.A., Froger N., Palacios-Prado N., Ezan P., Sáez P.J., Sáez J.C., Giaume C. Cx43 Hemichannels and Gap Junction Channels in Astrocytes Are Regulated Oppositely by Proinflammatory Cytokines Released from Activated Microglia. J. Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linsambarth S., Carvajal F.J., Moraga-Amaro R., Mendez L., Tamburini G., Jimenez I., Verdugo D.A., Gómez G.I., Jury N., Martínez P., et al. Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 2022;36:e22134. doi: 10.1096/fj.202100798RR. [DOI] [PubMed] [Google Scholar]
- 51.Maeda S., Nakagawa S., Suga M., Yamashita E., Oshima A., Fujiyoshi Y., Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 52.Nicholson B.J., Weber P.A., Cao F., Chang H.C., Lampe P., Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 2000;33:369–378. doi: 10.1590/S0100-879X2000000400002. [DOI] [PubMed] [Google Scholar]
- 53.Moore A.R., Zhou W.-L., Sirois C.L., Belinsky G.S., Zecevic N., Antic S.D. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. USA. 2014;111:E3919–E3928. doi: 10.1073/pnas.1405253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stehberg J., Moraga-Amaro R., Salazar C., Becerra A., Echeverría C., Orellana J.A., Bultynck G., Ponsaerts R., Leybaert L., Simon F., et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012;26:3649–3657. doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- 55.Orellana J.A. Advances in Experimental Medicine and Biology. Volume 949. Springer; Cham, Switzerland: 2016. Physiological Functions of Glial Cell Hemichannels; pp. 93–108. [DOI] [PubMed] [Google Scholar]
- 56.Quist A.P., Rhee S.K., Lin H., Lal R. Physiological role of gap-junctional hemichannels: Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamermans M., Fahrenfort I., Schultz K., Janssen-Bienhold U., Sjoerdsma T., Weiler R. Hemichannel-Mediated Inhibition in the Outer Retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 58.Dospinescu V.M., Nijjar S., Spanos F., Cook J., de Wolf E., Biscotti M.A., Gerdol M., Dale N. Structural determinants of CO2-sensitivity in the β connexin family suggested by evolutionary analysis. Commun. Biol. 2019;2:331. doi: 10.1038/s42003-019-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherian P.P., Siller-Jackson A.J., Gu S., Wang X., Bonewald L.F., Sprague E., Jiang J.X. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin. Mol. Biol. Cell. 2005;16:3100–3106. doi: 10.1091/mbc.e04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiori M.C., Figueroa V., Zoghbi M.E., Saéz J.C., Reuss L., Altenberg G.A. Permeation of calcium through purified connexin 26 hemichannels. J. Biol. Chem. 2012;287:40826–40834. doi: 10.1074/jbc.M112.383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sánchez H.A., Meşe G., Srinivas M., White T.W., Verselis V.K. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010;136:47–62. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li F., Sugishita K., Su Z., Ueda I., Barry W.H. Activation of connexin-43 hemichannels can elevate [Ca2+]i and[Na+]i in rabbit ventricular myocytes during metabolic inhibition. J. Mol. Cell. Cardiol. 2001;33:2145–2155. doi: 10.1006/jmcc.2001.1477. [DOI] [PubMed] [Google Scholar]
- 63.Gerido D.A., DeRosa A.M., Richard G., White T.W. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol.-Cell Physiol. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 64.De Vuyst E., Wang N., Decrock E., De Bock M., Vinken M., Van Moorhem M., Lai C., Culot M., Rogiers V., Cecchelli R., et al. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 65.De Vuyst E., Decrock E., Cabooter L., Dubyak G.R., Naus C.C., Evans W.H., Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris A.L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pang B., Neijssen J., Qiao X., Janssen L., Janssen H., Lippuner C., Neefjes J. Direct Antigen Presentation and Gap Junction Mediated Cross-Presentation during Apoptosis. J. Immunol. 2009;183:1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez V.H., Bortolozzi M., Pertegato V., Beltramello M., Giarin M., Zaccolo M., Pantano S., Mammano F. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat. Methods. 2007;4:353–358. doi: 10.1038/nmeth1031. [DOI] [PubMed] [Google Scholar]
- 69.Weber P.A., Chang H.C., Spaeth K.E., Nitsche J.M., Nicholson B.J. The Permeability of Gap Junction Channels to Probes of Different Size Is Dependent on Connexin Composition and Permeant-Pore Affinities. Biophys. J. 2004;87:958. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bevans C.G., Kordel M., Rhee S.K., Harris A.L. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg G.S., Moreno A.P., Lampe P.D. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J. Biol. Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- 72.Kanaporis G., Brink P.R., Valiunas V. Gap junction permeability: Selectivity for anionic and cationic probes. Am. J. Physiol.-Cell Physiol. 2011;300:C600–C609. doi: 10.1152/ajpcell.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodríguez-Sinovas A., Sánchez J.A., Valls-Lacalle L., Consegal M., Ferreira-González I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021;22:4413. doi: 10.3390/ijms22094413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leybaert L., De Smet M.A.J., Lissoni A., Allewaert R., Roderick H.L., Bultynck G., Delmar M., Sipido K.R., Witschas K. Connexin hemichannels as candidate targets for cardioprotective and anti-arrhythmic treatments. J. Clin. Investig. 2023;133:e168117. doi: 10.1172/JCI168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danik S.B., Liu F., Zhang J., Suk H.J., Morley G.E., Fishman G.I., Gutstein D.E. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ. Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lillo M.A., Muñoz M., Rhana P., Gaul-Muller K., Quan J., Shirokova N., Xie L.-H., Santana L.F., Fraidenraich D., Contreras J.E. Remodeled connexin 43 hemichannels alter cardiac excitability and promote arrhythmias. J. Gen. Physiol. 2023;155:e202213150. doi: 10.1085/jgp.202213150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andelova K., Benova T.E., Bacova B.S., Sykora M., Prado N.J., Diez E.R., Hlivak P., Tribulova N. Cardiac connexin-43 hemichannels and pannexin1 channels: Provocative antiarrhythmic targets. Int. J. Mol. Sci. 2021;22:260. doi: 10.3390/ijms22010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng C., Nowak R.B., Gao J., Sun X., Biswas S.K., Lo W.K., Mathias R.T., Fowler V.M. Lens ion homeostasis relies on the assembly and/or stability of large connexin 46 gap junction plaques on the broad sides of differentiating fiber cells. Am. J. Physiol. Cell Physiol. 2015;308:C835. doi: 10.1152/ajpcell.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beyer E.C., Berthoud V.M. Connexin hemichannels in the lens. Front. Physiol. 2014;5:20. doi: 10.3389/fphys.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson K.R., Patterson J.W. Localization of steady currents in the lens. Curr. Eye Res. 1982;2:843–847. doi: 10.3109/02713688209020020. [DOI] [PubMed] [Google Scholar]
- 81.Mathias R., Rae J., Baldo G. Physiological properties of the normal lens. Physiol. Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 82.Slavi N., Rubinos C., Li L., Sellitto C., White T.W., Mathias R., Srinivas M. Connexin 46 (Cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J. Biol. Chem. 2014;289:32694–32702. doi: 10.1074/jbc.M114.597898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berthoud V.M., Gao J., Minogue P.J., Jara O., Mathias R.T., Beyer E.C. Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization. Int. J. Mol. Sci. 2020;21:5822. doi: 10.3390/ijms21165822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berthoud V.M., Beyer E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal. 2009;11:339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ai Z., Fischer A., Spray D.C., Brown A.M., Fishman G.I. Wnt-1 regulation of connexin43 in cardiac myocytes. J. Clin. Investig. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spagnol G., Trease A.J., Zheng L., Gutierrez M., Basu I., Sarmiento C., Moore G., Cervantes M., Sorgen P.L. Connexin43 carboxyl-terminal domain directly interacts with β-catenin. Int. J. Mol. Sci. 2018;19:1562. doi: 10.3390/ijms19061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giepmans B.N.G., Moolenaar W.H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998;8:931–934. doi: 10.1016/S0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 88.Singh D., Solan J.L., Taffet S.M., Javier R., Lampe P.D. Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner. J. Biol. Chem. 2005;280:30416–30421. doi: 10.1074/jbc.M506799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhett J.M., Jourdan J., Gourdie R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell. 2011;22:1516–1528. doi: 10.1091/mbc.e10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghatnekar G.S., O’Quinn M.P., Jourdan L.J., Gurjarpadhye A.A., Draugh R.L., Gourdie R.G. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen. Med. 2009;4:205–223. doi: 10.2217/17460751.4.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miro-Casas E., Ruiz-Meana M., Agullo E., Stahlhofen S., Rodríguez-Sinovas A., Cabestrero A., Jorge I., Torre I., Vazquez J., Boengler K., et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009;83:747–756. doi: 10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- 92.Guo R., Si R., Scott B.T., Makino A. Mitochondrial connexin40 regulates mitochondrial calcium uptake in coronary endothelial cells. Am. J. Physiol. Cell Physiol. 2017;312:C398–C406. doi: 10.1152/ajpcell.00283.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sankaramoorthy A., Roy S. High Glucose-Induced Apoptosis Is Linked to Mitochondrial Connexin 43 Level in RRECs: Implications for Diabetic Retinopathy. Cells. 2021;10:3102. doi: 10.3390/cells10113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denuc A., Núñez E., Calvo E., Loureiro M., Miro-Casas E., Guarás A., Vázquez J., Garcia-Dorado D. New protein-protein interactions of mitochondrial connexin 43 in mouse heart. J. Cell. Mol. Med. 2016;20:794–803. doi: 10.1111/jcmm.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ul-Hussain M., Dermietzel R., Zoidl G. Connexins and Cap-independent translation: Role of internal ribosome entry sites. Brain Res. 2012;1487:99–106. doi: 10.1016/j.brainres.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 96.Salat-Canela C., Sesé M., Peula C., Ramón Y Cajal S., Aasen T. Internal translation of the connexin 43 transcript. Cell Commun. Signal. 2014;12:31. doi: 10.1186/1478-811X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leithe E., Mesnil M., Aasen T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta Biomembr. 2018;1860:48–64. doi: 10.1016/j.bbamem.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Joshi-Mukherjee R., Coombs W., Burrer C., Alvarez de Mora I., Delmar M., Taffet S.M. Evidence for the presence of a free C-Terminal fragment of Cx43 in cultured cells. Cell Commun. Adhes. 2007;14:75–84. doi: 10.1080/15419060701402320. [DOI] [PubMed] [Google Scholar]
- 99.Crespin S., Bechberger J., Mesnil M., Naus C.C., Sin W.C. The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J. Cell. Biochem. 2010;110:589–597. doi: 10.1002/jcb.22554. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y.W., Kaneda M., Morita I. The Gap Junction-independent Tumor-suppressing Effect of Connexin 43. J. Biol. Chem. 2003;278:44852–44856. doi: 10.1074/jbc.M305072200. [DOI] [PubMed] [Google Scholar]
- 101.Dang X., Doble B.W., Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell. Biochem. 2003;242:35–38. doi: 10.1023/A:1021152709313. [DOI] [PubMed] [Google Scholar]
- 102.Gangoso E., Thirant C., Chneiweiss H., Medina J.M., Tabernero A. A cell-penetrating peptide based on the interaction between c-Src and connexin43 reverses glioma stem cell phenotype. Cell Death Dis. 2014;5:e1023. doi: 10.1038/cddis.2013.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaraíz-Rodríguez M., Tabernero M.D., González-Tablas M., Otero A., Orfao A., Medina J.M., Tabernero A. A Short Region of Connexin43 Reduces Human Glioma Stem Cell Migration, Invasion, and Survival through Src, PTEN, and FAK. Stem Cell Rep. 2017;9:451–463. doi: 10.1016/j.stemcr.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaraíz-Rodríguez M., Talaverón R., García-Vicente L., Pelaz S.G., Domínguez-Prieto M., Álvarez-Vázquez A., Flores-Hernández R., Sin W.C., Bechberger J., Medina J.M., et al. Connexin43 peptide, TAT-Cx43266–283, selectively targets glioma cells, impairs malignant growth, and enhances survival in mouse models in vivo. Neuro. Oncol. 2020;22:493–504. doi: 10.1093/neuonc/noz243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuang J.-Y., Guo Y.-F., Chen Y., Wang J., Duan J.-J., He X.-L., Li L., Yu S.-C., Bian X.-W. Connexin 43 C-terminus directly inhibits the hyperphosphorylation of Akt/ERK through protein-protein interactions in glioblastoma. Cancer Sci. 2018;109:2611–2622. doi: 10.1111/cas.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kardami E., Dang X., Iacobas D., Nickel B., Jeyaraman M., Srisakuldee W., Makazan J., Tanguy S., Spray D. The role of connexins in controlling cell growth and gene expression. Prog. Biophys. Mol. Biol. 2007;94:245–264. doi: 10.1016/j.pbiomolbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 107.Kotini M., Barriga E.H., Leslie J., Gentzel M., Rauschenberger V., Schambony A., Mayor R. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat. Commun. 2018;9:3846. doi: 10.1038/s41467-018-06368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maqbool R., Rashid R., Ismail R., Niaz S., Chowdri N.A., Hussain M.U. The carboxy-terminal domain of connexin 43 (CT-Cx43) modulates the expression of p53 by altering miR-125b expression in low-grade human breast cancers. Cell. Oncol. 2015;38:443–451. doi: 10.1007/s13402-015-0240-x. [DOI] [PubMed] [Google Scholar]
- 109.Hebert C., Stains J.P. An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells. J. Cell. Biochem. 2013;114:2542–2550. doi: 10.1002/jcb.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Jimenez F.J., Alastrue A., Stojkovic M., Erceg S., Moreno-Manzano V. Connexin 50 modulates Sox2 expression in spinal-cord-derived ependymal stem/progenitor cells. Cell Tissue Res. 2016;365:295–307. doi: 10.1007/s00441-016-2421-y. [DOI] [PubMed] [Google Scholar]
- 111.Gago-Fuentes R., Fernández-Puente P., Megias D., Carpintero-Fernández P., Mateos J., Acea B., Fonseca E., Blanco F.J., Mayan M.D. Proteomic Analysis of Connexin 43 Reveals Novel Interactors Related to Osteoarthritis. Mol. Cell. Proteom. 2015;14:1831–1845. doi: 10.1074/mcp.M115.050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Campenhout R., Cooreman A., Leroy K., Rusiecka O.M., Van Brantegem P., Annaert P., Muyldermans S., Devoogdt N., Cogliati B., Kwak B.R., et al. Non-canonical roles of connexins. Prog. Biophys. Mol. Biol. 2020;153:35–41. doi: 10.1016/j.pbiomolbio.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Loewenstein W.R., Kanno Y. Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature. 1966;209:1248–1249. doi: 10.1038/2091248a0. [DOI] [PubMed] [Google Scholar]
- 114.Naus C.C., Elisevich K., Zhu D., Belliveau D.J., Del Maestro R.F. In vivo growth of C6 glioma cells transfected with connexin43 cDNA. Cancer Res. 1992;52:4208–4213. [PubMed] [Google Scholar]
- 115.Temme A., Buchmann A., Gabriel H.D., Nelles E., Schwarz M., Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 1997;7:713–716. doi: 10.1016/S0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- 116.Sinyuk M., Mulkearns-Hubert E.E., Reizes O., Lathia J. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front. Oncol. 2018;8:646. doi: 10.3389/fonc.2018.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aasen T., Leithe E., Graham S.V., Kameritsch P., Mayán M.D., Mesnil M., Pogoda K., Tabernero A. Connexins in cancer: Bridging the gap to the clinic. Oncogene. 2019;38:4429–4451. doi: 10.1038/s41388-019-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beckmann A., Hainz N., Tschernig T., Meier C. Facets of communication: Gap junction ultrastructure and function in cancer stem cells and tumor cells. Cancers. 2019;11:288. doi: 10.3390/cancers11030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aasen T., Mesnil M., Naus C.C., Lampe P.D., Laird D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer. 2016;16:775–788. doi: 10.1038/nrc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hopperstad M.G., Srinivas M., Spray D.C. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys. J. 2000;79:1954–1966. doi: 10.1016/S0006-3495(00)76444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaradat R., Li X., Chen H., Stathopulos P.B., Bai D. The Hydrophobic Residues in Amino Terminal Domains of Cx46 and Cx50 Are Important for Their Gap Junction Channel Ion Permeation and Gating. Int. J. Mol. Sci. 2022;23:11605. doi: 10.3390/ijms231911605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu X., Ma M., Dahl G. Conductance of connexin hemichannels segregates with the first transmembrane segment. Biophys. J. 2006;90:140–150. doi: 10.1529/biophysj.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valiunas V., Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflug. Arch. Eur. J. Physiol. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 124.Trexler E.B., Bennett M.V.L., Bargiello T.A., Verselis V.K. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Retamal M.A.M.A., Yin S., Altenberg G.A.G.A., Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am. J. Physiol.-Cell Physiol. 2009;296:C1356–C1363. doi: 10.1152/ajpcell.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Retamal M.A., Fiori M.C., Fernandez-Olivares A., Linsambarth S., Peña F., Quintana D., Stehberg J., Altenberg G.A. 4-Hydroxynonenal induces Cx46 hemichannel inhibition through its carbonylation. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids. 2020;1865:158705. doi: 10.1016/j.bbalip.2020.158705. [DOI] [PubMed] [Google Scholar]
- 127.Avanzo J.L., Mesnil M., Hernandez-Blazquez F.J., da Silva T.C., Fukumasu H., Mori C.M.C., Yamasaki H., Dagli M.L.Z. Altered expression of connexins in urethane-induced mouse lung adenomas. Life Sci. 2006;79:2202–2208. doi: 10.1016/j.lfs.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 128.Sanches D.S., Pires C.G., Fukumasu H., Cogliati B., Matsuzaki P., Chaible L.M., Torres L.N., Ferrigno C.R.A., Dagli M.L.Z. Expression of Connexins in Normal and Neoplastic Canine Bone Tissue. Vet. Pathol. 2009;46:846–859. doi: 10.1354/vp.08-VP-0263-S-FL. [DOI] [PubMed] [Google Scholar]
- 129.Banerjee D., Gakhar G., Madgwick D., Hurt A., Takemoto D., Nguyen T.A. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int. J. Cancer. 2010;127:839–848. doi: 10.1002/ijc.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burr D.B., Molina S.A., Banerjee D., Low D.M., Takemoto D.J. Treatment with connexin 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp. Eye Res. 2011;92:251–259. doi: 10.1016/j.exer.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Brien C.A., Kreso A., Jamieson C.H.M. Cancer stem cells and self-renewal. Clin. Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 132.Abbaszadegan M.R., Bagheri V., Razavi M.S., Momtazi A.A., Sahebkar A., Gholamin M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell. Physiol. 2017;232:2008–2018. doi: 10.1002/jcp.25759. [DOI] [PubMed] [Google Scholar]
- 133.Hitomi M., Deleyrolle L.P., Mulkearns-Hubert E.E., Jarrar A., Li M., Sinyuk M., Otvos B., Brunet S., Flavahan W.A., Hubert C.G., et al. Differential Connexin Function Enhances Self-Renewal in Glioblastoma. Cell Rep. 2015;11:1031–1042. doi: 10.1016/j.celrep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Acuña R.A., Varas-Godoy M., Herrera-Sepulveda D., Retamal M.A. Connexin46 Expression Enhances Cancer Stem Cell and Epithelial-to-Mesenchymal Transition Characteristics of Human Breast Cancer MCF-7 Cells. Int. J. Mol. Sci. 2021;22:12604. doi: 10.3390/ijms222212604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Acuña R.A., Varas-Godoy M., Berthoud V.M., Alfaro I.E., Retamal M.A. Connexin-46 contained in extracellular vesicles enhance malignancy features in breast cancer cells. Biomolecules. 2020;10:676. doi: 10.3390/biom10050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leithe E., Sirnes S., Omori Y., Rivedal E. Downregulation of gap junctions in cancer cells. Crit. Rev. Oncog. 2006;12:225–256. doi: 10.1615/CritRevOncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 137.Eghbali B., Kessler J.A., Reid L.M., Roy C., Spray D.C. Involvement of gap junctions in tumorigenesis: Transfection of tumor cells with connexin 32 cDNA retards growth in vivo. Proc. Natl. Acad. Sci. USA. 1991;88:10701–10705. doi: 10.1073/pnas.88.23.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamasaki H. Role of cell-cell communication in tumor suppression. Immunol. Ser. 1990;51:245–266. [PubMed] [Google Scholar]
- 139.Zhu D., Caveney S., Kidder G.M., Naus C.C.G. Transfection of C6 glioma cells with connexin 43 cDNA: Analysis of expression, intercellular coupling, and cell proliferation. Proc. Natl. Acad. Sci. USA. 1991;88:1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saez J.C., Connor J.A., Spray D.C., Bennett M.V.L. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc. Natl. Acad. Sci. USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valiunas V., Brink P.R., White T.W. Lens connexin channels have differential permeability to the second messenger cAMP. Investig. Ophthalmol. Vis. Sci. 2019;60:3821–3829. doi: 10.1167/iovs.19-27302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanaporis G., Mese G., Valiuniene L., White T.W., Brink P.R., Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J. Gen. Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandrasekhar A., Kalmykov E.A., Polusani S.R., Mathis S.A., Zucker S.N., Nicholson B.J. Intercellular redistribution of cAMP underlies selective suppression of cancer cell growth by connexin26. PLoS ONE. 2013;8:e82335. doi: 10.1371/journal.pone.0082335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y.W., Morita I., Ikeda M., Ma K.W., Murota S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene. 2001;20:4138–4149. doi: 10.1038/sj.onc.1204563. [DOI] [PubMed] [Google Scholar]
- 145.Ruch R.J. Connexin43 suppresses lung cancer stem cells. Cancers. 2019;11:175. doi: 10.3390/cancers11020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong X., Sin W.C., Harris A.L., Naus C.C. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. 2015;6:15566–15577. doi: 10.18632/oncotarget.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mulkearns-Hubert E.E., Torre-Healy L.A., Silver D.J., Eurich J.T., Bayik D., Serbinowski E., Hitomi M., Zhou J., Przychodzen B., Zhang R., et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019;27:1062–1072.e5. doi: 10.1016/j.celrep.2019.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schalper K.A., Sánchez H.A., Lee S.C., Altenberg G.A., Nathanson M.H., Sáez J.C. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol. Cell Physiol. 2010;299:C1504–C1515. doi: 10.1152/ajpcell.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Flora A., Zocchi E., Guida L., Franco L., Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N. Y. Acad. Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- 150.Song D., Liu X., Liu R., Yang L., Zuo J., Liu W. Connexin 43 Hemichannel Regulates H9c2 Cell Proliferation by Modulating Intracellular ATP and [Ca2+] Acta Biochim. Biophys. Sin. 2010;42:472–482. doi: 10.1093/abbs/gmq047. [DOI] [PubMed] [Google Scholar]
- 151.Franco L., Zocchi E., Usai C., Guida L., Bruzzone S., Costa A., De Flora A. Paracrine Roles of NAD+ and Cyclic ADP-ribose in Increasing Intracellular Calcium and Enhancing Cell Proliferation of 3T3 Fibroblasts. J. Biol. Chem. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- 152.Chi Y., Gao K., Li K., Nakajima S., Kira S., Takeda M., Yao J. Purinergic control of AMPK activation by ATP released through connexin 43 hemichannels—Pivotal roles in hemichannel-mediated cell injury. J. Cell Sci. 2014;127:1487–1499. doi: 10.1242/jcs.139089. [DOI] [PubMed] [Google Scholar]
- 153.Good M.E., Ek-Vitorín J.F., Burt J.M. Structural determinants and proliferative consequences of connexin 37 hemichannel function in insulinoma cells. J. Biol. Chem. 2014;289:30379–30386. doi: 10.1074/jbc.M114.583054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bao L., Sachs F., Dahl G. Connexins are mechanosensitive. Am. J. Physiol.-Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 155.Retamal M.A., Orellana V.P., Arévalo N.J., Rojas C.G., Arjona R.J., Alcaíno C.A., González W., Canan J.G., Moraga-Amaro R., Stehberg J., et al. Cx46 hemichannel modulation by nitric oxide: Role of the fourth transmembrane helix cysteine and its possible involvement in cataract formation. Nitric Oxide. 2019;86:54–62. doi: 10.1016/j.niox.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 156.Ebihara L., Tong J.-J., Vertel B., White T.W., Chen T.-L. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Investig. Ophthalmol. Vis. Sci. 2011;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou J.Z., Jiang J.X. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions—An update. FEBS Lett. 2014;588:1186–1192. doi: 10.1016/j.febslet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang J.X., Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim. Biophys. Acta Biomembr. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moorby C., Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp. Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- 160.Kanemitsu M.Y., Loo L.W., Simon S., Lau A.F., Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 161.Giepmans B.N.G., Verlaan I., Moolenaar W.H. Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun. Adhes. 2001;8:219–223. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- 162.Zhou Y., Yang W., Lurtz M.M., Ye Y., Huang Y., Lee H.-W., Chen Y., Louis C.F., Yang J.J. Identification of the calmodulin binding domain of connexin 43. J. Biol. Chem. 2007;282:35005–35017. doi: 10.1074/jbc.M707728200. [DOI] [PubMed] [Google Scholar]
- 163.Kawasaki Y., Omori Y., Li Q., Nishikawa Y., Yoshioka T., Yoshida M., Ishikawa K., Enomoto K. Cytoplasmic accumulation of connexin32 expands cancer stem cell population in human HuH7 hepatoma cells by enhancing its self-renewal. Int. J. Cancer. 2011;128:51–62. doi: 10.1002/ijc.25308. [DOI] [PubMed] [Google Scholar]
- 164.Yang J., Qin G., Luo M., Chen J., Zhang Q., Li L., Pan L., Qin S. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis. 2015;6:e1829. doi: 10.1038/cddis.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ableser M.J., Penuela S., Lee J., Shao Q., Laird D.W. Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo. J. Biol. Chem. 2014;289:1592–1603. doi: 10.1074/jbc.M113.507228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.High Glucose-Induced Hypertrophy of Mesangial Cells Is Reversed by Connexin43 Overexpression via PTEN/Akt/mTOR Signaling. [(accessed on 6 May 2020)]. Available online: https://www.semanticscholar.org/paper/High-glucose-induced-hypertrophy-of-mesangial-cells-Liu-Hu/bb327c95685df47fc3e71b1326c408f929e04c19.
- 167.Wang Y., Wang W., Wu X., Li C., Huang Y., Zhou H., Cui Y. Resveratrol Sensitizes Colorectal Cancer Cells to Cetuximab by Connexin 43 Upregulation-Induced Akt Inhibition. Front. Oncol. 2020;10:383. doi: 10.3389/fonc.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin D., Lobell S., Jewell A., Takemoto D.J. Differential Phosphorylation of connexin46 and connexin50 by H2O2 Activation of Protein Kinase Cgamma. Mol. Vis. 2004;10:688–695. [PubMed] [Google Scholar]
- 169.Dunia I., Recouvreur M., Nicolas P., Kumar N., Bloemendal H., Benedetti E.L. Assembly of Connexins and MP26 in Lens Fiber Plasma Membranes Studied by SDS-fracture Immunolabeling. Pt 15J. Cell Sci. 1998;111:2109–2120. doi: 10.1242/jcs.111.15.2109. [DOI] [PubMed] [Google Scholar]
- 170.Drissi R., Dubois M.L., Douziech M., Boisvert F.M. Quantitative proteomics reveals dynamic interactions of the minichromosome maintenance complex (MCM) in the cellular response to etoposide induced DNA damage. Mol. Cell. Proteom. 2015;14:2002–2013. doi: 10.1074/mcp.M115.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schubert A.L., Schubert W., Spray D.C., Lisanti M.P. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 172.Nielsen P.A., Baruch A., Shestopalov V.I., Giepmans B.N.G., Dunia I., Benedetti E.L., Kumar N.M. Lens Connexins α3Cx46 and α8Cx50 Interact with Zonula Occludens Protein-1 (ZO-1) Mol. Biol. Cell. 2003;14:2470–2481. doi: 10.1091/mbc.e02-10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teleki I., Krenacs T., Szasz M.A., Kulka J., Wichmann B., Leo C., Papassotiropoulos B., Riemenschnitter C., Moch H., Varga Z. The potential prognostic value of connexin 26 and 46 expression in neoadjuvant-treated breast cancer. BMC Cancer. 2013;13:50. doi: 10.1186/1471-2407-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Teleki I., Szasz A.M., Maros M.E., Gyorffy B., Kulka J., Meggyeshazi N., Kiszner G., Balla P., Samu A., Krenacs T. Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis. PLoS ONE. 2014;9:e112541. doi: 10.1371/journal.pone.0112541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reikvam H., Ryningen A., Sæterdal L.R., Nepstad I., Foss B., Bruserud O. Connexin expression in human acute myeloid leukemia cells: Identification of patient subsets based on protein and global gene expression profiles. Int. J. Mol. Med. 2015;35:645–652. doi: 10.3892/ijmm.2014.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kwak B.R., Mulhaupt F., Veillard N., Gros D.B., Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will send all the figures and results as requested.