Abstract
Poly(ADP-ribose) polymerase (PARP) enzymes have been shown to be essential for DNA repair pathways, including homologous recombination repair (HRR). Cancers with HRR defects (e.g., BRCA1 and BRCA2 mutations) are targets for PARP inhibitors (PARPis) based on the exploitation of “synthetic lethality”. As a result, PARPis offer a promising treatment option for advanced ovarian and breast cancers with deficiencies in HRR. However, acquired resistance to PARPis has been reported for most tumors, and not all patients with BRCA1/2 mutations respond to PARPis. Therefore, the formulation of effective treatment strategies to overcome resistance to PARPis is urgently necessary. This review summarizes the molecular mechanism of therapeutic action and resistance to PARPis, in addition to emerging combination treatment options involving PARPis.
Keywords: PARP-1, PARP inhibitors, resistance, combination therapy, homologous recombination deficiency, breast cancers, ovarian cancers
1. Introduction
Dysfunction in DNA repair often leads to genetic instability, which enables tumorigenesis [1]. In addition, malignancy and resistance to treatment are often linked to mutations of tumor suppressor genes that regulate DNA-damaging responses (e.g., BRCA1, BRCA2, PALB2, RAD51C, RAD51D, MLH1) or gatekeeper genes that trigger cell cycle arrest in response to DNA damage (e.g., p53, ATM, CHK1, CHK2) [2]. Although DNA repair dysfunction induces cancer development, it also opens the door for novel therapeutic development because cancers with DNA repair dysfunction tend to depend on alternative DNA repair systems to survive under genotoxic stress [3]. Therefore, it is important to identify cancer-specific vulnerabilities in cancers with DNA repair dysfunction to enhance the therapeutic potential and overcome drug resistance. The concept of “synthetic lethality” arises from a situation in which the impairment of more than two DNA repair components results in cell death, and it emerges as a promising strategy for novel therapies by targeting the DNA repair pathways in cancers [4].
As mentioned above, many cancers display a deficiency in one of the genes that are involved in homologous recombination (HR), which is an essential process to repair DNA double-stranded breakages (DSBs) [3]. The BRCA1 and BRCA2 proteins play important roles in HR by the error-free repair of DSBs [5]. In addition to BRAC1/2, there are several genes, such as PALB2 and RAD51, that contribute to HR [6]. Mutations of these genes are categorized as ‘BRCAness’ phenotypes represented by HR deficiency (HRD) [7]. ‘BRCAness’ phenotypes have served as biomarkers in predicting the therapeutic efficacy of HR inhibitors such as PARP inhibitors (PARPis) in breast, ovarian, pancreatic, and prostate cancers [8,9,10,11]. Currently, the next-generation sequencing (NGS)-based myChoice CDx and FoundationOne CDx assays are to validate the HRD status of cancers [12]. However, it remains challenging to establish a standardized test to understand the complex mechanisms leading to HRD. According to the Cancer Genome Atlas, roughly 50% of high-grade serous ovarian cancers (HGSOC) and 10–20% of triple-negative breast cancers (TNBCs) and metastatic prostate and pancreatic cancers may harbor BRCA1/2 mutations and other aberrations in HRD, qualifying them as potential candidates for treatment with PARPis [8,9,10,11]. However, it is common to observe acquired resistance to PARPis among cancer patients, even those with BRCA mutations [13]. Further, recent studies analyzing larger numbers of TNBC cell lines showed that sensitivity to PARPis is not necessarily related to the BRCA status [14], suggesting that BRCA-independent novel HR pathways may provide resistance to PARPis. Therefore, there is an urgent need to understand the nature of HRD in cancers resistant to PARPis.
2. Mechanisms of DNA-Damaging Responses
When genotoxic events produce DNA single-stranded breaks (SSBs) and DNA double-stranded breaks (DSBs), cells respond by activating DNA-damaging responses (DDR). Nucleotide excision repair (NER), base excision repair (BER), and mismatch repair (MMR) are the mechanisms that repair SSBs, whereas homologous recombination (HR) and non-homologous end joining (NHEJ) are the mechanisms that respond to DSBs [15,16]. HR usually occurs during the S and G2 phases of the cell cycle and uses the sister chromatid as a template to replace the DNA sequence and preserve genetic information [17]. On the other hand, NHEJ repairs the broken DNA ends through non-homologous end-joining and occurs throughout the cell cycle [17]. NHEJ is shown to be prone to errors in comparison to the high-fidelity editing of HR. The incorrect repair of DNA damage can lead to mutations and chromosomal aberration, which contribute to tumorigenesis.
During the process of HR, the Mre11-Rad50-Nbs1(MRN) complex initiates HR at the DSB by recruiting TIP60 and ATM to the DNA [18,19]. The phosphorylation of H2AX by TIP60/ATM allows phosphorylated H2AX to serve as an anchor for MDC1 [20]. Then, ATM also phosphorylates MDC1 so that phosphorylated MDC1 recruits E3 ligases RNF8 and RNF168, which ubiquitinate H2AX, which brings p53 binding protein (53BP1) and BRCA1 to the DDR foci [21]. The next step in HR involves the collaboration of nucleases such as EXO1, DNA2, and MUS8 with the MRN complex to resect the DSB ends, which leads to single-stranded DNA (ssDNA) formation [22]. Next, hyperphosphorylated replication protein A (RPA) coats the resected 3′ end, which is then replaced by RAD51 [23]. These events generate nucleoprotein filaments. BRCA2 and PALB2 also favor nucleoprotein filament formation as BRCA2 binds to BRCA1 and promotes the loading of recombinase RAD51 on ssDNA [24]. Once RAD51 is loaded on the ssDNA, it protects the DNA ends from degradation and mediates the invasion of homologous sequences and D-loops [25]. Therefore, we can assume that the restoration of the HR process plays an important role in resistance to PARPis based on their ability to repair DSBs, which otherwise lead to cell death.
3. The Mechanism by which PARP Inhibitors Act
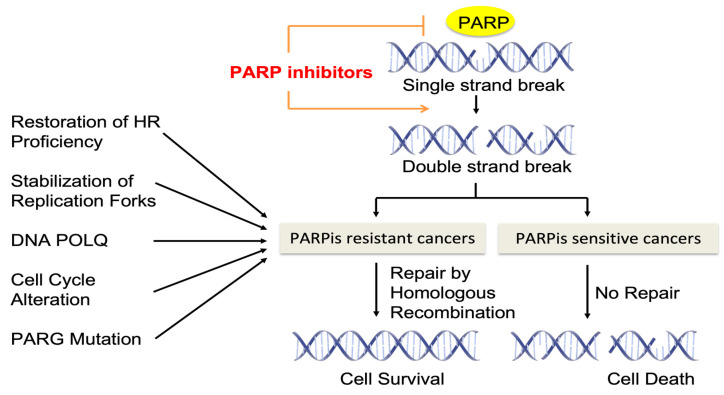
Poly(ADP-ribose) polymerase-1 (PARP-1) is an ADP-ribosylating nuclear enzyme that regulates diverse forms of DNA repair [26]. Among the 17 PARP family members of ADP-ribosyl transferases, PARP1 represents 80% of the poly ADP ribosylation (PAR) activity in cells as a founding member of the PARP family [27]. As PARP1 plays a key role in DNA repair and is reported to be upregulated in various cancer cell lines and tumor tissues, PARP1 has been a major target of PARPis in clinical trials [28]. PARP1 serves as a critical sensor and signal transducer of SSBs and DSBs and binds to exposed DNA. Upon binding to DNA, it undergoes conformational changes to be catalytically active and initiates the DNA repair processes [28]. PARP1 is eventually released from the repaired DNA region thorough self-PARylation. PARPis bind to key NAD+-binding and catalytic residues of PARP1 and inhibit the auto-PARylation of PARP1 at its auto-modification domain, which leads to ‘PARP trapping’ by blocking the release of PARP1 from DNA [29]. The consequence of PARP trapping leads to the crosslinking of DNA proteins, the collapse of replication forks, and a subsequent increase in DSBs [29,30,31] (Figure 1).
Figure 1.
Schematic of PARP inhibitor action and its resistance.
Four PARPis (Olaparib, Rucaparib, Niraparib, and Talazoparib) are approved by the FDA for the treatment of advanced ovarian and breast cancers with BRCA1/2 deficiency [32], and three PARPis (Veliparib, Pamiparib, and Fluzoparib) are currently under clinical trials for evaluation [33]. Different PARPis can induce various allosteric changes in PARP1 upon binding, which impacts PARP trapping and the subsequent cytotoxicity [34]. Among these PARPis, the most potent PARP trapping activity is reported in Talazoparib (nearly 100-fold more effective than the rest of the PARPis), followed by Niraparib, Olaparib, and Rucaparib [29,31]. The cytotoxic activity was also found to be highest in Talazoparib among PARPis. However, the relationship between the PARP trapping capability and efficacy of PARPis has not been clarified yet and additional studies are needed to understand the allosteric effects of different PARPis on PARP inhibition.
4. Mechanisms of Resistance to PARP Inhibitors
Although PARPis contributed to progression-free survival and overall survival, more than 40% of patients with a BRCA1/2 deficiency fail to respond to PARPis [35,36,37]. Furthermore, the prolonged administration of PARPis often leads to acquired PARPi resistance among patients. Therefore, resistance to PARPis is not uncommon in the clinic, and understanding the mechanisms of PARPi resistance in detail is necessary to increase PARPis’ sensitivity. We summarize the potential mechanisms of inherent and acquired resistance to PARPi therapy (Figure 1).
4.1. Restoration of Homologous Recombination Proficiency
The restoration of HR in HR-deficient tumors represents the most common acquired resistance mechanism to PARPis [37,38]. The restoration of HR activity can be achieved directly (direct effects on the HR machinery through genomic, epigenetic, and post-translational alterations) or indirectly (i.e., growth factor receptor-mediated signaling pathways that increase expression or activity of HR machinery) [38]. The direct restoration of HR includes genetic reversion mutations such as somatic insertion or deletion mutations that lead to the expression of functional proteins to increase HR proficiency [36]. For example, a germline or somatic reversion mutation in BRCA1/2, RAD51C, RAD51D, and PALB2 can restore protein functions relevant to DNA damage repair and thus confer up to 25% of resistance to PARPis among patients with BRCA1/2 deficiencies [38,39]. Another example of direct HR restoration is epigenetic regulation involving the promoter methylation of BRCA1 and RAD51C [40]. The loss of BRCA1 and RAD51C promoter methylation (de-methylation) restores the functional expression of these two proteins and leads to resistance to PARPis [40]. Oncogenic signaling pathways including the VEGF receptor, PI3 kinase, and heat-shock protein 90 (HSP90) were shown to promote HR proficiency indirectly by increasing the expression of DDR-associated genes [38]. Therefore, the therapeutic targeting of these pathways in combination with PARPis can be considered to overcome PARPi resistance.
Regaining HR proficiency can be achieved without affecting the BRCA1 mutation status, especially BRCA1 mutant cancers. The most studied example is the inactivation of the TP53BP1 gene that encodes the 53BP1 protein [41]. In BRCA1 mutated breast cancer cells, the loss of 53BP1 rescues the BRCA1 deficiency and prevents genomic instability by activating the ATM-mediated processing of broken DNA ends [41,42]. Then, 53BP1 recruits a protein complex called the Shieldin complex, composed of SHLD1, SHLD2, SHLD3, and REV7, to the DSB sites [43]. A number of recent studies indicate the role of the Shieldin complex as the key regulator of NHEJ repair and HR repair, both of which are associated with PARPi resistance [43,44,45].
4.2. Stabilization of Replication Forks
BRCA1 and BRCA2 are required for the protection of stalled replication forks by preventing them from being attacked by nucleases such as MRE11, DNA2, and MUS81 [46,47]. Therefore, cells need to rely on BRCA1 and BRCA2 for their survival when PARPis trap PARP on SSBs and stalled replication forks occur. PARPi-resistant BRCA mutant cells have developed an alternative mechanism to protect DNA replication forks from nucleases that attack the stalled replication forks [48,49]. PARPi-resistant BRCA mutant cells have low levels of EZH2, which leads to reduced H3K27 methylation and prevents the recruitment of nuclease MUS81 at stalled replication forks [49]. In addition, PTIP deficiency inhibits the recruitment of nuclease MRE11 to stalled replication forks and protects nascent DNA from degradation in BRCA2-deficient cells [50]. FANCD2 is highly overexpressed in BRCA1/2 mutant breast cancers and contributes to DNA fork stability by inhibiting MRE11-mediated DNA replication fork degradation in BRCA1/2 mutant breast cancer cells [51,52]. As fork degradation is an important synthetic lethality mechanism of PARPis, the increased stabilization of stalled replication forks by the various mechanisms mentioned above confers resistance to PARPis.
4.3. DNA POLQ
DNA polymerase θ, also known as DNA POLQ, is an enzyme that is involved in DSB repair by contributing to the error-prone “microhomology-mediated end joining (MMEJ) pathway” [53,54]. DNA POLQ is highly expressed in HR-deficient ovarian and breast cancers [53,54]. DNA POLQ inhibitors present synthetic lethality with PARPis in BRCA1/2 mutant tumor cells with acquired resistance to PARPis [55,56]. DNA POLQ is able to bind RAD51 and inhibits RAD51-mediated HR. Based on the potential of DNA POLQ inhibitors to overcome acquired resistance to PARPis in HR-deficient tumors, a phase I and II study that combines a DNA POLQ inhibitor, ART4215, with a PARP inhibitor, Talazoparib, was recently initiated in BRCA mutant breast cancer.
4.4. Alterations in Cell Cycle Control
The overexpression of cell cycle regulators such as cyclin-dependent kinase 12 (CDK12) and WEE1 is implicated in PARPi resistance by restoring HR [57]. Knocking down CDK12 expression increases the sensitivity to PARPis by downregulating the expression of DNA repair proteins [57]. CDK12 inhibitors reversed de novo and acquired resistance to PARPis in BRCA1 mutant and BRCA wild-type triple negative breast cancer cells [58]. In addition, an inhibitor of WEE1 kinase (WEE1i), Adavosertib, synergized well with PARPis in several preclinical studies by abrogating G2 cell cycle checkpoints. The inhibition of WEE1 activity allows HR-deficient cells to prematurely enter into mitosis, which increases DNA DSBs [59].
4.5. Inhibition of PARP Trapping by PAR Glycohydrolase (PARG) Downregulation
The depletion of poly(ADP-ribose) (PAR) glycohydrolase (PARG) expression by shRNA counteracts PARPi-mediated synthetic lethality by restoring PARP1 signaling and PARylation, even in the presence of PARPis [60]. As mentioned in Section 3, PARP trapping induced by PARPis leads to cell death via the accumulation of unrepaired SSBs and subsequent DSBs [31]. An increase in PARylation due to the loss of PARG expression includes PARP1 auto-PARylation, which allows PARP1 release from SSBs and the inhibition of PARP1 trapping. Consistent with this finding, the depletion of PARG expression is observed in PARPis resistant triple-negative breast cancers and serous ovarian cancers [60], suggesting that the loss of PARG leads to PARPi resistance.
4.6. Increased PARPi Efflux by P-gp Efflux Pumps
The chromosomal translocation of drug efflux transporter genes called Abcb1a and Abcb1b has been reported to increase the efflux of PARPis and contribute to PARPi resistance [61]. Abcb1a and Abcb1b encode P-glycoprotein (P-gp) efflux pumps, which are implicated in chemoresistance among various drugs and in the reduction of the efficiency of these compounds [62]. Consistent with these observations, Olaparib-resistant breast cancers showed a 2–85-fold increase in Abcb1a/b expression [62]. The P-glycoprotein (P-gp) efflux pump inhibitor Tariquidar effectively restored the sensitivity of PARPi-resistant ovarian cancer cells to PARPis [63,64]. Two common Abcb1a/b inhibitors, Verapamil and Elacridar, reversed the resistance of Paclitaxel- and Olaparib-resistant ovarian cancer cells [65].
4.7. ssDNA Gap Suppression
Recent evidence suggests that ssDNA gaps determine genotoxic lesions and PARPi sensitivity [66,67,68]. ssDNA gaps can occur in both the leading and lagging strands. ssDNA was indeed the predominate lesion identified after various chemo-treatments, and SSDNA gaps were considered in the toxicity of genotoxic agents [66]. Quinet et al. demonstrated that the primase-polymerase (PRIMPOL)-mediated repriming of DNA synthesis suppresses fork reversal, indicating a protective role of PRIMOL in BRCA-deficient cells [69]. They further determined that it was the primase activity of PRIMPOL, not the polymerase activity, that inhibited fork degradation in BRCA-deficient cells, thus leaving ssDNA gaps behind the replication forks. Kang et al. showed that PRIMPOL repriming and ssDNA gap accumulation are suppressed by BRCA2 and MCM10 [70]. These studies support the role of PRIMPOL-mediated ssDNA gap formation and the suppression of these gaps as an important mechanism to determine PARPi resistance in BRCA-deficient tumors.
5. The Feasible Combination Treatment Options to Overcome Resistance to PARP Inhibitors
Resistance to PARPis indicates that the combination of PARPis with a variety of existing treatment options may provide a solution for those tumors that fail to reach synthetic lethality with PARPi monotreatment. We summarize the various clinical trials that predict the best combination treatment to maximize the therapeutic efficacy of PARPis (Table 1).
Table 1.
A summary of the main current PARPi combination clinical trials.
| Trial Name | Combination Therapy | Phase | Cohort | Start Completion Date |
Ref | |
|---|---|---|---|---|---|---|
| PARPi + Chemotherapy | (STUDY41) NCT01081951 |
Olaparib + Paclitaxel + Carbopatin | II | Platinum sensitive advanced ovarian cancer | 4 Feb 2010– 29 Dec 2023 |
[59] |
| PARPi + Immunotherapy | (TOPACIO) NCT02657889 |
Niraparib + Pembrolizumab | II | Platinum-resistance ovarian cancer (regardless BRCA or HRD status) or BRCA mutated metastatic TNBC | 15 Apr 2016– 17 Sep 2021 |
[66,67] |
| (ENGOT-OV44) NCT03602859 | Niraparib + Dostarlimab | III | Stage III or IV nonmucinous Epithelial ovarin cancer | 11 Oct 2018– 22 Jun 2026 |
[71] | |
| (MEDIOLA) NCT02734004 |
Olaparib + Durvalumab | II | gBRCAm platinum-sensitive ovarian cancer | 28 Oct 2018– 2026 |
[68] | |
| (OPEB-1) NCT04361370 |
Olaparib + Durvalumab + Bevacizumab | II | Platinum-sensitive recurrent BRCA wt ovarian cancer | 28 Oct 2020– Aug 2026 |
[70] | |
| (ATHENA) NCT03522246 | Rucaparib + Nivolumab | III | Newly diagonsed advanced (FIGO stage III-IV) epithelial ovarin cancer. Fallopian Tube diseases. | 14 May 2018– 30 Dec2030 |
[72] | |
| (DUO-O) NCT03737643 | Olaparib + Durvalumab | III | Newly diagonsed advanced ovarain cancer (Regardless BRCA status) | 4 Jan 2019– 25 May 2028 |
[73] | |
| NCT02849496 | Olaparib + Atezolizumb (Anti-PD-L1) | II | Locally advanced unresectable or metastatic non-HER2 positive breast cancer | 30 Mar 2017– 31 Aug 2023 |
[74] | |
| PARPi + Anti-angiogenic agents |
NCT01116648 | Olaparib + Cediranib | I/II | Relapsed Platinum sensitive high-grade or endometrioid ovarian cancer | 25 Mar 2010– 31 Oct 2018 |
[75] |
| (NRG-GY005) NCT02502266 |
Olaparib + Cediranib | II/III | Recurrent Platinum-resistant ovarian cancer, Fallopian Tube diseases. | 3 May 2016– 30 Jun 2024 |
[76] | |
| (PAOLA-1) NCT02477644 |
Olaparib + Bevacizumab | III | HRD positive high-grade ovarian cancer | 6 May 2015– 22 Mar 2022 |
[77,78,79] | |
| NCT02354131 | Niraparib + Bevacizumab | I/II | platinum-sensitive ovarian cancer | 15 Feb 2015– 15 Dec 2021 |
[80] | |
| PARPi + PI3K/AKT pathway inhibitors | NCT01623349 | Olaparib + Alpelisib | I | Platinum-resistant ovarian cancer or recurrent triple negative breast cancer (regardless BRCA or HRD status) | Sep 2012– Dec 2020 |
[81,82] |
| (EPIK-O) NCT04729387 |
Olaparib + Alpelisib | III | Platinum-resistant or refractory high-grade serious epithelial ovarian cancer (HGSOC) with BRCA wt. | 2 Jul 2021– 29 Jul 2025 |
[83] | |
| NCT02208375 | Olaparib + Vistusertib or Olaparib + Capivasertib | I/II | Recurrent endometrial, triple negative breast cancer or ovarian cancer | 11 Nov 2014– 20 Jun 2024 |
[84] | |
| NCT02338622 | Olaparib + Capivasertib | I | Platinum-resistant HGSOC (regardless BRCA or HRD status) | 31 Mar 2014– 21 Mar 2017 |
[85] | |
| NCT03586661 | Niraparib + Copanlisib | I | Recurrent high-grade serous or BRCA mutant ovarian cancer | 29 Apr 2019– 31 Dec 23 |
[86] | |
| PARPi + MAPK pathway inhibitors | NCT03162627 | Olaprib + Selumetinib | I/II | PARPi resistant ovarian cancer or Solid tumor with RAS pathway alteration | 4 Aug 2017– 30 Aug 2026 |
[87] |
| PARPi + Epigenetic drugs | NCT02878785 | Talazoparib + Decitabine | I/II | Acute Myeloid Leukemia | Aug 2016– 19 Nov 2020 |
[88] |
| NCT03742245 | Olaprib + Vorinostat | I | Relapsed metastatic breast cancer | 11 Jun 2019– 1 Sep 2024 |
[89] | |
| PARPi + DDR inhibitors | (CAPRI) NCT03462342 | Olaparib + Ceralasertib | II | Platinum-resistant HGSOC | 9 Mar 2018– 31 Dec 2023 |
[90] |
| NCT04267939 | Niraparib + Elimsertib | I | Recurrent advanced solid tumors and ovarian cancer. | 26 Feb 2020– 3 Mar 2025 |
[91] | |
| NCT03057145 | Olaparib + Prexasertib | I | BRCA mutant PARPi resistant HGSOC | 10 Mar 2017– 9 Jun 2021 |
[92] | |
| (EFFORT) NCT03579316 |
Olaparib + Adavosertib | II | PARPis resistant ovarian cancer | 7 Dec 2018– 30 Dec 2023 |
[93] | |
| NCT04991480 | Talozoparib + ART4215 | I/II | BRCA deficient breast cancer | 13 Sep 2021– Aug 2025 |
[94] |
5.1. PARPis and Chemotherapy Drugs
PARPis available in the clinic are Olaparib (Lynparza, AsteraZeneca, Cambridge, UK), Rucaparib (Rubraca, Clovis Oncology, Boulder, CO, USA), Niraparib (Zejula, Gsk), and Talazoparib (Talzenna, Pfizer, New York, NY, USA). PARPi combination therapy with chemotherapy drugs (Cyclophosphamide, Carboplatin, and Paclitaxel) has been studied and used by many clinicians and researchers to treat patients with platinum-sensitive recurrent and advanced ovarian and breast cancers [32,71]. Among these PARPis, Olaparib effectively enhanced the efficacy of DNA-damaging chemotherapy drugs such as Carboplatin and Cisplatin [72,95]. In a randomized phase II trial involving recurrent platinum-sensitive ovarian cancer patients (NCT01081951), the addition of Olaparib (200 mg capsule) to Carboplatin (AUC 4 mg/mL per min) plus Paclitaxel (175 mg/(m2)) showed a significant improvement in progression-free survival (PFS) compared to Carboplatin plus Paclitaxel alone [73]. The PFS benefit of the combination of Olaparib with Carboplatin allowed the use of lower doses (Carboplatin AUC 4 mg/mL per min) than those used with chemotherapy alone (Carboplatin AUC 6 mg/mL per min), suggesting that Olaparib enhances the cytotoxic effects of Carboplatin [73]. In particular, ovarian cancer patients with BRCA mutations had the greatest clinical benefits [73]. In another example, Li C et al. compared and evaluated the clinical efficacy and safety of a combination treatment involving PARPis (Veliparib) versus chemotherapy (Carboplatin, Paclitaxel, Placebo, Cyclophosphamide) alone in patients with triple-negative breast cancer by using a meta-analysis through searches of seven databases [74]. The group treated with PARPis plus chemotherapy showed better PFS and overall survival (OS) than the group treated with chemotherapy alone. The studies demonstrated that the PARPis showed a synergy with chemotherapy in TNBC patients regardless of the BRCA mutational status [74]. However, in terms of safety, some specific adverse effects (AEs), including neutropenia, anemia, and diarrhea, were observed in relatively higher numbers in the combination group. The selection of the optimal dose of PARPis and chemotherapy in the combination therapy to maximize the clinical benefits and minimize AEs remains a challenge.
5.2. PARPis and Immunotherapy
Given the interaction between DNA damage in tumors and the activated immune system, the combination of PARPis with immune checkpoint inhibitors (ICI) has been considered as the most effective strategy to overcome PARPi resistance. The rationale of this combination is based on two main premises. Firstly, HRD cancers have a high tumor mutational burden (TMB) caused by the deficient DNA repair pathway, which elevates tumor-specific neoantigen loads and activates the antitumor immune response [75,96,97]. Secondly, PARPis suppress anticancer immunity through PD-L1 upregulation. PARPis also promote the accumulation of cytosolic DNA fragments, which activates the DNA-sensing cGAS-STING pathway and thereby induces a type 1 interferon (IFN)-mediated antitumor immune response with T cell recruitment [76,77].
The synergistic effect between PARPis and ICI has been confirmed in the clinical setting, such as the phase I/II TOPACIO (NCT02657889) with Niraparib and Pembrolizumab (PD-1 inhibitor), in platinum-resistant ovarian cancers and metastatic TNBCs [78,79]. The combination of Niraparib (200 mg) and Pembrolizumab (200 mg) showed improved clinical efficacy (ORR of 18% and disease control rate (DCR) of 65%) compared with monotherapy, particularly in patients with BRCA wild-type and non-HRD ovarian cancer [78]. The putative combination of Niraparib and Pembrolizumab was effective in ovarian cancer regardless of BRCA and HRD status, whereas its therapeutic synergistic effect was observed only in the breast cancer cohort with BRCA-mutated tumors among metastatic TNBCs (ORR of BRCAm vs. BRCAwt, 47% vs. 11% and PFS of BRCAm vs. BRCAwt, 8.3 months vs. 2.1 months) [79]. Another clinical trial in the platinum resistance setting is the phase I/II MEDIOLA (NCT02734004), which explored the combination of Olaparib and Durvalumab (PD-L1 inhibitor). Patients received 300 mg of Olaparib twice daily for 4 weeks, followed by a combination of Olaparib (300 mg) and Durvalumab (1.5 g) until tumor progression was observed [80]. In patients with gBRCAm platinum-sensitive relapsed ovarian cancer, the effect of combination therapy showed an ORR of 63% and a 12-week DCR of 81%. In patients with BRCAm HER2-negative metastatic breast cancer, the combination was tolerable, with an ORR of 63.3%, a 12-week DCR of 80%, and a 28-week DCR of 50%. The combination therapy had more effective efficacy compared to the monotherapy in gBRCAm. Recently, this clinical trial was further extensively studied with the BRCA wt cases. Triplet therapy with Olaparib (300 mg), Durvalumab (1.5 g), and Bevacizumab (10 mg/kg) showed enhanced tumor responses (ORR of 77.4%), compared with the doublet therapy (ORR of 31.3%) and PARP or VEGF inhibitor monotherapy [98]. In the OPEB-01 trial (NCT04361370), the synergistic effect and safety of the triplet combination was also evaluated as a maintenance treatment for BRCA wt patients with platinum-sensitive recurrent ovarian cancer [99].
Besides this, several larger randomized phase III trials with a combination of PARPis and ICI are currently being performed in the first-line setting and in pretreated patients. For example, the phase III ENGOT-OV44/FIRST trial (NCT03602859) is being performed to compare the clinical efficacy, such as PFS and OS, and safety of Niraparib (as a maintenance drug) + chemotherapy (Paclitaxel and Carboplatin or Bevacizumab) + Dostarlimab (PD-1-blocking antibody) vs. Niraparib (as a maintenance drug) + chemotherapy in patients with FIGO stage 3 or 4 non-mucinous epithelial ovarian cancer [81]. ENGOT-OV43/KEYLYNK-001 (NCT03740165) is currently evaluating the use of Olaparib as a first-line maintenance therapy after Pembrolizumab (PD-1 inhibitor) plus chemotherapy in BRCA1/2-nonmutated advanced epithelial ovarian cancer [82]. With a similar premise, the phase III ATHENA trial (NCT03522246) enrolling 1000 patients is evaluating the efficacy of the combination of Rucaparib with Nivolumab (PD-1-blocking antibody) as a maintenance therapy in newly diagnosed high-grade epithelial ovarian cancer patients. In this study, ATHENA COMBO aims to determine whether the addition of Nivolumab to Rucaparib increases the activity of Rucaparib and extends PFS compared to Rucaparib alone following standard platinum-based chemotherapy [83]. In addition, biomarkers such as PD-L1 expression and HR deficiency status, related to the prediction of PARPi resistance and response, were assessed in this combination study. Most recently, the results of the phase III DUO-O trial (NCT03737643) were presented at the 2023 ASCO Annual Meeting. This trial included 1130 patients and was designed to assess the efficacy and safety of triple treatment with Bevacizumab plus Olaparib and Durvalumab as a maintenance therapy in newly diagnosed advanced ovarian cancer patients without BRCA mutations [86]. They reported that the triplet maintenance therapy provided a statically significant and clinically meaningful improvement in PFS compared to Bevacizumab monotherapy in patients with HRD-positive tumors (median PFS of triplet arm, 37.3 months; median PFS of Bevacizumab alone arm, 23 months). In the intent-to-treat (ITT) population, the triplet arm showed a median PFS of 24.2 months vs. 19.3 months in the Bevacizumab alone arm (19.3 months). The final analysis including OS and other secondary endpoints is still awaiting a definitive conclusion. Numerous other clinical trials are now underway and are investigating the safety, toxicity, and optimal dose of combination PARPi and ICI agents in the target population (NCT02849496) [84].
5.3. PARPis and Anti-Angiogenic Agents
Preclinical studies have demonstrated that anti-angiogenic agents can increase PARPi susceptibility through HR deficiency (HRD) by downregulating homologous recombination repair genes such as BRCA1 and RAD51 under hypoxia stress [85]. This suggests that the combination of anti-angiogenic agents and PARPis may have at least additive or synergic effects in PARPi-resistant cancers. Cediranib, as an oral anti-angiogenic agent, is a VEGF receptor-targeted agent. In a randomized phase II trial (NCT01116648), the addition of Cediranib (30 mg) to Olaparib (200 mg) improved the PFS (from 9 months to 17.7 months) compared with Olaparib monotherapy (400 mg) in a population of women with platinum-sensitive high-grade or endometrioid ovarian cancer [100]. The survival benefit of the PARPi combination therapy with Cediranib was observed in both BRCA mutant and BRCA wild-type populations [101]. A subsequent study of Olaparib and Cediranib combination treatment in platinum-resistant ovarian cancer patients without BRCA1/2 mutation is currently underway in clinical trials (NRG-GY005:NCT02502266) [87]. In another clinical trial, Olaparib and Bevacizumab combination treatment effectively improved PFS compared to a placebo plus Bevacizumab in HRD-positive advanced ovarian cancer patients who received first-line platinum-based chemotherapy (PAOLA-1/ENGOT-ov25 trial; NCT02477644) [102]. In other study, patient cohorts that received Olaparib (300 mg for 24 months) plus Bevacizumab (15 kg for 15 months) showed significant improvements in PFS2 (second progression or death) and TSST (second subsequent therapy or death) [103]. A subsequent PAOLA-1 study recently updated the clinically meaningful OS efficacy in HRD-positive ovarian cancer patients who received Olaparib + Bevacizumab. This study showed the potential of Olaparib + Bevacizumab as one of the standard treatments for HRD+ newly diagnosed advanced ovarian cancer patients [104]. While the combination of Olaparib and Bevacizumab is approved only for HRD-positive cancers, the combination of Niraparib plus Bevacizumab has been reported to have clinical efficacy regardless of HRD status. The combination of Niraparib plus Bevacizumab is more effective than Niraparib alone in platinum-sensitive recurrent ovarian cancer (NTC02354131) [105].
5.4. PARPis and PI3K/AKT Pathway Inhibitors
The PI3K/AKT/mTOR pathway is known to contribute to DSB repair through HR. PI3K inhibitors induce HRD by downregulating BRCA1/2. mTOR inhibitors suppress DSB repair through decreasing homologous recombination repair (HRR) gene expression [88,106]. The synergistic effect has been confirmed in a phase I clinical trial with Olaparib and Alpelisib (FDA-approved α-specific PI3K inhibitor) in combination in BRCA wild-type platinum-resistant ovarian cancer and breast cancers [89,107]. The maximum tolerated dosage when combining Alpelisib (200mg once daily) and Olaparib (200 mg twice daily) was determined in a phase Ib dose-escalation/dose-expansion clinical trial (NCT01623349). The overall response rate (ORR) of the Olaparib and Alpelisib combination (33%) was higher than that of the Alpelisib (<5%) or Olaparib (4–5%) monotherapy in patients with platinum-resistant epithelial ovarian cancer [89]. A similar clinical study, EPIKK-O/ENGOT-ov61 (NCT04729387), is ongoing to assess the efficacy and safety of Olaparib + Alpelisib vs. single-agent chemotherapy in patients with platinum-resistant or refractory high-grade serious epithelial ovarian cancer (HGSOC) with BRCA wild type [108]. A recent study reported clinical efficacy with the combination treatment of Niraparib and Copanlisib (PI3K inhibitor) in recurrent high-grade serous or BRCA mutant ovarian cancer (NCT03586661) [109]. This treatment is now being evaluated for optimal doses and side effects. Several other ongoing clinical trials of PARPis in combination with mTOR inhibitors (Vistusertib;AZD2014) are also evaluating the clinical efficacy, safety, and best dose of Olaparib and Vistusertib in triple-negative breast cancer (NCT02208375) [110]. The ComPAKT trial evaluated the synergy in the combination of a pan-AKT inhibitor, Capivasertib (AZD5363), and Olaparib (NCT02338622) [111]. A 44.6% clinical benefit (RECIST complete response/partial response or stable disease ≥ 4 months) was achieved in platinum-resistant HGSOC patients regardless of BRCA or DDR status and PI3K-AKT pathway alterations [111].
5.5. PARPis and RAS/RAF/MEK Pathway Inhibitors
PARPi resistance is related to the upregulation of the RAS/MAPK pathway, suggesting that the MAPK pathway may have a certain influence on the re-sensitization to PARPis. MEKi decreases the HR capacity and causes increased DNA damage accumulation [112,113]. The combination of MEKi and PARPis served to induce more DNA damage and apoptosis in RAS mutant cell lines or BRCA2 proficient cancers [112,113]. The clinical benefits of this combination in patients are currently under investigation in an ongoing phase I/II trial of Olaparib and Selumetinib (MEKi), which includes a cohort with PARPi-resistant ovarian cancer (NCT03162627) [114].
5.6. PARPis and Epigenetic Drugs
DDR genes are epigenetically modified to cause transcriptional silencing and the loss of DNA repair capacity in cancers. These epigenetic modifications have been reported to affect PARPis’ sensitivity [115,116]. Therefore, epigenetic inhibitors including the DNA methyltransferase inhibitor (DMNTi) and histone deacetylation inhibitor (HDACi) have been considered to enhance PARPi treatment outcomes by modifying epigenetic marks associated with resistance. The combination of PARPis and epigenetic drugs has been tested in preclinical and clinical settings [117,118]. The addition of Decitabine (DMNTi) to Talazoparib enhanced the efficacy of Talazoparib by inducing the tight binding of PARP1 and DNMT to chromatin in BRCAness acute myeloid leukemia (AML) [119]. In a phase I clinical trial, the dose of the combination of Decitabine (20 mg/m2 intravenously daily for 5 or 10 days) and Talazoparib (1 mg orally daily for 28 days) was evaluated, based on tolerability, efficacy, and pharmacodynamic data (NCT02878785) [120]. At present, the NCT03742245 trial is ongoing to determine the safety and efficacy of the combinational treatment of Olaparib and Vorinostat (HDACi) in metastatic breast cancer [121].
5.7. PARPis and ATR/CHK1 Inhibitors
One of the strategies in modulating DNA repair activity in HR-deficient tumors is to disrupt cell cycle checkpoint signaling. Under elevated DNA replication stress and DNA-damaging conditions, activated ataxia telangiectasia and rad3-related (ATR) and its downstream checkpoint kinase 1 (CHK1) stabilize replication forks and simultaneously activate the S and G2-M checkpoints, resulting in DNA repair [122,123]. The ATR-CHK1 pathway also prevents collapse into DNA DSBs [122,123]. Thus, inhibition of the ATR-CHK1 pathway collapses replication forks and disrupts cell cycle progression. These events lead to chromosome aberrations, mitotic catastrophe, and ultimately apoptosis. Given that the HR-independent mechanism of PARPi resistance is associated with the increased stabilization of replication forks, a combination strategy targeting the ATR-CHK1 pathway is expected to be a rational approach to overcome PARPi resistance. Preclinical studies have demonstrated the synergistic effect of a PARPi + ATRi combination in in vitro and in vivo, where ATRi sensitized BRCA1/2-deficient ovarian cancer cells and tumors to PARPis by increasing DNA replication fork instability, double-strand breaks, and apoptosis [124,125]. Fonia et al. reported that Olaparib plus Ceralasertib (ATRi) was well-tolerated in platinum-resistant high-grade serous epithelial ovarian cancer (HGSOC) patients. The phase II study of Olaparib plus Ceralasertib (CAPRI: NCT03462342) showed their synergy in BRCA1 mutant ovarian cancers [90]. Moreover, their recent clinical study determined the tolerability, response rate, and safety of the combination treatment of Ceralasertib and Olaparib in three different cohorts (platinum-sensitive, platinum-resistant, and platinum-sensitive/PARPi-resistant) [126]. A great benefit from the addition of Ceralasertib was observed in HR-deficient platinum-sensitive/PARPi-resistant HGSOC, increasing the median PFS by 7.43 months (95% CI, 4.73–15.1), with a 57% partial response (PR: n = 4/7) and 50% ORR (n = 6/12) [126]. Ceralasertib re-sensitized PARPi-resistant HR-deficient ovarian cancer to Olaparib. This combination had dose reductions and low-grade toxicity. Other studies including the combination of Niraparib and Elimsertib (ATRi: BAY 1895344) in recurrent advanced solid tumors or ovarian cancer are underway (NCT04267939) [91]. CHK1 inhibitors (CHK1i) have also shown a benefit in overcoming PARPi resistance. A phase I clinical trial combining Prexasertib (CHK1i) and Olaparib has been completed in BRCA mutant PARPi-resistant HGSOC (NCT03057145) [92]. The addition of Prexasertib suppressed DNA repair via compromised HR and enhanced DNA damage [127]. Future studies are needed to assess the optimized dose for combination to minimize hematologic toxicity.
5.8. PARPis and Other DDR Inhibitors
Besides ATR-targeting drugs, the WEE1 inhibitor (WEE1i) is another modulator of DDR that increases replication stress and eventually promotes mitotic catastrophe by controlling CDK activity [128]. A number of preclinical studies have already shown that WEE1i may serve as a combination partner with PARPis. Indeed, the combination of PARPis and WEE1i has been demonstrated to be synergistic in small cell lung cancer PDX, ovarian cancer PDX, and breast cancer models [59,129,130]. The phase II EFFORT clinical study (NCT03579316) reported the efficacy of the combination of Olaparib and Adavosertib (WEE1i) in PARPi-resistant ovarian cancer. The combination therapy achieved clinical benefits compared to Olaparib monotherapy, with ORR (29% vs. 23%), clinical benefit (CBR: 89% vs. 63%), and median PFS (6.8 months vs. 5.5 months). However, due to the high grade 3 or 4 toxicity of this combination, additional studies including dose interruptions and reductions are underway [93].
POLQ inhibitors, known as potent inhibitors targeting tumor-specific DDR, have recently attracted attention as potential combination partners to overcome PARPi resistance. A phase I/II study (NCT04991480) is evaluating the safety and tolerability of a combination with Talzenna (Talazoparib) and ART4215 (selective small-molecule inhibitor of POLQ) in BRCA-deficient breast cancer [94].
6. Conclusions
FDA-approved PARPis have served as first-line and second-line maintenance and treatment options for breast, pancreatic, prostate, and ovarian cancers and benefited patients with these cancers with HRD. The recent data suggest the clinical benefits of PARPis in tumors regardless of BRCA1/2 mutation. However, prolonged treatment with PARPis highlights the emerging issue of PARPi resistance.
In this review, we discussed the restoration of homologous recombination proficiency, the stabilization of replication forks, DNA POLQ, increased drug efflux, PARG mutations, and cell cycle alteration as some of the known mechanisms that contribute to resistance to PARPis. Preclinical studies provided evidence to support these mechanisms, but clinical trials indicated that a combination of multiple mechanisms may be involved in the resistance to PARPis. Therefore, the optimization of combination treatment selection and dosing will be the key to overcome resistance to PARPis in multiple cancer types. We discussed a number of ongoing clinical trials evaluating the rationale of combination strategies to evade PARPi resistance. While the clinical benefits are obvious based on preliminary analyses of these trials, the establishment of the toxicity profiles and the development of reliable predictive biomarkers of the treatment responses will further enhance the use of PARPis.
Author Contributions
Conceptualization, Y.-H.S. and J.C.; writing—original draft preparation, Y.-H.S. and J.C.; writing—review and editing, Y.-H.S. and J.C.; visualization, Y.-H.S. and J.C.; project administration, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review was supported by NIH grant R01, grant number CA163657, and the Peter T. Rowley Breast Cancer Research Projects (Round 7) #C37903GG.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sieber O.M., Heinimann K., Tomlinson I.P.M. Genomic Instability--the Engine of Tumorigenesis? Nat. Rev. Cancer. 2003;3:701–708. doi: 10.1038/nrc1170. [DOI] [PubMed] [Google Scholar]
- 2.Chartron E., Theillet C., Guiu S., Jacot W. Targeting Homologous Repair Deficiency in Breast and Ovarian Cancers: Biological Pathways, Preclinical and Clinical Data. Crit. Rev. Oncol. Hematol. 2019;133:58–73. doi: 10.1016/j.critrevonc.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma I., Fleuren E.D.G., Williamson C.T., Lord C.J. Directing the Use of DDR Kinase Inhibitors in Cancer Treatment. Expert. Opin. Investig. Drugs. 2017;26:1341–1355. doi: 10.1080/13543784.2017.1389895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaelin W.G. The Concept of Synthetic Lethality in the Context of Anticancer Therapy. Nat. Rev. Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsdottir K., Ashworth A. The Roles of BRCA1 and BRCA2 and Associated Proteins in the Maintenance of Genomic Stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 6.Helleday T. The Underlying Mechanism for the PARP and BRCA Synthetic Lethality: Clearing up the Misunderstandings. Mol. Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lord C.J., Ashworth A. BRCAness Revisited. Nat. Rev. Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 8.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 9.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bono J., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K.N., Sartor O., Agarwal N., Olmos D., et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 11.Mateo J., Carreira S., Sandhu S., Miranda S., Mossop H., Perez-Lopez R., Nava Rodrigues D., Robinson D., Omlin A., Tunariu N., et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frampton G.M., Fichtenholtz A., Otto G.A., Wang K., Downing S.R., He J., Schnall-Levin M., White J., Sanford E.M., An P., et al. Development and Validation of a Clinical Cancer Genomic Profiling Test Based on Massively Parallel DNA Sequencing. Nat. Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norquist B., Wurz K.A., Pennil C.C., Garcia R., Gross J., Sakai W., Karlan B.Y., Taniguchi T., Swisher E.M. Secondary Somatic Mutations Restoring BRCA1/2 Predict Chemotherapy Resistance in Hereditary Ovarian Carcinomas. J. Clin. Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keung M.Y., Wu Y., Badar F., Vadgama J.V. Response of Breast Cancer Cells to PARP Inhibitors Is Independent of BRCA Status. J. Clin. Med. 2020;9:940. doi: 10.3390/jcm9040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeijmakers J.H. Genome Maintenance Mechanisms for Preventing Cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg E.C., Aguilera A., Gellert M., Hanawalt P.C., Hays J.B., Lehmann A.R., Lindahl T., Lowndes N., Sarasin A., Wood R.D. DNA Repair: From Molecular Mechanism to Human Disease. DNA Repair. 2006;5:986–996. doi: 10.1016/j.dnarep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Helleday T. Pathways for Mitotic Homologous Recombination in Mammalian Cells. Mutat. Res. 2003;532:103–115. doi: 10.1016/j.mrfmmm.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Stracker T.H., Petrini J.H.J. The MRE11 Complex: Starting from the Ends. Nat. Rev. Mol. Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y., Jiang X., Chen S., Fernandes N., Price B.D. A Role for the Tip60 Histone Acetyltransferase in the Acetylation and Activation of ATM. Proc. Natl. Acad. Sci. USA. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatti S., Kozlov S., Farooqi A.A., Naqi A., Lavin M., Khanna K.K. ATM Protein Kinase: The Linchpin of Cellular Defenses to Stress. Cell. Mol. Life Sci. 2011;68:2977–3006. doi: 10.1007/s00018-011-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altmeyer M., Lukas J. To Spread or Not to Spread—Chromatin Modifications in Response to DNA Damage. Curr. Opin. Genet. Dev. 2013;23:156–165. doi: 10.1016/j.gde.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Cejka P. DNA End Resection: Nucleases Team Up with the Right Partners to Initiate Homologous Recombination. J. Biol. Chem. 2015;290:22931–22938. doi: 10.1074/jbc.R115.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu V.F., Weaver D.T. The Ionizing Radiation-Induced Replication Protein A Phosphorylation Response Differs between Ataxia Telangiectasia and Normal Human Cells. Mol. Cell. Biol. 1993;13:7222–7231. doi: 10.1128/mcb.13.12.7222-7231.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sy S.M.H., Huen M.S.Y., Chen J. PALB2 Is an Integral Component of the BRCA Complex Required for Homologous Recombination Repair. Proc. Natl. Acad. Sci. USA. 2009;106:7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godin S.K., Sullivan M.R., Bernstein K.A. Novel Insights into RAD51 Activity and Regulation during Homologous Recombination and DNA Replication. Biochem. Cell Biol. 2016;94:407–418. doi: 10.1139/bcb-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai P., Cantó C. The Role of PARP-1 and PARP-2 Enzymes in Metabolic Regulation and Disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Richard I.A., Burgess J.T., O’Byrne K.J., Bolderson E. Beyond PARP1: The Potential of Other Members of the Poly (ADP-Ribose) Polymerase Family in DNA Repair and Cancer Therapeutics. Front. Cell Dev. Biol. 2021;9:801200. doi: 10.3389/fcell.2021.801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M.Y., Zhang T., Kraus W.L. Poly(ADP-Ribosyl)Ation by PARP-1: “PAR-Laying” NAD+ into a Nuclear Signal. Genes. Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 29.Murai J., Huang S.-Y.N., Renaud A., Zhang Y., Ji J., Takeda S., Morris J., Teicher B., Doroshow J.H., Pommier Y. Stereospecific PARP Trapping by BMN 673 and Comparison with Olaparib and Rucaparib. Mol. Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh M.S., Lindahl T. Role of Poly(ADP-Ribose) Formation in DNA Repair. Nature. 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 31.Murai J., Pommier Y. PARP Trapping Beyond Homologous Recombination and Platinum Sensitivity in Cancers. Annu. Rev. Cancer Biol. 2019;3:131–150. doi: 10.1146/annurev-cancerbio-030518-055914. [DOI] [Google Scholar]
- 32.Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Friedlander M., Balmaña J., Mitchell G., Fried G., Stemmer S.M., Hubert A., et al. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman R.L., Fleming G.F., Brady M.F., Swisher E.M., Steffensen K.D., Friedlander M., Okamoto A., Moore K.N., Efrat Ben-Baruch N., Werner T.L., et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandarashvili L., Langelier M.-F., Velagapudi U.K., Hancock M.A., Steffen J.D., Billur R., Hannan Z.M., Wicks A.J., Krastev D.B., Pettitt S.J., et al. Structural Basis for Allosteric PARP-1 Retention on DNA Breaks. Science. 2020;368:eaax6367. doi: 10.1126/science.aax6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaspers J.E., Kersbergen A., Boon U., Sol W., van Deemter L., Zander S.A., Drost R., Wientjens E., Ji J., Aly A., et al. Loss of 53BP1 Causes PARP Inhibitor Resistance in Brca1-Mutated Mouse Mammary Tumors. Cancer Discov. 2013;3:68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards S.L., Brough R., Lord C.J., Natrajan R., Vatcheva R., Levine D.A., Boyd J., Reis-Filho J.S., Ashworth A. Resistance to Therapy Caused by Intragenic Deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 37.Noordermeer S.M., van Attikum H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019;29:820–834. doi: 10.1016/j.tcb.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-Art Strategies for Targeting the DNA Damage Response in Cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondrashova O., Nguyen M., Shield-Artin K., Tinker A.V., Teng N.N.H., Harrell M.I., Kuiper M.J., Ho G.-Y., Barker H., Jasin M., et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2017;7:984–998. doi: 10.1158/2159-8290.CD-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nesic K., Kondrashova O., Hurley R.M., McGehee C.D., Vandenberg C.J., Ho G.-Y., Lieschke E., Dall G., Bound N., Shield-Artin K., et al. Acquired RAD51C Promoter Methylation Loss Causes PARP Inhibitor Resistance in High-Grade Serous Ovarian Carcinoma. Cancer Res. 2021;81:4709–4722. doi: 10.1158/0008-5472.CAN-21-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouwman P., Aly A., Escandell J.M., Pieterse M., Bartkova J., van der Gulden H., Hiddingh S., Thanasoula M., Kulkarni A., Yang Q., et al. 53BP1 Loss Rescues BRCA1 Deficiency and Is Associated with Triple-Negative and BRCA-Mutated Breast Cancers. Nat. Struct. Mol. Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunting S.F., Callén E., Wong N., Chen H.-T., Polato F., Gunn A., Bothmer A., Feldhahn N., Fernandez-Capetillo O., Cao L., et al. 53BP1 Inhibits Homologous Recombination in Brca1-Deficient Cells by Blocking Resection of DNA Breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noordermeer S.M., Adam S., Setiaputra D., Barazas M., Pettitt S.J., Ling A.K., Olivieri M., Álvarez-Quilón A., Moatti N., Zimmermann M., et al. The Shieldin Complex Mediates 53BP1-Dependent DNA Repair. Nature. 2018;560:117–121. doi: 10.1038/s41586-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta R., Somyajit K., Narita T., Maskey E., Stanlie A., Kremer M., Typas D., Lammers M., Mailand N., Nussenzweig A., et al. DNA Repair Network Analysis Reveals Shieldin as a Key Regulator of NHEJ and PARP Inhibitor Sensitivity. Cell. 2018;173:972–988.e23. doi: 10.1016/j.cell.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dev H., Chiang T.-W.W., Lescale C., de Krijger I., Martin A.G., Pilger D., Coates J., Sczaniecka-Clift M., Wei W., Ostermaier M., et al. Shieldin Complex Promotes DNA End-Joining and Counters Homologous Recombination in BRCA1-Null Cells. Nat. Cell Biol. 2018;20:954–965. doi: 10.1038/s41556-018-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlacher K., Christ N., Siaud N., Egashira A., Wu H., Jasin M. Double-Strand Break Repair-Independent Role for BRCA2 in Blocking Stalled Replication Fork Degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlacher K., Wu H., Jasin M. A Distinct Replication Fork Protection Pathway Connects Fanconi Anemia Tumor Suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taglialatela A., Alvarez S., Leuzzi G., Sannino V., Ranjha L., Huang J.-W., Madubata C., Anand R., Levy B., Rabadan R., et al. Restoration of Replication Fork Stability in BRCA1- and BRCA2-Deficient Cells by Inactivation of SNF2-Family Fork Remodelers. Mol. Cell. 2017;68:414–430.e8. doi: 10.1016/j.molcel.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rondinelli B., Gogola E., Yücel H., Duarte A.A., van de Ven M., van der Sluijs R., Konstantinopoulos P.A., Jonkers J., Ceccaldi R., Rottenberg S., et al. EZH2 Promotes Degradation of Stalled Replication Forks by Recruiting MUS81 through Histone H3 Trimethylation. Nat. Cell Biol. 2017;19:1371–1378. doi: 10.1038/ncb3626. [DOI] [PubMed] [Google Scholar]
- 50.Ray Chaudhuri A., Callen E., Ding X., Gogola E., Duarte A.A., Lee J.-E., Wong N., Lafarga V., Calvo J.A., Panzarino N.J., et al. Replication Fork Stability Confers Chemoresistance in BRCA-Deficient Cells. Nature. 2016;535:382–387. doi: 10.1038/nature18325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kais Z., Rondinelli B., Holmes A., O’Leary C., Kozono D., D’Andrea A.D., Ceccaldi R. FANCD2 Maintains Fork Stability in BRCA1/2-Deficient Tumors and Promotes Alternative End-Joining DNA Repair. Cell Rep. 2016;15:2488–2499. doi: 10.1016/j.celrep.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michl J., Zimmer J., Buffa F.M., McDermott U., Tarsounas M. FANCD2 Limits Replication Stress and Genome Instability in Cells Lacking BRCA2. Nat. Struct. Mol. Biol. 2016;23:755–757. doi: 10.1038/nsmb.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceccaldi R., Liu J.C., Amunugama R., Hajdu I., Primack B., Petalcorin M.I.R., O’Connor K.W., Konstantinopoulos P.A., Elledge S.J., Boulton S.J., et al. Homologous-Recombination-Deficient Tumours Are Dependent on Polθ-Mediated Repair. Nature. 2015;518:258–262. doi: 10.1038/nature14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceccaldi R., Rondinelli B., D’Andrea A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J., Gelot C., Pantelidou C., Li A., Yücel H., Davis R.E., Färkkilä A., Kochupurakkal B., Syed A., Shapiro G.I., et al. A First-in-Class Polymerase Theta Inhibitor Selectively Targets Homologous-Recombination-Deficient Tumors. Nat. Cancer. 2021;2:598–610. doi: 10.1038/s43018-021-00203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zatreanu D., Robinson H.M.R., Alkhatib O., Boursier M., Finch H., Geo L., Grande D., Grinkevich V., Heald R.A., Langdon S., et al. Polθ Inhibitors Elicit BRCA-Gene Synthetic Lethality and Target PARP Inhibitor Resistance. Nat. Commun. 2021;12:3636. doi: 10.1038/s41467-021-23463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajrami I., Frankum J.R., Konde A., Miller R.E., Rehman F.L., Brough R., Campbell J., Sims D., Rafiq R., Hooper S., et al. Genome-Wide Profiling of Genetic Synthetic Lethality Identifies CDK12 as a Novel Determinant of PARP1/2 Inhibitor Sensitivity. Cancer Res. 2014;74:287–297. doi: 10.1158/0008-5472.CAN-13-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson S.F., Cruz C., Greifenberg A.K., Dust S., Stover D.G., Chi D., Primack B., Cao S., Bernhardy A.J., Coulson R., et al. CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. Cell Rep. 2016;17:2367–2381. doi: 10.1016/j.celrep.2016.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ha D.-H., Min A., Kim S., Jang H., Kim S.H., Kim H.-J., Ryu H.S., Ku J.-L., Lee K.-H., Im S.-A. Antitumor Effect of a WEE1 Inhibitor and Potentiation of Olaparib Sensitivity by DNA Damage Response Modulation in Triple-Negative Breast Cancer. Sci. Rep. 2020;10:9930. doi: 10.1038/s41598-020-66018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gogola E., Duarte A.A., de Ruiter J.R., Wiegant W.W., Schmid J.A., de Bruijn R., James D.I., Guerrero Llobet S., Vis D.J., Annunziato S., et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell. 2018;33:1078–1093.e12. doi: 10.1016/j.ccell.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Dias M.P., Moser S.C., Ganesan S., Jonkers J. Understanding and Overcoming Resistance to PARP Inhibitors in Cancer Therapy. Nat. Rev. Clin. Oncol. 2021;18:773–791. doi: 10.1038/s41571-021-00532-x. [DOI] [PubMed] [Google Scholar]
- 62.Rottenberg S., Jaspers J.E., Kersbergen A., van der Burg E., Nygren A.O.H., Zander S.A.L., Derksen P.W.B., de Bruin M., Zevenhoven J., Lau A., et al. High Sensitivity of BRCA1-Deficient Mammary Tumors to the PARP Inhibitor AZD2281 Alone and in Combination with Platinum Drugs. Proc. Natl. Acad. Sci. USA. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leitner I., Nemeth J., Feurstein T., Abrahim A., Matzneller P., Lagler H., Erker T., Langer O., Zeitlinger M. The Third-Generation P-Glycoprotein Inhibitor Tariquidar May Overcome Bacterial Multidrug Resistance by Increasing Intracellular Drug Concentration. J. Antimicrob. Chemother. 2011;66:834–839. doi: 10.1093/jac/dkq526. [DOI] [PubMed] [Google Scholar]
- 64.Christie E.L., Pattnaik S., Beach J., Copeland A., Rashoo N., Fereday S., Hendley J., Alsop K., Brady S.L., Lamb G., et al. Multiple ABCB1 Transcriptional Fusions in Drug Resistant High-Grade Serous Ovarian and Breast Cancer. Nat. Commun. 2019;10:1295. doi: 10.1038/s41467-019-09312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaidyanathan A., Sawers L., Gannon A.-L., Chakravarty P., Scott A.L., Bray S.E., Ferguson M.J., Smith G. ABCB1 (MDR1) Induction Defines a Common Resistance Mechanism in Paclitaxel- and Olaparib-Resistant Ovarian Cancer Cells. Br. J. Cancer. 2016;115:431–441. doi: 10.1038/bjc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cantor S.B. Revisiting the BRCA-Pathway through the Lens of Replication Gap Suppression: “Gaps Determine Therapy Response in BRCA Mutant Cancer”. DNA Repair. 2021;107:103209. doi: 10.1016/j.dnarep.2021.103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cong K., Peng M., Kousholt A.N., Lee W.T.C., Lee S., Nayak S., Krais J., VanderVere-Carozza P.S., Pawelczak K.S., Calvo J., et al. Replication Gaps Are a Key Determinant of PARP Inhibitor Synthetic Lethality with BRCA Deficiency. Mol. Cell. 2021;81:3128–3144.e7. doi: 10.1016/j.molcel.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panzarino N.J., Krais J.J., Cong K., Peng M., Mosqueda M., Nayak S.U., Bond S.M., Calvo J.A., Doshi M.B., Bere M., et al. Replication Gaps Underlie BRCA Deficiency and Therapy Response. Cancer Res. 2021;81:1388–1397. doi: 10.1158/0008-5472.CAN-20-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinet A., Tirman S., Jackson J., Šviković S., Lemaçon D., Carvajal-Maldonado D., González-Acosta D., Vessoni A.T., Cybulla E., Wood M., et al. PRIMPOL-Mediated Adaptive Response Suppresses Replication Fork Reversal in BRCA-Deficient Cells. Mol. Cell. 2020;77:461–474.e9. doi: 10.1016/j.molcel.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang Z., Fu P., Alcivar A.L., Fu H., Redon C., Foo T.K., Zuo Y., Ye C., Baxley R., Madireddy A., et al. BRCA2 Associates with MCM10 to Suppress PRIMPOL-Mediated Repriming and Single-Stranded Gap Formation after DNA Damage. Nat. Commun. 2021;12:5966. doi: 10.1038/s41467-021-26227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.FDA Approves Olaparib Tablets for Maintenance Treatment in Ovarian Cancer. [(accessed on 17 August 2017)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-tablets-maintenance-treatment-ovarian-cancer.
- 72.Lee J.-M., Hays J.L., Annunziata C.M., Noonan A.M., Minasian L., Zujewski J.A., Yu M., Gordon N., Ji J., Sissung T.M., et al. Phase I/Ib Study of Olaparib and Carboplatin in BRCA1 or BRCA2 Mutation-Associated Breast or Ovarian Cancer with Biomarker Analyses. J. Natl. Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oza A.M., Cibula D., Benzaquen A.O., Poole C., Mathijssen R.H.J., Sonke G.S., Colombo N., Špaček J., Vuylsteke P., Hirte H., et al. Olaparib Combined with Chemotherapy for Recurrent Platinum-Sensitive Ovarian Cancer: A Randomised Phase 2 Trial. Lancet Oncol. 2015;16:87–97. doi: 10.1016/S1470-2045(14)71135-0. [DOI] [PubMed] [Google Scholar]
- 74.Li C., Hao M., Fang Z., Ding J., Duan S., Yi F., Wei Y., Zhang W. PARP Inhibitor plus Chemotherapy versus Chemotherapy Alone in Patients with Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Cancer Chemother. Pharmacol. 2023;91:203–217. doi: 10.1007/s00280-023-04506-x. [DOI] [PubMed] [Google Scholar]
- 75.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiao S., Xia W., Yamaguchi H., Wei Y., Chen M.-K., Hsu J.-M., Hsu J.L., Yu W.-H., Du Y., Lee H.-H., et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen J., Zhao W., Ju Z., Wang L., Peng Y., Labrie M., Yap T.A., Mills G.B., Peng G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2019;79:311–319. doi: 10.1158/0008-5472.CAN-18-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Konstantinopoulos P.A., Waggoner S., Vidal G.A., Mita M., Moroney J.W., Holloway R., Van Le L., Sachdev J.C., Chapman-Davis E., Colon-Otero G., et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination with Pembrolizumab in Patients with Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vinayak S., Tolaney S.M., Schwartzberg L., Mita M., McCann G., Tan A.R., Wahner-Hendrickson A.E., Forero A., Anders C., Wulf G.M., et al. Open-Label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drew Y., de Jonge M., Hong S., Park Y., Wolfer A., Brown J., Ferguson M., Gore M., Alvarez R., Gresty C., et al. An Open-Label, Phase II Basket Study of Olaparib and Durvalumab (MEDIOLA): Results in Germline BRCA-Mutated (gBRCAm) Platinum-Sensitive Relapsed (PSR) Ovarian Cancer (OC) Gynecol. Oncol. 2018;149:246–247. doi: 10.1016/j.ygyno.2018.04.555. [DOI] [Google Scholar]
- 81.Hardy-Bessard A.-C., Moore K.N., Mirza M.R., Asselain B., Redondo A., Pfisterer J., Pignata S., Provencher D.M., Cibula D., Reyners A.K.L., et al. ENGOT-OV44/FIRST Study: A Randomized, Double-Blind, Adaptive, Phase III Study of Standard of Care (SOC) Platinum-Based Therapy ± Dostarlimab Followed by Niraparib ± Dostarlimab Maintenance as First-Line (1L) Treatment of Stage 3 or 4 Ovarian Cancer (OC) J. Clin. Oncol. 2020;38:TPS6101. doi: 10.1200/JCO.2020.38.15_suppl.TPS6101. [DOI] [Google Scholar]
- 82.Fujiwara K., Vergote I.B., Sehouli J., Salutari V., Zola P., Madry R., Wenham R.M., Korach J., Pautier P., Cibula D., et al. ENGOT-Ov43/KEYLYNK-001: A Phase III Trial of Pembrolizumab plus Chemotherapy with Olaparib Maintenance for First-Line Treatment of BRCA¬-Nonmutated Advanced Epithelial Ovarian Cancer. Ann. Oncol. 2019;30:ix89. doi: 10.1093/annonc/mdz426.039. [DOI] [Google Scholar]
- 83.Monk B.J., Coleman R.L., Fujiwara K., Wilson M.K., Oza A.M., Oaknin A., O’Malley D.M., Lorusso D., Westin S.N., Safra T., et al. ATHENA (GOG-3020/ENGOT-Ov45): A Randomized, Phase III Trial to Evaluate Rucaparib as Monotherapy (ATHENA-MONO) and Rucaparib in Combination with Nivolumab (ATHENA-COMBO) as Maintenance Treatment Following Frontline Platinum-Based Chemotherapy in Ovarian Cancer. Int. J. Gynecol. Cancer. 2021;31:1589–1594. doi: 10.1136/ijgc-2021-002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LoRusso P., Pilat M.J.P., Santa-Maria C.A., Connolly R.M., Roesch E.E., Afghahi A., Han H.S., Nanda R., Wulf G.M., Assad H., et al. Trial in Progress: A Phase II Open-Label, Randomized Study of PARP Inhibition (Olaparib) Either Alone or in Combination with Anti-PD-L1 Therapy (Atezolizumab) in Homologous DNA Repair (HDR) Deficient, Locally Advanced or Metastatic Non-HER2-Positive Breast Cancer. J. Clin. Oncol. 2020;38:TPS1102. doi: 10.1200/JCO.2020.38.15_suppl.TPS1102. [DOI] [Google Scholar]
- 85.Kaplan A.R., Gueble S.E., Liu Y., Oeck S., Kim H., Yun Z., Glazer P.M. Cediranib Suppresses Homology-Directed DNA Repair through down-Regulation of BRCA1/2 and RAD51. Sci. Transl. Med. 2019;11:eaav4508. doi: 10.1126/scitranslmed.aav4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harter P., Trillsch F., Okamoto A., Reuss A., Kim J.-W., Rubio-Pérez M.J., Vardar M.A., Scambia G., Tredan O., Nyvang G.-B., et al. Durvalumab with Paclitaxel/Carboplatin (PC) and Bevacizumab (Bev), Followed by Maintenance Durvalumab, Bev, and Olaparib in Patients (Pts) with Newly Diagnosed Advanced Ovarian Cancer (AOC) without a Tumor BRCA1/2 Mutation (Non-tBRCAm): Results from the Randomized, Placebo (Pbo)-Controlled Phase III DUO-O Trial. J. Clin. Oncol. 2023;41:LBA5506. doi: 10.1200/JCO.2023.41.17_suppl.LBA5506. [DOI] [Google Scholar]
- 87.Lee J.-M., Moore R.G., Ghamande S., Park M.S., Diaz J.P., Chapman J., Kendrick J., Slomovitz B.M., Tewari K.S., Lowe E.S., et al. Cediranib in Combination with Olaparib in Patients without a Germline BRCA1/2 Mutation and with Recurrent Platinum-Resistant Ovarian Cancer: Phase IIb CONCERTO Trial. Clin. Cancer Res. 2022;28:4186–4193. doi: 10.1158/1078-0432.CCR-21-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mo W., Liu Q., Lin C.C.-J., Dai H., Peng Y., Liang Y., Peng G., Meric-Bernstam F., Mills G.B., Li K., et al. mTOR Inhibitors Suppress Homologous Recombination Repair and Synergize with PARP Inhibitors via Regulating SUV39H1 in BRCA-Proficient Triple-Negative Breast Cancer. Clin. Cancer Res. 2016;22:1699–1712. doi: 10.1158/1078-0432.CCR-15-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konstantinopoulos P.A., Barry W.T., Birrer M., Westin S.N., Cadoo K.A., Shapiro G.I., Mayer E.L., O’Cearbhaill R.E., Coleman R.L., Kochupurakkal B., et al. Olaparib and α-Specific PI3K Inhibitor Alpelisib for Patients with Epithelial Ovarian Cancer: A Dose-Escalation and Dose-Expansion Phase 1b Trial. Lancet Oncol. 2019;20:570–580. doi: 10.1016/S1470-2045(18)30905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah P.D., Wethington S.L., Pagan C., Latif N., Tanyi J., Martin L.P., Morgan M., Burger R.A., Haggerty A., Zarrin H., et al. Combination ATR and PARP Inhibitor (CAPRI): A Phase 2 Study of Ceralasertib plus Olaparib in Patients with Recurrent, Platinum-Resistant Epithelial Ovarian Cancer. Gynecol. Oncol. 2021;163:246–253. doi: 10.1016/j.ygyno.2021.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yap T.A., Konstantinopoulos P., Grisham R.N., Gupta D., Wilkinson G., Cao A., Jeffers M., Sharma N. 494TiP Phase Ib Study of Elimusertib (ATRi; BAY 1895344) in Combination with Niraparib (PARPi) in Patients with Advanced Solid Tumors. Ann. Oncol. 2022;33:S767–S768. doi: 10.1016/j.annonc.2022.07.622. [DOI] [Google Scholar]
- 92.Do K.T., Hill S.J., Kochupurakkal B., Supko J.G., Gannon C., Anderson A., Muzikansky A., Wolanski A., Hedglin J., Parmar K., et al. Abstract CT232: Phase I Combination Study of the CHK1 Inhibitor Prexasertib (LY2606368) and Olaparib in Patients with High-Grade Serous Ovarian Cancer and Other Advanced Solid Tumors. Cancer Res. 2019;79:CT232. doi: 10.1158/1538-7445.AM2019-CT232. [DOI] [Google Scholar]
- 93.Westin S.N., Coleman R.L., Fellman B.M., Yuan Y., Sood A.K., Soliman P.T., Wright A.A., Horowitz N.S., Campos S.M., Konstantinopoulos P.A., et al. EFFORT: EFFicacy Of Adavosertib in Parp ResisTance: A Randomized Two-Arm Non-Comparative Phase II Study of Adavosertib with or without Olaparib in Women with PARP-Resistant Ovarian Cancer. J. Clin. Oncol. 2021;39:5505. doi: 10.1200/JCO.2021.39.15_suppl.5505. [DOI] [Google Scholar]
- 94.Artios Pharma Ltd. A Phase I/IIa, Open-Label, Multi-Centre Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of the DNA Polymerase Theta Inhibitor ART4215 Administered Orally as Monotherapy and in Combination to Patients with Advanced or Metastatic Solid Tumors. [(accessed on 17 August 2017)]; Available online: https://clinicaltrials.gov/study/NCT04991480.
- 95.Evers B., Drost R., Schut E., de Bruin M., van der Burg E., Derksen P.W.B., Holstege H., Liu X., van Drunen E., Beverloo H.B., et al. Selective Inhibition of BRCA2-Deficient Mammary Tumor Cell Growth by AZD2281 and Cisplatin. Clin. Cancer Res. 2008;14:3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 96.Mouw K.W., Goldberg M.S., Konstantinopoulos P.A., D’Andrea A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017;7:675–693. doi: 10.1158/2159-8290.CD-17-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J.F., Garber J.E., Chowdhury D., Wu C.J., D’Andrea A.D., et al. Association and Prognostic Significance of BRCA1/2-Mutation Status with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes and Expression of PD-1/PD-L1 in High Grade Serous Ovarian Cancer. Oncotarget. 2016;7:13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drew Y., Penson R.T., O’Malley D.M., Kim J.-W., Zimmermann S., Roxburgh P., Sohn J., Stemmer S.M., Bastian S., Ferguson M., et al. 814MO Phase II Study of Olaparib (O) plus Durvalumab (D) and Bevacizumab (B) (MEDIOLA): Initial Results in Patients (Pts) with Non-Germline BRCA-Mutated (Non-gBRCAm) Platinum Sensitive Relapsed (PSR) Ovarian Cancer (OC) Ann. Oncol. 2020;31:S615–S616. doi: 10.1016/j.annonc.2020.08.953. [DOI] [Google Scholar]
- 99.Lee Y.J., Lim M.C., Kim B.-G., Ngoi N.Y., Choi C.H., Park S.-Y., Tan D.S., Go Y., Lee J.-Y. A Single-Arm Phase II Study of Olaparib Maintenance with Pembrolizumab and Bevacizumab in BRCA Non-Mutated Patients with Platinum-Sensitive Recurrent Ovarian Cancer (OPEB-01) J. Gynecol. Oncol. 2021;32:e31. doi: 10.3802/jgo.2021.32.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J.F., Barry W.T., Birrer M., Lee J.-M., Buckanovich R.J., Fleming G.F., Rimel B., Buss M.K., Nattam S., Hurteau J., et al. Combination Cediranib and Olaparib versus Olaparib Alone for Women with Recurrent Platinum-Sensitive Ovarian Cancer: A Randomised Phase 2 Study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J.F., Barry W.T., Birrer M., Lee J.-M., Buckanovich R.J., Fleming G.F., Rimel B.J., Buss M.K., Nattam S.R., Hurteau J., et al. Overall Survival and Updated Progression-Free Survival Outcomes in a Randomized Phase II Study of Combination Cediranib and Olaparib versus Olaparib in Relapsed Platinum-Sensitive Ovarian Cancer. Ann. Oncol. 2019;30:551–557. doi: 10.1093/annonc/mdz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ray-Coquard I., Pautier P., Pignata S., Pérol D., González-Martín A., Berger R., Fujiwara K., Vergote I., Colombo N., Mäenpää J., et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 103.González-Martín A., Desauw C., Heitz F., Cropet C., Gargiulo P., Berger R., Ochi H., Vergote I., Colombo N., Mirza M.R., et al. Maintenance Olaparib plus Bevacizumab in Patients with Newly Diagnosed Advanced High-Grade Ovarian Cancer: Main Analysis of Second Progression-Free Survival in the Phase III PAOLA-1/ENGOT-Ov25 Trial. Eur. J. Cancer. 2022;174:221–231. doi: 10.1016/j.ejca.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Ray-Coquard I., Leary A., Pignata S., Cropet C., González-Martín A., Marth C., Nagao S., Vergote I., Colombo N., Mäenpää J., et al. Olaparib plus Bevacizumab First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the PAOLA-1/ENGOT-Ov25 Trial. Ann. Oncol. 2023;34:681–692. doi: 10.1016/j.annonc.2023.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Mirza M.R., Lundqvist E., Birrer M.J., Christensen R.D., Nyvang G.-B., Malander S., Anttila M., Werner T.L., Lund B., Lindahl G., et al. Niraparib plus Bevacizumab versus Niraparib Alone for Platinum-Sensitive Recurrent Ovarian Cancer (NSGO-AVANOVA2/ENGOT-Ov24): A Randomised, Phase 2, Superiority Trial. Lancet Oncol. 2019;20:1409–1419. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 106.Ibrahim Y.H., García-García C., Serra V., He L., Torres-Lockhart K., Prat A., Anton P., Cozar P., Guzmán M., Grueso J., et al. PI3K Inhibition Impairs BRCA1/2 Expression and Sensitizes BRCA-Proficient Triple-Negative Breast Cancer to PARP Inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Batalini F., Xiong N., Tayob N., Polak M., Eismann J., Cantley L.C., Shapiro G.I., Adalsteinsson V., Winer E.P., Konstantinopoulos P.A., et al. Phase 1b Clinical Trial with Alpelisib plus Olaparib for Patients with Advanced Triple-Negative Breast Cancer. Clin. Cancer Res. 2022;28:1493–1499. doi: 10.1158/1078-0432.CCR-21-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konstantinopoulos P.A., Gonzalez-Martin A., Cruz F.M., Friedlander M., Glasspool R., Lorusso D., Marth C., Monk B.J., Kim J.-W., Hinson P., et al. EPIK-O/ENGOT-OV61: Alpelisib plus Olaparib vs Cytotoxic Chemotherapy in High-Grade Serous Ovarian Cancer (Phase III Study) Future Oncol. 2022;18:3481–3492. doi: 10.2217/fon-2022-0666. [DOI] [PubMed] [Google Scholar]
- 109.MD Anderson Cancer Center A Phase Ib Study of the Oral PARP Inhibitor Niraparib with the Intravenous PI3K Inhibitor Copanlisib for Recurrent Endometrial and Recurrent Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. [(accessed on 17 August 2017)]; Available online: https://clinicaltrials.gov/study/NCT03586661.
- 110.Westin S.N., Labrie M., Litton J.K., Blucher A., Fang Y., Vellano C.P., Marszalek J.R., Feng N., Ma X., Creason A., et al. Phase Ib Dose Expansion and Translational Analyses of Olaparib in Combination with Capivasertib in Recurrent Endometrial, Triple-Negative Breast, and Ovarian Cancer. Clin. Cancer Res. 2021;27:6354–6365. doi: 10.1158/1078-0432.CCR-21-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yap T.A., Kristeleit R., Michalarea V., Pettitt S.J., Lim J.S.J., Carreira S., Roda D., Miller R., Riisnaes R., Miranda S., et al. Phase I Trial of the PARP Inhibitor Olaparib and AKT Inhibitor Capivasertib in Patients with BRCA1/2- and Non-BRCA1/2-Mutant Cancers. Cancer Discov. 2020;10:1528–1543. doi: 10.1158/2159-8290.CD-20-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun C., Fang Y., Yin J., Chen J., Ju Z., Zhang D., Chen X., Vellano C.P., Jeong K.J., Ng P.K.-S., et al. Rational Combination Therapy with PARP and MEK Inhibitors Capitalizes on Therapeutic Liabilities in RAS Mutant Cancers. Sci. Transl. Med. 2017;9:eaal5148. doi: 10.1126/scitranslmed.aal5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vena F., Jia R., Esfandiari A., Garcia-Gomez J.J., Rodriguez-Justo M., Ma J., Syed S., Crowley L., Elenbaas B., Goodstal S., et al. MEK Inhibition Leads to BRCA2 Downregulation and Sensitization to DNA Damaging Agents in Pancreas and Ovarian Cancer Models. Oncotarget. 2018;9:11592–11603. doi: 10.18632/oncotarget.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MD Anderson Cancer Center Evaluation of the Combination of Selumetinib and Olaparib in Endometrial, Ovarian and Other Solid Tumors with Ras Pathway Alterations, and Ovarian Tumors with PARP Resistance. [(accessed on 17 August 2017)]; Available online: https://clinicaltrials.gov/study/NCT03162627.
- 115.Kondrashova O., Topp M., Nesic K., Lieschke E., Ho G.-Y., Harrell M.I., Zapparoli G.V., Hadley A., Holian R., Boehm E., et al. Methylation of All BRCA1 Copies Predicts Response to the PARP Inhibitor Rucaparib in Ovarian Carcinoma. Nat. Commun. 2018;9:3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lazo P.A. Targeting Histone Epigenetic Modifications and DNA Damage Responses in Synthetic Lethality Strategies in Cancer? Cancers. 2022;14:4050. doi: 10.3390/cancers14164050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abbotts R., Topper M.J., Biondi C., Fontaine D., Goswami R., Stojanovic L., Choi E.Y., McLaughlin L., Kogan A.A., Xia L., et al. DNA Methyltransferase Inhibitors Induce a BRCAness Phenotype That Sensitizes NSCLC to PARP Inhibitor and Ionizing Radiation. Proc. Natl. Acad. Sci. USA. 2019;116:22609–22618. doi: 10.1073/pnas.1903765116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pulliam N., Fang F., Ozes A.R., Tang J., Adewuyi A., Keer H., Lyons J., Baylin S.B., Matei D., Nakshatri H., et al. An Effective Epigenetic-PARP Inhibitor Combination Therapy for Breast and Ovarian Cancers Independent of BRCA Mutations. Clin. Cancer Res. 2018;24:3163–3175. doi: 10.1158/1078-0432.CCR-18-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muvarak N.E., Chowdhury K., Xia L., Robert C., Choi E.Y., Cai Y., Bellani M., Zou Y., Singh Z.N., Duong V.H., et al. Enhancing the Cytotoxic Effects of PARP Inhibitors with DNA Demethylating Agents—A Potential Therapy for Cancer. Cancer Cell. 2016;30:637–650. doi: 10.1016/j.ccell.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baer M.R., Kogan A.A., Bentzen S.M., Mi T., Lapidus R.G., Duong V.H., Emadi A., Niyongere S., O’Connell C.L., Youngblood B.A., et al. Phase I Clinical Trial of DNA Methyltransferase Inhibitor Decitabine and PARP Inhibitor Talazoparib Combination Therapy in Relapsed/Refractory Acute Myeloid Leukemia. Clin. Cancer Res. 2022;28:1313–1322. doi: 10.1158/1078-0432.CCR-21-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang J.C. Multicenter Phase I/Ib Trial of Olaparib in Combination with Vorinostat in Patients with Relapsed/Refractory and/or Metastatic Breast Cancer. [(accessed on 17 August 2017)]; Available online: https://clinicaltrials.gov/study/NCT03742245.
- 122.Karnitz L.M., Zou L. Molecular Pathways: Targeting ATR in Cancer Therapy. Clin. Cancer Res. 2015;21:4780–4785. doi: 10.1158/1078-0432.CCR-15-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ngoi N.Y.L., Pham M.M., Tan D.S.P., Yap T.A. Targeting the Replication Stress Response through Synthetic Lethal Strategies in Cancer Medicine. Trends Cancer. 2021;7:930–957. doi: 10.1016/j.trecan.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim H., George E., Ragland R.L., Rafail S., Zhang R., Krepler C., Morgan M.A., Herlyn M., Brown E.J., Simpkins F. Targeting the ATR/CHK1 Axis with PARP Inhibition Results in Tumor Regression in BRCA-Mutant Ovarian Cancer Models. Clin. Cancer Res. 2017;23:3097–3108. doi: 10.1158/1078-0432.CCR-16-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim H., Xu H., George E., Hallberg D., Kumar S., Jagannathan V., Medvedev S., Kinose Y., Devins K., Verma P., et al. Combining PARP with ATR Inhibition Overcomes PARP Inhibitor and Platinum Resistance in Ovarian Cancer Models. Nat. Commun. 2020;11:3726. doi: 10.1038/s41467-020-17127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wethington S.L., Shah P.D., Martin L., Tanyi J.L., Latif N., Morgan M., Torigian D.A., Rodriguez D., Smith S.A., Dean E., et al. Combination ATR (Ceralasertib) and PARP (Olaparib) Inhibitor (CAPRI) Trial in Acquired PARP Inhibitor-Resistant Homologous Recombination-Deficient Ovarian Cancer. Clin. Cancer Res. 2023;29:2800–2807. doi: 10.1158/1078-0432.CCR-22-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Do K.T., Kochupurakkal B., Kelland S., de Jonge A., Hedglin J., Powers A., Quinn N., Gannon C., Vuong L., Parmar K., et al. Phase 1 Combination Study of the CHK1 Inhibitor Prexasertib and the PARP Inhibitor Olaparib in High-Grade Serous Ovarian Cancer and Other Solid Tumors. Clin. Cancer Res. 2021;27:4710–4716. doi: 10.1158/1078-0432.CCR-21-1279. [DOI] [PubMed] [Google Scholar]
- 128.Forment J.V., O’Connor M.J. Targeting the Replication Stress Response in Cancer. Pharmacol. Ther. 2018;188:155–167. doi: 10.1016/j.pharmthera.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 129.Lallo A., Frese K.K., Morrow C.J., Sloane R., Gulati S., Schenk M.W., Trapani F., Simms N., Galvin M., Brown S., et al. The Combination of the PARP Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option for Small Cell Lung Cancer. Clin. Cancer Res. 2018;24:5153–5164. doi: 10.1158/1078-0432.CCR-17-2805. [DOI] [PubMed] [Google Scholar]
- 130.Fang Y., McGrail D.J., Sun C., Labrie M., Chen X., Zhang D., Ju Z., Vellano C.P., Lu Y., Li Y., et al. Sequential Therapy with PARP and WEE1 Inhibitors Minimizes Toxicity While Maintaining Efficacy. Cancer Cell. 2019;35:851–867.e7. doi: 10.1016/j.ccell.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.