Abstract
We investigated the influence of HMR 3004, a new ketolide antibiotic, on the pulmonary inflammation induced by heat-killed fluorescein isothiocyanate-labeled Streptococcus pneumoniae. HMR 3004 downregulated (P < 0.05) the pneumococcus-induced release of interleukin-6 (IL-6), IL-1β, and nitric oxide in bronchoalveolar lavage fluid. The drug limited (P < 0.05) neutrophil recruitment to lung tissues and alveoli but did not interfere with phagocytosis. HMR 3004 totally abrogated lung edema. By reducing inflammation in addition to possessing antimicrobial properties, HMR 3004 may participate in improving the outcome of bacterial pneumonia.
Despite the use of potent antibacterial agents, pneumococcal pneumonia still remains a deadly infectious disease in both immunocompetent and immunocompromised hosts. There is increasing evidence that although phagocytic cell recruitment to lung tissues plays a pivotal role in the killing of Streptococcus pneumoniae, excessive inflammatory reactions that result from this recruitment contribute to severe lung injury in immunocompetent hosts (3, 16). Antibiotics that may interact with the immune response in addition to possessing microbicidal properties might therefore contribute significantly to improving the outcome of pneumonia (12). Macrolides demonstrate anti-inflammatory properties, as they have been reported to reduce phagocytosis (26, 28), neutrophil (PMN) chemotaxis (17), and the release of proinflammatory cytokines, including interleukin-1 (IL-1) (25) and tumor necrosis factor (TNF) (10). HMR 3004 belongs to the ketolide family, which represents a new class of macrolide-like antimicrobial agents that are stable in weakly acidic environments. Recent studies indicated that HMR 3004 offers a potential alternative for the treatment of penicillin- and erythromycin-resistant S. pneumoniae (2, 6, 11). Using heat-killed S. pneumoniae, we evaluated the immunomodulating properties of HMR 3004 in a mouse model of pulmonary inflammation that mimics pneumococcal pneumonia. Inactivated bacteria have the advantage of being insensitive to the killing properties of antibiotics, so that alteration in bacterial clearance or inflammation may be attributed to drug-host cell interactions. In this study, we investigated the ability of HMR 3004 to modify PMN recruitment, phagocytosis by PMNs and alveolar macrophages, and release of TNF alpha (TNF-α), IL-1β, IL-6, and nitric oxide (NO).
S. pneumoniae serotype 3 was grown 12 h at 37°C in the presence of 5% CO2 in brain heart infusion broth supplemented with 5% horse serum. Bacteria were inactivated by heating at 60°C for 2 h and were labeled with fluorescein isothiocyanate (FITC) by stirring 108 CFU/ml (in 0.5 M carbonate-bicarbonate buffer, pH 9.5, containing 0.2 mg of FITC per ml) for 2 h at room temperature. Bacteria were washed and resuspended in phosphate-buffered saline (PBS) for inoculation to animals. The use of FITC-labeled bacteria allowed us to monitor phagocytosis through flow cytometry techniques. Lightly anesthetized male CD1 mice (Charles River, Québec, Canada) weighing 20 to 22 g received intranasal inoculations of 50 μl of PBS containing 108 CFU of heat-killed FITC-labeled S. pneumoniae every 12 h until four doses were administered (uninfected mice received PBS alone). HMR 3004 was given by gavage at a dose of 12.5 mg/kg of body weight in distilled water, starting 24 h before the initial bacterial inoculation and maintained every 12 h until the last inoculation (untreated animals received distilled water alone). This dosage ensures a 100% survival rate of pneumococcal pneumonia induced with live bacteria, even when therapy is started 48 h postinfection (data not shown). Multiple inoculations with the drug starting 24 h before the first infection appeared appropriate for the investigation of the drug’s potential interactions with immune cells. Mice received either bacteria alone, bacteria plus HMR 3004, HMR 3004 alone, or the appropriate control diluents. Animals were sacrificed by decerebration at either 1, 4, 12, 24, or 48 h after initiation of (the first) infection.
Bronchoalveolar lavage (BAL) was performed on six sacrificed mice per group per time. The skin was incised and the trachea was exposed, a catheter was inserted, and 3 1-ml aliquots of cold PBS were injected and recovered. After centrifugation at 3,400 × g for 10 min, supernatants were taken to detect TNF-α, IL-1β, and IL-6 with enzyme-linked immunosorbent assay kits (Genzyme Corporation, Cambridge, Mass.) and NO through the measurement of its oxidized nitrite and nitrate metabolites by the colorimetric method of Griess (7). The cell populations in the pellet were enumerated on Diff-Quick (Baxter, Pointe-Claire, Canada)-stained cytospin preparations. Cells were also fixed in 1% paraformaldehyde and subjected to flow cytometry for phagocytosis analysis. By selecting PMNs and macrophages on the forward-angle light scatter and the side scatter, we could determine the percentage of both populations that had a green fluorescence intensity greater than that of the control cells, thus obtaining the percentage of cells actively involved in the phagocytosis of FITC-labeled bacteria.
Six mice per group per time were also sacrificed for lung analysis. Lungs were taken, weighed, and infused with saline to remove blood. They were homogenized in 50 mM potassium phosphate buffer, pH 6.5. Six hundred microliters of phosphate buffer containing aprotinin (20 U) and CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (0.2%) were added to 600 μl of lung homogenate. Samples were centrifuged at 3,000 × g for 30 min, and cytokines and NO were quantified in supernatants as described above. Myeloperoxidase (MPO) was assayed in supernatants after the addition of 100 μl of hexadecyltrimethylammonium bromide to 100 μl of homogenate, sonication for 30 s, centrifugation, and reaction with O-dianisidine and hydrogen peroxide as previously described (3). Statistical analysis of the difference between groups was performed by the Mann-Whitney U test for nonparametric data and Fisher’s protected least-significant-difference test for normally distributed data. A P of <0.05 was considered significant. All data are presented as means ± standard errors of the means (SEM).
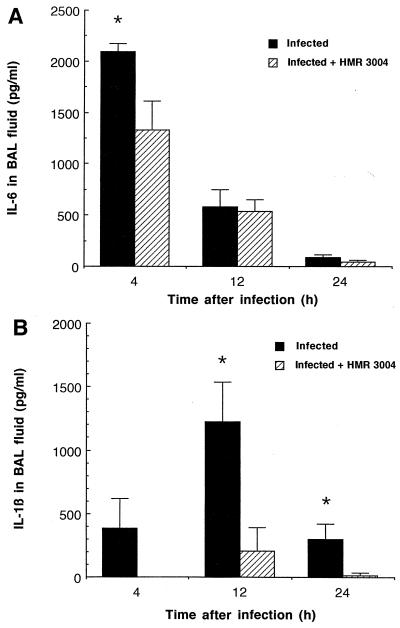
IL-6 levels rose sharply in BAL fluid of both infected groups as early as 4 h after inoculation, but significantly lower concentrations were seen at that time in animals treated with HMR 3004 (Fig. 1A; P < 0.05). IL-6 decreased rapidly thereafter in both groups. It remained undetectable in uninfected controls. The release of IL-1β in BAL fluid of untreated infected mice, although observable 4 h postinfection, occurred later than the release of IL-6, peaked at 12 h, and rapidly declined thereafter (Fig. 1B). HMR 3004 strongly inhibited IL-1β release, as this cytokine could not be detected at 4 h in treated infected mice and was significantly reduced at 12 and 24 h (P < 0.05). TNF-α expression was detected in BAL fluid of both infected groups, but no significant difference was noted between infected and treated infected mice. As an example, 1,560 ± 412 pg of TNF-α/ml of BAL fluid versus 1,829 ± 571 pg of TNF-α/ml of BAL fluid was recovered at 4 h in infected versus treated infected mice, respectively. IL-6, IL-1β, and TNF-α were below the limit of detection in lung homogenates of any group, as inactivated bacteria do not proliferate in tissues and do not induce bacteremia. They most likely activated alveolar macrophages for the expression of cytokines and chemokines.
FIG. 1.
Mean IL-6 (A) and IL-1β (B) levels in BAL fluid of CD1 mice infected with four doses of 108 CFU of heat-killed FITC-labeled S. pneumoniae and treated with placebo or HMR 3004 (12.5 mg/kg) every 12 h from 24 h preinfection until sacrifice of the animals. Both cytokines remained undetectable in uninfected controls. ∗, P < 0.05 between infected and infected plus HMR 3004-treated mice. Error bars, SEM.
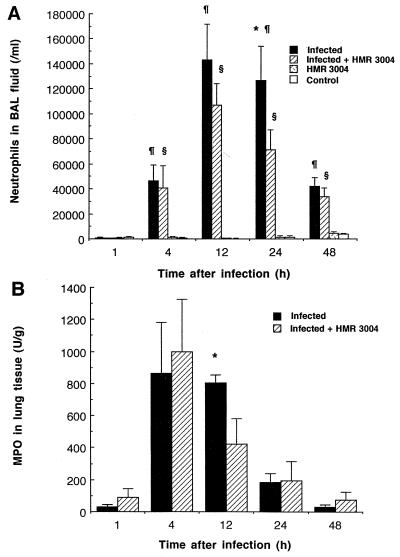
The profile of PMN recruitment in BAL fluid is reported in Fig. 2A. Compared to uninfected controls, in which negligible amounts of PMNs were harvested, both infected and treated infected mice experienced a significant increase in PMN counts from 4 to 48 h, peaking at 12 h. However, significantly lower counts were obtained after treatment of infected mice with HMR 3004 (24 h; P < 0.05). PMNs recruited to lung tissue before they reached the alveoli were detected in tissue homogenates earlier than in BAL fluid, as peak levels of MPO could be seen in both infected groups 4 h after infection (Fig. 2B). Here again HMR 3004 downregulated inflammation by significantly reducing MPO levels (12 h; P < 0.05). Monocytes were not recruited in this model, and the number of resident alveolar macrophages recovered in BAL remained stable (2.1 × 104 ± 0.1 × 104/ml of BAL fluid). As for the percentages of PMNs and macrophages that were actively involved in phagocytosis (detected as fluorescent cells by flow cytometry), of the total number of PMNs or macrophages present in BAL, no significant difference could be seen at any time of sacrifice between infected mice treated with HMR 3004 or a placebo. As an example, 75% of macrophages and 63% of PMNs were fluorescent at 12 h postinfection in untreated animals while treatment with HMR 3004 resulted in fluorescence in 82% of macrophages and 71% of PMNs.
FIG. 2.
(A) Mean PMN counts in BAL fluid of mice infected with four doses of 108 CFU of heat-killed FITC-labeled S. pneumoniae and treated with HMR 3004 (12.5 mg/kg) every 12 h from 24 h preinfection until sacrifice of the animals. Appropriate controls included uninfected mice treated with HMR 3004 or a placebo. Symbols: ∗, P < 0.05 between infected and infected plus HMR 3004-treated mice; ¶, P < 0.05 between infected and healthy control mice; §, P < 0.05 between infected plus HMR 3004-treated and HMR 3004 mice. (B) Mean MPO levels in lung tissue of animals treated as described for panel A. Negligible amounts of MPO were recovered in uninfected mice treated with HMR 3004 or placebo. ∗, P < 0.05 between infected and infected, HMR 3004-treated mice. Error bars, SEM.
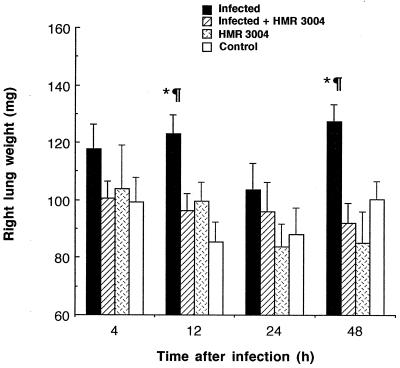
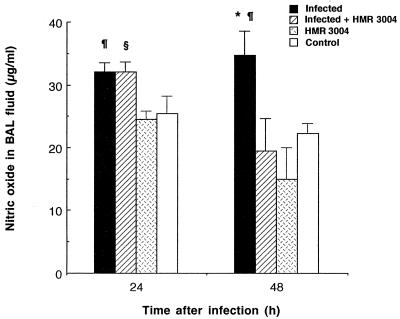
Edema was monitored as a criterion to evaluate additional host inflammatory reactions. Lung weight of untreated infected mice increased significantly after one or multiple inoculations with bacteria, as shown by high values at 12 and 48 h compared to those for healthy controls (Fig. 3; P < 0.05). HMR 3004 totally abrogated lung edema, as values similar to those in controls were obtained after treatment (P < 0.05). In addition, HMR 3004 significantly (P < 0.05) shortened late (24 to 48 h) pneumococcus-induced NO secretion by reducing the release of this proinflammatory mediator in BAL fluid at 48 h (Fig. 4).
FIG. 3.
Mean weight of right lung of mice infected with four doses of 108 CFU of heat-killed FITC-labeled S. pneumoniae and treated with HMR 3004 (12.5 mg/kg) every 12 h from 24 h preinfection until sacrifice of the animals. Appropriate controls included uninfected mice treated with HMR 3004 or placebo. Symbols: ∗, P < 0.05 between infected and infected plus HMR 3004-treated mice; ¶, P < 0.05 between infected and healthy control mice. Error bars, SEM.
FIG. 4.
Mean NO levels in BAL fluid of mice infected with four doses of 108 CFU of heat-killed FITC-labeled S. pneumoniae and treated with HMR 3004 (12.5 mg/kg) every 12 h from 24 h preinfection until sacrifice of the animals. Appropriate controls included uninfected mice treated with HMR 3004 or placebo. Symbols: ∗, P < 0.05 between infected and infected plus HMR 3004-treated mice; ¶, P < 0.05 between infected and healthy control mice; §, P < 0.05 between infected plus HMR 3004-treated and HMR 3004-treated mice. Error bars, SEM.
The present model of infection with repeated injections of heat-killed bacteria, although less potent at inducing inflammation than infection with live bacteria, is suitable for the investigation of the in vivo immunomodulator properties of antibiotics without regard to their direct effect on bacterial clearance. Heat-killed pneumococci have been shown in vitro to induce cytokine release by murine macrophages (22); we have shown in vivo by monitoring lung inflammation through BAL fluid that mice challenged with heat-killed S. pneumoniae secrete not only TNF-α, IL-1β, and IL-6 but also significant amounts of NO. We demonstrated in vivo anti-inflammatory effects of HMR 3004, as the drug downregulated IL-6, IL-1β, and NO release at the time of their peak production. The drug also diminished PMN recruitment in both lung tissue and alveoli without reducing the intrinsic phagocytic efficacy of either PMNs or alveolar macrophages, suggesting that HMR 3004 interferes with chemotaxis rather than with phagocytic processes. Although the mechanisms involved may not be fully clarified by the present experiment and possibly imply chemokine and additional host factor release, findings from other investigators provide support for a relationship between high intracellular uptake of ketolides or macrolides into phagocytes and resulting alterations in cytokine secretion and migration of inflammatory cells (1, 9, 15, 17, 25, 27). In the present experiment, the accumulation of HMR 3004 inside alveolar macrophages may have indirectly affected inflammation through variations in intracellular ion concentrations (23) or interaction with the expression of adhesion molecules (18, 26). In fact, alveolar macrophages, which are likely the primary immune cells in alveoli that recognize inhaled pneumococci, have been shown to secrete proinflammatory cytokines (4) which upregulate chemokine secretion for the recruitment of PMNs from the vascular compartment to infected tissues (13, 19). Thus, the observed reduction in PMN counts in BAL fluid might partly result from a decrease in cytokine (or chemokine) production (24).
The patterns of cytokine production and inflammatory cell recruitment differ substantially when heat-killed pneumococci are used instead of live bacteria, as can be seen by a comparison of the results in this manuscript and our previously reported data (3). BAL PMNs decline more rapidly with heat-killed bacteria, as does lung MPO, despite repeated inoculations with 10-fold-larger inoculum size than that used in single inoculation with live bacteria. There is also a much lower level of BAL TNF-α in the first 4 h when heat-killed bacteria are used, while there are higher levels of BAL IL-6 (IL-1β was measured in the present experiment, by contrast to IL-1α in the former one [3]). These observations suggest that bacterial proliferation and, most likely, toxin release greatly contribute to inflammation through TNF secretion and sustained PMN recruitment after infection with live bacteria. IL-6, by contrast, might reflect the severity of stress, whether of infectious or noninfectious origin (20), in animals exposed to repeated insults with various inoculum sizes of invasive agents.
It is now admitted that under septic conditions and in various pulmonary disorders, such as adult respiratory distress syndrome (5), overwhelming inflammatory reactions including TNF-α and IL-1β release participate in tissue injury and organ dysfunction. More recently NO was shown to participate in the pathogenesis of septic shock and of pneumococcal pulmonary infection (3, 29). Unrestrained NO secretion late in the course of infection appears to enhance edema (14) and cytotoxicity to tissue (3, 8). In addition, excessive PMN recruitment may exert deleterious effects through the release of oxidative intermediates and proteolytic enzymes (21). Thus, the development of drugs that optimize pulmonary inflammatory responses in addition to possessing bactericidal properties represent a therapeutic advantage for the treatment of pneumonia and other infectious diseases. Although studies of antibiotic-host cell interactions during infection with live bacteria are needed to corroborate our findings, our experimental approach allowed us to demonstrate a strong modulation of host response to S. pneumoniae by HMR 3004. These interesting properties of HMR 3004 provide support for the use of this agent against bacterial pneumonia. Future experiments with live pneumococci in untreated mice or mice treated with HMR 3004 or another antibiotic devoid of the in vitro immunomodulatory properties of ketolides are warranted. Both prophylactic and therapeutic regimens started at various stages of infection deserve to be investigated.
Acknowledgments
This work was supported by a grant from Hoechst Marion Roussel, Romainville, France.
We thank Martin Olivier and Denis Beauchamp for their kind participation in the project and Maurice Dufour for performing flow cytometry analysis.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Braham K, Collette P, Guitton M, Hochet A, Mauvais P, Chantot J F. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. RU 004: uptake by phagocytes, intracellular bioactivity and other immunomodulatory effects, abstr. F175; p. 102. [Google Scholar]
- 2.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehoux M S, Boutten A, Ostinelli J, Seta N, Dombret M C, Crestani B, Deschenes M, Trouillet J L, Aubier M. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 5.Demling R H. The role of mediators in human ARDS. J Crit Care. 1988;3:56–72. [Google Scholar]
- 6.Ednie L M, Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob Agents Chemother. 1997;41:1033–1036. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green L C, Tannenbaum S R, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981;212:56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- 8.Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 9.Honda, J., A. Keisuke, O. Yasumitu, N. Sin, and O. Kotaro. 1995. Effects of macrolides on cytokine mRNA expression, abstr. 3195. Can. J. Infect. Dis. 6(Suppl. C):423C.
- 10.Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol. 1992;101:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 11.Jamjian C, Biedenbach D, Jones R. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob Agents Chemother. 1997;41:454–459. doi: 10.1128/aac.41.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labro, M. T. 1997. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J. Chemother. 9(Suppl. 1):100–108. [PubMed]
- 13.Lukacs N W, Ward P A. Inflammatory mediators, cytokines and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–291. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 14.Lyons C R. The role of nitric oxide in inflammation. Adv Immunol. 1995;60:323–355. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- 15.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussa K, Michie H J, Cree I A, McCafferty A C, Winter J H, Dhillon D P, Stephens S, Brown R A. Phagocyte function and cytokine production in community acquired pneumonia. Thorax. 1994;49:107–111. doi: 10.1136/thx.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda H, Kadota J, Hara K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest. 1994;106:1116–1123. doi: 10.1378/chest.106.4.1116. [DOI] [PubMed] [Google Scholar]
- 18.Okubo, Y., J. Honda, K. Arikawa, and K. Oizumi. 1995. Macrolides reduce the expression of surface MAC-1 molecule on neutrophil, abstr. 3200. Can. J. Infect. Dis. 6(Suppl. C):424C. [DOI] [PubMed]
- 19.Oppenheim J, Zacharia C, Mukaida N, Matsushima K. Properties of the novel proinflammatory “intercrine” cytokine factor-alpha. Annu Rev Immunol. 1991;9:617–624. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 20.Puren A J, Feldman C, Savage N, Becker P J, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107:1342–1349. doi: 10.1378/chest.107.5.1342. [DOI] [PubMed] [Google Scholar]
- 21.Sibille Y, Reynolds H. Macrophages and polymorphonuclear cells in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 22.Simpson S Q, Singh R, Bice D E. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-alpha production by murine macrophages. Am J Respir Cell Mol Biol. 1994;10:284–289. doi: 10.1165/ajrcmb.10.3.8117447. [DOI] [PubMed] [Google Scholar]
- 23.Sugita, K., and T. Nishimura. 1995. Effects of antimicrobial agents on chemotaxis of human polymorphonuclear neutrophils, abstr. 3203. Can. J. Infect. Dis. 6(Suppl. C):424C.
- 24.Takeshi, F., J. I. Kadota, R. Shirai, K. Kawakami, K. Iida, M. Kaseda, S. Kawamoto, S. Kohno, and K. Hara. 1995. Inhibitory effect of roxithromycin on interleukin-8 production by vitamin D3-induced THP-1 cells, abstr. 3194. Can. J. Infect. Dis. 6(Suppl. C):423C.
- 25.Takeshita K, Yamagashi I, Harada M, Otomo S, Nakagawa T, Mizushima Y. Immunological and antiinflammatory effects of clarithromycin: inhibition of IL-1 production of murine peritoneal macrophages. Drugs Exp Clin Res. 1989;15:527–533. [PubMed] [Google Scholar]
- 26.Van Vlem B, Vanholder R, de Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 27.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenisch C, Parschalk B, Zedtwitz-Liebenstein K, Weihs A, El Menyawi I, Graninger W. Effect of single oral dose of azithromycin, clarithromycin, and roxithromycin on polymorphonuclear leukocyte function assessed ex vivo by flow cytometry. Antimicrob Agents Chemother. 1996;40:2039–2042. doi: 10.1128/aac.40.9.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright C E, Ress D, Mondaca S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992;26:48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]