Abstract
Cardiovascular diseases and diabetes mellitus are currently among the diseases with the highest morbidity and mortality. The pathogenesis and development of these diseases remain strongly connected, along with inflammation playing a major role. Therefore, the treatment possibilities showing a positive impact on both of these diseases could be especially beneficial for patients. SGLT-2 inhibitors and GLP-1 receptor agonists present this dual effect. Moreover, the hostile composition of the gut microbiota could influence the progression of these conditions. In this review, the authors present the latest knowledge on and innovations in diabetes mellitus and CVD—with the focus on the molecular mechanisms and the role of the microbiota.
Keywords: diabetes mellitus, cardiovascular diseases, microbiota
1. Introduction
Currently, both cardiovascular disorders and diabetes are among the diseases with the highest morbidity and mortality score, which are becoming an increasingly significant burden all over the world [1,2]. The term cardiovascular disease (CVD) includes, among others: coronary artery disease (CAD), stroke, heart failure, atrial fibrillation, and rheumatic and valvular heart disease [3]. Even though the knowledge about these diseases and the treatment options are constantly evolving, CVD remains the main cause of death worldwide [4].
The development of CVD remains strongly connected to the complications of diabetes. Metabolic disturbances occurring due to hyperglycaemia and insulin resistance induce proinflammatory phenotype and increase oxidative stress. These changes could lead to vascular and myocardial damage [5,6]. Consequently, proper prevention, diagnosis, and treatment of both diseases play a vital role in improving the quality of the patient’s life and extending the patient’s lifespan [7]. The connection between the pathophysiology of diabetes and CVD allows obtaining drugs that could have a beneficial effect on both of these conditions [6,8]. The current review focuses on the molecular mechanisms in the pathogenesis of diabetes and CVD and the molecular basis of the treatments used in these diseases.
2. What Is Diabetes Mellitus?
2.1. Definition and Types
Diabetes mellitus (DM) is a chronic disease characterised by a progressive decrease in the production of insulin or progressive tissue resistance to insulin [9,10]. These processes lead to higher blood glucose levels compared to the healthy population [11].
The American Diabetes Association (ADA) distinguishes four main types of diabetes mellitus: type 1 diabetes (T1D), type 2 diabetes (T2D), specific types of diabetes due to other causes, and gestational diabetes mellitus (GDM) [11]. Type 1 diabetes is triggered by a progressive destruction of the β-cells of the pancreas, usually as an effect of an autoimmunological mechanism, which results in a complete lack of insulin. Type 2 diabetes is characterised by progressive tissue insulin resistance [12]. Moreover, as an effect of the acceleration of the disease, patients develop a defect of insulin secretion. Usually T2D is part of a complex metabolic syndrome [13]. The development of medicine has allowed describing many subtypes of diabetes, which are collected by the ADA into a subgroup called “specific types of diabetes due to other causes”, which contains, for example: defects of β-cell function based on a genetic disorder (e.g., maturity-onset diabetes of the young (MODY) or neonatal diabetes), diabetes induced by drugs (glucocorticosteroids, medicines used after transplantation or in the treatment of human immunodeficiency virus), endocrinopathies, and diseases of the exocrine functions of the pancreas (e.g., cystic fibrosis (CF), hemochromatosis, pancreatitis) [14]. Furthermore, there are two main requirements to diagnose GDM: firstly, diabetes had not been diagnosed prior to gestation; secondly, diabetes has been diagnosed in the 2nd or 3red trimester of pregnancy [13].
2.2. Diagnosis
Guidelines specify that the diagnosis of diabetes should be based on one of four criteria:
Random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) with symptoms of hyperglycaemia.
Oral glucose tolerance test (OGTT) with a 75 g glucose: 2 h plasma glucose (2-hPG) level ≥ 200 mg/dL (11.1 mmol/L).
Fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L) in two separate tests.
Haemoglobin A1C level ≥ 6.5% (48 mmol/mol) [12].
Moreover, specific autoantibodies play a vital role in the diagnosis of T1D. The most-important ones are:
Antibodies against glutamic acid decarboxylase 65 (GAD65);
Islet cell antigen 2 (IA-2) antibodies;
Zinc transporter 8 (ZnT8) antibodies [15].
The detection of two or more of these autoantibodies enables diagnosing Stage 1 of diabetes (normoglycaemic, presymptomatic) [15,16].
2.3. Molecular Mechanism of Diabetes Mellitus
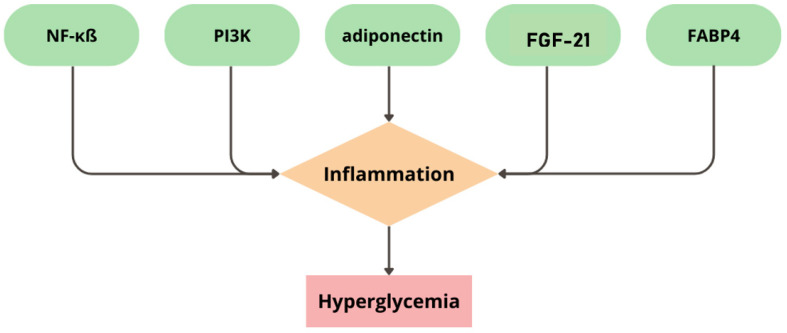
Inflammation is a topic covered by many scientific studies. This pathophysiological process appears as a reaction of the human immunological system to homeostasis imbalance. Inflammation is based on the signaling pathways that could lead to the expression of pro-inflammatory cytokines and immunological response [17]. The inflammatory process could also be the basis for the course of chronic diseases, e.g., diabetes mellitus [18,19,20]. This relationship applies both to T1D and T2D, but is due to different mechanisms [21,22]. The main pathological process that causes diabetes mellitus is inflammation, consequently leading to hyperglycaemia [18,19,20]. Scientists have revealed that the main factors that cause inflammation in diabetes mellitus are disorders of the following pathways: nuclear factor kappa ß (NF-κß), phosphoinositide 3-kinases (PI3Ks), adiponectin, fibroblast growth factor 21 (FGF-21), and fatty-acid-binding protein 4 (FABP4), which are presented in Figure 1 [18,23].
Figure 1.
The main pathways leading to inflammation in diabetes mellitus. NF-κß—nuclear factor kappa ß; PI3Ks—phosphoinositide 3-kinases; FGF-21—fibroblast growth factor 21; FABP4—fatty-acid-binding protein 4.
Nuclear factor kappa ß causes immunological response and inflammation since this molecule upregulates the genes responsible for coding inflammatory mediators, for example: tumour necrosis factor α (TNF-α) or first and sixth interleukin (IL-1 and IL-6). Apart from inflammation, these mediators can influence the growth, survival, and development of cells [24]. TNF-α disturbs the pathway of insulin function by lowering the expression of glucose transporter type 4 (GLUT-4), which results in lower glucose absorption by cells and increased resistance to insulin [25].
Phosphoinositide 3-kinases (PI3Ks) are a group of kinases for lipids and proteins. The pathway of PI3Ks is involved in the regulation of cells’ growth and metabolism by acting through the insulin-like growth factor receptor and the insulin receptor. Moreover, by the phosphorylation of protein kinase B (Akt/PKB), this pathway could affect the survival and proliferation of cells [26]. Furthermore, by affecting glucose transporters (GLUTs), PI3Ks could be the aim of treatment resulting in higher GLUT translocation and boosting insulin sensitivity [18,27].
Adipose tissue is made of adipocytes, which secrete adiponectin. This molecule could react via receptors named AdipoR1 and AdipoR2 with substrates originally intended for insulin receptors, affecting the PI3K pathway. Moreover, adiponectin might impact liver kinase, which influences NF-κß. Therefore, these reactions could probably prevent the development of insulin resistance and, furthermore, atherosclerosis [28].
Scientific research on animal models shows that fibroblast growth factor 21 could stimulate macrophages to present an anti-inflammatory effect on adipocytes, which leads to a decreased risk of insulin resistance. Moreover, FGF-21 might increase the storage of subcutaneous adipose tissue. Consequently, this process might raise the organism’s sensitivity to insulin. Furthermore, FGF-21 could enhance adiponectin with its activity against insulin resistance [29].
Fatty-acid-binding protein 4 is a molecule responsible for transporting lipids to cells and cells’ response to them. Moreover, FABP4 could impact peroxisome-proliferator-activated receptor gamma (PPAR-γ), which regulates insulin sensitivity and adipogenesis [30]. Therefore, FABP4 might influence insulin resistance and insulin secretion in diabetes mellitus type 2 [30,31].
The vasoactive intestinal peptide (VIP) pathway plays a vital role in immunological reactions through the activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH), which increases the production of reactive oxygen species (ROSs) by human phagocytes [32]. Yu et al. suggested that a lower VIP amount could be associated with the occurrence of diabetes mellitus in rodents [19].
2.4. Treatment
Achieving an HbA1C level under 7% is a desirable target in the treatment of diabetes mellitus [33]. Contemporary medicine gives compound therapeutic possibilities described in this section. It is worth pointing out that non-pharmacological therapy is the most-important in the treatment process of T2D. This type of diabetes is usually connected with metabolic syndrome. This leads to the conclusion that lifestyle interventions should be the main point of treatment in T2D and could be introduced with the help of. e.g., dietary and physiotherapy consultations [34]. Nevertheless, lifestyle changes alone are usually not sufficient to achieve proper glycaemic control, and pharmacotherapy is needed. The most-important groups of drugs used in the treatment of diabetes include:
Insulin—the main and the most-recognizable treatment of T1D and the last stage of treatment in T2D, when pancreas islets do not produce and secrete enough endogenic insulin. Adequate insulin therapy gives the opportunity to maintain the physiological level of glycaemia. The latest guidelines for treatment suggest using analogues of insulin in preference to human insulin because of their better profile of action [35,36].
Biguanide derivatives—with metformin as the main representative. The function of this drug is to increase the phosphorylation of the glucose transporter (GLUT), resulting in a boost of insulin sensitivity and uptake and, as consequence, leading to lover levels of glucose and HbA1c. Due to this function, metformin is used in the treatment of T2D. Moreover, metformin could support weight loss [37,38,39].
Thiazolidinediones (TZDs)—act through the peroxisome-proliferator-activated receptor γ (PPAR-γ), which leads to stimulation of insulin sensitivity. This group of medicines could also be used in T2D. Moreover, thiazolidinediones could decrease the patient’s weight as well [40,41,42].
Gliptins—work as inhibitors of dipeptidyl peptidase 4 (DPP-4), inhibiting the activation of incretin hormones such as gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). These hormones are responsible for the stimulation of insulin synthesis in response to a meal. To sum up, inhibitors of DPP-4 could help to achieve better glycaemia control in patients with T2D. It is worth mentioning that these medicines have a low hypoglycaemia risk and they are safe for the cardiovascular system (CVS) [43,44,45].
Glucagon-like peptide 1 (GLP-1) analogues. These medicines are glucose-dependent stimulators of insulin secretion. Moreover, GLP-1 analogues lower glucagon and hepatic glucose production, which could result in better control of glycaemia. Furthermore, they could slow down digestion and decrease food intake due to lower appetite. GLP-1 analogues, similar to DPP-4 inhibitors, could be safe for CVS and stimulate weight loss [46,47,48].
Gliflozins—inhibit sodium–glucose co-transporter-2 (SGLT-2), resulting in lower glucose reabsorption in renal tubules and higher glucose elimination through the kidneys (by glucosuria). Scientists have described that SGLT-2 inhibitors could have a cardiorenal protective effect coexisting with normoglycaemic function. Nevertheless, these medicines could increase the risk of urinary tract infections [49,50,51].
It is also worth noting that metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been associated with not only fat tissue reduction, but also the inhibition of epicardial adipose tissue deposition, alleviating adipose tissue inflammation, directly affecting cardiomyocytes [52].
2.5. Comorbidities
Diabetes mellitus, as a systemic disease, is related to abundant comorbidities.
2.5.1. Hypertension
Hypertension could accompany 2/3 patients with diabetes. Moreover, it is one of the risk factors of T2D, but also, T2D increases the risk of the occurrence of hypertension. This relationship might occur as a result of pathophysiological mechanisms such as the incorrect activity of the renin–angiotensin–aldosterone (RAA) system, vascular inflammation, hyperactivity of the sympathetic nervous system, and disturbed transport of sodium in the kidneys. Furthermore, diabetes associated with hypertension increases the risk of other cardiovascular diseases [53,54].
2.5.2. Obesity
Obesity is defined as abnormally high body fat deposition. The most-common obesity assessment scale is the body mass index (BMI). Obesity can be diagnosed when the BMI is over 30 kg/m2. Moreover, obesity is one of many modifiable risk factors of T2D. What is more, obesity could lead to insulin resistance and increase the level of inflammatory mediators, which could result in the progression of the prediabetes condition into diabetes mellitus [55,56,57].
2.5.3. Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is defined as a combination of endocrinological and gynaecological disturbances, which manifests as menstrual cycle disorder and infertility problems. Scientists have proven that PCOS is a nonmodifiable risk factor of T2D. This association occurs due to the fact that patients with PCOS are at a high risk of insulin resistance, so pharmacological and nonpharmacological prevention is necessary to protect against the development of the disease [58,59].
2.5.4. Dyslipidaemia
This lipid disorder is caused by abnormal metabolism and the quantity of lipoproteins such as triglycerides (TGs), low-density lipoproteins (LDLs), very-low-density lipoproteins (VLDLs), and high-density lipoproteins (HDLs). Patients with diabetes have higher amount of VLDLs and oxidative stress factors. Moreover, the simultaneous combination of diabetes and dyslipidaemia increases the risk of the occurrence of atherosclerosis [60,61,62].
2.5.5. Obstructive Sleep Apnoea
Obstructive sleep apnoea (OSA) is a disorder in which the patient presents periodic hypoxaemia during sleep as a consequence of partial obstruction of the airway. OSA is a risk factor of hypertension, cardiovascular diseases, and type 2 diabetes. The mechanism of the increased risk of diabetes is based on insulin resistance and chronic inflammation. Furthermore, recurring hypoxaemia induces higher catecholamine production and activity, which results in lower tissue sensitivity to insulin [63,64,65].
2.5.6. Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) is characterised by the increased storage of fat in the liver with no other concomitant causes. A biopsy is necessary to diagnose this condition. This disease could also lead to insulin resistance, but the specific relation and mechanism remain unclear [66,67,68,69].
The comparison between risk factors and the associated molecular pathways involved in the development of DM and CVD is presented in Table 1.
Table 1.
The comparison between the risk factors and associated molecular pathways involved in the development of diabetes (DM) and cardiovascular disease (CVD).
| Risk Factors | Diabetes | CVD |
|---|---|---|
| Inflammatory factors |
increased insulin resistance by IRS-1 [70] | endothelial injury, dysregulation of coagulation [71] |
| Oxidative stress | increased hexosamine pathway of glucose oxidation [72] | impaired mitochondrial electron transport, damage of DNA, leading to activation of stress-sensitive pathways [73] |
| Epigenetics | methylation of INS, PDX1, PPARGC1A, and GLP1R [74] | hydroxymethylation of myosin heavy chain 7, inhibition of BET protein [75] |
| Lipid dysregulations |
higher amount of VLDL [76] | increased risk of atherosclerosis [62,76] |
| Sleep apnoea | apnoea resulting in lower tissue sensitivity to insulin [63,64,65] | multifactorial pathophysiological processes resulting in HTN [77] |
| NAFLD | leading to bigger insulin resistance [68,69] | increased risk of myocardial infarction and brain stroke [78] |
| PCOS | higher risk of insulin resistance [76,77] | higher risk of hypertension in post-reproductive life [79] |
| RAAS | higher NADPH activity, resulting in cardiac dysfunction [80,81] | leading to local inflammation, which causes arterial thrombosis [82] |
| mRNA | m6A mRNA methylation [83] | m6A mRNA methylation [84] |
NAFLD—non-alcoholic fatty liver disease; PCOS—polycystic ovary syndrome; RAAS—renin–angiotensin–aldosterone system; mRNA—messenger ribonucleic acid; IRS-1—insulin receptor substrate-1; DNA—deoxyribonucleic acid; INS—insulin gene; PDX1—pancreatic and duodenal homeobox 1 gene; PPARGC1A—peroxisome-proliferator-activated receptor gamma coactivator 1-alpha gene; GLP1R—glucagon-like peptide-1 receptor gene; BET—bromodomain and extra-terminal domain; HTN—hypertension; VLDL—very-low-density lipoprotein; NADPH—nicotinamide adenine dinucleotide phosphate.
2.6. Morbidity of Diabetes Mellitus
Diabetes mellitus is one of the most-pervasive diseases all over the world [18,85]. In 2013, the authors established that there were approximately 382-million people suffering from diabetes worldwide [86]. In 2017, 451-million people had diabetes [87,88]. Moreover, the projection of the morbidity of diabetes mellitus has been included in scientific research. A progression to 591.9-million in 2035 and 693-million in 2045 in the 18–99-year-old group of people has been forecasted [86,87]. The growing amount of people with diabetes mellitus creates a disquieting perspective regarding the progression of mortality and morbidity, which could generate higher financial burdens on the healthcare system [89].
3. Cardiovascular Diseases in Patients with Diabetes
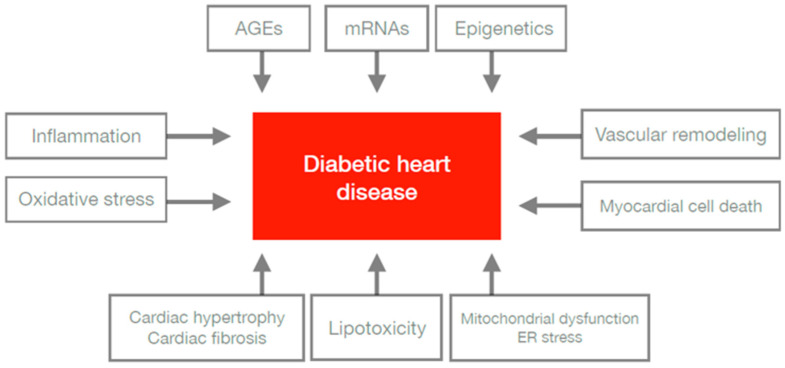
Patients living with diabetes are two-times more likely to develop and die from cardiovascular diseases (CVDs), such as myocardial infarctions, strokes, and heart failure, compared with non-diabetic subjects both in type 1 and 2 diabetes [90,91]. Heart diseases in diabetes may be characterised by an ischaemic etiology, leading to microvascular and macrovascular complications, as well as a muscle function disorder called diabetic cardiomyopathy [92,93,94]. Many studies have provided evidence for molecular changes, which, in combination, may affect the structure and function of the circulatory system. Metabolic anomalies, such as hyperglycaemia, hyperlipidaemia, inflammation, and insulin resistance, initiate a series of molecular events in the cardiovascular system involving structures such as vessels and the myocardium [5,93,95]. CVD is interlinked with diabetes due to the association with various mechanisms’ pathophysiologically. All undermentioned factors affecting the development of diabetic heart disease are presented in Figure 2.
Figure 2.
Factors affecting the development of diabetic heart disease. Factors affecting the development of diabetic heart disease: inflammation, increase of oxidative stress, increase of AGEs, expression of mRNAs, occurrence of epigenetic factors, vascular remodelling, imbalance of cell death, dysregulation of mitochondria, ER stress, lipotoxicity, cardiac hypertrophy, and cardiac fibrosis are included in the pathophysiology of cardiovascular diseases in patients with diabetes mellitus. AGEs—advanced glycation end products; mRNAs—messenger ribonucleic acids; ER—endoplasmic reticulum.
3.1. Inflammation and Vascular Remodelling
Increased inflammatory cytokines, such as C-reactive protein, interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor α (TNF-α), and endothelin-1, are assumed to cause endothelial injury and coagulation dysregulations, resulting in an increased risk for CVD [70]. These molecules can exacerbate systemic insulin resistance and contribute to cardiac insulin resistance mediated by insulin receptor substrate protein-1 (IRS-1) serine (Ser) phosphorylation [71]. Increased perivascular and intermyofibrillar fibrosis has been also observed in myocardial samples in the absence of coronary artery disease and hypertension [96]. Studies on animal models of insulin deficiency revealed further increased myocardial inflammation due to increased macrophage activation [97,98]. Other studies demonstrated intramyocardial inflammation including increased expression of cell adhesion molecules: intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), and an increased concentration of inflammatory cytokines and leukocytes [70,99]. Furthermore, hyperinsulinaemia promotes hepatic synthesis of prothrombotic factors and quantitative modifications in clotting factors, which combined, increase the thrombosis risk [100].
3.2. AGEs
It is worth mentioning advanced glycation end products (AGEs), which are advanced end products of protein glycosylation and are formed as a result of a multi-stage process that may disable protein function. Hyperglycaemia may impair the protein degradation process, collagen included, which leads to increased fibrosis and myocardial stiffness [101]. AGEs bind to the AGE receptors (RAGEs), whose expression increases in diabetic hearts due to oxidative stress [102]. AGEs also release key prosclerotic and proinflammatory cytokines and increase vascular stiffness. All these phenomena can be used to partly explain the atherosclerosis and diastolic dysfunction noted in diabetic patients [101,102].
3.3. Oxidative Stress
Hyperglycaemia-induced superoxide overproduction in the mitochondrial electron transport chain is thought to be a significant factor in diabetic vascular complications. The enhanced activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been linked to increased reactive oxygen species (ROS) production in diabetic cardiomyocytes. Uncoupling of mitochondrial adenosine triphosphate (ATP) synthesis from oxygen intake, followed by the leakage of the mitochondrial electron transport chain, may result in decreased heart function [82]. Uncoupling of nitric oxide synthase and activation of protein kinase C, lipoxygenase, and xanthine oxidase are all sources of ROSs [103]. The creation of the potent oxidant peroxynitrite causes DNA, protein, and lipid damage, as well as the activation of stress-sensitive pathways [82].
The main metabolic pathways induced by hyperglycaemia include the polyol pathway, the hexosamine pathway, the protein kinase C (PKC) pathway, and the advanced glycation end products (AGE) pathway. These processes could not only lead to increased ROS production, but also activate inflammatory response, alter gene expression, or induce osmotic and oxidative stress [104].
3.4. Lipotoxicity and Mitochondrial Dysfunction ER Stress
Fatty acid (FA) absorption and oxidation overwork diabetic hearts. Increased FA oxidation has been linked to higher myocardial oxygen demand and lower cardiac efficiency [82]. This is due to FA-induced mitochondrial uncoupling of adenosine triphosphate (ATP) production from oxygen consumption, which results in energy loss. In addition, cardiac lipotoxicity caused by myocardial accumulation of lipids impairs cardiomyocyte activity. Diabetes studies on animals showed increased lipid accumulation and usage [105]. An important underlying mechanism for lipotoxic damage may involve an increase in apoptotic cell death, ceramide biosynthesis, and ROS production, as well as remodelling of the mitochondrial membrane phospholipid composition, including a decrease in cardiolipin content and an increase in the endoplasmic reticulum (ER) saturated lipid content, resulting in ER stress [106,107].
3.5. Myocardial Cell Death
Cardiomyocyte injury in diabetes is also promoted by altered cell homeostatic processes such as autophagy. Autophagy is a physiological process in which damaged cell components, such as organelles, proteins, and metabolites from the cell, are removed or recycled. Any dysregulations of this process lead to an imbalance in cell homeostasis [108]. Not only repression, but also an increase of autophagy have been reported in diabetic hearts and their cardiomyocytes [73,109,110]. The activation of autophagy may be insufficient in the load-stressed heart and during postischaemic reperfusion [109]. Recent findings have provided compelling evidence that insulin signaling is an important regulator of myocardial autophagy [73]. More studies are required to elucidate whether autophagy is detrimental in diverse models of diabetes.
3.6. Renin–Angiotensin–Aldosterone System
Hyperglycaemia promotes angiotensinogen and angiotensin II production by activating p53 [86]. Angiotensin II directly increases vascular reactive oxygen species (ROS) production and reduces endothelial nitric oxide (NO) production. Both angiotensin II and aldosterone directly prompt oxidative stress by increasing NADPH oxidase activity [86]. Angiotensin II and aldosterone levels have also been implicated in the pro-fibrotic effects observed in patients with cardiac dysfunction induced by diabetes [87]. These abnormalities lead to the remodelling and fibrosis of diabetic hearts.
3.7. mRNA
Recently, micro-ribonucleic acids (miRNAs) have been defined as the micromanagers of gene expression. Many studies suggest that miRNAs take part in the pathogenesis of diabetes and cardiovascular complications such as endothelial dysfunction, angiogenesis, heart failure, and myocardial fibrosis by dysregulating the expression of multiple genes [111]. These regulators of genes are endogenous, noncoding, single-stranded ribonucleic acids (RNAs) with an average length of 22 nucleotides and are encoded by short inverted repeats within the genome [112]. Their regulation is based on the repression of translation and on promoting the degradation of target messenger ribonucleic acids (mRNAs). Changes in miRNA levels can play a crucial role in progressive dilated cardiomyopathy and heart failure [113].
3.8. Epigenetics
Histone modifications have also been found to play an important role in cardiac remodelling in patients with diabetes. Epigenetic factors could mediate the interplay between genes and the environment, resulting in the activation or repression of genetic transcription or even silencing genetic transcription by different types of reactions. Non-specific inhibitor-based silencing of histone deacetylases (HDACs) has been shown to promote cardiac hypertrophy and fibrosis by increasing glucose transporter-1 acetylation and mitogen-activated-protein-kinase (MAPK)-mediated phosphorylation in diabetic heart disease of mice, though their exact function should be confirmed [114,115]. Future studies may contribute to the specific molecular defects that characterise diabetic cardiomyopathy and changes in gene regulation by epigenetic mechanism and activation of transcription factors. All aforementioned factors affecting the development of diabetic heart disease are presented in Figure 2.
4. Drugs Used in Treatment of Diabetes and Cardiovascular Disease
The standard diabetes treatment includes different kinds of drugs, used in combination or as a monotherapy. Two of these groups, SGLT-2 inhibitors and GLP-1 receptor agonists, could be used in the treatment of both diabetes and cardiovascular disease, due to their beneficial effect on the cardiovascular system.
4.1. Sodium–Glucose Co-Transporter-2 Inhibitors
Sodium–glucose transporters (SGLTs) are located on the luminal membrane of the proximal tubules, where they reabsorb glucose from the glomerular filtrate. Oral SGLT-2 inhibitors (e.g., empagliflozin, dapagliflozin) are quickly absorbed into the bloodstream, where they remain for several hours. These molecules bind specifically to SGLT-2 in the luminal membrane of the early proximal tubules to reduce glucose reabsorption by 50–60% and, consequently, to reduce plasma glucose levels due to glucose excretion [116].
Although SGLT-2 inhibitors were originally developed as a glucose-lowering therapy in patients with diabetes, they present additional benefits such as lowering blood pressure [117]. Furthermore, the results of clinical trials have presented that these drugs show the ability to protect against cardiovascular disease, especially by reducing the risk of hospitalisation due to heart failure with both a reduced and preserved ejection fraction [117,118]. These beneficial effects turned out to be achievable in many subgroups of patients, regardless of diabetes diagnosis [6].
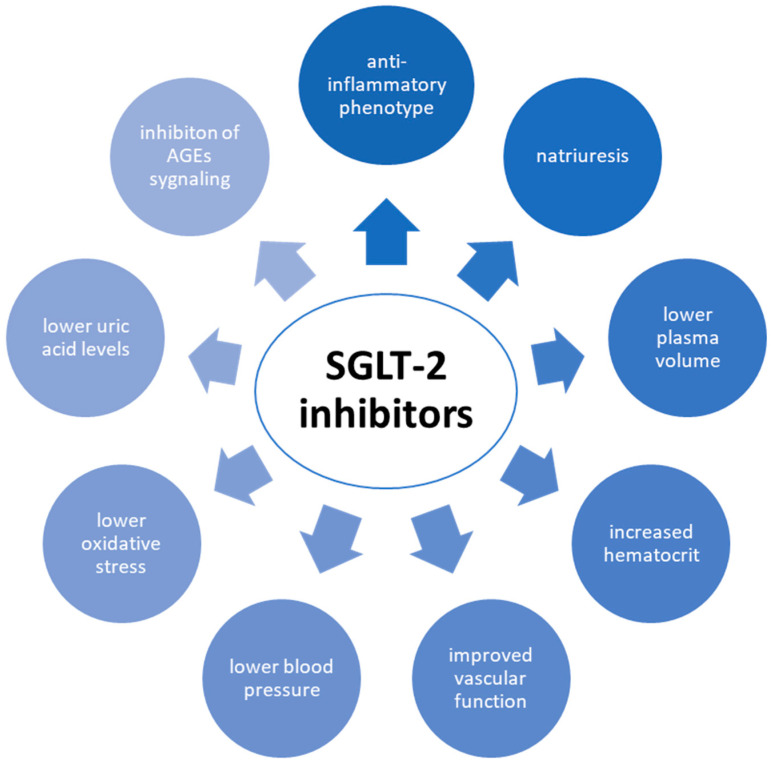
The mechanisms by which SGLT-2 inhibitors reduce the risk of CVD are compound and multifactorial. Early natriuresis with a decreased plasma volume, a subsequent increase in haematocrit, improved vascular function, lower blood pressure, and altered tissue handling of sodium may all play a role. Other mechanisms by which SGLT2 inhibitors may be beneficial include the reduction of adipose-tissue-mediated inflammation, the lower production of pro-inflammatory cytokines, the conversion of ketone bodies into cardiac and renal metabolic substrates, the reduction of oxidative stress, the reduction of serum uric acid levels, and the inhibition of advanced glycation end product (AGE) signaling [6]. These mechanisms are presented in Figure 3.
Figure 3.
Mechanisms by which SGLT-2 inhibitors reduce the risk of CVD. SGLT-2—sodium–glucose co-transporter-2; CVD—cardiovascular disease; AGEs—advanced glycation end products.
4.2. Glucagon-like Peptide-1 Receptor Agonists
Glucagon-like peptide-1 (GLP-1) belongs to the group of incretin hormones, which are involved in meal-induced insulin secretion and play a vital role in maintaining normal glucose tolerance via the gut–endocrine–pancreas axis [119]. The incretin effect causes increased insulin secretion after oral glucose intake compared to parenteral glucose administration [119]. Moreover, the activation of the GLP-1 receptor might present a protective function of pancreatic islet ß cells and promote their proliferation, act as an anti-inflammatory agent, improve the function of the heart, regulate lipid metabolism, and promote nerve growth [120]. Other biological functions of GLP-1 include a reduction of appetite and delayed gastric emptying and, as an effect, might lead to weight loss [8,120].
All those metabolic changes might be beneficial to general health as well. The cardioprotective effect of GLP-1 receptor agonists combined with lowered blood pressure, improved lipid profile, and reduced body weight suggest that this group of drugs could have the potential to decrease cardiovascular risk [121]. Studies have shown that, especially, semaglutide, liraglutide, and albiglutide are able to reduce the risk of major adverse cardiac events (MACEs) [8,122,123,124].
5. Potential Role of Microbiome in Diabetes and Cardiovascular Diseases
In diabetes mellitus, due to the reduced blood flow combined with atheromatic malformations and sclerotic alterations in blood vessels, peripherical neuropathy occurs, which could intensify the risk of creating ulcers and wounds. A high level of blood glucose functions as nutrition for microorganisms that can infect a wound or ulcer, then overgrow and, consequently, might lead to bacteriaemia/fungaemia. In severe cases, this may cause sepsis, which could lead to septic shock. In many cases, this condition might be extremely hard to overcome, which could even lead to the demise of the patient [125]. It is also worth noticing that the human gut consists of circa 1014 bacteria from over 1000 species and the dysbiosis of its structure or function is observed in serious diseases such as, e.g., diabetes mellitus, Crohn’s disease, irritable bowel syndrome (IBS), obesity, and colorectal cancer [126,127].
The human gut microbiota usually consists of six main phyla of bacteria: Acinetobacteria, Proteobacteria, Firmicutes, Bacteroidetes, Verrucomicrobia, and Cyanobacteria [128,129].
Type 1 diabetes mellitus is characterised by a lower amount of mucin-degrading bacteria such as Akkermansia municiphila, Bifidobacterium, Prevotella, Lactobacillus, and Firmicutes and, at the same time, an increased amount of Bacteroidetes and Clostridium [130,131]. What is also important to mention is that there are reports suggesting that exposure to the Rubella virus during pregnancy can increase the chance of acquiring T1D [132].
Microbial dysbiosis in T2D consists of a Clostridium sp. decrease and, simultaneously, an increase of Lactobacillus sp., Firmicutes sp., and Bacteroides sp. [125,133]. The Roseburia butyrate-producing genus, as well as Faecalibacterium prausnitzii are decreased; however, the amount of opportunistic pathogens is increased [134].
Even though it is not clear what is the one and only cause of diabetes mellitus, microbial dysbiosis has a detrimental effect on the patient’s homeostasis in general [135].
Cardiovascular diseases (CVDs) are the leading cause of death all over the world; however, there are multiple reasons for cardiovascular diseases to occur [136]. Likewise, the alterations in the composition of the gut microbiota could exert an impact on the cardiovascular system, which might be either beneficial or detrimental [137]. Several CVDs are associated with microbial dysbiosis such as atherosclerosis, heart failure, and hypertension [137]. The influence on the human body is exerted by bacterial metabolites such as trimethylamine-N-oxide (TMAO), short-chain fatty acids (SCFAs), and bile acids (BAs), which have been shown to exert an impact on the host’s homeostasis [138].
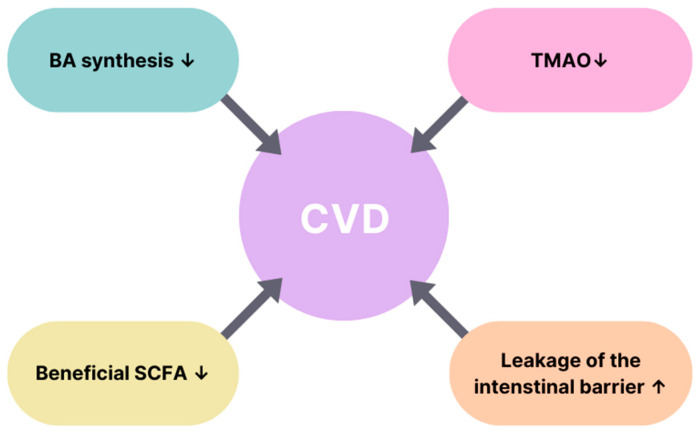
Cardiovascular diseases can be manifested by the alteration of the microbiota’s structure and its metabolites. The decrease of faecal butyrate and the increase of the TMAO level promote the development of strokes. A decreased level of SCFA production was observed to increase the chance of both carotid artery stenosis and mesenteric ischaemia. A decrease in bile acid levels and an increase of the TMAO level were linked to promoting carotid artery disease. A reduction of bile acid synthesis and an increase of the TMAO were observed to promote peripheral artery disease [139]. Cardiovascular diseases and the associated alterations of bacterial metabolites are presented in Table 2, and the connection between the alterations of bacterial metabolites and CVDs is presented in Figure 4.
Table 2.
Cardiovascular diseases and associated alterations of bacterial metabolites [139].
| Cardiovascular Disease | Alteration of Bacterial Metabolites |
|---|---|
| Stroke | Faecal butyrate decreased |
| TMAO increased | |
| Carotid artery stenosis | SCFA production by intestinal microbiota decreased |
| Mesenteric ischaemia | SCFA production by intestinal microbiota decreased |
| Carotid artery disease | BA decreased |
| TMAO increased | |
| Peripheral artery disease | Synthesis of BAs decreased |
| TMAO increased |
BAs—bile acids; SCFA—short-chain fatty acid; TMAO—trimethylamine-N-oxide.
Figure 4.
Connection between alterations of bacterial metabolites and CVDs [139]. The figure explains the correlation of factors leading to cardiovascular diseases. The increased leakage of the intestinal barrier alongside with a TMAO level decrease, the lowering of bile acid synthesis, and a reduction of beneficial SCFAs are the components that are related to the development of CVDs. ↑—increase; ↓—decrease; BA—bile acid; SCFA—short-chain fatty acid; TMAO—trimethylamine-N-oxide; CVD—cardiovascular disease.
In patients with atherosclerosis, an increased number of bacteria including Collinsella, E. coli, and Enterobacter aerogenes has been noticed. In regard to carotid artery disease (CAD), bacteria of the genera Lactobacillus, Streptococcus, the Escherichia/Shigella ratio, and Enterococcus were increased in number, whereas the amounts of Bacteroides, Faecalibacterium, Prevotella, Subdoligranulum, Eubacterium rectale, and Roseburia were lowered. When it comes to atheromatic plaques, an increase of Staphylococcus sp., Proteus vulgaris, Streptococcus sp., and Klebsiella pneumoniae was observed. However, it is vital to mention that the change of those microbes leads to inflammation, which exacerbates atherosclerosis, but is not definitive proof of being the cause of atherosclerotic plaques’ formation. Alterations in the composition of the gut microbiota in different CVDs are presented in Table 3.
Table 3.
Alterations in the composition of the gut microbiota in different CVDs.
| Cardiovascular Disease | Changes in the Composition of Microbiota |
|---|---|
| Atherosclerosis | Collinsella, Escherichia coli, Enterobacter aerogenes, Klebsiella sp. increased [134] |
| Carotid artery disease (CAD) |
Lactobacillus, Streptococcus, Escherichia/Shigella, Enterococcus increased Bacteroides, Prevotella, Faecalibacterium, Subdoligranulum, Roseburia, Eubacterium rectale decreased [140,141] |
| Atheromatous plaques | Proteus vulgaris, Staphylococcus sp., Klebsiella pneumoniae, Streptococcus sp. increased [142] |
sp.—species; CVD—cardiovascular disease.
Out of many possible ways of treating diabetes and CVDs, probiotic therapy is hoped to be both effective and safe from a long-term perspective. Probiotics act by inhibition of pathogen growth, stimulation of the immune system, and changing pH levels [128]. From the great variety of microorganisms, Sacharomycces boulardii is one that is being used in chronic heart failure and might have the ability to lower the uric acid level, lower the level of cholesterol, and also improve the ventricular ejection fraction [143].
The most popular type of probiotics consists of the four most-common species: Streptococcus sp. Bifidobacterium sp., Lactobacillus sp., and Enterococcus sp., and their sources might be dietary such as yogurt, cheese, or fermented products, which could present beneficial effects if consumed on a regular basis [144,145].
The comorbidities leading to CVDs that could potentially be reduced by the use of probiotics are presented in Table 4 [146]. This table presents strains of probiotic bacteria that might protect against the development of diseases that lead to CVDs.
Table 4.
The comorbidities leading to CVDs that could potentially be reduced using probiotics.
| Comorbidity | Genera of Probiotic Bacteria |
|---|---|
| Diabetes mellitus type 2 |
Lactobacillus reuteri
Lactobacillus casei Lactobacillus plantarum Bifidobacterium bifidum Lactobacillus rhamnosus Bifidobacterium lactis |
| Hypertension |
Lactobacillus bulgaricus
Lactobacillus casei Streptococcus thermophilus Lactobacillus helveticus Saccharomyces cerevisia |
| Obesity |
Bifidobacterium animalis sp. lactis Lactobacillus rhamnosus Lactobacillus curvatus Lactobacillus plantarum |
| Hypercholesterolaemia |
Lactobacillus reuteri
Lactobacillus curvatus Streptococcus thermophilus Enterococcus faecalis Lactobacillus casei Saccharomyces boulardii Bifidobacterium longum Lactobacillus acidophilius |
sp.—species.
6. Conclusions
Diabetes mellitus (DM) is a medical condition of endocrinological origin characterised by an elevated blood glucose level, which poses a serious threat to the public health system and can be perilous to the state of wellbeing for a patient, or even lethal [133,147]. The World Health Organization (WHO) states that diabetes mellitus is also a significant stimulus for developing cardiovascular diseases such as heart failure, stroke, renal failure, gastroparesis, or Charcot joint [125]. Other comorbidities of diabetes include hypertension, obesity, PCOS, dyslipidaemia, OSA, and NAFLD.
Scientists have proven that the pathological mechanism of diabetes mellitus could mostly be based on oxidative stress and inflammatory processes. What is more, other molecular mechanisms could also participate in the progression of the disease [89]. It has been shown that many different disturbances in molecular pathways might occur and exacerbate the proinflammatory phenotype (e.g., NF-κß, PI3Ks, FGF-21, FABP4, and adiponectin).
The diabetes treatment guidelines include several groups of drugs. Nonetheless, new chances for alleviating the complications of diabetes mellitus are based on sodium–glucose co-transporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists [127,130]. Besides, these medicines are used in the treatment of cardiovascular diseases [148,149]. This correlation is possible due to the interdependence of the pathogenesis of these diseases. Inflammation plays a significant role in the development of CVDs and DM; however, advanced glycation end products (AGEs), genetics, oxidative stress, and mitochondrial dysfunction are also involved in this compound process. Consequently, it has been found that a holistic treatment approach targeting a greater number of risk factors correlates with better CVD-free survival in patients with DM at high cardiovascular risk [150].
Moreover, the gut microbiota has a genuine effect on both diabetes and CVDs. Due to the fact that these diseases could lead to severe complications for the patient, relying entirely on probiotics may be insufficient; however, it is highly recommended to enrich the treatment with the intake of probiotics [145]. Further research on this topic is needed to fully establish the composition of the most-effective probiotic treatment in both CVDs and diabetes.
This literature review had certain limitations. Firstly, it relied entirely on previously published studies, and although great care was taken in the selection of the data included, some errors due to previously applied methods are possible. Secondly, the lack of external funding limited the availability of non-open-access research. Thirdly, only scientific papers written in English were evaluated.
A major positive outcome of this scientific study was that the key molecular mechanisms known to influence diabetes and CVDs were collected and discussed. The implementation of a treatment showing a positive impact on both diseases could be especially beneficial for patients. Therefore, further research towards the creation of novel drugs acting through common pathways of the progression of diabetes and CVDs could be the proper direction for the future.
Author Contributions
Conceptualisation: J.T., E.B. and E.M.; methodology: E.M., J.T. and E.B.; software: E.M.; validation: E.M., B.F. and J.R.; formal analysis: E.M., J.T., E.B., M.S., K.S. and E.W.; investigation: J.T., E.B., M.S., K.S., E.W. and E.M.; resources: E.M., B.F. and J.R.; data curation: E.M.; writing—original draft preparation: J.T., E.B., M.S., K.S., E.W. and E.M.; writing—review and editing: E.M.; visualisation: E.M., J.T. and E.B.; supervision: E.M.; project administration: E.M., B.F. and J.R.; funding acquisition: B.F. and J.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this article were sourced from materials mentioned in the References Section.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kleinberger J.W., Pollin T.I. Personalized Medicine in Diabetes Mellitus: Current Opportunities and Future Prospects: Personalized Medicine in Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2015;1346:45–56. doi: 10.1111/nyas.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend N., Kazakiewicz D., Lucy Wright F., Timmis A., Huculeci R., Torbica A., Gale C.P., Achenbach S., Weidinger F., Vardas P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022;19:133–143. doi: 10.1038/s41569-021-00607-3. [DOI] [PubMed] [Google Scholar]
- 3.Leong D.P., Joseph P.G., McKee M., Anand S.S., Teo K.K., Schwalm J.-D., Yusuf S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017;121:695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 4.Saeed A., Kampangkaew J., Nambi V. Prevention of Cardiovascular Disease in Women. Methodist DeBakey Cardiovasc. J. 2017;13:185. doi: 10.14797/mdcj-13-4-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun J.-S., Ko S.-H. Current Trends in Epidemiology of Cardiovascular Disease and Cardiovascular Risk Management in Type 2 Diabetes. Metabolism. 2021;123:154838. doi: 10.1016/j.metabol.2021.154838. [DOI] [PubMed] [Google Scholar]
- 6.Cowie M.R., Fisher M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit beyond Glycaemic Control. Nat. Rev. Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 7.Kivimäki M., Steptoe A. Effects of Stress on the Development and Progression of Cardiovascular Disease. Nat. Rev. Cardiol. 2018;15:215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 8.Andrikou E., Tsioufis C., Andrikou I., Leontsinis I., Tousoulis D., Papanas N. GLP-1 Receptor Agonists and Cardiovascular Outcome Trials: An Update. Hellenic J. Cardiol. 2019;60:347–351. doi: 10.1016/j.hjc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Al-awar A., Kupai K., Veszelka M., Szűcs G., Attieh Z., Murlasits Z., Török S., Pósa A., Varga C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016;2016:1–12. doi: 10.1155/2016/9051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerner W., Brückel J. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes. 2014;122:384–386. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 11.Khan R.M.M., Chua Z.J.Y., Tan J.C., Yang Y., Liao Z., Zhao Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina. 2019;55:546. doi: 10.3390/medicina55090546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Cusi K., Das S.R., Gibbons C.H., et al. Introduction and Methodology: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46:S1–S4. doi: 10.2337/dc23-Sint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punthakee Z., Goldenberg R., Katz P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes. 2018;42:S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Professional Practice Committee 2 Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45:S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 15.Insel R.A., Dunne J.L., Atkinson M.A., Chiang J.L., Dabelea D., Gottlieb P.A., Greenbaum C.J., Herold K.C., Krischer J.P., Lernmark Å., et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayan C.M., Korah M., Tatovic D., Bundy B.N., Herold K.C. Changing the Landscape for Type 1 Diabetes: The First Step to Prevention. Lancet. 2019;394:1286–1296. doi: 10.1016/S0140-6736(19)32127-0. [DOI] [PubMed] [Google Scholar]
- 17.Stewart A., Beart P. Inflammation: Maladies, Models, Mechanisms and Molecules: Inflammation: Maladies, Models, Mechanisms and Molecules. Br. J. Pharmacol. 2016;173:631–634. doi: 10.1111/bph.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehghan M., Ghorbani F., Najafi S., Ravaei N., Karimian M., Kalhor K., Movafagh A., Mohsen Aghaei Zarch S. Progress toward Molecular Therapy for Diabetes Mellitus: A Focus on Targeting Inflammatory Factors. Diabetes Res. Clin. Pract. 2022;189:109945. doi: 10.1016/j.diabres.2022.109945. [DOI] [PubMed] [Google Scholar]
- 19.Yu R., Zhang H., Huang L., Liu X., Chen J. Anti-Hyperglycemic, Antioxidant and Anti-Inflammatory Effects of VIP and a VPAC1 Agonist on Streptozotocin-Induced Diabetic Mice. Peptides. 2011;32:216–222. doi: 10.1016/j.peptides.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Oguntibeju O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Bending D., Zaccone P., Cooke A. Inflammation and Type One Diabetes. Int. Immunol. 2012;24:339–346. doi: 10.1093/intimm/dxs049. [DOI] [PubMed] [Google Scholar]
- 22.Lontchi-Yimagou E., Sobngwi E., Matsha T.E., Kengne A.P. Diabetes Mellitus and Inflammation. Curr. Diabetes Rep. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 23.Tucker W., McClelland R.L., Allison M.A., Szklo M., Rye K.-A., Ong K.L. The Association of Circulating Fibroblast Growth Factor 21 Levels with Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Metabolism. 2023;143:155535. doi: 10.1016/j.metabol.2023.155535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Z., Zhuang X., Luo M., Yin W., Xiong L. The Propionic Acid and Butyric Acid in Serum but Not in Feces Are Increased in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. BMC Gastroenterol. 2020;20:73. doi: 10.1186/s12876-020-01212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin Resistance: Review of the Underlying Molecular Mechanisms. J. Cell. Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 26.Li M., Murabito A., Ghigo A., Hirsch E. PI3Ks in Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2017;70:422–429. doi: 10.1097/FJC.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 27.Rubin B.R., Bogan J.S. Vitamins & Hormones. Volume 80. Elsevier; Amsterdam, The Netherlands: 2009. Chapter 7 Intracellular Retention and Insulin-Stimulated Mobilization of GLUT4 Glucose Transporters; pp. 155–192. [DOI] [PubMed] [Google Scholar]
- 28.Achari A., Jain S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Wu G., Fang Q., Zhang M., Hui X., Sheng B., Wu L., Bao Y., Li P., Xu A., et al. Fibroblast Growth Factor 21 Increases Insulin Sensitivity through Specific Expansion of Subcutaneous Fat. Nat. Commun. 2018;9:272. doi: 10.1038/s41467-017-02677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trojnar M., Patro-Małysza J., Kimber-Trojnar Ż., Leszczyńska-Gorzelak B., Mosiewicz J. Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus. Cells. 2019;8:227. doi: 10.3390/cells8030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura R., Okura T., Fujioka Y., Sumi K., Matsuzawa K., Izawa S., Ueta E., Kato M., Taniguchi S., Yamamoto K. Serum Fatty Acid-Binding Protein 4 (FABP4) Concentration Is Associated with Insulin Resistance in Peripheral Tissues, A Clinical Study. PLoS ONE. 2017;12:e0179737. doi: 10.1371/journal.pone.0179737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chedid P., Boussetta T., Dang P.M.-C., Belambri S.A., Marzaioli V., Fasseau M., Walker F., Couvineau A., El-Benna J., Marie J.-C. Vasoactive Intestinal Peptide Dampens Formyl-Peptide-Induced ROS Production and Inflammation by Targeting a MAPK-P47phox Phosphorylation Pathway in Monocytes. Mucosal Immunol. 2017;10:332–340. doi: 10.1038/mi.2016.51. [DOI] [PubMed] [Google Scholar]
- 33.Turner R.C. Glycemic Control With Diet, Sulfonylurea, Metformin, or Insulin in Patients With Type 2 Diabetes MellitusProgressive Requirement for Multiple Therapies (UKPDS 49) JAMA. 1999;281:2005. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 34.Tan S.Y., Mei Wong J.L., Sim Y.J., Wong S.S., Mohamed Elhassan S.A., Tan S.H., Ling Lim G.P., Rong Tay N.W., Annan N.C., Bhattamisra S.K., et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Sims E.K., Carr A.L.J., Oram R.A., DiMeglio L.A., Evans-Molina C. 100 Years of Insulin: Celebrating the Past, Present and Future of Diabetes Therapy. Nat. Med. 2021;27:1154–1164. doi: 10.1038/s41591-021-01418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson L.M., Castle J.R. Recent Advances in Insulin Therapy. Diabetes Technol. Ther. 2020;22:929–936. doi: 10.1089/dia.2020.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv Z., Guo Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020;11:191. doi: 10.3389/fendo.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020;41:868–881. doi: 10.1016/j.tips.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hostalek U., Campbell I. Metformin for Diabetes Prevention: Update of the Evidence Base. Curr. Med. Res. Opin. 2021;37:1705–1717. doi: 10.1080/03007995.2021.1955667. [DOI] [PubMed] [Google Scholar]
- 40.Cho E.-H. Oldies but Goodies: Thiazolidinedione as an Insulin Sensitizer with Cardioprotection. Diabetes Metab. J. 2022;46:827–828. doi: 10.4093/dmj.2022.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebovitz H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019;19:151. doi: 10.1007/s11892-019-1270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sameeh M.Y., Khowdiary M.M., Nassar H.S., Abdelall M.M., Amer H.H., Hamed A., Elhenawy A.A. Thiazolidinedione Derivatives: In Silico, In Vitro, In Vivo, Antioxidant and Anti-Diabetic Evaluation. Molecules. 2022;27:830. doi: 10.3390/molecules27030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love K.M., Liu Z. DPP4 Activity, Hyperinsulinemia, and Atherosclerosis. J. Clin. Endocrinol. Metab. 2021;106:1553–1565. doi: 10.1210/clinem/dgab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S.-Y., Kong X.-Q., Zhang K.-F., Luo S., Wang F., Zhang J.-J. DPP4 as a Potential Candidate in Cardiovascular Disease. J. Inflamm. Res. 2022;15:5457–5469. doi: 10.2147/JIR.S380285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deacon C.F. Dipeptidyl Peptidase 4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020;16:642–653. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 46.Müller T.D., Finan B., Bloom S.R., D’Alessio D., Drucker D.J., Flatt P.R., Fritsche A., Gribble F., Grill H.J., Habener J.F., et al. Glucagon-like Peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drucker D.J. GLP-1 Physiology Informs the Pharmacotherapy of Obesity. Mol. Metab. 2022;57:101351. doi: 10.1016/j.molmet.2021.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith N.K., Hackett T.A., Galli A., Flynn C.R. GLP-1: Molecular Mechanisms and Outcomes of a Complex Signaling System. Neurochem. Int. 2019;128:94–105. doi: 10.1016/j.neuint.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyama T., Neuen B.L., Jun M., Ohkuma T., Neal B., Jardine M.J., Heerspink H.L., Wong M.G., Ninomiya T., Wada T., et al. Effect of SGLT2 Inhibitors on Cardiovascular, Renal and Safety Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2019;21:1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 50.Taylor S.I., Yazdi Z.S., Beitelshees A.L. Pharmacological Treatment of Hyperglycemia in Type 2 Diabetes. J. Clin. Investig. 2021;131:e142243. doi: 10.1172/JCI142243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvatore T., Galiero R., Caturano A., Rinaldi L., Di Martino A., Albanese G., Di Salvo J., Epifani R., Marfella R., Docimo G., et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022;23:3651. doi: 10.3390/ijms23073651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y.-J., Dong G.-J., Guo M. Targeting Epicardial Adipose Tissue: A Potential Therapeutic Strategy for Heart Failure with Preserved Ejection Fraction with Type 2 Diabetes Mellitus. World J. Diabetes. 2023;14:724–740. doi: 10.4239/wjd.v14.i6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsimihodimos V., Gonzalez-Villalpando C., Meigs J.B., Ferrannini E. Hypertension and Diabetes Mellitus: Coprediction and Time Trajectories. Hypertension. 2018;71:422–428. doi: 10.1161/HYPERTENSIONAHA.117.10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teck J. Diabetes-Associated Comorbidities. Prim Care. 2022;49:275–286. doi: 10.1016/j.pop.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Tsoi M.-F., Li H.-L., Feng Q., Cheung C.-L., Cheung T.T., Cheung B.M.Y. Prevalence of Childhood Obesity in the United States in 1999-2018: A 20-Year Analysis. Obes. Facts. 2022;15:560–569. doi: 10.1159/000524261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.American Diabetes Association 8 Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S100–S110. doi: 10.2337/dc21-S008. [DOI] [PubMed] [Google Scholar]
- 57.Choi C.H.J., Cohen P. How Does Obesity Lead to Insulin Resistance? Elife. 2017;6:e33298. doi: 10.7554/eLife.33298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams T., Mortada R., Porter S. Diagnosis and Treatment of Polycystic Ovary Syndrome. Am. Fam. Physician. 2016;94:106–113. [PubMed] [Google Scholar]
- 59.Rodgers R.J., Avery J.C., Moore V.M., Davies M.J., Azziz R., Stener-Victorin E., Moran L.J., Robertson S.A., Stepto N.K., Norman R.J., et al. Complex Diseases and Co-Morbidities: Polycystic Ovary Syndrome and Type 2 Diabetes Mellitus. Endocr. Connect. 2019;8:R71–R75. doi: 10.1530/EC-18-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Last A.R., Ference J.D., Menzel E.R. Hyperlipidemia: Drugs for Cardiovascular Risk Reduction in Adults. Am. Fam. Physician. 2017;95:78–87. [PubMed] [Google Scholar]
- 61.Screening for Lipid Disorders in Children and Adolescents: Recommendation Statement. [(accessed on 30 May 2023)];Am. Fam. Physician. 2016 94:Online. Available online: https://pubmed.ncbi.nlm.nih.gov/28075093/ [PubMed] [Google Scholar]
- 62.Fan D., Li L., Li Z., Zhang Y., Ma X., Wu L., Qin G. Effect of Hyperlipidemia on the Incidence of Cardio-Cerebrovascular Events in Patients with Type 2 Diabetes. Lipids Health Dis. 2018;17:102. doi: 10.1186/s12944-018-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.American Diabetes Association 4 Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S40–S52. doi: 10.2337/dc21-S004. [DOI] [PubMed] [Google Scholar]
- 64.Subramanian A., Adderley N.J., Tracy A., Taverner T., Hanif W., Toulis K.A., Thomas G.N., Tahrani A.A., Nirantharakumar K. Risk of Incident Obstructive Sleep Apnea Among Patients With Type 2 Diabetes. Diabetes Care. 2019;42:954–963. doi: 10.2337/dc18-2004. [DOI] [PubMed] [Google Scholar]
- 65.Huang T., Lin B.M., Stampfer M.J., Tworoger S.S., Hu F.B., Redline S. A Population-Based Study of the Bidirectional Association Between Obstructive Sleep Apnea and Type 2 Diabetes in Three Prospective U.S. Cohorts. Diabetes Care. 2018;41:2111–2119. doi: 10.2337/dc18-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stefan N., Häring H.-U., Cusi K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 67.Powell E.E., Wong V.W.-S., Rinella M. Non-Alcoholic Fatty Liver Disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 68.Hazlehurst J.M., Woods C., Marjot T., Cobbold J.F., Tomlinson J.W. Non-Alcoholic Fatty Liver Disease and Diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desouza C.V., Bolli G.B., Fonseca V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care. 2010;33:1389–1394. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miranville A., Herling A., Biemer-Daub G., Voss M. Differential Adipose Tissue Inflammatory State in Obese Nondiabetic Zucker Fatty Rats Compared to Obese Diabetic Zucker Diabetic Fatty Rats. Horm. Metab. Res. 2012;44:273–278. doi: 10.1055/s-0032-1304581. [DOI] [PubMed] [Google Scholar]
- 72.Daniels M.C., McClain D.A., Crook E.D. Transcriptional Regulation of Transforming Growth Factor Β1 by Glucose: Investigation into the Role of the Hexosamine Biosynthesis Pathway. Am. J. Med. Sci. 2020;359:79–83. doi: 10.1016/j.amjms.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Bugger H., Abel E.D. Molecular Mechanisms of Diabetic Cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ling C., Rönn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019;29:1028–1044. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasher D., Greenway S.C., Singh R.B. The Impact of Epigenetics on Cardiovascular Disease. Biochem. Cell Biol. 2020;98:12–22. doi: 10.1139/bcb-2019-0045. [DOI] [PubMed] [Google Scholar]
- 76.US Preventive Services Task Force. Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., García F.A.R., Gillman M.W., Kemper A.R., Krist A.H., et al. Screening for Lipid Disorders in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:625. doi: 10.1001/jama.2016.9852. [DOI] [PubMed] [Google Scholar]
- 77.Brown J., Yazdi F., Jodari-Karimi M., Owen J.G., Reisin E. Obstructive Sleep Apnea and Hypertension: Updates to a Critical Relationship. Curr. Hypertens. Rep. 2022;24:173–184. doi: 10.1007/s11906-022-01181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deprince A., Haas J.T., Staels B. Dysregulated Lipid Metabolism Links NAFLD to Cardiovascular Disease. Mol. Metab. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Der Ham K., Koster M.P.H., Velthuis B.K., Budde R.P.J., Fauser B.C.J.M., Laven J.S.E., Louwers Y.V. Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. J. Clin. Mol. 2023;12:5226. doi: 10.3390/jcm12165226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiordaliso F., Leri A., Cesselli D., Limana F., Safai B., Nadal-Ginard B., Anversa P., Kajstura J. Hyperglycemia Activates P53 and P53-Regulated Genes Leading to Myocyte Cell Death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 81.Jia G., Whaley-Connell A., Sowers J.R. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologia. 2018;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poznyak A.V., Bharadwaj D., Prasad G., Grechko A.V., Sazonova M.A., Orekhov A.N. Renin-Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD. Int. J. Mol. Sci. 2021;22:6702. doi: 10.3390/ijms22136702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J., Wang K., Liu W., Cai Y., Jin H. m6A mRNA Methylation Regulates the Development of Gestational Diabetes Mellitus in Han Chinese Women. Genomics. 2021;113:1048–1056. doi: 10.1016/j.ygeno.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 84.Qin Y., Li L., Luo E., Hou J., Yan G., Wang D., Qiao Y., Tang C. Role of m6A RNA Methylation in Cardiovascular Disease (Review) Int. J. Mol. Med. 2020;46:1958–1972. doi: 10.3892/ijmm.2020.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayer-Davis E.J., Lawrence J.M., Dabelea D., Divers J., Isom S., Dolan L., Imperatore G., Linder B., Marcovina S., Pettitt D.J., et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017;376:1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Cho N.H., Shaw J.E., Karuranga S., Huang Y., Da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 88.Cole J.B., Florez J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yaribeygi H., Sathyapalan T., Atkin S.L., Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020;2020:1–13. doi: 10.1155/2020/8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bell D.S.H. Heart Failure. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 91.From A.M., Leibson C.L., Bursi F., Redfield M.M., Weston S.A., Jacobsen S.J., Rodeheffer R.J., Roger V.L. Diabetes in Heart Failure: Prevalence and Impact on Outcome in the Population. Am. J. Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 92.Bradley T.J., Slorach C., Mahmud F.H., Dunger D.B., Deanfield J., Deda L., Elia Y., Har R.L.H., Hui W., Moineddin R., et al. Early Changes in Cardiovascular Structure and Function in Adolescents with Type 1 Diabetes. Cardiovasc. Diabetol. 2016;15:31. doi: 10.1186/s12933-016-0351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forbes J.M., Cooper M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 94.Boudina S., Abel E.D. Diabetic Cardiomyopathy Revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 95.Cook S.A., Varela-Carver A., Mongillo M., Kleinert C., Khan M.T., Leccisotti L., Strickland N., Matsui T., Das S., Rosenzweig A., et al. Abnormal Myocardial Insulin Signalling in Type 2 Diabetes and Left-Ventricular Dysfunction. Eur. Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segar M.W., Khan M.S., Patel K.V., Butler J., Tang W.H.W., Vaduganathan M., Lam C.S.P., Verma S., McGuire D.K., Pandey A. Prevalence and Prognostic Implications of Diabetes With Cardiomyopathy in Community-Dwelling Adults. J. Am. Coll. Cardiol. 2021;78:1587–1598. doi: 10.1016/j.jacc.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 97.Jadhav A., Tiwari S., Lee P., Ndisang J.F. The Heme Oxygenase System Selectively Enhances the Anti-Inflammatory Macrophage-M2 Phenotype, Reduces Pericardial Adiposity, and Ameliorated Cardiac Injury in Diabetic Cardiomyopathy in Zucker Diabetic Fatty Rats. J. Pharmacol. Exp. Ther. 2013;345:239–249. doi: 10.1124/jpet.112.200808. [DOI] [PubMed] [Google Scholar]
- 98.Monji A., Mitsui T., Bando Y.K., Aoyama M., Shigeta T., Murohara T. Glucagon-like Peptide-1 Receptor Activation Reverses Cardiac Remodeling via Normalizing Cardiac Steatosis and Oxidative Stress in Type 2 Diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2013;305:H295–H304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 99.Westermann D., Rutschow S., Jäger S., Linderer A., Anker S., Riad A., Unger T., Schultheiss H.-P., Pauschinger M., Tschöpe C. Contributions of Inflammation and Cardiac Matrix Metalloproteinase Activity to Cardiac Failure in Diabetic Cardiomyopathy. Diabetes. 2007;56:641–646. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- 100.Alzahrani S., Ajjan R. Review Article: Coagulation and Fibrinolysis in Diabetes. Diabetes Vasc. Dis. Res. 2010;7:260–273. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]
- 101.Mittal A., Garg R., Bahl A., Khullar M. Molecular Mechanisms and Epigenetic Regulation in Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2021;8:725532. doi: 10.3389/fcvm.2021.725532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsuan C.-F., Teng S.I.F., Hsu C.-N., Liao D., Chang A.J.-W., Lee H.-L., Hee S.-W., Chang Y.-C., Chuang L.-M. Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice. Biomedicines. 2023;11:662. doi: 10.3390/biomedicines11030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murdoch C., Grieve D., Cave A., Looi Y., Shah A. NADPH Oxidase and Heart Failure. Curr. Opin. Pharmacol. 2006;6:148–153. doi: 10.1016/j.coph.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Kleibert M., Zygmunciak P., Łakomska K., Mila K., Zgliczyński W., Mrozikiewicz-Rakowska B. Insight into the Molecular Mechanism of Diabetic Kidney Disease and the Role of Metformin in Its Pathogenesis. Int. J. Mol. Sci. 2023;24:13038. doi: 10.3390/ijms241713038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Son N.-H., Yu S., Tuinei J., Arai K., Hamai H., Homma S., Shulman G.I., Abel E.D., Goldberg I.J. PPARγ-Induced Cardiolipotoxicity in Mice Is Ameliorated by PPARα Deficiency despite Increases in Fatty Acid Oxidation. J. Clin. Investig. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsushima K., Bugger H., Wende A.R., Soto J., Jenson G.A., Tor A.R., McGlauflin R., Kenny H.C., Zhang Y., Souvenir R., et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission. Circ. Res. 2018;122:58–73. doi: 10.1161/CIRCRESAHA.117.311307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Römer A., Linn T., Petry S.F. Lipotoxic Impairment of Mitochondrial Function in β-Cells: A Review. Antioxidants. 2021;10:293. doi: 10.3390/antiox10020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glick D., Barth S., Macleod K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munasinghe P.E., Riu F., Dixit P., Edamatsu M., Saxena P., Hamer N.S.J., Galvin I.F., Bunton R.W., Lequeux S., Jones G., et al. Type-2 Diabetes Increases Autophagy in the Human Heart through Promotion of Beclin-1 Mediated Pathway. Int. J. Cardiol. 2016;202:13–20. doi: 10.1016/j.ijcard.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 110.Dewanjee S., Vallamkondu J., Kalra R.S., John A., Reddy P.H., Kandimalla R. Autophagy in the Diabetic Heart: A Potential Pharmacotherapeutic Target in Diabetic Cardiomyopathy. Ageing Res. Rev. 2021;68:101338. doi: 10.1016/j.arr.2021.101338. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh N., Katare R. Molecular Mechanism of Diabetic Cardiomyopathy and Modulation of microRNA Function by Synthetic Oligonucleotides. Cardiovasc. Diabetol. 2018;17:43. doi: 10.1186/s12933-018-0684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li C., Wang D., Jiang Z., Gao Y., Sun L., Li R., Chen M., Lin C., Liu D. Non-Coding RNAs in Diabetes Mellitus and Diabetic Cardiovascular Disease. Front. Endocrinol. 2022;13:961802. doi: 10.3389/fendo.2022.961802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rawal S., Manning P., Katare R. Cardiovascular microRNAs: As Modulators and Diagnostic Biomarkers of Diabetic Heart Disease. Cardiovasc. Diabetol. 2014;13:44. doi: 10.1186/1475-2840-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen E., Diao X., Wang X., Chen R., Hu B. MicroRNAs Involved in the Mitogen-Activated Protein Kinase Cascades Pathway During Glucose-Induced Cardiomyocyte Hypertrophy. Am. J. Pathol. 2011;179:639–650. doi: 10.1016/j.ajpath.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liao W., Xu N., Zhang H., Liao W., Wang Y., Wang S., Zhang S., Jiang Y., Xie W., Zhang Y. Persistent High Glucose Induced EPB41L4A-AS1 Inhibits Glucose Uptake via GCN5 Mediating Crotonylation and Acetylation of Histones and Non-histones. Clin. Transl. Med. 2022;12:e699. doi: 10.1002/ctm2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wright E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360. 2021;2:2027–2037. doi: 10.34067/KID.0002772021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 Inhibitors and GLP-1 Receptor Agonists: Established and Emerging Indications. Lancet. 2021;398:262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 118.van der Aart-van der Beek A.B., de Boer R.A., Heerspink H.J.L. Kidney and Heart Failure Outcomes Associated with SGLT2 Inhibitor Use. Nat. Rev. Nephrol. 2022;18:294–306. doi: 10.1038/s41581-022-00535-6. [DOI] [PubMed] [Google Scholar]
- 119.Nauck M.A., Quast D.R., Wefers J., Pfeiffer A.F.H. The Evolving Story of Incretins (GIP and GLP-1) in Metabolic and Cardiovascular Disease: A Pathophysiological Update. Diabetes Obes. Metab. 2021;23((Suppl. 3)):5–29. doi: 10.1111/dom.14496. [DOI] [PubMed] [Google Scholar]
- 120.Zhao X., Wang M., Wen Z., Lu Z., Cui L., Fu C., Xue H., Liu Y., Zhang Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021;12:721135. doi: 10.3389/fendo.2021.721135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pedrosa M.R., Franco D.R., Gieremek H.W., Vidal C.M., Bronzeri F., de Cassia Rocha A., de Carvalho Cara L.G., Fogo S.L., Eliaschewitz F.G. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr. Atheroscler. Rep. 2022;24:867–884. doi: 10.1007/s11883-022-01062-2. [DOI] [PubMed] [Google Scholar]
- 122.Hernandez A.F., Green J.B., Janmohamed S., D’Agostino R.B., Granger C.B., Jones N.P., Leiter L.A., Rosenberg A.E., Sigmon K.N., Somerville M.C., et al. Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 123.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jódar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 124.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F.E., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang S., Cai Y., Meng C., Ding X., Huang J., Luo X., Cao Y., Gao F., Zou M. The Role of the Microbiome in Diabetes Mellitus. Diabetes Res. Clin. Pract. 2021;172:108645. doi: 10.1016/j.diabres.2020.108645. [DOI] [PubMed] [Google Scholar]
- 126.DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Joscelyn J., Kasper L.H. Digesting the Emerging Role for the Gut Microbiome in Central Nervous System Demyelination. Mult. Scler. 2014;20:1553–1559. doi: 10.1177/1352458514541579. [DOI] [PubMed] [Google Scholar]
- 128.Zabell A., Tang W.H.W. Targeting the Microbiome in Heart Failure. Curr. Treat. Options Cardiovasc. Med. 2017;19:27. doi: 10.1007/s11936-017-0528-4. [DOI] [PubMed] [Google Scholar]
- 129.Młynarska E., Gadzinowska J., Tokarek J., Forycka J., Szuman A., Franczyk B., Rysz J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients. 2022;14:1921. doi: 10.3390/nu14091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Goffau M.C., Fuentes S., van den Bogert B., Honkanen H., de Vos W.M., Welling G.W., Hyöty H., Harmsen H.J.M. Aberrant Gut Microbiota Composition at the Onset of Type 1 Diabetes in Young Children. Diabetologia. 2014;57:1569–1577. doi: 10.1007/s00125-014-3274-0. [DOI] [PubMed] [Google Scholar]
- 131.Wen L., Ley R.E., Volchkov P.Y., Stranges P.B., Avanesyan L., Stonebraker A.C., Hu C., Wong F.S., Szot G.L., Bluestone J.A., et al. Innate Immunity and Intestinal Microbiota in the Development of Type 1 Diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Menser M.A., Forrest J.M., Bransby R.D. Rubella infection and diabetes mellitus. Lancet. 1978;311:57–60. doi: 10.1016/S0140-6736(78)90001-6. [DOI] [PubMed] [Google Scholar]
- 133.Zimmet P., Alberti K.G., Magliano D.J., Bennett P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 134.Karlsson F.H., Tremaroli V., Nookaew I., Bergström G., Behre C.J., Fagerberg B., Nielsen J., Bäckhed F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 135.Beyan H., Wen L., Leslie R.D. Guts, Germs, and Meals: The Origin of Type 1 Diabetes. Curr. Diabetes Rep. 2012;12:456–462. doi: 10.1007/s11892-012-0298-z. [DOI] [PubMed] [Google Scholar]
- 136.Hulten E.A., Bittencourt M.S., Preston R., Singh A., Romagnolli C., Ghoshhajra B., Shah R., Abbasi S., Abbara S., Nasir K., et al. Obesity, Metabolic Syndrome and Cardiovascular Prognosis: From the Partners Coronary Computed Tomography Angiography Registry. Cardiovasc. Diabetol. 2017;16:14. doi: 10.1186/s12933-017-0496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lau K., Srivatsav V., Rizwan A., Nashed A., Liu R., Shen R., Akhtar M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients. 2017;9:859. doi: 10.3390/nu9080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smolinska A., Tedjo D.I., Blanchet L., Bodelier A., Pierik M.J., Masclee A.A.M., Dallinga J., Savelkoul P.H.M., Jonkers D.M.A.E., Penders J., et al. Volatile Metabolites in Breath Strongly Correlate with Gut Microbiome in CD Patients. Anal. Chim. Acta. 2018;1025:1–11. doi: 10.1016/j.aca.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 139.Ahmad A.F., Dwivedi G., O’Gara F., Caparros-Martin J., Ward N.C. The Gut Microbiome and Cardiovascular Disease: Current Knowledge and Clinical Potential. Am. J. Physiol.-Heart Circ. Physiol. 2019;317:H923–H938. doi: 10.1152/ajpheart.00376.2019. [DOI] [PubMed] [Google Scholar]
- 140.Emoto T., Yamashita T., Sasaki N., Hirota Y., Hayashi T., So A., Kasahara K., Yodoi K., Matsumoto T., Mizoguchi T., et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: A Possible Link between Gut Microbiota and Coronary Artery Disease. J. Atheroscler. Thromb. 2016;23:908–921. doi: 10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu Q., Gao R., Zhang Y., Pan D., Zhu Y., Zhang X., Yang R., Jiang R., Xu Y., Qin H. Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genomics. 2018;50:893–903. doi: 10.1152/physiolgenomics.00070.2018. [DOI] [PubMed] [Google Scholar]
- 142.Ott S.J., El Mokhtari N.E., Musfeldt M., Hellmig S., Freitag S., Rehman A., Kühbacher T., Nikolaus S., Namsolleck P., Blaut M., et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients With Coronary Heart Disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 143.Gan X.T., Ettinger G., Huang C.X., Burton J.P., Haist J.V., Rajapurohitam V., Sidaway J.E., Martin G., Gloor G.B., Swann J.R., et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat. Circ. Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 144.Parvez S., Malik K.A., Ah Kang S., Kim H.-Y. Probiotics and Their Fermented Food Products Are Beneficial for Health. J. Appl. Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 145.Tokarek J., Budny E., Saar M., Kućmierz J., Młynarska E., Rysz J., Franczyk B. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. Int. J. Mol. Sci. 2023;24:1377. doi: 10.3390/ijms24021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thushara R.M., Gangadaran S., Solati Z., Moghadasian M.H. Cardiovascular Benefits of Probiotics: A Review of Experimental and Clinical Studies. Food Funct. 2016;7:632–642. doi: 10.1039/C5FO01190F. [DOI] [PubMed] [Google Scholar]
- 147.Gardner D., Shoback D. In: Greenspan’s Basic & Clinical Endocrinology. 9th ed. Gardner D.G., Greenspan F.S., editors. McGraw Hill Medical; New York, NY, USA: 2011. A Lange Medical Book. [Google Scholar]
- 148.Ma X., Liu Z., Ilyas I., Little P.J., Kamato D., Sahebka A., Chen Z., Luo S., Zheng X., Weng J., et al. GLP-1 Receptor Agonists (GLP-1RAs): Cardiovascular Actions and Therapeutic Potential. Int. J. Biol. Sci. 2021;17:2050–2068. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li C., Liang S., Gao L., Liu H. Cardiovascular Outcomes Associated with SGLT-2 Inhibitors versus Other Glucose-Lowering Drugs in Patients with Type 2 Diabetes: A Real-World Systematic Review and Meta-Analysis. PLoS ONE. 2021;16:e0244689. doi: 10.1371/journal.pone.0244689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sasso F.C., Simeon V., Galiero R., Caturano A., De Nicola L., Chiodini P., Rinaldi L., Salvatore T., Lettieri M., Nevola R., et al. The Number of Risk Factors Not at Target Is Associated with Cardiovascular Risk in a Type 2 Diabetic Population with Albuminuria in Primary Cardiovascular Prevention. Post-Hoc Analysis of the NID-2 Trial. Cardiovasc. Diabetol. 2022;21:235. doi: 10.1186/s12933-022-01674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this article were sourced from materials mentioned in the References Section.