Abstract
The occurrence of hemolytic anemia in patients with active SARS-CoV-2 infection has been documented in medical literature. While relatively uncommon, there have been instances where this condition presents as a Coombs-negative hemolytic anemia. In this research study, we report a distinctive case of Coombs-negative hemolytic anemia and thrombocytopenia in a patient with a known history of COVID-19 infection. The patient demonstrated a favorable response to treatment involving the administration of steroids and intravenous immunoglobulin (IVIG) therapy. This case adds to the existing body of evidence regarding the hematological manifestations of SARS-CoV-2 infection, highlighting the importance of considering and managing hematological complications in patients with COVID-19.
Keywords: COVID-19, hemolytic anemia, thrombocytopenia
Background
Coronavirus disease 19 (COVID-19), caused by the SARS-CoV-2 virus, was first identified in December 2019 in Wuhan, China. 1 While its primary impact is on the respiratory system, COVID-19 has also been associated with various hematologic abnormalities. Coombs-negative hemolytic anemia cases linked to COVID-19 infection have been reported in studies conducted by Neerukonda et al 2 and Bae et al. 3 Lancman et al 4 conducted a study revealing elevated markers of hemolysis, particularly plasma hemoglobin (Hb) levels, in COVID-19 patients. Furthermore, platelet abnormalities have been observed in individuals infected with COVID-19. For instance, thrombocytopenia has been identified in 5% to 41.7% of COVID-19 patients. 5 Although severe thrombocytopenia is rare, a case associating COVID-19 with immune thrombocytopenic purpura (ITP) has been reported. 6
We present a case involving a patient who developed Coombs-negative hemolytic anemia and thrombocytopenia following COVID-19 infection. Extensive investigations were conducted to identify other potential causes for the patient’s hematologic abnormalities, but no other underlying source was revealed. Notably, the patient did not exhibit the characteristic end-organ damage at presentation. Treatment for the patient involved the administration of steroids and intravenous immunoglobulin (IVIG), which resulted in improvement of both the anemia and thrombocytopenia.
Case Presentation
Patient Information
The patient is a 35-year-old male with a medical history significant for asthma, depression, and chronic iron deficiency anemia. He presented to our hospital for evaluation of anemia and thrombocytopenia. The patient reported progressive worsening of symptoms following a recent COVID-19 infection. His symptoms included fatigue, light-headedness, generalized weakness, night sweats, exertional dyspnea, easy bruising, gum bleeding upon brushing teeth, and epistaxis. He had previously received 2 units of packed red blood cells at an outside facility.
Clinical and Lab Findings
On admission, the patient tested negative for COVID-19 and respiratory pathogens. Vital signs were stable with a blood pressure of 186/94 mmHg, heart rate of 82 beats per minute, respiratory rate of 18 breaths per minute, and oxygen saturation of 99% on room air. Initial laboratory tests revealed anemia and thrombocytopenia (see Table 1). Peripheral smear showed poikilocytosis, microcytosis, hypochromia, elliptocytes, stomatocytes, and nucleated red blood cells. A repeat smear showed 1+ schistocytes (see Figure 1). Further workup for the hemolytic anemia was performed (see Table 1). Lactate dehydrogenase and D-dimer levels were elevated. Liver function tests were unremarkable. Prothrombin time, partial thromboplastin time, and international normalized ratio were within normal limits. Haptoglobin was abnormally low. The initial anemia profile revealed an elevated iron saturation, ferritin, and iron level. His folate, vitamin B12, thyroid-stimulating hormone (TSH), and qualitative glucose-6-phosphate dehydrogenase (G6PD) testing yielded normal results.
Table 1.
Initial Laboratory Tests and Anemia Workup Upon Hospital Admission.
| Laboratory parameters | Results | Reference range |
|---|---|---|
| White blood cells (WBC) | 5.8 × 103/µL | 4-10 × 103/µL |
| Absolute neutrophil count | 2.53 × 103/µL | 1.8-7.0 × 103/µL |
| Lymphocyte count | 2.47 × 103/µL | 1.2-4.0 × 103/µL |
| Absolute myelocyte count | 0.06 × 103/µL | 0 |
| Hemoglobin (Hb) | 7.3 g/dL | 13.5-18 g/dL |
| Hematocrit | 26% | 41%-53% |
| Platelet count | 26 × 103/µL | 150-400 × 103/µL |
| Red cell distribution width | 21.9% | 11.5%-14.5% |
| Mean cell volume | 85.7% | 80-96 fL |
| Laboratory parameters | Results | Reference Range |
| Lactate dehydrogenase level | 1124 U/L | 122-225 U/L |
| Haptoglobin | <15 mg/dL | 30-200 mg/dL |
| D-dimer | 1.64 µg/mL | <0.50 µg/mL |
| % Iron saturation | 65.0% | 20%-55% |
| Immature reticulocyte fraction | 0.66% | 0.26%-0.52% |
| Ferritin | 814 ng/mL | 30-400 ng/mL |
| Iron | 225 µg/dL | 59-158 µg/dL |
| Total iron binding capacity | 344 µg/dL | 278-500 µg/dL |
| Transferrin | 248 mg/dL | 200-360 mg/dL |
| Folate | 12.40 ng/mL | >4.77 ng/mL |
| Vitamin B12 | 596 pg/mL | 211-946 pg/mL |
| Blood urea nitrogen (BUN) | 14 mg/dL | 2-20 mg/dL |
| Creatinine | 0.73 mg/dL | 0.70-1.20 mg/dL |
| Alanine transaminase (ALT) | 33 U/L | <41 U/L |
| Aspartate aminotransferase (AST) | 33 U/L | <40 U/L |
| Alkaline phosphatase | 81 U/L | 40-129 U/L |
| Total bilirubin | 0.5 mg/dL | <1.2 mg/dL |
| Direct bilirubin | <0.2 mg/dL | <0.3 mg/dL |
| ADAMTS13 activity assay | >100% | ≥70% |
Figure 1.
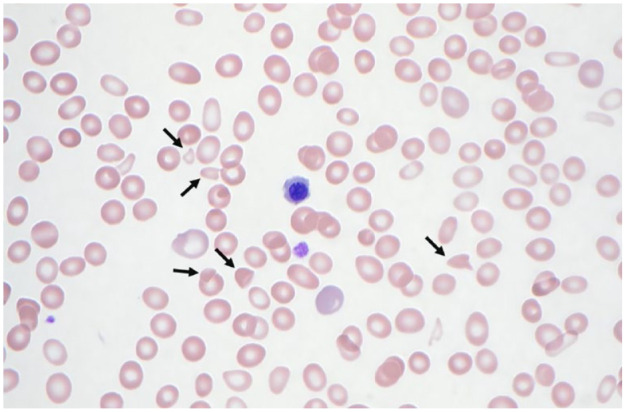
Peripheral smear (at 1000× magnification) exhibiting anisopoikilocytosis, hypochromia, polychromasia, anemia, and thrombocytopenia. Schistocytes are noted by arrows.
Diagnostic Evaluation
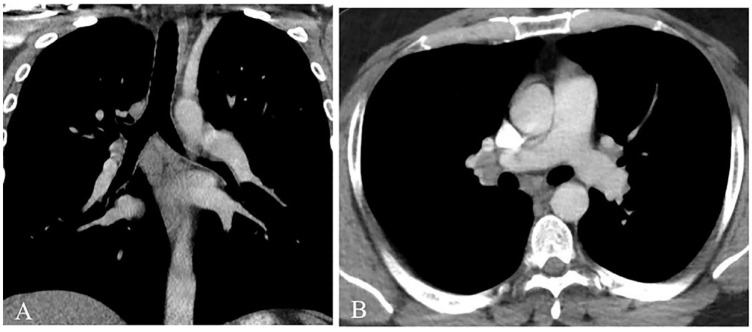
Imaging studies were performed to evaluate adenopathy and hepatosplenomegaly due to concern for primary hematologic pathology. A computerized tomography (CT) scan of the thorax revealed mediastinal and right hilar lymphadenopathy, with no evidence of focal airspace disease or acute cardiopulmonary pathology (see Figure 2). CT abdomen showed nonspecific top-normal sized mesenteric and retroperitoneal lymph nodes. Given the patient’s thrombocytopenia and the small size of the lymph nodes, the risk of bleeding outweighed the benefit of performing a lymph node biopsy, and it was deferred.
Figure 2.
Computed tomography of the thorax with contrast (soft tissue window settings) in coronal (A) and axial (B) planes. No nodules or areas of opacification were visualized within the lung parenchyma. Nonspecific subcarinal and right hilar lymphadenopathy was visualized in addition to prominent however nonenlarged left hilar lymph nodes.
Subsequently, a bone marrow biopsy was performed, which showed hypocellular marrow (5%-30%) with mildly decreased myeloid to erythroid ratio and mildly decreased megakaryocytes. Chromosome analysis was unremarkable. Flow cytometry of peripheral blood revealed normocytic anemia, frequent schistocytes, leukoerythroblastosis (1% circulating blasts), and frequent nucleated red blood cells with some dysplastic features. No evidence of acute leukemia was noted.
The differential diagnosis for the patient’s condition was extensive and included primary malignancy, G6PD deficiency, and autoimmune diseases such as systemic lupus erythematosus or antiphospholipid syndrome, thrombocytic thrombocytopenic purpura (TTP), disseminated intravascular coagulation (DIC), paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), or an infectious origin such as Lyme disease, cytomegalovirus (CMV), or Epstein-Barr virus (EBV) for the observed hemolytic anemia (MAHA).
An infectious workup was performed, including testing for Epstein-Barr virus (EBV), hepatitis C, Lyme disease, and HIV. The patient tested negative for Epstein-Barr virus VCA IgM antibodies, while the Epstein-Barr VCA IgG p18 antibody was positive, suggesting a past infection rather than a recent one. The workup for other infectious etiologies was unremarkable.
Autoimmune investigations, including antinuclear antibody (ANA) testing, complement levels (C3, C4, and total complement), immunoglobulin levels (IgA, IgM, kappa free light chain, and lambda free light chain), and testing for specific antibodies such as cardiolipin IgM and IgA, hex phase phospho neut, and beta 2 glycoprotein IgM and IgG, did not reveal any abnormalities. Fibrinogen levels were normal, and direct Coombs testing was negative. Flow cytometry for paroxysmal nocturnal hemoglobinuria (PNH) came back negative as well.
Treatment and Outcome
Considering suspicion for possible TTP, treatment was initiated with steroids and plasmapheresis. However, subsequent ADAMTS13 activity results were normal (see Table 1), leading to discontinuation of plasmapheresis.
Given the exclusion of other possible etiologies, it was hypothesized that the hemolysis was likely due to a COVID-19 infection the patient had experienced 1 month prior to admission. Based on a case report describing a positive response to IVIG therapy in a COVID-19-positive patient with Coombs-negative hemolytic anemia, the decision was made to administer IVIG therapy to our patient, considering his persistent anemia and thrombocytopenia. The patient underwent a course of IVIG therapy, receiving 1 g/kg daily for a duration of 3 days. This treatment resulted in an improvement in thrombocytopenia and stabilization of the patient’s anemia. After improvement in platelet counts, a consultation with the Pulmonology department was sought. The patient had shown improvement with steroids and IVIG, and it was determined that endobronchial ultrasound (EBUS) was unlikely to provide substantial additional information. The lymphadenopathy observed could be reactive and attributed to a recent infection. Following the above treatment intervention, the patient was discharged home on steroid with a plan to taper dose as outpatient.
During the posthospital discharge follow-up, laboratory tests demonstrated a sustained improvement in the patient’s hemoglobin levels, hematocrit, and platelet count (see Table 2). At the follow-up appointment, the patient did not report any episodes of bleeding, and it was decided to continue the patient on a gradual tapering regimen of steroid.
Table 2.
Follow-up Laboratory Tests on Outpatient Hematology Clinic Appointment After Completing Steroid Taper.
| Laboratory parameters | Results | Reference range |
|---|---|---|
| White blood cells (WBC) | 6.0 K/µL | 4.8-10.8 K/µL |
| Neutrophil percent | 64% | 37-80% |
| Lymphocyte percent | 20% | 10-50% |
| Hemoglobin (Hb) | 10.1 g/dL | 13.5-18.0 g/dL |
| Hematocrit | 32.2% | 41.0%-53.0% |
| Platelet count | 229 K/µL | 130-450 K/µL |
| Red cell distribution width | 21.4% | 11.0-15.0% |
| Mean Cell Volume | 99.7 fL | 80.0-100.0 fL |
| Lactate dehydrogenase | 291 U/L | 95-250 U/L |
| Haptoglobin | 108 mg/dL | 17-317 mg/dL |
Discussion
COVID-19 infection has been reported to cause hematologic abnormalities, including cases of Coombs-negative hemolytic anemia.2,3 Our patient presented with a unique combination of Coombs-negative hemolytic anemia and thrombocytopenia. Initially, we considered a broad range of differential diagnoses, including autoimmune, neoplastic, primary hematologic, and infectious causes. Thrombotic microangiopathies (TMAs) were also considered, as they manifest with microangiopathic hemolytic anemia, thrombocytopenia, and organ injury, commonly affecting the kidneys and other systems. 7 However, investigations for TMAs ruled out TTP and given normal renal function and complement level, we did not perform extensive workup for HUS.
In our case, considering the patient’s recent COVID-19 infection and the absence of other infectious etiologies identified during the hospital workup, it is likely that the Coombs-negative hemolytic anemia and thrombocytopenia were sequelae of the prior infection. The exact mechanism behind this phenomenon remains unclear. It has been suggested that SARS-CoV-2 might interact with erythrocytes, leading to invasion and potential hemolysis.3,4 This interaction could involve the CD147 spike protein, which facilitates binding to receptor cells and invasion. 8 However, further research is required to elucidate this mechanism and characterize it more comprehensively. It is crucial to consider hematologic manifestations in patients presenting with or having a history of COVID-19 infection.
While our patient demonstrated anemia and thrombocytopenia, there have been cases of pancytopenia and bone marrow suppression reported with COVID-19 infection. The presence of pancytopenia is not unique to COVID-19 and can be seen in other viral infections including HIV, parvovirus, CMV, and EBV. Sharma et al 9 describes a case of pancytopenia in a critically ill patient with COVID-19 with bone marrow results showing hypocellular marrow.
The mechanism behind bone marrow suppression in COVID-19 infection is unclear and further investigation is required. Some mechanisms for or bone marrow suppression have also been suggested, including inflammatory response during infection or immune system dysfunction. 10 A study done by Wang et al examined the effects of COVID-19 on bone marrow. In patients with severe COVID-19 infection (defined as requiring hospitalization with low or high flow oxygen), they found depleted lymphoid progenitors, and there were signs of increased apoptotic pathways in COVID-19 patients. While this study is limited by sample size and the patient population differs from our patient (who had prior COVID-19 infection), it does suggest that COVID-19 may lead to effects on hematopoiesis in bone marrow. 11
Moreover, COVID-19 has been associated with coagulopathy and thrombocytopenia. 5 There may be a correlation between the severity of the disease and the degree of thrombocytopenia, with more severe cases showing lower platelet counts. 12 Although the precise mechanisms of COVID-19-related thrombocytopenia are not fully understood, various hypotheses have been proposed. One theory suggests that infection via CD13 or CD66a on platelet progenitor cells may lead to reduced platelet production. 13 In some cases, sepsis-induced DIC might contribute to thrombocytopenia. 13 However, our patient did not exhibit the clinical features of DIC.
Limited case reports have described Coombs-negative hemolytic anemia associated with COVID-19 infection, and thus far, there have been no reports demonstrating concurrent thrombocytopenia. Given our patient’s persistent thrombocytopenia and declining hemoglobin levels necessitating transfusion support, we reviewed relevant cases to identify potential treatment options. Bae et al 3 reported a case of Coombs-negative hemolytic anemia that responded well to intravenous immunoglobulin (IVIG) therapy. Consequently, we administered IVIG to our patient as well. During follow-up, his anemia and thrombocytopenia stabilized, and he was managed with a gradual tapering regimen of steroids.
Conclusions
COVID-19 infection is recognized for its impact on the hematologic system, particularly in terms of coagulopathy and DIC. However, there are additional hematologic abnormalities that can arise in the context of COVID-19. Although the precise mechanisms remain elusive, Coombs-negative hemolytic anemia and thrombocytopenia have been observed in association with COVID-19. Treatment strategies for these hematologic manifestations typically involve the use of intravenous immunoglobulin (IVIG) and steroids, as demonstrated in our case. Further research is needed to elucidate the underlying mechanisms and optimize management approaches for these hematologic complications associated with COVID-19.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal and written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Alanna Glidden Siegenthaler  https://orcid.org/0000-0003-0376-2345
https://orcid.org/0000-0003-0376-2345
References
- 1. US Department of Health & Human Services, Centers for Disease Control and Prevention. About COVID-19. Published 2023. Accessed August 18, 2023. https://www.cdc.gov/coronavirus/2019-ncov/your-health/about-covid-19.html
- 2. Neerukonda T, Moughabel W, Chen J, et al. A case of Coombs-negative hemolytic anemia prompting diagnosis of SARS-COV-2. Cureus. 2021;13(11):e20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae JY, Jeon JE, Hussein KI, Lee MS. Coombs-negative hemolytic anemia in a male with COVID-19. Clin Case Rep. 2021;9(7):e04503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lancman G, Marcellino BK, Thibaud S, et al. Coombs-negative hemolytic anemia and elevated plasma hemoglobin levels in COVID-19. Ann Hematol. 2021;100(3):833-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zulfiqar AA, Lorenzo-Villalba N, Hassler P, et al. Immune thrombocytopenic purpura in a patient with covid-19. N Engl J Med. 2020;382:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brocklebank V, Wood KM, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018;13: 300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang K, Chen W, Zhang Z, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Sig Transduct Target Ther. 2020;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma N, Shukla R, Warrier R, et al. Pancytopenia secondary to SARS-CoV-2 infection—a case report. SN Compr Clin Med. 2022;4(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koromoki E, Gavriatopoulou M, Fotiou D, et al. Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist. Am J Hematol. 2022; 97(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Wen Y, Xie X, et al. Dysregulated hematopoiesis in bone marrow marks severe COVID-19. Cell Discov. 2021; 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang SQ, Huang QF, Xie WM, et al. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. 2020;190(1):e29-e33. [DOI] [PubMed] [Google Scholar]
- 13. Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. J Thromb Haemost. 2020;18(6):1514-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]