Abstract
Antimicrobial resistance is considered a “One-Health” problem, impacting humans, animals, and the environment. The problem of the rapid development and spread of bacteria resistant to multiple antibiotics is a rising global health threat affecting both rich and poor nations. Low- and middle-income countries are at highest risk, in part due to the lack of innovative research on the surveillance and discovery of novel therapeutic options. Fast and effective drug discovery is crucial towards combatting antimicrobial resistance and reducing the burden of infectious diseases. African medicinal plants have been used for millennia in folk medicine to cure many diseases and ailments. Over 10% of the Southern African vegetation is applied in traditional medicine, with over 15 species being partially or fully commercialized. These include the genera Euclea, Ficus, Aloe, Lippia. And Artemisia, amongst many others. Bioactive compounds from indigenous medicinal plants, alone or in combination with existing antimicrobials, offer promising solutions towards overcoming multi-drug resistance. Secondary metabolites have different mechanisms and modes of action against bacteria, such as the inhibition and disruption of cell wall synthesis; inhibition of DNA replication and ATP synthesis; inhibition of quorum sensing; inhibition of AHL or oligopeptide signal generation, broadcasting, and reception; inhibition of the formation of biofilm; disruption of pathogenicity activities; and generation of reactive oxygen species. The aim of this review is to highlight some promising traditional medicinal plants found in Africa and provide insights into their secondary metabolites as alternative options in antibiotic therapy against multi-drug-resistant bacteria. Additionally, synergism between plant secondary metabolites and antibiotics has been discussed.
Keywords: antimicrobial resistance, antibiotics, bioactive compounds, secondary metabolites, indigenous plants, Africa
1. Introduction
Antimicrobial resistance (AMR) is a well-recognized global health problem, affecting both rich and poor nations [1]. AMR is the ability of microorganisms to survive the effects of antimicrobials such as antifungals and antibiotics. Many pathogenic bacteria have developed resistance to almost all available antibiotics, and even newly developed antibiotics will ultimately become ineffective against the continuously evolving multi-drug-resistant (MDR) bacteria [1,2,3]. Some examples of clinically important MDR bacteria are Vancomycin-resistant Enterococci (VRE), Carbapenem-resistant Acinetobacter baumannii (CRAB), Carbapenem-resistant Enterobacteriales (CRE), XDR (extensively drug-resistant) Pseudomonas aeruginosa, extended-spectrum β-lactamase- (ESBL)-producing Enterobacteriales, and methicillin-resistant Staphylococcus aureus (MRSA) [4,5,6]. The E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter species, and E. coli (ESKAPEE) acronym is commonly used to refer to highly virulent bacterial pathogens known to “escape” treatment from multiple antibiotics and other traditional treatments [7,8]. The rapid development and spread of MDR bacteria is a rising One-Health challenge, impacting humans, animals, and the environment, which means that MDR bacteria and the associated AMR genes are capable of circulating among different habitats, making them difficult to control [4,9]. It has been estimated that 10 million deaths will be a result of AMR by the year 2050, and the World Health Organization (WHO) predicts over 24 million people globally will be condemned to poverty as result of the AMR burden [2]. The European Centre for Disease Prevention and Control (ECDC) estimates that today AMR is already responsible for ca. 25,000 deaths and €1.5 billion in health expenditures per year in Europe alone, and given the limitations of the availability of data, the death toll is estimated to be significantly higher in Africa, where the expenditure is much higher than in the first-world countries [1]. In fact, the Africa Centres for Disease Prevention and Control (CDC) has urged the public, academic institutions, farmers, veterinarians, and medical and professional organizations to become ‘antibiotic guardians’ by cutting the unnecessary use of antibiotics in order to slow resistance [10,11]. Some bacteria are naturally resistant to antibiotics via mutation and horizontal gene transfer; however, the frequent use of antibiotics to treat bacterial infections in healthcare, coupled with anthropogenic activities such as wastewater reuse, among other agricultural practices, contributes to the rapid spread of antibiotic resistance [2,7].
In low- and middle-income countries (LMICs), there is lack of innovative research on antibiotic usage, surveillance, and the discovery of novel therapeutic options; therefore, fast and effective drug discovery is crucial to help reduce the rise of deadly infections, which are now contributing to more deaths in these countries [12]. Thus, novel efficient antibacterial agents and alternative strategies are urgently required to fill the void of antibiotic discovery and development [13]. Medicinal plants have long been used as medicines to treat microbial infections, among other human diseases; hence, their antibiotic development should now be focused on, as they have proved to be the best alternatives for novel antibacterial targets and can be effective against MDR bacteria [3]. Compared to synthetic chemotherapeutic drugs, natural antibacterial agents and their analogues still dominate the multiple classes of antibiotics, such as β-lactams, tetracyclines, aminoglycosides, and polypeptides, which are routinely used in healthcare [13]. These natural antibacterial agents possess advantages in structural and chemical diversity, accessibility, robust activity, and peculiar modes of action, and present lower health risks associated with human toxicity or side effects [10,14,15]. Additionally, plants have a superior ability to assimilate genetic information and produce complex molecules that can be used to make more effective therapeutics. Moreover, there are significantly lower facility and production costs associated with plant-made drugs, as it costs significantly less to grow plants and mass-produce pharmaceutical compounds, which can allow more capital to be invested into the research and development of new therapeutics. Research focusing on plant secondary metabolites and their possible effectiveness against antibiotic-resistant bacteria could lead to much anticipated discoveries in terms of drug development.
In this paper, we highlight some promising traditional medicinal plants found in the African continent, review the current literature and studies on the therapeutic potential of traditional medicinal plants, and provide insights into their secondary metabolites and modes of action as alternative options in antibiotic therapy against MDR bacteria.
2. Materials and Methods
A comprehensive literature search was conducted on MDR bacteria of clinical relevance, along with their associated resistance mechanisms. A total of 36 selected medicinal plants indigenous to the continent of Africa were selected (refer to Figure 1). They were also reviewed together with their associated secondary metabolites known to have potential antibacterial properties and their modes of action. The reviewed literature included conference papers, books, theses, and papers published in peer-reviewed international journals, as well as reports from international, regional, and national organizations. The PubMed and Scopus databases, as well as the Google Scholar search engine, were used in mining the literature using the following keywords alone or in combination: muti-drug-resistant bacteria; plant bioactive compounds; plant secondary metabolites; indigenous plants; Africa. There was no restriction on the year of publication of the selected literature; the information in this article was collected in publications from both original and review articles spanning from 1994 to 2023.
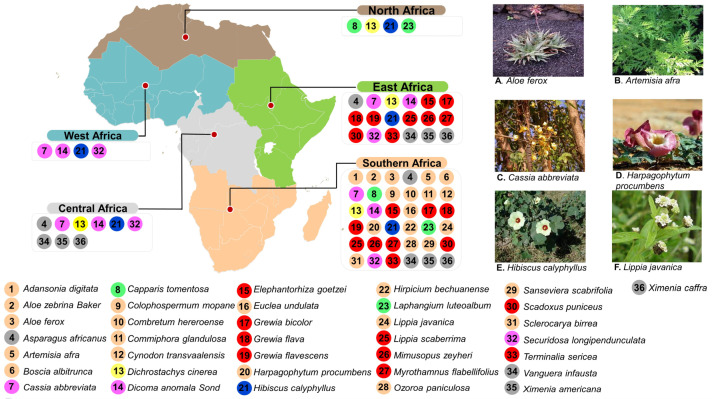
Figure 1.
Diverse plant species with potential antimicrobial properties widely distributed across different regions in Africa: (A–F) examples of plant species that have been commercialized as treatment for bacterial infections. See the Supplementary Materials (Table S1) for more details about species origins, studies, and sources.
3. Results and Discussion
3.1. Clinically Important Multi-Drug-Resistant Bacteria and Modes of Antibiotic Resistance
Antibiotic resistance is found in both Gram-negative and Gram-positive strains of bacteria, which are leading causes of hospital- and community-acquired infections, ranging from common infections such as skin and soft tissue infections to life-threatening infections [4]. MDR bacterial infections account for millions of global deaths annually, with over 40% being neonatal deaths and with that percentage expected to increase in the absence of effective therapeutic drugs [4]. Gram-negative bacteria belonging to the order Enterobacteriales have since developed resistance mechanisms posing a serious threat to human health, especially in hospitals and nursing homes [4,7,16]. Enterobacter species are also Gram-negative and are characterized as facultatively anaerobic, rod-shaped bacteria of the Enterobacteriales family, which includes E. coli and K. pneumoniae [17]. This group of pathogens are a major cause of urinary and respiratory tract infections, causing bacteremia and pneumonia in the immunocompromised [17,18]. Acinetobacter species (e.g., A. baumannii) are Gram-negative, aerobic, non-fermenting, non-fastidious, ubiquitous coccobacillus, or pleomorphic bacteria, and are responsible for bloodstream infections and ventilator-associated pneumonia [17,18]. Acinetobacter species have the ability to resist desiccation and form biofilms, and the presence of fundamental virulence factors, such as secretion systems, surface adhesins, and glycoconjugates, aggravate their pathogenicity [19,20]. Pseudomonas aureginosa, is an example of another Gram-negative bacterium that causes urinary tract infections, surgical site infections, pneumonia, septicemia, and bacteremia, especially in immunocompromised individuals [21]. P. aeruginosa displays innate resistance to a wide array of antibiotics. It is resistant to a wide spectrum of antibiotic classes, including penems and β-lactam, and its resistance to fluroquinolones is due to its ability to mutate on DNA gyrase or topoisomerase [21,22]. P. aeruginosa employs a variety of mechanisms such as alterations in porin channels, efflux pumps, targets modifications, and β-lactamases to exert resistance to antimicrobial agents [21,22,23]. A. baumannii, P. aeruginosa, and several members of the Enterobacteriales family exhibit broad resistance to carbapenems antibiotics; hence, at the top of the WHO priority list is research and development for new antibiotics, which are urgently needed [17,24].
The Gram-positive bacteria of clinical concern include the genera Bacillus (e.g., Enterococcus species, Staphylococcus aureus), Clostridium (C. botulinum, C. perfringens), Listeria (L. monocytogenes), Gardenella (G. vaginalis), and Corynebacterium (C. diphtheriae) [24]. Enterococcus faecalis is an enterococcal bacterium that is responsible for infections in the gut of humans, and is known to cause severe infections in immunocompromised individuals [13,16]. VRE readily accumulate mutations and exogenous genes (VanA, VanB, VanD, VanE, VanG, VanL) that confer resistance to vancomycin, including other antibiotics classes such as β-lactam [25,26]. S. aureus is a Gram-positive spherical bacterium that is normal microflora of the skin and the nasal mucosa, which can also be pathogenic [27,28]. Pathogenic strains of S. aureus normally cause life-threatening soft tissue abscesses, pneumonia, septicemia, and bacteremia, and can cause infections from contaminated medical implants. Its ability to form biofilms also poses a challenge in antibiotics-mediated treatments [29]. Additionally, due to the secretion of the TSST-1 exotoxin in some strains, S. aureus can also cause toxic shock syndrome [30,31]. S. aureus has evolved to develop resistance to vancomycin, methicillin, and many β-lactam classes of antibiotics [31]. MRSA harbors a mecA gene on the staphylococcal cassette chromosome mec (SCCmec) and codes for PBP2a [32]. A protective protein bound to the ribosomes of the bacterial cell inactivates the antibiotics via ‘target alteration’ by altering their structural confirmation [33].
The general mechanisms of antibacterial resistance range from alterations of binding sites, alterations of the bacterial porins’ structure, antibiotics efflux through the bacterial efflux pump structure, and destruction of antibacterial agents by hydrolytic enzymes [5,16]. Additionally, MDR bacteria can resist antibiotics via one mechanism or by combining more than one to produce their multiple resistance to antibiotics and other antimicrobials, including disinfectants and heavy metals in personal care products [7]. The examples of clinically relevant bacteria and their modes of action are summarized in Table 1.
Table 1.
Mechanisms of antibiotic resistance to common classes of antibiotics using healthcare.
| Bacterial Strain | Antibiotics | Mechanism of Resistance | Reference |
|---|---|---|---|
| A. baumanni | β-Lactams, aminoglycosides, carbapenems |
Altered membrane permeability. Enzyme inactivation by aminoglycoside-modifying enzymes |
[4,6,8] |
| E. coli | Tetracycline, gentamycin, amoxicillin, trimethoprim | AcrAB-TolC efflux pump | [5,8] |
| Enterobacter species | β-lactams, most antibiotics, carbapenem | Production of inactivation enzymes using extended-spectrum β-lactamases, carbapenemase acetyltransferases, phophotransferases, and adenyltransferase | [13,16] |
| K. pneumoniae | Third-generation cephalosporins, carbapenem, β-lactams | Changes in membrane permeability Alterations of target site Efflux pump Enzyme inactivation by β-lactamases |
[9,34] |
| S. aureus | Cephalosporins, methicillin, oxacillin, peniccilin, vancomycin, erythromycin | NorA efflux pumps Mutations in genes involved in cell wall synthesis Alterations of antibiotic binding site |
[13,16] |
| P. aeruginosa | Penem groups of antibiotics, β-lactams, fluoroquinolones | Structural changes in the cell’s surface casings Decreases in antibiotic porins MexAB-OprMM efflux pump Enzyme inactivation by β-lactamases Delay of the incoming flow by modifying the structure of the antibiotic |
[5,16] |
3.2. Diversity and Distribution of African Medicinal Plants with Potential Antimicrobial Properties
Thirty-six promising medicinal plant species are highlighted in this review. They are widely distributed across the African region. Southern Africa has the majority, followed by East Africa, then Central Africa, while West Africa and North Africa have the least (Figure 1). Hibiscus calyphyllus is common to all the regions; Cassia abbreviata, Dicoma anomala Sond, and Securidosa longipendunculata are also found in all the regions except North Africa. Dichrostachys cinerea is also universal to all regions except West Africa. Fifteen species (Adansonia digitata, Aloe zebrina Baker, Aloe ferox, Artemisia afra, Boscia albitrunca, Colophospermum mopane, Combretum hereroense, Commiphora glandulosa, Cynodon transvaalensis, Euclea undulata, Harpagophytum procumbens, Hirpicium bechuanense, Lippia javanica, Ozoroa paniculosa, Sanseviera scabrifolia, Sclerocarya birrea) were found to be unique to the Southern African region, nine (Elephantorhiza goetzei, Grewia bicolor, Grewia flava, Harpagophytum procumbens, Lippia scaberrima, Mimusopus zeyheri, Myrothamnus flabellifolius, Scadoxus puniceus, Terminalia sericea) are common to Southern and East Africa, and two (Capparis tomentosa, Laphangium luteoalbum) are found only in Southern and North Africa. Asparagus africanus, Vanguera infausta, Ximenia americana, and Ximenia caffra were found to be distributed in Central, East, and Southern Africa.
Over 10% of the Southern African vegetation is applied in traditional medicine, with over 15 species being partially or fully commercialized, which can be found in local pharmacies. These include Hibiscus calyphyllus, Harpagophytum procumbens, Cassia abbreviata, Aloe ferox, Lippia javanica, and Artemisia afra, amongst many others (Figure 1) [35,36,37,38,39,40]. C. abbreviata, which is also known as long-tail Cassia (or Monepenepe in Setswana, a native language of Botswana), belongs to the Caesalpiniaceae family and is characterized by thick bushes, brown bark, a rounded crown, yellowish leaves, and sweet-scented flowers, as well as long cylindrical dark brown fruits hanging in pods (Figure 1) [41]. The sun-dried bark is boiled in water and served as a hot tea to individuals with miscellaneous stomach ailments, skin problems, and STIs [38] (Table 2). H. calyphyllus, described as a large yellow hibiscus (or Motsididi in Setswana), is a leafy shrub with wide and simple serrate leaves and yellow flowers with a dark red center (Figure 1), belonging to the Malvaceae family [40]. The flowers, which have been reported to be rich in flavonoids and phenolic acids, are traditionally sun-dried, boiled, and served as a hot beverage to treat intestinal ailments in many sub-tropical parts of Africa [40,42] (Table 2). A. afra, also known an African wormwood, belongs to the Asteraceae family [43]. It is an erect, perennial woody shrub with oval-shaped, greyish-looking leaves (Figure 1). The leaves, stems, and roots are rich in terpenoids, tannins, saponins, and glycosides, which are active against colds, coughs, influenza, sore throat, malaria, asthma, pneumonia, and diabetes [44]. These parts of the plants are served pulverized as a hot beverage [45] (Table 1).
L. javanica (lemon bush), of the family Verbenaceae, is a woody shrub with aromatic leaves that gives a lemon-like smell, which is used as a culinary spice, as well as to treat coughs, colds, fever, chest ailments, kidney stones, measles, rashes, and stomach problems [37,46]. Small, dense spikes of white flowers are borne in the axils of leaves (Figure 1). Dried lemon bush leaves are boiled and consumed as is or applied on affected areas [47].
H. procumbens of the sesame seed or Pedaliaceae family, popularly known as devil’s claw, is rich in terpenoids, iridoid glycosides, glycosides, and acetylated phenolic compounds [47]. It is a tuberous perennial plant with creeping stems and dark pink flowers (Figure 1). Devil’s claw is used for a wide variety of health conditions in the form of hot or cooled infusions, decoctions, tinctures, powders, and extracts to treat blood diseases, urinary tract infections, postpartum pains, sprains, sores, sexually transmitted diseases, ulcers, and boils [48]. Commercially, the secondary tubers or roots are pulverized into capsules [47] (Table 2).
Aloe ferox (Xanthorrheaceae, previously Asphodelaceae, Aloaceae, or Liliaceae; commonly known as the bitter aloe in English and kgwaphane or mokhwapha in Setswana) is a cherished, popular, ornamental single-stemmed plant with erect racemes of red, orange, yellow, or rarely white flowers, with spreading or gracefully curved thorny leaves (Figure 1) [49,50]). Traditionally, the fresh leaf is cut up, the flesh is extracted and directly applied on the affected areas, and it is consumed as is or diluted in cold water [39]. Commercially, it is incorporated into different cosmetic products, health drinks, foods, and beverages to deal with various ailments [50] (Table 2). B. albitrunca is a medium-sized evergreen tree belonging to the Capparaceae or Caper family and is served as hot coffee or tea [51]. The bark, leaves, and roots are mainly used as herbal medicines for STIs and skin and stomach infections [51]. In fact, in a study by Pendota et al. in 2015, crude, dichloromethane, ethyl acetate, and butanol leaf extracts were evaluated and confirmed for antibacterial activities against B. subtilis, S. aureus, E. coli, and K. pneumoniae. Motlhanka et al. conducted a study to assess the antibacterial properties from the resin of C. glandulosa. This is a single-stemmed tree with greyish-green to yellowish-green flaking bark that belongs to the family Burseraceae [52]. Crude aqueous and chloroform extracts of the stem resin, as well as the isolated compound, exhibited good in vitro antibacterial activity against Gram-positive bacteria, B. subtilis, C. perfringens, and S. aureus, as well as multi-drug-resistant S. aureus, XU212-tetracycline-resistant, and SA1199B-norfloxacin-resistant strains [47].
X. caffra (Ximeniaceae), commonly known as “sour plum”, is traditionally used both topically and orally to treat a wide range of bacterial infections such as wounds, STIs, respiratory ailments, digestive tract ailments, colds, and coughs [53,54]. Phytochemical investigations of the bark, fruits, leaves, roots, and seeds of the sour plum revealed various compounds, including flavonoids, phenols, phytosterols, and tannins as active compounds against bacterial pathogens [55]. The methanol extracts of X. caffra roots exhibited antibacterial activities against S. aureus and S. epidermidis [56]. D. cinerea is a thornbush belonging to the Leguminosae subfamily Mimosoideae. It is a medicinal plant that is native to Africa and rich in tannins in its leaves, bark, and roots [57,58]. Tannins were isolated from D. cinerea and assayed against S. aureus, S. boydii, S. flexneri, E. coli, and P. aeruginosa using the agar diffusion method [57]. The associated tannins exhibited antibacterial activities against all test microorganisms. This explains why the dried bark, roots, and leaves are served as a hot tea to traditionally treat sexually transmitted, respiratory, dental, skin, and intestinal infections [58].
In a study aimed at investigating the in vitro antimicrobial activity of ethanolic extracts of seventeen species of Sansevieria, including S. scabrifolia, against E. coli using the agar disk diffusion method, a degree of inhibition was found [35]. The leaves of this species are used to treat ear infections, toothache, and diarrhea [54].
C. tomentosa (belonging to the family Capparaceae) is a scrambling shrub that grows as high as 10 m tall and is found across North Africa and Southern Africa (Table 2). It is used to treat pneumonia, coughs, headaches, tuberculosis, and gonorrhea [59]. The associated phytochemicals that are extracted from the hairy yellow-green twigs and leaves are linked to its unique biological, bactericidal, and bacteriostatic activities, which include alkaloids, L’stachydrine, saponin glycosides, phytosterols, terpenoids, tannins, and anthranoids [59]. Studies have shown that this species has good antimicrobial activity against antibiotic-resistant S. aureus, S. pyogenes, E. coli, and P. aeruginosa [60]. O. paniculosa (Anacardiaceae) is an evergreen, semideciduous, small- to medium-sized single-stemmed tree that is rich with phenols [61]. Phenolic-enriched leaf extracts of O. paniculosa were prepared using a mixture of 1% HCl-acidified 70% acetone and n-hexane, and then tested against S. aureus, P. aeruginosa, E. coli, and E. faecalis [61]. These extracts had good activities relating to diarrhea mechanisms or pharmacological relevance. E. undulata (belonging to the Ebenaceae or Ebony family) has egg-shaped to wide, bluntly pointed waxy leaves, yellowish fragrant flowers, and globose fleshy fruits that are all traditionally used for the treatment of body pains, chest complaints, cough, diarrhea, headaches, heart disease, and tooth aches because of their wealth of diterpenes, flavonoids, naphthoquinones, phytosterols, saponins, and tannins [62]. In a previous study, the antimicrobial activity of E. undulata chewing sticks against multi-drug-resistant S. mutans was determined [63]. The minimum inhibitory concentrations ranged from 0.385 to 11.22 mg/mL and the minimum bactericidal concentrations from 0.485 to 20.20 mg/mL. T. sericea (of the family Combretaceae) is a small to medium deciduous rounded flowering shrub whose roots are traditionally used to treat diarrhea, skin rashes, tuberculosis, and opportunistic infections associated with HIV/AIDS in Botswana [64]. It has been reported that dichloromethane/methanol (1:1) extracts of the stems, bark, leaves, and roots have antibacterial activity against B. subtilis, B. cereus, S. aureus, E. coli, K. pneumoniae, P. aeruginosa, S. sonnei, S. typhimurium, and S. epidermidis [65]. The compounds isolated from this species so far include a triterpene sericoside, resveratrol-3-O-β-D-rutinoside, and hydroxystilbene glycoside [66].
S. scabrifolia (also known as Mosokelatsebeng in Setswana) is a stemless evergreen perennial succulent that grows from a thick rhizome. Its fleshy leaves are warmed in a fire and the juice is squeezed into the ear or tooth to treat ear infections and cavities in Botswana [54]. In Namibia, the leaf sap is applied to wounds to prevent infection and accelerate healing [67]. This bactericidal capacity was also confirmed by Tkachenko et al., showing that the crude extracts had antibacterial activity against pathogenic E. coli [67]. The antibacterial activity in the Sansevieria genus may be due to the presence of alkaloids, saponins, terpenoids, steroids, glycosides, and tannins [65].
Table 2.
Summary of African medicinal plants, their uses in folk medicine, and their geographical locations.
| Scientific Name | Setswana Name (Common Name) | Manner of Administration |
Ailments Treated | Distribution in Africa |
References |
|---|---|---|---|---|---|
| Adansonia digitata | Mowana (African baobab) | Fruit is added to milk and consumed as is. Seeds are crushed and the oil is extracted. Bark, flowers, leaves, and roots are pulverized and consumed as a hot beverage |
Intestinal infections, respiratory infections, skin infections |
Mozambique | [68] |
| Namibia | |||||
| South Africa | |||||
| Aloe zebrina Baker/Aloe ferox | Kgophane/Mokhgwapha (variegated aloe) | Leaf flesh is cut up and juice extracted to be applied as is or diluted in water | Skin infections, STIs | Angola | [39] |
| Namibia | |||||
| Mozambique | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Asparagus africanus | Mhalatsamaru/wild Asparagus | Dried tubers or roots are boiled and served as a beverage | Genital sores and wounds | Eswatini | [47] |
| Lesotho | |||||
| Mozambique | |||||
| Namibia | |||||
| Zimbabwe | |||||
| Artemisia afra | Lengana (African woodworm) | Leaves, stems, and roots are boiled in water and served hot | Respiratory infections and related symptoms such as fevers | Cameroon | [45] |
| Chad | |||||
| Ethiopia | |||||
| Kenya | |||||
| Namibia | |||||
| Tanzania | |||||
| Uganda | |||||
| South Africa | |||||
| Zimbabwe | |||||
| Boscia albitrunca | Motopi (Shepherd’s tree) | Pulverized leaves are served in warm milk | Respiratory infections | Eswatini | [69] |
| Mozambique | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Capparis tomentosa | Motawana (African caper) | Dried bark, stems, leaves, or roots are boiled and consumed as tea | Respiratory system infections, STIs |
Tropical Africa | [38] |
| Namibia | |||||
| Mozambique | |||||
| South Africa | |||||
| Cassia abbreviata | Monepenepe (long pod cassia) | Sun dried bark or stem is boiled in water and served hot | Diarrhea, skin diseases, STIs, stomach infections | DRC | [38] |
| Eswatini | |||||
| Kenya | |||||
| Mozambique | |||||
| Namibia | |||||
| Somalia | |||||
| South Africa | |||||
| Tanzania | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Colophospermum mopane | Mophane (mopane tree) | Pulverized seeds are boiled and orally administered Gum from the stem is applied on affected areas. Roots are boiled to make a hot beverage |
Stomach infections, STIs, sore eyes |
Angola | [52] |
| Malawi | |||||
| Mozambique | |||||
| Namibia | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Combretum hereroense | Mokabi (russet bushwillow) | Fruits are eaten as is. Dried leaves are boiled to make tea |
Stomach infections | Eswatini | [47] |
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Zimbabwe | |||||
| Commiphora glandulosa | Moroka (tall common corkwood) | Several incisions are made to the plant during winter, and the resinous exudate is harvested and applied to affected areas. The bark is boiled and consumed as tea |
Skin and soft tissue infections | Angola | [70] |
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Cynodon transvaalensis | Monamane (Burtt-Davy) | Ground roots and root bark are sun-dried and pulverized for beverage preparation |
Stomach infections, fever |
Angola | [47] |
| Eswatini | |||||
| Lesotho | |||||
| Malawi | |||||
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Dichrostachys cinerea | Moselesele (sickle bush) | Dried bark, roots, and leaves are boiled to make the beverage. Seeds are crushed to extract the oil. Incisions on the stem are made to collect gum |
STIs, intestinal infections, dental infections, skin infections |
From Eastern Cape RSA to Tropical East Africa | [57] |
| Dicoma anomala Sond. | Tlhonya (fever/stomach bush | Dried roots are boiled in water and served as tea | Stomach infections and diarrhea | Angola | [71] |
| Burundi | |||||
| DRC | |||||
| Rwanda | |||||
| South Africa | |||||
| Tanzania | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Elephantorhiza goetzei | Mositsane/Mosidi (large been elephant root) | Bark and root decoctions are taken orally | Intestinal infections, urinary tract infections, STIs |
Malawi | [72] |
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Tanzania | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Euclea undulata | Motlhakola (common guarri) | The roots directly brush the teeth and mouth. Pulverized leaves make a hot beverage for oral ingestion. Bark is boiled and served as hot tea |
Mouth sores and wounds, tooth ache and sore throat, intestinal infections |
Namibia | [62] |
| Swaziland | |||||
| Zimbabwe | |||||
| Grewia bicolor; Grewia flava; Grewia flavescens | Moretlwa/Mogwana (wild currant/velvet raisins/brandy bush) | Fruit is eaten or prepared into a dry pulp. Fresh or dry roots are boiled to prepare a beverage |
Cavities Urinary tract infections, skin infections |
Angola | [73] |
| Eswatini | |||||
| Ethiopia | |||||
| Namibia | |||||
| Zimbabwe | |||||
| Harpagophytum procumbens | Sengaparile/Lengakapitsi (devil’s claw) | Sun-dried secondary tubers or roots are pulverized into capsules for average preparation | Intestinal infections, kidney infections |
Angola | [47] |
| Namibia | |||||
| South Africa | |||||
| Mozambique | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Hibiscus calyphyllus | Motsididi (Wild Hibiscus/Roselle) | Sun-dried flowers are boiled in water and served hot or cold | Stomach infections | West, North, East and some parts of Southern Africa |
[42] |
| Hirpicium bechuanense | Kgalemela (S. Moore-Rossler) | Dried tubers or roots are boiled and consumed as tea | Infections and diarrhea | South Africa | [42] |
| Mozambique | |||||
| Zimbabwe | |||||
| Mimosopus zeyheri | Mompudu (red milkwood) | Fruit is consumed fresh or as a dry pulp. Pulverized flowers are used to make snuff. Dried bark, leaves, and roots are boiled and served as tea |
Mouth wounds, Dental infections, STIs, soft tissue infections |
Eswatini | [74] |
| Mozambique | |||||
| South Africa | |||||
| Myrothamnus flabellifolius | Kgomodimetsing (resurrection plant) | Leaves are pulverized to make a hot beverage | Respiratory infections, soft tissue infections |
Angola | [75] |
| DRC | |||||
| Kenya | |||||
| Tanzania | |||||
| Lesotho | |||||
| Malawi | |||||
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Ozoroa paniculosa | Monokane (bushveld ozoroa/common resin tree) | Sun-dried bark, stems, leaves, or roots are pulverized for beverage preparation | Fevers associated with infections | Namibia | [47,76] |
| South Africa | |||||
| Zimbabwe | |||||
| Sanseviera scabrifolia | Mosokelatsebeng (bowstring hemp) | Fleshy leaves are warmed in a fire and the juice is squeezed into the ear | Ear infections | Namibia | [53,67] |
| Mozambique | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Scadoxus puniceus | Mathubadifhala (blood lily) | Dried tubers or roots are boiled and served as tea or applied on affected areas | Wound infections | Eswatini | [77] |
| Ethiopia | |||||
| Lesotho | |||||
| Malawi | |||||
| Mozambique | |||||
| South Africa | |||||
| Tanzania | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Sclerocarya birrea | Morula/Marula | Fruit is eaten as is Stem or bark is boiled in water and served hot or cold. Seeds are crushed to extract the oil for skin application |
Skin infections Mouth wounds |
Angola | [47] |
| Eswatini | |||||
| Malawi | |||||
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Zambia | |||||
| Zimbabwe | |||||
| Securidosa longipenduculata | Mmaba (violet tree) | Dried stem bark and roots are boiled and orally administered. Pulverized bark makes a paste and is applied to affected areas |
Skin infections, respiratory infections, urinary tract infections |
Tropical and Subtropical Africa | [78] |
| Terminalia sericea | Mogonono (silver cluster-leaf) | Leaves and roots are boiled and consumed as tea. Pulverized leaves are applied as a paste on affected area |
Respiratory infections, intestinal infections, soft tissue wounds |
Angola | [79] |
| DRC | |||||
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Tanzania | |||||
| Zimbabwe | |||||
| Vanguera infausta | Mmilo (wild medlar) | Fruit is eaten fresh or dry. Seeds are eaten as they are. Root and leaves are boiled and orally administered |
Mouth wounds, respiratory infections, intestinal infections |
Kenya | [80] |
| Madagascar | |||||
| Malawi | |||||
| Mozambique | |||||
| Namibia | |||||
| South Africa | |||||
| Tanzania | |||||
| Uganda | |||||
| Zimbabwe | |||||
| Ximenia americana/Ximenia caffra | Moretologa wa pudi/moretologa wa kgomo | Dried and crushed leaves are boiled in water for oral administration. The water extracts are applied on direct sores. The fruit is eaten as is | Body sores, STIs, diarrhea, sore eyes | Angola | [54] |
| Lesotho | |||||
| Malawi | |||||
| Mozambique | |||||
| South Africa | |||||
| Swaziland | |||||
| Tanzania |
3.3. Plant Secondary Metabolites with Antimicrobial Potential
Plants are known to synthesize and produce diverse groups of organic compounds that are involved in assorted metabolically related functions of the plant, known as secondary metabolites [81]. Their primary function to the plants is in the interaction of the plant with the environment, and they are mostly released in response to abiotic and biotic stresses, thereby supporting plant survival as molecules of defenses [82]. Examples of classes of secondary metabolites include terpenoids, phenols, and derivatives, as well as glucosinolates and alkaloids [83,84].
3.3.1. Alkaloids
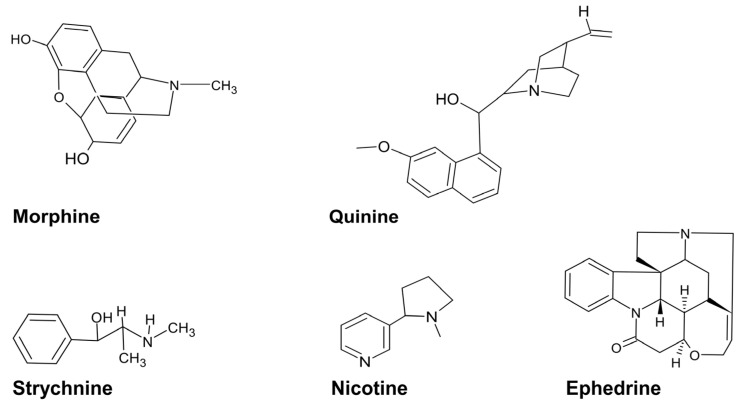
Alkaloids are nitrogenous compounds that can be classified as natural, semi-synthetic, and synthetic or based on their chemical structure into typical alkaloids with a heterocyclic ring or atypical alkaloid non-heterocyclic ring [5]. Additionally, they may be split into several classes: tropanes, indole, purines, imidazole, pyrrolidine, pyrrolizidine, isoquinoline, piperidine, and quinolizidine [85]. The antibacterial capacity of alkaloids has been documented and has been linked to efflux pump inhibition, bacterial cell wall synthesis inhibition, changes in cell membrane permeability, inhibition of bacterial metabolism, and nucleic acid and protein synthesis [85,86]. For example, strychnine from C. tomentosa has antibacterial activity against E. coli, P. aeruginosa, and K. pneumoniae (Table 3). Other plant-associated alkaloids include nicotine, ephedrine, morphine, and quinine (Figure 2).
Figure 2.
Examples of plant-associated alkaloids.
3.3.2. Polyphenols
Polyphenols are a large group of secondary metabolites that are classified according to their phenolic groups and structural elements as flavonoids, stilbenes, lignans, tannins, and phenolic acids [87]. The antibacterial capacity levels of polyphenols towards Gram-negative and positive MDR bacteria have been linked to their ability to bind to bacterial enzymes via a hydrogen bond, inducing several modifications in cell membrane permeability and cell wall integrity [86,88,89]. Examples include catechin from Adansonia digitata and vitexin from tannic acid from Dichrostachys cinerea (see Table 3). Tannin is a descriptive name for a group of polymeric phenolic substances capable of tanning leather or precipitating gelatin from solution [57].
3.3.3. Terpenes
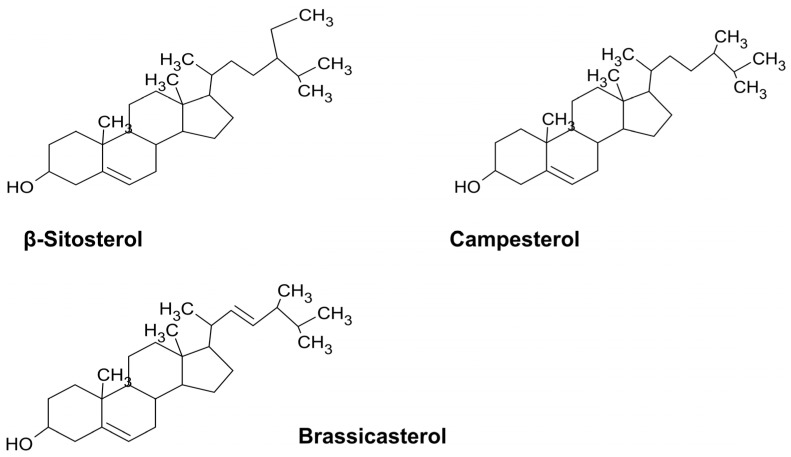
Terpenes are a large group of hydrocarbons synthesized from the 5-carbon precursor units of isopentenyl pyrophosphate and its functional isomer dimethylallyl pyprophosphate [90]. According to the number of isoprenes, they are classified into monoterpenes (e.g., limonene from A. afra (Table 3)), diterpenes (e.g., retinol), triterpenes (e.g., oleanolic acid from the Hibiscus spp.), and tetraterpenes (e.g., lutein, brassicasterol, campesterol, and β-sitosterol from Artemisia) [35,91,92] (Figure 3). The broad antibacterial activity of the terpenes includes efflux pump inhibition and the inhibition of bacterial growth and membrane properties towards MDR bacteria E. coli, S. aureus, and Enterobacter species [91,93,94] (see Table 3).
Figure 3.
Chemical structures of plant-associated terpenes.
Table 3.
Studied secondary metabolites from selected African medicinal plants with antimicrobial properties against MDR bacteria.
| Plant | Part | Active Compound | MDR Bacteria Active Against | Reference |
|---|---|---|---|---|
| Adansonia digitata | Leaves, stems, roots |
Rutin Catechin Gallic acid Caffeic acid |
E. coli
E. aerogenes K. pneumoniae P. aeruginosa S. aureus |
[95] |
| Aloe zebrina Baker/Aloe ferox | Leaves, roots, and stems | Stearic acid Palmitic acid |
E. coli
K. pneumoniae P. aeruginosa S. aureus |
[96] |
| Asparagus africanus | Roots | Gallic acid Hydroxybenzoic acid Hydroxycinnamic acids |
E. coli
P. aeruginosa A. baumannii |
[97] |
| Artemisia afra | Leaves, roots, and stems | Luteolin β-sitosterol Apigenin Myo-inositol |
E. faecalis
E. coli K. pneumoniae P. aeruginosa S. aureus |
[98] |
| Boscia albitrunca | Roots, leaves |
Anthraquinones, martynoside |
E. coli
K. pneumoniae S. aureus |
[69,99] |
| Capparis tomentosa | Roots, fruit |
Stachdrine Betane Strychnine 3-hydroxy-4methoxy-3-methyl-oxindole |
E. coli
P. aeruginosa K. pneumoniae |
[100] |
| Cassia abbreviata | Bark | Spectaline Iso-6-cassine |
E. coli
K. pneumoniae P. aeruginosa |
[101] |
| Colophospermum mopane | Seeds, husks, leaves |
Labdane, isolabdane, and clerodane diterpenoids |
E. coli
S. aureus Enterococcus |
[102] |
| Combretum hereroense | Bark, leaves, roots |
Vitexin, saponaretin, combretacin |
E. coli
S. aureus |
[103] |
| Commiphora glandulosa | Resin | 1β,2β,3β-trihyddoxy-urs-12-ene-23-oic-rhamnoside |
E. coli
K. aerogenes P. aeruginosa S. aureus |
[70] |
| Dichrostachys cinerea | Roots | Tanninic acid |
S. aureus
E. coli P. aeruginosa |
[57] |
| Dicoma anomala Sond. | Aerial parts of the plant | Gemacrene, β-farnasene, α-humulene |
E. coli
S. aureus P. aeruginosa |
[104] |
| Elephantorhiza goetzei | Rhizomes, roots |
Tanninc acid |
S. aureus
P. aeruginosa |
[72] |
| Euclea undulata | Bark, leaves, roots |
Diopyrin Lupeol 7-methyljuglone |
E. coli
P. aeruginosa |
[62] |
| Hibiscus calyphyllus | Aerial parts | Oleanolic acid β-amyrin |
S. aureus
P. aeruginosa |
[40] |
| Myrothamnus flabellifolius | Leaves, twigs |
Camphor 1,8-cineole α-pinene |
K. pneumoniae
S. aureus |
[105,106] |
| Ozoroa paniculosa | Leaves | Anacardic acid Quercetin Proanthocyanidin Gallotannin |
E. coli
S. aureus P. aeruginosa E. faecalis |
[61] |
| Scadoxus puniceus | Bulbs, roots, leaves, stems |
Haemanthamine Metolachlor |
E. coli
K. pneumoniae S. aureus |
[107] |
| Terminalia sericea | Roots, stems, bark, leaves | Hydroxystilbene glycoside, triterpene sericoside, resveratrol-3-O-β-D-rutinoside, Lupeol, Anolignan B |
E. coli
K. pneumoniae P. aeruginosa |
[64,66] |
| Ximenia americana/Ximenia caffra | Bark, fruit, roots, seeds, leaves |
Catechin Gallic acid Quercetin Proanthocyanidin |
E. coli
P. aeruginosa |
[53,55] |
3.4. Therapeutic Potential of Traditional Medicinal Plants against MDR Bacteria
Antibiotics are definitely the cornerstones of modern medicine; unfortunately, we are still experiencing the rapid spread of foodborne pathogens, emergence of antibiotic-resistant microbial strains, and increasing failure of available chemotherapeutics [108]. Hence, there is a need for efficient antibacterial agents to fill the gap for the discovery and development of anti-MDR agents. Natural products dominate the preferred chemical scaffolds for the discovery of antibacterial agents [13]. In fact, in Africa, traditional healers, herbalists, and individuals have used a variety of wild leaves, roots, barks, fruits, and seeds to combat miscellaneous bacterial illnesses and diseases [109]. Medicinal plants have a wide array of phytochemicals, alkaloids, phenolics, polyphenols, flavonoids, quinones, tannins, coumarins, terpenes, lectins, and saponin, which have been studied due to their mechanisms of action against drug-resistant pathogenic bacteria [2,110,111]. These plants can be proactively bactericidal or bacteriostatic because they have less nitrogen, sulfur, phosphorus, and halogens, and exhibit overall enhanced scaffold variety, molecular complexity, stereochemical abundance, diversity in the ring system, and carbohydrate contents [112,113]. These features allow plant products to modify or inhibit protein interactions, thereby presenting themselves as effective modulators of immune response, mitosis, apoptosis, and signal transduction [112,114]. In this way, the microbial cell can be affected in several ways, including the inhibition and disruption of cell membrane and cell wall functions and structures, interruption of nucleic acid replication and ATP synthesis, generation of reactive oxygen species, inhibition of the formation of biofilms, disruption of quorum sensing at all stages, and via synergy with other antimicrobial agents [94,115,116,117,118,119,120,121].
Antibacterial Activity of Plant-Derived Bioactive Compounds
Medicinal plants associated with low molecular weight antibiotics are classified into two types, phytoanticipins and phytoalexins [122]. Phytoanticipins are involved in microbial inhibitory actions while phytoalexins are generally antioxidative and are synthesized de novo by plants in response to microbial infections [122,123]. Many studies have shown that the antimicrobial activity of the plant-derived active compounds, namely alkaloids, phenylpropanoids, and terpenoids, have different capacities to promote cell wall disruption and lysis, induce reactive oxygen species production, inhibit biofilm formation, inhibit cell wall construction, inhibit microbial DNA replication, inhibit energy synthesis, inhibit bacterial toxins from effecting the hosts, and even prevent synergetic resistance to antibiotics [120,124,125,126,127,128].
Inhibition and Disruption of Cell Wall Construction
The cell wall is the principal stress-bearing and shape-maintaining element in bacteria. Cell walls can be broadly classified into two polyphyletic groups, Gram-positive and Gram-negative, each arising from structural differences linked to the bacterial interactions with the environment [129,130]. Gram-negative cell walls have an additional membrane covered in lipopolysaccharides, while Gram-positive walls contain only one membrane and have a thicker layer of peptidoglycan containing negatively charged teichoic acids [129]. The biosynthesis of bacterial cell wall peptidoglycan, also called murein, is a complex process that involves enzyme reactions that take place in the cytoplasm on the inner side and outer side of the cytoplasmic membrane [130,131]. Teichoic acid is synthesized as a lipid-linked precursor in the cytoplasm, translocated across the cytoplasmic membrane, and then covalently bonded to the peptidoglycan by the involvement of specific enzymes, namely TagF, TagG, and TagH [130,132]. Since the bacterial cell wall is responsible for many metabolic activities, both directly and indirectly, the integrity of the membrane is of paramount importance, and its disruption can lead to metabolic dysfunctions and ultimately bacterial lysis [129,130,132]
Phenolic compounds and quinones, which are aromatic compounds, are usually targeted to microbial cell surface adhesins and membrane-bound polypeptides, which may disrupt the development of the cell wall and lead to eventual cell lysis [116]. Catechins attract lipid bilayers of the membrane; they form hydrogen bonds, which attract polar head groups of lipids at the membrane edge, which causes structural changes in the cell membrane [133]. Phenolic compounds consist of a hydroxyl functional group, which leads to reactions of hydroxylation, while flavonoids, in particular, are able to complex with bacterial cell walls and disrupt microbial membranes [116,117]. Flavonoids, quercetin, rutin, naringenin, sophoraflavanone, chalcone, and tiliroside are linked to decreased lipid bilayer thickness and fluidity levels and increased membrane permeability and eventual leakage of intracellular proteins and ions in S. aureus and S. mutans [11,134]. Other active flavonoids, including acacetin, apigenin, morin, and rhamnetin, are known to cause the disarrangement and disorientation of the lipid bilayer, thereby weakening the bacterial cell wall and ultimately causing vesicle leakage [125,135]. Flavonoids such as EGCG, penta-hydroxy-flavones, and dimethoxyflavones inhibit the malonyl CoA-acyl carrier protein transacylase, which regulates bacterial fatty acid synthase-II [136,137].
Inhibition of Bacterial DNA Replication and ATP Synthesis
Plant-derived bioactive compounds have been proven to halt many enzymes that are linked, directly and indirectly, to DNA replication, transcription, and translation [138].
The replication of DNA is an ATP-dependent process, and the initiation of DNA replication thereof is linked to the growth-dependent accumulation of the ATP-bound form of the highly conserved protein DnaA [139]. As the bacterial cell grows and develops until it reaches a specified mass or size, DnaA-ATP accumulates. Adenosines, ATPase, and DnaA initiate DNA replication by mediating the unwinding of an ATP-rich stretch of DNA within the origin, facilitating the addition of the replication machinery [139]. Flavonoids such as baicalein, morin, quercetin, and silymarin can constrain the function of ATPases, which is the hydrolysis of a phosphate bond in ATP to generate energy, and can obstruct ATP synthesis [119,140]. This obstruction of the synthesis of ATP can disrupt the unwinding of DNA and halt DNA replication. Additionally, EGCG, flavones, and proanthocyanidins have been implicated in the inhibition of the enzymatic activity of ATPase in some bacteria [136,141]. Apigenin, genistein, and myricetin have been recognized as DNA gyrase inhibitors [138,142]. They have been proven to intercalate with the stacking of nucleic acid bases, as well as binding to the β subunit of gyrase and the corresponding blockage of the ATP binding pocket to halt DNA and RNA [122,143]. Helicase is responsible for unzipping the DNA double strand during the early stages of DNA replication [144]. Luteolin, morin, and myricetin have been demonstrated to inhibit the DNA-separating and -rearranging capacity of the helicases of E. coli [145,146].
Berberine and harmane, which are alkaloids, have been reported to intercalate with bacterial DNA and lead to the impairment of the cell membrane, consequently leading to cell death [147]. In another study, E. coli and L. monocytogenes were treated with trans cinnamaldehyde, an essential oil produced by cinnamon, and it was found that it can downregulate F1F0-ATPase, resulting in rapid reductions in ATP, thereby preventing the concentration of cellular ATP and reducing the growth of the bacterial cell [148]. According to Almuhayawi (2020) [149], the ATPase activity in E. coli was also inhibited as a result of quercetin binding to the bacterial DNA gyrase B subunit. The disruption of ATP synthesis and DNA replication in bacterial cells can lead to growth disturbances, a compromised cellular structure, and reduced resistance against conditions that may cause cell destruction and ultimate lysis [148,149].
Disruption of Pathogenicity Activities
The pathophysiology of a microbial infection in a host is mediated by multiple virulence factors, which are expressed at different stages of infection to cause the disease. These factors include capsule production, toxin production, and hydrolytic protein production; hence, virulence factors are the prime targets for therapeutic interventions and vaccine development [150]. Microbial toxins include exotoxins (secreted by bacteria) and endotoxins (released after bacterial lysis). Many phenolic compounds can affect enterotoxin production through several modes of action, including translation or transcription inhibition, the disruption of secretory mechanisms, the inhibition of quorum sensing regulatory systems, and toxin inactivation or neutralization [150,151,152]. RG tannin and apple phenols were reported to inhibit ADP–ribosyltranferase activity that is critical for cholera toxin action, leading to reductions in toxin-induced accumulation in mouse ileal loops [153,154]. Toxin-mediated pathogenesis is more aggressive because even post cell lysis the toxin remains behind and continues virulence [155]. Therefore, compounds that do not only cause cell death but also inactivate toxins are more effective against this kind of virulence. Phenolic hydroxylic compounds can inactivate or neutralize bacterial exo-proteins by forming hydrogen bonds with active sites of enzymes or toxins to inhibit their activity [151,156]. In some studies, resveratrol disrupted the toxin’s internalization and activity, epigallocatechin gallate and procyanidin blocked the toxin binding and occupied the binding sites, and kaempferol and quercitrin could directly inhibit the activity of the catalytic subunit [156,157]. Gallotannins have inhibitory effects on microorganisms. These effects are attributed to their complexing properties and ability to interact with proteins and inhibit enzyme activity via their chelation of the metal ions of the active sites [158].
Generation of Reactive Oxygen Species
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) can be formed from the partial reduction of molecular oxygen and nitrogen through many continuous metabolic pathways, involving both enzyme-catalyzed and non-enzymatic reactions [159,160,161]. ROS can attack diverse targets to exert antimicrobial activity, which helps to account for their versatility in mediating host defense against a vast range of disease-causing pathogenic organisms [160,161]. In fact, it is the host’s NADPH-dependent NOX2 phagocyte oxidase complex in the immune system that is responsible for the generation of ROS [160]. Therefore, many phenols that have the capacity to dabble between pro-oxidant and antioxidant activities because of their hydroxyl groups can be easily coupled with the human’s defense systems against pathogens [162,163]. Examples of these include quercetin and rutin; with their hydroxyl groups they carry out hydroxylation with the lipid bilayer of the bacteria and prevent the generation of ROS [11,116,117]. Upon penetrating the inner cell membrane, phenols can be oxidized by ROS, converting them into pro-oxidants, which are able to oxidize lipids, proteins, and DNA, leading to bacterial cell lysis [163].
ROS readily attack the polyunsaturated fatty acids of the fatty acid membrane to initiate lipid peroxidation, which compromises the integrity of the cell membrane and facilitates eventual cell lysis [164]. Some ROS include superoxide, hydrogen peroxide, hydroxyl radical, alkoxyl radicals, and singlet oxygen. They have different kinetics and levels of activity [162]. Hydrogen peroxide is a covalent and uncharged molecule that readily mixes with water, and is treated as such by cells, meaning it diffuses across the cell membrane with ease [161]. It can also form redox reactions and highly reactive free radicals, which may be aggressively oxidative and can impair the functions of many metabolic processes [161].
In this manner, plant-derived compounds with hydroxyl tails or double bonds can be oxidized to become phenoxyl radicals or quinone intermediates, meaning they behave as pro-oxidant systems [120,164]. In the presence of transition metals, they can fragment DNA and lead to mutation and cell death [165]. Catechins, for instance, enhance the production of oxidative stress as ROS and RNS [166]. In this way, they can cause altered membrane permeability, bond to the highly negatively charged lipids and liposomes, and facilitate cell damage and eventually death [167]. They are also linked to the leakage of intracellular proteins and ions, as they also disturb the membrane transport system [164,166,167]. Epigallocatechin gallate, in high concentrations, exerts its mode of action of killing bacteria via the generation of ROS, causing alterations of the membrane permeability and membrane damage [120].
Formation of Biofilms Inhibition
Biofilm formation is a process whereby microorganisms irreversibly attach to and grow on a surface and produce extracellular polymers that facilitate attachment and matrix formation, resulting in alterations of the phenotype of the organisms with respect to the growth rate and gene transcription [168]. These surfaces of attachment may take many forms, including the surface of the host that is colonized and infected [168,169]. The presence of mobility structures such as flagella, fimbriae, and pili is most important for microbial attachment [168,169]. Microbial cells, via these appendages, temporarily attach to the surface, as they prepare to form colonies and exude extracellular polymers that will then attach permanently to the surface [170,171].
Flavonoids have been described to cause the aggregation of multicellular composites of bacteria and cause psuedobacterial growth after aggregation, which indicates that flavonoids are potent antibiofilm compounds [122]. Flavonoids such isovitexin, galangin, EGCG, trihydroxyflavanols, and 3-O-octanoyl-epicatechin have been shown to inhibit the multicellular aggregation of pathogenic bacteria [122,124,127,172]. Moreover, chrysin, phloretin, naringenin, epicatechin gallate, and proanthocyanidins have been proven to inhibit N-acyl homoserine lactone-mediated quorum sensing [173,174,175]. The synthetic flavonoid lipophilic-3-arylidene was found to be very active against S. aureus, S. epidermidis, and E. faecalis due to a bacterial cell clump that influences the integrity of the cell wall as a result of biofilm disruption [155]. Phloretin was also proven to prevent the formation of fimbriae in E. coli by reducing the expression of the curli genes (csgA, csgB) and toxin genes (hemolysin E, Shiga toxin), eventually halting the formation of the biofilm [176,177].
In one study, the components of cranberry juice were hypothesized to interact with hydrophobic proteins on the surface of the bacterial cell [178]. Streptococcus mutans and Streptococcus sobrinus were treated with cranberry juice, and it was found that the cranberry juice reduced the surface hydrophobicity of the cells, interfering with adhesion and the initial stages of biofilm formation. Since hydrophobicity is an important factor in the initial attachment of the bacteria to a surface, reducing the hydrophobicity decreases the likelihood of adhesion [179]. Therefore, preventing biofilm formation is a potential method of curbing bacterial colonization and eventual pathogenicity, possibly through the engagement of plant-derived bioactive compounds.
Quorum Sensing (QS) Inhibition
The discovery of bacterial communication systems (QSsystems) that orchestrate important temporal events during the infection process has afforded a novel opportunity to ameliorate bacterial infections using compounds and possibly overriding bacterial signaling mechanisms [180]. Many plant species produce secondary metabolites and compounds that can control the growth of microbes and have traditionally been used to treat human microbial infections via anti-quorum sensing. The therapeutic substances in Centella asiatica L. Urban are saponin-containing triterpene acids and their sugar esters, e.g., Asiatic acid, madecassic acid, and asiaticosides, which have been shown to treat skin problems and heal wounds [181,182]. In fact, in a study by [183], the anti-quorum sensing potential of C. asiatica was investigated using Chromobacterium violaceum and P. aeruginosa PA01. The anti-quorum sensing activity of an ethanolic extract and ethyl acetate fraction of the herb were investigated against C. violaceum. The ethanol extract showed quorum sensing inhibition against violacein production, while the ethyl acetate fraction was four times more active against violacein production, without significantly affecting the growth [183]. The ethyl acetate fraction also inhibited quorum-sensing-regulated phenotypes, pyocyanin production, elastolytic and proteolytic activities, swarming motility, and biofilm formation in P. aeruginosa PA01 in a concentration-dependent manner [183].
Bacteria are consistently subjected to environmental stimuli such as nutrient availability, temperature, and pH changes. They in turn have developed multiple systems that form cell-to-cell communication via the use of autoinducers of N-acyl-homoserine lactone (AHLs) for Gram-negative bacteria and oligopeptides for Gram-positive bacteria in order to activate specific gene expression and QS systems [184,185,186]. The AHL-mediated QS function requires three major components: an AHL signal molecule, an AHL synthase protein to make the AHL signal (LuxI), and a regulatory protein that responds to the surrounding concentration of AHL [187,188]. At high AHL concentrations, the sigma receptor protein (LuxR) forms a complex with AHL and becomes activated; this activation triggers the expression of genes that are linked to biofilm formation and virulence during pathogenesis [185,187].
The traditional treatment of bacterial infections is anchored on compounds that either kill or inhibit bacterial growth [189]. With the development of resistance against and tolerance for these antibacterial agents, hacking into the quorum sensing systems using plant-derived substances may pose a potential novel opportunity to amend bacterial virulence [190,191]. This is because many compounds that have been proven to override bacterial signaling are present in medicinal plants, and bacterial communication systems are responsible for the orchestration of the important progressive events during the infection process [186,188,190,191]. Therefore, the strategies aiming to interrupt bacterial quorum sensing circuits possibly include the inhibition of AHL or oligopeptide signal generation, inhibition of AHL or oligopeptide signal distribution by antagonization or degradation, and prevention of AHL signal reception via competitive and non-competitive inhibition [184,190]. The plant-derived molecules that affect these AHL-mediated quorum sensing stages include sulfur-containing compounds, monoterpenes, monoterpenoids, phenylpropanoids, benzoic acid derivatives, diarylheptanoids, coumarins, flavonoids, and tannins [189,192].
Inhibition of AHL or Oligopeptide Signal Generation
The AHL signal is synthesized at a low basal level by the AHL synthase. Often the quorum sensor is subject to autoinduction because the gene encoding the signal synthase is among the target genes; hence, a positive feedback regulatory loop is created [189,192]. The autoinduction allows a rapid increase in signal production and dissemination, which in turn induces a quorum-sensing-controlled phenotype throughout the bacterial population [189]. AI-1 is an AHL that is involved in intraspecies communication, whereas AI-2, a furanosyl borate diester, has been suggested to play an important role in interspecies communication [193]. Genome sequencing revealed the presence of luxS homologs (encoding the AI-2 signal synthase) in many pathogens, including E. coli, Helibacter, Neisseria, Porphyromonas, Proteus, Salmonella, E. faecalis, S. pyogenes, and S. aureus, and in some of the pathogens luxS is required for virulence [194,195]. The luxS gene responsible for the production of AI-2 is produced from S-ribosylhomocysteine, a product in S-adenosylmethione utilization [194,196]. LuxS cleaves S-ribosylhomocysteine to form homocysteine and 4,5-dihydroxy-2,3-pentanedione, which is subsequently converted to AI-2 through an unknown biochemical pathway [193,197]. LasI, which is part of one of the quorum sensing systems in P. aeruginosa (LasR/I and RhII/R), is essential for the production of the AHL molecule, N-(3oxododecanoyl)-L-homoserine lactone (3O-C12-HSL), and lasR is the transcriptional regulator [118,198]. The second QS system comprises the RhII and RhIR proteins [118,198]. The RhII synthase mediates the synthesis of the signal molecule, N-butyryl-homoserine lactone C4-HSL, while RhIR is the transcriptional regulator [198,199].
The majority of bacteria that produce AHL signals encode one or more genes homologous to luxI of Vibrio fischeri [189]. The expression of these genes in a heterologous host background has demonstrated that the LuxI-type protein is required and sufficient for the production of AHL signals. Additionally, the catalysis of AHL synthesis involves a sequentially ordered reaction mechanism that uses S-adenosyl methionine (SAM) as the amino donor for the generation of the homoserine lactone ring moiety and an appropriately charged acyl carrier protein (ACP) as the precursor for the acyl side chain of the AHL signal [200]. Various analogs of SAM, such as S-adenosylcysteine and sinefungin, have been demonstrated to be potent inhibitors of AHL synthesis catalyzed by the P. aeruginosa RhII protein [189]. Moreover, such inhibition can be due to interference with acyl homesrine lactone production (luxI effect) or a transcription response (luxR effect) [191]. Protein inhibition at the ribosomal level can be a means of hacking into the quorum sensing system of the pathogen.
Trans-cinnamaldehyde was reported to reduce the expression of luxR, which codes for the transcriptional regulator of quorum sensing in C. sakazakii [201]. Similarly, garlic extract and p-coumaric acid inhibited quorum sensing in quorum sensing strains, indicating that plant compounds potentially modulate virulence by affecting quorum sensing in microbes [150]. In one study, S. aromaticum exhibited high quorum sensing inhibition ability and concentration-dependent violacein pigment inhibition of C. violaceum, which is also correlated with biofilm formation inhibition [202,203]. The inhibition of biofilm formation indicated that EPS was not formed and that the bacteria were unable to attach to the host for the formation of biofilms [204].
Decreasing the active signal molecule concentration in the environment can inhibit bacterial cell-to-cell communication. The reversible hydrolysis of AHLs at high pH levels has been recorded in some instances and was linked purely to alkaline conditions [205]. In other cases, the degradation of the AHLs was linked to enzymatic activity. Bacillus species produce an enzyme, termed AiiA, which catalyzes the hydrolysis of AHL molecules. The expression of this gene in the plant pathogen Erwinia carotovora resulted in the reduced release of AHL signals, decreased extracellular pectolytic enzyme activity, and attenuated soft rot disease symptoms in all plants tested [206]. Moreover, transgenic plants expressing AiiA have been shown to be significantly less susceptible to infection by E. carotovora [168]. Therefore, AHL-degrading or antagonizing proteins are of great clinical potential for use in the prevention of diseases caused by quorum-sensing-proficient bacterial populations.
Blocking of the quorum sensing signal transduction can be achieved by an antagonist molecule capable of competing or interfering with the native AHL signal for binding to the LuxR-type receptor [180,192]. Competitive inhibitors share structural similarities with the native AHL signal; in that way they bind and occupy the AHL binding site and block the LuxR-type receptor [180,189,207]. The non-competitive inhibitors, on the other hand, show no or little structural similarities to AHL signals, as these molecules bind to different sites on the receptor protein. In this manner, it would be more effective to locate competitive inhibitors. AHLs have acyl side chains, whose length and flexibility are also crucial in the effective binding of the AHL to the LuxR-type receptor [188,208]. Receptors also have ion ends that are respective to their partners when coupling.
Several plants secrete bioactive compounds that mimic bacterial AHL signal activities and affect quorum-sensing-regulated behaviors in associated pathogens [209,210]. Quercetin, among other phenols, was implicated to bind the LuxR-type receptor proteins (LasR, RhIR, and CviR) and inhibit quorum-sensing-regulated bioluminescence in V. harveyi [211]. The Australian red macroalga D. pulchra, Hoslundia opposita, and Lippia javanica produce ranges of furanone compounds that display antifouling and antimicrobial properties [212,213]. Furanones were implicated in the displacement of radiolabeled AHL molecules from LuxR, suggesting competitive inhibition by binding on the LuxR receptor site [180,207]. Moreover, these furanones have been linked to the inhibition of AHL-controlled virulence factor production and pathogenesis in P aeruginosa. The furanones are also linked to the inhibition of the quorum-sensing-controlled luminescence and virulence of the black tiger prawn pathogen Vibrio harveyi, as well as the inhibition of the quorum-sensing-controlled virulence of E. carotovora [189,192,207,214]. Anti-quorum-sensing activity against S. aureus was also linked to L. javanica-derived limonene, carvone, geranial, and neral essential oils [189,192,207,209,214]. Other flavonoids such as apigenin, kaempferol, naringenin, and quercetin were also proven to possess anti-quorum-sensing capacities by inhibiting enteroaggregative biofilm formation in a concentration-dependent manner [215,216].
Synergy with Other Antimicrobial Agents
The synergistic behavior of traditional antibiotics with compounds obtained from plants offers many advantages, such as increased efficiency, decreased undesirable effects, increased stability or bioavailability of free agents, and the ability to obtain suitable therapeutic effects at lower doses [217]. Many studies have demonstrated that the synergism between different antibacterial agents can yield better effects than merely one. This can include the combined effects of plant-derived compounds alone or plant-derived compounds with antibiotics. Mixing plant extracts with different commercial antibiotics enhanced and synergized their antibacterial effects [218]. Additionally, the synergic effects between different single compounds could trigger the antimicrobial effectiveness of the plant-derived compounds and may reduce the resistance of many pathogenic microorganisms.
In one study, anti-bacterial activity against Listeria monocytogenes biofilm was more effective where the synergism between a-pinene, limonene, and linalool was at play than when each single component was used [219]. Carvacrol (Table 1), γ-terpinene, and ρ-cymene can be effective and have synergistic effects when combined; this is due to the action of ρ-cymene, which works as a mediator for the transportation of carvacrol and γ-terpinene across the cell walls and cytoplasmic membranes of pathogenic microorganisms [220]. In another study, plant extracts from Mezoneuron benthamianum, Securinega virosa, and Microglossa pyrifolia increased the susceptibility of major drug-resistant bacteria such as S. aureus and P. aeruginosa to antibiotic norfloxacin [221]. Similarly, geraniol, extracted from Helichrysum italicum, was found to restore the efficacy of quinolones, chloramphenicol, and β-lactams against multi-drug-resistant pathogens, including Acinetobacter baumannii [222]. The flavonoid chalcone can also be used as a therapeutic agent against infections of methicillin-resistant S. aureus strains [223]. A bioactive macrocyclic spermine alkaloid from Albizia schimperiana was linked to the inhibitory activity against methicillin-resistant S. aureus and E. coli [224].
Essential oils from different plants demonstrated activities against the emerging multi-drug-resistant bacteria in human skin; this could be used to combat the problem of untreatable skin diseases. In a study by Haroun and Al-Kayali [225], T. spicata extracts showed the ability to increase the susceptibility of multi-drug-resistant strains of S. aureus and K. pneumoniae to various antibiotics. Essential oils and organic extracts of A. afra, A. betulina, and S. frutescens, in combination with ciprofloxacin, also displayed synergistic interactions against E. coli [225]. Arasu et al. evaluated the antibacterial activities of essential oils of Sesamum indicum, Allium sativum, and Acorus calamus; they found out that essential oils alone and in combination with antibiotics had high activity levels against some food spoilage bacteria [226]. Indeed, medicinal plants, unlike pharmacological drugs, commonly have several chemicals working together catalytically and synergically to produce a combined effect that surpasses the total activity of the individual constituents [227]. The combined effect of these substances tends to increase the activity of the main medicinal constituents by speeding up or slowing down their assimilation in the body. In one study, plant extracts from Mezoneuron benthamianum, Securinega virosa, and Microglossa pyrifolia increased the susceptibility of major drug-resistant bacteria such as S. aureus and P. aeruginosa to antibiotic norfloxacin [221]. Similarly, geraniol, extracted from Helichrysum italicum, was found to restore the efficacy of quinolones, chloramphenicol, and β-lactams against multi-drug-resistant pathogens, including Acinetobacter baumannii [222].
4. Conclusions and Future Research
Novel therapeutic options are crucial in combatting AMR and reducing the burden of infectious diseases. Bioactive compounds from indigenous medicinal plants alone or in combination with existing antimicrobials offer promising solutions towards overcoming global AMR threats. As already shown in this review, the African continent is rich in diverse medicinal plant species, and the current research should focus on new discoveries of plant secondary metabolites with antimicrobial properties. There have already been advances in the development of new antimicrobial therapies, which are currently supported by technological advancements in “omics” techniques such as proteomics and metabolomics. Microbial genomics has contributed globally to the understanding of bacterial resistance mechanisms, evolution, and dissemination, allowing better treatment response, prevention, and control strategies. However, Africa is still lagging behind in “omics” research due to socioeconomic factors a lack of access to and sustainable use of genomic sequencing technologies. The economic challenges, limited infrastructure for “omics“ research, and shortage of well-trained staff in the African continent have evidently been the downfall of efforts towards combating AMR and the development of new antimicrobial drugs. Despite these challenges, there is promising research in some parts of Africa, such as Botswana, aimed at addressing AMR problems using secondary metabolites derived from medicinal plants. The preliminary results from some of the indigenous plant extracts studied in Botswana revealed great potential when tested against MDR bacteria. Therefore, extensive research in this area of antimicrobial discovery remains critical towards combating AMR in Africa and globally.
Acknowledgments
We acknowledge the Department of Biological Sciences and Biotechnology and Botswana International University of Science and Technology. Additionally, we would like to thank and acknowledge Kathleen Hefferon for proofreading this manuscript.
Abbreviations
| AMR | Antimicrobial resistance |
| MDR | Multi-drug-resistant |
| VRE | Vancomycin-resistant Enterococci |
| CRAB | Carbapenem-resistant Acinetobacter baumannii |
| CRE | Carbapenem-resistant Enterobacteriales |
| ESBL | Extended-spectrum β-lactamase |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| XDR | Extensively drug-resistant |
| WHO | World Health Organization |
| ECDC | European Centre for Disease Prevention and Control |
| CDC | Centres for Disease Prevention and Control |
| LMICs | Low- and middle-income countries |
| ESKAPEE | E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter species, and E. coli |
| SCCmec | Staphylococcal cassette chromosome mec |
| EGCG | Epigallocatechin gallate |
| PBP2a | Modified penicillin-binding protein |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| AHL | Autoinducers of N-acyl-homoserine lactone |
| QS | Quorum sensing |
| SAM | S-adenosyl methionine |
| ACP | Acyl carrier protein |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11102605/s1. Table S1: Additional informations about species origins, studies, and sources.
Author Contributions
Conceptualization, A.M. and T.O.R.; validation, A.M., T.O.R. and G.R.; formal analysis and investigation, B.N.M. and K.P.P.M.; writing—original draft preparation, T.O.R. and A.M.; writing—review and editing, T.O.R., A.M., B.N.M. and K.P.P.M.; supervision, A.M. and G.R.; project administration, T.O.R. and A.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Littmann J., Buyx A., Cars O. Antibiotic resistance: An ethical challenge. Int. J. Antimicrob. Agents. 2015;46:359–361. doi: 10.1016/j.ijantimicag.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Kongkham B., Prabakaran D., Puttaswamy H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia. 2020;147:104762. doi: 10.1016/j.fitote.2020.104762. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim N., Kebede A. In vitro antibacterial activities of methanol and aqueous leave extracts of selected medicinal plants against human pathogenic bacteria. Saudi J. Biol. Sci. 2020;27:2261–2268. doi: 10.1016/j.sjbs.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demgne O.M., Tchinda C.F., Mbaveng A.T., Beng V.P., Kuete V. Antibacterial and antibiotic-potentiating activities of nine Cameroonian medicinal plants against multidrug-resistant bacteria expressing active efflux pumps. Investig. Med. Chem. Pharmacol. 2022;5:58. doi: 10.31183/imcp.2022.00058. [DOI] [Google Scholar]
- 5.Jubair N., Rajagopal M., Chinnappan S., Abdullah N.B., Fatima A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR) Evid.-Based Complement. Altern. Med. 2021;2021:3663315. doi: 10.1155/2021/3663315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Duin D., Paterson D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia P., Sharma A., George A.J., Anvitha D., Kumar P., Dwivedi V.P., Chandra N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon. 2021;7:e06310. doi: 10.1016/j.heliyon.2021.e06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake K.S., Choi J., Dantas G. Approaches for characterizing and tracking hospital-associated multidrug-resistant bacteria. Cell Mol. Life Sci. 2021;78:2585–2606. doi: 10.1007/s00018-020-03717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kebede T., Gadisa E., Tufa A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE. 2021;16:e0249253. doi: 10.1371/journal.pone.0249253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskin I., Ribnicky D.M., Komarnytsky S., Ilic N., Poulev A., Borisjuk N., Brinker A., Moreno D.A., Ripoll C., Yakoby N., et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/S0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 11.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013;10:210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soulaimani B., El Hidar N., El Fakir S.B., Mezrioui N., Hassani L., Abbad A. Combined antibacterial activity of essential oils extracted from Lavandula maroccana (Murb.), Thymus pallidus Batt. and Rosmarinus officinalis L. against antibiotic-resistant Gram-negative bacteria. Eur. J. Integr. Med. 2021;43:101312. doi: 10.1016/j.eujim.2021.101312. [DOI] [Google Scholar]
- 13.Song M., Liu Y., Li T., Liu X., Hao Z., Ding S., Panichayupakaranant P., Zhu K., Shen J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021;8:2100749. doi: 10.1002/advs.202100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. EHP. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rout S., Choudary K., Kar D., Das L., Jain A. Plants in traditional medicinal system-future source of new drugs. [(accessed on 14 July 2023)];Int. J. Pharm.Sci. 2009 1:1–23. Available online: https://www.researchgate.net/publication/26626847_Plants_in_traditional_medicinal_system-Future_source_on_new_drugs. [Google Scholar]
- 16.Terreni M., Taccani M., Pregnolato M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules. 2021;26:2671. doi: 10.3390/molecules26092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballot D.E., Bandini R., Nana T., Bosman N., Thomas T., Davies V.A., Cooper P.A., Mer M., Lipman J. A review of-multidrug-resistant Enterobacteriaceae in a neonatal unit in Johannesburg, South Africa. BMC Pediatr. 2019;19:320. doi: 10.1186/s12887-019-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adesanya O.A., Igwe H.A. Carbapenem-resistant Enterobacteriaceae (CRE) and Gram-negative bacterial infections in south-west Nigeria: A retrospective epidemiological surveillance study. AIMS Pub. Health. 2020;7:804. doi: 10.3934/publichealth.2020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orababa O.Q., Arowolo M.T., Olaitan M.O., Osibeluwo B.V., Essiet U.U., Batholomew O.H., Ogunrinde O.G., Lagoke O.A., Soriwe J.D., Ishola O.D., et al. Prevalence Of carbapenem resistance in Acinetobacter baumanii and Pseudomonas aeruginosa in sub-Saharan Africa: A systematic review and meta-analysis. medRxiv. 2022;11:22282516. doi: 10.1101/2022.11.29.22282516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolba S., El Shatoury E.H., Abo AlNasr N.M. Prevalence of carbapenem resistant acinetobacter baumannii (CRAB) in some Egyptian hospitals: Evaluation of the use of blaOXA-51-like gene as species specific marker for CRAB. Egypt. J. Bot. 2019;59:723–733. doi: 10.21608/ejbo.2019.10095.1297. [DOI] [Google Scholar]
- 21.Hosu M.C., Vasaikar S.D., Okuthe G.E., Apalata T. Detection of extended spectrum beta-lactamase genes in Pseudomonas aeruginosa isolated from patients in rural Eastern Cape Province, South Africa. Sci. Rep. 2021;11:7110. doi: 10.1038/s41598-021-86570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindu M., Derseh L., Gelaw B., Moges F. Carbapenemase-producing non-glucose-fermenting Gram-negative Bacilli in Africa, Pseudomonas aeruginosa and Acinetobacter baumannii: A systematic review and meta-analysis. Int. J. Microbiol. 2020;2020:9461901. doi: 10.1155/2020/9461901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Orphaly M., Hadi H.A., Eltayeb F.K., Al-Hail H., Samuel B.G., Sultan A.A., Skariah S. Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. Msphere. 2021;6:e00202–e00221. doi: 10.1128/mSphere.00202-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrivastava S.R., Shrivastava P.S., Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018;32:76–77. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 25.Melese A., Genet C., Andualem T. Prevalence of Vancomycin resistant enterococci (VRE) in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2020;20:124. doi: 10.1186/s12879-020-4833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orababa O.Q., Soriwei J.D., Akinsuyi S.O., Essiet U.U., Solesi O.M. A systematic review and meta-analysis on the prevalence of vancomycin-resistant enterococci (VRE) among Nigerians. Porto. Biomed. J. 2021;6:e125. doi: 10.1097/j.pbj.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mkhize S., Amoako D.G., Shobo C.O., Zishiri O.T., Bester L.A. Genotypic and Phenotypic Characterizations of Methicillin-Resistant Staphylococcus aureus (MRSA) on Frequently Touched Sites from Public Hospitals in South Africa. Int. J. Microbiol. 2021;2021:6011045. doi: 10.1155/2021/6011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omoshaba E., Ojo O., Oyekunle M., Sonibare A., Adebayo A. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from raw milk and nasal swabs of small ruminants in Abeokuta, Nigeria. Trop. Anim. Health. Prod. 2020;52:2599–2608. doi: 10.1007/s11250-020-02301-x. [DOI] [PubMed] [Google Scholar]
- 29.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goda K., Kenzaka T., Hoshijima M., Yachie A., Akita H. Toxic shock syndrome with a cytokine storm caused by Staphylococcus simulans: A case report. BMC Infect. Dis. 2021;21:19. doi: 10.1186/s12879-020-05731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor T.A., Unakal C.G. Staphylococcus aureus Infection. StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 14 July 2023)]. StatPearls [Internet] Available online: https://www.ncbi.nlm.nih.gov/books/NBK441868/ [PubMed] [Google Scholar]
- 32.Adeiza S.S., Onaolapo J.A., Olayinka B.O. Prevalence, risk-factors, and antimicrobial susceptibility profile of methicillin-resistant Staphylococcus aureus (MRSA) obtained from nares of patients and staff of Sokoto state-owned hospitals in Nigeria. GMS Hyg. Infect. Control. 2020;15:Doc25. doi: 10.2139/ssrn.3704591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parmanik A., Das S., Kar B., Bose A., Dwivedi G.R., Pandey M.M. Current treatment strategies against multidrug-resistant bacteria: A review. Curr. Microbiol. 2022;79:388. doi: 10.1007/s00284-022-03061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jernigan J.A., Hatfield K.M., Wolford H., Nelson R.E., Olubajo B., Reddy S.C., McCarthy N., Paul P., McDonald L.C., Kallen A., et al. Multidrug-resistant bacterial infections in US hospitalized patients, 2012–2017. N. Engl. J. Med. 2020;382:1309–1319. doi: 10.1056/NEJMoa1914433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bora K.S., Sharma A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 36.Bromley A., Cock I. Antibacterial Activity of Harpagophytum procumbens (Burch.) DC. ex Meisn. Root Extracts against Gastrointestinal Pathogens and Bacterial Triggers of Autoimmune Diseases. Pharmacogn. Commn. 2022;12:14–22. doi: 10.5530/pc.2022.1.4. [DOI] [Google Scholar]
- 37.Endris A., Asfaw N., Bisrat D. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Lippia javanica leaves from Ethiopia. J. Essenti. Oil. Res. 2016;28:221–226. doi: 10.1080/10412905.2015.1108880. [DOI] [Google Scholar]
- 38.Hikaambo C.N., Chisanga T., Kampamba M., Akapelwa T.M., Chimombe T., Chulu M., Nanyangwe N., Kabuka R., Mudenda S. Antibacterial Activity of Cassia abbreviata Oliv Bark Extract against Escherichia coli and Staphylococcus aureus. J. Pharma. Res. Sci. Technol. 2022;6:76–83. doi: 10.31531/jprst.1000161. [DOI] [Google Scholar]
- 39.Kambiz L., Afolayan A. Extracts from Aloe ferox and Withania somnifera inhibit Candida albicans and Neisseria gonorrhoea. Afr. J. Biotechnol. 2008;7:12–15. [Google Scholar]
- 40.Siddiqui N.A., Al-Yousef H.M., Alhowiriny T.A., Alam P., Hassan W., Amina M., Hussain A., Abdelaziz S., Abdallah R.H. Concurrent analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi. Pharm. J. 2018;26:266–273. doi: 10.1016/j.jsps.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mongalo N., Mafoko B. Cassia abbreviata Oliv. A review of its ethnomedicinal uses, toxicology, phytochemistry, possible propagation techniques and Pharmacology. Afr. J. Pharm. Pharmacol. 2013;7:2901–2906. doi: 10.5897/AJPP12.1017. [DOI] [Google Scholar]
- 42.Danley K. Letters of the bush: A case study of traditional Setswana herbal medicine. [(accessed on 14 July 2023)];ISP Collect. 2006 270:1–30. Available online: https://digitalcollections.sit.edu/isp_collection/270. [Google Scholar]
- 43.du Toit A., van der Kooy F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnoph. 2019;244:112127. doi: 10.1016/j.jep.2019.112127. [DOI] [PubMed] [Google Scholar]
- 44.Kane N., Kyama M., Nganga J., Hassanali A., Diallo M., Kimani F. Comparison of phytochemical profiles and antimalarial activities of Artemisia afra plant collected from five countries in Africa. Afr. J. Bot. 2019;125:126–133. doi: 10.1016/j.sajb.2019.07.001. [DOI] [Google Scholar]
- 45.Liu C., Huan H., Zhou Q., Liu B., Wang Y., Li P., Liao K., Su W. Antibacterial and antibiotic synergistic activities of the extract from Pithecellobium clypearia against clinically important multidrug-resistant gram-negative bacteria. Eur. J. Integr. Med. 2019;32:100999. doi: 10.1016/j.eujim.2019.100999. [DOI] [Google Scholar]
- 46.Asowata-Ayodele A.M., Otunola G.A., Afolayan A.J. Assessment of the polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of acetone and aqueous extracts of Lippia javanica (Burm. F.) spreng. Pharmacogn. Mag. 2016;12:S353. doi: 10.4103/0973-1296.185770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motlhanka D.M., Makhabu S.W. Medicinal and edible wild fruit plants of Botswana as emerging new crop opportunities. J. Med. Plants Res. 2011;5:1836–1842. [Google Scholar]
- 48.Mncwangi N., Chen W., Vermaak I., Viljoen A.M., Gericke N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharnacol. 2012;143:755–771. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Chen W., Van Wyk B.E., Vermaak I., Viljoen A.M. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 2012;5:1–12. doi: 10.1016/j.phytol.2011.09.001. [DOI] [Google Scholar]
- 50.Nalimu F., Oloro J., Kahwa I., Ogwang P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Futur. J. Pharm. Sci. 2021;7:145. doi: 10.1186/s43094-021-00296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maroyi A. Boscia albitrunca: Review of its botany, medicinal uses, phytochemistry, and biological activities. Asian. Pac. J. Trop. Med. 2019;12:51–56. doi: 10.22159/ajpcr.2019.v12i10.35337. [DOI] [Google Scholar]
- 52.Madzibane J., Potgieter M. Uses of Colophospermum mopane (Leguminosae: Caesalpinioideae) by the Vhavenda. S. Afr. J. Bot. 1999;65:440–444. doi: 10.1016/S0254-6299(15)31039-5. [DOI] [Google Scholar]
- 53.Bakrim W.B., Nurcahyanti A.D.R., Dmirieh M., Mahdi I., Elgamal A.M., El Raey M.A., Wink M., Sobeth M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxid. Med. Cell Longev. 2022;2022:3486257. doi: 10.1155/2022/3486257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majinda R.R., Motswaledi M.S. Antibiotic activity of selected Botswana medicinal plants. Botsw. Notes Rec. 1998;30:157–162. [Google Scholar]
- 55.Maroyi A. Ximenia caffra Sond.(Ximeniaceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016;184:81–100. doi: 10.1016/j.jep.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 56.Steenkamp V., Fernandes A.C., Jansen van Rensburg C.E. Antibacterial activity of Venda medicinal plants. Fitoterapia. 2007;78:561–564. doi: 10.1016/j.fitote.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Banso A., Adeyemo S. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol. 2007;6:15. doi: 10.5897/AJB2007.000-2262. [DOI] [Google Scholar]
- 58.Neondo J.O., Mbithe C.M., Njenga P.K., Muthuri C.W. Phytochemical characterization, antibacterial screening and toxicity evaluation of Dichrostachys cinerea. Int. J. Med. Plant. Res. 2012;1:32–37. [Google Scholar]
- 59.Gebrehiwot S., Chaithanya K.K. Traditional uses, phytochemistry, and pharmacological properties of Capparis tomentosa Lam.: A review. Drug Invent. 2020;13:1006–10011. [Google Scholar]
- 60.Steenkamp V., Mathivha E., Gouws M., Van Rensburg C. Studies on antibacterial, antioxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J. Ethnopharmacol. 2004;95:353–357. doi: 10.1016/j.jep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed A.S., McGaw L.J., Moodley N., Naidoo V., Eloff J.N. Cytotoxic, antimicrobial, antioxidant, antilipoxygenase activities and phenolic composition of Ozoroa and Searsia species (Anacardiaceae) used in South African traditional medicine for treating diarrhoea. S. Afr. J. Bot. 2014;95:9–18. doi: 10.1016/j.sajb.2014.07.013. [DOI] [Google Scholar]
- 62.Maroyi A. Euclea undulata Thunb.: Review of its botany, ethnomedicinal uses, phytochemistry and biological activities. Asian Pac. J. Trop. Med. 2017;10:1030–1036. doi: 10.1016/j.apjtm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Mbanga J., Ncube M., Magumura A. Antimicrobial activity of Euclea undulata, Euclea divinorum and Diospyros lycioides extracts on multi-drug resistant Streptococcus mutans. J. Med. Plant. Res. 2013;7:2741–2746. doi: 10.5897/JMPR12.1180. [DOI] [Google Scholar]
- 64.Mongalo N., McGaw L., Segapelo T., Finnie J., Van Staden J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia sericea Burch. ex DC. (Combretaceae)—A review. J. Ethnopharmacol. 2016;194:789–802. doi: 10.1016/j.jep.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 65.Akindele A.J., Wani Z.A., Sharma S., Mahajan G., Satti N.K., Adeyemi O.O., Mondhe D.M., Saxena A.K. In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae) Evid.-Based Complement. Altern. Med. 2015;2015:560404. doi: 10.1155/2015/560404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moshi M., Mbwambo Z. Some pharmacological properties of extracts of Terminalia sericea roots. J. Ethnopharmacol. 2005;97:43–47. doi: 10.1016/j.jep.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 67.Tkachenko H., Buyun L., Osadowski Z., Maryniuk M. The Antibacterial Activity of Certain Sansevieria Thunb. species against Escherichia coli. Agrobiodivers. Improv. Nutr. Health Life Qual. 2017:446–453. doi: 10.15414/agrobiodiversity.2017.2585-8246.446-453. [DOI] [Google Scholar]
- 68.Rahul J., Jain M.K., Singh S.P., Kamal R.K., Naz A., Gupta A.K., Mritunjay S.K. Adansonia digitata L.(baobab): A review of traditional information and taxonomic description. Asian Pac. J. Trop. Biomed. 2015;5:79–84. doi: 10.1016/S2221-1691(15)30174-X. [DOI] [Google Scholar]
- 69.Pendota S., Aderogba M., Van Staden J. In vitro antimicrobial activity of extracts and an isolated compound from Boscia albitrunca leaves. S. Afr. J. Bot. 2015;96:91–93. doi: 10.1016/j.sajb.2014.11.005. [DOI] [Google Scholar]
- 70.Motlhanka D., Houghton P., Miljkovic-Brake A., Habtemariam S. A Novel Pentacyclic Triterpene Glycoside from a Resin of Commiphora glandulosa from Botswana. 2010. [(accessed on 14 July 2023)]. Available online: http://moodle.buan.ac.bw:80/handle/123456789/45.
- 71.Maroyi A. Dicoma capensis less: A review of its botany, ethno medicine, phytochemistry and pharmacology. Asian J. Agri. Biol. 2018;6:287–294. [Google Scholar]
- 72.Maroyi A. Phytochemical and ethnopharmacological review of Elephantorrhiza goetzei (Harms) Harms. Asian Pac. J. Trop. Med. 2017;10:107–113. doi: 10.1016/j.apjtm.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Lamola S.M., Dzoyem J.P., Botha F., Van Wyk C. Anti-bacterial, free radical scavenging activity and cytotoxicity of acetone extracts of Grewia flava. Afri. Health Sci. 2017;17:790–796. doi: 10.4314/ahs.v17i3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monyela S. Characterisation of Mmupudu (Mimusops zeyheri) Leaf Rust in Limpopo Province. 2021. [(accessed on 14 July 2023)]. Available online: http://www.secheresse.info/spip.php?article123237.
- 75.Kwape T.E., Majinda R.R., Chaturvedi P. Antioxidant and antidiabetic potential of Myrothamnus flabellifolius found in Botswana. Cogent Biol. 2016;2:1275403. doi: 10.1080/23312025.2016.1275403. [DOI] [Google Scholar]
- 76.Semenya S., Maroyi A., Potgieter M., Erasmus L. Herbal medicines used by Bapedi traditional healers to treat reproductive ailments in the Limpopo Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2013;10:331–339. doi: 10.4314/ajtcam.v10i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naidoo D., Slavětínská L.P., Aremu A.O., Gruz J., Biba O., Doležal K., Van Staden J., Finnie J.F. Metabolite profiling and isolation of biologically active compounds from Scadoxus puniceus, a highly traded South African medicinal plant. Phytother. Res. 2018;32:625–630. doi: 10.1002/ptr.6000. [DOI] [PubMed] [Google Scholar]
- 78.Mongalo N.I., McGaw L., Finnie J., Van Staden J. Securidaca longipedunculata Fresen (Polygalaceae): A review of its ethnomedicinal uses, phytochemistry, pharmacological properties and toxicology. J. Ethnopharmacol. 2015;165:215–226. doi: 10.1016/j.jep.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 79.Raimondo D., Von Staden L.V., Foden W., Victor J.E., Helme N.A., Turner R.C., Kamundi D.A., Manyama P.A. Red List of South African Plants 2009, SANBI. South African National Biodiversity Institute; Pretoria, South Africa: 2009. [Google Scholar]
- 80.Maroyi A. Nutraceutical and ethnopharmacological properties of Vangueria infausta subsp. infausta. Molecules. 2018;23:1089. doi: 10.3390/molecules23051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hübsch Z., Van Zyl R., Cock I., Van Vuuren S. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern African medicinal plants. S. Afr. J. Bot. 2014;93:185–197. doi: 10.1016/j.sajb.2014.04.005. [DOI] [Google Scholar]
- 82.Viljoen A.M., Subramoney S., van Vuuren S.F., Başer K., Demirci B. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J. Ethnopharmacol. 2005;96:271–277. doi: 10.1016/j.jep.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Ding J., Wang L., He C., Zhao J., Si L., Huang H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 2021;273:113960. doi: 10.1016/j.jep.2021.113960. [DOI] [PubMed] [Google Scholar]
- 84.Hussain M.S., Fareed S., Ansari S., Rahman M.A., Ahmad I.Z., Saeed M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012;4:10. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Y., Li X., Zhang C., Lv L., Gao B., Li M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics. 2021;10:318. doi: 10.3390/antibiotics10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019;10:911. doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rana A., Samtiya M., Dhewa T., Mishra V., Aluko R.E. Health benefits of polyphenols: A concise review. J. Food. Biochem. 2022;46:e14264. doi: 10.1111/jfbc.14264. [DOI] [PubMed] [Google Scholar]
- 88.Ali A., Parisi A., Normanno G. Polyphenols as emerging antimicrobial agents. In: Akhtar N., Singh K.S., Prerna Goyal D., editors. Emerging Modalities in Mitigation of Antimicrobial Resistance. Springer; Berlin/Heidelberg, Germany: 2022. pp. 219–259. [DOI] [Google Scholar]
- 89.Álvarez-Martínez F.J., Barrajón-Catalán E., Encinar J.A., Rodríguez-Díaz J.C., Micol V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020;27:2576–2606. doi: 10.2174/0929867325666181008115650. [DOI] [PubMed] [Google Scholar]
- 90.Boncan D.A.T., Tsang S.S., Li C., Lee I.H., Lam H.M., Chan T.F., Hui J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020;21:7382. doi: 10.3390/ijms21197382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdallah I.I., Quax W.J. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sci. 2017;3:81–98. doi: 10.18502/kls.v3i5.981. [DOI] [Google Scholar]
- 92.Gach K., Długosz A., Janecka A. The role of oxidative stress in anticancer activity of sesquiterpene lactones. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:477–486. doi: 10.1007/s00210-015-1096-3. [DOI] [PubMed] [Google Scholar]
- 93.Yang W., Chen X., Li Y., Guo S., Wang Z., Yu X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020;15:1934578X20903555. doi: 10.1177/1934578X20903555. [DOI] [Google Scholar]
- 94.Guimarães A.C., Meireles L.M., Lemos M.F., Guimarães M.C.C., Endringer D.C., Fronza M., Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ngwaneu L.S.M., Mbaveng A.T., Nayim P., Wamba B.E., Youmbi L.M., Bonsou I.N., Ashu F., Kuete V. Antibacterial and antibiotic-potentiation activity of Coffea arabica and six other Cameroonian edible plants against multidrug-resistant phenotypes. Investig. Med. Chem. Pharmacol. 2022;5:2. [Google Scholar]
- 96.Baloyi I.T., Adeosun I.J., Yusuf A.A., Cosa S. Antibacterial, antiquorum sensing, antibiofilm activities and chemical profiling of selected South African medicinal plants against multi-drug resistant bacteria. J. Med. Plants Res. 2022;16:52–65. doi: 10.5897/JMPR2021.7192. [DOI] [Google Scholar]
- 97.Guo Y., Liu Z., Wan Y., Zhang Y., Abdu H.I., Yang M., Pei J., Yue T., Zhang X., Hacimuftuoglu A., et al. Literature analysis on asparagus roots and review of its functional characterizations. Front. Nutr. 2023;9:1024190. doi: 10.3389/fnut.2022.1024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Motshudi M., Olaokun O., Mkolo N. Evaluation of GC × GC-TOF-MS untargeted metabolomics, cytotoxicity and antimicrobial activity of leaf extracts of Artemisia afra (Jacq.) purchased from three local vendors. J. King. Saud. Univ. Sci. 2021;33:101422. doi: 10.1016/j.jksus.2021.101422. [DOI] [Google Scholar]
- 99.Okwu M.U., Olley M., Akpoka A.O., Izevbuwa O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019;5:117. doi: 10.3934/microbiol.2019.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Annaz H., Sane Y., Bitchagno G.T.M., Ben Bakrim W., Drissi B., Mahdi I., El Bouhssini M., Sobeh M. Caper (Capparis spinosa L.): An updated review on its phytochemistry, nutritional value, traditional uses, and therapeutic potential. Front. Pharmacol. 2022;13:878749. doi: 10.3389/fphar.2022.878749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ambadiang M.M., Atontsa B.C., Tankeo S.B., Nayim P., Wamba B.E., Bitchagno G.T.M., Mpetga J.D.S., Penlap V.B., Kuete V. Bark extract of Cassia sieberiana DC.(Caesalpiniaceae) displayed good antibacterial activity against MDR gram-negative phenotypes in the presence of phenylalanine-arginine β-naphthylamide. BMC Complemen. Med. Ther. 2020;20:342. doi: 10.1186/s12906-020-03148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Du K. Ph.D. Thesis. University of the Free State; Bloemfontein, South Africa: 2015. [(accessed on 14 July 2023)]. Hplc-Based Activity Profiling for hERG Channel Inhibitors from Galenia africana and Gnidia polycephala, and Counter-Current Chromatographic Isolation of Antimicrobials from Colophospermum mopane. Available online: https://scholar.ufs.ac.za/handle/11660/2286. [Google Scholar]
- 103.Anokwuru C.P., Chen W., van Vuuren S., Combrinck S., Viljoen A.M. Bioautography-guided HPTLC–MS as a rapid hyphenated technique for the identification of antimicrobial compounds from selected South African Combretaceae species. Phytochem. Anal. 2022;33:1177–1189. doi: 10.1002/pca.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mangisa M., Peter X.K., Khosa M.C., Fouche G., Nthambeleni R., Senabe J., Tarirai C., Tembu V.J. Ethnomedicinal and phytochemical properties of sesquiterpene lactones from Dicoma (Asteraceae) and their anticancer pharmacological activities: A review. Sci. Afr. 2021;13:e00919. doi: 10.1016/j.sciaf.2021.e00919. [DOI] [Google Scholar]
- 105.Chukwuma C.I., Matsabisa M.G., Rautenbach F., Rademan S., Oyedemi S.O., Chaudhary S.K., Javu M. Evaluation of the nutritional composition of Myrothamnus flabellifolius (Welw.) herbal tea and its protective effect against oxidative hepatic cell injury. J. Food Biochem. 2019;43:e13026. doi: 10.1111/jfbc.13026. [DOI] [PubMed] [Google Scholar]
- 106.Gufe C., Mugabe T.N., Makuvara Z., Marumure J., Benard M. In-vitro assessment of the efficacy of herb-herb combinations against multidrug-resistant mastitis-causing bacteria: Staphylococcus aureus and Klebsiella pneumoniae. Cogent. Food Agric. 2023;9:2187250. doi: 10.1080/23311932.2023.2187250. [DOI] [Google Scholar]
- 107.Das R., Barman A., Ray S. Traditional knowledge, phytochemistry, and pharmacological properties of the African genus Scadoxus (Amaryllidaceae) S. Afr. J. Bot. 2022;151:565–577. doi: 10.1016/j.sajb.2022.10.005. [DOI] [Google Scholar]
- 108.Erhabor C., Erhabor J., McGaw L. The potential of South African medicinal plants against microbial biofilm and quorum sensing of foodborne pathogens: A review. S. Afr. J. Bot. 2019;126:214–231. doi: 10.1016/j.sajb.2019.07.024. [DOI] [Google Scholar]
- 109.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alamgir A. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacogn. Springer; Cham, Switzerland: 2017. Pharmacognostical Botany: Classification of medicinal and aromatic plants (maps), botanical taxonomy, morphology, and anatomy of drug plants; pp. 177–293. [DOI] [Google Scholar]
- 111.Katz L., Baltz R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016;43:155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 112.Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 114.Vadhana P., Singh B.R., Bharadwaj M., Singh S.V. Emergence of herbal antimicrobial drug resistance in clinical bacterial isolates. Pharm. Anal. Acta. 2015;6:10. doi: 10.4172/2153-2435.1000434. [DOI] [Google Scholar]
- 115.Chabán M.F., Karagianni C., Joray M.B., Toumpa D., Sola C., Crespo M.I., Palacios S.M., Athanassopoulos C.M., Carpinella M.C. Antibacterial effects of extracts obtained from plants of Argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant Lepechinia meyenii. J. Ethnopharmacol. 2019;239:111930. doi: 10.1016/j.jep.2019.111930. [DOI] [PubMed] [Google Scholar]
- 116.Ganesan K., Xu B. A critical review on polyphenols and health benefits of black soybeans. Nutrients. 2017;9:455. doi: 10.3390/nu9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ganesan K., Xu B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata) Food. Sci. Hum. Wellness. 2018;7:11–33. doi: 10.1016/j.fshw.2017.11.002. [DOI] [Google Scholar]
- 118.Ganesh P.S., Rai V.R. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J. Tradit. Complement. Med. 2017;8:170–177. doi: 10.1016/j.jtcme.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gledhill J.R., Montgomery M.G., Leslie A.G., Walker J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Górniak I., Bartoszewski R., Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019;18:241–272. doi: 10.1007/s11101-018-9591-z. [DOI] [Google Scholar]
- 121.Gregoire S., Singh A., Vorsa N., Koo H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J. Appl. Micribiol. 2007;103:1960–1968. doi: 10.1111/j.1365-2672.2007.03441.x. [DOI] [PubMed] [Google Scholar]
- 122.Mickymaray S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019;8:257. doi: 10.3390/antibiotics8040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mickymaray S., Al Aboody M.S., Rath P.K., Annamalai P., Nooruddin T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian. Pac. J. Trop. Biomed. 2016;6:185–191. doi: 10.1016/j.apjtb.2015.12.005. [DOI] [Google Scholar]
- 124.Cushnie T., Hamilton V., Chapman D., Taylor P., Lamb A. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007;103:1562–1567. doi: 10.1111/j.1365-2672.2007.03393.x. [DOI] [PubMed] [Google Scholar]
- 125.Ollila F., Halling K., Vuorela P., Vuorela H., Slotte J.P. Characterization of flavonoid–biomembrane interactions. Arch. Biochem. Biophys. 2002;399:103–108. doi: 10.1006/abbi.2001.2759. [DOI] [PubMed] [Google Scholar]
- 126.Sanver D., Murray B.S., Sadeghpour A., Rappolt M., Nelson A.L. Experimental modeling of flavonoid–biomembrane interactions. Langmuir. 2016;32:13234–13243. doi: 10.1021/acs.langmuir.6b02219. [DOI] [PubMed] [Google Scholar]
- 127.Stapleton P.D., Shah S., Hamilton-Miller J.M., Hara Y., Nagaoka Y., Kumagai A., Uesato S., Taylor P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents. 2004;24:374–380. doi: 10.1016/j.ijantimicag.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 128.Stepanović S., Antić N., Dakić I., Švabić-Vlahović M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003;158:353–357. doi: 10.1078/0944-5013-00215. [DOI] [PubMed] [Google Scholar]
- 129.Cremin K., Jones B.A., Teahan J., Meloni G.N., Perry D., Zerfass C., Asally M., Soyer O.S., Unwin P.R. Scanning ion conductance microscopy reveals differences in the ionic environments of gram-positive and negative bacteria. Anal. Chem. 2020;92:16024–16032. doi: 10.1021/acs.analchem.0c03653. [DOI] [PubMed] [Google Scholar]
- 130.Scheffers D.J., Pinho M.G. Bacterial cell wall synthesis: New insights from localization studies. Microbiol. Mol. Biol. Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barreteau H., Kovač A., Boniface A., Sova M., Gobec S., Blanot D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:168–207. doi: 10.1111/j.1574-6976.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 132.Neuhaus F.C., Baddiley J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reygaert W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014;5:434. doi: 10.3389/fmicb.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsuchiya H., Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine. 2000;7:161–165. doi: 10.1016/S0944-7113(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 135.Wang M., Firrman J., Liu L., Yam K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed. Res. Int. 2019;2019:7010467. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elmasri W.A., Zhu R., Peng W., Al-Hariri M., Kobeissy F., Tran P., Hamood A.N., Hegazy M.F., Pare P.W., Mechref Y. Multitargeted flavonoid inhibition of the pathogenic bacterium Staphylococcus aureus: A proteomic characterization. J. Proteome. Res. 2017;16:2579–2586. doi: 10.1021/acs.jproteome.7b00137. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y.M., Rock C.O. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J. Biol. Chem. 2004;279:30994–31001. doi: 10.1074/jbc.M403697200. [DOI] [PubMed] [Google Scholar]
- 138.Ulanowska K., Tkaczyk A., Konopa G., Węgrzyn G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006;184:271–278. doi: 10.1007/s00203-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 139.Hill N.S., Kadoya R., Chattoraj D.K., Levin P.A. Cell size and the initiation of DNA replication in bacteria. PLoS Genet. 2012;8:e1002549. doi: 10.1371/journal.pgen.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chinnam N., Dadi P.K., Sabri S.A., Ahmad M., Kabir M.A., Ahmad Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010;46:478–486. doi: 10.1016/j.ijbiomac.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Plaper A., Golob M., Hafner I., Oblak M., Šolmajer T., Jerala R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commu. 2003;306:530–536. doi: 10.1016/S0006-291X(03)01006-4. [DOI] [PubMed] [Google Scholar]
- 142.Wu D., Kong Y., Han C., Chen J., Hu L., Jiang H., Shen X. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents. 2008;32:421–426. doi: 10.1016/j.ijantimicag.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 143.Gorlenko C.L., Kiselev H.Y., Budanova E.V., Zamyatnin A.A., Jr., Ikryannikova L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics. 2020;9:170. doi: 10.3390/antibiotics9040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rocha E.P. The replication-related organization of bacterial genomes. Microbiology. 2004;150:1609–1627. doi: 10.1099/mic.0.26974-0. [DOI] [PubMed] [Google Scholar]
- 145.Shadrick W.R., Ndjomou J., Kolli R., Mukherjee S., Hanson A.M., Frick D.N. Discovering new medicines targeting helicases: Challenges and recent progress. J. Biomo. Screen. 2013;18:761–781. doi: 10.1177/1087057113482586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu H., Ziegelin G., Schröder W., Frank J., Ayora S., Alonso J.C., Lanka E., Saenger W. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 2001;29:5058–5066. doi: 10.1093/nar/29.24.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gupta P.D., Birdi T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017;8:266–275. doi: 10.1016/j.jaim.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vasconcelos N., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018;120:198–203. doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 149.Almuhayawi M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020;27:3079–3086. doi: 10.1016/j.sjbs.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. BioMed Res. Int. 2014;2014:761741. doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khameneh B., Iranshahy M., Soheili V., FazlyBazzaz B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019;8:118. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Takó M., Kerekes E.B., Zambrano C., Kotogán A., Papp T., Krisch J., Vágvölgyi C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants. 2020;9:165. doi: 10.3390/antiox9020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morinaga N., Iwamaru Y., Yahiro K., Tagashira M., Moss J., Noda M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005;280:23303–23309. doi: 10.1074/jbc.M502093200. [DOI] [PubMed] [Google Scholar]
- 154.Saito T., Miyake M., Toba M., Okamatsu H., Shimizu S., Noda M. Inhibition by apple polyphenols of ADP—Ribosyltransferase activity of cholera toxin and toxin—Induced fluid accumulation in mice. Microbiol. Immunol. 2002;46:249–255. doi: 10.1111/j.1348-0421.2002.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 155.Burnett J.C., Schmidt J.J., McGrath C.F., Nguyen T.L., Hermone A.R., Panchal R.G., Vennerstrom J.L., Kodukula K., Zaharevitz D.W., Gussio R., et al. Conformational sampling of the botulinum neurotoxin serotype A light chain: Implications for inhibitor binding. Bioorg. Med. Chem. 2005;13:333–341. doi: 10.1016/j.bmc.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 156.Cherubin P., Garcia M.C., Curtis D., Britt C.B., Craft J.W., Jr., Burress H., Berndt C., Reddy S., Guyette J., Zheng T., et al. Inhibition of cholera toxin and other AB toxins by polyphenolic compounds. PLoS ONE. 2016;11:e0166477. doi: 10.1371/journal.pone.0166477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reddy S., Taylor M., Zhao M., Cherubin P., Geden S., Ray S., Francis D., Teter K. Grape extracts inhibit multiple events in the cell biology of cholera intoxication. PLoS ONE. 2013;8:e73390. doi: 10.1371/journal.pone.0073390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Engels C., KnÖdler M., Zhao Y.-Y., Carle R., Gänzle M.G., Schieber A. Antimicrobial activity of gallotannins isolated from mango (Mangifera indica L.) kernels. J. Agric. Food. Chem. 2009;57:7712–7718. doi: 10.1021/jf901621m. [DOI] [PubMed] [Google Scholar]
- 159.Fang F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 160.Fang F.C. Antimicrobial actions of reactive oxygen species. mBio. 2011;2:e00141-11. doi: 10.1128/mBio.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vatansever F., de Melo W.C., Avci P., Vecchio D., Sadasivam M., Gupta A., Chandran R., Karimi M., Parizotto N.A., Tegos G.P., et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Mocrobiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bouayed J., Bohn T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Christaki E., Bonos E., Giannenas I., Florou-Paneri P. Aromatic plants as a source of bioactive compounds. Agriculture. 2012;2:228–243. doi: 10.3390/agriculture2030228. [DOI] [Google Scholar]
- 164.Mishra J., Joshi A., Rajput R., Singh K., Bansal A., Misra K. Phenolic rich fractions from mycelium and fruiting body of Ganoderma lucidum inhibit bacterial pathogens mediated by generation of reactive oxygen species and protein leakage and modulate hypoxic stress in HEK 293 cell line. Adv. Pharmacol. Sci. 2018;2018:6285615. doi: 10.1155/2018/6285615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Treml J., Šmejkal K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 166.Fathima A., Rao J.R. Selective toxicity of Catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016;100:6395–6402. doi: 10.1007/s00253-016-7492-x. [DOI] [PubMed] [Google Scholar]
- 167.Cushnie T., Taylor P., Nagaoka Y., Uesato S., Hara Y., Lamb A. Investigation of the antibacterial activity of 3-O-octanoyl-(–)-epicatechin. J. Appl. Microbiol. 2008;105:1461–1469. doi: 10.1111/j.1365-2672.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 168.Donlan R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 169.Abebe G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020;2020:1705814. doi: 10.1155/2020/1705814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bowler P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care. 2018;27:273–277. doi: 10.12968/jowc.2018.27.5.273. [DOI] [PubMed] [Google Scholar]
- 171.Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.-F., Alarcon E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Awolola G.V., Koorbanally N.A., Chenia H., Shode F.O., Baijnath H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr. J. Tradit. Complement. Altern. Med. 2014;11:124–131. doi: 10.4314/ajtcam.v11i3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paczkowski J.E., Mukherjee S., McCready A.R., Cong J.-P., Aquino C.J., Kim H., Henke B.R., Smith C.D., Bassler B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 2017;292:4064–4076. doi: 10.1074/jbc.M116.770552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roy R., Tiwari M., Donelli G., Tiwari V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ulrey R.K., Barksdale S.M., Zhou W., van Hoek M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC complement. Altern. Med. 2014;14:499. doi: 10.1186/1472-6882-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Budzynska A., Wieckowska-Szakiel M., Sadowska B., Kalemba D., Rozalska B. Antibiofilm activity of selected plant essential oils and their major components. Pol. J. Microbiol. 2011;60:35–41. doi: 10.33073/pjm-2011-005. [DOI] [PubMed] [Google Scholar]
- 177.Vasconcelos M.A., Arruda F.V.S., De Alencar D.B., Saker-Sampaio S., Albuquerque M.R.J.R., Dos Santos H.S., Bandeira P.N., Pessoa O.D.L., Cavada B.S., Henriques M., et al. Antibacterial and antioxidant activities of derriobtusone A isolated from Lonchocarpus obtusus. Biomed Res. Int. 2014;2014:248656. doi: 10.1155/2014/248656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yamanaka A., Kimizuka R., Kato T., Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 2004;19:150–154. doi: 10.1111/j.0902-0055.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 179.Yoo S., Murata R., Duarte S. Antimicrobial traits of tea-and cranberry-derived polyphenols against Streptococcus mutans. Caries. Res. 2011;45:327–335. doi: 10.1159/000329181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N., Kumar N., Schembri M.A., Song Z., Kristoffersen P., et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brinkhaus B., Lindner M., Schuppan D., Hahn E. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine. 2000;7:427–448. doi: 10.1016/S0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 182.Hamid A.A., Shah Z.M., Muse R., Mohamed S. Characterisation of antioxidative activities of various extracts of Centella asiatica (L) Urban. Food. Chem. 2002;77:465–469. doi: 10.1016/S0308-8146(01)00384-3. [DOI] [Google Scholar]
- 183.Vasavi H., Arun A., Rekha P. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Infect. 2016;49:8–15. doi: 10.1016/j.jmii.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 184.Bacha K., Tariku Y., Gebreyesus F., Zerihun S., Mohammed A., Weiland-Bräuer N., Schmitz R.A., Mulat M. Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents. BMC Microbiol. 2016;16:139. doi: 10.1186/s12866-016-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Kievit T. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2009;11:279–288. doi: 10.1111/j.1462-2920.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 186.Whitehead N.A., Barnard A.M., Slater H., Simpson N.J., Salmond G.P. Quorum-sensing in Gram-negative bacteria. FEMSMicrobiol. Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 187.Feng L., Wu Z., Yu X. Quorum sensing in water and wastewater treatment biofilms. J. Environ. Biol. 2013;34:437. [PubMed] [Google Scholar]
- 188.Nievas F.L., Bogino P.C., Giordano W. Programmed Lab Experiments for Biochemical Investigation of Quorum-Sensing Signal Molecules in Rhizospheric Soil Bacteria. Biochem. Mol. Biol. 2016;44:256–262. doi: 10.1002/bmb.20949. [DOI] [PubMed] [Google Scholar]
- 189.Hentzer M., Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003;112:1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rodrigues A.C., Oliveira B.D., Silva E.R., Sacramento N.T.B., Bertoldi M.C., Pinto U.M. Anti-quorum sensing activity of phenolic extract from Eugenia brasiliensis (Brazilian cherry) Food Sci. Technol. 2016;36:337–343. doi: 10.1590/1678-457X.0089. [DOI] [Google Scholar]
- 191.Shukla V., Bhathena Z. Broad spectrum anti-quorum sensing activity of tannin-rich crude extracts of Indian medicinal plants. Scientifica. 2016;2016:5823013. doi: 10.1155/2016/5823013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hentzer M., Riedel K., Rasmussen T.B., Heydorn A., Andersen J.B., Parsek M.R., Rice S.A., Eberl L., Molin S., Høiby N., et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 193.Chen X., Schauder S., Potier N., Van Dorsselaer A., Pelczer I., Bassler B.L., Hughson F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 194.Kim S.Y., Lee S.E., Kim Y.R., Kim C.M., Ryu P.Y., Choy H.E., Chung S.S., Rhee H.J. Regulation of Vibrio vulnificus virulence by the LuxS quorum—Sensing system. Mol. Microbiol. 2003;48:1647–1664. doi: 10.1046/j.1365-2958.2003.03536.x. [DOI] [PubMed] [Google Scholar]
- 195.Xavier K.B., Bassler B.L. LuxS quorum sensing: More than just a numbers game. Curr. Opin. Microbiol. 2003;6:191–197. doi: 10.1016/S1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 196.Bouyahya A., Dakka N., Et-Touys A., Abrini J., Bakri Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac. J. Trop. Med. 2017;10:729–743. doi: 10.1016/j.apjtm.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 197.Schauder S., Shokat K., Surette M.G., Bassler B.L. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 198.Pearson J.P., Gray K.M., Passador L., Tucker K.D., Eberhard A., Iglewski B.H., Greenberg E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pearson J.P., Passador L., Iglewski B.H., Greenberg E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parsek M.R., Val D.L., Hanzelka B.L., Cronan J.E., Jr., Greenberg E. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Amalaradjou M.A.R., Narayanan A., Baskaran S.A., Venkitanarayanan K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010;184:358–363. doi: 10.1016/j.juro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 202.Khan M.S.A., Zahin M., Hasan S., Husain F.M., Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009;49:354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 203.Mutungwa W., Alluri N., Majumdar M. Anti-quorum sensing activity of some commonly used traditional indian spices. Int. J. Pharm. Pharm. Sci. 2015;7:80–83. [Google Scholar]
- 204.Krishnan T., Yin W., Chan K.-G. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium Aromati cum) bud extract. Sensors. 2012;12:4016–4030. doi: 10.3390/s120404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yates E.A., Philipp B., Buckley C., Atkinson S., Chhabra S.R., Sockett R.E., Goldner M., Cámara M., Smith H., Williams P. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002;70:5635–5646. doi: 10.1128/IAI.70.10.5635-5646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dong Y.-H., Xu J.-L., Li X.-Z., Zhang L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA. 2000;97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Manefield M., de Nys R., Kumar N., Read R., Michael G., Steinberg P., Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 208.Churchill M.E., Sibhatu H.M., Uhlson C.L. Defining the structure and function of acyl-homoserine lactone autoinducers. Methods Mol. Biol. 2011;692:159–171. doi: 10.1007/978-1-60761-971-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Olivero-Verbel J., Barreto-Maya A., Bertel-Sevilla A., Stashenko E.E. Composition, anti-quorum sensing and antimicrobial activity of essential oils from Lippia alba. Braz. J. Microbiol. 2014;45:759–767. doi: 10.1590/S1517-83822014000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teplitski M., Robinson J.B., Bauer W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant. Microbe Interact. 2000;13:637–648. doi: 10.1094/MPMI.2000.13.6.637. [DOI] [PubMed] [Google Scholar]
- 211.Manner S., Fallarero A. Screening of natural product derivatives identifies two structurally related flavonoids as potent quorum sensing inhibitors against gram-negative bacteria. Int. J. Mol. Sci. 2018;19:1346. doi: 10.3390/ijms19051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mujovo S.F., Hussein A.A., Meyer J.M., Fourie B., Muthivhi T., Lall N. Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat. Prod. Res. 2008;22:1047–1054. doi: 10.1080/14786410802250037. [DOI] [PubMed] [Google Scholar]
- 213.Salame R., Cheikh-Ali Z., Bories C., Adiko M., Poupon E., Champy P. Pyrone and unusually furanone-substituted flavones from the leaves of Hoslundia opposita. Planta Med. 2012;78:1777–1779. doi: 10.1055/s-0032-1315256. [DOI] [PubMed] [Google Scholar]
- 214.Manefield M., Harris L., Rice S.A., de Nys R., Kjelleberg S. Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl. Environ. Microbiol. 2000;66:2079–2084. doi: 10.1128/AEM.66.5.2079-2084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.de Souza Barboza T.J., Ferreira A.F., Ignà A.C.d.P.R., Albarello N. Cytotoxic, antibacterial and antibiofilm activities of aqueous extracts of leaves and flavonoids occurring in Kalanchoe pinnata (Lam.) Pers. J. Med. Plants Res. 2016;10:763–770. doi: 10.5897/JMPR2016.6260. [DOI] [Google Scholar]
- 216.Ouyang J., Sun F., Feng W., Sun Y., Qiu X., Xiong L., Liu Y., Chen Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016;120:966–974. doi: 10.1111/jam.13073. [DOI] [PubMed] [Google Scholar]
- 217.Aiyegoro O., Okoh A. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plants Res. 2009;3:1147–1152. doi: 10.5897/JMPR.9001277. [DOI] [Google Scholar]
- 218.Maia N.L., De Barros M., De Oliveira L.L., Cardoso S.A., Dos Santos M.H., Pieri F.A., Ramalho T.C., Da Cunha E.F.F., Moreira M.A.S. Synergism of plant compound with traditional antimicrobials against Streptococcus spp. isolated from bovine mastitis. Front. Microbiol. 2018;9:1203. doi: 10.3389/fmicb.2018.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sandasi M., Leonard C., Viljoen A. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010;50:30–35. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 220.Sousa L.G., Castro J., Cavaleiro C., Salgueiro L., Tomás M., Palmeira-Oliveira R., Martinez-Oliveira J., Cerca N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022;12:4417. doi: 10.1038/s41598-022-08217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dickson R.A., Houghton P., Hylands P.J., Gibbons S. Antimicrobial, resistance-modifying effects, antioxidant and free radical scavenging activities of Mezoneuron benthamianum Baill., Securinega virosa Roxb. &Wlld. and Microglossa pyrifolia Lam. Phytother. Res. 2006;20:41–45. doi: 10.1002/ptr.1799. [DOI] [PubMed] [Google Scholar]
- 222.Lorenzi V., Muselli A., Bernardini A.F., Berti L., Pagès J.-M., Amaral L., Bolla J.-M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009;53:2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alcaraz L., Blanco S., Puig O., Tomas F., Ferretti F. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2005;205:231–240. doi: 10.1006/jtbi.2000.2062. [DOI] [PubMed] [Google Scholar]
- 224.Samoylenko V., Jacob M.R., Khan S.I., Zhao J., Tekwani B.L., Midiwo J.O., Walker L.A., Muhammad I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. [(accessed on 14 July 2023)];Nat. Prod. Commun. 2009 4:791–796. doi: 10.1177/1934578X0900400611. Available online: https://pubmed.ncbi.nlm.nih.gov/19634324/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haroun M.F., Al-Kayali R.S. Synergistic effect of Thymbra spicata L. extracts with antibiotics against multidrug-resistant Staphylococcus aureus and Klebsiella pneumoniae strains. Iran. J. Basic. Med. Sci. 2016;19:1193. [PMC free article] [PubMed] [Google Scholar]
- 226.Arasu M.V., Viayaraghavan P., Ilavenil S., Al-Dhabi N.A., Choi K.C. Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops. Prod. 2019;133:54–62. doi: 10.1016/j.indcrop.2019.03.018. [DOI] [Google Scholar]
- 227.Mahomoodally M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid.-Based. Complement. Alternat. Med. 2013;2013:617459. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.