Abstract
Four peptides, cecropin P1, magainin II, indolicidin, and ranalexin, were evaluated against 202 clinical isolates of gram-positive and gram-negative aerobic bacteria by a microbroth dilution method. The gram-negative isolates were more susceptible to cecropin P1. Ranalexin was the most active compound against the gram-positive strains. The bactericidal activity of each peptide was equivalent to, or 1 dilution above, the MIC. In conclusion, the four peptides exhibited different in vitro activities and rapid time-dependent killing.
In the last few years many cationic peptides have been isolated from a wide range of animal, plant, and bacterial species (1–6, 9–12, 15, 19). These compounds comprise a diverse class of molecules used in host defense by plants, insects, fish, crustaceans, amphibians, birds, mammals, and humans. These peptides, because of their small sizes (11 to 39 amino acids) and antimicrobial potencies, may have therapeutic potential in the treatment of infections in humans. In mammals, including humans, they are the predominant protein species in the neutrophil; they are found on the surfaces of the tongue, trachea, lungs, and upper intestine; and they are thought to be major factors in antibacterial defense on mucosal surfaces (10).
It has been suggested that the modes of action of these compounds on the membranes of bacteria, fungi, protozoa, and artificial lipid bilayers may be similar and that they may involve the formation of ion channel pores that span membranes without requiring a specific target receptor (18). Recent reports demonstrated that the site for the antibacterial action of the peptides is the cytoplasmic membrane (13, 16). Therefore, these compounds must initially be able to cross or disintegrate the outer membranes of gram-negative bacteria. The lethal event which occurs at the cytoplasmic membrane is not fully understood. It has been shown that the peptides cause channel formation in the cytoplasmic membrane, resulting in cell death (9). In this study we investigated the in vitro activities of four membrane-active peptides against gram-positive and gram-negative bacteria that grow aerobically. The peptides evaluated belonged to different groups of molecules: magainin II, a basic peptide; cecropin P1, a peptide with two α-helices joined by regions containing glycine and proline; indolicidin, a tryptophan-rich peptide; and ranalexin, a polymyxin-related peptide (7, 17).
A total of 202 nonduplicate, clinical isolates were tested and consisted of methicillin-resistant (MR) Staphylococcus aureus (15 strains), methicillin-susceptible (MS) S. aureus (15 strains), MR Staphylococcus hominis (5 strains), MS S. hominis (5 strains), MS Staphylococcus epidermidis (5 strains), MR S. epidermidis (5 strains), MS Staphylococcus saprophyticus (5 strains), MR S. saprophyticus (5 strains), MS Staphylococcus haemolyticus (2 strains), Streptococcus pneumoniae (10 strains), Streptococcus sanguis (5 strains), Gemella morbillorum (5 strains), Lactococcus cremoris (2 strains), Lactococcus lactis (2 strains), Enterococcus faecalis (5 strains), Rhodococcus equi (5 strains), Pseudomonas aeruginosa (20 strains), Pseudomonas cepacia (5 strains), Pseudomonas fluorescens (5 strains), Stenotrophomonas maltophilia (5 strains), Klebsiella pneumoniae (5 strains), Enterobacter cloacae (5 strains), Serratia marcescens (5 strains), Salmonella typhi (5 strains), Salmonella paratyphi A (2 strains), Salmonella typhimurium (5 strains), Salmonella choleraesuis (2 strains), Salmonella arizonae (5 strains), Proteus mirabilis (5 strains), Escherichia coli (10 strains), Shigella flexneri (2 strains), Shigella sonnei (2 strains), Yersinia enterocolitica (3 strains), Acinetobacter species (5 strains), and Brucella species (5 strains).
Cecropin P1, magainin II, indolicidin, and ranalexin were obtained from Sigma-Aldrich S.r.l. (Milan, Italy). The peptides were solubilized in phosphate-buffered saline (pH 7.2), yielding 1,000 mg per liter of stock solution. Solutions of drugs were made fresh on the day of assay or stored at −80°C in the dark for short periods. The MIC of each peptide was determined by a microbroth dilution method with Mueller-Hinton broth (Becton Dickinson Italia, Milan, Italy) and an initial inoculum of 5 × 105 CFU/ml, according to the procedures outlined by the National Committee for Clinical Laboratory Standards (14). For streptococci and enterococci the medium was supplemented with 5% lysed horse blood. Polystyrene 96-well plates (Becton Dickinson and Co., Franklin Lakes, N.J.) were incubated for 18 h at 37°C in air, and since several peptides have a tendency to precipitate, plates were shaken throughout the study. The MIC was considered the lowest peptide concentration at which observable growth was inhibited. The MBC was considered the lowest concentration of each peptide that resulted in a more than 99.9% reduction of the initial inoculum. Peptide concentrations required to inhibit 50 and 90% of the strains and those required to kill 50 and 90% of the strains, as well as the ranges of the MICs of each peptide, are listed in Tables 1 and 2.
TABLE 1.
MICs and MBCs of lytic peptides for gram-negative bacteria
| Organism (no. of strains) | Agentc | MIC (μg/ml)a
|
MBC (μg/ml)b
|
||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | ||
| Escherichia coli (10) | CP1 | 0.25–1 | 0.25 | 1 | 0.25–2 | 0.50 | 2 |
| MGII | 0.25–1 | 0.50 | 1 | 0.25–2 | 1 | 2 | |
| IND | 0.50–16 | 2 | 8 | 1–16 | 4 | 16 | |
| RNL | 1–32 | 4 | 16 | 2–32 | 8 | 32 | |
| Klebsiella pneumoniae (5) | CP1 | 0.25–2 | 0.50–4 | ||||
| MGII | 1–4 | 1–8 | |||||
| IND | 2–8 | 2–16 | |||||
| RNL | 4–16 | 4–32 | |||||
| Enterococcus cloacae (5) | CP1 | 1–8 | 2–8 | ||||
| MGII | 1–8 | 2–16 | |||||
| IND | 4–16 | 4–32 | |||||
| RNL | 8–32 | 8–64 | |||||
| Proteus mirabilis (5) | CP1 | 4–32 | 4–32 | ||||
| MGII | 8–32 | 8–64 | |||||
| IND | 16–64 | 16–128 | |||||
| RNL | >128 | >128 | |||||
| Serratia marcescens (5) | CP1 | 2–16 | 4–16 | ||||
| MGII | 2–32 | 4–32 | |||||
| IND | 4–32 | 8–64 | |||||
| RNL | 8–128 | 8–128 | |||||
| Salmonella typhi (5) | CP1 | 0.50–8 | 1–8 | ||||
| MGII | 1–16 | 1–16 | |||||
| IND | 2–32 | 4–32 | |||||
| RNL | 8–128 | 16–128 | |||||
| Salmonella spp. (14) | CP1 | 2–16 | 4 | 16 | 2–32 | 8 | 32 |
| MGII | 2–32 | 8 | 16 | 2–32 | 8 | 32 | |
| IND | 4–64 | 16 | 32 | 8–64 | 32 | 64 | |
| RNL | 8–128 | 32 | 64 | 16–128 | 64 | 128 | |
| Shigella spp. (4) | CP1 | 1–32 | 2–32 | ||||
| MGII | 2–32 | 4–32 | |||||
| IND | 8–64 | 16–64 | |||||
| RNL | 16–>128 | 32–>128 | |||||
| Yersinia enterocolitica (3) | CP1 | 0.50–2 | 1–4 | ||||
| MGII | 1–8 | 2–8 | |||||
| IND | 4–16 | 8–32 | |||||
| RNL | 16–64 | 16–128 | |||||
| Pseudomonas aeruginosa (20) | CP1 | 4–64 | 16 | 64 | 8–128 | 64 | 128 |
| MGII | 8–128 | 32 | 128 | 8–128 | 64 | 128 | |
| IND | 8–128 | 64 | 128 | 16–128 | 128 | 128 | |
| RNL | >128 | >128 | >128 | >128 | >128 | >128 | |
| Pseudomonas spp. (10) | CP1 | 2–64 | 8 | 64 | 4–128 | 16 | 128 |
| MGII | 2–128 | 16 | 128 | 4–128 | 32 | 128 | |
| IND | 8–>128 | 64 | 128 | 16–>128 | 128 | 128 | |
| RNL | >128 | >128 | >128 | >128 | >128 | >128 | |
| Stenotrophomonas maltophilia (5) | CP1 | 8–128 | 16–>128 | ||||
| MGII | 16–>128 | 32–>128 | |||||
| IND | 32–>128 | 32–>128 | |||||
| RNL | >128 | >128 | |||||
| Acinetobacter spp. (5) | CP1 | 0.50–2 | 1–4 | ||||
| MGII | 1–8 | 2–16 | |||||
| IND | 2–16 | 2–32 | |||||
| RNL | 4–64 | 8–64 | |||||
| Brucella spp. (5) | CP1 | 0.25–2 | 0.50–2 | ||||
| MGII | 0.50–4 | 0.50–8 | |||||
| IND | 2–16 | 4–16 | |||||
| RNL | 4–64 | 8–64 | |||||
50% and 90%, MICs at which 50 and 90% of the strains, respectively, are inhibited.
50% and 90%, MBCs at which 50 and 90% of the strains, respectively, are killed.
CP1, cecropin P1; MGII, megainin II; IND, indolicidin; RNL, ranalexin.
TABLE 2.
MICs and MBCs of lytic peptides for gram-positive bacteria
| Organism (no. of strains) | Agentc | MIC (μg/ml)a
|
MBC (μg/ml)b
|
||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | ||
| MS Staphylococcus aureus (15) | CP1 | 32–>128 | 64 | 128 | 32–>128 | 128 | >128 |
| MGII | 16–128 | 32 | 64 | 32–>128 | 64 | >128 | |
| IND | 4–32 | 16 | 16 | 8–64 | 32 | 64 | |
| RNL | 1–32 | 4 | 8 | 2–32 | 8 | 16 | |
| MR Staphylococcus aureus (15) | CP1 | >128 | >128 | >128 | >128 | >128 | >128 |
| MGII | 16–>128 | 64 | 128 | 32–>128 | 128 | >128 | |
| IND | 4–32 | 16 | 32 | 8–64 | 32 | 64 | |
| RNL | 2–32 | 8 | 16 | 4–64 | 16 | 64 | |
| MR CoNSd (17) | CP1 | 8–128 | 32 | 64 | 16–>128 | 64 | 128 |
| MGII | 2–64 | 16 | 32 | 4–128 | 32 | 64 | |
| IND | 0.5–16 | 8 | 16 | 1–32 | 16 | 32 | |
| RNL | 0.0625–8 | 2 | 4 | 0.125–16 | 4 | 8 | |
| MR CoNS (15) | CP1 | >128 | >128 | >128 | >128 | >128 | >128 |
| MGII | 8–>128 | 64 | 128 | 16–>128 | 64 | >128 | |
| IND | 4–128 | 32 | 64 | 8–128 | 64 | 128 | |
| RNL | 1–128 | 8 | 32 | 1–128 | 16 | 64 | |
| Streptococcus pneumoniae (10) | CP1 | 8–128 | 32 | 128 | 8–>128 | 64 | >128 |
| MGII | 4–128 | 32 | 64 | 8–128 | 32 | 128 | |
| IND | 4–64 | 8 | 16 | 4–128 | 16 | 64 | |
| RNL | 1–32 | 2 | 8 | 2–32 | 4 | 16 | |
| Streptococcus sanguis (5) | CP1 | 16–128 | 16–>128 | ||||
| MGII | 16–128 | 16–>128 | |||||
| IND | 4–64 | 8–64 | |||||
| RNL | 1–8 | 2–16 | |||||
| Gemella spp. (5) | CP1 | 8–64 | 8–128 | ||||
| MGII | 8–64 | 8–128 | |||||
| IND | 2–64 | 4–64 | |||||
| RNL | 0.5–8 | 1–8 | |||||
| Lactococcus spp. (4) | CP1 | 16–128 | 32–128 | ||||
| MGII | 8–64 | 8–128 | |||||
| IND | 4–64 | 8–64 | |||||
| RNL | 1–16 | 2–32 | |||||
| Enterococcus faecalis (5) | CP1 | >128 | >128 | ||||
| MGII | >128 | >128 | |||||
| IND | 32–>128 | 64–>128 | |||||
| RNL | 16–128 | 32–128 | |||||
| Rhodococcus equi (5) | CP1 | >128 | >128 | ||||
| MGII | 32–128 | >128 | |||||
| IND | 4–32 | 16–128 | |||||
| RNL | 4–16 | 16–64 | |||||
50% and 90%, MICs at which 50 and 90% of the strains, respectively, are inhibited.
50% and 90%, MBCs at which 50 and 90% of the strains, respectively, are killed.
CP1, cecropin P1; MGII, magainin II; IND, indolicidin; RNL, ranalexin.
CoNS, coagulase-negative staphylococci.
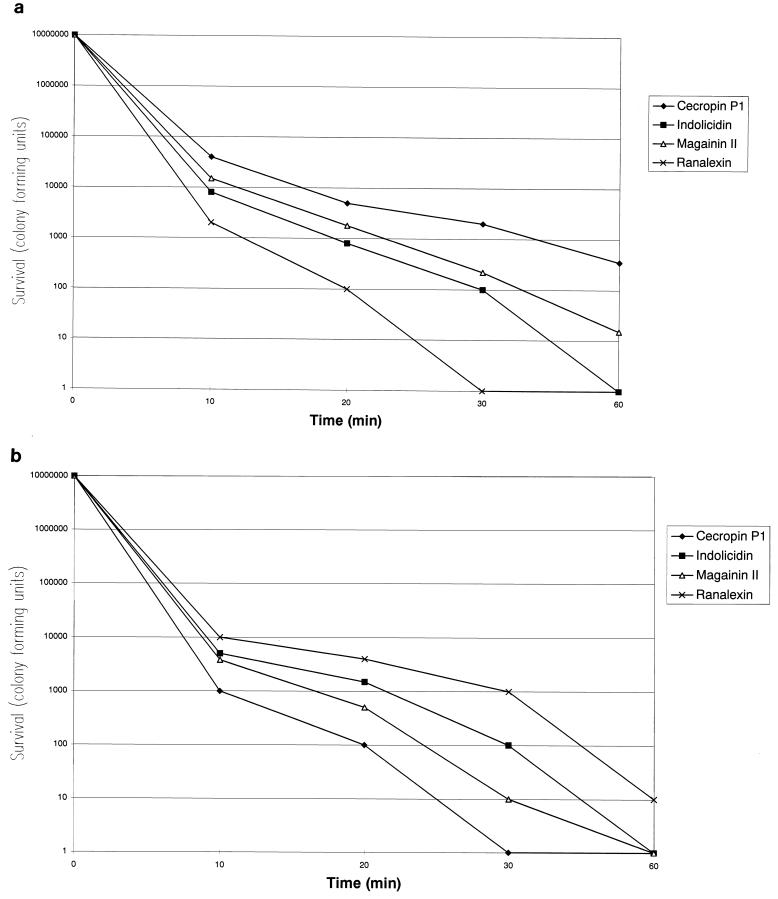
S. aureus ATCC 25923 and E. coli ATCC 25922 were grown at 37°C in Mueller-Hinton broth. Aliquots of exponentially growing bacteria were resuspended in fresh Mueller-Hinton broth at approximately 107 cells/ml and exposed to each peptide (final concentration, 32 μg/ml) for 0, 10, 20, 30, and 60 min at 37°C. After these times, samples were serially diluted and plated onto Mueller-Hinton agar plates to obtain viable colonies. Killing by ranalexin was shown to be the most rapid against S. aureus ATCC 25923 (Fig. 1a): its activity was complete after a 30-min exposure period. Killing by cecropin P1 was shown to be the most rapid against E. coli ATCC 25922 (Fig. 1b): its activity was also complete after a 30-min exposure period.
FIG. 1.
Time-kill kinetics of membrane-active peptides against S. aureus ATCC 25923 (a) and E. coli ATCC 25922 (b). Peptides were tested at a concentration of 32 μg/ml.
Membrane-active peptides are known to have variable antibacterial, antifungal, and antiprotozoan in vitro activities. These compounds provide a host defense system to combat infections. In the present study we evaluated the activities of four structurally different peptides against gram-positive and gram-negative bacteria that grow aerobically. The comparative in vitro activities of cecropin P1, magainin II, indolicidin, and ranalexin against gram-negative aerobic species are shown in Table 1. Overall, the peptides had different ranges of inhibitory values for most species. All the strains were more susceptible to cecropin P1 than to ranalexin. The peptides were effective against all the members of the family Enterobacteriaceae except Proteus mirabilis. They were scarcely effective against the clinical isolates of P. aeruginosa, P. cepacia, P. fluorescens, and Stenotrophomonas maltophilia. On the other hand, with the exception of ranalexin, they showed activity against Acinetobacter species and Brucella species.
Ranalexin was the most active compound against the gram-positive clinical isolates. It inhibited MS S. aureus strains at concentrations of 1 to 32 μg/ml and MR S. aureus strains at concentrations of 2 to 32 μg/ml. It showed similar activities against MS and MR strains of S. epidermidis, S. hominis, S. saprophyticus, and S. haemolyticus. Overall, the activities of cecropin P1, magainin II, and indolicidin against coagulase-negative MS staphylococci were similar to their activities against MS S. aureus strains and lower than those of ranalexin (Table 2). Cecropin P1, magainin II, and indolicidin also inhibited Streptococcus species, Gemella species, and Lactococcus species, but they were less active than ranalexin. The least sensitive of the gram-positive group of bacteria to the four peptides was Enterococcus faecalis; ranalexin and indolicidin at concentrations of 16 to 128 and 32 to 128 μg/ml, respectively, inhibited the five isolates of Enterococcus faecalis which were resistant to cecropin P1 and magainin II at concentrations of ≥128 μg/ml. All peptides showed activities against R. equi similar to those exerted against MR S. aureus.
Our data show that the bactericidal activities of each peptide against both gram-negative and gram-positive bacteria were equivalent to, or 1 dilution above, their MICs, with the exception of those against R. equi: the MBCs for R. equi were fourfold higher than the MICs. There are few data on the concentration- or time-dependent killing kinetics of bacteria by membrane-active peptides; nevertheless, our observations are in agreement with recent reports which showed that killing by peptides was very rapid and resulted in log orders of cell death within minutes of peptide addition (9, 10, 13). In conclusion, the four peptides showed different activities against gram-positive and gram-negative organisms in vitro and exhibited rapid time-dependent killing of the two control strains.
The membrane-active peptides are interesting compounds: their antibacterial activities make these agents potentially valuable as adjuvants in antimicrobial chemotherapy. Furthermore, the emergence of antimicrobial resistance is an increasing problem in human medicine. Although many molecules have been subjected to chemical manipulation to yield novel derivatives with extended activities and increased resistance to enzymatic inactivation, antibiotic-resistant mutants have usually emerged quickly. The membrane-active peptides represent a conserved theme in host antimicrobial defenses throughout nature; they are potentially the first new structural class of antimicrobial agents in 3 decades. This study has shown that this group of peptides possesses a broad spectrum of antibacterial activity; nevertheless, further studies are needed to elucidate their in vivo efficacies and toxicities and their utility in clinical practice.
REFERENCES
- 1.Aley S B, Zimmerman M, Hetsko M, Selsted M E, Gillin F D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreu D, Merrifield R B, Steiner H, Boman H G. N-terminal analogues of cecropin A: synthesis, antibacterial activity and conformational properties. Biochemistry. 1985;24:1683–1688. doi: 10.1021/bi00328a017. [DOI] [PubMed] [Google Scholar]
- 3.Bevins C L, Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- 4.Blondelle S E, Houghton R A. Hemolytic and antimicrobial activities in the twenty-four individual omission analogues of mellitin. Biochemistry. 1991;30:4671–4678. doi: 10.1021/bi00233a006. [DOI] [PubMed] [Google Scholar]
- 5.Boman HG, Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 6.Cannon M. A family of wound healers. Nature. 1987;328:478. doi: 10.1038/328478a0. [DOI] [PubMed] [Google Scholar]
- 7.Clark D P, Durell S, Maloy W L, Zasloff M. Ranalexin: a novel antimicrobial peptide from bullfrog (Rana catasbeiana) skin, structurally related to the bacterial antibiotic polymyxin. J Biol Chem. 1994;269:10849–10855. [PubMed] [Google Scholar]
- 8.DeLucca A J, Blond J M, Jacks T J, Grimm C, Cleveland T E, Walsh T J. Fungicidal activity of cecropin A. Antimicrob Agents Chemother. 1997;41:481–483. doi: 10.1128/aac.41.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falla T J, Hancock R E W. Improved activity of a synthetic indolicidin analog. Antimicrob Agents Chemother. 1997;41:771–775. doi: 10.1128/aac.41.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock R E W. Antibacterial peptides and the outer membranes of gram-negative bacilli. J Med Microbiol. 1997;46:1–3. doi: 10.1099/00222615-46-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee J Y, Boman A, Chuanxin S, Andersson M, Jörnall H, Mutt V, Boman H G. Antimicrobial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci USA. 1989;86:9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrer R I, Barton A, Daher K A, Harwig S S L, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli. J Clin Investig. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore A J, Beazley W D, Bibby M C, Devine D A. Antimicrobial activity of cecropins. J Antimicrob Chemother. 1996;37:1077–1089. doi: 10.1093/jac/37.6.1077. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Approved standard M7-A3. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 15.Sawyer J G, Martin N L, Hancock R E W. The interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1988;56:693–698. doi: 10.1128/iai.56.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaara M, Porro M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob Agents Chemother. 1996;40:1801–1805. doi: 10.1128/aac.40.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viljanen P, Matsunaga H, Kimura Y, Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot. 1991;44:517–523. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- 18.Wade D, Boman A, Wåhlin B, Drain C M, Andreu D, Boman H G, Merrifield R B. All-d amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci USA. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]