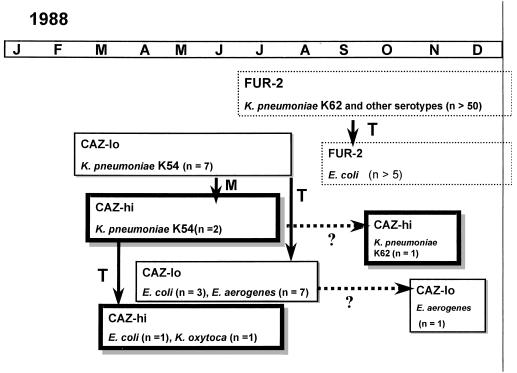
Ceftazidime was introduced into our hospital during the second half of 1987. It was aimed especially for use in the intensive care unit to treat infections with gram-negative bacilli with diminished sensitivity for cefuroxime, at that time the first-line β-lactam antibiotic for serious infections. At that time Escherichia coli and Klebsiella sp. isolates were susceptible to ceftazidime. An increase in cefuroxime-resistant isolates was noted, resulting in an increase in the use of ceftazidime. A few months later ceftazidime-resistant E. coli and Klebsiella pneumoniae were isolated. It could be shown that these isolates harbored different plasmid-mediated extended-spectrum β-lactamases (ESBLs), at that time a rare phenomenon (7). The phenotypic characteristics of the isolates and the β-lactamases were reported (10), and the epidemiology is summarized in Fig. 1. From a patient treated with ceftazidime, and in whom the first K. pneumoniae producing an ESBL, called “Caz-lo” (Isoelectric focusing point [pl], 5.6), was observed, 10 days later a more resistant K. pneumoniae, of the same serotype, with an identical arbitrarily primed PCR profile and with a “Caz-hi” enzyme, was isolated. This was indicative of Caz-hi being an in vivo-selected mutant of Caz-lo. Starting from the Caz-lo K. pneumoniae, in vitro selection with ceftazidime resulted in a strain that produced an ESBL with the Caz-hi phenotype. This reinforced the hypothesis of in vivo selection, initially based on epidemiological data only.
FIG. 1.
Epidemiology of ESBLs. M, mutation of enzyme; T, transfer of plasmid to another serotype or another species; ?, period of apparent disappearance of the enzyme. Months are abbreviated at the top.
The CAZ-lo and CAZ-hi enzymes were detected in other patients, in other K. pneumoniae serotypes, and other species but disappeared after a few months and were replaced by other ESBL enzymes (designated FUR-2) (Fig. 1). Here we report newly obtained data on the Caz-hi ESBL enzyme.
The Caz-lo ESBL gene has been sequenced previously and is characterized by a Glu-to-Lys transition at amino acid position 39 (numbering according to Ambler et al. [1]) and an Arg-to-His transition at position 164 (8), with respect to the sequence of TEM-1. According to the standardized nomenclature of β-lactamases, Caz-lo has been named TEM-11 (6).
The sequencing data reported here reveal that Caz-hi (designated TEM-61) differs from TEM-11 by a single transition from Glu to Lys at amino acid position 240 (2). This transition has been shown to occur in other ESBL enzymes (TEM-5, TEM-10, TEM-24, TEM-27, TEM-28, TEM-42, TEM-46, and TEM-49) (3–5) but was never observed in combination with the mutations which are characteristic for TEM-11. It is interesting that a single mutation from an acidic to a basic amino acid at position 240, which also readily explains the shift in pl from 5.6 to 6.5, causes an increase in ceftazidime resistance from a MIC of 8 mg/liter (TEM-11) to a MIC of 256 mg/liter (TEM-61).
REFERENCES
- 1.Ambler R P, Coulson A F W, Frère J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet G, Brami G, Décrè D, Flippo A, Gaillot O, Lagrange P H, Philippon A. Molecular characterisation by PCR-restriction fragment length polymorphism of TEM β-lactamases. FEMS Lett. 1995;134:203–208. doi: 10.1111/j.1574-6968.1995.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1997;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Gniadkowski M, Schneider I, Jungwirth R, Hryniewicz W, Bauemfeind A. Ceftazidime-resistant Enterobacteriaceae isolated from three Polish hospitals: identification of three novel TEM- and SHV-5 type extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1998;42:514–520. doi: 10.1128/aac.42.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knothe H, Shah P, Kremery V, Antal M, Mitsuhasi S. Transferrable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 8.Mabilat C, Courvalin P. Development of “oligotyping” for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990;34:2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medeiros, A. A. 1997. β-Lactamases: quality and resistance. Clin. Microbiol. Infection 3(Suppl. 4):2–9. [PubMed]
- 10.Vuye A, Verschraegen G, Claeys G. Plasmid-mediated β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli resistant to ceftazidime. Antimicrob Agents Chemother. 1989;33:757–761. doi: 10.1128/aac.33.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]