Abstract
Background
Previous studies demonstrated the efficacy of a rifampicin-based regimen in the treatment of acute staphylococcal periprosthetic joint infections (PJIs) treated with surgical debridement. However, evidence is lacking to support the use of rifampicin in cases where the implant is exchanged during revision.
Methods
We included all consecutive cases of staphylococcal PJIs treated from January 2013 to December 2018 with revision surgery in this international, retrospective, multicenter observational cohort study. PJI was defined according to the European Bone and Joint Infection Society diagnostic criteria. A relapse or reinfection during follow-up, the need for antibiotic suppressive therapy, the need for implant removal, and PJI-related death were defined as clinical failure. Cases without reimplantation or with follow-up <12 months were excluded.
Results
A total of 375 cases were included in the final analysis, including 124 1-stage exchanges (33.1%) and 251 2-stage exchanges (66.9%). Of those, 101 cases failed (26.9%). There was no statistically significant difference in failure of patients receiving rifampicin (22.5%, 42/187) and those not receiving rifampicin (31.4%, 59/188; P = .051). A subanalysis of chronic PJIs treated by 2-stage exchange arthroplasty demonstrated a lower failure rate in cases treated with rifampicin (15%) compared with the no-rifampicin group (35.5%; P = .005). In this subgroup, the use of rifampicin and an antibiotic holiday of >2 weeks were independent predictors of clinical success (odds ratio [OR], 0.36; 95% CI, 0.15–0.88; and OR, 0.19; 95% CI, 0.04–0.90; respectively).
Conclusions
Combination treatment with rifampicin increases treatment success in patients with chronic staphylococcal PJI treated with 2-stage exchange arthroplasty.
Keywords: periprosthetic joint infection, PJI, rifampicin, rifampin, staphylococci
Device-associated infections are a known complication after orthopedic surgeries. The majority of these infections are caused by staphylococci [1], and treatment success largely depends on surgical and antimicrobial treatment strategies [2] due to the formation of biofilm on foreign devices [3, 4]. The additional use of rifampicin is recommended for the treatment of orthopedic device–related infections (ODIs) caused by staphylococci [5]. These recommendations are largely based on findings regarding the efficacy of rifampicin combined with a fluoroquinolone in the treatment of ODI that were demonstrated in a randomized controlled trial by Zimmerli et al. in the late 1990s [6]. Additional observational studies have demonstrated the benefit of rifampicin in the treatment of acute staphylococcal periprosthetic joint infections (PJIs) managed with debridement antibiotics and implant retention (DAIR) [7, 8]. While the DAIR approach is traditionally used in cases with acute infection and a relatively short duration of symptoms, 1- or 2-stage revision surgeries are recommended in cases with chronic infections or as a salvage therapy [9]. An additional benefit of rifampicin is believed to exist in treatment strategies such as DAIR or 1-stage exchange, in which the bacterial biofilm is theoretically not fully removed during surgery [10]. Subsequently, its role has been extrapolated to staphylococcal infections treated with revision surgery in some clinical settings [11].
However, limited evidence is available about the utility of rifampicin in the treatment of staphylococcal PJI when the implant is extracted and exchanged. The objective of this study was to investigate if there is an influence of rifampicin combination treatment on the failure rate of staphylococcal PJIs treated by revision surgery.
METHODS
Study Design
This was a retrospective, multicenter, observational cohort study in which staphylococcal PJIs treated with revision surgery from January 2013 until December 2018 were analyzed. A PJI caused by staphylococci was diagnosed according to the European Bone and Joint Infection Society diagnostic criteria [12]. Hip, knee, and shoulder PJIs undergoing 1-stage or 2-stage revision arthroplasty were included, either performed for chronic or acute infections (with or without a prior DAIR procedure). An acute infection was defined as an early postsurgical infection occurring within the first 3 months after the index arthroplasty or as a late acute, hematogenous infection, defined as a sudden onset of acute symptoms in a prior asymptomatic joint existing for <3 weeks. A chronic infection was defined as an infection occuring >3 months after the index arthroplasty and presenting with longstanding pain or stifness of the joint with or without loosening of the implant. Patients were excluded if reimplantation was not performed or if they had <1 year of follow-up after reimplantation (unless they failed within that time period). Ethical approval was obtained individually by participating centers depending on local requirements.
Outcome
Clinical failure was defined as a relapse or reinfection during follow-up, the need for antibiotic suppressive therapy because of persistent clinical and biochemical signs of infection, the need for implant removal for any cause (infection or noninfection), and PJI-related death.
Microbiological failure was defined as a relapse of infection during follow-up, defined as isolation of the same staphylococcal species that caused the initial infection (ie, with the same antibiogram, with the exception of rifampicin susceptibility).
Statistical Analysis
Continuous variables were presented as mean and SD or as median and interquartile range (IQR) when not normally distributed. A chi-square test was used to analyze the difference between groups for categorical variables, and a Student t test (or Mann-Whitney U test when data were not normally distributed) was used for continuous variables. Logistic regression and Cox regression analyses were performed to identify independent risk factors for treatment failure. Variables with a difference between groups, defined as a P value <.1 in the univariate analysis, were included in the multivariate analysis. Kaplan-Meier curves were performed to evaluate clinical failure in time. Statistical significance was defined as a 2-tailed P value <.05. Statistical analyses were performed using IBM SPSS Statistics (version 24.0; Chicago, IL, USA).
RESULTS
Patient Population
A total of 375 cases from 13 centers were included in the final analysis, including 124 1-stage exchanges (33.1%) and 251 2-stage exchanges (66.9%). The majority of cases were hip (48.8%) and knee (49.1%) replacements. Among the 375 cases, 160 PJIs were caused by S. aureus (42.7%) and 201 by coagulase-negative staphylococci (CoNS; 53.6%), and in 14 cases both were isolated (3.7%). The indication for revision surgery was chronic infection in 240 cases (64.0%) and acute infection in 135 cases (36.0%). Among the acute infections, revision surgery was the initial surgery in 66 cases and after a failed surgical debridement in 69 cases. In the total cohort, rifampicin was prescribed in 187 cases (49.9%). The reasons rifampicin was not prescribed were common practice in 70.4%, rifampicin resistance in 15.9%, intolerance in 9%, interaction with other drugs in 4.2%, and other reasons in 0.5% of cases. The median follow-up of the total cohort (range) was 115 (52–403) weeks.
Outcome
Total Cohort
From the total cohort of 375 cases, 101 cases had clinical failure (26.9%), occurring in 22.5% of the rifampicin group (42/187) vs 31.4% of the nonrifampicin group (59/188; P = .051). Cox regression showed increased failure for patients not receiving rifampicin (Supplementary Figure 1). Table 1 shows the results of the univariate and multivariate analysis for clinical failure. The only independent significant risk factor for clinical failure was renal insufficiency (odds ratio [OR], 2.92; 95% CI, 1.24–6.88). Withholding rifampicin was not a predictor for clinical failure (Supplementary Table 1). Microbiological failure was observed in 5.3% of cases; microbiological failure occurred in 4.8% of the rifampicin group (9/187) vs 5.9% of the no-rifampicin group (11/188; P = .51). In the rifampicin group, 1 of the 9 relapses was due to a rifampicin-resistant strain.
Table 1.
Risk Factors for Clinical Failure; Univariate and Multivariate Analysis
| Nonfailures (n = 264), % | Failures (n = 101), % | P Value | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Male sex | 58.0 | 61.4 | .56 | ||
| Age >80 y | 9.1 | 5.9 | .32 | ||
| BMI >30 kg/m2 | 46.9 | 56.1 | .12 | ||
| Medical history | |||||
| Diabetes | 24.1 | 31.7 | .14 | ||
| Renal failure | 4.7 | 12.9 | .01a | 2.92 (1.24–6.88) | .02 |
| COPD | 10.2 | 12.9 | .47 | .053 | |
| Liver cirrhosis | 2.2 | 5.9 | .07a | 3.21 (0.99–10.47) | |
| Rheumatoid arthritis | 4.4 | 10.9 | .02a | 1.35 (0.46–4.03) | .59 |
| Medication | |||||
| Immune-suppressive drugs | 9.5 | 18.0 | .03a | 1.53 (0.68–3.45) | .30 |
| Characteristics of infected implant | |||||
| Knee | 45.8 | 58.4 | .03a | 1.63 (0.99–2.67) | .053 |
| Revision prosthesis | 20.5 | 37.6 | .001a | 0.95 (0.55–1.66) | .86 |
| Cemented | 61.3 | 59.7 | .82 | ||
| Clinical presentation | |||||
| Sinus tract | 20.4 | 22.0 | .75 | ||
| Intraoperative pus | 47.4 | 59.4 | .04a | 1.35 (0.80–2.28) | .26 |
| Serum CRP >50 mg/L | 27.3 | 24.1 | .57 | ||
| Identified staphylococci | |||||
| Staphylococcus aureus | 40.1 | 49.5 | .10 | ||
| Coagulase-negative staphylococci | 56.9 | 44.6 | .03a | 0.75 (0.45–1.25) | .27 |
| Methicillin resistance | 35.2 | 38.4 | .57 | ||
| Type of infection | |||||
| Chronic infection | 66.4 | 57.4 | .11 | ||
| Surgical treatment | |||||
| One-stage revision surgery | 35.0 | 27.7 | .18 | ||
| Antibiotic treatment | |||||
| Rifampicin used | 52.9 | 41.6 | .051a | 0.68 (0.42–1.11) | .13 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; OR, odds ratio.
aVariables included in the multivariate binary logistic regression analysis.
Acute vs Chronic PJIs
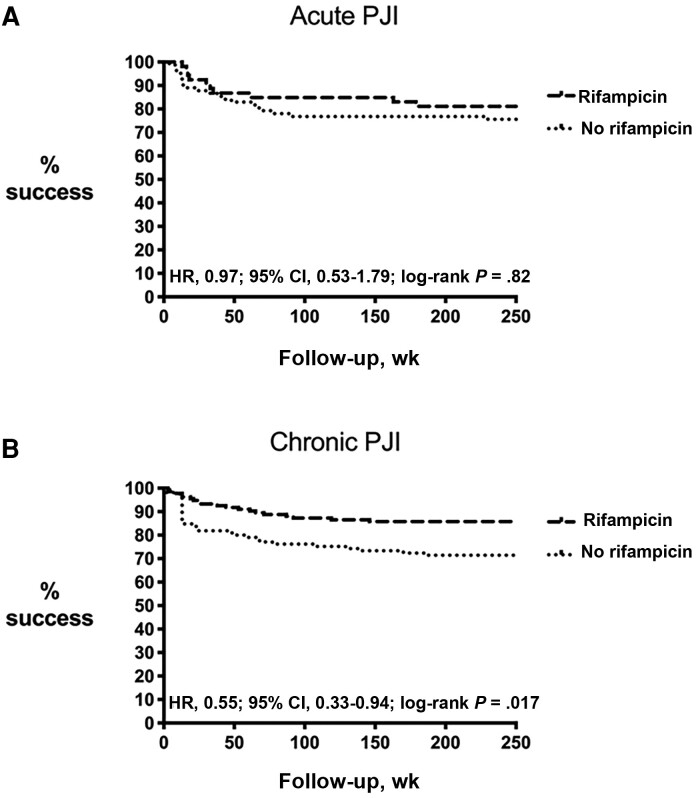
To investigate whether there was a difference between acute and chronic PJIs, we performed a subgroup analysis on both types of infections. There was a clear benefit of rifampicin in chronic cases, but not in acute cases (Figure 1). Clinical failure in chronic infections treated with rifampicin was 18.7% (25/134) vs 31.1% (33/106) in those not treated with rifampicin (P = .025). The clinical benefit of rifampicin in chronic infections was most prominently observed in infections caused by S. aureus, as the failure rate was 24.2% in the rifampicin group (9/37) vs 51.6% (16/31) in the nonrifampicin group (P = .02). This was less prominent in CoNS infections, as the failure rate in the rifampicin group was 12.9% (12/93) vs 22.5% (16/71) in the nonrifampicin group (P = .10). The mean follow-up (SD) was 144 (75) weeks in S. aureus cases vs 133 (75) weeks in CoNS cases (P = .30). Two-stage exchange arthroplasties were performed to the same extent in acute and chronic infections (69.6% vs 65.4%; P = .40).
Figure 1.
Clinical success of patients treated with rifampicin vs no rifampicin in acute PJI (A) and chronic PJI (B). Abbreviation: PJI, periprosthetic joint infection.
One-Stage vs Two-Stage Exchange Arthroplasty
To analyze whether the potential benefit of rifampicin differed between 1- and 2-stage exchanges, we analyzed these surgeries separately. Table 2 shows the patient characteristics. In the 124 cases who were treated with a 1-stage exchange arthroplasty, 76 were treated with rifampicin (61%). Clinical failure was 23.7% in the rifampicin group (18/76) vs 20.8% in the nonrifampicin group (10/48; P = .71). Microbiological failure was 6.6% in the rifampicin group (5/76) vs 4.2% in the nonrifampicin group (2/48; P = .57).
Table 2.
Baseline Characteristics of One-Stage and Two-Stage Revisions According to Treatment With Rifampicin
| One-Stage Revisions (n = 124) | Two-Stage Revisions (n = 251) | |||||
|---|---|---|---|---|---|---|
| Rifampicin (n = 76), % | No Rifampicin (n = 48), % | P Value | Rifampicin (n = 111), % | No Rifampicin (n = 140), % | P Value | |
| Baseline characteristics | ||||||
| Male sex | 53.9 | 58.3 | .63 | 57.7 | 62.3 | .46 |
| Age >80 y | 11.8 | 14.6 | .66 | 4.5 | 7.2 | .37 |
| BMI >30 kg/m2 | 49.3 | 44.7 | .63 | 42.5 | 57.5 | .02 |
| Medical history | ||||||
| Diabetes | 32.9 | 16.7 | .05 | 21.6 | 29.7 | .15 |
| Renal failure | 5.3 | 6.3 | .82 | 5.4 | 9.4 | .24 |
| COPD | 7.9 | 8.3 | .93 | 15.3 | 10.1 | .22 |
| Liver cirrhosis | 1.3 | 0 | .45 | 4.5 | 4.3 | .95 |
| Rheumatoid arthritis | 2.6 | 2.1 | .85 | 7.2 | 8.7 | .67 |
| Medication | ||||||
| Immune-suppressive drugs | 9.2 | 8.3 | .87 | 13.5 | 13.2 | .95 |
| Characteristics of infected implant | ||||||
| Hip | 58.7 | 62.5 | .71 | 37.8 | 47.1 | .37 |
| Revision prosthesis | 19.7 | 44.7 | .003 | 21.6 | 23.9 | .67 |
| Cemented | 46.7 | 59.4 | .16 | 64.4 | 68.1 | .62 |
| Loosened implant | 47.1 | 36.4 | .05 | 48.6 | 33.0 | .02 |
| Clinical presentation | ||||||
| Sinus tract | 7.7 | 25.5 | .009 | 23.7 | 24.0 | .95 |
| Intraoperative pus | 28.0 | 38.3 | .38 | 53.6 | 66.2 | .05 |
| Serum CRP >50 mg/L | 32.7 | 20.1 | .008 | 34.6 | 20.7 | .02 |
| Identified microorganism | ||||||
| Staphylococcus aureus | 34.2 | 31.3 | .73 | 36.0 | 56.5 | .001 |
| Coagulase-negative staphylococci | 64.5 | 66.7 | .80 | 58.6 | 39.1 | .001 |
| Both | 1.3 | 2.1 | .74 | 5.4 | 4.3 | .70 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein.
Of the 251 cases who were treated with a 2-stage exchange procedure, 111 were treated with rifampicin (44%), with a lower percentage of S. aureus cases receiving rifampicin (36% vs 56.5%). Clinical failure was lower in cases treated with rifampicin: 21.6% in the rifampicin group (24/111) vs 35.0% in the nonrifampicin group (49/140; P = .02). Microbiological failure was 3.6% in the rifampicin group (4/111) vs 6.4% in the nonrifampicin group (9/140; P = .32).
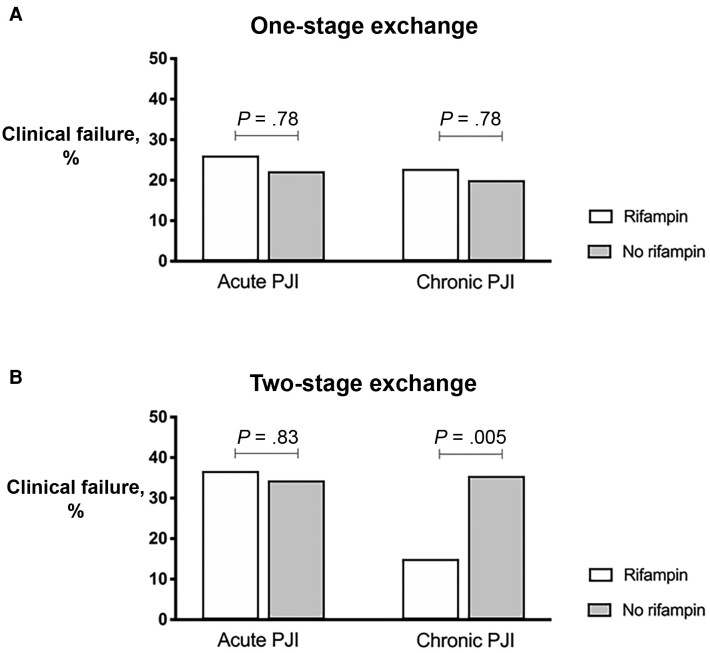
Figure 2 shows the effect of rifampicin on clinical failure in 1- vs 2-stage revision according to the type of infection (acute vs chronic). Interestingly, a statistically significant benefit of rifampicin was only observed in chronic cases treated with 2-stage exchange, but no benefit was observed in 1-stage exchanges. In the majority of chronic cases treated with 2-stage exchange (86.4%), rifampicin was administered after prosthesis extraction (during the spacer period). Table 3 shows risk factors for clinical failure in chronic PJIs treated with 2-stage exchange surgery. The only independent risk factor for clinical failure was diabetes mellitus (OR, 2.98; 95% CI, 1.17–7.56). Use of rifampicin and an antibiotic holiday of >2 weeks were independent predictors of clinical success (OR, 0.36; 95% CI, 0.15–0.88; and OR, 0.19; 95% CI, 0.04–0.90; respectively).
Figure 2.
Clinical failure rifampicin vs no rifampicin in 1-stage (A), vs 2-stage exchanges (B) according to the type of infection (acute or chronic). Abbreviation: PJI, periprosthetic joint infection.
Table 3.
Risk Factors for Clinical Failure in Chronic PJIs Treated With Two-Stage Exchange Surgery (n = 157)
| Nonfailures (n = 117), % |
Failures (n = 40), % |
P Value | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Male sex | 55.6 | 55.0 | .95 | ||
| Age >80 y | 6.0 | 5.0 | .82 | ||
| BMI >30 kg/m2 | 40.7 | 60.0 | .04 | 2.36 (0.99–5.63) | .05 |
| Medical history | |||||
| Diabetes | 19.7 | 37.5 | .02 | 2.98 (1.17–7.56) | .02 |
| Renal failure | 3.4 | 17.5 | .003 | 2.67 (0.55–13.03) | .23 |
| COPD | 13.7 | 20.0 | .34 | ||
| Liver cirrhosis | 3.4 | 7.5 | .28 | ||
| Rheumatoid arthritis | 2.6 | 10.0 | .05 | 3.92 (0.57–27.09) | .17 |
| Medication | |||||
| Immune-suppressive drugs | 8.5 | 15.0 | .24 | ||
| Characteristics of infected implant | |||||
| Knee | 45.3 | 60.0 | .11 | ||
| Revision prosthesis | 18.8 | 32.5 | .07 | 2.34 (0.88–6.21) | .09 |
| Cemented | 60.0 | 73.1 | .52 | ||
| Clinical presentation | |||||
| Sinus tract | 24.3 | 29.7 | .52 | ||
| Intraoperative pus | 50.4 | 50.0 | .96 | ||
| Serum CRP >50 mg/L | 25.5 | 20.0 | .51 | ||
| Identified staphylococci | |||||
| Staphylococcus aureus | 28.2 | 45.0 | .05 | 2.17 (0.89–5.24) | .08 |
| Surgical treatment | |||||
| Antibiotic-loaded cement spacer | 91.5 | 92.5 | .84 | ||
| Antibiotic holiday >2 wk | 77.8 | 57.5 | .01 | 0.19 (0.04–0.90) | .04 |
| Spacer exchange | 13.9 | 12.1 | .88 | ||
| Antibiotic treatment | |||||
| Rifampicin used | 58.1 | 32.5 | .005 | 0.36 (0.15–0.88) | .02 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; OR, odds ratio; PJI, periprosthetic joint infection.
Variables included in the multivariate binary logistic regression analysis.
Statistically significant variables are presented in bold.
DISCUSSION
In this cohort of acute and chronic staphylococcal PJIs that were treated with 1- or 2-stage exchange arthroplasty, adjunctive use of rifampicin showed a modest but statistically nonsignificant effect on the clinical and microbiological outcomes of patients. In multivariable regression analysis, renal insufficiency was the only independent risk factor identified as contributing to clinical failure. While renal insufficiency is a well-described risk factor for the development of PJI, a recent meta-analysis did not identify it to be a risk factor for treatment failure in PJI [13]. However, local and systemic antibiotic treatment can be a cause of acute renal insufficiency [14]. In addition, adapted dosing could lead to insufficient drug levels at the site of infection, promoting treatment failure [15].
A major benefit of rifampicin was observed in chronic PJIs, in particular when the PJI was caused by S. aureus and treated with 2-stage exchange arthroplasty. Our data support its use in this patient group. Benefits for the use of rifampicin in staphylococcal PJIs are described to a varying extent in the literature. Especially in early acute (postsurgical) and late acute (hematogenous) infections that are frequently treated with surgical debridement, the addition of rifampicin appears to reduce the failure rate and improve the outcomes of patients [7, 8]. However, in our cohort of patients, no difference in outcome was observed in patients with an acute infection when treated with revision surgery, indicating that the addition of rifampicin may be unnecessary when the implant is removed. This finding is in accordance with the results of a recent study on S. aureus PJIs treated with implant removal, in which the majority of cases were acute infections [16]. Also in this cohort, patients treated with a rifampicin-based regimen did not demonstrate a higher success rate compared with those in whom rifampicin was withheld. More than half of the patients with an acute infection in our cohort had a prior DAIR that failed. Some studies suggest that these patients have a higher risk of failure when compared with patients initially treated with a total exchange [17].
In contrast to acute infections, we observed a clear benefit from rifampicin in patients with a chronic infection. This benefit was predominantly observed in patients with PJI due to S. aureus, a microorganism known to invade osteoblasts [18, 19]. The added value of rifampicin in this category of patients could be explained by the activity of rifampicin against intracellular staphylococci [20–22]. Indeed, these intracellular bacteria are known to be associated with treatment failure in chronic staphylococcal PJIs [23, 24].
Surprisingly, the association between clinical success and rifampicin was only observed in chronic PJIs treated with 2-stage revision surgery. Senneville et al. observed similar findings in a previous study focusing on PJIs caused by S. aureus [25]. We cannot fully explain this difference observed between 1- vs 2-stage revision surgery. An explanation could lie within potential differences in chosen surgical strategies in regards to the complexity of cases. One could speculate that patients with a more complex and extensive PJI could be more likely to be a treated with a 2-stage revision, thereby shifting comparability. Interestingly, the majority of 2-stage revisions treated with rifampicin were treated after prosthesis extraction, during the spacer period without any implant in situ. An additional multivariable regression analysis in cases with chronic PJI treated with 2-stage exchange revealed that an antibiotic drug holiday of >2 weeks and the use of rifampicin were independently associated with treatment success. Discontinuation of antimicrobial treatment was initially used in 2-stage exchange arthroplasty before reimplantation in order to improve sensitivity of culture diagnostics upon reimplantation and to allow for identification of persistent infection. Recently, continuation of antibiotic treatment was identified as a potential target to improve outcomes [26, 27].
There are limitations to this study, which need to be considered when interpreting its findings. Due to the retrospective nature of this study, inherent limitations with the design, the occurrence of confounding, and selection and information biases cannot be ruled out. In particular, this could apply to the selection of the patients included in this study. Insufficient documentation and follow-up resulted in exclusion of potential cases. This could have artificially increased the failure rate determined in this study, which would have influenced the cohorts’ representativeness, limiting the study’s external validity. Furthermore, we did not investigate the influence of the antibiotic substances used individually or in combination with rifampicin on the outcome. In addition, we cannot rule out bias by indication. Rifampicin might have been used at a higher rate in cases that already showed treatment success in patients who were treated with DAIR previously, while rifampicin might have been withheld in those cases for whom the treating physician considered it likely that a reoperation would be indicated to control infection. However, considering the fact that its main benefit was observed in cases after prosthesis extraction without an implant in situ, this potential bias seems unlikely. Lastly, the total duration of rifampicin was not sufficiently collected by centers, a factor that has been shown to be important in terms of efficacy [28, 29]. The rifampicin dosing used was also not reported.
In conclusion, we were unable to show a benefit of rifampicin combination therapy on the outcome of acute staphylococcal PJI in patients treated with exchange of their prosthesis. However, the addition of rifampicin could potentially increase the clinical success of patients with chronic staphylococcal PJI treated with 2-stage exchange arthroplasty, especially when caused by S. aureus. Future efforts should prospectively validate these findings.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Collaborators. Rares Mircea Birlutiu, Marc Trojanowski, Lansing Sugita, Haijun Xu, Montserrat Sanmarti Vilamala, Laura Morata, Luisa Sorli, Juan Pablo Horcajada, Marie Dorel, Nicolo Rossi, Ashley Barnes, Björn Wandhoff, Vincent Derdour.
Author contributions. Tobias Siegfried Kramer, Alex Soriano, and Marjan Wouthuyzen-Bakker formulated the research question. Tobias Siegfried Kramer and Wouthuyzen-Bakker drafted the manuscript with the input of all of the coauthors. Tobias Siegfried Kramer, Alex Soriano, Sarah Tedeschi, Antonia F. Chen, Pierre Tattevin, Eric Senneville, Joan Gomez-Junyent, Victoria Birlutiu, Sabine Petersdorf, Vicens Diaz de Brito, Ignacio Sancho Gonzalez, Katherine A. Belden, and Marjan Wouthuyzen-Bakker collected and validated data on regional cases. ESGIAI provided the platform for planning and exchange for this study. All authors agreed to the final version of the manuscript and its submission for publication.
Patient consent. The design of the work and the use of pseudonymized patient data have been approved by local ethical committees of the participating centers. A written consent from patients was not required for this retrospective study.
Data availability. Data are available upon reasonable request.
Contributor Information
Tobias Siegfried Kramer, Institute for Hygiene and Environmental Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany; Clinic for Orthopedic Surgery and Traumatology, Evangelisches Waldkrankenhaus Berlin, Berlin, Deutschland; LADR der Laborverbund Dr. Kramer & Kollegen, Geesthacht, Germany.
Alex Soriano, Department of Infectious Diseases, University of Barcelona, IDIBAPS, Hospital Clinic of Barcelona, Barcelona, Spain.
Sarah Tedeschi, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy; Infectious Diseases Unit, IRCCS Azienda Ospedaliero-Universistaria di Bologna, Bologna, Italy.
Antonia F Chen, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Pierre Tattevin, Infectious Diseases and Intensive Care Unit, Pontchaillou University Hospital, Rennes, France.
Eric Senneville, French National Referent Centre for Complex Bone and Joint Infections, CRIOAC Lille-Tourcoing, Lille, France.
Joan Gomez-Junyent, Department of Infectious Diseases, Hospital del Mar, Infectious Pathology and Antimicrobial Research Group (IPAR), Institut Hospital del Mar d'Investigacions Mèdiques (IMIM), Universitat Autònoma de Barcelona (UAB), CEXS-Universitat Pompeu Fabra, Barcelona, Spain.
Victoria Birlutiu, County Clinical Emergency Hospital of Sibiu, Faculty of Medicine, Lucian Blaga University of Sibiu, Romania.
Sabine Petersdorf, Institute for Medical Laboratory Diagnostics, Helios University Clinic Wuppertal, Wuppertal, Germany.
Vicens Diaz de Brito, Department of Infectious Diseases, Parc Sanitari Sant Joan de Deu, Sant Boi (Barcelona), Spain.
Ignacio Sancho Gonzalez, Servicio de Cirugía Ortopédica y Traumatología, Hospital Universitario de Navarra, Pamplona, España.
Katherine A Belden, Division of Infectious Diseases, Sidney Kimmel Medical College at Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA.
Marjan Wouthuyzen-Bakker, Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
References
- 1. Tai DBG, Patel R, Abdel MP, et al. Microbiology of hip and knee periprosthetic joint infections: a database study. Clin Microbiol Infect 2022; 28:255–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Josse J, Valour F, Maali Y, et al. Interaction between staphylococcal biofilm and bone: how does the presence of biofilm promote prosthesis loosening? Front Microbiol 2019; 10:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maduka-Ezeh AN, Greenwood-Quaintance KE, Karau MJ, et al. Antimicrobial susceptibility and biofilm formation of Staphylococcus epidermidis small colony variants associated with prosthetic joint infection. Diagn Microbiol Infect Dis 2012; 74:224–9. [DOI] [PubMed] [Google Scholar]
- 5. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in Vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother 2019; 63:e01746–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351:1645–54. [DOI] [PubMed] [Google Scholar]
- 7. Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56:182–94. [DOI] [PubMed] [Google Scholar]
- 8. Beldman M, Löwik C, Soriano A, et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis 2021; 73:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anemüller R, Belden K, Brause B, et al. Hip and knee section, treatment, antimicrobials: proceedings of international consensus on orthopedic infections. Arthroplasty 2019; 34(2S):S463–75. [DOI] [PubMed] [Google Scholar]
- 10. Zimmerli W, Trampuz A, Ochsner P. Prosthetic joint infections. N Engl J Med 2004; 351:1645–54. [DOI] [PubMed] [Google Scholar]
- 11. Le Marechal M, Cavalli Z, Batailler C, et al. Management of prosthetic joint infections in France: a national audit to identify key situations requiring innovation and homogenization. BMC Infect Dis 2021; 21:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNally M, Sousa R, Wouthuyzen-Bakker M, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021; 103-B:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim CW, Kim HJ, Lee CR, et al. Effect of chronic kidney disease on outcomes of total joint arthroplasty: a meta-analysis. Knee Surg Relat Res 2020; 32:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dagneaux L, Limberg AK, Osmon DR, et al. Renal toxicity associated with resection and spacer insertion for chronic hip PJI. J Arthroplasty 2021; 36:3289–93. [DOI] [PubMed] [Google Scholar]
- 15. Cojutti PG, Rinaldi M, Gatti M, et al. Usefulness of therapeutic drug monitoring in estimating the duration of dalbavancin optimal target attainment in staphylococcal osteoarticular infections: a proof-of-concept. Int J Antimicrob Agents 2021; 58:106445. [DOI] [PubMed] [Google Scholar]
- 16. Gómez-Junyent J, Lora-Tamayo J, Baraia-Etxaburu J, et al. Implant removal in the management of prosthethic joint infection by Staphylococcus aureus: outcome and predictors of failure in a large retrospective multicenter study. Antibiotics (Basel) 2021; 10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajgopal A, Panda I, Rao A, et al. Does prior failed debridement compromise the outcome of subsequent two-stage revision done for periprosthetic joint infection following total knee arthroplasty? J Arthroplasty 2018; 33:2588–94. [DOI] [PubMed] [Google Scholar]
- 18. Valour F, Rasigade J-P, Trouillet-Assant S, et al. Delta-toxin production deficiency in Staphylococcus aureus: a diagnostic marker of bone and joint infection chronicity linked with osteoblast invasion and biofilm formation. Clin Microbiol Infect 2015; 21:568.e1–11. [DOI] [PubMed] [Google Scholar]
- 19. Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol 2015; 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher C, Patel R. Rifampin, rifapentin, and rifabutin are active against intracelular periprosthetic joint infection associated Staphylococcus epidermidis. Antimicrob Agents Chemother 2021; 65:e01275–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marro FC, Abad L, Blocker AJ, et al. In vitro antibiotic activity against intraosteoblastic Staphylococcus aureus: a narrative review of the literature. J Antimicrob Chemother 2021; 76:3091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valour F, Trouillet-Assant S, Rasigade JP, et al. Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One 2013; 8:e67240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masters EA, Ricciardi BF, de Mesy Bently KL, et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol 2022; 20:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang G, Wijenyaka AR, Solomon LB, et al. Novel insights into Staphylococcus aureus deep bone infections: the involvement of osteocytes. mBio 2018; 9:e00415–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 2011; 53:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan TL, Kheir MM, Rondon AJ, et al. Determining the role and duration of the “antibiotic holiday” period in periprosthetic joint infection. J Arthroplasty 2018; 33:2976–80. [DOI] [PubMed] [Google Scholar]
- 27. Ascione T, Balato G, Mariconda M, et al. Continuous antibiotic therapy can reduce recurrence of prosthetic joint infection in patients undergoing 2-stage exchange. J Arthroplasty 2019; 34:704–9. [DOI] [PubMed] [Google Scholar]
- 28. Yang JW, Parvizi J, Hansen EN, et al. 2020 Mark Coventry Award: microorganism-directed oral antibiotics reduce the rate of failure due to further infection after two-stage revision hip or knee arthroplasty for chronic infection: a multicentre randomized controlled trial at a minimum of two years. Bone Joint J 2020; 102-B:3–9. [DOI] [PubMed] [Google Scholar]
- 29. Becker A, Kreitmann L, Triffaut-Fillit C, et al. Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. Bone Jt Infect 2020; 5:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.