Abstract
Simple Summary
The liver is a common site of metastasis across multiple solid organ malignancies. Liver metastases are a known site of treatment resistance, regardless of the site of primary tumour, and their presence is associated with a poor prognosis. This meta-analysis of 4445 patients from 25 randomized controlled trials demonstrated that the addition of vascular endothelial growth factor inhibitors to standard of care improved survival in patients with liver metastases across cancer types. This study highlights the efficacy of vascular endothelial growth factor inhibitors in liver metastases and suggests a treatment approach for clinicians with a focus on sites of metastasis rather than the established primary-specific approach.
Abstract
Background: Liver metastases are associated with poor prognosis across cancers. Novel treatment strategies to treat patients with liver metastases are needed. This meta-analysis aimed to assess the efficacy of vascular endothelial growth factor inhibitors in patients with liver metastases across cancers. Methods: A systematic search of PubMed, Cochrane CENTRAL, and Embase was performed between January 2000 and April 2023. Randomized controlled trials of patients with liver metastases comparing standard of care (systemic therapy or best supportive care) with or without vascular endothelial growth factor inhibitors were included in the study. Outcomes reported included progression-free survival and overall survival. Results: A total of 4445 patients with liver metastases from 25 randomized controlled trials were included in this analysis. The addition of vascular endothelial growth factor inhibitors to standard systemic therapy or best supportive care was associated with superior progression-free survival (HR = 0.49; 95% CI, 0.40–0.61) and overall survival (HR = 0.83; 95% CI, 0.74–0.93) in patients with liver metastases. In a subgroup analysis of patients with versus patients without liver metastases, the benefit with vascular endothelial growth factor inhibitors was more pronounced in the group with liver metastases (HR = 0.44) versus without (HR = 0.57) for progression-free survival, but not for overall survival. Conclusion: The addition of vascular endothelial growth factor inhibitors to standard management improved survival outcomes in patients with liver metastasis across cancers.
Keywords: drug resistance, immunotherapy resistance, liver metastases, meta-analysis, overall survival, progression-free survival, randomized controlled trials, VEGF inhibitor
1. Introduction
The liver is a common site of metastasis, and the presence of liver metastases is a poor prognostic factor in several cancers [1,2]. Furthermore, in melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC), the presence of liver metastases has been associated with poorer response and survival in patients treated with immunotherapy [1,3,4,5].
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is known to be resistant to chemotherapy [6,7]. Nevertheless, in the last decade, targeting angiogenesis with vascular endothelial growth factor (VEGF) inhibitors (VEGFi) has improved clinical outcomes in patients with advanced HCC [8,9,10]. Additionally, immunotherapy as monotherapy for HCC has seen modest responses [11]; however, combination immunotherapy with VEGFi in recent years has demonstrated more robust responses [10]. HCC is characterized by an immunosuppressive, hypoxic, and highly vascularized tumour microenvironment [12,13]. In the presence of oxygen, hypoxia-inducible factor-1α (HIF1α) is degraded; however, in a hypoxic microenvironment (e.g., in the context of an aggressive tumour), HIF1α binds to hypoxia-inducible factor-1β (HIF1β), leading to the transcription of target genes, including VEGF, which plays a key role in angiogenesis [14]. A high level of VEGF in the plasma is a poor prognostic feature in several cancer types, and the blockade of the VEGF–VEGFR signalling pathway has demonstrated the significant improvement of clinical outcomes in some cancers [15] besides HCC [8,9,10], including renal cell carcinoma (RCC) [16,17,18] and colorectal cancer (CRC) [19,20]. Over the years, multiple drugs have been developed to block the VEGF–VEGFR signalling pathway. These encompass different classes of drugs, including monoclonal antibodies against VEGF (e.g., bevacizumab) [21], and tyrosine kinase inhibitors (TKIs), which target multiple pathways, including VEGF–VEGFR (e.g., sunitinib) [22,23], amongst others.
The liver is the most common site of metastasis in CRC, with 25–50% of patients presenting with liver metastases at the time of diagnosis [24], and the addition of bevacizumab to FOLFOX/CAPOX (5-fluorouracil (5-FU) or capecitabine in combination with oxaliplatin) or FOLFIRI/CAPIRI (5-FU or capecitabine in combination with irinotecan) has shown significant improvement in objective response rate (ORR) and survival in these patients [19]. Whether this strategy is also effective in liver metastases in patients with other cancer types is unknown.
In this study, we aimed to assess the efficacy of VEGFi in cancer patients with liver metastases in a meta-analysis including randomized–controlled clinical trials (RCTs) testing the efficacy of VEGFi, regardless of primary cancer site. We also compared VEGFi efficacy in patients with versus without liver metastases.
2. Methods
2.1. Search Strategy and Selection Criteria
Systematic searches of PubMed, Cochrane CENTRAL, and Embase were conducted from 1 January 2000 to 30 April 2023, based on the following criteria: Population, stage IV solid organ malignancy with liver metastasis. Hepatocellular carcinoma was excluded. Intervention, backbone of systemic therapy (chemotherapy and/or immunotherapy and/or non-VEGFi targeted therapy) or best supportive care (BSC) with a VEGFi (tyrosine kinase inhibitors (TKI) (sunitinib, pazopanib, sorafenib, lenvatinib, vandetanib, regorafenib, cabozantinib, axitinib, cediranib, ponatinib, aflibercept, vatalanib, tivozanib, motesanib, linifanib, anlotinib, fruquintinib, nintedanib, apatinib) or monoclonal antibody (bevacizumab, ramucirumab, vanucizumab)) (Supplementary Table S1). TKIs that targeted multiple pathways were included as long as the inhibition of the VEGF–VEGFR pathway was part of the mechanism of action. Comparator, backbone of systemic therapy (chemotherapy and/or immunotherapy and/or non-VEGFi targeted therapy) or best supportive care without VEGFi. Outcome, progression-free survival (PFS) and/or overall survival (OS). Study design, published randomized clinical trial (RCTs) (Supplementary Table S2).
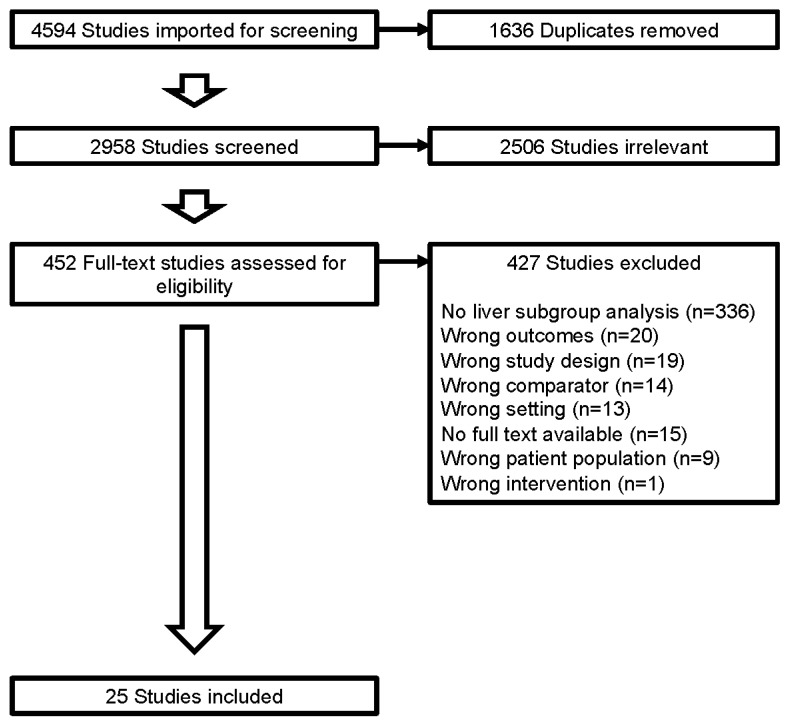
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Figure 1) and was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (registration number; INPLASY 202390034).
Figure 1.
Study selection. Studies selected based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
2.2. RCT Quality Assessment and Mitigation of Bias
Study inclusion criteria were established prior to commencing database searches. Three authors (JWC, JB, and IPdS) conducted independent database searches. Each study underwent individual assessment for eligibility by the respective reviewing authors before cross-referencing any shared final selections. Any studies not unanimously identified then underwent independent evaluation by the remaining authors to determine their eligibility for inclusion. All studies included in the final analysis were deemed to meet eligibility criteria by consensus of all authors.
We utilized the JADAD scale [25] to assess the quality of all RCTs. A score of 3 or higher defined a good-quality RCT and this cut off was required for studies to be included in this analysis (Supplementary Table S3).
2.3. Outcomes
The two primary outcomes of this study were the PFS and OS of the addition of VEGFi to a backbone of systemic therapy (chemotherapy and/or immunotherapy and/or non-VEGFi targeted therapy) or best supportive care, measured in terms of the PFS and/or OS differences compared with no VEGFi. Preplanned subgroups of analysis included: (a) cancer type, “colorectal cancer” and “non-colorectal cancers”; (b) backbone systemic therapy, “chemotherapy” and “non-chemotherapy”; (c) VEGFi type, “bevacizumab” and “non-bevacizumab”; (d) line of treatment, “first line” and “subsequent line”; and (e) liver metastases, “presence” and “absence”. Information extracted included the first author’s name, study name, journal and year of publication, study design, National Clinical Trials (NCT) identification number, study phase, cancer type, number of patients, lines of treatment, study drugs, and hazard ratios (HRs) with 95% CIs for OS and for PFS. In two trials, the HR for PFS was estimated from the figures on the manuscript, and in another trial, the 95% CI for HR for PFS was not provided in the manuscript.
2.4. Statistical Analysis
The selected studies were summarized, including the total number of patients (patients with liver metastases) and the estimated effect (HR for PFS, OS or both). The overall effects of the addition of VEGFi to standard therapy or BSC in patients with liver metastases across different cancer types were estimated by pooling HRs from individual studies using a random effect model with inverse variance. Forest plots of pooled results were generated, along with their 95% confidence intervals (CI). Heterogeneity between studies was assessed using I2, a statistical metric that estimates the percentage of total variation across studies [26]. An I2 > 75% indicates high heterogeneity between studies. The presence of potential publication bias was assessed graphically using a funnel plot.
Subgroup analyses were performed considering four pre-specified factors: cancer type, backbone systemic therapy, VEGFi type, and line of treatment. The categories within each factor are defined in the Outcomes subsection. We performed a separate analysis which included studies with data on liver metastasis versus no liver metastasis.
All statistical analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing).
3. Results
3.1. Systematic Review and Characteristics
Of a total of 4594 studies identified from the literature review, 1636 duplicates were removed. A total of 2958 studies were screened (title and abstract reviewed) and 2506 were considered irrelevant due to the topic, non-randomized controlled trials, or no usable data available. A total of 452 full-text studies were assessed for eligibility, and of these, 427 studies were excluded due to no liver subgroup analysis (n = 336), wrong outcomes (n = 20), wrong study design (n = 19), wrong comparator (n = 14), wrong setting (n = 13), no full text available (n = 15), wrong patient population (n = 9), or wrong intervention (n = 1) (Figure 1).
Twenty-five RCTs were eligible and included in this meta-analysis, involving 4445 patients with liver metastases (Table 1). The 25 RCTs selected included 10 trials performed in patients with CRC [27,28,29,30,31,32,33,34,35,36], 6 trials in patients with NSCLC [37,38,39,40,41,42], 5 trials in patients with RCC or urothelial cancer [18,43,44,45,46], 1 trial in patients with pancreatic cancer [47], 1 trial in patients with gastrointestinal stromal tumour [48], 1 trial in patients with gastric cancer [49] and 1 trial in patients with melanoma [50]. The backbone of systemic therapy in these trials included chemotherapy in 13 trials, targeted therapy in 3 trials, immunotherapy in 2 trials, chemotherapy combined with targeted therapy in 1 trial, and BSC in 6 trials.
Table 1.
Randomized clinical trial characteristics.
| Trial | NCT ID 1 Number Trial Phase | Cancer Type | Backbone Treatment Type | VEGF 2 Inhibitor (Dose) |
1st Line Treatment | Liver Metastases Only | Number of Patients with Liver Metastases | PFS 3 HR 4 (95%CI) |
OS 5 HR 4 (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Escudier et al. JCO 2010 (AVOREN) [43] |
NCT02056587 III |
Renal Cell Carcinoma | Immunotherapy | Bevacizumab (10 mg/kg IV q2weekly) |
Yes | No | 138 | 1.61 (1.09–2.37) |
|
| Rini et al. JCO 2010 (CALGB 90206) [44] |
NCT00072046 III |
Renal Cell Carcinoma | Immunotherapy | Bevacizumab (10 mg/kg IV q2weekly) |
Yes | No | 147 | 0.727 (0.507–1.043) |
|
| Van Cutsem et al. JCO 2009 [47] | III | Pancreatic Cancer | Chemotherapy + Targeted therapy | Bevacizumab (5 mg/kg IV q2weekly) |
Yes | No | 462 | 0.83 (0.68–1.02) |
|
| Mir et al. Lancet Oncology 2016 (PAZOGIST) [48] |
NCT01323400 II |
GIST | Best supportive care | Pazopanib (800 mg PO OD) |
No | No | 34 | 0.29 (0.13–0.67) |
|
| Fuchs et al. Lancet Oncology 2019 (RAINFALL) [49] |
NCT02314117 III |
Gastric orJunctional Adenocarcinoma | Chemotherapy | Ramucirumab (8 mg/kg IV D1,8 q3weekly) |
Yes | No | 236 6 | 0.605 (0.433–0.847) |
0.907 (0.674–1.219) |
| Petrylak et al. JCO 2016 [45] |
NCT01282463 II |
Urothelial Carcinoma | Chemotherapy | Ramucirumab (10 mg/kg IV q3weekly) |
No | No | 28 | 0.59 (0.25–1.41) |
0.88 (0.39–1.96) |
| Petrylak et al. Lancet Oncology 2020 (RANGE) [46] |
NCT02426125 III |
Urothelial Carcinoma | Chemotherapy | Ramucirumab (10 mg/kg IV q3weekly) |
No | No | 147 | 0.885 (0.614–1.276) |
|
| Nakagawa et al. Lancet Oncology 2019 (RELAY) [37] |
NCT02411448 III |
Non-small Cell Lung Cancer | Targeted therapy | Ramucirumab (10 mg/kg IV q2weekly) |
Yes | No | 45 | 0.48 (0.23–1.02) |
|
| Tabernero et al. Clinical Cancer Research 2013 (RESPECT) [27] |
NCT00865709 II |
Colorectal Cancer | Chemotherapy | Sorafenib (400 mg PO BD) |
Yes | No | 160 | 0.86 (0.60–1.24) |
1.06 (0.72–1.56) |
| Escudier et al. NEJM 2007 (TARGET) [18] |
NCT00073307 III |
Renal Cell Carcinoma | Best supportive care | Sorafenib (400 mg PO BD) |
No | No | 233 | 0.44 7 (0.29–0.68) |
|
| Sandler et al. NEJM 2006. (NCT00021060) [38] |
NCT00021060 II/III |
Non-small Cell Lung Cancer | Chemotherapy | Bevacizumab (15 mg/kg IV q3weekly) |
Yes | No | 163 | 0.68 (0.49–0.96) |
|
| Scagliotti et al. JCO 2012 [39] |
NCT00457392 III |
Non-small Cell Lung Cancer | Targeted therapy | Sunitinib (37.5 mg PO OD) |
No | No | 182 | 0.957 (0.689–1.329) |
0.980 (0.711–1.351) |
| Cunningham et al. Lancet Oncology 2013. (AVEX) [28] |
NCT00484939 III |
Colorectal Cancer | Chemotherapy | Bevacizumab (7.5 mg/kg IV q3weekly) |
Yes | Yes | 106 | 0.54 (0.35–0.83) |
|
| Tabernero et al. EJC 2014 (VELOUR) [29] |
NCT00561470 III |
Colorectal Cancer | Chemotherapy | Aflibercept (4 mg/kg IV q2weekly) |
No | Yes | 299 | 0.547 (0.413–0.725) |
0.649 (0.492–0.855) |
| Tang et al. JCO 2020 (BECOME) [30] |
NCT01972490IV | Colorectal Cancer | Chemotherapy | Bevacizumab (5 mg/kg IV q2weekly) |
Yes | Yes | 241 | 0.49 (0.38–0.65) |
0.71 (0.52–0.97) |
| Tebbutt et al. JCO 2010 (MAX) [31] |
ACTRN12605000025639 | Colorectal Cancer | Chemotherapy | Bevacizumab (7.5 mg/kg IV q3weekly) |
Yes | Yes | 61 | 0.25 8 | |
| Li et al. Future Oncology 2018 [32] |
NCT01661270 III |
Colorectal Cancer | Chemotherapy | Aflibercept4 mg/kg IV q2weekly) | No | Yes | 71 | 0.54 (0.3–0.971) |
|
| Tabernero et al. Lancet Oncology 2015 (RAISE) [33] |
NCT01183780 III |
Colorectal Cancer | Chemotherapy | Ramucirumab (8 mg/kg IV q2weekly) |
No | Yes | 187 | 0.801 (0.590–1.089) |
0.963 (0.679–1.367) |
| Chi et al. The Oncologist 2021 (ALTER0703) [34] |
NCT02332499 II/III |
Colorectal Cancer | Chemotherapy | Anlotinib (12 mg PO D1-14 q3weekly) |
No | No | 312 | 0.27 (0.20–0.36) |
0.92 (0.71–1.2) |
| Doebele et al. Cancer 2015 [40] |
NCT01160744 II |
Non-small Cell Lung Cancer | Chemotherapy | Ramucirumab (10 mg/kg IV q3weekly) |
Yes | No | 24 | 0.45 9 (0.25–1.05) |
|
| Li et al. Jama 2018 (FRESCO) [35] |
NCT02314819 III |
Colorectal Cancer | Best supportive care | Fruquintinib (5 mg PO OD D1-21 q3weekly) |
No | No | 287 | 0.22 (0.17–0.30) |
0.59 (0.45–0.77) |
| Van Cutsem et al. Annals of Oncology 2018 (LUME-Colon 1) [36] |
NCT02149108 III |
Colorectal Cancer | Best supportive care | Nintedanib (200 mg PO BD) |
No | No | 543 | 0.53 (0.44–0.64) |
0.95 (0.79–1.14) |
| Zhao et al. Journal of Thoracic Oncology 2021 (CTONG1706) [41] |
NCT02824458 III |
Non-small Cell Lung Cancer | Targeted therapy | Apatinib (500 mg PO OD) |
Yes | No | 40 | 0.42 (0.15–1.17) |
|
| Kim et al. JCO 2012 (BEAM) [50] |
NCT00434252. II |
Melanoma | Chemotherapy | Bevacizumab (15 mg/kg IV q3weekly) |
Yes | No | 96 | 0.73 (0.46–1.16) | 0.60 (0.36–1.00) |
| Shen et al. Journal of Cancer Research and Clinical Oncology 2013 (ALTER 0303) [42] |
NCT02388919 III |
Non-small Cell Lung Cancer | Best supportive care | Anlotinib (12 mg PO OD) |
No | No | 78 | 0.23 (0.12–0.42) | 0.61 (0.36–1.02) |
1 NCT ID number, National Clinical Trials (NCT) identification number; 2 VEGF, vascular endothelial growth factor; 3 PFS, progression-free survival; 4 HR, hazard ratio; 5 OS, overall survival; 6 Fuchs et al., Lancet Oncology 2019 (RAINFALL)—number of patients with liver metastasis reported in OS analysis n = 236; number of patients with liver metastasis reported in PFS n = 189. 7 Escudier et al. NEJM 2007 (TARGET)—HR estimated from Figure 3 of the manuscript. 8 Tebbutt et al. JCO 2010 (MAX)—95% CI for HR for PFS was not provided in the manuscript. 9 Doebele et al. Cancer 2015—HR estimated from Figure 4 of the manuscript.
3.2. Progression-Free Survival Comparison
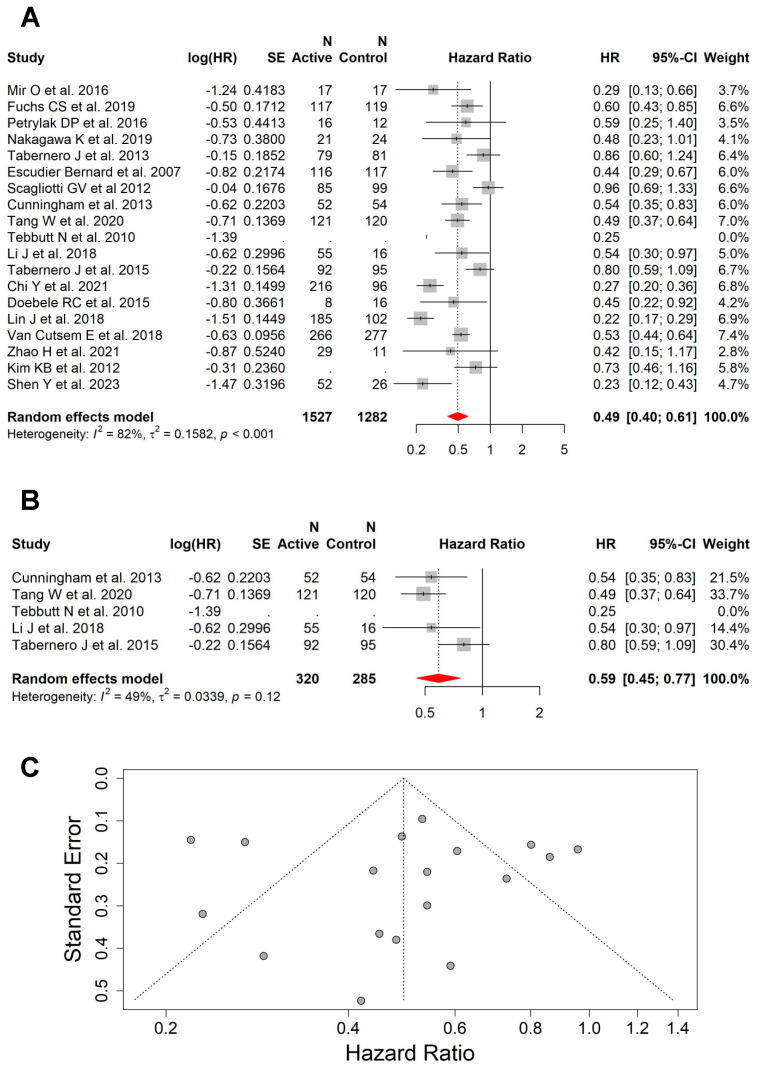
The pooled results from 19 studies with an available HR for PFS showed that the addition of VEGFi to backbone systemic therapy or BSC was associated with superior PFS (HR = 0.49; 95% CI, 0.40–0.61) compared with no VEGFi in this group of patients (Figure 2A). The same effect was seen in the subgroup of studies (n = 5) that included patients with liver metastases only (without other sites of metastases) (HR = 0.59; 95% CI, 0.45–0.77) (Figure 2B). There was high heterogeneity between all studies for PFS (I2 = 82%, p < 0.001) (Figure 2C), but moderate heterogeneity between the studies including patients with liver metastases only (I2 = 49%, p = 0.12).
Figure 2.
The addition of VEGFi to a backbone of systemic therapy or BSC was associated with superior PFS. (A) Forest plot and pooled HRs for PFS comparing the backbone systemic therapy or BSC with versus without VEGFi (HR = 0.49; 95% CI, 0.40–0.61). (B) Forest plot and pooled HRs for PFS comparing the backbone systemic therapy or BSC with versus without VEGFi (HR = 0.59; 95% CI, 0.45–0.77) from studies that included patients with liver as the only site of metastasis. (C) Funnel plot showing high heterogeneity between all studies for PFS (I2 = 82%, p < 0.001) [18,27,28,30,31,32,33,34,35,36,37,39,40,41,42,45,48,49,50].
The benefit of the addition of VEGFi in PFS was seen across all preplanned subgroups, including (a) cancer type, “colorectal cancer” (HR = 0.48; 95% CI, 0.34–0.68; high heterogeneity: I2 = 89%, p < 0.0001) (Supplementary Figure S1A) and “non-colorectal cancers” (HR = 0.51; 95% CI, 0.39–0.68; high heterogeneity: I2 = 62%, p = 0.005) (Supplementary Figure S1B); (b) backbone systemic therapy, “chemotherapy” (HR = 0.63; 95% CI, 0.53–0.75; low heterogeneity: I2 = 25%, p = 0.22) (Supplementary Figure S2A) and “non-chemotherapy” (HR = 0.39; 95% CI, 0.27–0.55; high heterogeneity: I2 = 87%, p < 0.001) (Supplementary Figure S2B); (c) VEGFi type, “bevacizumab” (HR = 0.54; 95% CI, 0.44–0.67; low heterogeneity: I2 = 6%, p = 0.34) (Supplementary Figure S3A) and “non-bevacizumab” (HR = 0.48; 95% CI, 0.37–0.62; high heterogeneity: I2 = 85%, p < 0.001) (Supplementary Figure S3B); and (d) line of treatment, “first line” (HR = 0.62; 95% CI, 0.52–0.74; low heterogeneity: I2 = 30%, p = 0.18) (Supplementary Figure S4A) and “subsequent line” (HR = 0.40; 95% CI, 0.29–0.57; high heterogeneity: I2 = 88%, p < 0.001) (Supplementary Figure S4B).
3.3. Overall Survival Comparison
This pooled analysis included 17 studies with available HR for OS, which showed that the addition of VEGFi to the backbone systemic therapy or BSC is associated with improved OS (HR = 0.83; 95% CI, 0.74–0.93) in the subset of patients with liver metastases (Figure 3A). This effect was also seen within the group of studies (n = 3) that included patients with liver metastases as the only site of disease (HR = 0.75; 95% CI, 0.60–0.94) (Figure 3B). There was moderate heterogeneity between all studies for OS (I2 = 51%, p = 0.008) (Figure 3C), but low heterogeneity between the studies including patients with liver metastases only (I2 = 36%, p = 0.21).
Figure 3.
The addition of VEGFi to a backbone of systemic therapy or BSC was associated with superior OS. (A) Forest plot and pooled HRs for OS comparing the backbone systemic therapy or BSC with versus without VEGFi (HR = 0.83; 95% CI, 0.74–0.93). (B) Forest plot and pooled HRs for OS comparing the backbone systemic therapy or BSC with versus without VEGFi (HR = 0.75; 95% CI, 0.60–0.94) from studies that included patients with liver as the only site of metastasis. (C) Funnel plot showing moderate heterogeneity between all studies for OS (I2 = 51%, p = 0.008) [27,29,30,33,34,35,36,38,39,42,43,44,45,46,47,49,50].
There was a trend (most were statistically significant) towards better OS with the addition of VEGFi in all preplanned subgroups, including: (a) cancer type, “colorectal cancer” (HR = 0.81; 95% CI, 0.69–0.96; moderate heterogeneity: I2 = 60%, p = 0.02) (Supplementary Figure S5A) and “non-colorectal cancers” (HR = 0.85; 95% CI, 0.72–1.00; moderate heterogeneity: I2 = 48%, p = 0.05) (Supplementary Figure S5B); (b) backbone systemic therapy, “chemotherapy” (HR = 0.79; 95% CI, 0.70–0.91; low heterogeneity: I2 = 11%, p = 0.34) (Supplementary Figure S6A) and “non-chemotherapy” (HR = 0.86; 95% CI, 0.71–1.05; high heterogeneity: I2 = 68%, p = 0.002) (Supplementary Figure S6B); (c) VEGFi type, “bevacizumab” (HR = 0.81; 95% CI, 0.63–1.05; high heterogeneity: I2 = 68%, p = 0.009) (Supplementary Figure S7A) and “non-bevacizumab” (HR = 0.84; 95% CI, 0.74–0.96; moderate heterogeneity: I2 = 41%, p = 0.08) (Supplementary Figure S7B); (d) line of treatment, “first line” (HR = 0.86; 95% CI, 0.73–1.02; moderate heterogeneity: I2 = 55%, p = 0.02) (Supplementary Figure S8A) and “subsequent line” (HR = 0.80; 95% CI, 0.68–0.94; moderate heterogeneity: I2 = 51%, p = 0.05) (Supplementary Figure S8B).
3.4. Role of Anti-VEGF in Patients with vs. without Liver Metastases
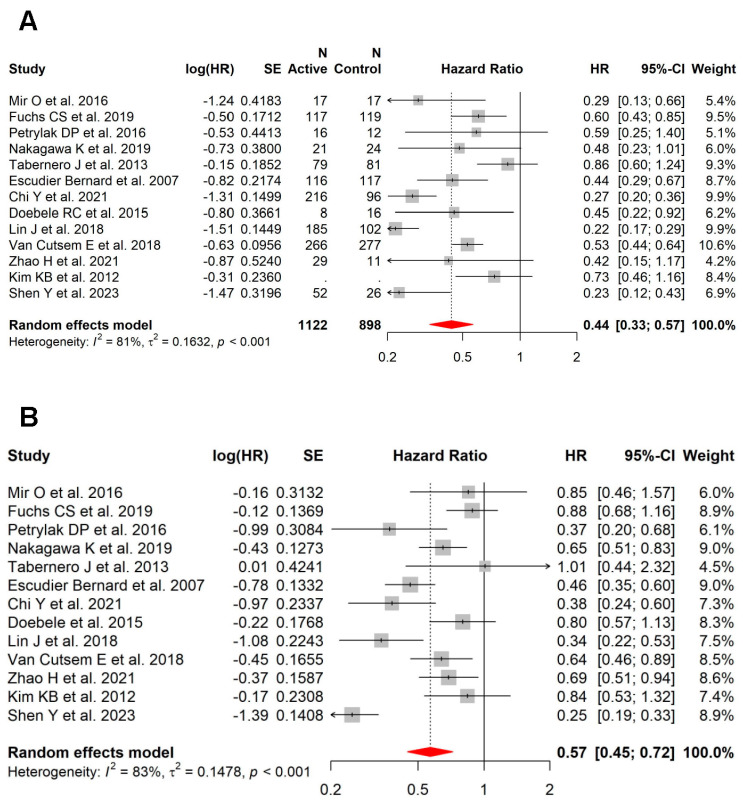
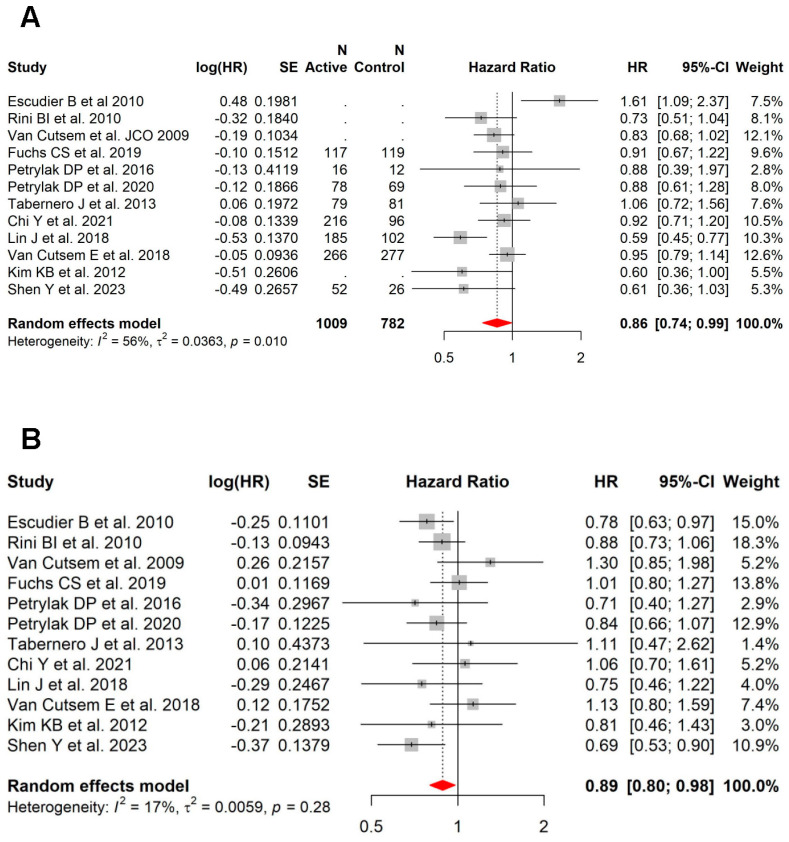
In this meta-analysis, 17 studies also had available PFS (n = 13) and/or OS (n = 12) data on patients without liver metastases. Within this subset of studies, the benefit of the addition of VEGFi was more pronounced in patients with liver metastases (HR = 0.44; 95% CI, 0.33–0.57; high heterogeneity: I2 = 81%, p < 0.001) (Figure 4A) compared to those without liver metastases (HR = 0.57; 95% CI, 0.45–0.72; high heterogeneity: I2 = 83%, p < 0.001) for PFS (Figure 4B). In contrast, this was not seen for OS (patients with liver metastases (HR = 0.86; 95% CI, 0.74–0.99; moderate heterogeneity: I2 = 56%, p = 0.010; Figure 5A) versus patients without liver metastases (HR = 0.89; 95% CI, 0.80–0.98; low heterogeneity: I2 = 17%, p = 0.28; Figure 5B)).
Figure 4.
In the subset of RCTs with data on patients with and without liver metastases, the benefit with VEGFi was more pronounced in patients with liver metastases vs. those without liver metastases for PFS. (A) Forest plot and pooled HRs for PFS in patients with liver metastases (HR = 0.44; 95% CI, 0.33–0.57; high heterogeneity: I2 = 81%, p < 0.001). (B) Forest plot and pooled HRs for PFS in patients without liver metastases (HR = 0.57; 95% CI, 0.45–0.72; high heterogeneity: I2 = 83%, p < 0.001) [18,27,34,35,36,37,40,41,42,45,48,49,50].
Figure 5.
In the subset of RCTs with data on patients with and without liver metastases, the similar benefit with VEGFi was seen in patients with liver metastases vs. those without liver metastases for OS. (A) Forest plot and pooled HRs for OS in patients with liver metastases (HR = 0.86; 95% CI, 0.74–0.99; moderate heterogeneity: I2 = 56%, p = 0.010). (B) Forest plot and pooled HRs for OS in patients without liver metastases (HR = 0.89; 95% CI, 0.80–0.98; low heterogeneity: I2 = 17%, p = 0.28) [27,34,35,36,42,43,44,45,46,47,49,50].
4. Discussion
In this meta-analysis, including RCTs where patients were treated with a backbone of systemic therapy or BSC and randomized into groups with or without VEGFi, we have shown that the addition of VEGFi improved PFS and OS in patients with liver metastases across multiple cancer types. Remarkably, that benefit was more pronounced in patients with liver metastases compared to those without liver metastases, suggesting that VEGFi might be a treatment option for patients with liver metastases resistant to standard treatment.
Several studies have shown that the presence of liver metastases confers a poor prognosis across different cancers [1,2,51,52], and that patients with liver metastases are more likely to be resistant to immune checkpoint inhibitor immunotherapy compared with other sites of metastases [1,3,4,5,53,54]. This was shown in patients with metastatic melanoma, NSCLC, RCC, and urothelial cancer, treated with anti-programmed cell death 1 (anti-PD-1) monotherapy or anti-PD-1 in combination with anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), where the presence of liver metastases was associated with a lower response and a shorter progression-free and overall survival [4,5,13,53,55]. This might be a consequence of more aggressive cancer biology that has a higher likelihood to spread to the liver, but there is also recent evidence suggesting that the presence of liver metastases negatively influences the systemic anti-tumour immune response [56,57]. The liver tolerogenic microenvironment was shown for the first time when MHC-mismatched liver allografts were grafted successfully [58]. Several immunosuppressive mechanisms have been postulated. These include the tolerogenic way of antigen presentation in the liver by Kupffer cells, stellate cells, and liver sinusoidal endothelial cells (LSECs) [59], the induction of regulatory T cells by LSECs [60], and the clonal deletion of activated T cells in the liver [61].
Tumeh and colleagues showed there was a lower density of CD8+ T cells at the invasive margins in liver versus non-liver metastases in patients with advanced melanoma in an effort to understand the liver-specific mechanisms of resistance to checkpoint inhibitor immunotherapy. They also showed that a lower density of CD8+ T cells was associated with poorer response [4]. More recently, our group compared the immune infiltrate within the tumour microenvironment of melanoma liver metastases with other metastatic sites, including lung, brain, subcutis, and lymph nodes. We described a lower density of T cells, in particular of PD1+ and CD103+ T cells, but higher Tim-3+ T cells in the tumour microenvironment of liver metastases, compared with the other sites of metastases [62]. A recent study used an in vivo colon adenocarcinoma model to demonstrate that the presence of liver metastases had a systemic immunosuppressive effect [57]. In this study, Lee and colleagues used immunocompetent C57BL/6 mice and showed that a subcutaneous tumour (MC38 cells injected subcutaneously) had a significantly higher growth in the presence of liver tumours (subcapsular injection of MC38 cells into the liver), but not in the presence of lung tumours (MC38 cells intravenously delivered into the lung). Moreover, liver tumours were less responsive to anti-PD-1 compared to subcutaneous tumours, and these subcutaneous tumours appeared less responsive to anti-PD-1 in the presence of liver tumours (while there was no difference in the presence of lung tumours), confirming that liver tumours constitute a site of resistance to immunotherapy, which negatively affects the response at distant sites of disease. In addition, the authors have shown that the presence of regulatory T cells (Tregs) was responsible for liver-specific resistance to anti-PD-1, and that by depleting these immunosuppressor cells with anti-CTLA-4 monoclonal antibodies, resistance was completely reversed. Even though the presence of Tregs is a possible liver-specific mechanism of resistance, this is not the only one in humans. Firstly, Treg depletion by anti-CTLA-4 has not been clearly shown in humans [63]. Furthermore, in patients with advanced melanoma, even though there is a subset of patients who are free of progression at 5 years (36%) when treated with the combination PD1+CTLA4 [64], 64% of patients still progress with this therapy.
Hypoxia, defined as low oxygen tensions, is a hallmark of the tumour microenvironment across cancers, which leads to local immunosuppression [65]. In hypoxic conditions, HIF1α stabilizes and binds to HIF1β, which induces the transcription of several angiogenic factors responsible for abnormal vascularization, including vascular endothelial growth factor, angiopoietin-2 (ANGPT-2), and IL-8, amongst others [66,67]. These factors have been postulated to inhibit the normal differentiation of key anti-tumour immune cells (e.g., dendritic cells) [68]. This has been clearly shown in the context of HCC, where HIF1 induces the overexpression of ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2/CD39L1) in cancer cells, which impairs the myeloid-derived suppressor cell differentiation, leading to their accumulation in the tumour microenvironment [12]. One way of overcoming hypoxia is by normalizing the vessels that feed the tumour with anti-VEGF agents, which has been successfully used in HCC and other cancers, such as CRC [19] and RCC [17]. Little work has been conducted regarding the role of hypoxia in liver metastases across cancers. Our group has recently shown that T cells are excluded from hypoxic areas within melanoma liver, lung, and subcutaneous metastases (glucose transporter 1 [Glut1] positive), which was not seen in other sites of metastases, such as brain and lymph node metastases [69]. Nevertheless, the impact of adding VEGFi to standard treatment for liver metastases across cancer types is yet to be studied. Why VEGFi may be more efficacious in patients with liver metastases is unclear. Since the liver receives a dual blood supply, in contrast to other organs, it appears that mechanisms beyond hypoxia may play a role.
Hepatocellular carcinoma is known to have a hypoxic and immunosuppressive tumour microenvironment [70], and to be resistant to chemotherapy, but responsive to VEGFi. In a phase III trial (SHARP trial) comparing sorafenib with placebo in patients with advanced HCC, there was a significant difference in time to radiologic progression (5.5 months vs. 2.8 months) and in OS (10.7 months vs. 7.9 months) favouring the sorafenib arm [8]. In another phase III trial, comparing lenvatinib with sorafenib in patients with unresectable HCC, lenvatinib showed non-inferiority in overall survival compared to sorafenib (13.6 months vs. 12.3 months) [9]. More recently, the IMBrave150 trial compared the combination of bevacizumab and atezolizumab (anti-programmed cell death ligand 1 (anti-PD-L1)) with sorafenib in unresectable HCC, and showed that the combination was associated with better clinical outcomes, including PFS (6.8 months vs. 4.3 months) and OS (not reached vs. 13.2 months) [10].
Enhancing our understanding of the potential mechanisms underlying the response to VEGFi and how these mechanisms can influence other processes, like tumour hypoxia and cell signalling, may open up opportunities for novel therapeutic agents. These agents may be aimed at targeting tumour hypoxia (e.g., targeting HIF1α pathways) or cell signalling pathways. Sanguinarine is one of such novel agents, which inhibits VEGF, induces AKT phosphorylation, and reduces angiogenesis [71,72].
From the 25 selected studies included in this meta-analysis, not all of them had PFS and OS data, which constitutes a limitation. Further to this, only a subset of these studies provided data on patients with and without liver metastases. The authors note a high degree of heterogeneity across several of the subgroup analyses, which is a limitation of this study; however, the overall heterogeneity across all studies was moderate. Such heterogeneity is not unexpected given the differences in studies, including differences in cancer type, the backbone of systemic therapy, the line of treatment, and the VEGFi type. Nevertheless, we performed subgroup analysis, and observed that the addition of VEGFi to the backbone of systemic therapy in patients with liver metastases consistently improved PFS across all subsets of patients, and there were trends, with the majority being statistically significant, towards better OS across these subgroups of patients.
5. Conclusions
VEGFi added to standard systemic therapy or BSC showed promising results in patients with liver metastases. For patients with liver metastases resistant to standard systemic therapy, such as checkpoint inhibitor immunotherapy, these findings suggest VEGFi may be an appropriate target as a further line of systemic therapy. This study specifically emphasizes VEGFi as a potential treatment choice, particularly for patients with liver metastases, regardless of primary tumour type, who might otherwise face an increased risk of developing resistance to standard-of-care therapy options. Translational studies are ongoing to address this and understand the biological basis of this response, and also to better identify patients with liver metastases who are resistant to standard treatment but responsive to VEGFi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15205012/s1, Table S1: VEGF inhibitors. Table S2: Search Strategy. Table S3: RCT quality assessment. Figure S1: The addition of VEGFi to a backbone of systemic therapy or BSC was associated with superior PFS in patients with liver metastases from “colorectal” and “non-colorectal” cancers. Forest plot and pooled HRs for PFS comparing the backbone systemic therapy or BSC with versus without VEGFi in patients with liver metastases from “colorectal cancer” (HR = 0.48; 95% CI, 0.34–0.68; high heterogeneity: I2 = 89%, p < 0.001) (A) and with liver metastases from “non-colorectal cancer” (GIST, gastric or junctional adenocarcinoma, urothelial carcinoma, non-small cell lung cancer, renal cell carcinoma, melanoma) (HR = 0.51; 95% CI, 0.39–0.68; high heterogeneity: I2 = 62%, p = 0.005) (B). Figure S2: The addition of VEGFi to “chemotherapy” and “non-chemotherapy” was associated with superior PFS in patients with liver metastases across cancers. Forest plot and pooled HRs for PFS comparing “chemotherapy” with versus without VEGFi in patients with liver metastases across cancers (HR = 0.63; 95% CI, 0.53–0.75; low heterogeneity: I2 = 25, p = 0.22) (A) and “non-chemotherapy” (non-VEGFi targeted therapy or BSC) with versus without VEGFi in patients with liver metastases across cancers (HR = 0.39; 95% CI, 0.27–0.55; high heterogeneity: I2 = 87%, p < 0.001) (B). Figure S3: The addition of VEGFi (“bevacizumab” and “non-bevacizumab”) to a backbone of systemic therapy or BSC was associated with superior PFS in patients with liver metastases across cancers. Forest plot and pooled HRs for PFS comparing a backbone of systemic therapy with versus without VEGFi (“bevacizumab”) in patients with liver metastases across cancers (HR = 0.54; 95% CI, 0.44–0.67; low heterogeneity: I2 = 6%, p = 0.34) (A) and with versus without VEGFi (“non-bevacizumab” [pazopanib, ramucirumab, sorafenib, sunitinib, aflibercept, anlotinib, fruquintinib, nintedanib, apatinib) in patients with liver metastases across cancers (HR = 0.48; 95% CI, 0.37–0.62; high heterogeneity: I2 = 85%, p < 0.001) (B). Figure S4: The addition of VEGFi to a backbone of systemic therapy or BSC as “1st line” and “subsequent line” of treatment was associated with superior PFS in patients with liver metastases across cancers. Forest plot and pooled HRs for PFS comparing a backbone of systemic therapy with versus without VEGFi as “1st line treatment” in patients with liver metastases across cancers (HR = 0.62; 95% CI, 0.52–0.74; low heterogeneity: I2 = 30%, p = 0.18) (A) and as “subsequent line treatment” in patients with liver metastases across cancers (HR = 0.40; 95% CI, 0.29–0.57; high heterogeneity: I2 = 88%, p < 0.001) (B). Figure S5: The addition of VEGFi to a backbone of systemic therapy or BSC was associated with superior OS in patients with liver metastases from “colorectal” and “non-colorectal” cancers. Forest plot and pooled HRs for OS comparing the backbone systemic therapy or BSC with versus without VEGFi in patients with liver metastases from “colorectal cancer” (HR = 0.81; 95% CI, 0.69–0.96; moderate heterogeneity: I2 = 60%, p = 0.02) (A) and with liver metastases from “non-colorectal cancer” (GIST, gastric or junctional adenocarcinoma, urothelial carcinoma, non-small cell lung cancer, renal cell carcinoma, melanoma) (HR = 0.85; 95%CI, 0.72–1.00; moderate heterogeneity: I2 = 48%, p = 0.05) (B). Figure S6: The addition of VEGFi to “chemotherapy” and “non-chemotherapy” was associated with superior OS in patients with liver metastases across cancers. Forest plot and pooled HRs for OS comparing “chemotherapy” with versus without VEGFi in patients with liver metastases across cancers (HR = 0.79; 95% CI, 0.70–0.91; low heterogeneity: I2 = 11%, p = 0.34) (A) and “non-chemotherapy” (targeted therapy or BSC) with versus without VEGFi in patients with liver metastases across cancers (HR = 0.86; 95% CI, 0.71–1.05; high heterogeneity: I2 = 68%, p = 0.002) (B). Figure S7: The addition of VEGFi (“bevacizumab” and “non-bevacizumab”) to a backbone of systemic therapy or BSC was associated with superior OS in patients with liver metastases across cancers. Forest plot and pooled HRs for OS comparing a backbone of systemic therapy with versus without VEGFi (“bevacizumab”) in patients with liver metastases across cancers (HR = 0.81; 95% CI, 0.63–1.05; high heterogeneity: I2 = 68%, p = 0.009) (A) and with versus without VEGFi (“non-bevacizumab” [pazopanib, ramucirumab, sorafenib, sunitinib, aflibercept, anlotinib, fruquintinib, nintedanib, apatinib) in patients with liver metastases across cancers (HR = 0.84; 95% CI, 0.74–0.96; moderate heterogeneity, I2 = 41%, p = 0.08) (B). Figure S8: The addition of VEGFi to a backbone of systemic therapy or BSC as 1st or subsequent line of treatment was associated with superior OS in patients with liver metastases across cancers. Forest plot and pooled HRs for OS comparing a backbone of systemic therapy with versus without VEGFi as “1st line treatment” in patients with liver metastases across cancers (HR = 0.86; 95% CI, 0.73–1.02; moderate heterogeneity: I2 = 55%, p = 0.02) (A) and as “subsequent line treatment” in patients with liver metastases across cancers (HR = 0.80; 95% CI, 0.68–0.94; moderate heterogeneity: I2 = 51%, p = 0.05) (B).
Author Contributions
J.W.C., J.B., G.V.L. and I.P.d.S. conceived and designed the study. J.W.C., J.B. and I.P.d.S. extracted the data for the study. J.W.C., J.B., S.N.L. and I.P.d.S. analysed the data. J.W.C., J.B., G.V.L. and I.P.d.S. wrote the manuscript. R.A.S., M.S.C. and A.M.M. all contributed to the review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
R.A.S. has received fees for professional services from MetaOptima Technology Inc., F. Hoffmann-La Roche Ltd., Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline. M.S.C. has served on advisory boards or as a consultant for Amgen, BMS, Eisai, Ideaya, MSD, Nektar, Novartis, Oncosec, Pierre-Fabre, Qbiotics, Regeneron, Roche, Merck, and Sanofi, and received honoraria from BMS, MSD, and Novartis. A.M.M. has served on advisory boards for BMS, MSD, Novartis, Roche, Pierre-Fabre, and QBiotics. G.V.L. is consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA, Merck Sharpe & Dohme, Novartis, PHMR Ltd., Pierre Fabre, Provectus, Qbiotics, and Regeneron. I.P.d.S. has served on advisory board for MSD, and received honoraria from Roche, BMS, and MSD. J.W.C., J.B., and S.N.L. have no conflicts of interest.
Funding Statement
S.N.L is supported by Melanoma Institute Australia. R.A.S. is supported by a National Health and Medical Research Council of Australia (NHMRC) Investigator Grant (APP2018514). A.M.M. is supported by an NHMRC Investigator Grant, Nicholas and Helen Moore, and Melanoma Institute Australia. G.V.L. is supported by an NHMRC Investigator Grant and the University of Sydney Medical Foundation. I.P.d.S. is supported by the early-career CINSW fellowship. This project was supported by Lady Mary Fairfax Charitable Trust.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pires da Silva I., Ahmed T., McQuade J.L., Nebhan C.A., Park J.J., Versluis J.M., Serra-Bellver P., Khan Y., Slattery T., Oberoi H.K., et al. Clinical Models to Define Response and Survival With Anti-PD-1 Antibodies Alone or Combined with Ipilimumab in Metastatic Melanoma. J. Clin. Oncol. 2022;40:1068–1080. doi: 10.1200/JCO.21.01701. [DOI] [PubMed] [Google Scholar]
- 2.Ren Y., Dai C., Zheng H., Zhou F., She Y., Jiang G., Fei K., Yang P., Xie D., Chen C. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7:53245–53253. doi: 10.18632/oncotarget.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pires da Silva I., Lo S., Quek C., Gonzalez M., Carlino M.S., Long G.V., Menzies A.M. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer. 2020;126:86–97. doi: 10.1002/cncr.32522. [DOI] [PubMed] [Google Scholar]
- 4.Tumeh P.C., Hellmann M.D., Hamid O., Tsai K.K., Loo K.L., Gubens M.A., Rosenblum M., Harview C.L., Taube J.M., Handley N., et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Sosman J.A., Atkins M.B., Leming P.D., et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cidon E.U. Systemic treatment of hepatocellular carcinoma: Past, present and future. World J. Hepatol. 2017;9:797–807. doi: 10.4254/wjh.v9.i18.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez P.M., Villanueva A., Llovet J.M. Systematic review: Evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment. Pharmacol. Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 8.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M., Finn R.S., Qin S., Han K.-H., Ikeda K., Piscaglia F., Baron A., Park J.-W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 10.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 11.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 12.Chiu D.K., Tse A.P., Xu I.M., Di Cui J., Lai R.K., Li L.L., Koh H.Y., Tsang F.H., Wei L.L., Wong C.M., et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017;8:517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M.S., Cui J.D., Lee D., Yuen V.W., Chiu D.K., Goh C.C., Cheu J.W., Tse A.P., Bao M.H., Wong B.P.Y., et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat. Commun. 2022;13:954. doi: 10.1038/s41467-022-28618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook K.M., Figg W.D. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J. Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva I.P., Salhi A., Giles K.M., Vogelsang M., Han S.W., Ismaili N., Lui K.P., Robinson E.M., Wilson M.A., Shapiro R.L., et al. Identification of a Novel Pathogenic Germline KDR Variant in Melanoma. Clin. Cancer Res. 2016;22:2377–2385. doi: 10.1158/1078-0432.CCR-15-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sternberg C.N., Davis I.D., Mardiak J., Szczylik C., Lee E., Wagstaff J., Barrios C.H., Salman P., Gladkov O.A., Kavina A., et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2010;28:1061. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 17.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O., Oudard S., Negrier S., Szczylik C., Kim S.T., et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M., Negrier S., Chevreau C., Solska E., Desai A.A., et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 19.Saltz L.B., Clarke S., Diaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.S., Rivera F., et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 20.Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouche O., Mineur L., Barone C., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 21.Kazazi-Hyseni F., Beijnen J.H., Schellens J.H. Bevacizumab. Oncologist. 2010;15:819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polena H., Creuzet J., Dufies M., Sidibe A., Khalil-Mgharbel A., Salomon A., Deroux A., Quesada J.L., Roelants C., Filhol O., et al. The tyrosine-kinase inhibitor sunitinib targets vascular endothelial (VE)-cadherin: A marker of response to antitumoural treatment in metastatic renal cell carcinoma. Br. J. Cancer. 2018;118:1179–1188. doi: 10.1038/s41416-018-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D., Ding Y., Li Y., Luo W.M., Zhang Z.F., Snider J., Vandenbeldt K., Qian C.N., Teh B.T. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H., Liu Z., Wang Y., Wen X., Amador E.H., Yuan L., Ran X., Xiong L., Ran Y., Chen W., et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022;7:70. doi: 10.1038/s41392-022-00922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Deeks J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) Cochrane Group; London, UK: 2022. Chapter 10: Analysing Data and Undertaking Meta-Analyses. [Google Scholar]
- 27.Tabernero J., Garcia-Carbonero R., Cassidy J., Sobrero A., Van Cutsem E., Kohne C.-H., Tejpar S., Gladkov O., Davidenko I., Salazar R., et al. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: The RESPECT trial. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2013;19:2541–2550. doi: 10.1158/1078-0432.CCR-13-0107. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham D., Lang I., Lorusso V., Ocvirk J., Shin D., Jonker D.J., Osborne S., Andre N.A., Waterkamp D., Saunders M.P. Bevacizumab (bev) in combination with capecitabine (cape) for the first-line treatment of elderly patients with metastatic colorectal cancer (mCRC): Results of a randomized international phase III trial (AVEX) J. Clin. Oncol. 2013;31:337. doi: 10.1200/jco.2013.31.4_suppl.337. [DOI] [Google Scholar]
- 29.Tabernero J., Van Cutsem E., Lakomy R., Prausov J., Ruff P., van Hazel G.A., Moiseyenko V.M., Ferry D.R., McKendrich J.J., Soussan-Lizard K., et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: Prespecified subgroup analyses from the VELOUR trial. Eur. J. Cancer. 2014;50:320. doi: 10.1016/j.ejca.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Tang W., Ren L., Liu T., Ye Q., Wei Y., He G., Lin Q., Wang X., Wang M., Liang F., et al. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J. Clin. Oncol. 2020;38:3175–3184. doi: 10.1200/JCO.20.00174. [DOI] [PubMed] [Google Scholar]
- 31.Tebbutt N.C., Wilson K., Gebski V.J., Cummins M.M., Zannino D., van Hazel G.A., Robinson B., Broad A., Ganju V., Ackland S.P., et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Xu R., Qin S., Liu T., Pan H., Xu J., Bi F., Lim R., Zhang S., Ba Y., et al. Aflibercept plus FOLFIRI in Asian patients with pretreated metastatic colorectal cancer: A randomized Phase III study. Future Oncol. 2018;14:2031–2044. doi: 10.2217/fon-2017-0669. [DOI] [PubMed] [Google Scholar]
- 33.Tabernero J., Yoshino T., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.-E., Portnoy D.C., Van Cutsem E., Grothey A., et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 34.Chi Y., Shu Y., Ba Y., Bai Y., Qin B., Wang X., Xiong J., Xu N., Zhang H., Zhou J., et al. Anlotinib Monotherapy for Refractory Metastatic Colorectal Cancer: A Double-Blinded, Placebo-Controlled, Randomized Phase III Trial (ALTER0703) Oncologist. 2021;26:e1693–e1703. doi: 10.1002/onco.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., Qin S., Xu R.-H., Shen L., Xu J., Bai Y., Yang L., Deng Y., Chen Z.-D., Zhong H., et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Cutsem E., Yoshino T., Lenz H.J., Lonardi S., Falcone A., Limon M.L., Saunders M., Sobrero A., Park Y.S., Ferreiro R., et al. Nintedanib for the treatment of patients with refractory metastatic colorectal cancer (LUME-Colon 1): A phase III, international, randomized, placebo-controlled study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018;29:1955. doi: 10.1093/annonc/mdy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa K., Garon E.B., Seto T., Nishio M., Ponce Aix S., Paz-Ares L., Chiu C.-H., Park K., Novello S., Nadal E., et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 38.Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., Lilenbaum R., Johnson D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 39.Scagliotti G.V., Krzakowski M., Szczesna A., Strausz J., Makhson A., Reck M., Wierzbicki R.F., Albert I., Thomas M., Miziara J.E., et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: A phase III trial. J. Clin. Oncol. 2012;30:2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 40.Doebele R.C., Spigel D., Tehfe M., Thomas S., Reck M., Verma S., Eakle J., Bustin F., Goldschmidt J., Cao D., et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer. 2015;121:883–892. doi: 10.1002/cncr.29132. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H., Yao W., Min X., Gu K., Yu G., Zhang Z., Cui J., Miao L., Zhang L., Yuan X., et al. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706) J. Thorac. Oncol. 2021;16:1533–1546. doi: 10.1016/j.jtho.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y., Lu J., Hu F., Qian J., Zhang X., Zhong R., Zhong H., Chu T., Han B. Effect and outcomes analysis of anlotinib in non-small cell lung cancer patients with liver metastasis: Results from the ALTER 0303 phase 3 randomized clinical trial. J. Cancer Res. Clin. Oncol. 2023;149:1417–1424. doi: 10.1007/s00432-022-03964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escudier B., Bellmunt J., Négrier S., Bajetta E., Melichar B., Bracarda S., Ravaud A., Golding S., Jethwa S., Sneller V. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J. Clin. Oncol. 2010;28:2144. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 44.Rini B.I., Halabi S., Rosenberg J.E., Stadler W.M., Vaena D.A., Archer L., Atkins J.N., Picus J., Czaykowski P., Dutcher J., et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J. Clin. Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrylak D.P., Tagawa S.T., Kohli M., Eisen A., Canil C., Sridhar S.S., Spira A., Yu E.Y., Burke J.M., Shaffer D., et al. Docetaxel As Monotherapy or Combined With Ramucirumab or Icrucumab in Second-Line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma: An Open-Label, Three-Arm, Randomized Controlled Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016;34:1500–1509. doi: 10.1200/JCO.2015.65.0218. [DOI] [PubMed] [Google Scholar]
- 46.Petrylak D.P., de Wit R., Chi K.N., Drakaki A., Sternberg C.N., Nishiyama H., Castellano D., Hussain S.A., Fléchon A., Bamias A., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): Overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2020;21:105. doi: 10.1016/S1470-2045(19)30668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Cutsem E., Vervenne W.L., Bennouna J., Humblet Y., Gill S., Van Laethem J.L., Verslype C., Scheithauer W., Shang A., Cosaert J., et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 48.Mir O., Cropet C., Toulmonde M., Cesne A.L., Molimard M., Bompas E., Cassier P., Ray-Coquard I., Rios M., Adenis A., et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): A randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17:632. doi: 10.1016/S1470-2045(16)00075-9. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs C.S., Shitara K., Di Bartolomeo M., Lonardi S., Al-Batran S.-E., Van Cutsem E., Ilson D.H., Alsina M., Chau I., Lacy J., et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420–435. doi: 10.1016/S1470-2045(18)30791-5. [DOI] [PubMed] [Google Scholar]
- 50.Kim K.B., Sosman J.A., Fruehauf J.P., Linette G.P., Markovic S.N., McDermott D.F., Weber J.S., Nguyen H., Cheverton P., Chen D., et al. BEAM: A randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J. Clin. Oncol. 2012;30:34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam R., Aloia T., Krissat J., Bralet M.P., Paule B., Giacchetti S., Delvart V., Azoulay D., Bismuth H., Castaing D. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann. Surg. 2006;244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b. discussion 907–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riihimaki M., Hemminki A., Fallah M., Thomsen H., Sundquist K., Sundquist J., Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Balar A.V., Castellano D., O’Donnell P.H., Grivas P., Vuky J., Powles T., Plimack E.R., Hahn N.M., de Wit R., Pang L., et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 54.Sonpavde G., Manitz J., Gao C., Tayama D., Kaiser C., Hennessy D., Makari D., Gupta A., Abdullah S.E., Niegisch G., et al. Five-Factor Prognostic Model for Survival of Post-Platinum Patients with Metastatic Urothelial Carcinoma Receiving PD-L1 Inhibitors. J. Urol. 2020;204:1173–1179. doi: 10.1097/JU.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waninger J.J., Ma V.T., Journey S., Skvarce J., Chopra Z., Tezel A., Bryant A.K., Mayo C., Sun Y., Sankar K., et al. Validation of the American Joint Committee on Cancer Eighth Edition Staging of Patients With Metastatic Cutaneous Melanoma Treated With Immune Checkpoint Inhibitors. JAMA Netw. Open. 2021;4:e210980. doi: 10.1001/jamanetworkopen.2021.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J., Green M.D., Li S., Sun Y., Journey S.N., Choi J.E., Rizvi S.M., Qin A., Waninger J.J., Lang X., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J.C., Mehdizadeh S., Smith J., Young A., Mufazalov I.A., Mowery C.T., Daud A., Bluestone J.A. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. 2020;5:eaba0759. doi: 10.1126/sciimmunol.aba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calne R.Y., Sells R.A., Pena J.R., Davis D.R., Millard P.R., Herbertson B.M., Binns R.M., Davies D.A. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 59.Limmer A., Ohl J., Wingender G., Berg M., Jungerkes F., Schumak B., Djandji D., Scholz K., Klevenz A., Hegenbarth S., et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 2005;35:2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 60.Jinushi M., Takehara T., Tatsumi T., Yamaguchi S., Sakamori R., Hiramatsu N., Kanto T., Ohkawa K., Hayashi N. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J.C., Green M.D., Huppert L.A., Chow C., Pierce R.H., Daud A.I. The Liver-Immunity Nexus and Cancer Immunotherapy. Clin. Cancer Res. 2022;28:5–12. doi: 10.1158/1078-0432.CCR-21-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conway J.W., Rawson R.V., Lo S., Ahmed T., Vergara I.A., Gide T.N., Attrill G.H., Carlino M.S., Saw R.P.M., Thompson J.F., et al. Unveiling the tumor immune microenvironment of organ-specific melanoma metastatic sites. J. Immunother. Cancer. 2022;10:e004884. doi: 10.1136/jitc-2022-004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma A., Subudhi S.K., Blando J., Scutti J., Vence L., Wargo J., Allison J.P., Ribas A., Sharma P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2019;25:1233–1238. doi: 10.1158/1078-0432.CCR-18-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 65.Abou Khouzam R., Brodaczewska K., Filipiak A., Zeinelabdin N.A., Buart S., Szczylik C., Kieda C., Chouaib S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2020;11:613114. doi: 10.3389/fimmu.2020.613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fei M., Guan J., Xue T., Qin L., Tang C., Cui G., Wang Y., Gong H., Feng W. Hypoxia promotes the migration and invasion of human hepatocarcinoma cells through the HIF-1alpha-IL-8-Akt axis. Cell. Mol. Biol. Lett. 2018;23:46. doi: 10.1186/s11658-018-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simon M.P., Tournaire R., Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J. Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 68.Corzo C.A., Condamine T., Lu L., Cotter M.J., Youn J.I., Cheng P., Cho H.I., Celis E., Quiceno D.G., Padhya T., et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paver Elizabeth G.T., Wilmott J. Carbonic Anhydrase IX expression as a predictor of resistance to immune checkpoint inhibitor therapy in melanoma. Pigment. Cell Melanoma Res. 2022;35:97–184. doi: 10.1111/pcmr.13018. [DOI] [Google Scholar]
- 70.Mo Z., Liu D., Rong D., Zhang S. Hypoxic Characteristic in the Immunosuppressive Microenvironment of Hepatocellular Carcinoma. Front. Immunol. 2021;12:611058. doi: 10.3389/fimmu.2021.611058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J., Su Q., Wu Q., Chen K., Ullah A., Ghauri M.A., Zhang Y. Sanguinarine impairs lysosomal function and induces ROS-dependent mitophagy and apoptosis in human hepatocellular carcinoma cells. Arch. Pharm. Res. 2021;44:1025–1036. doi: 10.1007/s12272-021-01356-0. [DOI] [PubMed] [Google Scholar]
- 72.Qi X., Chen Y., Liu S., Liu L., Yu Z., Yin L., Fu L., Deng M., Liang S., Lu M. Sanguinarine inhibits melanoma invasion and migration by targeting the FAK/PI3K/AKT/mTOR signalling pathway. Pharm. Biol. 2023;61:696–709. doi: 10.1080/13880209.2023.2200787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article.