Abstract
Background: Genetic and epigenetic changes, oxidative stress and inflammation influence the rate of aging, which diseases, lifestyle and environmental factors can further accelerate. In accelerated aging (AA), the biological age exceeds the chronological age. Objective: The objective of this study is to reappraise the AA concept critically, considering its weaknesses and limitations. Methods: We reviewed more than 300 recent articles dealing with the physiology of brain aging and neurodegeneration pathophysiology. Results: (1) Application of the AA concept to individual organs outside the brain is challenging as organs of different systems age at different rates. (2) There is a need to consider the deceleration of aging due to the potential use of the individual structure–functional reserves. The latter can be restored by pharmacological and/or cognitive therapy, environment, etc. (3) The AA concept lacks both standardised terminology and methodology. (4) Changes in specific molecular biomarkers (MBM) reflect aging-related processes; however, numerous MBM candidates should be validated to consolidate the AA theory. (5) The exact nature of many potential causal factors, biological outcomes and interactions between the former and the latter remain largely unclear. Conclusions: Although AA is commonly recognised as a perspective theory, it still suffers from a number of gaps and limitations that assume the necessity for an updated AA concept.
Keywords: aging, accelerated aging, brain aging, neurodegeneration, epigenetics, biological clocks, molecular biomarkers, rejuvenation
1. Introduction
Aging is associated with structural and physiological changes that increase the risk of developing diseases and death [1,2,3]. Aging processes are influenced by different factors, including genetic mutations, epigenetic modifications, oxidative stress and inflammation [4], resulting in the accumulation of damage and dysfunction at all biological levels [5]. Biological age (BA), also called physiological age, can be defined as the current state of an individual as a biological system, characterized by a combination of detectable life time-dependent biological parameters (determination criteria), for example, by the current profile of genomic DNA methylation, the present status of aging-associated structures in the brain, etc.
Normal aging corresponds to all monitored aging-associated processes where BA equals the chronological age. Accelerated aging (AA) is observed when the BA exceeds the chronological age and, vice versa, decelerated aging is observed when the chronological age exceeds the BA [6,7,8]. AA shares common features with normal aging, but it is also characterised by specific processes such as protein aggregation and excitotoxicity [9,10,11]. Understanding the mechanisms of aging can open opportunities for targeted therapies to slow it [9].
AA is an area of active research with unresolved issues, including the unstandardised terminology [12] and understudied mechanisms [13] including neurodegeneration (ND) described either as a type of AA [14,15] or as its outcome [14,16,17,18]. The latter view argues that certain biomarkers (BM) are ND-specific and do not detect AA [17]. Different theories have been proposed to explain the pathogenesis of AA, including genetic theory [19], the multi-proteinopathies theory [20] and mitochondrial theory [21]. Genetic theory assumes the accumulation of DNA mutations and/or gene dysregulation in AA and has certain limitations [19,22]. It considers random DNA changes but ignores chromosomal, multifactorial and monogenic alterations [13,23,24]. The multi-proteinopathies theory is based on the accumulation/aggregation of misfolded proteins leading to cell dysfunction and causing age-related diseases [20,25]. The free radical theory considers the oxidative damage to DNA and proteins by reactive oxygen species (ROS) as the primary accelerator of aging [15,26,27,28]; however, it has the problem of segregating between the normal and abnormal levels of ROS [21,29]. However, these aging theories lack reliable diagnostic BM for early identification and prognostication [20,30].
2. Biomolecular Aspects of Aging
Aging theories differ in their approaches to describing aging at cellular, supracellular and subcellular levels. ND aetiology can be approached with the neurocentric (NC) or neurovascular (NV) view. The latter refers to the dynamic multicellular structure called the neurovascular unit (NVU) that includes astrocytes, microglia, oligodendrocytes, precursor cells, excitatory and inhibitory neurons, endothelial cells and pericytes, and mediates the functional interactions between brain tissues per se and the blood vessels [31]. The NV hypothesis proposes that neural cells in the NVU and circulating immune cells secrete proinflammatory mediators, therefore, contributing to age-related neuroinflammation [32], cell degeneration [33,34] and endothelial impairment [34,35]. These changes disrupt molecular networks, induce damage to the blood–brain barrier [36,37] and lead to NVU dysfunction, a major cause of ND [38]. However, the exact role of NVU in ND remains unclear [39]. Accumulated evidences of the high complexity and molecular heterogeneity of the NVU network makes the search for associated BM difficult and requires whole genome studies, e.g., global transcriptome analysis followed by hierarchical data clustering [40] or single-cell/single-nucleus transcriptomics [41,42].
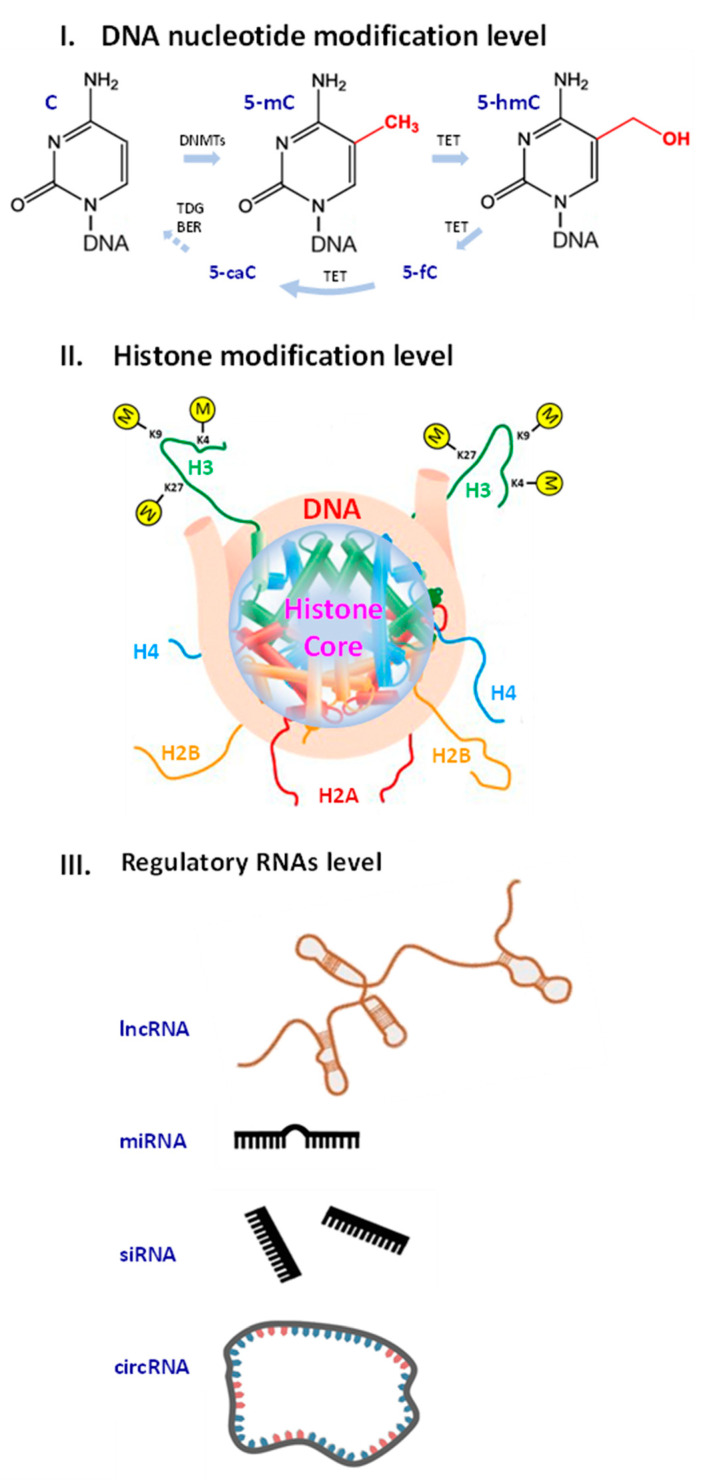
Molecular biomarkers (MBM) are biomolecules, their components, fragments or modifications with associated measurable parameters that serve as a tool to diagnose pathologies and monitor biological processes. MBM can be used to evaluate aging, particularly to estimate the rate of its progression [43]. Aging MBM include mRNA transcripts, proteins [44], the length of telomeres, serum markers of DNA damage [45], DNA methylation profiles [46,47], histone modifications [48,49,50,51,52,53,54,55,56,57,58,59], differentially expressed genes [42,60], non-coding RNAs [61,62,63,64] and other biomolecules (Figure 1).
Figure 1.
Examples of epigenetic changes and factors affected by accelerated aging at different levels that could serve as potential biomarkers of aging. (I) DNA nucleotide modification level: cytosine (C) can be converted to 5-methylcytosine (5-mC) and oxidised further to 5-hydroxymethylcytosine (5-hmC). (II) Histone modification level: Three examples of histone 3 (H3) lysine methylation marks are indicated. Lysine 4 (K4) H3K4me3 mark is associated with active genes, lysine 9 (K9) H3K9me3 is an established mark of transcriptionally repressed heterochromatin and lysine 27 (K27) H3K27me3 mark is linked to both transcriptional activation and repression. (III) Regulatory RNAs level: several types of ncRNAs, long non-coding RNA (lnc RNA), microRNA (miRNA), small interfering RNA (siRNA), circular RNA (circRNA), shown schematically.
Despite the large number of suggested MBM of ND, only a few of them have been validated. In most studies, the sample sizes have been too small to justify the accuracy and reproducibility of MBM data. For example, a recent study aimed at determining whether age affects different cell types in NVU resulted in the model discriminating Alzheimer’s disease (AD) from healthy control (HC) samples, revealing 15 genes related to accelerated aging (AAG): IGF1R, MXI1, RB1, PPARA, NFE2L2, STAT5B, FOS, PRKCD, YWHAZ, HTT, MAPK9, HSPA9, SDHC, PRKDC and PDPK1 [42]. Of these genes, differential expression of IGF1R, MXI1, PPARA, YWHAZ and MAPK9 correlate with the progression of ND and may function as facilitators or inhibitors of AD. However, questions remain on the cell-specific roles of the discovered AAGs, and their contribution to AD pathogenesis and interactions in NVU. Moreover, the study cohort included only 11 AD patients and seven HC, which is insufficient for justifying AAGs as MBM in AD [42]. ND results from multiple structural changes at different genetic loci over a period of time [65,66]. AD represents 90% of ND cases, and the chances of it developing increase with changes in the 15 indicated genes predisposing to ND (NDG): GBA1, APP, PSEN1, MAPT, GRN, SETX, SPAST, CSF1R, C9orf72 [67], TET2 [68], TBK1 [69], TOMM40, APOC1 [70], APOE [70,71] and TREM2 [72,73,74,75,76,77,78]. Surprisingly, there is no overlap between both sets of genes resulting from different studies: AAGs and NDGs. In dementia with Lewy bodies, multi-cognitive decline and corticobasal degeneration, the risk factors shown to be involved are the APOE e4 allele and the mutation spectrum for TREM2 gene [78,79,80,81,82,83,84,85,86,87].
DNA methylation rate reflects the rate of aging. Approximately 1.5% of genomic DNA contains 5-methylcytosine (5-mC) that decreases during ontogenesis [88]. The level of 5-mC is found to be highest in embryos and it decreases gradually with age [89,90]. Global genomic DNA hypomethylation in aging proceeds along with hypermethylation of CpG islands (CGIs) in the mammalian genome where 60% of CGIs are associated with gene promoters and involved in the regulation of gene transcription [91]. Changes in DNA methylation patterns known as “epigenetic drift” are associated with aging across the entire lifespan [92].
Age-predictive models demonstrate gradual linear changes in the DNA methylation profile in normal aging; however, environmental or genetic risk factors accelerate aging [93]. In monozygotic twins, the divergence of the methylome increases at different rates [94]. Furthermore, the DNA methylation profile was proposed as mechanisms of the epigenetic clock [95,96,97] by analogy with the biological clock [98,99]. Monitoring deviations between biological and chronological age helps to study development and aging across the lifespan [100]. Horvath [101], Hannum [93] and PhenoAge [102] epigenetic clocks serve as markers of ND [102,103,104,105,106], with the first of these showing the strongest correlation between epigenetics and chronological age [107].
Histone modifications can be used as potential aging MBM; however, the heterogeneity of the studies and the animal models limited the applicability of the findings. For highly abundant transcription activation mark H3K4me3 [48], the decrease in its level was shown to correlate with an extended lifespan in Caenorhabditis elegans [49], while an increase has been linked with AA and a reduced lifespan in Drosophila melanogaster [50]. For the heterochromatin-associated histone transcription repression mark H3K9me3 that is gradually lost during human and mouse aging in haematopoietic stem cells [51], the most significant changes occurred in the repressive regions in C. elegans [52] and models of AA [53]. The role of the repressive histone mark H3K27me3 associated with transcriptional silencing [54] in aging is controversial, as studies have shown both an increase and a decrease in its level during aging [55,56,57,58,59].
Increased H4K20me3 and H3K4me3 and decreased H3K9me1 and H3K27me3 histone modification levels have been described as common age-associated epigenetic marks [108,109,110]. In AD, an increase in the gene activation-related histone mark H3K4me3 was shown in both the CK-p25 tauopathy mouse model and in the hippocampus of AD patients [111,112]. Other histone methylation marks identified in AD patients’ brain included: H4K20me2, H3K4me2, H3K27me3, H3K79me1, H3K79me2, H3K36me2, H4K20me3, H3K27me1 and H3K56me1 [113,114]. In addition to methylation, histone acetylation marks H3K9ac, H3K14ac and H4K16ac have been shown to be associated with aging and AD [110,111,113,114,115]. Among other ND-associated histone modifications in AD, histone phosphorylation H4S47p and H3S10p {chaput2016potential} and histone ubiquitination H2BK120ub were reported [114,116,117]. The exact regulatory modes of both the modifications and mechanisms of their interaction and interplay with other factors in such complex processes as aging and ND are still unclear and require further systematic research.
Non-coding RNAs (ncRNAs) are used as aging MBM [118,119,120,121]. Long non-coding (lncRNA), for example, the growth-arrest-specific transcript 5 (GAS5), plays a significant role in cell proliferation and apoptosis [122,123,124], and its down-regulation leads to the phosphorylation of the tau protein in ND [125,126]. Long intergenic brain cytoplasmic RNA 1 (BCYRN1) expressed in the dendritic domains of neurons is down-regulated in aging [127].
MicroRNAs (miRNAs) mediate brain aging through the regulation of gene expression, impact neuronal plasticity and influence tau protein metabolism [128,129,130,131,132,133,134,135,136,137]. The regulation of MiR145a and MiR-375 was shown to be age-dependent in mouse brains [138,139,140]. The MIR29 family, MIR339-5p, MIR195 and MIR107, regulate the expression of beta-secretase 1 that is responsible for proteolysis of the amyloid precursor protein [141,142,143,144,145,146]. Interestingly, Mir34 was shown to play a protective role in Drosophila [147], and MIR144/MIR451 was found to regulate ADAM metallopeptidase domain 10 in AD [148]. Over 20 miRNAs secreted into the cerebrospinal fluid by hypothalamic stem cells were found to control the aging rate in mice [149], which is especially important as the hypothalamus was placed at the base of human brain aging [150]. As most miRNA studies have been conducted on non-human models, their relevance to human data needs to be verified; hence, future studies will be required to define the roles of miRNAs [151].
Circular RNAs (circRNAs) are a recently described type of ncRNA with age-dependent expression in skeletal muscles [152] and an abundance in the brain [153], playing a role in ND through their interaction with miRNAs. For example, CIRS-7 potentially functions as a sponge for MIR7-1 [154] and its level is dramatically reduced in the AD brain [155]. Cerebral circRNAs are associated with neurotransmitter function, synaptic activities and neuronal maturation and target the expression and availability of specific age-related mRNAs in the brain. At least four circRNAs were found to be involved in postoperative neurocognitive disorders [156]. Another study revealed nearly 1200 cerebral circRNAs in a rat aging model [157]; however, these circRNAs still await their complete characterisation.
3. Aging of Organs and Systems beyond Neurodegeneration
Aging affects organs and systems with different rates of change; therefore, the AA concept needs to be adjusted when applied to individual organs. For example, ovarian aging implies a loss of follicle numbers and decreased oocyte viability. Typically, an accelerated decline in fertility begins around the age of 38 years and continues until the climacteric [158]; however, a non-uniform decrease in follicle numbers results in a large variation in menopause onset. The BA of the male reproduction system can also be assessed by fertility, but the arrest of reproductive capacity is reversible in older men, with lifestyle and disease factors prevailing over other determinants of aging [159]. In mice, oxidative stress, inflammation, DNA damage and de novo mutations accelerate testicular aging [160,161,162,163], while enhancing antioxidant enzyme activities with growth differentiation factor 11 protects the testes [164]. A progressive age-related drop in Leydig and Sertoli cell function [165], testicular size [166] or testosterone levels was demonstrated in older elite men [167]; however, no decrease in testicle size or the levels of testosterone was observed in a cohort of older elite men without comorbidities [168,169].
The cumulative effect of disease rather than age may account for changes in the male reproductive system throughout life. Obviously, chronological age does not always determine reproductive BA, and adopting the AA concept to the reproductive system is not allowed, which illustrates the challenges in assessing BA at the organ and system levels.
Sex hormones that affect fertility are part of the endocrine system. Data on the susceptibility of the endocrine system and metabolism to aging differ between organs and sex. The hypothalamic–pituitary–testicular axis in men does not undergo dramatic chronobiological changes, accounting for only 35–50% of men over 80 with reduced testosterone levels [170,171]. Conversely, diabetes mellitus and obesity predispose to accelerated adipose tissue dysfunction, affecting telomere length [172,173]. Adrenal and thyroid functions undergo less prominent age-related changes than their hypothalamic regulation [174]; therefore, assessing BA from hormonal findings is challenging. With aging, hormone activity decreases and endocrine alterations are established [175]. BA is affected by the level of glycosylated haemoglobin, glucose, triglycerides, and low-density and total cholesterol [176,177,178]. The modulation of these parameters, lifestyle and environmental factors can prevent or contribute to AA [179]. The effectiveness of hormone replacement therapy for aging reversal is questionable though [180].
Environmental and endocrinological factors affect the BA of connective tissue. The status of the skeletal system reflects the individual endocrine profile and micronutrient balance [181,182,183] as well as environmental and occupational attainments [184]. For example, bone resorption in astronauts prevails over its formation due to the effects of microgravity; however, bone density normalises after the flight [185,186]. Skin elasticity serves as a marker of aging, whose rate can be modified due to estrogen deficiency, metabolic alterations and exogenous factors (burns) [187,188,189,190,191]. Fibroblasts constitute a natural cell stock that allows skin rejuvenation, repair and decelerated aging [192]. In connective tissue, a combinatory effect of internal and external factors determines BA more accurately than the chronological one [193,194]. Therefore, the inability to account for the decreased aging rate reveals the weakness of the AA concept.
Studies in other systems have also reported the reversibility of age-related changes in them. For example, physical training can rejuvenate the respiratory system by expanding the alveolar space. However, studies on these issues did not comprehensively evaluate the BA of the lung since the impact of muscle atrophy on the results in the spirometry test was not considered [195,196,197]. Lifestyle changes (e.g., calorie restriction and physical activity) could also reverse aging in patients with early stages of chronic kidney disease [198]. Another example is shown by the discovered potential to rejuvenate the kidneys with up-regulation of the Klotho gene [199]. These evidences speak for a limited generalisation of the AA concept. AA affects various systems and cross-organ communication. The interaction between systems can impede the atrophy of an organ through compensatory mechanisms in other organs. Several studies have demonstrated the role of the central nervous system in reversing the aging of other systems and organs [200,201,202]. Endocrine and cardiovascular diseases promote renal aging [203]. Conversely, kidney transplantation can revive other parts of the body [204,205]. The characterisation of organ- and system-specific aging processes is challenging and will require combinatory approaches that are largely missing in the AA concept.
4. Limitations of the AA Concept
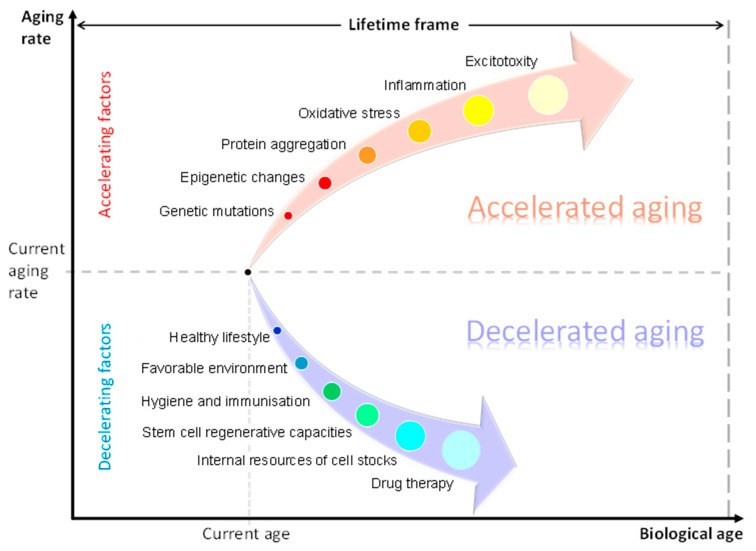
The AA concept is closely linked with and needs to be considered within the context of individual capacities and personalised structure–functional reserve mechanisms (Figure 2). The generalized term “structure-functional reserves” is introduced to approach the observed variability in multiple structure–functional parameters at different levels (expression of genetically and epigenetically regulated genes, number, viability and functionality of cells, number of synapses and intercellular contacts, secretion of cytokines, potency of physiological responses, etc.) in a population or group of subjects. The description of this term could be further developed and linked to the statistical distributions of measurable biological parameters in the population and to the norm of reaction resulting from the influence of environmental factors on trait variation.
Figure 2.
Factors influencing the rate of aging Factors accelerating aging include: genetic mutations, epigenetic changes, protein aggregation, oxidative stress, inflammation and excitotoxity. Factors decelerating aging include: a healthy lifestyle, favourable environment, hygiene and immunisation, stem cell regenerative capacities, internal resources of cell stocks and drug therapy.
Physiological reserves reflect the remaining capacity of an organ to perform its function. Aging and diseases lead to atrophy, reducing the number of cells and supracellular structures [206,207]. In the context of brain aging, its physiological cognitive reserve is assumed by the level of education, occupational and environmental attainments and the performance of cognitive tests [206]. Reversible forms of mild cognitive impairment (MCI) and dementia represent clinical examples of restoring individual reserve potential that are not in line with AA theory [208,209]. Neural compensation in the elderly leads to the formation of secondary brain networks [210], which decelerate the aging of the brain [206,211]. Reversion to MCI in elderly patients has been reported to be as a result of specific lifestyle activities and cognitive stimulation throughout life [212,213].
Appraising age requires an accurate estimation of individual reserves that account for biological and chronological age differences. In neuroscience, machine learning models establish an association between the number of years lived in good health and brain-imaging data with an accuracy of 2.1–4.9 years [214,215]. An individual’s brain age can also be calculated as the difference between chronological age and the predicted BA [216]. In obstetrics, the evaluation of gynaecological status takes into account both reproductive health and potential fertility. Therefore, an overall BA depends on the reserve capacities of individual systems and organs [217,218].
Variance in individual reserve capacities complicates the precise assessment of BA. Brain AA criteria are unclear since normal aging indicators are still missing [219], and no reference curves for brain changes have been created yet. Moreover, the rate of aging is hard to assess due to the lack of a standardised methodology that could take into account individual reserve potentials. Furthermore, methodological discrepancies lead to contradictory findings between different studies that vary between laboratories. For example, published studies report that AD results in the addition of 1.5 years to the brain age; MCI adds 1 year; multiple sclerosis (MS), 0.41 years; Parkinson’s disease (PD), 3 years; and schizophrenia, 5.5 years. However, the last two pathologies impact cognition in a milder and slower way than AD [220,221,222]. Other studies have reported an added brain age of between 6 and 9 years in AD, which is more consistent with the findings on MCI and PD [223].
The weaknesses and limitations of certain studies show the need for caution when assessing results. For example, the studies on age-related brain atrophy commonly have a cross-sectional design that is less accurate compared to the longitudinal one [224]. Many studies are based on small non-representative cohorts [225,226,227]; therefore, the usage of the resulting mathematical models is low. Another challenge for the AA concept is due to the focus on middle-aged adults and elderly patients in certain brain aging studies. These studies should not ignore individual prenatal pathologies and childhood trauma affecting the brain’s health and BA [228]. Applying the concept of AA to localised degeneration is complicated since different parts of the brain age unevenly [229]. For example, in localised ND, the BA assessment reflects the level of damage to the most vulnerable brain parts (e.g., substancia nigra and ruber nuclei in PD) [230,231,232]; however, one should also consider the brain resources that can minimise the atrophy effects [233]. In systematic ND, the brain ages faster than within localised ND [234,235], showing an apparent difference in the speed of atrophic changes [236].
Contemporary neuroscience lacks a clear explanation of the interaction between different causal factors due to the polyetiological nature of ND. It is still unclear whether chronic diseases lead to or result from ND [237,238] since the genetic, environmental and lifestyle factors interact in an undefined way [18,239,240]. Several articles have revealed a misalignment between dementia risk, cognitive performance and MBM levels [241,242]. Another disadvantage of AA studies is the inability to account for the influence of medications used by participants on study results [243]. Last but not least, AA represents a diagnostic but not pathognomonic signature in ND and in psychiatric diseases: schizophrenia, bipolar disorder and major depressive disorder [222,244,245], since the entire range of symptoms observed in these patients cannot be explained by brain aging only [222,246,247].
Finally, drug therapy could extend reserve abilities, for instance, in psychiatric conditions related to MCI and brain AA. Antidepressant medication has been reported to help convert MCI to true reversible MCI [248]. Certain cognitive disorders have demonstrated a reversible pattern in cognitive performance upon treatment [248,249]. Sex hormone replacement in ND reduced the risk, delayed the onset and slowed the progression of cognitive impairment [250]. Antioxidant-based therapy also alleviated the severity of the disease [251,252]. Recent ND studies have described a number of novel therapeutic options including specific antibodies, inducers of cell proliferation and NAD+ supplementation that are able to target mitophagy, protein aggregation and cellular senescence [9,18,253]. The observed treatment effects question the irreversible changes claimed by the AA concept.
5. Recommendations for Further Development and Improvement of AA Concept
5.1. Statistical Models
Building highly accurate machine learning models is the most common solution to distinguishing AA from normal aging. Recent articles suggest a set of approaches for improving the quality of these statistical solutions by reviewing the research data on MBM, the identification of potential gaps or inconsistencies, the incorporation of additional data sources or adjusting of research methodology, and the conduction of more rigorous statistical analyses, which will reveal any trends or correlations in the data that may have been overlooked. These suggestions have been commonly made by peers and experts who can provide valuable insights and suggestions. The following is the list of parameters that should be controlled to improve the diagnostic model of accelerated aging based on the use of specific molecular biomarkers:
-
○
Sample size. The number of individuals in the study can affect the statistical power of the analysis, and larger sample sizes generally provide more robust results.
-
○
Biomarker types. The choice of biomarkers can impact the diagnostic model used, as different types of biomarkers may require different statistical analyses.
-
○
Age range. The age range of the study population can influence the types of biomarkers identified, as some biomarkers may be more prevalent in certain age groups.
-
○
Data normalization. Normalization of the data is critical to ensure that data collection or processing differences do not affect the analysis.
-
○
Statistical methods. The choice of statistical methods used can impact the sensitivity and specificity of the analysis, and different methods may be more appropriate for different types of data.
Careful consideration of these parameters will be critical in developing diagnostic models based on specific molecular biomarkers of accelerated aging. Recommendations on future developments of diagnostic models also include the functional characterisation of organ-tissue-specific, single-cell-specific and disease-specific molecular clocks, the integration of epigenetics into diverse large longitudinal studies, the exploration of additional epigenomic marks of aging, and the establishing and generation of data in robust non-human aging models.
5.2. Molecular Clocks
Consideration of organ-specific and tissue-specific aging molecular clocks in non-human models would further resolve the complexity of aging processes. Several useful molecular clocks in mice have already been reported [254], including those specific to organs and tissues: the liver [255,256,257], lung [255,256], blood [256,258], heart and cortex [255], adipose, kidney, muscle [256], and multi-tissue [259].
5.3. Single-Cell Epigenomics
The study of aging at single-cell resolution represents an important direction in aging science. In particular, the variation in gene expression and the corresponding changes in aging tissues and organs [260,261] suggest that single-cell methods will be needed for a detailed analysis of accelerated aging. For instance, a rise in cell-to-cell variability with age (“epigenomic noise”) associated with methylation increase in both H3K4me3 and H3K27me3 and with transcriptional heterogeneity was shown in immune cells in blood [262] and in muscle stem cells [263], respectively. Although arranging epigenetic clocks at the single-cell level will be technically challenging, new emerging methods [264,265] and deep-learning-based computer algorithms [266,267,268] may help to construct them.
5.4. New Epigenetic Biomarkers
The search for new epigenetic marks of aging represents another challenge and opens up new exciting research opportunities. The connections between aging and DNA modifications other than methylation are puzzling. Nevertheless, the signs of such associations are obvious. For example, deregulation of histone H4 acetylation (H4K12) [269] and accumulation of histone variant H2A.Z [270] with age were observed in a mouse hippocampus. Changes related to the aging-related acetylation of H3 (H3K9ac) and H4 (H4K16ac) histones were found in the brain of AD patients [113,271]. Furthermore, investigations on the modulation of histone acetylation by SIRT6 HDAC linked to longevity in mammals could lead to potential pharmacological developments to target AA [272,273,274].
5.5. Consideration of Ageotypes
Diagnostic models can be adjusted to different personalised physiological subsets of aging, ageotypes [44]. This approach considers various factors such as genetics, molecular clock parameters, other epigenetic changes, lifestyle habits and environmental exposures that may influence individual aging rates. Researchers can further adjust the diagnostic model of accelerated aging by incorporating numerous potential biomarkers of aging and health metrics [44,275,276] to more accurately classify ageotypes and monitor the effectiveness of future interventions on each subset.
5.6. Genetic Predisposition to AA
Certain diseases can be considered as clinically important models of human genetic predisposition to AA. In particular, the phenotypes of genome instability disorders that result from autosomal recessive mutations are associated with ineffective genome maintenance systems, deficiencies in DNA helicase activity or an aberrant nuclear architecture. Three groups of these disorders include the sunlight hypersensitivity disorders: Xeroderma pigmentosum (XP), Cockayne syndrome (CS) and trichothiodystrophy; the ionizing radiation hypersensitivity disorders including Ataxia telangiectasia (AT) and Nijmegen breakage syndrome (NBS); and the progeroid disorders including Werner syndrome, Hutchinson–Gilford progeria syndrome, Bloom syndrome, Rothmund–Thompson syndrome and Fanconi anemia [277,278,279].
5.7. Application of AA Animal Models
The emergence of new animal models exhibiting aging-related features (accelerated senescence, damage of nuclear envelope, increased accumulation of genomic lesions) can significantly contribute to AA research [280]. Well-developed mouse aging models provide the possibility of testing interventions and modulators [254]. For example, acceleration of the epigenetic clock by a high-fat diet, and the effects of caloric restriction and rapamycin were demonstrated in mice models [255,257]. Emerging new animal aging models include a vertebrate with the shortest captive lifespan, killifish (Nothobranchius furzeri) [281,282,283,284,285,286,287], longevity models such as naked mole rats (Heterocephalus glaber, Fukomys mechowii) [288,289,290], Brandt’s bat (Myotis brandtii) [291,292,293], olm (Proteus anguinus) [294,295,296], bivalve (Arctica islandica) [297,298] and non-aging organisms: Hydra (Hydra vulgaris/Hydra magnipapillata) [299,300,301,302] and Planaria (Schmidtea mediterranea) [303,304,305]. The use of these models can provide the generation of new robust aging data.
6. Conclusions
-
i.
The concept of an increased rate of age-related changes has certain weaknesses and limitations that are considered in the current review. In particular, so far, no unified methodology and terminology has been established in the field. The studies that justify the AA concept have too low sample sizes. Some age-related changes appear to be reversible under certain conditions.
-
ii.
Aging MBM help to estimate the aging rate increase due to a developed pathology or the exhaustion of individual reserves. A large variety of MBM candidates in different combinations can be associated with the aging brain; however, their validation, clinical interpretation and use in disease subtyping remain a challenge.
-
iii.
Activation of the regenerative mechanisms, and restoring metabolic and energy molecular reserves with novel therapeutic options could be potential ways to decelerate aging in the CNS. For example, sex hormone replacement, antioxidant-based and target therapy, and environmental and lifestyle factors’ improvement may delay ND. Future longitudinal studies could provide clinics and society with more options to prevent AA and slow the aging rate.
7. Afterword: Aging Science History and Theories
Several theories have been postulated to explain the possible biological meaning or evolutionary role of aging [306]: the evolutionary advantage of the species (1890s, Weisman); the accumulated mutation theory (1952, Medawar); the antagonistic pleiotropy (1957, Williams); the replicative senescence (1965, Hayflick); and the disposable soma theory (1972, Kirkwood). Furthermore, the causative theories of aging can be arranged in two groups: (I) Genetic (programmed) and (II) Stochastic (damage) theories. Programmed theories include programmed longevity theory, endocrine theory and immunological theory. Stochastic theories include wear-and-tear theory, rate of living theory, cross-linking theory, free radicals theory and somatic DNA damage theory [307].
Finally, the theories of aging can be classified by biological level and divided into: molecular level theories including gene regulation, codon restriction, error catastrophe, somatic mutation and dysdifferentiation theories; cellular level theories including cellular senescence–telomere theory, free radical theory, wear-and-tear theory and apoptosis theory; and system level theories including neuroendocrine theory, immunologic theory and rate of living theory [308].
The first publications of AA experiments performed at the Laboratories of the Rockefeller Institute for Medical Research date back to the 1920s with “normal aging” and “rate of aging” terms applied to the effects of light on Drosophila inbred in the dark [309,310]. Since then, the numbers of references on “aging”, “aging rate” and “AA” has reached 614,132; 56,088 and 21,401, respectively [311].
In 1928, the Professor of Neurology of the Columbia University, Frederick Tilney, published the work “The aging of the human brain”, where, in particular, the AD patient brain was compared to the normally aged one in the diagnostic context of the number of plaques. In this work, he also stated the abundance of senile plaques in all human brains after the age of 90 years, the influence of unfavourable factors and diseases on the brain and the importance of aging brain research. Recorded a century ago, his words are worth repeating today: “It is amazing how little general or particular interest man has shown in the most important organ of his body and life. Up to the present time he has devoted relatively little attention and much less capital to the understanding of that part of his machinery which is the secret of his success and the only hope for his future progress, if not his actual salvation… The ridiculous stupidity of annually consecrating appalling sums of money to the savage purposes of destruction should in time shock human intelligence out of patronizing such futilities and into wiser realizations. Certainly, one liberally supported and effective brain institute would prove an incomparably more profitable investment for civilization than the most powerful battle fleet that ever sailed the seas.” (Tilney, 1928 [312]).
Abbreviations
The following abbreviations are used in this manuscript:
| 5-mC | 5-methylcytosine |
| AA | accelerated aging |
| AAG | gene related to accelerated aging |
| AD | Alzheimer’s disease |
| BA | biological age |
| BM | biomarker |
| circRNA | circular RNA |
| HC | healthy control |
| lncRNA | long non-coding RNA |
| MBM | molecular biomarkers |
| MCI | mild cognitive impairment |
| miRNA | microRNA |
| ncRNA | non-coding RNA |
| ND | neurodegeneration |
| NDG | gene that predispose to neurodegeneration |
| NV | neurovascular |
| NVU | neurovascular unit |
| PD | Parkinson’s disease |
Author Contributions
Y.S., S.A.A., B.S.E. and M.L. contributed to the conceptual idea of the paper. Y.S., K.N.-V.G. and R.H. formulated the objectives. Y.S., N.V.K., D.M. and K.L. wrote the manuscript. N.V.K., D.M. and K.L. prepared the figures for data presentation. N.V.K., G.L.S., D.S., A.K., S.M. and F.I. contributed to the literature review. All authors contributed to the writing of the original draft article. N.V.K. and B.S.E. performed the review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the ASPIRE, the technology program management pillar of Abu Dhabi’s Advanced Technology Research Council (ATRC), via the ASPIRE Precision Medicine Research Institute Abu Dhabi (VRI-20-10).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Isaev N.K., Stelmashook E.V., Genrikhs E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019;30:573–580. doi: 10.1515/revneuro-2018-0084. [DOI] [PubMed] [Google Scholar]
- 2.Brivio P., Paladini M.S., Racagni G., Riva M.A., Calabrese F., Molteni R. From healthy aging to frailty: In search of the underlying mechanisms. Curr. Med. Chem. 2019;26:3685–3701. doi: 10.2174/0929867326666190717152739. [DOI] [PubMed] [Google Scholar]
- 3.Feltes B.C., de Faria Poloni J., Bonatto D. Development and aging: Two opposite but complementary phenomena. Aging Health-A Syst. Biol. Perspect. 2015;40:74–84. doi: 10.1159/000364932. [DOI] [PubMed] [Google Scholar]
- 4.Bogeska R., Mikecin A.M., Kaschutnig P., Fawaz M., Büchler-Schäff M., Le D., Ganuza M., Vollmer A., Paffenholz S.V., Asada N., et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell. 2022;29:1273–1284. doi: 10.1016/j.stem.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelman E.R., Figueroa M.E. Human hematopoiesis: Aging and leukemogenic risk. Curr. Opin. Hematol. 2021;28:57. doi: 10.1097/MOH.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooten N.N., Pacheco N.L., Smith J.T., Evans M.K. The accelerated aging phenotype: The role of race and social determinants of health on aging. Ageing Res. Rev. 2022;73:101536. doi: 10.1016/j.arr.2021.101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester S.N., Zmora R., Schreiner P.J., Jacobs D.R., Jr., Roger V.L., Thorpe R.J., Jr., Kiefe C.I. Accelerated aging: A marker for social factors resulting in cardiovascular events? SSM-Popul. Health. 2021;13:100733. doi: 10.1016/j.ssmph.2021.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamczyk M.R., Nevado R.M., Barettino A., Fuster V., Andres V. Biological versus chronological aging: Jacc focus seminar. J. Am. Coll. Cardiol. 2020;75:919–930. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- 9.Vaquer-Alicea J., Diamond M.I. Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 2019;88:785–810. doi: 10.1146/annurev-biochem-061516-045049. [DOI] [PubMed] [Google Scholar]
- 10.Armada-Moreira A., Gomes J.I., Pina C.C., Savchak O.K., Gonçalves-Ribeiro J., Rei N., Pinto S., Morais T.P., Martins R.S., Ribeiro F.F., et al. Going the extra (synaptic) mile: Excitotoxicity as the road toward neurodegenerative diseases. Front. Cell. Neurosci. 2020;14:90. doi: 10.3389/fncel.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Margolick J.B., Ferrucci L. Accelerating aging research: How can we measure the rate of biologic aging? Exp. Gerontol. 2015;64:78–80. doi: 10.1016/j.exger.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melzer D., Pilling L.C., Ferrucci L. The genetics of human ageing. Nat. Rev. Genet. 2020;21:88–101. doi: 10.1038/s41576-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller M.W., Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh C., De A. Basics of aging theories and disease related aging-an overview. PharmaTutor. 2017;5:16–23. [Google Scholar]
- 16.Wadhwa R., Gupta R., Maurya P.K. Oxidative stress and accelerated aging in neurodegenerative and neuropsychiatric disorder. Curr. Pharm. Des. 2018;24:4711–4725. doi: 10.2174/1381612825666190115121018. [DOI] [PubMed] [Google Scholar]
- 17.Bersani F.S., Mellon S.H., Reus V.I., Wolkowitz O.M. Accelerated aging in serious mental disorders. Curr. Opin. Psychiatry. 2019;32:381. doi: 10.1097/YCO.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Ma Z., Cheng J., Lv Z. A genetic program theory of aging using an RNA population model. Ageing Res. Rev. 2014;13:46–54. doi: 10.1016/j.arr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2018;145:301–307. doi: 10.1016/B978-0-12-802395-2.00021-3. [DOI] [PubMed] [Google Scholar]
- 21.Sanz A., Stefanatos R.K. The mitochondrial free radical theory of aging: A critical view. Curr. Aging Sci. 2008;1:10–21. doi: 10.2174/1874609810801010010. [DOI] [PubMed] [Google Scholar]
- 22.Libertini G., Shubernetskaya O., Corbi G., Ferrara N. Is evidence supporting the subtelomere–telomere theory of aging? Biochemistry. 2021;86:1526–1539. doi: 10.1134/S0006297921120026. [DOI] [PubMed] [Google Scholar]
- 23.Xie L., Wu S., He R., Li S., Lai X., Wang Z. Identification of epigenetic dysregulation gene markers and immune landscape in kidney renal clear cell carcinoma by comprehensive genomic analysis. Front. Immunol. 2022;13:901662. doi: 10.3389/fimmu.2022.901662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Růžička M., Kulhánek P., Radová L., Čechová A., Špačková N., Fajkusová L., Réblová K. Dna mutation motifs in the genes associated with inherited diseases. PLoS ONE. 2017;12:e0182377. doi: 10.1371/journal.pone.0182377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korb M.K., Kimonis V.E., Mozaffar T. Multisystem proteinopathy: Where myopathy and motor neuron disease converge. Muscle Nerve. 2021;63:442–454. doi: 10.1002/mus.27097. [DOI] [PubMed] [Google Scholar]
- 26.Barja G. The mitochondrial free radical theory of aging. Prog. Mol. Biol. Transl. Sci. 2014;127:1–27. doi: 10.1016/B978-0-12-394625-6.00001-5. [DOI] [PubMed] [Google Scholar]
- 27.Amorim J.A., Coppotelli G., Rolo A.P., Palmeira C.M., Ross J.M., Sinclair D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022;18:243–258. doi: 10.1038/s41574-021-00626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esmaeili Y., Yarjanli Z., Pakniya F., Bidram E., Łos M.J., Eshraghi M., Klionsky D.J., Ghavami S., Zarrabi A. Targeting autophagy, oxidative stress, and er stress for neurodegenerative diseases treatment. J. Control. Release. 2022;345:147–175. doi: 10.1016/j.jconrel.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Pomatto L.C., Davies K.J. Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018;124:420–430. doi: 10.1016/j.freeradbiomed.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson D.J., Chandra T. Epigenetic age prediction. Aging Cell. 2021;20:e13452. doi: 10.1111/acel.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeffer S., Iadecola C. Revisiting the neurovascular unit. Nat. Neurosci. 2021;24:1198–1209. doi: 10.1038/s41593-021-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- 33.Zlokovic B.V. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics. 2008;5:409–414. doi: 10.1016/j.nurt.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X., De Silva T.M., Chen J., Faraci F.M. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ. Res. 2017;120:449–471. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lähteenvuo J., Rosenzweig A. Effects of aging on angiogenesis. Circ. Res. 2012;110:1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson A.R., Sweeney M.D., Sagare A.P., Zlokovic B.V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862:887–900. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm I., Nyúl-Tóth Á., Kozma M., Farkas A.E., Krizbai I.A. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H1000–H1012. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z.D., Wang D.Q., Tan E.K. The role of neurovascular unit in neurodegeneration. Front. Cell. Neurosci. 2022;16:870631. doi: 10.3389/fncel.2022.870631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer D., Guérit S., Puetz T., Khel M.I., Armbrust M., Dunst M., Macas J., Zinke J., Devraj G., Jia X., et al. Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood–brain barrier in acute ischemic stroke. Acta Neuropathol. 2022;144:305–337. doi: 10.1007/s00401-022-02452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong H.W., Diéguez-Hurtado R., Arf H., Song J., Park H., Kruse K., Sorokin L., Adams R.H. Single-cell transcriptomics reveals functionally specialized vascular endothelium in brain. eLife. 2022;11:e57520. doi: 10.7554/eLife.57520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y., Xie Y.Z., Liu Y.S. Accelerated aging-related transcriptome alterations in neurovascular unit cells in the brain of Alzheimer’s disease. Front. Aging Neurosci. 2022;14:949074. doi: 10.3389/fnagi.2022.949074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia X., Chen W., McDermott J., Han J.D.J. Molecular and phenotypic biomarkers of aging. F1000Research. 2017;6:860. doi: 10.12688/f1000research.10692.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahadi S., Zhou W., Schüssler-Fiorenza Rose S.M., Sailani M.R., Contrepois K., Avina M., Ashland M., Brunet A., Snyder M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020;26:83–90. doi: 10.1038/s41591-019-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Z., von Figura G., Liu Y., Kraus J.M., Torrice C., Dillon P., Rudolph-Watabe M., Ju Z., Kestler H.A., Sanoff H., et al. Lifestyle impacts on the aging-associated expression of biomarkers of dna damage and telomere dysfunction in human blood. Aging Cell. 2010;9:607–615. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvath S., Zhang Y., Langfelder P., Kahn R.S., Boks M.P., van Eijk K., van den Berg L.H., Ophoff R.A. Aging effects on dna methylation modules in human brain and blood tissue. Genome Biol. 2012;13:1–18. doi: 10.1186/gb-2012-13-10-r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day K., Waite L.L., Thalacker-Mercer A., West A., Bamman M.M., Brooks J.D., Myers R.M., Absher D. Differential dna methylation with age displays both common and dynamic features across human tissues that are influenced by cpg landscape. Genome Biol. 2013;14:1–19. doi: 10.1186/gb-2013-14-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greer E.L., Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer E.L., Maures T.J., Hauswirth A.G., Green E.M., Leeman D.S., Maro G.S., Han S., Banko M.R., Gozani O., Brunet A. Members of the h3k4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L., Greer C., Eisenman R.N., Secombe J. Essential functions of the histone demethylase lid. PLoS Genet. 2010;6:e1001221. doi: 10.1371/journal.pgen.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Djeghloul D., Kuranda K., Kuzniak I., Barbieri D., Naguibneva I., Choisy C., Bories J.C., Dosquet C., Pla M., Vanneaux V., et al. Age-associated decrease of the histone methyltransferase suv39h1 in hsc perturbs heterochromatin and b lymphoid differentiation. Stem Cell Rep. 2016;6:970–984. doi: 10.1016/j.stemcr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C.L., Pu M., Wang W., Chaturbedi A., Emerson F.J., Lee S.S. Region-specific h3k9me3 gain in aged somatic tissues in caenorhabdi-tis elegans. PLoS Genet. 2021;17:e1009432. doi: 10.1371/journal.pgen.1009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J.H., Kim E.W., Croteau D.L., Bohr V.A. Heterochromatin: An epigenetic point of view in aging. Exp. Mol. Med. 2020;52:1466–1474. doi: 10.1038/s12276-020-00497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R.S., Zhang Y. Role of histone h3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 55.Siebold A.P., Banerjee R., Tie F., Kiss D.L., Moskowitz J., Harte P.J. Polycomb repressive complex 2 and trithorax modulate Drosophila longevity and stress resistance. Proc. Natl. Acad. Sci. USA. 2010;107:169–174. doi: 10.1073/pnas.0907739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni Z., Ebata A., Alipanahiramandi E., Lee S.S. Two set domain containing genes link epigenetic changes and aging in caenorhabditis elegans. Aging Cell. 2012;11:315–325. doi: 10.1111/j.1474-9726.2011.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maures T.J., Greer E.L., Hauswirth A.G., Brunet A. The h3k27 demethylase utx-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell. 2011;10:980–990. doi: 10.1111/j.1474-9726.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L., Cheung T.H., Charville G.W., Hurgo B.M., Leavitt T., Shih J., Brunet A., Rando T.A. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumgart M., Groth M., Priebe S., Savino A., Testa G., Dix A., Ripa R., Spallotta F., Gaetano C., Ori M., et al. Rna-seq of the aging brain in the short-lived fish N. furzeri–conserved pathways and novel genes associated with neurogenesis. Aging Cell. 2014;13:965–974. doi: 10.1111/acel.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters M.J., Joehanes R., Pilling L.C., Schurmann C., Conneely K.N., Powell J., Reinmaa E., Sutphin G.L., Zhernakova A., Schramm K., et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015;6:8570. doi: 10.1038/ncomms9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Khanna A., Li N., Wang E. Circulatory mir-34a as an RNA-based, noninvasive biomarker for brain aging. Aging. 2011;3:985. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhahbi J.M. Circulating small noncoding rnas as biomarkers of aging. Ageing Res. Rev. 2014;17:86–98. doi: 10.1016/j.arr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Grammatikakis I., Panda A.C., Abdelmohsen K., Gorospe M. Long noncoding rnas (lncrnas) and the molecular hallmarks of aging. Aging. 2014;6:992. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kour S., Rath P.C. Long noncoding rnas in aging and age-related diseases. Ageing Res. Rev. 2016;26:1–21. doi: 10.1016/j.arr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Finkel D., Pedersen N.L., Plomin R., McClearn G.E. Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: The swedish adoption/twin study of aging. Dev. Psychol. 1998;34:1400. doi: 10.1037/0012-1649.34.6.1400. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds C.A., Finkel D. A meta-analysis of heritability of cognitive aging: Minding the “missing heritability” gap. Neuropsychol. Rev. 2015;25:97–112. doi: 10.1007/s11065-015-9280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blauwendraat C., Pletnikova O., Geiger J.T., Murphy N.A., Abramzon Y., Rudow G., Mamais A., Sabir M.S., Crain B., Ahmed S., et al. Genetic analysis of neurodegenerative diseases in a pathology cohort. Neurobiol. Aging. 2019;76:214.e1–214.e9. doi: 10.1016/j.neurobiolaging.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cochran J.N., Geier E.G., Bonham L.W., Newberry J.S., Amaral M.D., Thompson M.L., Lasseigne B.N., Karydas A.M., Roberson E.D., Cooper G.M., et al. Non-coding and loss-of-function coding variants in tet2 are associated with multiple neurodegenerative diseases. Am. J. Hum. Genet. 2020;106:632–645. doi: 10.1016/j.ajhg.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cirulli E.T., Lasseigne B.N., Petrovski S., Sapp P.C., Dion P.A., Leblond C.S., Couthouis J., Lu Y.F., Wang Q., Krueger B.J., et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung S.J., Kim M.J., Kim J., Kim Y.J., You S., Koh J., Kim S.Y., Lee J.H. Exome array study did not identify novel variants in Alzheimer’s disease. Neurobiol. Aging. 2014;35:1958.e13–1958.e14. doi: 10.1016/j.neurobiolaging.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Nikolac Perkovic M., Pivac N. Genetic Markers of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019;1192:27–52. doi: 10.1007/978-981-32-9721-0_3. [DOI] [PubMed] [Google Scholar]
- 72.Song W., Hooli B., Mullin K., Jin S.C., Cella M., Ulland T.K., Wang Y., Tanzi R.E., Colonna M. Alzheimer’s disease-associated trem2 variants exhibit either decreased or in-creased ligand-dependent activation. Alzheimer’s Dement. 2017;13:381–387. doi: 10.1016/j.jalz.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz A., Dols-Icardo O., Bullido M.J., Pastor P., Rodríguez-Rodríguez E., López de Munain A., de Pancorbo M.M., Pérez-Tur J., Alvarez V., Antonell A., et al. Assessing the role of the trem2 p. r47h variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiol. Aging. 2014;35:444.e1–444.e4. doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Mehrjoo Z., Najmabadi A., Abedini S.S., Mohseni M., Kamali K., Najmabadi H., Khorram Khorshid H.R. Association study of the trem2 gene and identification of a novel variant in exon 2 in iranian patients with late-onset Alzheimer’s disease. Med. Princ. Pract. 2015;24:351–354. doi: 10.1159/000430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S., Younkin S., et al. Trem2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., et al. Variant of trem2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang T., Tan L., Chen Q., Tan M.S., Zhou J.S., Zhu X.C., Lu H., Wang H.F., Zhang Y.D., Yu J.T. A rare coding variant in trem2 increases risk for Alzheimer’s disease in han chinese. Neurobiol. Aging. 2016;42:217.e1–217.e3. doi: 10.1016/j.neurobiolaging.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 78.Jin S.C., Carrasquillo M.M., Benitez B.A., Skorupa T., Carrell D., Patel D., Lincoln S., Krishnan S., Kachadoorian M., Reitz C., et al. Trem2 is associated with increased risk for Alzheimer’s disease in african amer-icans. Mol. Neurodegener. 2015;10:1–7. doi: 10.1186/s13024-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berge G., Sando S.B., Rongve A., Aarsland D., White L.R. Onset of dementia with lewy bodies is delayed for carriers of the apolipoprotein e ε2 genotype in a norwegian cohort. Mov. Disord. 2014;29:S220. doi: 10.1136/jnnp-2013-307228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calvo A., Chiò A. Sclerosi laterale amiotrofica come modello di gestione interdisciplinare. SALUTE E SOCIETÀ. 2015;3:173–184. doi: 10.3280/SES2015-003014. [DOI] [Google Scholar]
- 81.Borroni B., Ferrari F., Galimberti D., Nacmias B., Barone C., Bagnoli S., Fenoglio C., Piaceri I., Archetti S., Bonvicini C., et al. Heterozygous trem2 mutations in frontotemporal dementia. Neurobiol. Aging. 2014;35:934.e7–934.e10. doi: 10.1016/j.neurobiolaging.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 82.Rayaprolu S., Mullen B., Baker M., Lynch T., Finger E., Seeley W.W., Hatanpaa K.J., Lomen-Hoerth C., Kertesz A., Bigio E.H., et al. Trem2 in neurodegeneration: Evidence for association of the p. r47h variant with frontotemporal dementia and parkinson’s disease. Mol. Neurodegener. 2013;8:1–5. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cady J., Koval E.D., Benitez B.A., Zaidman C., Jockel-Balsarotti J., Allred P., Baloh R.H., Ravits J., Simpson E., Appel S.H., et al. Trem2 variant p. r47h as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:449–453. doi: 10.1001/jamaneurol.2013.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slattery C.F., Beck J.A., Harper L., Adamson G., Abdi Z., Uphill J., Campbell T., Druyeh R., Mahoney C.J., Rohrer J.D., et al. R47h trem2 variant increases risk of typical early-onset Alzheimer’s disease but not of prion or frontotemporal dementia. Alzheimer’s Dement. 2014;10:602–608. doi: 10.1016/j.jalz.2014.05.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez Murcia J.D., Schmutz C., Munger C., Perkes A., Gustin A., Peterson M., Ebbert M.T., Norton M.C., Tschanz J.T., Munger R.G., et al. Assessment of trem2 rs75932628 association with Alzheimer’s disease in a population-based sample: The cache county study. Neurobiol. Aging. 2013;34:2889. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walton R.L., Soto-Ortolaza A.I., Murray M.E., Lorenzo-Betancor O., Ogaki K., Heckman M.G., Rayaprolu S., Rademakers R., Ertekin-Taner N., Uitti R.J., et al. Trem2 p. r47h substitution is not associated with dementia with lewy bodies. Neurol. Genet. 2016;2:e85. doi: 10.1212/NXG.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun J., Zhu Z., Chen K., Wei D., Li X., Li H., Zhang J., Chen X., Chen Y., Zhang Z. Apoe ε4 allele accelerates age-related multi-cognitive decline and white matter damage in non-demented elderly. Aging. 2020;12:12019. doi: 10.18632/aging.103367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goel N., Karir P., Garg V.K. Role of DNA methylation in human age prediction. Mech. Ageing Dev. 2017;166:33–41. doi: 10.1016/j.mad.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Jung M., Pfeifer G.P. Aging and DNA methylation. BMC Biol. 2015;13:1–8. doi: 10.1186/s12915-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lister R., Pelizzola M., Dowen R.H., Hawkins R.D., Hon G., Tonti-Filippini J., Nery J.R., Lee L., Ye Z., Ngo Q.M., et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 92.Zampieri M., Ciccarone F., Calabrese R., Franceschi C., Bürkle A., Caiafa P. Reconfiguration of DNA methylation in aging. Mech. Ageing Dev. 2015;151:60–70. doi: 10.1016/j.mad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., Klotzle B., Bibikova M., Fan J.B., Gao Y., et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fraga M.F., Ballestar E., Paz M.F., Ropero S., Setien F., Ballestar M.L., Heine-Suñer D., Cigudosa J.C., Urioste M., Benitez J., et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryan C.P. “Epigenetic clocks”: Theory and applications in human biology. Am. J. Hum. Biol. 2021;33:e23488. doi: 10.1002/ajhb.23488. [DOI] [PubMed] [Google Scholar]
- 96.Martino D., Loke Y.J., Gordon L., Ollikainen M., Cruickshank M.N., Saffery R., Craig J.M. Longitudinal, genome-scale analysis of dna methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biol. 2013;14:R42. doi: 10.1186/gb-2013-14-5-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bjornsson H.T., Sigurdsson M.I., Fallin M.D., Irizarry R.A., Aspelund T., Cui H., Yu W., Rongione M.A., Ekström T.J., Harris T.B., et al. Intra-individual change over time in dna methylation with familial clustering. JAMA. 2008;299:2877–2883. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wynford-Thomas D. Telomeres, p53 and cellular senescence. Oncol Res. 1996;8:387–398. [PubMed] [Google Scholar]
- 99.von Zglinicki T. Telomeres: Influencing the rate of aging. Ann. N. Y. Acad. Sci. 1998;854:318–327. doi: 10.1111/j.1749-6632.1998.tb09912.x. [DOI] [PubMed] [Google Scholar]
- 100.Teschendorff A.E., West J., Beck S. Age-associated epigenetic drift: Implications, and a case of epigenetic thrift? Hum. Mol. Genet. 2013;22:R7–R15. doi: 10.1093/hmg/ddt375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horvath S. Dna methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levine M.E., Lu A.T., Quach A., Chen B.H., Assimes T.L., Bandinelli S., Hou L., Baccarelli A.A., Stewart J.D., Li Y., et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marioni R.E., Shah S., McRae A.F., Ritchie S.J., Muniz-Terrera G., Harris S.E., Gibson J., Redmond P., Cox S.R., Pattie A., et al. The epigenetic clock is correlated with physical and cognitive fitness in the lothian birth cohort 1936. Int. J. Epidemiol. 2015;44:1388–1396. doi: 10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levine M.E., Lu A.T., Bennett D.A., Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging. 2015;7:1198. doi: 10.18632/aging.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horvath S., Langfelder P., Kwak S., Aaronson J., Rosinski J., Vogt T.F., Eszes M., Faull R.L., Curtis M.A., Waldvogel H.J., et al. Huntington’s disease accelerates epigenetic aging of human brain and disrupts dna methylation levels. Aging. 2016;8:1485. doi: 10.18632/aging.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grodstein F., Lemos B., Yu L., Klein H.U., Iatrou A., Buchman A.S., Shireby G.L., Mill J., Schneider J.A., De Jager P.L., et al. The association of epigenetic clocks in brain tissue with brain pathologies and common aging phenotypes. Neurobiol. Dis. 2021;157:105428. doi: 10.1016/j.nbd.2021.105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grodstein F., Lemos B., Yu L., Iatrou A., De Jager P.L., Bennett D.A. Characteristics of epigenetic clocks across blood and brain tissue in older women and men. Front. Neurosci. 2021;14:555307. doi: 10.3389/fnins.2020.555307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fraga M.F., Esteller M. Epigenetics and aging: The targets and the marks. Trends Genet. 2007;23:413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Han S., Brunet A. Histone methylation makes its mark on longevity. Trends Cell Biol. 2012;22:42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gjoneska E., Pfenning A.R., Mathys H., Quon G., Kundaje A., Tsai L.H., Kellis M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao Q., Wang W., Williams J.B., Yang F., Wang Z.J., Yan Z. Targeting histone K4 trimethylation for treatment of cognitive and synaptic deficits in mouse models of Alzheimer’s disease. Sci. Adv. 2020;6:eabc8096. doi: 10.1126/sciadv.abc8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nativio R., Donahue G., Berson A., Lan Y., Amlie-Wolf A., Tuzer F., Toledo J.B., Gosai S.J., Gregory B.D., Torres C., et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018;21:497–505. doi: 10.1038/s41593-018-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santana D.A., Smith MD A.C., Chen E.S. Histone modifications in alzheimer’s disease. Genes. 2023;14:347. doi: 10.3390/genes14020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang B., Dean B., Thomas E. Disease-and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl. Psychiatry. 2011;1:e64. doi: 10.1038/tp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chaput D., Kirouac L., Stevens S.M., Jr., Padmanabhan J. Potential role of PCTAIRE-2, PCTAIRE-3 and P-Histone H4 in amyloid precursor protein-dependent Alzheimer pathology. Oncotarget. 2016;7:8481. doi: 10.18632/oncotarget.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ogawa O., Zhu X., Lee H.G., Raina A., Obrenovich M.E., Bowser R., Smith M.A. Ectopic localization of phosphorylated histone H3 in Alzheimer’s disease: A mitotic catastrophe? Acta Neuropathol. 2003;105:524–528. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- 118.D’haene E., Vergult S. Interpreting the impact of noncoding structural variation in neurodevelopmental disorders. Genet. Med. 2021;23:34–46. doi: 10.1038/s41436-020-00974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sherazi S.A.M., Abbasi A., Jamil A., Uzair M., Ikram A., Qamar S., Olamide A.A., Arshad M., Fried P.J., Ljubisavljevic M., et al. Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases. Neural Regen. Res. 2023;18:959–968. doi: 10.4103/1673-5374.355751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang D.Q., Fu P., Yao C., Zhu L.S., Hou T.Y., Chen J.G., Lu Y., Liu D., Zhu L.Q. Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseas-es. Mol. Ther. Nucleic Acids. 2018;10:269–276. doi: 10.1016/j.omtn.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mishra P., Kumar S. Association of lncRNA with regulatory molecular factors in brain and their role in the pathophysiology of schizophrenia. Metab. Brain Dis. 2021;36:849–858. doi: 10.1007/s11011-021-00692-w. [DOI] [PubMed] [Google Scholar]
- 122.Coccia E.M., Cicala C., Charlesworth A., Ciccarelli C., Rossi G.B., Philipson L., Sorrentino V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biol. 1992;12:3514–3521. doi: 10.1128/mcb.12.8.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pickard M., Mourtada-Maarabouni M., Williams G. Long non-coding RNA gas5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 124.Mourtada-Maarabouni M., Pickard M., Hedge V., Farzaneh F., Williams G. Gas5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 125.Tang S., Buchman A.S., De Jager P.L., Bennett D.A., Epstein M.P., Yang J. Novel variance-component TWAS method for studying complex human diseases with applications to Alzheimer’s dementia. PLoS Genet. 2021;17:e1009482. doi: 10.1371/journal.pgen.1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang W.S., Dunckley T., Beach T.G., Grover A., Mastroeni D., Walker D.G., Caselli R.J., Kukull W.A., McKeel D., Morris J.C., et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genom. 2007;28:311–322. doi: 10.1152/physiolgenomics.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mus E., Hof P.R., Tiedge H. Dendritic bc200 RNA in aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maoz R., Garfinkel B.P., Soreq H. Alzheimer’s disease and ncRNAs. Neuroepigenomics Aging Dis. 2017;978:337–361. doi: 10.1007/978-3-319-53889-1_18. [DOI] [PubMed] [Google Scholar]
- 129.Fiore R., Khudayberdiev S., Saba R., Schratt G. Micro-RNA function in the nervous system. Prog. Mol. Biol. Transl. Sci. 2011;102:47–100. doi: 10.1016/B978-0-12-415795-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 130.Goodall E.F., Heath P.R., Bandmann O., Kirby J., Shaw P.J. Neuronal dark matter: The emerging role of microRNAs in neurodegeneration. Front. Cell. Neurosci. 2013;7:178. doi: 10.3389/fncel.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dickson J.R., Kruse C., Montagna D.R., Finsen B., Wolfe M.S. Alternative polyadenylation and mir-34 family members regulate tau expression. J. Neurochem. 2013;127:739–749. doi: 10.1111/jnc.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith P.Y., Hernandez-Rapp J., Jolivette F., Lecours C., Bisht K., Goupil C., Dorval V., Parsi S., Morin F., Planel E., et al. Mir-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015;24:6721–6735. doi: 10.1093/hmg/ddv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Santa-Maria I., Alaniz M.E., Renwick N., Cela C., Fulga T.A., Van Vactor D., Tuschl T., Clark L.N., Shelanski M.L., McCabe B.D., et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Investig. 2015;125:681–686. doi: 10.1172/JCI78421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hébert S.S., Papadopoulou A.S., Smith P., Galas M.C., Planel E., Silahtaroglu A.N., Sergeant N., Buée L., De Strooper B. Genetic ablation of dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 2010;19:3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 135.Cai Z., Zhao Y., Zhao B. Roles of glycogen synthase kinase 3 in Alzheimer’s disease. Curr. Alzheimer Res. 2012;9:864–879. doi: 10.2174/156720512802455386. [DOI] [PubMed] [Google Scholar]
- 136.Mohamed J.S., Lopez M.A., Boriek A.M. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3β. J. Biol. Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao Z.B., Wu L., Xiong R., Wang L.L., Zhang B., Wang C., Li H., Liang L., Chen S.D. MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase l1 (uchl1) expression in the pathogenesis of Alzheimer’s disease. Neuroscience. 2014;275:232–237. doi: 10.1016/j.neuroscience.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 138.Law P.T., Ching A.K., Chan A.W., Wong Q.W., Wong C.K., To K.F., Wong N. Mir-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis. 2012;33:1134–1141. doi: 10.1093/carcin/bgs130. [DOI] [PubMed] [Google Scholar]
- 139.El Ouaamari A., Baroukh N., Martens G.A., Lebrun P., Pipeleers D., Van Obberghen E. mir-375 targets 3-phosphoinositide–dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic β-cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Inukai S., de Lencastre A., Turner M., Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS ONE. 2012;7:e40028. doi: 10.1371/journal.pone.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang G., Song Y., Zhou X., Deng Y., Liu T., Weng G., Yu D., Pan S. MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol. Med. Rep. 2015;12:3081–3088. doi: 10.3892/mmr.2015.3728. [DOI] [PubMed] [Google Scholar]
- 142.Lei X., Lei L., Zhang Z., Zhang Z., Cheng Y. Downregulated mir-29c correlates with increased bace1 expression in sporadic Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2015;8:1565. [PMC free article] [PubMed] [Google Scholar]
- 143.Zong Y., Wang H., Dong W., Quan X., Zhu H., Xu Y., Huang L., Ma C., Qin C. mir-29c regulates bace1 protein expression. Brain Res. 2011;1395:108–115. doi: 10.1016/j.brainres.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 144.Hébert S.S., Horré K., Nicolaï L., Bergmans B., Papadopoulou A.S., Delacourte A., De Strooper B. MicroRNA regulation of Alzheimer’s amyloid precursor protein expression. Neurobiol. Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 145.Zhu H.C., Wang L.M., Wang M., Song B., Tan S., Teng J.F., Duan D.X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting bace1. Brain Res. Bull. 2012;88:596–601. doi: 10.1016/j.brainresbull.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 146.Wang W.X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA mir-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kennerdell J.R., Liu N., Bonini N.M. Mir-34 inhibits polycomb repressive complex 2 to modulate chaperone expression and promote healthy brain aging. Nat. Commun. 2018;9:4188. doi: 10.1038/s41467-018-06592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng C., Li W., Zhang Z., Yoshimura S., Hao Q., Zhang C., Wang Z. MicroRNA-144 is regulated by activator protein-1 (ap-1) and decreases expression of Alzheimer disease-related a disintegrin and metalloprotease 10 (adam10) J. Biol. Chem. 2013;288:13748–13761. doi: 10.1074/jbc.M112.381392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y., Kim M.S., Jia B., Yan J., Zuniga-Hertz J.P., Han C., Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang G., Li J., Purkayastha S., Tang Y., Zhang H., Yin Y., Li B., Liu G., Cai D. Hypothalamic programming of systemic ageing involving ikk-β, nf-κb and gnrh. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mohammed C.P.D., Park J.S., Nam H.G., Kim K. MicroRNAs in brain aging. Mech. Ageing Dev. 2017;168:3–9. doi: 10.1016/j.mad.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 152.Abdelmohsen K., Panda A.C., De S., Grammatikakis I., Kim J., Ding J., Noh J.H., Kim K.M., Mattison J.A., de Cabo R., et al. Circular RNAs in monkey muscle: Age-dependent changes. Aging. 2015;7:903. doi: 10.18632/aging.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynami-cally expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 154.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 155.Lukiw W.J. Circular RNA (circRNA) in Alzheimer’s disease (ad) Front. Genet. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bao N., Liu J., Peng Z., Zhang R., Ni R., Li R., Wu J., Liu Z., Pan B. Identification of circRNA-miRNA-mRNA networks to explore the molecular mechanism and immune regulation of postoperative neurocognitive disorder. Aging. 2022;14:8374. doi: 10.18632/aging.204348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahmoudi E., Fitzsimmons C., Geaghan M.P., Shannon Weickert C., Atkins J.R., Wang X., Cairns M.J. Circular RNA biogenesis is decreased in postmortem cortical gray matter in schizophrenia and may alter the bioavailability of associated miRNA. Neuropsychopharmacology. 2019;44:1043–1054. doi: 10.1038/s41386-019-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Broekmans F., Soules M., Fauser B. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 159.Frungieri M.B., Calandra R.S., Bartke A., Matzkin M.E. Male and female gonadal ageing: Its impact on health span and life span. Mech. Ageing Dev. 2021;197:111519. doi: 10.1016/j.mad.2021.111519. [DOI] [PubMed] [Google Scholar]
- 160.De la Rochebrochard E., Thonneau P. Paternal age 40 years: An important risk factor for infertility. Am. J. Obstet. Gynecol. 2003;189:901–905. doi: 10.1067/S0002-9378(03)00753-1. [DOI] [PubMed] [Google Scholar]
- 161.Brahem S., Mehdi M., Elghezal H., Saad A. The effects of male aging on semen quality, sperm dna fragmentation and chromosomal abnormalities in an infertile population. J. Assist. Reprod. Genet. 2011;28:425–432. doi: 10.1007/s10815-011-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paul C., Robaire B. Ageing of the male germ line. Nat. Rev. Urol. 2013;10:227–234. doi: 10.1038/nrurol.2013.18. [DOI] [PubMed] [Google Scholar]
- 163.Frungieri M.B., Calandra R.S., Bartke A., Matzkin M.E. Ageing and inflammation in the male reproductive tract. Andrologia. 2018;50:e13034. doi: 10.1111/and.13034. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Y., Ni S., Li C., Song L., Zhang S. Gonadal rejuvenation of mice by growth differentiation factor 11. J. Gerontol. Ser. A. 2022;77:892–901. doi: 10.1093/gerona/glab343. [DOI] [PubMed] [Google Scholar]
- 165.Mularoni V., Esposito V., Di Persio S., Vicini E., Spadetta G., Berloco P., Fanelli F., Mezzullo M., Pagotto U., Pelusi C., et al. Age-related changes in human Leydig cell status. Hum. Reprod. 2020;35:2663–2676. doi: 10.1093/humrep/deaa271. [DOI] [PubMed] [Google Scholar]
- 166.Mahmoud A., Goemaere S., El-Garem Y., Van Pottelbergh I., Comhaire F., Kaufman J. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J. Clin. Endocrinol. Metab. 2003;88:179–184. doi: 10.1210/jc.2002-020408. [DOI] [PubMed] [Google Scholar]
- 167.Golan R., Scovell J.M., Ramasamy R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. Aging Male. 2015;18:201–204. doi: 10.3109/13685538.2015.1052392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Handelsman D.J., Staraj S. Testicular size: The effects of aging, malnutrition, and illness. J. Androl. 1985;6:144–151. doi: 10.1002/j.1939-4640.1985.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 169.Ilacqua A., Izzo G., Emerenziani G.P., Baldari C., Aversa A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018;16:1–11. doi: 10.1186/s12958-018-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaufman J. Ageing of the hypothalamo-pituitary-testicular axis in men. Horm. Res. Paediatr. 1995;43:25–28. doi: 10.1159/000184233. [DOI] [PubMed] [Google Scholar]
- 171.Harman S.M., Metter E.J., Tobin J.D., Pearson J., Blackman M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J. Clin. Endocrinol. Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 172.Spinelli R., Parrillo L., Longo M., Florese P., Desiderio A., Zatterale F., Miele C., Raciti G.A., Beguinot F. Molecular basis of ageing in chronic metabolic diseases. J. Endocrinol. Investig. 2020;43:1373–1389. doi: 10.1007/s40618-020-01255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Emami M., Agbaedeng T.A., Thomas G., Middeldorp M.E., Thiyagarajah A., Wong C.X., Elliott A.D., Gallagher C., Hendriks J.M.L., Lau D.H., et al. Accelerated biological aging secondary to cardiometabolic risk factors is a predictor of cardiovascular mortality: A systematic review and meta-analysis. Can. J. Cardiol. 2022;38:365–375. doi: 10.1016/j.cjca.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 174.Russell S.J., Kahn C.R. Endocrine regulation of ageing. Nat. Rev. Mol. Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 175.Van den Beld A.W., Kaufman J.M., Zillikens M.C., Lamberts S.W., Egan J.M., van der Lely A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018;6:647–658. doi: 10.1016/S2213-8587(18)30026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park J., Cho B., Kwon H., Lee C. Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in korean men. Arch. Gerontol. Geriatr. 2009;49:7–12. doi: 10.1016/j.archger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 177.Nakamura E., Moritani T., Kanetaka A. Effects of habitual physical exercise on physiological age in men aged 20–85 years as estimated using principal component analysis. Eur. J. Appl. Physiol. Occup. Physiol. 1996;73:410–418. doi: 10.1007/BF00334417. [DOI] [PubMed] [Google Scholar]
- 178.Nakamura E., Moritani T., Kanetaka A. Biological age versus physical fitness age in women. Eur. J. Appl. Physiol. Occup. Physiol. 1990;61:202–208. doi: 10.1007/BF00357600. [DOI] [PubMed] [Google Scholar]
- 179.Nunn A.V., Bell J.D., Guy G.W. Lifestyle-induced metabolic inflexibility and accelerated ageing syndrome: Insulin resistance, friend or foe? Nutr. Metab. 2009;6:1–26. doi: 10.1186/1743-7075-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chahal H., Drake W. The endocrine system and ageing. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 181.Chandra A., Rajawat J. Skeletal aging and osteoporosis: Mechanisms and therapeutics. Int. J. Mol. Sci. 2021;22:3553. doi: 10.3390/ijms22073553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gaffney-Stomberg E., Hughes J.M., Guerriere K.I., Staab J.S., Cable S.J., Bouxsein M.L., McClung J.P. Once daily calcium (1000 mg) and vitamin d (1000 iu) supplementation during military training prevents increases in biochemical markers of bone resorption but does not affect tibial microarchitecture in army recruits. Bone. 2022;155:116269. doi: 10.1016/j.bone.2021.116269. [DOI] [PubMed] [Google Scholar]
- 183.Wang S., Luo Z., Luo H., Li Z., Yuan Z., Tang J., Lin L., Du Z., Zhou J.R. Effects of a calcium/vitamin d/zinc combination on anti-osteoporosis in ovariectomized rats. J. Trace Elem. Med. Biol. 2023;77:127138. doi: 10.1016/j.jtemb.2023.127138. [DOI] [PubMed] [Google Scholar]
- 184.Sfeir J.G., Drake M.T., Khosla S., Farr J.N. Skeletal aging. Mayo Clin. Proc. 2022;97:1194–1208. doi: 10.1016/j.mayocp.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Carter M.I., Hinton P.S. Physical activity and bone health. MO Med. 2014;111:59. [PMC free article] [PubMed] [Google Scholar]
- 186.Orwoll E.S., Adler R.A., Amin S., Binkley N., Lewiecki E.M., Petak S.M., Shapses S.A., Sinaki M., Watts N.B., Sibonga J.D. Skeletal health in long-duration astronauts: Nature, assessment, and management recommendations from the NASA bone summit. J. Bone Miner. Res. 2013;28:1243–1255. doi: 10.1002/jbmr.1948. [DOI] [PubMed] [Google Scholar]
- 187.Boismal F., Serror K., Dobos G., Zuelgaray E., Bensussan A., Michel L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. M/S. 2020;36:1163–1172. doi: 10.1051/medsci/2020232. [DOI] [PubMed] [Google Scholar]
- 188.Baumann L. Skin ageing and its treatment. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007;211:241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 189.Kohl E., Steinbauer J., Landthaler M., Szeimies R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011;25:873–884. doi: 10.1111/j.1468-3083.2010.03963.x. [DOI] [PubMed] [Google Scholar]
- 190.Brincat M., Muscat Baron Y., Galea R. Estrogens and the skin. Climacteric. 2005;8:110–123. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 191.Park H.Y., Kim J.H., Jung M., Chung C.H., Hasham R., Park C.S., Choi E.H. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 2011;20:969–974. doi: 10.1111/j.1600-0625.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 192.Bonté F., Girard D., Archambault J.C., Desmoulière A. Skin changes during ageing. Biochem. Cell Biol. Ageing Pt II Clin. Sci. 2019;91:249–280. doi: 10.1007/978-981-13-3681-2_10. [DOI] [PubMed] [Google Scholar]
- 193.Shpichka A., Butnaru D., Bezrukov E.A., Sukhanov R.B., Atala A., Burdukovskii V., Zhang Y., Timashev P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019;10:1–16. doi: 10.1186/s13287-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bae C.Y., Kang Y.G., Piao M.H., Cho B., Cho K.H., Park Y.K., Yu B.Y., Lee S.W., Kim M.J., Lee S.H., et al. Models for estimating the biological age of five organs using clinical biomarkers that are commonly measured in clinical practice settings. Maturitas. 2013;75:253–260. doi: 10.1016/j.maturitas.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 195.Naskalova S., Shatilo V., Pisaruk A., Antoniuk-Shcheglova I., Bondarenko O., Bodretska L., Shapovalenko I. Estimating the functional age of the respiratory system. Ageing Longev. 2022;3:71–76. doi: 10.47855/jal9020-2022-3-1. [DOI] [Google Scholar]
- 196.Sprung J., Gajic O., Warner D.O. Age related alterations in respiratory function–anesthetic considerations. Can. J. Anesth. 2006;53:1244. doi: 10.1007/BF03021586. [DOI] [PubMed] [Google Scholar]
- 197.Charansonney O.L. Physical activity and aging: A life-long story. Discov. Med. 2011;12:177–185. [PubMed] [Google Scholar]
- 198.Negri A.L. The klotho gene: A gene predominantly expressed in the kidney is a fundamental regulator of aging and calcium/phosphorus metabolism. J. Nephrol. 2005;18:654–658. [PubMed] [Google Scholar]
- 199.Wei S.Y., Pan S.Y., Li B., Chen Y.M., Lin S.L. Rejuvenation: Turning back the clock of aging kidney. J. Formos. Med. Assoc. 2020;119:898–906. doi: 10.1016/j.jfma.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 200.Alcedo J., Flatt T., Pasyukova E.G. The role of the nervous system in aging and longevity. Front. Genet. 2013;4:124. doi: 10.3389/fgene.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bouchard J., Villeda S.A. Aging and brain rejuvenation as systemic events. J. Neurochem. 2015;132:5–19. doi: 10.1111/jnc.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kimura K., Ieda M., Kanazawa H., Yagi T., Tsunoda M., Ninomiya S., Kurosawa H., Yoshimi K., Mochizuki H., Yamazaki K., et al. Cardiac sympathetic rejuvenation: A link between nerve function and cardiac hypertrophy. Circ. Res. 2007;100:1755–1764. doi: 10.1161/01.RES.0000269828.62250.ab. [DOI] [PubMed] [Google Scholar]
- 203.Martin J., Sheaff M. Renal ageing. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007;211:198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- 204.Harciarek M., Biedunkiewicz B., Lichodziejewska-Niemierko M., Dębska-Ślizień A., Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 2011;79:1353–1360. doi: 10.1038/ki.2011.40. [DOI] [PubMed] [Google Scholar]
- 205.Griva K., Thompson D., Jayasena D., Davenport A., Harrison M., Newman S.P. Cognitive functioning pre-to post-kidney transplantation—A prospective study. Nephrol. Dial. Transplant. 2006;21:3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 206.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Amanvermez R., Tosun M. An update on ovarian aging and ovarian reserve tests. Int. J. Fertil. Steril. 2016;9:411. doi: 10.22074/ijfs.2015.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chilovi B.V., Caratozzolo S., Mombelli G., Zanetti M., Rozzini L., Padovani A. Does reversible mci exist? Alzheimer’s Dement. 2011;7:5. doi: 10.1016/j.jalz.2011.05.1547. [DOI] [Google Scholar]
- 209.Vermunt L., van Paasen A.J.L., Teunissen C.E., Scheltens P., Visser P.J., Tijms B.M., Alzheimer’s Disease Neuroimaging Initiative Alzheimer disease biomarkers may aid in the prognosis of mci cases initially reverted to normal. Neurology. 2019;92:e2699–e2705. doi: 10.1212/WNL.0000000000007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zarahn E., Rakitin B., Abela D., Flynn J., Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol. Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 211.Oosterhuis E.J., Slade K., May P.J., Nuttall H.E. Towards an understanding of healthy cognitive ageing: The importance of lifestyle in cognitive reserve and the scaffolding theory of aging and cognition. J. Gerontol. Ser. B. 2022;78:777–788. doi: 10.1093/geronb/gbac197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shimada H., Doi T., Lee S., Makizako H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimer’s Res. Ther. 2019;11:1–9. doi: 10.1186/s13195-019-0480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Valenzuela M.J., Sachdev P., Wen W., Chen X., Brodaty H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE. 2008;3:e2598. doi: 10.1371/journal.pone.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Aycheh H.M., Seong J.K., Shin J.H., Na D.L., Kang B., Seo S.W., Sohn K.A. Biological brain age prediction using cortical thickness data: A large scale cohort study. Front. Aging Neurosci. 2018;10:252. doi: 10.3389/fnagi.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Franke K., Gaser C. Ten years of brainage as a neuroimaging biomarker of brain aging: What insights have we gained? Front. Neurol. 2019;10:789. doi: 10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Elliott M.L., Belsky D.W., Knodt A.R., Ireland D., Melzer T.R., Poulton R., Ramrakha S., Caspi A., Moffitt T.E., Hariri A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry. 2021;26:3829–3838. doi: 10.1038/s41380-019-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saxon S.V., Etten M.J., Perkins E.A. Physical Change and Aging: A Guide for Helping Professions. Springer Publishing Company; Berlin/Heidelberg, Germany: 2021. [Google Scholar]
- 218.Berezina T.N., Rybtsov S.A. Use of personal resources may influence the rate of biological aging depending on individual typology. Eur. J. Investig. Health Psychol. Educ. 2022;12:1793–1811. doi: 10.3390/ejihpe12120126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bethlehem R.A.I., Seidlitz J., White S.R., Vogel J.W., Anderson K.M., Adamson C., Adler S., Alexopoulos G.S., Anagnostou E., Areces-Gonzalez A. Brain charts for the human lifespan. Nature. 2022;604:525–533. doi: 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gaser C., Franke K., Klöppel S., Koutsouleris N., Sauer H., Initiative A.D.N. Brainage in mild cognitive impaired patients: Predicting the conversion to Alzheimer’s disease. PLoS ONE. 2013;8:e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Eickhoff C.R., Hoffstaedter F., Caspers J., Reetz K., Mathys C., Dogan I., Amunts K., Schnitzler A., Eickhoff S.B. Advanced brain ageing in Parkinson’s disease is related to disease duration and individual impairment. Brain Commun. 2021;3:fcab191. doi: 10.1093/braincomms/fcab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Koutsouleris N., Davatzikos C., Borgwardt S., Gaser C., Bottlender R., Frodl T., Falkai P., Riecher-Rössler A., Möller H.J., Reiser M., et al. Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophr. Bull. 2014;40:1140–1153. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Franke K., Gaser C. Longitudinal changes in individual brainage in healthy aging, mild cognitive impairment, and Alzheimer’s disease. GeroPsych. 2012;25:235–245. doi: 10.1024/1662-9647/a000074. [DOI] [Google Scholar]
- 224.Scahill R.I., Frost C., Jenkins R., Whitwell J.L., Rossor M.N., Fox N.C. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 225.Coffey C.E., Wilkinson W.E., Parashos I.A., Soady S.A., Sullivan R.J., Patterson L.J., Figiel G.S., Webb M.C., Spritzer C.E., Djang W.T. Quantitative cerebral anatomy of the aging human brain: A cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527. doi: 10.1212/WNL.42.3.527. [DOI] [PubMed] [Google Scholar]
- 226.Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 227.Iwasaki A., Foxman E.F., Molony R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017;17:7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sarkar T., Patro N., Patro I.K. Cumulative multiple early life hits-a potent threat leading to neurological disorders. Brain Res. Bull. 2019;147:58–68. doi: 10.1016/j.brainresbull.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 229.Hawkes C.H., Del Tredici K., Braak H. A timeline for parkinson’s disease. Park. Relat. Disord. 2010;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 230.Turknett J., Wood T.R. Demand coupling drives neurodegeneration: A model of age-related cognitive decline and dementia. Cells. 2022;11:2789. doi: 10.3390/cells11182789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schneider C.B., Donix M., Linse K., Werner A., Fauser M., Klingelhoefer L., Löhle M., von Kummer R., Reichmann H., Storch A., et al. Accelerated age-dependent hippocampal volume loss in parkinson disease with mild cognitive impairment. Am. J. Alzheimer’s Dis. Other Dement. 2017;32:313–319. doi: 10.1177/1533317517698794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Smith S.M., Elliott L.T., Alfaro-Almagro F., McCarthy P., Nichols T.E., Douaud G., Miller K.L. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. eLife. 2020;9:e52677. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bayati A., Berman T. Localized vs. systematic neurodegeneration: A paradigm shift in understanding neurodegenerative diseases. Front. Syst. Neurosci. 2017;11:62. doi: 10.3389/fnsys.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Beheshti I., Mishra S., Sone D., Khanna P., Matsuda H. T1-weighted mri-driven brain age estimation in Alzheimer’s disease and parkinson’s disease. Aging Dis. 2020;11:618. doi: 10.14336/AD.2019.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.McCartney D.L., Stevenson A.J., Walker R.M., Gibson J., Morris S.W., Campbell A., Murray A.D., Whalley H.C., Porteous D.J., McIntosh A.M., et al. Investigating the relationship between dna methylation age acceleration and risk factors for Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018;10:429–437. doi: 10.1016/j.dadm.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Norrara B., Doerl J.G., Guzen F.P., Cavalcanti J.R.L.P., Freire M.A.M. Commentary: Localized vs. systematic neurodegeneration: A paradigm shift in understanding neurodegenerative diseases. Front. Syst. Neurosci. 2017;11:91. doi: 10.3389/fnsys.2017.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim Y.E., Jung Y.S., Ock M., Yoon S.J. Daly estimation approaches: Understanding and using the incidence-based approach and the prevalence-based approach. J. Prev. Med. Public Health. 2022;55:10. doi: 10.3961/jpmph.21.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gao T., Wang X.C., Chen R., Ngo H.H., Guo W. Disability adjusted life year (daly): A useful tool for quantitative assessment of environmental pollution. Sci. Total Environ. 2015;511:268–287. doi: 10.1016/j.scitotenv.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 239.Jonsson B.A., Bjornsdottir G., Thorgeirsson T.E., Ellingsen L.M., Walters G.B., Gudbjartsson D.F., Stefansson H., Stefansson K., Ulfarsson M.O. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 2019;10:5409. doi: 10.1038/s41467-019-13163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hung C.W., Chen Y.C., Hsieh W.L., Chiou S.H., Kao C.L. Ageing and neurodegenerative diseases. Ageing Res. Rev. 2010;9:S36–S46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 241.Sibbett R.A., Altschul D.M., Marioni R.E., Deary I.J., Starr J.M., Russ T.C. Dna methylation-based measures of accelerated biological ageing and the risk of dementia in the oldest-old: A study of the lothian birth cohort 1921. BMC Psychiatry. 2020;20:1–15. doi: 10.1186/s12888-020-2469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Soldan A., Pettigrew C., Li S., Wang M.C., Moghekar A., Selnes O.A., Albert M., O’Brien R. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol. Aging. 2013;34:2827–2834. doi: 10.1016/j.neurobiolaging.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fries G.R., Bauer I.E., Scaini G., Valvassori S.S., Walss-Bass C., Soares J.C., Quevedo J. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Disord. 2020;22:498–507. doi: 10.1111/bdi.12876. [DOI] [PubMed] [Google Scholar]
- 244.Tønnesen S., Kaufmann T., de Lange A.G., Richard G., Doan N.T., Alnæs D., van der Meer D., Rokicki J., Moberget T., Maximov I.I., et al. Brain age prediction reveals aberrant brain white matter in schizophrenia and bipolar disorder: A multisample diffusion tensor imaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:1095–1103. doi: 10.1016/j.bpsc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 245.Heather C.W., Jude G., Riccardo M., Rosie M.W., Toni-Kim C., David M.H., Mark J.A., Lynsey H., Stewart M., Ian J.D., et al. Accelerated epigenetic ageing in major depressive disorder. bioRxiv. 2017 doi: 10.1101/210666. [DOI] [Google Scholar]
- 246.Dudley J.A., Lee J.H., Durling M., Strakowski S.M., Eliassen J.C. Age-dependent decreases of high energy phosphates in cerebral gray matter of patients with bipolar i disorder: A preliminary phosphorus-31 magnetic resonance spectroscopic imaging study. J. Affect. Disord. 2015;175:251–255. doi: 10.1016/j.jad.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 247.Masuda Y., Okada G., Takamura M., Shibasaki C., Yoshino A., Yokoyama S., Ichikawa N., Okuhata S., Kobayashi T., Yamawaki S., et al. Age-related white matter changes revealed by a whole-brain fiber-tracking method in bipolar disorder compared to major depressive disorder and healthy controls. Psychiatry Clin. Neurosci. 2021;75:46–56. doi: 10.1111/pcn.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muangpaisan W., Petcharat C., Srinonprasert V. Prevalence of potentially reversible conditions in dementia and mild cognitive impairment in a geriatric clinic. Geriatr. Gerontol. Int. 2012;12:59–64. doi: 10.1111/j.1447-0594.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 249.Gauthier S., Touchon J. Mild cognitive impairment is not a clinical entity and should not be treated. Arch. Neurol. 2005;62:1164–1166. doi: 10.1001/archneur.62.7.1164. [DOI] [PubMed] [Google Scholar]
- 250.Bates K., Harvey A.R., Carruthers M., Martins R. Androgens, andropause and neurodegeneration: Exploring the link between steroidogenesis, androgens and Alzheimer’s disease. Cell Mol. Life Sci. 2005;62:281–292. doi: 10.1007/s00018-004-4383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bordoni M., Scarian E., Rey F., Gagliardi S., Carelli S., Pansarasa O., Cereda C. Biomaterials in neurodegenerative disorders: A promising therapeutic approach. Int. J. Mol. Sci. 2020;21:3243. doi: 10.3390/ijms21093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bell C.G., Lowe R., Adams P.D., Baccarelli A.A., Beck S., Bell J.T., Christensen B.C., Gladyshev V.N., Heijmans B.T., Horvath S., et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019;20:1–24. doi: 10.1186/s13059-019-1824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stubbs T.M., Bonder M.J., Stark A.K., Krueger F., von Meyenn F., Stegle O., Reik W. Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 2017;18:68. doi: 10.1186/s13059-017-1203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Thompson M.J., Chwiałkowska K., Rubbi L., Lusis A.J., Davis R.C., Srivastava A., Korstanje R., Churchill G.A., Horvath S., Pellegrini M. A multi-tissue full lifespan epigenetic clock for mice. Aging. 2018;10:2832. doi: 10.18632/aging.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang T., Tsui B., Kreisberg J.F., Robertson N.A., Gross A.M., Yu M.K., Carter H., Brown-Borg H.M., Adams P.D., Ideker T. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18:57. doi: 10.1186/s13059-017-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Petkovich D.A., Podolskiy D.I., Lobanov A.V., Lee S.G., Miller R.A., Gladyshev V.N. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–960. doi: 10.1016/j.cmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Meer M.V., Podolskiy D.I., Tyshkovskiy A., Gladyshev V.N. A whole lifespan mouse multi-tissue DNA methylation clock. eLife. 2018;7:e40675. doi: 10.7554/eLife.40675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bahar R., Hartmann C.H., Rodriguez K.A., Denny A.D., Busuttil R.A., Dollé M.E., Calder R.B., Chisholm G.B., Pollock B.H., Klein C.A., et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 261.Rimmelé P., Bigarella C.L., Liang R., Izac B., Dieguez-Gonzalez R., Barbet G., Donovan M., Brugnara C., Blander J.M., Sinclair D.A., et al. Aging-like phenotype and defective lineage specification in sirt1-deleted hematopoietic stem and progenitor cells. Stem Cell Rep. 2014;3:44–59. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cheung P., Vallania F., Warsinske H.C., Donato M., Schaffert S., Chang S.E., Dvorak M., Dekker C.L., Davis M.M., Utz P.J., et al. Single-cell chromatin modification profiling reveals increased epigenetic variations with aging. Cell. 2018;173:1385–1397. doi: 10.1016/j.cell.2018.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hernando-Herraez I., Evano B., Stubbs T., Commere P.H., Jan Bonder M., Clark S., Andrews S., Tajbakhsh S., Reik W. Ageing affects DNA methylation drift and transcriptional cell-to-cell variability in mouse muscle stem cells. Nat. Commun. 2019;10:4361. doi: 10.1038/s41467-019-12293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Trapp A., Kerepesi C., Gladyshev V.N. Profiling epigenetic age in single cells. Nat. Aging. 2021;1:1189–1201. doi: 10.1038/s43587-021-00134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hu Y., An Q., Guo Y., Zhong J., Fan S., Rao P., Liu X., Liu Y., Fan G. Simultaneous profiling of mRNA transcriptome and DNA methylome from a single cell. Single Cell Methods Seq. Proteom. 2019;1979:363–377. doi: 10.1007/978-1-4939-9240-9_21. [DOI] [PubMed] [Google Scholar]
- 266.Angermueller C., Lee H.J., Reik W., Stegle O. Deepcpg: Accurate prediction of single-cell DNA methylation states using deep learning. Genome Biol. 2017;18:1–13. doi: 10.1186/s13059-017-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hamidouche Z., Rother K., Przybilla J., Krinner A., Clay D., Hopp L., Fabian C., Stolzing A., Binder H., Charbord P., et al. Bistable epigenetic states explain age-dependent decline in mesenchymal stem cell heterogeneity. Stem Cells. 2017;35:694–704. doi: 10.1002/stem.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.de Lima Camillo L.P., Lapierre L.R., Singh R. A pan-tissue DNA-methylation epigenetic clock based on deep learning. npj Aging. 2022;8:4. doi: 10.1038/s41514-022-00085-y. [DOI] [Google Scholar]
- 269.Peleg S., Sananbenesi F., Zovoilis A., Burkhardt S., Bahari-Javan S., Agis-Balboa R.C., Cota P., Wittnam J.L., Gogol-Doering A., Opitz L., et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 270.Stefanelli G., Azam A.B., Walters B.J., Brimble M.A., Gettens C.P., Bouchard-Cannon P., Cheng H.M., Davidoff A.M., Narkaj K., Day J.J., et al. Learning and age-related changes in genome-wide h2a.z binding in the mouse hippocampus. Cell Rep. 2018;22:1124–1131. doi: 10.1016/j.celrep.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Klein H.U., McCabe C., Gjoneska E., Sullivan S.E., Kaskow B.J., Tang A., Smith R.V., Xu J., Pfenning A.R., Bernstein B.E., et al. Epigenome-wide study uncovers tau pathology-driven changes of chromatin organization in the aging human brain. Biorxiv. 2018;22:273789. [Google Scholar]
- 272.Roichman A., Elhanati S., Aon M.A., Abramovich I., Di Francesco A., Shahar Y., Avivi M.Y., Shurgi M., Rubinstein A., Wiesner Y., et al. Restoration of energy homeostasis by sirt6 extends healthy lifespan. Nat. Commun. 2021;12:3208. doi: 10.1038/s41467-021-23545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Grootaert M.O., Finigan A., Figg N.L., Uryga A.K., Bennett M.R. Sirt6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ. Res. 2021;128:474–491. doi: 10.1161/CIRCRESAHA.120.318353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Soto-Palma C., Niedernhofer L.J., Faulk C.D., Dong X. Epigenetics, DNA damage, and aging. J. Clin. Investig. 2022;132:e158446. doi: 10.1172/JCI158446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hartmann A., Hartmann C., Secci R., Hermann A., Fuellen G., Walter M. Ranking biomarkers of aging by citation profiling and effort scoring. Front. Genet. 2021;12:686320. doi: 10.3389/fgene.2021.686320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bürkle A., Moreno-Villanueva M., Bernhard J., Blasco M., Zondag G., Hoeijmakers J.H., Toussaint O., Grubeck-Loebenstein B., Mocchegiani E., Collino S., et al. MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 2015;151:2–12. doi: 10.1016/j.mad.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 277.Mirzayans R., Murray D. Human Genetic Disorders Associated with Genome Instability, Premature Aging and Cancer Predisposition. Open Cancer J. 2008;2:42–52. doi: 10.2174/1874079000802010042. [DOI] [Google Scholar]
- 278.Tiwari V., Wilson D.M. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019;105:237–257. doi: 10.1016/j.ajhg.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rizza E.R., DiGiovanna J.J., Khan S.G., Tamura D., Jeskey J.D., Kraemer K.H. Xeroderma pigmentosum: A model for human premature aging. J. Investig. Dermatol. 2021;141:976–984. doi: 10.1016/j.jid.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Han Z.Z., Fleet A., Larrieu D. Can accelerated ageing models inform us on age-related tauopathies? Aging Cell. 2023;22:e13830. doi: 10.1111/acel.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Platzer M., Englert C. Nothobranchius furzeri: A model for aging research and more. Trends Genet. 2016;32:543–552. doi: 10.1016/j.tig.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 282.Reichwald K., Petzold A., Koch P., Downie B.R., Hartmann N., Pietsch S., Baumgart M., Chalopin D., Felder M., Bens M., et al. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163:1527–1538. doi: 10.1016/j.cell.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 283.Valenzano D.R., Benayoun B.A., Singh P.P., Zhang E., Etter P.D., Hu C.K., Clément-Ziza M., Willemsen D., Cui R., Harel I., et al. The African turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell. 2015;163:1539–1554. doi: 10.1016/j.cell.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Petzold A., Reichwald K., Groth M., Taudien S., Hartmann N., Priebe S., Shagin D., Englert C., Platzer M. The transcript catalogue of the short-lived fish Nothobranchius furzeri provides insights into age-dependent changes of mRNA levels. BMC Genom. 2013;14:185. doi: 10.1186/1471-2164-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Genade T., Benedetti M., Terzibasi E., Roncaglia P., Valenzano D.R., Cattaneo A., Cellerino A. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell. 2005;4:223–233. doi: 10.1111/j.1474-9726.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 286.Di Cicco E., Tozzini E.T., Rossi G., Cellerino A. The shortlived annual fish Nothobranchius furzeri shows a typical teleost aging process reinforced by high incidence of age-dependent neoplasias. Exp. Gerontol. 2011;46:249–256. doi: 10.1016/j.exger.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 287.Dodzian J., Kean S., Seidel J., Valenzano D.R. A protocol for laboratory housing of Turquoise Killifish (Nothobranchius furzeri) J. Vis. Exp. 2018;134:e57073. doi: 10.3791/57073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tan L., Ke Z., Tombline G., Macoretta N., Hayes K., Tian X., Lv R., Ablaeva J., Gilbert M., Bhanu N.V., et al. Naked mole rat cells have a stable epigenome that resists iPSC reprogramming. Stem Cell Rep. 2017;9:1721–1734. doi: 10.1016/j.stemcr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Dammann P., Šumbera R., Massmann C., Scherag A., Burda H. Extended longevity of reproductives appears to be common in Fukomys molerats (Rodentia. Bathyergidae) PLoS ONE. 2011;6:e18757. doi: 10.1371/journal.pone.0018757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ruby J.G., Smith M., Buffenstein R. Naked mole-rat mortality rates defy gompertzian laws by not increasing with age. Elife. 2018;7:e31157. doi: 10.7554/eLife.31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Munshi-South J., Wilkinson G.S. Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Res. Rev. 2010;9:12–19. doi: 10.1016/j.arr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 292.Seim I., Fang X., Xiong Z., Lobanov A.V., Huang Z., Ma S., Feng Y., Turanov A.A., Zhu Y., Lenz T.L., et al. Genome analysis reveals insights into physiology and longevity of the Brandt’s bat Myotis brandtii. Nat. Commun. 2013;4:2212. doi: 10.1038/ncomms3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Podlutsky A.J., Khritankov A.M., Ovodov N.D., Austad S.N. A new field record for bat longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1366–1368. doi: 10.1093/gerona/60.11.1366. [DOI] [PubMed] [Google Scholar]
- 294.Holtze S., Lukač M., Cizelj I., Mutschmann F., Szentiks C.A., Jelić D., Hermes R., Göritz F., Braude S., Hildebrandt T.B. Monitoring health and reproductive status of olms (Proteus anguinus) by ultrasound. PLoS ONE. 2017;12:e0182209. doi: 10.1371/journal.pone.0182209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mulec J. Welcome to the -omics era of the 21st century: Will Proteus anguinus finally reveal all its mysteries? Acta Carsol. 2020;1:49. doi: 10.3986/ac.v49i1.8009. [DOI] [Google Scholar]
- 296.Voituron Y., de Fraipont M., Issartel J., Guillaume O., Clobert J. Extreme lifespan of the human fish (Proteus anguinus): A challenge for ageing mechanisms. Biol. Lett. 2011;7:105–107. doi: 10.1098/rsbl.2010.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Philipp E.E., Wessels W., Gruber H., Strahl J., Wagner A.E., Ernst I.M., Rimbach G., Kraemer L., Schreiber S., Abele D., et al. Gene expression and physiological changes of different populations of the long-lived bivalve Arctica islandica under low oxygen conditions. PLoS ONE. 2012;7:e44621. doi: 10.1371/journal.pone.0044621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lutz R.A., Goodsell J.G., Mann R., Castagna M. Experimental culture of the ocean quahog, Arctica islandica. J. World Maricult. Soc. 1981;12:196–205. doi: 10.1111/j.1749-7345.1981.tb00255.x. [DOI] [Google Scholar]
- 299.Chapman J.A., Kirkness E.F., Simakov O., Hampson S.E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P.G., Borman J., Busam D., et al. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Schaible R., Sussman M., Kramer B.H. Aging and potential for self-renewal: Hydra living in the age of aging—A mini-review. Gerontology. 2014;60:548–556. doi: 10.1159/000360397. [DOI] [PubMed] [Google Scholar]
- 301.Bellantuono A.J., Bridge D., Martínez D.E. Hydra as a tractable, long-lived model system for senescence. Invert. Reprod. Dev. 2015;59:39–44. doi: 10.1080/07924259.2014.938196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Klimovich A., Rehm A., Wittlieb J., Herbst E.-M., Benavente R., Bosch T.C.G. Non-senescent Hydra tolerates severe disturbances in the nuclear lamina. Aging. 2018;10:951–972. doi: 10.18632/aging.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Grohme M.A., Schloissnig S., Rozanski A., Pippel M., Young G.R., Winkler S., Brandl H., Henry I., Dahl A., Powell S., et al. The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature. 2018;554:56–61. doi: 10.1038/nature25473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Fincher C.T., Wurtzel O., de Hoog T., Kravarik K.M., Reddien P.W. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science. 2018;360:eaaq1736. doi: 10.1126/science.aaq1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Merryman M.S., Alvarado A.S., Jenkin J.C. Culturing Planarians in the Laboratory. Methods Mol. Biol. 2018;1774:241–258. doi: 10.1007/978-1-4939-7802-1_5. [DOI] [PubMed] [Google Scholar]
- 306.Lipsky M.S., King M. Biological theories of aging. Dis.-A-Mon. 2015;61:460–466. doi: 10.1016/j.disamonth.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 307.Jin K. Modern biological theories of aging. Aging Dis. 2010;1:72. [PMC free article] [PubMed] [Google Scholar]
- 308.Weinert B.T., Timiras P.S. Invited review: Theories of aging. J. Appl. Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- 309.Northrop J.H. The influence of the intensity of light on the rate of growth and duration of life of Drosophila. J. Gen. Physiol. 1925;9:81. doi: 10.1085/jgp.9.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Northrop J.H. Duration of life of an aseptic Drosophila culture inbred in the dark for 230 generations. J. Gen. Physiol. 1926;9:763–765. doi: 10.1085/jgp.9.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.NCBI PubMed. [(accessed on 31 August 2023)]; Available online: https://pubmed.ncbi.nlm.nih.gov/
- 312.Tilney F. The Aging of the Human Brain. Bull. N. Y. Acad. Med. 1928;4:1125–1143. [PMC free article] [PubMed] [Google Scholar]