Abstract
Objectives
The purpose of this study was to analyze the National Institute of Neurological Disorders and Stroke (NINDS) Request for Information (RFI) input from the public—including health care providers, researchers, patients, patient advocates, caregivers, advocacy organizations, professional societies, and private and academic stakeholders with an interest in health disparities (HDs) in neurologic disease. RFI questions were structured to solicit input on what stakeholders believe are neurologic disease HD research priorities, drivers of health inequity, and potential interventions. Furthermore, these stakeholder insights were examined within the context of contemporary scientific literature and research frameworks on health equity and health disparities.
Background
The NINDS published a RFI from March 31 to July 15, 2020. The RFI analysis presented here is part of a larger strategic planning process aimed to guide future NINDS efforts in neurologic disorder health equity (HE) research and training. The public commented on facilitators of HDs, populations that experience HDs (HDPs), potential interventions, and research opportunities related to HDs in neurologic disease and/or care in the United States across the lifespan. Responses were analyzed using qualitative methodology. Frequently suggested interventions were thematically clustered using the interpretive phenomenological analysis methodology and are presented in this article to provide a stakeholder-identified roadmap for advancing HE.
Results
Respondents identified socioecological factors as driving HDs in 89% of determinants reported. Stakeholder-reported HD determinants and subsequent interventions could be classified into the following conceptual categories: HDP neurospecialty care access, innovative HDP engagement and research inclusion strategies, and development of a well-trained clinician-scientist HD workforce. Clustering of the feedback from patient and patient-adjacent respondents (i.e., caretakers and patient advocates) highlighted the prevalence of patient-provider interpersonal factors and limited resources driving access-to-care barriers among their sentiments.
Discussion
Respondent sentiments suggest prioritization of social determinants of health (SDOH) research, shifting away from the common target of biological and behavioral themes addressed in the existing body of HE research provided by the stakeholder. Overall, respondents suggest focusing research prioritization on access to care, engagement across the HE research and care landscape, and HE workforce development.
Introduction
The National Institute of Neurological Disorders and Stroke (NINDS) is committed to reducing the disproportionate burden of neurologic disease borne by underserved groups of society, including racial and ethnic minoritized, rural, and socioeconomically disadvantaged populations. The tragic murder of George Floyd and the effect of the global COVID-19 pandemic have catapulted the dire consequences of structural and social inequities on health outcomes and opportunity. Within this landscape, NINDS carried out its health equity (HE) and health disparities strategic planning process to direct research and research training investments over the next 5–10 years. In alignment with the US Department of Health and Human Services' Healthy People 2030 initiative HE definition,1 NINDS research efforts are aimed to promote health by facilitating the elimination of obstacles that create unfair and unjust access to health opportunity. The NINDS mission is to advance HE by funding a spectrum of research from basic science through clinical studies and training the next generation of health disparities investigators.
HE can be characterized as the state in which all individuals in a society have access to the highest level of health opportunity. Communities are excluded from this opportunity when they experience marginalization based on their identities, associations, environments, or other socioecological characteristics linked to discrimination.2-8 Therefore, understanding how intersecting socioecological influences like individual, interpersonal, community, and societal factors contextually shape health opportunity for populations that experience HDs (HDPs) is vital to advancing HE in neurologic disease.
Although there is mounting evidence that disparities in neurologic disorders, care, and outcomes exist for HDPs,9-13 there is a gap in interventions to address these disparities. To better understand the current neurologic HE state of science and incorporate a wide range of diverse perspectives from the public into our strategic planning, we published a Request for Information (RFI) on research opportunities related to the Health Disparities and Inequities in Neurological Disease (Notice Number: NOT-NS-20-026).14 A RFI is a formal mechanism fully open to the public by which the US Government gathers information from multiple stakeholders for planning purposes. It is not a solicitation for proposals and does not obligate the Government. In this qualitative RFI analysis, we include a thematically clustered narrative review of respondent-provided HE determinants, gaps, and interventions supported by respondent-provided sources. The summary of findings presented here link stakeholder input to the contemporary state of science in neurologic disorder HE research and reflect the context provided by respondents.
Methods
Dissemination and Data Collection
The NINDS RFI: Soliciting Input on Areas of Health Disparities and Inequities in Neurological Disease and/or Care in the United States Across the Lifespan14 was published in the NIH Guide and made accessible for public input from March 31 to July 15, 2020. The public was provided an opportunity to comment on facilitators of HDs, populations that experience HDs (HDPs), potential interventions, and research opportunities related to HDs in neurologic disease and/or care in the United States across the lifespan. In addition, in collaboration with the NINDS Office of Neuroscience Communications and Engagement, direct solicitations were sent to 376 nonprofit organizations and to 92 professional societies. The RFI contained multiple-choice and short-answer questions developed to seek input from scientists, clinicians, patients, families, caregivers, advocates, and the broader community. Responses to this RFI were collected electronically using a web-based form. We received 142 responses. Duplicate responses were excluded (n = 2). The exclusion criterion was that if a respondent did not answer at least one short-answer RFI question, their response was not included in the coding analysis (n = 16). International responses were not included in the analysis (n = 3). The final analysis included 121 responses.
Respondents were asked to provide input on the demographic multiple-choice questions designed to give context to individual responses. The demographic questions asked respondents to (1) describe their relationship or interest in HD in neurologic disease (that is, patient, advocate, researcher, caregiver, health care provider (HCP), or government official), (2) identify the region of the country in which they reside, and (3) identify the size of the community in which they reside (i.e., urban population >50,000, suburban population 2,500–50,000, and rural <2,500). Organizational responses included advocacy organizations (i.e., primary mission to support, inform, or advocate for patients/science/equity), biomedical society (i.e., professional science and/or medicine collaboratives promoting and investing in academic, scholarly, or clinical activities), and academic institutions.
Respondents were prompted to respond to short-answer questions designed to solicit stakeholder input on health inequity and disparity knowledge gaps, vulnerable populations, health determinants, and intervention opportunities in neurologic care and research. Stakeholders often cited scientific literature to support their suggestions, and those references were included throughout the report as applicable.
Qualitative Code Development by Reviewers: Thematic Clustering
Qualitative methodology was adopted in this analysis to provide reviewers an opportunity to gain in-depth understanding of each respondent's unique perceptions and find themes across individual narratives.15 Three reviewers read and evaluated each RFI transcript submitted by respondents. Prevalent themes appearing across responses were extracted and used to develop response codes within the designated categories: neurologic disorder, vulnerable population, and determinants. Through an iterative process, prevalent themes were clustered and code descriptions refined. The neurologic disorder and vulnerable population codes were directly extracted from reviewer responses. Codes used to describe operational organization of HD influences and determinant factors driving HDs were developed by reviewers' identification of connections across respondent sentiments. The prevalent themes that emerged from responses closely aligned and could be well-described using definitions captured within widely accepted HDs and SDOH contemporary literature and resources. Apart from the NINDS strategic planning process, the National Institute on Minority Health and Health Disparities (NIMHD) had published a well-known research framework for categorization of HD determinants. The framework organizes a sample of determinant categories into a schematic depicting a layered structure of influence.16,17 The domains of influence (DOIs; Biological, Behavioral, Physical/Built Environment, Sociocultural Environment, Health Care System) and levels of influence (LOIs; Individual, Interpersonal, Community, Societal) within those domains were extracted from the NIMHD framework and used to code RFI respondent themes describing the organization of HD influences. HD determinants, or factors that drive health difference from within the DOIs and LOIs within which they operate, were generated de novo using contemporary HE research literature.1,2,5,6,13,16-19
Response Analysis
Three reviewers read and evaluated each RFI transcript submitted by respondents using the finalized codes for each category: neurologic disorder, vulnerable population, and determinants. Reviewers applied combinatory codes to individual responses as appropriate (i.e., one response could receive 2 codes for neurologic disease area). Majority consensus was required to finalize the codes for each response. A master table of codes assigned to each respondent was compiled, and the data were used to derive the relative frequency distribution graphs presented within this report. Response coding is reported as relative frequency distribution expressed as a percentage of the total codes applied. A detailed explanation of standard coding procedures used to evaluate RFI responses and detailed quantitative reporting data are provided in supplemental materials (links.lww.com/WNL/C929).
Potential interventions and evidenced interventions (i.e., respondent interventions that included a reviewer-verified source) supplied by stakeholders were clustered by theme. Themes that reflected most respondent intervention sentiments and spanned across most neurologic diseases reported by respondents were grouped into overarching concepts and linked to provided determinant targets. These concepts were organized into a neurologic disorder HD domain pyramid to convey the frequency of concepts across RFI responses. Intervention examples illustrating intervention themes most frequently identified by respondents are summarized in a narrative format.
Selected patient and patient-adjacent respondent (i.e., patient, caregiver, and individual patient advocate) direct quotes reflecting themes repeatedly described by patients, irrespective of neurologic disease, are also reported.
Results
RFI Summary of Key Findings
Geographically and vocationally diverse respondents described more than 14 neurologic disease areas affected by health disparities. Five prominent take-home points emerged from the NINDS RFI analysis (Table 1). Respondents primarily described socioecological determinants as driving health inequity in communities most vulnerable to experiencing HDs. Stakeholders also provided a wealth of evidence-based strategies to attenuate HDs in neurologic disease. The RFI findings are described in further detail below.
Table 1.
Summary of Neurologic Disorder HD RFI Findings
RFI Findings Represent a Diverse Perspective
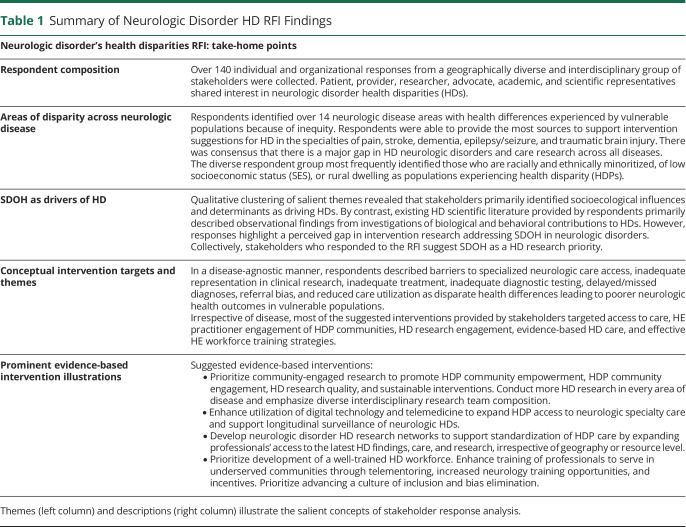
Stakeholders represented diverse backgrounds and experience with HDs in neurologic disorders. Respondents were well-distributed across the United States (Figure 1A), but were predominantly urban dwelling (Figure 1B). While rural residents represent nearly 14% of the national population,20 only 9% of RFI respondents identified themselves as dwelling in rural communities. Stakeholders were distributed across patient-centric, research/provider-centric, and advocacy-centric respondent categories (Figure 1C). Respondents described at least one specific neurologic disease area with perceived HDs (n = 133 areas were reported by n = 100 responders). Ninety-five percent of respondents characterized vulnerable populations which experience health disparities. Seventy-five percent of respondents described explicit health differences caused by health inequity in neurologic disease (n = 91 respondents). Seventy percent of respondents provided evidence-based HD intervention suggestions (n = 69), and these suggestions addressed HDs in over 84% of the neurologic disease areas reported by all respondents.
Figure 1. Diverse RFI Perspectives.
(A) Respondents geographic region distribution, (B) community size distribution (dark gray = urban population >50,000, light gray = suburban population 2,500–50,000, and black = rural population <2,500), and (C) relationship with neurologic disorder health disparity research, responder type. Data expressed as percent of respondents. n, number of respondents; see eAppendix 1 (links.lww.com/WNL/C929).
SDOH Driving Neurologic Disorder Health Inequities
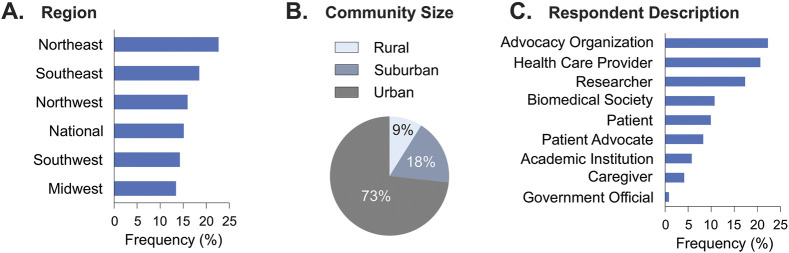
Over 14 health equity research priority neurologic disease areas were identified by respondents (Figure 2A). Stroke, pain, dementia, epilepsy, traumatic brain injury (TBI), and seizure dominated responder health disparity areas of interest, representing 73% of the neurologic disorders identified (Figure 2B). The emphasis on addressing HDs among low socioeconomic status (SES), minoritized racial/ethnic status, and geographically disadvantaged populations was identified in a neurologic disease-agnostic manner. Low socioeconomic status and/or racial/ethnic minoritized populations were identified nearly 50% of the time (Figure 2B). Geographically disadvantaged populations were identified 18% of the time (Figure 2B).
Figure 2. Neurologic Disease and HDP HD Areas Reported.
(A) Reviewers applied combinatorial qualitative codes to responses that identified at least one neurologic disorder and (B) vulnerable population that experiences HDs. *, rare diseases (n = 9). HDP, population that experience HDs. ALS, amyotrophic lateral sclerosis. The category of pain included headache, migraine, and chronic pain. Dementia includes Alzheimer disease and related dementias (ADRD). Neurodevelopmental Disease includes autism and intellectual and developmental disabilities (IDDs). Data expressed as distribution frequency (%). n, number of respondents; see eAppendix 1 (links.lww.com/WNL/C929).
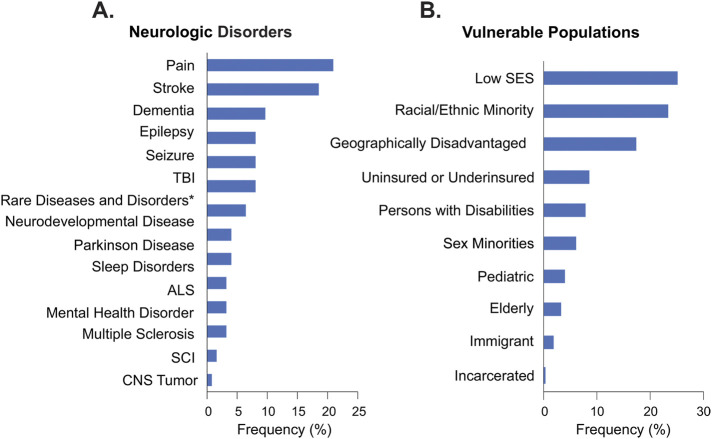
Factors related to sociodemographic characteristics and health care access dominated levels and domains of influence (DOIs and LOIs) when stakeholder neurologic disorder HD interests were mapped to the NIMHD research framework.16,17 Per response, a combination of multiple DOIs (Figure 3A) and LOIs (Figure 3B) were often described as contributing to the context that drives HDs in neurologic disorders. Specifically, HD drivers operating within Health Care System and Sociocultural Environment DOIs (Figure 3A) and Community and Individual LOIs (Figure 3B) were frequently characterized.
Figure 3. Frequently Identified Determinants.
(A) Reviewers applied combinatorial qualitative codes to responses to characterize domains of influence (DOIs), (B) levels of influence (LOIs), and (C) determinant categories. Data expressed as distribution frequency (%). n, number of respondents; see eAppendix 1 (links.lww.com/WNL/C929).
Factors influencing neurospecialty care access, engagement among a broad range of stakeholders across the HE research landscape, and the HD workforce were often characterized as determinants driving disparity in neurologic disorders. Categories describing socioecological determinants were most frequently described within responses (89%) (Figure 3C). Only Research Gap and Biological Risk Factor determinant category codes lie outside of the SDOH landscape.
Respondent-Suggested HD Interventions
HDPs were often described as experiencing disparate health differences, including higher disease risk, poorer outcomes, access barriers to specialized neurologic care, inadequate representation in clinical research, inadequate treatment, inadequate diagnostic testing, delayed/missed diagnoses, referral bias, treatment bias, and reduced care utilization (supplemental material, links.lww.com/WNL/C929). In a disease-agnostic manner, respondents described the need for interventions that target the abovementioned health differences (supplemental material) by addressing determinants driving these disparities.
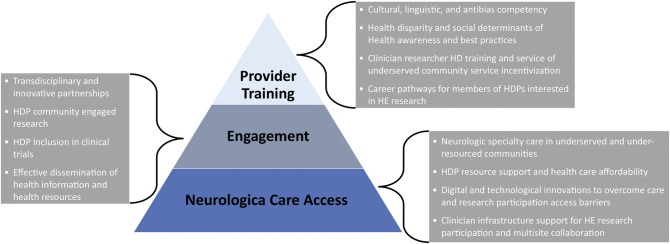
A large proportion of applicable evidence-based interventions were suggested by researchers (16%), health care professionals (26%), and professional entities (49%; societies, academic institutions, and advocacy organizations). Most of the interventions targeted determinants influencing access to care, HE practitioner (i.e., providers, researchers, advocates) engagement of HDP communities, HD research engagement, evidence-based HD care, and the HE workforce. Respondent-identified interventions most frequently included strategies to enhance HD research, to expand access to care for HDPs, and to guide HD competent workforce development. A unique three-tier neurologic disorder HD domain pyramid describes the combination of operational determinants and subsequent potential interventions most frequently described by respondents (Figure 4; neurologic care access, engagement = HE cross-landscape engagement, and provider training = workforce HD competency development).
Figure 4. Neurologic Disorder HD Domain Pyramid.
Respondent-identified neurologic disorder health disparity (HD) determinants and subsequent health equity (HE) intervention concepts were distributed across 3 neurologic disorder HD domains with hierarchy weighted by frequency (bottom of pyramid = most frequent). Gray text boxes describe common intervention themes per category. HDP = population that experiences health disparity.
Explicit Respondent Intervention Illustrations
Strategies to Enhance HD Research
Stakeholders identified evidence-based clinical research interventions for potential application to address neurologic disorder HDs by enhancing HD research strategies.
Respondent Illustrations
Respondents described the importance of engaging a broad range of stakeholders in HE research to simultaneously improve scientific rigor and care quality for HDPs. Community-engaged research (CEr) was described as a strategy to increase HDP clinical trial participation by using the collaboration as a vehicle to build trust and promote health empowerment. Utilization of the following CEr strategies were frequently described: community-based peer educator training programs, community-based patient navigators, community awareness and education programs, availability of multilingual research coordinators, and community-driven material adaptation/translation. Respondents frequently recommended that funding agencies support research to create a compendium of assessment tools validated in differing languages and across differing cultures. They pointed to examples where CEr strategies were used to develop HDP clinical disease surveys21,22 and educational materials.23
To normalize HDP CEr, respondents suggested that infrastructure to sustainably integrate strategies in clinical research be widely adopted in neurologic disorder research. Several respondents suggested using a widely applicable engagement rubric24 or developing standing formal community engagement advisory boards.25 The advisory board infrastructure could, for example, support training of community representatives and resources to support bi-directional knowledge transfer. The patient advisory board could serve as a CEr community partner source and increase HDP awareness about the importance of participation in clinical research studies. Respondents suggest conducting implementation research to evaluate the effectiveness of incorporating community social needs practitioners onto multidisciplinary care coordination referral committees at sites enrolling HDP research participants. The decision-making team could comprise physicians, nurses, and social workers who consider treatment and services referred to each patient based on identification of needs and prioritization of the services. Programs could model features of existing referral acceptance programs used to support clinician decision making as an intervention to reduce potential health care bias.26
Respondents suggested that research to identify scalable strategies to leverage longitudinally collected electronic health record (EHR) data for study recruitment, monitoring, and outcome reporting would be highly valued. Development of national registries of deidentifiable SDOH data collected from HDP patients in the clinical settings to streamline HE research was frequently described. Standardized collection of SDOH common data elements was a common feature of surveillance-related intervention recommendations. Stakeholders pointed to existing models of the multisite institutional infrastructure that has been created to standardize capture of social and behavioral data elements in EHRs during standard clinical protocols.27
Enhance Access to Specialized Neurology Care, Specialists, and Clinical Research
Stakeholders identified evidence-based clinical research interventions to expand access to care among HDPs through telemedicine and technology.
Respondent Illustrations
Respondents recommended adoption of mobile health delivery technology.28,29 It was suggested that broad use of low-cost mobile units with trained care providers and real-time physician consultation capabilities monitoring a wide catchment area could deliver specialized neurologic care in a timely fashion to the geographically disadvantaged populations. Remote device-enhanced teleconsultation can connect prehospital to emergency department care and allow clinicians to triage remotely.
Respondents recommended the development of portable lightweight videoconferencing and rapid evaluation technology. The videoconferencing technology would support synchronous communication between specialists, emergency medical service providers, nurses, physicians, and technicians in emergent care settings. Respondents highlighted examples where portable rapid evaluation technology was currently being used to reduce time in diagnosis.30 They suggested equipping ambulances, critical access hospital emergency departments, and intensive care units with this technology to enhance prehospital and acute care coordination in HDP communities. In addition, respondents called for the development and adoption of software technology to reduce barriers to collecting HE research data in clinical settings. Respondents described clinical research software technology that could easily support care coordination and clinical research integration into the clinical setting.31,32 Implementation research evaluating the effectiveness of technologies to reduce care disparities in neurologic disorders was frequently described by stakeholders.
Respondents also suggested the use of web-based digital platforms, mobile technology, and telehealth to increase access to care and health empowerment for HDPs. Stakeholders suggested that streamlining patient engagement and care provider communication through technology would increase HDP clinical trial inclusion and provide infrastructure for remote trial participation. Respondents described adapting features of existing online clinical trial matching tools33 and remote intervention delivery technology. The remote clinical trial technology recommendations combined the utilization of hybrid technology, incorporating both virtual care and mobile applications.34-36 Stakeholders suggested that integrating mobile technology in care and clinical research would promote medication adherence and enhance communication about medication management between HDPs and primary caregivers.
HD Clinical Research Networks
Interventions designed to increase HDP access to specialized neurology care, provider HD competency, provider bias, and HE clinical research through the development of HD research networks were frequently described.
Respondent Illustrations
Respondents suggested that national HD research networks supporting multisite clinical care coordination, research collaboration, resource sharing, and provider training be developed. To fast-track quality disparity studies, respondents suggested that efforts to recruit HDPs into the existing subspecialty multisite research collaboratives be prioritized.37-39 It was suggested that developing HD research networks would enhance the neurologic research quality by facilitating HDP recruitment, bolstering study design expertise, and enhancing the validity of the output.
HD clinical research networks were also described as an opportunity to improve clinician-scientist training and continued medical education. Respondents suggested that this will dramatically affect providers practicing in resource-limited environments and those serving rural communities.40,41 Stakeholders suggest that HD networks can provide the infrastructure to support remote telementoring and provider-provider consultation to geographically disadvantaged health centers. Community providers could learn from specialists through real-time case-based learning and mentorship learning collaboratives and expand health care provider training to rural, underserved, and under-resourced areas.
HD clinical research networks were also described as an opportunity to improve HDP quality of care through standardizing care protocols and access to telehealth resources.42,43 This would standardize quality-of-care protocols, patient access to expertise, and provider access to up-to-date medical education required to support a diverse HE clinical research workforce. This would build infrastructure to examine implementation and best practice strategies, provide technical assistance, and disseminate information about successful interventions.
HD networks were also described as an opportunity to enhance HDP patient engagement and standardize implementation of community-engaged research strategies across HE research. Respondents emphasized the need to invest in sustainable partnerships with HDP community members, apart from any individual study. Respondents highlighted the importance of developing equitable community partner relationships by addressing SDOH needs of community members in addition to providing information. Stakeholders suggested that such networks could standardize implementation strategies used by existing engagement networks44 across sites to promote community and clinician-scientist bi-directional information sharing and health empowerment. Most of the network examples identified through the RFI were not focused on HDP engagement. However, these engagement networks have established sustainable relationships with the communities and families within neurologic subspecialties.45,46
Training
Training interventions frequently described efforts to support, recruit, retain, and train a diverse neurologic disorder HD research workforce. Respondents infrequently provided evidence-based HD training illustrations, suggesting that training represents a HD gap area.
Respondents described strategies to incentivize recruitment of neurospecialists to conduct HE research in diverse settings serving HDPs in under-resourced communities. Stakeholders suggested recruiting experienced clinicians and high-effect clinician-scientists into the HE field by incentivizing their transition to practice and study in geographic locations with limited neurospecialists. Respondents emphasized the need for research funding agencies to create pathways to retain talented members of the HE research workforce who are discouraged from participating in HE research because of dependent and family care barriers (i.e., providing childcare or assistance with home schooling). These intervention opportunities also described pathways for recruiting and retaining individuals who are members of marginalized communities in the neurologic disorder workforce.
Stakeholders made clear the importance of broad investment in changing the culture of the neurologic research workforce to make it more inclusive. Respondents suggested standardizing development of the neurologic disorder workforce through increasing training and continuing education funding opportunities in HE research, effective diverse community/patient engagement, health disparities awareness, and SDOH health outcomes influence. Respondents suggested that development of HD research networks could support infrastructure to increase HE training of clinical house staff and deliver continuing medical education (CME) opportunities for practitioners to improve skills. Stakeholders also described the need for the development of HE-relevant Accreditation Council for Continuing Medical Education–accredited CME training across neurologic disorder subspecialties and pointed to existing examples.44
Patient Perspective on Neurologic Disorder HDs
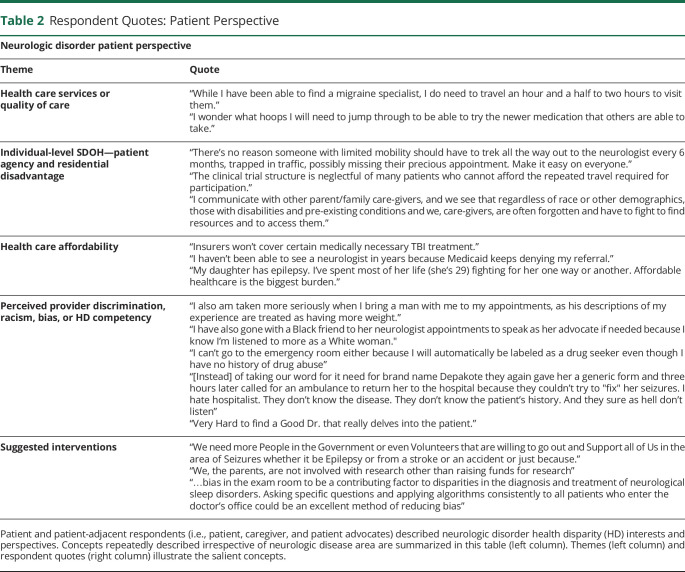
Patient, patient advocate, and caregiver respondents represented more than 20% of RFI stakeholders. When determinant categories (Figure 3C) were stratified by responder type, there was consensus among patient-centric stakeholders that SDOH are the primary drivers of HDs. Patient and patient-adjacent (i.e., caregivers and individual patient advocates) respondents frequently identified Health Care Services or quality of care, individual-level SDOH (patient agency and geographic disadvantage), and perceived provider discrimination as the primary drivers of HDs (data not shown). Across neurologic diseases, respondent descriptions of patient experiences and barriers to care often described determinants related to patient agency, structural barriers, medical mistrust, provider bias, health care service availability, and health care affordability (Table 2). Respondents highlighted a common sentiment of HDPs—many feel that the interpersonal relationship with the provider is not built on mutual respect.47 This creates trust barriers, which, in turn, reduce care quality and utilization.
Table 2.
Respondent Quotes: Patient Perspective
Discussion
We conducted a RFI for the public to comment on barriers and facilitators related to HD research in neurologic disease and care. The RFI attracted stakeholders with interest across over 14 neurologic disease areas, with stroke, pain, dementia, TBI, and epilepsy/seizure having the highest representation. Low SES, racial/ethnic minoritized, and geographically disadvantaged populations were most frequently identified as populations disproportionately experiencing HDs. They perceived socioecological drivers of HDs as primary determinants driving inequity. Most of the HD determinants and subsequent interventions could be classified into the following concepts: neurospecialty care access, innovative HDP engagement and research inclusion strategies, and development of a well-trained clinician and researcher HD workforce. Finally, the patient perspective shows deep stakeholder concern regarding cultural competence, provider bias, medical mistrust, neurospecialty care access, and affordability driving HDs in neurologic disorders. While we report key themes that consistently emerged from the analysis of respondent perspectives, inferences drawn from respondent narratives cannot be generalized because of low sample size and limitations inherent to the process (see eAppendix 1, links.lww.com/WNL/C929—Limitations section).
Across all short-answer responses, barriers related to access to care for HDPs were consistently discussed. Like specialty care utilization and access across medicine,48 respondents report that access to neurospecialty care is lower in populations experiencing low socioeconomic status and racial/ethnic minoritization and the underinsured. High geographic distance from neurologic centers with neurospecialization was suggested to be a primary determinant underlying care disparities (i.e., limited referrals to specialists and long wait times to see specialists) for low-income individuals and those living in health deserts. However, respondents suggested that even proximity to a specialist is not beneficial if you are a low-income member or member of a minoritized racial/ethnic group. Stakeholders suggested that it is critical for research funding agencies to develop opportunities to address SDOH to reduce HDs in neurologic disorders.
Stakeholder responses suggest that the lack of diverse participant inclusion in neurologic research drives the lack of community engagement and lack of patient health literacy and serves as a root cause of medical mistrust among vulnerable populations. Respondents propose that the incorporation of CEr into HE neurologic disorder research could promote the creation of effective culturally sensitive strategies, best practices in patient education, HDP health empowerment, and development of appropriate interventions. They emphasized that researchers learn to develop and foster partnerships with trusted community-based organizations, ensure that members of their research team reflect under-represented groups, and budget specifically for recruitment and retention efforts. The NIH has historically invested in research to test the effect of implementing the community-based participatory research (CBPR) conceptual model and has begun to identify promising practices associated with improved equitable outcomes associated with implementation of the CBPR model.49 This presents an opportunity for NINDS to emphasize incorporation of innovative CEr implementation solutions in future research directions.
In addition to telehealth and digital technologies' potential to reduce barriers to access to care for HDPs, they are also described as research opportunities to improve HDP quality of care and advance HE research integration into clinical settings. Embedding point-of-care research within regular clinical care settings could accelerate longitudinal HDP neurologic disease surveillance and implementation of HD interventions. Technology that streamlines point-of-care research by supporting researchers’ ability to create custom workflows32 and incorporating SDOH data collection into EHRs21 likely represent the future wave of innovative HE interventions to improve HE research. Telehealth can reduce cost burden to HDP participants and help overcome structural barriers to care. Another promising practice discussed was the development of hybrid comprehensive care models consisting of remote monitoring of training at patients' homes.50
Respondents affirm the importance of creating pathways for interested HDPs identifying trainees, clinicians, and scientists to enter the HE workforce. Collectively, respondents were enthusiastic about developing an innovative research network and highlighted potential benefits. For example, a HE network could facilitate the incorporation of interdisciplinary research teams into HE research by fostering collaboration between a diverse coalition of disciplines. These teams would be able to innovatively develop interventions that incorporate important contextual factors (i.e., structural racism, food insecurity, unemployment). Respondents suggest that training programs could build on or model components of the existing HD network infrastructure (i.e., NIH StrokeNet). Training collaboratives could model the existing NIH StrokeNet Training Core,41 which supports a multidisciplinary training committee and cross-institutional mentorship by StrokeNet investigators from over 500 hospitals and ensures that trainees can dedicate at least 50% of their time to effectively train and engage in stroke research. Stakeholders recommend that HE networks incorporate tools to facilitate training and engagement of HDP community partners. This has implications for strengthening community relationships and accelerating health empowerment. NIH programs, such as Faculty Institutional Recruitment for Sustainable Transformation (FIRST) and the NIH-wide UNITE Initiative, create an opportunity to diversify the workforce and develop research teams that reflect the diversity of HDP participants within HD clinical research.
Congruent with current HD findings outside of neurologic disorders, SDOH affect health opportunity and outcomes are a primary concern of stakeholders. These findings provide insight into how diverse stakeholders conceptualize the hierarchy of SDOH determinant and intervention prioritization in neurologic disorder clinical research. By contrast, much of the existing HD evidence provided by respondents primarily described observational findings from investigations of biological and behavioral contribution to HD. Thematic clustering of stakeholder sentiments through a HE research lens gives contextual insight to support NINDS strategic planning efforts to advance HE. This RFI input will help inform planning and priorities as NINDS and NIH work to eliminate health disparities in neurologic disease and disorders.
Acknowledgment
The authors thank individuals and organizations who provided source materials for this article through their RFI responses. They thank the Health Equity Coordinating Committee and the Division of Clinical Research at NINDS for feedback and input on this manuscript draft (especially Lauren E. Ullrich, Kristina K. Hardy, and Sara Dodson). This article does not intend to imply or summarize the interest of the National Institute of Health or Office of Global Health and Health Disparities or any other grant funding entity. It comprises suggestions and opinions of experts and community stakeholders across the fields of neurological disease and health disparities.
Glossary
- CBPR
community-based participatory research
- CEr
community engaged research
- CME
continuing medical education
- EHR
electronic health records
- HD
health disparity
- HDP
population that experience HD
- NIMHD
National Institute on Minority Health and Health Disparities
- NINDS
National Institute of Neurological Disorders and Stroke
- RFI
request for information
- SDOH
social determinants of health
- SES
socioeconomic status
- TBI
traumatic brain injury
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Gómez CA, Kleinman DV, Pronk N, et al. . Addressing health equity and social determinants of health through healthy people 2030. J Public Health Manag Pract. 2021;27(suppl 6):S249-s257. doi: 10.1097/phh.0000000000001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine. 2017. Communities in Action: Pathways to Health Equity. Washington, DC: The National Academies Press. 10.17226/24624. [DOI] [PubMed] [Google Scholar]
- 3.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099-1104. doi: 10.1016/s0140-6736(05)71146-6 [DOI] [PubMed] [Google Scholar]
- 4.Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(1_suppl2):19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113-2121. doi: 10.2105/ajph.2005.077628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchwell K, Elkind MSV, Benjamin RM, et al. . Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454-e468. doi: 10.1161/cir.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 7.Grabovschi C, Loignon C, Fortin M. Mapping the concept of vulnerability related to health care disparities: a scoping review. BMC Health Serv Res. 2013;13:94. doi: 10.1186/1472-6963-13-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46(1):15-31. doi: 10.1177/002214650504600103 [DOI] [PubMed] [Google Scholar]
- 9.Feigin VL, Vos T, Alahdab F, et al. . Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi: 10.1001/jamaneurol.2020.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients' social and economic needs. Am J Prev Med. 2017;53(5):719-729. doi: 10.1016/j.amepre.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Green CR, Anderson KO, Baker TA, et al. . The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277-294. doi: 10.1046/j.1526-4637.2003.03034.x [DOI] [PubMed] [Google Scholar]
- 12.Benson RT, Koroshetz WJ. Health disparities: research that matters. Stroke. 2022;29(2):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skolarus LE, Sharrief A, Gardener H, Jenkins C, Boden-Albala B. Considerations in addressing social determinants of health to reduce racial/ethnic disparities in stroke outcomes in the United States. Stroke. 2020;51(11):3433-3439. doi: 10.1161/strokeaha.120.030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Neurological Disorders and Stroke (NINDS). Request for Information: Soliciting Input on Areas of Health Disparities and Inequities in Neurological Disease and/or Care in the United States Across the Lifespan. Accessed May 16, 2022. grants.nih.gov/grants/guide/notice-files/NOT-NS-20-026.html. [Google Scholar]
- 15.Smith JA, Shinebourne P. Interpretative Phenomenological Analysis. American Psychological Association; 2012. [Google Scholar]
- 16.National Institute on Minority Health and Health Disparities (2017). NIMHD Research Framework. Accessed May 12, 2022. nimhd.nih.gov/researchFramework. [Google Scholar]
- 17.Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and Health Disparities Research Framework. Am J Public Health. 2019;109(S1):S16-S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Secretary’s Advisory Committee on National Health Promotion and Disease Prevention Objectives for 2030. Issue Briefs to Inform Development and Implementation of Healthy People 2030. Accessed January 12, 2023. health.gov/our-work/national-health-initiatives/healthy-people/healthy-people-2030/secretarys-advisory-committee-2030/secretarys-advisory-committee-2030-committee-reports-and-meetings. [Google Scholar]
- 19.Israel BA, Coombe CM, Cheezum RR, et al. . Community-based participatory research: a capacity-building approach for policy advocacy aimed at eliminating health disparities. Am J Public Health. 2010;100(11):2094-2102. doi: 10.2105/AJPH.2009.170506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Economic Research Service USDA. ERS Charts of Note. Accessed December 12, 2022. ers.usda.gov/data-products/charts-of-note/charts-of-note/?topicId=4e8a0642-e40d-4299-906e-906bbaaf9e4d. [Google Scholar]
- 21.Grinspan ZM, Patel AD, Shellhaas RA, et al. . Design and implementation of electronic health record common data elements for pediatric epilepsy: Foundations for a learning health care system. Epilepsia. 2021;62(1):198-216. doi: 10.1111/epi.16733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah PD, Yun M, Wu A, et al. . Pediatric Epilepsy Learning Healthcare System Quality of Life (PELHS-QOL-2): a novel health-related quality of life prompt for children with epilepsy. Epilepsia. 2022;63(3):672-685. doi: 10.1111/epi.17156 [DOI] [PubMed] [Google Scholar]
- 23.Hussein HM, Droegemueller C, Xiong P, et al. . Hmong cross-cultural adaptation of stroke educational material. WMJ. 2020;119(2):115-118. [PubMed] [Google Scholar]
- 24.Sheridan S, Schrandt S, Forsythe L, Hilliard TS, Paez KA. The PCORI engagement rubric: promising practices for partnering in research. Ann Fam Med. 2017;15(2):165-170. doi: 10.1370/afm.2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feeney M, Evers C, Agpalo D, Cone L, Fleisher J, Schroeder K. Utilizing patient advocates in Parkinson's disease: a proposed framework for patient engagement and the modern metrics that can determine its success. Health Expect. 2020;23(4):722-730. doi: 10.1111/hex.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung J, Aguila F, Harris O. Validity assessment of referral decisions at a VA health care system polytrauma system of care. Cureus. 2015;7(1):e240. doi: 10.7759/cureus.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamberty GJ, Nakase-Richardson R, Farrell-Carnahan L, et al. . Development of a traumatic brain injury model system within the department of veterans affairs polytrauma system of care. J Head Trauma Rehabil. 2014;29(3):E1-E7. doi: 10.1097/HTR.0b013e31829a64d1 [DOI] [PubMed] [Google Scholar]
- 28.Walter S, Zhao H, Easton D, et al. . Air-Mobile Stroke Unit for access to stroke treatment in rural regions. Int J Stroke. 2018;13(6):568-575. doi: 10.1177/1747493018784450 [DOI] [PubMed] [Google Scholar]
- 29.Chapman Smith SN, Govindarajan P, Padrick MM, et al. . A low-cost, tablet-based option for prehospital neurologic assessment: the iTREAT Study. Neurology. 2016;87(1):19-26. doi: 10.1212/wnl.0000000000002799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman-Toker DE, Curthoys IS, Halmagyi GM. Diagnosing stroke in acute vertigo: the HINTS family of eye movement tests and the future of the "Eye ECG". Semin Neurol. 2015;35(5):506-521. doi: 10.1055/s-0035-1564298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajeevan N, Niehoff KM, Charpentier P, et al. . Utilizing patient data from the veterans administration electronic health record to support web-based clinical decision support: informatics challenges and issues from three clinical domains. BMC Med Inform Decis Mak. 2017;17(1):111. doi: 10.1186/s12911-017-0501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhond R, Elbers D, Majahalme N, et al. . ProjectFlow: a configurable workflow management application for point of care research. JAMIA Open. 2021;4(3):ooab074. doi: 10.1093/jamiaopen/ooab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocker C, Cappelletti L, Marshall C, et al. . Use of an online portal to facilitate clinical trial recruitment: a preliminary analysis of Fox Trial Finder. J Parkinsons Dis. 2015;5(1):55-66. doi: 10.3233/jpd-140522 [DOI] [PubMed] [Google Scholar]
- 34.Parkinson's Foundation. PD GENEration: Mapping the Future of Parkinson's Disease. Accessed March 7, 2022. parkinson.org/PDGENEration. [Google Scholar]
- 35.Shellmer DA, Dew MA, Mazariegos G, DeVito Dabbs A. Development and field testing of Teen Pocket PATH(®), a mobile health application to improve medication adherence in adolescent solid organ recipients. Pediatr Transpl. 2016;20(1):130-140. doi: 10.1111/petr.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modi AC, Mann KA, Urso L, Peugh J. Preliminary feasibility and efficacy of text messaging and application-based adherence interventions in adolescents with epilepsy. Epilepsy Behav. 2016;63:46-49. doi: 10.1016/j.yebeh.2016.07.036 [DOI] [PubMed] [Google Scholar]
- 37.Sorin LM, Knupp KG, Berg AT. New-onset seizure survey of epilepsy centers in the United States. Epilepsy Behav. 2019;101(Pt A):106579. doi: 10.1016/j.yebeh.2019.106579 [DOI] [PubMed] [Google Scholar]
- 38.Broderick JP, Palesch YY, Janis LS. The National Institutes of Health StrokeNet: a user's guide. Stroke. 2016;47(2):301-303. doi: 10.1161/strokeaha.115.011743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stickel A, McKinnon A, Ruiz J, Grilli MD, Ryan L. The impact of cardiovascular risk factors on cognition in Hispanics and non-Hispanic whites. Learn Mem. 2019;26(7):235-244. doi: 10.1101/lm.048470.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandt L, Warne-Griggs M, Hoffman K, Popejoy L, Mutrux ER. Embracing the power of show-Me ECHO learning communities to transform clinical practice in Missouri. Mo Med. 2020;117(3):216-221. [PMC free article] [PubMed] [Google Scholar]
- 41.Vahidy FS, Sozener CB, Meeks JR, et al. . National Institutes of Health StrokeNet Training Core. Stroke. 2020;51(1):347-352. doi: 10.1161/STROKEAHA.119.027946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly AG, Hellkamp AS, Olson D, Smith EE, Schwamm LH. Predictors of rapid brain imaging in acute stroke: analysis of the Get with the Guidelines-Stroke program. Stroke. 2012;43(5):1279-1284. doi: 10.1161/strokeaha.111.626374 [DOI] [PubMed] [Google Scholar]
- 43.Thielst CB. At the crossroads: NRTRC white paper examines trends driving the convergence of telehealth, EHRs and HIE. World Hosp Health Serv. 2010;46(4):17-23. [PubMed] [Google Scholar]
- 44.Lunn MR, Lubensky M, Hunt C, et al. . A digital health research platform for community engagement, recruitment, and retention of sexual and gender minority adults in a national longitudinal cohort study—The PRIDE Study. J Am Med Inform Assoc. 2019;26(8-9):737-748. doi: 10.1093/jamia/ocz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahoo SS, Zhang GQ, Bamps Y, et al. . Managing information well: toward an ontology-driven informatics platform for data sharing and secondary use in epilepsy self-management research centers. Health Inform J. 2016;22(3):548-561. doi: 10.1177/1460458215572924 [DOI] [PubMed] [Google Scholar]
- 46.Hong X, Liu C, Momotaz H, Cassidy K, Sajatovic M, Sahoo SS. Enhancing multi-center patient cohort studies in the managing epilepsy well (MEW) network: integrated data integration and statistical analysis. AMIA Annu Symp Proc. 2020;2019:1071-1080. [PMC free article] [PubMed] [Google Scholar]
- 47.Ighodaro ET, Littlejohn EL, Akhetuamhen AI, Benson R. Giving voice to Black women in science and medicine. Nat Med. 2021;27(8):1316-1317. doi: 10.1038/s41591-021-01438-y [DOI] [PubMed] [Google Scholar]
- 48.Saadi A, Himmelstein DU, Woolhandler S, Mejia NI. Racial disparities in neurologic health care access and utilization in the United States. Neurology. 2017;88(24):2268-2275. doi: 10.1212/WNL.0000000000004025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallerstein N, Oetzel JG, Sanchez-Youngman S, et al. . Engage for equity: a long-term study of community-based participatory research and community-engaged research practices and outcomes. Health Educ Behav. 2020;47(3):380-390. doi: 10.1177/1090198119897075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piotrowicz E, Pencina MJ, Opolski G, et al. . Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 2020;5(3):300-308. doi: 10.1001/jamacardio.2019.5006 [DOI] [PMC free article] [PubMed] [Google Scholar]