Abstract
Asian freshwater clams, Corbicula fluminea, exposed for 24 h to 38 liters of water contaminated with infectious Cryptosporidium parvum oocysts (1.00 × 106 oocysts/liter; approximately 1.9 × 105 oocysts/clam) were examined (hemolymph, gills, gastrointestinal [GI] tract, and feces) on days 1, 2, 3, 7, and 14 postexposure (PE). No oocysts were detected in the water 24 h after the contamination event. The percentage of oocyst-containing clams varied from 20 to 100%, depending on the type of tissue examined and the technique used—acid-fast stain (AFS) or immunofluorescent antibody (IFA). The oocysts were found in clam tissues and feces on days 1 through 14 PE; the oocysts extracted from the tissues on day 7 PE were infectious for neonatal BALB/c mice. Overall, the highest number of positive samples was obtained when gills and GI tracts were processed with IFA (prevalence, 97.5%). A comparison of the relative oocyst numbers indicated that overall, 58.3% of the oocysts were found in clam tissues and 41.7% were found in feces when IFA was used; when AFS was used, the values were 51.9 and 48.1%, respectively. Clam-released oocysts were always surrounded by feces; no free oocysts or oocysts disassociated from fecal matter were observed. The results indicate that these benthic freshwater clams are capable of recovery and sedimentation of waterborne C. parvum oocysts. To optimize the detection of C. parvum oocysts in C. fluminea tissue, it is recommended that gill and GI tract samples be screened with IFA (such as that in the commercially available MERIFLUOR test kit).
Cryptosporidium parvum-associated cryptosporidiosis is a zoonotic diarrheal disease which is life threatening for immunocompromised or immunosuppressed humans (7) and can severely debilitate immunocompetent people (20). The infectious stage, the oocyst, is transmitted via the fecal-oral route or via water. Contamination of surface waters is caused by agricultural and urban runoff (11, 16, 21). Waterborne transmission is facilitated by the long-lasting infectivity of oocysts, their small size (3.5 to 6.0 μm), and their low sedimentation rate (0.5 μm/s) (21).
The Asian freshwater clam, Corbicula fluminea, introduced to North America in the early 1900s (18), is well adapted to life in unstable and unpredictable habitats (17). The species is highly successful in drainage systems with periodic human interference (17, 19). This benthic clam can survive in waters receiving agricultural and industrial pollution and urban waste (17). C. fluminea serves as a bioindicator of major, minor, and trace constituents (15), pollution with organochlorine insecticides and metals (22, 23), and waterborne polycyclic mutagens (14).
C. fluminea is a suspension feeder able to filter detrital particles of 1.5 to 10.0 μm at a rate of up to 2.50 liters/h (17). It was demonstrated that hemocytes of C. fluminea phagocytosed in vitro infectious C. parvum oocysts and Giardia duodenalis cysts (12, 13). By mathematical extrapolation it was determined that a single clam can retain in its tissue over 3 million C. parvum oocysts (12) or G. duodenalis cysts (13). Although in vitro phagocytosis of C. parvum oocysts was efficient (12), it remains unknown if intact clams are capable of recovering oocysts from contaminated water and if waterborne oocysts can be subsequently detected in clam tissue.
The purpose of the present study was to determine if C. fluminea clams could recover and sediment waterborne C. parvum oocysts and if in vivo-phagocytosed oocysts retained their infectivity. Additional aims of the study were to determine which tissue of a clam, e.g., hemolymph or gills and gastrointestinal (GI) tract, was best suited for the detection of C. parvum oocysts and which technique, e.g., acid-fast stain (AFS) or immunofluorescent antibody (IFA), was optimal for the detection of oocysts in clam tissue.
MATERIALS AND METHODS
Oocysts of C. parvum (AUCP-1 strain) were obtained and purified as described previously (4). Oocysts resuspended in phosphate-buffered saline (PBS) (pH 7.4) were counted with a hemacytometer (4) and stored at 4°C for no longer than 1 week.
A 38-liter (approximately 10-gal) aquarium was filled with dechlorinated drinking water filtered by a Filterite 10-μm-pore-size, yarn-wound cartridge (Memtec America Corp., Baltimore, Md.). Aliquots of the water were tested for Cryptosporidium oocysts by the cellulose acetate membrane (CAM) filter dissolution method (10) and were found to be oocyst free. The aquarium was equipped with a Fluval filter (model 403; Askoll) and an air stone, and the system had been working continuously for 1 week prior to clam introduction. Two hundred C. fluminea clams with shell lengths of 1.5 to 2.5 cm were collected from Lake Cheston (Franklin County, Tenn.) and placed in the aquarium. The clams were fed on alternate days with a 20-ml suspension of algae (Chlorella pyrenoidosa; Carolina Biological Supply Co., Burlington, N.C.) cultured in a 3.9-liter all-glass aquarium according to the manufacturer’s instructions. The filter system (but not the air stone) had been off for 5 h at the moment of addition of the algae to the aquarium with the clams. Dead C. fluminea clams were removed from the aquarium.
Thirty randomly selected control clams were removed from the aquarium. The filter (but not the air stone) was turned off, and the water was seeded with 3.8 × 107 oocysts of C. parvum (1.00 × 106 oocysts/liter; approximately 1.9 × 105 oocysts/clam). After 24 h (1 day postexposure [PE]), five water samples of 3.8 liters each were collected from the aquarium and the remaining water was removed. The aquarium was filled with 38 liters of dechlorinated drinking water processed as described previously, and the clams were maintained as prior to the exposure to C. parvum oocysts. Another five water samples of 3.8 liters each were collected from the aquarium on day 3 PE; a similar volume of water was added to the aquarium. The water samples were individually processed by the CAM filter dissolution method for the detection of waterborne Cryptosporidium oocysts (10).
Thirty randomly selected clams were examined on days 1, 2, 3, 7, and 14 PE. While the clam was kept on ice, the anterior and posterior adductor muscles were cut by a scalpel inserted gently below the anterior and posterior lateral teeth (17). The shell was opened, and the hemolymph (approximately 0.5 ml) was aspirated from the blood sinuses with a 1.0-ml pipette. Hemocyte monolayers were prepared by filling one 12-mm-diameter well on a slide of the MERIFLUOR Cryptosporidium/Giardia test kit (Meridian Diagnostics, Inc., Cincinnati, Ohio) with 50 μl of the hemolymph from a clam. The remaining hemolymph samples were each spread evenly on glass slides which were processed with AFS following air drying and fixation with methanol. The MERIFLUOR slides were processed with IFA as described previously (5). The concentration of hemocytes was determined as described previously (12) for the first 10 control clams and the first 10 test clams at each time point.
After hemolymph collection, the gills and GI tract were excised from each clam and placed individually in 0.25 ml of PBS in a Kimble pellet Eppendorf tube with a plastic grinding pin fitting the bottom of the tube (VWR, Piscataway, N.J.); grains were removed from the samples, which were then vortexed, and the fluid was aspirated with a pipette. One MERIFLUOR slide and one AFS slide were prepared from the fluid in the same manner as that described for the hemolymph.
Clam feces were aspirated daily from the bottom of the aquarium on days 1 through 7 PE and on day 14 PE. Efforts were made to collect all feces. The water (approximately 0.5 liters) containing the feces was left overnight at 4°C in a Kimax conical graduated container (VWR). Each daily feces sediment collection was used for the preparation of 10 AFS and 10 IFA slides in the manner described for the hemolymph.
Examination of the fluorescein-stained slides followed a previously described protocol (9), and the confirmation approach (2) was used to rule out presumptive Cryptosporidium oocysts. The number of C. parvum oocysts on IFA and AFS slides was determined during a 5-min examination with a ×40 objective. To ensure that C. pyrenoidosa algae were not mistakenly identified as C. parvum oocysts in AFS and IFA reactions, direct wet smears of C. pyrenoidosa were stained with IFA and AFS and examined microscopically.
On day 7 PE, the hemolymph and the gill and GI tract samples collected from 30 clams were pooled separately. Tubes with pooled samples were centrifuged at 2,000 × g for 15 min at 4°C, and the supernatant was discharged. The hemolymph and the gill and GI tract pellets were resuspended in 300 and 400 μl of PBS, respectively. Hemolymph-derived and gill- and GI tract-derived suspensions were administered by gastric intubation to three and four neonatal BALB/c mice, respectively. Two control mice were inoculated with 100 μl of PBS. Mice were euthanized by overexposure to CO2 96 h postinoculation and necropsied, and their ilea were recovered and processed for histologic examination (3). Hematoxylin- and eosin-stained sections were examined for developmental stages of C. parvum by light microscopy (3), and the severity of infection was scored as described previously (3).
Statistical analysis was carried out with Statistix 4.1 (Analytical Software, St. Paul, Minn.). The variables were examined by the Runs test to determine conformity to a normal distribution. A nonparametric test included a Kruskal-Wallis analysis of variance (ANOVA). The degree of linear association between variables was assessed with the regression test. Prevalences and fractions were compared with a G-heterogeneity test and a χ2 test, respectively, and mean values were compared with a two-sample t test. Mean values were associated with standard deviations. Statistical significance was set at P ≤ 0.05.
RESULTS
The mean hemocyte concentrations varied from 3.3 × 105 to 6.7 × 105 cells/ml, with an overall mean of 4.6 × 105 cells/ml (±9.9 × 104 cells/ml). Fluctuations in the mean hemocyte concentrations were not significant (G-heterogeneity test; G = 1.70, P > 0.05).
Overall, the clam mortality rate was 10%.
No C. parvum oocysts were detected in the water samples collected on days 1 and 3 PE. The filtration rates were considerably accelerated compared with drinking water filtration rates, indicating that the filtered water was substantially depleted of particulate matter.
C. pyrenoidosa algae did not produce a positive IFA or AFS reaction.
Four of 30 (13%) hemolymph monolayers from control clams contained one presumptive Cryptosporidium oocyst each, as determined with IFA and AFS.
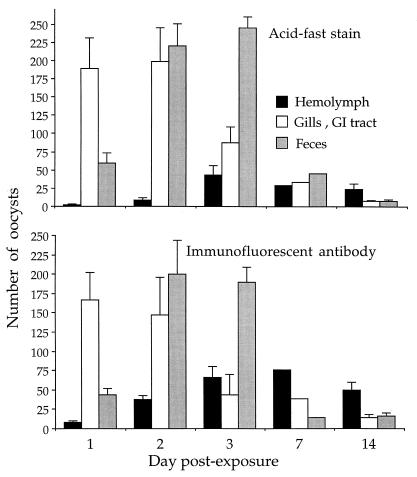
At 24 h after exposure of clams to waterborne oocysts of C. parvum, the pathogen was observed, although at various concentrations, in the hemolymph, gills and GI tract, and feces (Fig. 1). The oocysts were detected in clam tissues and feces as late as 14 days PE (Fig. 1). As determined by IFA, the number of phagocytosed oocysts progressively increased up to day 7 PE and then decreased (Fig. 1). When AFS preparations were used, the peak in the number of phagocytosed oocysts occurred on day 3 PE (Fig. 1). Hemocyte-internalized C. parvum oocysts were clearly visible in both IFA and AFS preparations. On AFS slides, the oocysts displayed nonuniform, bright red coloration with characteristic black granules. Viewed by fluorescence microscopy, the oocysts were bright green. No free oocysts were observed in hemocyte monolayers. The density of oocysts detected in the gill and GI tract samples was highest on days 1 and 2 PE and then progressively decreased (Fig. 1). The IFA patterns of oocyst concentrations in clam tissues and feces did not differ significantly (Kruskal-Wallis ANOVA; F = 3.00, P > 0.05) from the results obtained by AFS.
FIG. 1.
Mean numbers (and standard deviations) of C. parvum oocysts detected in tissues of Asian freshwater clams, C. fluminea, and in their feces. Clams were exposed for 24 h to 38 liters of water contaminated with 3.8 × 107 C. parvum oocysts and examined in batches of 30. Ten fecal specimens were obtained.
The number of C. parvum-positive clams varied depending on the type of tissue examined and the technique used for oocyst detection (Table 1). The most apparent differences were observed on day 1 PE, when all 30 samples of gills and GI tract were found oocyst positive by either AFS or IFA and only 6 and 14 hemolymph samples were found positive by AFS and IFA, respectively (Table 1). The differences between the numbers of oocyst-positive hemolymph samples and gills and GI tract samples were significant only on day 1 PE (χ2 test; χ2 = 3.84, P < 0.05) for either AFS or IFA. Also, only on day 1 PE, the number of IFA-positive determinations was significantly higher than the number of AFS-positive readings (χ2 test; χ2 = 4.31, P < 0.05). Overall, the highest number of oocyst-positive samples was obtained when gill and GI tract samples were screened with IFA (prevalence, 97.5%) (Table 1). This prevalence value was significantly higher (G-heterogeneity test; G = 7.9, P < 0.05) than the values obtained for hemolymph samples examined by AFS and IFA, 72.5 and 86.7%, respectively (Table 1).
TABLE 1.
Numbers of Asian freshwater clams (C. fluminea) positive for C. parvum oocysts after exposure for 24 h to 38 liters of C. parvum-contaminated watera
| Tissue | No. of clams with a positive result on day PE
|
Total no. (%) of clams found positive by:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
14
|
|||||||
| AFS | IFA | AFS | IFA | AFS | IFA | AFS | IFA | AFS | IFA | |
| Hemolymph | 6 | 14 | 27 | 30 | 29 | 30 | 25 | 30 | 87 (72.5) | 104 (86.7) |
| Gills and stomach | 30 | 30 | 29 | 30 | 27 | 30 | 27 | 27 | 113 (94.2) | 117 (97.5) |
The water contained 1.00 × 106 oocysts/liter. Clams were examined in batches of 30.
Based on the results presented in Table 1, the sensitivities of detection by AFS of C. parvum oocysts were 83.6% for hemolymph and 96.5% when the gills and GI tract were examined. If we assume the superiority of gill and GI tract testing, the sensitivities of oocyst detection in hemolymph were 77.0% for AFS and 88.8% for IFA.
When the relative numbers of C. parvum oocysts in the hemolymph and the gill and GI tract samples were summarized, it appeared that most of the oocysts were detected on days 1 and 2 PE and that the oocyst numbers then progressively decreased (Fig. 1). For AFS (but not for IFA), the decrease in the mean oocyst number per clam was significantly related to the length of PE time (regression test; F = 13.95, P < 0.03). Interestingly, on days 7 and 14 PE, when the numbers of detected oocysts were low (Fig. 1), IFA produced significantly higher levels of positive determinations than AFS (two-sample t test; t = 5.13, P < 0.03).
Not all C. parvum oocysts were retained in clam tissues; some were released in feces. All fecal samples contained oocysts (Fig. 1). Peak numbers of oocysts in feces were found on days 2 and 3 PE (Fig. 1). The oocysts were always surrounded by feces and were frequently observed in clusters. No free oocysts or oocysts disassociated from fecal matter were observed. On day 14 PE, over 30% of all detected oocysts were found in clam feces (Table 2). The patterns of oocyst numbers detected in clam tissues and feces did not differ significantly between IFA and AFS readings (Kruskal-Wallis ANOVA; F = 0.25, P > 0.05). A comparison of the relative numbers of detected oocysts indicated that overall, 58.3% of oocysts were found in clam tissues and 41.7% were found in feces when the IFA technique was used; these values were 51.9 and 48.1%, respectively, when the AFS method was used.
TABLE 2.
Mean numbers and percentages of C. parvum oocysts detected in tissues and feces of Asian freshwater clams (C. fluminea)a
| Day PE | No. (%) with oocysts detected in indicated samples by:
|
|||
|---|---|---|---|---|
| IFA
|
AFS
|
|||
| Tissues | Feces | Tissues | Feces | |
| 1 | 87.6 (66.6) | 44.0 (33.4) | 95.8 (61.6) | 59.8 (38.4) |
| 2 | 92.5 (31.7) | 200.1 (68.3) | 103.8 (32.1) | 220.0 (67.9) |
| 3 | 55.0 (22.5) | 190.3 (77.5) | 65.7 (21.2) | 245.0 (78.8) |
| 7 | 57.5 (76.7) | 14.0 (23.3) | 31.0 (40.8) | 45.0 (59.2) |
| 14 | 32.0 (66.7) | 16.0 (33.3) | 15.5 (68.6) | 7.1 (31.4) |
Clams were exposed for 24 h to 38 liters of water contaminated with 3.8 × 107 C. parvum oocysts and were examined in batches of 30. Ten fecal specimens were obtained.
The ilea of all seven neonatal BALB/c mice inoculated with C. parvum oocysts recovered from clam hemolymph and gill and GI samples on day 7 PE contained large numbers of Cryptosporidium life-cycle stages. Over 65% of the epithelial cells harbored the developmental stages of C. parvum. Control mice were negative for C. parvum developmental stages.
DISCUSSION
As demonstrated in the present study, the Asian freshwater clam, C. fluminea, can remove C. parvum oocysts from water. Based on negative findings from the CAM filter dissolution method, 200 clams removed 3.8 × 107 oocysts from 38 liters of water within 24 h. To achieve such removal, an average clam had to recover and retain within its body approximately 1.9 × 105 oocysts. As C. fluminea can filter as much as 2.5 liters/h (17), it is assumed that under the conditions given in the present experiment (approximately 0.2 liter/clam), the water in the aquarium was filtered multiple times. A similar phenomenon was described in a natural situation in the Trinity River, Clear Fork, W.Va. (average depth, 0.25 m; current flow, 18.5 m/min), where the entire water volume overlying C. fluminea beds was filtered every 16 min (17).
The present study showed that in addition to in vitro phagocytosis (12), waterborne oocysts of C. parvum could be phagocytosed in vivo. The hemocyte-internalized oocysts could be detected by IFA or AFS for at least 14 days after exposure of clams to contaminated water. However, as oocyst size and morphology do not vary considerably within the genus Cryptosporidium, we conclude that oocysts of other Cryptosporidium species (11) might also be recovered from water and retained in the clam tissue. The mechanism of particle circulation in the branched GI system of C. fluminea ensures the most efficient utilization of water-recovered matter. In C. fluminea, before particles filtered from water are accepted for extracellular or intracellular digestion or are rejected as feces, they may pass through the gut several times (17).
C. fluminea clams are preferential filter feeders rather than detritus feeders (they will sporadically ingest detrital particles from substrata, e.g., sediments) (17). Thus, the presence of oocysts in the clam tissue would be indicative of water contamination rather than contamination of sediments. These bivalves are abundantly prevalent in Cryptosporidium-contaminated waters; have a wide geographical range (17), facilitating the comparison of water contamination; can survive in wastewaters (1); can be collected throughout the year; and can be held in field enclosures (17). Also, their small size and consequently the small amount of tissue to be tested facilitate more accurate detection of oocysts.
Examination of gill and GI tract samples for the presence of C. parvum oocysts was superior to examination of hemocyte monolayers based on overall higher oocyst concentrations in the former samples. Sensitivities of oocyst detection obtained for AFS and IFA for either type of clam tissue compare favorably for IFA. Thus, it is recommended that gill and GI tract samples be screened with IFA. However, technician time and the costs of IFA and AFS compare favorably for AFS. Given 5 min for slide examination (either AFS or IFA) and assuming similar times allocated for the processing of clam tissue by both techniques, an IFA slide has to be processed for 40 min, versus 10 min for an AFS slide. The cost of a single IFA determination is approximately six times higher than that of an AFS determination, but IFA costs can be reduced as much as two times by use of the approach taken for the monitoring of Eastern oyster (Crassostrea virginica) tissue for C. parvum oocysts (5).
C. fluminea clams develop high-density beds (up to 3,750 clams/m2) in agricultural drainages in North America (17), which may carry as many as 5,500 Cryptosporidium oocysts/liter (21). Because these benthic clams can substantially reduce (up to 75%) the amount of seston (17), it was suggested (12) that they may have significant epidemiological and epizootiological importance in recovering C. parvum and thereby provide an efficient reduction of the waterborne oocyst load. The present study confirms this suggestion. Waterborne oocysts were efficiently removed from the water by the clams even when present in high concentrations. The concentration of oocysts in the present study exceeded by over 180 times the number reported for heavily contaminated agricultural drainage water (11, 21). Negative results for water filtering on day 3 PE and the absence of free oocysts in the water or oocysts disassociated from fecal matter indicate that C. fluminea clams are able to sediment waterborne oocysts of C. parvum. Sedimentation of recovered particles is characteristic for these benthic clams. All particles recovered by C. fluminea gills are bound to the mucus rope before entering the stomach (17). Also, mucus secreted by clam hindgut and rectal cells tightly binds the particles before egestion, preventing recirculation of fecal matter with inhalant currents (17). In an aquarium with mild water circulation enforced by air stone and filter actions, clam feces adhered to the bottom of the aquarium and were never observed in the water volume.
In some parts of the world, Corbicula bivalves are consumed raw and serve as reservoirs of intestinal helminths (8). In the United States, C. fluminea clams are not commercially offered for human consumption; however, they are collected from the wild by some ethnic groups and consumed raw (6). Mammals, e.g., otters, minks, muskrats, raccoons, and even wild hogs, have been reported to feed extensively on C. fluminea (17). As demonstrated by the mouse bioassay results, C. parvum oocysts retain their infectivity for at least 1 week after being recovered by clams. Thus, these benthic clams may play an epidemiological and epizootiological role in food-borne cryptosporidiosis.
ACKNOWLEDGMENTS
We thank H. Miller, L. Campbell, S. David, B. Haynie, J. Trout, and C. Carpenter for technical assistance.
This study was supported by the Maryland Zoological Society (Baltimore, Md.) and by the AKC Fund of New York (New York, N.Y.).
REFERENCES
- 1.Aldridge D W, McMahon R F. Growth, fecundity, and bioenergetics in a natural population of Asiatic freshwater clam, Corbicula manilensis Phillippi, from north central Texas. J Mollusk Stud. 1978;44:49–70. [Google Scholar]
- 2.Clancy J L, Gollnitz W D, Tabib W D. Commercial labs: how accurate are they? J Am Water Works Assoc. 1994;86:89–97. [Google Scholar]
- 3.Fayer R. Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol. 1994;60:2732–2735. doi: 10.1128/aem.60.8.2732-2735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayer R, Ellis W. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J Parasitol. 1993;79:771–774. [PubMed] [Google Scholar]
- 5.Fayer R, Farley C A, Lewis E J, Trout J M, Graczyk T K. The potential role of the oyster Crassostrea virginica in the epidemiology of Cryptosporidium parvum. Appl Environ Microbiol. 1997;63:2086–2088. doi: 10.1128/aem.63.5.2086-2088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried B, Emili S. Experimental infection of Corbicula fluminea (Bivalvia: Corbiculidae) with Echinostoma revolutum cercariae. J Parasitol. 1987;73:655–656. [PubMed] [Google Scholar]
- 7.Goodgame R W, Genta R M, White A C, Chappbell C L. Intensity of infection in AIDS-associated cryptosporidiosis. J Infect Dis. 1993;167:704–709. doi: 10.1093/infdis/167.3.704. [DOI] [PubMed] [Google Scholar]
- 8.Graczyk, T. K., and B. Fried. Echinostomiasis: a common but forgotten foodborne disease. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 9.Graczyk T K, Cranfield M R, Fayer R. A comparative assessment of direct fluorescence antibody, modified acid fast stain, and sucrose flotation techniques for detection of Cryptosporidium serpentis oocysts in snake fecal specimens. J Zoo Wildl Med. 1995;26:396–402. [Google Scholar]
- 10.Graczyk T K, Cranfield M R, Fayer R. Recovery of waterborne oocysts of Cryptosporidium from water samples by the membrane-filter dissolution method. Parasitol Res. 1997;83:121–125. doi: 10.1007/s004360050221. [DOI] [PubMed] [Google Scholar]
- 11.Graczyk T K, Fayer R, Cranfield M R. Zoonotic potential of Cryptosporidium parvum: implications for waterborne cryptosporidiosis. Parasitol Today. 1997;13:348–351. doi: 10.1016/s0169-4758(97)01076-4. [DOI] [PubMed] [Google Scholar]
- 12.Graczyk T K, Fayer R, Cranfield M R, Conn D B. In vitro interactions of Asian freshwater clam (Corbicula fluminea) hemocytes and Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1997;63:2910–2912. doi: 10.1128/aem.63.7.2910-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graczyk T K, Cranfield M R, Conn D B. In vitro phagocytosis of Giardia duodenalis cysts by hemocytes of the Asian freshwater clam Corbicula fluminea. Parasitol Res. 1997;83:743–745. doi: 10.1007/s004360050333. [DOI] [PubMed] [Google Scholar]
- 14.Kira S, Nogami Y, Taketa K, Hayatsu H. Comparison of techniques for monitoring water-borne polycyclic mutagens: efficiency of blue rayon, Sep-Pak C18, and a biota, Corbicula, in concentrating benzo(a)pyrene in a model water system. Bull Environ Contam Toxicol. 1996;57:278–283. doi: 10.1007/s001289900187. [DOI] [PubMed] [Google Scholar]
- 15.Leland H V, Scudder B C. Trace elements in Corbicula fluminea from the San Joaquin River, California. Sci Total Environ. 1990;97–98:641–672. doi: 10.1016/0048-9697(90)90267-x. [DOI] [PubMed] [Google Scholar]
- 16.Lisle J T, Rose J B. Cryptosporidium contamination of the water in the USA and UK: a mini-review. Aqua. 1995;44:103–117. [Google Scholar]
- 17.McMahon R F. Mollusca: Bivalvia. In: Thorp J H, Covich A P, editors. Ecology and classification of North American freshwater invertebrates. San Diego, Calif: Academic Press, Inc., Hartcourt Brace Jovanovich, Publishers; 1991. pp. 315–401. [Google Scholar]
- 18.McMahon R F. The occurrence and spread of the introduced Asiatic freshwater clam, Corbicula fluminea (Muller), in North America: 1924–1982. Nautilus. 1982;96:134–141. [Google Scholar]
- 19.McMahon R F. Ecology of an invasive pest bivalve, Corbicula. In: Russell-Hunter W D, editor. The Mollusca. 6. Ecology. New York, N.Y: Academic Press, Inc.; 1983. pp. 505–561. [Google Scholar]
- 20.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 21.Rose, J. B., J. T. Lisle, and M. LeChevallier. Waterborne cryptosporidiosis: incidence, outbreaks, and treatment strategies. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis, in press. CRC Press, Inc., Boca Raton, Fla.
- 22.Tatem H E. Bioaccumulation of polychlorinated biphenyls and metals from contaminated sediment by freshwater prawns, Macrobrachium resenbergii, and clams, Corbicula fluminea. Arch Environ Contam Toxicol. 1986;15:171–183. doi: 10.1007/BF01059966. [DOI] [PubMed] [Google Scholar]
- 23.Winger P V, Sieckman C, May T W, Johnson W W. Residues of organochlorine insecticides, polychlorinated biphenyls, and heavy metals in biota from Apalachicola River, Florida, 1978. J Assoc Off Anal Chem. 1984;67:325–333. [PubMed] [Google Scholar]