Abstract
Background: Numerous studies have demonstrated a close relationship between antioxidant-rich diets and comorbidities as well as mortality. However, the relationship between such diets and aging remains unclear. The purpose of this study was to investigate the association between the Composite Dietary Antioxidant Index (CDAI) and aging. Methods: All participants were from the National Health and Nutrition Examination Survey (NHANES) 2001–2010. Phenotypic age was calculated using a formula and subtracted from the chronological age to determine the aging. When the phenotypic age exceeded the chronological age, it was considered as aging. A weighted logistic regression model was employed to explore the relationship between CDAI and aging. Restricted cubic splines (RCSs) were used to examine the potential nonlinear relationship between them. Subgroup analysis and joint analysis were conducted to explore the effect of modifiers in these relationships. Results: A total of 19,212 participants (weighted: 165,285,442 individuals) were included in this study. The weighted logistic regression model showed a significant correlation between CDAI and the risk of aging (OR = 0.90, 95% CI: 0.84–0.96). RCS analysis revealed an L-shaped dose–response relationship between CDAI and the risk of aging. Subgroup analysis indicated that the association between CDAI and aging was more pronounced in middle-aged individuals and non-smokers. The joint analysis demonstrated that although smoking accelerated aging among participants, a high CDAI diet could still offset these damages. Conclusions: The association between high CDAI and reduced risk of aging is particularly significant in young and middle-aged individuals and non-smokers. Consuming foods rich in CDAI components may potentially lower the risk of aging.
Keywords: dietary antioxidant index, aging, phenotypic age, NHANES
1. Introduction
With the development of society and the improvement in medical standards, human life expectancy is continuously increasing. According to the latest report by the World Health Organization (WHO), by 2030 approximately one-sixth of the global population may reach the age of 60 or above. Furthermore, by 2050 this population is projected to double [1]. Generally, aging is often accompanied by a decline in physiological function and an increase in chronic diseases. Research suggests that 23% of the global disease burden can be attributed to diseases among individuals aged 60 and above. Moreover, as age increases, the burden of disease progressively intensifies [2]. Therefore, the issue of population aging poses a significant challenge to public health security. However, aging is not merely the result of increased chronological age and declining organ function; it also involves genetic alterations and disruptions in protein functionality at the molecular level, as well as metabolic dysregulation and an increased prevalence of chronic inflammation [3]. Due to the complexity of the aging process, it requires a multifaceted approach for assessment and evaluation. Merely relying on the increase in chronological age seems insufficient to accurately determine physiological aging within the body [4]. In recent years, a novel concept called “PhenoAge” has been introduced. It is a measurement method that incorporates multiple indicators to calculate an individual’s age, providing a better representation of their actual age and physical condition [5,6]. Multiple studies have validated the relationship between PhenoAge and comorbidity and mortality [6,7].
Biologists aim to intervene, slow down, or even reverse the aging process through various measures. It has been proven that environmental interventions have a significant impact on aging. In particular, dietary modifications have shown to be particularly effective in combating aging [8]. We have observed that supplementation with individual nutrients may be closely associated with anti-aging effects. Eugenio et al. demonstrated that supplementation of vitamin E can effectively counteract aging and the inflammatory burden associated with aging [9]. We acknowledge that while individual nutrient supplementation can be useful, people’s diets are holistic and require proper combinations and compositions. It is necessary to explore effective dietary patterns and scoring systems for anti-aging purposes. The Composite Dietary Antioxidant Index (CDAI) is widely used to assess the antioxidant capacity of dietary components. It is calculated based on the levels of vitamin A, C, and E, as well as manganese, selenium, and zinc [10]. However, there has been limited exploration of the relationship between CDAI and aging. Therefore, the aim of this study is to investigate the potential association between CDAI and aging using the National Health and Nutrition Examination Survey (NHANES) database. This study aims to provide robust dietary guidance for anti-aging purposes.
2. Materials and Methods
2.1. Study Participants
All patients in this study were sourced from NHANES, an ongoing research initiative conducted by the National Center for Health Statistics aimed at investigating the health and nutrition of the U.S. population. NHANES employs a multi-stage, complex probability sampling methodology to collect representative health data for the U.S. population, as described earlier [11]. For this cross-sectional study, we included individuals from five survey cycles conducted between 2001 and 2010 who had complete dietary information. Exclusion criteria included the following. (1) Participants lacking covariates required for the calculation of phenotypic age (albumin, creatinine, glucose, C-reactive protein [CRP], lymphocyte percentage, mean cell volume, red cell distribution width, alkaline phosphatase, and white blood cell count). (2) Participants lacking covariates for model adjustments (factors such as smoking, alcohol consumption, comorbidity information, health insurance, and income, Supplementary method S1). After applying the strict inclusion and exclusion criteria, a total of 19,212 participants were included in this study (Figure S1).
2.2. Exposures and Covariates
Dietary data were obtained through 24 h dietary recall interviews conducted with participants. They were asked detailed questions about their diet at the mobile examination center (MEC), where trained staff recorded the participants’ dietary information. To ensure the reliability of the data source, all participants were asked to provide a second report via telephone 3–10 days later. The mean values of both reports were included in this study. The calculation of CDAI was performed using a previously validated formula. This index incorporates six minerals or vitamins associated with oxidative processes [12]. The formula is as follows:
Total nutrient intake represents the average values of each individual’s CDAI components from the two visits. Mean represents the average values of each CDAI component across the entire cohort, while SD represents the standard deviation of each CDAI component across the entire cohort.
The covariates included in the study are age, gender, race, waist circumference, education level, poverty income ratio (PIR), health insurance, smoking, alcohol consumption, diabetes mellitus (DM), cardiovascular disease (CVD), hypertension, history of cancer, CRP, and albumin. The race categories include Non-Hispanic Black, Non-Hispanic White, and other races (Mexican American, Other Hispanic, or Other Race—Including Multi-Racial). Diabetes was defined as fasting blood glucose levels ≥ 7 mmol/L or the use of oral hypoglycemic medications.
2.3. Outcome
Morgan E. Levine et al. utilized the NHANES III to calculate phenotypic age and validated it using the NHANES IV [5]. Phenotypic age is determined by computing nine hematological parameters (albumin, creatinine, glucose, [log] CRP, lymphocyte percentage, mean cell volume, red cell distribution width, alkaline phosphatase, and white blood cell count) along with chronological age. The formula for calculating phenotypic age is as follows:
-
(1)
xb = −19.907 − 0.0336 × albumin − 0.0095 × creatinine − 0.0195 × glucose + 0.0954 × ln (CRP) − 0.0120 × lymphocyte percent + 0.0268 × mean cell volume + 0.3356 × red blood cell distribution width + 0.00188 × alkaline phosphatase + 0.0554 × white blood cell count + 0.0804 × chronological age.
-
(2)
Phenotypic Age = 141.50 +ln [−0.0053 × ln (1 − xb)]/0.09165.
Finally, in order to differentiate between individual’s chronological age and phenotypic age difference, Zuyun Liu et al. also calculated a metric called phenotypic age acceleration: PhenoAgeAccel = Phenotypic Age − Chronological Age. We define phenotypic aging as when PhenoAgeAccel is greater than 0, and phenotypic rejuvenation as when it is less than or equal to 0 [6].
2.4. Statistic Analysis
Since NHANES is a stratified and multi-stage sampling survey, we applied appropriate sampling weights to each participant during the statistical analysis to ensure the representativeness of the population. Descriptive statistics were used to summarize the data, where normally distributed variables were presented as mean ± standard deviation, and skewed variables were expressed as median (interquartile range) or frequency (percentage). In cases of skewed distribution, the values were transformed using the natural logarithm. To examine the linear relationship between CDAI and phenotypic age, Pearson correlation analysis was conducted. Furthermore, the relationship between CDAI or its components and aging was assessed using restrictive cubic spline regression (RCS). Weighted logistic regression analysis was employed to describe the association between CDAI or its components and aging, presented as odds ratio (OR) with corresponding 95% confidence intervals (CI). In the logistic regression analysis, we examined the relationship between CDAI as a continuous variable, as a binary variable (based on the median), and as a categorical variable with five groups (based on quintiles) and aging. We constructed three models to comprehensively evaluate the relationship between them. Model 1 was a crude model. Model 2 was adjusted for sex, race, BMI, education level, PIR, health insurance, smoke, and alcohol. Model 3 was adjusted for model 2 and DM, CVD, hypertension, cancer history, CRP, and albumin.
Interaction analysis was employed to explore the potential modifiers in these relationships, including age, sex, race, PIR, health insurance, smoking, alcohol consumption, DM, hypertension, cancer, and CRP (with a cutoff of 1 mg/L). Subgroup analyses were conducted based on these factors to describe the relationship between CDAI and aging across different strata. Furthermore, joint analyses were performed to further elucidate the role of the modifiers in the presence of interaction effects.
Sensitivity analyses were conducted to assess the robustness of the findings. These included excluding participants with a history of cancer (as tumor burden may significantly impact nutritional status and levels of inflammation, thereby affecting phenotypic age [13]), excluding participants older than 75 years, and including data from three examinations conducted between 2001 and 2006. The statistical analysis was conducted using R 4.2.0 software, and statistical significance was determined with a two-sided p-value of less than 0.05.
3. Results
3.1. Baseline Characteristics
A total of 19,212 participants (weighted: 165,285,442 individuals) were included in this study (Table 1). The average age was 46.49 ± 16.2 years, with 49.7% (9821 individuals) being male. The average level of CDAI was 0.52. Participants were divided into five groups (Q1 to Q5) based on quintiles of CDAI: Q1 (−7.35 to −2.63), Q2 (−2.63 to −1.01), Q3 (−1.01 to 0.65), Q4 (0.65 to 3.06), and Q5 (3.06 to 36.70).
Table 1.
Baseline characteristics.
| Characteristic | Level | CDAI | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p | ||
| N | 3842 (28,879,833) | 3843 (31,351,729) | 3841 (33,353,289) | 3843 (35,393,618) | 3843 (36,306,973) | ||
| Age (Year) | 47.25 (17.03) | 47.21 (16.81) | 47.06 (16.01) | 46.34 (15.91) | 44.89 (15.32) | <0.001 | |
| Phenoage (Year) | 42.19 (19.92) | 41.66 (19.37) | 40.87 (18.30) | 39.93 (18.04) | 37.92 (17.35) | <0.001 | |
| Sex | Men | 1759 (41.8) | 1952 (47.9) | 2046 (51.9) | 1989 (50.6) | 2066 (54.5) | <0.001 |
| Race | Non-Hispanic Black | 928 (14.2) | 710 (10.1) | 670 (9.3) | 658 (8.7) | 654 (8.5) | <0.001 |
| Non-Hispanic White | 1752 (67.5) | 1970 (72.3) | 2059 (74.8) | 2104 (74.8) | 2140 (76.2) | ||
| Other | 1162 (18.3) | 1163 (17.5) | 1112 (16.0) | 1081 (16.5) | 1049 (15.3) | ||
| BMI (kg/m2) | 28.56 (6.55) | 28.62 (6.59) | 28.62 (6.48) | 28.48 (6.28) | 28.22 (6.73) | 0.083 | |
| Waist circumference (cm) | 97.37 (15.65) | 98.02 (15.98) | 98.11 (15.59) | 97.68 (15.82) | 96.88 (16.36) | 0.015 | |
| Education level | High school and above | 1400 (45.2) | 1721 (53.4) | 1947 (59.8) | 2046 (61.3) | 2194 (65.7) | <0.001 |
| PIR | <1 | 1433 (27.3) | 1142 (20.2) | 998 (17.1) | 943 (16.4) | 878 (15.1) | <0.001 |
| 1-2.99 | 1536 (39.2) | 1539 (38.4) | 1502 (35.3) | 1429 (34.7) | 1412 (33.1) | ||
| ≥3 | 873 (33.5) | 1162 (41.4) | 1341 (47.5) | 1471 (48.9) | 1553 (51.8) | ||
| Health insurance | Yes | 2876 (78.1) | 2994 (81.5) | 3055 (83.4) | 3051 (83.2) | 3017 (82.7) | <0.001 |
| Smoke | Yes | 1124 (32.5) | 899 (24.7) | 851 (23.4) | 754 (19.8) | 765 (19.1) | <0.001 |
| Alcohol use | Yes | 2276 (65.5) | 2478 (69.6) | 2626 (73.8) | 2684 (73.8) | 2806 (77.2) | <0.001 |
| DM | Yes | 1035 (20.0) | 967 (19.7) | 900 (17.9) | 826 (16.9) | 742 (15.8) | <0.001 |
| CVD | Yes | 572 (11.5) | 496 (10.2) | 417 (8.0) | 353 (7.2) | 285 (5.4) | <0.001 |
| Hypertension | Yes | 1800 (40.6) | 1643 (36.5) | 1637 (37.0) | 1531 (34.5) | 1398 (32.6) | <0.001 |
| Cancer history | Yes | 355 (9.8) | 376 (9.5) | 396 (9.4) | 339 (8.3) | 318 (7.4) | 0.012 |
| C reactive protein(mg/L) | 0.48 (0.88) | 0.44 (0.85) | 0.39 (0.77) | 0.37 (0.68) | 0.35 (0.74) | <0.001 | |
| Albumin(g/dL) | 42.45 (3.25) | 42.47 (3.19) | 42.76 (3.10) | 42.85 (3.12) | 43.11 (3.13) | <0.001 | |
Abbreviation: CDAI: composite dietary antioxidant index, PIR: poverty income ratio, DM: diabetes mellitus, CVD: cardiovascular disease.
Compared to participants in Q1, those in Q5 had a lower phenotypic age; a higher proportion of males; smaller waist circumference; higher education levels; higher income levels; a lower prevalence of smoking; fewer cases of cardiovascular disease (CVD), DM, and hypertension; lower inflammation levels; and higher levels of albumin.
3.2. Association of Composite Dietary Antioxidant Index and Aging
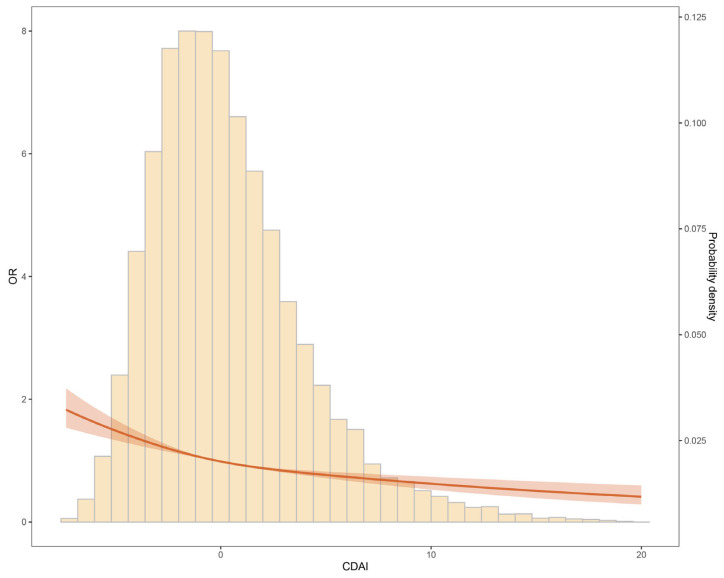
We initially investigated the correlation between phenotypic age and CDAI (Figure S2). The results showed a negative correlation between CDAI and phenotypic age across different age groups, genders, BMI categories, and races. The results from the restrictive cubic spline regression (RCS) analysis also demonstrated an L-shaped dose–response relationship between CDAI and the risk of aging (Figure 1).
Figure 1.
Association between CDAI and aging.
Table 2 presents the results of the multivariable logistic analysis assessing the association between CDAI and the risk of aging. When treated as a continuous variable and adjusted for confounding factors (sex, race, BMI, education level, PIR, health insurance, smoking, alcohol, DM, CVD, hypertension, cancer history, CRP, and albumin), each standard deviation increase in CDAI was associated with an OR of 0.90 (95% CI: 0.84–0.96) for aging. Even after categorizing CDAI using cutoff values and quintiles, a negative correlation between CDAI and aging persisted. Compared to the low CDAI group, the high CDAI group had a lower risk of aging (OR: 0.82, 95% CI: 0.72–0.93). Additionally, there was a decreasing trend in aging risk across the different quintiles: Q1 (reference), Q2 (OR: 0.89, 95% CI: 0.76–1.04), Q3 (OR: 0.87, 95% CI: 0.70–1.06), Q4 (OR: 0.82, 95% CI: 0.69–0.98), and Q5 (OR: 0.68, 95% CI: 0.57–0.81). The trend test showed statistically significant differences (p = 0.014).
Table 2.
Association of composite dietary antioxidant index and aging.
| CDAI | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Per SD | 0.81 (0.77, 0.87) | <0.001 | 0.89 (0.84, 0.95) | 0.001 | 0.90 (0.84, 0.96) | 0.001 |
| Cutoff | ||||||
| Low | Ref. | Ref. | Ref. | |||
| High | 0.69 (0.62, 0.78) | <0.001 | 0.81 (0.71, 0.91) | 0.001 | 0.82 (0.72, 0.93) | 0.003 |
| Quantiles | ||||||
| Q1 | Ref. | Ref. | Ref. | |||
| Q2 | 0.83 (0.72, 0.94) | 0.005 | 0.90 (0.77, 1.04) | 0.146 | 0.89 (0.76, 1.04) | 0.141 |
| Q3 | 0.73 (0.61, 0.86) | <0.001 | 0.84 (0.70, 1.01) | 0.061 | 0.87 (0.70, 1.06) | 0.163 |
| Q4 | 0.66 (0.56, 0.79) | <0.001 | 0.80 (0.67, 0.96) | 0.016 | 0.82 (0.69, 0.98) | 0.032 |
| Q5 | 0.52 (0.44, 0.60) | <0.001 | 0.66 (0.56, 0.78) | <0.001 | 0.68 (0.57, 0.81) | <0.001 |
| P for trend | <0.001 | <0.001 | 0.014 | |||
Model 1 was a crude model. Model 2 was adjusted for sex, race, BMI, education level, PIR, health insurance, smoke, and alcohol. Model 3 was adjusted for model 2 and DM, CVD, hypertension, cancer history, CRP, and albumin.
In addition, to further elucidate the relationship between CDAI and aging, we also examined the associations between CDAI components and the risk of aging. We found that, unlike CDAI as a whole, only vitamin C, vitamin E, selenium, and manganese showed an L-shaped relationship with the risk of aging, while vitamin A and zinc did not demonstrate such a pattern (Figure S3). The results of COX regression analysis further supported these findings. Vitamin C (OR: 0.94, 95% CI: 0.89–0.99), vitamin E (OR: 0.86, 95% CI: 0.80–0.92), selenium (OR: 0.89, 95% CI: 0.83–0.96), and manganese (OR: 0.89, 95% CI: 0.84–0.94) were significantly associated with a decreased risk of aging (Table S1).
3.3. Subgroup Analysis and Joint Analysis
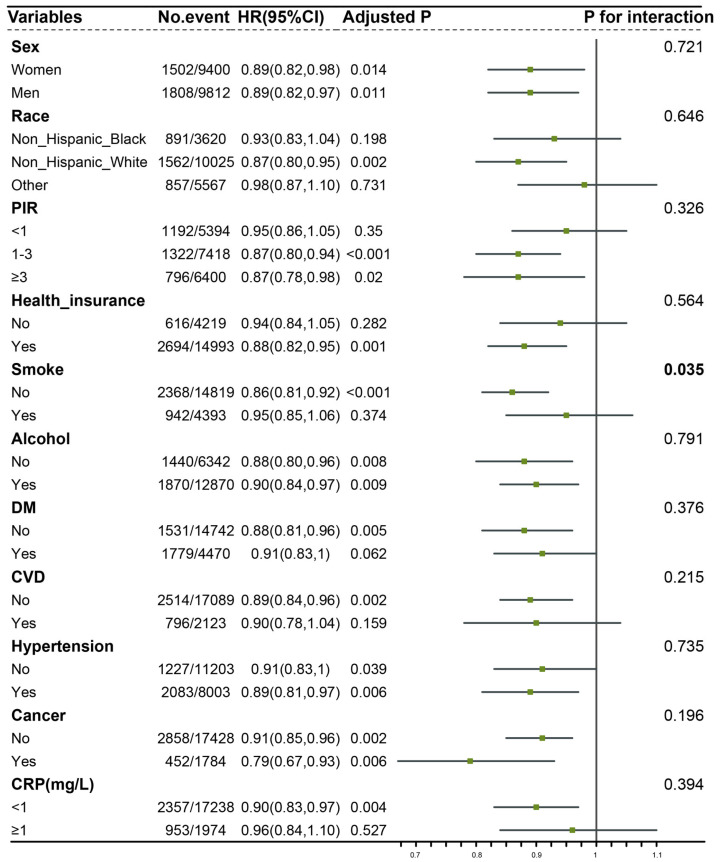
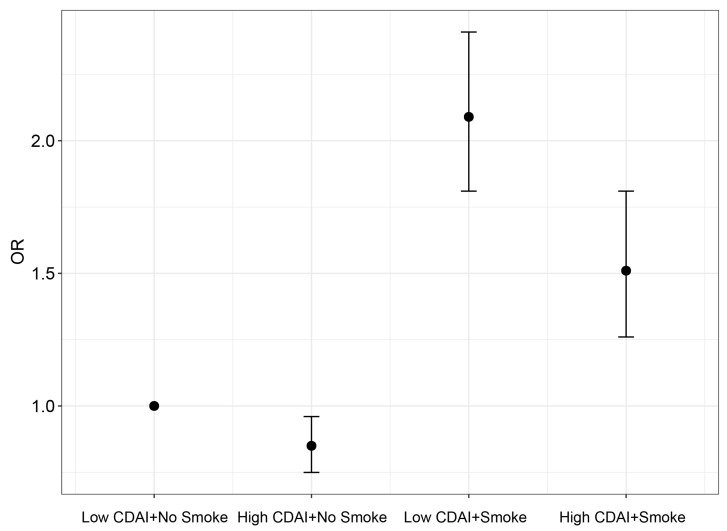
We conducted a detailed subgroup analysis based on age and found an interesting observation. The relationship between CDAI and the risk of aging was significant in the middle-aged and younger population, rather than in individuals aged over 60 years (Table 3). When considering CDAI as a continuous variable, the odds ratios (ORs) for the risk of aging associated with CDAI were 0.89 (95% CI: 0.80–0.99) and 0.90 (95% CI: 0.80–1.00) in participants aged <45 years and those aged 45–59 years, respectively. However, in participants aged 60–74 years and 75–84 years, the ORs for the risk of aging associated with CDAI were 0.91 (95% CI: 0.80–1.05) and 0.95 (95% CI: 0.8–1.10), respectively. The results when considering CDAI as quintiles were similar. Compared to participants in Q1, in the Q5 group of participants aged <45 years and those aged 45–59 years, the odds ratios (ORs) for the risk of aging associated with CDAI were 0.65 (95% CI: 0.48–0.89) and 0.69 (95% CI: 0.48–0.98), respectively. However, in the Q5 group of participants aged 75–84 years, the OR for the risk of aging associated with CDAI was 0.85 (95% CI: 0.52–1.38). Furthermore, we performed subgroup and interaction analyses based on gender, race, PIR, health insurance, smoking, alcohol consumption, diabetes, hypertension, cancer, and CRP (Figure 2). The results indicated an interaction between smoking and the relationship between CDAI and the risk of aging (p = 0.035). In non-smokers, the negative association between CDAI and the risk of aging was more pronounced. Once the interaction effect was determined, we conducted joint analyses (Figure 3). The results demonstrated that although smoking accelerates aging and increases the risk of aging, a high CDAI can partially counteract these negative effects.
Table 3.
Association of composite dietary antioxidant index and aging stratified by age.
| CDAI | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| <45 years | ||||||
| Per SD | 0.83 (0.75, 0.91) | <0.001 | 0.90 (0.81, 1.00) | 0.045 | 0.89 (0.80, 0.99) | 0.040 |
| Cutoff | ||||||
| Low | Ref. | Ref. | Ref. | |||
| High | 0.67 (0.56, 0.80) | <0.001 | 0.76 (0.62, 0.92) | 0.005 | 0.73 (0.60, 0.89) | 0.002 |
| Quantiles | ||||||
| Q1 | Ref. | Ref. | Ref. | |||
| Q2 | 0.79 (0.61, 1.00) | 0.053 | 0.87 (0.66, 1.15) | 0.323 | 0.89 (0.67, 1.17) | 0.393 |
| Q3 | 0.77 (0.60, 1.00) | 0.048 | 0.89 (0.67, 1.17) | 0.385 | 0.88 (0.66, 1.16) | 0.354 |
| Q4 | 0.63 (0.47, 0.83) | 0.002 | 0.78 (0.57, 1.07) | 0.120 | 0.80 (0.58, 1.11) | 0.173 |
| Q5 | 0.54 (0.42, 0.70) | <0.001 | 0.68 (0.51, 0.91) | 0.010 | 0.65 (0.48, 0.89) | 0.007 |
| 45–59 years | ||||||
| Per SD | 0.84 (0.75, 0.94) | 0.004 | 0.89 (0.8, 1.00) | 0.046 | 0.90 (0.80, 1.00) | 0.052 |
| Cutoff | Ref. | Ref. | Ref. | |||
| Low | ||||||
| High | 0.76 (0.61, 0.94) | 0.012 | 0.84 (0.66, 1.06) | 0.144 | 0.87 (0.67, 1.12) | 0.280 |
| Quantiles | ||||||
| Q1 | Ref. | Ref. | Ref. | |||
| Q2 | 0.85 (0.61, 1.17) | 0.316 | 0.98 (0.68, 1.40) | 0.891 | 0.99 (0.68, 1.44) | 0.957 |
| Q3 | 0.70 (0.51, 0.97) | 0.033 | 0.86 (0.61, 1.22) | 0.394 | 0.92 (0.63, 1.34) | 0.645 |
| Q4 | 0.70 (0.51, 0.96) | 0.028 | 0.84 (0.60, 1.18) | 0.316 | 0.87 (0.62, 1.23) | 0.423 |
| Q5 | 0.55 (0.42, 0.73) | <0.001 | 0.68 (0.50, 0.92) | 0.013 | 0.69 (0.48, 0.98) | 0.041 |
| 60–74 years | ||||||
| Per SD | 0.79 (0.69, 0.91) | 0.001 | 0.89 (0.79, 1.01) | 0.069 | 0.91 (0.8, 1.05) | 0.192 |
| Cutoff | ||||||
| Low | Ref. | Ref. | Ref. | |||
| High | 0.66 (0.54, 0.80) | <0.001 | 0.82 (0.67, 1.00) | 0.051 | 0.84 (0.68, 1.04) | 0.104 |
| Quantiles | ||||||
| Q1 | Ref. | Ref. | Ref. | |||
| Q2 | 0.87 (0.67, 1.14) | 0.324 | 0.93 (0.69, 1.25) | 0.621 | 0.87 (0.64, 1.19) | 0.389 |
| Q3 | 0.71 (0.56, 0.90) | 0.005 | 0.83 (0.64, 1.06) | 0.137 | 0.80 (0.60, 1.07) | 0.131 |
| Q4 | 0.64 (0.48, 0.85) | 0.002 | 0.83 (0.62, 1.10) | 0.192 | 0.83 (0.62, 1.12) | 0.217 |
| Q5 | 0.47 (0.33, 0.67) | <0.001 | 0.61 (0.42, 0.89) | 0.011 | 0.64 (0.43, 0.95) | 0.029 |
| 75–84 years | ||||||
| Per SD | 0.92 (0.81, 1.05) | 0.234 | 0.94 (0.82, 1.08) | 0.367 | 0.95 (0.83, 1.10) | 0.480 |
| Cutoff | ||||||
| Low | Ref. | Ref. | Ref. | |||
| High | 0.98 (0.77, 1.24) | 0.837 | 1.02 (0.79, 1.31) | 0.877 | 1 (0.78, 1.28) | 0.999 |
| Quantiles | ||||||
| Q1 | Ref. | Ref. | Ref. | |||
| Q2 | 0.82 (0.57, 1.2) | 0.302 | 0.74 (0.51, 1.08) | 0.116 | 0.71 (0.48, 1.03) | 0.067 |
| Q3 | 0.85 (0.60, 1.19) | 0.330 | 0.8 (0.56, 1.15) | 0.218 | 0.80 (0.54, 1.16) | 0.234 |
| Q4 | 0.91 (0.65, 1.27) | 0.573 | 0.9 (0.64, 1.27) | 0.557 | 0.88 (0.63, 1.23) | 0.455 |
| Q5 | 0.84 (0.53, 1.33) | 0.453 | 0.84 (0.54, 1.32) | 0.440 | 0.85 (0.52, 1.38) | 0.495 |
Model 1 was a crude model. Model 2 was adjusted for sex, race, BMI, education level, PIR, health insurance, smoke, and alcohol. Model 3 was adjusted for model 2 and DM, CVD, hypertension, cancer history, CRP, and albumin.
Figure 2.
Subgroup analysis and interaction analysis. Figure legend: Model was adjusted for sex, race, BMI, education level, PIR, health insurance, smoke, alcohol, DM, CVD, hypertension, cancer history, CRP, and albumin. Abbreviation: PIR: poverty income ratio, DM: diabetes mellitus, CVD: cardiovascular disease, CRP: C-reactive protein.
Figure 3.
Joint analysis. Figure legend: Model was adjusted for sex, race, BMI, education level, PIR, health insurance, smoke, alcohol, DM, CVD, hypertension, cancer history, CRP, and albumin.
3.4. Sensitivity Analyses
To assess the robustness of the results, sensitivity analyses were conducted. After excluding participants with a history of cancer, the association between CDAI and the risk of aging remained significant (OR: 0.91, 95% CI: 0.85–0.96). Similarly, when individuals aged over 75 years were excluded, the results remained robust (OR: 0.89, 95% CI: 0.84–0.96). Furthermore, an internal validation was performed using participants from the years 2001–2006 as a validation set. Although the odds ratio for CDAI as a continuous variable was at the margin (OR: 0.92, 95% CI: 0.83–1), the trend of a decreased risk of aging with higher CDAI values persisted when considering CDAI as a categorical variable (Table S2).
4. Discussion
In this large cross-sectional study using data from NHANES between 2001 and 2010, we found that higher levels of CDAI were associated with a decreased risk of aging. Importantly, this relationship was more pronounced in the middle-aged and younger population. Subgroup and joint analyses revealed that the association between CDAI and the risk of aging was influenced by smoking. However, overall, despite the detrimental effects of smoking on aging, CDAI was able to partially counteract these effects. Overall, our findings suggest that CDAI may serve as a potential indicator of anti-aging effects. Additionally, our study underscores the importance of considering lifestyle factors, such as smoking, in understanding the complex relationship between CDAI and the risk of aging.
Several studies have highlighted the importance of CDAI in various aspects. Wang et al. demonstrated that higher CDAI levels were associated with a reduced risk of all-cause mortality and cardiovascular mortality. They suggested that a dietary intake rich in antioxidants may significantly lower the mortality rate from cardiovascular diseases [12]. A study from Italy has indicated that a high intake of antioxidant-rich diet may reduce the risk of HPV infection and HPV-related diseases [14]. Regarding cancer, Yu et al. demonstrated that higher levels of CDAI were associated with a reduced risk of colorectal cancer [15]. Consistent with our study, they also found that this inverse association was primarily observed in individuals who never smoked and showed a significant interaction effect. However, to our knowledge, our study is the first specifically to investigate the relationship between CDAI and aging.
The relationship between oxidative stress and aging has been extensively studied in both clinical and basic research, as oxidative stress is considered a significant factor in the aging process [16]. Oxidative stress refers to a state of cellular oxidative damage caused by the accumulation of excessive reactive oxygen species in both intracellular and extracellular environments. Inside of cells, sources of oxidative stress include the mitochondrial respiratory chain, cytochrome P450 enzyme system, and nucleic acid metabolism [17,18]. Outside of cells, oxidative stress can be induced by various factors such as inflammation, external environmental factors, and free radicals generated by metabolic activities within the organism [19]. Oxidative stress triggers a series of molecular mechanisms that can profoundly affect cellular function and viability. Among these mechanisms, oxidative damage to DNA, proteins, and lipids plays a crucial role in the aging process. DNA oxidative damage can lead to gene mutations and chromosomal instability, thereby impairing the cell’s ability to repair and regenerate itself [20]. Oxidative damage to proteins can result in functional dysregulation and abnormal aggregation, thereby affecting cellular metabolism and signaling pathways [21]. Additionally, the accumulation of lipid peroxidation products can cause damage to cellular membranes and functional abnormalities [22]. This intricate web of oxidative stress and its multifaceted impact on cellular components underscores its pivotal role in the aging process.
Oxidative stress exerts its influence not only by directly damaging cellular components but also by triggering a series of intricate signaling pathways that impact the cellular aging process. One such pathway that has been extensively studied is the nuclear factor-kappa B (NF-κB) pathway. When cells encounter oxidative stress, NF-κB is activated and subsequently translocates into the cell nucleus. In this nuclear location, NF-κB assumes its role as a regulator of gene expression, particularly for genes associated with inflammation and apoptosis. This regulation has significant consequences for cell survival and the initiation of inflammatory responses [23]. Another pivotal pathway linking oxidative stress and aging is the mitochondrial signaling pathway. Mitochondria, while essential for cellular energy production, are both a primary source of oxidative stress and highly susceptible to oxidative damage themselves. Any impairment in mitochondrial function can exacerbate oxidative stress within the cell, setting in motion a chain reaction that accelerates the aging process [24]. In summary, the relationship between oxidative stress and aging is a multifaceted and tightly interwoven one. Not only does oxidative stress directly damage cellular components, but it also sets off intricate signaling cascades involving pathways like NF-κB and mitochondria. Given the complexity of these interactions, the quest for potential interventions and the development of therapeutic strategies to counteract the aging process are of paramount importance. Such endeavors hold promise for extending healthy lifespans and enhancing overall well-being.
Diet plays a significant role in the anti-aging process, and its antioxidant-rich properties are crucial for mitigating oxidative stress damage and promoting healthy aging [25]. Antioxidants are bioactive compounds found in food that have the ability to neutralize free radicals and reduce oxidative damage [26]. Vitamin C, vitamin E, selenium, and other antioxidants are commonly recognized as key constituents of CDAI. Taking vitamin C as an example, research has shown that vitamin C is involved in the anti-aging process through multiple mechanisms. Importantly, it has the ability to neutralize free radicals and reduce oxidative stress-induced damage to cells. By scavenging and neutralizing free radicals, vitamin C helps maintain the cellular redox balance and mitigate the detrimental effects of oxidative stress on cells [27]. In addition, selenium also plays a role in antioxidant stress and anti-aging. It is a component of various enzymes that participate in regulating the redox balance and combating oxidative stress within cells. These enzymes include glutathione peroxidase (GPx), selenoprotein P (SelP), and selenoprotein W (SelW), among others. Selenium acts as a cofactor for these enzymes, enhancing their antioxidant capacity and contributing to the overall protection against oxidative damage and aging [28,29]. GPx, as an crucial antioxidant enzyme, plays a essential role in cellular defense against oxidative stress. It catalyzes the reaction between substrates such as glutathione (GSH) and hydrogen peroxide, converting harmful peroxides into harmless water and alcohol, thus reducing oxidative stress within cells. By reducing free radical levels, GPx helps protect the structure and function of cells from oxidative damage and contributes to delaying the aging process [30].
This study also has the following limitations. Firstly, as with all cross-sectional studies, we only observed the relationship between CDAI and the risk of aging, and causality cannot be determined. Prospective studies are needed to establish causal relationships. Secondly, we did not account for the use of antioxidants, which is a general term for drugs with antioxidant properties [31] This could potentially lead to an underestimation of the risk of aging and the ability of CDAI. Importantly, we only included dietary data from a single time point, which may introduce bias. Long-term dietary investigations are necessary to provide more accurate assessments.
5. Conclusions
The association between high CDAI and reduced risk of aging is particularly significant in young and middle-aged individuals and non-smokers. Therefore, consuming foods rich in CDAI components may potentially lower the risk of aging. In the future, larger-scale randomized controlled trials and further exploration in basic experiments are necessary to investigate its effectiveness and underlying mechanisms more comprehensively.
Acknowledgments
We would like to express our gratitude to all participants and staff involved in the NHANES.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11202722/s1, Figure S1: Flow chart. Figure S2: The correlation between CDAI and phenotypic age. Figure S3: RCS of CDAI components and aging risk. References [32,33,34,35] are cited in the supplementary materials.
Author Contributions
Conceptualization, H.W.; Methodology, H.W.; Software, H.W.; Validation, H.W.; Formal analysis, H.W.; Investigation, H.W.; Resources, H.W.; Data curation, H.W.; Writing—original draft, H.W.; Writing—review & editing, H.W.; Supervision, Y.C.; Project administration, Y.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of National Center for Health Statistics Research Ethics Review Board (Protocol #98-12 and Protocol #2005-06 and approved in 1998).
Informed Consent Statement
The data of this studies involving human participants were approved by National Center for Health Statistics.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1. [(accessed on 20 July 2023)]. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/ageing-and-health.
- 2.Prince M.J., Wu F., Guo Y., Robledo L.M.G., O’Donnell M., Sullivan R., Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Sebastiani P., Thyagarajan B., Sun F., Schupf N., Newman A.B., Montano M., Perls T.T. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi: 10.1111/acel.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine M.E., Lu A.T., Quach A., Chen B.H., Assimes T.L., Bandinelli S., Hou L., Baccarelli A.A., Stewart J.D., Li Y., et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z., Kuo P.-L., Horvath S., Crimmins E., Ferrucci L., Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Med. 2018;15:e1002718. doi: 10.1371/journal.pmed.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Zhao Y., Liu F., Chen H., Tan T., Yao P., Tang Y. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 2022;20:207. doi: 10.1186/s12916-022-02403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge L., Deelen J., Slagboom P.E. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8. [DOI] [PubMed] [Google Scholar]
- 9.Mocchegiani E., Costarelli L., Giacconi R., Malavolta M., Basso A., Piacenza F., Ostan R., Cevenini E., Gonos E.S., Franceschi C., et al. Vitamin E-gene interactions in aging and inflammatory age-related diseases: Implications for treatment. A systematic review. Ageing Res. Rev. 2014;14:81–101. doi: 10.1016/j.arr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Wright M.E., Mayne S.T., Stolzenberg-Solomon R.Z., Li Z., Pietinen P., Taylor P.R., Virtamo J., Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004;160:68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 11.Liu C.-A., Liu T., Ge Y.-Z., Song M.-M., Ruan G.-T., Lin S.-Q., Xie H.-L., Shi J.-Y., Zheng X., Chen Y., et al. Muscle distribution in relation to all-cause and cause-specific mortality in young and middle-aged adults. J. Transl. Med. 2023;21:154. doi: 10.1186/s12967-023-04008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Yi Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study. Front. Cardiovasc. Med. 2022;9:993930. doi: 10.3389/fcvm.2022.993930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C.-A., Zhang Q., Ruan G.-T., Shen L.-Y., Xie H.-L., Liu T., Tang M., Zhang X., Yang M., Hu C.-L., et al. Novel Diagnostic and Prognostic Tools for Lung Cancer Cachexia: Based on Nutritional and Inflammatory Status. Front. Oncol. 2022;12:890745. doi: 10.3389/fonc.2022.890745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barchitta M., Maugeri A., La Mastra C., La Rosa M.C., Favara G., Lio R.M.S., Agodi A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients. 2020;12:1384. doi: 10.3390/nu12051384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y., Paragomi P., Wang R., Jin A., Schoen R.E., Sheng L., Pan A., Koh W., Yuan J., Luu H.N. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the Singapore Chinese Health Study. Int. J. Cancer. 2022;150:1599–1608. doi: 10.1002/ijc.33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J., Mills K., le Cessie S., Noordam R., van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020;57:100982. doi: 10.1016/j.arr.2019.100982. [DOI] [PubMed] [Google Scholar]
- 17.Abdelmegeed M.A., Choi Y., Ha S.K., Song B.J. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic. Biol. Med. 2016;91:188–202. doi: 10.1016/j.freeradbiomed.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabibzadeh S. Signaling pathways and effectors of aging. Front. Biosci. 2021;26:50–96. doi: 10.2741/4889. [DOI] [PubMed] [Google Scholar]
- 19.Hajam Y.A., Rani R., Ganie S.Y., Sheikh T.A., Javaid D., Qadri S.S., Pramodh S., Alsulimani A., Alkhanani M.F., Harakeh S., et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells. 2022;11:552. doi: 10.3390/cells11030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jauhari A., Baranov S.V., Suofu Y., Kim J., Singh T., Yablonska S., Li F., Wang X., Oberly P., Minnigh M.B., et al. Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Investig. 2020;130:3124–3136. doi: 10.1172/JCI135026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H., Liu Y., Men H., Zheng Y. Protective Mechanism of Humanin against Oxidative Stress in Aging-Related Cardiovascular Diseases. Front. Endocrinol. 2021;12:683151. doi: 10.3389/fendo.2021.683151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Y., Li J., Jia C., He Y., Deng C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020;11:312. doi: 10.1186/s13287-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Reest J., Nardini Cecchino G., Haigis M.C., Kordowitzki P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021;70:101378. doi: 10.1016/j.arr.2021.101378. [DOI] [PubMed] [Google Scholar]
- 25.Aleksandrova K., Koelman L., Rodrigues C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021;42:101869. doi: 10.1016/j.redox.2021.101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Q., Wei B., Wang S., Ke S., Chen J., Zhang H., Wang H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs. 2019;17:674. doi: 10.3390/md17120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J.C., Caballero Arredondo M., Braakhuis A.J., Donaldson P.J. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients. 2020;12:3142. doi: 10.3390/nu12103142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Z., Zhang J., Li H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019;31:1035–1047. doi: 10.1007/s40520-018-1086-7. [DOI] [PubMed] [Google Scholar]
- 29.Bjørklund G., Shanaida M., Lysiuk R., Antonyak H., Klishch I., Shanaida V., Peana M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules. 2022;27:6613. doi: 10.3390/molecules27196613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samarghandian S., Azimi-Nezhad M., Farkhondeh T., Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed I., Hollenberg M.D., Ding H., Triggle C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021;12:718942. doi: 10.3389/fendo.2021.718942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernhard D., Moser C., Backovic A., Wick G. Cigarette smoke--an aging accelerator? Exp. Gerontol. 2007;42:160–165. doi: 10.1016/j.exger.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Nunes P.T., Kipp B.T., Reitz N.L., Savage L.M. Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction. Int. Rev. Neurobiol. 2019;148:101–168. doi: 10.1016/bs.irn.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pothisiri W., Prasitsiriphon O., Aekplakorn W. Extent of aging across education and income subgroups in Thailand: Application of a characteristic-based age approach. PLoS ONE. 2020;15:e0243081. doi: 10.1371/journal.pone.0243081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker S.W., Saenz J., Wong R. Health Insurance and the Aging: Evidence from the Seguro Popular Program in Mexico. Demography. 2018;55:361–386. doi: 10.1007/s13524-017-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.