Abstract
Lacticin 3147 is a broad-spectrum bacteriocin produced by Lactococcus lactis subsp. lactis DPC3147 (M. P. Ryan, M. C. Rea, C. Hill, and R. P. Ross, Appl. Environ. Microbiol. 62:612–619, 1996). Partial purification of the bacteriocin by hydrophobic interaction chromatography and reverse-phase fast protein liquid chromatography revealed that two components are required for full activity. Lacticin 3147 is bactericidal against L. lactis, Listeria monocytogenes, and Bacillus subtilis; at low concentrations of the bacteriocin, bactericidal activity is enhanced when target cells are energized. This finding suggests that the presence of a proton motive force promotes the interaction of the bacteriocin with the cytoplasmic membrane, leading to the formation of pores at these low lacticin 3147 concentrations. These pores were shown to be selective for K+ ions and inorganic phosphate. The loss of these ions resulted in immediate dissipation of the membrane potential and hydrolysis of internal ATP, leading to an eventual collapse of the pH gradient at the membrane and ultimately to cell death. Our results suggest that lacticin 3147 is a pore-forming bacteriocin which acts on a broad range of gram-positive bacteria.
Bacteriocins produced by lactic acid bacteria (LAB) are typically defined as proteinaceous compounds with activity against related species, including organisms involved in food-borne disease and food spoilage (17, 18). The commercial potential for these inhibitory compounds has fueled an intensive research effort into their exploitation as food preservatives. To date, nisin remains the only purified bacteriocin approved for use in food products (11). However, it is believed that the inclusion of bacteriocin-producing starter cultures in food fermentations will be more widely accepted than the use of pure bacteriocin preparations, which could be considered food additives.
Lacticin 3147 is a bacteriocin produced by Lactococcus lactis subsp. lactis DPC3147, a strain isolated from an Irish kefir grain. The bacteriocin has a broad inhibition spectrum, similar to that of nisin, but is both genetically and biologically distinct from nisin (29). It is a heat-stable compound which is active at physiological pH. The genetic determinants for production and immunity are encoded on pMRC01, a 60.2-kb plasmid which can be conjugally transferred to strains with industrially important characteristics. A pMRC01 transconjugant of a commercial cheese-making strain, L. lactis subsp. cremoris DPC4268, was used as a single-strain starter to manufacture cheddar cheese. The presence of lacticin 3147 in the cheese could be detected throughout the 6-month ripening period and resulted in significantly lower levels of nonstarter LAB (29). Lacticin 3147 has also been used in the veterinary field, where a prophylactic role in the prevention of mastitis in cattle was recently developed (30).
Most of the characterized LAB bacteriocins appear to have a common mechanism of action in that they dissipate the proton motive force (PMF), i.e., the membrane potential (Δψ) and the pH gradient (ΔpH), in target organisms through the formation of pores in the cytoplasmic membrane (1, 2, 7, 14, 15, 24, 31, 32). Nisin, a broad-spectrum lantibiotic, acts at the membrane without the requirement for a specific receptor protein and results in the efflux of ions, amino acids, and ATP (3, 12–14). Class II bacteriocins, such as lactococcin A (16, 31), lactococcin B (32), and the pediocin-like bacteriocins (7, 9, 10), have narrow host ranges and appear to have receptor-mediated action which leads to leakage of ions, PMF dissipation, and ATP depletion. While the antimicrobial activity of these bacteriocins is due to the action of a single peptide, others require the complementary action of two peptides to inhibit target organisms. For example, lactococcin G (26) is a two-component bacteriocin which acts by forming small pores which allow K+ efflux, resulting in ATP hydrolysis and the dissipation of the membrane potential. A model presented for the action of lactococcin G suggested that the rapid hydrolysis of internal ATP was due to K+ uptake by a K+ ATPase in the cell membrane in an attempt to compensate for the loss of this ion through the bacteriocin pore (24). Another two-component bacteriocin, lacticin F, causes efflux of phosphate and K+ ions as well as PMF dissipation (2, 25). Like other class II bacteriocins, lactococcin G and lacticin F have narrow host ranges (25, 26). Recently, a broad-host-range, two-component bacteriocin, thermophilin 13, was isolated from Streptococcus thermophilus; this bacteriocin acts on target organisms through dissipation of the PMF (23).
Here, we present evidence that the broad-host-range bacteriocin lacticin 3147 is a membrane-active, two-component bacteriocin and report its effects on the energetic parameters of cells of L. lactis subsp. cremoris, Listeria monocytogenes, and Bacillus subtilis, including ATP levels, Δψ, ΔpH, and K+ and phosphate levels. These species vary in their sensitivity to the bacteriocin but, strikingly, the membrane potential is selectively dissipated at the same low levels of the bacteriocin. However, the killing efficiency is associated with a reduction in cellular ATP levels, and these correlate with the amounts of K+ and phosphate retained in the cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacteriocin producer L. lactis subsp. lactis DPC3147 was grown at 30°C without aeration in M17 (Oxoid Ltd., Basingstoke, Hampshire, England) supplemented with 0.5% (wt/vol) glucose (GM17 broth). L. lactis subsp. cremoris HP was used as the standard sensitive strain and was cultured in the same manner. Other sensitive strains used included L. monocytogenes Scott A, which was grown in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with 0.6% (wt/vol) yeast extract (Oxoid) at 37°C, and B. subtilis ATCC 6051, which was grown in brain heart infusion broth (Difco) containing 1% (wt/vol) glucose at 37°C with shaking.
Bacteriocin assay.
Bacteriocin activity was estimated by the agar well diffusion assay as described by Parente and Hill (27). Molten agar was cooled to 48°C and seeded with the sensitive strain (approximately 2 × 107 fresh overnight-grown cells). The inoculated medium was dispensed into sterile petri plates, allowed to solidify, and dried. Wells (approximately 4.6 mm in diameter) were made in the seeded agar plates. Aliquots (50 μl) of a twofold serial dilution of the bacteriocin preparation were dispensed into the wells, and plates were incubated overnight at 30°C. The arbitrary units (AU) per milliliter were obtained as described by Ryan et al. (29).
Preparation of lacticin 3147.
For the purposes of purification, L. lactis subsp. lactis DPC3147 was propagated in 8 liters of tryptone-yeast broth (containing, per liter, tryptone at 2.5 g, yeast extract at 5 g, glucose at 10 g, β-glycerol phosphate at 19 g, MgSO4 · 7H2O at 0.25 g, and MnSO4 · 4H2O at 0.05 g; pH 6.75) that had been passed through a column containing 50 g of XAD-16 beads to clear contaminating hydrophobic peptides from the broth medium. Following autoclaving, the resulting medium was inoculated with 1% L. lactis subsp. lactis DPC3147 and incubated at 30°C for 16 h. The cells were removed by centrifugation at 10,000 × g for 15 min, and the supernatant was applied to a column (2 by 30 cm) containing 50 g of XAD-16 beads at a flow rate of approximately 15 ml/min. The column was washed with 40% ethanol (15 ml/min), and the bacteriocin was eluted with 70% isopropanol–10 mM acetic acid (pH 2) in 20-ml fractions. The isopropanol was removed by evaporation with oxygen-free nitrogen gas (BOC Gases, Cork, Ireland). The bacteriocin was assayed after each step as described above with L. lactis subsp. cremoris HP as the sensitive indicator.
The most active bacteriocin fractions were pooled (approximately seven fractions) and applied to a C18 reverse-phase Varian Bond Elut cartridge (JVA Analytical Ltd., Dublin, Ireland), which was activated by being rinsed with 1 volume of methanol and 1 volume of 5 mM sodium phosphate buffer (pH 7). The column was washed with 30% ethanol (approximately 4 ml), and the active bacteriocin was eluted with approximately 2 ml of 70% isopropanol–10 mM acetic acid (pH 2). Active fractions thus obtained were concentrated by evaporation with N2 gas, pooled, and applied to a fast protein liquid chromatography (FPLC) C18 reverse-phase column (Pharmacia). The column was equilibrated with 0.1% trifluoroacetic acid in water and eluted with a linear gradient of 0 to 90% acetonitrile containing 0.1% trifluoroacetic acid at a flow rate of 1 ml/min, and 1-ml fractions were collected. The fractions thus obtained were assayed, and the bacteriocin peptides were isolated.
Effect of lacticin 3147 on sensitive cells.
Cells were grown to an optical density at 620 nm (OD620) of 0.7, harvested, washed, and resuspended to approximately 107 cells per ml in 2.5 mM sodium phosphate buffer (pH 7.0), in 2.5 mM sodium phosphate buffer (pH 7.0) supplemented with 10 mM glucose, or in GM17 broth. The bacteriocin was added at different concentrations, and samples were taken at appropriate times to determine the viable cell count and the OD620.
Measurement of ATP levels.
Intracellular and extracellular ATP levels were determined as described previously (6) with some modifications. Cells were suspended in 2.5 mM sodium phosphate buffer (pH 7.0) with 10 mM glucose and 1,200 AU of lacticin 3147 per ml. At various times, 20- and 50-μl samples were taken to determine the total and extracellular ATP concentrations, respectively. The 20-μl samples were immediately mixed with 80 μl of dimethyl sulfoxide. The 50-μl samples were spun down immediately for 2 min, and 20 μl of the supernatant was removed and mixed with 80 μl of dimethyl sulfoxide. All samples were diluted with 5 ml of water filtered by nanopure filtration. ATP concentrations were determined with a Lumac/3M biocounter M2010 by use of the Lumac luciferin-luciferase enzyme assay. Enzyme (100 μl) was added to 200 μl of sample, and luminescence was measured.
Measurement of the Δψ.
The transmembrane electrical potential (inside negative) was determined by the quenching of the potential-sensitive fluorescent probe 3,3′-dipropylthiacarbocyanine [diSC3(5); Molecular Probes Inc., Eugene, Oreg.]. Fluorescence was measured with a Perkin-Elmer LS50 spectrofluorometer at 30°C with continuous stirring. An excitation wavelength of 643 nm and an emission wavelength of 666 nm were used. Cells were suspended in 50 mM potassium HEPES buffer (pH 7.0) with 10 mM glucose. On addition of the K+/H+ exchanger nigericin (Sigma Chemical Co., St. Louis, Mo.), the ΔpH was completely dissipated.
Measurement of the ΔpH.
The transmembrane pH gradient (inside alkaline) was measured by loading cells with the pH-sensitive fluorescent probe 5 (and 6-)-carboxyfluorescein diacetate succinimidyl ester (cFDASE; Molecular Probes) as described previously (5). The cells were concentrated threefold in 1 ml of potassium HEPES buffer (pH 8.0). The cells were then incubated at 30°C for 10 min in the presence of 1.0 μM cFDASE, washed, and resuspended in the same volume of 50 mM potassium phosphate buffer (pH 7.0). Nonconjugated probe was eliminated by incubating the cells with 10 mM glucose at 30°C for 30 min. The cells were washed twice, resuspended in the same volume of potassium phosphate buffer, and placed on ice until used. The intracellular pH was determined by diluting the loaded cells to a concentration of 107 cells per ml in a 3-ml glass cuvette, and fluorescence was measured with a spectrofluorometer.
Determination of K+ and inorganic phosphate contents of cells.
Intracellular and extracellular K+ contents were determined as follows. Cells were suspended in 2.5 mM sodium HEPES buffer (pH 7.0) to an OD620 of 1.0. Glucose was added to a final concentration of 10 mM, lacticin 3147 was added to a concentration of 1,200 AU/ml, and valinomycin (Sigma) was added to a concentration of 1.0 μM. Samples (1 ml) were taken at intervals and immediately chilled on ice. The samples were centrifuged at 10,000 × g at 0°C for 7 min. The supernatant was removed and stored for the determination of extracellular K+. The cell pellet was resuspended in 1 ml of 5% trichloroacetic acid and frozen overnight at −20°C. The samples were thawed and incubated at 95°C for 10 min. Demineralized water (4 ml) was added to each sample, which was then centrifuged for 15 min at 10,000 × g. The supernatant was retained for intracellular K+ determination. The K+ concentration in the samples was determined by flame photometry (Jenway PFP7).
To determine inorganic phosphate concentrations, the supernatants were assayed for phosphate as described by Chen et al. (8).
Protein determination.
Protein concentrations were determined by the bicinchoninic acid method (Sigma procedure TRPO-562) with bovine serum albumin as a standard.
RESULTS
Lacticin 3147 is a two-component bacteriocin.
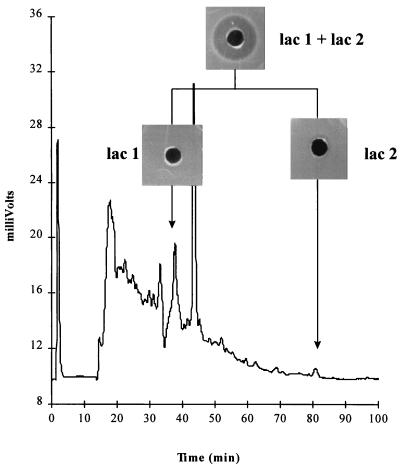
Application of an active, partially purified lacticin 3147 preparation to a C18 reverse-phase column with an acetonitrile gradient resulted in the FPLC chromatogram shown in Fig. 1. Agar well diffusion assays of each of the eluted fractions showed negligible inhibition of the sensitive indicator, L. lactis subsp. cremoris HP. When the fractions corresponding to lac 1 and lac 2 (Fig. 1) were combined and assayed, bacteriocin activity was restored. Therefore, it appears that the FPLC purification step separated two components required for activity, and it may be concluded that lacticin 3147 requires the complementary action of two peptides, lac 1 and lac 2, for full activity.
FIG. 1.
FPLC chromatogram (C18 reverse-phase column) of partially purified lacticin 3147. Also shown are the results of an agar well diffusion assay of fractions (lac 1 and lac 2) which combined to form an active bacteriocin.
Lacticin 3147 is bactericidal.
Lacticin 3147 has been shown to have a broad inhibition spectrum (29), inhibiting all gram-positive bacteria which have been tested thus far. To investigate the mode of action of lacticin 3147 on target organisms, the bacteriocin was added to suspensions of sensitive cells, and the viable count and optical density were determined over time.
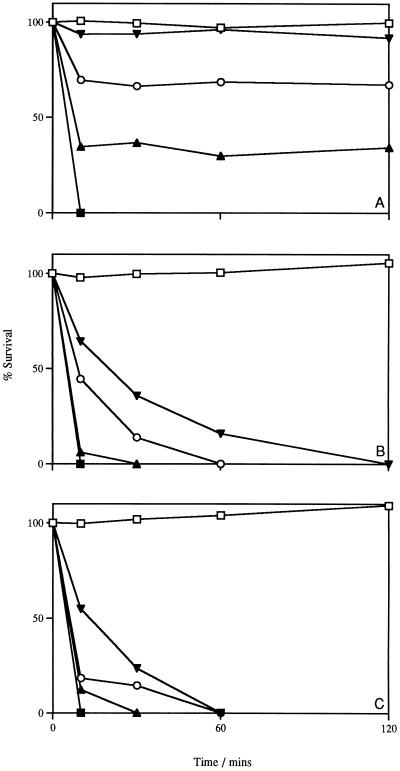
L. lactis subsp. cremoris HP cells in buffer were incubated with increasing concentrations of lacticin 3147. In a concentration-dependent manner, an initial rapid decline in viability was observed (Fig. 2A). However, prolonged incubation did not result in further killing. A similar experiment was performed with cells incubated with the bacteriocin in the presence of glucose, i.e., buffer containing 10 mM glucose (Fig. 2B) and GM17 broth (Fig. 2C). Under these conditions, the killing efficiency of the bacteriocin was dramatically increased; even at the lowest concentration, no viable cells could be detected after 2 h. In all cases, the optical density of the bacteriocin-treated cultures did not change during the experiments (data not shown). These results indicate that lacticin 3147 has bactericidal, rather than bacteriolytic or bacteriostatic, activity and that, at low lacticin 3147 concentrations, energized cells are more sensitive to the bacteriocin. Energized cells have a PMF which may favor the insertion of the bacteriocin molecules into the membrane, as is the case with the pore-forming lantibiotic nisin (3, 12, 14).
FIG. 2.
Effect of lacticin 3147 addition on the viability of L. lactis subsp. cremoris HP in buffer (A), buffer supplemented with 10 mM glucose (B), and GM17 broth (C). Symbols: □, no addition; ▾, addition of 75 AU/ml; ○, addition of 300 AU/ml; ▴, addition of 600 AU/ml; ▪, addition of 1,200 AU/ml. Data points along the horizontal axis represent 0% survival. The initial cell number was 107 CFU/ml. Data points represent the average of at least three experiments done in duplicate.
Effect of lacticin 3147 on ATP levels.
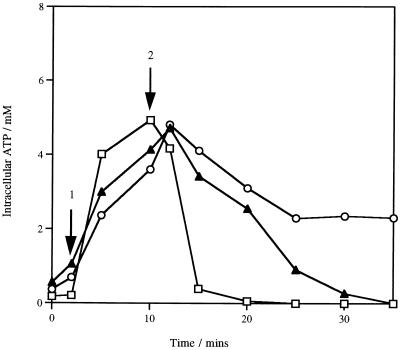
The impact of lacticin 3147 on the energetic condition of sensitive cells was determined by measuring the cellular ATP levels. On the addition of glucose, the intracellular ATP concentration in L. lactis subsp. cremoris HP cells increased approximately 10-fold; the addition of 1,200 AU of lacticin 3147 per ml resulted in a rapid decrease in the cellular ATP levels. After 10 min of incubation with the bacteriocin, no ATP could be detected inside the cells (Fig. 3). However, an increase in the extracellular ATP concentration was not observed (data not shown), indicating that the bacteriocin-induced depletion of ATP was not due to the release of ATP from cells.
FIG. 3.
Intracellular ATP levels in cells of L. lactis subsp. cremoris HP (□), L. monocytogenes Scott A (▴), and B. subtilis ATCC 6051 (○) treated with lacticin 3147. At arrow 1, 10 mM glucose was added; at arrow 2, 1,200 AU of lacticin 3147 per ml was added. Data points represent the average of at least three experiments done in duplicate.
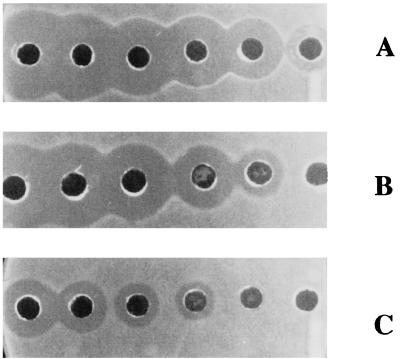
These results suggest that the pores formed by the bacteriocin are not large enough to allow the leakage of large compounds, such as ATP, and that the rapid decrease in intracellular ATP levels is probably due to hydrolysis. Since lacticin 3147 is a broad-host-range bacteriocin (29), this experiment was also performed with two different gram-positive bacteria, L. monocytogenes Scott A and B. subtilis ATCC 6051. As with L. lactis subsp. cremoris HP, intracellular ATP levels decreased (Fig. 3), while no leakage of ATP was observed (data not shown). The different levels of sensitivity of the three strains to the bacteriocin (Fig. 4) were reflected in the different rates of ATP hydrolysis.
FIG. 4.
Inhibitory action of lacticin 3147 against the gram-positive species L. lactis subsp. cremoris HP (A), L. monocytogenes Scott A (B), and B. subtilis ATCC 6051 (C) illustrating the difference in sensitivity of the test strains. A twofold serial dilution of the bacteriocin was used as described in Materials and Methods.
Lacticin 3147 selectively dissipates the Δψ component of the PMF.
To determine if lacticin 3147 acts on the cytoplasmic membrane of target organisms, the effect of the bacteriocin on the components of the PMF, i.e., Δψ and ΔpH, was examined.
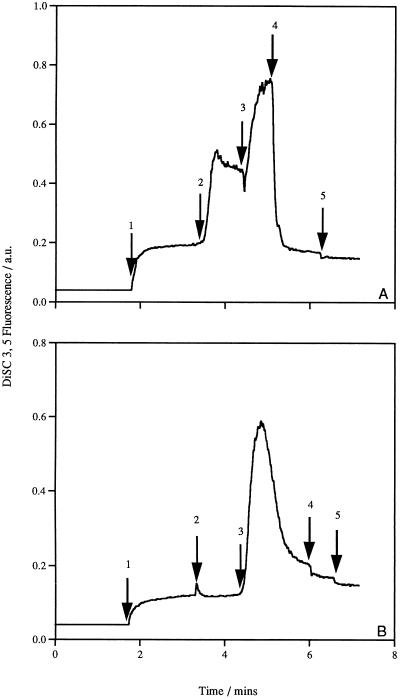
The Δψ was measured qualitatively with the potential-sensitive fluorescent cyanine dye diSC3(5). Generation of a Δψ (inside negative) on addition of glucose to the cells resulted in quenching of the fluorescent signal. In the presence of the K+/H+ exchanger nigericin (1.0 μM), the cells were able to maintain a maximum Δψ, which could be completely dissipated by the addition of the K+ ionophore valinomycin (1.0 μM) (data not shown). The addition of lacticin 3147 to cells maintaining a maximum Δψ led to immediate depolarization of the cytoplasmic membrane (Fig. 5A). No further dissipation was observed when valinomycin was added to cells treated with the bacteriocin, indicating that complete dissipation had occurred as a result of bacteriocin action. In fact, lacticin 3147 was as effective at dissipating the Δψ as valinomycin. Figure 5B demonstrates that lacticin 3147 may require an energized membrane for insertion. The bacteriocin was added to cells prior to glucose addition; when glucose was added, a transient Δψ was rapidly dissipated. Apparently, upon creation of a potential difference across the membrane, the insertion of the bacteriocin molecules is enhanced. When the experiment was performed with L. monocytogenes Scott A and B. subtilis ATCC 6051, similar results were obtained; the Δψ was completely dissipated at the same lacticin 3147 concentration (data not shown). Regardless of the different sensitivities of the strains, the depolarization of the membrane was immediate in all cases. This result is in contrast to the results obtained for internal ATP levels, where the rate of decrease on addition of lacticin 347 was strain dependent.
FIG. 5.
Effect of lacticin 3147 on the Δψ of L. lactis subsp. cremoris HP cells. The following additions were made as indicated by the arrows: (A) arrow 1, L. lactis subsp. cremoris HP cells; arrow 2, 10 mM glucose; arrow 3, 1.0 μM nigericin; arrow 4, 1,200 AU of lacticin 3147 per ml; arrow 5, 1.0 μM valinomycin; and (B) arrow 1, L. lactis subsp. cremoris HP cells; arrow 2, 1,200 AU of lacticin 3147 per ml; arrow 3, 10 mM glucose; arrow 4, 1.0 μM valinomycin; arrow 5, 1.0 μM nigericin. The fluorescence of the diSC3(5) probe is depicted inversely.
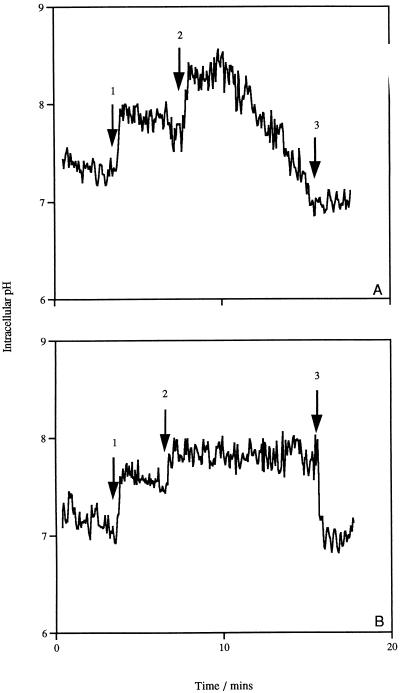
The generation of a ΔpH in L. lactis subsp. cremoris HP was monitored by measuring the changes in the intracellular pH with the pH-sensitive fluorescent probe cFDASE (5). The fluorescence of the probe was rapidly increased on addition of glucose, indicating an increase in the internal pH due to the extrusion of H+ ions. The addition of nigericin caused a rapid and complete collapse of the ΔpH, leading to an equilibration of the internal pH with the outside environment. The addition of lacticin 3147 resulted in an increase in the internal pH and not a collapse of the ΔpH (Fig. 6A). This increase in the internal pH was maintained for approximately 4 min before dissipation of the ΔpH occurred. The gradual dissipation of the ΔpH in the presence of lacticin 3147 is best explained by the depletion of the ATP pool (Fig. 3). Therefore, lacticin 3147 does not cause immediate dissipation of the ΔpH of sensitive cells, as the membrane does not become permeable to H+ ions; therefore, the bacteriocin is selective for the Δψ component of the PMF. A similar increase in the intracellular pH can be seen when valinomycin is added to cells that have generated a ΔpH; however, valinomycin-treated cells can maintain this internal pH until nigericin is added (Fig. 6B).
FIG. 6.
Effect of lacticin 3147 (A) and valinomycin (B) on the ΔpH of glucose-energized cells of L. lactis subsp. cremoris HP. Glucose (10 mM) was added at arrow 1; 1,200 AU of lacticin 3147 per ml (A) and 1.0 μM valinomycin (B) were added at arrow 2; and 1.0 μM nigericin was added at arrow 3 to completely dissipate the ΔpH.
Efflux of K+ ions and inorganic phosphate is the result of bacteriocin action.
The bactericidal action of lactococcin G (24), lactostrepcin 5 (33), and lacticin F (2) results in the leakage of K+ ions from susceptible cells. To determine whether lacticin 3147 has an impact on the internal K+ pool of L. lactis subsp. cremoris HP, control cells and cells treated with the bacteriocin were permeabilized with trichloroacetic acid, and the intracellular and extracellular K+ concentrations were measured by flame photometry.
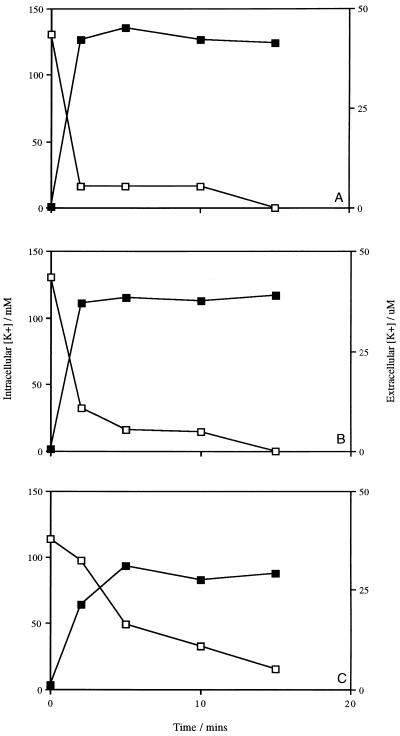
In the absence of the bacteriocin, energized cells of L. lactis subsp. cremoris HP maintained an intracellular concentration of K+ of approximately 130 mM. The subsequent addition of lacticin 3147 caused a dramatic loss of cellular K+ (Fig. 7A). Measurement of the K+ content outside the cells indicated that the bacteriocin had induced massive leakage of K+ from the cells, as the concentration outside had increased dramatically (Fig. 7A). The efflux of K+ was immediate, and after 15 min of treatment with 1,200 AU of lacticin 3147 per ml, no trace could be detected inside the cells. Similar results were observed for L. monocytogenes Scott A (Fig. 7B), whereas the efflux of K+ from lacticin 3147-treated cells of B. subtilis ATCC 6051 was somewhat slower (Fig. 7C). The same experiments were carried out with cells which were not energized; the concentrations of intracellular K+ were similar to those in cells in the presence of glucose, and the rates of efflux of K+ were comparable to those shown in Fig. 7 for all three organisms (data not shown). Nisin and the K+ ionophore valinomycin also induced K+ leakage (data not shown).
FIG. 7.
Lacticin 3147-induced K+ efflux from energized L. lactis subsp. cremoris HP (A), L. monocytogenes Scott A (B), and B. subtilis ATCC 6051 (C) cells. Symbols: □, intracellular K+ concentration; ▪, extracellular K+ concentration. Data points represent the average of at least two experiments done in duplicate.
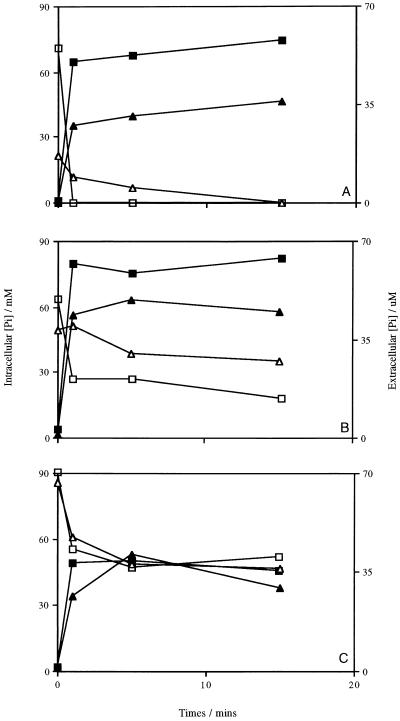
Lacticin F was shown to make cells permeable to inorganic phosphate as well as K+ ions (2); therefore, the samples were assayed for internal and external inorganic phosphate contents. Lacticin 3147 induced the loss of intracellular phosphate from L. lactis subsp. cremoris HP at a concentration of 1,200 AU/ml. In unenergized cells, the release was very rapid, as was found with K+ (Fig. 8A). A reduced rate of efflux was observed in energized cells, but the intracellular levels of phosphate were much lower here than in unenergized cells, approximately 20 mM versus 75 mM. In both cases, no trace of phosphate could be detected intracellularly after 15 min of incubation with the bacteriocin (Fig. 8A). When L. monocytogenes Scott A was treated with lacticin 3147, a lower initial level of phosphate and a reduced efflux rate were measured for unenergized cells, but the cells retained a low level of free phosphate internally (Fig. 8B). However, the difference between the unenergized and energized cells was not as obvious in this case. For B. subtilis ATCC 6051, there was little difference in the effect of lacticin 3147 on phosphate levels in the unenergized and energized cells (Fig. 8C). The levels of intracellular phosphate were comparable, as were the rates of efflux. Experiments with valinomycin revealed that no phosphate was released when cells were treated with this ionophore (data not shown), since valinomycin is K+ specific.
FIG. 8.
Inorganic phosphate (Pi) efflux from cells of L. lactis subsp. cremoris HP (A), L. monocytogenes Scott A (B), and B. subtilis ATCC 6051 (C). Symbols: □ and ▵, intracellular Pi concentrations in unenergized and energized cells, respectively; ▪ and ▴, extracellular Pi concentrations in unenergized and energized cells, respectively. Data points represent the average of at least two experiments done in duplicate.
DISCUSSION
The results presented in this paper suggest that the broad-host-range lacticin 3147 is a membrane-active, two-component bacteriocin that is hydrophobic in nature. The data suggest that the bacteriocin induces cell death by making sensitive cell membranes permeable, allowing for the efflux of K+ ions and phosphate. This action results in the dissipation of the Δψ component of the PMF, the hydrolysis of intracellular ATP, and ultimately cell death.
Two peptides, lac 1 and lac 2, were separated by FPLC in the final purification step. In isolation, these components have negligible activity; the complementary action of both peptides is required for full activity. The combined action of two peptides has been demonstrated for other bacteriocins, including lacticin F (1, 25), lactococcin G (24, 26), and thermophilin 13 (23). It has been observed for these bacteriocins that equivalent amounts of both peptides are required for an interaction with target cells. Therefore, a preparation of lacticin 3147 purified to the stage prior to the separation of the individual components by FPLC was used in this study to mimic the natural composition of the bacteriocin secreted by the producing organism.
As with other LAB bacteriocins, the primary site of action of lacticin 3147 appears to be the cytoplasmic membrane (1, 2, 7, 14, 15, 19, 23, 24, 31, 32). Results from killing assays performed with lacticin 3147 suggest that the action of the bacteriocin is enhanced when target cells are energized, leading to the conclusion that the presence of a PMF may favor insertion into the membrane of the bacteriocin at low concentrations. Lacticin 3147 can also induce cell death in the absence of an energized membrane, although somewhat less effectively. However, at high concentrations the bacteriocin appears to be as effective against unenergized cells; this result suggests that saturating amounts of the bacteriocin can overcome the requirement for an energized membrane.
It was observed that lacticin 3147 makes the membranes of sensitive cells permeable, allowing the efflux of K+ ions and phosphate but not larger compounds, such as ATP. This movement of ions results in a collapse of the Δψ; however, the ΔpH of L. lactis subsp. cremoris HP is not immediately dissipated, suggesting that lacticin 3147 does not induce proton leakage and that the pores formed by the bacteriocin are selective. Over time, however, the ΔpH is dissipated, possibly a secondary effect resulting from the inability of the cells to maintain the activity of the F0F1-ATPase (the membrane-bound enzyme responsible for generating the ΔpH by pumping protons across the membrane in L. lactis [4, 20]) in the absence of ATP, which is rapidly hydrolyzed on addition of lacticin 3147. This mechanism of action resembles that of the narrow-spectrum, two-component bacteriocins, e.g., lactococcin G (24) and lacticin F (2), which form selective ion pores, but differs from that of the broad-spectrum bacteriocin nisin, which forms much larger pores as a result of the aggregation of several bacteriocin molecules; the latter activity makes the membrane permeable to larger compounds, such as ATP and amino acids (14, 21, 22).
All three species tested in this study, L. lactis subsp. cremoris HP, L. monocytogenes Scott A, and B. subtilis ATCC 6051, lost a large percentage of intracellular K+ within minutes of treatment with lacticin 3147. Treatment of L. lactis subsp. cremoris HP with lacticin 3147 also resulted in the release of all detectable internal phosphate; as with K+, this release occurred almost immediately. The level of free phosphate in energized cells of L. lactis subsp. cremoris HP was much lower than that in unenergized cells, presumably due to the formation of phosphorylated sugar intermediates of the glycolytic pathway. Also, the rate of efflux of phosphate was somewhat reduced under these conditions, perhaps due to the lower initial levels. It is known that for L. lactis ML3 the uptake of phosphate is driven by a unidirectional ATP-dependent transport system (28). Therefore, reaccumulation of phosphate under conditions where this transport system is active, i.e., in the presence of an energy source, may explain the reduction in the rate of efflux. The loss of these essential ions may also explain the ATP hydrolysis that occurs as a result of lacticin 3147 action; the cells utilize the available ATP in a futile attempt to reaccumulate K+ and phosphate by ATP-dependent uptake systems, resulting in an eventual collapse of the ΔpH. Similar transport mechanisms in Listeria and Bacillus species are, however, not well understood. Both organisms have the ability to retain a certain level of intracellular phosphate, even after prolonged incubation with lacticin 3147. It is possible that these organisms also have phosphate bond-dependent uptake systems for K+ and phosphate and that the various rates of ATP hydrolysis can be explained by the differences in the retention or reaccumulation of these ions by the organisms. Overall, this results in a lower rate of ATP hydrolysis and therefore less efficient killing by the bacteriocin. These differences in ion retention and reaccumulation may explain why L. monocytogenes Scott A and B. subtilis ATCC 6051 are more resistant to lacticin 3147 than L. lactis subsp. cremoris HP.
There are other possible explanations for the observed variations in the levels of sensitivity to lacticin 3147. For example, the ability of the bacteriocin to interact with the cytoplasmic membrane is influenced by factors such as the composition of the cell envelope, including the peptidoglycan layer, and the lipid composition of the membrane, as has been demonstrated with nisin (13, 14). The presence of K+ and/or phosphate ATPases may also affect the efficiency of the bacteriocin, since these systems appear to be involved in ATP hydrolysis. Furthermore, the type of ATP-generating pathway, i.e., via substrate-level phosphorylation in the glycolysis of L. lactis subsp. cremoris and via oxidative phosphorylation in the electron transfer chain of B. subtilis, may affect the capacity to synthesize ATP, resulting in differences in the killing efficiency of lacticin 3147 among the three organisms studied here.
On the basis of these results, we propose that lacticin 3147 forms pores which allow K+ and phosphate to leak; the resulting change in electrical charge across the membrane causes the immediate dissipation of the Δψ. In an attempt to recover these ions, the cells use phosphate bond-dependent transport, resulting in rapid ATP hydrolysis and leading to cell death. We have shown that this two-component bacteriocin acts against L. lactis subsp. cremoris HP, L. monocytogenes Scott A, and B. subtilis ATCC 6051 despite the differences in the lipid compositions of the cytoplasmic membranes of these organisms. The broad range of lacticin 3147, as well as its activity at a neutral pH, paves the way for the use of this bacteriocin in food systems for which no natural alternative to artificial preservatives is presently available.
REFERENCES
- 1.Abee T. Pore-forming bacteriocins of gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–10. doi: 10.1016/0378-1097(95)00137-T. [DOI] [PubMed] [Google Scholar]
- 2.Abee T, Klaenhammer T R, Letellier L. Kinetic studies of the action of lacticin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl Environ Microbiol. 1994;60:1006–1013. doi: 10.1128/aem.60.3.1006-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz R, Jung G, Sahl H-G. Mechanism of channel-formation by lantibiotics in black lipid membranes. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991. pp. 359–372. [Google Scholar]
- 4.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breeuwer P, Drocourt J L, Rombouts F M, Abee T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol. 1996;62:178–183. doi: 10.1128/aem.62.1.178-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breeuwer P. Assessment of viability of microorganisms employing fluorescence techniques. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1996. [Google Scholar]
- 7.Bruno M E C, Montville T J. Common mechanistic action of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1993;59:3003–3010. doi: 10.1128/aem.59.9.3003-3010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P S, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 9.Chikinidas M L, Garcia-Garcera M J, Driessen A J M, Ledeboer A M, Nissen-Meyer J, Nes I F, Abee T, Konings W N, Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.O, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cintas L M, Rodriguez J M, Fernandez M F, Sletten K, Nes I F, Hernandez P E, Holo H. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol. 1995;61:2643–2648. doi: 10.1128/aem.61.7.2643-2648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delves-Broughton J. Nisin and its use as a food preservative. Food Technol. 1990;44:100–117. [Google Scholar]
- 12.Driessen A J M, van den Hooven H W, Kuiper W, van de Kamp M, Sahl H-G, Konings R N H, Konings W N. Mechanistic studies of lantibiotic-induced permeabilisation of phospholipid vesicles. Biochemistry. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 13.Gao F H, Abee T, Konings W N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991;57:2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Garcera M J, Elferink M G L, Driessen A J M, Konings W N. In vitro pore-forming activity of the lantibiotic nisin: role of protonmotive-force and lipid composition. Eur J Biochem. 1993;212:417–422. doi: 10.1111/j.1432-1033.1993.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez B, Glaasker E, Kunji E R S, Driessen A J M, Suarez J E, Konings W N. Bactericidal mode of action of plantaricin C. Appl Environ Microbiol. 1996;62:2701–2709. doi: 10.1128/aem.62.8.2701-2709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holo H, Nilssen O, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover D G, Stevenson L R, editors. Bacteriocins of lactic acid bacteria. London, England: Academic Press Ltd.; 1993. [Google Scholar]
- 18.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez-Diaz R, Ruiz-Barba J L, Cathcart D P, Holo H, Nes I F, Sletten K H, Warner P J. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl Environ Microbiol. 1995;61:4459–4463. doi: 10.1128/aem.61.12.4459-4463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konings W N, Poolman B, Driessen A J M. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16:419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- 21.Kordel M, Schuller F, Sahl H-G. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energised liposomes. FEBS Lett. 1989;244:99–102. doi: 10.1016/0014-5793(89)81171-8. [DOI] [PubMed] [Google Scholar]
- 22.Kordel M, Sahl H-G. Susceptibility of bacterial, eukaryotic and artificial membranes to the disruptive action of the cationic peptides Pep 5 and nisin. FEMS Microbiol Lett. 1986;34:139–144. [Google Scholar]
- 23.Marciset O, Jeronimus-Stratingh M C, Mollet B, Poolman B. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin that functions without a receptor. J Biol Chem. 1997;272:14277–14284. doi: 10.1074/jbc.272.22.14277. [DOI] [PubMed] [Google Scholar]
- 24.Moll G, Ubbink-Kok T, Hildeng-Hauge H, Nissen-Meyer J, Nes I F, Konings W N, Driessen A J M. Lactococcin G is a potassium ion-conducting, two-component bacteriocin. J Bacteriol. 1996;178:600–605. doi: 10.1128/jb.178.3.600-605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muriana P M, Klaenhammer T R. Purification and partial characterization of lacticin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol. 1991;57:114–121. doi: 10.1128/aem.57.1.114-121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nissen-Meyer J, Holo H, Havarstein L S, Sletten K, Nes I F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J Bacteriol. 1992;174:5686–5692. doi: 10.1128/jb.174.17.5686-5692.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parente E, Hill C. A comparison of factors affecting the production of two bacteriocins from lactic acid bacteria. J Appl Bacteriol. 1992;73:290–298. [Google Scholar]
- 28.Poolman B, Nijssen R M J, Konings W N. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J Bacteriol. 1987;169:5373–5378. doi: 10.1128/jb.169.12.5373-5378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan M P, Rea M C, Hill C, Ross R P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol. 1996;62:612–619. doi: 10.1128/aem.62.2.612-619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan, M. P., W. J. Meaney, R. P. Ross, and C. Hill. Submitted for publication.
- 31.van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venema K, Abee T, Haandrikman A J, Leenhouts K J, Kok J, Konings W N, Venema G. Mode of action of lactococcin B, a thiol-activated bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993;59:1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zajdel J K, Ceglowski P, Dobrzanski W T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985;49:969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]