Abstract
A specially designed resistometer was constructed, and the lethal effect on Yersinia enterocolitica of ultrasonic waves (UW) at different static pressures (manosonication [MS]) and of combined heat-UW under pressure treatments (manothermosonication [MTS]) was investigated. During MS treatments at 30°C and 200 kPa, the increase in the amplitude of UW of 20 kHz from 21 to 150 μm exponentially decreased decimal reduction time values (DMS) from 4 to 0.37 min. When pressure was increased from 0 to 600 kPa at a constant amplitude (150 μm) and temperature (30°C), DMS values decreased from 1.52 to 0.20 min. The magnitude of this decrease in DMS declined progressively as pressure was increased. The influence of pressure on DMS values was greater with increased amplitude of UW. Pressure alone of as much as 600 kPa did not influence the heat resistance of Y. enterocolitica (D60 = 0.094; z = 5.65). At temperatures of as much as 58°C, the lethality of UW under pressure was greater than that of heat treatment alone at the same temperature. At higher temperatures, this difference disappeared. Heat and UW under pressure seemed to act independently. The lethality of MTS treatments appeared to result from the added effects of UW under pressure and the lethal effect of heat. The individual contributions of heat and of UW under pressure to the total lethal effect of MTS depended on temperature. The inactivating effect of UW was not due to titanium particles eroded from the sonication horn. The addition to the MS media of cysteamine did not increase the resistance of Y. enterocolitica to MS treatment. MS treatment caused cell disruption.
Heat can impair the organoleptic properties and nutritional values of foods. To avoid the unwanted effects of heat, efforts are being made to find new methods of food preservation, either based on new inactivation procedures (22, 35) or by combining others already known (21, 28). High pressures and high electric field pulses are among the most intensively studied. The long-known inactivating effect of ultrasound waves (UW) has drawn very little attention.
UW are generated by mechanical vibrations of frequencies higher than 15 kHz. When these waves propagate into liquid media, alternating compression and expansion cycles are produced. During the expansion cycle, high-intensity UW make small bubbles grow in a liquid. When they attain a volume at which they can no longer absorb enough energy, they implode violently. This phenomenon is known as cavitation. During implosion, very high temperatures (approximately 5,500°C) and pressures (approximately 50 MPa) are reached inside these bubbles (31, 42, 43). In most authors’ opinions (15, 38), this is the ultimate reason for the bactericidal effect of high-intensity UW.
Some authors have proposed the use of UW in food preservation (2, 12, 16, 26, 44). However, the use of UW for this purpose has not been adopted, probably because of the adverse effects on food quality of such high-intensity treatments as those required to inactivate the most resistant bacterial species and spores (25). The increase in lethality of UW by increasing static pressure (manosonication [MS]) was not explored until the 1960s by Neppiras and Hughes (32). Their investigation was carried out with very weak acoustic fields, and, as a result, the effect observed was very slight. More recently, the lethal effect of a combination of heat with UW (thermoultrasonication) has been reported (17, 33). The lethality of a combined treatment of heat and UW under pressure (manothermosonication [MTS]) remains unexplored. The influence of the amplitude on the lethality of high-intensity UW is also unknown.
The purpose of this investigation was to determine the lethality at different temperatures of UW under pressure with the food-borne pathogen Yersinia enterocolitica as a model microorganism. This objective required the design and construction of an instrument that allowed measurement by the multipoint method of the lethality of these treatments. With this instrument, measurement and control of the different influencing parameters (temperature, pressure, and amplitude of UW) were possible.
MATERIALS AND METHODS
Bacterial culture and media.
Y. enterocolitica (ATCC 9610) was supplied by the Spanish Type Culture Collection (catalog no. 4315) and during this investigation was maintained on tryptic soy agar slants (Biolife, Milan, Italy).
A 250-ml Erlenmeyer flask containing 50 ml of sterile tryptic soy broth (Biolife) was inoculated to a final concentration of 106 cells/ml in the flask, with a 12-h broth subculture in tryptic soy broth at 37°C (obtained from a single colony). This flask was incubated at 4°C under agitation (130 rpm) until the stationary phase of growth was reached (2 weeks) and was then stored for 1 month in the same medium at 4°C, but without agitation. Cultures grown at 4°C exhibited no loss of viability or of resistance to heat or UW during storage.
Heat, MS, and MTS treatments: the instrument.
Heat, MS, and MTS treatments were carried out in a specially designed resistometer (Fig. 1) built in our laboratory. This instrument was a modified version of the thermoresistometer TR-SC (7, 8).
FIG. 1.
MTS resistometer. A, Main vessel; B, agitation motor; C, cooling coil; D, fraction collector; E, pH meter; F, MTS resistometer main unit; G, ultrasound generator; 1 and 3, top and bottom caps, respectively; 2, pH electrode; 4, vacuum inlet; 5, pressure inlet; 6, pH electrode pressurized housing; 7, main agitation shaft; 8, temperature sensor; 9, heating element; 10, temperature sensor; 11, manometer; 12, automatic injection syringe; 13, sonication horn and housing; 14, treatment chamber; 15, filling-emptying tube; 16, one-way inverted sense valve; 17, solenoid sampling valve; 18, photocell detector.
This MTS resistometer, built of stainless steel and Teflon, consisted of a main vessel (650-ml capacity) with removable top and bottom caps with o rings. Caps were held in place by stainless steel rings screwed onto the wall of the main vessel. The top cap, as in the original instrument, had a security pressure release valve and connections to vacuum and pressure. This cap also had a pressurized housing to hold, when needed, a pH electrode and held an agitation shaft, a 1,200-W heating element, and a temperature sensor.
The bottom cap had a filling and emptying tube with a valve and a cooling coil to dissipate heat generated by ultrasound. A small treatment chamber (23 ml) was screwed onto this cap. This chamber also had a small agitation shaft and blade, coupled to the agitation shaft of the main vessel through a Teflon friction bearing, to prevent any leakage to or from the main vessel. This agitation device ensured a quick and homogeneous distribution of the inoculum during the experiment. This small treatment chamber was connected to a capillary sampling tube that had a solenoid sampling valve activated by a timer. This chamber also had two one-way inverted sense valves in the top and three hermetical ports with o rings in the wall to hold a temperature sensor, a tube connected to a manometer, and an automatic injection syringe holder. One of the valves allowed the filling of the chamber by vacuum through the sampling tube before the experiment started, and the other allowed refilling it with menstruum from the main vessel after every sample extraction.
The bottom of the chamber was reached by the tip of the sonication horn of an ultrasound generator held in place by a housing screwed on to the bottom cap of the main vessel. The remaining elements of the MTS resistometer were as described in the original thermoresistometer TR-SC (7, 8).
Pressure during the experiments was supplied by a nitrogen cylinder and was monitored by the manometer of the treatment chamber.
Temperature control during ultrasonication experiments was achieved by dissipating excess heat evolved during ultrasonication by circulating cold tapwater through the cooling coil.
As in the original instrument, during heat treatments, samples could be taken manually (as many as 30 samples/min) or automatically (as many as 32 samples/s) with a fraction collector. To compensate for the decrease in pressure in the treatment chamber during automatic sampling, the fraction collector was fitted with a photocell detector (OMRON E32; Tokyo, Japan) (18) that opened the solenoid sampling valve only during the preset time (0.1 s) every time a tube passing under the sampling tip was detected. During MTS treatments, the maximum sampling speed used was five samples/s.
Ultrasound generators.
In this investigation, two Branson Sonifier ultrasound generators (Branson Ultrasonics, Danbury, Conn.) with maximum outputs of 450 and 2,000 W were used. These constant-frequency instruments (20 kHz) keep the amplitude constant by automatically supplying the amount of power needed to maintain the set amplitude. The higher the pressure applied to the horn, the greater the power required to maintain a given amplitude. The maximum intensity that a given generator is capable of delivering depends on its maximum output. The 2,000-W instrument was used to widen the operational range. No statistically significant differences (P ≥ 0.05) were observed between the DMS values (where D is decimal + reduction time) obtained with either instrument under the same operational parameters.
Heat, MS, and MTS resistance measurements: the method.
The general handling procedure was as already described for the thermoresistometer TR-SC (7, 8). Once temperature and pressure (and amplitude of UW in MS and MTS treatments) had attained stability, 0.2 ml of the Y. enterocolitica suspension was injected into the treatment chamber containing citrate-phosphate buffer (pH 7) (13). At preset intervals, one 0.1-ml sample for each treatment time was directly collected into tubes of melted sterile nutrient agar (Biolife) with 500 mg of Bacto-Dextrose (Difco, Detroit, Mich.) per liter. These tubes were immediately plated. Survival curves (obtained by plotting the log of the number of survivors versus time of treatment) were plotted with 8 to 15 separate samples collected over time.
Incubation of heated samples and survival counting.
Colonies were counted after incubation at 37°C for 48 h. Previous experiments showed that longer incubation times did not influence the slope of survival curves. After incubation, the plates were counted with an Image Analyzer Automatic Counter (Protos; Analytical Measuring Systems, Cambridge, United Kingdom) fitted with a 70-mm objective to facilitate the count of plates with high numbers of CFU (as much as 3 × 104 CFU per plate) (9).
Microscopic observations.
Microscopic counts and observations of the effects of heat, MS, and MTS treatments were carried out with a Nikon (Nippon Kogaku KK, Tokyo, Japan) microscope and Thoma counting chamber.
Heat, MS, and MTS resistance parameters.
The lethality of treatments, single or combined, was measured by D values, which were defined as minutes of a given treatment for the number of survivors to decrease one log cycle (DT, DMS, and DMTS, values for heat, MS, and MTS, respectively). D values were calculated from the slope of the regression line plotted with the counts of the straight portion of the survival curve. Only survival curves with a correlation coefficient (r0) of ≥0.98 and with more than four values in the straight portion were used. Survival curves with a straight portion including less than one log cycle were rejected.
Decimal reduction time curves (DRTC) were obtained by plotting D values versus their corresponding heating temperatures. z values (measured as the increase in temperature [in degrees Celsius] for the DT value to decrease by one log cycle) were calculated from the slope of DRTC.
r0s and 95% confidence limits (CL) were calculated by the appropriate statistical package (StatView SE plus Graphics; Abacus Concepts Inc., Berkeley, Calif.). The coefficients of variation (CV%) and the statistical significance of differences (P ≥ 0.05) among DT, DMS, and DMTS values were calculated and checked, respectively, as described by Steel and Torrie (40).
The individual contributions of heat and of UW to the lethal effect of MTS treatments at different temperatures were evaluated by the degree to which experimental DMTS values matched the theoretical DRTC. DMTS values used to plot theoretical DRTC were calculated by assuming that heat and UW acted independently and that heat, MS, and MTS destruction of Y. enterocolitica were single reactions ruled by first-order kinetics. In this way the logarithmic order of death of microorganisms would be expressed by the following equations: log Nt = log N0 − (1/DT) × t for heat treatments, log Nt = log N0 − (1/DMS) × t for MS treatments, and log Nt = log N0 − (1/DMTS) × t for MTS treatments, where Nt is the number of surviving cells after t minutes of treatment, N0 is the number of living cells at t = 0, and DT, DMS, and DMTS are the corresponding decimal reduction times.
If heat and UW acted independently, MTS inactivation rates could be calculated by adding the inactivation rate of UW under pressure to the heat inactivation rate by the following equations: 1/DMTS = 1/DT + 1/DMS and DMTS = (DT × DMS)/(DT + DMS).
RESULTS
Performance of the MTS resistometer.
The temperature stability of the MTS resistometer instrument during heat treatments was ±0.05°C and during experiments including UW was ±0.2°C. The heating-up rate was 10°C/min. In the range of 40 to 140°C, the instrument stabilized to a temperature 10°C higher in approximately 3 min.
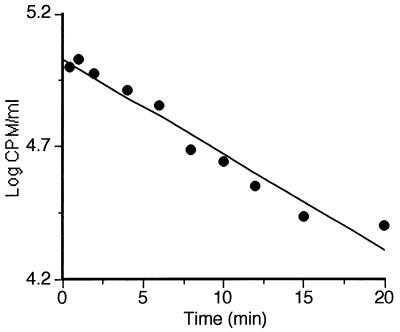
The alternative compression and decompression cycles generated by UW might force the contents of the treatment chamber to leak out to the main vessel. An experiment was carried out to evaluate the significance for the results of possible leaks occurring during an MS treatment at the highest amplitude (250 kPa, 30°C, and 150 μm of amplitude). Since microorganisms were unsuitable for this purpose, the detection of leaks was performed with radioactive [14C]ascorbic acid. After 0.2 ml of a concentrated solution of radioactive ascorbate had been injected into the treatment chamber, one 0.1-ml sample per each treatment time was extracted. These samples were counted in a liquid scintillation counter (LKB-Wallac; 1211ß) as described elsewhere (34). The results of this experiment are shown in Fig. 2. As seen in this figure, radioactivity (in counts per minute) decreased exponentially with time (t) according to the equation log CPM/ml = 5.3 − 0.036 t.
FIG. 2.
Concentration of [14C]ascorbic acid (in counts per minute per milliliter) in the treatment chamber during MS treatment (250 kPa, 30°C, 150 μm of amplitude, 20 kHz).
The regression line in Fig. 2 had an r0 of 0.98, and the 95% CL of the slope ranged from −0.030 to −0.042. According to these results, the expected leak to the main vessel was evaluated to be approximately 10% of the contents of the treatment chamber/minute of treatment.
The lowest D value measurable by a mixing method (3) is limited by the time needed for the inoculum to attain a homogeneous distribution and also by its sampling speed. Time required for a homogeneous distribution was determined in three consecutive experiments by the procedure already described (8). The homogeneous distribution of inoculum was attained in less than 0.2 s (data not shown).
During MTS experiments, to keep the treatment parameters stable during automatic sampling and to minimize the dilution effect caused by menstruum from the main vessel replenishing the chamber after every sample extraction, a photocell tube detector was installed. This device opened the sampling valve during a preset time (0.1 s) when a tube was detected. To check the performance of this sampling device, 0.2 ml of a suspension of Y. enterocolitica was automatically injected into the menstruum at room temperature. After injection, one 0.1-ml sample per treatment time was automatically collected. Counts obtained in two successive experiments of 2-s duration with a sampling speed of five tubes/s had a CV of 1% (data not shown). This variation is considered normal in microbiological counts (24). Therefore, this sampling method should not influence precision of results.
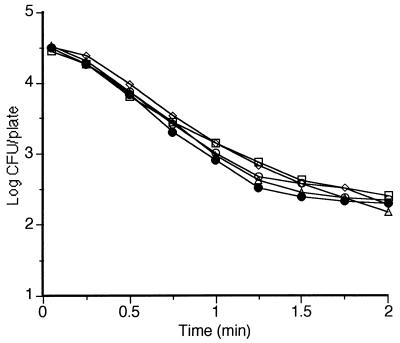
Figure 3 shows reproducibility obtained in five consecutive MS treatments at room temperature, at 200 kPa, and at 117 μm of amplitude. The CV% of D values obtained in these experiments was 7%.
FIG. 3.
Survival curves of Y. enterocolitica corresponding to five different experiments under the same experimental conditions (200 kPa, 30°C, 150 μm of amplitude, 20 kHz).
Lethal effect of heat, MS, and MTS treatments.
Our strain of Y. enterocolitica had a D59 of 0.138 min (95% CL ranged from 0.122 to 0.154) and a z value of 5.65°C (95% CL ranged from 5.32 to 6.02) as calculated from the corresponding DRTC (see below). Pressure (to as much as 600 kPa) did not influence heat resistance (data not shown).
At ambient temperature and pressure, the D value of Y. enterocolitica corresponding to an ultrasonication treatment at the highest amplitude setting (150 μm) was 1.5 min.
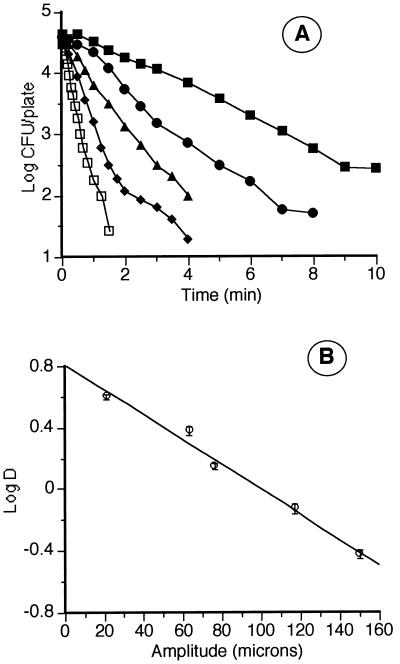
Figure 4A shows the survival curves of Y. enterocolitica corresponding to MS treatments of different amplitudes, at 200 kPa and 30°C. In panel B, the relationship between amplitude and log D value is shown. The lethality of these treatments increased exponentially with amplitude (A) according to a regression line (r0 = 0.98; 95% CL of the slope ranged from −0.01 to −0.006) that followed the equation log DMS = 0.806 − 0.008A.
FIG. 4.
Influence of amplitude of ultrasonication on the lethality of MS treatment. (A) Survival curves of Y. enterocolitica corresponding to MS treatments (200 kPa, 30°C, 20 kHz) at the following amplitudes of ultrasonication: 21 (▪), 63 (•), 76 (▴), 117 (♦), and 150 (□) μm. (B) Relationship between amplitude of ultrasonication and lethality of MS treatments (log D).
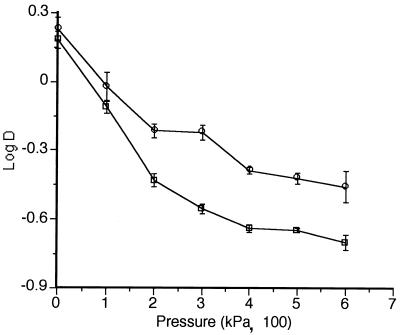
The influence of pressure on the lethality of UW of 117 and 150 μm of amplitude is shown in Fig. 5. At 150 μm of amplitude, an increase in pressure from 0 to 300 kPa reduced the DMS from 1.5 to 0.28 min. A further increase from 300 to 600 kPa reduced DMS only from 0.28 to 0.20 min. The relationship between amplitude and pressure is also illustrated in this figure. While at 100 kPa the increase of amplitude from 117 to 150 μm reduced DMS from 0.95 to 0.77 min, at 300 kPa this increase reduced DMS from 0.60 to 0.28 min.
FIG. 5.
Relationship between pressure and lethality of MS treatments (log D) at the following amplitudes: 117 (○) and 150 (□) μm.
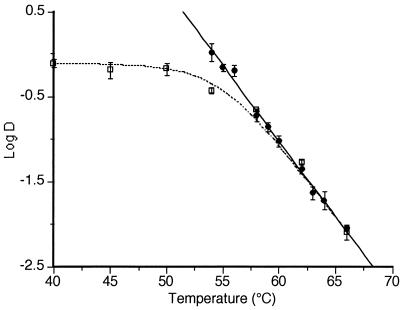
The lethality of UW of 117 μm of amplitude at 200 kPa but at different temperatures was investigated. The results of these experiments are shown in Fig. 6, which shows the DRTC corresponding to heat treatments as well as DMTS values at different temperatures. The theoretical DRTC corresponding to MTS treatment has been included (dotted line) to illustrate the discussion. This theoretical DRTC was calculated as described in Materials and Methods.
FIG. 6.
Influence of temperature on the lethality of MS treatment. Experimental values were for heat (•) and MS (□) (117 μm, 200 kPa, and 20 kHz) treatments. Curved line, theorical inactivation curve obtained by the formula DMTS = (DT × DMS)/(DT + DMS).
Inactivation mechanisms of MS and MTS treatments.
The ultimate reason for the lethal effect on microorganisms of high-intensity UW is thought to be the very high temperatures and pressures developed (15, 38) and/or free radicals released into the medium (20, 36) by cavitation. Although titanium is supposed to be a very stable material, titanium particles eroded from the tip of the horn during ultrasonication treatments might also somehow contribute to the lethal effect of MS and MTS treatments.
Several experiments were performed to explore the inactivation mechanisms of MS and MTS treatments. In the first one, an aliquot of a Y. enterocolitica cell suspension was kept for 15 min in a medium that previously had been intensely MS treated for 10 min (200 kPa, 117 μm). At preset intervals, samples of menstruum were extracted to assess the number of survivors. No lethal effect from the presence of titanium particles in this medium was observed. This experiment did not rule out the possible contribution to lethality of short-lived free radicals. In another experiment, the lethality of an MS treatment with a medium containing different concentrations (2, 50, and 100 mM) of the free radical scavenger cysteamine was measured. Cysteamine did not increase D values (P ≥ 0.05) and did not influence the profile of survival curves (data not shown).
The cell suspension was given the following three different treatments: one heat treatment at 63°C for 7 s; one MS treatment at 30°C, 200 kPa, and 117 μm of amplitude during 3 min; and one MTS treatment at 63°C, 200 kPa, and 117 μm of amplitude for 7 s. A microscopic observation of suspensions was carried out after each treatment to try to establish a relationship between percentages of disrupted and live cells. Percentages of disrupted cells were calculated by measuring the difference between the number of undisrupted cells before and after treatment. The percentage of inactivated cells in all three treatments was very high (>99%). This percentage was calculated by the difference between plate counts before and after each treatment. The percentage of disrupted cells was different in each treatment. No disruption was observed after heat treatment. After MS treatment, no undisrupted cell could be seen. After MTS treatment, approximately 80% of cells were undisrupted.
DISCUSSION
Performance of the instrument.
The temperature stability of this instrument in heat resistance experiments was similar to the best reported result for other heat resistance determination methods (5, 41). In MS and MTS experiments, stability was slightly less since it required manual adjustment of the flow of cold water through the cooling coil. However, it was still better than that of other heat resistance determination methods (18, 23).
DT values at successively higher temperatures can be obtained by reinoculating the same menstruum. Since stabilization to a higher temperature is fast, the DRTC could be obtained in the same working session. By this procedure, the possible influence of uncontrolled factors was avoided. The temperature probe and the manometer of the treatment chamber allowed the monitoring of temperature and pressure during the experiments. The agitation system ensured a quick and homogeneous distribution of the inoculum and a fast heating-up rate and temperature stabilization.
During heat treatments under pressure, no influence of dilution and possible leaks on the DT values obtained was observed. No statistically significant differences (P ≥ 0.05) were detected between DT values measured with this instrument and the TR-SC (7, 8). Under the most drastic ultrasonication conditions used during this research, the concentration of the contents of the chamber decreased by 10% per min (Fig. 2). Of course, the significance of dilution plus leaks on the accuracy of D values obtained would be greater for longer experiments. The duration of the experiment will be greater to the extent that the resistance of the microorganism involved and the number of decimal reductions of the survival curve to be obtained are higher. Under the most frequent ultrasonication conditions in this study (150 μm, 250 kPa), the maximum D value measured was 0.77 min. Therefore, the largest expected error was a 10% underestimation of the D value. This error is within the precision range of most heat resistance determination methods (11, 18, 29). Only when much larger D values are measured would a correction be required.
In mixing methods, the menstruum at treatment temperatures was inoculated with a microbial suspension. In these methods, the lowest measurable D value was limited by the distribution time of the inoculum and by the sampling speed. With this instrument, the homogeneous distribution of the inoculum (0.2 ml) was obtained in less than 0.2 s. This distribution speed was even greater than that of the original TR-SC thermoresistometer (8). This was most probably due to the much smaller size of the treatment chamber (23 ml instead of 350 ml).
The precision of D values obtained with this instrument is among the greatest reported by authors using different methods (11, 18, 29) as judged by the CV% of D values of the five survival curves of Fig. 3 (7%) and the 95% CL of D and z values shown in the figures.
This instrument proved to be a very versatile instrument to measure with accuracy, by the multipoint method, the sensitivities of microorganisms to heat, UW, MS, and MTS treatments in a wide range of temperatures, pressures, and amplitudes. The small volume of the treatment chamber makes it especially suitable to deal with small volume samples or expensive compounds (some enzymes).
Lethal effect of heat, MS, and MTS treatments.
Survival curves in this investigation sometimes had shoulders and tails. The rather frequent appearance of shoulders and tails has prompted some authors to question the logarithmic order of death and to propose alternative mathematical models (6, 37). However, none of these models has been widely accepted. Most authors still use the traditional logarithmic death rate model. In this investigation, resistance to heat, MS, and MTS treatments is shown as D and z values.
The heat resistance of our strain was much less (DT/5 − DT/8) than that reported for Y. enterocolitica by other authors, but the z value was similar (27, 39). Perhaps our DT values were less because our strain had been grown at a lower temperature. It is known that, in most species, lower growth temperatures lead to lower DT values (14, 19).
At ambient temperature and pressure, the lethal effect of UW on microorganisms is small. This is probably why UW have not been used as a method for microbial inactivation. The D value of 1.5 min obtained with our strain with UW at 150 μm is similar to those obtained for other gram-negative species such as Pseudomonas aeruginosa (38) and Escherichia coli (1). By increasing static pressure, the lethality of UW increased drastically. At 30°C, 600 kPa, and the same amplitude (150 μm), the D value was 0.22 min.
It has been reported that the energy of the UW decreases with the distance from the emission source and increases with amplitude (10). However, there are no data in the literature on the influence of amplitude on the lethal effect of UW on microorganisms. As shown by our results (Fig. 4), the resistance of Y. enterocolitica decreased exponentially when the amplitude was increased linearly. Ten-micrometer increments in amplitude led to steady decreases of approximately 20% in DMS.
The effect of pressure on the lethality of UW (MS) has been reported (with yeasts) only with UW of low intensity (32). The mechanism by which pressure increases lethality is not known (4, 30). As shown in Fig. 5, the lethality of UW of 117 and 150 μm of amplitude increased with pressure. However, these increments became progressively smaller as the pressure increased, tending to disappear. No statistically significant differences (P ≥ 0.05) were found between DMS values obtained at 400 and 600 kPa. Our results are partly in agreement with those of the very few investigations reported in the literature (4, 30). The authors in these investigations also reported an increase in the physicochemical effects of UW when pressure was increased. However, after an optimum pressure at which maximum effect was obtained, any further pressure increase led to a decrease in efficacy. We did not observe the occurrence of any decrease in our working range (of as much as 600 kPa).
No reports are found in the literature on the interaction between amplitude and pressure on the effects of UW. As shown in Fig. 5, the increase in lethality by increasing pressure (to as much as 600 kPa) was greater when the amplitude of UW was higher.
The individual contributions of heat and of UW under pressure (MS) to the lethal effect of MTS can be deduced from Fig. 6. As seen in this figure, the D value of Y. enterocolitica corresponding to UW under pressure was the same (up to 50°C) regardless of the temperature of treatment. From this temperature to 58°C, D values decreased rapidly and were always smaller than D values for heat treatment alone at the same temperature. At temperatures higher than 58°C, D values corresponding to heat and to MTS were equal.
The results of the experiments on the contribution to the lethal effect of UW of free radicals and of titanium particles and the microscopical observation of suspensions after heat, MS, or MTS treatments appeared to indicate that the inactivation of MS was due to one single mechanism, the mechanical disruptions of cells. This mechanism of action has also been suggested by other authors (15, 31).
Unlike MS, the lethality of MTS treatments appeared to be due to two different mechanisms acting independently, one of heat and the other of MS. The lethal effect of MTS treatments would be the result of adding the lethal effect of MS to the lethal effect of heat. The inactivation rate of each mechanism would be determined by temperature. Therefore, which one would prevail would depend on the temperature of treatment.
Up to 50°C, the D value of MS treatments was constant (Fig. 6), because in this range the inactivation rate of UW under pressure was not influenced by temperature and because, at these temperatures, the lethality of heat would be negligible. The rapid decrease in D value at temperatures higher than 50°C (MTS) would be due only to the exponential increase in the lethality of heat by linear increases in temperature. This exponential increase in the lethality of heat (while that of MS treatment remained constant) would finally make (at 58°C) the lethality of UW under pressure negligible. At temperatures higher than 58°C, D values of MTS and of heat would be equal. At these temperatures, the inactivating effect would be solely due to heat.
The good match of D values corresponding to MS-MTS treatments with the theoretical DRTC (dotted line in Fig. 6) supports this hypothesis. The values of this curve were calculated by adding the lethal effect of MS treatment to that of heat. Furthermore, microscopic observations of cell disruption are also in agreement. After an MS treatment at 30°C that caused a three-log reduction in the number of survivors, all cells were disrupted. However, an MTS treatment at 63°C that caused the same reduction in survivors disrupted only 20% of cells.
As shown by our results, static pressure is a very efficient means of increasing lethality of UW (MS). This increase becomes greater when the amplitude of UW is higher. Between 50 and 58°C, the lethality of heat can be increased by combining heat treatments with UW under pressure (MS). The lethality of this treatment (MTS) is equivalent to the additive lethal effect of heat and UW. MS and MTS treatments could become an alternative for the inactivation, in heat-sensitive media (i.e., liquid egg), of Y. enterocolitica and possibly other microorganisms. It might also find applications in foods in which the high intensity of heat treatments required (i.e., low-water-activity foods) would impair food quality.
ACKNOWLEDGMENTS
This study was supported by the CICYT (Project ALI90-900) and by the Ministerio Español de Educación y Ciencia which provided J. Raso and R. Pagán with a grant to carry out this investigation.
Our thanks to S. Kennelly and R. Levene for their collaboration in the English correction of this work.
REFERENCES
- 1.Allison D G, D’Emanuele A, Eginton P, Williams A R. The effect of ultrasound on Escherichia coli viability. J Basic Microbiol. 1996;1:3–11. doi: 10.1002/jobm.3620360102. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, R. M. G. July 1980. Process for ultrasonic pasteurisation. U.S. patent 4,211,744.
- 3.Brown K L. Spore resistance and ultra heat treatment processes. J Appl Bacteriol Symp Suppl. 1994;76:67–80. doi: 10.1111/j.1365-2672.1994.tb04359.x. [DOI] [PubMed] [Google Scholar]
- 4.Chendke P K, Fogler H S. Effect of static pressure on the intensity and spectral distribution on the sonoluminiscence of water. J Phys Chem. 1983;87:1644–1648. [Google Scholar]
- 5.Cole M B, Jones M V. A submerged-coil heating apparatus for investigating thermal inactivation of micro-organisms. Lett Appl Microbiol. 1990;11:233–235. [Google Scholar]
- 6.Cole M B, Davies K W, Munro G, Holyoak C D, Kilsby D C. A vitalistic model to describe the thermal inactivation of Listeria monocytogenes. J Ind Microbiol. 1993;12:232–239. [Google Scholar]
- 7.Condón S, López P, Oria R, Sala F J. Thermal death determination: design and evaluation of a thermoresitometer. J Food Sci. 1989;54:451–457. [Google Scholar]
- 8.Condón S, Arrizubieta M J, Sala F J. Microbial heat resistance determinations by the multipoint system with the thermoresistometer TR-SC. J Microbiol Methods. 1993;18:357–366. [Google Scholar]
- 9.Condón S, Palop A, Raso J, Sala F J. Influence of the incubation temperature after heat treatment upon the estimated heat resistance values of spores of Bacillus subtilis. Lett Appl Microbiol. 1996;22:149–152. [Google Scholar]
- 10.Cracknell A P. Ultrasonidos. Madrid, Spain: Paraninfo, S. A.; 1983. [Google Scholar]
- 11.David J R D, Merson R L. Kinetic parameters for inactivation of Bacillus stearothermophilus at high temperatures. J Food Sci. 1990;55:488–493. [Google Scholar]
- 12.Davies, P. W., J. Donnelly, and E. Stentiford. October 1993. Treatment of water with ultrasound. European patent 93302120.6.
- 13.Dawson R M C, Elliot D C, Elliot W H, Jones K M. Data for biochemical research. Oxford, United Kingdom: Oxford at the Clarendon Press; 1974. [Google Scholar]
- 14.Elliker P R, Frazier W C. Influence of time and temperature of incubation on the heat resistance of Escherichia coli. J Bacteriol. 1938;36:83–98. doi: 10.1128/jb.36.1.83-98.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frizzell L A. Biological effects of acoustic cavitation. In: Suslick K, editor. Ultrasound: its chemical, physical, and biological effects. New York, N.Y: VCH Publishers, Inc.; 1988. pp. 287–301. [Google Scholar]
- 16.Gaboriaud, P. L. F. May 1984. Stérilisation de liquides par ultra-sons. French patent 84 07122.
- 17.García M L, Burgos J, Sanz B, Ordoñez J A. Effect of heat and ultrasonic waves on the survival of two strains of Bacillus subtilis. J Appl Bacteriol. 1989;67:619–628. doi: 10.1111/j.1365-2672.1989.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaze E J, Brown K L. The heat resistance of spores of Clostridium botulinum 213B over the temperature range 120 to 140°C. Int J Food Sci Technol. 1988;23:373–378. [Google Scholar]
- 19.Hansen N H, Riemann H. Factors affecting the heat resistance of nonsporing organisms. J Appl Bacteriol. 1963;26:314–333. [Google Scholar]
- 20.Jacobs S E, Thornley M J. The lethal action of ultrasonic waves on bacteria suspended in milk and other liquids. J Appl Bacteriol. 1954;17:38–56. [Google Scholar]
- 21.Kim A, Thayer W. Mechanism by which gamma irradiation increases the sensitivity of Salmonella typhimurium ATCC 14028 to heat. Appl Environ Microbiol. 1996;62:1759–1763. doi: 10.1128/aem.62.5.1759-1763.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knorr D. Effects of high-hydrostatic-pressure processes on food safety and quality. Food Technol. 1993;46:156–161. [Google Scholar]
- 23.Kooiman W J. The screw cap tube technique: a new and accurate technique for the determination of the heat resistance of bacterial spores. In: Barker A N, Gould G, Wolf J, editors. Spore research. London, United Kingdom: Academic Press; 1974. pp. 87–92. [Google Scholar]
- 24.Kramer J M, Gilbert R J. Enumeration of microorganisms in food: a comparative study of five methods. J Hyg. 1978;81:151–159. doi: 10.1017/s0022172400053857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyzlink V. Principles of food preservation. Amsterdam, The Netherlands: Elsevier Science Publishers; 1990. [Google Scholar]
- 26.Lee B H, Kermasha S, Baker B E. Thermal, ultrasonic and ultraviolet inactivation of Salmonella in thin films of aqueous media and chocolate. Food Microbiol. 1989;6:143–152. [Google Scholar]
- 27.Lovett J, Bradshaw J G, Peeler J T. Thermal inactivation of Yersinia enterocolitica in milk. Appl Environ Microbiol. 1982;44:517–519. doi: 10.1128/aem.44.2.517-519.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallidis C G, Drizou D. Effect of simultaneous application of heat and pressure on the survival of bacterial spores. J Appl Bacteriol. 1991;71:285–288. doi: 10.1111/j.1365-2672.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 29.Mikolajcik E M, Rajkowski K T. Simple technique to determine heat resistance of Bacillus stearothermophilus spores in fluid systems. J Food Prot. 1980;43:799–804. doi: 10.4315/0362-028X-43.10.799. [DOI] [PubMed] [Google Scholar]
- 30.Neppiras E A. Acoustic cavitation thresholds and cyclic processes. Ultrasonics. 1980;9:201–209. [Google Scholar]
- 31.Neppiras E A. Acoustic cavitation. Phys Rep. 1980;61:159–251. [Google Scholar]
- 32.Neppiras E A, Hughes D E. Some experiments on the disintegration of yeast by high intensity ultrasound. Biotechnol Bioeng. 1964;4:247–270. [Google Scholar]
- 33.Ordoñez J A, Aguilera M A, Garcia M L, Sanz B. Effect of combined ultrasonic and heat treatment (thermoultrasonication) on the survival of a strain of Staphylococcus aureus. J Dairy Res. 1987;54:61–67. doi: 10.1017/s0022029900025206. [DOI] [PubMed] [Google Scholar]
- 34.Puyol P, Perez M D, Mata L, Calvo M. Study on interaction between b-lactoglobulin and other bovine whey proteins with ascorbic acid. Milchwissenschaft. 1994;49:25–26. [Google Scholar]
- 35.Qin B L, Pothakamury U S, Barbosa-Canovas G V, Swanson G. Nonthermal pasteurization of liquid foods using high-intensity pulsed electric fields. Crit Rev Food Sci Nutr. 1996;36:603–627. doi: 10.1080/10408399609527741. [DOI] [PubMed] [Google Scholar]
- 36.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radical Biol Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez A C, Smerage G H, Teixeira A A, Lindsay J A, Busta F F. Population model of bacterial spores for validation of dynamic thermal processes. J Food Process Eng. 1992;15:1–30. [Google Scholar]
- 38.Scherba G, Weigel R M, O’Brien J W D. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl Environ Microbiol. 1991;57:2079–2084. doi: 10.1128/aem.57.7.2079-2084.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sörquist S. Heat resistance of Campylobacter and Yersinia strains by three methods. J Appl Bacteriol. 1989;67:543–549. doi: 10.1111/j.1365-2672.1989.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 40.Steel R G, Torrie J H. Principles and procedures of statistics. New York, N.Y: McGraw-Hill Book Company Inc.; 1960. [Google Scholar]
- 41.Stumbo C R. Thermobacteriology in food processing. 2nd ed. New York, N.Y: Academic Press, Inc.; 1973. [Google Scholar]
- 42.Suslick K S. Ultrasound: its chemical, physical, and biological effects. New York, N.Y: VCH Publishers, Inc.; 1988. [Google Scholar]
- 43.Suslick K S. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 44.Wrigley D M, Llorca N G. Decrease of Salmonella typhimurium in skim milk and egg by heat and ultrasonic wave treatment. J Food Prot. 1992;55:678–680. doi: 10.4315/0362-028X-55.9.678. [DOI] [PubMed] [Google Scholar]