ABSTRACT
Pollution in the world and exposure of humans and nature to toxic substances is continuously worsening at a rapid pace. In the last 60 years, human and domestic animal health has been challenged by continuous exposure to toxic substances and pollutants because of uncontrolled growth, modernization, and industrialization. More than 350,000 new chemicals have been introduced to our lives, mostly without any reasonable control of their health effects and toxicity. A plethora of studies show exposure to these harmful substances during this period with their implications on the skin and mucosal epithelial barrier and increasing prevalence of allergic and autoimmune diseases in the context of the “epithelial barrier hypothesis”. Exposure to these substances causes an epithelial injury with peri-epithelial inflammation, microbial dysbiosis and bacterial translocation to sub-epithelial areas, and immune response to dysbiotic bacteria. Here, we provide scientific evidence on the altered human exposome and its impact on epithelial barriers.
KEYWORDS: Detergents, epithelial barrier, microplastics, nanoparticles, ozone, particulate matter, exposome
1. Introduction
The recently proposed epithelial barrier hypothesis suggests that disruption of the epithelial barrier due to exposure to harmful environmental agents along with genetic factors is responsible for up to two billion chronic, non-communicable diseases. The hypothesis attributes the rapid increase in allergic and autoimmune diseases in recent decades to industrialization, urbanization and the western lifestyle1–3 It is important in preserving the structural and functional integrity of the body by protecting the host from pathogen invasion and foreign substance infiltration. Maintaining the physiological epithelial barrier integrity is of utmost importance since its dysfunction is implicated with many chronic conditions such as allergic, autoimmune, and metabolic diseases.1 Exposure to toxic/barrier damaging agents results in an impaired epithelial barrier in affected tissue. This epithelial barrier damage leads to a vicious cycle continuing with opportunistic pathogen colonization, translocation of microbiota to subepithelial tissues, immune response to the microbiome and colonized pathogens, microbial dysbiosis and chronic tissue inflammation which eventually leads to defective epithelial barrier healing (Figure 1).1 In the context of the epithelial barrier hypothesis, the epithelial barrier impairment could cause local and systemic pathologies.1 The local epithelial damage in affected tissues such as skin and mucosal sites leads to the development of allergic diseases.4 It should be noted that epithelial barrier impairment and microbial dysbiosis in affected tissues usually coincide. It is known that the gut epithelial barrier impairment and microbial dysbiosis are associated with systemic pathologies such as diabetes, obesity, rheumatoid arthritis, multiple sclerosis and ankylosing spondylitis, and even neurodegenerative and psychiatric diseases such as Parkinson’s disease, Alzheimer’s disease, autism spectrum disorders and chronic depression.1
Figure 1.
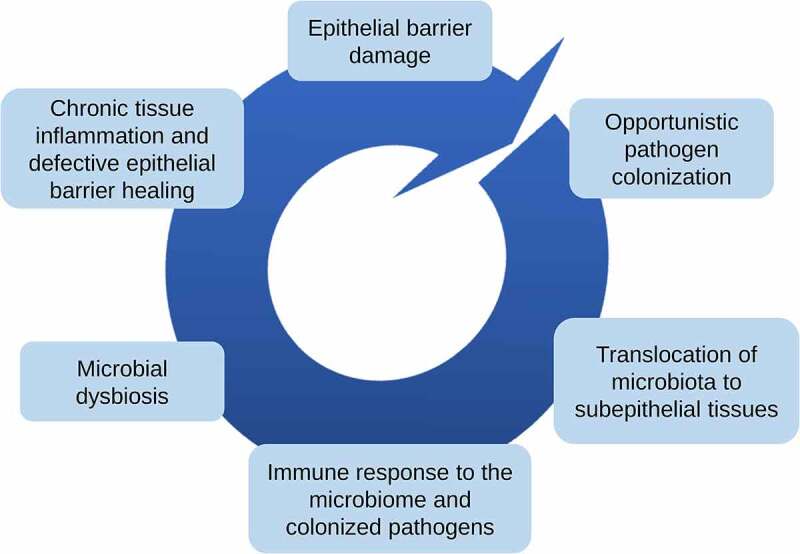
The epithelial barrier hypothesis. The hypothesis attributes the rapid rise of allergic and autoimmune diseases to the increased exposure to hazardous substances, such as chemical sensitizers and air pollutants. The epithelial barrier impairment causes a vicious cycle of pathological events.
Human exposure, defined as the sum of the factors to which an individual is exposed during her lifetime, has changed alarmingly in recent years due to anthropogenic activities.4,5 Today humans, domestic animals and wild animals are exposed to a wide range of hazardous environmental factors, such as particulate matter (PM), diesel exhaust particulates (DEP), ozone, industrial toxic gases, nanoparticles, nano (NP), and microplastics (MP), cigarette smoke, household cleaning agents, enzymes and emulsifiers in processed food and pesticides.4,6–9 Exposure to these hazardous compounds, along with global warming and viral pandemics like COVID-19, is one of the greatest threats to humanity. Detergents used for laundry and dishwashing, household cleaners, cosmetics, conservatives and emulsifiers in packaged food, disinfectants and toothpastes represent complex mixtures that humans are confronted that apparently contain several toxic substances. In addition, several hundred thousands of new chemicals have been introduced to our lives during the last 60 years, without any meaningful toxicity control as a part of industrialization, urbanization, and modernization.1,4 As an estimation, 350,000 chemicals are used in Europe.10 Global chemicals sales have been doubled during the last two decades and end up in the environment as a pollutant.10 By volume, three quarters of chemicals produced in Europe are hazardous.11 The full extent of the effect of these environmental factors on our health remains to be elucidated, however, there is a plethora of studies demonstrating their damage to the epithelial barrier integrity that can culminate into chronic health problems.1 Synergistic effect of these substances and how they would behave in inflammatory and affected tissues in certain diseases is an important aspect to be considered. It is crucial to improve our understanding of the underlying mechanisms affecting the integrity of the epithelial barrier at the skin and mucosal sites to develop novel treatments to restore its physiological function and to provide policymakers with science-based information to steer them in implementing new measures to reduce human exposure to these toxic agents. Herein, we provide a comprehensive overview of the impact of exogenous factors on respiratory, gastrointestinal, and skin barriers.
2. The change in the human exposome: The main threats to nature and human health
Anthropocene refers to the current geological epoch in which human activities significantly affect the Earth’s geology and ecosystems. Since the industrial revolution, the proposed beginning of anthropogenic influences, the human impact on the nature has become more evident and the consequences such as anthropogenic climate change, habitat destruction, and environmental pollution are now threatening the Earth’s ecosystem and human health.1,6,12–20 According to current reports, population sizes of species in the nature have decreased by 60% between 1970 and 2014 and extinction rates are 100 to 1000 times higher than previous background rates.21
The term exposome describes all the environmental exposures individuals are facing during their lifetime.4 These factors can be divided into three categories: general external environment, specific external environment, and host-dependent internal environment.22 The general external environment includes a wider socioeconomic environment such as climate, urban-rural environment, and educational level. However, specific external environment includes more individual factors such as lifestyle, pollutant exposure, and infections. However, the host-dependent internal environment includes both biological effects of external exposure and biological responses such as metabolic factors, inflammation, and oxidative stress.4,23,24 There have been alarming changes in the exposome in the last 70 years brought about by industrialization, urbanization, and modernization as exemplified by studies indicating human exposure to nearly 200,000–350,000 new chemicals.4,5,10 Since the 1950s, the amount of plastic production has increased nearly 200 times, and it is estimated that the total amount of plastic produced worldwide by 2017 reached approximately 8.3 billion metric tons.13,15,25 Today the human body is continuously exposed to a wide range of potentially harmful substances, such as PM, DEP, cigarette smoke, NP, MP, nanoparticles, ozone, nitric oxide (NO), nitrogen dioxide(NO2), carbon monoxide (CO), sulfur dioxide(SO2), household cleaners, laundry and dishwasher detergents, toothpaste, surfactants and emulsifiers in processed food, and pesticides (Figure 2). 1 Annual global deaths from pollution-related diseases are estimated at 9 million, still more than COVID-19-related deaths, mostly in underdeveloped countries.6,17
Figure 2.
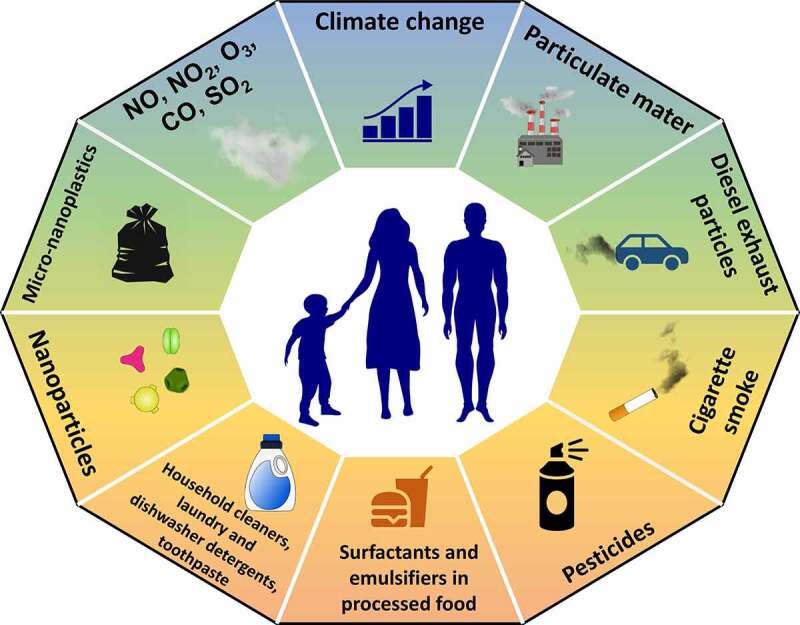
Our modernized lifestyle has dramatically altered the human exposome, the collection of exogenous factors that an individual is exposed to in its lifetime. Anthropogenic activities are responsible for the increased exposure to hazardous substances, such as particulate matter, diesel exhaust particulates, cigarette smoke, pesticides, emulsifiers in processed food, detergents, micro and nanoplastics, and traffic and industrial gas emissions.
Ambient and indoor air pollution
Air pollution is one of our era’s biggest menaces as it contributes to climate change and is a leading cause of respiratory diseases.26 According to the World Health Organization’s (WHO) World Global Ambient Air Quality Database, 91% of the global population is exposed to poor air quality.27,28 Ambient air pollution alone is responsible for an estimated 3.7 to 4.2 million annual deaths worldwide.6 Air pollution is a complex mixture of gaseous and particulate components. Gaseous components include NO, NO2, SO2, CO and ozone.29 Air and aquatic PM originates from both natural and anthropogenic sources. Natural sources include dust, including desert dust, sea salt, and forest fires. In contrast, anthropogenic sources include traffic, power plants, factories, wood, and coal-burning emissions.30 Studies suggest that air pollution aggravates cardiovascular and respiratory diseases and is associated with the development or progression of asthma, diabetes, reproductive, and various neurocognitive diseases.31–33 PM is classified according to particle size (PM0.1, PM2.5, and PM10). Epidemiological studies indicate that exposure to ambient PM pollution has increased over the past ten years coinciding with increased drug use, high fasting plasma glucose, and high body mass index.14 In the last 40 years, atmospheric black carbon concentration (which is a PM2.5) generated as a by-product of incomplete combustion of fossil fuels, biofuels, and biomass, has shown a substantial increase of 1.57 times (from 0.70 μg/m3 in 1980 to 1.10 ± 0.22 μg/m3 in 2019) with an annual increase by 1.52% in China.34 Moreover, it was reported that there was an independent association between short-term exposure to PM10 and PM2.5 and daily all-cause, cardiovascular, and respiratory mortality.35 According to a meta-analysis, there is a positive and statistically significant link between the onset of asthma and exposure to black carbon, NO2, PM2.5 and PM10, well-known traffic-related air pollution constituents.36 Ambient air pollution, especially PM, is considered a neurotoxicant and impairs cognitive functions, learning abilities, and neurodevelopment. It is thought to be associated with depression, vascular dementia, and stroke. Along with urban PM, wildfire PM has also been associated with morbidity and mortality. An increased risk of hospitalization and emergency room visits for asthma, chronic obstructive pulmonary disease (COPD), and respiratory tract infection has been reported in individuals exposed to wildfire PM.19 Among other air pollutants, exposure to oxides of nitrogen affects the central nervous system and contributes to neurological disorders.37 During COVID-19 restrictions, the atmospheric levels of air pollutants associated with traffic emissions including oxides of nitrogen, SO2, and CO, and primary PM levels decreased due to the quarantine and lock-down measures imposed by the governments. In contrast, secondary PM levels remained unchanged or even increased during the same period.38
Indoor pollution is a major problem, as pollution levels are often twice as high then outdoors and people spend 80–90% of their lives indoors.39 Household air pollution is estimated to be responsible for 2.9–4.3 million deaths per year worldwide.2 Thanks to social and economic development, household air pollution has declined in the last ten years.14 Although the risk exposure to tobacco use is declining, it is still responsible for more than 8 million deaths annually of which 1.2 million are from secondhand smoking,40 mostly affecting low- and middle-income countries.5,40
Micro- and nano- plastic pollution
The use of plastics has rapidly grown as these materials offer a low production cost and high stability and durability. However, plastic waste poses a threat to nature as most plastics are non-biodegradable. It is estimated that in 2015 globally 66–90 million metric tonnes of miss managed plastic waste are produced and every year 8 million tonnes of plastic waste are escaped to the oceans.25,41 When plastic waste enters the environment, it breaks down into small fragments and particles such as MPs (1 mm to 5 mm) and NPs (1 nm to 1000 nm).13,42 The degradation products can be detected in the air, water, and sediment.13,16 It is reported that nano- and MPs are harmful to aquatic species, such as zooplankton, bivalves, and small fish.13 Moreover, NPs can penetrate living organisms and eventually enter the human food chain.13,15 In addition, humans are exposed to airborne NPs through the airways and in contact with the skin.15
Processed foods: harmful additives in foods
The modern food industry provides a wealth of supply and diversity made possible by the incorporation of food additives, such as synthetic colorants, preservatives, stabilizers, surfactants, emulsifiers, and texturizers. There is mounting evidence suggesting that processed foods that contain food additives and advanced glycation endproducts (AGEs) due to heat processing disrupt the integrity of the epithelial barrier, a key pathological feature in the development of allergic and autoimmune diseases.1,43 The consumption of processed food has been associated with all-cause mortality, obesity, metabolic syndrome and depression.44 In addition, food contamination is possible by contact with dishware that has residues from cleaning products, such as detergents and anionic surfactants.7,45,46
Anthropogenic climate change
Anthropogenic activities are responsible for global warming,47–49 with a mean temperature increase of 0.2°C per decade.18 A recent study predicts global warming of 2.6°C (1.9°C to 3.7°C) by 2100, taking into account only the current energy policies and measures being developed by the countries participating in the Paris agreement. Nonetheless, the authors noted that warming can be limited to 1.9–2.0°C if all the conditional and unconditional pledges of the Paris agreement are met in full and on time.50 The generation of large quantities of greenhouse gases has been a key driver of climate change, in particular carbon dioxide emissions. Moreover, deforestation cripples the Earth’s natural ability to remove atmospheric CO2, further aggravating global warming and causing extreme weather events.26,51 In the future, increases in morbidity and mortality are estimated due to climate change-related adverse effects such as heat-related illnesses, poor air quality, and undernutrition due to reduced food quality and security.18 It should be noted that the global risks from toxic pollution and climate change are highly correlated, with low- and middle-income countries being the most affected by both.17
3. Diseases associated with epithelial barrier impairment
The number of patients with chronic non-communicable diseases has been estimated to be less than 50 million in 1960s, which increased to almost 2 billion patients in 2020s. The first group of diseases is allergies, asthma, atopic dermatitis, colitis and celiac in which the affected skin and mucosa show inflammation.52–58 The second group of diseases are autoimmune and metabolic diseases with the affected organ is distant from the damaged epithelial barriers.59–64 The third group is chronic neuropsychiatric conditions with gut barrier leakiness and microbial dysbiosis in the gut.65–68 The epithelial barrier is the primary constituent of the internal and external surfaces of the human body. Epithelial barriers in the gastrointestinal and respiratory tracts act as selective barriers that allow gas or nutrient translocation while maintaining a healthy microbiota and preventing pathogens from entering the host.7,69,70 The mucosal barrier integrity is mainly maintained by tight junctions (TJ), adherens junctions, and desmosomes that seal off the paracellular space and prevents the diffusion of soluble mediators, proteins, or pathogens between apical and basolateral cell surfaces.56,70 TJs seal the apical side of the neighboring epithelial cells and form the physical barrier even water cannot pass through, because of homodimeric covalent bonds. They are composed of transmembrane proteins, such as claudins, occludin, junctional adhesion molecules, and an intracellular protein called zonula occludens (ZO) and are the main regulators of the paracellular permeability.70
The skin epithelium has a unique structure comprised of four layers: the stratum corneum, stratum granulosum, stratum spinosum, and the stratum basale.52 The stratum corneum is the outermost layer of the epidermis, which is composed of enucleated keratinocytes known as corneocytes. It is maintained by the intricate interaction of a complex lipid mixture in the extracellular space, the intracytoplasmic moisturizing factors, and the cornified envelope. Filaggrin (FLG) is an important structural protein of the cornified envelope, acts as a natural moisturizing factor and plays a role in the alignment of keratin filaments.71–73 TJs are mainly located in the stratum granulosum of the epidermis and regulate cell permeability.
In addition to the physical barrier function, the epithelial barrier also exhibits antimicrobial activity against exogenous insults as it can secrete antimicrobial peptides, proteases, and antioxidants.74 Membrane-bound and cytosolic pattern recognition receptors in epithelial cells recognize various pathogen components and activate downstream signaling, promoting the release of pro-inflammatory cytokines/chemokines and attracting and activating cells from the innate and adaptive immune system.74,75
Maintaining a healthy epithelial barrier is considered a crucial first step in preventing many chronic inflammatory conditions. An extended “epithelial barrier hypothesis” has been recently proposed that attributes epithelial barrier dysfunction to the development of allergic, autoimmune, and metabolic diseases.1,76 Disruption of epithelial barrier homeostasis leads to subsequent pathophysiological events such as microbial dysbiosis, colonization of opportunistic pathogens, translocation of microbiota, inflammation and immune response to commensals and colonizing opportunistic pathogens, and eventually, the defective barrier healing capacity of the epithelium (Figure 3). In the context of the epithelial barrier hypothesis, damage to the epithelial barrier integrity is due to genetic factors in a small fraction of patients but is mainly attributed to exposure to environmental insults introduced during industrialization, modernization, and westernization. Further research on the effect of environmental factors on our health is warranted as the humans are in continuous contact with these barrier-damaging agents at unprecedented concentrations.1 The epithelial barrier is responsible for maintaining microbiota homeostasis. A healthy microbiome limits pathogenic microorganism colonization over epithelial surfaces and provides barrier strengthening substances such as short-chain fatty acids and tryptophan metabolites.77,78 Other microbiome related products such as bile acid, conjugated fatty acids, polyamines, and polyphenolic derivatives have many regulatory functions essential for maintaining the integrity of the intestinal epithelial barrier.79 The link between dysbiosis and diseases characterized by an impaired epithelial barrier is well documented.1,80–88 Although they are often found together, it is unclear which one occurs first. Nevertheless, an impaired epithelial barrier eventually leads to microbial translocation to subepithelial tissues and inflammation (Figure 4).1
Figure 3.
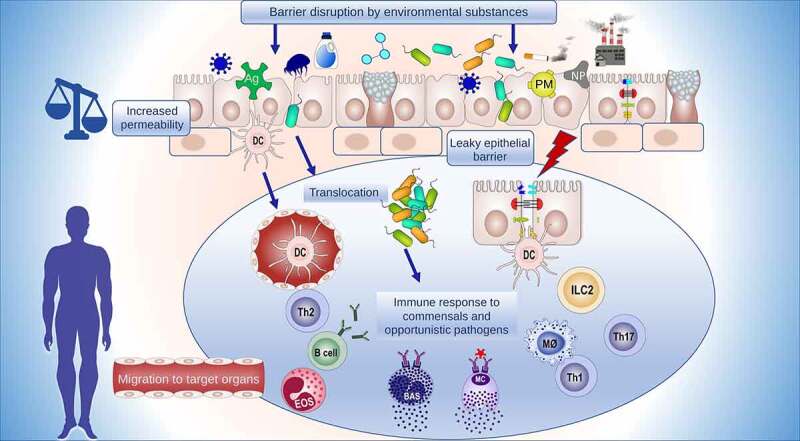
Epithelial barrier damaging factor cause series of pathological events. The external barrier damaging stimuli impair the epithelial barrier, increasing its permeability and facilitating the entry of foreign substances and microbial translocation to interepithelial and subepithelial regions. A cascade of events follows including continuous immune responses against allergens and the development of tissue microinflammation, which have been implicated in the development of various diseases due to a vicious cycle of a leaky barrier and chronic inflammation.
Figure 4.
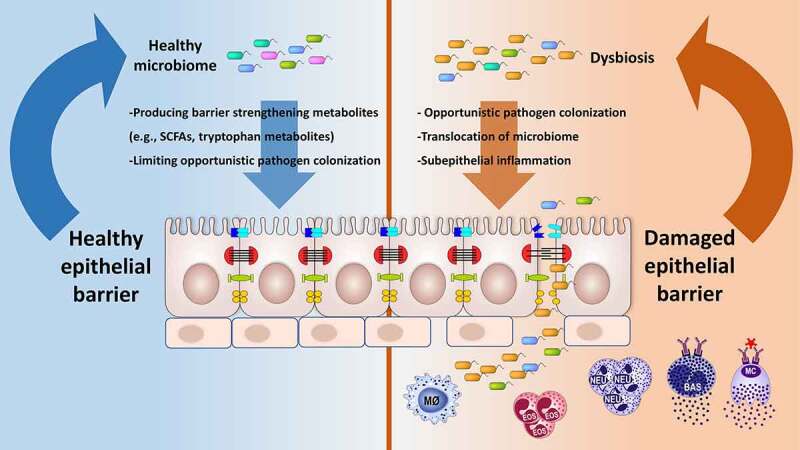
The microbiome has an important role in epithelial barrier physiology. Generally, a diverse microbiome is associated with a healthy epithelial barrier. Microbiota metabolites with immunosuppressive activities, such as short-chain fatty acids and tryptophan, play a key role in maintaining the barrier function. Dysbiosis has been linked to allergic and autoimmune diseases characterized by an impaired epithelial barrier. Loss of microbial diversity can lead to an impaired barrier and vice versa.
Disruption of the epithelial barrier contributes to the pathogenesis or development of chronic lung, skin and intestinal diseases characterized by epithelial barrier impairment in affected tissues. In addition, culminating evidence shows gut barrier defects in various autoimmune diseases affecting tissues other than gut, metabolic and neuropsychiatric diseases (Table 1).
Table 1.
Diseases associated with epithelial barrier impairment.
| Diseases with epithelial barrier impairment in affected tissues |
|---|
|
| Autoimmune and metabolic diseases linked with gut epithelial barrier defect |
|
| Neuropsychiatric disease linked with gut epithelial barrier defect |
|
Diseases with epithelial barrier impairment in affected tissues
It is well established that epithelial barrier impairment is involved in the pathogenesis of chronic respiratory diseases such as asthma, allergic rhinitis (AR) and chronic rhinosinusitis. The barrier function of the epithelium is impaired through decreased TJ molecule expression in asthmatic patients. Decreased expression of E-cadherin, β- catenin, occludin, and ZO-189–91 was observed in the airway epithelium of asthma patients all of which cause epithelial barrier leakiness induced by T helper (Th)-2 cells, interleukin (IL)-4, and IL-13.92,93 Downregulation of ZO-1 and claudin-18 were demonstrated in eosinophilic, mixed, and neutrophilic experimental mouse models of asthma.94 Moreover, lower occludin and ZO-1 expressions in patients with house dust mite-induced AR56 and decreased expression of claudins was observed in the nasal mucosa epithelium of AR patients which was accompanied by an impaired barrier function.95 Soyka et al. showed that primary epithelial cell cultures from patients with chronic rhinosinusitis with nasal polyps had an impaired epithelial barrier evidenced by low transepithelial resistance, as well as decreased occludin and claudin-4 expressions.55
Skin barrier deficiency in atopic dermatitis (AD) is related to loss of functions mutations in the FLG gene,96 and the reduced expression of FLG2 has been demonstrated in the epidermis of AD patients.97 The FLG mutations were also found in AD patients with eosinophilic asthma.98 Skin biopsies of allergic contact dermatitis patients in the presence of p-phenylenediamine showed reduced expression of claudin-1, claudin-8, claudin-11, occludin, FLG1, FLG2, and loricrin.99 The compromised epidermal barrier activates a local and systemic type 2-predominant response, characterized by elevated levels of IL-4, IL-5, IL-13, and IgE.100,101
The intestinal epithelium is crucial in maintaining intestinal homeostasis. Impairment of the epithelial barrier results in elevated intestinal permeability and promotes a leaky barrier resulting in abnormal interactions between intestinal epithelial cells and immune cells residing in the lamina propria. Inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, have been linked to a disrupted intestinal epithelium.102 In Crohn’s disease, the epithelial barrier function is impaired due to the altered TJ structure. Upregulation of claudin-2 and downregulation and redistribution of claudin-5 and claudin-8 were associated with impaired intestinal barrier function in mild to moderately active Crohn’s disease.103 An impaired barrier function is also implicated in the pathogenesis of the Celiac disease, characterized by a decreased expression and redistribution of occludins, claudins, and junctional adhesion molecules.104 Pro-inflammatory responses relating to a complex network of cytokines further aggravate barrier dysfunction in patients with inflammatory bowel disease.105 Gluten ingestion can trigger celiac disease as one of its proteins, gliadin, induces the release of zonulin, a protein involved in opening the TJ.106 In addition to the barrier impairment regarding intestinal epithelium, there are also other gastrointestinal tract-related barrier disorders as exemplified in eosinophilic esophagitis, an atopic disease involving the esophagus.107,108 The dysregulation of transcription factor hypoxia-inducible factor-1α impairs claudin-1 expression resulting in dysfunction of the stratified squamous esophageal epithelial barrier in eosinophilic esophagitis patients.109
Autoimmune and metabolic diseases linked with gut barrier defect
Impairment of the intestinal barrier is associated with various autoimmune and metabolic diseases. Elevated serum zonulin levels are accompanied by a leaky intestinal barrier, microbial dysbiosis, and inflammation as exemplified in ankylosing spondylitis, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes.62,63,110,111 Zonulin induces a decrease in occludin and cadherin expression in the gut vascular barrier of ankylosing spondylitis patients, accompanied by downregulated claudin, occludin, and ZO-1 expression modulated by ileal bacteria.63 Barrier disruption through zonulin upregulation appears to precede the onset of type 1 diabetes111 and arthritis.62 The development of insulin resistance in type 2 diabetes has been linked to gut microbial dysbiosis and the impairment of the intestinal barrier.112,113 In addition hyperglycemia causes increased intestinal barrier permeability in mouse models of obesity and diabetes through a GLUT2-dependent decrease of tight and adherence junction integrity.114 Obesity is associated with higher gastroduodenal permeability (but not small intestine and colonic) with a pro-inflammatory systemic and intestinal profile.115 Impaired intestinal barrier function has a critical role in the pathogenesis of gut-liver-associated diseases, including fatty liver disease and cirrhosis.116–118 Immunohistochemical analysis in duodenal biopsy specimens showed lower ZO-1 staining compared to healthy subjects and a significant increase of small intestinal bacterial overgrowth in nonalcoholic liver disease.116 In a mouse model of alcoholic steatohepatitis, disruption of intestinal TJ was associated with enhanced intestinal endocytosis of occludin.119
Neuropsychiatric disease linked with gut barrier defect
Recent studies revealed that there are numerous neuropsychiatric diseases associated with an impaired gut barrier. Alzheimer’s disease is associated with intestinal inflammation, barrier deficiency, and microbial dysbiosis.66,67 Similarly, barrier impairment was found in intestinal biopsy samples from people with Parkinson’s disease along with elevated zonulin concentrations in the blood and feces.66,120 An amyotrophic lateral sclerosis mouse model shows a disrupted gut TJ structure and increased permeability, as well as decreased ZO-1 and E-cadherin expression levels.121 Patients with autism spectrum disorder shows an altered gut permeability through the decreased expression of barrier-forming TJ components.65 Acute stress has been shown to disrupt the intestinal barrier in animal models.68 Kelly et al. showed that depression is associated with decreased gut microbiota richness and diversity. Moreover, the behavioral and physiological characteristics of depression can be induced in microbiota-depleted rats with fecal microbiota transplantation from depressed individuals.122 Microbial dysbiosis, bacterial translocation in the gut, and an inflammatory response to commensals are common in patients with chronic depression.123
4. Epithelial barrier damaging environmental factors
Air pollution and related substances
Air pollution a complex mixture of hazardous gases and PM has detrimental effects on mucosal and skin epithelial barriers. Ambient air pollution accounts for health problems such as COPD, asthma, AR, bronchiolitis, cardiovascular disorders, reproductive and central nervous system malfunction, and lung cancer.26,124 Exposure to air pollutants, such as PM, DEP, ozone and NO2, induces the generation of reactive oxygen species (ROS) and impairs the epithelial barrier function in human epithelial cells (Table 2).29,30,125–131 The human microbiome and its immunomodulatory potential are negatively impacted by an increased exposure to the natural environment.132 Cavaleiro Rufo et al. found that at the age of seven, living close to a rural area has a preventive effect on the development of allergy disorders and asthma.133 The symbiotic relationship between the microbiota in the airways and the host is crucial for maintaining airway immune homeostasis.134Air pollutants can also alter the innate immune cells in airways, causing asthma symptoms to worsen.135 Recent studies demonstrate the importance of Toll-like receptor (TLR) and nucleotide-binding oligomerization domain-like receptor signaling in air pollutant-induced responses in the lung.136
Table 2.
In vitro and in vivo models of the respiratory epithelial barrier disruption triggered by air pollution-related substances.
| Substance | Evidence | Reference(s) |
|---|---|---|
| Particulate matter | PM2.5 exposure in a BEAS-2B cell line increased apoptosis, decreased viability, and decreased ZO-1 and e-cadherin mRNA expression.125 | 125 |
| PM2.5 exposure cause ROS activation and apoptosis.138,140 | 138,140 | |
| PM2.5 and urban PM disrupt TJ molecules of the human nasal epithelium and exacerbate AR.141–143 | 141–143 | |
| PM10 induces epithelial barrier damage in a primary rat alveolar epithelial cell culture.147 | 147 | |
| Micro- and nano-plastics | Polystyrene micro and nanoparticle exposure induce cytotoxicity, apoptosis, and autophagy in human bronchial and alveolar cell culture models.131,200 | 131,200 |
| Polystyrene micro and nanoparticle exposure damage the bronchial epithelial barrier by reducing ZO-1 mRNA expression. | 131,200 | |
| DEP | DEP induce epithelial barrier damage in human bronchial epithelial cell line.154,155 | 154,155 |
| DEP increase IL-33, IL-25, and TSLP cytokine production with Th-2 activation in allergic severe asthma patients. | 151–153 | |
| Ozone | Ozone exposure causes respiratory barrier injury in mouse models.126,159 | 126,159 |
IL: Interleukin; PM: Particulate matter; Th: T helper; TJ: Tight junction; TSLP: Thymic stromal lymphopoietin; ZO: Zonula occludens
Particulate matter
PMs generated from anthropogenic sources have a higher oxidative potential, especially secondary organic aerosols from residential biomass burning and vehicular non-exhaust emissions.137 As one of the main components of air pollution, PM2.5 is known to induce apoptosis, airway barrier dysfunction, breaking of inhalational tolerance, and sensitization to allergic antigens.125,138 Moreover, PM can pass through the epithelial barrier and accumulate in the endothelium.139 PM2.5 exposure has been demonstrated to have deleterious effects on the lung function of mice, including decreased total lung capacity, residual volume, and vital capacity.140 Exposure to PM2.5 is implicated in apoptosis, decreased TJ protein expression, and airway inflammation by NF-κB and MAPK activation,94 and exacerbates asthma in an OVA-induced mouse model of allergic asthma.125,141 Recent evidence indicates that PMs of various sizes, including urban PM (5.85 μm and 10.5 μm) and PM2.5 disrupt TJ barriers of the human nasal epithelium and exacerbate AR, suggesting an increased risk of rhinitis and rhinosinusitis in areas with high PM pollution.69,142–144 PM2.5 and black carbon exposure, was associated with the development of asthma in childhood.145 Exposure to PMs from biomass fuels has been reported as a major risk factor for COPD development,146 and epithelial barrier damage.147 A recent study has shown that exposure to PM10 and DEP causes occludin dissociation from ZO-1, resulting in an impaired alveolar epithelial barrier.148 Exposure to traffic-related air pollutants, oxides of nitrogen, and PM is associated with increased odds of incident eczema and is more pronounced with nonatopic eczema in elderly women.149 Prenatal exposure to PM2.5 during weeks 7 to 17 of pregnancy was significantly associated with an increased risk of childhood eczema by 6 years of age. The risk was increased in children who were not breastfed or exposed to prenatal environmental tobacco smoke.150
Diesel exhaust particles
DEPs, traffic-related air pollutants, are regarded as environmental factors that exacerbate allergic diseases. Studies demonstrated that DEP promotes Th2 cell responses and mediates up-regulation of IL-33, IL-25 and thymic stromal lymphopoietin with increased IgE and Th-2 activation, potentially linking environmental pollution and severe allergic asthma.151–153 Van den Broucke et al. reported DEP causes bronchial epithelial barrier impairment without directly or indirectly activating human mast cells in vitro.154 The effect of DEP exposure on the airway epithelial barrier junctions and its function was demonstrated using in vitro and in vivo models. Exposure to DEP may produce long-term alterations in airway epithelial barrier function.142,155,156 DEP exposure resulted in a significant concentration-dependent decrease in barrier integrity as well as decreased tricellulin mRNA expression in a bronchial epithelial cell line culture.155
Epicutaneous exposure to DEP or DMBA (7,12-dimethylbenz[a]anthracene) leads to an AD-like phenotype by activation of the aryl hydrocarbon receptor (AhR) transcription factor in a transgenic mouse model that expresses a constitutively active AhR.157
Air pollution related hazardous gases
Ozone and oxides of nitrogen such as NO and NO2 induce the generation of ROS and reactive nitrogen species and impair the epithelial barrier function in human epithelial cells.126,128,158,159 In asthmatic patients presenting to more than one emergency department for asthma, ambient NO2 concentrations have been shown to be associated with death from all causes.160 And a study estimated that NO2 pollution is responsible for 4 million new pediatric asthma cases per year.161 A population-based prospective study demonstrated that NO2 is associated with an increased risk of atopic eczema in male adults.162 In a mouse model SO2 exposure via inhalation caused increased airway epithelial permeability without histological changes.163
Ozone, a major air pollutant, is highly toxic to the human respiratory system. Its exposure can cause airway hyperreactivity, airway inflammation, and epithelial barrier disruption by inducing ROS production and it is thought to have an important role in the pathogenesis of chronic respiratory diseases such as asthma and COPD.128,130,164 Moreover, studies revealed that ozone exposure causes lung epithelial barrier injury in mouse models and primary normal human bronchial epithelial cells (NHBECs) cultures.126,159,165 Although further studies are warranted, there is early evidence suggesting that exposure to ozone increases lung epithelial barrier permeability in healthy subjects.166,167 A recent study demonstrated that the co-exposure of ozone and carbon black (which is different from black carbon) causes lung function decline along with increased inflammation (high epithelial alarmin, IL-1β, IL-6, IL-13, Muc5b secretion) and bronchiolar epithelial necrosis.168 Malondialdehyde collected from nasal fluid was proposed as a biomarker of the oxidative stress attributed to PM2.5 and ozone exposures and can be used to monitor asthma status in relation to air pollution.169
Volatile organic compounds
Volatile organic compounds (VOCs) are carbon-based molecules with a low boiling point and so readily vaporize at room temperature. Some of these volatile substances are cytotoxic and/or carcinogenic solvents such as benzene, toluene, and formaldehyde, present in cleaning products, wallpaper, paints, and plastics.170,171 Certain VOCs can react with NO2 to form ozone under UV light from the sun.127 Using an airborne formaldehyde provocation test, Kim et al. demonstrated that topical stimulation of the skin with formaldehyde gas for 1 hour aggravated transepidermal water loss and increased skin pH in both healthy and AD children. The observed skin barrier impairment was more prominent in the AD group compared to controls and was further disrupted with an additional 1 h of continuous exposure in a time-dependent manner at a constant dose.172
Anthropogenic climate change
Greenhouse gas emissions, especially CO2, have rapidly increased during the last 60 years due to anthropogenic effects resulting in global warming of the Earth’s atmosphere173 There is mounting scientific evidence linking climate change to the emergence and exacerbation of AR, asthma, and other chronic respiratory disorders.174 Climate change alters vegetation distribution patterns and aggravates the intensity and frequency of extreme weather events.174,175
The elevated CO2 and NO2 levels in the atmosphere enhance the allergenicity of some plants by increasing the rate of photosynthesis, pollen production and prolong the pollen season.176 The higher pollen concentrations can increase respiratory symptoms during the first year of life.177 Floods provide ideal conditions for mold growth further aggravating allergic diseases. Global warming is intensifying the severity of thunderstorms that can trigger severe asthma attacks. Notably, the Melbourne asthma outbreak in 1992 coincided with a severe thunderstorm with a surge in hospital admissions. Hydration of the pollen grains fragments it into an aerosol carrying the allergen of sufficiently small size to easily enter the lungs and aggravate respiratory allergies and asthma.178–181
Global warming and climate change have caused an increase in the extension and severity of desert dust storms, which can travel several thousand kilometers and affect the northern lands of the Saharan Desert and Arabian Peninsula. Desert dust causes an increase in mortality and morbidity due to airway diseases such as lower respiratory tract infection, COPD and asthma.182,183 Itazawa et al. showed that inhaling transborder desert dust raised the risk of developing nasal, ocular, and respiratory symptoms in infants.184 Desert dust can locally-increase PM10 levels, and together with long-term changes in maximum temperature, they impact morbidity and mortality due to respiratory diseases.182
Cigarette smoke and e-cigarettes
Inhalation of cigarette smoke disrupts the airway epithelial barrier and may contribute to the pathogenesis of chronic lung diseases such as asthma, AR and COPD.185–187 Toxic agents in cigarette smoke disrupt the epithelial barrier function by inducing oxidative stress in the airway epithelium.186 Lung tissue sections of patients with COPD and NHBECs from healthy subjects were used to demonstrate that repeated cigarette smoke exposure reduces E-cadherin and ß-catenin expression and impacts the tension of epithelial cells by increasing actin polymer levels to further destabilize cell adhesion.188 Tatsuta et al. also observed airway epithelial barrier dysfunction and enhanced permeability in Calu-3 cells treated with cigarette smoke extract in a concentration-dependent manner.185 Furthermore, cigarette smoke exposure elevated the number of inflammatory dendritic cells in the lung and compromised epithelial barrier integrity, both of which were aggravated by H1N1 infection.189 A cross-sectional study of house dust mite-sensitized AR patients demonstrated that living in urban areas and exposure to secondhand smoke was associated with a defective nasal epithelial barrier attributed to a reduced expression of occludin and claudin-7.187
Electronic cigarettes (e-cigarettes) have a nicotine delivery system and have become increasingly popular during the last decade. The aerosol generated when smoking e-cigarettes varies in composition and mainly consists of propylene glycol (PG) and vegetable glycerin (VG) as solvents, nicotine, and optional flavoring,190 all of which are potentially toxic substances.190 The adverse effect of repeated inhalation of these products on the airways has been evaluated in preclinical models. E-cigarette exposure impairs the airway epithelium, including abnormal mucus composition and epithelial barrier function, and alters the cytokine profile of lung macrophages.191 Short-term daily exposure to e-cigarette vapor weakened the human airway epithelial barrier function as demonstrated in air-liquid interface cultures of primary NHBECs, increasing its permeability and facilitating the passage of external stimuli.192 In vitro experiments where PG/VG was vaped onto the apical surface of HBECs demonstrated a reduction in glucose transport by these e-cigarette vapors.193
Micro- and nano-plastics
There are three potential routes by which MPs and NPs can enter our body, namely inhalation, ingestion, and skin contact. The potential health hazards of inhalation and ingestion of MPs and NPs are attracting attention as they are increasingly detected in the air and food products, entering both our respiratory and digestive systems, respectively. MPs are present in possibly every food, as demonstrated in honey, milk, beer, fish, table salt, drinking water, and air.15,42,194 Current safety hazard assessments of MPs and NPs on human health mostly pertain to gut and lung toxicity through mechanisms that involve oxidative stress, inflammatory reactions, and metabolic disorders.195
Atmospheric micro- and nano-plastic pollution is now a global health concern affecting both urban and suburban areas. Moreover, significant amounts of MPs have been detected in remote regions, suggesting the capability of atmospheric MPs to travel long distances.13,196 Indoor MP and NP pollution are significantly higher compared to outdoor levels, and so its effect on health warrants further investigation.196 Humans are continuously inhaling toxic MPs and NPs present in polluted air throughout their lives.42,196
Inhaled MPs and NPs are known to induce inflammation, oxidative stress, and cytotoxicity. In addition, these particles can translocate to subepithelial tissues passing through the epithelial barriers.13,197 The toxicity is dependent on the particle size, density, surface area, and its microenvironment such as pH, ionic strength, clearance capacity of the mucosa, and the amount of secreted mucin.197,198 Both MPs and NPs can reach the alveolar surface of the lungs, but additionally, NPs can enter the bloodstream by crossing the alveolar-capillary barrier.197 It has been shown that polystyrene NPs down to 50 nm can reach the lung parenchyma and lung draining lymph nodes of mice after intranasal administration.199 Additionally, polystyrene micro-and nanoparticle exposure is known to cause cytotoxicity, apoptosis, and autophagy in human bronchial and alveolar epithelial cell lines via endoplasmic reticulum stress caused by misfolded protein aggregation and mitochondrial disruption. Polystyrene particles also induce ROS and proinflammatory cytokine production such as IL-6 and IL-8.13,131,200 Moreover, polystyrene MPs and NPs damage the bronchial epithelial barrier by decreasing ZO-1 expression in a dose-dependent manner.131,200 All of the adverse events discussed above can disrupt the epithelial barrier integrity.
In vivo studies have demonstrated an inflammatory response to MPs, contributing to intestinal leakage and immunotoxicity.201 Jin et al. reported that polystyrene MPs accumulate in the gut and lead to intestinal barrier dysfunction, gut microbiota dysbiosis, and bile acid metabolism disorder in mice.202 Recently, Lee et al. reported loose glands in the colon and duodenum, visible swelling in the lamina propria, lymphocyte and plasma cell infiltration, and other inflammatory events in mice after being exposed to polyethylene MPs for five weeks. In addition, MPs decreased the percentage of CD4+ regulatory T and Th17 cells. Their results also indicated that a high concentration of MPs induced small intestinal inflammation through TLR4 signaling.203 A recent meta-regression study revealed that exposure duration, concentration, and irregular morphology are the major factors determining the cytotoxicity of MPs.204 Recently, Domenech et al. showed that although micro and nano polystyrene particles can enter and cross the intestinal barrier.205 Forte et al. suggested that the polystyrene NPs accumulated in the cytoplasm of human gastric adenocarcinoma cells and upregulated IL-6 and IL-8 genes involved in gastric pathologies.206 Mahler et al. used in vitro and in vivo intestinal loop models of the epithelium and reported that acute oral exposure to polystyrene NPs could interfere with iron transport. Chronic exposure can cause remodeling of the intestinal villi.207
Nanoparticles
Nanoparticles are considered particle matter with a diameter less than 1000 nm. With unique physical and chemical properties, nanoparticles can be tailored to a wide range of applications, including as additives in food products and in the pharmaceutical industry as drug delivery systems.208 There are increasing concerns about the toxicity of nanoparticles on the epithelial barrier as they can activate an inflammatory response in a dose-dependent manner. The generation of damage-associated molecular patterns induces a cascade of events that disrupts the gut microbiota homeostasis. Examples of potentially hazardous nanoparticles include titanium dioxide (TiO2), zinc oxide (ZnO), silica dioxide (SiO2), iron oxide (Fe3O4), silver (Ag), and cationic liposomes. Several studies have demonstrated disruption of the barrier integrity and an increase in cell leakage in Caco-2 cells following administration with TiO2 and SiO2.198,208–210 Their respiratory epithelial damaging effects were demonstrated in in vitro and in vivo studies. TiO2 nanoparticles disrupt the barrier integrity of NHBECs211 and exacerbate respiratory syncytial virus induced airway barrier damage in a mouse model.212 In an in vitro model of the intestine using co-cultured Caco-2/THP-1 cells, Kampfer et al. demonstrated the increased vulnerability of the epithelial barrier integrity toward Ag nanoparticles when exposed in its inflammatory state.213 In addition, Ag nanoparticles cause alterations on intestinal epithelial barrier permeability genes on ileal tissue explants.214
Carbon nanomaterials (CNMs) are used in agriculture to activate seed germination, growth, and fruit production. However, the potential toxicity of ingested CNMs on animals and humans raises concerns.215 Several in vivo studies have demonstrated adverse effects linked to the ingestion of CNMs.216,217 Lahiani et al. reported the cytotoxicity of multi-walled carbon nanotubes (CNT) at a high concentration of 10 µg/mL impaired intestinal epithelial integrity and altered the gene expression pattern in an in vitro model of T-84 human intestinal epithelial cells. However, no significant toxicity was observed when the T-84 human intestinal epithelial cells were exposed to extracts from fruits containing CNTs or pure CNT at the same concentration.215
Pesticides
Pesticides refer to all compounds (insecticides, herbicides, and fungicides) that are applied to destroy pests.218 Synthetic organic pesticides, one of the most commonly used pesticide types, are chemically classified as organochlorine, organophosphate, carbamate and pyrethroid pesticides.218 Although organochlorine pesticides are banned due to their high toxicity, slow degradation and bioaccumulation properties in most developed countries they are still widely used worldwide.219,220 Half the lifetime of various organochlorines can be reached 15 years and it is still can be detected in environment.218,221 Pesticides especially organochlorines can contaminate soil, water, and foods such as meat, fish, poultry, and dairy products. Humans are exposed to pesticides mostly by eating contaminated food, inhalation and skin absorption, and various studies show that pesticides especially organochlorines can be detected in different human tissues, mostly in tissues with high lipid content.222–226 Many pesticides from different chemical groups cause ROS activation on various human cell types such as human intestinal cell line, Caco-2, immortalized keratinocyte cell line, HaCaT, and human liver cancer cell line, HepG2.227–229 Organochlorine pesticides have cancerogenic and neurotoxic effects on humans. Their exposure is associated with blood pressure disorders, and endocrine, reproductive, liver and immune disfunction.219,230 Organochlorine pesticides such as dieldrin, endosulfan, heptachlor and lindane cause ROS activation in the HaCaT cell line, in addition, endosulfan and heptachlor have cytotoxic effects.228 A group of pesticides previously shown as food contaminants such as deltamethrin (pyrethroid pesticide), lambda-cyhalothrin (pyrethroid pesticide), fenitrothion (an organophosphate pesticide), fipronil (phenylpyrazole pesticide) and teflubenzuron has cytotoxic effects on Caco-2 cells. In addition, fenitrothion and teflubenzuron exposure cause increased epithelial barrier permeability on Caco-2 cells.229 Neonicotinoid insecticides which is another group of pesticides being used on large scales have been found to contaminate fruits and vegetables.231 A recent study demonstrated that a neonicotinoid pesticide imidacloprid exposure impairs intestinal epithelial barrier integrity both in vitro in Caco-2 cells and in vivo on a rat oral toxicity model. Exposure to imidacloprid in Caco-2 cells resulted in decreased expression of occludin, claudin-1 and ZO-1.232
Detergents and household cleaners
Bleach, ammonia-containing detergents, and other substances present in disinfectants (e.g., chloramine-T, quaternary ammonium compounds, and ethanolamine), irritate the lungs and can exacerbate asthma, rhinitis, or respiratory symptoms.233–235 An epidemiological study demonstrated that occupational exposure to disinfectants, including formaldehyde, glutaraldehyde, enzymatic cleaners, hypochlorite bleach, and hydrogen peroxide, was related to an increased risk of poor control of asthma in nurses.236
Inhalation of irritants can induce lung permeability and remodeling of the airway epithelium by promoting a pro-inflammatory response, oxidative stress, and neurogenic inflammation due to exposed nerve endings.236–238 Inhalation of chlorine vapors from sodium hypochlorite solutions irritates the airway and contributes to respiratory symptoms, asthma, and chronic bronchitis.234 Acute chlorine gas exposure in mice resulted in severe injury of the bronchial epithelium.239 In an ovalbumin-induced mouse model of allergic asthma, OVA-sensitized/challenged mice subjected to a low dose of chlorine vapors exhibited enhanced airway hyperresponsiveness and eosinophilic inflammation with the presence of Th2 cells, an increase in innate lymphoid cell (ILC)-2s and ILC-3s, and CD11cint monocyte-derived macrophages, compared to controls.240
Laundry detergents contain phosphates, surfactants, and various enzymes (proteases, lipases, amylases, cellulase).241 Cellulase, lipase, and amylase, derived from Bacillus licheniformis, are considered as potent allergens as proteases. The addition of surfactants and enzymes to detergents dates back to the 1960s. They are widely used in detergent washing powders and are suspected to cause occupational asthma in the detergent industry.242,243 Even at low concentrations, laundry detergents and rinse residue are cytotoxic in a dose-dependent manner and directly impair TJ barrier integrity of NHBECs while upregulating lipid metabolism and apoptosis-related genes.244
Identifying biomarkers of occupational asthma associated with exposure to cleaning products is warranted to monitor disease progression and develop a stratified treatment strategy. Potential biomarkers of exposure to alkaline products and oxidative stress in exhaled breath condensate were identified in occupational cleaners without clinical symptoms of respiratory diseases.245Laundry detergents contain phosphates, surfactants, and various enzymes (proteases, lipases, amylases, cellulase).241 Cellulase, lipase, and amylase, derived from Bacillus licheniformis, are considered as potent allergens as proteases. The addition of surfactants and enzymes to detergents dates back to the 1960s. They are widely used in detergent washing powders and are suspected to cause occupational asthma in the detergent industry.242,243 Even at low concentrations, laundry detergents and rinse residue are cytotoxic in a dose-dependent manner and directly impair TJ barrier integrity of NHBECs while upregulating lipid metabolism and apoptosis-related genes.244
Processed food: surfactants, emulsifiers and advanced glycation endproducts
The modern Western diet is characterized by its high intake of processed foods with potentially harmful ingredients that have been implicated in the development of several chronic inflammatory diseases, including inflammatory bowel disease and metabolic syndrome.246 A common feature of highly processed foods is the presence of AGEs and artificial additives such as emulsifiers. Among its many uses, emulsifiers are frequently added to food products to prevent phase separation and extend shelf-life.247
The food additives such as synthetic colorants, preservatives, stabilizers, surfactants, emulsifiers, texturizers and AGEs -due to heat processing step- work together to cause significant damage to the epithelial cells and epithelial barrier and cause microbial dysbiosis at the same time (Table 3).4,43,248 They show direct epithelial cell toxicity and change in surface tension stretch the epithelial cells and open the barriers. Anti-microbial molecules that act as preservatives increase the shelf life of packaged food but in the meantime show the same bacteria killing activity to commensals in the gastrointestinal tract.43,248,249 Emulsifiers thicken the mucosal lining fluid and trap the microbiome and cause the death of commensals. In this way, the healthy interaction of commensal bacteria with the epithelium such as healthy and physiological, metabolite, vitamin and nutrient exchange cannot take place.250 There is accumulating evidence on the health hazards of dietary emulsifiers as they can increase intestinal barrier permeability, most extensively evaluated with the synthetic emulsifiers polysorbate 80 (P80) and carboxymethylcellulose (CMC). Roberts et al. generated M-cell monolayers using a co-culture of Caco-2 and Raji B cells and reported that P80 promoted translocation of E. coli across M-cells, an event that may be implicated in the pathogenesis of Crohn's disease.251 Further in vivo studies by Zhu et al. indicate that chronic exposure to P80 decreases the expressions of mucus and TJ proteins and disturbs the intestinal epithelial cell integrity and mucosal barrier.252 Similarly, in vivo and in vitro studies have demonstrated that P80 and CMC alter the amount and thickness of mucus in rat intestinal tissue and in mucus-producing cell cultures.250 In addition, administration of these two emulsifiers altered microbiota composition to a more pro-inflammatory state, as indicated by increased levels of bioactive flagellin, and increased intestinal permeability in mice,253 and in a mucosal simulator of the human intestinal microbial ecosystem model.254 In a recent randomized controlled study, consumption of a diet containing CMC induced gut microbiota dysbiosis in humans and reduced SCFA levels in the fecal metabolome, a metabolite from bacteria known for its anti-inflammatory properties.255
Table 3.
In vitro and in vivo models of gastrointestinal system epithelial barrier disruption.
| Substance | Evidence | Reference(s) |
|---|---|---|
| Microplastics | Polystyrene MPs affect gut microbiota dysbiosis, intestinal epithelial barrier dysfunction and bile acid metabolism disorders in vivo.202 | 202 |
| Polyethylene MPs induce visible swelling in the lamina propria and increase the inflammatory response in vivo. | 203 | |
| MPs show cytotoxic effects on Caco-2 cells in vitro. | 204 | |
| Nanoplastics | Polystyrene NPs upregulate IL-6 and IL-8 genes in gastric adenocarcinoma cells in vitro.206 | 206 |
| Polystyrene NPs cause remodeling of intestinal villi in vitro and in vivo intestinal loop models.207 | 207 | |
| Nanoparticles | TiO2 nanoparticles disrupts epithelial barrier integrity of Caco-2 cells. | 209 |
| Silica nanoparticles causes oxidative stress and epithelial barrier integrity impairment on Caco-2 cells. | 210 | |
| Ag nanoparticles causes alterations on intestinal epithelial barrier permeability genes ex vivo. | 214 | |
| Carbon nanotubes are toxic and disrupt the intestinal epithelial barrier at high concentrations. | 215 | |
| Surfactant and emulsifiers | P80 induces translocation of E. coli across M-cells.251 | 251 |
| P80 and CMC alter intestinal epithelial barrier in vivo and in vitro. | 250, 252 | |
| P80 and CMC alter mice and human microbiota composition. | 253, 254 | |
| CMC modifies the microbiota composition and metabolome in humans. | 255 | |
| Heat processed foods (AGEs containing foods) | AGE-containing foods impairs the intestinal epithelial barrier integrity and cause gut dysbiosis in vivo.43 | 43 |
Ag: silver; CMC: carboxymethylcellulose; IL: interleukin; MP: microplastics, NP: nanoplastics; P80: polysorbate 80; SiO2: silicon dioxide; TiO2: titanium dioxide; ZnO: zinc oxide
The ubiquitously used thermal processing methods in the food industry induce chemical changes in the food that generate potentially toxic AGEs.256 Snelson et al. demonstrate that consuming AGE-containing foods increases the risk of chronic kidney disease, disrupting the intestinal epithelial barrier integrity and reshaping of the gut microbiota in a mouse model.43 These events drive inflammation and promote kidney injury. In a mouse model of diabetes, a high resistant starch fiber diet mitigated the inflammatory events and restored gut microbiota homeostasis.43
Contact allergens, hand sanitizers, and disinfectants
Isothiazolinones are added as preservatives to prevent the growth of microorganisms in cosmetic, household, and industrial products. In the past, isothiazolinones were also used very often in our daily life products like shampoos, cleaning detergents, and cosmetics that elicit (airborne) allergic contact dermatitis.257–259 The usage of isothiazolinones is now restricted in consumer products in Europe, but occupational allergic contact dermatitis related to isothiazolinones is still on the rise.260,261
Daily detergent use directly affects the integrity of the skin (Table 4). Enzyme detergents may pose a safety hazard due to their multienzyme formulation and surfactant properties. Surfactants can act as an adjuvant for immune response to enzymes in animal studies.265 After 72 h of stimulation, even nontoxic doses of anionic surfactants (SDS and sodium dodecylbenzene sulfonate [SDBS]), increase the paracellular flux in normal human epidermal keratinocytes in a dose-dependent manner. Moreover, disruption to the TJ barrier integrity of normal human epidermal keratinocytes was also observed through downregulation of occludin, and claudin-1 as measured by immunofluorescence staining and Western blotting.262 A recent study demonstrated that sodium dodecyl sulfate exposure causes eosinophilic inflammation and epithelial barrier damage in the esophagus in vivo.266
Table 4.
In vitro and in vivo studies of skin barrier disruption with detergents and disinfectants.
| Substance | Evidence | Reference(s) |
|---|---|---|
| Sodium dodecyl sulfate and sodium dodecyl benzene sulfonate |
Increase the paracellular flux in normal human epidermal keratinocytes with downregulation of occludin and claudin-1.262 | 263 |
| Professional cleaning detergents | High skin transepidermal water loss in cleaning professionals.263 | 265 |
| Hand sanitizers and disinfectants | Increased prevalence of hand eczema in children and health care workers during the COVID-19 pandemic.264 | 266, 267 |
COVID-19: Coronavirus disease 2019
In a prospective study, high transepidermal water loss was found in detergent-exposed skin of professional cleaners due to a disrupted skin barrier, which was associated with an increased risk of work-related hand dermatitis in occupational cleaners.263
During the COVID-19 pandemic, the WHO recommended frequent hand hygiene practices as a sanitary measure to prevent contact transmission of severe acute respiratory syndrome coronavirus 2. Unfortunately, this has also increased the prevalence of hand eczema in children and health care workers during the COVID-19 pandemic.264,267 In a randomized clinical trial, soap- or alcohol-based disinfectants were found to cause less transepidermal water loss increases than disinfectant-based wipes.268
Detection of skin barrier function
The assessment of skin barrier function is of great clinical value to monitor AD severity and responsiveness to treatment. Infantile eczema is one of the epithelial barrier-related diseases and its prevalence is increasing.269 Early prediction of infantile eczema development at the early months of the life of the babies such as the first and fourth months and taking protective measures is an important research aim in this area.270,271 We recently reported a novel method to assess the in vivo status of the epithelial barrier directly using electrical impedance spectroscopy (EIS).272,273 The skin EIS score provided a direct measurement of the epithelial barrier damage triggered by tape stripping, cholera toxin, papain, and trypsin. EIS could also distinguish the skin of healthy individuals without AD from the non-lesional skin of AD patients with precision using artificial intelligence-based models. Furthermore, EIS could assess skin lesion healing in response to treatment, correlating with the Alzheimer’s disease score, pruritus score, and inflammatory serum biomarkers. Confocal Raman spectroscopy has also been used as a noninvasive tool for skin characterization. Recently, this technique was used to elucidate the molecular mechanisms by which nonionic PEG-20 ethers reduce the lipid content of the skin and disrupt lipid organization.274
5. Conclusion
Environmental exposure to hazardous substances and their health-related effects and global warming and climate change are the two main challenges in front of humanity and nature of the world. The detrimental impact of anthropogenic influences on the environment has in turn adversely affected human health by damaging the epithelial barriers present in the respiratory tract, intestine and skin. The evidence presented in this review article points to the disruption of the epithelial barrier integrity as a key driver of microbial dysbiosis, pathological bacterial colonization, immune response to opportunistic pathogens and commensals and tissue inflammation. The inflammatory cascade of events originating from an impaired epithelial barrier is strongly implicated in the development of allergic diseases, autoimmune diseases, and metabolic disorders1,7,80 Taking an interdisciplinary holistic perspective focusing on planetary health is imperative.20 There is a current need to find novel therapeutic strategies to restore the barrier function, strengthening tissue-specific barriers, inhibiting dysbiosis and opportunistic pathogen colonization. It is essential to implement governmental policies first to fully avoid exposure and then to mitigate the cellular and molecular effects of exposure to toxic/barrier-damaging substances (Table 5). One of the main aims in this context will be to discover nontoxic substances as alternatives to toxic substances. Awareness and education of the public and political bodies is an essential in this fight. Identification of novel biomarkers for leaky barriers would facilitate personalized therapeutic interventions is also a major part of identifying barrier leaky individuals, substances and conditions. The scientific evidence presented in this review article stresses the need to raise awareness of the important role of the epithelial barrier on the pathogenesis of chronic immune-related diseases.
Table 5.
Future steps to prevent exposure to epithelial barrier damaging substances.
| (1) Dose control and avoidance |
| (2) Development of less-toxic products |
| (3) Studies on the epithelial barrier to advance our understanding of its mechanisms |
| (4) Development of novel therapeutic approaches for strengthening the mucosal and skin epithelial barrier |
| (5) Biomarker discovery for the identification of individuals with a leaky epithelial barrier |
| (6) Raising awareness (conferences, community talks, social media networks) |
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Abbreviations
- AGEs
Advanced glycation endproducts
- AR
Allergic rhinitis
- AhR
Aryl hydrocarbon receptor
- AD
Atopic dermatitis
- CO
Carbon monoxide
- CNMS
Carbon nanomaterials
- CNT
Carbon nanotubes
- CMC
Carboxymethylcellulose
- COPD
Chronic obstructive pulmonary disease
- DEP
Diesel exhaust particulates
- EIS
Electrical impedance spectroscopy
- E-cigarette
Electronic cigarettes
- FLG
Filaggrin
- ILC
Innate lymphoid cell
- IL
Interleukin
- Fe3
Iron oxide
- MP
Microplastic
- NP
Nanoplastic
- NO
Nitric oxide
- NO2
Nitrogen dioxide
- NHBECS
Normal human bronchial epithelial cells
- PM
Particulate matter
- P80
Polysorbate 80
- PG
Propylene glycol
- ROS
Reactive oxygen species
- SiO
Silica dioxide
- SDS
Sodium dodecyl sulphate
- SDBS
Sodium dodecylbenzene sulfonate
- SO2
Sulphur dioxide
- TJ
Tight junction
- Th
T helper
- TiO
Titanium dioxide
- TLR
Toll-like receptor
- VG
Vegetable glycerine
- VOCS
Volatile organic compounds
- WHO
World Health Organization
- ZnO
Zinc oxide
- ZO
Zonula occludens
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):332–360. doi: 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 2.Peng C, Yu N, Ding Y, Shi Y.. Epidemiological variations in global burden of atopic dermatitis: an analysis of trends from 1990 to 2019. Allergy. 2022;77(9):2843–2845. doi: 10.1111/all.15380. [DOI] [PubMed] [Google Scholar]
- 3.Haahtela T, Jantunen J, Saarinen K, Tommila E, Valovirta E, Vasankari T, Mäkelä MJ. Managing the allergy and asthma epidemic in 2020s-lessons from the Finnish experience. Allergy. 2022;77(8):2367–2380. doi: 10.1111/all.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celebi Sozener Z, Ozdel Ozturk B, Cerci P, Turk M, Gorgulu Akin B, Akdis M, Altiner S, Ozbey U, Ogulur I, Mitamura Y, et al. Epithelial barrier hypothesis: effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy. 2022;77(5):1418–1449. doi: 10.1111/all.15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters A, Nawrot TS, Baccarelli AA. Hallmarks of environmental insults. Cell. 2021;184(6):1455–1468. doi: 10.1016/j.cell.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet. 2018;391(10119):462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 7.Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517–1528. doi: 10.1016/j.jaci.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Alkotob SS, Cannedy C, Harter K, Movassagh H, Paudel B, Prunicki M, Sampath V, Schikowski T, Smith E, Zhao Q, et al. Advances and novel developments in environmental influences on the development of atopic diseases. Allergy. 2020;75(12):3077–3086. doi: 10.1111/all.14624. [DOI] [PubMed] [Google Scholar]
- 9.Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75(1):63–74. doi: 10.1111/all.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol. 2020;54(5):2575–2584. doi: 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- 11.EEB . The great detox – largest ever ban of toxic chemicals announced by EU. European Environmental Bureau. Published 2022. Accessed 05.july.2022, 2022.
- 12.Karlsson O, Rocklöv J, Lehoux AP, Bergquist J, Rutgersson A, Blunt MJ, Birnbaum LS. The human exposome and health in the anthropocene. Int J Epidemiol. 2021;50(2):378–389. doi: 10.1093/ije/dyaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol. 2019;53(4):1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- 14.Collaborators GRF. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kik K, Bukowska B, Sicińska P. Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262:114297. doi: 10.1016/j.envpol.2020.114297. [DOI] [PubMed] [Google Scholar]
- 16.Prata JC. Airborne microplastics: consequences to human health? Environ Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Marcantonio R, Javeline D, Field S, Fuentes A. Global distribution and coincidence of pollution, climate impacts, and health risk in the anthropocene. PLoS One. 2021;16(7):e0254060. doi: 10.1371/journal.pone.0254060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines A, Ebi K, Solomon CG. The imperative for climate action to protect health. N Engl J Med. 2019;380(3):263–273. doi: 10.1056/NEJMra1807873. [DOI] [PubMed] [Google Scholar]
- 19.Xu R, Yu P, Abramson MJ, Johnston FH, Samet JM, Bell ML, Haines A, Ebi KL, Li S, Guo Y, et al. Wildfires, global climate change, and human health. N Engl J Med. 2020;383(22):2173–2181. doi: 10.1056/NEJMsr2028985. [DOI] [PubMed] [Google Scholar]
- 20.Prescott SL, Logan AC, Bristow J, Rozzi R, Moodie R, Redvers N, Haahtela T, Warber S, Poland B, Hancock T. Exiting the Anthropocene: achieving personal and planetary health in the 21st century. Allergy. 2022. doi: 10.1111/all.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WWF . Living planet report - 2018: aiming higher. In: Grooten M, Almond REA editors. Gland (Switzerland): WWF; 2018. [Google Scholar]
- 22.Kim KN, Hong YC. The exposome and the future of epidemiology: a vision and prospect. Environ Health Toxicol. 2017;32:e2017009. doi: 10.5620/eht.e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agache I, Miller R, Gern JE, Hellings PW, Jutel M, Muraro A, Phipatanakul W, Quirce S, Peden D. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a practall document. Allergy. 2019;74(3):449–463. doi: 10.1111/all.13690. [DOI] [PubMed] [Google Scholar]
- 24.Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21(1):5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 25.Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tandon A, Kanchan T, Tandon A. The poison we breathe. Lancet. 2020;395(10221):e18. doi: 10.1016/S0140-6736(19)32547-4. [DOI] [PubMed] [Google Scholar]
- 28.WHO . Global ambient air quality database. https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/ambient-air-pollution. Accessed 28.11.21, 2021.
- 29.Diao P, He H, Tang J, Xiong L, Li L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed Pharmacother. 2021;138:111534. doi: 10.1016/j.biopha.2021.111534. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Li C, Tang X. The impact of PM(2.5) on the host defense of respiratory system. Front Cell Dev Biol. 2020;8:91. doi: 10.3389/fcell.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goshua A, Akdis C, Nadeau KC. World Health Organization global air quality guideline recommendations: executive summary. Allergy. 2022;77(7):1955–1960. doi: 10.1111/all.15224. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 33.Luo X, Hong H, Lu Y, Deng S, Wu N, Zhou Q, Chen Z, Feng P, Zhou Y, Tao J, et al. Impact of air pollution and meteorological factors on incidence of allergic rhinitis: a low-latitude multi-city study in China. Allergy. 2022. doi: 10.1111/all.15469. [DOI] [PubMed] [Google Scholar]
- 34.Cao S, Zhang S, Gao C, Yan Y, Bao J, Su L, Liu M, Peng N, Liu M. A long-term analysis of atmospheric black carbon MERRA-2 concentration over China during 1980–2019. Atmospheric Environment. 2021;264:118662. doi: 10.1016/j.atmosenv.2021.118662. [DOI] [Google Scholar]
- 35.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, Coelho MSZS, Saldiva PHN, Lavigne E, Matus P, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381(8):705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environment International. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Kim WH, Kim YY, Park HY. Air pollution and central nervous system disease: a review of the impact of fine particulate matter on neurological disorders. Front Public Health. 2020;8:575330. doi: 10.3389/fpubh.2020.575330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam MG, Tran PTM, Balasubramanian R. Air quality changes in cities during the COVID-19 lockdown: a critical review. Atmos Res. 2021;264:105823. doi: 10.1016/j.atmosres.2021.105823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Martín J, Kraakman NJR, Pérez C, Lebrero R, Muñoz R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere. 2021;262:128376. doi: 10.1016/j.chemosphere.2020.128376. [DOI] [PubMed] [Google Scholar]
- 40.WHO . Tobacco 2021 Available at: https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed 31.10, 2021.
- 41.Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Communications. 2019;5(1):6. doi: 10.1057/s41599-018-0212-7. [DOI] [Google Scholar]
- 42.Zhang Q, Xu EG, Li J, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54(7):3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- 43.Snelson M, Tan SM, Clarke RE, et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci Adv. 2021;7(14). doi: 10.1126/sciadv.abe4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane MM, Davis JA, Beattie S, Gómez‐Donoso C, Loughman A, O’Neil A, Jacka F, Berk M, Page R, Marx W, et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021;22(3):e13146. doi: 10.1111/obr.13146. [DOI] [PubMed] [Google Scholar]
- 45.Arenas-Jal M, Suñé-Negre JM, Pérez-Lozano P, García-Montoya E. Trends in the food and sports nutrition industry: a review. Crit Rev Food Sci Nutr. 2020;60(14):2405–2421. doi: 10.1080/10408398.2019.1643287. [DOI] [PubMed] [Google Scholar]
- 46.Sproesser G, Ruby MB, Arbit N, Akotia CS, Alvarenga MDS, Bhangaokar R, Furumitsu I, Hu X, Imada S, Kaptan G, et al. Understanding traditional and modern eating: the TEP10 framework. BMC Public Health. 2019;19(1):1606. doi: 10.1186/s12889-019-7844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agache I, Sampath V, Aguilera J, et al. Climate change and global health: a call to more research and more action. Allergy. 2022;77(5):1389–1407. doi: 10.1111/all.15229. [DOI] [PubMed] [Google Scholar]
- 48.D’Amato G, Akdis CA. Global warming, climate change, air pollution and allergies. Allergy. 2020;75(9):2158–2160. doi: 10.1111/all.14527. [DOI] [PubMed] [Google Scholar]
- 49.Atwoli L, Baqui AH, Benfield T, Bosurgi R, Godlee F, Hancocks S, Horton R, Laybourn‐Langton L, Monteiro CA, Norman I, et al. Call for emergency action to limit global temperature increases, restore biodiversity and protect health: wealthy nations must do much more, much faster: wealthy nations must do much more, much faster. Allergy. 2022;77(3):730–733. doi: 10.1111/all.15059. [DOI] [PubMed] [Google Scholar]
- 50.Meinshausen M, Lewis J, McGlade C, et al. Realization of Paris Agreement pledges may limit warming just below 2°C. Nature. 2022;604(7905):304–309. [DOI] [PubMed] [Google Scholar]
- 51.Nadeau KC, Agache I, Jutel M, et al. Climate change: a call to action for the united nations. Allergy. 2022;77(4):1087–1090. [DOI] [PubMed] [Google Scholar]
- 52.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773–786.e771–777. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 54.Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128(3):549–556.e541–512. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 55.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130(5):1087–1096.e1010. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 56.Steelant B, Farré R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137(4):1043–1053. e1045. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 57.Toedter G, Li K, Sague S, Ma K, Marano C, Macoritto M, Park J, Deehan R, Matthews A, Wu GD, et al. Genes associated with intestinal permeability in ulcerative colitis: changes in expression following infliximab therapy. Inflamm Bowel Dis. 2012;18(8):1399–1410. doi: 10.1002/ibd.22853. [DOI] [PubMed] [Google Scholar]
- 58.Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Lucianò R, Rocchi M, Ferrarese R, Sanvito F, Canducci F, et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci U S A. 2019;116(30):15140–15149. doi: 10.1073/pnas.1814558116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590(3):441–446. doi: 10.1113/jphysiol.2011.222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995. doi: 10.1038/s41467-020-15831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciccia F, Guggino G, Rizzo A, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–1132. doi: 10.1136/annrheumdis-2016-210000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdelhamid L, Luo XM. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients. 2018;10(8):1016. doi: 10.3390/nu10081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiorentino M, Sapone A, Senger S, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7(1):49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136(3):345–361. doi: 10.1007/s00401-018-1856-5. [DOI] [PubMed] [Google Scholar]
- 67.Köhler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt K, Carvalho A. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in alzheimer’s disease. Curr Pharm Des. 2016;22(40):6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 68.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukuoka A, Yoshimoto T. Barrier dysfunction in the nasal allergy. Allergol Int. 2018;67(1):18–23. doi: 10.1016/j.alit.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Adil MS, Narayanan SP, Somanath PR. Cell-cell junctions: structure and regulation in physiology and pathology. Tissue Barriers. 2021;9(1):1848212. doi: 10.1080/21688370.2020.1848212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 72.Riethmuller C, McAleer MA, Koppes SA, Abdayem R, Franz J, Haftek M, Campbell LE, MacCallum SF, McLean WHI, Irvine AD, et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol. 2015;136(6):1573–1580.e1572. doi: 10.1016/j.jaci.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270(30):17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 74.Heijink IH, Kuchibhotla VNS, Roffel MP, Maes T, Knight DA, Sayers I, Nawijn MC. Epithelial cell dysfunction, a major driver of asthma development. Allergy. 2020;75(8):1902–1917. doi: 10.1111/all.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Constant DA, Nice TJ, Rauch I. Innate immune sensing by epithelial barriers. Curr Opin Immunol. 2021;73:1–8. doi: 10.1016/j.coi.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pat Y, Ogulur I. The epithelial barrier hypothesis: a 20-year journey. Allergy. 2021;76(11):3560–3562. doi: 10.1111/all.14899. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microbial Cell Factories. 2020;19(1):23. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, Marasco G, Stanghellini V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8:718356. doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11(5):1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitamura Y, Ogulur I, Pat Y, Rinaldi AO, Ardicli O, Cevhertas L, Brüggen M-C, Traidl‐Hoffmann C, Akdis M, Akdis CA, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85(6):615–626. doi: 10.1111/cod.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh LF, Ong RY, Common JE. Skin microbiome of atopic dermatitis. Allergol Int. 2021;71(1):31–39. doi: 10.1016/j.alit.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L. Microbiome and asthma. Asthma Res Pract. 2018;4(1):1. doi: 10.1186/s40733-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22(5):1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy. 2018;73(12):2314–2327. doi: 10.1111/all.13634. [DOI] [PubMed] [Google Scholar]
- 85.Lee JJ, Kim SH, Lee MJ, Kim B-K, Song W-J, Park H-W, Cho S-H, Hong S-J, Chang Y-S, Kim B-S, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy. 2019;74(4):709–719. doi: 10.1111/all.13608. [DOI] [PubMed] [Google Scholar]
- 86.Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, Sandel M, Bacharier LB, Zeiger R, Sodergren E, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy. 2018;73(1):145–152. doi: 10.1111/all.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fazlollahi M, Chun Y, Grishin A, Wood RA, Burks AW, Dawson P, Jones SM, Leung DYM, Sampson HA, Sicherer SH, et al. Early-life gut microbiome and egg allergy. Allergy. 2018;73(7):1515–1524. doi: 10.1111/all.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: how early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103–2115. doi: 10.1111/all.13812. [DOI] [PubMed] [Google Scholar]
- 89.Trautmann A, Kruger K, Akdis M, Müller-Wening D, Akkaya A, Bröcker E-B, Blaser K, Akdis CA. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005;138(2):142–150. doi: 10.1159/000088436. [DOI] [PubMed] [Google Scholar]
- 90.de Boer Wi, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ, de Boer WI. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86(3):105–112. doi: 10.1139/Y08-004. [DOI] [PubMed] [Google Scholar]
- 91.Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 92.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 93.Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, Kubo T, Wawrzyniak P, Rückert B, Sudo K, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300–310.e311. doi: 10.1016/j.jaci.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 94.Tan HT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, Eljaszewicz A, Moniuszko M, Akdis M, Akdis CA, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2019;74(2):294–307. doi: 10.1111/all.13619. [DOI] [PubMed] [Google Scholar]
- 95.Ms J W, Ms X K, Ms ZQ H, Shen, MS L, Luo, MD Q, Li, MS M-Y, Luo, MS L-P, Tu, MS J-H, Han, MS M, Ye J, et al. Protease-activated receptor-2 decreased zonula occlidens-1 and claudin-1 expression and induced epithelial barrier dysfunction in allergic rhinitis. Am J Rhinol Allergy. 2021;35(1):26–35. doi: 10.1177/1945892420932486. [DOI] [PubMed] [Google Scholar]
- 96.Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018;67(1):3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Méchin M-C, Hansmann B, Rodriguez E, Weindinger S, Schmitt A-M, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131(4):1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 98.Gao W, Gong J, Mu M, Zhu Y, Wang W, Chen W, Han G, Hu H, Bao P. The pathogenesis of eosinophilic asthma: a positive feedback mechanism that promotes th2 immune response. Front Immunol. 2021;12:672312. doi: 10.3389/fimmu.2021.672312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meisser SS, Altunbulakli C, Bandier J, Opstrup MS, Castro-Giner F, Akdis M, Bonefeld CM, Johansen JD, Akdis CA. Skin barrier damage after exposure to paraphenylenediamine. J Allergy Clin Immunol. 2020;145(2):619–631.e612. doi: 10.1016/j.jaci.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 100.Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity. 2019;50(4):975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 101.Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman‐Yassky E, Kabashima K, Mitamura Y, Vian L, Wu J, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. doi: 10.1111/all.14318. [DOI] [PubMed] [Google Scholar]
- 102.Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med (Lausanne). 2014;1:24. doi: 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke J-D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3(1–2):e977176. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schoultz I, Keita Å. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells. 2019;8(2):193. doi: 10.3390/cells8020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vanuytsel T, Tack J, Farre R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front Nutr. 2021;8:717925. doi: 10.3389/fnut.2021.717925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greuter T, Straumann A, Fernandez-Marrero Y, Germic N, Hosseini A, Yousefi S, Simon D, Collins MH, Bussmann C, Chehade M, et al. Characterization of eosinophilic esophagitis variants by clinical, histological and molecular analyses: a cross-sectional multi-center study. Allergy. 2022;77(8):2520–2533. doi: 10.1111/all.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simon D, Page B, Vogel M, Bussmann C, Blanchard C, Straumann A, Simon H-U. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid-treated eosinophilic esophagitis. Allergy. 2018;73(1):239–247. doi: 10.1111/all.13244. [DOI] [PubMed] [Google Scholar]
- 109.Masterson JC, Biette KA, Hammer JA, Nguyen N, Capocelli KE, Saeedi BJ, Harris RF, Fernando SD, Hosford LB, Kelly CJ, et al. Epithelial HIF-1α/claudin-1 axis regulates barrier dysfunction in eosinophilic esophagitis. J Clin Invest. 2019;129(8):3224–3235. doi: 10.1172/JCI126744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, Marchi N, van Boxel-dezaire AHH. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. 2018;507(1–4):274–279. doi: 10.1016/j.bbrc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 112.Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human type 2 diabetes. Diabet Med. 2014;31(5):559–563. doi: 10.1111/dme.12360. [DOI] [PubMed] [Google Scholar]
- 113.Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? The Journal of Nutritional Biochemistry. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 114.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 115.Wilbrink J, Bernards N, Mujagic Z, van Avesaat M, Pijls K, Klaassen T, van Eijk H, Nienhuijs S, Stronkhorst A, Wilms E, et al. Intestinal barrier function in morbid obesity: results of a prospective study on the effect of sleeve gastrectomy. International Journal of Obesity. 2020;44(2):368–376. doi: 10.1038/s41366-019-0492-z. [DOI] [PubMed] [Google Scholar]
- 116.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 117.Lin R, Zhou L, Zhang J, Wang B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int J Clin Exp Pathol. 2015;8:5153–5160. [PMC free article] [PubMed] [Google Scholar]
- 118.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 119.Wang HY, Chi C, Xu YQ, Wang C, Wang TY, Lv D, Li X. Occludin endocytosis is involved in the disruption of the intestinal epithelial barrier in a mouse model of alcoholic steatohepatitis. Journal of Digestive Diseases. 2019;20(9):476–485. doi: 10.1111/1751-2980.12800. [DOI] [PubMed] [Google Scholar]
- 120.van Ijzendoorn, van Iscd P, van Ijzendoorn SCD. The Intestinal Barrier in Parkinson’s Disease: current State of Knowledge. J Parkinsons Dis. 2019;9(s2):S323–s329. doi: 10.3233/JPD-191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu S, Yi J, Zhang YG, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;3(4):e12356. doi: 10.14814/phy2.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelly JR, Borre Y, OB C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 123.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 124.Galán C, Thibaudon M. Climate change, airborne pollen, and pollution. Allergy. 2020;75(9):2354–2356. doi: 10.1111/all.14538. [DOI] [PubMed] [Google Scholar]
- 125.Zhao C, Wang Y, Su Z, Pu W, Niu M, Song S, Wei L, Ding Y, Xu L, Tian M, et al. Respiratory exposure to PM2.5 soluble extract disrupts mucosal barrier function and promotes the development of experimental asthma. Sci Total Environ. 2020;730:139145. doi: 10.1016/j.scitotenv.2020.139145. [DOI] [PubMed] [Google Scholar]
- 126.Michaudel C, Mackowiak C, Maillet I, Fauconnier L, Akdis CA, Sokolowska M, Dreher A, Tan HTT, Quesniaux VF, Ryffel B, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol. 2018;142(3):942–958. doi: 10.1016/j.jaci.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 127.Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest. 2019;129(4):1504–1515. doi: 10.1172/JCI124612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sokolowska M, Quesniaux VFJ, Akdis CA, Chung KF, Ryffel B, Togbe D. Acute respiratory barrier disruption by ozone exposure in mice. Front Immunol. 2019;10:2169. doi: 10.3389/fimmu.2019.02169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu T, Zhang X, Chen X, et al. Nasal DNA methylation differentiates severe from nonsevere asthma in African American children. Allergy. 2021; 76(6):1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, Ma J, Zhou Y, Zhang L. Short-term exposure to ambient air pollution and asthma mortality. Am J Respir Crit Care Med. 2019;200(1):24–32. doi: 10.1164/rccm.201810-1823OC. [DOI] [PubMed] [Google Scholar]
- 131.Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575. doi: 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- 132.Xing Y, Wang MH, Leung T-F, Wong C-K, Roponen M, Schaub B, Li J, Wong GWK. Poultry exposure and environmental protection against asthma in rural children. Allergy. 2022;77(10):2949–2960. doi: 10.1111/all.15365. [DOI] [PubMed] [Google Scholar]
- 133.Cavaleiro Rufo J, Paciência I, Hoffimann E, Moreira A, Barros H, Ribeiro AI. The neighbourhood natural environment is associated with asthma in children: a birth cohort study. Allergy. 2021;76(1):348–358. doi: 10.1111/all.14493. [DOI] [PubMed] [Google Scholar]
- 134.Xue Y, Chu J, Li Y, Kong X. The influence of air pollution on respiratory microbiome: a link to respiratory disease. Toxicol Lett. 2020;334:14–20. doi: 10.1016/j.toxlet.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 135.Kim J, Kim YC, Ham J, Sohn K-H, Lee S-Y, Chung DH, Cho S-H, Kang HR, Kim HY. The effect of air pollutants on airway innate immune cells in patients with asthma. Allergy. 2020;75(9):2372–2376. doi: 10.1111/all.14323. [DOI] [PubMed] [Google Scholar]
- 136.Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129(1):14–24; quiz 25–16. doi: 10.1016/j.jaci.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Daellenbach KR, Uzu G, Jiang J, Cassagnes L-E, Leni Z, Vlachou A, Stefenelli G, Canonaco F, Weber S, Segers A, et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature. 2020;587(7834):414–419. doi: 10.1038/s41586-020-2902-8. [DOI] [PubMed] [Google Scholar]
- 138.Dagher Z, Garçon G, Billet S, Gosset P, Ledoux F, Courcot D, Aboukais A, Shirali P. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225(1):12–24. doi: 10.1016/j.tox.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 139.Wang G, Zhang X, Liu X, Zheng J, Chen R, Kan H. Ambient fine particulate matter induce toxicity in lung epithelial-endothelial co-culture models. Toxicol Lett. 2019;301:133–145. doi: 10.1016/j.toxlet.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 140.Han X, Zhuang Y. PM2.5 induces autophagy-mediated cell apoptosis via PI3K/AKT/mTOR signaling pathway in mice bronchial epithelium cells. Exp Ther Med. 2021;21(1):1. doi: 10.3892/etm.2020.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang L, He X, Xiong Y, et al. Transcriptome-wide profiling discover: PM2.5 aggravates airway dysfunction through epithelial barrier damage regulated by Stanniocalcin 2 in an OVA-induced model. Ecotoxicol Environ Saf. 2021;220:112408. doi: 10.1016/j.ecoenv.2021.112408. [DOI] [PubMed] [Google Scholar]
- 142.Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46(1):142–152. doi: 10.1111/cea.12597. [DOI] [PubMed] [Google Scholar]
- 143.Xian M, Ma S, Wang K, et al. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol Res. 2020;12(1):56–71. doi: 10.4168/aair.2020.12.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park SK, Yeon SH, Choi M-R, et al. Urban particulate matters may affect endoplasmic reticulum stress and tight junction disruption in nasal epithelial cells. Am J Rhinol Allergy. 2021;35(6):817–829. doi: 10.1177/19458924211004006. [DOI] [PubMed] [Google Scholar]
- 145.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 146.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 147.Stapleton EM, Kizhakke Puliyakote A, Metwali N, Jeronimo M, Thornell IM, Manges RB, Bilas M, Kamal Batcha MA, Kumaravel MS, Durairaj K, et al. Lung function of primary cooks using LPG or biomass and the effect of particulate matter on airway epithelial barrier integrity. Environ Res. 2020;189:109888. doi: 10.1016/j.envres.2020.109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340–349. doi: 10.1111/j.1440-1843.2010.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hüls A, Abramson MJ, Sugiri D, Fuks K, Krämer U, Krutmann J, Schikowski T. Nonatopic eczema in elderly women: effect of air pollution and genes. J Allergy Clin Immunol. 2019;143(1):378–385.e379. doi: 10.1016/j.jaci.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 150.Yao TC, Huang HY, Pan WC, Wu C-Y, Tsai S-Y, Hung C-Y, Lu K-L, Chang‐Chien J, Tseng C-L, Wu C-D, et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy. 2021;76(7):2241–2245. doi: 10.1111/all.14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weng CM, Wang CH, Lee MJ, He J-R, Huang H-Y, Chao M-W, Chung KF, Kuo H-P. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy. 2018;73(11):2192–2204. doi: 10.1111/all.13462. [DOI] [PubMed] [Google Scholar]
- 152.Brandt EB, Bolcas PE, Ruff BP, Khurana Hershey GK. IL33 contributes to diesel pollution-mediated increase in experimental asthma severity. Allergy. 2020;75(9):2254–2266. doi: 10.1111/all.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J Allergy Clin Immunol. 1995;95(1 Pt 1):103–115. doi: 10.1016/S0091-6749(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 154.Van Den Broucke S, Vanoirbeek J, Alfaro-Moreno E, Hoet P. Contribution of mast cells in irritant-induced airway epithelial barrier impairment in vitro. Toxicol Ind Health. 2020;36(10):823–834. doi: 10.1177/0748233720948771. [DOI] [PubMed] [Google Scholar]
- 155.Smyth T, Veazey J, Eliseeva S, Chalupa D, Elder A, Georas SN. Diesel exhaust particle exposure reduces expression of the epithelial tight junction protein Tricellulin. Particle and Fibre Toxicology. 2020;17(1):52. doi: 10.1186/s12989-020-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim N, Han DH, Suh M-W, Lee JH, Oh S-H, Park MK. Effect of lipopolysaccharide on diesel exhaust particle-induced junctional dysfunction in primary human nasal epithelial cells. Environ Pollut. 2019;248:736–742. doi: 10.1016/j.envpol.2019.02.082. [DOI] [PubMed] [Google Scholar]
- 157.Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Fujimura T, Aiba S, Nakayama K, Okuyama R, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18(1):64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 158.Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem. 2002;234-235(1–2):71–80. doi: 10.1023/A:1015973530559. [DOI] [PubMed] [Google Scholar]
- 159.Michaudel C, Fauconnier L, Julé Y, Ryffel B. Functional and morphological differences of the lung upon acute and chronic ozone exposure in mice. Sci Rep. 2018;8(1):10611. doi: 10.1038/s41598-018-28261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sunyer J, Basagaña X, Belmonte J, Antó JM. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax. 2002;57(8):687–693. doi: 10.1136/thorax.57.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Achakulwisut P, Brauer M, Hystad P, Anenberg SC. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: estimates from global datasets. The Lancet Planetary Health. 2019;3(4):e166–e178. doi: 10.1016/S2542-5196(19)30046-4. [DOI] [PubMed] [Google Scholar]
- 162.Lopez DJ, Lodge CJ, Bui DS, et al. Association between ambient air pollution and development and persistence of atopic and non-atopic eczema in a cohort of adults. Allergy. 2021;76(8):2524–2534. doi: 10.1111/all.14783. [DOI] [PubMed] [Google Scholar]
- 163.Joelsson JP, Kricker JA, Arason AJ, et al. Azithromycin ameliorates sulfur dioxide-induced airway epithelial damage and inflammatory responses. Respir Res. 2020;21(1):233. doi: 10.1186/s12931-020-01489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uysal N, Schapira RM. Effects of ozone on lung function and lung diseases. Curr Opin Pulm Med. 2003;9(2):144–150. doi: 10.1097/00063198-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 165.Kim BG, Lee PH, Lee SH, Park CS, Jang AS. Impact of ozone on claudins and tight junctions in the lungs. Environ Toxicol. 2018;33(7):798–806. doi: 10.1002/tox.22566. [DOI] [PubMed] [Google Scholar]
- 166.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol. 2011. 2011;111(3):679–687. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smyth T, Georas SN. Effects of ozone and particulate matter on airway epithelial barrier structure and function: a review of in vitro and in vivo studies. Inhal Toxicol. 2021;33(5):177–192. doi: 10.1080/08958378.2021.1956021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Majumder N, Goldsmith WT, Kodali VK, Velayutham M, Friend SA, Khramtsov VV, Nurkiewicz TR, Erdely A, Zeidler-Erdely PC, Castranova V, et al. Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure. Redox Biology. 2021;46:102092. doi: 10.1016/j.redox.2021.102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He L, Cui X, Li Z, et al. Malondialdehyde in nasal fluid: a biomarker for monitoring asthma control in relation to air pollution exposure. Environmental Science & Technology. 2020;54(18):11405–11413. [DOI] [PubMed] [Google Scholar]
- 170.Hendricks AJ, Eichenfield LF, Shi VY. The impact of airborne pollution on atopic dermatitis: a literature review. Br J Dermatol. 2020;183(1):16–23. doi: 10.1111/bjd.18781. [DOI] [PubMed] [Google Scholar]
- 171.Farraia M, Cavaleiro Rufo J, Paciencia I, et al. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy. 2020;75(7):1630–1639. doi: 10.1111/all.14207. [DOI] [PubMed] [Google Scholar]
- 172.Kim J, Han Y, Ahn JH, et al. Airborne formaldehyde causes skin barrier dysfunction in atopic dermatitis. Br J Dermatol. 2016;175(2):357–363. doi: 10.1111/bjd.14357. [DOI] [PubMed] [Google Scholar]
- 173.D’Amato G, Chong-Neto HJ, Monge Ortega OP, Vitale C, Ansotegui I, Rosario N, Haahtela T, Galan C, Pawankar R, Murrieta‐Aguttes M, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy. 2020;75(9):2219–2228. doi: 10.1111/all.14476. [DOI] [PubMed] [Google Scholar]
- 174.Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJH, Fuertes E, Comberiati P, Cai YS, Tomazic PV, Diamant Z, Vestbo J, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75(9):2170–2184. doi: 10.1111/all.14177. [DOI] [PubMed] [Google Scholar]
- 175.Cecchi L, D’Amato G, Annesi-Maesano I. Climate change and outdoor aeroallergens related to allergy and asthma: taking the exposome into account. Allergy. 2020;75(9):2361–2363. doi: 10.1111/all.14286. [DOI] [PubMed] [Google Scholar]
- 176.Rauer D, Gilles S, Wimmer M, Frank U, Mueller C, Musiol S, Vafadari B, Aglas L, Ferreira F, Schmitt‐Kopplin P, et al. Ragweed plants grown under elevated CO 2 levels produce pollen which elicit stronger allergic lung inflammation. Allergy. 2021;76(6):1718–1730. doi: 10.1111/all.14618. [DOI] [PubMed] [Google Scholar]
- 177.Gisler A, Eeftens M, de Hoogh K, Vienneau D, Salem Y, Yammine S, Jakob J, Gorlanova O, Decrue F, Gehrig R, et al. Pollen exposure is associated with risk of respiratory symptoms during the first year of life. Allergy. 2022. doi: 10.1111/all.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D’Amato G, Liccardi G, Frenguelli G. Thunderstorm-asthma and pollen allergy. Allergy. 2007;62(1):11–16. doi: 10.1111/j.1398-9995.2006.01271.x. [DOI] [PubMed] [Google Scholar]
- 179.Hew M, Lee J, Susanto NH, Prasad S, Bardin PG, Barnes S, Ruane L, Southcott AM, Gillman A, Young A, et al. The 2016 Melbourne thunderstorm asthma epidemic: risk factors for severe attacks requiring hospital admission. Allergy. 2019;74(1):122–130. doi: 10.1111/all.13609. [DOI] [PubMed] [Google Scholar]
- 180.Hew M, Lee J, Varese N, Aui PM, McKenzie CI, Wines BD, Aumann H, Rolland JM, Mark Hogarth P, Zelm MC, et al. Epidemic thunderstorm asthma susceptibility from sensitization to ryegrass (Lolium perenne) pollen and major allergen Lol p 5. Allergy. 2020;75(9):2369–2372. doi: 10.1111/all.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.D’Amato G, Annesi-Maesano I, Cecchi L, D’Amato M. Latest news on relationship between thunderstorms and respiratory allergy, severe asthma, and deaths for asthma. Allergy. 2019;74(1):9–11. doi: 10.1111/all.13616. [DOI] [PubMed] [Google Scholar]
- 182.Boğan M, Kul S, Al B, Oktay MM, Akpinar Elçi M, Pinkerton KE, Bayram H. Effect of desert dust storms and meteorological factors on respiratory diseases. Allergy. 2022;77(7):2243–2246. doi: 10.1111/all.15298. [DOI] [PubMed] [Google Scholar]
- 183.D’Amato G, Akdis CA. Desert dust and respiratory diseases: further insight on the epithelial barrier hypothesis. Allergy. 2022. doi: 10.1111/all.15392. [DOI] [PubMed] [Google Scholar]
- 184.Itazawa T, Kanatani KT, Hamazaki K, Inadera H, Tsuchida A, Tanaka T, Nakayama T, Go T, Onishi K, Kurozawa Y, et al. The impact of exposure to desert dust on infants’ symptoms and countermeasures to reduce the effects. Allergy. 2020;75(6):1435–1445. doi: 10.1111/all.14166. [DOI] [PubMed] [Google Scholar]
- 185.Tatsuta M, Kan-O K, Ishii Y, Yamamoto N, Ogawa T, Fukuyama S, Ogawa A, Fujita A, Nakanishi Y, Matsumoto K, et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir Res. 2019;20(1):251. doi: 10.1186/s12931-019-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58(2):157–169. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- 187.Nur Husna SM, Siti Sarah CO, Tan HT, Md Shukri N, Mohd Ashari NS, Wong KK. Reduced occludin and claudin-7 expression is associated with urban locations and exposure to second-hand smoke in allergic rhinitis patients. Sci Rep. 2021;11(1):1245. doi: 10.1038/s41598-020-79208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nishida K, Brune KA, Putcha N, Mandke P, O’Neal WK, Shade D, Srivastava V, Wang M, Lam H, An SS, et al. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am J Physiol Lung Cell Mol Physiol. 2017;313(3):L581–L591. doi: 10.1152/ajplung.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Danov O, Wolff M, Bartel S, Böhlen S, Obernolte H, Wronski S, Jonigk D, Hammer B, Kovacevic D, Reuter S, et al. Cigarette smoke affects dendritic cell populations, epithelial barrier function, and the immune response to viral infection with H1N1. Front Med (Lausanne). 2020;7:571003. doi: 10.3389/fmed.2020.571003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee YJ, Na CJ, Botao L, Kim KH, Son YS. Quantitative insights into major constituents contained in or released by electronic cigarettes: propylene glycol, vegetable glycerin, and nicotine. Sci Total Environ. 2020;703:134567. doi: 10.1016/j.scitotenv.2019.134567. [DOI] [PubMed] [Google Scholar]
- 191.Kalininskiy A, Kittel J, Nacca NE, Misra RS, Croft DP, McGraw MD. E-cigarette exposures, respiratory tract infections, and impaired innate immunity: a narrative review. Pediatr Med. 2021;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R834–R847. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Woodall M, Jacob J, Kalsi KK, Schroeder V, Davis E, Kenyon B, Khan I, Garnett JP, Tarran R, Baines DL, et al. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2020;319(6):L957–L967. doi: 10.1152/ajplung.00123.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fournier E, Etienne-Mesmin L, Grootaert C, Jelsbak L, Syberg K, Blanquet-Diot S, Mercier-Bonin M. Microplastics in the human digestive environment: a focus on the potential and challenges facing in vitro gut model development. J Hazard Mater. 2021;415:125632. doi: 10.1016/j.jhazmat.2021.125632. [DOI] [PubMed] [Google Scholar]
- 195.Yee MS, Hii LW, Looi CK, Lim W-M, Wong S-F, Kok -Y-Y, Tan B-K, Wong C-Y, Leong C-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials (Basel). 2021;11(2):496. doi: 10.3390/nano11020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Y, Kang S, Allen S, Allen D, Gao T, Sillanpää M. Atmospheric microplastics: a review on current status and perspectives. Earth-Science Reviews. 2020;203:103118. doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- 197.Facciolà A, Visalli G, Pruiti Ciarello M, Di Pietro A. Newly emerging airborne pollutants: current knowledge of health impact of micro and nanoplastics. Int J Environ Res Public Health. 2021;18(6):2997. doi: 10.3390/ijerph18062997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Das Neves J, Sverdlov Arzi R, Sosnik A. Molecular and cellular cues governing nanomaterial-mucosae interactions: from nanomedicine to nanotoxicology. Chem Soc Rev. 2020;49(14):5058–5100. doi: 10.1039/c8cs00948a. [DOI] [PubMed] [Google Scholar]
- 199.Blank F, Stumbles PA, Seydoux E, Holt PG, Fink A, Rothen-Rutishauser B, Strickland DH, von Garnier C. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am J Respir Cell Mol Biol. 2013;49(1):67–77. doi: 10.1165/rcmb.2012-0387OC. [DOI] [PubMed] [Google Scholar]
- 200.Yang S, Cheng Y, Chen Z, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicology and Environmental Safety. 2021;226:112837. doi: 10.1016/j.ecoenv.2021.112837. [DOI] [PubMed] [Google Scholar]
- 201.Huang Z, Weng Y, Shen Q, Zhao Y, Jin Y. Microplastic: a potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci Total Environ. 2021;785:147365. doi: 10.1016/j.scitotenv.2021.147365. [DOI] [PubMed] [Google Scholar]
- 202.Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 203.Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji X, Zhang Y, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/j.chemosphere.2019.125492. [DOI] [PubMed] [Google Scholar]
- 204.Danopoulos E, Twiddy M, West R, Rotchell JM. A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells. J Hazard Mater. 2021;427:127861. doi: 10.1016/j.jhazmat.2021.127861. [DOI] [PubMed] [Google Scholar]
- 205.Domenech J, Hernández A, Rubio L, Marcos R, Cortés C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Archives of Toxicology. 2020;94(9):2997–3012. doi: 10.1007/s00204-020-02805-3. [DOI] [PubMed] [Google Scholar]
- 206.Forte M, Iachetta G, Tussellino M, et al. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol In Vitro. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 207.Mahler GJ, Esch MB, Tako E, et al. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat Nanotechnol. 2012;7(4):264–271. doi: 10.1038/nnano.2012.3. [DOI] [PubMed] [Google Scholar]
- 208.Vita AA, Royse EA, Pullen NA. Nanoparticles and danger signals: oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J Leukoc Biol. 2019;106(1):95–103. doi: 10.1002/JLB.3MIR1118-414RR. [DOI] [PubMed] [Google Scholar]
- 209.Pedata P, Ricci G, Malorni L, Venezia A, Cammarota M, Volpe MG, Iannaccone N, Guida V, Schiraldi C, Romano M, et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res Int. 2019;119:634–642. doi: 10.1016/j.foodres.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 210.Cornu R, Chrétien C, Pellequer Y, Martin H, Béduneau A. Small silica nanoparticles transiently modulate the intestinal permeability by actin cytoskeleton disruption in both Caco-2 and Caco-2/HT29-MTX models. Archives of Toxicology. 2020;94(4):1191–1202. doi: 10.1007/s00204-020-02694-6. [DOI] [PubMed] [Google Scholar]
- 211.Lee YG, Lee SH, Hong J, Lee PH, Jang AS. Titanium dioxide particles modulate epithelial barrier protein, Claudin 7 in asthma. Mol Immunol. 2021;132:209–216. doi: 10.1016/j.molimm.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 212.Smallcombe CC, Harford TJ, Linfield DT, Lechuga S, Bokun V, Piedimonte G, Rezaee F. Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2020;319(3):L481–l496. doi: 10.1152/ajplung.00104.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kampfer AAM, Urban P, La Spina R, Jiménez IO, Kanase N, Stone V, Kinsner-Ovaskainen A. Ongoing inflammation enhances the toxicity of engineered nanomaterials: application of an in vitro co-culture model of the healthy and inflamed intestine. Toxicol In Vitro. 2020;63:104738. doi: 10.1016/j.tiv.2019.104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gokulan K, Williams K, Orr S, Khare S. Human intestinal tissue explant exposure to silver nanoparticles reveals sex dependent alterations in inflammatory responses and epithelial cell permeability. Int J Mol Sci. 2020;22(1):9. doi: 10.3390/ijms22010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lahiani MH, Khare S, Cerniglia CE, Boy R, Ivanov IN, Khodakovskaya M. The impact of tomato fruits containing multi-walled carbon nanotube residues on human intestinal epithelial cell barrier function and intestinal microbiome composition. Nanoscale. 2019;11(8):3639–3655. doi: 10.1039/C8NR08604D. [DOI] [PubMed] [Google Scholar]
- 216.Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Moller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect. 2009;117(5):703–708. doi: 10.1289/ehp.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Philbrook NA, Walker VK, Afrooz AR, Saleh NB, Winn LM. Investigating the effects of functionalized carbon nanotubes on reproduction and development in drosophila melanogaster and CD-1 mice. Reprod Toxicol. 2011;32(4):442–448. doi: 10.1016/j.reprotox.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 218.Hassaan MA, El Nemr A. Pesticides pollution: classifications, human health impact, extraction and treatment techniques. The Egyptian Journal of Aquatic Research. 2020;46(3):207–220. doi: 10.1016/j.ejar.2020.08.007. [DOI] [Google Scholar]
- 219.Jayaraj R, Megha P, Sreedev P. Review article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol. 2016;9(3–4):90–100. doi: 10.1515/intox-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Keswani C, Dilnashin H, Birla H, Roy P, Tyagi RK, Singh D, Rajput VD, Minkina T, Singh SP. Global footprints of organochlorine pesticides: a pan-global survey. Environmental Geochemistry and Health. 2022;44(1):149–177. doi: 10.1007/s10653-021-00946-7. [DOI] [PubMed] [Google Scholar]
- 221.Maisano M, Cappello T, Oliva S, Natalotto A, Giannetto A, Parrino V, Battaglia P, Romeo T, Salvo A, Spanò N, et al. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the mediterranean sea. Mar Environ Res. 2016;121:40–48. doi: 10.1016/j.marenvres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 222.Aktar MW, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116(11):1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pastor Belda M, González-Franco JA, Rubio R, Campillo N, Hernández-Córdoba M, Torres C, Pérez-Cárceles MD, Viñas P. Occurrence of organochlorine pesticides in human tissues assessed using a microextraction procedure and gas chromatography–mass spectrometry. Journal of Analytical Toxicology. 2020;45(1):84–92. doi: 10.1093/jat/bkaa036. [DOI] [PubMed] [Google Scholar]
- 225.Wang N, Shi L, Kong D, Cai D, Cao Y, Liu Y, Pang G, Yu R. Accumulation levels and characteristics of some pesticides in human adipose tissue samples from Southeast China. Chemosphere. 2011;84(7):964–971. doi: 10.1016/j.chemosphere.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 226.Bräuner EV, Raaschou-Nielsen O, Gaudreau E, Leblanc A, Tjønneland A, Overvad K, Sørensen M. Predictors of adipose tissue concentrations of organochlorine pesticides in a general Danish population. Journal of Exposure Science & Environmental Epidemiology. 2012;22(1):52–59. doi: 10.1038/jes.2011.39. [DOI] [PubMed] [Google Scholar]
- 227.Barrón Cuenca J, de Oliveira Galvão Mf, Ünlü Endirlik B, Tirado N, Dreij K, de Oliveira Galvão MF. In vitro cytotoxicity and genotoxicity of single and combined pesticides used by Bolivian farmers. Environ Mol Mutagen. 2022;63(1):4–17. doi: 10.1002/em.22468. [DOI] [PubMed] [Google Scholar]
- 228.Ledirac N, Antherieu S, d’Uby AD, Caron J-C, Rahmani R, d’Uby AD. Effects of organochlorine insecticides on map kinase pathways in human hacat keratinocytes: key role of reactive oxygen species. Toxicological Sciences. 2005;86(2):444–452. doi: 10.1093/toxsci/kfi192. [DOI] [PubMed] [Google Scholar]
- 229.Ilboudo S, Fouche E, Rizzati V, Toé AM, Gamet-Payrastre L, Guissou PI. In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicology Reports. 2014;1:474–489. doi: 10.1016/j.toxrep.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Russo M, Humes ST, Figueroa AM, Tagmount A, Zhang P, Loguinov A, Lednicky JA, Sabo-Attwood T, Vulpe CD, Liu B, et al. Organochlorine pesticide dieldrin suppresses cellular interferon-related antiviral gene expression. Toxicol Sci. 2021;182(2):260–274. doi: 10.1093/toxsci/kfab064. [DOI] [PubMed] [Google Scholar]
- 231.Craddock HA, Huang D, Turner PC, Quirós-Alcalá L, Payne-Sturges DC. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environmental Health. 2019;18(1):7. doi: 10.1186/s12940-018-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhao GP, Wang XY, Li JW, Wang R, Ren F-Z, Pang G-F, Li Y-X. Imidacloprid increases intestinal permeability by disrupting tight junctions. Ecotoxicol Environ Saf. 2021;222:112476. doi: 10.1016/j.ecoenv.2021.112476. [DOI] [PubMed] [Google Scholar]
- 233.Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma. 2014;51(1):18–28. doi: 10.3109/02770903.2013.833217. [DOI] [PubMed] [Google Scholar]
- 234.Medina-Ramón M, et al. Asthma, chronic bronchitis, and exposure to irritant agents in occupational domestic cleaning: a nested case-control study. Occup Environ Med. 2005;62(9):598–606. doi: 10.1136/oem.2004.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zock JP, Plana E, Jarvis D, Antó JM, Kromhout H, Kennedy SM, Künzli N, Villani S, Olivieri M, Torén K, et al. The use of household cleaning sprays and adult asthma: an international longitudinal study. Am J Respir Crit Care Med. 2007;176(8):735–741. doi: 10.1164/rccm.200612-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dumas O, Wiley AS, Quinot C, Varraso R, Zock J-P, Henneberger PK, Speizer FE, Le Moual N, Camargo CA. Occupational exposure to disinfectants and asthma control in US nurses. Eur Respir J. 2017;50(4):1700237. doi: 10.1183/13993003.00237-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Siracusa A, De Blay F, Folletti I, Moscato G, Olivieri M, Quirce S, Raulf-Heimsoth M, Sastre J, Tarlo SM, Walusiak-Skorupa J, et al. Asthma and exposure to cleaning products - a European academy of allergy and clinical immunology task force consensus statement. Allergy. 2013;68(12):1532–1545. doi: 10.1111/all.12279. [DOI] [PubMed] [Google Scholar]
- 238.Couto M, Bernard A, Delgado L, Drobnic F, Kurowski M, Moreira A, Rodrigues‐Alves R, Rukhadze M, Seys S, Wiszniewska M, et al. Health effects of exposure to chlorination by-products in swimming pools. Allergy. 2021;76(11):3257–3275. doi: 10.1111/all.15014. [DOI] [PubMed] [Google Scholar]
- 239.Tuck SA, Ramos-Barbón D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res. 2008;9(1):61. doi: 10.1186/1465-9921-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shim JS, Lee HS, Park DE, Won Lee J, Bae B, Chang Y, Kim J, Kim HY, Kang H-R. Aggravation of asthmatic inflammation by chlorine exposure via innate lymphoid cells and CD11c intermediate macrophages. Allergy. 2020;75(2):381–391. doi: 10.1111/all.14017. [DOI] [PubMed] [Google Scholar]
- 241.Cullinan P, Harris JM, Newman Taylor AJ, Hole AM, Jones M, Barnes F, Jolliffe G. An outbreak of asthma in a modern detergent factory. Lancet. 2000;356(9245):1899–1900. doi: 10.1016/S0140-6736(00)03264-5. [DOI] [PubMed] [Google Scholar]
- 242.Brant A, Hole A, Cannon J, et al. Occupational asthma caused by cellulase and lipase in the detergent industry. Occup Environ Med. 2004;61(9):793–795. doi: 10.1136/oem.2003.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hole AM. Occupational asthma caused by bacillary amylase used in the detergent industry. Occup Environ Med. 2000;57(12):840–842. doi: 10.1136/oem.57.12.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, Altunbulakli C, Westermann P, Dreher A, Yan L, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892–1903. doi: 10.1016/j.jaci.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 245.Corradi M, Gergelova P, Di Pilato E, Folesani G, Goldoni M, Andreoli R, Selis L, Mutti A. Effect of exposure to detergents and other chemicals on biomarkers of pulmonary response in exhaled breath from hospital cleaners: a pilot study. Int Arch Occup Environ Health. 2012;85(4):389–396. doi: 10.1007/s00420-011-0686-8. [DOI] [PubMed] [Google Scholar]
- 246.Halmos EP, Mack A, Gibson PR. Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Aliment Pharmacol Ther. 2019;49(1):41–50. doi: 10.1111/apt.15045. [DOI] [PubMed] [Google Scholar]
- 247.Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G589–G608. doi: 10.1152/ajpgi.00245.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Miclotte L, Van de Wiele T. Food processing, gut microbiota and the globesity problem. Crit Rev Food Sci Nutr. 2020;60(11):1769–1782. doi: 10.1080/10408398.2019.1596878. [DOI] [PubMed] [Google Scholar]
- 249.Hrncirova L, Machova V, Trckova E, Krejsek J, Hrncir T. Food preservatives induce proteobacteria dysbiosis in human-microbiota associated nod2-deficient mice. Microorganisms. 2019;7(10):383. doi: 10.3390/microorganisms7100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lock JY, Carlson TL, Wang CM, Chen A, Carrier RL. Acute exposure to commonly ingested emulsifiers alters intestinal mucus structure and transport properties. Sci Rep. 2018;8(1):10008. doi: 10.1038/s41598-018-27957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Roberts CL, Keita AV, Duncan SH, O’Kennedy N, Soderholm JD, Rhodes JM, Campbell BJ. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59(10):1331–1339. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu YT, Yuan YZ, Feng QP, Hu M-Y, Li W-J, Wu X, Xiang S-Y, Yu S-Q. Food emulsifier polysorbate 80 promotes the intestinal absorption of mono-2-ethylhexyl phthalate by disturbing intestinal barrier. Toxicol Appl Pharmacol. 2021;414:115411. doi: 10.1016/j.taap.2021.115411. [DOI] [PubMed] [Google Scholar]
- 253.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66(8):1414–1427. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chassaing B, Compher C, Bonhomme B, et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology. 2022;162(3):743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hellwig M, Henle T. Baking, ageing, diabetes: a short history of the Maillard reaction. Angew Chem Int Ed Engl. 2014;53(39):10316–10329. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- 257.Aerts O, Goossens A, Lambert J, Lepoittevin JP. Contact allergy caused by isothiazolinone derivatives: an overview of non-cosmetic and unusual cosmetic sources. Eur J Dermatol. 2017;27(2):115–122. doi: 10.1684/ejd.2016.2951. [DOI] [PubMed] [Google Scholar]
- 258.Van Steenkiste E, Goossens A, Meert H, Apers S, Aerts O. Airborne-induced lymphomatoid contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2015;72(4):237–240. doi: 10.1111/cod.12359. [DOI] [PubMed] [Google Scholar]
- 259.Recke A, Recke AL, Jappe U. Periorbital contact dermatitis caused by octylisothiazolinone in a floor-cleaning agent. Contact Dermatitis. 2015;72(5):339–341. doi: 10.1111/cod.12351. [DOI] [PubMed] [Google Scholar]
- 260.Lee EB, Lobl M, Ford A, DeLeo V, Adler BL, Wysong A. What Is New in Occupational Allergic Contact Dermatitis in the Year of the COVID Pandemic? Curr Allergy Asthma Rep. 2021;21(4):26. doi: 10.1007/s11882-021-01000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sandvik A, Holm J. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80(4):243–245. doi: 10.1111/cod.13182. [DOI] [PubMed] [Google Scholar]
- 262.Xian M, Wawrzyniak P, Rückert B, Duan S, Meng Y, Sokolowska M, Globinska A, Zhang L, Akdis M, Akdis CA, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890–893.e899. doi: 10.1016/j.jaci.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 263.Douwes J, Slater T, Shanthakumar M, McLean D, Firestone RT, Judd L, Pearce N. Determinants of hand dermatitis, urticaria and loss of skin barrier function in professional cleaners in New Zealand. Int J Occup Environ Health. 2017;23(2):110–119. doi: 10.1080/10773525.2018.1427307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Erdem Y, Inal S, Sivaz O, Copur S, Boluk KN, Ugurer E, Kaya HE, Gulsunay IE, Sekerlisoy G, Vural O, et al. How does working in pandemic units affect the risk of occupational hand eczema in healthcare workers during the coronavirus disease-2019 (COVID-19) pandemic: a comparative analysis with nonpandemic units. Contact Dermatitis. 2021;85(2):215–224. doi: 10.1111/cod.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Adisesh A, Murphy E, Barber CM, Ayres JG. Occupational asthma and rhinitis due to detergent enzymes in healthcare. Occup Med (Lond). 2011;61(5):364–369. doi: 10.1093/occmed/kqr107. [DOI] [PubMed] [Google Scholar]
- 266.Doyle AD, Masuda MY, Pyon GC, Luo H, Putikova A, LeSuer WE, Flashner S, Rank MA, Nakagawa H, Kita H, et al. Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy. 2022. doi: 10.1111/all.15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Simonsen AB, Ruge IF, Quaade AS, Johansen JD, Thyssen JP, Zachariae C. Increased occurrence of hand eczema in young children following the Danish hand hygiene recommendations during the COVID-19 pandemic. Contact Dermatitis. 2021;84(3):144–152. doi: 10.1111/cod.13727. [DOI] [PubMed] [Google Scholar]
- 268.Montero-Vilchez T, Martinez-Lopez A, Cuenca-Barrales C, Quiñones‐Vico MI, Sierra‐Sanchez A, Molina‐Leyva A, Gonçalo M, Cambil‐Martin J, Arias‐Santiago S. Assessment of hand hygiene strategies on skin barrier function during COVID-19 pandemic: a randomized clinical trial. Contact Dermatitis. 2022;86(4):276–285. doi: 10.1111/cod.14034. [DOI] [PubMed] [Google Scholar]
- 269.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 270.Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, Ridd MJ, Lawton S, Simpson EL, Cork MJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet. 2020;395(10228):962–972. doi: 10.1016/S0140-6736(19)32984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brough HA, Lanser BJ, Sindher SB, Teng JMC, Leung DYM, Venter C, Chan SM, Santos AF, Bahnson HT, Guttman‐Yassky E, et al. Early intervention and prevention of allergic diseases. Allergy. 2022;77(2):416–441. doi: 10.1111/all.15006. [DOI] [PubMed] [Google Scholar]
- 272.Rinaldi AO, Korsfeldt A, Ward S, Burla D, Dreher A, Gautschi M, Stolpe B, Tan G, Bersuch E, Melin D, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy. 2021;76(10):3066–3079. Online ahead of print. doi: 10.1111/all.14842. [DOI] [PubMed] [Google Scholar]
- 273.Rinaldi AO, Morita H, Wawrzyniak P, Dreher A, Grant S, Svedenhag P, Akdis CA. Direct assessment of skin epithelial barrier by electrical impedance spectroscopy. Allergy. 2019;74(10):1934–1944. doi: 10.1111/all.13824. [DOI] [PubMed] [Google Scholar]
- 274.Liu Y, Lunter DJ. Systematic investigation of the effect of non-ionic emulsifiers on skin by confocal raman spectroscopy—a comprehensive lipid analysis. Pharmaceutics. 2020;12(3):223. doi: 10.3390/pharmaceutics12030223. [DOI] [PMC free article] [PubMed] [Google Scholar]


