Abstract
The lipid compositions of barophilic bacterial strains which contained docosahexaenoic acid (DHA [22:6n-3]) were examined, and the adaptive changes of these compositions were analyzed in response to growth pressure. In the facultatively barophilic strain 16C1, phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) were major components which had the same fatty acid chains. However, in PE, monounsaturated fatty acids such as hexadecenoic acid were major components, and DHA accounted for only 3.7% of the total fatty acids, while in PG, DHA accounted for 29.6% of the total fatty acids. In response to an increase in growth pressure in strain 16C1, the amounts of saturated fatty acids in PE were reduced, and these decreases were mainly balanced by an increase in unsaturated fatty acids, including DHA. In PG, the decrease in saturated fatty acids was mainly balanced by an increase in DHA. Similar adaptive changes in fatty acid composition were observed in response to growth pressure in obligately barophilic strain 2D2. Furthermore, these adaptive changes in response were also observed in response to low temperature in strain 16C1. These results confirm that the general shift from saturated to unsaturated fatty acids including DHA is one of the adaptive changes in response to increases in pressure and suggest that DHA may play a role in maintaining the proper fluidity of membrane lipids under high pressure.
Lipids, in particular, phospholipids, are the main components of cell membranes, in which many important biological functions occur. The dynamic states of lipids, such as the fluidity and the order, are known to be closely related to the functions of biological membranes (9). The dynamic states are dependent on factors such as temperature and pH (16). Thus, it is well known that a number of bacteria, plants, and poikilotherms regulate the lipid compositions of their membranes in response to changes in the environmental temperature so as to maintain the membrane lipid fluidity necessary for proper biological function (9). This phenomena is known as homeoviscous adaptation (25). For example, a decrease in growth temperature generally results in an increase in the level of unsaturation and a decrease in the average chain length of fatty acids in bacterial lipids (16, 24). Furthermore, in cold-adapted species, the levels of unsaturated fatty acids or polyunsaturated fatty acids (PUFAs) are known to be relatively high (9, 31).
Barophiles are defined as organisms which grow optimally or preferentially at pressures greater than atmospheric pressure (0.1 MPa). Barophilic bacteria have been isolated from various deep-sea environments and have been shown to grow rapidly at low temperatures and high pressures (11, 35, 36). High pressure and low temperature in deep-sea environments theoretically decrease the fluidity of lipids and possibly depress the functions of biological membranes (9, 14). Thus, barophiles seem to have some mechanism which allows their lipids to adapt to deep-sea environments. In fact, in a barophilic strain, it was shown that an increase in growth pressure resulted in an increase in the level of unsaturated fatty acids in the lipids, suggesting a homeoviscous adaptation in response to pressure (3). In general, bacteria have been considered to be unable to produce PUFAs (5, 7). A great number of bacterial strains isolated from terrestrial and shallow-sea environments are reported to produce fatty acids which have chain lengths of less than 20 (19, 20, 30). However, marine bacteria such as deep-sea isolates and barophilic isolates have been reported to contain PUFAs such as docosahexaenoic acid (DHA [22:6n-3]) and eicosapentaenoic acid (EPA [20:5n-3]) in their lipids (4, 12, 22). PUFAs have relatively low melting points (16), and so they may assist in maintaining the proper fluidity of membrane lipids that the marine bacteria require to adapt to deep-sea environments. Furthermore, it was found that the relative amounts of PUFAs were higher during both medium- and high-pressure incubations, than during low-pressure incubations (4, 32). From these studies, we assumed that PUFAs may be involved in the adaptation of barophiles to deep-sea environments. However, the details of the lipid compositions remain unknown in bacteria containing PUFAs, and the adaptive changes of lipid compositions in barophilic bacteria have been reported only for the fatty acid composition of total lipids.
In a previous study (33), we showed that the barophilic strains isolated from the intestinal contents of deep-sea fish contained DHA and EPA. Furthermore, we found that bacteria containing DHA were generally and abundantly distributed in the intestines of deep-sea fish, compared with shallow-sea poikilothermic animals (34), suggesting the involvement of DHA in the adaptation to deep-sea environments. Thus, in the present study, we initially examined the lipid compositions of a barophilic strain which contained DHA in its lipids. Next, we examined the effect of growth pressure on the phospholipid and the fatty acid compositions in facultatively and obligately barophilic strains containing DHA.
MATERIALS AND METHODS
Bacterial strains and medium.
Strain 16C1 was facultatively barophilic and was originally isolated from the intestinal contents of the deep-sea fish Coryphaenoides armatus, which was retrieved from a depth of 3,100 m (34). Strain 2D2 was obligately barophilic and was isolated from the intestinal contents of the deep-sea fish Coryphaenoides yaquinae, which was retrieved from a depth of 6,100 m (18). These strains were maintained in retortable pouches (nylon-CPP; Sanei Chemical industry, Tokyo, Japan) containing marine broth (Difco, Detroit, Mich.) at 5°C and at in situ pressures (strain 16C1, 41.1 MPa; strain 2D2, 62.1 MPa) by using pressure vessels (18). Growth characteristics of strains 16C1 and 2D2 in relation to pressure were examined by epifluorescence microscopy with 4′,6-diamidino-2-phenylindole (DAPI) (21), as described previously (18).
Fatty acid composition of strain 16C1.
For analyses of the fatty acid composition of strain 16C1, the culture was grown in defatted marine broth. Defatted marine broth consisted of (per liter) 5 g of Bacto Peptone (Difco), 1 g of Bacto yeast extract (Difco), 0.1 g of ammonium ferric citrate, 20 mM MOPS (morpholinepropanesulfonic acid [pH 7.0]), and 30.0 g of artificial seawater salts (Senju, Osaka, Japan). Before the preparation of the medium, peptone and yeast extract were extracted with chloroform-methanol (2:1 [vol/vol]) to remove the lipids. The culture of strain 16C1 was grown in the medium at 5°C and 41.4 MPa. After a 7-day incubation period which led the strain to the stationary phase, bacterial cells were harvested by centrifugation at 9,000 × g for 15 min under 4°C, immediately frozen at −20°C, freeze-dried, and used for lipid analyses.
Total lipids were extracted from dried cells with chloroform-methanol (2:1 [vol/vol]) by the method of Folch et al. (6). The organic phase was filtered and washed with one-quarter its volume of distilled water. After removal of the solvent by evaporation, the total lipid extracts were redissolved in a known volume of chloroform-methanol (2:1 [vol/vol]) and stored at −20°C under nitrogen for further analysis. The total lipids were added to 2.5% methanolic HCl and heated at 85°C for 2.5 h for preparation of fatty acid methyl esters (FAMEs). The resulting FAMEs were extracted three times with n-hexane and stored at −20°C under nitrogen for further analysis. The analyses of the FAMEs were performed with a G-5000 gas chromatograph (Hitachi, Tokyo, Japan) equipped with an Omega wax 320 capillary column (30 m by 0.32 mm [inside diameter]; Supelco, Bellefonte, Pa.) and a flame ionization detector. The oven temperature was programmed from 170 to 215°C at a rate of 1°C per min. Helium was used as the carrier gas, and the injector and the detector were maintained at 255 and 260°C, respectively. The samples were further analyzed with an SP-2330 capillary column (30 m by 0.25 mm [inside diameter]; Supelco) with the oven temperature programmed as described above. The FAMEs were identified by comparison with authentic standards and measured with a recording integrator attached to the gas chromatograph.
The unsaturated nature of fatty acids was confirmed by hydrogenation with platinum oxide and reanalysis with the gas chromatograph (2). The FAMEs were dissolved with methanol in a test tube, and PtO2 was added. The tube was flushed with hydrogen to remove any air, and then was vigorously shaken and maintained for 2 h. The solvent was then evaporated, and the resulting saturated FAMEs were taken up in n-hexane. The resulting saturated FAMEs were analyzed for gas chromatography and compared with the original FAMEs.
Positions of double bonds in fatty acids were determined by gas chromatography-mass spectrometry (GC-MS) according to the procedure (the pyrrolidine method) of Anderson et al. (1, 2). The FAMEs were dissolved with pyrrolidine in a test tube, and acetic acid was added. The mixture was heated at 100°C for 30 min. The resulting derivatives were taken up in dichloromethane and washed with 2 N HCl and then with water. GC-MS analyses of derivatized FAME samples were performed on a JMS-DX303 instrument (JEOL, Tokyo, Japan) equipped with an Omega wax 320 capillary column (30 m by 0.32 mm [inside diameter]; Supelco). The oven temperature was 260°C. Electron impact mass spectra were measured at 70 eV.
For analysis of constituent fatty acids, known amounts of the total lipids were spotted on thin-layer chromatography plates and separated with chloroform-methanol-water (65:25:4 [vol/vol/vol]) as a mobile phase. Developed chromatograms were sprayed with 0.01% (wt/vol) primurin reagent (Tokyo Kasei, Tokyo, Japan) and viewed under UV light. Bands containing phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) were identified by reference to the standard, scraped from the plates, and then subjected to transesterification as described above. A known amount of 23:0 methyl ester was added as an internal standard. After cooling, the resulting FAMEs were extracted and stored at −20°C under nitrogen until GC analyses. GC analyses were performed under the conditions described above. Phospholipid composition was determined from the weight of the constituent fatty acids by using the internal standard.
Effect of pressure and temperature on lipid compositions.
Chilled marine broth (1,000 ml) was inoculated with 1.0 ml of strain cultures which grew at 5°C and at in situ pressures for a week. This was distributed to four retortable pouches (250 ml each) and then heat-sealed. Next, the pouches were placed in pressure vessels and incubated at 5°C and at various pressures. After incubation, bacterial cells were harvested in early stationary phase in a manner similar to that described above. That is, in the case of strain 16C1, bacteria cells were harvested after 4 days from both the 20.7- and 41.4-MPa incubations and after 5 days from the 0.1-MPa incubation. In the case of strain 2D2, harvesting was conducted after 4 days from the 41.4-MPa incubation and after 5 days from both the 20.7- and 62.1-MPa incubations. Bacterial cells were immediately frozen at −20°C, freeze-dried, and used for lipid analyses as described above. Furthermore, in the case of strain 16C1, in order to examine the effect of growth temperature on fatty acid composition, the pouches containing the medium were incubated at 1°C and atmospheric pressure (0.1 MPa) at the same time with the pressure experiment. After a 5-day incubation, the bacterial cells were harvested, frozen at −20°C, freeze-dried, and used for lipid analyses as described above. In all of these experiments, the cell numbers of the cultures were counted by epifluorescence microscopy at the harvesting time.
RESULTS
Growth characteristics.
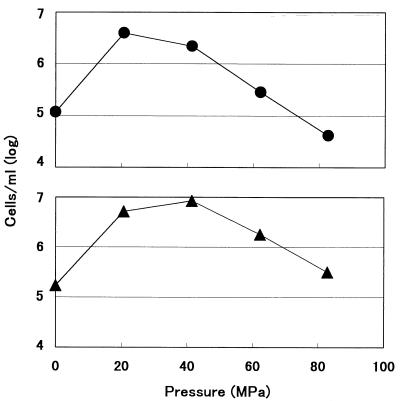
Figure 1 shows the cell numbers of strains 16C1 and 2D2 after a 2-day incubation period when the cells were grown at 5°C and at various pressures. Strain 16C1 optimally grew at 20.4 MPa. Because the strain was capable of growing at 0.1 MPa, it was considered to be facultatively barophilic. Strain 16C1 grew to the early stationary phase for 4 days at both 20.7 and 41.4 MPa, and for 5 days at 0.1 MPa (data not shown). Strain 2D2 optimally grew at 41.4 MPa, as shown in the previous study (18). Furthermore, the strain was confirmed to be obligately barophilic, because it was not capable of growing at 0.1 MPa. Strain 2D2 grew to the early stationary phase for 4 days at 41.4 MPa and for 5 days at both 20.7 and 62.1 MPa (data not shown).
FIG. 1.
Growth of barophilic strains 16C1 (•) and 2D2 (▴) at different pressures and 5°C. Markers show the cell numbers of the cultures at 2 days of incubation, respectively. The cell numbers at the beginning of incubation were 4.2 × 104 cells/ml in strain 16C1 and 1.7 × 105 cells/ml in strain 2D2.
Fatty acid composition of strain 16C1.
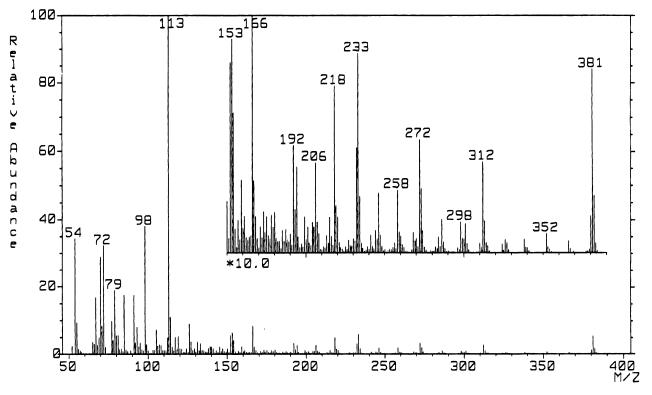
The representative fatty acid compositions of total lipids, PE, and PG in strain 16C1 are shown in Table 1. In the total lipids of strain 16C1, the major fatty acids were tetradecanoic acid (14:0), tetradecenoic acid (14:1), hexadecanoic acid (16:0), hexadecenoic acid (16:1), and DHA, according to the comparison of retention time with the standards on the gas chromatogram. Octadecanoic acid (18:0) and octadecenoic acid (18:1) were minor components. The PUFAs of C18 (the carbon length is 18; this notation is also used below) and C20 were also found as minor fatty acids in total lipids, but those of C22, except for DHA, were not detected. After hydrogenation, all peaks which were regarded as unsaturated fatty acids disappeared on the gas chromatogram, and the resulting fatty acid composition was almost the same as the composition calculated from the fatty acid composition before hydrogenation (data not shown). Furthermore, on the mass spectrum of DHA, the characteristic peaks of m/z 381, 366, 352, 338, and 326 were found (Fig. 2), and the spectrum was the same as that of the authentic 22:6n-3 (data not shown). The positions of double bonds in monounsaturated fatty acids of C14 and C16 and 20:5n-3 were also confirmed (data not shown).
TABLE 1.
Fatty acid compositions of total lipids, PE, and PG in strain 16C1
| Fatty acid | Fatty acid composition (%)
|
||
|---|---|---|---|
| Total lipids | PE | PG | |
| 14:0 | 9.5 | 10.7 | 6.8 |
| 14:1n-7a | 6.1 | 7.6 | 1.7 |
| 15:0 | 0.3 | —b | — |
| 16:0 | 12.6 | 9.6 | 12.6 |
| 16:1n-9a | 9.4 | 9.4 | 7.7 |
| 16:1n-7a | 48.2 | 55.6 | 35.9 |
| 18:0 | 1.1 | 0.2 | 0.8 |
| 18:1n-9a | 0.9 | 0.4 | 0.7 |
| 18:1n-7a | 0.7 | 1.0 | 1.0 |
| 18:2n-6 | 0.7 | 0.7 | 1.0 |
| 18:3n-6 | Trc | Tr | Tr |
| 18:3n-3 | 0.1 | 0.3 | 0.1 |
| 18:4n-3 | Tr | Tr | Tr |
| 20:3n-6 | Tr | Tr | 0.1 |
| 20:4n-6a | 0.2 | 0.2 | 0.3 |
| 20:4n-3 | 0.2 | 0.2 | 0.5 |
| 20:5n-3a | 0.5 | 0.3 | 1.3 |
| 22:6n-3a | 9.3 | 3.7 | 29.6 |
The double bond positions were confirmed.
—, not detected.
Tr, trace (<0.1%).
FIG. 2.
Mass spectra of pyrrolidide derivatives prepared from DHA of strain 16C1.
In PE, the fatty acid composition was, in general, similar to that of the total lipids; however, it is notable that 16:1n-7 was present at higher levels (55.6% of the total fatty acids) and that DHA amounted to only 3.7% of the total fatty acids. In contrast, 16:1n-7 was present at a lower level (35.9% of the total fatty acids), and DHA accounted for 29.6% of the total fatty acids in PG.
Effect of pressure on phospholipid composition.
Phospholipid compositions of strain 16C1 and obligately barophilic strain 2D2, grown at different pressures, are shown in Table 2. In the case of strain 16C1, the proportion of PE was greater with higher pressure incubations than that with the 0.1-MPa incubation. However, this proportion was not different between the 20.7- and 41.4-MPa incubations. Phospholipids in strain 2D2 were also generally PE and PG, and the change in composition was not distinctly observed among cells grown at any given pressure, while PE appeared to increase slightly in cells grown at higher pressures.
TABLE 2.
Effect of growth pressure on phospholipid composition in strains 16C1 and 2D2
| Strain | Phospholipid | Composition (%) at growth pressure (MPa) ofa:
|
|||
|---|---|---|---|---|---|
| 0.1 | 20.7 | 41.4 | 62.1 | ||
| 16C1 | PE | 77.3 | 85.1 | 84.4 | —b |
| PG | 22.7 | 14.9 | 15.6 | — | |
| 2D2 | PE | — | 77.0 | 78.1 | 81.8 |
| PG | — | 23.0 | 21.9 | 18.2 | |
Values are means (strain 16C1, n = 3; strain 2D2, n = 2).
—, not detected.
Effect of pressure on fatty acid composition.
The fatty acid compositions of total lipids, PE, and PG from strain 16C1, grown at different pressures, are shown in Table 3. The major fatty acids were 14:0, 14:1n-7, 16:0, 16:1n-9, 16:1n-7, and DHA, regardless of the growth pressures. In total lipids, the change in response to growth pressure was observed in the C14 and C16 acids. The proportions of 14:0 and 14:1n-7 in the cells grown at 0.1 MPa were 26.9 and 19.2% of the total fatty acids, respectively, and these proportions decreased in the cells grown at 20.7 MPa (10.0 and 6.2% of total fatty acids, respectively) and 41.4 MPa (9.0 and 5.8% of total fatty acids, respectively). This decrease was largely balanced by an increase in 16:0 and 16:1. DHA accounted for 6.6% of the total fatty acids at 0.1 MPa and increased to 8.4% at 20.7 MPa. However, this change in fatty acid composition was not distinct between the cultures at 20.7 and 41.4 MPa. Thus, in the total lipids, the proportions of saturated fatty acids decreased with an increase in growth pressure, while the proportions of monounsaturated fatty acids increased. In addition, the proportions of PUFAs slightly increased.
TABLE 3.
Effect of growth pressure on fatty acid composition in strain 16C1
| Fatty acid | Composition (%) at incubation pressure (MPa) ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total lipids
|
PE
|
PG
|
|||||||
| 0.1 | 20.7 | 41.4 | 0.1 | 20.7 | 41.4 | 0.1 | 20.7 | 41.4 | |
| 14:0 | 26.9 | 10.0 | 9.0 | 28.2 | 10.7 | 9.7 | 23.6 | 10.2 | 6.2 |
| 14:1n-7 | 19.2 | 6.2 | 5.8 | 22.6 | 7.5 | 6.3 | 4.3 | 2.0 | 1.3 |
| 15:0 | Trb | 0.4 | —c | — | — | — | — | — | — |
| 16:0 | 8.4 | 12.4 | 11.4 | 8.7 | 9.6 | 8.9 | 14.7 | 12.9 | 12.6 |
| 16:1n-9 | 8.8 | 9.5 | 10.0 | 9.4 | 9.5 | 10.6 | 9.7 | 7.7 | 8.3 |
| 16:1n-7 | 27.1 | 48.4 | 50.3 | 27.3 | 55.7 | 57.2 | 28.1 | 32.7 | 32.4 |
| 18:0 | 0.6 | 1.0 | 1.1 | 0.6 | 0.3 | 0.3 | 2.6 | 1.1 | 1.2 |
| 18:1n-9 | 0.3 | 1.0 | 0.7 | 0.4 | 0.6 | 0.5 | 1.4 | 0.6 | 0.7 |
| 18:1n-7 | 0.2 | 0.8 | 0.9 | 0.3 | 0.8 | 0.8 | 0.8 | 1.0 | 1.5 |
| 18:2n-6 | 1.0 | 0.7 | 0.6 | 0.4 | 0.4 | 0.8 | 1.1 | 0.8 | 1.0 |
| 18:3n-6 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr |
| 18:3n-3 | Tr | 0.1 | 0.2 | Tr | 0.1 | 0.1 | Tr | 0.1 | 0.2 |
| 18:4n-3 | Tr | Tr | Tr | Tr | Tr | 0.1 | Tr | — | Tr |
| 20:3n-6 | — | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr |
| 20:4n-6 | 0.4 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.4 | 0.2 | 0.4 |
| 20:4n-3 | 0.2 | 0.2 | 0.3 | — | 0.3 | 0.3 | 0.4 | 0.6 | 0.6 |
| 20:5n-3 | 0.2 | 0.6 | 0.6 | 0.1 | 0.3 | 0.4 | 0.3 | 1.4 | 1.3 |
| 22:6n-3 | 6.6 | 8.4 | 9.0 | 1.6 | 3.9 | 3.9 | 12.5 | 28.6 | 32.4 |
| Total saturated fatty acids | 35.9 | 23.8 | 21.4 | 37.5 | 20.6 | 18.8 | 40.9 | 24.3 | 20.0 |
| Total monounsaturated fatty acids | 55.6 | 66.0 | 67.7 | 60.1 | 74.1 | 75.4 | 44.3 | 44.0 | 44.2 |
| Total PUFAs | 8.4 | 10.2 | 10.2 | 2.4 | 5.2 | 5.8 | 14.7 | 31.7 | 35.9 |
Values are means (n = 3).
Tr, trace (<0.1%).
—, not detected.
The change in fatty acid composition of PE was generally similar to that observed in the total lipids. With increasing growth pressure, the proportions of saturated fatty acids decreased, while the proportions of monounsaturated fatty acids and PUFAs increased.
In PG, the change in fatty acid composition in response to growth pressure was most markedly observed in the level of 14:0 and DHA. The proportion of 14:0 was 23.6% of the total fatty acids at 0.1 MPa, and this decreased to 10.2% at 20.7 MPa. DHA accounted for 12.5% of the total fatty acids at 0.1 MPa and increased to 28.6% at 20.7 MPa. The proportion of 16:1 was generally constant. The change was not distinct between the culture at 20.7 MPa and the culture at 41.4 MPa. Thus, the decrease in saturated fatty acids in PG associated with increasing growth pressure was balanced by an increase in PUFAs.
The change in fatty acid composition of the obligately barophilic strain 2D2 in response to growth pressure is shown in Table 4. In this strain, the fatty acid compositions of total lipids, PE, and PG were generally similar to those of strain 16C1, and the major fatty acids were 14:0, 14:1, 16:0, 16:1, and DHA. However, the fatty acid compositions in strain 2D2 were notably different from those of strain 16C1, in that strain 2D2 contained a lower proportion of 16:1 and a far higher proportion of DHA. In particular, DHA accounted for about 50% of the fatty acids of PG. The change in fatty acid composition of the 2D2 strain was, in general, similar to that in strain 16C1. In the total lipids and PE, the proportions of 14:0 and 14:1n-7 decreased and those of 16:0 and 16:1 increased, with increasing growth pressure. Thus, with increasing growth pressure, the proportions of saturated fatty acids decreased, while those of monounsaturated fatty acids and PUFAs increased. In PG, the proportion of 14:0 decreased and that of DHA increased with increasing growth pressure. The degree of change in fatty acid composition, however, was not as great as that observed in the PG of strain 16C1. The proportion of 16:1 remained constant. Thus, the proportions of saturated fatty acids decreased in PG, while those of PUFAs increased.
TABLE 4.
Effect of growth pressure on fatty acid composition in strain 2D2
| Fatty acid | Composition (%) at incubation pressure (MPa) ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total lipids
|
PE
|
PG
|
|||||||
| 20.7 | 41.4 | 62.1 | 20.7 | 41.4 | 62.1 | 20.7 | 41.4 | 62.1 | |
| 14:0 | 16.6 | 11.7 | 9.1 | 18.1 | 13.2 | 10.6 | 13.1 | 7.6 | 7.3 |
| 14:1n-7 | 11.9 | 7.9 | 6.5 | 15.3 | 10.3 | 8.3 | 1.6 | 1.1 | 1.4 |
| 15:0 | 0.6 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.6 | 0.5 | 0.5 |
| 16:0 | 6.8 | 6.7 | 6.9 | 5.9 | 6.0 | 6.4 | 8.4 | 8.1 | 7.9 |
| 16:1n-7 | 8.3 | 8.6 | 8.4 | 8.7 | 9.2 | 9.3 | 6.9 | 7.0 | 6.9 |
| 16:1n-9 | 35.3 | 40.6 | 41.9 | 40.4 | 48.3 | 50.8 | 21.4 | 21.7 | 21.5 |
| 18:0 | 0.5 | 0.4 | 0.4 | 0.1 | 0.1 | 0.2 | 0.3 | 0.4 | 1.0 |
| 18:1n-9 | 0.4 | 0.4 | 0.5 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| 18:1n-7 | 0.8 | 1.2 | 1.8 | 0.6 | 0.8 | 1.2 | 1.3 | 1.6 | 1.5 |
| 18:2n-6 | 0.2 | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 | 0.4 | 0.2 |
| 18:3n-6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | Tr | Tr | 0.1 |
| 18:3n-3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 18:4n-3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| 20:3n-6 | Trb | Tr | Tr | Tr | Tr | Tr | Tr | 0.1 | 0.1 |
| 20:4n-6 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 0.3 |
| 20:3n-3 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 |
| 20:4n-3 | 0.4 | 0.6 | 1.2 | 0.3 | 0.5 | 0.7 | 0.6 | 0.8 | 1.3 |
| 20:5n-3 | 0.5 | 0.4 | 1.5 | 0.3 | 0.4 | 0.5 | 0.9 | 1.0 | 1.4 |
| 22:6n-3 | 17.2 | 19.2 | 20.3 | 8.7 | 9.4 | 10.2 | 43.5 | 48.8 | 47.7 |
| Total saturated fatty acids | 24.4 | 19.3 | 16.9 | 24.7 | 19.8 | 17.7 | 22.5 | 16.5 | 16.8 |
| Total monounsaturated fatty acids | 56.6 | 58.8 | 59.0 | 65.3 | 68.9 | 69.9 | 31.5 | 31.6 | 31.7 |
| Total PUFAs | 19.0 | 22.0 | 24.1 | 10.0 | 11.3 | 12.4 | 46.0 | 51.8 | 51.5 |
Values are means (n = 2).
Tr, trace (<0.1%).
Effect of temperature on fatty acid composition.
The fatty acid compositions of total lipids, PE, and PG from strain 16C1 grown at 1°C and 0.1 MPa are shown in Table 5. For the convenience of comparison, the table includes the data for cells grown at 5°C and 0.1 MPa, previously shown in Table 3. The major fatty acids of the total lipids were 14:0, 14:1, 16:0, 16:1 and DHA, regardless of the respective growth temperature. In response to an increase in growth temperature, changes were observed in C14 acids, C16 acids, and DHA. These changes, in general, corresponded with the changes caused by growth pressure for the same fatty acids. Thus, with decreasing growth temperature, the proportions of saturated fatty acids decreased and those of unsaturated fatty acids increased.
TABLE 5.
Effect of growth temperature on fatty acid composition in strain 16C1
| Fatty acid | Composition (%) at a growth temp (°C) ofa:
|
|||||
|---|---|---|---|---|---|---|
| Total lipids
|
PE
|
PG
|
||||
| 1 | 5 | 1 | 5 | 1 | 5 | |
| 14:0 | 12.8 | 26.9 | 13.5 | 28.2 | 8.5 | 23.6 |
| 14:1n-7 | 8.7 | 19.2 | 9.5 | 22.6 | 1.8 | 4.3 |
| 15:0 | Trb | Tr | —c | — | — | — |
| 16:0 | 11.2 | 8.4 | 10.4 | 8.7 | 12.3 | 14.7 |
| 16:1n-9 | 8.2 | 8.8 | 8.5 | 9.4 | 6.8 | 9.7 |
| 16:1n-7 | 47.8 | 27.1 | 53.6 | 27.3 | 39.5 | 28.1 |
| 18:0 | 0.7 | 0.6 | 0.2 | 0.6 | 1.0 | 2.6 |
| 18:1n-9 | 0.5 | 0.3 | 0.3 | 0.4 | 0.5 | 1.4 |
| 18:1n-7 | 0.5 | 0.2 | 0.9 | 0.3 | 1.6 | 0.8 |
| 18:2n-6 | 0.6 | 1.0 | 0.6 | 0.4 | 0.8 | 1.1 |
| 18:3n-6 | Tr | Tr | Tr | Tr | — | Tr |
| 18:3n-3 | 0.2 | Tr | 0.1 | Tr | 0.5 | Tr |
| 18:4n-3 | Tr | Tr | Tr | Tr | — | Tr |
| 20:3n-6 | Tr | — | Tr | Tr | Tr | Tr |
| 20:4n-6 | 0.3 | 0.4 | 0.2 | 0.3 | 0.7 | 0.4 |
| 20:4n-3 | 0.3 | 0.2 | 0.2 | — | 0.5 | 0.4 |
| 20:5n-3 | 0.6 | 0.2 | 0.3 | 0.1 | 2.1 | 0.3 |
| 22:6n-3 | 7.6 | 6.6 | 1.9 | 1.6 | 23.4 | 12.5 |
| Total saturated fatty acids | 24.6 | 35.9 | 24.1 | 37.5 | 21.8 | 40.9 |
| Total monounsaturated fatty acids | 65.7 | 55.6 | 72.7 | 60.1 | 50.2 | 44.3 |
| Total PUFAs | 9.6 | 8.4 | 3.3 | 2.4 | 28.0 | 14.7 |
Incubations were done at atmospheric pressure. Values are means (n = 3).
Tr, trace (<0.1%).
—, not detected.
DISCUSSION
Production of DHA in procaryotes was first reported in deep-sea isolates, including barophilic bacteria (4). The present study showed that the DHA observed in the barophilic strain 16C1 was 22:6n-3, which contained methylene-interrupted double bonds and was normally observed in lipids of eucaryotes (17, 26). Furthermore, it was found that the double-bond positions of monounsaturated fatty acids were n-9 and n-7 in the strain. Generally, unsaturated fatty acids in bacteria have been considered to be produced by two possible mechanisms: an oxygen-independent (anaerobic) pathway and an oxygen-dependent (aerobic) pathway (7). In the anaerobic pathway, which is catalyzed by a fatty acid synthetase, palmitoleic acid (16:1n-7) and cis-vaccenic acid (18:1n-7) are produced. In the aerobic pathway, which involves fatty acid desaturation analogous to that of eucaryotes, palmitoleic acid (16:1n-7) and oleic acid (18:1n-9) are commonly produced from saturated fatty acids by position-specific desaturase. A production mechanism for PUFA, such as DHA, however, is unknown in bacteria. In procaryotic algae producing 18:4 (28) and eucaryote-synthesized DHA (17), it was reported that C18 and C20 PUFAs of the n-3 and n-6 series were present in their lipids. Also in strain 16C1, those PUFAs were shown to be contained in the lipids, although no previous reports have been made of the presence of C20 PUFAs, even EPA, in bacteria containing DHA (4, 8). Furthermore, according to the known elongation system of fatty acid, it is difficult to imagine that 22:6n-3 containing methylene-interrupted double bonds is formed by means of an anaerobic pathway. Thus, we suppose that DHA in the bacterial strain 16C1 may be also synthesized via C18 and C20 PUFAs of the n-3 and n-6 series, as is the case in eucaryotes. However, C22 PUFAs (with the except of DHA) were not detected in strain 16C1 or in other bacteria containing DHA. In fish, DHA is considered to be formed by the addition of C2 to EPA, followed by desaturation (13). However, in rat hepatocytes, it has been reported that elongation of 22:5n-3 to 24:5n-3 is followed by desaturation to 24:6n-3, and then this is metabolized, via β-oxidation, to 22:6n-3 (27). Thus, further investigations are needed to clarify the synthetic pathway of DHA in bacteria.
Phospholipids in strain 16C1 were mainly PE and PG, as described previously (34). PE was found to be abundant in monounsaturated fatty acids (74% of total fatty acids) and lacking in PUFAs, including DHA (5.4% of total fatty acids), compared with PG. In PG, monounsaturated fatty acids were present at a lower level (47% of total fatty acids) and DHA accounted for 29.6% of the total fatty acids. This tendency was also observed in strain 2D2, as shown in Table 4, and the proportion of DHA in the PG accounted for about 50% of the total fatty acids. Although there have been no reports on the fatty acid composition of phospholipids in bacteria containing DHA, it has been reported that PG has higher levels of EPA than PE in two EPA-producing bacterial strains isolated from freshwater fish and shallow-sea fish (10, 29).
The change in phospholipid composition in relation to growth pressure was not distinct, except that the proportion of PE became high when strain 16C1 underwent pressurized incubation. It is well known that the phospholipid compositions of bacteria and the changes related to incubation conditions vary with bacterial species (24). In a bacterium containing EPA, which has PE and PG as the major lipids, the proportion of PE was reported to increase at a lower growth temperature (10), although what the change meant was unknown.
With increasing growth pressure, DHA levels increased in the fatty acids of the total lipids in strains 16C1 and 2D2. This indicates that DHA was more necessary at high pressures and confirms that DHA plays some roles in the growth and biological functions of barophilic bacteria at high pressures, suggested by DeLong and Yayanos (4). Furthermore, the present study found that the increases in the level of DHA occurred in phospholipids, especially PG. This finding suggests that the role of DHA may be closely related to the functions of the membrane.
In PE, in the present study, the proportions of 14:0 and 14:1 were reduced with an increase in pressure, and these decreases were mainly balanced by a increase in 16:1. In PG, the decrease of 14:0 was mainly balanced by an increase in DHA. Generally speaking, with increasing growth pressure, saturated fatty acids decreased and unsaturated fatty acids, including DHA, increased in major phospholipids of barophilic strains. It is well known that the increase in the level of unsaturation of fatty acids occurs with the lowering of growth temperature in bacteria and poikilotherms (15, 23). In the present study also, the lowering of growth temperature caused the increase in unsaturated fatty acids. These results suggest that the barophilic strains compensate for pressure increases through homeoviscous adaptation in a fashion similar to the response to lowering of temperature. Thus, the present study further confirms the suggestion of DeLong and Yayanos (3, 4). That is to say, one of the roles of DHA may be in maintaining the fluidity of lipids at high pressure.
In the present study, the changes in fatty acid composition in phospholipids were slight or ambiguous between medium pressure and high pressure. It has also been reported that in the barophilic strain MT41, there was a decrease in the relative amount of DHA at the highest growth pressure compared with that at the medium pressure (4). If these strains always regulate their lipid states when pressure increases, it is difficult to understand slight or no change in the fatty acid composition at high pressure, as observed in the present study. Furthermore, although it is known that a shortening of carbon length in fatty acids is observed at lower temperatures (9, 24), the proportions of 14:0 and 14:1 were reduced at higher pressures in the present study. These results may suggest that there were changes in the lipid composition which were not detected by the analysis of fatty acid composition. This indicates the need for more detailed analysis of the molecular species of phospholipids.
ACKNOWLEDGMENTS
We thank Y. Ezura, K. Takahashi, H. Shinano, and K. Yoshida for valuable advice.
This study was partially supported by grant BRP-97-I-A-4 from the Ministry of Agriculture, Forestry, and Fisheries.
REFERENCES
- 1.Anderson B A, Christie W W, Holman R T. Mass spectrometric determination of positions of double bonds in polyunsaturated fatty acid pyrrolidides. Lipids. 1975;10:215–219. doi: 10.1007/BF02532483. [DOI] [PubMed] [Google Scholar]
- 2.Christie W W. Preparation of methyl ester and derivatives. In: Christie W W, editor. Gas chromatography and lipids. Glasgow, United Kingdom: The Oily Press; 1992. pp. 64–84. [Google Scholar]
- 3.DeLong E F, Yayanos A A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985;228:1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- 4.DeLong E F, Yayanos A A. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl Environ Microbiol. 1986;51:730–737. doi: 10.1128/aem.51.4.730-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erwin J, Bloch K. Biosynthesis of unsaturated fatty acids in microorganisms. Science. 1964;143:1006–1012. doi: 10.1126/science.143.3610.1006. [DOI] [PubMed] [Google Scholar]
- 6.Folch J, Lees M, Sloane-Stanley G H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 7.Fulco A J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22:133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 8.Hamamoto T, Takata N, Kudo T, Horikoshi K. Characteristic presence of polyunsaturated fatty acids in marine psychrophilic vibrios. FEMS Microbiol Lett. 1995;129:51–56. [Google Scholar]
- 9.Hazel J R, Williams E E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 10.Henderson R J, Millar R M, Sargent J R, Jostensen J P. trans-Monoenoic and polyunsaturated fatty acids in phospholipids of a Vibrio species of bacterium in relation to growth conditions. Lipids. 1993;28:389–396. doi: 10.1007/BF02535935. [DOI] [PubMed] [Google Scholar]
- 11.Jannasch H W, Wirsen C O. Variability of pressure adaptation in deep sea bacteria. Arch Microbiol. 1984;139:281–288. [Google Scholar]
- 12.Johns R B, Perry G J. Lipids of the marine bacterium Flexibacter polymorphus. Arch Microbiol. 1977;114:267–271. [Google Scholar]
- 13.Kayama M. Essential fatty acids, their metabolism and function. In: Kayama M, editor. AA, EPA, DHA-highly unsaturated fatty acids. Tokyo, Japan: Koseisha Koseikaku; 1995. pp. 44–81. [Google Scholar]
- 14.MacDonald A G. The role of membrane fluidity in complex processes under high pressure. In: Marquis R E, Zimmerman A M, Jannasch H W, editors. Current perspective in high pressure biology. London, United Kingdom: Academic Press; 1987. pp. 207–223. [Google Scholar]
- 15.Marr A G, Ingraham J L. Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol. 1962;84:1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh D, editor. Handbook of lipid bilayers. Boca Raton, Fla: CRC Press; 1990. pp. 211–225. [Google Scholar]
- 17.Morris R J, Culkin F. Fish. In: Ackman R G, editor. Marine biogenic lipids, fats, and oils. Boca Raton, Fla: CRC Press; 1989. pp. 146–166. [Google Scholar]
- 18.Nakayama A, Yano Y, Yoshida K. New method for isolating barophiles from intestinal contents of deep-sea fishes retrieved from the abyssal zone. Appl Environ Microbiol. 1994;60:4210–4212. doi: 10.1128/aem.60.11.4210-4212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oleary W M, Wilkinson S G. The family Bacillaceae. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 1. London, United Kingdom: Academic Press; 1988. pp. 155–164. [Google Scholar]
- 20.Oliver J D, Colwell R R. Extractable lipids of gram-negative marine bacteria: fatty-acid composition. Int J Syst Bacteriol. 1973;23:442–458. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 22.Ringø E, Sinclair P D, Birkbeck H, Barbour A. Production of eicosapentaenoic acid (20:5 n-3) by Vibrio pelagius isolated from turbot (Scophthalmus maximus (L.)) larvae. Appl Environ Microbiol. 1992;58:3777–3778. doi: 10.1128/aem.58.11.3777-3778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose A H. Influence of the environment on microbial lipid composition. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 2. London, United Kingdom: Academic Press; 1988. pp. 255–278. [Google Scholar]
- 24.Russell N J, Fukunaga N. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. FEMS Microbiol Rev. 1990;75:171–182. [Google Scholar]
- 25.Sinensky M. Homeoviscous adaptation: a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkman J K, Jeffrey S W, Nichols P D, Rogers G I, Garland C D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol. 1989;128:219–240. [Google Scholar]
- 27.Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7, 10, 13, 16, 19-docosapentaenoic acid to 4, 7, 10, 13, 16, 19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991;266:19995–20000. [PubMed] [Google Scholar]
- 28.Wada H, Murata N. Temperature-induced changes in the fatty acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990;92:1062–1069. doi: 10.1104/pp.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe K, Ishikawa C, Yazawa K, Kondo K, Kawaguchi A. Fatty acid and lipid composition of an eicosapentaenoic acid-producing marine bacterium. J Mar Biotechnol. 1996;4:104–112. [Google Scholar]
- 30.Wilkinson S G. Gram-negative bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 2. London, United Kingdom: Academic Press; 1988. pp. 299–457. [Google Scholar]
- 31.Williams E E, Hazel J R. Thermal adaptation in fish membranes; temporal resolution of adaptive mechanisms. In: Cossins R R, editor. Temperature adaptation of biological membranes. London, United Kingdom: Portoland Press; 1994. pp. 91–106. [Google Scholar]
- 32.Wirsen C O, Jannasch H W, Wakeham S G, Canuel E A. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr Microbiol. 1987;14:319–322. [Google Scholar]
- 33.Yano Y, Nakayama A, Saito H, Ishihara K. Production of docosahexaenoic acid by marine bacteria isolated from deep sea fish. Lipids. 1994;29:527–528. doi: 10.1007/BF02578252. [DOI] [PubMed] [Google Scholar]
- 34.Yano Y, Nakayama A, Yoshida K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl Environ Microbiol. 1997;63:2572–2577. doi: 10.1128/aem.63.7.2572-2577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yayanos A A, Dietz A S, Van Boxtel R. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979;205:808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- 36.Yayanos A A, Dietz A S, Van Boxtel R. Dependence of reproduction rate on pressure as a hallmark of deep-sea bacteria. Appl Environ Microbiol. 1982;44:1356–1361. doi: 10.1128/aem.44.6.1356-1361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]