Abstract
COVID-19, caused by SARS-CoV-2, has had a significant global impact. While vaccines and treatments have reduced severe cases and deaths, the long-term effects are not yet well understood. Current models used for research, such as non-human primates and transgenic mice, are expensive and require scarce Biosafety Level-3 (BSL-3) laboratories, thereby limiting their practicality. However, the Murine Hepatitis Virus-1 (MHV-1) mouse model offers a promising alternative. This surrogate model can be investigated in more widely available Biosafety Level-2 (BSL-2) laboratories. Furthermore, mice are affordable and easy to handle, and utilizing MHV-1 as a surrogate for SARS-CoV-2 eliminates the need for costly transgenic mice. Importantly, the MHV- 1 model successfully recapitulates COVID-19-related clinical symptoms, weight loss, multiorgan pathological changes and failure in acute stages, and irreversible neurological complications and other organ dysfunction, long-term post-infection, which are similar to available human data post- COVID-19. To assist researchers in establishing and employing the MHV-1 mouse model, this protocol offers comprehensive guidance encompassing procedures for animal preparation, induction of viral infection, clinical observation, pathological changes, and tissue analysis (for mechanistic studies), thereby yielding valuable insights into disease mechanisms and progression. By adopting the MHV-1 model and the provided protocols, researchers can effectively circumvent financial constraints and the limited availability of BSL-3 laboratories, thus facilitating a more accessible and cost-effective approach to investigating the underlying mechanisms of SARS-CoV-2 pathophysiology and exploring potential therapeutic interventions.
Basic Protocol: Induction of Mouse Hepatitis Virus 1 (MHV-1) infection in A/J mice
Support Protocol 1: Histological evaluation
Support Protocol 2: Liver enzymes measurement
Support Protocol 3: Western blot analysis
Support Protocol 4: mRNA measurement
Support Protocol 5: Immunohistochemistry/Immunofluorescence
Support Protocol 6: Tissue water measurement
Keywords: COVID-19, SARS-CoV-2, mice, MHV-1, surrogate model
INTRODUCTION:
COVID-19 is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first emerged in 2019 in Wuhan, China. This infectious disease has had a profound impact on various aspects of society, including public health, economics, and daily living. As of June 2023, the World Health Organization (WHO) has reported nearly 768 million confirmed cases of COVID-19 worldwide, with a staggering death toll surpassing 6.9 million people (World Health Organization, 2020). There are about 219 thousand confirmed cases per week as of June 12, 2023. Although extensive research has been conducted on COVID-19, resulting in the development of vaccines and treatments that have significantly mitigated severe infections and fatalities, our understanding of the long-term consequences of viral infection remains limited. To comprehend this crucial aspect, it is imperative to identify an animal model that is accessible, cost-effective, and relevant to humans.
Establishing an effective animal model for studying SARS-CoV-2 presents significant challenges. First, many proposed models require working directly with the SARS-CoV-2 virus and therefore necessitate the use of Biosafety Level-3 (BSL-3) laboratories. Yet only 434 institutions worldwide and 148 institutions in the United States possess such facilities (Schuerger et al., 2022). Consequently, research involving direct exposure to SARS-CoV-2 is restricted to a limited pool of researchers with access to these facilities. Financial considerations associated with operating BSL-3 facilities further curtail their general use. Second, selecting an appropriate animal model poses its own set of challenges. Models involving non-human primates, recommended for their physiologic and immunologic similarity to humans, are costly and challenging to handle. Conversely, mouse models offer affordability and manageability; however, wild-type and standard laboratory mouse strains lack the expression of ACE2, the receptor necessary for viral entry and replication, rendering them resistant to SARS-CoV-2 infection. Therefore, mice appropriate for study of SARS-CoV-2 infection must be genetically modified to express human ACE2 receptors. While certain modified strains (such as K18-hACE2, mACE2-hACE2, and endogenous mACE2 mice) have been or can be produced, they often face issues of limited availability or high production costs. Further, these genetically modified mice demonstrate a more severe disease progression than is commonly observed in humans (Caldera-Crespo et al., 2021).
One mouse model that addresses the aforementioned challenges for COVID-19 studies is predicated upon the infection of mice using a surrogate coronavirus, specifically the Murine Hepatitis Virus-1 (MHV-1) (Caldera-Crespo et al., 2021). The pathology elicited by MHV-1-infected mice closely mimics the clinical manifestations observed in humans infected with SARS-CoV-2, encompassing both acute and long-COVID (Paidas et al., 2021, 2022a, 2022b). Notably, it successfully recapitulates the histopathological changes in the infected tissues analogous to those observed in SARS-CoV-2-infected patients (Caldera-Crespo et al., 2021). The utilization of MHV-1 as a surrogate of SARS-CoV-2 enables research to be conducted within the less stringent Biosafety Level-2 (BSL-2) laboratories and, moreover, permits the use of relatively affordable mice that are simple to manage and maintain.
This article provides a comprehensive exposition of the establishment, sustenance, and application of the MHV-1 mouse model for investigating COVID-19. The fundamental methodology delineates a range of pivotal facets, encompassing the preparation and upkeep of the experimental animals, the induction of MHV-1 viral infection, the observation of clinical manifestations, and the extraction of organ tissue for subsequent analysis (see Basic Protocol). In addition, this article provides detailed protocols for conducting various analyses on the collected tissue samples, ensuring a comprehensive understanding of the experimental outcomes. These analyses encompass histological evaluation (see Support Protocol 1), liver enzyme measurement (see Support Protocol 2), western blot analysis (see Support Protocol 3), mRNA measurement (see Support Protocol 4), immunohistochemistry/immunofluorescence (see Support Protocol 5), and tissue water measurement (see Support Protocol 6). By employing these diverse techniques, researchers can obtain a multidimensional characterization of the MHV-1 mouse model, enhancing the elucidation of COVID-19 pathogenesis and associated mechanisms.
CAUTION: Murine Hepatitis Virus-1 (MHV-1) is a Biosafety Level-2 (BSL-2) pathogen. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms.
NOTE: All animal care and experimental protocols received approval from the University of Miami Institutional Animal Care and Use Committee (IACUC) under protocol number 20–131 LF and were performed in accordance with both national and institutional guidelines.
BASIC PROTOCOL: Induction of Mouse Hepatitis Virus 1 (MHV-1) infection in A/J mice
MATERIALS:
Female A/J wildtype mice (8 weeks old, ~22g) (Jackson Laboratories, cat# 000646, Bar Harbor, ME)
MHV-1 (American Type Culture Collection, cat# VR-261, Manassas, VA)
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, cat# 11965–092, lot# 2186816, ThermoFisher Scientific, Waltham, MA).
Ketamine/Xylazine cocktail (see recipe in Reagents and Solutions)
10% formalin (Azer Scientific, cat# 22–026-449, lot# 22315, Morgantown, PA) or similar Standard lab chow (Envigo 2918 irradiated) (Teklad Diets, Madison, WI)
Water (50:50, autoclaved to tap water)
Micro-isolated cages (BCU-2) (Allentown LLC, Allentown, NJ) or similar Plastic mouse holder (see Fig. 1)
Electronic scale (Taylor, ref# 3807BK21, St. Louis, MO) or similar
Figure 1.

(A) A micropipette tip containing the viral inoculation solution is presented near a mouse’s nares for inhalation. (B) Mice are placed within a plastic holder, angled strategically to guarantee complete inhalation of the viral inoculation solution.
PROTOCOL STEPS:
Preparation and maintenance of mice
-
1
Divide the mice into 3 groups - (1) healthy controls (non-sham), (2) infusion of healthy controls with DMEM (sham), (3) infusion with MHV-1 (experimental).
A control (non-sham) group is added to compare and investigate pathological changes with DMEM alone (sham) group.
-
2
Place mice into micro-isolated cages.
-
3
Feed the mice a standard lab chow diet and water ad libitum.
Preparation of mice infected with MHV-1
-
4
Weigh the mice to establish a baseline.
-
5
Anesthetize the mice by intraperitoneal injection with Ketamine/Xylazine solution at a ratio of 0.1 mL per 20g of mouse weight.
Ensure the successful anesthetization of the mice using the pedal reflex. Using this method of anesthetization, dose, and injection site ensures that mice are anesthetized for a minimum of 20 minutes and a maximum of 35 minutes (thus allotting adequate time for viral inoculation).
-
6
Prepare viral inoculation solution by mixing 5000 PFU MHV-1 with 50 μL of ice-cold Dulbecco’s modified Eagle’s medium (DMEM).
To investigate the milder effects on mice, conducting dose-response experiments would be beneficial. For instance, the mice can be administered different concentrations of MHV-1, such as 1000, 2000, 3000 or 4000 PFU, through infusion. Lower viral loads can be employed to simulate the conditions observed in non-hospitalized COVID patients, thereby providing an opportunity to study the long-term consequences of infection.
-
7
Administer the viral inoculation solution to the area located just under the nares of the mice. For the experimental group, transfer 50 μL of the prepared viral inoculation solution using a 100 or 200 μL micropipette and deliver it near the nasal region. In the case of the sham group, administer 50 μL of DMEM alone.
Avoid administration of the viral inoculate into the nose of the mice as that will cause ejection of the solution.
-
8
Monitor the mice until the complete inhalation of the solution. Once the last drop has been inhaled, place the animal in a slanted position within a plastic holder (Fig. 1) and maintain this position for a minimum of 10 minutes to make sure the solutions are inhaled completely.
Monitoring of mice for weight changes and clinical signs of infection
-
9
Record the body weight of the mice 24 hours after inoculation and continue this assessment daily until the conclusion of the study period.
For the acute studies, maintain daily weight measurements for 12 days following the inoculation. For the chronic studies, conduct daily weight measurements for 12 days post-inoculation, followed by weekly measurements for a duration of 1 year.
-
10
Monitor mice daily for clinical signs and record disease stage: (0) no clinical signs; (I) drowsy and lack of movement; (II) slightly ruffled fur and altered hind limb posture; (III) ruffled fur and mildly labored breathing; (IV) ruffled fur, inactive, and moderately labored breathing; V) ruffled fur, labored breathing and lethargy; and (VI) moribund and death. This monitoring regimen is based on the FELASA Working Group Report.
Euthanization of mice, collection of blood, and harvesting of organs
-
11
Weigh the mice that have reached a disease score of V or VI or reached the end of the study period, and then administer anesthesia using ketamine/xylazine as described above.
Mice for acute studies can be evaluated for up to 14 days, while longer durations spanning from 3 weeks to years can be utilized for chronic studies. Mice surviving after inoculation with MHV-1 can be monitored for extended periods to analyze injury to the CNS and other organs.
-
12
Draw blood via cardiac puncture (up to 0.60 ml) through the left ventricle using a 23–25- gauge needle (non-survival method) to determine the extent of liver failure following viral inoculation (see Support Protocol 2).
Serum can also be utilized for viral titer analysis. Note that viral titer measurements were not performed given the parent virus obtained from American Type Culture Collection (ATCC) (parent virus) was administered immediately upon arrival. Instead, the virus was directly measured in tissues based on reviewers’ advice in all our published manuscripts.
-
13
Euthanize the mice with CO2 and collect various organs, including the lung, liver, kidney, heart, and brain.
Avoid decapitation due to the potential spillage and spread of the virus, not only within the guillotine but also in the surrounding area.
-
14
Place one half of each organ immediately in 10% formalin (15 ml in 50 ml plastic tubes) to ensure proper fixation for subsequent histopathological examination. Freeze the other half of each organ in 15 ml plastic tubes and store them at -80°C for future molecular assays and other relevant studies.
SUPPORT PROTOCOL 1: Histological evaluation
INTRODUCTORY PARAGRAPH:
The histopathological study of tissues constitutes a major component in assessing the ramifications of MHV-1 viral infection. Although this evaluation is inherently semi-quantitative, it facilitates an in-depth analysis of cytopathological and morphological alterations across various organs that can then be correlated with necropsy findings, clinical chemistry data, and clinical observations. For preliminary assessment of tissue morphological changes, conventional hematoxylin/eosin staining of paraffin-embedded tissue sections or immunohistochemistry techniques are preferred. However, for granular investigations, more specific staining techniques may be subsequently employed.
MATERIALS:
Tissue samples preserved in formalin
Hematoxylin (Harris Formula) (Leica Biosystems Inc, cat# 3801560, Deer Park, IL) Eosin (Leica Biosystems Inc, cat# 3801600, Deer Park, IL)
Tissue processor (ASP300S Fully Enclosed Tissue Processor) (Leica Biosystems Inc, Deer Park, IL) or similar
Paraffin embedding station (HistoCore Arcadia H - Heated Paraffin Embedding Center, Leica Biosystems Inc, Deer Park, IL) or similar
Automated rotary microtome (RM2255 Fully Automated Rotary Microtome, Leica Biosystems Inc, Deer Park, IL or a similar instrument)
Microscope (Olympus VS120 Automated Slide Scanner, Olympus, Pittsburg, PA or a similar instrument).
Slides (X-tra Slides, cat# 3800050, Lecia Biosystems Inc, Deer Park, IL)
Paraffin (Paraplast) (Leica Biosystem Inc, cat# 39601006, Deer Park, IL)
PROTOCOL STEPS:
Collect the necessary samples that have been previously preserved in formalin.
-
Process and embed tissue into paraffin blocks using standard histologic technique.
This may require help and input from a pathology lab technician.
Cut into 10-μm sections with a microtome, place sections on microscope slides, and stain with hematoxylin and eosin (H&E) according to standard procedures (Paidas et al., 2021, 2022a, 2022b).
-
Capture images of H&E-stained tissue slides at 20x magnification using a microscope and evaluate with a pathologist unaware of the treatment protocol.
The expertise of multiple pathologists (neuropathologist, anatomical and cytopathologist) can be engaged for blinded evaluation of tissue samples.
SUPPORT PROTOCOL 2: Liver enzyme measurement
INTRODUCTORY PARAGRAPH:
Quantification of liver enzyme levels serves as an indicator of the impact of MHV-1 viral infection on its target organ. Concurrently, such measurement is equally pertinent for evaluating the impact of SARS-CoV-2 viral infection across various tissues, despite the liver not being a direct target and the viral damage being more ubiquitously distributed.
MATERIALS:
Blood samples
Centrifuge Tubes (Eppendorf Safe-Lock Tubes, 1.5 ml, cat# 0030123611, Enfield, CT)
Automatic analyzer system (Cobes c501 Automatic Analyzer) (Roche Diagnostics, Indianapolis, IN or a similar instrument)
Mini Centrifuge (ThermoFisher Scientific mySPIN™, cat# 75004083, Waltham, MA or a similar instrument)
Serum storage equipment (e.g., freezers)
PROTOCOL STEPS:
Centrifuge the blood samples at 1000 RPM for 5 minutes.
-
Remove the supernatant (clear fluid) and store separated serum appropriately.
Separated serum should not be exposed to temperatures higher than 10–20 °C for more than 8 hours. If assays cannot be completed within 2 days or if separated samples need to be stored for more than 2 days, it is recommended to freeze the samples. Once frozen, the samples can be thawed only once.
-
Measure the liver enzymes – aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin – in separated serum using the automatic analyzer system. Record the values as units per liter.
There is no limitation in using this method or interfering substances or conditions. The lower limit for the analysis is 10 units while there is no upper limit. However, when the values are high, the samples can be diluted appropriately if needed.
SUPPORT PROTOCOL 3: Western blot analysis of aquaporin expression.
INTRODUCTORY PARAGRAPH:
One potential outcome of the immune and inflammatory responses elicited during various viral infections, including SARS-CoV-2, is the progressive onset of generalized edema (Wiggli et al., 2013; Rump et al., 2018). Utilizing a Western blot technique, it has been demonstrated that this phenomenon is due to upregulation of aquaporins (AQPs), or endothelial water channels, in multiple organs (Rump et. Al., 2018). Furthermore, evidence suggests that the emergence of these channels and subsequent edema is implicated in organ malfunction within the context of the MHV-1 viral infection model. Such observations underscore the legitimacy of this model as a representative COVID analog, particularly given that pulmonary and systemic edema are salient pathological outcomes of SARS-CoV-2 infection.
MATERIALS:
AQP1 Polyclonal Antibody (cat# A53228–020, EpiGentek, Farmingdale, NY) AQP4 Polyclonal Antibody (cat# A50672–020, EpiGentek, Farmingdale, NY)
AQP5 Polyclonal Antibody (cat# BS-1554R-TR, Bioss Antibodies, Woburn, MA)
AQP8 Polyclonal Antibody (cat# PA1511, Boster Biological Technology, Pleasanton, CA)
β-actin Mouse Monoclonal Antibody (C4, cat# SC-47778, Santa Cruz Biotechnology, Dallas, TX)
Horseradish Peroxidase-conjugated Anti-rabbit Secondary Antibody (1:1000) (Ref# PI- 1000, LOT# ZH0309, Vector Laboratories, Newark, CA)
Horseradish Peroxidase-conjugated Anti-mouse Secondary Antibodies (1:1000) (Ref# PI- 2000, LOT# ZG1208, Vector Laboratories, Newark, CA)
SigmaScan Pro software (Systat Software, San Jose, CA)
PROTOCOL STEPS:
Preform gel electrophoresis and immunoblotting with the selected tissues from all groups as described previously (Jayakumar, Tong, et al., 2014; Jayakumar, Valdes, et al., 2014).
Prepare and use the primary antibodies in separate lanes – AQP1 Polyclonal Antibody, AQP4 Polyclonal Antibody, AQP5 Polyclonal Antibody, AQP8 Polyclonal Antibody, and β-actin mouse monoclonal antibody – at a 1:2000 dilution.
Prepare and use horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies at a 1:5000 dilution.
-
Use the SigmaScan Pro software to quantify the optical density of the bands relative to the signal of β-actin, or a similar housekeeping protein.
Make sure to calibrate the intensity using the 2-point linear remapping setting in SigmaScan Pro (from uncalibrated to calibrated intensity 1 and 2) before acquiring the total area and intensity measurements. Remember to normalize the intensity obtained relative the housekeeping protein band, in this case β-actin.
SUPPORT PROTOCOL 4: Measurement of mRNAs
INTRODUCTORY PARAGRAPH:
Evaluating mRNA levels offers a comprehensive methodology for gauging an array of infection-associated abnormalities. These encompass viral levels, the activity of various signaling pathways, and the expression of critical immunologic factors within bodily fluids and tissues.
MATERIALS:
Frozen samples
RNAqueous®-4 PCR kit (cat# AM1914, Ambion, Austin, TX)
High-Capacity cDNA reverse transcription kit (cat# 4368814, Applied Biosystems, Foster City, CA)
RT-qPCR SYBR GREEN reagents (Brilliant® II SYBR® Green QPCR Master Mix, cat# 600828, Agilent Technologies, Santa Clara, CA)
Mx3005P qPCR system (cat# 401513, Agilent Technologies, Santa Clara, CA) or similar MxPro-Mx3005P qPCR software (v4.10, Agilent Technologies, Santa Clara, CA)
PROTOCOL STEPS:
Thaw previously frozen tissue.
Isolate the mRNA as previously described (Jayakumar, Valdes, et al., 2014).
Use the RNAqueous®-4PCR kit to isolate the mRNA (following the instructions provided by the manufacturer).
Generate the cDNA using the High-Capacity cDNA Reverse Transcription kit (following the instructions provided by the manufacturer).
-
Conduct the RT-qPCR analysis using the Mx3005P Multiplex Quantitative PCR System with the RT-qPCR SYBR GREEN reagents. Utilize ROX as the reference dye and maintain a final reaction volume of 25 μL. The cycling conditions are as follows: (a) 1 cycle at 95°C for 10 minutes; (b) 40 cycles at 95°C for 30 seconds; (c) 1 cycle at 58°C for 30 seconds; and (d) 1 cycle at 72°C for 15 seconds.
Incorporate negative controls by substituting the cDNA templates with nuclease-free water.
-
Determine the crossing points for each amplification reaction by using the MxPro- Mx3005P v4.10 software.
The NMDAR1 forward primer sequence consists of 5′-GCAAGAATGAGTCAGCCCAC-3′. The NMDAR1 reverse primer consists of 5′-CAGTCACTCCGTCCGCATAC-3′.
Normalize the RT-qPCR data against glyceraldehyde 3-phosphate dehydrogenase.
Repeat each RT-qPCR experiment at least three times to document its reproducibility.
SUPPORT PROTOCOL 5: Immunohistochemistry/Immunofluorescence
INTRODUCTORY PARAGRAPH:
While assessing viral titers in serum derived from infected animals can be informative, quantifying the virus directly within tissues facilitates a more precise characterization of viral pathogenesis. Utilizing immunohistochemistry or immunofluorescence methods – especially when combined with confocal microscopy – enhances the precision in localizing viral particles within tissue samples. Additionally, this approach aids in discerning cell-specific expression patterns of various signaling molecules in relation to infection severity.
MATERIALS:
Paraffin-embedded brain tissue sections (see Support Protocol 1)
Purified anti-TDP43 Phospho Antibody (1:100) (Ser409/410, cat# 829901, BioLegend, San Diego, CA)
Phospho-PHF-tau pSer202 + Thr205 Antibody (AT8, Life Technologies Corporation, cat# MN1020, 1:100 dilution)
Anti-synaptophysin antibody (rabbit monoclonal, YE269, 1: 150 dilution, cat# 32,127, Abcam, Cambridge, MA, USA)
Ionized calcium-binding adapter molecule 1 (Iba1, FL-147: sc-98468, 1:200 dilution, Santa Cruz Biotechnology, Inc. Dallas, TX, USA)
Purified mouse Anti-GFAP (Cat# 556,328, 1:100 dilution, BD Pharmingen™, BA1 1BE UK)
Alexa Flour-488/Alexa Flour-546 goat anti-mouse/rabbit or appropriate IgG (H + L) (Life Technologies)
Duolink® In Situ Mounting Medium with DAPI (Sigma-Aldrich, Cat. No. DUO82040– 5ML, St. Louis, MO)
Zeiss LSM510/UV Axiovert 200 M confocal microscope with a Plan Apochromat (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA) or a similar instrument
Volocity 6.0 High-Performance Cellular Imaging Software (PerkinElmer, Waltham, MA, USA)
PROTOCOL STEPS:
Inoculate paraffin-embedded brain tissue sections from healthy control (non-sham) and MHV-1-inoculated (experimental) mice with 10 microns of each of the following: anti- TDP43 Phospho (Ser409/410) antibody, Phospho-PHF-tau pSer202 + Thr205 Antibody, anti-synaptophysin antibody, ionized calcium-binding adapter molecule 1 (Iba1), and purified mouse Anti-GFAP.
Wash sections and incubate in respective Alexa Flour-488/Alexa Flour-546 goat anti- mouse/rabbit or appropriate IgG (H + L) at a concentration of 1:200.
Acquire immunofluorescent images with a Zeiss LSM510/UV Axiovert 200 M confocal microscope with a Plan Apochromat 40 × objective lens and 2 × zoom, resulting in images of 125 × 125 μm in area and 1.0-μm optical slice thickness (1.0 Airy units for Alexa Fluor 546 or 568 emission channel).
-
Obtain a random collection of images from sections of healthy control (non-sham) and MHV-1-inoculated (experimental) mice by systematically capturing each image in a blinded manner by moving the microscope stage approximately 5 mm in four different directions.
Capture at least 14 fluorescent images per sample to improve statistical power.
Quantify images using the Volocity 6.0 High-Performance Cellular Imaging software as described previously (Jayakumar et al., 2014a; 2014b) and normalize to the number of DAPI-positive cells and the area and intensity of DAPI.
SUPPORT PROTOCOL 6: Tissue water measurement
INTRODUCTORY PARAGRAPH:
As previously mentioned, tissue edema is a well-documented sequela of viral infections, notably in the context of SARS-CoV-2. This pathological phenomenon correlates with the intensity of the immune and inflammatory processes at play and, in turn, may contribute to progressive generalized edema and subsequent mortality. (Paidas et al., 2022a).
MATERIALS:
Freshly excised tissue samples
Oven (10GC ANALOG LAB OVEN, Quincy Lab, Burr Ridge, IL) or similar Electronic scale (Taylor, 3807BK21, St. Louis, MO) or similar
PROTOCOL STEPS:
Measure the tissue water content by the wet/dry weight method as described previously (Jayakumar et al., 2011; Jayakumar et al., 2014b; Paidas et al., 2022a):
-
Ascertain 10 mg of the tissue of interest.
8 pieces of tissue from each mouse is recommended.
Weigh the tissue samples using an electronic scale to determine the wet weights.
Dry the tissue samples in an oven at 100°C overnight.
Weigh the tissue samples using an electronic scale to determine the dry weights.
Calculate the differences between the wet and dry weights and express as percentages.
REAGENTS AND SOLUTIONS:
- Ketamine/Xylazine Cocktail
- 87.5 mg/kg Ketamine
- 12.5 mg/kg Xylazine
COMMENTARY: BACKGROUNDINFORMATION:
Numerous animal models suitable for studying SARS-CoV-2 infection, vaccine efficacy, and therapeutic interventions harbor limitations that curtail their utility. A paramount challenge is the requisite for Biosafety Level-3 (BSL-3) facilities to safeguard the investigators and staff from potential self-infection. As mentioned in the introduction, such facilities are either scarce or entail significant expenditure.
The selection of an appropriate species poses yet another challenge in determining a suitable animal model. While employing non-human primates presents obvious financial and managerial difficulties, they might also fail to recapitulate predominant COVID-19 symptoms observed in humans. This is particularly relevant to pulmonary symptoms, as SARS-CoV-2 viral replication in certain primate species is restricted to the gastrointestinal tract. Mouse models, with their affordability and ease of handling, appear to circumvent many of these challenges; however, mice inherently lack the ACE2 receptor expression necessary for SARS-CoV-2 infection, necessitating genetic alterations. In practical terms, this approach may be financially limiting, and theoretically, the ectopic nature of ACE2 may modify tissue or cellular viral tropism, leading to COVID-19 manifestations inconsistent with human presentation. Among the considered mouse models, the Murine Hepatitis Virus-1 (MHV-1) mouse model has emerged as a highly promising research tool for COVID-19 studies. Importantly, this model retains the intrinsic advantages of a mouse model and the use of less stringent Biosafety Level-2 (BSL-2) laboratory settings, while supporting an infection in normal (un-modified) mice that closely mimics the acute and chronic characteristics of human SARS-CoV-2 infection (Paidas et al., 2021, 2022a, 2022b). This resemblance stems from the taxonomic kinship between SARS-CoV-2 and MHV-1 as members of the beta coronaviruses family, differentiated only by their cellular entry mechanisms: SARS-CoV-2 via ACE2 and MHV-1 via CEACAM-1. Thus, both viruses induce analogous histopathological changes in infected tissues – including the lungs (Paidas et al., 2021, 2022a, 2022b).
CRITICAL PARAMETERS:
For an accurate evaluation of infection severity under various conditions, it is imperative that the assessment of clinical symptoms and pathological alterations be performed by separate and independent investigators. This method ensures that the relatively subjective clinical parameters remain uninfluenced by the comparatively objective pathological findings.
TROUBLESHOOTING:
When mice fail to exhibit symptoms or show disease progression, researchers should consider the accuracy of dosage/viral load, the age of the animals, and the possibility of covert infections.
Accurate dosage administration can be assessed through viral titers, ensuring precise determination of viral particle concentration. Notably, a lower viral load may not induce symptoms or illness. This consideration is particularly relevant when working with newly acquired or propagated viruses or in uncertain source/handling situations. However, if the parent virus is obtained from reputable sources like the American Type Culture Collection (ATCC) or meticulously cultivated under stringent quality control measures and promptly utilized upon receipt, viral titer determination may not be necessary.
Additionally, the age of the animals should be taken into account as it can contribute to the absence of symptoms or disease progression. Thoroughly analyzing the age distribution of the animal cohort and identifying potential age-related confounding factors is crucial to understanding their impact on the observed lack of symptoms or disease progression.
Researchers should also be alert to the possibility of covert infections, where animals may be sick without obvious changes. Conducting a careful clinical evaluation, monitoring physiological parameters, and employing appropriate diagnostic techniques can help detect subtle indications of illness or distress, ensuring animal welfare and reliable experimental outcomes.
UNDERSTANDING RESULTS:
Effects on bodyweight
MHV-1 infection in mice results in distinct weight change phases depending on severity of infection. Post-inoculation with a dose of virus described in this protocol and with clinical signs of usual severity, a 20% reduction in initial weight is observed by day 3, followed by a reduction of 25–30% by days 4 and 5 and a reduction of 35–40% by days 7 to 12 (Fig. 2). However, mice displaying more severe clinical signs may experience more rapid weight loss by days 6–7, with a drop of approximately 25% to 40% below initial weight. The precise mechanisms underlying this rapid weight loss remain unclear but may involve factors commonly observed in SARS-CoV-2-infected humans, such as fluid loss, reduced fluid intake, and muscle or fat depletion. Body weight loss has of this magnitude has, in fact, been identified in severely affected COVID-19 individuals (Paidas et al., 2021).
Figure 2.
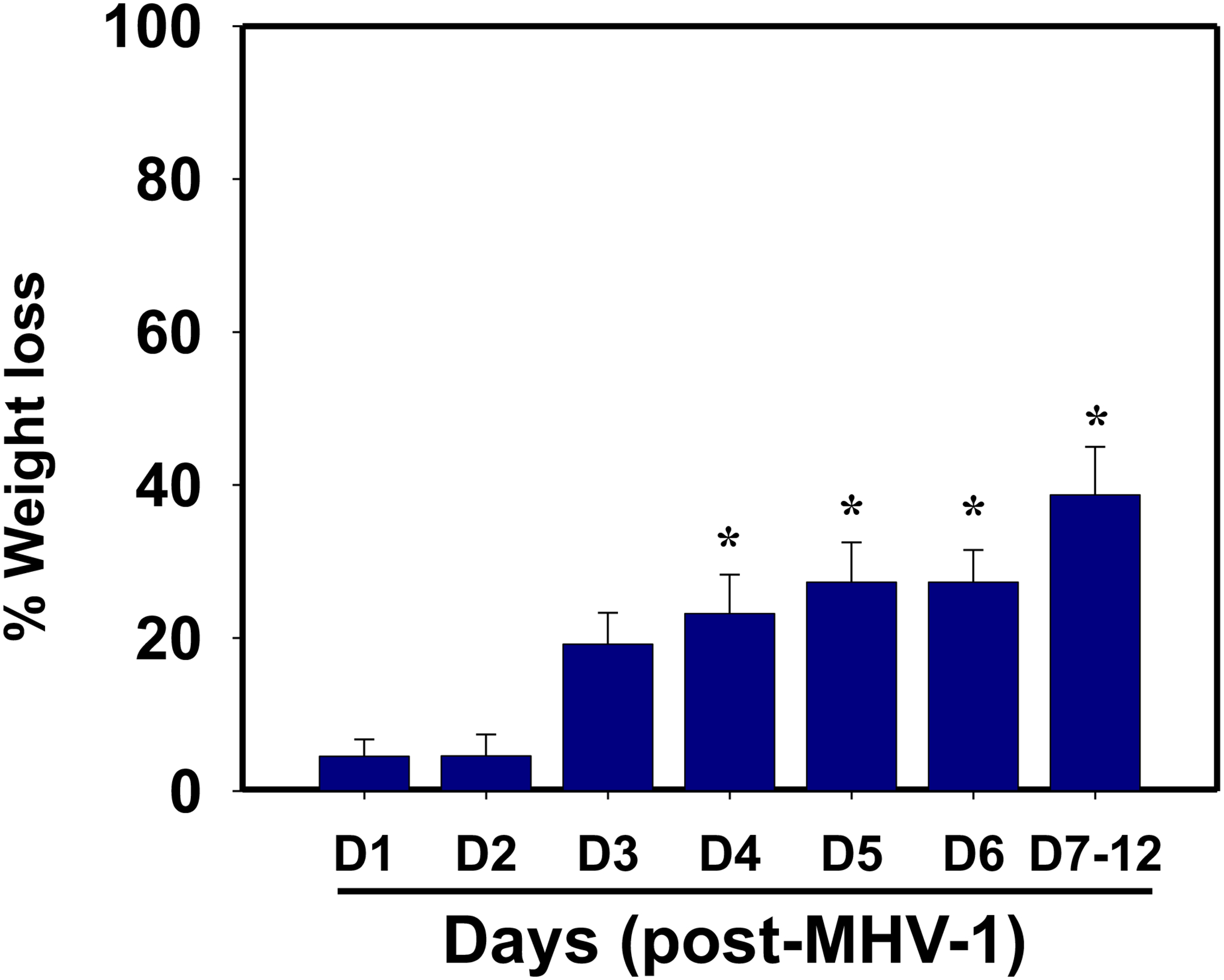
Body weight losses in MHV-1 infected mice. MHV-1 inoculated mice lost 20–40% of body weight over days 3–12, corresponding well with the severity of the disease (n=16). *p<0.05 versus control.
NOTE: Special permission from the IACUC is required for the conduct of studies resulting in weight loss of this magnitude since more than 15% body weight reduction is not ordinarily permittable.
In the subacute and chronic phases, there is a gradual recovery of weight starting after three weeks, but complete restoration to pre-infection weight levels is not achieved. Interestingly, MHV-1- infected mice showed a significant weight gain of 40% compared to uninfected mice up to three months post-inoculation. However, no further weight gain occurred beyond this period, extending up to twelve months (Paidas et al., 2022b).
Effects on liver enzyme levels
MHV-1 infection in mice exhibit distinct patterns of liver enzyme changes during different phases of the disease. In the acute phase, significant elevations in liver enzymes are observed, resembling the patterns seen in acute drug-induced liver failure or patients with SARS- CoV-2 infection. Specifically, MHV-1-infected mice displayed a substantial increase in aspartate transaminase (AST) levels compared to the control group (3459.2 ± 684.1 units/L in infected mice vs. 96.8 ± 14.2 units/L in controls, representing a 34.7-fold increase). Similarly, alanine transaminase (ALT) levels were significantly elevated in infected mice (3068.5 ± 861.3 units/L in infected mice vs. 31.5 ± 11.6 units/L in controls, corresponding to a 96.4-fold increase). Elevated alkaline phosphatase (ALP) and bilirubin levels were also observed in infected mice (986.3 ± 178.4 units/L of ALP in infected mice vs. 589.1 ± 108.7 units/L in controls, a 67% increase; and 0.86 ± 0.2 mg/L of bilirubin in infected mice vs. 0.075 ± 0.02 mg/L in uninfected mice, a 10.4-fold increase) (Table 1) (Paidas et al., 2021). Importantly, exposure of mice to DMEM did not result in liver enzyme alterations, indicating that the changes were specifically associated with MHV-1 infection. These findings strongly support the notion that MHV-1 induces severe liver injury, similar to what has been observed in patients with SARS- CoV-2 infection.
Table 1.
Acute and long-term effect of MHV-1 infection on liver enzymes.
| Uninfected mice | MHV-1 infected mice (7 day) | MHV-1 infected mice (12 month) | |
|---|---|---|---|
| AST (units/l) | 96.8 ± 14.2 | 3459.2 ± 684.1* | 412.8 ± 70.9† |
| ALT (units/l) | 31.5 ± 11.6 | 3068.5 ± 861.3* | 316.4 ± 56.1† |
| ALP (units/l) | 589.1 ± 108.7 | 986.3 ± 158.4* | 610.3 ± 106.8† |
| Bilirubin (mg/l) | 0.075 ± 0.02 | 086 ± 0.2* | 0.27 ± 0.05† |
Mean values ± SD.
statistically significant difference from uninfected mice.
AST, Aspartate amino transferase; ALT, Alanine amino transferase; ALP, alkaline phosphatase.
During the subacute and chronic phases of MHV-1 infection, the elevations in liver enzyme levels are less pronounced compared to those in the acute phase (Table 1) (Paidas et al., 2022b). Moreover, clinical signs of disease at 12 months post-infection are milder than those observed during the acute phase, characterized at this point by stage II-III clinical signs such as drowsiness, decreased movement, mildly ruffled fur, and mild respiratory distress. These findings resemble those seen in patients who, having recovered from SARS- CoV-2 infection, exhibit chronic liver injury.
Effects on clinical symptoms
During the acute phase, mice that have been inoculated with MHV-1 virus exhibit initial signs of illness such as drowsiness and reduced movement starting on day 2 post-inoculation. In a study of the symptom timeline, approximately 43.75% of the mice (n=16) showed signs of illness on the second day post-inoculation, and the percentage of mice displaying clinical signs gradually increased over time (56.25%, 62.5%, and 75.0% on days 3, 4, and 5, respectively) (Fig. 3A) (Paidas et al., 2021). It is important to note that the remaining 25% of mice did not exhibit any clinical signs throughout the acute observation period, which lasted up to 91 days.
Figure 3.
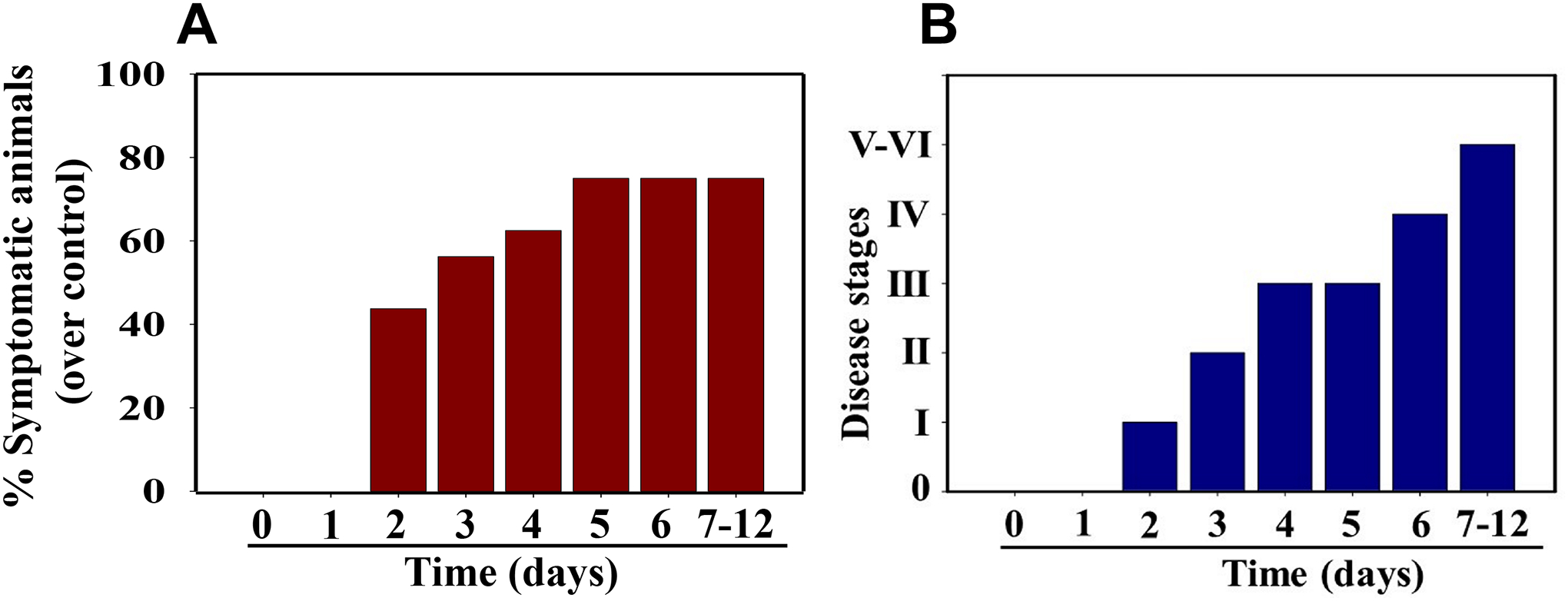
MHV-1 inoculated mice displayed signs of sickness. (A) About 40% of mice showed signs of sickness on day 2 post-MHV-1 inoculation, and the number of mice exhibiting clinical signs increased gradually (up to 75%). The remaining 25% of mice did not show any clinical signs (observed for up to 91 days). (B) MHV-1 inoculated mice showed clinical signs (from stages I to VI) from days 2 to 12 and beyond. Mild to moderate clinical signs (stages I–III) were observed from days 2 to 4. The MHV-1 infected mice showed severe sickness (stages IV-VI) from day 6 onward. Further, 60% of animals died from days 7 to 12 (n=16).
In the same study, the timeline of acute disease progression was as follows: Stage I symptoms (drowsiness + lack of movement) were observed on day 2 post-inoculation, while stage II symptoms (slightly ruffled fur + altered hind limb posture) were observed on day 3. Stage III symptoms (ruffled fur + mildly labored breathing) were observed on days 4 and 5, and stage IV symptoms (ruffled fur, inactivity, moderately labored breathing, and tremors) were observed on day 6. This was followed by stages V and VI (ruffled fur, obvious labored breathing, lethargy, a moribund state, and death) on days 7 to 12 (Fig. 3B) (Paidas et al., 2021). Figure 3A,B represent data from the same animals. For example, in Figure 3A, approximately 40% of the animals displayed clinical signs of infection, all of which were in stage I. On day 3, approximately 55–60% of the animals showed clinical signs of infection at stage II. The additional mice (10–20%) that exhibited clinical signs at later stages showed a similar severity to those that exhibited signs at earlier stages. Notably, mild diarrhea, characterized by a few loose stools in a single day, was observed during the early stages of illness (from day 2 to 4). However, diarrhea ceased between days 5 and 12 and was only observed in a few animals (5 out of 16 mice), which is consistent with observations in humans with SARS-CoV-2 infection. Additionally, severe venous thrombosis, a characteristic feature of SARS-CoV-2 infection, was observed in MHV-1 inoculated mice (4 out of 16 mice) from days 5 to 7 (Fig. 4) (Paidas et al., 2021). Less severe forms of thrombosis were also observed in MHV-1 inoculated mice from days 5 to 7 (7 out of 16).
Figure 4.

MHV-1 inoculated mice displayed severe disease. (A) Normal mice. (B) A representative mouse from the MHV-1 inoculated group showed venous thrombosis consistent with symptoms in patients with COVID-19 (n=11).
During the chronic phase (3 to 12 months post-inoculation), the clinical signs of the disease were less pronounced compared to the acute stages of infection. The mice displayed a range of stage II and stage III symptoms, which included drowsiness, lack of movement, ruffled fur, and mildly labored breathing (Paidas et al., 2022b). Importantly, exposing mice to DMEM alone had no impact on animal survival both acutely and chronically.
Effects on histopathology
Brain
The histopathological examination of brain samples obtained from mice infected with MHV-1 during the acute phase of infection reveals pathological changes that closely resemble those observed in humans during the acute stages of SARS-CoV-2 infection. Noteworthy observations included congested blood vessels, perivascular cavitation indicative of edema, pericellular halos, vacuolation of neuropils, darkly stained nuclei, pyknotic nuclei accompanied by vacuolation of the neuropil, and acute eosinophilic necrosis. Furthermore, the brain hippocampus of MHV-1-infected mice exhibited necrotic neurons displaying fragmented nuclei and vacuolation (Fig. 5) (Paidas et al., 2021).
Figure 5.
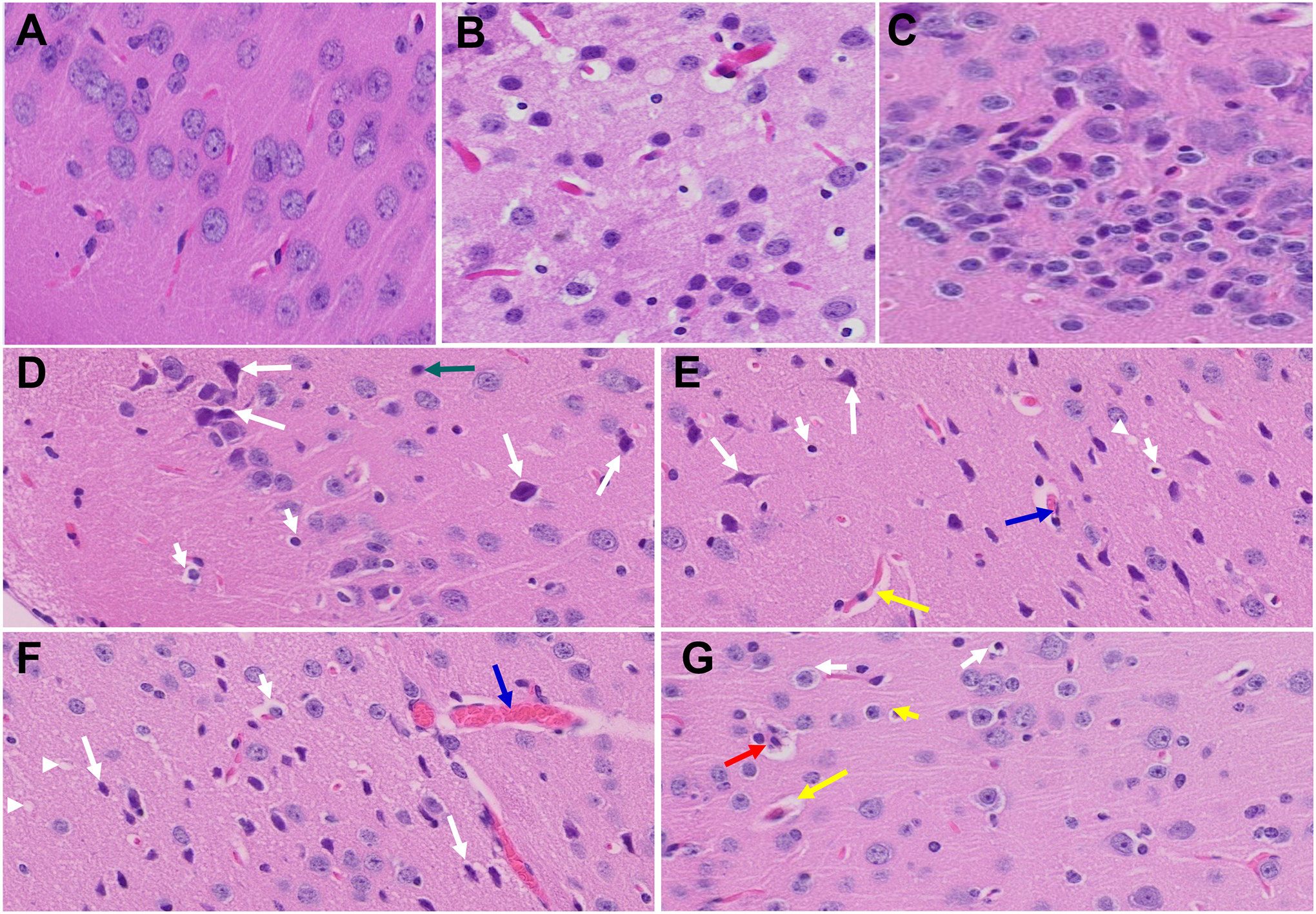
Acute and long-term changes in brain post-MHV-1 coronavirus infection. (A) Normal mouse brain cortex. (B) Representative image from MHV-1-infected mouse brain cortex showed “perivascular cavitation, congested blood vessel, pericellular halos, darkly stained nuclei, vacuolation of neuropil, pyknotic nuclei, and acute eosinophilic necrosis at 7 days (acute phase)” (Paidas et al., 2021). (C) MHV-1-infected mouse brain cortex (12 months post-infection). (D- G) Enlarged images of (C) showed widespread neuronal necrosis (long arrows), pyknotic nuclei/neuronal clearing (short arrows), vacuolation of neuropil (arrowhead), congested blood vessels (blue arrows), perivascular cavitation (yellow arrows, Virchow–robin space), darkly stained nuclei (green arrow), neuronophagia (red arrow, presence of necrotic neurons surrounded by invaded hypertrophic microglia (G)). (H&E, original magnification 400 × (A-C), and (D-G) are enlarged images of (C))
Subsequent examination of the brains of MHV-1-inoculated mice after a duration of 12 months post-infection revealed the persistence of nearly all the aforementioned changes observed during the acute stages of infection (Fig. 5) (Paidas et al., 2022b). Additionally, a wide spectrum of necrotic neurons was identified. Thus, if MHV-1 virus infection is indeed a mimic of SARS-CoV-2 infection, the latter has the potential to cause irreversible neurological damage.
Lung
The histopathological examination of the lungs of MHV-1-infected mice during the acute phase of infection is marked by inflammation characterized by the presence of cellular granular degeneration and leukocyte infiltration accompanied by an accumulation of proteinaceous debris in alveolar spaces consisting of fibrillar to granular eosinophilic protein strands. These changes can be attributed to deterioration of both capillary wall and epithelial integrity, thus enabling the leakage of protein-rich edematous fluid into the alveoli. The lungs at this stage also exhibit pulmonary congestion indicated by the presence of dilated capillaries, leakage of blood into the alveolar spaces and hemosiderin-laden macrophages (Fig. 6) (Paidas et al., 2021). Importantly, these histopathological features align with the observed pulmonary manifestations in humans infected with SARS-CoV-2 virus such as peribronchiolar interstitial cellular infiltration, necrosis of bronchiole epithelial cells, presence of necrotic cell debris within alveolar lumens, formation of hyaline membranes, alveolar hemorrhage and interstitial edema.
Figure 6.
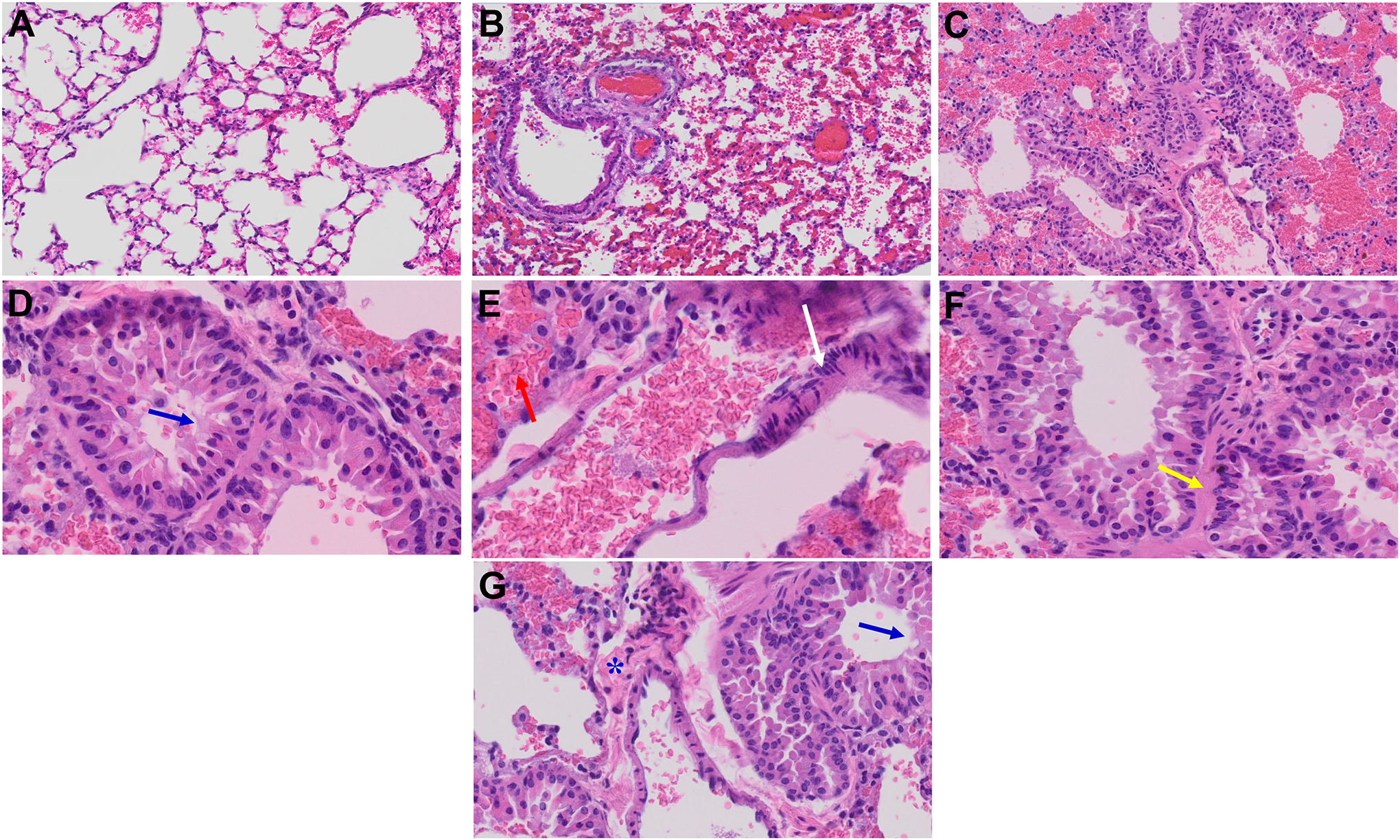
Acute and long-term changes in lung post-MHV-1 coronavirus infection. (A) Normal mouse lung. (B) Representative image from MHV-1-infected mouse lung showed “inflammation (i.e., granular degeneration of cells, and migration of leukocytes into the lungs), along with proteinaceous debris filling of the alveolar spaces with fibrillar to granular eosinophilic protein strands caused by the progressive breakdown of the capillary wall and epithelial integrity, permitting leakage of protein-rich edematous fluid into the alveoli, and the presence of hemosiderin-laden macrophages (indicating pulmonary congestion with dilated capillaries and leakage of blood into alveolar spaces). Furthermore, peribronchiolar interstitial infiltration, bronchiole epithelial cell necrosis, necrotic cell debris within alveolar lumens, alveolar exudation, hyaline membrane formation, alveolar hemorrhage with red blood cells within the alveolar space, and interstitial edema, characteristic features of infected lungs in humans with SARS-CoV-2 infection are observed in MHV-1-infected mice at acute phase (at 7 days)” (Paidas et al., 2021). (C) MHV-1-infected mouse lung (12 months post-infection). (D-G) Enlarged images of (C). Blue arrows, airspaces of alveolar ducts, and alveoli are lined by hyaline membranes; yellow arrow pulmonary edema located in bronchiolar and alveolar airspaces, along with congestion of capillaries in the septal wall, and in the perivascular interstitial spaces; white arrow, nuclear atypia and lack of polarity. Asterisks, intraluminal fibrosis. (H&E, original magnification 400 × (A-C), and (D-G) are enlarged images of (C))
After a lapse of 12 months post-MHV-1 infection, mice continue to exhibit a range of findings previously observed during the acute phase of infection (severe lung inflammation, peribronchiolar interstitial infiltration, bronchiolar epithelial cell necrosis, intra-alveolar necrotic debris, alveolar exudation, mononuclear cell infiltration, the presence of hemosiderin-laden macrophages, and interstitial edema). In addition, several new changes can be observed. Notably, the bronchioles now display thickened airway walls, a consequence of fibrotic remodeling likely attributable to excessive deposition of collagen bundles. Moreover, bronchioles now contain a significant intra-luminal mucous plugging and exhibit an increased numbers of goblet cells in the epithelial lining. Finally, the walls of bronchioles contain an increased number of inflammatory cells (Fig. 6) (Paidas et al., 2022b). Collectively, these findings strongly suggest two important points: (1) the acute changes observed after infection may be irreversible, and (2) the infected animals develop pathological features similar to those observed in chronic pulmonary disease (COPD). Consequently, if left untreated, it is plausible that COPD may develop in the future.
Heart
The histopathological examination of MHV-1-infected mice hearts during the acute phase reveal pathological changes that parallel those observed in humans with SARS-CoV-2 infection in its acute stage. These include severe interstitial edema, vascular congestion, dilation, and the infiltration of red blood cells between degenerative myocardial fibers (Paidas et al., 2021).
MHV-1-infected mice hearts that remained untreated for a year exhibited additional pathological features. These include the presence of inflammatory cells and apoptotic bodies in the cardiac tissue as well as acute myocyte necrosis, myocyte hypertrophy, and fibrosis, indicative of advanced severe heart disease (Paidas et al., 2022b). These findings underscore the enduring cardiac consequences of SARS-CoV-2 infection and its potential to cause heart failure.
Liver
The histopathological examination of liver samples from mice infected with MHV-1 during the acute phase reveal pathological changes closely resemble those observed in humans during acute stages of SARS-CoV-2 infection. These changes include degeneration of hepatocytes, severe peri-portal hepatocellular necrosis characterized by pyknotic nuclei, pronounced hepatic congestion, ballooned hepatocytes, vacuolation, piecemeal necrosis, and hemorrhagic alterations. Notably, ground glass hepatocytes exhibited enlarged cytoplasm with abundant granules, peripheral cytoplasmic clearing, central nuclei, and apoptotic bodies, accompanied by the absence of hepatocytes their replacement by inflammatory cells. Other features include condensed and darkly stained cytoplasm, nuclear absence, fatty changes, binucleated hepatocytes, and activated Kupffer cells. These findings are indicative of macrovesicular steatosis, mild acute hepatitis, and minimal-to-mild portal inflammation (Paidas et al., 2021).
Upon observation of MHV-1-inoculated mice over a year-long period, the liver enzyme levels begin to return to normal. However, acute phase-associated pathological changes persist along with the emergence of new pathological alterations. These include increased infiltration of small lymphocytes in sinusoidal spaces, multifocal hepatic necrosis both in the periportal area and near the terminal hepatic veins, an elevated number of portal veins associated with severe luminal dilatation, activated Kupffer cells displaying enlarged cytoplasm containing necrotic debris, eosinophilic bodies, mitotic cells, and liver cells resembling balloons. Additionally, mild inflammation of lobular lymphocytic and portal tract, as well as mild hydropic degeneration of liver parenchymal cells, is noted (Paidas et al., 2022b). These findings strongly indicate that despite the normalization of liver enzyme levels, the liver does not fully recover even 1-year post-infection.
Kidney
The histopathological examination of MHV-1-infected mice kidneys during the acute phase unveiled pathological changes that exhibit striking similarities to those observed in humans during the acute stages of SARS-CoV-2 infection. These changes include loss of the proximal tubule brush border, vacuolar degeneration with debris consisting of necrotic epithelium in tubular lumens, infiltration of inflammatory cells in tubules and the arcuate artery, occasional presence of hemosiderin granules, deposits of calcium in tubules, and sporadic pigmented casts. Additionally, segmental fibrin thrombi are observed in glomeruli, accompanied by ischemic glomerular contraction and the accumulation of leaked plasma in Bowman’s space (Paidas et al., 2021).
Upon observation of MHV-1-inoculated mice over a year-long period, edema and inflammation of the renal parenchyma, severe acute tubular necrosis, and infiltration of macrophages and lymphocytes are evident in addition to the changes observed during the acute phase (Paidas et al., 2022b). These collective findings suggest that the structural alterations in the kidneys following SARS-CoV-2 infection may also demonstrate a degree of irreversibility, although some changes in the kidney of these chronically infected mice are comparatively less severe than those observed during the acute phase.
Western blot results
Semi-quantitative Western blot analysis of aquaporin expression following MHV-1 viral infection generally provide results that are consistent with immunofluorescence-based studies of such expression. Specifically, a significantly increased levels of AQP 1, 4, 5, and 8 is observed in all examined organs, following initiation of MHV-1 infection (except for a decrease in AQP-1 levels in the lungs) (Fig. 7). However, minor variations have been identified in the measurements of AQP levels obtained from both the Western blot and immunofluorescence methods (Paidas et al., 2022a). These variations are likely attributable to the differing sensitivities of these respective methods and that immunofluorescence-based method possess greater sensitivity compared to Western blots (Paidas et al., 2022a). Overall, these findings strongly suggest that alterations in AQP levels play a pivotal role in the pathogenesis of SARS-CoV-2 infection by mediating generalized edema.
Figure 7.
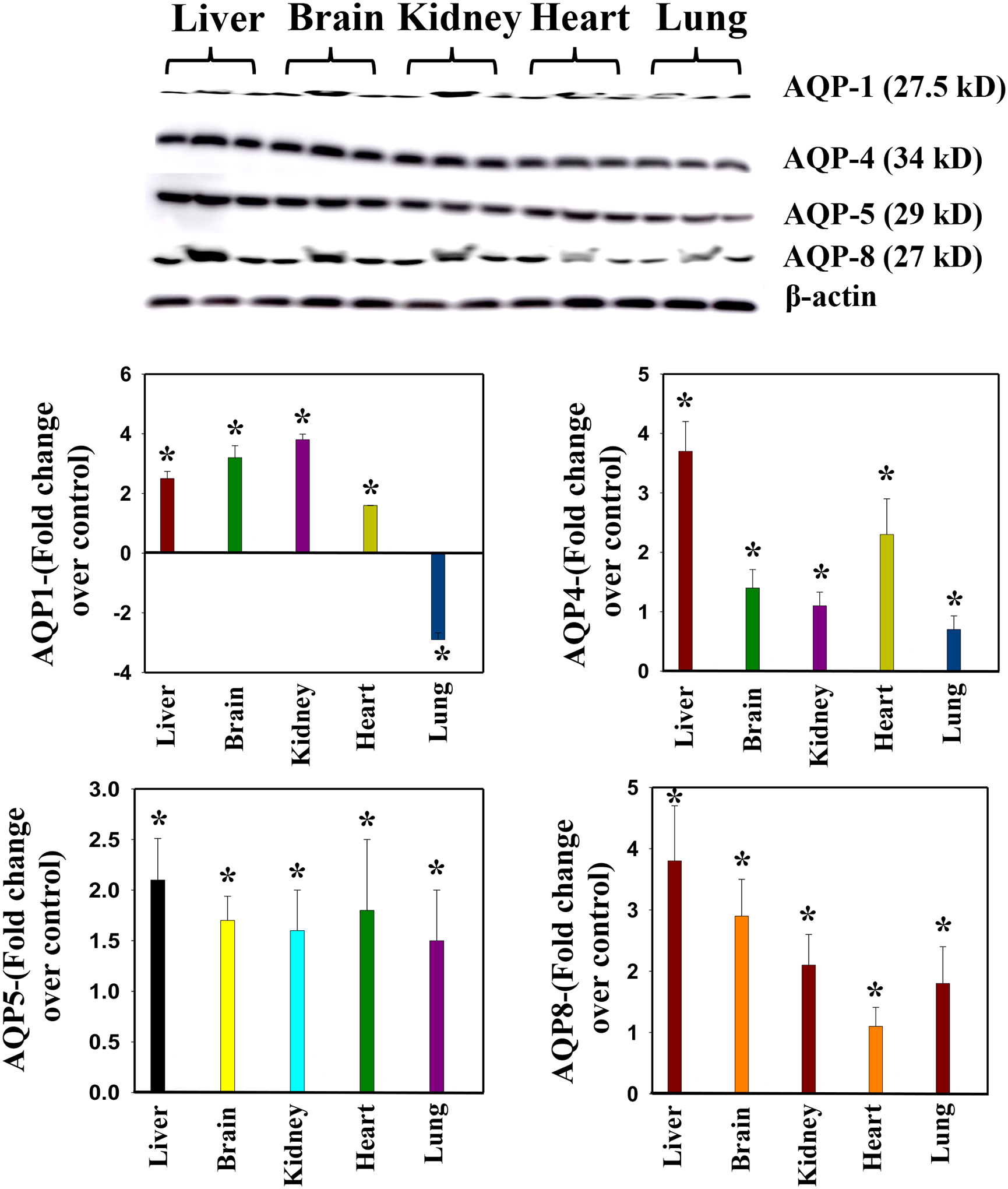
Altered AQP levels were identified in various organs post-MHV-1 inoculation. Representative immunoblots showed an increase in AQPs 1, 4, 5, and 8 in various organs, while AQP1 levels were decreased in lungs post-MHV-1. AQP levels are normalized against β-actin. ANOVA, n=4. *p<0.0.5 versus control. Error bars represent mean ± SEM.
mRNA measurements
mRNA measurements via RT-qPCR can conceivably be used to determine levels of the many factors influencing viral infections, particularly when protein measurements are not feasible. To overcome inconsistent results obtained from Western blots and other methods, mRNA measurements were used to determine NMDA receptor levels in the cerebral cortex of MHV-1-innoculated mice. As determined by RT-qPCR, there was a slight reduction in the NR1 subunit of NMDA receptor mRNA at 7 days post-infection followed by a significant decrease at 12 months post-infection. Normalization of the measurementw was performed relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Concurrently, immunofluorescence results demonstrated a reduction in neuronal proteins, such as synaptophysin and PSD95. These findings suggest a potential loss of neuronal proteins/neurotransmitters in the acute and chronic post-infection stages, commonly known as long-COVID.
Immunohistochemistry results
Aquaporins (AQPs)
AQP levels within the plasma membrane have been implicated in the maintenance of intra- and extracellular water balance. In particular, over-expression of such water channels appears to be linked to development of edema. Increased levels of AQPs 4, 5, and 8 were observed in the lung, brain, liver, kidney, and heart, as determined by immunohistochemical studies (Fig. 8). Notably, AQP-1 exhibited heightened level exclusively in the brain, liver, kidney, and heart, while demonstrating a significant decrease in the lungs.
Figure 8.
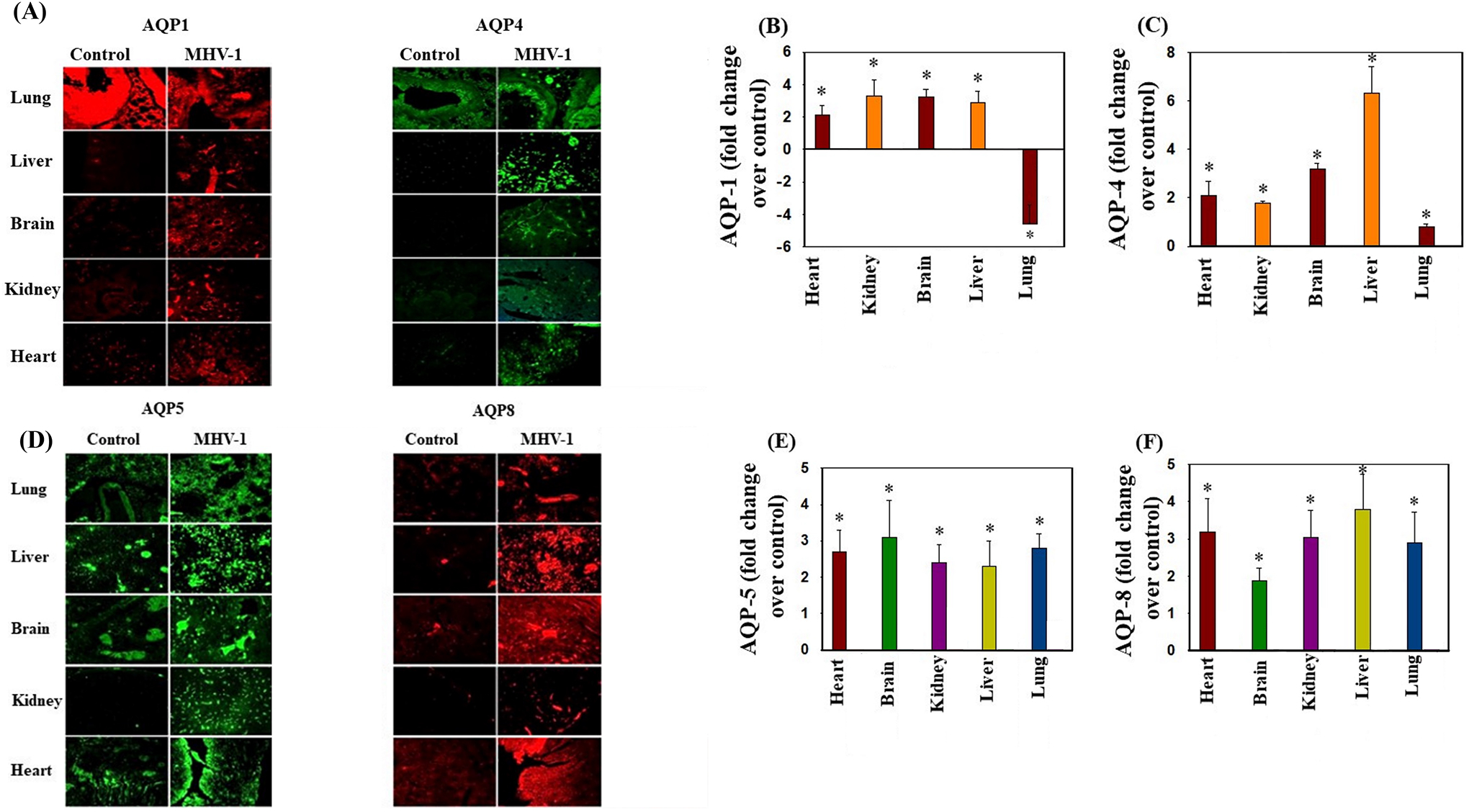
Altered AQP levels were identified in various organs post-MHV-1 inoculation. Immunofluorescences showed an increase in AQPs 1, 4, 5, and 8 in various organs, while AQP1 levels were decreased in lungs post-MHV-1. Scale bar = 25 μm. Quantitation of AQPs 1, 4, 5, and 8 immunofluorescence images. ANOVA, n=5 (Control) and 16 (MHV-1). *p<0.05 versus control. Error bars represent mean ± SEM.
Post MHV-1 infection mice exhibit severe and widespread edema, an abnormality that contributes to organ failure and mortality (Paidas et al., 2022a). The extent of edema in various organs correlates with levels of APQs in those organs. In addition, treatment aimed at inhibiting AQPs yields a notable reduction in edema and abrogation of animal death (Paidas et al., 2022a). These findings suggests that the development of edema is in part due to altered aquaporin expression.
Neuronal proteins
A number of neuronal proteins are potentially involved in the pathogenesis of long-term SARS-CoV-2 infection. These include GAFP (a marker for astrogliosis), Iba1 (a marker for activated microglia), phosphorylated Tau and TDP43 (positive markers for AD and other neurological complications), and synaptophysin (a pre-synaptic protein implicated in neuronal synaptic regulation). Mouse CNS tissue, examined by RT-qPCR in the late phase of MHV-1 infection exhibit elevated levels of GAFP, Iba1, phosphorylated Tau and TDP43 mRNA as well as reduced levels of synaptophysin. This suggests that the infection results in the development of a spectrum of neurological abnormalities including compromised neuronal synaptic regulation (Paidas et al., 2022b).
Evidence of oxidative stress
While it has been proposed that alterations in the redox system influence the pathophysiology of general infection, little is currently known about the involvement of oxidative stress and its impact on SARS-CoV-2 infection. At 6 days post-infection MHV-1-inoculated mice identified the presence of lipid peroxidation-derived aldehydes, including 4-hydroxynonenol (4-HNE) and malondialdehyde (MDA), within various tissues (Paidas et al., 2022a). These findings suggest that oxidative stress does occur in MHV-1 infection and possibly in SARS-CoV-2 infection as well.
Viral particles
Viral particles, detected by immunofluorescence studies of S1 and nucleocapsid protein were present in all organs of MHV-1-infected animals, predominately around the nucleus and within the cytoplasm of the infected cells, (Paidas et al., 2022a).
TIME CONSIDERATIONS:
In studies of acute disease, mice should be evaluated and analyzed for up to 14 days. In studies of chronic disease, evaluations should span from 3 weeks to several years. If lower dosages of the virus are used, it is important to allocate longer study periods to account for the potential delay in the onset of symptoms or illness.
ACKNOWLEDGMENTS:
Dr. Paidas has been awarded grant funding from various sources for conducting research on Covid, and brain injury. Dr. Paidas is the University of Miami Principal Investigator of an NICHD- sponsored grant to BioIncept, LLC for the study of hypoxic-ischemic encephalopathy with PreImplantation Factor (PIF), in a preclinical model. Dr. Paidas served as Principal Investigator of a NIAID sponsored grant with BioIncept, LLC to evaluate PIF in acute radiation injury. Dr. Paidas is the Principal Investigator of funding from the Muriel Murray and Robert Smith Foundation to study pregnancy loss associated with COVID-19. Dr. Paidas is the Principal Investigator of a grant from the Charles M. Vallee Foundation to study brain injury in Long Covid in preclinical models. Dr. Paidas is the Principal Investigator of a University of Miami Team Science Funding Program to study brain injury, in preclinical models. Dr. Paidas is a Co-Investigator for a University of Miami COVID-19 Rapid Response Grant to investigate the impact of COVID-19 on maternal and neonatal outcomes (No salary support). Dr. Paidas is Co-Investigator for a University of Miami study using umbilical cord-derived mesenchymal stem cells for COVID-19 Patients with ARDS (No salary support).
Footnotes
CONFLICT OF INTEREST STATEMENT:
Dr. Paidas is a Scientific Advisory Board Member of BioIncept, LLC with stock options. The other authors have no conflict of interest or competing interest to declare.
DATA AVAILABILITY STATEMENT:
The data presented in this protocol are available on request from the corresponding author. The data are not publicly available due to University of Miami Miller School of Medicine privacy policy.
LITERATURE CITED
- Caldera-Crespo LA, Paidas MJ, Roy S, Schulman CI, Kenyon NS, Daunert S, and Jayakumar AR 2021. Experimental Models of COVID-19. Front. Cell. Infect. Microbiol 11, 792584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Valdes V, and Norenberg MD 2011. The Na-K-Cl cotransporter in the brain edema of acute liver failure. J. Hepatol 54(2):272–278. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Tong XY, Curtis KM, Ruiz-Cordero R, Shamaladevi N, Abuzamel M, Johnstone J, Gaidosh G, Rama Rao KV, and Norenberg MD 2014a. Decreased astrocytic thrombospondin-1 secretion after chronic ammonia treatment reduces the level of synaptic proteins: in vitro and in vivo studies. J. Neurochem 131(3):333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Valdes V, Tong XY, Shamaladevi N, Gonzalez W, and Norenberg MD 2014b. Sulfonylurea receptor 1 contributes to the astrocyte swelling and brain edema in acute liver failure. Transl. Stroke Res 5(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas MJ, Mohamed AB, Norenberg MD, Saad A, Barry AF, Colon C, Kenyon NS, and Jayakumar AR 2021. Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19. Viruses, 13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas MJ, Sampath N, Schindler EA, Cosio DS, Ndubizu CO, Shamaladevi N, Kwal J, Rodriguez S, Ahmad A, Kenyon NS, and Jayakumar AR 2022a. Mechanism of Multi-Organ Injury in Experimental COVID-19 and Its Inhibition by a Small Molecule Peptide. Front. Pharmacol 13, 864798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidas MJ, Cosio DS, Ali S, Kenyon NS, and Jayakumar AR 2022b. Long-Term Sequelae of COVID-19 in Experimental Mice. Mol. Neurobiol 59(10), 5970–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump K, and Adamzik M 2018. Function of Aquaporins in Sepsis: a Systematic Review. Cell. Biosci 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger C, Abdulla S, and Puglisi A 2022. Mapping Biosafety Level-3 Laboratories by Publications. Center for Security and Emerging Technology. Retrieved from https://cset.georgetown.edu/publication/mapping-biosafety-level-3-laboratories-by-publications/. [Google Scholar]
- Wiggli B, Imhof E, Meier CA, and Laifer G 2013. Water, Water, Everywhere. Acute Parvovirus B19 Infection. Lancet 381 (9868), 776. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2020. WHO COVID-19 Dashboard. Retrieved from https://covid19.who.int/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this protocol are available on request from the corresponding author. The data are not publicly available due to University of Miami Miller School of Medicine privacy policy.


