Abstract
The lack of rigorous quality standards in pre-clinical radiation dosimetry has renewed interest in the development of anthropomorphic phantoms. Using 3D printing customisable phantoms can be created to assess all parts of pre-clinical radiation research: planning, image guidance and treatment delivery. We present the full methodology, including material development and printing designs, for the production of a high spatial resolution, anatomically realistic heterogeneous small animal phantom. A methodology for creating and validating tissue equivalent materials is presented. The technique is demonstrated through the development of a bone-equivalent material. This material is used together with a soft-tissue mimicking ABS plastic filament to reproduce the corresponding structure geometries captured from a CT scan of a nude mouse. Air gaps are used to represent the lungs. Phantom validation was performed through comparison of the geometry and x-ray attenuation of CT images of the phantom and animal images. A 6.6% difference in the attenuation of the bone-equivalent material compared to the reference standard in softer beams (0.5 mm Cu HVL) rapidly decreases as the beam is hardened. CT imaging shows accurate (sub-millimetre) reproduction of the skeleton (Distance-To-Agreement 0.5 mm ± 0.4 mm) and body surface (0.7 mm ± 0.5 mm). Histograms of the voxel intensity profile of the phantom demonstrate suitable similarity to those of both the original mouse image and that of a different animal. We present an approach for the efficient production of an anthropomorphic phantom suitable for the quality assurance of pre-clinical radiotherapy. Our design and full methodology are provided as open source to encourage the pre-clinical radiobiology community to adopt a common QA standard.
Abbreviations
ABS – acrylonitrile butadiene styrene, CBCT – cone beam computed tomography, FDM – fused deposition modelling, HVL – half value layer, HU – Hounsfield units, ICRU - International Commission on Radiation Units and Measurements, NIST – National Institute of Standards and Technology, NPL – National Physical Laboratory, QA – quality assurance, ROI – region of interest, SARRP – small animal radiation research platform, STL – stereolithography.
Keywords: radiotherapy, small animal, preclinical, quality assurance, phantom, 3D printing, tissue equivalent
1. Introduction
Pre-clinical radiobiology and radiotherapy drug development facilities have undergone a transformation in recent years, from single wide field irradiation units to high precision, image guided systems able to much more closely mimic the complex radiation treatments delivered to patients. A thorough review of these developments is given by Verhaegen et al (2011).
The cornerstone of radiotherapy and radiobiological research is an accurate knowledge of the radiation dose distribution delivered to a patient or experiment. Whilst there are rigorous quality standards in place for clinical radiotherapy e.g. Thwaites et al (1995) and bodies such as the UK’s Radiotherapy Trials Quality Assurance dedicated to ensuring quality in radiotherapy clinical trials, historically the same has not been true for pre-clinical studies. Desrosiers et al (2013) report that basic dosimetry information, necessary for the accurate repetition of experiments, is missing in the majority of pre-clinical radiobiology publications in 2010/11. Whether through lack of reporting or poor dosimetry practice, the inability to repeat measurements undermines the experimental basis of radiobiology (Yoshizumi et al 2011, Pederson et al 2016, Kazi et al 2014) and hampers the translation of pre-clinical findings to clinical practice.
As a consequence, and in an effort to match the advances in pre-clinical radiotherapy delivery, there is renewed interest in the area of small animal dosimetry (Biglin et al 2019). In keeping with complex clinical treatments, modern small field targeted preclinical irradiations cannot be prescribed using a single dose value, instead individual organ doses are required to prevent unacceptably high dosimetric uncertainty (Belley et al 2014). Anthropomorphic phantoms have long been used in the clinic to verify the overall radiotherapy treatment planning and delivery process (Low et al 2011), as well as the quality of image guidance systems (Jaffray et al 2002) as they can reveal problems not evident in simple geometric phantoms. Until recently phantoms for preclinical work had not developed much beyond the simple cylindrical geometry (Welch et al 2015) used for single dose value reporting.
Perks et al (2015) report on the use of 3D printing to replicate a toy mouse model that was subsequently machined to accept either an ionization chamber or metal-oxide semiconductor field-effect transistor dosimeter. However, the phantom is homogeneous, without internal structure, limiting its usefulness for imaging verification. Furthermore, conventional dosimeters have been shown to be ineffective with small radiation fields due to partial volume and positioning error effects (Newton et al 2011).
Bache et al (2015), Zhang et al (2018) and Soultanidis et al (2019) use 3D printing to create moulds in which to cast phantoms. Bache et al use flexible moulds to form Presage 3D radiochromic dosimeters of a rat section including a linear spine insert. Zhang et al and Soultanidis et al both 3D print a hollow skin shell and full rodent skeleton. The skeleton is placed within the skin shell allowing the soft tissue elements to be moulded around it. Zhang et al use an agarose-based gel that has similar x-ray attenuation and relaxation time to those of a real mouse, making the phantom compatible with both CT and MRI imaging. Soultanidis et al additionally include CNC milled lung components and fill the body with epoxy resin or ICRU soft tissue reference material (WT1 (White et al 1989)). Following manufacture they further machine the phantom to enable the accommodation of alanine pellet dosimeters. Such mould-based processes enable the production of realistic anatomies but may suffer from variability in the production process and present challenges to the incorporation of detectors.
Welch et al (2015, 2017) describe a heterogeneous mouse phantom constructed from 2 mm slices of tissue equivalent material machined to anatomical cross-sections modelled from either Digimouse atlas data or a mouse CT scan. The phantom permits dosimetry film to be sandwiched between the different sections for the accurate verification of planned dose distributions. The milling resolution of 400 µm and slice thickness of 2 mm, however, constrains the geometric realism of the phantom and thereby reduces its applicability as an imaging phantom. Additionally the manufacturing process reported in (Welch et al 2015) is more involved and intricate than that reported using other approaches.
Printing all aspects of the phantom allows the incorporation of detectors, increases reproducibility and requires no manual assembly. The model designed by Esplen et al (2019) segmented the skeleton and lungs from the body and used materials of varying density to represent each one. However, the same material was used for both skeleton and soft tissue, meaning that there was no x-ray contrast between the two, limiting its suitability as an imaging and dosimetric phantom.
Ideally, pre-clinical phantoms would combine the flexibility, high resolution and low cost of 3D printing with the anatomically realistic materials provided by other manufacturing techniques. In this article we report on the development of a technique for producing high spatial resolution, anatomically realistic heterogeneous small animal phantoms that can be used to verify the imaging, treatment planning, and radiation delivery process of modern preclinical precision radiotherapy units. We propose that due to its relative ease of manufacture, a standard phantom produced using the presented technique should be adopted by the preclinical radiation therapy community as a Quality Assurance (QA) standard. We offer our reported design as an open source option—it may be downloaded from https://github.com/gpricechristie/mousePhantom.
2. Methods
2.1. Tissue equivalent materials
2.1.1. Determination of mixture compositions for tissue equivalency
In order to manufacture a phantom suitable for radiology and dosimetry assessments, it is mandatory that the materials used are able to simulate appropriate tissues with a high level of accuracy. Specifically, for radiology and dosimetry applications, the key parameter is the mass attenuation coefficient, , as this impacts on both the contrast and segmentation of the radiology investigation as well as the dose scattering and absorption. There are three fundamental classes of material necessary to accurately model mammalian anatomy: soft tissues, bones and lungs. As the linear attenuation for lung materials (White et al 1989) is similar to air (< 10% difference from NIST (National Institute of Standards and Technology) defined x-ray mass attenuation coefficients between 100 keV and 300 keV (Hubbell and Seltzer 2004)), a void in the 3D printing process can be appropriate in first instance.
For soft tissues and bones, bespoke mixtures can be developed taking the commonly used 3D printing material Acrylonitrile Butadiene Styrene (ABS) as the main element. ABS is a mainstay of fused deposition modelling (FDM), particularly in the context of modern consumer grade printers, due to the intersection of its thermoplastic and material properties, chemically inert nature and low price point (Mccullough and Yadavalli 2013). Native ABS plastic has an electron density, physical density, and mass attenuation very similar to water at pre-clinical treatment x-ray energies (mass attenuation difference of ∼1% at 200 keV) (Kumar et al 2010), but this diverges at the lower energies (∼6.5% difference at 80 keV) typically used in the pre-clinical imaging setting, highlighting the need to modify the base material to better simulate biological matter.
The fundamental methodology is to dope the ABS base with selected materials at the appropriate ratio to achieve mass attenuation profiles similar to that of the desired tissue. Highly purified doping materials can be easily obtained from commercially available powders containing chemical elements which are dominant in the tissue of interest (i.e. CaTiO3 for calcium rich cortical bone). The ratios of ABS and doping powder can be calculated in first approximation by minimizing the difference between the mass attenuation coefficients of the newly formed mixture and the ICRU (International Commission on Radiation Units and Measurements) reference materials described in report 44 (Hubbell and Seltzer 2004) (e.g. materials SB5 and WT1 for bone and soft tissue respectively). As pre-clinical radiology and radiobiology studies use medium energy x-ray sources, this comparison must incorporate energies below 300 keV. Finally, in order to obtain a final mixture that is predominantly ABS and therefore amenable to 3D printing, the volume of doping material must be limited to some threshold. We established, through empirical experimentation, a workable threshold to be < 20% dopant by volume.
Using the above approach we developed a cortical bone equivalent material consisting of ABS doped with CaTiO3 powder (maximum particle size 45 μm) at a ratio of 38% by weight of the total material mass (14% by volume). Figure 1 shows the mass attenuation coefficients of the SB5 reference bone material, our bone mixture constituents, and the final simulated bone mixture with the corresponding mass densities and estimated effective atomic number (at 150 keV) listed in table 1. The theoretical density for the ABS:CaTiO3 mixture (0.62:0.38 ratio by weight) is 1.46 g cm−3 with the measured density of a 3D printed sample 1.27 g cm−3. The difference results from the 3D printing process (e.g. overflow, deposition pattern).
Figure 1.
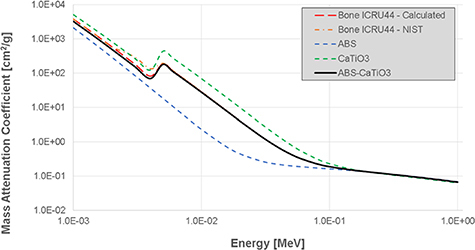
Calculated mass attenuation coefficients for SB5 cortical bone ICRU44, ABS, CaTiO3 and ABS-CaTiO3 mixtures as a function of x-ray energy plotted from the National Institute of Standards and Technology (NIST) database using the presented material weightings.
Table 1.
The mass density and estimated effective atomic number of cortical bone, native ABS and our bone substitute. Effective atomic numbers were estimated using the direct approach of Manohara et al (2008).
| Mass density (g cm−3) | Effective atomic number (150 keV) | |
|---|---|---|
| ICRU Cortical bone | 1.92 | 6.26 |
| ABS | 1.06–1.08 | 3.46 |
| Bone material | 1.27 measureda 1.46 theoretical | 8.91 |
Nominal density value based on dimensions measured with digital calliper and calibrated balance.
2.1.2. Production of doped ABS filament
The following process was developed to manufacture 3D printer filaments containing a homogeneously distributed dopant. Throughout the rest of the paper the manufacturing process is described for the bone material we use in the presented phantom. The process, however, is generic and the dopant can be substituted according to the mixture desired.
Commercially available Red ABS filament is cut into approximately 1 cm long pieces and dissolved in acetone (65 g ABS per 500 ml acetone). The powdered doping agent (36 g CaTiO3 for bone) is then added and mixed at maximum impeller speed to maintain a uniform particle suspension in the dissolved ABS whilst the acetone solvent evaporates and the plastic reforms. The mixture is then transferred to a 40 °C drying oven for 1 week to ensure complete evaporation of the acetone. The dried ABS is then blended into course granules before being extruded to form a new printing filament. We used a Noztek Touch 1 extruder fitted with a bespoke nozzle to produce 2.85 mm diameter filament with the heating element set to 200° C and the winder at 60 rpm.
2.1.3. Verification of tissue equivalent material properties
The attenuation properties of slabs of ICRU reference material and the doped ABS can be measured and compared experimentally using the experimental setup previously described by Soultanidis et al (2019). A 5 mm thick slab of the reference material, or an equivalent printed volume of the doped material, is sandwiched between 1 cm build-up and 20 cm backscatter WT1 slabs. The materials are then irradiated with 0.5, 1, 2, 4 mm Cu Half Value Layer (HVL) beams generated by x-ray tube with tube potentials and filtration given in table 2. A calibrated TW30012 Farmer chamber, traceable to the UK primary standard 300 kV free air chamber, is positioned at 2 cm depth to record the transmitted beam intensity (figure 2). The attenuation (D) difference between doped ABS and the reference ICRU material can then be calculated as:
Table 2.
X-ray tube (Comet MXR-321) settings and filtration used to measure the attenuation of the bone equivalent materials. The x-ray tube has an inherent filtration of 3 mm Be. Additionally, the system is equipped with a monitor chamber that is used throughout exposures to record the output of the x-rays tube (0.3 mm Al and 4.8 mm Perspex). Full details of the radiation set up are available in (Bass et al 2019). HVL measurements were performed using 99.99% pure Cu sheets of variable thickness (measured by a calibrated high-performance coordinate measuring machine, CMM, with 0.01 μm resolution).
| HVL [mm Cu] | Nominal generating tube potential [kV] | Additional filtration | ||
|---|---|---|---|---|
| [mm Sn] | [mm Cu] | [mm Al] | ||
| 0.5 | 135 | — | 0.27 | 1.2 |
| 1 | 180 | — | 0.54 | 1.0 |
| 2 | 220 | — | 1.40 | 0.9 |
| 4 | 280 | 1.5 | 0.26 | 1.0 |
Figure 2.

Experimental setup used for attenuation measurements (Soultanidis et al 2019).
X-ray tube source-to-surface distance is 75 cm resulting in an approximately 7 cm diameter beam size at the phantom surface. We used this set-up to test a 50 × 50 x 5 mm3 slab of SB5 against a volume of the same dimensions printed from our bone equivalent CaTiO3 doped ABS at the National Physical Laboratory (NPL). Although the radiation beam is larger than the samples, the radiation scattering contribution from the edges of the samples is expected to be the same due to the same physical dimensions of the samples.
2.2. Anatomical phantom model
The anatomical model we used to create the phantom geometry we present was segmented from a cba nude mouse (20–25 g) image obtained using the on-board Cone Beam CT (CBCT) system in a Small Animal Radiation Research Platform (SARRP) pre-clinical precision irradiation unit 2 (conducted under project licence 70/7760). The mouse was anesthetized and imaged (65 kV/1.0 mA, 360° 1024 × 1024 pixel projections at 1° increments) and reconstructed with FDK filtered back-projection using the system’s inbuilt software. The resulting image had an isotropic voxel size of 0.275 mm and dimensions 398 × 398 × 408 voxels. A volumetric rendering of the CBCT image is shown in figure 3.
Figure 3.
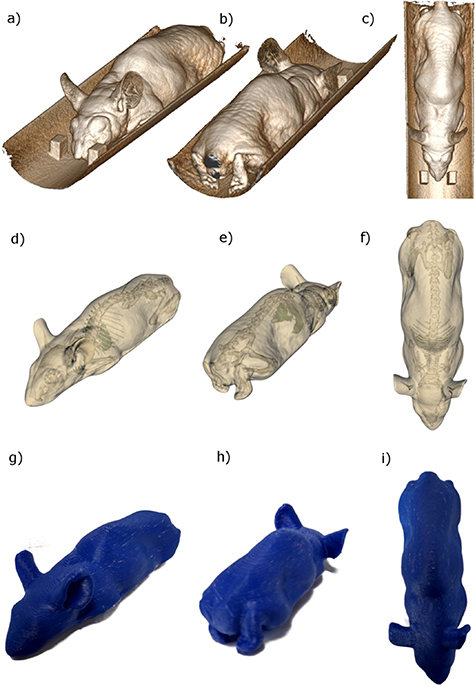
Volumetric renderings of the CBCT image used to construct the mouse phantom (a)–(c), the body, skeleton and lung segmentation extracted from the volumetric data, including removal of the imaging and treatment couch (d)–(f), and the heterogeneous printed phantom (g)–(i). The design structures necessary to print the phantom can be downloaded from https://github.com/gpricechristie/mousePhantom.
The mouse image was segmented using the Python implementations of the Visualization Toolkit (Schroeder et al 2006) and Simple Insight Toolkit (Lowekamp et al 2013). A region of interest (ROI) was manually defined to remove the cylindrical couch bed and simple connected thresholding used to segment the body, skeleton and lung structures. The segmented ROIs were saved in STL (stereolithography) format and cleaned using MeshLab (Cignoni et al 2008) for export to the 3D printer (figure 3). The STL files can then be edited using commonly available tools such as Meshmixer (http://www.meshmixer.com) or Autodesk Netfabb (https://www.autodesk.co.uk/products/netfabb/overview) to, for example, split the phantom into sections to accommodate dosimetric film. The base phantom geometries may be downloaded from https://github.com/gpricechristie/mousePhantom in STL format.
The mouse model was manufactured using an Ultimaker 3 3 FDM printer loaded with the differently doped ABS filaments manufactured using the above process. The gcode for the mouse model was produced using the Cura software package (Ultimaker 2018). The phantom we present has a skeleton printed using our CaTiO3 doped ABS bone equivalent material with the base ABS alone used for soft tissues and air cavities for lungs.
2.3. Phantom validation
2.3.1. Geometry and composition
The phantom was imaged using both the SARRP CBCT system and a Hounsfield unit (HU) calibrated CBCT image from a micro PET-CT (Siemens Inveon 4 ). The skeletal and tissue regions of the mouse phantom were compared to published values of attenuation coefficients and both the original mouse CBCT scan used to define the phantom geometry and an Inveon CT scan of a different mouse of the same strain. Inveon CT scans were acquired at 80 kVp, 500 µA with an isotropic voxel size of 0.205 mm and reconstruction dimensions of 496 × 496 × 634.
The phantom images were registered with the original data using a rigid registration algorithm implemented in the Insight Toolkit (Lowekamp et al 2013) (6 degrees of freedom rigid registration with Mutual Information similarity function and regular step gradient descent optimizer) and the skeletal and body surface geometry compared.
3. Results
3.1. Assessment of the bone equivalent material
Results of the attenuation evaluation measurements are reported in figure 4. The figure shows the percentage difference, evaluated according to equation (1), related to change in the beam attenuation between the ABS + CaTiO3 mixture (1.27 g cm−3) and the SB5 reference material.
Figure 4.
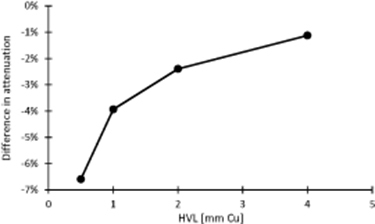
Differences in attenuation measured using the experimental set-up shown in figure 2 between the ABS + CaTiO3 mixture and the ICRU reference material (SB5).
A measured 6.6% difference in the attenuation of the ABS + CaTiO3 material compared to the SB5 reference standard in softer beams (0.5 mm Cu HVL) rapidly decreases as the beam is hardened, reducing to 1.1% at 4 mm Cu HVL.
3.2. Validation of mouse phantom
3.2.1. Geometry
The phantom image from the Inveon system registered with the original mouse SARRP CBCT image is shown in figure 5. The skeletal structures of both images, extracted using connected thresholding are shown ((d) and (e)) demonstrating the accuracy with which the 3D printing methodology we describe can reproduce realistic animal anatomy. We assess the quality of the geometric agreement as the Distance-To-Agreement (DTA) of the registered surfaces (excluding the anaesthetic pipe visible in figures 5(d) and (e)). The phantom geometry agreed to within 0.5 mm, 1 mm and 1.5 mm for 61%, 87% and 98% of the skeletal surface, and 39%, 74% and 90% of the body surface, respectively. DTA mean and standard deviations are 0.5 ± 0.4 mm and 0.7 ± 0.5 mm for the skeleton and body surfaces with the corresponding histograms shown in figures 5(f) and (g).
Figure 5.
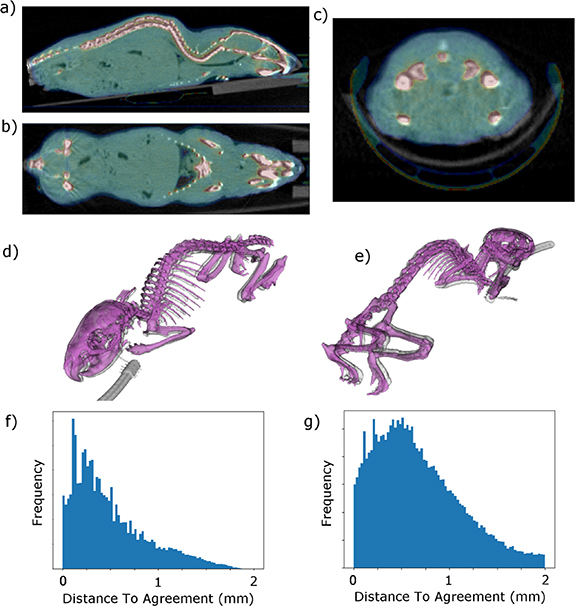
Results of rigid registration of phantom imaged in the Siemens Inveon PET-CT and the original SARRP CBCT mouse image from which the phantom structures were segmented. The skeletons segmented from the registered images are shown in (d) and (e). The mouse skeleton is in purple, the phantom in grey. Histograms of the Distance-To-Agreement of all points on the phantom surfaces are shown for the skeleton (f) and body (g). A portion of the Inveon anaesthesia system can be seen in the images.
3.2.2. CT number
Figure 6 shows histograms of the CT numbers of the mouse phantom imaged using the SARRP Cone Beam CT (CBCT) system and compared to the original mouse image (figure 3) captured using the same system ((a) and (b)); and imaged using an HU calibrated PET-CT device and compared to the intensity profile of a different mouse of the same strain ((c) and (d)). These plots demonstrate both the similarity of the phantom heterogeneity to the original image and the broad generalizability of the phantom anatomy to similar mouse models. The figure also shows how the use of the same threshold levels results in appropriately labelled tissue images in the phantom and an example mouse when using the histogram based segmentation methodology employed by the SARRP treatment planning system.
Figure 6.

Histogram comparison of the voxel intensities of the phantom and different mice. Histograms (a) and (b) show the intensities from the original mouse image from which the phantom was created (green solid line) and the phantom (blue dotted line) both imaged on the SARRP CBCT system. Histograms (c) and (d) show the intensity profiles of the phantom (red dashed line) and a different mouse (black solid line) imaged in an HU calibrated Siemens Inveon PET-CT system, demonstrating the broad generalizability of the phantom to similar mouse models. The CBCT data have been normalized to the magnitude of the soft tissue peak and a linear transform used to map the CT number air and soft tissue peaks to − 1000HU and 0HU respectively. Panels (e) and (f) show how applying the thresholds indicated in (c) effectively segment the phantom (g) and mouse (h) images into soft tissue, bone and air components.
4. Discussion and conclusion
We have presented a heterogeneous mouse phantom for use in the QA of small animal radiobiology experiments, in particular the new generation of high precision image guided radiotherapy platforms. We have demonstrated the workflow for the design and manufacture of 3D printable tissue equivalent materials and shown how these can be used to develop a simple and reproducible heterogeneous small animal phantom. The phantom has been demonstrated to be a suitable mimic of generic mouse skeletal, body surface and lung geometry (figure 5) and anatomical composition (figure 6) for use as an x-ray imaging and dosimetry phantom.
The use of 3D printing technology means that the phantom geometry can be easily manipulated using commonly available software to customize it to accept a variety of different inserts. The encapsulation of high resolution radiochromic films, emerging micro-array devices, or gel dosimeters will allow the dosimetric verification of the highly conformal plans that it is now possible to deliver in the pre-clinical setting. Furthermore, the simplicity of construction, coupled with the plentiful availability of inexpensive FDM equipment means that an open source community standard design could easily be replicated at centres around the world. The use of common reference phantoms is an important aspect of the standardized QA approaches needed in pre-clinical laboratories to ensure experiment inter-comparability and repeatability. Previous small animal phantoms have either been geometrically unrepresentative (Stenner et al 2007, Belley et al 2014), challenging to manufacture and machine to accommodate different detectors (Welch et al 2015, Zhang et al 2018, Soultanidis et al 2019), internally homogeneous (Perks et al 2015), or not tissue equivalent (Esplen et al 2019).
There are still some challenges with the tissue equivalent tissue manufacturing process we report. For instance, losses of CaTiO3 during the process of making the doped filament and the effects of spaces between deposits in the printed model still need to be thoroughly assessed. Furthermore, the manufactured material differed in general to the reference SB5 standard owing to the presence of elements such as Na, Mg and O in the SB5 material (White et al 1989) which have higher attenuation coefficients for low x-ray energies than carbon, the main elemental constituent of the ABS base plastic. However, these differences are small and disappear in harder x-ray beams.
The methodology is directly extensible to other 3D printable base plastics and additives to mimic different organs of interest. Future work of interest should include formulation of homogeneous mixtures for other tissues (e.g. muscle and fat) not included in the presented example as well as the lung tissues as this is currently achieved by inserting air gaps in a soft tissue equivalent material. Some groups have used low density spacers (Welch et al 2017) to yield lung equivalent material at a macroscopic level (i.e. bulk density and attenuation) but this approach may be not suitable for small animal irradiator platforms as the air bubbles can result in unrealistic dose distributions, particularly for an irradiation isocentre placed in the lungs. A printable homogeneous lung equivalent material similar to the ICRU LN330/LN10 milling material used by Soultanidis et al (2019) is needed.
Finally, the process has been focused on medium energy x-rays (below 300 kV) as these are routinely used in the majority of radiobiological investigations. Recently, in vivo radiobiological investigations with proton beams have started to attract considerable interest from the research community and experiments have started in several institutions worldwide. As for x-rays, a phantom for validating the positioning and dosimetry for proton beam experiments with small animals would be extremely valuable. Our methodology should be adapted, using the proton stopping power ratios rather than the mass attenuation coefficients for the formulation of suitable 3D printable mixtures.
Consumer level 3D printing is mature enough that the radiobiology community is in a position to take advantage of it to standardize experiment quality assurance. Such standardization is vital if the 3 R principles of replacing, reducing and refining the use of animals in research are to be addressed to ensure that each experiment contributes to the advancement of scientific knowledge. The recent advent of conformal treatment platforms provides an impetus to accelerate the process. We suggest that the model we share here could be adopted as a standard geometry and our tissue equivalent material manufacturing process used to replicate it in laboratories across the world. The spirit of open science, with design modifications shared openly, can be used to directly address one of the persistent issues in this field of research.
Acknowledgments
This work was supported by the NC3Rs (Training Grant No.: NC/P00203X/1). Gareth Price acknowledges the support of Cancer Research UK via funding to the Cancer Research Manchester Centre [C147/A18083] and [C147/A25254]. This work was also supported by an STFC-IAA grant and the UK National Physical Laboratory through the National Measurement System Programmes Unit of the UK’s Department of Business, Energy and Industrial Strategy. Amy Chadwick and Karen Kirkby are supported by the NIHR Manchester Biomedical Research Centre.
Footnotes
Noztek.com, Shoreham, UK.
XStrahl Ltd., Surry, UK.
Ultimaker BV, Geldermalsen, The Netherlands.
Siemens PLC, Surrey UK.
References
- Bache S T, et al. Investigating the accuracy of microstereotactic-body-radiotherapy utilizing anatomically accurate 3D printed rodent-morphic dosimeters. Med. Phys. 2015;42:846–55. doi: 10.1118/1.4905489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass G, et al. NPL Report IR 54: the NPL air kerma primary standard free-air chamber for medium energy x-rays: summary of factors incorporating ICRU report 90 recommendations . Teddington: National Physical Laboratory (NPL); 2019. [Google Scholar]
- Belley M D, et al. Toward an organ based dose prescription method for the improved accuracy of murine dose in orthovoltage x-ray irradiators. Med. Phys. 2014;41:034101. doi: 10.1118/1.4864237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglin E R, Price G J, Chadwick A L, Aitkenhead A H, Williams K J, Kirkby K J. Preclinical dosimetry: exploring the use of small animal phantoms. Radiat. Oncol. 2019;14:134. doi: 10.1186/s13014-019-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignoni P, et al. MeshLab: an open-source mesh processing tool. In: Scarano V, De Chiara R, Erra U, editors. Proc. 6th Eurographics Italian Chapter Conf.; 2008. pp. p 129–36. [DOI] [Google Scholar]
- Desrosiers M, et al. The importance of dosimetry standardization in radiobiology. J. Res. Natl Inst. Stand. Technol. 2013;118:403–18. doi: 10.6028/jres.118.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplen N, Alyaqoub E, Bazalova-Carter M. Technical note: manufacturing of a realistic mouse phantom for dosimetry of radiobiology experiments. Med. Phys. 2019;46:1030–36. doi: 10.1002/mp.13310. [DOI] [PubMed] [Google Scholar]
- Hubbell J H, Seltzer S M. NISTIR 5632 x-ray mass attenuation coefficients. National Institute of Stanadards and Technology Physical Measurement Laboratory. 2004. www.nist.gov/pml/x-ray-mass-attenuation-coefficients .
- Jaffray D A, Siewerdsen J H, Wong J W, Martinez A A. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:1337–49. doi: 10.1016/S0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- Kazi A M, Macvittie T J, Lasio G, Lu W, Prado K L. The MCART radiation physics core: the quest for radiation dosimetry standardization. Health Phys. 2014;106:1–20. doi: 10.1097/HP.0b013e3182a2a987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, et al. Acrylonitrile Butadiene Styrene (ABS) plastic-based low cost tissue equivalent phantom for verification dosimetry in IMRT. J. Appl. Clin. Med. Phys. 2010;11:24–32. doi: 10.1120/jacmp.v11i1.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D A, Moran J M, Dempsey J F, Dong L, Oldham M. Dosimetry tools and techniques for IMRT. Med. Phys. 2011;38:1313–38. doi: 10.1118/1.3514120. [DOI] [PubMed] [Google Scholar]
- Lowekamp B C, Chen D T, Ibáñez L, Blezek D. The design of simpleITK. Front. Neuroinf. 2013;7:45. doi: 10.3389/fninf.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohara S R, Hanagodimath S M, Thind K S, Gerward L. On the effective atomic number and electron density: a comprehensive set of formulas for all types of materials and energies above 1 keV. Nucl. Instrum. Methods Phys. Res., Sect. B . 2008;266:3906–12. doi: 10.1016/j.nimb.2008.06.034. [DOI] [Google Scholar]
- Mccullough E J, Yadavalli V K. Surface modification of fused deposition modeling ABS to enable rapid prototyping of biomedical microdevices. J. Mater. Process. Technol. 2013;213:947–54. doi: 10.1016/j.jmatprotec.2012.12.015. [DOI] [Google Scholar]
- Newton J, et al. Commissioning a small-field biological irradiator using point, 2D, and 3D dosimetry techniques. Med. Phys. 2011;38:6754–62. doi: 10.1118/1.3663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K H, Kunugi K A, Hammer C G, Culberson W S, Dewerd L A. Radiation biology irradiator dose verification survey. Radiat. Res. 2016;185:163–8. doi: 10.1667/RR14155.1. [DOI] [PubMed] [Google Scholar]
- Perks J R, Lucero S, Monjazeb A M, Li J J. Anthropomorphic phantoms for confirmation of linear accelerator-based small animal irradiation. Cureus. 2015;7:e254. doi: 10.7759/cureus.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W, Martin K, Lorensen B. The Visualization Toolkit. New York: Kitware; 2006. [Google Scholar]
- Soultanidis G, et al. Development of an anatomically correct mouse phantom for dosimetry measurement in small animal radiotherapy research. Phys. Med. Biol. 2019;64:12NT02. doi: 10.1088/1361-6560/ab215b. [DOI] [PubMed] [Google Scholar]
- Stenner P, Berkus T, Kachelriess M. Empirical dual energy calibration (EDEC) for cone-beam computed tomography. Med. Phys. 2007;34:3630–41. doi: 10.1118/1.2769104. [DOI] [PubMed] [Google Scholar]
- Thwaites D, Scalliet P, Leer J W, Overgaard J. Quality assurance in radiotherapy. Radiother. Oncol. 1995;35:61–73. doi: 10.1016/0167-8140(95)01549-V. [DOI] [PubMed] [Google Scholar]
- Ultimaker B V. Cura. 2018. https://ultimaker.com/en/products/ultimaker-cura-software .
- Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys. Med. Biol. 2011;56:R55. doi: 10.1088/0031-9155/56/12/R01. [DOI] [PubMed] [Google Scholar]
- Welch D, Harken A D, Randers-Pehrson G, Brenner D J. Construction of mouse phantoms from segmented CT scan data for radiation dosimetry studies. Phys. Med. Biol. 2015;60:3589. doi: 10.1088/0031-9155/60/9/3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D, Turner L, Speiser M, Randers-Pehrson G, Brenner D J. Scattered dose calculations and measurements in a life-like mouse phantom. Radiat. Res. 2017;187:433–42. doi: 10.1667/RR004CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D R, Booz J, Griffith R V, Spokas J J, Wilson I J. ICRU Report 44: tissue substitutes in radiation dosimetry and measurement. J. Int. Comm. Radiat. Units Meas. 1989;os23 doi: 10.1093/jicru/os23.1.Report44. [DOI] [Google Scholar]
- Yoshizumi T, Brady S L, Robbins M E, Bourland J D. Specific issues in small animal dosimetry and irradiator calibration. Int. J. Radiat. Biol. 2011;87:1001–10. doi: 10.3109/09553002.2011.556178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Fabrication of an anthropomorphic heterogeneous mouse phantom for multimodality medical imaging. Phys. Med. Biol. 2018;63:195011. doi: 10.1088/1361-6560/aadf2b. [DOI] [PubMed] [Google Scholar]


