Abstract
A psychrotrophic bacterium producing a cold-adapted lipase upon growth at low temperatures was isolated from Alaskan soil and identified as a Pseudomonas strain. The lipase gene (lipP) was cloned from the strain and sequenced. The amino acid sequence deduced from the nucleotide sequence of the gene (924 bp) corresponded to a protein of 308 amino acid residues with a molecular weight of 33,714. LipP also has consensus motifs conserved in other cold-adapted lipases, i.e., Lipase 2 from Antarctic Moraxella TA144 (G. Feller, M. Thiry, J. L. Arpigny, and C. Gerday, DNA Cell Biol. 10:381–388, 1991) and the mammalian hormone-sensitive lipase (D. Langin, H. Laurell, L. S. Holst, P. Belfrage, and C. Holm, Proc. Natl. Acad. Sci. USA 90:4897–4901, 1993): a pentapeptide, GDSAG, containing the putative active-site serine and an HG dipeptide. LipP was purified from an extract of recombinant Escherichia coli C600 cells harboring a plasmid coding for the lipP gene. The enzyme showed a 1,3-positional specificity toward triolein. p-Nitrophenyl esters of fatty acids with short to medium chains (C4 and C6) served as good substrates. The enzyme was stable between pH 6 and 9, and the optimal pH for the enzymatic hydrolysis of tributyrin was around 8. The activation energies for the hydrolysis of p-nitrophenyl butyrate and p-nitrophenyl laurate were determined to be 11.2 and 7.7 kcal/mol, respectively, in the temperature range 5 to 35°C. The enzyme was unstable at temperatures higher than 45°C. The Km of the enzyme for p-nitrophenyl butyrate increased with increases in the assay temperature. The enzyme was strongly inhibited by Zn2+, Cu2+, Fe3+, and Hg2+ but was not affected by phenylmethylsulfonyl fluoride and bis-nitrophenyl phosphate. Various water-miscible organic solvents, such as methanol and dimethyl sulfoxide, at concentrations of 0 to 30% (vol/vol) activated the enzyme.
Lipases catalyze the hydrolysis of acylglycerides and other fatty acid esters. They resemble esterases, but differ markedly from them in their ability to act on water-insoluble esters (5). Lipases and esterases have been recognized as very useful biocatalysts because of their wide-ranging versatility in industrial applications.
A variety of microbial lipases with different enzymological properties and substrate specificities have been found (22). The temperature stability of lipases has been regarded as the most important characteristic for use in industry (16). However, low stability is favorable for some purposes. For example, heat-labile enzymes can be easily inactivated by treatment for short periods of time at relatively low temperatures after being used for processing of food and other materials (32). One can therefore prevent the materials from damage during heat inactivation of the enzymes. Cold-adapted microorganisms, which are expected to produce cold-adapted enzymes, have been isolated. These microorganisms usually grow only slowly even under appropriate conditions (19). However, recent advances in genetic engineering have enabled efficient production of heterologous enzyme genes in an appropriate host strain such as Escherichia coli. Thus, cold-adapted enzymes from psychrotrophic microorganisms showing high catalytic activity at low temperatures can be highly expressed in such recombinant strains. The cold-adapted enzymes are expected to be applicable as additives to detergents used at low temperatures and biocatalysts for biotransformation of labile compounds at cold temperatures (32).
Recently, the genes of cold-adapted lipases from psychrotrophic bacteria Moraxella TA144 (10) and Psychrobacter immobilis B10 (1) isolated in Antarctica were cloned and sequenced. The enzymes showed high activities at temperatures as low as 3°C. None of these recombinant enzymes, however, have been purified to homogeneity due to strong interaction of the enzymes with lipopolysaccharides secreted by the bacterial cells (1).
Our group has also isolated cold-adapted microorganisms producing cold-adapted lipases from Alaskan and Siberian soils and cloned the lipase gene, lipP, from an Alaskan psychrotroph, Pseudomonas sp. strain B11-1. In this study, we report the cloning and sequencing of the lipP gene and the purification and characterization of the recombinant enzyme LipP.
MATERIALS AND METHODS
Bacterial strains and media.
Psychrotrophic bacteria were isolated from tundra soils of Alaska and Siberia on agar plates of Luria-Bertani (LB) medium (pH 7.6) at 4°C. Lipase activity of the bacteria was detected through the formation of halos around the colonies in three kinds of agar plates as follows. The basal medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl, pH 7.2) was supplemented with medium A, 0.01% CaCl2 · 2H2O and 1% Tween 80 (40); medium B, 0.01% CaCl2 · 2H2O and 1% tributyrin (26); or medium C, 2.5% olive oil and 0.001% rhodamine B (25). Lipase production on medium C plates was monitored by fluorescence with UV light at 350 nm.
Construction of a genomic DNA library and screening for a lipase gene from Pseudomonas sp. strain B11-1.
DNA manipulation was carried out according to the methods described by Sambrook et al. (38). Restriction enzymes and DNA-modifying enzymes were purchased from Takara Shuzo, Kyoto, Japan. E. coli C600 was used as a host cell with pUC118 (Takara Shuzo, Kyoto, Japan) as a cloning vector. The chromosomal DNA was isolated from Pseudomonas sp. strain B11-1 cells by phenol treatment (37) and was partially digested with Sau3AI at 37°C. The resulting DNA fragments were electrophoresed in 0.8% agarose gel, and fragments of 1 to 10 kbp were electroeluted and then ligated with pUC118 which had been previously digested with BamHI and dephosphorylated with bacterial alkaline phosphatase. The resultant plasmids were introduced into E. coli C600 according to a previously described method (33), providing a gene library containing 35,000 recombinant E. coli clones. The clone cells producing a lipase were detected due to the formation of halos around the colonies on LB agar plates supplemented with ampicillin (0.1 mg/ml), 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), 1% tributyrin, and 1% gum arabic. After incubation at 28°C for 16 h, the plates were incubated further at 4°C for 4 days.
Assays.
Lipase activity was determined with a lipase assay kit (Dainippon Pharmaceutical, Osaka, Japan) at 25°C. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol of 2,3-dimercaptopropan-1-ol from 2,3-dimercaptopropan-1-ol tributyl ester per min (27). The amount of protein was determined with a Bio-Rad protein assay kit with bovine serum albumin used as the standard (3). Esterase activity was determined by measuring p-nitrophenol formed from fatty acid p-nitrophenyl esters at 25°C. The following molar extinction coefficients of p-nitrophenol at 400 nm were used: 14,775 M−1 cm−1 in 0.1 M sodium phosphate buffer containing 0.1 M NaCl (pH 7.25) (36) and 15,100 M−1 cm−1 in Tris-HCl (pH 8.0) (24). The assay mixture (1 ml) contained p-nitrophenyl butyrate, 0.1 M sodium phosphate buffer containing 0.1 M NaCl (pH 7.25), and 1 μg of enzyme. Long-chain fatty acid esters of p-nitrophenol (e.g., p-nitrophenyl laurate and p-nitrophenyl palmitate) are not soluble in this assay mixture, and substrate specificity for various p-nitrophenyl esters was examined in a mixture containing 0.5 mM p-nitrophenyl esters, 0.1 M sodium phosphate buffer (pH 7.25), 0.1 M NaCl, 15% acetonitrile, and 0.038 mM Triton X-100. After preincubation for 10 min at the designated temperatures (from 4 to 70°C), the reaction was initiated by addition of the substrate.
Purification of LipP.
All operations were performed at 4°C, and 20 mM Tris-HCl (pH 8.0) was used as the standard buffer unless otherwise stated.
E. coli C600 cells harboring pPL2-1, which encodes the cloned lipase gene lipP, were grown aerobically at 37°C for 14 h in 2 liters of the LB medium containing ampicillin (0.2 mg/ml). The cells harvested (10 g [wet weight]) were disrupted by sonication. The supernatant solution was fractionated with ammonium sulfate, and a fraction of 20 to 65% saturation was collected. After dialysis, the enzyme solution was applied to a DEAE-Cellulofine column (3 by 50 cm). The column was washed with 1 liter of the buffer supplemented with 0.2 M NaCl, and the enzyme was eluted with a linear gradient of 0.2 to 1.0 M NaCl with a total volume of 1.0 liter. The active fractions were concentrated with 65% saturation of ammonium sulfate. The enzyme solution, dialyzed against 5 mM potassium phosphate buffer (KPB) (pH 6.8), was applied to a Gigapite column (3 by 30 cm) equilibrated with the same buffer. The enzyme was eluted with a linear gradient of 0.005 to 1.0 M KPB (pH 6.8) with a total volume of 500 ml. The active fractions were concentrated with an Amicon 30 PM ultrafiltration membrane.
DNA sequencing.
All bases were sequenced at least once in each direction by the chain termination method with a Dye Primer or a Dye Terminator sequencing kit, by using an Applied Biosystem model 370A DNA sequencer. Sequence analysis was carried out with software from DNASTAR, Inc. (Madison, Wis.).
Nucleotide sequence accession number.
The nucleotide sequence reported in this work has been assigned GenBank accession no. AF034088.
RESULTS AND DISCUSSION
Isolation of Pseudomonas sp. strain B11-1.
We screened various psychrotrophic bacteria isolated from tundra soils of Alaska and Siberia (total, 88 strains) for bacterial strains showing high lipase activity at cold temperatures. The psychrotrophic bacteria were examined with a screening system suitable for detection of lipase producers as described in Materials and Methods. Strain B11-1 was selected as the best producer of lipase and was identified as a pseudomonad by the German Collection of Microorganisms, Braunschweig, Germany, on the basis of its taxonomic characteristics: motile by polar flagella; number of flagella, >1; rod shaped (0.5 to 0.8 by 1.5 to 3.0 mm); gram negative; anaerobic growth, negative; spore formation, negative; oxidase test, positive; catalase test, positive; O-F test, oxidative; urease test, negative; denitrification, positive; diffusible pigment formation, negative; gelatin hydrolysis, negative; carbon sources utilized: glucose, malate, adonitol, mannitol, sorbitol, β-alanine, and l-valine; and nonutilized carbon sources: arabinose, l-rhamnose, adipate, citraconate, and erythritol. The 16S rRNA sequence of strain B11-1 showed a similarity of 99.3% to that of Pseudomonas syringae, but they were distinct from each other in a few physiological characteristics, in particular, those shown by the oxidase and denitrification tests (15). No strain identical to strain B11-1 could be found, and we named strain B11-1 Pseudomonas sp. strain B11-1.
Cloning and nucleotide sequencing of the lipase gene.
A library of the chromosomal genes of Pseudomonas sp. strain B11-1 was constructed with vector plasmid pUC118 and host strain E. coli C600. About 35,000 recombinant colonies were isolated on the agar plates with medium B. Six clones showed clear halos around the colonies at 4°C due to hydrolysis of tributyrin in the medium. We selected the clone forming the clearest and largest halo and designated the cloned gene lipP. The cloned plasmid encoding lipP was named pPL2-1; it contained an insert DNA of approximately 2.5 kbp. Since the recombinant E. coli cells harboring pPL2-1 formed a clear halo in the presence IPTG, LipP is probably expressed under the control of its inherent promoter in E. coli. We determined the nucleotide sequence of the fragment and found a single open reading frame comprising 924 bp, which encodes a putative protein of 308 amino acids with a predicted molecular weight of 33,714. A putative Shine-Dalgarno sequence (5′-GAAGGA) was found seven bases upstream from the initiation codon ATG. The sequence GDSAG starting at residue 153 fits the GXSXG motif shared with various lipases, esterases, and other hydrolytic enzymes. Therefore, Ser155 probably acts as the nucleophile to form an acyl intermediate with the substrate as do many hydrolytic enzymes. The active-site serine is encoded by AGC in the same manner as various other lipases: AGY (Y, a pyrimidine) is known as the common code for the active-site serine residue of most lipases (34).
Comparison of amino acid sequences.
The amino acid sequence of LipP was compared with those of other lipases. LipP shows a high sequence similarity to Lipase 2 from psychrotrophic Moraxella TA144 (12) and the mammalian hormone-sensitive lipase (28, 29), with homologies of 28.5 and 22.7%, respectively. The putative lipolytic proteins such as the E. coli lipase-like protein and Bacillus acidocaldarius ORF3 protein also show some sequence similarity to those of the three above-mentioned enzymes (28). However, the functions of these hypothetical lipases have not yet been clarified. LipP probably uses a catalytic triad (Ser-His-Asp/Glu) and contains an oxyanion hole to stabilize the tetrahedral intermediate during the acetylation and deacetylation steps (8). The consensus sequence of GDSAG occurs in LipP, and the central serine (Ser155) is probably a member of the triad. The enzyme has another conserved sequence in the region including Asp250, Asp254, and Glu255, and one of these acidic amino acid residues presumably participates in catalysis as a member of the triad. Two conserved histidine residues, His81 and His280, occur, and one of them is thought to form the triad. The histidine residue constituting the triad is quite often followed by a glycine. Both His81 and His280 precede a glycine residue, and either of them could be the catalytic His residue, although we have at present no information to predict which is more plausible.
Purification of LipP.
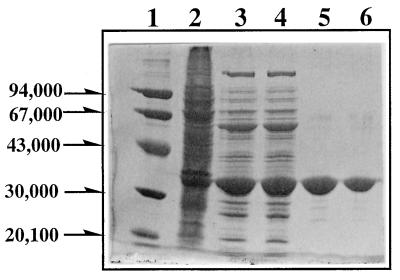
LipP was purified with a 17% yield by 38-fold purification (Table 1). When the purity was judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, only a single major protein band, whose molecular mass (about 33 kDa) is consistent with the subunit molecular weight (33,714) of LipP deduced from the nucleotide sequence of lipP, was observed (Fig. 1).
TABLE 1.
Purification of LipP from E. coli C600 harboring pPL2-1
| Purification method | Total protein (mg) | Total activity (Ua) | Sp act (U/mg) | Purifi- cation (fold) | Yield (%) |
| Cell extract | 180 | 5,200 | 29 | 1 | 100 |
| DEAE-Cellulofine | 5.3 | 1,300 | 250 | 8.6 | 25 |
| Gigapite | 0.8 | 860 | 1,100 | 38 | 17 |
Activity was measured by determination of 2,3-dimercaptopropan-1-ol formed from 2,3-dimercaptopropan-1-ol tributyl ester with 5,5′-dithio-bis(2-nitrobenzoic acid). The definition of unit is given in the text.
FIG. 1.
Polyacrylamide gel electrophoresis of LipP. Lane 1, molecular weight markers; lane 2, the preparation after ammonium sulfate precipitation (amount of protein loaded, 25 μg); lanes 3 and 4, the preparation after DEAE-Cellulofine chromatography (amount of protein loaded, 15 μg); lanes 5 and 6, the preparation after Gigapite chromatography (amount of protein loaded, 10 μg).
Substrate specificity.
Substrate specificity of LipP was examined with p-nitrophenyl esters of various fatty acids. The rates of hydrolysis for the following fatty acids were as indicated (in units/milligram): butyrate, 164; valerate, 64; caproate, 119; caprylate, 41; laurate, 12; and palmitate, 8. The enzyme showed high activity towards short- to medium-chain (C4 and C6) fatty acids, and the esters of longer-chain fatty acids were poor substrates. A similar specificity was found for the crude preparation of Lipase 2 from Moraxella sp. strain TA144 (10) and lipases of psychrotrophic pseudomonads isolated from refrigerated milk (23). The recombinant lipase from psychrotrophic Pseudomonas fluorescens SIK W1 also acted on the esters of medium-chain (C6 and C8) fatty acids (30). Positional specificity of LipP for triolein was examined by thin-layer chromatography (7). The products were analyzed after incubation for 10, 30, 60, and 120 min at 30°C: 1,2-diolein and monoolein were formed, but only a small amount of 1,3-diolein was accumulated. The results indicate that LipP has a 1,3-positional specificity toward the fatty acid triglyceride.
Effect of pH on lipase activity.
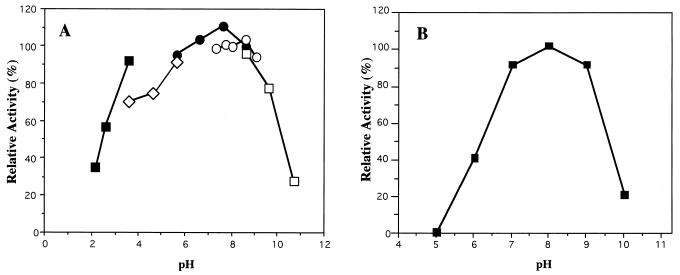
Figure 2A shows the pH stability of LipP at 25°C with 0.5 mM p-nitrophenyl butyrate as a substrate. The enzyme was stable between pH 6 and 9 at the indicated pH range when incubated at 0°C for 24 h, but its activity decreased at acidic and alkaline pH values. The recombinant lipases from Pseudomonas pseudoalcaligenes and Pseudomonas mendocina were reported to be stable between pH 5 and 10, whereas the enzyme from Pseudomonas cepacia DSM 50181 was reported to be stable under acidic (pH < 2.0) and alkaline (pH > 12.0) conditions (44). Figure 2B shows the optimum pH for the LipP reaction with tributyrin as a substrate. The optimal pH was found to be around 8.0. The pH optima for the reactions catalyzed by Pseudomonas cepacia, Pseudomonas pseudoalcaligenes, and Pseudomonas mendocina enzymes were reported to be between 8 and 9 (44).
FIG. 2.
Effect of pH on the stability (A) and activity (B) of LipP. (A) The enzyme was incubated at various pH values at 4°C for 24 h, and the activity was measured. The enzyme activity after treatment with Tris-HCl (pH 9.0) was taken as 100%. Buffers used (final concentration, 20 mM) were glycine-HCl (▪) (pH 2.2 to 3.6); sodium acetate (◊) (pH 3.6 to 5.6); Tris-malate (•) (pH 5.6 to 8.6); Tris-HCl (○) (pH 7.4 to 9.0); glycine-NaOH (□) (pH 8.6 to 10.6). (B) The enzyme was incubated with 100 mM tributyrin as a substrate in NaH2PO4-NaOH buffer at various pH values at 25°C for 10 min, and free fatty acid formed was titrated with 0.05 M NaOH.
Effect of temperature on enzyme activity.
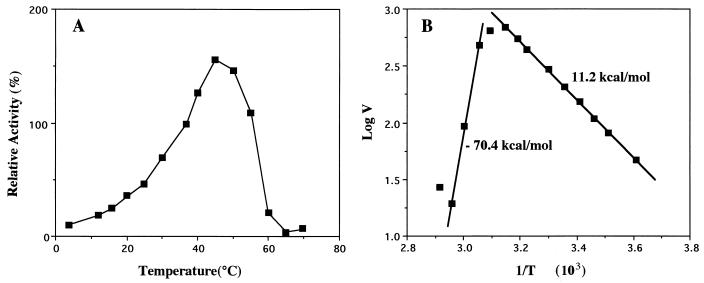
The enzyme showed maximal activity at 45°C toward p-nitrophenyl butyrate (Fig. 3A) and at 37°C toward p-nitrophenyl laurate (data not shown). The activation energy of an enzyme reaction reflects the catalytic efficiency of the enzyme; low activation energy is due to the high catalytic efficiency of the enzyme. The reactions catalyzed by the enzymes derived from cold-adapted organisms are usually lower than those catalyzed by the corresponding enzymes from their mesophilic counterparts (11). Therefore, we determined the activation energy for the hydrolysis of p-nitrophenyl butyrate catalyzed by LipP. It was about 11.2 kcal/mol in the range 5 to 35°C (Fig. 3B) and constant over the assay temperatures below 35°C. Such a monophasic feature suggests that LipP does not undergo structural changes in this temperature range, although at higher temperatures LipP was inactivated irreversibly. Lower activation energy (7.7 kcal/mol) was observed toward p-nitrophenyl laurate. This value is much lower than those for the same substrate shown by enzymes from other sources: Antarctic bacteria, 12 to 17 kcal/mol, and mesophilic Pseudomonas aeruginosa, 25 kcal/mol (9). This indicates that the catalytic efficiency is much higher for LipP than for these enzymes. However, at temperatures above 40°C, enzyme activity fell drastically at an inactivation energy of 70.4 kcal/mol.
FIG. 3.
Effect of temperature on the activity of LipP. (A) The enzyme was incubated with a mixture containing 20 mM phosphate buffer (pH 7.25), 5% acetonitrile, and 0.5 mM p-nitrophenyl butyrate at various temperatures for 10 min, and p-nitrophenol formed was measured. The value obtained at 37°C was taken as 100%. (B) The logarithm of the specific activity (V) (in micromoles per milligram per minute) was plotted against the reciprocal of absolute temperature (T). The values shown are activation energy calculated from the linear part of the plot.
Thermostability.
The enzyme was incubated at various temperatures (30, 40, 50, 60, and 70°C) for 30 min, and then the residual activity was measured at 25°C (100, 88, 66, 25, and 0%, respectively). Remaining activities after 60-min incubations at the same temperatures were 92, 85, 47, 0, and 0%, respectively. LipP is a little more stable than enzymes from other psychrotrophs, e.g., Moraxella TA144 (9) and Acinetobacter O16 (4).
Effect of inhibitors.
The enzyme was incubated with various compounds that may inhibit the enzyme, and the remaining activity was measured with p-nitrophenyl butyrate as the substrate at 25°C. The enzyme was not affected by phenylmethylsulfonyl fluoride, EDTA, and 2-mercaptoethanol but was strongly inhibited by Zn2+, Cu2+, Fe3+, and Hg2+ ions (Table 2). Hormone-sensitive lipase of rat adipose tissue is also inhibited by Hg2+ ions, indicating the occurrence of an essential thiol group of this family of lipases (13). Both Fe2+ and Fe3+ ions were found to inhibit the lipase from Aspergillus niger (20). On the other hand, the lipases from A. niger and Humicola lanuginosa were activated by Ca2+ ions, which facilitated the removal of free fatty acids formed in the reaction at the water-oil interface (21, 31). However, LipP was not activated by the addition of Ca2+ ions. Human liver arylacetamide deacetylase, which shares the sequences GDSAG and HGGG with LipP, was inhibited by bis-p-nitrophenyl phosphate (35), whereas LipP was not inhibited by this compound.
TABLE 2.
Inhibition of LipP by various compounds
| Compound | Remaining activity (%)a at a concn (mM) of:
|
|||
|---|---|---|---|---|
| 0.1 | 1.0 | 5.0 | 10 | |
| Phenylmethylsulfonyl fluoride | 89 | |||
| EDTA | 109 | |||
| Na2N3 | 67 | |||
| 2-Mercaptoethanol | 100 | |||
| Bis-p-nitrophenyl phosphate | 90 | |||
| CaCl2 | 70 | 58 | ||
| MgCl2 | 100 | 90 | ||
| ZnCl2 | 12 | 9 | ||
| CuCl2 | 4 | 4 | ||
| CoCl2 | 58 | 58 | ||
| FeCl2 | 86 | 55 | ||
| Fe2Cl3 | 44 | 0 | ||
| MnCl2 | 70 | 29 | ||
| HgCl2 | 0 | 0 | 0 | |
| RbCl | 83 | 72 | ||
LipP (0.05 mg/ml) was incubated in 20 mM Tris-HCl (pH 8.0) containing each compound at 25°C for 1 h. Remaining activity was determined with 0.5 mM p-nitrophenyl butyrate at 25°C and expressed as the percent of the control value (with no addition).
Effect of temperature on the Michaelis constant.
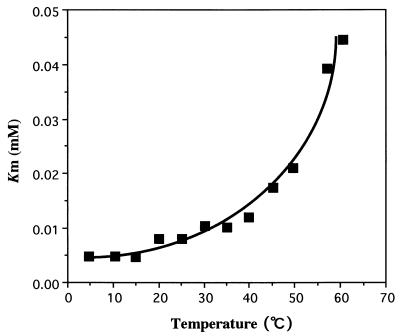
Somero suggested that enzyme-ligand interactions are disrupted by both increases and decreases in temperature (41, 42). Various enzymes from poikilothermal organisms, including thermophilic and psychrophilic microorganisms, show the lowest Km values for their substrates at the physiological temperatures of the source organisms. Trout produce the warm- and cold-adapted forms of acetylcholinesterase and citrate synthase according to their habitat temperature, and the Km values of the enzymes are at a minimum at their acclimated temperatures (2, 17). Urocanase from a psychrotroph, Pseudomonas putida, shows a low Km at low temperatures (18). On the other hand, the Km of the thermostable enolase from the thermophile Thermus aquaticus YT-1 increases at low temperatures, and the lowest Km is at high temperatures, near the optimal growth temperature for the strain (43). We examined the apparent Km of LipP for p-nitrophenyl butyrate at various temperatures (Fig. 4). The Km decreased with decreases in temperature, and the lowest Km was observed in the range 5 to 15°C; this is consistent with the physiological temperature of the source organism.
FIG. 4.
Effect of temperature on the Michaelis constant of LipP. The enzyme was assayed with a mixture containing 0.1 M phosphate buffer (pH 7.25), 0.1 M NaCl, and various concentrations of p-nitrophenyl butyrate (0.005 to 0.5 mM) at 25°C. The Km for p-nitrophenyl butyrate was obtained from a plot of velocity versus substrate concentration with KaleidaGraph software (Synergy Software, Reading, Pa.). The values of four separate experiments were averaged and plotted against temperature.
Effect of organic solvents on enzyme activity.
LipP was incubated with various water-miscible organic solvents at a concentration of either 15 or 30% (vol/vol) at 25°C for 1 h. The enzyme was not inhibited; rather, it was slightly activated by all the solvents examined except acetonitrile (Table 3). The enzyme was completely inactivated either by 30% acetonitrile at 25°C for 1 h or by 15% acetonitrile at 37°C for 5 min (data not shown). The lipase from Fusarium heterosporum is also a solvent-resistant enzyme but was completely inactivated in 50% acetonitrile (39). Other lipases from Pseudomonas and Bacillus were also activated in the presence of several water-miscible organic solvents (39). Although the three-dimensional structure of LipP has not yet been clarified, the solvents probably convert the closed form of the enzyme to the open form by affecting a lid or flap in LipP to activate the enzyme. The Candida rugosa lipase is also known to be activated by organic solvents, which keep the enzyme in the open conformation; the lid of the enzyme does not cover the active site-crevice, thus keeping a flexible conformation (6, 14). Whatever the mechanism of the activation of LipP by organic solvents is, stability to solvents is a useful characteristic of the enzyme.
TABLE 3.
Effects of organic solvents on LipP activity
| Organic solvent | Relative activity (%)a at a concn (%) of:
|
|
|---|---|---|
| 15 | 30 | |
| Acetonitrile | 34 | |
| Ethanol | 132 | 38 |
| Methanol | 194 | 107 |
| Dimethyl sulfoxide | 142 | 149 |
| Dimethyl formamide | 130 | 80 |
LipP (final concentration, 0.05 mg/ml) was incubated in 20 mM Tris-HCl (pH 8.0) buffer containing each organic solvent at 25°C for 1 h. Remaining activity was determined with 0.5 mM p-nitrophenyl butyrate in 20 mM Tris-HCl (pH 8.0) buffer at 25°C and expressed as the percent of the control value (with no addition of organic solvent).
REFERENCES
- 1.Arpigny J L, Feller G, Gerday C. Cloning, sequence and structural features of a lipase from the Antarctic facultative psychrophile Psychrobacter immobilis B10. Biochim Biophys Acta. 1993;1171:331–333. doi: 10.1016/0167-4781(93)90078-r. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin J, Hochachka P W. Functional significance of isoenzymes in thermal acclimatization; acetylcholinesterase from trout brain. Biochem J. 1970;116:883–887. doi: 10.1042/bj1160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Breuil C, Kushner D J. Partial purification and characterization of the lipase of a facultative psychrophilic bacterium (Acinetobacter O16) Can J Microbiol. 1975;21:434–441. doi: 10.1139/m75-062. [DOI] [PubMed] [Google Scholar]
- 5.Brockerhoff H, Jensen R G, editors. Lipolytic enzymes. New York, N.Y: Academic Press; 1974. pp. 1–3. [Google Scholar]
- 6.Colton I J, Ahmed S N, Kazlauskas R J R. A 2-propanol treatment increases the enantioselectivity of Candida rugosa lipase toward esters of chiral carboxylic acids. J Org Chem. 1995;60:212–217. [Google Scholar]
- 7.Commenil P, Belingheri L, Sancholle M, Dehorter B. Purification and properties of an extracellular lipase from the fungus Botrytis cinerea. Lipids. 1995;30:351–356. doi: 10.1007/BF02536044. [DOI] [PubMed] [Google Scholar]
- 8.Derewenda Z S. Structure and function of lipases. Adv Protein Chem. 1993;45:1–52. doi: 10.1016/s0065-3233(08)60637-3. [DOI] [PubMed] [Google Scholar]
- 9.Feller G, Thiry M, Arpigny J L, Mergeay M, Gerday C. Lipases from psychrotrophic antarctic bacteria. FEMS Microbiol Lett. 1990;66:239–244. [Google Scholar]
- 10.Feller G, Thiry M, Arpigny J L, Gerday C. Cloning and expression in Escherichia coli of three lipase-encoding genes from the psychrotrophic antarctic strain Moraxella TA144. Gene. 1991;102:111–115. doi: 10.1016/0378-1119(91)90548-p. [DOI] [PubMed] [Google Scholar]
- 11.Feller G, Narinix E, Arpigny J L, Aittaleb M, Baise E, Genicot S, Gerday C. Enzymes from psychrophilic organisms. FEMS Microbiol Rev. 1996;18:189–202. [Google Scholar]
- 12.Feller G, Thiry M, Arpigny J L, Gerday C. Nucleotide sequence of the lipase gene Lip2 from the antarctic psychrotrophic Moraxella TA144 and site-specific mutagenesis of the conserved serine and histidine resides. DNA Cell Biol. 1991;10:381–388. doi: 10.1089/dna.1991.10.381. [DOI] [PubMed] [Google Scholar]
- 13.Fredrikson G, Stralfors P, Nilsson N O, Belfrage P. Hormone-sensitive lipase of rat adipose tissue: purification and some properties. J Biol Chem. 1981;256:6311–6320. [PubMed] [Google Scholar]
- 14.Grochulski P, Li Y, Schrag J P, Bouthillier F, Smith P, Harrison D, Rubin B, Cygler M. Insight into interfacial activation from an open structural of Candida rugosa lipase. J Biol Chem. 1993;268:12843–12847. [PubMed] [Google Scholar]
- 15.Hensyl W R. Gram-negative aerobic/microaerophilic rods and cocci. In: Holt J G, Krieg N R, Sneath P H A, Staley J T, Williams S T, editors. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore, Md: The Williams & Wilkins Co.; 1994. pp. 71–174. [Google Scholar]
- 16.Herbert R A. The perspective on the biotechnological potential of extremophiles. Trends Biotechnol. 1992;10:395–402. doi: 10.1016/0167-7799(92)90282-z. [DOI] [PubMed] [Google Scholar]
- 17.Hochachka P W, Lewis J K. Enzyme variants in thermal acclimation. Trout liver citrate synthase. J Biol Chem. 1970;245:6567–6573. [PubMed] [Google Scholar]
- 18.Hug D H, Hunter J K. Effect of temperature on urocanase from a psychrophile, Pseudomonas putida. Biochemistry. 1974;13:1427–1430. doi: 10.1021/bi00704a017. [DOI] [PubMed] [Google Scholar]
- 19.Ingraham J L, Strokes J L. Psychrophilic bacteria. Bacteriol Rev. 1959;23:97–108. doi: 10.1128/br.23.3.97-108.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai M, Tsujisaka Y, Fukumoto J. Studies on lipase. III. Effect of calcium ion on the action of the crystalline lipase from Aspergillus niger. J Gen Appl Microbiol. 1964;10:87–93. [Google Scholar]
- 21.Iwai M, Tsujisaka Y, Fukumoto J. Studies on lipase. V. Effect of iron ions on the Aspergillus niger lipase. J Gen Appl Microbiol. 1970;16:81–90. [Google Scholar]
- 22.Jaeger K E, Ransac S, Dijkstra B W, Colson C, van Heuvel M, Misset O. Bacterial lipases. FEMS Microbiol Rev. 1994;15:29–63. doi: 10.1111/j.1574-6976.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson L A, Beacham I R, MacRae I C, Free M L. Degradation of triglycerides by a pseudomonad isolated from milk: molecular analysis of a lipase-encoding gene and its expression in Escherichia coli. Appl Environ Microbiol. 1992;58:1776–1779. doi: 10.1128/aem.58.5.1776-1779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kok R G, Christoffels V M, Vosma B, Hellugwerf K G. Growth-phase-dependent expression of the lipolytic system of Acinetobacter calcoaceticus BD413: cloning of a gene encoding one of the esterases. J Microbiol. 1993;139:2329–2342. doi: 10.1099/00221287-139-10-2329. [DOI] [PubMed] [Google Scholar]
- 25.Kouker G, Jaeger K E. Specific and sensitive plate assay for bacterial lipase. Appl Environ Microbiol. 1987;53:211–213. doi: 10.1128/aem.53.1.211-213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kugiyama W, Otani Y, Hashimoto Y, Tagagi Y. Molecular cloning and nucleotide sequence of lipase gene. Biochem Biophys Res Commun. 1980;14:185–190. doi: 10.1016/s0006-291x(86)80352-7. [DOI] [PubMed] [Google Scholar]
- 27.Kurooka S, Okamoto S, Hashimoto M. A novel and simple colorimetric assay for human serum lipase. J Biochem (Tokyo) 1977;81:361–369. doi: 10.1093/oxfordjournals.jbchem.a131467. [DOI] [PubMed] [Google Scholar]
- 28.Langin D, Holm C. Sequence similarities between hormone-sensitive lipase and five prokaryotic enzymes. Trends Biochem Sci. 1993;18:466–467. doi: 10.1016/0968-0004(93)90007-a. [DOI] [PubMed] [Google Scholar]
- 29.Langin D, Laurell H, Holst L S, Belfrage P, Holm C. Gene organization and primary structure of human hormone-sensitive lipase: possible significance of a sequence homology with a lipase of Moraxella TA 144, an antarctic bacterium. Proc Natl Acad Sci USA. 1993;90:4897–4901. doi: 10.1073/pnas.90.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y P, Chung G H, Rhee J S. Purification and characterization of Pseudomonas fluorescens SIK W1 lipase expressed in Escherichia coli. Biochim Biophys Acta. 1993;1169:156–164. doi: 10.1016/0005-2760(93)90200-s. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Beppu T, Arima K. Effect of various inhibitors on lipase action of thermophilic fungus Humicola lanuginosa S-38. Agric Biol Chem. 1973;37:2487–2492. [Google Scholar]
- 32.Margesin R, Schinner F. Properties of cold-adapted microorganisms and their potential role in biotechnology. J Biotechnol. 1994;33:1–14. [Google Scholar]
- 33.Nishimura A, Morita A, Nishimura Y, Sugino Y. A rapid and highly efficient method for preparation of competent Escherichia coli cells. Nucleic Acids Res. 1990;18:6169. doi: 10.1093/nar/18.20.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen S B, Drabløs F. A sequence analysis of lipases, esterases and related proteins. In: Woolley P, Petersen S B, editors. Lipases: their structure, biochemistry and application. New York, N.Y: Cambridge University Press; 1994. pp. 23–48. [Google Scholar]
- 35.Probst M R, Beer M, Beer D, Jenö D, Meter U A, Randolfo G. Human liver arylacetamide deacetylase: molecular cloning of a novel esterase involved in the metabolic activation of arylamine carcinogens with high sequence similarity to hormone-sensitive lipase. J Biol Chem. 1994;269:21650–21656. [PubMed] [Google Scholar]
- 36.Quinn D M, Shirai K, Jackson R L, Harmony J A K. Lipoprotein lipase catalyzed hydrolysis of water-soluble p-nitrophenyl ester: inhibition by apolipoprotein-II. Biochemistry. 1982;21:6872–6879. doi: 10.1021/bi00269a038. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, Miura K. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Shimada Y, Koga C, Sugihara A, Nagao T, Takada N, Tsunasawa S, Tominaga Y. Purification and characterization of a novel solvent-tolerant lipase from Fusarium heteroporum. J Ferment Bioeng. 1993;75:349–352. [Google Scholar]
- 40.Smibert R M, Krieg N R. General characterization. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 409–443. [Google Scholar]
- 41.Somero G N. Temperature adaptation of enzymes: role of free energy, the enthalpy, and the entropy of activation. Proc Natl Acad Sci USA. 1973;70:430–432. doi: 10.1073/pnas.70.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somero G N. Temperature as a selective factor in protein evolution: the strategy of “compromise”. J Exp Zool. 1977;194:175–188. doi: 10.1002/jez.1401940111. [DOI] [PubMed] [Google Scholar]
- 43.Stellwagen E, Cronlund M M, Barnes L D. A thermostable enolase from the extreme thermophile Thermus aquaticus YT-1. Biochemistry. 1973;12:1552–1559. doi: 10.1021/bi00732a014. [DOI] [PubMed] [Google Scholar]
- 44.Svendsen A, Borch K, Barfoed M, Nielsen T B, Gormsen E, Patkar S A. Biochemical properties of cloned lipases from the Pseudomonas family. Biochim Biophys Acta. 1995;259:9–17. doi: 10.1016/0005-2760(95)00117-u. [DOI] [PubMed] [Google Scholar]