Abstract
Simple Summary
As the two major vectors of mosquito-borne pathogens, yellow fever (Aedes aegypti) and Asian tiger (Ae. albopictus) mosquitos are greatly threatening human health globally. However, their niche and range shifts remain little known. Using the largest occurrence record datasets to date, the present study examined the niche and range shifts between the native and invasive Ae. aegypti and Ae. albopictus populations. We detected substantial niche and range expansions in both species. Additionally, compared to the introduced Ae. aegypti, the more recent invader Ae. albopictus had greater niche and range expansions over its shorter invasion history, making it a more invasive vector of global mosquito-borne pathogens.
Abstract
The yellow fever (Aedes aegypti) and Asian tiger (Ae. albopictus) mosquitos are major vectors of global mosquito-borne pathogens. However, their niche and range shifts, the underlying mechanisms, and related relative invasion rates remain scarcely known. We examined the niche and range shifts between the native and invasive Ae. aegypti and Ae. albopictus populations through dynamic niche and range models and the largest occurrence record datasets to date. We detected substantial niche and range expansions in both species, probably because the introduced populations have more opportunities to acclimate to diverse environmental conditions than their native counterparts. Mitigating climate change could effectively control their future invasions, given that future climate changes could promote their invasiveness. Additionally, compared to the introduced Ae. aegypti, the more recent invader Ae. albopictus had greater niche and range expansion over its shorter invasion history. In terms of the range shifts, Ae. albopictus had an invasion rate approximately 13.3 times faster than that of Ae. aegypti, making it a more invasive vector of global mosquito-borne pathogens. Therefore, considering its higher invasion rate, much more attention should be paid to Ae. albopictus in devising our strategies against prevailing global mosquito-borne pathogens than Ae. aegypti. Since small niche shifts could result in their large range shifts, niche shifts might be a more important indicator for biological invasion assessments.
Keywords: Aedes aegypti, Aedes albopictus, climate factor, invasiveness, niche shift, range shift
1. Introduction
The yellow fever (Aedes aegypti) and Asian tiger (Ae. albopictus) mosquitos are two major vectors of mosquito-borne pathogens [1,2], including the viruses responsible for Zika virus disease, yellow fever, dengue fever, and chikungunya fever [3,4,5,6], which greatly threaten human health globally [7,8,9,10,11,12,13]. Native to Africa, Ae. aegypti has proliferated and spread into subtropical and tropical regions outside its native continent since the 16th and 17th centuries, a hectic time of global slave trade [14,15]. Unlike Ae. aegypti, Ae. albopictus, a mosquito native to Southeast Asia, has a shorter invasion history. It has proliferated and expanded its range from tropic and subtropical regions into temperate ones over the last 30–40 years [14,15,16]. However, studies comparing the invasion rates of the two Aedes species have been scarcely reported.
The ecological niche, which delimits environmental ranges under which a particular species could survive [17], is an essential conception in invasion ecology, probably due to its close associations with environmental conditions and the range shifts in alien invasive species under global change scenarios [18,19]. Recently, species distribution models (SDMs) have been widely used to project the potential ranges of alien invasive species and their shifts [20,21]. Nevertheless, one of their key presuppositions is the niche conservatism hypothesis, i.e., the alien invasive species conserves the niche inherited from its native counterpart [22,23]. However, until now, this hypothesis is still intensely debated. Some studies detected niche conservatism in alien invasive species [24,25,26,27], while others rejected it [28,29,30]. Niche shifts in alien invasive species might be an indicator of their invasiveness, i.e., alien invasive species with considerable niche expansions might have higher invasiveness. Thus, niche shifts in alien invasive species are also one of the important topics in invasion ecology [31,32,33]. However, to the best of our knowledge, few specific studies on the niche shift of Ae. aegypti and Ae. albopictus were reported.
Climatic factors, e.g., temperature and precipitation, are closely associated with the life histories of these mosquitos [34], including growth [35], development [36,37], reproduction [38,39], and even behavior [39,40]. For example, many studies have detected potential influences the temperature has on the life histories of these two Aedes species and their epidemiological features [41,42,43]. Notably, temperature could shorten the extrinsic incubation time (the time needed for a pathogen to develop inside Ae. albopictus or Ae. aegypti before it can be transmitted) [41,42]. These observations suggested that climatic factors could modify their roles as major vectors of global mosquito-borne pathogens [44]. Therefore, their invasion potentials and distribution patterns might be closely linked to their adaptability to changing climate conditions [45,46]. For example, Cunze et al. projected an increase in their suitable habitats in Europe under future climate change scenarios, in which their adaptability to climatic conditions played an essential role [47]. Recently, Laporta et al. predicted that future climate change could promote their proliferation at a global scale [46]. These findings undoubtedly offered important information for devising strategies against their future invasions.
In addition to climate conditions, anthropogenic factors also play an important role in their potential ranges, probably because both have an inheritably anthropophilic nature [1,2,3,4]. Anthropogenic factors could affect their thermal regulation behaviors and exploitation ability for man-made habitats [48,49]. Therefore, studies on the influence of anthropogenic factors on their potential ranges have attracted much attention. For example, Dickens et al. observed that human and trade movement could facilitate range expansions in both vectors [50]. Abílio et al. found that the water container index significantly modified the ranges of both vectors in the urban/peri-urban regions of Mozambique [51]. Recently, Holeva-Eklund et al. observed that human population density was a principal predictor of the ranges of Ae. aegypti in Maricopa County, Arizona [52]. These studies have furthered our understanding of the roles anthropogenic factors have in determining the ranges of both vectors.
Although climatic and anthropogenic factors could be responsible for their potential ranges, their relative roles in determining the ranges of these vectors remain debated. For example, while Dickens et al. found that anthropogenic factors had stronger associations with the potential ranges of both vectors than climatic factors [50], Liu et al. observed the opposite [45]. Therefore, the relative influences of anthropogenic and climatic factors on the potential ranges of Ae. aegypti and Ae. albopictus should be further investigated.
Topographical factors (e.g., elevation, slope, and aspect) are closely associated with the radiation, energy, and water conditions, forming various macro- and micro-habitats. Additionally, topographical patterns, such as deep canyons and lofty mountain ranges, could act as barriers against the spread of such invasive species [53,54,55]. Although the potential ranges of the two Aedes species might be strongly associated with climatic and anthropogenic factors, the effects of topographical patterns should not be neglected. However, the relative impacts of topographical, climatic, and anthropogenic factors on their potential ranges remain largely unknown.
The potential ranges of Ae. aegypti and Ae. albopictus have attracted considerable attention [46,56,57,58,59,60,61]. For instance, Kamal et al. predicted a marked increase in the potential ranges of Ae. aegypti across tropical and subtropical regions and an increase in the potential ranges of Ae. albopictus in the temperate regions of the United States and Europe based on future climate scenarios [62]. We hypothesized that since Ae. aegypti started its invasions about 300–400 years ago [14,15], it has a long time to adapt to novel conditions in the introduced regions. Therefore, there might be large range shifts between native and introduced Ae. aegypti, while smaller range shifts could be detected in Ae. albopictus due to its relatively shorter invasion history (30–40 years) [14,15,16]. However, most studies paid little attention to the range dynamics between the native and introduced Aedes species that might offer novel and essential information for controlling their invasions. For example, determining their potential range expansions (ranges only occupied by the introduced populations) during a certain period could provide essential information for assessing their invasiveness. Although Ae. aegypti and Ae. albopictus played major roles in the worldwide spread of chikungunya, dengue, and Zika fevers, their relative invasion rates in terms of the range shifts remain unknown.
In the present study, the niche and potential range shifts of an invasive species in its introduced regions relative to those of its native counterpart were used to calibrate its invasion potentials. Therefore, we grouped the population of each species into two populations: i.e., native and introduced populations, represented by those occurrences in native and introduced regions, respectively. We hypothesized that substantial niche and range shifts occurred between the native and introduced populations of each species. We developed models to detect niche and range shifts between native and introduced populations of each Aedes species and estimated the relative influences of climatic, anthropogenic, and topographical factors on their potential ranges and range shifts. Additionally, we also compared the invasion rates of the two Aedes species based on their range shifts. Our study could enrich and advance our understanding of the invasion potential of the two Aedes species.
2. Materials and Methods
2.1. Occurrence Record Datasets
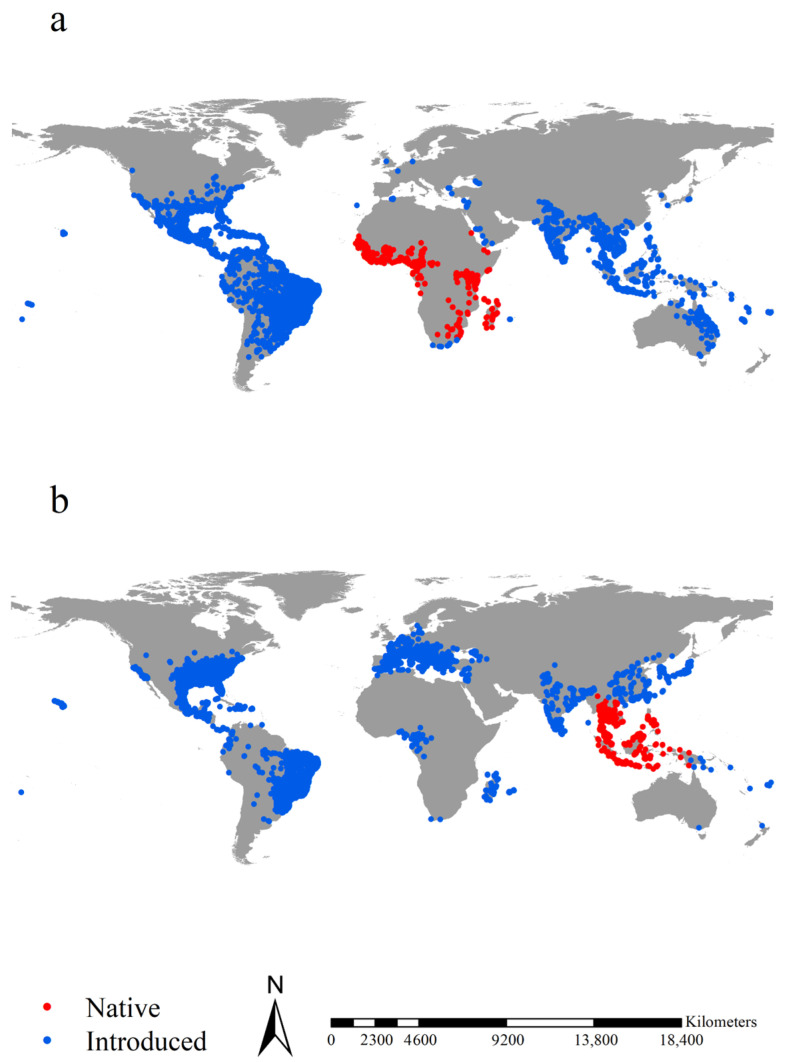
We retrieved the occurrence records of Ae. aegypti and Ae. albopictus from four sources: (1) the Global Biodiversity Information Facility (www.gbif.org, accessed on 2 March 2023), from which we obtained 60,197 and 78,619 records of Ae. aegypti and Ae. albopictus, respectively, with clear geographical coordinates; (2) a literature survey, primarily the paper by Kraemer et al. [63], from which we obtained 19,929 and 22,137 records with clear geographical coordinates for Ae. aegypti and Ae. albopictus, respectively; (3) the Early Detection and Distribution Mapping System (https://www.eddmaps.org/, accessed on 3 March 2023), from which we obtained 10,337 and 10,421 records of Ae. aegypti and Ae. albopictus, respectively; (4) the VectorMAP (https://vectormap.si.edu/, accessed on 4 March 2023), from which we obtained 37,545 and 42,167 occurrence records of Ae. aegypti and Ae. albopictus, respectively. Combined, our occurrence record datasets contained 128,008 and 153,344 records for Ae. aegypti and Ae. albopictus, respectively. We refine the occurrence record dataset by removing duplicate records and those with a geographical coordinate uncertainty of over 5 km, resulting in a dataset with 25,170 and 38,457 distinct records for Ae. aegypti and Ae. albopictus, respectively. We used SDMtoolbox by Brown et al. [64] to spatially rarefy the dataset with a radius of 5 km to reduce the effects of sampling bias on our models. Finally, we retained 7606 and 7921 occurrence records of Ae. aegypti and Ae. albopictus, respectively. Subsequently, we divided the dataset into four sub-datasets based on the native regions of Ae. aegypti and Ae. albopictus [65], i.e., 7239 records of Ae. aegypti in its introduced regions and 367 in its native regions and 7461 records of Ae. albopictus in its introduced regions and 460 in its native regions (Figure 1, Online datasets 1). The native and introduced occurrence records were input into species distribution models (SDMs) to project their native and introduced potential ranges, respectively.
Figure 1.
Occurrence records of the two Aedes species. Red and blue points in (a) indicated the native and introduced records of Aedes aegypti, respectively. Red and blue points in (b) indicated the native and introduced records of Aedes albopictus, respectively. After spatial rarefication, we retrieved 367 and 7239 native and introduced records for Ae. aegypti, respectively, and 460 and 7461 native and introduced records for Ae. albopictus, respectively, in total.
2.2. Niche Dynamic Analysis
Following the COUE scheme (Centroid shift, Overlap, Unfilling, and Expansion) [66], we utilized ecospat, an R package for niche dynamic analysis [67], to calibrate the niche dynamics of the two Aedes species. We used occurrence records of the native and invasive Ae. aegypti and the spatial layers of the 32 predictors to extract values of each predictor for each occurrence record. Then, through an embedded principal component analysis (PCA) in the ecospat R package [66], we generated two PCA axes to delimit the niche space of Ae. aegypti in invaded and native regions. We divided the total environmental space into 100 × 100 grid cells [22,23,66]. We also utilized Kernel density functions to calibrate the smoothed density of occurrence records and available environment space along the first two PCA axes. According to the COUE scheme, the niche spaces were grouped into three elements: niche stabilized (S), niche unfilled (U), and niche expanded (E). Niche unfilled represented the niche space exploited only by the native Ae. aegypti; niche stabilized was the niche space exploited both by native and introduced Ae. Aegypti; and niche expanded indicated the niche space exploited only by introduced Ae. aegypti. The niche breadth of the native Ae. aegypti (NB) was the sum of S and U, and that of the introduced Ae. aegypti was the sum of S and E (IB). Breadth ratio (BR), indicating the ratio of the niche breadth of the introduced Ae. aegypti to that of the native Ae. aegypti, was calibrated as follows:
If breadth ratio (BR) > 1, the niche breadth of the introduced Ae. aegypti was wider than that of the native counterpart and vice versa.
We also estimated the index of niche similarity (SI) to measure niche position shifts between the native and introduced Ae. aegypti as follows:
If index of niche similarity (SI) > 0.5, native and introduced Ae. aegypti occupied similar niche positions and vice versa. When index of niche similarity < 0.5 and breadth ratio > 1, the introduced Ae. aegypti did not conserve the niche inherited from its native counterpart, and the niche conservatism hypothesis was rejected [26]. In addition to the investigations on breadth ratio and index of niche similarity, we also conducted niche equivalency test and niche similarity test.
Using this method, we also examined the niche dynamics between the native and introduced Ae. aegypti at the continental scale, separately, as well as those of Ae. albopictus at global and continental scales.
2.3. Predictors Used in SDMs
Our study retrieved 32 predictors to develop the SDMs, including 3 topographical, 10 anthropogenic, and 19 climatic predictors. As most (>85%) Ae. aegypti and Ae. albopictus occurrences were recorded after 1990, all predictors used in our study except the topographical ones had a time stamp of 1990–2020. We extracted a spatial elevation layer at a global scale from a digital elevation model (with a spatial resolution of 30 arc seconds) downloaded from Worldclim [68] and generated global spatial slope and aspect layers. The 1990–2020 anthropogenic predictors included eight land-use variables, gross domestic product per capita (GDP), and population density. The eight land-use predictors, retrieved from Land-Use Harmonization (http://luh.umd.edu/, accessed on 4 March 2023), had a spatial resolution of 0.25° and were divided into primary forested land, primary non-forested land, potentially secondary forested land, potentially secondary non-forested land, managed pasture, rangeland, urban land, and cropland. The GDP per capita and population density had a spatial resolution of 0.5 arc minutes and were retrieved from the Socioeconomic Data and Applications Center (https://sedac.ciesin.columbia.edu/, accessed on 4 March 2023). Instead of using the near-current (1970–2000) climatic predictors offered by Worldclim [69], we retrieved the most-updated monthly datasets of temperature and precipitation during 1990–2020 from the Climatic Research Unit (https://crudata.uea.ac.uk/, accessed on 6 March 2023) and applied the Biovars R package by Fick and Hijmans [69] to generate 19 climatic variables at a spatial resolution of 2.5 arc minutes. All 19 climatic variables were consistent with those in Worldclim [69]. Our 19-climatic-variable dataset included temperature and precipitation variables related to climatic factors at the month, quarter, and annual time scales. It must be noted that all 32 predictors were at or resampled into a spatial resolution of 2.5 arc minutes.
We used the methodology proposed recently [70] to reduce the collinearity among the predictors. First, biomod2 [71], an assembled SDM platform, was used to develop the preliminary SDMs and calibrate important values of each predictor (S1). We utilized VarImport, a function in Biomod2, to calculate predictors’ importance values calibrated by each algorithm in species distribution models [71]. Then, the averaged importance values of predictors were adopted. Subsequently, we used Pearson correlation analysis to calibrate the collinearity among the 32 predictors, using a threshold of >0.7 or <−0.7(S2) [72]. We retained the predictor with the higher importance values in each collinear pair. This process was repeated until no strong collinearity was observed. The retained predictors are presented in Table 1 and were included in the final SDMs to generate potential ranges for the two Aedes species and retrieve their importance values in the final SDMs.
Table 1.
The importance values of the retained predictors in the final species distribution models for Ae. aegypti and Ae. albopictus.
| Aedes aegypti | Aedes albopictus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Native Ae. aegypti | Introduced Ae. aegypti | Native Ae. albopictus | Introduced Ae. albopictus | ||||||||
| Category | Predictors | Importance Values | Category | Predictors | Importance Values | Category | Predictors | Importance Values | Category | Predictors | Importance Values |
| Climate | Bio3 | 0.351 | Climate | Bio4 | 0.369 | Climate | Bio1 | 0.230 | Climate | Bio10 | 0.150 |
| GDP | GDP | 0.196 | GDP | GDP | 0.194 | Climate | Bio4 | 0.197 | Climate | Bio4 | 0.149 |
| POP | POP | 0.113 | POP | POP | 0.112 | Climate | Bio12 | 0.195 | Climate | Bio13 | 0.108 |
| Climate | Bio7 | 0.052 | Climate | Bio10 | 0.099 | Climate | Bio18 | 0.148 | Land-use | Pastr | 0.082 |
| Land-use | Primf | 0.030 | Climate | Bio9 | 0.096 | POP | POP | 0.136 | Land-use | Urban | 0.056 |
| Land-use | Range | 0.028 | Land-use | Crop | 0.045 | Land-use | Range | 0.059 | POP | POP | 0.056 |
| Climate | Bio13 | 0.024 | Climate | Bio13 | 0.033 | Climate | Bio5 | 0.050 | Climate | Bio17 | 0.050 |
| Land-use | Secdn | 0.024 | Land-use | Primf | 0.015 | GDP | GDP | 0.049 | Climate | Bio18 | 0.03 |
| Land-use | Primn | 0.020 | Land-use | Urban | 0.011 | Land-use | Pastr | 0.032 | Land-use | Crop | 0.032 |
| Climate | Bio1 | 0.019 | Climate | Bio19 | 0.011 | Land-use | Crop | 0.021 | Climate | Bio8 | 0.030 |
| Climate | Bio14 | 0.017 | Topography | Ele | 0.010 | Land-use | Primf | 0.018 | Climate | Bio19 | 0.028 |
| Land-use | Pastr | 0.015 | Climate | Bio18 | 0.010 | Topography | Ele | 0.018 | Land-use | Primf | 0.025 |
| Climate | Bio18 | 0.012 | Climate | Bio17 | 0.008 | Climate | Bio14 | 0.018 | Topography | Ele | 0.022 |
| Land-use | Crop | 0.011 | Land-use | Pastr | 0.007 | Topography | Slop | 0.011 | Land-use | Range | 0.020 |
| Climate | Bio15 | 0.007 | Land-use | Range | 0.007 | Climate | Bio2 | 0.007 | Land-use | Primn | 0.016 |
| Topography | Slop | 0.007 | Land-use | Primn | 0.007 | Land-use | Secdn | 0.003 | Climate | Bio2 | 0.010 |
| Land-use | Secdf | 0.004 | Topography | Slop | 0.005 | Land-use | Secdf | 0.003 | Land-use | Secdn | 0.010 |
| Topography | Asp | 0.003 | Land-use | Secdf | 0.004 | Land-use | Urban | 0.003 | Topography | Slop | 0.007 |
| Land-use | Urban | 0.002 | Land-use | Secdn | 0.003 | Land-use | Primn | 0.002 | Land-use | Secdf | 0.005 |
| Topography | Asp | 0.003 | Topography | Asp | 0.002 | Topography | Asp | 0.001 | |||
| Climate | Bio2 | 0.002 | |||||||||
Note: Bio1: annual mean temperature (°C); Bio2: mean diurnal range (°C); Bio3: isothermality; Bio4: temperature seasonality; Bio5: max temperature of the warmest month (°C); Bio7: temperature annual range (°C); Bio8: mean temperature of the wettest quarter (°C); Bio9: mean temperature of the driest quarter (°C); Bio10: mean temperature of the warmest quarter (°C); Bio12: annual precipitation (mm); Bio13: precipitation of the wettest month (mm); Bio14: precipitation of the driest month (mm); Bio15: precipitation seasonality (mm); Bio17: precipitation of the driest quarter (mm); Bio18: precipitation of the warmest quarter (mm); Bio19: precipitation of the coldest quarter (mm); Asp: aspect (°); Ele: elevation (m); Slop: slope (°); Urban: fractions of urban land; Primf: fractions of forested primary land; Crop: fractions of cropland; Pastr: fractions of managed pasture; Secdf: fractions of potentially forested secondary land; Range: fractions of rangeland; Primn: fractions of non-forested primary land; Secdn: fractions of potentially non-forested secondary land; GDP: gross domestic product; and POP: population density. The importance values were calibrated by eight algorithms, i.e., Artificial Neural Network, Classification Tree Analysis, Generalized Linear Model, Flexible Discriminant Analysis, Multiple Adaptive Regression Splines, Generalized Boosting Model, Random Forest for Classification and Regression, and Maximum Entropy Modeling, and the averaged importance values of each predictor were adopted.
2.4. Potential Ranges of the Two Aedes Species
We projected the potential ranges of the two Aedes species using biomod2, an assembled platform for SDMs [71]. We used the retained predictors and adopted nine algorithms from biomod2 to project the potential ranges of Ae. aegypti and Ae. albopictus. The nine algorithms included Artificial Neural Network, Classification Tree Analysis, Generalized Linear Model, Flexible Discriminant Analysis, Multiple Adaptive Regression Splines, Generalized Boosting Model, Random Forest for Classification and Regression, Maximum Entropy Modeling, and Surface Range Envelope. Only algorithms showing an area under the curve (AUC) > 0.8 or true skill statistic (TSS) > 0.6 were included in the assembled SDMs to obtain reliable and central tendencies of the potential ranges’ projections [73]. A weight proportional to their TSS evaluation was given to each model’s projection [71]. As required by presence-only SDMs, we conducted a three-time random selection at a global terrestrial scale (except Antarctica) as follows: equal numbers of pseudo absences when the number of real occurrence records was over 1000, or 1000 pseudo absences randomly were selected, following Cao et al. [74]. We used the maximization sensitivity–specificity sum thresholds to calibrate the potential ranges of the two Aedes species, as suggested by Liu et al. (2016) [75].
To evaluate the reliability of SDMs, a five-time cross-validation was utilized, in which we randomly selected 70% of the total occurrences to develop the SDMs and used the remaining 30% to calibrate the models’ reliability [71]. We also applied null models to evaluate our model’s reliability following a robust methodology [76,77], in which we randomly selected virtual occurrence records (equal in number to 70% of the total occurrences) to generate SDMs and used 30% of the real occurrences to assess the null SDMs’ performances (S3).
2.5. Range Shifts of the Two Aedes Species
We decomposed the total potential ranges of Ae. aegypti into three parts, following Yang et al. [78]: range expansion (RE), potential ranges only occupied by introduced Ae. aegypti; range stability (RS), potential ranges shared by native and introduced Ae. aegypti; and range unfilling (RU), potential ranges occupied by native Ae. aegypti only. The potential ranges of native Ae. aegypti (PRN) were the sum of RS and RU, whereas those of introduced Ae. aegypti (PRI) were the sum of RE and RS. We constructed a range ratio index (RRI) to compare the Ae. aegypti PRI and PRN as follows:
When the potential ranges of the introduced Ae. Aegypti (PRI) were greater than its native counterpart (PRN), the range ratio index (RRI) was >1 [78,79].
We also constructed a range similarity index (RSI) to calibrate the range position shifts between the introduced and native Ae. aegypti, rendered as follows:
When the native and introduced Ae. aegypti were in similar range positions, the range similarity index (RSI) was >0.5 [78,79].
Similar methods were adopted to calibrate the range shifts between the introduced and native Ae. albopictus.
3. Results
3.1. Major Predictors for the Potential Ranges
Our results showed that SDMs calibrated by Artificial Neural Network, Classification Tree Analysis, Generalized Linear Model, Flexible Discriminant Analysis, Multiple Adaptive Regression Splines, Generalized Boosting Model, Random Forest for Classification and Regression and Maximum Entropy Modeling were included in the assembled SDMs, whereas those calibrated by Surface Range Envelope were removed because their AUC and TSS were less than 0.8 and 0.6, respectively (S4). The importance values of the most important predictors of potential ranges in decreasing order were isothermality (0.351), GDP per capita (0.196), and population density (0.113) for native Ae. aegypti, temperature seasonality (0.369), GDP per capita (0.194), and population density (0.112) for introduced Ae. aegypti, mean annual temperature (0.230), Temperature seasonality (0.197), and annual precipitation (0.195) for native Ae. albopictus, and the mean temperature of the warmest quarter (0.150), temperature seasonality (0.149), and precipitation of the wettest month (0.108) for introduced Ae. albopictus (Table 1 and Table S5). Our results also indicated that none of the topographical predictors had an importance value in the top ten list for any of the potential ranges (Table 1). While GDP per capita and population density were the second and third most important predictors in the SDMs for Ae. aegypti, they were not in the top three among the SDMs for Ae. albopictus. In summary, climatic predictors showed the highest importance values for all potential ranges, followed by anthropogenic and topographical factors (Table 1).
3.2. Niche Dynamics of the Two Aedes Species
The global scale investigations into niche dynamics of Ae. aegypti showed that at the global scale, the niche expanded, niche stabilized, and niche unfilled were 0.0045, 0.955, and 0.027, respectively; the breadth ratio and niche similarity index were 1.018 and 0.964, respectively (Table 2). Therefore, at the global scale, the niche shifts in Ae. aegypti supported the niche conservatism hypothesis. At the continental scale, smaller niche shifts were detected between the native Ae. aegypti and the introduced counterpart in South America relative to those between the native Ae. aegypti and the introduced counterparts in Asia and North America (Table 2). At the global scale, the niche expanded, niche stabilized, and niche unfilled of Ae. albopictus were 0.382, 0.618, and 0.101, respectively; the breadth ratio and niche similarity index were 1.391 and 0.719, respectively (Table 2). Therefore, at the global scale, the introduced Ae. albopictus conserved the niche inherited from its native counterpart. At the continental scale, relatively larger niche expanded, niche stabilized, and niche unfilled were detected between the native Ae. albopictus and the introduced counterparts in Europe, South America, and Europe, respectively (Table 2). Additionally, all of our equivalency tests and similarity tests showed that the two introduced Aedes species have niches equivalent to their native counterparts but similar by chance, which, to a certain extent, was consistent with our observations on their shifts in breadth ratio and niche similarity, which indicated the niche conservatism of the two introduced Aedes species.
Table 2.
Niche dynamics of the two Aedes species.
| Species (Native Range) | Introduced Population | Expan | Stable | Unfill | Breadth | EquaT | Similar | SimiT |
|---|---|---|---|---|---|---|---|---|
| Aedes aegypti (Africa) | Global | 0.045 | 0.955 | 0.027 | 1.017 | ns | 0.964 | ns |
| Asia | 0.087 | 0.913 | 0.005 | 1.090 | ns | 0.952 | ns | |
| North America | 0.137 | 0.863 | 0.058 | 1.085 | ns | 0.899 | ns | |
| South America | 0.008 | 0.992 | 0.148 | 0.877 | ns | 0.927 | ns | |
| Ae. albopictus (Asia) | Global | 0.382 | 0.618 | 0.101 | 1.391 | ns | 0.719 | ns |
| Africa | 0.055 | 0.945 | 0.208 | 0.867 | ns | 0.878 | ns | |
| Europe | 0.499 | 0.501 | 0.806 | 0.765 | ns | 0.434 | ns | |
| North America | 0.332 | 0.668 | 0.359 | 0.974 | ns | 0.659 | ns | |
| South America | 0.022 | 0.978 | 0.286 | 0.791 | ns | 0.864 | ns |
Note: EquaT: equivalency test; SimiT: similarity test; Epan: niche expanded; Stable: niche stabilized; Unfill: niche unfilled; Breadth: breadth ratio; and Similar: similarity index. We utilized the COUE scheme to calibrate niche dynamics of the two Aedes species. ns: p > 0.05.
3.3. Potential Ranges of the Two Aedes Species
All four SDMs for the potential ranges showed high performance. Our SDMs for projecting the potential ranges of native and introduced Ae. aegypti had AUCs of 0.993 and 0.984 and TSS values of 0.936 and 0.878 (S3). The SDMs for projecting the potential ranges of native and introduced Ae. albopictus had AUCs of 0.997 and 0.978 and TSS values of 0.967 and 0.852 (S3). All four SDMs performed better than the null SDMs (all p < 0.001) (S3). The maximization sensitivity–specificity sum thresholds for calibrating the potential ranges were 0.58 and 0.45 for introduced and native Ae. aegypti and 0.50 and 0.47 for introduced and native Ae. albopictus, respectively.
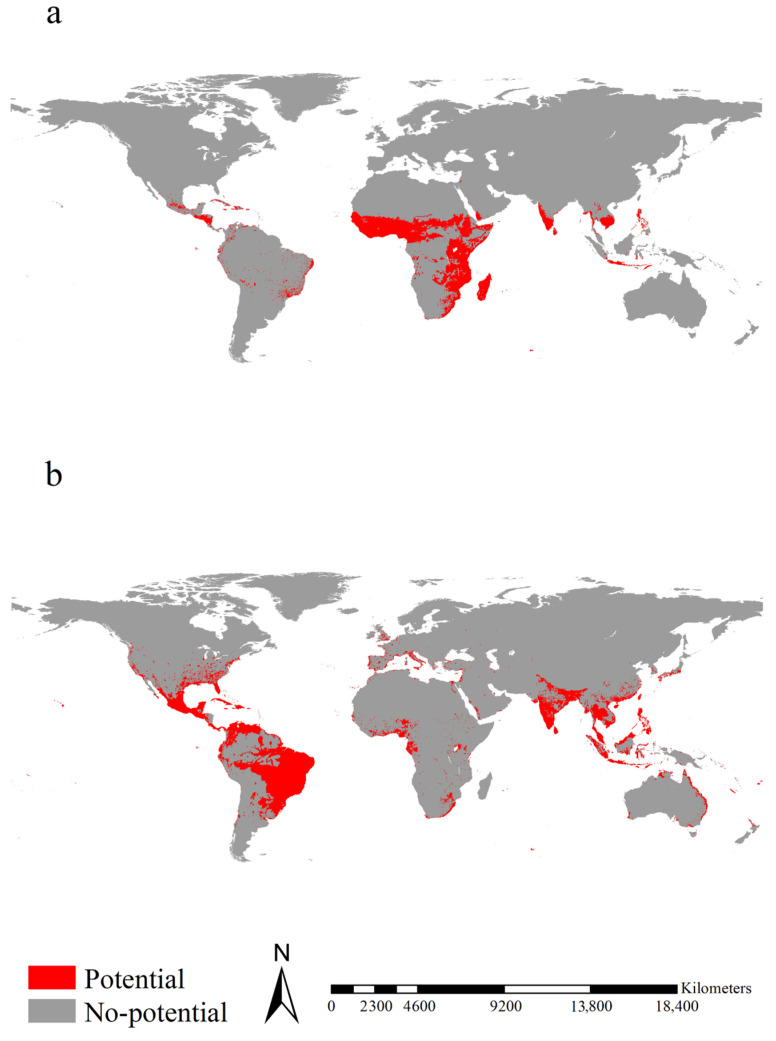
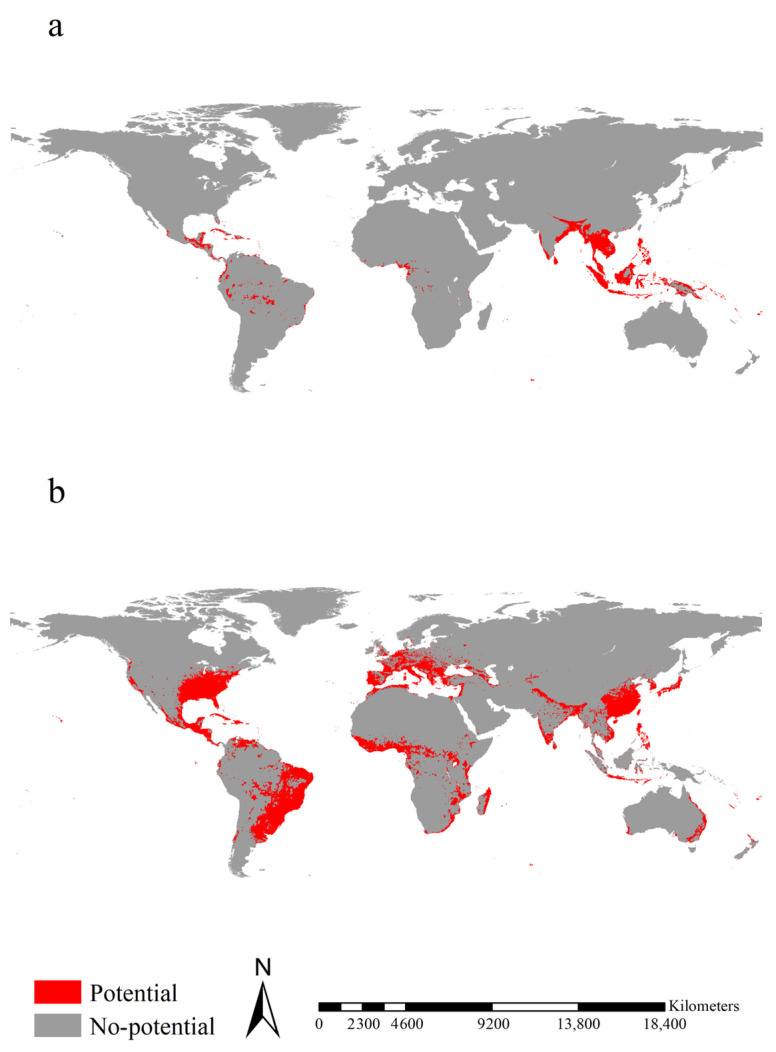
Potential ranges for native Ae. aegypti were mainly observed in Cambodia, Cameroon, Central Africa, Chad, East Africa from Ethiopia and Somalia to South Africa, Honduras, Indonesia, Madagascar, Mexico, Nicaragua, South India, Sri Lanka, the Philippines, tropical West Africa, and Vietnam, covering 9.38 × 106 km2 (Figure 2a). Potential ranges of introduced Ae. aegypti were detected in Bangladesh, Brazil, Colombia, India, Indonesia, Japan, Malaysia, Nepal, Thailand, the Philippines, the southeastern coastal regions of China, vast regions of Mexico and Venezuela, covering 14.53 × 106 km2 (Figure 2b). Potential ranges for native Ae. albopictus were mainly projected in Bangladesh, Bolivia, Brazil, Cambodia, Ecuador, India, Indonesia, Laos, Malaysia, Mexico, Sri Lanka, Thailand, and Vietnam, covering 4.71 × 106 km2 (Figure 3a). Potential ranges of introduced Ae. albopictus were mainly projected in Cambodia, eastern China, eastern United States, Europe, India, Indonesia, Japan, Mexico, the Philippines, tropical regions of West Africa, and Vietnam, covering 18.01 × 106 km2 (Figure 3b).
Figure 2.
Potential ranges of Aedes aegypti. (a,b) represented the potential ranges of the native and introduced Ae. aegypti, respectively. The potential ranges of native Ae. aegypti were mainly observed in Cambodia, Cameroon, Central Africa, Chad, East Africa from Ethiopia and Somalia to South Africa, Honduras, Indonesia, Madagascar, Mexico, Nicaragua, South India, Sri Lanka, the Philippines, tropical West Africa, and Vietnam. The potential ranges of introduced Ae. aegypti were mainly detected in Bangladesh, Brazil, Colombia, India, Indonesia, Japan, Malaysia, Nepal, Thailand, the Philippines, the southeastern coastal regions of China, and vast regions of Mexico and Venezuela. The potential ranges were calibrated by eight algorithms, i.e., Artificial Neural Network, Classification Tree Analysis, Generalized Linear Model, Flexible Discriminant Analysis, Multiple Adaptive Regression Splines, Generalized Boosting Model, Random Forest for Classification and Regression, and Maximum Entropy Modeling, and a weight proportional to their TSS evaluation was given to each model’s projection.
Figure 3.
Potential ranges of Aedes albopictus. (a,b) represented the potential ranges of the native and introduced Aedes albopictus, respectively. The potential ranges of native Ae. Albopictus were mainly observed in Bangladesh, Bolivia, Brazil, Cambodia, Ecuador, India, Indonesia, Laos, Malaysia, Mexico, Sri Lanka, Thailand, and Vietnam. The potential ranges of introduced Ae. Albopictus were mainly observed in Cambodia, eastern China, eastern United States, Europe, India, Indonesia, Japan, Mexico, the Philippines, tropical regions of West Africa, and Vietnam. The potential ranges were calibrated by eight algorithms, i.e., Artificial Neural Network, Classification Tree Analysis, Generalized Linear Model, Flexible Discriminant Analysis, Multiple Adaptive Regression Splines, Generalized Boosting Model, Random Forest for Classification and Regression, and Maximum Entropy Modeling, and a weight proportional to their TSS evaluation was given to each model’s projection.
3.4. Range Shifts of the Two Aedes Species
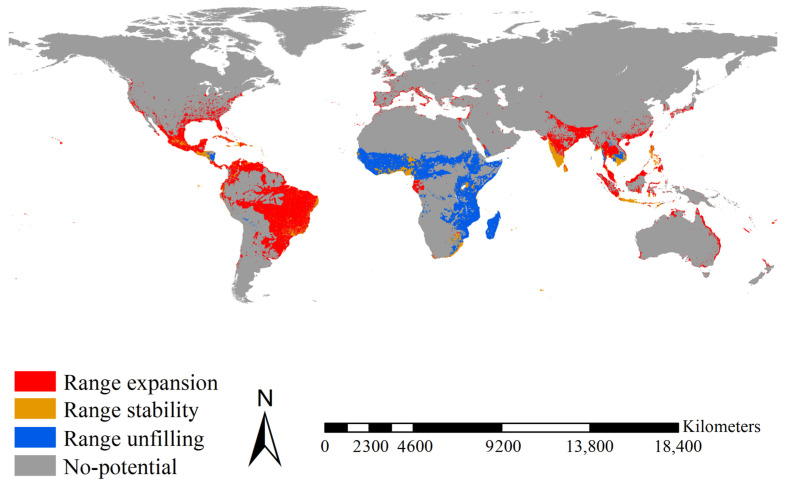
Range expansion for Ae. aegypti was mainly projected for Bangladesh, Brazil, Colombia, Guatemala, India, Indonesia, Malaysia, Mexico, the southeastern United States, Thailand, and Venezuela, covering 12.03 × 106 km2 (Figure 4). Range stability for Ae. aegypti was mainly projected for Cameroon, Honduras, India, Nigeria, South Africa, Sri Lanka, Togo, and Vietnam, covering 2.50 × 106 km2 (Figure 4). Range unfilling was mainly anticipated in the tropical regions of East Africa from Ethiopia and Somalia to South Africa, Madagascar, and West Africa, covering 6.88 × 106 km2 (Figure 4). Accordingly, the range ratio index was 1.549, i.e., the introduced Ae. aegypti had 1.55 times more potential ranges than the native one. The range similarity index was 0.209, i.e., the native and introduced Ae. aegypti occupied different range positions.
Figure 4.
Range shifts between the native and introduced Aedes aegypti. Range expansions between native and introduced Ae. aegypti were mainly detected in Bangladesh, Brazil, Colombia, Guatemala, India, Indonesia, Malaysia, Mexico, southeastern United States, Thailand, and Venezuela. Range stability between native and introduced Ae. aegypti was mainly detected in Honduras, Cameroon, Togo and Nigeria, South Africa, India, Sri Lanka, and Vietnam. Range unfilling was mainly anticipated in Madagascar, the tropical regions of East Africa from Ethiopia and Somalia to South Africa and West Africa. Red, blue, and orange indicated the range expanded, range unfilled, and range stabilized, respectively.
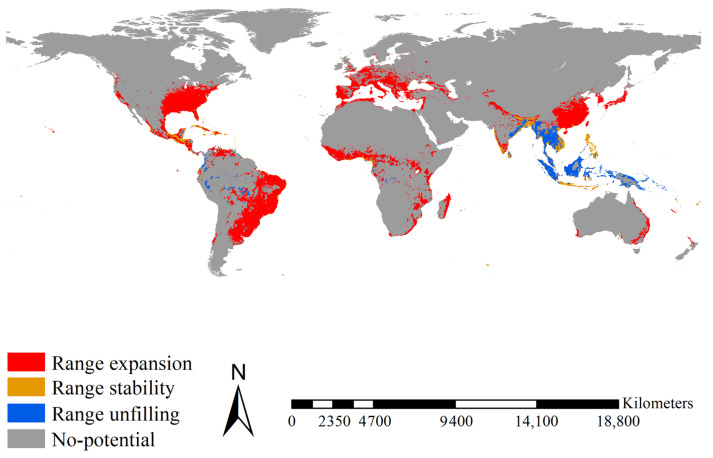
Range expansion for Ae. albopictus was mainly projected for Brazil, eastern China, Europe, India, Japan, Madagascar, Mexico, the eastern United States, and the tropical regions of East Africa, covering 16.10 × 106 km2 (Figure 5). Range stability for Ae. albopictus was mainly projected for Bangladesh, India, Indonesia, Mexico, and the Philippines, covering 1.91 × 106 km2 (Figure 5). Range unfilling was mainly anticipated for India, Indonesia, Malaysia, and Thailand, covering 2.80 × 106 km2 (Figure 5). Accordingly, the range ratio index was 3.824, i.e., the introduced Ae. albopictus had ca. 3.83 times more potential ranges than the native one. The range similarity index was 0.168, i.e., the native and introduced Ae. albopictus occupied different range positions. Additionally, as a more recent invader, introduced Ae. albopictus showed a larger range expansion (16.10 × 106 km2 vs. 12.03 × 106 km2, ca. 1.33 times) than the introduced Ae. aegypti over its shorter invasion history (ca. one tenth). Therefore, in terms of range shifts, Ae. albopictus had an invasion rate ca. 13.3 times that of Ae. aegypti.
Figure 5.
Range shifts between the native and introduced Ae. Albopictus. Range expansions between native and introduced Ae. Albopictus were mainly detected in Brazil, eastern China, Europe, India, Japan, Madagascar, Mexico, the eastern United States, and the tropical regions of East Africa. Range stability between native and introduced Ae. Albopictus was mainly detected in Bangladesh, India, Indonesia, Mexico, and the Philippines. Range unfilling was mainly projected in India, Indonesia, Malaysia, and Thailand. Red, blue, and orange indicated the range expanded, range unfilled, and range stabilized, respectively.
4. Discussion
Our study detected substantial niche and range shifts that occurred between the native and introduced populations of both species. We observed stronger roles of climatic factors in their potential ranges relative to those of anthropogenic and topographical ones. Our study also suggested that in terms of the niche and range shifts, Ae. albopictus, as a latecomer, exhibited higher invasiveness than Ae. aegypti. Therefore, our study could enrich and further our understanding of their invasion potential and risk assessment.
Although the niche conservatism hypothesis on alien invasive species has received much attention in the past decades, this hypothesis is still under intense debate. Recently, Liu et al. (2020) argued that most invasive species largely conserve their climatic niche [26], which, to a great extent, supported our observations that the two introduced Aedes species conserved the niche spaces inherited from their native counterparts. Ae. albopictus had relatively shorter invasions than Ae. aegypti, i.e., 30–40 vs. 300–400 years [14,15,16]. However, our study showed that compared with Ae. aegypti, Ae. albopictus had larger niche expansions (0.382 vs. 0.045), indicating that the latter has evolved stronger adaptability to novel environmental conditions in a relatively shorter history and has higher invasiveness. Our study also showed that the niche dynamics of the two Aedes species differed among continents. For example, larger niche expansions of Ae. albopictus were detected in Europe, while smaller ones were observed in South America (0.499 vs. 0.022). The introduced Ae. albopictus in Europe and South America survived in temperate and tropical regions, respectively, and the native Ae. albopictus originated from tropical regions in Asia. Therefore, climatic differences between the native Ae. albopictus and the introduced counterpart in Europe were larger than those between the native Ae. albopictus and the introduced counterpart in South America, resulting in larger niche expansions. Additionally, previous studies have shown that Ae. albopictus had strong adaptability to novel environmental conditions. For example, Lacour et al. (2015) detected adaptive synchronization of the diapause process of Ae. albopictus in adverse winter conditions [80]. Marini et al. (2020) observed the ability of Ae. albopictus to quickly adapt to colder environments [81]. Therefore, the differences in niche dynamics among the continents might not only be closely associated with the climatic differences between the regions where the introduced and native Ae. albopictus survived, but also with a strong adaptability to novel conditions.
Over the last decades, various studies have examined the potential ranges or range shifts of Ae. aegypti and Ae. albopictus [45,47,62,82,83,84,85]. However, even though most of the occurrences were recorded after 1990, most previous studies used the near-current condition climatic datasets (1970–2000) from Worldclim [69] to probe the range shifts of the two Aedes species [46,56]. Conversely, we constructed the climatic factors’ datasets for 1990–2020, potentially achieving a better match between the climatic datasets and the occurrence records. Additionally, most previous global-scale studies retrieved far fewer occurrence records than we did, even though they did their best to retrieve as many occurrence records as possible. First, most of them built datasets without sampling bias correction with fewer records than our study, e.g., 19,930 records of Ae. aegypti and 22,137 Ae. albopictus [5], 9735 Ae. aegypti and 13,093 Ae. albopictus [46], 19,930 Ae. aegypti and 22,137 Ae. albopictus [50], 4251 Ae. aegypti and 3341 Ae. albopictus [62], and 6599 Ae. albopictus [82]. However, the present study built a dataset without sampling bias correction with 25,170 and 38,457 distinct occurrence records for Ae. aegypti and Ae. albopictus, respectively. To our knowledge, this was the largest occurrence record dataset of the two Aedes species. Second, most of them built sampling bias-corrected datasets with fewer records than our study, e.g., 2303 Ae. aegypti and 1427 Ae. albopictus [62] and 673 Ae. albopictus [82]. Additionally, we developed a sampling bias-corrected dataset with 7606 and 7921 occurrence records of Ae. aegypti and Ae. albopictus, respectively. Moreover, we constructed SDMs using nine algorithms in Biomod2 [71] to probe their range shifts with the advantage of reducing the arbitrariness of fewer algorithms. Therefore, the present study might be more reliable than previous studies due to its large dataset, time-matching between climatic predictors and occurrence records, and the use of diverse algorithms. However, we have to acknowledge that the potential ranges in our study are just the results of theoretical projections without biotic factors in the SDMs. Therefore, our projections might not be fully consistent with the realized ranges of the two Aedes species, and further investigations should be needed in the future.
In 2018, Dickens et al. predicted that the potential ranges of the two Aedes species were similar and restricted mainly to the subtropical and tropical regions, with the range of Ae. albopictus extending further into higher latitudes [50]. Recently, Laporta et al. projected that the ranges of the two Aedes species would expand in the Northern Hemisphere and shrink in the Southern Hemisphere [46]. Although these relevant studies enhanced our understanding of their potential invasiveness, the present study advances this knowledge even further. Unlike these previous studies, we built models to examine range shifts between the native and introduced Ae. aegypti and Ae. albopictus. We identified their respective potential ranges (PRIs and PRNs), estimated their ratios (RRIs), and examined the effects of controlling factors. Moreover, our study examined the range expansions of the two introduced Aedes species and compared their invasion rates. Therefore, our study offers some novel and important information on the invasiveness of the investigated Aedes species.
Our study showed that climatic factors had a stronger influence on the potential ranges of the two Aedes species than anthropogenic and topographical factors. This finding suggested that although anthropogenic factors such as population density and GDP per capita could modify their exploitation of man-made habitats [48,49], their ability to occupy potential ranges might be determined by their adaptation to the local climatic conditions. Additionally, well-developed transportation networks might shadow the barrier effects of topographical factors such as huge and lofty mountain ranges and deep valleys. These observations suggested that climate change mitigation could effectively control the potential invasions of the two Aedes species [45,62]. Although we observed important roles for climatic factors in predicting the potential ranges of the two Aedes species, the GDP per capita and population density played a stronger role in determining the potential ranges of Ae. aegypti than Ae. albopictus. This difference might be because while Ae. aegypti feeds primarily on humans [86], Ae. albopictus feeds on diverse mammalian and avian species [48,87,88,89].
Sirami et al. (2017) argued that the relative effects of anthropogenic and climatic factors on the potential ranges of invasive species largely depend on the spatial scale used [90], i.e., a stronger role is found for climatic factors at large scales and a stronger role for anthropogenic factors at small scales, somewhat supporting the findings of Liu et al. [45] and Ding et al. [91] and our observation of a stronger role for climatic factors at the global scale. However, Dickens et al., who also investigated at a global scale, found that anthropogenic factors had a stronger role than climatic factors in determining the potential ranges of both vectors [50]. Additionally, a small-scale field study by Tsuda et al. [92] detected a stronger role for climatic predictors than anthropogenic ones in determining the ranges of the two vectors in three villages in northern Thailand. Conversely, a small-scale field study by Holeva-Eklund et al. found that population density, an anthropogenic factor, had a stronger effect on the potential ranges of Ae. aegypti than climatic ones [52]. Therefore, the argument that the relative roles of anthropogenic and climatic factors in determining the potential ranges depended on the spatial scale might not be a general pattern, leaving their relative effects on determining the potential ranges of the two Aedes species under debate and in need of further investigation.
Although both Aedes species are competent vectors of several diseases, their invasion history durations differ. The global invasions of Ae. aegypti started about 300–400 years ago, triggered by the global slave trade in the 16th and 17th centuries [14,15], whereas it started only 30–40 years ago for Ae. albopictus induced by accidental introduction [14,16]. Our study showed that the range expansion of Ae. albopictus was larger than that of Ae. aegypti (16.10 vs. 12.03 × 106 km2, i.e., 1.33 times larger) in a relatively shorter invasion history (ca. one tenth). Therefore, from a range shift perspective, the invasion rate of Ae. albopictus was about 13.3 times higher than that of Ae. aegypti. This finding was somewhat supported by a consensus report arguing that Ae. albopictus was the most invasive mosquito in the world [93] and one of the world’s 100 worst invasive species [94], whereas Ae. aegypti was not. The higher invasion rate of Ae. albopictus might be due to the rapid growth of international trade in used tires over the past decades [95] and its strong ecophysiological plasticity [96]. However, we must acknowledge that invasion rates of Ae. albopictus and Ae. aegypti may be high at first and then drop and remain more stable at the final stage. Ae. albopictus might be in the first stage with a high invasion rate, whereas Ae. aegypti might be at the final stage, showing a stable and low invasion rate. Therefore, our observation of the invasion rates concerned just overall invasion rates across all stages up to now and could not reflect temporal variations of invasion rates across all stages.
Potential ranges of Ae. aegypti and Ae. albopictus and their shifts have attracted much attention in the past decades [52,62]. Undoubtedly, these studies have offered important information for devising strategies to fight their invasions. For example, Laporta et al. found that under future climate change scenarios, the potential ranges of Ae. aegypti and Ae. albopictus might expand in the Northern Hemisphere, whereas in the Southern Hemisphere, their potential range might show a decreasing trend, suggesting stricter strategies against their invasions should be needed in the Northern Hemisphere [46]. At the global scale, we detected a larger range ratio in Ae. aegypti and Ae. albopictus relative to their niche breadth ratios, i.e., 1.549 vs. 1.018 and 3.824 vs. 1.391, respectively. This might indicate that small niche shifts in them could induce their large range shifts and that niche shifts might be a more important indicator for biological invasion assessments [74].
5. Conclusions
Our study detected substantial niche and range expansions in introduced Ae. aegypti and Ae. albopictus relative to their respective native counterparts, probably because the introduced populations have much more opportunities to adapt to novel climatic conditions. Climate change mitigation could effectively control their invasions, given that climatic factors played strong roles in determining their potential ranges, and future climate changes could promote their invasions. Ae. albopictus underwent larger niche range expansions over its relatively short invasion history than Ae. aegypti. In terms of the niche and range shifts, Ae. albopictus had an invasion rate about 13.3 times faster than that of Ae. aegypti. Therefore, compared with Ae. aegypti, the niche and range shifts of Ae. albopictus suggested that the latecomer showed higher invasiveness over its relatively shorter invasion history. Since small niche shifts in them could induce their large range shifts, niche shifts might be a more important indicator for biological invasion assessments.
Acknowledgments
We are grateful to Xiaoli Yu for her time and energy in the data collection. We would also like to thank the anonymous reviewers’ valuable comments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14100810/s1, Table S1: Correlations among the 32 predictors; Table S2: Importance values of predictors in the preliminary species distribution models; Table S3: Comparisons of TSS and AUC between the real and null species distribution models; Table S4: Importance values of the predictors calibrated by eight algorithms; Table S5: AUC and TSS calibrated by eight algorithm.
Author Contributions
Conceptualization, J.F.; methodology, J.F. and P.N.; software, P.N.; validation, P.N.; formal analysis, P.N.; investigation, P.N.; resources, J.F. and P.N.; data curation, P.N.; writing—original draft preparation, J.F. and P.N.; writing—review and editing, J.F. and P.N.; visualization, P.N.; supervision, J.F.; project administration, J.F.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data (Online dataset 1) that support the findings of this study are available at https://doi.org/10.6084/m9.figshare.23584812.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the National Natural Science Foundation of China (Grant ID: 31560178).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jentes E.S., Poumerol G., Gershman M.D., Hill D.R., Lemarchand J., Lewis R.F., Staples J.E., Tomori O., Wilder-Smith A., Monath T.P. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- 2.Simmons C.P., Farrar J.J., Nguyen V.V., Wills B. Dengue. N. Engl. J. Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Leparc-Goffart I., Nougairede A., Cassadou S., Prat C., de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 4.Campbell L.P., Luther C., Moo-Llanes D., Ramsey J.M., Danis-Lozano R., Peterson A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B. 2015;370:20130554. doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraemer M.U., Sinka M.E., Duda K.A., Mylne A.Q., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samy A.M., Thomas S.M., Wahed A.A., Cohoon K.P., Peterson A.T. Mapping the global geographic potential of Zika virus spread. Mem. Inst. Oswaldo Cruz. 2016;111:559–560. doi: 10.1590/0074-02760160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., Moyes C.L., Farlow A.W., Scott T.W., Hay S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffner F., Medlock J.M., Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin. Microbiol. Infec. 2013;19:685–692. doi: 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- 10.Garske T., Van Kerkhove M.D., Yactayo S., Ronveaux O., Lewis R.F., Staples J.E., Perea W., Ferguson N.M., Yellow Fever Expert Committee Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11:e1001638. doi: 10.1371/journal.pmed.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers A.M. Chikungunya virus control: Is a vaccine on the horizon? Lancet. 2014;384:2008–2009. doi: 10.1016/S0140-6736(14)61290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp T.M., Roth N.M., Torres J., Ryff K.R., Rodriguez N.M.P., Mercado C., Diaz Padró M.D.P., Ramos M., Phillips R., Lozier M. Chikungunya cases identified through passive surveillance and household investigations—Puerto Rico, May 5–August 12, 2014. MMWR-Morb. Mortal. Wkly. Rep. 2014;63:500–501. [PMC free article] [PubMed] [Google Scholar]
- 13.Staples J.E., Fischer M. Chikungunya virus in the Americas—What a vectorborne pathogen can do. N. Engl. J. Med. 2014;371:887–889. doi: 10.1056/NEJMp1407698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabachnick W.J. Evolutionary genetics and arthropod-borne disease: The Yellow Fever Mosquito. Am. Entomol. 1991;37:14–26. doi: 10.1093/ae/37.1.14. [DOI] [Google Scholar]
- 15.Kaplan L., Kendell D., Robertson D., Livdahl T., Khatchikian C. Aedes aegypti and Aedes albopictus in Bermuda. Extinction, invasion, invasion and extinction. Biol. Invasions. 2010;12:3277–3288. doi: 10.1007/s10530-010-9721-z. [DOI] [Google Scholar]
- 16.Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson G.E. Concluding remarks of Cold Spring Harbor Symposium. Quant. Biol. 1957;22:415–427. doi: 10.1101/SQB.1957.022.01.039. [DOI] [Google Scholar]
- 18.Davies S.J., Hill M.P., McGeoch M.A., Clusella-Trullas S. Niche Shift and Resource Supplementation Facilitate an Amphibian Range Expansion. Divers. Distrib. 2019;25:154–165. doi: 10.1111/ddi.12841. [DOI] [Google Scholar]
- 19.MacDougall A.S., Gilbert B., Levine J.M. Plant Invasions and the Niche. J. Ecol. 2009;97:609–615. doi: 10.1111/j.1365-2745.2009.01514.x. [DOI] [Google Scholar]
- 20.Fang Y.Q., Zhang X.H., Wei H.Y., Wang D.J., Chen R.D., Wang L.K., Gu W. Predicting the Invasive Trend of Exotic Plants in China Based on the Ensemble Model under Climate Change: A Case for Three Invasive Plants of Asteraceae. Sci. Total Environ. 2021;756:143841. doi: 10.1016/j.scitotenv.2020.143841. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W.G., Chen X.Y., Liu R.L., Song X.J., Liu G., Zou J.B., Qian Z.Q., Zhu Z., Cui L. Realized Niche Shift Associated with Galinsoga quadriradiata (Asteraceae) Invasion in China. J. Plant Ecol. 2022;15:538–548. doi: 10.1093/jpe/rtab086. [DOI] [Google Scholar]
- 22.Broennimann O., Fitzpatrick M.C., Pearman P.B., Petitpierre B., Pellissier L., Yoccoz N.G., Thuiller W., Fortin M.-J., Randin C., Zimmermann N.E., et al. Measuring Ecological Niche Overlap from Occurrence and Spatial Environmental Data. Glob. Ecol. Biogeogr. 2012;21:481–497. doi: 10.1111/j.1466-8238.2011.00698.x. [DOI] [Google Scholar]
- 23.Petitpierre B., Kueffer C., Broennimann O., Randin C., Daehler C., Guisan A. Climatic Niche Shifts Are Rare among Terrestrial Plant Invaders. Science. 2012;335:1344–1348. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez-Ortega J.S., Salinas-Rodriguez M.M., Ito T., Perez-Farrera M.A., Vovides A.P., Martinez J.F., Molina-Freaner F., Hernández-López A., Kawaguchi L., Nagano A.J., et al. Niche Conservatism Promotes Speciation in Cycads: The Case of Dioon merolae (Zamiaceae) in Mexico. New Phytol. 2020;227:1872–1884. doi: 10.1111/nph.16647. [DOI] [PubMed] [Google Scholar]
- 25.Da Re D., Olivares A.P., Smith W., Vallejo-Marín M. Global analysis of ecological niche conservation and niche shift in exotic populations of monkeyflowers (Mimulus guttatus, M. luteus) and their hybrid (M.× robertsii) Plant Ecol. Divers. 2020;13:133–146. doi: 10.1080/17550874.2020.1750721. [DOI] [Google Scholar]
- 26.Liu C.L., Wolter C., Xian W.W., Jeschke J.M. Most Invasive Species Largely Conserve Their Climatic Niche. Proc. Natl. Acad. Sci. USA. 2020;117:23643–23651. doi: 10.1073/pnas.2004289117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X., Jarvie S., Guo W.Y., Deng T., Mao L.F., Zhang M.H., Chu C.J., Qian H., Svenning J., He F. Niche Overlap and Divergence Times Support Niche Conservatism in Eastern Asia-Eastern North America Disjunct Plants. Glob. Ecol. Biogeogr. 2021;30:1990–2003. doi: 10.1111/geb.13360. [DOI] [Google Scholar]
- 28.Atwater D.Z., Ervine C., Barney J.N. Climatic Niche Shifts Are Common in Introduced Plants. Nat. Ecol. Evol. 2018;2:34–43. doi: 10.1038/s41559-017-0396-z. [DOI] [PubMed] [Google Scholar]
- 29.Loerch M., Mutke J., Weigend M., Luebert F. Historical Biogeography and Climatic Differentiation of the Fulcaldea-Archidasyphyllum-Arnaldoa Clade of Barnadesioideae (Asteraceae) Suggest a Miocene, Aridity-Mediated Andean Disjunction Associated with Climatic Niche Shifts. Glob. Planet. Change. 2021;201:103495. doi: 10.1016/j.gloplacha.2021.103495. [DOI] [Google Scholar]
- 30.Kelly C.L., Gordon I.J., Schwarzkopf L., Pintor A., Pople A., Hirsch B.T. Invasive wild deer exhibit environmental niche shifts in Australia: Where to from here? Ecol. Evol. 2023;13:e10251. doi: 10.1002/ece3.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates O.K., Ollier S., Bertelsmeier C. Smaller Climatic Niche Shifts in Invasive Than Non-Invasive Alien Ant Species. Nat. Commun. 2020;11:5213. doi: 10.1038/s41467-020-19031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escoriza D., Ben Hassine J., Boix D. Factors Regulating the Invasive Success of an Alien Frog: A Comparison of the Ecology of the Native and Alien Populations. Hydrobiologia. 2014;730:127–138. doi: 10.1007/s10750-014-1827-3. [DOI] [Google Scholar]
- 33.Wan J.Z., Wang C.J., Tan J.F., Yu F.H. Climatic Niche Divergence and Habitat Suitability of Eight Alien Invasive Weeds in China under Climate Change. Ecol. Evol. 2017;7:1541–1552. doi: 10.1002/ece3.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piovezan-Borges A.C., Valente-Neto F., Urbieta G.L., Laurence S.G.W., Roque F.D. Global trends in research on the effects of climate change on Aedes aegypti: International collaboration has increased, but some critical countries lag behind. Parasite Vector. 2022;15:346. doi: 10.1186/s13071-022-05473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padmanabha H., Bolker B., Lord C.C., Rubio C., Lounibos L.P. Food availability alters the effects of larval temperature on Aedes aegypti Growth. J. Med. Entomol. 2011;48:974–984. doi: 10.1603/ME11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rueda L.M., Patel K.J., Axtell R.C., Stinner R.E., Carolina N. Temperature dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) Entomol. Soc. Am. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 37.Carrington L.B., Armijos M.V., Lambrechts L., Barker C.M., Scott T.W. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS ONE. 2013;8:e58824. doi: 10.1371/journal.pone.0058824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa E., Santos E., Correia J., Albuquerque C. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev. Bras. Entomol. 2010;54:488–493. doi: 10.1590/S0085-56262010000300021. [DOI] [Google Scholar]
- 39.Armbruster P., Conn J.E. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2006;99:1234–1243. doi: 10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2. [DOI] [Google Scholar]
- 40.Paaijmans K.P., Blanford S., Bell A.S., Blanford J.I., Read A.F., Thomas M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonel B.F., Koroiva R., Hamada N., Ferreira-Keppler R.L., Roque F.O. Potential effects of climate change on ecological interaction outcomes between two disease-vector mosquitoes: A mesocosm experimental study. J. Med. Entomol. 2015;52:866–872. doi: 10.1093/jme/tjv068. [DOI] [PubMed] [Google Scholar]
- 42.Mordecai E.A., Cohen J.M., Evans M.V., Gudapati P., Johnson L.R., Lippi C.A., Miazgowicz K., Murdock C.C., Rohr J.R., Ryan S.J., et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl. Trop. Dis. 2017;11:1–18. doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piovezan-Borges A.C., Valente-Neto F., Tadei W.P., Hamada N., Roque F.O. Simulated climate change, but not predation risk, accelerates Aedes aegypti emergence in a microcosm experiment in western Amazonia. PLoS ONE. 2020;15:1–12. doi: 10.1371/journal.pone.0241070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winokur O.C., Main B.J., Nicholson J., Barker C.M. Impact of temperature on the extrinsic incubation period of Zika virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2020;14:1–15. doi: 10.1371/journal.pntd.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B.Y., Gao X., Ma J., Jiao Z.H., Xiao J.H., Hayat M.A., Wang H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019;664:203–214. doi: 10.1016/j.scitotenv.2019.01.301. [DOI] [PubMed] [Google Scholar]
- 46.Laporta G.Z., Potter A.M., Oliveiram J.F.A., Bourke B.P., Pecor D.B., Linton Y.M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects. 2023;14:49. doi: 10.3390/insects14010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunze S., Koch L.K., Kochmann J., Klimpel S. Aedes albopictus and Aedes japonicus—Two invasive mosquito species with different temperature niches in Europe. Parasite Vector. 2016;9:573. doi: 10.1186/s13071-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gratz N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 49.Simard F., Nchoutpouen E., Toto J.C., Fontenille D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005;42:726–731. doi: 10.1093/jmedent/42.5.726. [DOI] [PubMed] [Google Scholar]
- 50.Dickens B.L., Sun H., Jit M., Cook A.R., Carrasco L.R. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob. Health. 2018;3:e000801. doi: 10.1136/bmjgh-2018-000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abílio A.P., Abudasse G., Kampango A., Candrinho B., Sitoi S., Luciano J., Tembisse D., Sibindy S., de Almeida A.P.G., Garcia G.A., et al. Distribution and breeding sites of Aedes aegypti and Aedes albopictus in 32 urban/peri-urban districts of Mozambique: Implication for assessing the risk of arbovirus outbreaks. PLoS Negl. Trop. Dis. 2018;12:e0006692. doi: 10.1371/journal.pntd.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holeva-Eklund W.M., Young S.J., Will J., Busser N., Townsend J., Hepp C.M. Species distribution modeling of Aedes aegypti in Maricopa County, Arizona from 2014 to 2020. Front. Environ. Sci. 2022;10:1001190. doi: 10.3389/fenvs.2022.1001190. [DOI] [Google Scholar]
- 53.Chardon N.I., Cornwell W.K., Flint L.E., Flint A., Ackerly D. Topographic, latitudinal and climatic distribution of Pinus coulteri: Geographic range limits are not at the edge of the climate envelope. Ecography. 2015;38:590–601. doi: 10.1111/ecog.00780. [DOI] [Google Scholar]
- 54.Michalak J.L., Lawler J.J., Roberts D.R., Carroll C. Distribution and protection of climatic refugia in North America. Conserv. Biol. 2018;32:1414–1425. doi: 10.1111/cobi.13130. [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Li X., Sandel B., Blank D., Liu Z.T., Liu X. Climate and topography explain range sizes of terrestrial vertebrates. Nat. Clim. Chang. 2016;6:498–502. doi: 10.1038/nclimate2895. [DOI] [Google Scholar]
- 56.Mweya C.N., Kimera S.I., Stanley G., Misinzo G., Mboera L.E.G. Climate Change Influences Potential Distribution of Infected Aedes aegypti Co-Occurrence with Dengue Epidemics Risk Areas in Tanzania. PLoS ONE. 2016;11:e0162649. doi: 10.1371/journal.pone.0162649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estallo E.L., Sangermano F., Grech M., Luduena-Almeida F., Frias-Cespedes M., Ainete M., Almirón W., Livdahl T. Modelling the distribution of the vector Aedes aegypti in a central Argentine city. Med. Vet. Entomol. 2018;32:451–461. doi: 10.1111/mve.12323. [DOI] [PubMed] [Google Scholar]
- 58.Echeverry-Cárdenas E., LÓpez-Castañeda C., Carvajal-Castro J.D., Aguirre-Obando O.A. Potential geographic distribution of the tiger mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in current and future conditions for Colombia. PLoS Negl. Trop. Dis. 2021;15:e0008212. doi: 10.1371/journal.pntd.0008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurjanah S., Atmowidi T., Hadi U.K., Solihin D.D., Priawandiputra W., Santoso B., Asmarani D., Setiawan T., Meidaliyantisyah Distribution modelling of Aedes aegypti in three dengue-endemic areas in Sumatera, Indonesia. Trop. Biomed. 2022;39:373–383. doi: 10.47665/tb.39.3.007. [DOI] [PubMed] [Google Scholar]
- 60.Yang B., Borgert B.A., Alto B.W., Boohene C.K., Brew J., Deutsch K., DeValerio J.T., Dinglasan R.R., Dixon D., Faella J.M., et al. Modelling distributions of Aedes aegypti and Aedes albopictus using climate, host density and interspecies competition. PLoS Negl. Trop. Dis. 2021;15:e0009063. doi: 10.1371/journal.pntd.0009063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wint W., Jones P., Kraemer M., Alexander N., Schaffner F. Past, present and future distribution of the yellow fever mosquito Aedes aegypti: The European paradox. Sci. Total Environ. 2022;847:157566. doi: 10.1016/j.scitotenv.2022.157566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamal M., Kenawy M.A., Rady M.H., Khaled A.S., Samy A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE. 2018;13:e0210122. doi: 10.1371/journal.pone.0210122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A., Shearer F.M., Brady O.J. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data. 2015;2:150035. doi: 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown J.L., Bennett J.R., French C.M. SDMtoolbox 2.0: The next generation python–based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. 2017;5:e4095. doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilkerson R.C., Linton Y.M., Strickman D. Mosquitoes of the World. Johns Hopkins University Press; Baltimore, MD, USA: 2020. [Google Scholar]
- 66.Guisan A., Petitpierre B., Broennimann O., Daehler C., Kueffer C. Unifying Niche Shift Studies: Insights from Biological Invasions. Trends Ecol. Evol. 2014;29:260–269. doi: 10.1016/j.tree.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Di Cola V., Broennimann O., Petitpierre B., Breiner F.T., D’Amen M., Randin C., Engler R., Pottier J., Pio D., Dubuis A., et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography. 2017;40:774–787. doi: 10.1111/ecog.02671. [DOI] [Google Scholar]
- 68.Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 69.Fick S.E., Hijmans R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 70.Gong X., Chen Y.J., Wang T., Jiang X.F., Hu X.K., Feng J.M. Double—Edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020;740:139933. doi: 10.1016/j.scitotenv.2020.139933. [DOI] [PubMed] [Google Scholar]
- 71.Thuiller W., Lafourcade B., Engler R., Araujo M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. doi: 10.1111/j.1600-0587.2008.05742.x. [DOI] [Google Scholar]
- 72.Dormann C.F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., Marquéz J.R.G., Gruber B., Lafourcade B., Leitão P.J., et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:7–46. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- 73.Gallien L., Douzet R., Pratte S., Zimmermann N.E., Thuiller W. Invasive species distribution models—How violating the equilibrium assumption can create new insights. Glob. Ecol. Biogeogr. 2012;21:1126–1136. doi: 10.1111/j.1466-8238.2012.00768.x. [DOI] [Google Scholar]
- 74.Cao R.Y., Gong X., Feng J.M., Yang R.J. Niche and range dynamics of Tasmanian blue gum (Eucalyptus globulus Labill.), a globally cultivated invasive tree. Ecol. Evol. 2022;12:e9305. doi: 10.1002/ece3.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C., Newell G., White M. On the selection of thresholds for predicting species occurrence with presence–only data. Ecol. Evol. 2016;6:337–348. doi: 10.1002/ece3.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gotelli N.J., Ulrich W. Statistical challenges in null model analysis. Oikos. 2012;121:171–180. doi: 10.1111/j.1600-0706.2011.20301.x. [DOI] [Google Scholar]
- 77.Bohl C.L., Kass J.M., Anderson R.P. A new null model approach to quantify performance and significance for ecological niche models of species distributions. J. Biogeogr. 2019;46:1101–1111. doi: 10.1111/jbi.13573. [DOI] [Google Scholar]
- 78.Yang R.J., Yu X.L., Nie P.X., Cao R.Y., Feng J.M. Climatic niche and range shifts of grey squirrels (Sciurus carolinensis Gmelin) in Europe: An invasive pest displacing native squirrels. Pest Manag. Sci. 2023;79:3731–3739. doi: 10.1002/ps.7554. [DOI] [PubMed] [Google Scholar]
- 79.Nie P.X., Yang R.J., Cao R.Y., Hu X.K., Feng J.M. Niche and range shifts of the fall webworm (Hyphantria cunea Dury) in Europe imply its huge invasion potential in the future. Insects. 2023;14:316. doi: 10.3390/insects14040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lacour G., Chanaud L., L’Ambert G., Hance T. Seasonal synchronization of diapause phases in Aedes albopictus (Diptera: Culicidae) PLoS ONE. 2015;10:e0145311. doi: 10.1371/journal.pone.0145311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marini G., Manica M., Arnoldi D., Inama E., Rosà R., Rizzoli A. Influence of temperature on the life-cycle dynamics of Aedes albopictus population established at temperate latitudes: A laboratory experiment. Insects. 2020;11:808. doi: 10.3390/insects11110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medley K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010;19:122–133. doi: 10.1111/j.1466-8238.2009.00497.x. [DOI] [Google Scholar]
- 83.Diaz-Nieto L.M., Macia A., Perotti M.A., Beron C.M. Geographical limits of the Southeastern distribution of Aedes aegypti (Diptera, Culicidae) in Argentina. PLoS Negl. Trop. Dis. 2013;7:e1963. doi: 10.1371/journal.pntd.0001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seixas G., Salgueiro P., Bronzato-Badial A., Goncalves Y., Reyes-Lugo M., Gordicho V. Origin and expansion of the mosquito Aedes aegypti in Madeira Island (Portugal) Sci. Rep. 2019;9:2241. doi: 10.1038/s41598-018-38373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helmersson J., Brännström Å., Sewe M., Semenza J.C., Rocklöv J. Estimating past, present and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health. 2019;7:148. doi: 10.3389/fpubh.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bargielowski I.E., Lounibos L.P., Carrasquilla M.C. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA. 2013;110:2888–2892. doi: 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109:141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Juliano S.A., Philip L.L. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Kamara F., Zhou G., Puthiyakunnon S., Li C., Liu Y., Zhou Y., Yao L., Yan G., Chen X.-G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014;8:e3301. doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sirami C., Caplat P., Popy S., Clamens A., Arlettaz R., Jiguet F., Brotons L., Martin J.-L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017;26:385–394. doi: 10.1111/geb.12555. [DOI] [Google Scholar]
- 91.Ding F.Y., Fu J.Y., Jiang D., Hao M.M., Lin G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018;178:155–162. doi: 10.1016/j.actatropica.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 92.Tsuda Y., Suwonkerd W., Chawprom S., Prajakwong S., Takagi M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control. 2006;22:222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 93.Benedict M.Q., Levine R.S., Hawley W.A., Lounibos L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Invasive Species Specialist Group 100 of the World’s Worst Invasive Alien Species. 2023. [(accessed on 4 March 2023)]. Available online: http://www.iucngisd.org/gisd/species.php?sc=109.
- 95.Reiter P., Sprenger D. The used tire trade: A mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control. Assoc. 1987;3:494–501. [PubMed] [Google Scholar]
- 96.Kramer I.M., Pfeiffer M., Steffens O., Schneider F., Gerger V., Phuyal P. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci. Total Environ. 2021;778:146128. doi: 10.1016/j.scitotenv.2021.146128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data (Online dataset 1) that support the findings of this study are available at https://doi.org/10.6084/m9.figshare.23584812.