Abstract
We studied the concentration of hepatitis A virus (HAV) from environmental samples by membrane filter-based urea-arginine phosphate buffer and its detection by using immunomagnetic capture (IC) reverse transcription (RT)-PCR (IC PCR). Magnetic beads coated with anti-HAV rabbit antibodies were used for enrichment and concentration of HAV from environmental samples. IC PCR is sensitive enough to detect as few as 0.04 PFU of cell culture-adapted HAV in inoculated water and sewage samples. IC PCR is specific and does not yield positive reactions with poliovirus 1, HAV RNA, or selected bacteriophages. IC concentrates viruses suspended in small volumes to microliter volumes that can be used directly in RT-PCR. IC concentration of viruses from sewage samples without concentration of inhibitory substances is important for successful RT-PCR detection. In a field trial, 2 of 18 raw sewage samples tested by IC PCR were positive for HAV.
Hepatitis A virus (HAV) has been a major cause of outbreaks of food-borne illness in the United States (3) and of sporadic waterborne epidemics worldwide (1). HAV is a 27-nm-diameter, nonenveloped particle containing a polyadenylated positive-strand RNA genome of 7,400 nucleotides and is a member of the picornaviruses.
The variety of concentration techniques still being proposed by researchers for the isolation of HAV from water samples shows that there is room for improvement in available methods. We recently described a positively charged membrane filter-based adsorption-elution method for concentrating coliphages in water samples, with subsequent assay of the concentrated material by the plaque technique (17). The sensitivity of the method encouraged its application to water samples for the concentration of HAV.
Molecular methods for detecting HAV have largely superseded infectivity tests performed with cell cultures. The adaptation of HAV from environmental samples to cell culture propagation is difficult; it may take several weeks and sometimes ends in failure because viral replication is slow and does not shut off host cell synthesis so as to cause cytopathic effects (2, 21, 22). No cell line is currently recommended for the detection of HAV from environmental samples. With the advent of PCR, even viruses that are not amenable to culturing in vitro can be detected (16).
Reverse transcription (RT)-PCR is not suitable for sample volumes above a few microliters, so it depends on previous treatment of samples to reduce volumes and remove inhibitors (14). Antigen capture PCR with human anti-HAV immunoglobulin G (IgG)-coated microcentrifuge tubes has been demonstrated by several researchers (7, 12, 15, 22) and has the advantage of detecting intact and hence potentially infectious viruses from environmental samples. Some of the major drawbacks of this method are the limitation to small sample volumes (ca. 100 μl) and long incubation periods (12 to 24 h at 4°C) to form the antigen-antibody complexes. An alternative strategy is to concentrate viruses from large-volume water samples to 1 to 5 ml and subsequently to use immunomagnetic capture (IC) for detection. IC is an effective tool for the separation and isolation of viruses from heterogeneous environmental samples. Many immunoassays exploiting immunomagnetic separation have been described (23). This technique has also been used for the rapid separation and subsequent detection of bacteria such as Escherichia coli O157:H7 (4, 5, 11, 20) and even of protozoa such as Cryptosporidium parvum (8). IC for the detection of HAV and rotavirus has been reported and tested with 1- to 20-ml samples, mainly of clinical specimens and foods (13, 18, 19).
In the present study, the use of IC with RT-PCR (IC PCR) was developed with the objective of detecting HAV from environmental samples that were previously concentrated by adsorption of the virus to positively charged membrane filters and elution with urea-arginine phosphate buffer. The present communication describes the concentration of HAV from water and sewage samples and the subsequent capture of virus by interaction of the viral capsid antigen with homologous antibody coupled with magnetic beads for easier separation.
MATERIALS AND METHODS
HAV.
HAV HM-175/18f was chosen as a model virus for optimizing the protocol and has the advantage of being easy to quantitate by the plaque assay with a continuous line of fetal rhesus monkey kidney (FRhK-4) cells (9). After 16 days at 37°C, the overlay medium was removed, the cells were fixed with 12.3% formaldehyde at room temperature for 2 h, and the plaques were visualized by staining with crystal violet solution (0.5% crystal violet plus 0.85% NaCl dissolved in 69% distilled water–26% ethanol [95%] solution–5% formaldehyde [37%] solution) for 1 to 2 min.
Preparation of rabbit antibody and immunomagnetic beads.
Inactivated HAV vaccine (0.5 ml; SmithKline Beecham, Philadelphia, Pa.) was thoroughly mixed with 0.5 ml of Freund’s adjuvant and injected subcutaneously into rabbits. After a series of injections, blood was collected and the serum was separated and stored at −20°C. Antibody was purified with a MabTrap G II kit (Pharmacia Biotech, Uppsala, Sweden) and biotinylated with a biotinylation kit (Pierce, Rockford, Ill.) according to the manufacturer’s instructions. The biotinylated protein was stored at 4°C in 0.1% sodium azide.
Streptavidin Magnesphere paramagnetic particles (Promega, Madison, Wis.) were washed three times; 1 mg was suspended in biotinylated rabbit anti-HAV IgG (5.8 mg/ml) and incubated for 2 h at room temperature, and the beads were separated. About 100 μg of biotinylated rabbit anti-HAV IgG is bound per milligram of magnetic particles. Unbound IgG was removed by washing four times with 1 ml of phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA), and the beads were stored in 600 μl of PBS containing 0.1% BSA at 4°C. Uncoated magnetic Dynabeads (M-450; Dynal, Lake Success, N.Y.) were coated with antibody by a covalent coupling mechanism according to the manufacturer’s instructions.
In order to learn whether the antibody-coated beads would capture other viruses, a cocktail mixture of HAV (4 × 102 PFU/ml), poliovirus 1 (6.5 × 102 PFU/ml), and coliphages φX174 (ATCC 13706B; 2.8 × 103 PFU/ml), MS2 (ATCC 15597; 4.3 × 103 PFU/ml), Qβ (ATCC 23631B; 7.9 × 103 PFU/ml), and T1 (ATCC 11303B; 1.2 × 103 PFU/ml) was added to 1.5 ml of sterile PBS–150 μg of magnetic beads coated with HAV antibody. The suspension was incubated for 1 h at room temperature with occasional stirring. The beads were collected with a magnetic separator (Promega), washed four times with 250 μl of PBS containing 0.1% BSA, and finally suspended in 1.5 ml of PBS. Each suspension (250 μl, equivalent to 25 μg) was then plated on soft-agar medium with the following hosts: φX174 with host E. coli C (ATCC 13706), MS2 with host E. coli (ATCC 15597), Qβ with host E. coli (ATCC 23631), T1 with host E. coli (ATCC 11303), and poliovirus 1 with host cells of the FRhK-4 cell line. HAV was detected by IC PCR. In a parallel experiment, beads exposed to the heterologous agents and washed were tested by IC PCR. In a further experiment to evaluate the specificity of IC PCR, poliovirus 1 (6.5 × 105, 6.5 × 104, 6.5 × 103, 6.5 × 102, and 0 PFU of poliovirus, the last two with 400 PFU of HAV) was added to sterile 500-μl water samples, which were then subjected to IC PCR.
Optimization of magnetic beads for detection of HAV.
A set of experiments was performed to establish the optimal conditions for the separation of HAV from the concentrated samples. To test the magnetic antibody capture of HAV, different amounts of Promega magnetic beads (25 to 100 μg) were added to 250 μl of distilled water in a 2-ml sterile microcentrifuge tube at a constant HAV titer (400 PFU) and incubated at room temperature for 1 h with occasional mixing. The beads were collected from the suspension with a magnetic separator (Promega), washed four times with 100 μl of PBS containing 0.1% BSA, and transferred to a 500-μl microcentrifuge tube. Parallel experiments were done with Dynabeads.
Concentration of inoculated HAV from water and sewage samples.
Dechlorinated tap water samples (100 ml; pH 7.4 to 8.1) were contaminated with 0.004 to 400 PFU of HAV and concentrated and tested by IC PCR as shown in Fig. 1.
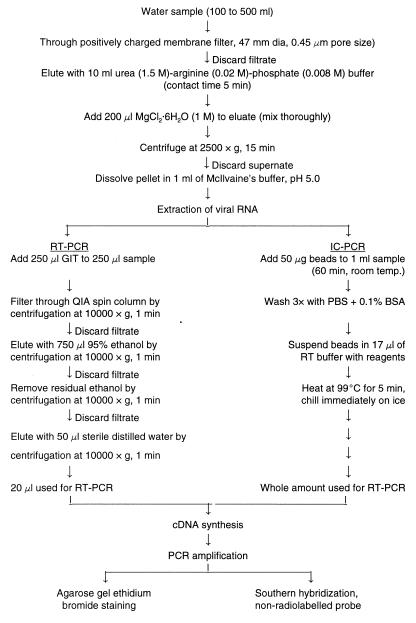
FIG. 1.
Protocol for concentration and detection of HAV from environmental samples. A conventional RT-PCR detection procedure is compared with the IC PCR method.
Sewage samples were collected from sewage treatment facilities at the University of California, Davis, campus and processed as described by Jothikumar et al. (16) with the modification that no pH adjustment of clarified samples was carried out. Briefly, the raw sewage sample, which was negative for HAV as evidenced by negative IC PCR testing and cell culturing, was heat treated at 85°C for 1 h and cooled to room temperature. The heat-treated sewage (100 ml) was stirred for 30 min at room temperature. The coarse material was removed by centrifugation (1,500 × g for 20 min), and the supernatant fluid was inoculated with a known HAV stock and concentrated and tested as described for water samples (Fig. 1).
Detection of HAV in raw sewage samples.
Raw sewage samples (200 ml) were collected during the first and third weeks of every month from April to December 1996, and 100 ml of each was concentrated to 1 ml by the membrane adsorption-elution method and subjected to detection of HAV by IC PCR. PCR products were further purified and concentrated with a QIAquick spin column (Qiagen, Chatsworth, Calif.) to 20 μl, of which 10 μl representing an initial raw sewage sample volume of 100 ml was further analyzed by Southern blotting and oligonucleotide probe testing.
RNA extraction from water samples.
To compare a more conventional detection procedure to IC PCR, water samples (100 ml) were inoculated with 10-fold dilutions of HAV and concentrated to 1 ml by the membrane adsorption-elution method. The guanidinium isothiocyanate (GIT) protocol was followed to extract RNA from 250 μl of water samples for RT-PCR by a slight modification of the method of Chomczynski and Sacchi (6) (Fig. 1).
RNA extraction from beads, RT, and PCR.
RNA was obtained from the magnetic beads by heating at 99°C for 5 min in 17 μl of 1× PCR buffer (10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2 [pH 8.3]–1 mM each deoxynucleoside triphosphate–1.5 μM primer 2 (nucleotides 2413 to 2389; 5′-GGAAA TGTCT CAGGT ACTTT CTTTG-3′). There was no difference between adding the primer before or after the denaturation step. Following denaturation of the virus at 99°C for 5 min and rapid chilling on ice, the buffer was separated from the beads and transferred to a sterile tube containing 1 μl of RNase inhibitor (20 U/μl; Perkin-Elmer Cetus, Foster City, Calif.) and 1 μl of Moloney murine leukemia virus reverse transcriptase (50 U/μl; Perkin-Elmer Cetus). Assuming that a residual volume of 1.0 μl was associated with the immunobead-virus isolates, the final total volume was 20 μl. The entire mixture was incubated at 42°C for 30 min in a Progene DNA Thermocycler (Techne, Princeton, N.J.) and used as a substrate for PCR at a final total volume of 50 μl. Reverse transcriptase in the RT mixture was inactivated by heating at 95°C for 5 min, after which 30 μl of 1× PCR buffer–1.5 μM primer 1 (nucleotides 2167 to 2192; 5′-GTTTT GCTCC TCTTT ATCAT GCTAT G-3′)–2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus) was added. To increase the sensitivity and specificity of the PCR, hot-start PCR was performed by adding MaXWax Nuggets (Nebraska Diagnostics & Biologicals, Omaha, Nebr.). The PCR cycle carried out in the Thermocycler was as follows: 5 min of denaturation at 94°C, 1 min of annealing at 55°C, and 1 min of extension at 72°C for 35 cycles, with an additional 7 min of extension at 72°C. The amplified product of 247 bp was visualized by UV light after electrophoresis in a 2% agarose gel (Gibco BRL) in the presence of ethidium bromide (0.5 μg/ml). A 100-bp DNA ladder (Invitrogen, San Diego, Calif.) was used as a size marker. The 247-base amplified region of the HAV genome corresponds to a highly conserved region encoding the carboxyl terminus of capsid protein VP3 and the amino terminus of protein VP1 (7).
PCR products were also analyzed after Southern blotting (24) to a nylon membrane (Amersham Life Science, Arlington, Ill.) with a digoxigenin (DIG)-labelled oligonucleotide probe (nucleotides 2232 to 2251; 5′-TCAAC AACAG TTTCT ACAGA-3′) by a nonradioactive method of detection. Bound DIG-labelled probe was detected with the Genius 3 nucleic acid detection kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.).
Sequencing of purified PCR products.
Fluorescence-based cycle sequencing reactions were carried out on purified PCR fragments with dye-labelled terminators by use of the ABI PRISM dye terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase (Fluorescent Sequencing, Perkin-Elmer Cetus); the manufacturer’s protocol was followed. Extension products were purified with Centri-Sep columns (Princeton Separations, Adelphia, N.J.) according to the manufacturer’s protocol and analyzed with an ABI PRISM 377 DNA sequencer (Perkin-Elmer Cetus).
RESULTS
Specificity of IC.
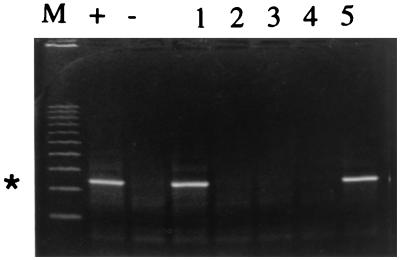
All phages and poliovirus 1 were negative in their homologous hosts, indicating a lack of IC, as well as in IC PCR (data not shown). Amplification produced a 247-base DNA fragment for HAV with the same intensity in the presence or absence of poliovirus 1; there was no nonspecific amplification, as observed by ethidium bromide staining, with poliovirus 1 alone (Fig. 2). IC beads stored at 4°C were assayed monthly for stability and sensitivity; the sensitivity of detection was the same for the first 4 months and was 1 log lower during months 5 and 6 (data not shown).
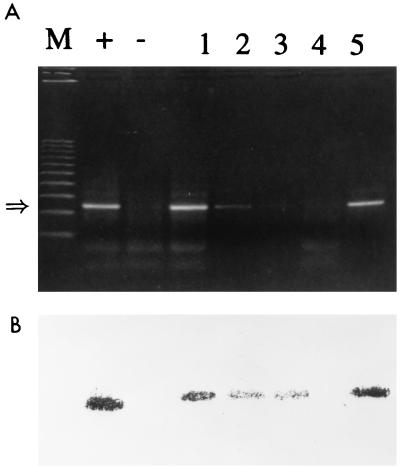
FIG. 2.
Specificity of IC PCR. HAV (400 PFU) in the presence of poliovirus 1 in 500 μl of water was processed by the IC method with 25 μg of magnetic beads. Lanes: M, markers; +, positive control (reaction mixture with 400 PFU of HAV); −, negative control (reaction mixture without virus); 1, 400 PFU of HAV plus 6.5 × 102 PFU of poliovirus 1; 2, 6.5 × 105 PFU of poliovirus 1 without HAV; 3, 6.5 × 104 PFU of poliovirus 1 without HAV; 4, 6.5 × 103 PFU of poliovirus 1 without HAV; 5, 400 PFU of HAV in 500 μl of water. The asterisk at the left indicates the 247-base position of the HAV amplicon.
Optimal quantity of immunomagnetic beads in water samples for concentration of HAV.
We next established the optimal dilution of immunomagnetic beads for binding virus in different volumes of water samples; it was clear that the greatest amount of HAV was bound when immunomagnetic bead/water dilution ratios of 1:20, 1:40, and 1:60 were used at an HAV dilution of 4 × 102 PFU (data not shown). At an immunomagnetic bead/water dilution ratio of 1:80, the sensitivity decreased dramatically, probably due to poor separation of beads or to poor adsorption of viruses, with rapid settling in the larger volume of the water sample. The ratio of 25 μg of beads to 500 μl of environmental sample was kept constant in all subsequent experiments. Increasing the amount of magnetic beads tested to 50, 75, and 100 μg in a 500-μl volume of water containing 40 PFU of HAV did not increase the amount of bound HAV, as evidenced by IC PCR (data not shown). Viruses captured on beads evidently were not dislodged by three to five washes with gentle resuspension after each wash (data not shown).
Sensitivity of IC PCR.
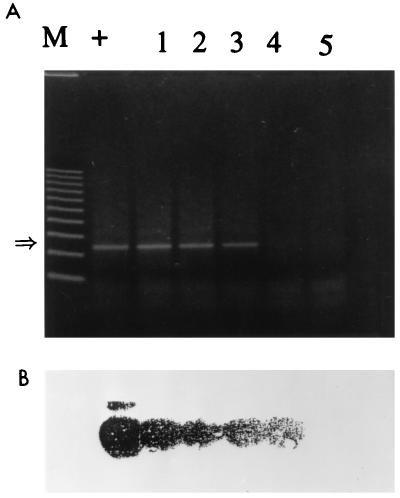
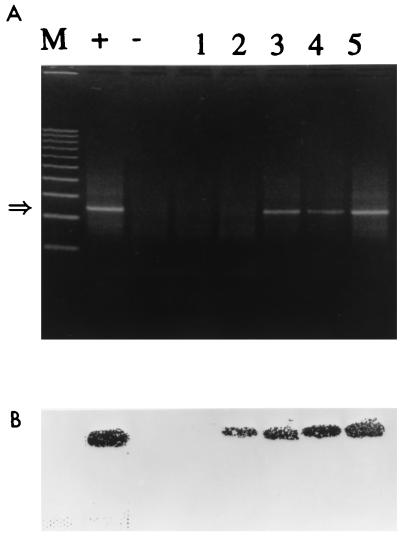
The sensitivities of detection of HAV diluted in water samples by the IC PCR assay and the conventional RT-PCR assay were studied and compared. Tap water samples (100 ml, pH 7.4 to 8.1) seeded with 10-fold dilutions of HAV were subjected to concentration on positively charged membrane filters, elution, and reconcentration with urea-arginine-phosphate buffer (Fig. 1). Further extraction of viral RNA by the GIT procedure was necessary for RT-PCR. For IC PCR, exposure of the beads to 1 ml of concentrate for 1 h followed by heat treatment to release viral RNA was sufficient. The preparation of viral RNA and RT-PCR are the most critical steps for the detection of HAV. The highest dilution that gave an HAV-positive PCR signal was interpreted as the end point of detection. The detection of 0.04 PFU was achieved with IC PCR (Fig. 3); conventional RT-PCR detected 0.4 PFU (Fig. 4). The sensitivity attained with immunomagnetic beads was 10-fold higher than that without the beads. Similar results were obtained with 25 μl of Dynabeads (4 × 108 beads/ml), with no difference in sensitivity (data not shown). Hence, further study was carried out with Promega beads coated with HAV antiserum because they cost less than Dynabeads. When water samples of 100, 250, and 500 ml were seeded with 0.4 PFU, the larger water volumes did not affect the sensitivity of detection (data not shown). Southern hybridization with a DIG-labelled probe verified that the PCR product actually represented the HAV genome.
FIG. 3.
Detection of HAV by IC PCR. (A) Water samples (100 ml) were inoculated with 10-fold dilutions of HAV, concentrated to 1 ml by the membrane adsorption-elution method, processed for IC with 50 μg of magnetic beads, and resuspended to 20 μl in 1× PCR buffer for RT-PCR. Lanes: M, markers; +, positive control (reaction mixture with 400 PFU of HAV); 1 through 5, HAV at 40, 4, 0.4, 0.04, and 0.004 PFU, respectively. The arrow at the left indicates the 247-base position of the HAV amplicon. (B) Confirmation of the bands by Southern transfer and hybridization with an internal oligonucleotide probe.
FIG. 4.
Detection of HAV by RT-PCR. (A) Water samples (100 ml) were inoculated with 10-fold dilutions of HAV, and concentrated to 1 ml by the membrane adsorption-elution method, RNA was extracted as described in Materials and Methods by the GIT procedure, and a 20-μl sample was subjected to RT-PCR. Lanes: M, markers; +, positive control (reaction mixture with 400 PFU of HAV); −, negative control (reaction mixture without virus); 1 through 5, HAV at 0.04, 0.4, 4, 40, and 400 PFU, respectively. The arrow at the left indicates the 247-base position of the HAV amplicon. (B) Confirmation of the bands by Southern transfer and hybridization with an internal oligonucleotide probe.
Detection of inoculated HAV in sewage samples.
Experiments were performed in triplicate according to the protocol described for heat-treated sewage samples (shown to contain no indigenous cytopathic viruses by testing in FRhK-4 cells) inoculated with HAV to determine the sensitivity of the test (Fig. 5). The IC PCR methods used were the same as those used for water samples and had the same sensitivity, with an end point of detection at 0.04 PFU. The presence of any inhibitory substances did not alter the sensitivity of detection; the presence of inhibitors that would have interfered with RT-PCR was not determined.
FIG. 5.
Detection of HAV inoculated into heat-treated sewage samples. (A) Heat-treated sewage samples (100 ml) were inoculated with 10-fold dilutions of HAV, concentrated to 1 ml by the membrane adsorption-elution method, processed for IC with 50 μg of magnetic beads, and resuspended to 20 μl in 1× PCR buffer for RT-PCR. Lanes: M, markers; +, positive control (reaction mixture with 400 PFU of HAV); −, negative control (reaction mixture without virus); 1 through 4, HAV at 4, 0.4, 0.04, and 0.004, PFU, respectively; 5, 100 ml of water with 40 PFU of HAV, concentrated to 1 ml by the membrane adsorption-elution method (as in Fig. 3, lane 1). The arrow at the left indicates the 247-base position of the HAV amplicon. (B) Confirmation of the bands by Southern transfer and hybridization with an internal oligonucleotide probe.
Detection of HAV in sewage samples.
Direct testing of environmental samples by standard PCR methods has been severely hampered by the presence of inhibitory compounds in sewage and similar materials. In trials with 100 ml of raw sewage, 2 of 18 samples (collected on 6 May 1996 [S1] and 15 November 1996 [S2]) were found positive for HAV by the IC PCR method (data not shown), a result which enabled sequencing of a portion of the VP3-VP1 region after PCR amplification (Fig. 6). Partial genomic comparison of the sequences of two isolates of HAV with that of wild-type HAV reported by Cohen et al. (7) suggests that the genome sequence is relatively stable; the sequences were 94.59% and 96.85% in agreement for strains from samples S1 and S2, respectively. The observed difference in these strains isolated from a campus sewage treatment plant may reflect the ethnic and geographical diversities of the university population.
FIG. 6.
Sequence alignment for a 222-bp sequence from the region encoding the carboxyl terminus of capsid protein VP3 and the amino terminus of protein VP1 of HAV strains isolated from sewage samples S1 and S2. Dashes represent nucleotides identical to those of the consensus sequence (I). Duplicate determinations yielded identical results.
DISCUSSION
In recent years, IC PCR-based technologies have proved to be very important tools in the detection of waterborne and food-borne pathogens; in the present study, we adapted IC PCR to the detection of HAV from water and wastewater samples. RNases and substances that inhibit RT reactions often occur in environmental samples and are difficult to remove (14); these problems can be overcome by IC, thus avoiding false-negative results. Our adaptation of IC PCR entails three major steps: (i) virus concentration to a small volume, (ii) immunomagnetic separation of adsorbed viruses, and (iii) heat treatment for RNA extraction followed by RT-PCR. In that the positively charged filter medium that we used (17) for adsorption-elution concentration of viruses from water is the same as that used in cartridge form for larger-volume samples (10), this method could probably be scaled up readily. The easy second-stage [of step (i)] concentration of virus by precipitation on the phosphate floc that is formed by the addition of MgCl2 may improve on the results obtained with beef extract.
Monceyron and Grinde (19) reported that the sensitivity of HAV detection by direct PCR was lower than that of immunomagnetic separation followed by PCR for samples of polluted water, seawater, or fecal extracts. They also reported that even increasing the volume from 1 to 20 ml without increasing the amount of beads (0.2 mg) did not drastically influence the sensitivity of detection; however, in this study we observed that if the sample volume was increased beyond 1.5 ml without an increase in the amount of beads (0.025 mg), the sensitivity of detection was drastically reduced because of poor separation from the mixture. It is possible that nonspecific inhibitors became limiting in this situation.
Comparative recovery trials with HAV in water and sewage showed that Dynabeads (4.5 μm in diameter) had a sensitivity equal to that of the smaller Promega beads, probably due to the larger surface area of the latter. However, HAV separation from shellfish samples was more effective with Dynabeads than with Promega beads (data not shown), perhaps because the extremely small size and the flake form of the Promega beads promoted the attachment of shellfish proteins or other solids that obscured the antibody.
The immunomagnetic beads did not collect heterologous viruses or HAV RNA, and the presence of heterologous viruses did not affect IC PCR detection of HAV. The limit of HAV detection by conventional RT-PCR was 1 log unit higher than that by IC PCR. This difference may have been the result of a loss of viral RNA during the GIT extraction procedure or the ability of IC PCR to process 1 ml of sample concentrate in a single microcentrifuge tube, an amount which is fourfold higher than that in the GIT procedure. In an earlier study, Deng et al. (9) reported a ratio of 1 PFU per 79 viral particles; since the same HAV strain was used in the present study, in which the detection limit was 0.04 PFU, a positive result may have been obtained with 3.16 viral particles in 100 ml of environmental sample. This level of detection is, of course, far more sensitive than that of the plaque assay, assuming that the HAV present was capable of producing plaques in cell cultures. Although the present method does not directly test for infectivity, it is likely that a positive result in IC PCR at least shows that the HAV RNA is still coated by capsid protein. Despite the few nucleotide differences observed in the environmental isolates of HAV, IC PCR was able to detect them, probably because of the high conservation found in VP3-VP1 protein sequences. The study of HAV sequence variations in environmental isolates will provide potentially useful information for addressing epidemiological questions such as pathways for viral spread and viral pathogenicity.
In summary, we have developed a rapid and sensitive IC PCR technique for the identification of HAV in water and sewage samples; it also appears applicable to testing of foods and to studying the molecular epidemiology of HAV. This procedure can be carried out in less than 24 h. If an appropriate mixture of magnetic beads coated with antibodies to different viruses could be developed, this procedure might permit the simultaneous detection of other enteric viruses in environmental samples.
ACKNOWLEDGMENTS
N. Jothikumar was supported by a fellowship from the Department of Biotechnology, Government of India. This study was supported by the Livestock Disease Research Laboratory, School of Veterinary Medicine, University of California, Davis, and by U.S. Department of Agriculture NRICGP agreement 96-35201-3370.
We thank Ming Qi Deng for technical assistance.
REFERENCES
- 1.Advisory Committee on Immunization Practices. License of inactivated hepatitis A vaccine and recommendations for use among international travelers. Morbid Mortal Weekly Rep. 1995;44:559–560. [PubMed] [Google Scholar]
- 2.Agnes F, Crance J M, Leveque F. Separate detection of the two complementary RNA strands of hepatitis A virus. J Virol Methods. 1994;49:323–330. doi: 10.1016/0166-0934(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 3.Bean N H, Goulding J S, Lao C, Angulo F J. Surveillance for foodborne-disease outbreaks—United States, 1988–1992. Morbid Mortal Weekly Rep. 1996;45(SS-5):1–66. [PubMed] [Google Scholar]
- 4.Bennet A R, Macphee S, Betts R P. The isolation and detection of Escherichia coli O157 by use of immunomagnetic separation and immunoassay procedures. Lett Appl Microbiol. 1996;22:237–243. doi: 10.1111/j.1472-765x.1996.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapman P A, Siddons C A. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from cases of bloody diarrhoea, non-bloody diarrhoea and asymptomatic contacts. J Med Microbiol. 1996;44:267–271. doi: 10.1099/00222615-44-4-267. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J I, Ticehurst J R, Purcell R H, Buckler-White A, Baroudy B M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987;61:50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng M Q, Cliver D O, Mariam T W. Immunomagnetic capture PCR to detect viable Cryptosporidium parvum oocysts from environmental samples. Appl Environ Microbiol. 1997;63:3134–3138. doi: 10.1128/aem.63.8.3134-3138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng M Y, Day S P, Cliver D O. Detection of hepatitis A virus in environmental samples by antigen capture PCR. Appl Environ Microbiol. 1994;60:1927–1933. doi: 10.1128/aem.60.6.1927-1933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton A D, Clesceri L S, Greenberg A E, editors. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C: American Public Health Association; 1995. pp. 9-92–9-95. [Google Scholar]
- 11.Fratamico P M, Schultz F L, Buchanan R L. Rapid isolation of Escherichia coli O157:H7 from enrichment cultures of food using immunomagnetic separation method. Food Microbiol. 1992;9:105–113. [Google Scholar]
- 12.Graff J, Ticehurst J, Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Appl Environ Microbiol. 1993;59:3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinde B, Jonassen T O, Ushijima H. Sensitive detection of group A rotaviruses by immunomagnetic separation and reverse transcription polymerase chain reaction. J Virol Methods. 1995;55:327–338. doi: 10.1016/0166-0934(95)00070-x. [DOI] [PubMed] [Google Scholar]
- 14.Ijzerman M M, Dahling D R, Fout G S. A method to remove environmental inhibitors prior to the detection of waterborne enteric viruses by reverse transcription-polymerase chain reaction. J Virol Methods. 1997;63:145–153. doi: 10.1016/s0166-0934(96)02123-4. [DOI] [PubMed] [Google Scholar]
- 15.Jansen R W, Siegel G, Lemon S M. Molecular epidemiology of hepatitis A defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci USA. 1990;87:2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jothikumar N, Aparna K, Kamatchiammal S, Paulmurugan R, Saravanadevi S, Khanna P. Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:2558–2562. doi: 10.1128/aem.59.8.2558-2562.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jothikumar N, Cliver D O. Elution and reconcentration of coliphages in water from positively charged membrane filters with urea-arginine phosphate buffer. J Virol Methods. 1997;65:281–286. doi: 10.1016/s0166-0934(97)02195-2. [DOI] [PubMed] [Google Scholar]
- 18.López-Sabater E I, Deng M Y, Cliver D O. Magnetic immunoseparation PCR assay (MIPA) for detection of hepatitis A virus (HAV) in American oyster (Crassostrea virginica) Lett Appl Microbiol. 1996;24:101–104. doi: 10.1046/j.1472-765x.1997.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.Monceyron C, Grinde B. Detection of hepatitis A virus in clinical and environmental samples by immunomagnetic separation and PCR. J Virol Methods. 1994;46:157–166. doi: 10.1016/0166-0934(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 20.Mortlock S. Recovery of Escherichia coli O157:H7 from mixed suspension: evaluation and comparison of pre-coated immunomagnetic beads and direct plating. Br J Biomed Sci. 1994;51:207–214. [PubMed] [Google Scholar]
- 21.Nasser A M. Prevalence and fate of hepatitis A virus in water. Crit Rev Water Sci Technol. 1994;24:281–323. [Google Scholar]
- 22.Prevot J S, Dubrou S, Marechal J. Detection of human hepatitis A virus in environmental water by an antigen-capture polymerase chain reaction. Water Sci Technol. 1993;27:227–233. [Google Scholar]
- 23.Safarik I, Safarikova M, Forsythe S J. The application of magnetic separations in applied microbiology. J Appl Bacteriol. 1995;78:575–585. doi: 10.1111/j.1365-2672.1995.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 24.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]